Abstract
The functional recovery following non-severing peripheral nerve injury (PNI) is often incomplete. Erythropoietin (EPO) is a pleiotropic hormone and it has been shown to protect peripheral nerves following mild and even moderate severity injuries. However, the effectiveness of EPO in severe PNI is largely unknown. In this study, we sought to investigate the neuroprotective effect of a new dose regimen of EPO in severe sciatic nerve crush injury (SSCI). Adult male mice (8 animals/group) were randomly assigned to sham (normal saline, 0.1ml/mouse), SSCI (normal saline, 0.1ml/mouse) and SSCI with EPO (5000 IU/kg) groups. SSCI was performed using calibrated forceps for 30 sec. EPO or normal saline was administered intraperitoneally immediately after the SSCI and at post-injury day 1 and 2. The functional recovery after injury was assessed by sciatic function index (SFI), von Frey Test (VFT), and grip strength test. Mice were euthanized on day 7 and 21 and nerves at injury/peri-injury site were processed for gene (quantitative real-time PCR) and protein (immunohistochemistry) expression analysis. EPO significantly improved SFI, VFT, and hind limb paw grip strength from post-injury day 7. EPO demonstrated significant regulatory effects on mRNA expression of inflammatory (IL-1β and TNF-α), anti-inflammatory (IL-10), angiogenesis (VEGF and eNOS), and myelination (MBP) genes. The protein expression of IL-1β, F4/80, CD31, NF-κB p65, NF-H, MPZ, and DHE (redox-sensitive probe) was also significantly modulated by EPO treatment. In conclusion, the new dose regimen of EPO augments sciatic nerve functional recovery by mitigating inflammatory, anti-inflammatory, oxidative stress, angiogenesis, and myelination components of SSCI.
Keywords: Erythropoietin, sciatic nerve, crush injury, inflammatory, angiogenesis, myelination
1. Introduction
Peripheral nerve injuries (PNI) caused by an accident or trauma can result in physiological and functional disabilities. PNI occurs along a wide spectrum of severity and its recovery is directly dependent on the type and severity of the injury [1–3]. Despite advanced microsurgical repair, the functional recovery after severe PNI is often unsatisfactory and there is no effective medical or adjunctive therapy is available [4,5]. Even microsurgical approaches fail to address the complex cellular and molecular events associated with PNI. While there is an unmet need for new therapeutic and adjunctive strategies to promote functional recovery after PNI, molecular and cellular events within the injured nerve microenvironment remain unclear.
An expanding body of evidence suggests that non-surgical strategies may improve PNI recovery following mild injury through cellular regeneration of nerve structure, which supports function [4,6]. Erythropoietin (EPO) is a U.S. Food and Drug Administration (FDA) approved drug for the treatment of anemia with a minimal side-effect profile [4]. In addition to its well-known erythropoietic function, EPO also has potent anti-inflammatory, vascular, neurotrophic and neuroprotective effects [7–12]. Considering its multiple functions, EPO has gained a tremendous attention as an adjunct to bolster neuronal function, especially in the peripheral nervous system [12–16]. Experimental and clinical studies have demonstrated that EPO can function as a neuroprotective molecule by mediating angiogenesis and mitigating oxidative stress, inflammation, autophagy, and apoptosis [11,13–16]. Although we and others have shown that EPO improves post-PNI functional recovery following mild injuries [17–22], the effectiveness of EPO to protect a nerve following severe injury is not well defined. We hypothesized that a new dose regimen of EPO could improve post-PNI recovery by altering pro-inflammatory, anti-inflammatory, oxidative stress, and angiogenesis regulatory mechanisms following severe sciatic crush injury (SSCI). The aim of this study was to explore the protective effect of a new dose regimen of EPO in a mouse model of SSCI. PNI has significant healthcare implications and there is no current medical treatment option for PNI. Therefore, we aimed to demonstrate the beneficial effects of a new dose regimen of EPO in the complex pathophysiological events following severe PNI and its usefulness as a potential therapeutic adjunctive agent.
2. Materials and methods
2.1. Mouse model of severe sciatic nerve crush injury (SSCI) and experimental design
The experimental design and animal protocols were approved by the ethical committee on animal research at The Pennsylvania State University College of Medicine, Hershey, PA. Ten-week-old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) weighing 25 ± 3g were used in this study. Sciatic nerve crush injury was performed as previously described [17]. Briefly, after intraperitoneal (IP) ketamine (100 mg/kg)/xylazine (10 mg/kg) anesthesia, the right hindlimb was shaved and prepared with alcohol swabs and povidone-iodine (Betadine). Under a precision stereo zoom binocular microscope (Model PZMIII, World Precision Instruments), a lateral skin incision (~2.5 cm) was made along the length of the femur and the sciatic nerve (SN) was bluntly exposed through the iliotibial band. Crush injury was performed ~3 mm proximal to the SN trifurcation using calibrated forceps (5 mm tip width; 18-1107, Miltex Instruments) for 30s. The skin was closed by surgical staples and post-operative slow release buprenorphine (0.05 mg/kg) was given subcutaneously to all animals as an analgesic. The experimental animals (8 animals/group) were randomly assigned to Sham (normal saline, 0.1ml/mouse), SN crush injury (normal saline, 0.1ml/mouse) and SN crush injury with EPO (5000 IU/kg; Epoetin alfa, PROCRIT®) groups. EPO was given intraperitoneally (IP) immediately after surgery and on post-surgery day 1 and 2. The dose of 5000 IU/kg EPO was based on previous studies by us and others (17,18,20–22) and the new dose-regimen of EPO was selected based on our recent dose-escalation study with EPO on mouse severe sciatic nerve crush injury (data not shown) in addition to the fact that similar regimens have been used in humans. Post-injury functional recovery was assessed by walking track analysis (WTA), sensory nerve test (SNT), grip strength test, and hematological test on day 3, 7, 14 and/or 21. The animals were euthanized on post-injury day 7 and 21 to harvest nerves (flash-frozen in liquid nitrogen) for gene (quantitative real-time PCR, qRT-PCR) and protein (immunohistochemistry, IHC) expression analysis.
2.2. Sciatic functional index (SFI)
To evaluate in vivo global motor function recovery, sciatic function index (SFI) was determined by WTA as previously described [17]. Briefly, mice were trained to walk freely along a 77 cm by 7 cm corridor lined with white paper and individual footprint of the hind limbs were obtained by painting each foot with ink before surgery and on post-surgery day 3, 7, 14, and 21. At least three measurable footprints for each hind limb were obtained. Two blinded observers selected three footprints per hind limb, which were measured by digital calipers. SFI was calculated using three parameters of footprints: (1) toe spread (TS, first to the fifth toe), (2) total print length (PL), and (3) intermediate toe spread (IT, second to the fourth toe) and following formula: SFI = −38.3 {(EPL-NPL)/NPL} + 109.5 {(ETS-NTS)/NTS} + 13.3 {(EIT-NIT)/NIT} − 8.8, where E for experimental (injured) and N for normal (contralateral uninjured) sides.
2.3. Hindlimb grip strength test
The grip strength meter (BIO-GS3; Bioseb-In Vivo Research Instruments) was used to measure hindlimb grip strength [23]. Briefly, the mice were restrained properly by holding the scruff and base of the tail and allowed to grasp the angled grid. Mice were then gently pulled along the length of the sensor grid until the grip was released. The displayed value in the “kg” force was recorded with each animal tested 5 times to obtain an average value of the grip strength. Attention was paid to the animals to avoid paw injury and habit formation during each trial.
2.4. Von Frey test (VFT)
Mice were placed in a transparent polycarbonate chamber (~10 × 10 cm) equipped with a metallic mesh floor, ~25 cm above the bench. Animals were allowed to acclimatize prior to testing. SNT was then performed as previously described using Von Frey filament unit (NC12775-08, Touch Test® Sensory Evaluators) [24,25]. In brief, the filament pressure (1g force) was applied to the plantar surface of the hind limb through the mesh floor, until the filament bends (maximum 3 seconds) or animal withdraws the paw. The withdrawal reflex of the hind limb was recorded and each animal tested three times to calculate the percent response of sensory nerve.
2.5. Hematological evaluation
Mice were anesthetized using isoflurane (IsoSol™, VEDCO) and blood samples (125 μl) were collected into the K2EDTA anticoagulant tubes (07 601; Safe-T-Fill, RAM Scientific) via retro-orbital plexus using heparinized microhematocrit capillary tubes (22-260950; Fisher Scientific). Blood samples were immediately processed for hematological evaluations [Hemoglobin (Hb g/dl) and hematocrit (HCT %)] using automatic blood cell counter (Element HT5 Veterinary hematology analyzer).
2.6. RNA isolation and qRT-PCR analysis
Total RNA was extracted from the SN (at injury and peri-injury site) using the miRNeasy mini kit (217004; Qiagen) and the RNA reverse transcribed to cDNA using iScript™ reverse transcription supermix (1708840; Bio-Rad), according to the manufacturer’s instructions. Primers were purchased from Invitrogen (Life Technologies) and the sequences are listed in Supplementary Table 1. qRT-PCR was performed using Fast SYBR Green Master Mix (4385612; Applied Biosystems) with a Step One Plus Real-Time PCR System (Applied Biosystems) for detection of gene expression. Relative mRNA expression of the target genes was normalized to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene. The data were represented as fold change versus respective control.
2.7. Nerve processing and IHC analysis
SN processing and immunofluorescence (IF) staining was performed as previously described with slight modification [20]. Briefly, on day 7 and 21, SN was harvested from the ipsilateral hindlimbs of mice. Nerves were fixed in 4% paraformaldehyde (PFA) solution overnight, washed with 70% alcohol 3 times and embedded in paraffin. The serial 5μm thick longitudinal sections were taken from the embedded blocks using a microtome (Model RM2235, Leica) to evaluate targeted antibodies. Prior to staining, tissue sections were deparaffinized and serially rehydrated using xylene and alcohol respectively. Antigen retrieval was performed using 10 mM sodium citrate buffer (pH 6.0) for 20 min at 95°C. Permeabilization and blocking of nonspecific binding were performed using 1% Triton X-100 and 5% goat serum respectively. Primary antibody staining was performed with anti-NF-H (1:1000; NB300-135, Novus biologicals), anti-MP0 (1:1000; PZ0, AvesLabs), anti-IL-1β (1:250; GTX74034, GeneTex), F4/80 (1:100; MCA497GA, Bio-Rad), anti-NF-κB p65 (1:500; ab86299, abeam) and anti-CD31 (1:100; 553370, BD Pharmingen) followed by incubation with the appropriate secondary antibody Alexa Fluor 488 (1:1000; A11008, Invitrogen) or Alexa Fluor 647 (1:1000; A21449; Invitrogen). Staining without primary antibodies was used as a control for non-specific fluorescence. Nuclei were counter-stained using ProLong™ Gold anti-fade reagent with DAPI (P36935; Invitrogen) and sections were examined under a fluorescent microscope (ZEISS Apotome 2). Stained nerve tissues were analyzed using NIH-ImageJ software.
2.8. Dihydroethidinm (DHE) staining for reactive oxygen species (ROS)
DHE staining was performed to detect the intensity of ROS (superoxide and hydrogen peroxide) as described previously [26]. In brief, SN tissue sections were deparaffinized and serially rehydrated as described above. SN was incubated with DHE (5 μmol/l, Cat# D1168, Invitrogen) in deionized water in a light-protected, humidified chamber (37°C) for 10 min. Slides were washed three times with deionized water for 1 min. Nuclei were counterstained with ProLong™ Gold anti-fade reagent with DAPI and DHE fluorescence was measured using Cy3 (548 nm excitation and 561nm emission; 5-mm slits).
2.9. Hematoxylin and Eosin (H & E) staining
SN tissue sections were deparaffinized and serially rehydrated as described above. The sections were stained with Mayer’s hematoxylin (Cat # MHS32, Sigma-Aldrich) for 8 min and then incubated in 0.2% ammonia water for 10 min. The sections were stained with eosin (71204; Thermo Scientific) for 40s, followed by dehydration with 95% and 100 % alcohol for 5 min, two times. Sections were then cleared in xylene for 5 min, 2 times and mounted with Permount™ medium (SP15-500; Fisher Scientific). H & E stained slides were visualized on the Olympus BX53 microscope.
2.10. Statistical Analyses
The data were analyzed using Student’s t-Test, One-way and two-way analysis of variance (ANOVA) using GraphPad Prism Version 8.2.0. All values are presented as mean ± SEM. Probability (P) values of < 0.05 were considered statistically significant
3. Results
3.1. An effective therapeutic dose regimen of EPO in SSCI
3.1.1. EPO improves post-injury SFI
In previous studies, we showed that the systemic administration of EPO can accelerate sciatic functional recovery after mild and moderate crush injuries but not after severe crush injury [5,9]. It was unknown whether an effective therapeutic dose of EPO would be able to overcome the severity of injury and improve the function. In this study we show that systemic IP administration of EPO (vs. saline) at three doses (5000 IU/kg/day) can treat the increased injury severity and significantly improve post-injury functional recovery (SFI) on day 7 (37.21% vs. 18.54%) and day 14 (85.18% vs. 67.29%) (Fig. 1A, *P<0.05). While EPO treatment restored the SFI to normal level (95.6%) on post-injury day 21, SFI in the saline group remained persistently impaired (85.4%). These findings demonstrate that an effective dose regimen of EPO can overcome the functional deficit produced by SSCI.
Fig. 1.
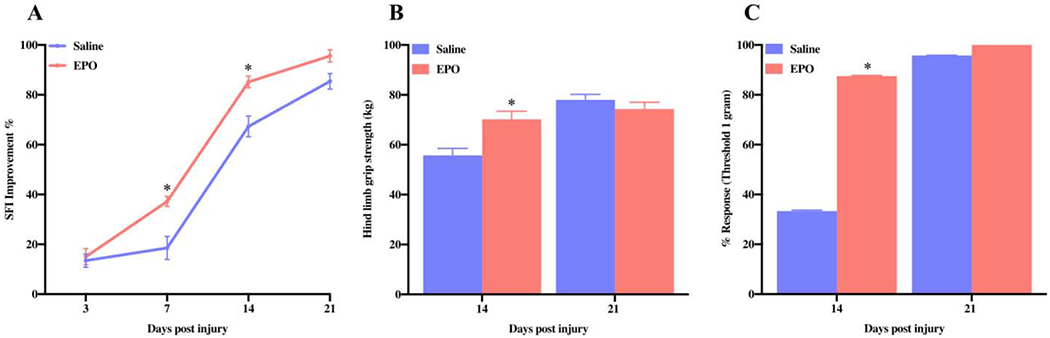
EPO improves nerve function after SSCI. The systemic dosage of EPO (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) significantly enhanced SFI on day 7 and day 14 (A), hind limb grip strength (kg) on day 14 (B), and percent response of nerve on day 14 (C). Data are expressed as the mean ± SEM, *P < 0.05 vs. saline group, n=8 per group.
3.1.2. EPO improves muscle grip strength
Grip strength examines neuromuscular function in the mouse by determining the maximum volitional force produced by the animal [27]. Compared to the saline group, EPO treatment significantly improved hind limb muscle grip strength at post-injury day 14 and it was 70.18 kg vs. 55.73 kg (Fig. 1B, *P<0.05). The muscle strength in both groups on post-injury day 21 was comparable between the groups (EPO, 74.34 kg vs. saline, 77.98 kg). This suggests that EPO treatment improves volitional muscle strength in proportion to improved global motor function (SFI) following SSCI.
3.1.3. EPO accelerates sensory nerve function recovery
Sensory testing (Touch Test®; North Coast Medical) provides a non-invasive assessment of cutaneous sensations by evaluating paw withdrawal thresholds [28]. To determine the effect of EPO on sensory nerve recovery, we performed Von Frey filament testing (Fig. 1C, *P<0.05). Similar to muscle grip strength, EPO treatment also significantly improved withdrawal reflex (percent response to filament) as compared to the saline group (87.50 % vs. 33.33%,*P<0.05) on post-injury day 14 and sensory reflexes in both groups returned to the baseline level on post-injury day 21. These findings demonstrate that EPO can improve sensory nerve function after SSCI.
3.2. Effect of new EPO dose regimen on hematological parameter
The primary function of EPO is to stimulate red blood cells (RBC) production in the bone marrow [29,30]. But too much RBC production can lead to polycythemia. To determine the possible erythropoietic effect of our new EPO dose regimen in mice, we checked the hematological parameter before (day 0) and after EPO treatment (day 7, 14 and 21). We observed a small significant increase in HCT % on day 7 (54.78%) as compared to day 0 (49.92%) (Fig. 2, *P<0.05). However, this increase was transient and HCT% returned to the baseline level by day 14 (49.42%) and day 21 (47.94%). Of note, we did not observe any adverse events in mice with transient rise in HCT%, such as hunched posture, inactivity, respiratory difficulty, leg edema or bleeding issues, with animals remaining normally active all the time, they demonstrated significant improvements in both motor and sensory function. This indicates that our new EPO dose regimen is safe, effective, and non-toxic to the animal, and the effects do not depend on a higher HCT.
Fig. 2.
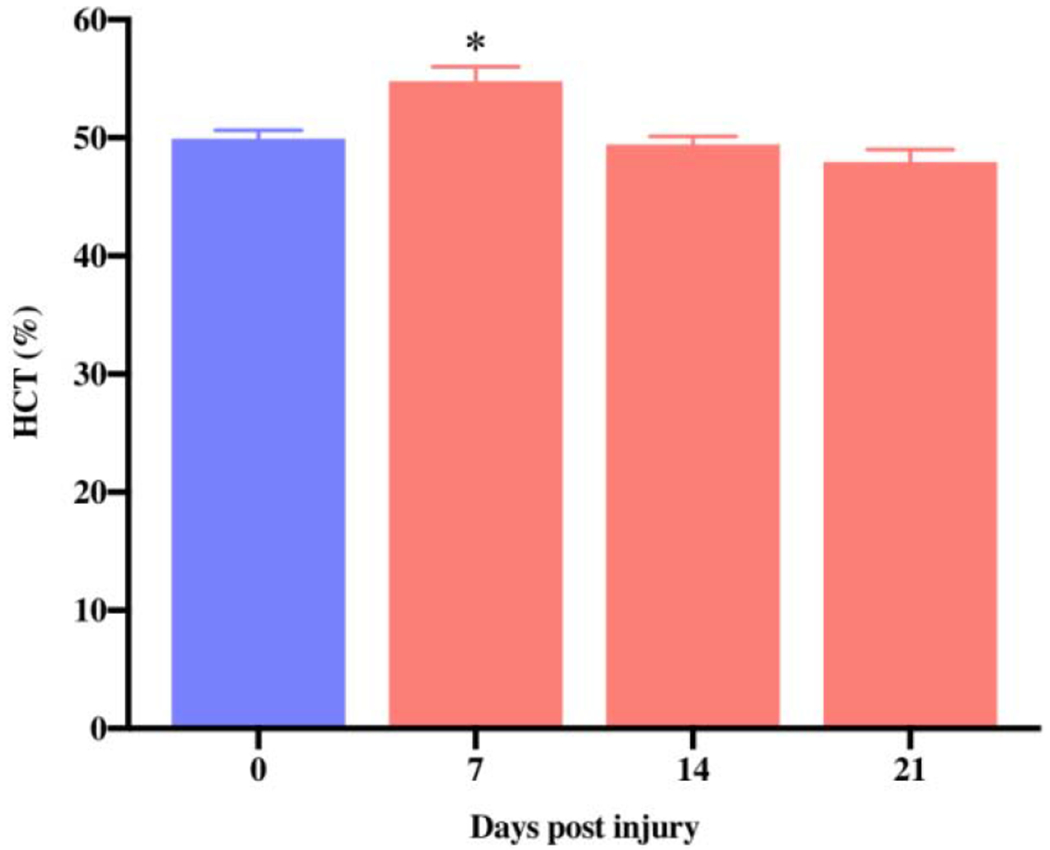
Effect of new EPO dose regimen on HCT level. The systemic dosage of EPO (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) significantly increased HCT% on day 7 and the values returned to the normal level afterwards. Data are expressed as the mean ± SEM, *P < 0.05 vs. before EPO treatment, n=5 per group.
3.3. EPO modulates inflammatory, angiogenesis and myelination components in the injured nerve
EPO is a pleiotropic cytokine reported to mediate neuroprotective effects by regulating inflammatory, oxidative stress, and angiogenesis in PNI [31,32]. To determine the role of these factors in our SSCI model, we analyzed the gene expression profile in the injury or preinjury site of SN using qRT-PCR. The qRT-PCR data on day 7 and day 21 revealed significantly increased mRNA expression level of the inflammatory genes IL-1β (Figs. 3A and 4A, *P <0.05) and TNF-α (Figs. 3B and 4B, *P <0.05) in saline group as compared to the sham group, and EPO treatment totally abolished these increased expressions. However, compared to the sham group, the expression level of anti-inflammatory gene IL-10 at day 7 (Fig. 3C) and 21(Fig. 4C) was significantly increased in both saline and EPO groups (*P <0.05), and there was no significant difference between the saline and EPO groups.
Fig. 3.
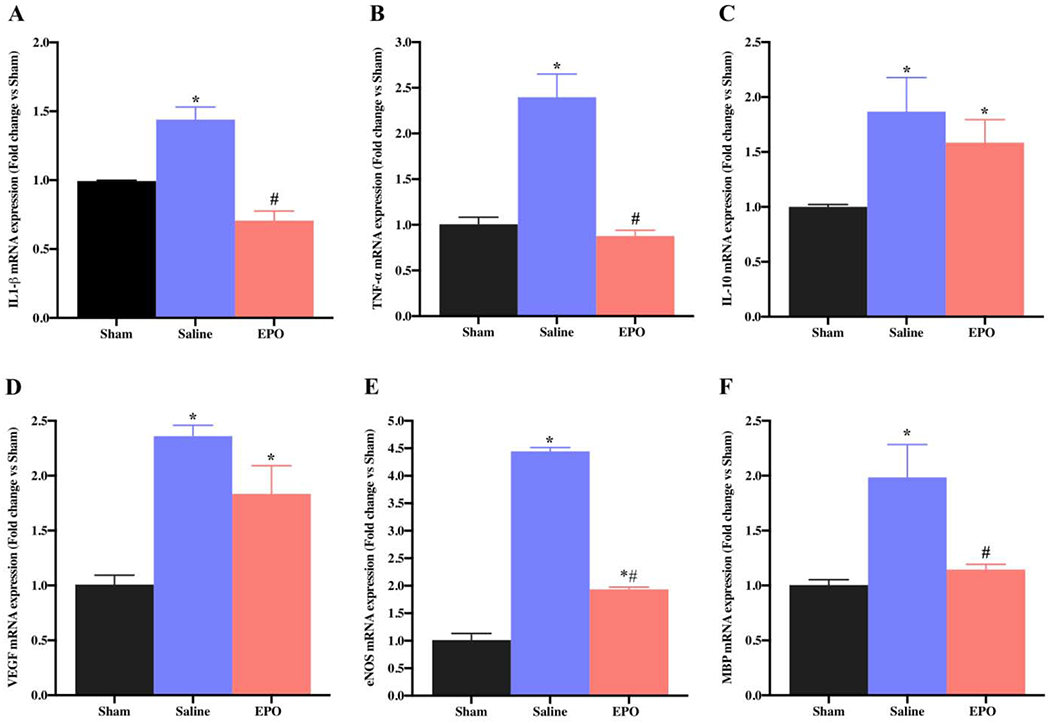
EPO regulates the expression of SN genes after SSCI on post-injury day 7. The systemic dosage of EPO (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) significantly modulated mRNA expression of inflammatory (IL-1β and TNF-α), anti-inflammatory (IL-10), angiogenesis (VEGF and eNOS), and myelination (MBP) genes. Data are expressed as the mean ± SEM, *P < 0.05 vs. sham, #P < 0.05 vs. saline, n=3 per group.
Fig. 4.
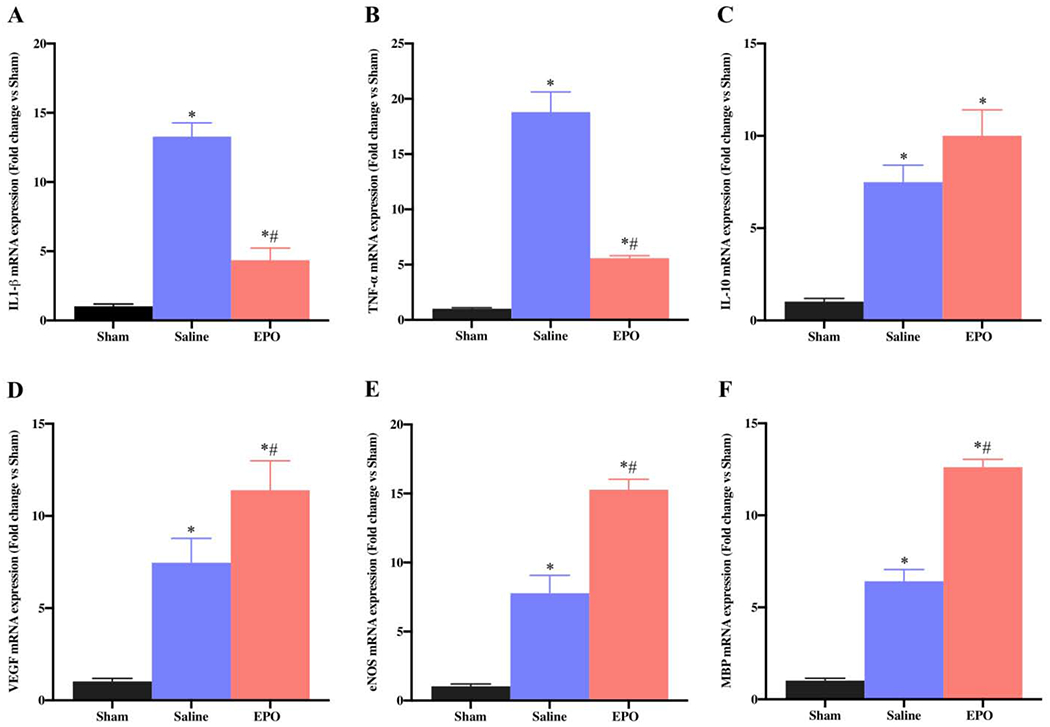
EPO regulates the expression of SN genes after SSCI on post-injury day 21. The systemic dosage of EPO (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) significantly modulated mRNA expression of inflammatory (IL-1β and TNF-α), anti-inflammatory (IL-10), angiogenesis (VEGF and eNOS), and myelination (MBP) genes. Data are expressed as the mean ± SEM, *P < 0.05 vs. sham, #P < 0.05 vs. saline, n=3 per group.
Angiogenesis plays an important role during tissue injury, inflammation and repair, and EPO is known to induce angiogenesis in different experimental models [11,33]. On day 7, compared to sham group, we observed a significantly increased expression levels of vascular endothelial growth factor (VEGF) (Fig. 3D, *P <0.05) and endothelial nitric oxide synthase (eNOS) (Fig. 3E, * P <0.05) in both saline and EPO groups; however, the expression levels were more pronounced in the saline group. In contrast, compared to sham and saline groups on day 21, EPO significantly augmented the upregulation of angiogenic VEGF (Fig. 4D, *#P <0.05) and this upregulation was associated with increased expression of eNOS (Fig. 4E, *#P <0.05). Myelination status of the nerve is a key factor for nerve conduction and function, and myelin basic protein (MBP) is a major constituent of the myelin sheath. On day 7, while MBP gene expression was significantly increased in saline group as compared to sham group (Fig. 3F, * P <0.05), MBP gene expression with EPO treatment was comparable to the sham group (Fig. 3F, * P <0.05). In contrast, compared to sham and saline groups on day 21, EPO significantly augmented the upregulation of MBP gene (Fig. 4F, *# P <0.05).
To evaluate the effect of EPO on post-injury morphological alterations, cellular infiltration/inflammation, and molecular changes, H&E and IF staining were performed with SN. H&E staining of SNs on day 21 in Fig. 5 showed increased infiltration of inflammatory cells and ill-defined myelin sheaths in the saline group as compared to the sham group. Compared to the saline group, EPO treatment markedly reduced the infiltration of inflammatory cells and effectively preserved myelin sheaths. Immunofluorescence analysis on day 7 (Fig. 6) and day 21 (Fig. 7) revealed significantly increased intensity of IL-1β, F4/80 (macrophage marker), and DHE in the saline group as compared to the sham group. Compared to the saline group, EPO treatment either totally abolished or significantly attenuated the increased intensity of IL-1β and F4/80 (Figs. 6A, 6B and Figs. 7A, 7B, # P <0.05) and DHE (Figs. 8A, 8B and Figs. 8C and 8D, # P <0.05) at day 7 and day 21. Figure 9 shows the area fraction % of CD31 (endothelial cell marker) and it was significantly increased in saline group as compared to the sham group on day 7 (Figs. 9A and 9B, *P <0.05) and day 21 (Figs. 9C and 9D, * P <0.05). While EPO treatment had no effect on the area fraction % of CD31 on day 7 (Figs. 9A and 9B), it was significantly augmented by EPO by day 21 (Figs. 9C and 9D, #P <0.05).
Fig. 5.
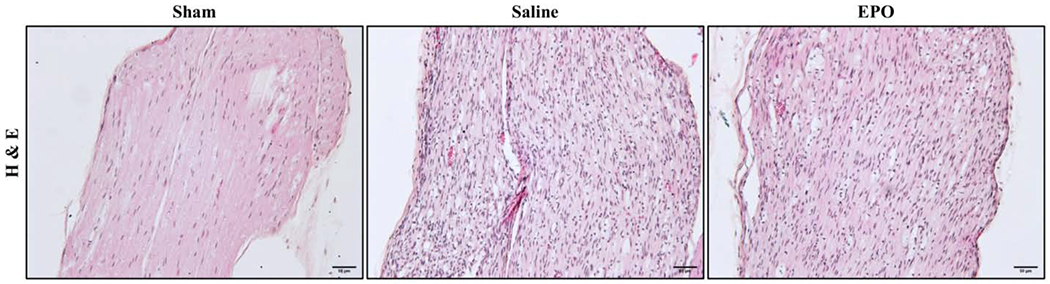
Effect of EPO on SN morphology and cellular infiltrations on day 21 after SSCI. Representative H&E images of SNs showing that EPO treatment (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) markedly attenuated mononuclear cell infiltration and improved the structural organization of SN. Each image represents 9 images from 3 different mice. Scale bar, 50 μm; magnification, 20x.
Fig. 6.
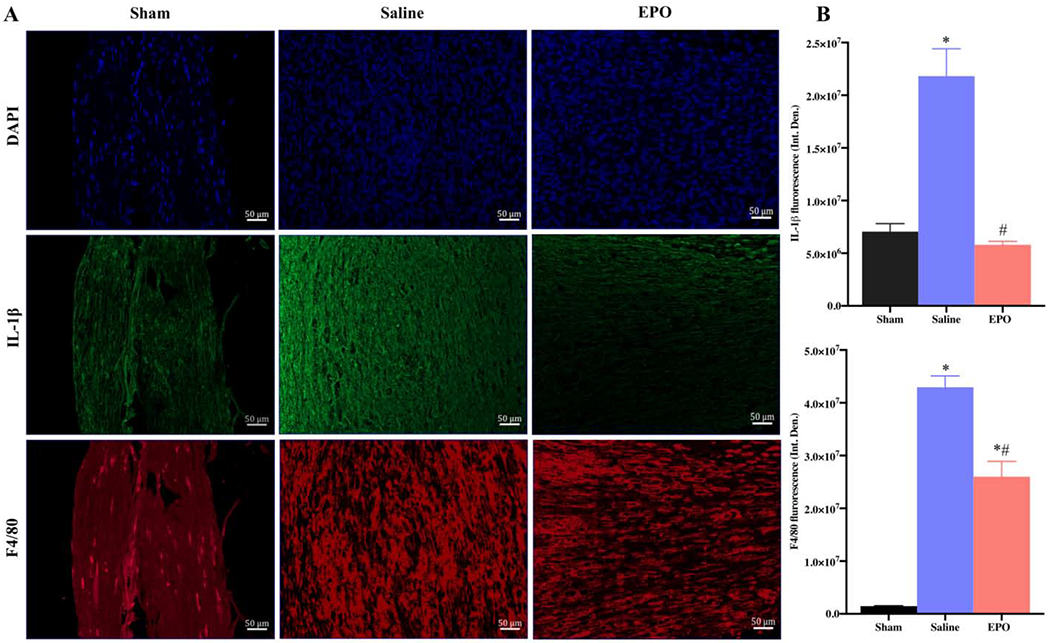
EPO modulates inflammatory processes after SSCI on post-injury day 7. Representative IF images of SNs showing that EPO treatment (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) significantly attenuated IL-1β and F4/80 (macrophage marker) expression (A) and their integrated density of fluorescence (B). Each image represents 9 images from 3 different mice. Scale bar, 50 μm; magnification, 20x. Data are expressed as means ± SEM, * P < 0.05 vs. sham, # P < 0.05 vs. saline, n = 3/group.
Fig. 7.
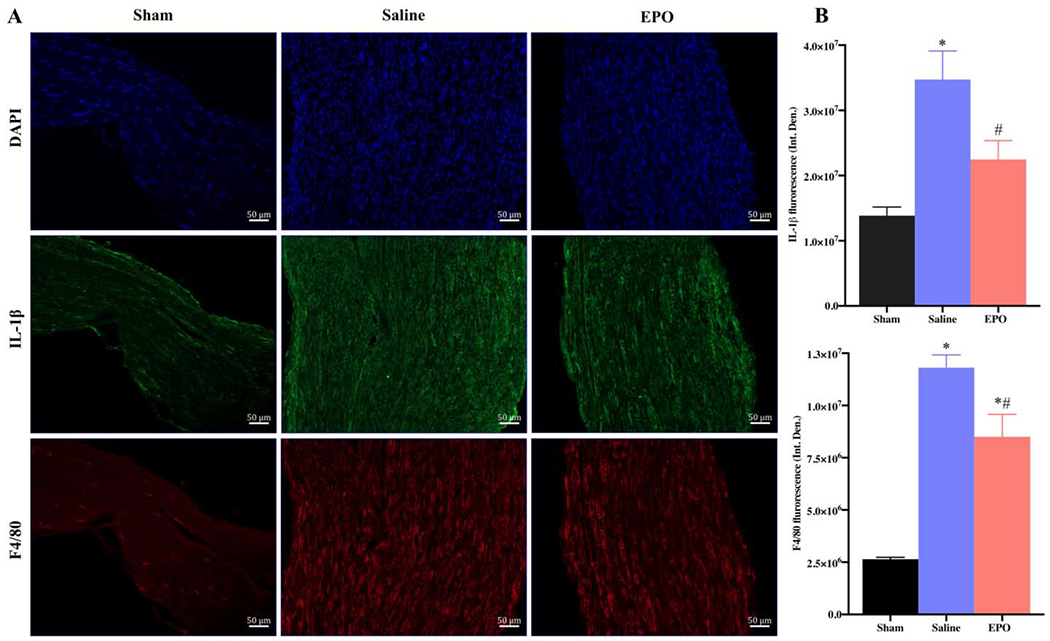
EPO modulates inflammatory processes after SSCI on post-injury day 21. Representative IF images of SNs showing that EPO treatment (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) significantly attenuated IL-1β and F4/80 (macrophage marker) expression (A) and their integrated density of fluorescence (B). Each image represents 9 images from 3 different mice. Scale bar, 50 μm; magnification, 20x. Data are expressed as means ± SEM, * P < 0.05 vs. sham, # P < 0.05 vs. saline, n = 3/group.
Fig. 8.
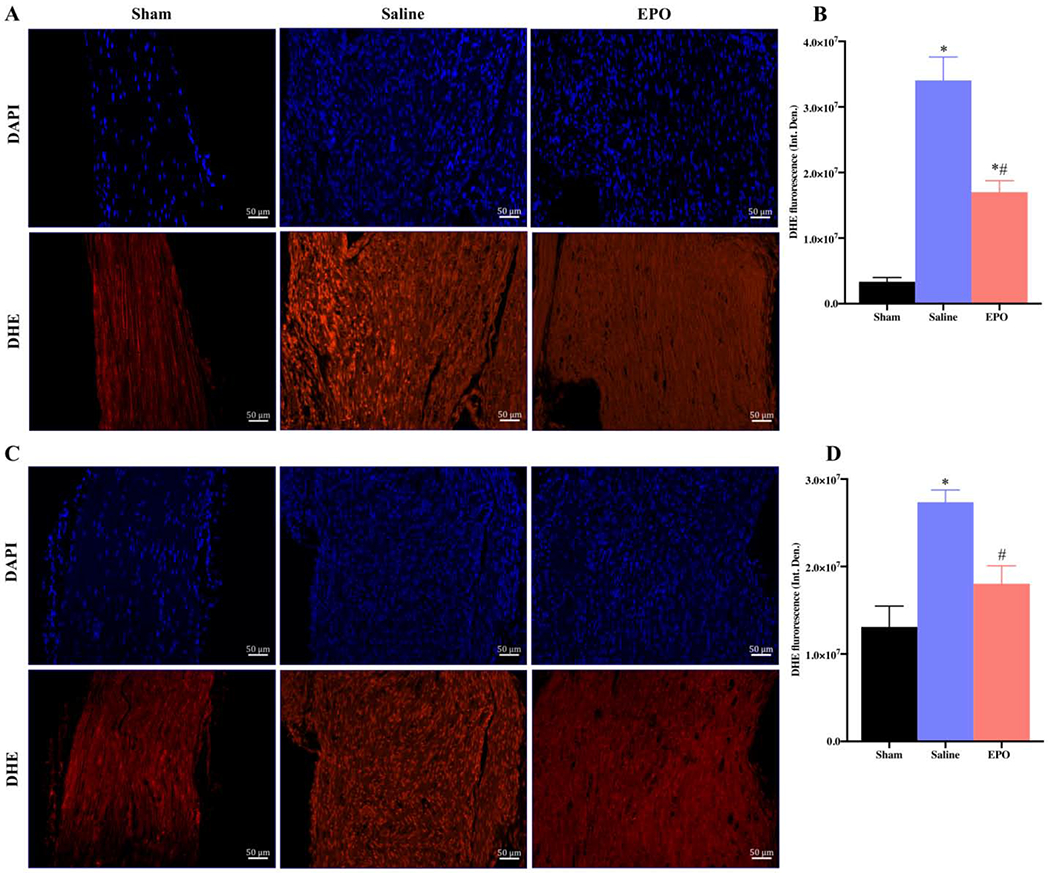
EPO decreases oxidative stress after SSCI. Representative IF images of SNs showing the effect of EPO treatment (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) on DHE expression intensity on post-injury day 7 (A) and day 21 (C). Each image represents 9 images from 3 different mice. Scale bar, 50 μm; magnification, 20x. Quantification of DHE integrated density (Int. Den.) on day 7 (B) and day 21 (D). Data are expressed as means ± SEM, * P < 0.05 vs. sham, # P < 0.05 vs. saline, n = 3/group.
Fig. 9.

EPO augments angiogenesis after SSCI. Representative IF images of SNs showing that the effect of EPO treatment (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) on CD31 expression intensity on post-injury day 7 (A and B) and day 21 (C and D). Each image represents 9 images from 3 different mice. Scale bar, 50 μm; magnification, 20x. Quantification of CD31 area fraction % on day 7 (B) and day 21 (D). Data are expressed as means ± SEM, * P < 0.05 vs. sham, # P < 0.05 vs. saline, n = 3/group.
Nuclear factor-κB (NF-κB) is a transcription factor that regulates the expression of genes/proteins and influences diverse biological processes including cell proliferation, apoptosis, inflammation, oxidative stress, and angiogenesis [34]. The phosphorylated form of NF-κB, NF-κB p65, activates the expression of target genes [35]. We observed that the expression level of NF-κB p65 was significantly increased on day 7 as compared to the sham group (Figs. 10A and 10B, * P <0.05) and EPO had no effect on this increased level of NF-κB. However, by day 21, EPO treatment significantly attenuated the intensity of NF-κB p65 as compared to the saline group (Figs. 10C and 10D, # P <0.05). We also checked the expression level of NF-H and MPZ to evaluate the neuroprotective effect of EPO after nerve injury. Figures 11 and 12 show the expression level of NF-H and MPZ at day 7 and day 21, respectively. On day 7, both NF-H and MPZ levels were significantly reduced in saline group as compared to the sham group (Figs. 11A and 11B, *P <0.05). While EPO treatment had no effect on the reduced levels of NF-H and MPZ on day 7 (Figs. 11A and 11B), the levels of NF-H and MPZ were significantly rescued with EPO treatment by day 21 as compared to the drastically reduced level in the saline group (Figs. 12A and 12B, # P <0.05).
Fig. 10.
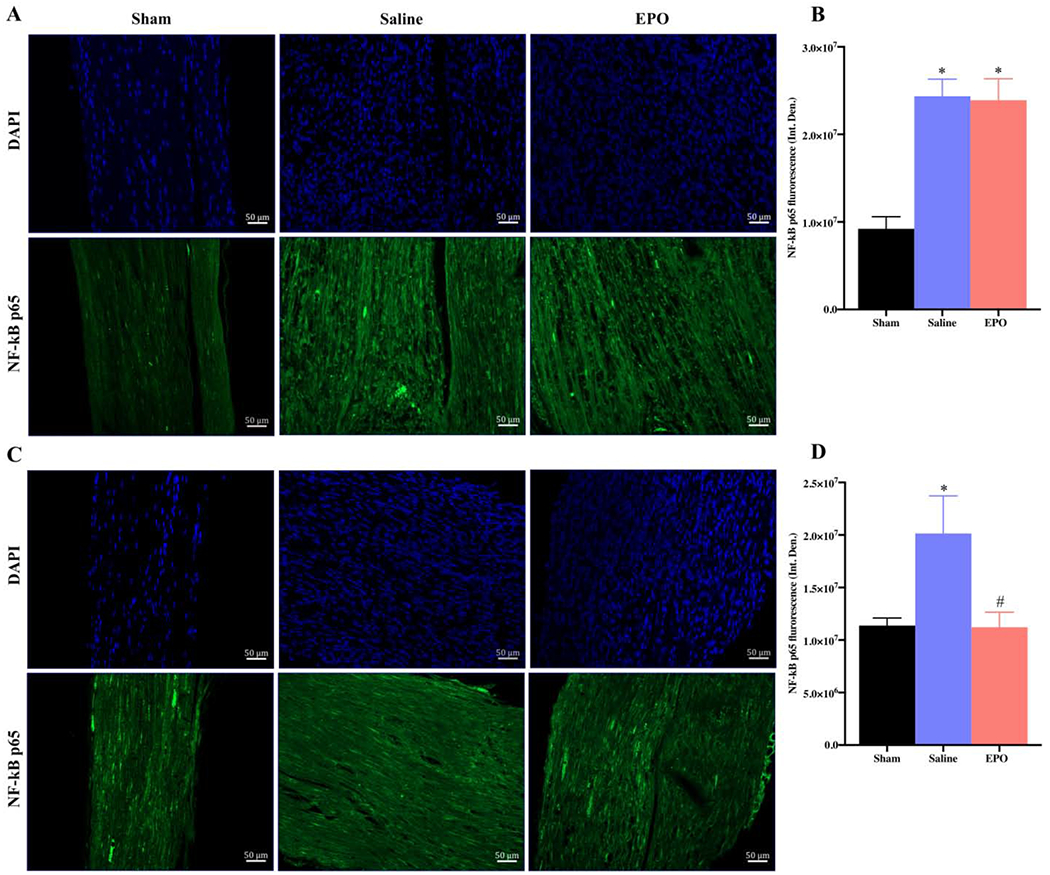
EPO modulates Nuclear Factor-κB after SSCI. Representative IF images of SNs showing that the effect of EPO treatment (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) on NF-κB p65 expression on post-injury day 7 (A and B) and day 21 (C and D). Each image represents 9 images from 3 different mice. Scale bar, 50 μm; magnification, 20x. Quantification of NF-κB p65 integrated density (Int. Den.) on day 7 (B) and day 21 (D). Data are expressed as means ± SEM, * P < 0.05 vs. sham, # P < 0.05 vs. saline, n = 3/group.
Fig. 11.
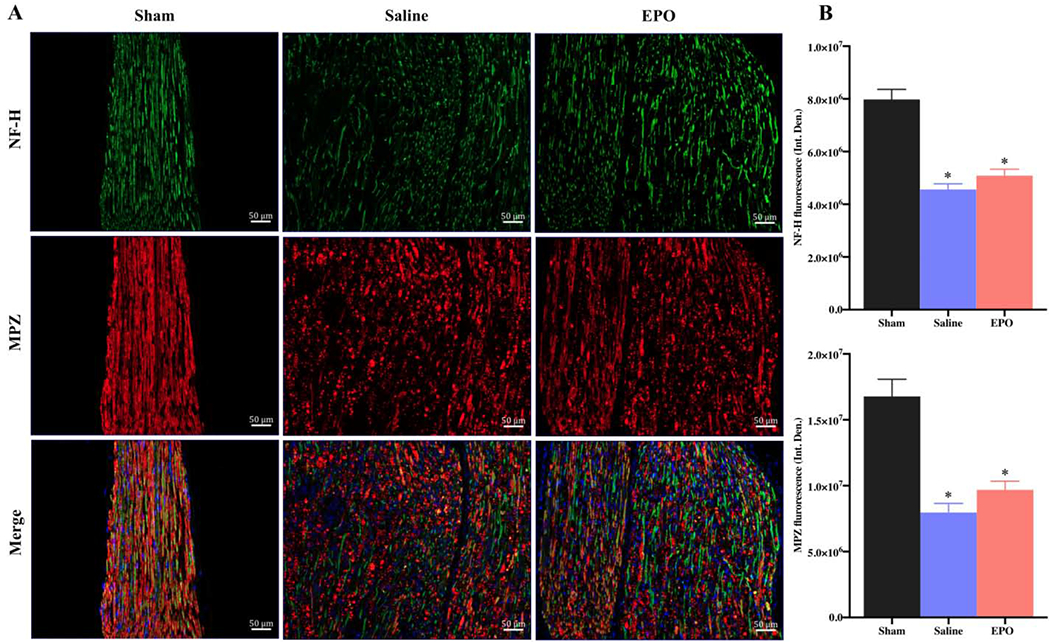
The expression level of neurofilament (NH-F) and myelination (MBZ) proteins after 7 days of SSCI. Representative IF images of SNs showing that the effect of EPO treatment (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) on NF-H and MBZ expression on post-injury day 7 (A). Each image represents 9 images from 3 different mice. Scale bar, 50 μm; magnification, 20x. Quantification of NF-H and MPZ integrated density (Int. Den.) (B). Data are expressed as means ± SEM, * P < 0.05 vs. sham, # P < 0.05 vs. saline, n = 3/group.
Fig. 12.
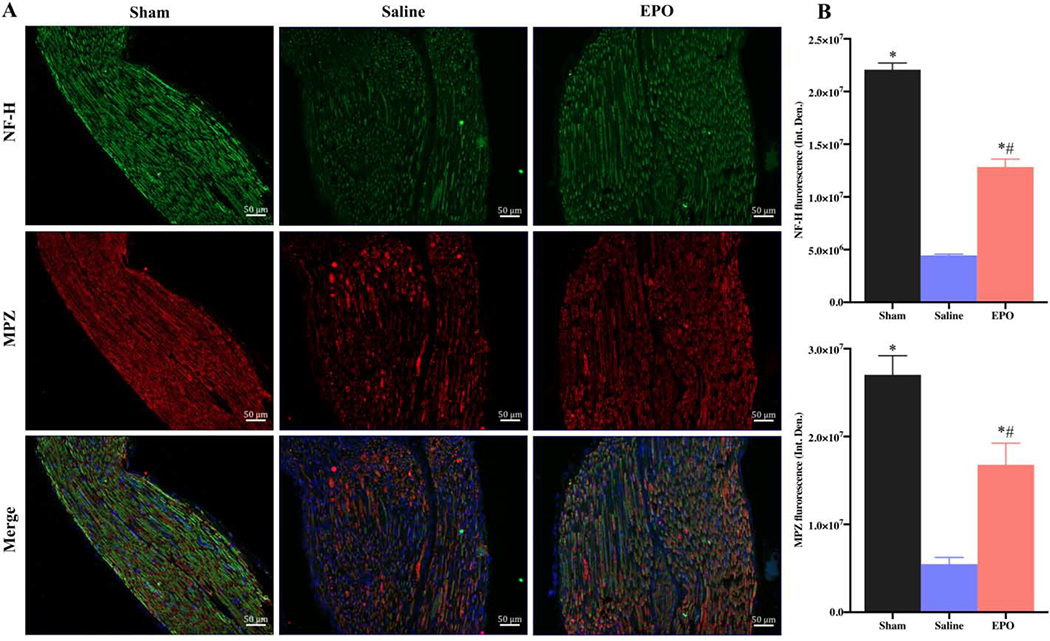
EPO restores neurofilament and myelination proteins after 21 days of SSCI. Representative IF images of SNs showing that EPO treatment (5000 IU/kg/IP, immediately after surgery and on post-surgery day 1 and 2) significantly increased NF-H and MPZ expression (A) on post-injury day 21. Each image represents 9 images from 3 different mice. Scale bar, 50 μm; magnification, 20x. Quantification of NF-H and MPZ integrated density (Int. Den.) (B). Data are expressed as means ± SEM, * P < 0.05 vs. sham, # P < 0.05 vs. saline, n = 3/group.
4. Discussion
The key finding of this study is that a new augmented dose regimen of EPO can overcome severe crush injury-induced nerve pathophysiology and improve post-injury functional recovery by modulating inflammatory, oxidative stress, angiogenesis, and myelination components of nerve injury. We provide new mechanistic insights into the beneficial effects of EPO in severe PNI-induced neural gene expression and functional dysfunction, and further confirm the potent neuroprotective effect of EPO in PNI.
PNI remains an important health problem that often leads to significant functional impairment and permanent disability. Although preclinical and clinical studies have focused on several novel therapeutic approaches for the treatment of PNI, there is no reliable or effective treatment option employed clinically [4,5]. We recently showed that a single dose EPO treatment could promote functional recovery and enhance nerve regeneration after mild to moderate sciatic nerve crush injuries, but not after severe crush injury [18]. In this context, we sought to investigate the effect of a multi-dose augmented EPO regimen in a mouse model of severe PNI. With a new EPO dose regimen, we demonstrate a significant improvement in motor functional recovery from post-injury day 7, and improvements in hind limb grip strength and sensory function from post-injury day 14. Although we observed a minor transient increase in HCT on post-injury day 7, HCT returned to normal level afterwards and we did not observe any adverse events or toxic effects (such as hunched posture, inactivity, respiratory difficulty, leg edema or bleeding problems) associated with this transient elevation of HCT. Notably HCT was not found to exit the normal range even with this augmented EPO dose regimen. Functional recovery with this new EPO dose regimen in SSCI was consistent with the beneficial effects of single dose EPO in milder injuries [17,18,20] and supports the therapeutic importance of the multi-dose EPO regimen in severe PNI treatment.
PNI occurs along a spectrum from injuries in which some axonal continuity is maintained with demyelination (crush or compression injuries) to injuries involving complete nerve transection [1–3]. A complex multicellular event takes place after PNI and the functional recovery is usually slow and incomplete [4,36,37]. The nerve damage that occurs after the severe crushing injury using forceps (mechanical pressure) might be associated with several pathophysiological complexities, such as: A) deformation of the tissues at crush zone, B) ischemic conditions and intraneural edema, C) changes in the electrolytes and nerve conduction, D) inflammation and oxidative stress, E) apoptosis and necrosis, and F) myelin and axonal degradation. The extent of these complexities is directly related to the type and severity of injury [1,37,38]. To address these circumstances, we looked at the expression level of inflammatory, anti-inflammatory, angiogenesis, and myelination genes at injury and peri-injury sites. Our qRT-PCR data demonstrated that in both early (day 7) and late (day 21) stages of injury EPO significantly attenuated mRNA expression of pro-inflammatory genes (IL-1β and TNF-α) and also effectively modulated anti-inflammatory (IL-10) gene. Nerve injury induced hypoxia promotes angiogenesis by upregulating multiple angiogenic factors that mediates key aspects of endothelial cell biology [39,40]. Our result supports an effect of hypoxic microenvironment with increased mRNA expression of VEGF and eNOS in both saline and EPO groups. Interestingly, on day 21, EPO treatment significantly augmented the expression of angiogenic genes compared to the saline group supporting the role of EPO in tissue repair process [41]. It is also evident that EPO’s effective modulation of inflammation, anti-inflammation, and angiogenesis processes can significantly protect against nerve injury by promoting myelination (MBP). The overall findings in this study are in agreement with EPO’s well-recognized potent anti-inflammatory, angiogenic, and neuroprotective effects [7,8,12,42] and thus support the beneficial effects of our new EPO dose regimen in severe PNI.
The beneficial neuroprotective effect of our new EPO dose regimen was also evident in the histopathological evaluation of the injured nerves. We observed that the injured sciatic nerves had intense cellular infiltrations and disrupted myelin sheaths compared to the intact myelin sheaths and negligible cellular infiltrations in control nerves. EPO treatment markedly reduced the extent of cellular infiltrations and maintained the intactness of myelin sheaths. These findings are consistent with the neuroprotective effects of EPO in rats and mice where myelin sheaths were well preserved [21,22,43]. To further investigate the cellular and molecular processes involved in PNI, we performed immunofluorescence staining for IL-1β, F4/80, CD31, NF-κB p65, NF-H, and MPZ proteins. Our findings are very interesting in demonstrating significantly reduced expression of IL-1β and F4/80 with EPO treatment in both early and late stages of nerve injury and significantly increased expression of CD31, NF-H, and MPZ with EPO treatment in the late stage of nerve injury. NF-κB is one of the most important transcription factors regulating gene expression of proinflammatory cytokines and NF-κB is activated following peripheral nerve injuries [44,45]. Several studies have shown that EPO (5000 units/kg) improves neuroinflammation, neuropathic pain, and neural injury by modulating NF-κB activation [45,46,47]. Consistent with these reports, we were also able to show that EPO treatment can significantly reduce crush injury-induced neuronal NF-κB activation on day 21 with near complete functional recovery. In addition, the significantly reduced level of DHE fluorescence in the injured nerves on day 7 and day 21 with EPO treatment demonstrated the potent anti-oxidative effect EPO in PNI [16]. Our findings on day 7 and day 21 also demonstrated an interesting spatially-localized and temporal neuronal effects of EPO where anti-inflammatory and anti-oxidative effects occurred early after treatment compared to its late tissue-regenerative effect (myelination). The overall results in this study are in good agreement with the reported immunomodulatory, angiogenic, and neuroregenerative properties of EPO [9,10,12,18,48,49]. Taken together, we provide robust preclinical evidence for the use of an augmented multi-dose EPO regimen in severe PNI recovery.
In conclusion, this study demonstrates a novel and effective EPO dose regimen that can address increased severity of nerve injury and promote post-injury functional recovery by a complex but coordinated modulation of inflammation, oxidative stress, angiogenesis, and myelination components of nerve injury. Although our findings provide new insight for the potential therapeutic application of EPO in non-severing severe nerve injuries, its effects on nerve transection and gap-graft injuries are largely unknown in preclinical models. EPO is a multifunctional molecule that triggers diverse biological responses. The intracellular cross-talk between different sub-cellular and molecular signaling cascades is poorly understood in relation to EPO. In addition to the spatially-localized effects of EPO in tissues, there is also temporal relationship of its beneficial effects [50]. Moreover, it is also unknown how other cytokines, growth factors, and disease conditions may modify the actions of EPO and the risks of potential adverse effects. While recombinant human EPO has been safely used at haematopoietic doses in many patients, the successful translation of positive preclinical neuroprotective effects of other drugs has failed in clinical trials [51]. Therefore, there is an absolute need for more robust preclinical models of nerve injury. Future studies directed to address, characterize and understand the non-haematopoietic effects of EPO in relation to the above-mentioned factors may contribute to the optimization of its use in diverse peripheral nerve injuries.
Supplementary Material
Highlights.
Characterization of severe sciatic nerve crush injury improving effects of EPO
EPO downregulates pro-inflammatory and upregulates anti-inflammatory neural genes
EPO induces an upregulation of angiogenic and myelination genes in injured nerve
EPO-induced gene expression and protein expression profiles are comparable
EPO attenuates macrophage activation and oxidative stress, and improves function
Acknowledgments
This study was supported by the National Institutes of Health (K08 AR060164-01 A), Department of Defense (W81XWH-16-1-0725) and Pennsylvania State University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- [1].Campbell WW, Evaluation and management of peripheral nerve injury, Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 119 (2008) 1951–1965. 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- [2].Menorca RMG, Fussell TS, Elfar JC, Nerve physiology: mechanisms of injury and recovery, Hand Clin. 29 (2013) 317–330. 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Robinson LR, Traumatic injury to peripheral nerves, Muscle Nerve. 23 (2000) 863–873. . [DOI] [PubMed] [Google Scholar]
- [4].Modrak M, Talukder MAH, Gurgenashvili K, Noble M, Elfar JC, Peripheral nerve injury and myelination: Potential therapeutic strategies, J. Neurosci. Res (2019). 10.1002/jnr.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Houschyar KS, Momeni A, Pyles MN, Cha JY, Maan ZN, Duscher D, Jew OS, Siemers F, van Schoonhoven J, The Role of Current Techniques and Concepts in Peripheral Nerve Repair, Plast. Surg. Int 2016 (2016) 4175293 10.1155/2016/4175293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Martinez de Albornoz P, Delgado PJ, Forriol F, Maffulli N, Non-surgical therapies for peripheral nerve injury, Br. Med. Bull 100 (2011) 73–100. 10.1093/bmb/ldr005. [DOI] [PubMed] [Google Scholar]
- [7].Kowalczyk M, Banach M, Mikhailidis DP, Rysz J, Erythropoietin update 2011, Med. Sci. Monit. Int. Med. J. Exp. Clin. Res 17 (2011) RA240–247. 10.12659/msm.882037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maiese K, Chong ZZ, Shang YC, Wang S, Erythropoietin: new directions for the nervous system, Int. J. Mol. Sci 13 (2012) 11102–11129. 10.3390/ijms130911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Arcasoy MO, The non-haematopoietic biological effects of erythropoietin, Br. J. Haematol 141 (2008) 14–31. 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- [10].Byts N, Siren A-L, Erythropoietin: a multimodal neuroprotective agent, Exp. Transl. Stroke Med 1 (2009) 4 10.1186/2040-7378-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lombardero M, Kovacs K, Scheithauer BW, Erythropoietin: a hormone with multiple functions, Pathobiol. J. Immunopathol. Mol. Cell. Biol 78 (2011) 41–53. 10.1159/000322975. [DOI] [PubMed] [Google Scholar]
- [12].Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H, Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research, Best Pract. Res. Clin. Anaesthesiol 24 (2010) 573–594. 10.1016/j.bpa.2010.10.005. [DOI] [PubMed] [Google Scholar]
- [13].Lykissas MG, Korompilias AV, Vekris MD, Mitsionis GI, Sakellariou E, Beris AE, The role of erythropoietin in central and peripheral nerve injury, Clin. Neurol. Neurosurg 109 (2007) 639–644. 10.1016/j.clineuro.2007.05.013. [DOI] [PubMed] [Google Scholar]
- [14].Rey F, Balsari A, Giallongo T, Ottolenghi S, Di Giulio AM, Samaja M, Carelli S, Erythropoietin as a Neuroprotective Molecule: An Overview of Its Therapeutic Potential in Neurodegenerative Diseases, ASN Neuro. 11 (2019) 1759091419871420 10.1177/1759091419871420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ghezzi P, Brines M, Erythropoietin as an antiapoptotic, tissue-protective cytokine, Cell Death Differ. 11 Suppl 1 (2004) S37–44. 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- [16].Katavetin P, Tungsanga K, Eiam-Ong S, Nangaku M, Antioxidative effects of erythropoietin, Kidney Int. Suppl (2007) S10–15. 10.1038/sj.ki.5002482. [DOI] [PubMed] [Google Scholar]
- [17].Elfar JC, Jacobson JA, Puzas JE, Rosier RN, Zuscik MJ, Erythropoietin accelerates functional recovery after peripheral nerve injury, J. Bone Joint Surg. Am 90 (2008) 1644–1653. 10.2106/JBJS.G00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Geary MB, Li H, Zingman A, Ketz J, Zuscik M, De Mesy Bentley KL, Noble M, Elfar JC, Erythropoietin accelerates functional recovery after moderate sciatic nerve crush injury, Muscle Nerve. 56 (2017) 143–151. 10.1002/mus.25459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grasso G, Meli F, Fodale V, Calapai G, Buemi M, Iacopino DG, Neuroprotective potential of erythropoietin and darbepoetin alfa in an experimental model of sciatic nerve injury. Laboratory investigation, J. Neurosurg. Spine. 7 (2007) 645–651. 10.3171/SPI-07/12/645. [DOI] [PubMed] [Google Scholar]
- [20].Sundem L, Chris Tseng K-C, Li H, Ketz J, Noble M, Elfar J, Erythropoietin Enhanced Recovery After Traumatic Nerve Injury: Myelination and Localized Effects, J. Hand Surg 41 (2016) 999–1010. 10.1016/jjhsa.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang C-T, Chen S-H, Lin S-C, Chen W-T, Lue J-H, Tsai Y-J, Erythropoietin reduces nerve demyelination, neuropathic pain behavior and microglial MAPKs activation through erythropoietin receptors on Schwann cells in a rat model of peripheral neuropathy, Glia. 66 (2018) 2299–2315. 10.1002/glia.23461. [DOI] [PubMed] [Google Scholar]
- [22].Yin Z-S, Zhang H, Bo W, Gao W, Erythropoietin promotes functional recovery and enhances nerve regeneration after peripheral nerve injury in rats, AJNR Am. J. Neuroradiol 31 (2010) 509–515. 10.3174/ajnr.A1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hwee DT, Kennedy A, Ryans J, Russell AJ, Jia Z, Hinken AC, Morgans DJ, Malik FI, Jasper JR, Fast skeletal muscle troponin activator tirasemtiv increases muscle function and performance in the B6SJL-SOD1G93A ALS mouse model, PloS One 9 (2014) e96921 10.1371/journal.pone.0096921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bonin RP, Bories C, De Koninck Y, A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments, Mol. Pain 10 (2014) 26 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhao W-J, Gao Z-Y, Wei H, Nie H-Z, Zhao Q, Zhou X-J, Wang Y-X, Spinal D-amino acid oxidase contributes to neuropathic pain in rats, J. Pharmacol. Exp. Ther 332 (2010) 248–254. 10.1124/jpet.109.158816. [DOI] [PubMed] [Google Scholar]
- [26].Wang Q, Zou M-H, Measurement of Reactive Oxygen Species (ROS) and Mitochondrial ROS in AMPK Knockout Mice Blood Vessels, Methods Mol. Biol. Clifton NJ. 1732 (2018) 507–517. 10.1007/978-1-4939-7598-3_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Takeshita H, Yamamoto K, Nozato S, Inagaki T, Tsuchimochi H, Shirai M, Yamamoto R, Imaizumi Y, Hongyo K, Yokoyama S, Takeda M, Oguro R, Takami Y, Itoh N, Takeya Y, Sugimoto K, Fukada S-I, Rakugi H, Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice, Sci. Rep 7 (2017) 42323 10.1038/srep42323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Martinov T, Mack M, Sykes A, Chatterjea D, Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von Frey apparatus, J. Vis. Exp. JoVE. (2013) e51212 10.3791/51212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chateauvieux S, Grigorakaki C, Morceau F, Dicato M, Diederich M, Erythropoietin, Erythropoiesis and beyond, Biochem. Pharmacol 82 (2011) 1291–1303. 10.1016/j.bcp.2011.06.045. [DOI] [PubMed] [Google Scholar]
- [30].Robinson N, Giraud S, Saudan C, Baume N, Avois L, Mangin P, Saugy M, Erythropoietin and blood doping, Br. J. Sports Med 40 Suppl 1 (2006) i30–34. 10.1136/bjsm.2006.027532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nairz M, Sonnweber T, Schroll A, Theurl I, Weiss G, The pleiotropic effects of erythropoietin in infection and inflammation, Microbes Infect. 14 (2012) 238–246. 10.1016/j.micinf.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Heitrich M, de DM Garcia LA, Stoyanoff TR, Rodriguez JP, Todaro JS, Aguirre MV , Erythropoietin attenuates renal and pulmonary injury in polymicrobial induced-sepsis through EPO-R, VEGF and VEGF-R2 modulation, Biomed. Pharmacother. Biomedecine Pharmacother 82 (2016) 606–613. 10.1016/j.biopha.2016.05.045. [DOI] [PubMed] [Google Scholar]
- [33].Henry DH, Bowers P, Romano MT, Provenzano R, Epoetin alfa. Clinical evolution of a pleiotropic cytokine, Arch. Intern. Med 164 (2004) 262–276. 10.1001/archinte.164.3.262. [DOI] [PubMed] [Google Scholar]
- [34].Oeckinghaus A, Ghosh S, The NF-kappaB family of transcription factors and its regulation, Cold Spring Harb. Perspect. Biol 1 (2009) a000034 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Christian F, Smith EL, Carmody RJ, The Regulation of NF-κB Subunits by Phosphorylation, Cells. 5 (2016). 10.3390/cells5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Grinsell D, Keating CP, Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies, BioMed Res. Int 2014 (2014) 698256 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Burnett MG, Zager EL, Pathophysiology of peripheral nerve injury: a brief review, Neurosurg. Focus. 16 (2004) El 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- [38].Gordon T, The physiology of neural injury and regeneration: The role of neurotrophic factors, J. Commun. Disord 43 (2010) 265–273. 10.1016/jjcomdis.2010.04.003. [DOI] [PubMed] [Google Scholar]
- [39].Chen H, Li J, Liang S, Lin B, Peng Q, Zhao P, Cui J, Rao Y, Effect of hypoxia-inducible factor-1/vascular endothelial growth factor signaling pathway on spinal cord injury in rats, Exp. Ther. Med 13 (2017) 861–866. 10.3892/etm.2017.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Krock BL, Skuli N, Simon MC, Hypoxia-induced angiogenesis: good and evil, Genes Cancer. 2(2011) 1117–1133. 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang L, Chopp M, Gregg SR, Zhang RL, Teng H, Jiang A, Feng Y, Zhang ZG, Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF, J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 28 (2008) 1361–1368. 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Campana WM, Li X, Shubayev VT, Angert M, Cai K, Myers RR, Erythropoietin reduces Schwann cell TNF-alpha, Wallerian degeneration and pain-related behaviors after peripheral nerve injury, Eur. J. Neurosci 23 (2006) 617–626. 10.1111/j.1460-9568.2006.04606.x. [DOI] [PubMed] [Google Scholar]
- [43].Zhang W, Gao Y, Zhou Y, Liu J, Zhang L, Long A, Zhang L, Tang P, Localized and sustained delivery of erythropoietin from PLGA microspheres promotes functional recovery and nerve regeneration in peripheral nerve injury, BioMed Res. Int 2015 (2015) 478103 10.1155/2015/478103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jia H, Feng X, Li W, Hu Y, Zeng Q, Liu J, Xu J, Recombinant human erythropoietin attenuates spinal neuroimmune activation of neuropathic pain in rats, Ann. Clin. Lab. Sci 39 (2009)84–91. [PubMed] [Google Scholar]
- [45].Ma W, Bisby MA, Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries, Brain Res. 797 (1998) 243–254. 10.1016/s0006-8993(98)00380-1. [DOI] [PubMed] [Google Scholar]
- [46].Jia H, Jin Y, Ji Q, Hu Y, Zhou Z, Xu J, Yang J, Effects of recombinant human erythropoietin on neuropathic pain and cerebral expressions of cytokines and nuclear factor-kappaB, Can. J. Anaesth. J. Can. Anesth 56 (2009) 597–603. 10.1007/s12630-009-9111-0. [DOI] [PubMed] [Google Scholar]
- [47].Tegeder I, Niederberger E, Schmidt R, Kunz S, Giihring H, Ritzeler O, Michaelis M, Geisslinger G, Specific Inhibition of IkappaB kinase reduces hyperalgesia in inflammatory and neuropathic pain models in rats, J. Neurosci. Off. J. Soc. Neurosci 24 (2004) 1637–1645. 10.1523/JNEUROSCI.3118-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mausberg AK, Meyer Zu Horste G, Dehmel T, Stettner M, Lehmann HC, Sheikh KA, Kieseier BC, Erythropoietin ameliorates rat experimental autoimmune neuritis by inducing transforming growth factor-β in macrophages, PloS One. 6 (2011) e26280 10.1371/journal.pone.0026280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Luo B, Jiang M, Yang X, Zhang Z, Xiong J, Schluesener HJ, Zhang Z, Wu Y, Erythropoietin is a hypoxia inducible factor-induced protective molecule in experimental autoimmune neuritis, Biochim. Biophys. Acta 1832 (2013) 1260–1270. 10.1016/j.bbadis.2013.04.015. [DOI] [PubMed] [Google Scholar]
- [50].Brines M, Cerami A, Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 264 (2008) 405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- [51].O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW, 1,026 experimental treatments in acute stroke, Ann. Neurol 59 (2006) 467–477. 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


