Abstract
Directional and positional information is essential for the diverse neuronal morphology and connectivity during development. The direction of axon growth helps build the correct networks among neurons sometimes from far away. Neuronal synapses are asymmetric cell-cell junctions with distinct pre- and postsynaptic structures to convey neural activity in a directional fashion. Recent studies show that some of the key asymmetry is mediated by highly conversed cell polarity signaling pathways. These pathways, planar cell polarity and apical-basal polarity, are not required for the global axon-dendrite polarity. Therefore, the apparent distinct types of morphological asymmetry in the nervous system, growth cone turning and synaptic junctions, are mediated by similar cell polarity signaling mechanisms widely used in cellular and tissue morphogenesis.
Planar cell polarity and apical basal polarity components in axon guidance
Brain wiring relies on directed growth of axons to find the correct synaptic partners. During development, the main axon tracks form along the major body axes. Wnts were the first identified guidance cues for axon growth along the anterior-posterior axis [1]. Multiple Wnts, expressed in an anterior-posterior decreasing gradient along the length of the rodent spinal cord, attract ascending commissural axons to turn anteriorly (rostrally) after midline (floor plate) crossing (Figure 1A, 1B) [2]. Along the same longitudinal axis, Wnts repel descending corticospinal tract axons to grow posteriorly down along the spinal cord (caudally) (Figure 1A) [3]. Subsequent work showed that Wnts are conserved guidance cues along the anterior-posterior axis in C. elegans, zebrafish and chick (Figure 1C) [4] [5] [6] [7] [8]. In the brainstem, the Wnt gradients are more complex along the A-P axis. However, Wnts also provide directionality for dopaminergic and serotonergic axons (Figure 1D) [9]. In addition to providing direction along the A-P axis, Wnt gradients control dorsal-ventral topographic mapping in the chick visual system [10], as well as retinotopic mapping in the Drosophila visual system also along the dorsal-ventral axis [11].
Figure 1. Wnt/planar cell polarity is a conserved axon guidance signaling system.
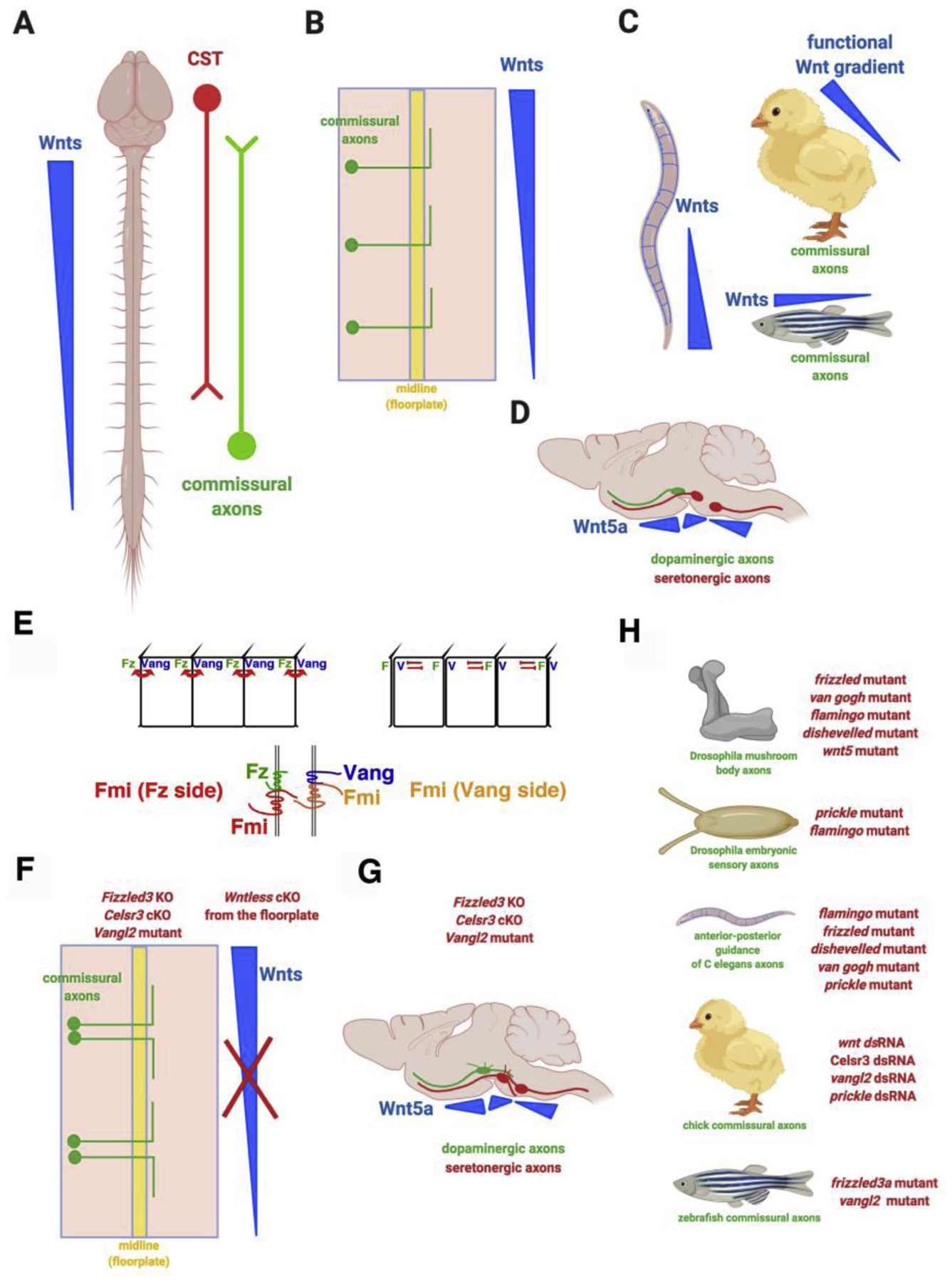
A. Wnts are guidance cues for anterior-posterior (rostral-caudal) pathfinding of ascending and descending axons in rodent spinal cord. CST: corticospinal tract.
B. Ascending spinal cord commissural axons turn anteriorly after they cross the midline, the floor plate.
C. Wnts are anterior-posterior axon guidance cues in C elegans. Wnts guidance spinal cord commissural axons to turn anteriorly after midline crossing in chick and zebrafish.
D. Wnt gradients guide the anterior-posterior growth of dopaminergic and serotonergic axons in the brainstem.
E. Schematics of the distribution of and the interactions among the planar cell polarity signaling components within the same epithelial cell or between the neighboring cells: Fmi (Flamingo or Celsr), Fz (Frizzled), Vang (Van Gogh).
F. Spinal cord commissural axons lose directionality along the anterior-posterior axis, turning randomly along the A-P axis after midline crossing in Frizzled3, Ceslr3 and Vangl2 mutants and in floor plate-specific conditional knockout of Wntless, encoding the Wnt cargo receptor required for the secretion of all Wnt proteins
G. Wnt gradients guide the anterior-posterior growth of dopaminergic and serotonergic axons in the brainstem via the planar cell polarity components.
H. PCP components are also required for the guidance of mushroom body axons in Drosophila, sensory axons in Drosophila, mechanosensory axons in C. elegans, chick spinal cord commissural axons and zebrafish spinal cord commissural axons.
It was surprising that the Wnt family morphogens, better known to activate different sets of gene expression at different concentrations, can act as guidance cues to provide the direction to guide axon growth. This prompted the investigation of the signaling mechanisms and lead to the finding that a non-canonical Wnt signaling pathway, planar cell polarity (PCP), mediates axon attraction in rodents within the axonal growth cones (Figure 1E–1G) [9] [12] [13]. Many epithelial tissues show planar cell polarity (PCP), which is the global morphological and structural asymmetry of cells along the plane of the tissues [14,15]. PCP establishes the orientations of hairs, bristles and ommatidia in Drosophila. It also orients stereociliary bundles within the inner ear and directs cell movement in convergent extension during gastrulation in mammals [16] [17]. The Frizzled/Flamingo core PCP components involve three transmembrane proteins, Frizzled (Fzd), Flamingo/starry night (Fmi/Stan or Celsr in vertebrates), and Van Gogh/Strabismus (Vang/Stbm or Vangl), as well as three cytoplasmic proteins, Dishevelled (Dvl), Diego (Dgo) and Prickle (Prkl) (Figure 1E) [18]. PCP signaling was first shown to mediate growth cone turning in response to Wnt gradients in rodent commissural axons and serotonergic and dopaminergic axons in the brainstem [2,9,12,13] (Figure 1F, 1G). Subsequent work confirmed the conserved function of PCP signaling in A-P axon guidance in invertebrates and other vertebrate species [19] [20] [21] [22] [23] [24] [8]. (Figure 1H).
The identification of the role of Wnt/PCP pathway in growth cone guidance provides great opportunities to understand how growth cones detect guidance cues and then signal to turn in directed ways. This then prompted subsequent studies of the biochemical mechanism of how PCP components function in growth cone guidance (Figure 2A, 2B). The first breakthrough was the finding that Frizzled3 phosphorylation, which is antagonistically regulated by Vangl2 and Dishevelled1, regulates its subcellular localization [12]. Frizzled3 hyperphosphorylation, promoted by Dishevlled1, increases its localization on the plasma membrane. Vangl2 inhibits Frizzled3 hyperphosphorylation and promotes Frizzled3 endocytosis and thus less plasma membrane localization. It was subsequently found that Frizzled3 endocytosis is mediated by Arf6, which binds to unphosphorylated Frizzled3, and occurs at the tips of growth cone filopodia [13]. Vangl2 is enriched at the tips of growth cone filopodia what are growing longer but not at those which are shrinking (Figure 2C) [12]. Dishevelled2, surprisingly, antagonizes Dishevelled1 and inhibits Frizzled3 hyperphosphorylation and promotes Frizzled3 endocytosis (Figure 2A, 2B) [13]. Like Vangl2 and Dvl2, an apical-basal polarity component, atypical PKC, also inhibits Frizzled3 hyperphosphorylation and promotes Frizzled3 endocytosis [13].
Figure 2. Antagonistic interactions of planar cell polarity components in axon guidance and spatial and temporal regulation by Sonic Hedgehog.
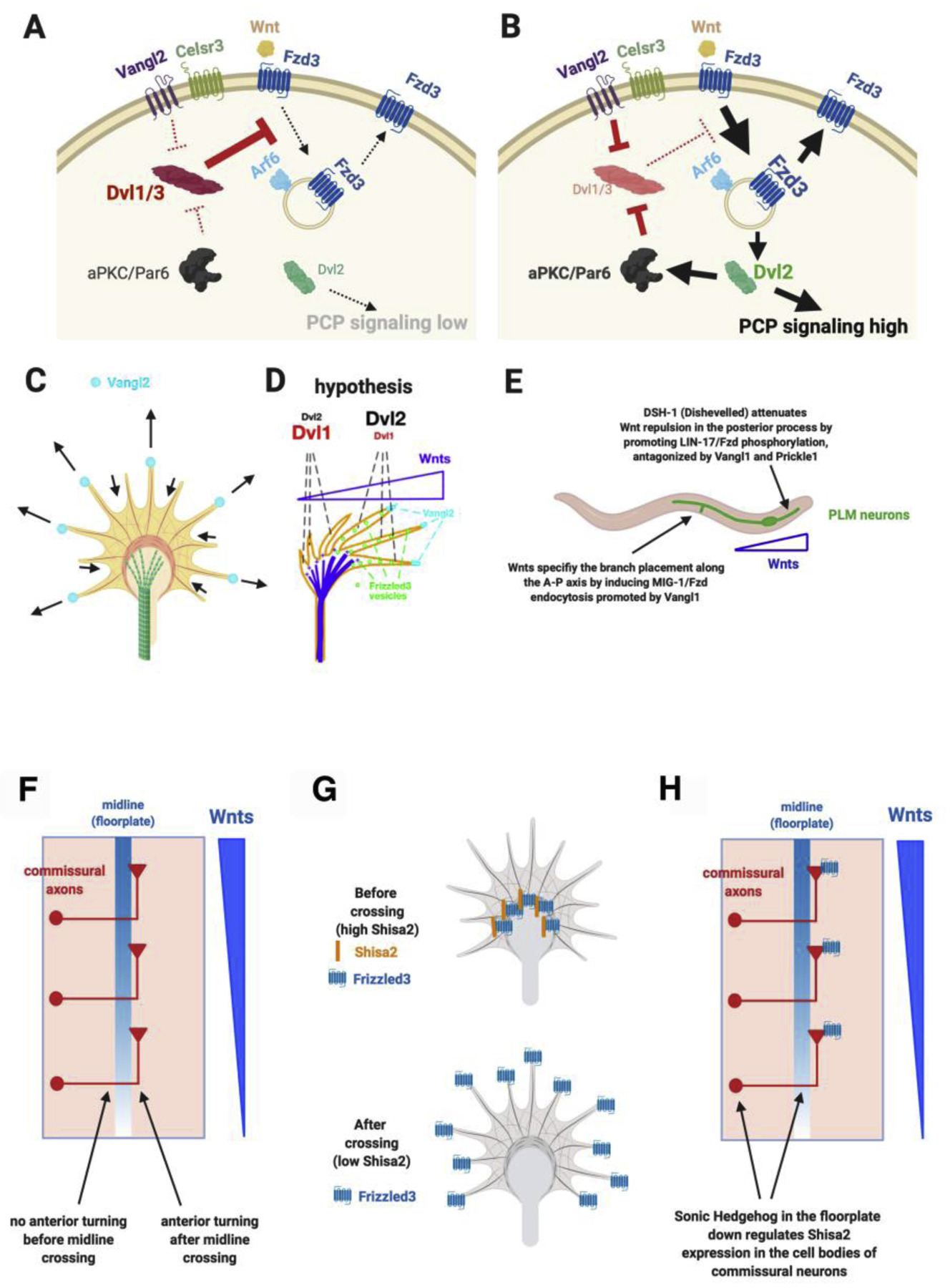
A. Dishevelled1 and 3 induce Frizzled3 hyperphosphorylation and increase the cell surface presence of Frizzled3, reducing the activation of downstream signals such as JNK.
B. Vangl2 and Dishevelled2 can antagonize Dishevelled1 and 3 by inhibiting Frizzled3 phosphorylation. The unphosphorylated Frizzled3 binds to Arf6, which mediates Frizzled3 endocytosis. aPKC is required for Dishevelled2 to inhibit Dishevelled1/3-induced Frizzled3 phosphorylation.
C. Vangl2 protein is enriches on the tips of the filopodia that are extending but not on the tips of filopodia that are shrinking.
D. A hypothesis for how the antagonistic interactions of planar cell polarity signaling components may amplify the shallow Wnt gradient into highly spatially asymmetric and polarized signals inside the growth cone to cause turning.
E. The antagonism between Dishevelled and Vangl in Frizzled phosphorylation and endocytosis and dual functions of Dishevelled are conserved in C. elegans.
F. Spinal cord commissural axons turn anteriorly only after midline crossing even though their growth cones are exposed to the anterior-high-posterior-low Wnt gradient when they reach the floor plate before they cross.
G. Shisa2 binds to Frizzzled3 and retains Frizzzled3 in the endoplasmic reticulum, preventing Frizzled3 from being trafficked to the growth cone surface. Sonic Hedgehog down regulates the expression of Shisa2, allowing Frizzled3 to come to the growth cone surface.
H. Sonic Hedgehog is highly but specifically expressed in the floor plate. When spinal cord commissural axons reach midline, their growth cones detect Sonic Hedgehog, and via smoothened signaling, retrogradely signal to the cell body to down regulate the expression of Shisa2 so that Frizzled3 can move to the cell surface to detect Wnt proteins and their gradient.
In a Wnt gradient, activated aPKC is observed more on the side facing higher Wnt concentration and Frizzled3 endocytosis also occurs more frequently on the side of growth cone facing higher Wnt concentration [13]. This suggests that Frizzled3 phosphorylation/endocytosis may be part of a key regulatory mechanism for detecting concentration difference and signal asymmetrically in the growth cone. It was also hypothesized that these opposing groups of proteins that regulate Frizzled3 endocytosis may be not only the substrate for establishing asymmetry but also amplifying asymmetric signaling activities by bringing polarizing signals into the growth cone via endocytosis from the filopodia tips and by repeated cycles of signaling (Figure 2D) [13] [25]. The importance of Frizzled phosphorylation and dual functions of Dvl have now been shown conserved in A-P guidance of C. elegans axons (Figure 2E) [26]. A requirement for Frizzled endocytosis promoted by Vangl was also recently confirmed in PCP signaling mediating axon branching in C. elegans [27]. (Figure 2E).
An important question is how Wnt/PCP signaling is used with high spatial resolution to ensure precise wiring. For example, commissural axons do not turn to higher Wnt concentration when they arrive at the floor plate before crossing. Only after they have crossed the floorplate, they make a sharp anterior turn (Figure 2F). It turned out that the midline morphogen, Sonic Hedgehog, is a key switch. Sonic Hedgehog, down regulates the expression of a protein called Shisa2 in commissural neurons while their axons enter and cross the floor plate [28]. Shisa2 retains Frizzled3 in the endoplasmic reticulum by blocking Frizzled3 glycosylation and thus prevents Frizzled3 from being translocated to the cell surface. (Figure 2G). Therefore, Frizzled3 is retained in the ER of commissural neurons by Shisa2 before midline crossing. But during midline crossing, their axons detect high levels of Sonic Hedgehog, which is only expressed in the floorplate and inhibit the expression of Shisa2 in the cell body. Frizzled3 is then able to be expressed on the surface of the growth cone to detect the Wnt gradient (Figure 2H).
Wnts and Wnt receptors are up regulated and reactivated after spinal cord injury. Blocking Wnt repulsion can promote the regeneration of dorsal root ganglion axons in a conditioning lesion model and the growth of collateral branches corticospinal tract axons in a dorsal column lesion model in the adult spinal cord [29] [30]. Blocking repulsive Wnt signaling can also promote recovery of proprioceptive sensory function and fine motor skills [31] [32]. In particular, genetic evidence for a role of an axon guidance system in adult spinal cord injury was provided by analyzing a conditional Ryk knockout [32]. This expands the function of axon guidance molecules in adult CNS injury, suggesting that the knowledge about axon guidance may benefit the efforts in spinal cord repair.
Planar Cell Polarity and Apical Basal Polarity signaling components in glutamatergic synapse formation
The glutamatergic synapses are the main excitatory synapses in the brain and are made of distinct but highly organized pre- and postsynaptic structures involving hundreds to over a thousand different proteins across the 20-nm synaptic cleft [33–36]. Abnormal synapse formation and function are responsible for numerous diseases, such as intellectual disability, autism and other neuropsychiatric as well as degenerative disorders. The signaling mechanisms, especially those which directly control their assembly and disassembly, have not been well understood.
The asymmetric pre- and postsynaptic structures strikingly resemble the asymmetric cell-cell junctions localized on the apical side of the epithelium in planar polarized epithelial tissues. The conserved core PCP components form asymmetric protein complexes at the Cadherin-mediated adherens junctions that connect neighboring epithelial cells (Figure 3A) [14,15]. These asymmetric intercellular complexes are essential for establishing/maintaining planar cell polarity. These interactions are also thought to be part of an amplification mechanism that increase the fidelity of cell and tissue polarization along the tissue plane [17].
Figure 3. Planar cell polarity and apical-basal polarity signaling are key regulators of glutamatergic synapse formation.
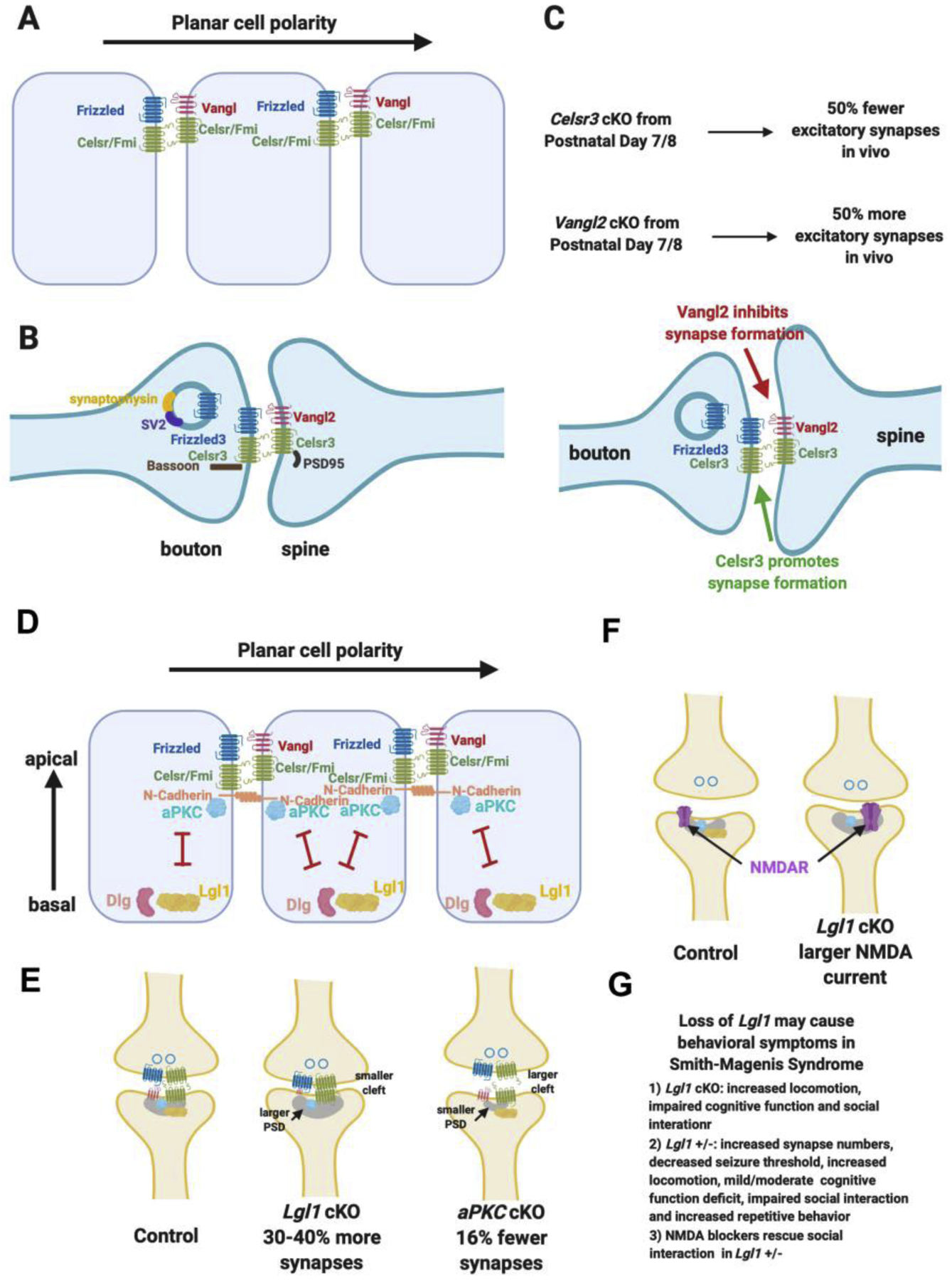
A. Schematics of the asymmetric localization of planar cell polarity components in epithelial cells and the asymmetric intercellular complexes.
B. Planar cell polarity components are localized in the glutamatergic synapse in similar ways as in epithelial polarization signaling and interact with key synaptic proteins.
C. Loss of Celsr3 (encoding one of the two Celsrs found in glutamatergic synapses) lead to reduction of the formation of 50% of the glutamatergic synapses in vivo when conditionally knocked out from postnatal day 7/8. Loss of Vangl2 lead to a large increase of glutamatergic synapse numbers in vivo.
D. Apical-basal polarity is perpendicular to planar cell polarity and is mediated by the antagonistic interactions and localization of aPKC and Lgl.
E. Lgl1 cKO lead to a large increase of glutamatergic synapse numbers, larger postsynaptic density, smaller synaptic cleft and reduced Vangl2 protein in the synaptosome. aPKC double cKO lead to a decrease of glutamatergic synapse numbers, smaller postsynaptic density and larger synaptic cleft.
F. Lgl1 +/− animals display behavioral deficit mimicking human Smith-Magenis Syndrome and the behavioral deficit can be rescued by NDMAR blockers.
Components of both planar cell polarity (PCP) are localized in developing excitatory synapses in similar fashions as in PCP signaling and interact with multiple key presynaptic and postsynaptic proteins (Figure 3B). Conditionally knocking out Celsr3 in hippocampal pyramidal neurons at postnatal day 7 lead to a reduction of approximately 50% of glutamatergic synapses measured at postnatal day 14. Conditionally knocking out Vangl2 in hippocampal pyramidal neurons at postnatal day 7, another PCP component, which together with Prickle antagonizes the Frizzled/Dishevelled complex, lead to approximately 50% increase of the glutamatergic synapse numbers measured at postnatal day 14 (Figure 3C) [37]. Therefore, PCP signaling components can control synapse numbers in opposite directions, suggesting that this mechanism allows both positive and negative regulations in development and, potentially in plasticity if this pathway continues to function in mature synapses. It should be noted that it is critical to knockout PCP signaling components at a later stage of development in order to correctly assign their functions in synapse formation. One of the reasons is that PCP signaling components are very important in axon guidance and are involved in the development of axons of many types of neurons. Germline mutation or early conditional KO using Cre lines are expressed in neuronal progenitors or in postmitotic early neurons will lead to earlier axon guidance defects and complicate the results, which will likely misinform their functions in synapse formation. The functions of Celsr3 and Vangl2 described here were from conditional KO in pyramidal neurons one week after birth right before the peak of synaptogenesis in vivo.
Perpendicular to the tissue plane, apical-basal polarity proteins, aPKC and lethal giant larvae (Lgl), specify the apical-basal polarity and limit PCP signaling only on the apical side of each epithelial cell [38]. (Figure 3D). Lgl1 is a basolateral protein, which antagonizes the apical kinase, aPKC and PCP signaling [39]. Lgl1 forms complex with Discs Large (Dlg), homolog of the membrane-associated guanylate kinases (MAGUKs), which traffic and cluster glutamate receptors [40–42].
Lgl1 cKO in approximately 60% of the hippocampal pyramidal neurons lead to 30–40 % increase of glutamatergic synapses in vivo with defects in plasticity, whereas aPKC double cKO in approximately 60% of the hippocampal pyramidal neurons lead to 16% fewer glutamatergic synapses in vivo (Figure 3E) [43]. In Lgl1, aPKC double cKO (triple cKO), normal numbers of glutamatergic synapses were formed, suggesting that the antagonism between Lgl1 and aPKC occurs in synapse number control. Importantly, in Lgl1 mutant, Vangl2 protein is significantly reduced, consistent with the inhibitory function of Vangl2 in synapse numbers (Figure 3E) [43] [37]. In Lgl1 cKO, the synapses also appear more stable with smaller synaptic cleft, was aPKC cKO were the opposite (Figure 3E). Lgl1 is also important for NMDA receptor trafficking, as cKO in postnatal or in adulthood both caused reduction of AMPA/NMDA ratio, which resulted from a large increase of NMDA current (Figure 3F).
Lgl1 is one of the 25 deleted genes in Smith-Magenis Syndrome (SMS), a chromosome microdeletion disorder occurring early in embryonic development [44] [45]. Individuals with this deletion, Chromosome 17 p11.2, are frequently diagnosed with autism (80–100%). Lgl1 cKO animals showed increased locomotor activity, travelling 20% further during the 10-minute test than control mice, impaired novel object recognition (NOR) and defects in sociability in the 3-chamber social interaction task (Figure 3G). Because SMS arises from heterozygous deletion, the effects of germline heterozygous deletion of Lgl1 were tested. An increase of excitatory synapse numbers, decrease seizure threshold, increase of locomotion, mild/moderate novel object recognition defect, impaired social interaction and increased repetitive behavior were observed in Lgl1 +/− animals. NMDA blockers were shown to rescue social interaction behavior (Figure 3G).
In mutations of both PCP and A-BP signaling components, strong phenotypes of synapse number changes were observed. These mutations were conditional KOs from one week after birth. Therefore, these results suggest that the cell polarity pathways are likely the key signaling pathways that directly regulate synapse assembly. These two pathways interact with each other. aPKC is required for Frizzled3 trafficking and likely essential for PCP signaling [13]. This is also consistent with the fact that PCP signaling is only active on the apical side not on the basal side. The fact that the A-BP signaling can also affect synapse numbers is reassuring, suggesting that PCP signaling is truly essential in controlling synapse assembly. More importantly, in the P2 fraction of Lgl1 −/−, Vangl2 protein level is significantly reduced, again consistent with a large increase of synapse numbers. Therefore, one way that Lgl1 may regulate synapse numbers is to influence Vangl2 protein trafficking or stability [43].
PCP as a common mechanism for growth cone polarity and asymmetric synaptic junction
The findings that the planar cell polarity pathway mediates growth cone guidance as well as glutamatergic synapse formation are fascinating. If one looks into the history and the progression of development, a potential logic may start to emerge, at least for some neurons, particularly the ones that are regulated by Wnts. Neurons are born through asymmetric cell division from neural epithelia, which are apical-basal polarized and likely already planar polarized as neural tube closure is a planar polarity-dependent process and occurs earlier than neurogenesis. During epithelial PCP signaling, the PCP components form intercellular complexes at the E-Cadherin-dependent adherens junctions of epithelia on the apical side (Figure 4A). After neurons are born and delaminate from the epithelia, they start to migrate and/or grow axons, which are tipped by a highly dynamic structure, the growth cone (Figure 4B). Our studies show that the growth cones share the same molecules that are present in the adherens junctions in the epithelium, except neurons and axonal growth cones use N-Cadherin. Therefore, we propose that the growth cone can be considered “half adhesion junctions” and growth cone navigation can be viewed as a search for the other half to form new junctions again [46] (Figure 4B). In addition to the adherens junction proteins, the growth cones also express various receptors for axon guidance molecules, that are expressed in specific patterns to guide the axon navigation. At least for axons that are guided by Wnts, the PCP components in the axonal growth cone will mediate the detection and interpretation of directional information to guide the growth of axons. Once the axons or growth cones find the right new half, correct postsynaptic partners, the PCP components then direct glutamatergic synapse assembly (Figure 4C). Therefore, the glutamatergic synapses can be viewed as a new union of two halves of adherens junctions, which are polarized by the PCP components. The process before the new union, axon pathfinding, axon targeting and synapse recognition, is the history these “half adherens junctions” experience (arranged by the developmental program). These experiences are determined by spatial and temporally arranged axon guidance molecules (for example, Wnts) and synapse adhesion molecules, which provide the important directional information and synapse specificity, respectively. Because PCP is a ubiquitous signaling pathway, it is appealing to propose that PCP is a strong candidate for a convergent signaling mechanism for many classes of axon guidance molecules and synapse-matching adhesion molecules (Figure 4D). A-BP is also important as it is intimately involved in PCP signaling and regulates the location and activation of PCP signaling. These cell-polarity signaling pathways, which are best studied in epithelial cell and tissue polarity, do not regulate the axon-dendrite polarity in earlier neuronal development. Instead, they appear to directly regulate axon guidance and synapse formation.
Figure 4. Planar cell polarity signaling in growth cone guidance and synapse formation.
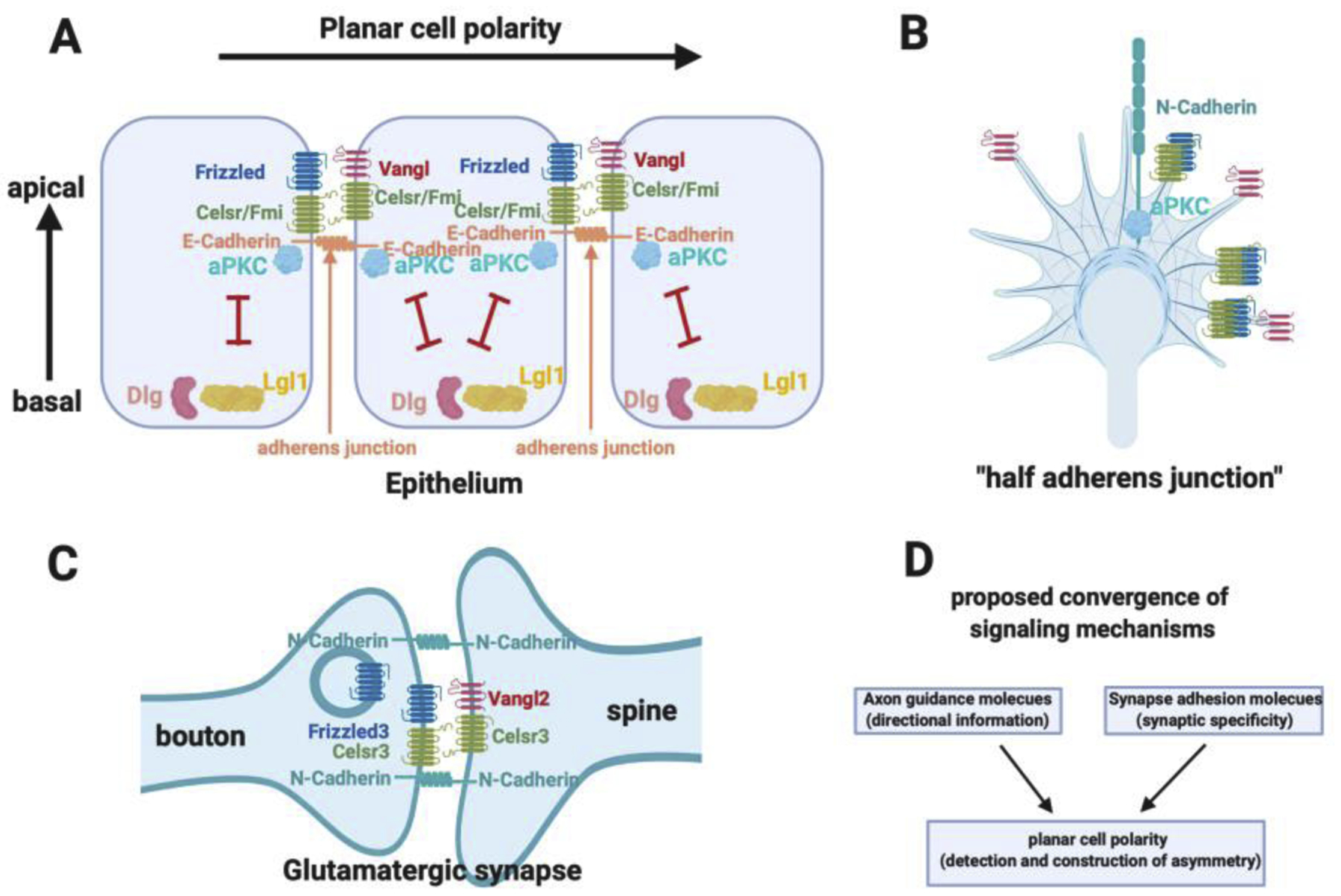
A. Schematics of planar cell polarity and apical-basal polarity and their interactions. E-Cadherin is located in the adherens junctions where aPKC is localized and activated. Planar cell polarity complexes are built at the adherens junctions.
B. The growth cone has the same planar cell polarity and apical basal polarity components and similar molecular organizations as the adherens junctions. The growth cone has N-Cadherin.
C. The glutamatergic synapses have the same planar cell polarity and apical basal polarity components and similar molecular organization as the adherens junctions.
D. The hypothesis that cell polarity pathways may be a common mechanism mediating the function of axon guidance molecules and synapse adhesion molecules, which provide directional and positional information to provide specificity for brain wiring.
This proposed logic is a testable hypothesis at least for some neurons and their axons and synapses. This may not be applicable to inhibitory synapses because PCP proteins are only found in glutamatergic synapses and regulate their assembly. They are absent from GABAergic synapses. At least for excitatory synapses, it will be interesting to test whether PCP signaling actually mediates the function of guidance molecules other than Wnts and whether PCP signaling in synapse formation is regulated by synapse adhesion molecules (Figure 4D). Finding a convergent signaling mechanism, if it exists, will not only allow us to have deeper understanding of the molecular logic of brain wiring but also provide guidance to develop new tools for repairing damaged neural circuits due to injury or neurogenerative diseases.
Highlights.
Planar cell polarity components mediate Wnt functions in growth cone guidance
Planar polarity components are required for glutamatergic synapse formation
Apical basal polarity components regulate planar cell polarity components
These pathways may be a convergent mechanism of growth cone guidance
These pathways may be a convergent mechanism of synapse formation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
Yimin Zou is the founder of VersaPeutics and VersaChem and has equity, compensation and interim managerial role. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
References
special interest (*)
outstanding interest (**)
- 1.Dickson BJ: Wnts send axons up and down the spinal cord. Nat Neurosci 2005, 8:1130–1132. [DOI] [PubMed] [Google Scholar]
- 2.Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y: Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 2003, 302:1984–1988. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song X, Zou Y: Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci 2005, 8:1151–1159. [DOI] [PubMed] [Google Scholar]
- 4.Pan CL, Howell JE, Clark SG, Hilliard M, Cordes S, Bargmann CI, Garriga G: Multiple Wnts and Frizzled Receptors Regulate Anteriorly Directed Cell and Growth Cone Migrations in Caenorhabditis elegans. Dev Cell 2006, 10:367–377. [DOI] [PubMed] [Google Scholar]
- 5.Hilliard MA, Bargmann CI: Wnt Signals and Frizzled Activity Orient Anterior-Posterior Axon Outgrowth in C. elegans. Dev Cell 2006, 10:379–390. [DOI] [PubMed] [Google Scholar]
- 6.Prasad BC, Clark SG: Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development 2006, 133:1757–1766. [DOI] [PubMed] [Google Scholar]
- 7.Domanitskaya E, Wacker A, Mauti O, Baeriswyl T, Esteve P, Bovolenta P, Stoeckli ET: Sonic hedgehog guides post-crossing commissural axons both directly and indirectly by regulating Wnt activity. J Neurosci 2008, 30:11167–11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun SD, Purdy AM, Walsh GS: Planar cell polarity genes Frizzled3a, Vangl2, and Scribble are required for spinal commissural axon guidance. BMC Neurosci 2016, 17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, Zou Y, Pasterkamp RJ: Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci 2010, 30:16053–16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y: Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature 2006, 439:31–37. [DOI] [PubMed] [Google Scholar]
- 11.Sato M, Umetsu D, Murakami S, Yasugi T, Tabata T: DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat Neurosci 2006, 9:67–75. [DOI] [PubMed] [Google Scholar]
- 12.Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y: Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell 2011, 20:177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onishi K, Shafer B, Lo C, Tissir F, Goffinet AM, Zou Y: Antagonistic Functions of Dishevelleds Regulate Frizzled3 Endocytosis via Filopodia Tips in Wnt-Mediated Growth Cone Guidance. J Neurosci 2013, 33:19071–19085. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper identifies key antagonistic functions of PCP components and the role and mechanism of aPKC in regulating PCP signaling. The rich antagonistic interactions among PCP and apical-basal polarity components may be the substrate of growth cone polarization and signal amplification necessary for precisely directed navigation of growth cones.
- 14.Wang Y, Nathans J: Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 2007, 134:647–658. [DOI] [PubMed] [Google Scholar]
- 15.Zallen JA: Planar polarity and tissue morphogenesis. Cell 2007, 129:1051–1063. [DOI] [PubMed] [Google Scholar]
- 16.Simons M, Mlodzik M: Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet 2008, 42:517–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler MT, Wallingford JB: Planar cell polarity in development and disease. Nat Rev Mol Cell Biol 2017, 18:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler PN: The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol 2012, 101:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu K, Sato M, Tabata T: The Wnt5/planar cell polarity pathway regulates axonal development of the Drosophila mushroom body neuron. J Neurosci 2011, 31:4944–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mrkusich EM, Flanagan DJ, Whitington PM: The core planar cell polarity gene prickle interacts with flamingo to promote sensory axon advance in the Drosophila embryo. Dev Biol 2011, 358:224–230. [DOI] [PubMed] [Google Scholar]
- 21.Huarcaya Najarro E, Ackley BD: C. elegans fmi-1/flamingo and Wnt pathway components interact genetically to control the anteroposterior neurite growth of the VD GABAergic neurons. Dev Biol 2013, 377:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gombos R, Migh E, Antal O, Mukherjee A, Jenny A, Mihaly J: The Formin DAAM Functions as Molecular Effector of the Planar Cell Polarity Pathway during Axonal Development in Drosophila. J Neurosci 2015, 35:10154–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aviles EC, Stoeckli ET: Canonical wnt signaling is required for commissural axon guidance. Dev Neurobiol 2016, 76:190–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung V, Iliescu A, Jolicoeur C, Gravel M, Apuzzo S, Torban E, Cayouette M, Gros P: The planar cell polarity protein Vangl2 is required for retinal axon guidance. Dev Neurobiol 2016, 76:150–165. [DOI] [PubMed] [Google Scholar]
- 25.Onishi K, Hollis E, Zou Y: Axon guidance and injury-lessons from Wnts and Wnt signaling. Curr Opin Neurobiol 2014, 27:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng C, Diaz-Cuadros M, Chalfie M: Dishevelled attenuates the repelling activity of Wnt signaling during neurite outgrowth in Caenorhabditis elegans. Proc Natl Acad Sci U S A 2015, 112:13243–13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CH, He CW, Liao CP, Pan CL: A Wnt-planar polarity pathway instructs neurite branching by restricting F-actin assembly through endosomal signaling. PLoS Genet 2017, 13:e1006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onishi K, Zou Y: Sonic Hedgehog switches on Wnt/planar cell polarity signaling in commissural axon growth cones by reducing levels of Shisa2. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper identifies one of the key switches of Wnt/PCP signaling and a link between Shh and PCP signalling, which may be generally applicable in other contexts of PCP signaling. The spatial and temporal control of PCP signaling (in tissue polarization and growth cone wiring, which lead to axon patterning) is essential for proper development and function.
- 29.Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y: Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci 2008, 28:8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollis ER, 2nd, Zou Y: Reinduced Wnt signaling limits regenerative potential of sensory axons in the spinal cord following conditioning lesion. Proc Natl Acad Sci U S A 2012, 109:14663–14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollis ER 2nd, Ishiko N, Pessian M, Tolentino K, Lee-Kubli CA, Calcutt NA, Zou Y: Remodelling of spared proprioceptive circuit involving a small number of neurons supports functional recovery. Nat Commun 2015, 6:6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollis ER 2nd, Ishiko N, Yu T, Lu CC, Haimovich A, Tolentino K, Richman A, Tury A, Wang SH, Pessian M, et al. : Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci 2016, 19:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper provides genetic evidence that Wnt/PCP/Ryk signaling plays a role in regulating axon growth in adulthood after spinal cord injury.
- 33.Sheng M, Kim E: The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol 2011, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudhof TC: The presynaptic active zone. Neuron 2012, 75:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loh KH, Stawski PS, Draycott AS, Udeshi ND, Lehrman EK, Wilton DK, Svinkina T, Deerinck TJ, Ellisman MH, Stevens B, et al. : Proteomic Analysis of Unbounded Cellular Compartments: Synaptic Clefts. Cell 2016, 166:1295–1307 e1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uezu A, Kanak DJ, Bradshaw TW, Soderblom EJ, Catavero CM, Burette AC, Weinberg RJ, Soderling SH: Identification of an elaborate complex mediating postsynaptic inhibition. Science 2016, 353:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakar S, Wang L, Yu T, Ye M, Onishi K, Scott J, Qi J, Fernandes C, Han X, Yates JR 3rd, et al. : Evidence for opposing roles of Celsr3 and Vangl2 in glutamatergic synapse formation. Proc Natl Acad Sci U S A 2017, 114:E610–E618. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper identifies PCP signaling components as key regulators of glutamatergic synapse formation. Celsr3 is responsible for the assembly 50% of the glutamatergic synapses in hippocampal pyramidal neurons and Vangl2 negatively regulate synapse numbers.
- 38.Karner C, Wharton KA, Carroll TJ: Apical-basal polarity, Wnt signaling and vertebrate organogenesis. Semin Cell Dev Biol 2006, 17:214–222. [DOI] [PubMed] [Google Scholar]
- 39.Dollar GL, Weber U, Mlodzik M, Sokol SY: Regulation of Lethal giant larvae by Dishevelled. Nature 2005, 437:1376–1380. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Shang Y, Wan Q, Xia Y, Chen J, Du Q, Zhang M: Phosphorylation-dependent interaction between tumor suppressors Dlg and Lgl. Cell Res 2014, 24:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamanaka T, Ohno S: Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci 2008, 13:6693–6707. [DOI] [PubMed] [Google Scholar]
- 42.Zhu J, Shang Y, Zhang M: Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat Rev Neurosci 2016, 17:209–223. [DOI] [PubMed] [Google Scholar]
- 43.Scott J, Thakar S, Mao Y, Qin H, Hejran H, Lee SY, Yu T, Klezovitch O, Cheng H, Mu Y, et al. : Apical-Basal Polarity Signaling Components, Lgl1 and aPKCs, Control Glutamatergic Synapse Number and Function. iScience 2019, 20:25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper identifies apical-basal signaling components also play important roles in glutamatergic synapse formation in part by regulating PCP signaling. Loss of Lgl1 may be the cause of some of the neurological symtoms of Smith-Magenis Syndrome.
- 44.Smith AC, McGavran L, Robinson J, Waldstein G, Macfarlane J, Zonona J, Reiss J, Lahr M, Allen L, Magenis E: Interstitial deletion of (17)(p11.2p11.2) in nine patients. Am J Med Genet 1986, 24:393–414. [DOI] [PubMed] [Google Scholar]
- 45.Vlangos CN, Yim DK, Elsea SH: Refinement of the Smith-Magenis syndrome critical region to approximately 950kb and assessment of 17p11.2 deletions. Are all deletions created equally? Mol Genet Metab 2003, 79:134–141. [DOI] [PubMed] [Google Scholar]
- 46.Zou Y: Does planar cell polarity signaling steer growth cones? Curr Top Dev Biol 2012, 101:141–160. [DOI] [PubMed] [Google Scholar]


