Abstract
Small cell lung cancer (SCLC) represents ~15% of all lung cancer diagnoses in the US and has a particularly poor prognosis. We hypothesized that kinases regulating SCLC survival pathways represent therapeutically targetable vulnerabilities whose inhibition may improve SCLC outcome. A shRNA library targeting all human kinases was introduced into seven chemonaive patient derived xenografts (PDX), and cells cultured in vitro and in vivo. On harvest, lost, or depleted, shRNAs were considered as regulating cell survival pathways, and deemed essential kinases. Unsupervised hierarchical cluster analysis of recovered shRNAs separated the PDX into two clusters suggesting kinase based heterogeneity among the SCLC PDX. Twenty-three kinases were identified as essential in two or more PDX, with mTOR a candidate essential kinase in four. mTOR phosphorylation (p-mTOR) status correlated with PDX sensitivity to mTOR kinase inhibition, and mTOR inhibition sensitized PDX to cisplatin/etoposide. In PDX where mTOR was defined as essential, mTOR inhibition caused a 43% decrease in tumor volume at 21 days (P<0.01). Combining mTOR inhibition with cisplatin/etoposide decreased PDX tumor volume 96% compared to cisplatin/etoposide alone at 70 days (P<0.002). Chemoresistance did not develop with the combination of mTOR inhibition and cisplatin/etoposide in mTOR essential PDX over 105 days. The prevalence of p-mTOR in a tissue microarray of chemonaive SCLC was 27% identifying a significant SCLC subtype that might benefit by the addition of mTOR inhibition to standard chemotherapy.
These studies show that kinases can define SCLC subgroups, can identify therapeutic vulnerabilities, and can potentially be used to optimize therapeutic approaches.
Keywords: Functional genomics, kinome, kinase, mTOR, small cell lung cancer
Introduction
Lung cancer accounts for more deaths worldwide than any other cancer in both men and women. In 2020 the American Cancer Society estimates there will be over 228,000 new lung cancer diagnoses, and over 135,000 deaths [1]. Small cell lung cancer (SCLC) makes up ~13% of these cases, representing 30,000 new diagnoses and over 20,000 deaths [2]. Only ~10% of SCLC patients are cured with combined chemotherapy and radiation therapy [2–4]. In the overwhelming majority of cases, relapse occurs with the emergence of chemotherapy resistant disease. Standard first-line chemotherapy consists of a platinum agent with etoposide [5], and recently, the addition of an immune checkpoint inhibitor in extensive stage disease [6]. SCLC is remarkably responsive to therapy initially with a 70–85% response rate, but disease relapse is nearly universal. As a result, long-term survival has improved only marginally [2, 3]. Comparing the 1986–1999 time period to 2000–2008, the median survival increased from 11.3 months (95% CI 10.5–12.7) to 15.2 months (95% CI 13.6–16.6) [7], and the overall 5-year survival rate minimally improved from 8.3% to 11.0% with the majority of long term survivors having limited stage disease. The poor outcomes in SCLC are due, in part, to a critical knowledge gap in our understanding of mechanisms regulating SCLC growth and chemotherapy resistance.
Histologically, SCLC appears fairly homogeneous, and SCLC has been generally treated as a biologically homogeneous disease, clinically distinguished only by limited versus extensive stage. However, classification of SCLC into subtypes has been proposed. The classic and variant SCLC phenotypes are defined by in vitro biochemical, growth, and differential gene expression characteristics [8, 9]. Subtypes have been further defined by DNA methylation and gene expression patterns, identifying subtypes in histologically indistinguishable patient samples [10, 11]. Importantly, differential NEUROD1 vs. ASCL1 expression has defined distinct subtypes, with differential therapeutic responses [10, 12–14]. A recent synthesis of human and mouse molecular data proposed four SCLC subtypes based on relative expression of key transcription factors defining each subtype – achaete-scute homologue (ASCL1), neurogenic differentiation factor 1 (NeuroD1), yes-associated protein 1 (YAP1) and Pou class 2 homeobox 3 (Pou2F3) [11]. Thus, SCLC is being increasingly recognized as a heterogeneous disease, and identification of subtypes may carry mechanistic and therapeutic information to better understand the disease and guide therapy.
The expressed set of protein kinases, or kinome, may also define functional subtypes of SCLC. The role of kinase signaling in non-small cell lung cancer (NSCLC) is well known, where kinase activation plays a central role in carcinogenesis, can define therapeutic targets, contribute to acquired drug resistance, and is associated with differential clinical outcomes [15–17]. In contrast, much less is known about kinase-dependency in SCLC. Changes in kinases are known to occur, with mutations in MET, KIT and PIK3CA [18–20], and amplification and overexpression of FGFR1 and RICTOR [20–22]. Kinase signaling has also been implicated in SCLC development and metastasis [23–26]. In some cases, targeting kinases in vitro along with standard cytotoxic agents in SCLC models has demonstrated a strong combinatorial effect, interacting synergistically in a synthetic lethal fashion [13, 27]. However, few studies have evaluated the entire kinome to understand routes of kinase pathway activation [28]. The lack of a true kinase target in the SCLC kinome may have contributed to the failure of kinase inhibitor clinical trials [29, 30]. The possibility of defining SCLC subgroups based on unique kinase characteristics may provide a new understanding of SCLC pathobiology, and identify therapeutic vulnerabilities that can be exploited based on the subgroups essential kinase profile.
We hypothesized that there are critical kinases in SCLC that regulate cell survival pathways, or essential kinases. To define a tumor’s entire kinome, its essential kinases, and any dynamic response to therapy, we employed a functional genomics approach using a kinase specific shRNA library to identify essential kinases regulating SCLC survival in human chemonaive patient derived xenografts (PDX). These studies identified subtypes of SCLC based on the PDX’s kinome and essential kinases. The mechanistic Target of Rapamycin (mTOR) was identified as a leading essential kinase in one SCLC subtype. In this subtype, mTOR inhibition suppressed tumor growth as monotherapy, and sensitized SCLC PDX to cisplatin/etoposide, profoundly inhibiting SCLC growth in vivo and preventing the emergence of acquired chemoresistance.
Materials and Methods
Patient-Derived Xenografts (PDX)
PDX (JHU-LX22, JHU-LX33, MSK-LX40, JHU-LX48, MSK-LX95, JHU-LX108, JHU-LX110) were developed as previously described [31], and maintained subcutaneously in the flanks of NSG mice (NSG NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ). Tumor size was measured weekly and volume calculated (Volume = 4/3 × π × (Length × Width × Height)/2)). When needed, tumors were harvested, and dissociated into individual cells for study (Tumor Dissociation Kit, Miltenyi Biotech, Inc.). PDX authenticity was confirmed at the beginning and end of their use by SNP genotyping and comparison to the parental tumor.
Cell Culture
Kinome essential screen
A short-hairpin RNA (shRNA) kinome library consisting of 3,113 shRNAs targeting known human kinases (670 kinases) (TRC1.5 lentivirus-based shRNA library, The RNAi Consortium, Broad Institute) was obtained from the University of Colorado Cancer Center Functional Genomics Shared Resource Core. Seventy-four (74) shRNAs targeting 13 murine genes were added as controls, and the library packaged in lentivirus. Single cell PDX preparations were transduced with the shRNA kinome library (MOI=0.3) based on achieving a minimum final threshold of 500 reads/shRNA clone [33]. Infected cells were placed in culture, and at 24–48H following infection, DNA was harvested and shRNAs PCR amplified representing all shRNA clones that were initially infected into the cell pool (Baseline shRNAs). Infected cells were then divided for in vitro and in vivo studies. In vitro, cells were placed in culture, and on Days 7 and 14 following infection, DNA was isolated and shRNAs PCR amplified representing all surviving shRNA clones at those time points. In vivo, 15 × 106 viable cells were injected subcutaneously with Matrigel into the flanks of NSG mice. Tumors were harvested at specified times or tumor volumes, and DNA isolated, shRNAs PCR amplified, representing all surviving shRNA clones in vivo. Three separate aliquots of DNA were taken from each sample and independent libraries constructed. PCR products were digested at the hairpin’s XhoI site, the shRNA sense target sequence was gel purified, and dual, in-line, bar codes added by PCR along with Illumina adaptors [33]. Libraries were subjected to 1×75 single-end reads on a HiSeq 2500 platform to quantify shRNA abundance.
Bioinformatics Analysis
Bioinformatics for Next Generation Sequencing! (BiNGS!) was used to analyze and interpret sequencing data as previously described [33, 34]. In brief, a preprocessing step filtered out erroneous and low-quality reads. Remaining reads were mapped against the shRNA reference library using Bowtie [35]. The output from this step is a P × N matrix, where P and N represent shRNA counts and samples, respectively. We used edgeR to model the negative binomial count distribution of the sequencing data and performed statistical analysis using this model. The q-value of false discovery rate for multiple comparisons was computed, and a meta-analysis was performed by combining adjusted P values for all shRNAs representing the same gene using weighted Z transformation. For each gene, a P-value, P(wZ), was computed and used to sort the kinase list as previously described [27, 36]. shRNA reads/clone were quantified, pairwise comparisons of each of the samples were performed and compared to reads obtained for the same shRNA clone in the Baseline shRNA library. shRNA clones with a 2-fold or greater decrease in reads (P<0.05) when compared to the Baseline shRNA library were considered an essential kinase candidate. To ensure infection efficiency, all shRNA clones recovered from Baseline shRNA samples were compared to the clones in the TRC1.5 shRNA library listing. Identification of >95% of the TRC1.5 library shRNAs was required to verify efficiency of shRNA library transduction. On average, 99% (3,093) of the shRNAs targeting the kinome were detected in all samples. Efficiency of shRNA recovery was verified by identifying the 74 control murine shRNA clones in all samples. All control shRNAs (100%) were detected supporting full shRNA recovery. MORPHEUS (Broad Institute, https://software.broadinstitute.org/morpheus/) was used to generate hierarchical clustering of the kinase profiles. We used “one minus spearman rank correlation” as the metric and performed linkage analysis using “Complete” method.
Drug Cytotoxicity/Proliferation assays
Cisplatin and etoposide were obtained from the National Jewish Health pharmacy. AZD-8055 was obtained from MedChem Express (Monmouth Junction, NJ). Cytotoxic/proliferation effects were determined using the Water Soluble Tetrazolium salt assay (WST-1, Sigma-Aldrich, St. Louis, MO). The half maximal inhibitory concentration (IC50) of individual drugs was determined from cell viability curves.
Immunoblot assays
Protein expression and phosphorylation levels were determined by western blotting as previously described [37, 38] using mTOR, p-MTOR, and beta-actin antibodies.(Cell Signaling Technology, Danvers, MA).
Tissue Micro-Array and Immunohistochemical Analysis
For PDX analysis, formalin-fixed paraffin-embedded (FFPE) blocks were prepared from PDX harvested from NSG mice to perform immunohistochemistry (IHC) as previously described [39].
To construct tissue microarrays, sections (5 μm) were prepared from SCLC FFPE blocks using a Microm HM315 microtome and stained by hematoxylin and eosin for pathology review. Stained slides were marked for tumor area and 2 mm cores were removed for construction of a tissue microarray (TMA) block.
IHC was performed on slides (5 μm) and stained on a Leica Bond RX autostainer. Primary antibodies from Cell Signaling included anti-human mTOR rabbit monoclonal antibody (#2983, diluted 1:100), anti-human phospho-mTOR rabbit monoclonal antibody (#2976, diluted 1:100), anti-human S6 ribosomal protein rabbit monoclonal antibody (#2217, diluted 1:100), anti-human phospho-S6 ribosomal protein rabbit monoclonal antibody (#5364, diluted 1:100) and anti-human p70 S6 Kinase rabbit monoclonal antibody (#2708, diluted 1:480). Anti-human phospho-p70 S6 Kinase mouse monoclonal antibody was from Santa Cruz Biotechnology (#8416, diluted 1:500). All antibodies were diluted with Cell Signaling antibody diluent (#8112). Staining on the Bond RX autostainer used the Bond Polymer Refine Detection kit (#DS9800) using Leica IHC Protocol F and exposed to epitope retrieval 1 (low pH) for twenty minutes. For DLL3, slides were stained on a Ventana Benchmark XT autostainer using the DLL3 (SP347) Antibody Assay from Ventana (#790–7016). Slides were stained using the OptiView DAB kit (#253–4582) with Ventana IHC Protocol 118-DLL3 Protocol, and exposed to epitope retrieval 1 (low pH) for eighty-eight minutes. Following staining, all slides were cleared and dehydrated on an automated Tissue-Tek Prisma platform and cover slipped using a Tissue-Tek Film cover slipper. Staining was assessed based on H-score = (% of tumor 1+) + (2 × % of tumor cells with 2+ intensity) + (3 × % of tumor cells with 3+ intensity).
Animal modelling
All animal studies were performed under protocol AS2808 approved by the National Jewish Health Institutional Animal Care and Use Committee using 6–10 week old male and female mice. For drug studies, cisplatin and etoposide were given weekly; Day 1 = 1mL normal saline subcutaneously, cisplatin, 5mg/kg intraperitoneal (IP), Days 1–3 = etoposide 8mg/kg IP. AZD-8055 was administered by daily oral gavage (20 mg/kg).
Statistical Analyses
All in vitro studies consisted of at least three independent replicates, and duplicated. In vivo studies consisted of at least 6 mice. Individual cohorts were compared against controls or each other using a Student T test, accepting P<0.05 as significant and reported as an average ± standard deviation for in vitro studies, or average ± standard error for in vivo studies.
Nomenclature
Sample labels reflect; JHU or MSK – source of PDX (Johns Hopkins University, Memorial Sloan Kettering), I/II-experiment number, Day 1/7/14=in vitro day of harvest post-shRNA infection, Tumor=shRNA infected tumor grown in NSG mice, 1–3/4–6/7–9=sample triplicate libraries.
Results
SCLC Kinase Profiles
Seven chemonaive patient derived xenografts (PDX) were expanded from frozen stocks as previously described, grown in immunocompromised mice, histologically confirmed as SCLC [31], and taken through our screening and validation strategy (Figure 1). In brief, single cell preparations were made from the PDX and infected with a kinase specific shRNA library. At defined points (in vitro Day 1, 7, 14, in vivo tumor volume >300mm3), total DNA was harvested and shRNAs were amplified by PCR to construct the sequencing libraries. On average, three million (range two to four million) sequencing reads were obtained per tumor sample. Sequencing reads were analyzed by the BiNGS! algorithm [34] as previously described [27, 33, 36, 40]. On average, >90% sequences were mapped to the TRC 1.5 library and 99% (3,093) of the shRNAs targeting the kinome were detected in all samples with a shRNA coverage of 840 reads/clone. The mapped sequencing reads were normalized by edgeR and the kinase profiles for these samples were used for subsequent analyses.
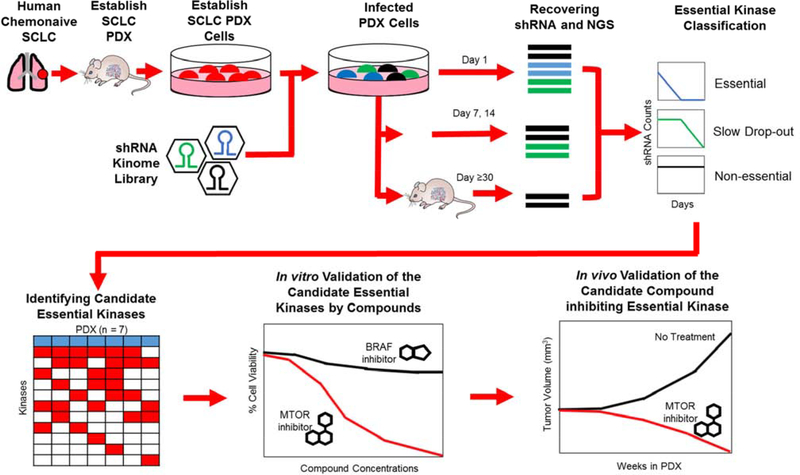
Figure 1.
Overview of the essential kinase screening strategy. SCLC tumor specimens were injected subcutaneously into NSG mice to obtain primary PDX. PDX were harvested, infected with the shRNA kinome library and cultured in vitro, or reimplanted into NSG mice. DNA was extracted from shRNA infected cells at specified time points (in vitro) or tumor volumes (in vivo), shRNAs PCR amplified, and next generation sequencing performed to quantify the retained shRNAs. Lost, or depleted, shRNAs were considered as essential kinase candidates and validated for their essential function in vitro and in vivo.
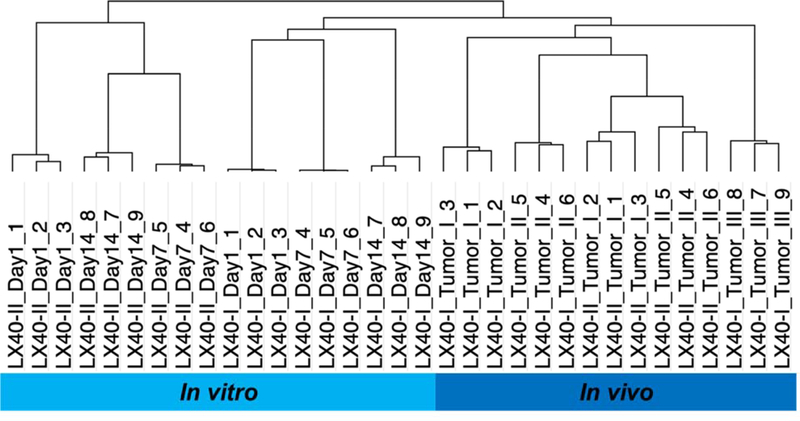
To investigate the reproducibility of the essential screen, unsupervised hierarchical clustering was used to cluster the kinase profiles for each sample. As illustrated in Figure 2, the results from the hierarchical clustering showed that triplicate libraries obtained from an individual sample clustered together confirming data reproducibility of the screen. A representative analysis of the chemonaive PDX MSK-LX-40 shows triplicate libraries (libraries 1–3, 4–6, 7–9) clustered together by day of harvest (Day 1, 7, 14), experiment (I, II) or culture condition (Day vs Tumor) reflecting in vivo vs. in vitro conditions. In addition, in vitro kinase profiles were separable from in vivo kinase profiles. Different kinase dependencies may reflect the differing in vitro vs. in vivo growth conditions (three-dimensional growth, lower oxygen tension, lower glucose and nutrient availability, requirement for angiogenic stimulus). Alternatively, it is possible that kinase function defined by the assay is time dependent, with longer in vivo shRNA exposure (~30 days) leading to differences in the kinase profile when compared to shorter in vitro exposure times (7–14 days). Given the greater similarities to tumor growth conditions in patients, further analyses focused on in vivo samples.
Figure 2.
Unsupervised hierarchical clustering of essential kinase profiles in MSK-LX-40. I/II-experiment number, Day 1/7/14=day of harvest post-shRNA infection of PDX cultured in vitro, Tumor=shRNA infected tumor grown in vivo in NSG mice, 1–3/4–6/7–9=sample triplicate libraries
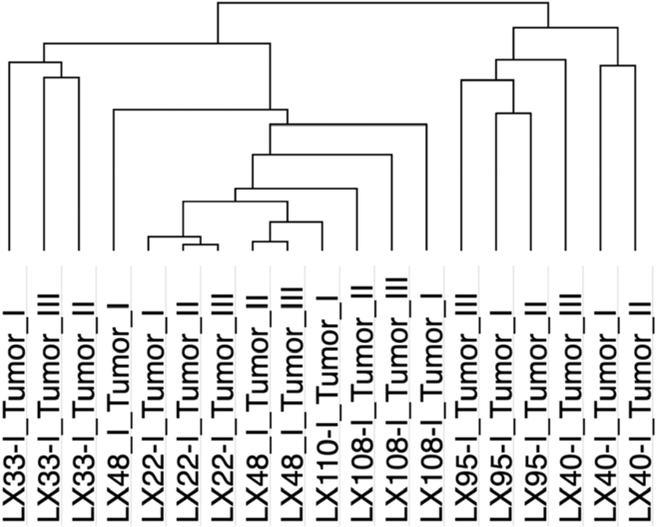
Performing unsupervised hierarchical clustering of the kinase profiles of all 7 PDX again found the results to be reproducible across specimens with profiles from multiple individual tumors of each PDX clustering together (Figure 3). PDX segregated into two major clusters, with MSK-LX-95 and MSK-LX-40 formed one cluster, and the remaining PDX comprising a second cluster. These data are consistent with kinase profiling capable of identifying subtypes of SCLC.
Figure 3.
Unsupervised hierarchical clustering of essential kinases from all PDX. I= experiment number, Tumor I/II/III=shRNA infected tumors grown in vivo in NSG mice.
SCLC Essential Kinases
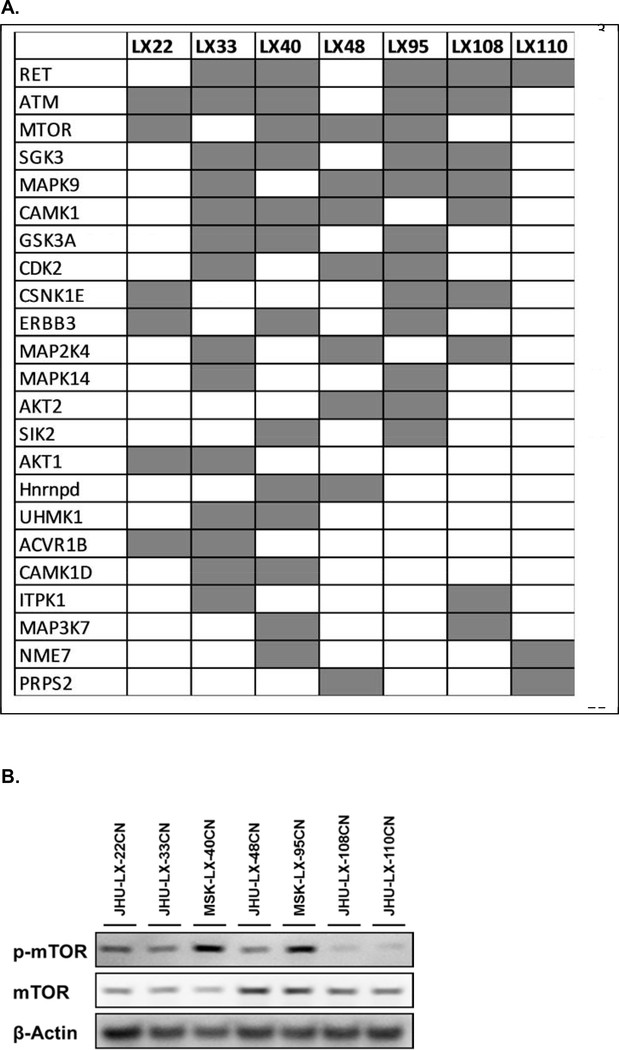
Individual PDX were analyzed to identify candidate essential kinases, defined as shRNA clones with a 2-fold or greater decrease in shRNA reads (P<0.05) when compared to the corresponding Baseline shRNA library [27, 33, 34, 36]. One hundred and twenty-eight (128) candidate essential kinases were identified including data across all seven PDX (Supplementary Data, Table 1, Table 2). However, many of these candidates were relevant only in a single PDX. Filtering results for essential kinase candidates that were present in two or more PDX identified twenty-three (23) kinases (Figure 4A). Mapping these 23 candidate kinases to KEGG pathways found them predominantly involved in Cellular Senescence, Pathways in Cancer and MAPK Signaling. The most prevalent candidate essential kinases in the PDX were RET, ATM, mTOR, SGK3, MAPK9, and CAMK1, identified in >50% of the PDX screened.
Figure 4.
SCLC Essential Kinases present in 2 or more PDX (A). mTOR and p-mTOR identified by western blotting in chemonaive SCLC PDX (B).
Validation of Essential Kinases
Two further conditions were established to consider a candidate kinase as essential; 1) evidence of kinase activation as defined by phosphorylation, and 2) pharmacologic kinase inhibition leading to decreased cell viability. The most divergent clusters found in Figure 3 were LX-33 vs. LX40 and LX-95, with mTOR being the most significant candidate essential kinase difference (Figure 4A). Therefore, mTOR was taken forward for validation as an essential kinase. Western blotting identified mTOR expression in all PDX, and it was variably active as defined by phospho-mTOR-Ser-2448 (p-mTOR) (Figure 4B). P-mTOR Ser-2448 is the Akt and S6 Kinase-β1 (S6K) phosphorylation site, and provides a net analysis of mTOR phosphorylation by both Akt and S6K, reflecting mTOR’s catalytic activity [41]. Higher levels of p-mTOR were found in MSK-LX-40 and MSK-LX-95, no mTOR phosphorylation was seen in JHU-LX-108 and JHU-LX-110, and intermediate levels of mTOR phosphorylation were identified in JHU-LX-22, JHU-LX-33, and JHU-LX-48. These results correlated with the results of our shRNA analysis, nominating mTOR as essential in MSK-LX-40 and MSK-LX-95, and not essential in JHU-LX-108 and JHU-LX-110. Immunohistochemical analysis for mTOR and p-mTOR (Table 1), correlated with western blotting, with discordance only in MSK-LX-110. The phosphorylation status of the mTOR downstream targets p70-S6K and rpS6 were also defined immunohistochemically, but did not correlate with p-mTOR levels suggesting that there are other phosphorylation mechanisms beside mTOR leading to their activation, and therefore, may not serve as a reproducible biomarker for mTOR activity.
Table 1.
Immunohistochemical Analysis of the mTOR Pathway in Chemonaive SCLC PDX.
| Biomarkers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tumor | mTOR | Phospho-mTOR | P70 S6 Kinase | Phospho-p70 S6 Kinase | S6 Ribosomal Protein | Phospho-S6 Ribosomal Protein | DLL3 | PVR |
| JHU-LX22 | 190 | 0 | 300 | 300 | 300 | 70 | 20 | 0 |
| JHU-LX33 | 230 | 0 | 300 | 300 | 300 | 150 | 180 | 300 |
| MSK-LX40 | 110 | 5 | 300 | 300 | 300 | 100 | 300 | |
| JHU-LX48 | 170 | 0 | 300 | 300 | 300 | 20 | 300 | 300 |
| MSK-LX95 | 190 | 20 | 300 | 300 | 300 | 175 | 110 | 300 |
| JHU-LX108 | 180 | 0 | 300 | 300 | 300 | 225 | 220 | 300 |
| JHU-LX110 | 250 | 30 | 300 | 290 | 300 | |||
Using our kinase inhibitor database K-map [42], a connectivity map that connects kinases to kinase inhibitors based on quantitative profiles of the kinase inhibitor activities, AZD-8055 was identified as having a high connectivity score with mTOR. AZD-8055 is an ATP-competitive mTOR kinase inhibitor that inhibits both mTORC1 and mTORC2 with ~ 1,000-fold selectivity for mTOR vs. phosphoinositide 3-kinase isoforms or phosphatidylinositol 3-kinase–related family members. AZD-8055 is currently being explored in phase I and I/II clinical trial in patients with advanced solid tumors (ClincalTrials.gov Identifier: NCT00999882, NCT01194193), recurrent gliomas (ClinicalTrials.gov Identifier; NCT013168098), and hepatic cancer (ClinicalTrials.gov Identifier: NCT00999882). AZD-8055 effectively inhibited mTOR phosphorylation in vitro, and the sensitivity of each PDX to mTOR inhibition, as defined by its IC50, correlated with essential kinase identification and mTOR phosphorylation (Supplementary Data, Figure 1). In MSK-LX-40 and MSK-LX-95, mTOR was essential based on the shRNA screen and phosphorylation status, and the AZD-8055 IC50 was 53nM and 45nM, respectively. In JHU-LX-108 and JHU-LX-110, mTOR was not essential, and the IC50 for AZD-8055 was 81nM and 233nM. Thus, the lowest IC50 values were found in PDX with the highest levels of p-mTOR (MSK-LX-40, MSK-LX-95) and the highest IC50s were found in PDX with low/no p-mTOR (JHU-LX-108, JHU-LX-110). These results confirmed mTOR as an essential kinase in MSK-LX-40 and MSK-LX-95, while non-essential in JHU-LX-108 and JHU-LX-110 supporting our shRNA analysis and identifying subtypes of SCLC PDX based on mTOR activation.
mTOR inhibition decreases SCLC PDX growth in vivo
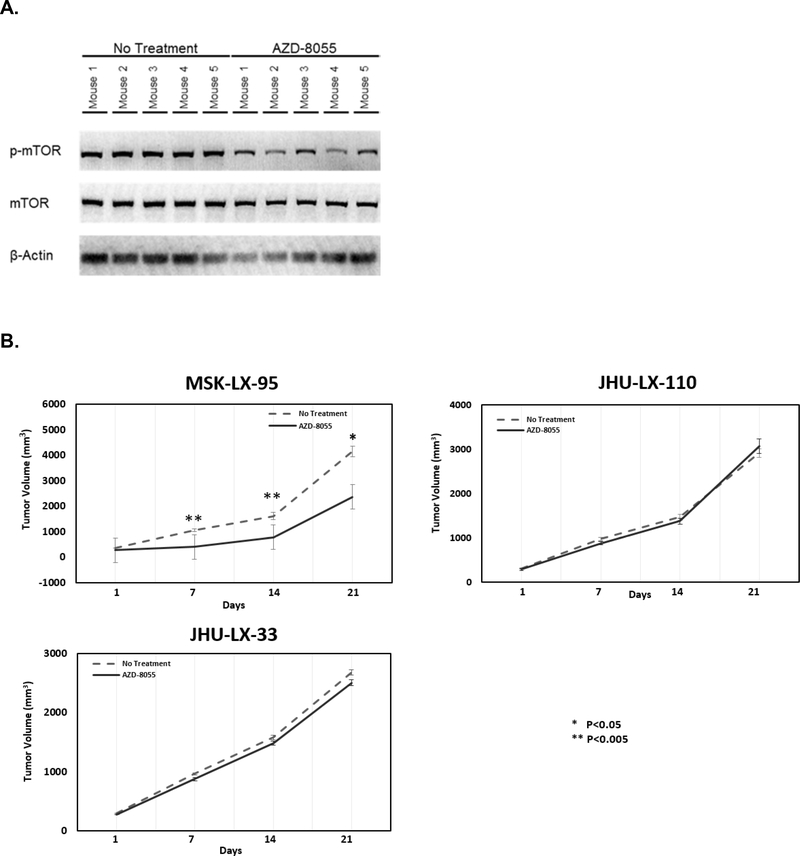
PDX in which mTOR was validated as essential (MSK-LX-95), not essential (JHU-LX-110), and intermediate (JHU-LX-33) were studied in vivo for the effect of mTOR inhibition on SCLC growth. PDX were established in NSG mice and treated with or without AZD-8055 (20 mg/kg, daily gavage). AZD-8055 effectively inhibited mTOR phosphorylation in vivo in MSK-LX-95 with a significant decrease in p-mTOR (Figure 5A). At the end of 14 days, mTOR inhibition with AZD-8055 decreased SCLC growth and tumor volume in MSK-LX-95 by at least 43% over days 7–21 (P≤0.01) (day 21, 2,375±309 mm3 vs. 4,154±599 mm3), yet had no effect in JHU-LX-110 (day 21, 3,074±169 mm3 vs 2,917±93 mm3) and JHU-LX-33 (day 21, 2,509±69 mm3 vs 2,680±104 mm3 respectively) (Figure 5B). Thus, our essential kinase analysis and validation correctly identified mTOR as regulating SCLC growth and survival in this subset of SCLC, and defined mTOR as a possible therapeutic target.
Figure 5.
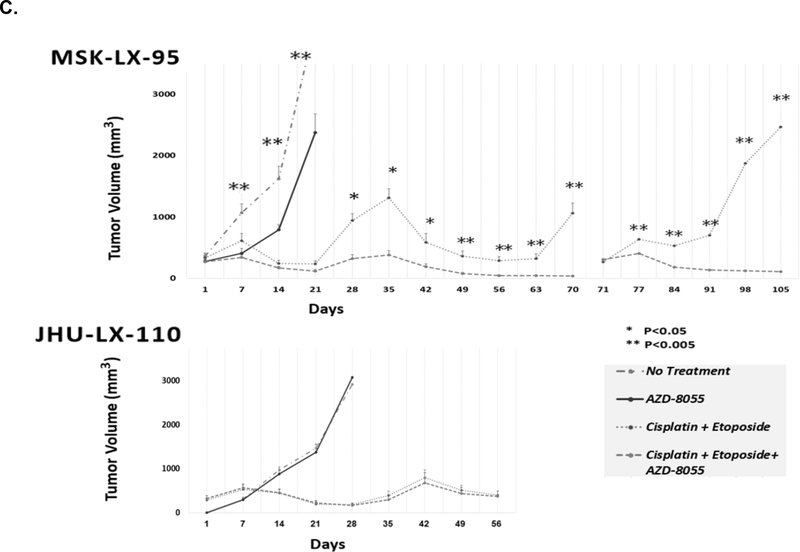
AZD-8055 inhibited mTOR phosphorylation in MSK-LX-95 (A), and impacted PDX growth in vivo when given alone (B). When given in combination with cisplatin and etoposide, mTOR inhibition sensitized SCLC to cisplatin etoposide (C) in MSK-LX-95, a PDX where mTOR was defined as essential and predicted to be responsive to mTOR inhibition, but not JHU-LX-110, a PDX where mTOR was defined as not essential and predicted to be unresponsive to mTOR inhibition.
mTOR inhibition sensitized SCLC to cisplatin/etoposide therapy
As other studies have found kinase inhibition synergizes with chemotherapy in SCLC [13, 27], we studied the effect of mTOR inhibition in combination with cisplatin/etoposide treatment in vitro. The IC50 for the combination of cisplatin and etoposide was defined for cell lines derived from MSK-LX-95, predicted to be sensitive to mTOR inhibition, and JHU-LX-110 where mTOR was defined as non-essential. Then, the cell lines were exposed to the IC50 concentration of cisplatin/etoposide combined, with or without AZD-8055. The addition of AZD-8055 (10 nM) significantly decreased cell viability compared to cisplatin/etoposide alone by 33% (p<0.001) in MSK-LX-95, while no effect was seen in JHU-LX-110 (Supplementary Data, Figure 2).
The efficacy of mTOR inhibition in combination with cisplatin and etoposide was next studied in vivo using the same PDX. Mice were assigned to two groups; cisplatin/etoposide vs. cisplatin/etoposide with AZD-8055. In administering chemotherapy, cisplatin/etoposide was held when tumor volume fell below 300mm3, and restarted when tumor volume was above 300mm3 to determine whether tumor regrowth would occur and generate chemoresistance [31]. AZD-8055 was given continuously (Figure 5C.). In MSK-LX-95, the addition of AZD-8055 to cisplatin/etoposide decreased growth at all-time points. At 5 weeks (Day 35), the combination therapy decreased SCLC growth by 71% compared to cisplatin/etoposide therapy alone (380±72 mm3 vs. 1,314±145 mm3, P=0.0001). At 10 weeks (Day 70), combination therapy reduced growth 96% (37±8 mm3 vs 1,061±169 mm3) (P<0.0005) identifying mTOR’s ability to sensitize SCLC to cisplatin/etoposide. No significant effect was seen in JHU-LX-110 as predicted.
Transplanting MSK-LX-95 into new NSG hosts confirmed that it had become chemoresistant to cisplatin and etoposide and no longer responded to therapy. However, treatment with AZD-8055 and cisplatin/etoposide did not lead to chemoresistance, and there was no tumor growth during an additional 5 weeks (Day 71–105) (Figure 5C). Thus, in PDX where mTOR is defined as essential and predicted to be responsive to mTOR inhibition, the addition of AZD-8055 to the therapeutic regimen significantly decreased growth and delayed, if not prevented, acquired chemoresistance.
Prevalence of mTOR activation in chemonaive SCLC
A tissue microarray of chemonaive SCLC samples was stained for p-mTOR to determine the prevalence of mTOR activation and whether it could potentially serve as a biomarker to identify patients who might respond to mTOR inhibition. An array of 22 chemonaive SCLC samples with duplicate tissue cores, 5 SCLC cell lines, and 9 normal tissue controls was stained for p-mTOR (Supplemental Data, Table 3). Positive staining was defined as an H-score >10 in both tissue cores. Six of the 22 SCLC microarray samples were positive for p-mTOR for a prevalence of 27%. Average H-score among all positive SCLC samples was 88 ± 33, with H-scores ranging from 10–150. All seven SCLC cell lines included on the microarray were positive for p-mTOR with an average H-score of 115 ± 14, ranging from 90–140.
Discussion
Our studies show that kinase profiling and identification of essential kinases can functionally classify SCLC into distinct subgroups, with therapeutic implications seen in variable SCLC PDX sensitivity to mTOR inhibitors. SCLC, traditionally thought of as a homogeneous disease, is heterogeneous based on its essential kinome. mTOR was specifically identified as an essential kinase in a subset of chemonaive SCLC. mTOR inhibition significantly decreased SCLC growth when used alone, and sensitized SCLC to cisplatin and etoposide when used in combination, and delayed, if not prevented, the development of chemoresistance, supporting its essential kinase function in vivo. Phosphorylated mTOR predicted responsiveness to mTOR inhibition as monotherapy, and sensitization of SCLC PDX to cisplatin/etoposide. Finally, p-mTOR was identified in a tissue microarray of chemonaive SCLC, and potentially could serve as a response biomarker for the therapeutic use of mTOR inhibitors.
Our screen strongly identified mTOR as a critical node in SCLC. This fits well with prior observations that the PI3K/PTEN/mTOR axis is frequently mutationally altered in both murine [43, 44] and human [45, 46] SCLC, and has been implicated as a promising therapeutic target [27, 32]. A recent National Cancer Institute drug screen profiled the effect of 526 small molecule inhibitors, including AZD-8055, on the proliferation of 65 SCLC cell lines [47]. This screen also demonstrated SCLC sensitivity to mTORC1, mTORC2, and PI3K/mTOR inhibitors. It is notable, however, that these data are in contrast to clinical trials in SCLC that have shown limited efficacy using rapamycin or its analogues as mTOR inhibitors. Phase II clinical trials using the rapalogue inhibitors temsirolimus (CCI-779), and everolimus (RAD001) did not find significant improvement in progression-free survival in patients with extensive-stage SCLC when combined with chemotherapy or as monotherapy [29, 48, 49]. However, these studies have limitations. Temsirolimus and everolimus are weak allosteric mTOR inhibitors, only partially inhibiting TORC1 and not affecting TORC2. Patients enrolled in the studies were not selected on the basis of mTOR activation. Previously treated patients were included which may have altered mTOR status. mTOR inhibitors were studied as monotherapy, and combinations with cisplatin and etoposide was not considered. Finally, chemotherapy-induced signal adaptation and feedback mechanisms that could result in further suboptimal mTOR pathway inhibition were not considered in study designs. Alternatively, our studies show that in a biomarker selected population of chemonaive PDX, mTOR inhibition alone significantly slowed SCLC growth in vivo, and a combinatorial strategy including mTOR inhibition, sensitized chemonaive SCLC to cisplatin/etoposide therapy. Based on these data, p-mTOR biomarker driven clinical trials with an mTOR kinase inhibitor combined with cisplatin/etoposide may be a better therapeutic strategy for SCLC treatment. Our immunohistochemical analysis of a tissue microarray consisting of chemonaive SCLC identified a 27% prevalence of p-mTOR. Thus, mTOR phosphorylation status might identify the patient population that could benefit from mTOR inhibition. In addition, an essential kinase screen approach could be used to further identify novel combinatorial strategies as a way to define drug:drug synthetic lethality in SCLC.
This work adds to the concept of SCLC as a heterogeneous disease, and suggests that identification of SCLC with distinct kinase vulnerabilities can define new therapeutic strategies. Specifically, the use of mTOR kinase inhibitors in SCLC with active mTOR (p-mTOR) may delay, or possibly prevent, resistance and improve patient outcome. In addition, these studies provide a discovery mechanism to define the SCLC kinome, and in particular to identify essential kinases. The integration of functional genomic studies with genomic [20, 22, 50], epigenomic and transcriptomic [10, 51] data may further subdivide SCLC into discrete functional subsets with defined therapeutic vulnerabilities. The number of PDX samples studied in our analysis may under-represent the transcriptionally defined SCLC subgroups and additional PDX samples representing each of these subsets [11] are needed to fully understand the extent to which SCLC subtypes as defined by key transcriptional regulators are also reflective of essential kinase SCLC subtype classification.
Supplementary Material
Significance.
We used functional genomics to identify kinases regulating SCLC survival. mTOR was identified as essential in a subset of PDX. mTOR inhibition decreased PDX growth, sensitized PDX to cisplatin/etoposide, and prevented chemoresistance.
Acknowledgements
The authors wish to acknowledge support by the Center for Genes, Environment and Health at National Jewish Health, and Ms. Kendra Walton and Dr. Brian O’Connor in the Center for assistance in developing our sequencing strategy. The Functional Genomics Shared Resource of the University of Colorado provided assistance in the development of the shRNA kinase library. Gary Wildey assisted with the use of the tissue microarray. The authors thank the Antoinette E. (“Mimi”) & Herman Boehm Foundation Inc. for its generous support of the studies, the Cancer League of Colorado, and the David F. and Margaret T. Grohne Family Foundation. This work was also supported by the National Cancer Institute through R21CA209121, R01CA197936, U24CA213274, the University of Colorado Cancer Center Support Grant (P30CA046934), the University of Colorado SPORE in Lung Cancer (P50CA058187), and the National Heart Lung Blood Institute (HL111674).
DISCLOSURES
Dr. Kern reports support from the Antoinette E. (“Mimi”) & Herman Boehm Foundation Inc, and grants from the National Cancer Institute during the conduct of the study. Other financial activities outside the submitted work include Biodesix, and Cireca, LLC.
Dr. Finigan reports personal fees from EMD Serono, personal fees from Astra Zeneca, outside the submitted work;.
Dr. Dowlati reports other from Glaxo Smith Kline, non-financial support from Glaxo Smith Kline, during the conduct of the study; grants from EMD Serono, grants from Tesaro, grants and personal fees from Abbvie, grants from Roche, grants from Vertex, grants and personal fees from Astra Zeneca, grants from Regeneron, grants and personal fees from Millenium, grants from Eli Lilly, grants from Takeda, grants from Ipsen, grants from United Therapeutics, grants from Mirati, grants from Bristol Myers Squibb, grants from Incuron, grants and personal fees from Seattle Genetics, personal fees from Ariad, grants from Bayer, outside the submitted work;.
Dr. Rudin reports personal fees from AbbVie, Amgen, Ascentage, AstraZeneca, Bicycle, Celgene, Daiichi Sankyo, Genentech/Roche, Ipsen, Jansen, Jazz, Lilly/Loxo, Pfizer, PharmaMar, Syros, Vavotek, Bridge Medicines, and Harpoon Therapeutics, outside the submitted work.
Remaining authors have nothing to disclose.
Footnotes
The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2020. CA Cancer J Clin, 2020. 70(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Govindan R, et al. , Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol, 2006. 24(28): p. 4539–44. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen M, et al. , Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2010. 21 Suppl 5: p. v120–5. [DOI] [PubMed] [Google Scholar]
- 4.Gazdar AF, Bunn PA, and Minna JD, Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer, 2017. 17(12): p. 725–737. [DOI] [PubMed] [Google Scholar]
- 5.William WN Jr. and Glisson BS, Novel strategies for the treatment of small-cell lung carcinoma. Nat Rev Clin Oncol, 2011. 8(10): p. 611–9. [DOI] [PubMed] [Google Scholar]
- 6.Horn L, et al. , First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med, 2018. 379(23): p. 2220–2229. [DOI] [PubMed] [Google Scholar]
- 7.Schabath MB, et al. , Temporal trends from 1986 to 2008 in overall survival of small cell lung cancer patients. Lung Cancer, 2014. 86(1): p. 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazdar AF, et al. , Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res, 1985. 45(6): p. 2924–30. [PubMed] [Google Scholar]
- 9.Carney DN, et al. , Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res, 1985. 45(6): p. 2913–23. [PubMed] [Google Scholar]
- 10.Poirier JT, et al. , DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudin CM, et al. , Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer, 2019. 19(5): p. 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirier JT, et al. , Selective tropism of Seneca Valley virus for variant subtype small cell lung cancer. J Natl Cancer Inst, 2013. 105(14): p. 1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollaoglu G, et al. , MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell, 2017. 31(2): p. 270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borromeo MD, et al. , ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep, 2016. 16(5): p. 1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson TR, et al. , Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature, 2012. 487(7408): p. 505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosell R, et al. , Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol, 2012. 13(3): p. 239–46. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi S, et al. , EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med, 2005. 352(8): p. 786–92. [DOI] [PubMed] [Google Scholar]
- 18.Ma PC, et al. , Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res, 2005. 65(4): p. 1479–88. [DOI] [PubMed] [Google Scholar]
- 19.Ma PC, et al. , c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res, 2003. 63(19): p. 6272–81. [PubMed] [Google Scholar]
- 20.George J, et al. , Comprehensive genomic profiles of small cell lung cancer. Nature, 2015. 524(7563): p. 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakre N, et al. , RICTOR amplification identifies a subgroup in small cell lung cancer and predicts response to drugs targeting mTOR. Oncotarget, 2017. 8(4): p. 5992–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudin CM, et al. , Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet, 2012. 44(10): p. 1111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn KA, et al. , Insulin-like growth factor expression in human cancer cell lines. J Biol Chem, 1996. 271(19): p. 11477–83. [DOI] [PubMed] [Google Scholar]
- 24.Badzio A, et al. , Increased insulin-like growth factor 1 receptor protein expression and gene copy number in small cell lung cancer. J Thorac Oncol, 2010. 5(12): p. 1905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakanishi Y, et al. , Insulin-like growth factor-I can mediate autocrine proliferation of human small cell lung cancer cell lines in vitro. J Clin Invest, 1988. 82(1): p. 354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon MC, et al. , Paracrine signaling between tumor subclones of mouse SCLC: a critical role of ETS transcription factor Pea3 in facilitating metastasis. Genes Dev, 2015. 29(15): p. 1587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singleton KR, et al. , Kinome RNAi Screens Reveal Synergistic Targeting of MTOR and FGFR1 Pathways for Treatment of Lung Cancer and HNSCC. Cancer Res, 2015. 75(20): p. 4398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, et al. , Target Identification in Small Cell Lung Cancer via Integrated Phenotypic Screening and Activity-Based Protein Profiling. Mol Cancer Ther, 2016. 15(2): p. 334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandya KJ, et al. , A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the Eastern Cooperative Oncology Group (E1500). J Thorac Oncol, 2007. 2(11): p. 1036–41. [DOI] [PubMed] [Google Scholar]
- 30.Tarhini A, et al. , Phase II study of everolimus (RAD001) in previously treated small cell lung cancer. Clin Cancer Res, 2010. 16(23): p. 5900–7. [DOI] [PubMed] [Google Scholar]
- 31.Gardner EE, et al. , Chemosensitive Relapse in Small Cell Lung Cancer Proceeds through an EZH2-SLFN11 Axis. Cancer Cell, 2017. 31(2): p. 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner EE, et al. , Rapamycin rescues ABT-737 efficacy in small cell lung cancer. Cancer Res, 2014. 74(10): p. 2846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, et al. , Bioinformatics-driven discovery of rational combination for overcoming EGFR-mutant lung cancer resistance to EGFR therapy. Bioinformatics, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J and Tan AC, BiNGS!SL-seq: a bioinformatics pipeline for the analysis and interpretation of deep sequencing genome-wide synthetic lethal screen. Methods Mol Biol, 2012. 802: p. 389–98. [DOI] [PubMed] [Google Scholar]
- 35.Langmead B, et al. , Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol, 2009. 10(3): p. R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casas-Selves M, et al. , Tankyrase and the canonical Wnt pathway protect lung cancer cells from EGFR inhibition. Cancer Res, 2012. 72(16): p. 4154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finigan JH, et al. , HER2 activation results in beta-catenin-dependent changes in pulmonary epithelial permeability. Am J Physiol Lung Cell Mol Physiol, 2015. 308(2): p. L199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nethery DE, et al. , Expression of mutant human epidermal receptor 3 attenuates lung fibrosis and improves survival in mice. J Appl Physiol, 2005. 99(1): p. 298–307. [DOI] [PubMed] [Google Scholar]
- 39.Suda K, et al. , Therapy-induced E-cadherin downregulation alters expression of programmed death ligand-1 in lung cancer cells. Lung Cancer, 2017. 109: p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan KD, et al. , ATM and MET kinases are synthetic lethal with nongenotoxic activation of p53. Nat Chem Biol, 2012. 8(7): p. 646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang GG and Abraham RT, Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem, 2005. 280(27): p. 25485–90. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, et al. , K-Map: connecting kinases with therapeutics for drug repurposing and development. Hum Genomics, 2013. 7(1): p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McFadden DG, et al. , Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell, 2014. 156(6): p. 1298–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui M, et al. , PTEN is a potent suppressor of small cell lung cancer. Mol Cancer Res, 2014. 12(5): p. 654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umemura S, et al. , Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J Thorac Oncol, 2014. 9(9): p. 1324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibata T, et al. , Oncogenic mutation of PIK3CA in small cell lung carcinoma: a potential therapeutic target pathway for chemotherapy-resistant lung cancer. Cancer Lett, 2009. 283(2): p. 203–11. [DOI] [PubMed] [Google Scholar]
- 47.Polley E, et al. , Small Cell Lung Cancer Screen of Oncology Drugs, Investigational Agents, and Gene and microRNA Expression. J Natl Cancer Inst, 2016. 108(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marinov M, et al. , AKT/mTOR pathway activation and BCL-2 family proteins modulate the sensitivity of human small cell lung cancer cells to RAD001. Clin Cancer Res, 2009. 15(4): p. 1277–87. [DOI] [PubMed] [Google Scholar]
- 49.Besse B, et al. , A phase Ib dose-escalation study of everolimus combined with cisplatin and etoposide as first-line therapy in patients with extensive-stage small-cell lung cancer. Ann Oncol, 2014. 25(2): p. 505–11. [DOI] [PubMed] [Google Scholar]
- 50.Peifer M, et al. , Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet, 2012. 44(10): p. 1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christensen CL, et al. , Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell, 2014. 26(6): p. 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.