Abstract
Objective:
To determine the association of Nine Hole Peg Test (9HPT), Box and Block Test (BBT), Jebsen Taylor Hand Function Test (JTHFT), and kinematic measures of a simple reaching task with ataxia severity in adults with degenerative cerebellar disease.
Design:
Fourteen adults with cerebellar degeneration were recruited, and ataxia severity was determined using the Scale for the Assessment and Rating of Ataxia (SARA). The median SARA score was used to divide participants into less and more severe ataxia groups. The two groups’ average scores on the hand function tests were compared, and correlation of each test with ataxia severity was determined.
Results:
The 9HPT, BBT, and JTHFT all differentiated between less and more severe ataxia groups, and the 9HPT performed with the participant’s dominant hand had the highest correlation with ataxia severity (rs=.92, p<.01). Although accuracy, precision, and number of sub-movements were statistically different between healthy individuals and the more ataxic participant group, most kinematic measures were not significantly different between the less and more severe ataxic groups.
Conclusion:
Overall, our results indicate that all three clinical tests correlate with ataxia severity. Larger future studies should examine the reliability and validity of these hand function measures in adults with degenerative cerebellar disease.
Keywords: Ataxia, Spinocerebellar ataxia, Hand function, Arm kinematics
Introduction:
Although a rare disease affecting approximately 150,000 Americans, degenerative cerebellar diseases are serious disorders that progress to severe disability and can be fatal.1 Rehabilitation is commonly used to maintain function and improve motor skills. The lower extremities are usually first to be affected, making walking difficult and falls common.2,3 Hand function is retained for a longer duration, although difficulty with writing, eating, and dressing eventually develop.3,4
The Scale for the Assessment and Rating of Ataxia (SARA) is the most common primary outcome measure to evaluate the effectiveness of rehabilitative techniques in the treatment of cerebellar degeneration. Although validated in determining ataxia severity with excellent inter-rater and test/retest reliability,5,6 this scale is not designed to detect functional changes that may occur for ataxic individuals as a result of rehabilitation. As a result, most studies use gait analysis and clinical balance scales as secondary outcome measures to detect changes that occur in the lower extremity as a result of rehabilitation.7–10 Upper extremity function is usually not monitored in these studies.
One possible reason that hand function is not routinely monitored in ataxic patients is due to the lack of knowledge regarding which clinical scales to use. Up to now, only a few studies have evaluated upper limb functional scales in ataxic patients. In 2010, Corben et al evaluated three measures [Nine Hole Peg Test (9HPT), Box and Block Test (BBT), and Jebsen Taylor Hand Function Test (JTHFT)] of arm function in Friedreich’s ataxia. They concluded that the 9HPT performed with the non-dominant limb was the best test to detect change in arm function in these ataxic patients.11 In 2015, Germanotta et al used an InMotion Arm Robot to assess the kinematics of upper limb reaching tasks in Friedreich’s ataxia subjects. Compared to healthy controls, participants with Friedreich’s ataxia exhibited slower movements as well as loss of accuracy and smoothness. Duration of movement and number of sub-movements were the best indicators in that they correlated with the status of disease as measured by SARA.12
A limitation of these prior studies is that they were conducted only in patients with Friedreich’s ataxia, and it is unclear if the results would be the same in other degenerative cerebellar diseases. Moreover, both of the studies included children in their assessment, which may have also influenced the results. In the one study evaluating arm kinematic function solely in other degenerative cerebellar diseases, investigators did not examine if kinematic measures correlated with ataxia severity. Moreover, the study only consisted of seven subjects with dominant-type cerebellar degeneration.13 In this study, we used known-groups validity, in which a good test will be able to discriminate between two groups known to differ on the variable of interest,14 as a preliminary step to evaluate the utility of the 9HPT, BBT, and JTHFT to test hand function in adults with dominant type cerebellar degeneration. We also sought to examine the usefulness of testing arm kinematics of reaching tasks in these patients.
Methods:
This study ran from September 2017 to August 2019. It was conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent according to the human studies guidelines of the Institutional Review Boards of Columbia University (IRB AAAQ9629), and the Biomedical Research Alliance of New York (19-08-218-512). This study conforms to all STROBE guidelines and reports the required information accordingly (see Supplemental Checklist, Supplemental Digital Content 1, http://links.lww.com/PHM/A969).
Individuals with degenerative cerebellar diseases were recruited from the adult ataxia clinic at the Neurological Institute at New York Presbyterian-Columbia. After one year of recruitment, fourteen subjects had been recruited. Although a smaller sample size than originally anticipated, fourteen subjects was deemed large enough for this pilot study as a prior study looking at arm kinematics in Friedreich’s ataxia used this number of subjects and had found statistically significant results.12 Inclusion criteria for the study were comprised of cerebellar atrophy on magnetic resonance imaging and prevalence of ataxia on clinical exam. Participants were excluded if they had other neurological diseases, cognitive impairment (Mini-Mental State exam score15 <22), or if they were medically unstable. Results of genetic testing were obtained when available. Subjects with different types of cerebellar degeneration were allowed to participate in the study as long as their predominant deficit was ataxia. Clinically, all participants had evidence of at least mild ataxia (SARA >3)5 for more than 3 months.
The three clinical measures that were examined in this study were chosen after extensive review of the literature. The 9HPT is a timed measure of finger dexterity commonly used in a range of neurological conditions. The task involves placing and removing nine pegs from a pegboard as quickly as possible. The test has excellent intra and inter-rate reliability and normative reference values.16 The BBT measures manual dexterity by counting the number of blocks transported from one section of a box to another in one minute. This test also has high inter-rater and test-retest reliability with normative reference values.17 Finally, the JTHFT is a measure of arm and hand function. There are seven test items including 1) writing a short sentence, 2) turning over five index cards, 3) picking up six small objects and placing them in a container, 4) simulated feeding, 5) stacking four standard checker pieces, 6) lifting five light cans, and 7) lifting five heavy cans. Performance time for each task is recorded and added. The JTHFT also has established normative reference values and good test-retest reliability.18
Participants also underwent clinical assessment by the SARA, a 40-point scale that evaluates the degree of ataxia, according to the published protocol. A score of zero indicates no signs of ataxia whereas a score of 40 indicates the most severe ataxia.19 After all 14 participants were assessed, the median score was determined to be 15. This was then used as the cutoff to differentiate between less severe (SARA <15) and more severe ataxia (SARA ≥15) groups. A SARA score around 15 is a reasonable cut-point given the findings in a prior study on ataxic stroke patients; individuals with a SARA score of less than 14.97 were able to ambulate independently whereas those with higher scores required a wheelchair for mobility. Moreover, patients with SARA scores greater than 16.25 were classified as being severely dependent in activities of daily living.20
For the kinematic analysis, 14 age and gender-matched healthy individuals with no neurological or musculoskeletal disorders were recruited for kinematic evaluation in addition to the 14 participants with cerebellar degeneration (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/PHM/A970).
Participants were seated comfortably with the trunk restrained using a five-point seatbelt to minimize compensatory movements. The participant’s hand and forearm were gently strapped into a thermoplastic splint. A system of air jets lifted the frame supporting the arm above the glass surface to reduce friction during reaching movements (Figure 1). The kinematic evaluation consisted of two blocks of 80 planar reaching movements with each arm to one of four circular targets (Northeast (NE); Southeast (SE); Southwest (SW); Northwest (NW)) located 8 cm from a central start circle. Targets were projected in pseudo-random order onto the plane of the hand and forearm using a mirror obscuring the participant viewing their arm and hand. Participants were instructed to move a cursor corresponding to the hand position from the start circle to the target and stop. Movement speed feedback was provided in the form of a velocity bar (with target peak velocity range 20–40 centimeters (cm) per second (s)) and automated verbal feedback to increase or reduce movement speed if the velocity fell outside of this target range. The location of the hand at the end of each trajectory was frozen on the screen for 1s to provide feedback regarding reaching accuracy.
Figure 1:
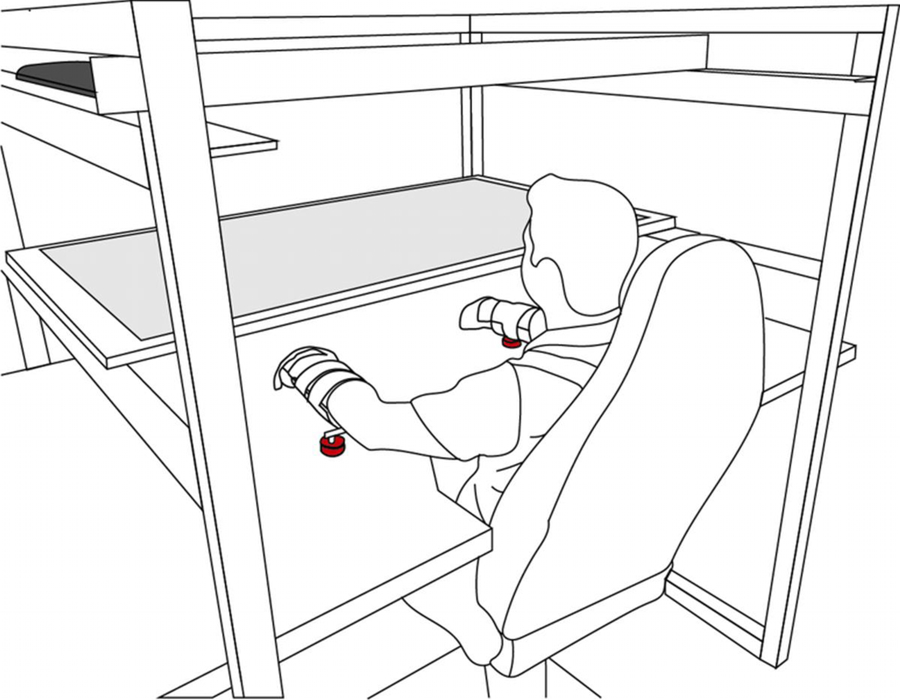
Experimental setup. Participants sat comfortably with the trunk restrained by a five-point seatbelt. The participants hands and forearms were strapped into thermoplastic splints attached to a frame with a system of air jets (red) that lifted the arm above the glass surface, thus reducing friction during reaching movements. A screen (black) was reflected onto a mirror (grey) that obstructed the participant’s view of their arm. Participants were instructed to move a cursor, that represented the hand, towards a target and then back to the center point.
Hand position as recorded at 130 Hertz (Hz) using a pair of six degree of freedom magnetic sensors (Flock of Birds, Ascension Technologies, Burlington, VT). Kinematic data were processed offline to obtain quantitative indices related to clinical features of cerebellar degeneration using custom routines in MATLAB 2018 (MathWorks, Inc., Natick, MA). Hand position was filtered with a 6th order zero phase shift low-pass Butterworth filter, with cut-off frequency of 10 Hz, and then differentiated to obtain speed, acceleration and jerk. Left arm trajectories were transformed into right arm coordinates to match joint configurations during analysis. Movement start was defined as the point at which the velocity magnitude became greater than two standard deviation above the mean baseline velocity, calculated during the rest period prior to the appearance of the target. Similarly, reaching movements were considered to end when the velocity dropped and remained below this threshold for longer than 1s (Figure 2A).
Figure 2:
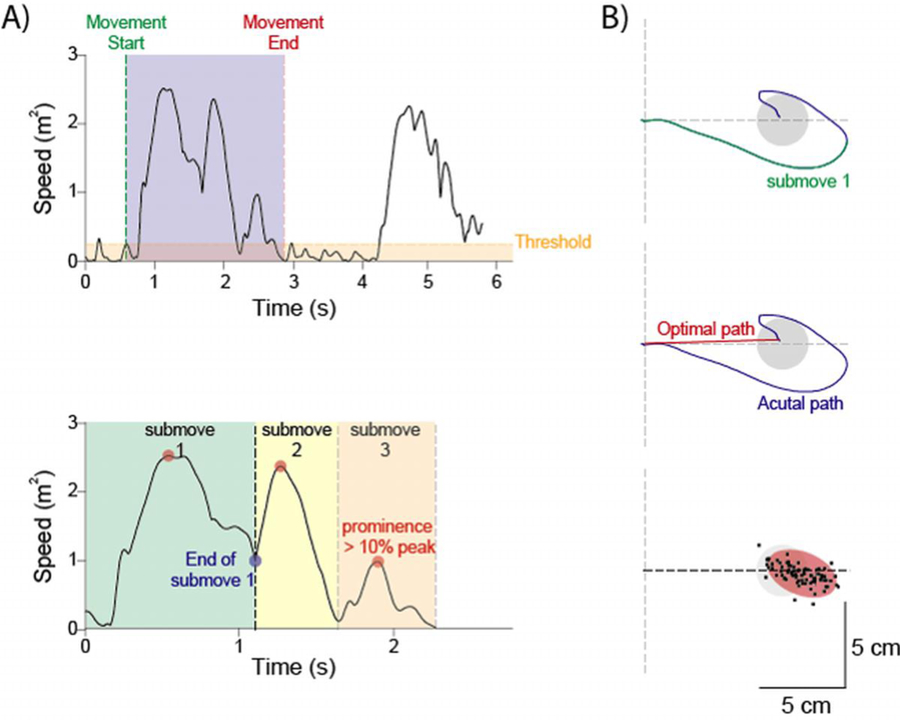
Kinematic analysis. A) The start of a movement determined to the point when speed was greater than 2 standard deviation of baseline away. The movement towards the target (blue shaded area) was deemed to have ended when speed dropped below the threshold for more than one second (top). Trials were broken down into sub-movements which were calculated by the number of oeaks that had a prominence greater than 10% of peak speed (red circles). Sub-movement 1 (green shaded area) was later used for subsequent analysis (bottom). B) Hand position during movement towards the target is shown in blue, while the first sub-movement is seen overlaid in green (top). Length ratio was calculated as the actual path length (blue trace) divided by the optimal path length (red trace) (middle). Once all trials were completed an ellipse (red shaded oval) was fitted to the endpoints of all trajectories and then used to calculate accuracy and precision.
For each trial length ratio, endpoint accuracy, endpoint precision, and the number of sub-movements were calculated. Length ratio, endpoint accuracy, and endpoint precision were also calculated for the first sub-movement of each trial. The number of sub-movements was defined as the number of peaks in the velocity profile that had a prominence of 20% of peak velocity. Lower number of sub-movements represent smoother trajectories (Figure 2A). Length ratio is defined as the ratio between the path actually traveled by the hand and a straight line (i.e. the minimum distance between the beginning and the end of the trajectory (Figure 2B). Higher values of length ratio represent trajectories with greater curvature. Accuracy and precision were calculated by fitting an ellipse to the endpoints of the first sub-movement, and the endpoints of each trajectory. Accuracy was calculated as the distance between the center of the ellipse and the center of the desired target, while precision was obtained by calculating the area of the ellipse (Figure 2B). Indices were averaged for trajectories of all targets combined.
For statistical analysis of the clinical tests, the average scores for less and more severe ataxia groups were determined and compared using the two sample t-test. We examined the distribution of our data for the two groups using the Shapiro-Wilk test. Since data were not normally distributed, Spearman rank correlation was used to detect relationship between SARA score and clinical tests. The partial correlation coefficient was also used to examine the relationship between age, a possible confounder, SARA scores, and the clinical tests. For categorical demographic variables such as sex and diagnoses, we compared proportions between mild and severe SARA groups using either chi-squared or Fisher’s exact test.
For statistical analysis of the kinematic analysis, the average scores for healthy controls, mild ataxic subjects, and severe ataxic subjects was determined and compared using a one-way ANOVA test. Tukey post hoc analysis was done to confirm where the differences were present between groups. For the measurements that were statistically different between all three groups (endpoint precision measurement with the dominant hand and sub-movement end precision with the non-dominant hand), the distribution of the data was determined using the Shapiro-Wilk test. Since the data were normally distributed, Pearson correlation was used to detect the relationship between these measures and ataxia severity.
Results:
The demographic data for the cohort can be examined in Table 1. The mean age of participants was 50.3 years with a range from 20 to 78 years. There were no statistical differences between groups (Table 1).
Table 1.
Demographic data
| Mild Ataxia | Severe Ataxia | Total | P-Value | |
|---|---|---|---|---|
| Number of Patients | 7 | 7 | 14 | |
| Age in years (SD)/range | 51 (18.0)/20–71 | 49.6 (20.5)/23–78 | 50.3 (18.5)/20–78 | .92 |
| Female/Male | 3/4 | 3/4 | 6/8 | 1.0 |
| MSA-C/SCA/Idiopathic | 4/1/2 | 2/3/2 | 6/4/4 | .43 |
| SARA (SD) | 8.1 (2.6) | 20.1 (4.0) | 14.1 (7.0) | NA |
| SARA hand sub-group (SD) | 3.2 (.7) | 3.9 (1.2) | 3.6 (1.0) | .20 |
SD: standard deviation; MSA-C: multiple system atrophy-cerebellar type; SCA: spinocerebellar ataxia; SARA: Scale for the Assessment and Rating of Ataxia; NA: Not Applicable
Scores for both the dominant and non-dominant hand of the 9HPT, BBT, and JTHFT can be seen in Table 2. There was a statistically significant difference with large effect sizes between mild and severely ataxic subjects for all three tests using their dominant hand. A significant difference with a medium effect size was also seen between groups for the BBT conducted with the non-dominant hand, but not the 9HPT and JTHFT (Table 2). All three clinical tests, whether conducted with the dominant or non-dominant hand, correlated with SARA score, although the 9HPT performed with the dominant hand exhibited the highest degree of correlation (Figure 3). The correlation between SARA scores and clinical tests remained statistically significant when adjusting for age as a possible confounder (Supplemental Table 2, Supplemental Digital Content 3, http://links.lww.com/PHM/A971).
Table 2.
Results of clinical tests for healthy subjects, mild ataxic and more severe ataxic subjects.
| Average Healthy Controls Score (SD) | Average Mild Ataxia Score (SD) | Average Severe Ataxia Score (SD) | Difference between mild and severe ataxia averages (95% CI) | P-Value | Effect Size | |
|---|---|---|---|---|---|---|
| BBT Dominant | 77.7 (10.7) | 42.1 (7.1) | 27.4 (10.9) | 14.7 (4.0 to 25.4) | .01 | .80 |
| BBT Non-Dominant | 74.3 (9.9) | 40.0 (9.5) | 26.6 (10.9) | 13.4 (1.5 to 25.3) | .03 | .66 |
| 9HPT Dominant in seconds | 17.4 (1.7) | 25.7 (2.4) | 62.4 (30.5) | 36.7 (11.5 to 61.9) | <.01 | .85 |
| 9HPT Non-Dominant in seconds | 18.9 (2.3) | 34.0 (5.4) | 71.3 (61.7) | 37.3 (−13.7 to 88.3) | .14 | .43 |
| JTHFT Dominant in seconds | 39.3 (1.6) | 41.2 (9.0) | 71.2 (33.5) | 30.0 (1.4 to 58.6) | .04 | .61 |
| JTHFT Non-Dominant in seconds | 41.7 (1.5) | 55.1 (12.1) | 82.5 (42.6) | 27.4 (−9.1 to 63.9) | .13 | .44 |
SD: standard deviation; 95% CI: 95% confidence interval; BBT: Box and Block Test; 9HPT: Nine Hole Peg Test; JTHFT: Jebsen Taylor Hand Function Test.
Figure 3:
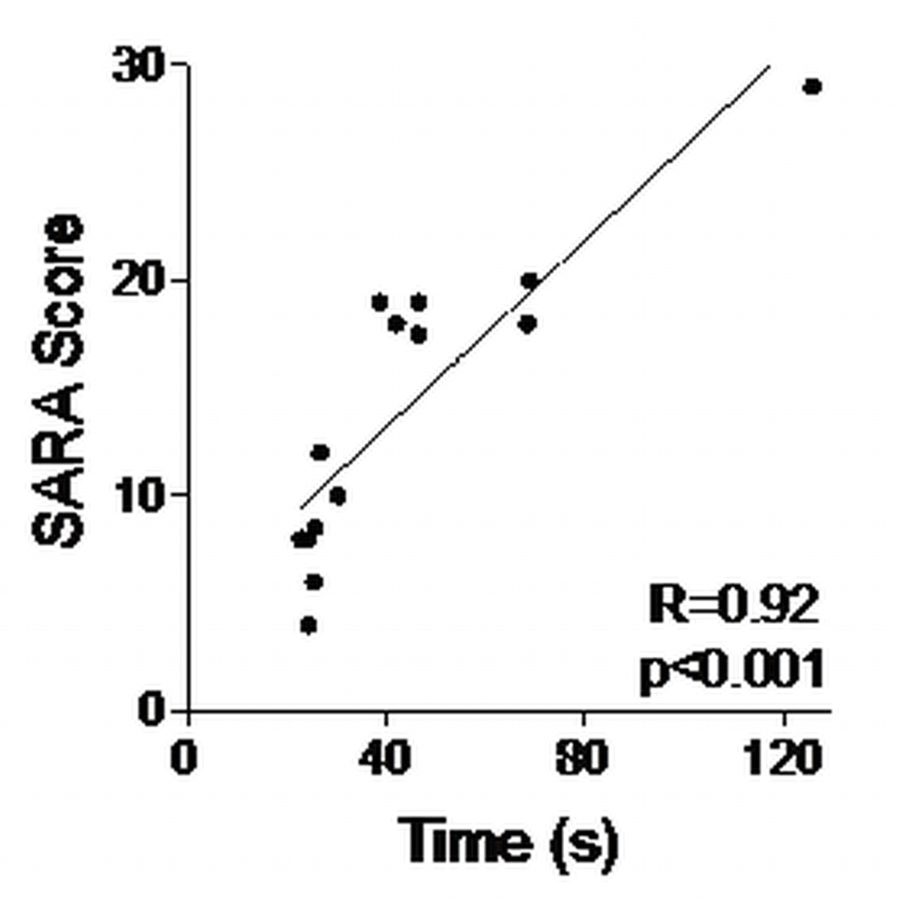
Correlation between SARA score and Time (s) to complete Nine Hole Peg Test with dominant hand. Correlation of other tests not graphed, but results shown below. 9HPT: Nine Hole Peg Test; BBT: Box and Block test; JTHFT: Jebsen-Taylor Hand Function Test.
9HPT Non-dominant r=.86 p<.01
BBT Dominant r=−.79 p<.01
BBT Non-dominant r=−.74 p<.01
JTHFT Dominant r=.63 p=.02
JTHFT Non-dominant. r=.69 p=.01
A sample of typical kinematic data for each group can be seen in Figure 4. Table 3 shows the average results of length ratio, number of sub-movements, precision, accuracy, first sub-movement precision, and first sub-movement accuracy for participants’ dominant hands. Table 4 shows these same measures for the non-dominant hand. Although all measures besides accuracy were statistically different between healthy controls and severe ataxic patients, only endpoint precision with the dominant hand and first sub-movement precision with the non-dominant hand differentiated between all three groups. Neither first sub-movement precision nor endpoint precision, however, correlated with ataxia severity as determined by Pearson correlation coefficients (p>0.05).
Figure 4:
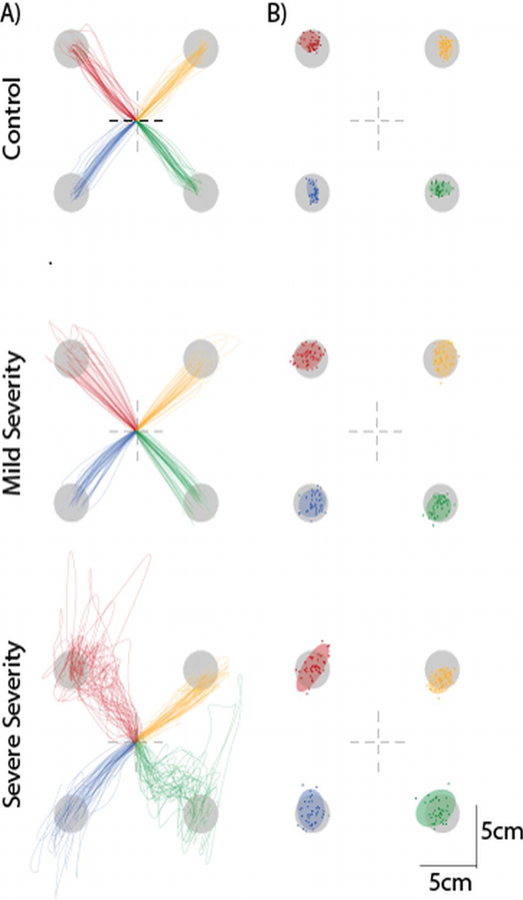
Representative participant data. A) Dominant right arm trajectories for a control (top), a patient with mild (SARA Score 4, middle), and severe ataxia (SARA score 20, bottom). B) An ellipse was fitted with the endpoints of each movement to provide measure of accuracy and precision.
Table 3.
Results of kinematic arm reaching with dominant hand.
| Average Healthy Control Score (SD) | Average Mild Ataxia Score (SD) | Average Severe Ataxia Score (SD) | P-Value | Effect Size | |
|---|---|---|---|---|---|
| Length Ratio* | 1.18 (.08) | 1.17 (.13) | 1.59 (.41) | <.01 | .71 |
| Number of submovements* | 1.22 (.10) | 1.39 (.26) | 1.91 (.60) | <.01 | .74 |
| Precision in mm2** | .18 (.07) | .34 (.17) | .48 (.15) | <.01 | .91 |
| Accuracy in mm | 6.1 (1.32) | 6.0 (1.70) | 6.4 (3.82) | .95 | .06 |
| Submovement Precision in mm2* | .38 (.13) | 1.02 (.58) | 4.4 (5.54) | .01 | .52 |
| Submovement Accuracy in mm*** | 6.3 (5.21) | 15.0 (6.34) | 40.5 (51.38) | .03 | .47 |
SD: standard deviation; mm: millimeters
Means of healthy control verse severe ataxia and mild ataxia verse severe ataxia differ. Means of healthy control verse mild ataxia not significantly different.
Means of healthy control verse mild and severe ataxia differ. Means of mild and severe ataxia differ.
Means of healthy control verse severe ataxia differ. Means of mild ataxia verse healthy control and severe ataxia not significantly different.
Means of healthy control verse mild and severe ataxia differ. Means of mild and severe ataxia are not significantly different
Table 4.
Results of kinematic arm reaching with non-dominant hand.
| Average Healthy Control Score (SD) | Average Mild Ataxia Score (SD) | Average Severe Ataxia Score (SD) | P-Value | Effect Size | |
|---|---|---|---|---|---|
| Length Ratio* | 1.25 (.07) | 1.29 (.17) | 1.47 (.24) | .01 | .52 |
| Number of submovements*** | 1.33 (.18) | 1.77 (.75) | 1.89 (.51) | .03 | .55 |
| Precision in mm2**** | .18 (.04) | .51 (.35) | .64 (.16) | <.01 | .91 |
| Accuracy in mm | 6.3 (1.43) | 8.8 (5.92) | 7.1 (1.62) | .24 | .28 |
| Submovement Precision in mm2** | .47 (.19) | 1.30 (.44) | 2.05 (.92) | <.01 | 1.10 |
| Submovement Accuracy in mm**** | 5.7 (3.68) | 18.0 (14.03) | 25.8 (12.41) | <.01 | .78 |
SD: standard deviation; mm: millimeters
Means of healthy control verse severe ataxia and mild ataxia verse severe ataxia differ. Means of healthy control verse mild ataxia not significantly different.
Means of healthy control verse mild and severe ataxia differ. Means of mild and severe ataxia differ.
Means of healthy control verse severe ataxia differ. Means of mild ataxia verse healthy control and severe ataxia not significantly different.
Means of healthy control verse mild and severe ataxia differ. Means of mild and severe ataxia are not significantly different
Discussion:
In this article, we demonstrate that results of the 9HPT, JTHFT, and BBT for 7 mildly ataxic and 7 severely ataxic patients correlate with ataxia severity. The 9HPT performed with the dominant hand showed the highest degree of correlation. Kinematic analysis showed that precision with the dominant hand and first sub-movement precision of the non-dominant hand could differentiate between healthy controls, mildly ataxic, and severely ataxic patients. However, neither of these measures correlated with ataxia severity.
SARA is the main outcome measure for most rehabilitative studies evaluating treatments for degenerative cerebellar disease. This scale has been validated in monitoring disease severity in degenerative cerebellar disease as well as shown to have excellent test-retest reliability, inter-rater reliability, and internal consistency.5 However, the test was not designed to detect functional changes as a result of rehabilitation, and we speculate that this test lacks the sensitivity to accurately assess hand function. Indeed, hand function SARA sub-scores were not statistically different between our mild and severe ataxia groups (Table 1). Although this may be due to the small sample size in our study, there is a need to identify other clinical scales to monitor hand function in these patients; a measurement with more sensitivity may be required to monitor disease progression.
In 2010, researchers performed the 9HPT, JTHFT, and BBT on Friedrich ataxia patients. They found the BBT and 9HPT performed similarly, while the JTHFT performed the worst. Since the 9HPT is easily portable and the most widely used tool for assessment of upper limb function,21 they recommend that 9HPT to be used to monitor hand function in Friedreich ataxia. In our study performed in adults with other types of cerebellar degeneration, we found similar results. All three tests were able to differentiate between mild and severe ataxic patients, but the 9HPT had the highest correlation with ataxia severity.
Small trials have shown benefits of rehabilitative training in degenerative cerebellar disease, but there is a need for larger, more definitive trials to be conducted.10 It is vital that the outcome measures chosen to examine efficacy in these trials to be the most responsive to change over time.22 As such, the true benefit of treatments can be determined for individuals with degenerative cerebellar disease. Thus, confirming that the 9HPT is a valid and reliable measure of hand function in this population would be useful, and future studies with more participants are needed.
The kinematic results showed differences between ataxic patients and healthy controls as seen in previous studies.11,12 It was surprising that none of the measured parameters correlated with ataxia severity as measured by the SARA score. There are a few possible causes for this result. First, it is possible that some of the measurements would have correlated with ataxia severity, but there was not enough power with the small sample size in the study. Another possibility is that the task was not able to capture more subtle manifestations of upper limb ataxia in the mildly impaired group. For the reaching task, participants performed 2D movements with the arm fully supported against gravity, and 2 degrees of freedom at the shoulder and elbow. The tasks within the clinical scales required more complex 3D movements, thus requiring the ability to adjust for multiple interaction torques, which has been previously demonstrated to be impaired in ataxic patients.23 This may be the reason why better results were seen with the 9HPT, BBT, and JTHFT. Finally, since more ataxia is typically more evident at end range of motion, results may have been different if participants were required to perform longer reaching movements.
Future studies should consider performing kinematic analyses on more complex upper extremity tasks. Moreover, the next study can follow subjects over time to see how measurements vary over time and see if they continue to correlate with SARA scores. Overall, continued evaluation of the clinical tests as valid and reliable measures of hand function in degenerative cerebellar disease is warranted.
Study Limitations:
There are a variety of limitations in this study. First, out sample size was relatively small, comprising of fourteen subjects. Moreover, subjects with various causes of cerebellar degeneration were used in this study to increase recruitment. We also did not follow these subjects over time to see how the different clinical scales and kinematic measures change as disease progresses. As noted above, the reaching task for the kinematic analysis was only a two-dimensional movement which may have affected results. Finally, the reaching task was over a short distance and did not include end range of motion.
Conclusions:
The BBT, 9HPT, and JTHFT all correlated with ataxia severity. The 9HPT performed with the dominant hand had the highest correlation with ataxia severity. Kinematic analysis of simple reaching tasks showed differences between healthy controls and ataxic individuals, but measurements did not correlate with SARA score, probably due to the small sample size in this study.
Supplementary Material
Summary Text:
What is Known:
Although the Nine Hole Peg Test is used to evaluate patients with Friedreich’s Ataxia, there is no consensus on which clinical scales to use to monitor hand function in other degenerative cerebellar diseases. As a result, hand function assessment is often neglected in these patients.
What is New:
In adults with degenerative cerebellar disease, we evaluate clinical scales used to assess hand function in other neurological diseases and determine their correlation with ataxia severity.
Acknowledgments
Funding
No funding was received for this research.
Footnotes
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References:
- 1.Salman MS “Epidemiology of cerebellar diseases and therapeutic approaches.” The Cerebellum. 2018; 17: 4–11. [DOI] [PubMed] [Google Scholar]
- 2.Parodi L, Coarelli G, Stevanin G, Brice A, Durr A. “Hereditary ataxias and paraparesias: clinical and genetic update.” Curr Opin Neurol. 2018; 31: 462–471. [DOI] [PubMed] [Google Scholar]
- 3.Luo L, Wang J et al. “The initial symptom and motor progression in spinocerebellar ataxias.” Cerebellum. 2017; 16:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashizawa T, Oz G, Paulson HL. “Spinocerebellar ataxias: prospects and challenges for therapy development.” Nat Rev Neurol. 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 5.Yabe I, Matsushima M, Soma H, Basri R, Sasaki H. “Usefulness of the Scale for Assessment and Rating of Ataxia (SARA).” J Neurol Sci. 2008; 266: 164–166. [DOI] [PubMed] [Google Scholar]
- 6.Weyer et al. “Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients.” Mov Disord. 2007; 22: 1633–1637. [DOI] [PubMed] [Google Scholar]
- 7.Trujillo-Martin MM, Serrano-Aguilar P, Monton-Alvarez F, Carrillo-Fumero R. Effectiveness and safety of treatments for degenerative ataxias: a systematic review. Mov Disord. 2009; 24: 1111–1124. [DOI] [PubMed] [Google Scholar]
- 8.Miyai I, Mizuki I, Hattori N, Mihara M, et al. Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil Neural Repair. 2012; 26: 515–522. [DOI] [PubMed] [Google Scholar]
- 9.Bastian A and Keller J. A home balance exercise program improves walking in people with cerebellar ataxia. Neurorehabil Neural Repair. 2014; 28: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilg W, Synofzik M, Brotz D, Burkard S, et al. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009; 73: 1823–1830. [DOI] [PubMed] [Google Scholar]
- 11.Corben LA, Tai G, Wilson C, Collins V, Churchyard AJ, Delatycki MB. “A comparison of three measures of upper limb function in Friedrich ataxia.” J Neurol. 2010; 257: 518–523. [DOI] [PubMed] [Google Scholar]
- 12.Germanotta M, Vasco G, Petrarca M, Rossi S, et al. “Robotic and clinical evaluation of upper limb motor performance in patients with Friedrich’s Ataxia: an observational study.” Journal of NeuroEngineering and Rehabilitation. 2015; 12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastian AJ, Martin TA, Keating JG, Thach WT. “Cerebellar ataxia: abnormal control of interaction torques across multiple joints.” J Neurophysiol. 1996; 76:492–509. [DOI] [PubMed] [Google Scholar]
- 14.Mokkink LB, Terwee CB, Patrick DL, et al. “The COSMIN study reached international consensus on taxonomy terminology, and definitions of measurement properties of health related patient-reported outcomes.” J Clinical Epidemiology. 2010; 63: 737–745. [DOI] [PubMed] [Google Scholar]
- 15.Pangman VC, Sloan J, Guse L (2000). “An Examination of Psychometric Properties of the Mini-Mental Status Examination and the Standardized Mini-Mental Status Examination: Implications for Clinical Practice.” Applied Nursing Research. 2000; 13;4: 209–213. [DOI] [PubMed] [Google Scholar]
- 16.Oxford GK, Vogel KA, Le V, et al. “Adult norms for the commercially available nine hole peg test for finger dexterity.” Am J Occup Ther. 2003; 57: 570–573. [DOI] [PubMed] [Google Scholar]
- 17.Mathiowetz V, Volland G, Kashman N, Weber K. “Adult norms for the box and block test of manual dexterity.” Am J Occup Ther. 1985; 39: 386–391. [DOI] [PubMed] [Google Scholar]
- 18.Lynch K, Bridle M. “Validity of the Jebsen-Taylor Hand Function Test in predicting activities of daily living.” Occup Ther J Res. 1989; 9:316–319. [Google Scholar]
- 19.Schitz-hubsch T, Du montcel ST, Baliko L, et al. “Scale for the assessment and rating of ataxia: development of a new clinical scale.” Neurology. 2006; 66:1717–1720. [DOI] [PubMed] [Google Scholar]
- 20.Kim BR, Lim JH, Lee S, Park S, Koh SE, Lee IS, Jung H, Lee J. “Usefulness of the Scale for the Assessment and Rating of Ataxia (SARA) in Ataxic Stroke Patients.” Ann Rehabil Med. 2011; 35: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudman D, Hannah S. “An instrument evaluation framework: description and application to assessments of hand function.” J Jand Ther. 1998; 11:266–277. [DOI] [PubMed] [Google Scholar]
- 22.Delatycki MB. “Evaluating progression of Friedreich ataxia and its treatment.” J Neurol. 2009; 256:36–41. [DOI] [PubMed] [Google Scholar]
- 23.Bastian AJ, Zackowski KM, Thach WT. “Cerebellar ataxia: torque deficiency or torque mismatch between joints?” J Neurophysiol. 2000; 83:3019–3030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


