Abstract
Contrary to tandem autologous transplant (auto-auto), autologous followed by reduced intensity conditioning allogenic transplantation (auto-allo) offers graft-versus-myeloma (GVM) effect but with higher toxicity. Trials comparing these two strategies relied on availability of HLA-matched sibling donors for arm allocation (biological randomization) and yielded conflicting results. A pooled analysis of multiple trials with extended follow up provides an opportunity to compare these strategies. We obtained individual patient data from participants of 4 trials comparing auto-auto vs. auto-allo after induction therapy. There were 899 patients in auto-auto and 439 in auto-allo. Median follow up of survivors was 118.5 months. Median overall survival (OS) was 78.0 months in auto-auto and 98.3 months in auto-allo (HR= 0.84, P=0.02). OS was 36.4% vs. 44.1% at 10 years (P=0.01) for auto-auto and auto-allo respectively. Progression-free survival (PFS) was also improved in auto-allo (HR= 0.84, P=0.004). Risk of non-relapse mortality (NRM) was higher in auto-allo (10 year 8.3% vs. 19.7%, P<0.001), while risk of disease progression was higher in auto-auto (10 year 77.2% vs. 61.6%, P<0.001). Median post relapse survival (PRS) was 41.5 months in auto-auto and 62.3 months in auto-allo (HR= 0.71, P<0.001). This supports the existence of durable GVM effect enhancing myeloma control with subsequent therapies.
Introduction
Disease control and survival of MM patients has improved steadily over the last two decades thanks to incorporation of proteasome inhibitors (PI) and immunomodulatory agents (IMiDs) in the upfront management, greater utilization of hematopoietic cell transplantation and incorporation of new PIs, IMiDs and more recently monoclonal antibodies to treat relapsed and refractory disease.
Although autologous hematopoietic cell transplantation (AHCT) prolongs PFS and OS in newly diagnosed MM patients, near all patients will eventually relapse. Allogeneic transplantation carries the potential benefit of GVM effect1–4, but at the risk of increased NRM driven by conditioning regimen toxicity and graft versus host disease (GVHD)5–7. The use of reduced intensity instead of fully myeloablative regimens mitigated conditioning toxicity at the expense of reduced cytoreductive effect.
Two strategies to improve transplantation in MM are the use of tandem autologous transplant (auto-auto) and autologous followed by reduced intensity conditioning (RIC) allogeneic transplant (auto-allo) from HLA identical sibling donors. Multiple clinical trials were performed assigning patients to auto-auto or auto-allo based on the availability of an HLA identical sibling donor (biological randomization) and yielded conflicting conclusions with relatively short term follow up. A prior meta-analysis of published results indicated higher NRM but also higher chance of obtaining complete response (CR) with auto-allo but no significant difference between these two approaches in terms of PFS or OS8.
Here we describe an individual patient data pooled analysis inquiring whether long term follow up affected the comparison between those two transplant strategies.
Methods
Study selection
We included prospective trials that enrolled only patients with newly diagnosed MM (typically after conventional induction therapy) who were allocated to auto-auto or auto-allo based exclusively on the availability or not of a HLA-identical matched sibling donor (biological assignment). Conditioning regimen for the allogeneic transplant had to meet the center for international blood and marrow transplant research (CIBMTR) criteria for reduced intensity9.
We utilized a comprehensive search strategy to capture available relevant data. A search in MEDLINE (PubMed) was performed utilizing the terms “allogeneic”, “myeloma” and “clinical trial” or “allograft”, “myeloma” and “clinical trial”. There was no filtering for type of publication, language, country of publication or year of publication. We invited for participation investigators from all trials with these characteristics and with basic patient and disease characteristics and data on OS and PFS available for both arms. Authors were asked to share the most up to date dataset even if not yet published.
Outcomes
The primary objectives of this study were to compare OS and PFS between auto-auto and auto-allo. Secondary objectives were comparison of NRM, cumulative incidence of relapse/progression and post-relapse survival. Analysis was performed according to intention to treat (ITT) principle. We also aimed at comparing OS and PFS between auto-auto and auto-allo arms among high-risk patients. Because different studies employed different definitions of high-risk, we utilized the definition from the largest study, BMT CTN 010210, that defined as high-risk patients with β2 microglobulin level at diagnosis ≥ mg/L and/or presence of deletion of chromosome 13 by metaphase karyotyping.
Statistical methods
For time-to event outcomes OS, PFS, NRM and cumulative incidence of relapse/progression, time was counted from first autologous transplant until event of interest. For post-relapse survival (PRS) time was counted from relapse/progression until death from any cause. For all time-to event outcomes patients not experiencing the event were censored at the most recent follow up.
We estimated survival distributions using the Kaplan-Meier method while NRM and relapse/progression were estimated using cumulative incidence. NRM and relapse/progression were analyzed as competing events. For point wise comparisons we employed Gray’s test. For time-to-event analysis we evaluated the proportional hazards assumption using the log (-log) survival plots. When there was no violation of proportional hazards assumption, we compared arms using log-rank test. When we found that associations varied over the follow up period, hazard ratios were calculated by adding an interaction term between arm of the study and the natural logarithm of time.
Results
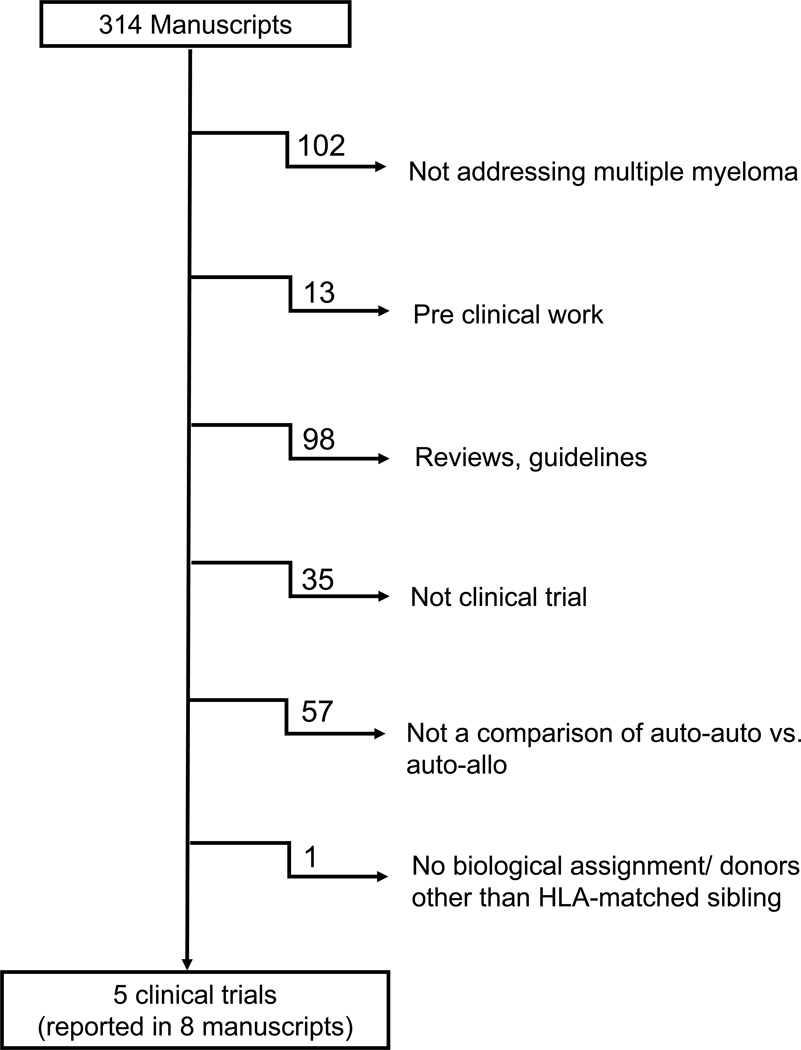
Utilizing the comprehensive search strategy as described above we identified 314 manuscripts in the initial screening. Figure 1 displays the reason for exclusion of the reports not meeting all the eligibility criteria. Overall, there were 9 manuscripts corresponding to 5 trials that met eligibility criteria10–18. Investigators from 4 of these trials accepted the invitation to share data and collaborate in this pooled analysis 10–14, 17, 18.
Figure 1-.
Flow chart showing the outcomes of the comprehensive search strategy to identify studies meeting entry criteria for the pooled analysis.
There were 899 patients analyzed in the auto-auto arm and 439 patients in the auto-allo arm. Median follow up of survivors approached 10 years (118.5 months). Characteristics of individual trials and the entire population included in the analysis are displayed in Table 1. The treatment assigned by study design was completed in 56% of auto-auto and 73% of auto-allo patients.
Table 1-.
Characteristics of the studies included
| Krishnan, Giralt | Gahrton, Bjorkstrand | Giacone, Bruno | Rosinol | Total | |
|---|---|---|---|---|---|
| BMT CTN | EBMT | Torino | GEM-PETHEMA | ||
| Population | Newly diagnosed MM patients | Newly diagnosed MM patients | Newly diagnosed MM patients | Not achieving CR/nCR after first autologous | |
| Years of accrual | 2003–2007 | 2001 to 2005 | 1998–2004 | 1999–2004 | |
| Number of participants | 709 | 357 | 162 | 110 | 1,338 |
| Auto-auto | 483 | 249 | 82 | 85 | 899 |
| Auto-allo | 226 | 108 | 80 | 25 | 439 |
| Median follow up (mo) | 75.5 | 75.5 | 57.6 | 87.7 | 75.3 |
| Median follow up of survivors (mo) | 122.6 | 94.2 | 146.9 | 140.1 | 118.5 |
| Age criteria | ≤70 | <70 | ≤65 | <65 | |
| Male patients | 418 (59%) | 211 (59%) | 88 (54%) | 59 (54%) | 776 (58%) |
| Median age (years) | |||||
| Auto-auto | 55 | 57 | 55 | 55 | 56 |
| Auto-allo | 52 | 54 | 55 | 52 | 53 |
| Age ≥ 60 years | |||||
| Auto-auto | 133 (28%) | 81 (33%) | 18 (22%) | 30 (35%) | 262 (29%) |
| Auto-allo | 32 (14%) | 18 (17%) | 15 (19%) | 3 (12%) | 68 (15%) |
| Conditioning regimen | |||||
| Auto-auto | Mel200 | Mel200 | Mel200, Mel100– 200 | Mel200 or CVB | |
| Auto-allo | TBI 200cGy | Flu+TBI 200cGy | TBI 200cGy | Flu+Mel | |
| GVHD prophylaxis | CSA + MMF | CSA + MMF | CSA + MMF | CSA + MTX |
HR= high risk, Mel200= Melphalan 200 mg/m2, TBI = total body irradiation; CSA = Cyclosporine A; MMF = Mycophenolate mofetil; MTX = Methotrexate. Bu= Busulfan; Flu= Fludarabine; ATG= Anti-thymocyte globulin; CVB = Cyclophosphamide, etoposide, BCNU.
NRM and relapse/progression
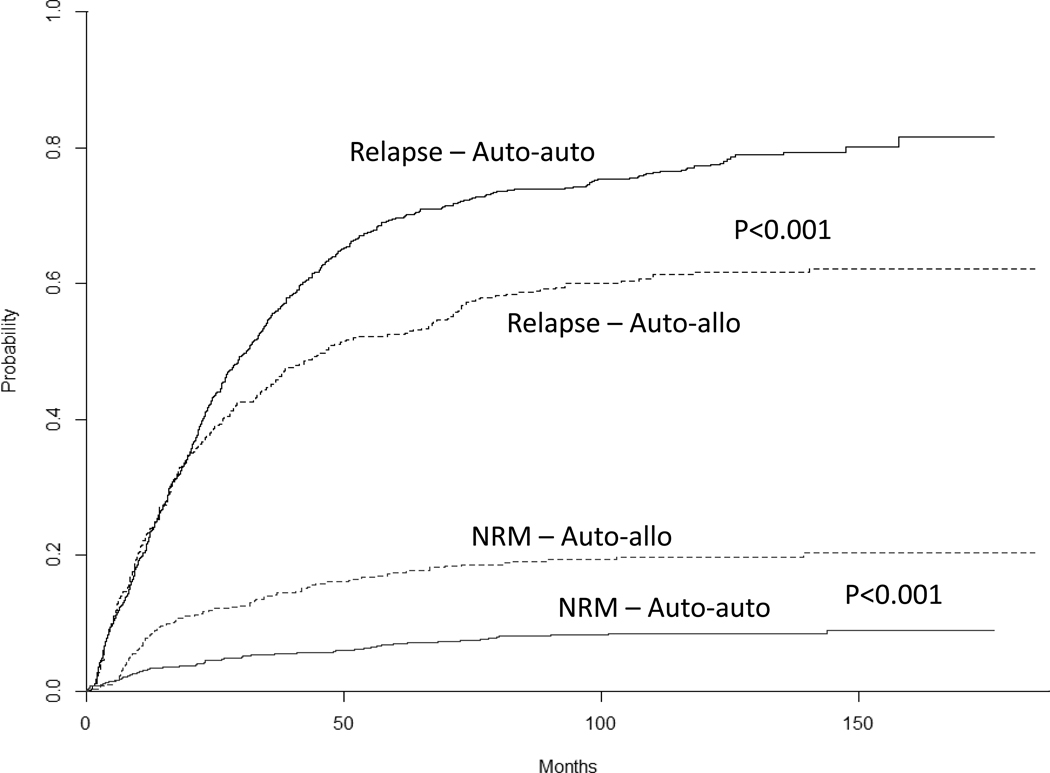
Overall, 40.0% in the auto-auto and 54.9% of patients in auto-allo achieved complete response. The risk of NRM was lower in auto-auto than in auto-allo (P<0.001). Five-year risk of NRM was 6.9% (95% C.I. 5.3%–8.5%) vs. 17.4% (95% C.I. 13.9%–20.9%), P<0.001, while 10-year risk of NRM was 8.3% (95% C.I. 6.5%–10.0%) vs. 19.7% (95% C.I. 16.0%–23.4%), P<0.001, for auto-auto and auto-allo respectively (Figure 2). The risk of relapse/progression was higher in auto-auto arm. Five-year risk of relapse/progression was 69.7% (95% C.I. 66.8%–72.6%) vs. 52.4% (95% C.I. 47.9%–56.9%), P<0.001, while 10-year risk of relapse/progression was 77.2% (95% C.I. 74.5%–79.9%) vs. 61.6% (95% C.I. 56.9%–66.3%), P<0.001, for auto-auto and auto-allo respectively (Figure 2).
Figure 2-.
Risks of relapse and non-relapse mortality in auto-auto and auto-allo arms.
Progression-free survival
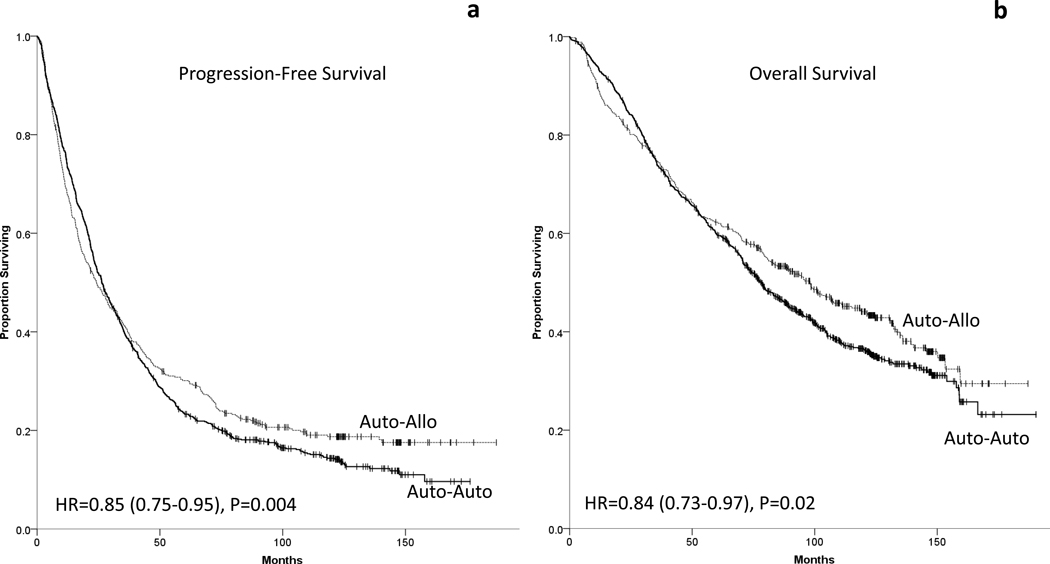
Overall, 759 of the 899 patients in auto-auto, and 352 of the 439 patients in auto- allo died or had disease relapse/progression. Patients in auto-allo had improved PFS (HR 0.85, 95% PFS 0.75–0.95, P=0.004), figure 3. Median PFS was 26.4 months (95% C.I. 23.8–28.9) in auto-auto and 24.4 months (95% C.I. 18.8–30.0) in auto-allo. The 5-year PFS was 23.4% (95% C.I. 20.7–26.1%) vs. 30.1% (95% C.I. 25.8%–34.4%), P=0.010, and the 10-year PFS was 14.4% (95% C.I. 11.8–16.9%) vs. 18.7% (95% C.I. 15.0%–33.4%), P=0.060, for auto-auto and auto-allo arms respectively.
Figure 3-.
Comparisons of (a) progression free survival and (b) overall survival between auto-auto and auto-allo arms.
Overall survival
There were 553 deaths among the 899 patients in auto-auto, and 249 deaths among the 439 patients in auto-allo. Patients in auto-allo had improved OS (HR 0.84, 95% C.I. 0.73–0.97, P=0.02), figure 3. Median OS was 78.0 months (95% C.I. 71.5–84.5) in auto-auto and 98.3 months (95% C.I. 81.8–114.7) in auto-allo. The 5-year OS was 59.8% (95% C.I. 56.6–63.0%) vs. 62.3% (95% C.I. 57.8%–66.8%), P=0.370, and the 10-year OS was 36.4% (95% C.I. 32.9–40.0%) vs. 44.1% (95% C.I. 39.2%–49.0%), P=0.010, for auto-auto and auto-allo arms respectively. There was no sex or age subset with clear increased benefit from either strategy (Supplemental figure 1).
High risk patients
Among high-risk patients median PFS was 22.9 months in auto-auto and 21.5 months in auto-allo. PFS was superior in auto-allo arm at 5 years (17.0% vs. 31.5%, P=0.015) and at 10 years (8.9% vs. 22.3%, P=0.008). Median OS was 64.4 months for auto-auto and 73.2 months for auto-allo. There was no difference in OS at 5 years (50.8% vs. 51.7%, P=0.897) and 10 years (28.6% vs. 39.0%, P=0.120), Supplemental Figure 2.
Post relapse survival
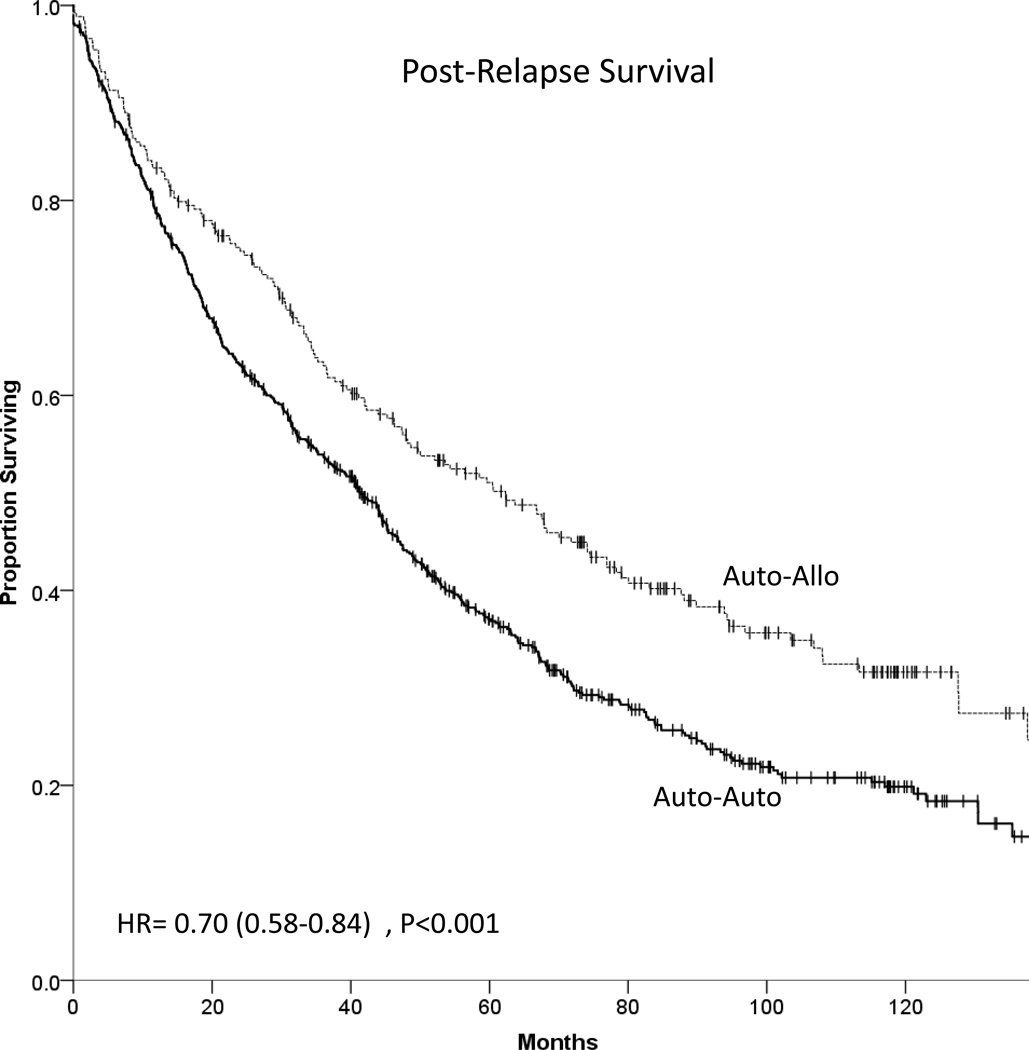
In auto-auto arm, 478 of the 685 patients who relapsed/progressed subsequently died while in auto-allo arm there were 162 deaths among 266 patients who relapsed. Patients in auto-allo had improved PRS (HR 0.71, 95% C.I. 0.59–0.85), P<0.001 (Figure 4). Median PRS was 41.5 months (95% C.I. 36.5–46.4) in auto-auto and 62.3 months (95% C.I. 47.7–76.9) in auto-allo. The 5-year PRS was 37.0% (95% C.I. 33.1–40.9%) vs. 51.1% (95% C.I. 44.8%–57.4%) in auto-auto and auto-allo respectively, P<0.001.
Figure 4 -.
Post relapse survival of auto-auto and auto-allo patients.
Discussion
Trials comparing auto-auto vs. auto-allo in patients with NDMM using biological randomization yielded divergent results for reasons that remain unclear, but may be related to population included, conditioning regimen employed and duration of follow up. While auto-allo has clearly higher NRM, mostly driven by risk of GVHD, it also leads to deeper responses8 and may carry long term benefit due to the existence of GVM effect1, 2 hence the importance of long term follow up.
The present analysis combines 4 studies designed in the early 2000s, before the introduction of IMiDs, PIs and monoclonal antibodies, in order to determine the relative benefit of auto-allo in patients with available HLA-matched sibling donors. We found that although auto-allo carries higher risk of NRM, it also substantially reduces the risk of relapse/progression leading to improved PFS and OS.
One important observation, previously noted in some studies 11, 19–21 is the clear and robust improvement in post-relapse survival in patients who received prior allogeneic transplantation when compared with patients who did not. While information on specific therapies administered post relapse is not available, we believe this finding indicates that in some cases GVM does not fully prevent a relapse but may enhance the effect of therapies utilized at progression, particularly PI and IMiDs. In fact, the IMiD lenalidomide has been found to magnify alloreactivity of donor T cells and even drive GVHD22–25. The improved survival of auto-allo patients treated for relapsed disease also argues that MM patients recipients of prior allogeneic transplantation should not be arbitrarily exclude from clinical trials exploring new MM agents.
One important limitation of the present study is that it does not support analysis by risk subsets as determined by modern criteria. These trials were designed prior to adoption of the international staging system for MM26 and prior to broader use of fluorescence in situ hybridization for risk classification27, 28. We did report outcomes on a subset of patients identified as high risk based on the criteria defined in the largest of the studies. In alignment with the overall study, we found improved PFS for high-risk patients receiving auto-allo but no significant difference in OS. This result however cannot be used to inform a risk-based transplant strategy for patients with NDMM given the evolution of risk classification that has since occurred. In recent years, minimal residual disease (MRD) has also extensively been applied to predict clinical outcomes in myeloma. Although small, a meaningful prospective study on MRD after auto-allo reported the long-term outcomes of molecular monitoring on 26 patients. At a median follow up of 12.1 years, median OS and EFS were not reached in patients who achieved MRD negativity by nested-PCR. Of note, patients in continuous molecular remission were never exposed to newer agents underlying a durable GVM effect that could potentially lead to disease eradication29.
Absent from the present analysis are the Intergroupe Francophone du Myelome (IFM) studies IFM99–03 and IFM99–0416, 30. Patients with high risk NDMM defined by deletion in chromosome 13 or high β2 microglobulin received an autologous HCT and were assigned to a RIC allogeneic transplant (IFM99–03, N=65) or a second autologous HCT (IFM99–04, N=219) according to the availability of an HLA-matched sibling. A comparison between these two studies was last published in 2008 with a median follow up of 56 months and showing no difference in event-free survival between the two transplant approaches. Unfortunately, patient-level updated information was not available to integrate the current analysis. Another important trial not included in the analysis is the HOVON-50. Patients with NDMM received induction with one of two different regimens followed by AHCT. After transplantation, patients were assigned to maintenance with thalidomide or interferon-alpha. Patients with an HLA identical sibling could be enrolled in the HOVON-54, a phase II trial of RIC allogeneic transplantation. Even though a donor vs. no donor analysis has been presented31, this trial did not meet entry criteria for the present analysis since a second AHCT was not part of the design.
In summary, the present study stresses the need for long term follow up in trials appraising transplant strategies in MM. This combined analysis with long term follow up confirms the existence of meaningful GVM effect that prevents and delays progression, improves outcomes post relapse, improves OS and may lead to cure in a subset of patients. This analysis does not inform what subsets of patients, if any, would currently benefit from an upfront auto-allo strategy given the multiplication of treatment options since these trials were designed and conducted. It also does not indicate whether the potential benefit in OS would justify the morbidity and impairment in quality of life expected from graft versus host disease or how the presented auto-allo strategy would compare with modern induction and maintenance strategies that lead to higher 10 year OS than presented in either arm of the present analysis. Instead, the findings invite continuous exploration of allogeneic transplantation in MM with approaches such as use of post-transplantation cyclophosphamide32, unrelated and related haploidentical donors and the deployment of new MM immunotherapeutic agents as post allogeneic HCT maintenance strategy. Along with better risk stratification and patient selection, these innovations could mitigate regimen toxicity, minimize risk of GVHD and reduce risk of relapse ultimately improving the safety and applicability of allogeneic HCT in MM particularly in young patients with high-risk disease or early relapse, where prognosis remains very poor.
Supplementary Material
Acknowledgements
This work was presented as oral abstract at the 61th Annual Meeting of the American Society of Hematology, Orlando, FL, USA, December 7-10, 2019. This study was not funded commercially.
Support for this study was provided to the Blood and Marrow Transplant Clinical Trials Network by grant #U01HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, along with funding from the Southwest Oncology Group (SWOG grant award U10CA180888). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or SWOG.
Footnotes
Conflicts of Interest
LJC: Consultancy (Karyopharm, Celgene, Amgen, Sanofi, Abbvie, GSK), Research Funding (Amgen, Janssen); Honoraria (Celgene, Janssen, Amgen). MCP Consultancy (Pfizer, Medigene, Amgen, Novartis), Research Funding (Kite Pharmaceuticals, BMS). JB: Advisory committee (Janssen, Celgene, Amgen, Takeda). SS Research Funding (Prothena, Takeda, Janssen), Honoraria (Prothena, Medac, Janssen). PH: Consultancy (Celgene, Takeda, BMS, Janssen, Kite Pharmaceuticals, Amgen, Spectrum, Abbvie), Honoraria (Celgene, Takeda, Janssen, Kite Pharmaceuticals, Sanofi, Abbvie), Research Funding (Celgene, Takeda, BMS, Kite Pharmaceuticals, Spectrum). SAG: Consultancy (Jazz Pharmaceuticals, Novartis, Takeda, Celgene, Kite pharmaceuticals, Johnson & Johnson, Actinium, Amgen, Spectrum Pharmaceuticals), Research Funding (Takeda, Celgene, Johnson & Johnson, Actinium, Miltenyi, Amgen). FP: Membership on Advisory Committee (Takeda, Celgene, Janssen). AYK: Consultancy (Abbvie, Novartis, Celgene, Takeda), Research Funding (Abbvie, Novartis, Tmunity). GG: Consultancy (Fujimoto Pharmaceutical Corporation). The remaining authors have no conflict of interest to disclose.
References
- 1.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus- myeloma effect: proof of principle. Blood 1996. February 01; 87(3): 1196–1198. [PubMed] [Google Scholar]
- 2.Lokhorst HM, Schattenberg A, Cornelissen JJ, van Oers MH, Fibbe W, Russell I, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol 2000. August; 18(16): 3031–3037. [DOI] [PubMed] [Google Scholar]
- 3.Lokhorst HM, Wu K, Verdonck LF, Laterveer LL, van de Donk NW, van Oers MH, et al. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood 2004. June 1; 103(11): 4362–4364. [DOI] [PubMed] [Google Scholar]
- 4.Aschan J, Lonnqvist B, Ringden O, Kumlien G, Gahrton G. Graft-versus- myeloma effect. Lancet 1996. August 3; 348(9023): 346. [DOI] [PubMed] [Google Scholar]
- 5.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol 2006. February 20; 24(6): 929–936. [DOI] [PubMed] [Google Scholar]
- 6.Gahrton G, Tura S, Ljungman P, Belanger C, Brandt L, Cavo M, et al. Allogeneic bone marrow transplantation in multiple myeloma. European Group for Bone Marrow Transplantation. N Engl J Med 1991. October 31; 325(18): 1267–1273. [DOI] [PubMed] [Google Scholar]
- 7.Gahrton G, Svensson H, Cavo M, Apperly J, Bacigalupo A, Bjorkstrand B, et al. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983−−93 and 1994−−8 at European Group for Blood and Marrow Transplantation centres. Br J Haematol 2001. April; 113(1): 209–216. [DOI] [PubMed] [Google Scholar]
- 8.Armeson KE, Hill EG, Costa LJ. Tandem autologous vs autologous plus reduced intensity allogeneic transplantation in the upfront management of multiple myeloma: meta-analysis of trials with biological assignment. Bone Marrow Transplant 2013. April; 48(4): 562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009. March; 15(3): 367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E 3rd, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol 2011. December; 12(13): 1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gahrton G, Iacobelli S, Bjorkstrand B, Hegenbart U, Gruber A, Greinix H, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood 2013. June 20; 121(25): 5055–5063. [DOI] [PubMed] [Google Scholar]
- 12.Giaccone L, Storer B, Patriarca F, Rotta M, Sorasio R, Allione B, et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood 2011. June 16; 117(24): 6721–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorkstrand B, Iacobelli S, Hegenbart U, Gruber A, Greinix H, Volin L, et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow- up. JClin Oncol 2011. August 1; 29(22): 3016–3022. [DOI] [PubMed] [Google Scholar]
- 14.Rosinol L, Perez-Simon JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood 2008. November 1; 112(9): 3591–3593. [DOI] [PubMed] [Google Scholar]
- 15.Moreau P, Garban F, Attal M, Michallet M, Marit G, Hulin C, et al. Long-term follow-up results of IFM99–03 and IFM99–04 trials comparing nonmyeloablative allotransplantation with autologous transplantation in high-risk de novo multiple myeloma. Blood 2008. November 1; 112(9): 3914–3915. [DOI] [PubMed] [Google Scholar]
- 16.Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, et al. Prospective comparison of autologous stem cell transplantation followed by dose- reduced allograft (IFM99–03 trial) with tandem autologous stem cell transplantation (IFM99–04 trial) in high-risk de novo multiple myeloma. Blood 2006. May 1; 107(9): 3474–3480. [DOI] [PubMed] [Google Scholar]
- 17.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. NEngl J Med 2007. March 15; 356(11): 1110–1120. [DOI] [PubMed] [Google Scholar]
- 18.Giralt S, Costa LJ, Maloney D, Krishnan A, Fei M, Antin JH, et al. Tandem Autologous-Autologous versus Autologous-Allogeneic Hematopoietic Stem Cell Transplant for Patients with Multiple Myeloma: Long-Term Follow-Up Results from the Blood and Marrow Transplant Clinical Trials Network 0102 Trial. Biol Blood Marrow Transplant 2019. November 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Htut M, D’Souza A, Krishnan A, Bruno B, Zhang MJ, Fei M, et al. Autologous/Allogeneic Hematopoietic Cell Transplantation versus Tandem Autologous Transplantation for Multiple Myeloma: Comparison of Long-Term Postrelapse Survival. Biol Blood Marrow Transplant 2018. March; 24(3): 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maffini E, Storer BE, Sandmaier BM, Bruno B, Sahebi F, Shizuru JA, et al. Long-term follow up of tandem autologous-allogeneic hematopoietic cell transplantation for multiple myeloma. Haematologica 2019. February; 104(2): 380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giaccone L, Evangelista A, Patriarca F, Sorasio R, Pini M, Carnevale-Schianca F, et al. Impact of New Drugs on the Long-Term Follow-Up of Upfront Tandem Autograft-Allograft in Multiple Myeloma. Biol Blood Marrow Transplant 2018. January; 24(1): 189–193. [DOI] [PubMed] [Google Scholar]
- 22.Kneppers E, van der Holt B, Kersten MJ, Zweegman S, Meijer E, Huls G, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial. Blood 2011. September 01; 118(9): 2413–2419. [DOI] [PubMed] [Google Scholar]
- 23.Kroger N, Zabelina T, Klyuchnikov E, Kropff M, Pfluger KH, Burchert A, et al. Toxicity-reduced, myeloablative allograft followed by lenalidomide maintenance as salvage therapy for refractory/relapsed myeloma patients. Bone Marrow Transplant 2013. March; 48(3): 403–407. [DOI] [PubMed] [Google Scholar]
- 24.Wolschke C, Stubig T, Hegenbart U, Schonland S, Heinzelmann M, Hildebrandt Y, et al. Postallograft lenalidomide induces strong NK cell-mediated antimyeloma activity and risk for T cell-mediated GvHD: Results from a phase I/II dose- finding study. Exp Hematol 2013. February; 41(2): 134–142 e133. [DOI] [PubMed] [Google Scholar]
- 25.Alsina M, Becker PS, Zhong X, Adams A, Hari P, Rowley S, et al. Lenalidomide maintenance for high-risk multiple myeloma after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2014. August; 20(8): 1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol 2005. May 20; 23(15): 3412–3420. [DOI] [PubMed] [Google Scholar]
- 27.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol 2015. September 10; 33(26): 2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia 2009. December; 23(12): 2210–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladetto M, Ferrero S, Drandi D, Festuccia M, Patriarca F, Mordini N, et al. Prospective molecular monitoring of minimal residual disease after non-myeloablative allografting in newly diagnosed multiple myeloma. Leukemia 2016. May; 30(5): 1211–1214. [DOI] [PubMed] [Google Scholar]
- 30.Moreau P, Hullin C, Garban F, Yakoub-Agha I, Benboubker L, Attal M, et al. Tandem autologous stem cell transplantation in high-risk de novo multiple myeloma: final results of the prospective and randomized IFM 99–04 protocol. Blood 2006. January 1; 107(1): 397–403. [DOI] [PubMed] [Google Scholar]
- 31.Lokhorst HM, van der Holt B, Cornelissen JJ, Kersten MJ, van Oers M, Raymakers R, et al. Donor versus no-donor comparison of newly diagnosed myeloma patients included in the H0V0N-50 multiple myeloma study. Blood 2011. June 28; 119(26): 6219–6225; quiz 6399. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh N, Ye X, Tsai HL, Bolanos-Meade J, Fuchs EJ, Luznik L, et al. Allogeneic Blood or Marrow Transplantation with Post-Transplantation Cyclophosphamide as Graft-versus-Host Disease Prophylaxis in Multiple Myeloma. Biol Blood Marrow Transplant 2017. November; 23(11): 1903–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.