Abstract
Alcohol use and alcohol use disorders (AUDs) are an increasing concern among veterans, particularly those from recent conflicts in Iraq and Afghanistan. The study of biomarkers in alcohol use and AUD has moved to enhancing the understanding of the development and maintenance of AUDs, as well as investigating its association with clinical severity and potential predictors of treatment response. Cortisol, a glucocorticoid known as a stress hormone, has been linked with both stress and trauma, as well as increased alcohol suppression effects. The present review summarizes existing literature and presents suggestions for future research to evaluate whether cortisol may be a possible biomarker of alcohol use disorder risk in combat veterans. Specifically, aspects of combat deployments and high levels of PTSD, coupled with the stress of reintegration may dysregulate cortisol and increase risk to AUD. There may also be bidirectional impacts, such that alcohol is used as a coping mechanism and can dysregulate hypothalamic pituitary adrenal (HPA) axis functioning and cortisol. In the context of this framework, cortisol may serve as a biomarker for the development of AUD, as well as a biomarker of risk or relapse. This review ends with both theoretical and clinical implications, as well as directions for future research.
Keywords: cortisol, biomarker, stress, trauma, combat, Veterans, alcohol use, alcohol abuse
Introduction
Increased alcohol use and alcohol use disorders (AUD) are more prevalent in veterans than civilians, with some estimates suggesting that combat veterans are more likely to engage in heavy and binge drinking than their civilian counterparts by 26.8% and 54.8% respectively (Teeters, Lancaster, Brown & Back, 2017). Extant theories regarding the underlying mechanisms of problematic alcohol use do not fully account for prevalence among veterans. Objective biological markers, or biomarkers, can enhance understanding of vulnerability, severity and prognosis of different disorders. Given that alcohol is often used as a coping mechanism during periods of prolonged stress, one promising specific biomarker may be cortisol, a glucocorticoid released from the adrenal gland following the activation of the hypothalamic-pituitary-adrenal (HPA) axis in response to stress (Smith & Vale, 2006). Indeed, previous studies have posited that glucocorticoids and the HPA axis may have an important role for the development and chronicity for AUDs due to their connection with limbic and prefrontal cortex circuits (Blaine & Sinha, 2017). Recent literature in civilians suggests associations between stress and both behavioral motivation to drink and past alcohol use (Blaine et al., 2019). Additionally, higher basal cortisol has been observed in heavy drinkers compared to moderate drinkers, but basal cortisol has been negatively associated with increased alcohol consumption in a laboratory consumption task (Blaine et al., 2019). Hence it is plausible that cortisol can serve as a biomarker that can enhance previous theoretical frameworks of vulnerability to AUDs (Milivojevic & Sinha, 2018). This would be particularly relevant in cases of co-occurring prolonged stress and alcohol use, like in active duty and former active duty servicemembers with combat exposure, hereafter referred to as veterans, who have unique experiences related to combat and military service (e.g., trauma and posttraumatic stress disorder [PTSD]) that may increase vulnerability. The current paper reviews recent research to propose a theoretical framework on the role of cortisol in problematic alcohol use among those with co-occurring prolonged stress.
Although problematic alcohol use can be distinguished from the formal diagnosis of AUD due to frequency, intensity, or functional impairment, research suggests that both have negative health and psychosocial consequences (Rehm et al., 2006). Further, considering that problematic alcohol use has one of the largest mental health treatment gaps suggesting that it is not often diagnosed and treated, it is likely that problematic alcohol use and AUD have shared mechanisms (Kohn et al., 2004). Vulnerability to AUDs is both genetically complex and behaviorally heterogeneous, such that multiple genes convey risk for AUD and multiple behavioral risk factors and functional uses of alcohol have been identified (i.e., delay discounting, self-soothing, executive dysfunction; Salvatore et al., 2015). Endophenotypes, or measurable components of psychological or medical morbidity that may not be physically apparent, along with neurobiological correlates may be valuable to distill specific subgroups and enhance understanding of otherwise intricate presentations, like those typical of veterans (Gottesman & Gould, 2003). Historically, biomarkers of alcohol use focused on liver function, limiting the information that they provided to detection or prognosis. While the utility for these markers is immense, more recently, the study of biomarkers in alcohol use and AUD has expanded to include prediction by enhancing the understanding of the development and maintenance of AUDs, as well as investigating its association with clinical severity and potential predictors of treatment response. Given the complexity of substance use vulnerability, it is likely that alternative biomarkers need to be considered to fully capture diverse domains of biological and genetic vulnerability, particularly in populations with high rates of comorbidity like veterans. With these goals in mind, the present review extends recent research on individual differences in responses to alcohol and cortisol to the emerging research on the impact of combat and other veteran – specific risk factors on AUD and provides directions for future research.
Alcohol Use and AUD among Veterans
Problematic alcohol use (e.g., binge drinking and heavy drinking) and alcohol use disorders are a growing and highly relevant struggle among veterans (Bray et al., 2013; Teeters et al., 2017). Problematic alcohol use is associated with mental health problems and functional impairment, interpersonal violence, and risk of poor health, including chronic pancreatitis, liver dysfunction, cardiovascular damage, cancer, damage to the nervous systems, stroke and even death (Bridevaux, Bradley, Bryson, McDonell, & Fihn, 2004; National Institute on Alcohol Abuse and Alcoholism, 2000; Rehm et al., 2010). According to the Center for Disease Control and Prevention, the combined yearly cost associated with AUD in the US is over $249 billion (CDC, 2018). Although there are no specific estimates of the yearly cost for veterans, research suggests that over 40% of US military veterans have a lifetime history of AUD (Fuehrlein et al., 2016) and approximately 40% of post-9/11 veterans serving in Iraq and Afghanistan report potentially hazardous alcohol use (Calhoun et al., 2008), which suggests that problematic alcohol use is likely associated with substantial comorbid psychiatric, and likely economic, burden among veterans
To address the high rates of alcohol use among veterans, the Department of Veterans Affairs (VA) offers behavioral evidence-based psychotherapy, like cognitive behavioral therapy for substance use disorders, behavioral couples therapy for substance use, motivational interviewing, motivational enhancement therapy, contingency management, and brief behavioral intervention, as well as pharmacological interventions, like acamprosate, disulfiram, and naltrexone, which have been approved by the Food and Drug Administration (Teeters et al., 2017; U.S. Department of Veterans Affairs, 2019). Behavioral interventions focus on helping veterans identify and modify maladaptive thoughts and behaviors associated with increased craving, use, or relapse to alcohol, or facilitate their ability to successfully manage stress without alcohol. Pharmacotherapy approaches can help reduce cravings and manage withdrawal symptoms, thus reducing the likelihood for relapse. Nonetheless, despite the diverse and comprehensive treatment options available to veterans struggling with AUD, there is variability in efficacy of these interventions (Litten et al., 2016). For instance, while behavioral couples therapy, cognitive behavioral coping skills training, and motivational enhancement therapy have received strong support for their efficacy, research does not unequivocally support any one intervention as superior, either in general or when matched by patient characteristics (Hawkins et al., 2012; Imel et al., 2008) Furthermore, recent data from the Department of Veterans Affairs suggests that pharmacotherapy for veterans is currently underutilized, with only 7–11% of veterans with AUDs receiving medication as a treatment for AUD (Rubinsky et al., 2015).
Given the rates of underutilization in pharmacotherapy, the variability in efficacy of these interventions, and the fact that, due to limited resources, substance use specialty-care clinics are often unable to offer multiple psychological treatment options (Litten et al., 2016), using biomarkers to promptly and properly identify the treatment that would be maximally helpful could minimize the taxing cost of care, accelerate rate of treatment, and possibly increase the precision of individualized treatment. Similarly, understanding biologically why combat veterans are at a higher risk to develop alcohol use disorders, and strategizing what can be done to enhance prevention and early detection could further improve access to care and treatment outcomes. Although AUD has a strong genetic component, as evidenced by high heritability (Verhulst et al., 2015), the genetics of AUD are beyond the scope of this review and have been summarized elsewhere (Dick & Bierut, 2006; Enoch & Goldman, 2001; Tawa et al., 2016). However, it is important to note that evidence supports the idea of a gene-environment interaction on both the consumption and misuse of alcohol (Young-Wolff et al., 2011), thus environmental stressors may be critical to the understanding of individual differences in likelihood to develop an AUD and how veterans, in particular, are at increased risk of AUD. Therefore, a biomarker that can tap stress responses could contribute to the broader efforts of developing personalized treatment programs.
Stress and Cortisol: A brief overview
Stress is considered to be a state of threat to homeostasis, alternately described as perception of a novel, uncontrollable, unpredictable stimulus in which an organism expects negative physical or psychological outcomes (Kudielka, Hellhammer, & Kirschbaum, 2007; Smith & Vale, 2006). Stress can be triggered by a multitude of events – physical and psychological. For instance, both internal (e.g., physiological reaction such as disease and infection) and external states (e.g., exercising or physical trauma) can cause physical stress, while psychological or mental stress can often be caused by a range of experiences, including trauma, anxiety and emotional dysregulation. Critically, regardless of the type of trigger, stress can cause an increase in HPA axis activation, which can be indexed by cortisol.
During situations of stress, corticotropin-releasing factor (CRF), released from the paraventricular nucleus of the hypothalamus, initiates a biological cascade that includes the release of adrenocorticotropic hormone (ACTH) from the pituitary gland and culminates in the release of glucocorticoids, including cortisol, from the adrenal glands (Smith & Vale, 2006; Figure 1A). Rising cortisol levels block the release of CRF and ACTH creating a negative feedback loop, eventually leading to the return of HPA axis homeostasis (Guilliams & Edwards, 2010). Activation of the HPA axis improves cognition and increases awareness, cardiovascular tone, respiratory rate, and intermediate metabolism (Sapolsky, Romero & Munck, 2000). Cortisol plays a role in inhibiting HPA axis activation and is often used as a biomarker, or objective quantification, of degree of HPA axis activation. While this stress response can be beneficial for acute situations, chronic elevations have been associated with cortical atrophy, changes in neuronal plasticity, and structural changes in dendritic structures within the prefrontal cortex (Radley et al., 2004; Radley & Morrison, 2005), as well as overall health problems, including impaired immune function (McGregor, Murphy, Albano, & Ceballos, 2016; Sale, Ridding, & Nordstrom, 2008; Sapolsky, 2000; Figure 1B).
Figure 1.
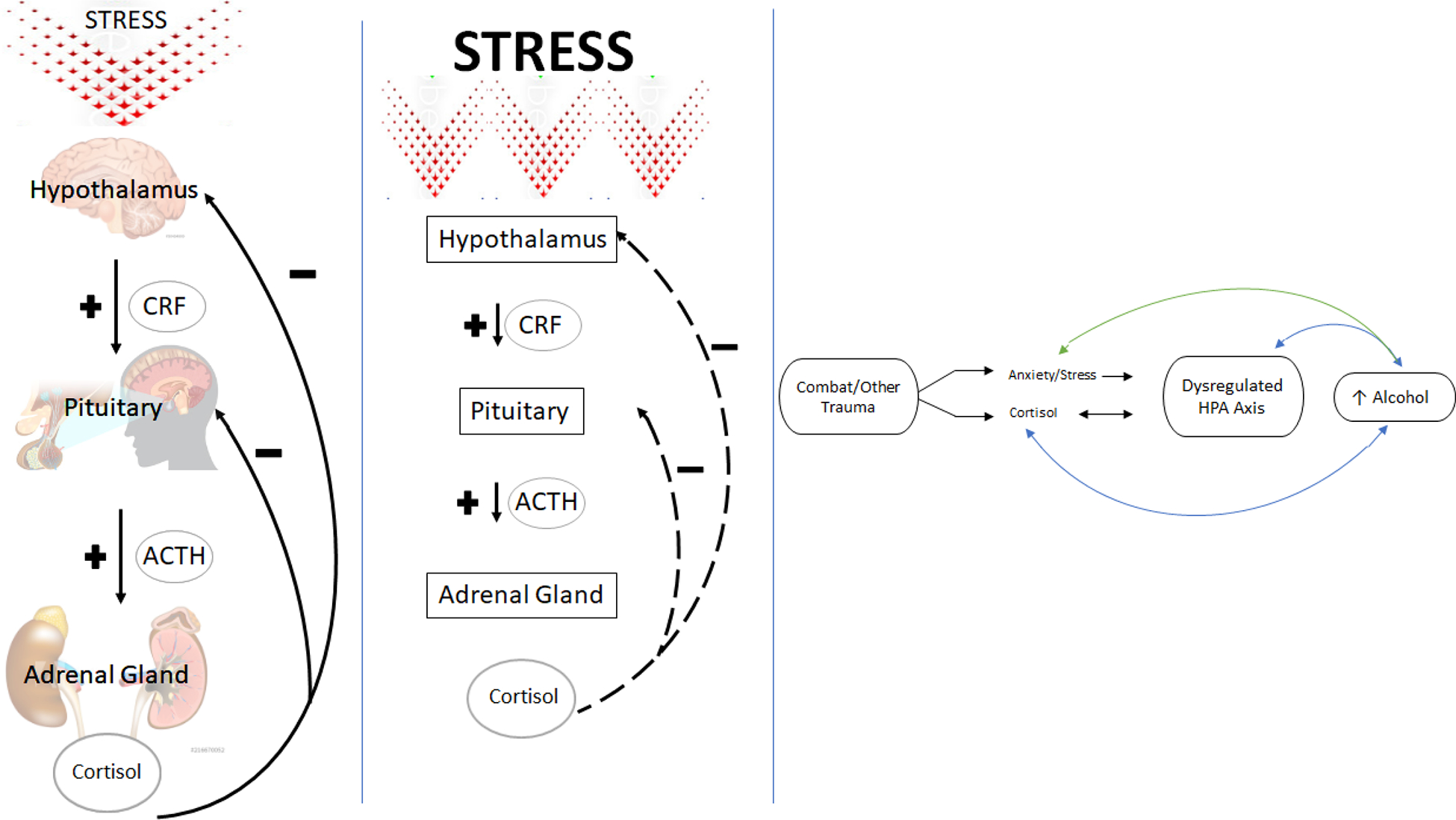
Conceptual Model. A. Overview of HPA axis in times of acute stress in healthy functioning. Cortisol serves as a negative feedback mechanism. B. With repeated or chronic stressors, a stunted negative feedback loop occurs, resulting in potentially higher circulating basal cortisol. C. Conceptual framework. Combat Veterans often experience dysregulated cortisol and increases in negative emotion in response to combat trauma. Over time, this results in a dysregulated HPA axis and increased alcohol craving. Proposed bi-directional positive reinforcement pathway (blue) and bi-directional negative reinforcement pathway (green) are highlighted.
The literature examining associations between stressful and traumatic life events and cortisol and a dysregulated HPA axis have been mixed, with often inconsistent findings observed. For example, cortisol awakening response, the change in cortisol concentration that occurs in the first hour after waking from sleep, has been found to be higher in those with recent life stress and job stress in one meta-analysis (Chida & Steptoe, 2009). Burnout, fatigue and posttraumatic stress symptoms were all associated with a lower cortisol awakening response, though the latter was only observed among high quality studies, underscoring that both elevated and blunted responses can be tied to psychosocial factors. In addition, meta-analytic findings suggested adulthood trauma alone does not result in altered basal cortisol levels but may be associated with increased suppression to dexamethasone exposure tests (Klaassens et al., 2012). However, childhood trauma and maltreatment may lead to long lasting changes in the HPA axis’ regulation of cortisol (Van Voorhees et al., 2014). Recent research among German military personnel found that long-term measures of cortisol (e.g., hair cortisol concentration) were blunted or decreased as the number of lifetime trauma exposures increased (Trautmann et al., 2018). These seemingly conflicting findings may be due to dysregulation of the HPA axis given the many measured and unmeasured moderators, and thus represent opportunities for future research to build upon.
Behavioral Reinforcement of Alcohol – A possible role for cortisol?
Beyond its role in stress responses, cortisol levels and dysregulation are also believed to contribute to the reinforcing effects of alcohol via both positive and negative reinforcement. Indeed, a prior review of AUD in adolescents connects HPA axis and its influence on the limbic system with problematic alcohol use due to the potentially reinforcing nature of glucocorticoids, such as cortisol (Schepis et al., 2011). The acute effects of alcohol include initial stimulation overlapping with increased autonomic nervous system and HPA axis activation, including raised cortisol levels. Alcohol is positively reinforcing when cortisol levels are increased, leading to a range of responses, such as calming feelings, increased energy, and inducing a general feeling of euphoria. This is an important consideration for those with histories of trauma and abuse, who often have low or blunted cortisol responses to acute stressors (Suzuki et al., 2014). As a result, these individuals may exhibit a lower response to alcohol and report less sedation to high doses of alcohol, which could lead to greater tolerance and consumption (Kwako et al., 2018). Taken together, the degree to which one experiences the initial stimulating effects of alcohol has been posited to be an endophenotype for alcohol use disorder (Ray et al., 2010), and may determine how positively reinforcing it is.
Though positive reinforcement can promote early alcohol use, research has consistently noted the role of negative reinforcement in alcohol use, by reducing negative moods and facilitating avoidance of thoughts or memories. The biological mechanisms through which these uncomfortable affective states motivate alcohol use, such as dysregulation of HPA axis and structural changes in the prefrontal cortex (PFC), further highlight the plausible role of cortisol as a biomarker. More specifically, prefrontal regulation is critical for effective coping in times of stress yet, as mentioned previously, high levels of basal cortisol have been linked to structural change within dendrites in the PFC (McEwen et al., 2016). Research suggests that PFC regulatory dysfunction can disinhibit the amygdala and aggravate severity of emotional distress (Li & Sinha, 2008), which in turn can both perpetuate trauma-related symptoms and increase alcohol craving in order suppress or avoid uncomfortable cognitions and emotions. For instance, trauma history has been linked with greater PFC activation while processing negative emotions (McLaughlin et al., 2015), therefore this may be one way that effective emotion regulation breaks down in times of stress and increases vulnerability to alcohol’s negatively reinforcing aspects on mood. Critically, alcohol can stimulate the HPA axis and impact glucocorticoid receptors, including in the limbic system and PFC. This creates bidirectional problems whereby cortisol and uncomfortable affective states motivate alcohol use and then alcohol use can dysregulate HPA axis functioning. Taken together, it is plausible that cortisol can impact the risk of engaging in problematic alcohol use and/or the relapse to problematic alcohol use by facilitating alcohol motivation. Although research has not directly examined the role of cortisol and prefrontal dysfunction in combat veterans, it is important to note here that veterans with genetic risk for AUD show associations between combat exposure and behavioral indicators of prefrontal dysfunction (i.e., decreased executive functioning abilities; Sadeh et al., 2016) suggesting that those veterans with possible PFC dysregulation may be especially vulnerable to use alcohol to cope with and reduce negative emotions.
Veterans at increased risk for AUD due to cortisol dysregulation
We posit that characteristics about combat experience or military service that can prime the HPA axis and result in long-term dysregulation of cortisol place veterans at increased risk for problematic alcohol use (Figure 1C). Combat deployments can be thought of as a chronic exposure to frequent acute stressors with little opportunity for recovery and regulation of the HPA axis between events. As an example, a service member may have missions that last minutes, hours or days. During these times, the exposure to potentially traumatic events or conflict would activate the HPA axis to adaptively increase awareness and vigilance to latent threat. Repeated activation of this response can result in chronic, maladaptive elevations of basal or circulating cortisol, perhaps due to enhanced sensitivity from childhood or adulthood trauma, insufficient recovery between missions, or due to HPA dysfunction. Both childhood rearing experiences and cortisol in response to prolonged stress are associated with alcohol consumption in rhesus monkeys (Fahlke et al., 2000), suggesting that both early experiences and ongoing stress can motivate alcohol use. Further, a study of German armed forces veterans that found decreases in alcohol use from pre-deployment to one year after were less likely in individuals with histories of childhood neglect (Trautmann et al., 2015).
Although little is known of the effects of combat exposure, or long periods of adversity in young adulthood or midlife, cortisol reactivity has been shown to be primed by both childhood and adulthood trauma (Heim & Binder, 2012). This is particularly noteworthy for veterans who in addition to having increased odds of trauma during their military service also report higher rates of childhood trauma compared to the general population (Blosnich et al., 2014), which has been associated with greater HPA dysregulation in response to stress (Heim & Binder, 2012). In addition, blunted basal and post-stress cortisol levels in women veterans, relative to civilian women, were associated with military status independent of PTSD diagnosis (Pierce & Pritchard, 2016). This may suggest that military stress may be uniquely important for cortisol responses, though other potential contributions such as childhood abuse history were not considered. Another study found that although childhood trauma was not a significant predictor of cortisol or alcohol use at baseline, there was an interaction between baseline levels of hair cortisol and combat exposure during a 12-month deployment to predict alcohol use, such that those with low hair cortisol had a positive association with alcohol use and trauma exposure but this was not found in those with high hair cortisol concentrations (Trautmann et al., 2018). Figure 1C illustrates possible ways through which the HPA axis and alcohol use can hold bidirectional relationships among veterans who have experienced trauma and combat.
We offer that when veterans transition from military status and combat missions to civilian life, there may be an increased desire to use alcohol to cope, particularly driven by a desire to numb emotions, reduce arousal, and self-medicate sleeping problems. This is supported by the positive reinforcement of euphoric effects of alcohol and negatively reinforced anxiolytic effects (Koob, 2013) as well as some research showing short-term hypnotic effects (i.e., reduced sleep latency and reduced wakefulness during the first half of the night after drinking; Feige et al., 2006). Alcohol use may then become a habitual coping response to a variety of cues, which may include trauma-related stimuli, uncomfortable situations or self-conscious emotions (Heinz et al., 2009). In support of this conceptualization, a study of United Kingdom Armed Forces found that problems at home during and after deployment are associated with heavy drinking, as were military related problems such as unit leadership, comradeship and unit characteristics (Browne et al., 2008). Notably, acute stress or high levels of stress and negative mood are associated with increased likelihood to use alcohol to cope (Dvorak et al., 2014; Serre et al., 2015). Over time, the use of alcohol to cope becomes habitual and worsens one’s ability to cope with stress. In this way, dysregulated levels of cortisol may contribute to this motivational pattern through a myriad of factors, to include childhood trauma, military trauma, and daily stressors associated with reintegration, a noted stressful period of adjustment (Orazem et al., 2017; Zamorski & Britt, 2010).
Another way that combat veterans are at increased risk for problematic alcohol use is by the prevalence of comorbid mental health conditions. For example, PTSD has been named one of the invisible wounds of war from the post-9/11 wars in Iraq and Afghanistan, with some estimates of a prevalence of 23% (Fulton et al., 2015). Recent literature has synthesized the highly co-occurring relationship between alcohol use disorder and PTSD in veterans (Norman et al., 2018), with some research suggesting emotional numbing symptoms to be most associated with alcohol misuse (Jakupcak et al., 2010). We note that associations with basal cortisol and PTSD are mixed, with one meta-analysis finding no overall difference between those with and without PTSD (Meewisse et al., 2007). However, an association with PTSD diagnostic status was found when samples were assessed in the afternoon, in serum or plasma, and when samples included women or individuals with physical or sexual abuse histories, suggesting that abuse history and methodological factors may be critical in assessing this association. The limited research in associations between cortisol and heavy combat is mixed – with some research in veterans of other eras suggesting lower morning cortisol in those with heavy combat exposure (Boscarino, 1996), while other research suggests great variability or lability of cortisol in subsets of veterans with PTSD that may indicate individual differences in vulnerability (Mason et al., 2002). Given the circadian rhythm of cortisol with levels peaking shortly after waking and decreasing throughout the day, future clarification is needed to understand the contributions of trauma and PTSD. We posit that PTSD is an additional vulnerability factor to dysregulated cortisol and subsequent AUD, but additional research is needed to elucidate the unique contributions and consider potential bidirectional associations. Moreover, depression is highly comorbid with PTSD and a common psychiatric complaint for post-9/11 Veterans (Haskell et al., 2010). Recent research suggests that cortisol insensitivity, or a resistance to glucocorticoids, is associated with flatter diurnal cortisol slopes and may be a particular vulnerability for both negative attentional bias and depression (Gaffey et al., 2019), which would could be related to an individual difference in circulating or basal cortisol levels. Another common comorbid condition that could act as a transdiagnostic vulnerability is sleep problems, which can include sleep quality, disturbed sleep, difficulty falling asleep or awakening problems. In a study of Danish civil servants, sleep problems are associated with low awakening cortisol and low evening cortisol, and that the cortisol awakening response and diurnal slope in turn predicted disturbed sleep and awakening problems (Hansen et al., 2012). Veterans often use alcohol as means of facilitating sleep (Cucciare et al., 2011) and increases in alcohol use before deployment and one year after are correlated with sleep problems (Trautmann et al., 2015). Indeed, alcohol can be hypnotic in the short-term acting as a negative reinforcer by temporarily reducing sleep latency creating an increased reliance on its use as a self-medication. However rebound effects begin during the latter parts of sleep each night (Feige et al., 2006), and is contraindicated for those with sleep problems due to its negative impact on sleep architecture ultimately contributing to exacerbated sleep difficulties. Taken together research appears to suggest that, over and above the stressors inherently associated with combat deployments, common psychiatric symptoms may place veterans at risk for dysregulated cortisol and increased risk for problematic alcohol use.
Future Research and Implications
Thus far, we have extended existing literature on stress, trauma, and individual differences in responses to alcohol and cortisol to veteran-specific risk factors of AUD. Though supported by preliminary evidence and theoretical plausibility, future research is needed to test the conceptualizations presented here and begin to examine the potential implications for veterans. Many studies examining associations between acute stress and consumption or sedation to alcohol have been conducted in civilian samples and replication in veterans is needed. Much of the literature on trauma and cortisol has focused on trauma in children and is confounded by issues of development. Veterans experience high amounts of exposure to stressful and potentially traumatic events in young adulthood through midlife and examining associations between combat exposure, cortisol and alcohol use is imperative. Longitudinal or prospective studies with military servicemembers that assessed cortisol, alcohol use and trauma prior to deployment, as well as exposure to deployment stressors would provide the strongest evidence in support of this framework. In the absence of this, laboratory or experimental studies would improve the ability to make causal claims and to delineate whether cortisol is a mechanism that mediates between stress/trauma and AUDs. Literature has shown in acute stress paradigms that subjects with a high cortisol response, especially those at high risk for using alcohol problematically, show increased sedation to the effects of alcohol and thus put them at a higher risk for abuse due to the desirability of alcohol’s calming ability (Brkic et al., 2016). Additional research is needed with veterans that utilize acute stress paradigms, both standardized, validated psychosocial stressors (e.g., Trier Social Stress Test; Kirschbaum, Pirke, & Hellhammer, 1993; Kudielka et al., 2007) as well as potential trauma paradigms (Holmes & Bourne, 2008), which would help to determine the specificity of associations with stress and trauma.
Some initial evidence on the associations between trauma exposure, PTSD, alcohol use and cortisol present mixed findings – such that elevated cortisol is a risk factor in one study, but low cortisol is connected in others (e.g., Blaine et al., 2019; Klaassens et al., 2012). Future research would benefit from using longitudinal designs and considering both basal cortisol levels and reactivity to inform the degree to which each is impacted. Additional methodological considerations include fluid of measurement (saliva, serum, plasma or urine) and time of day samples are taken (i.e., morning samples likely capture the cortisol awakening response). Further, some research has suggested that associations between cortisol and chronicity of PTSD symptoms (i.e., non-remitted symptom trajectory) are due to history of childhood trauma (Galatzer-Levy et al., 2017) suggesting group level analyses may mask important associations. Sophisticated models that allow for individual level or trajectory-based associations to emerge may be helpful to uncover associations.
One possible future direction is to examine cortisol in relation to other biomarkers. Two plausible biomarkers to examine with cortisol might be ACTH and alcohol-focused genetics. Though one of cortisol’s primary functions is to inhibit HPA axis activation, it does not act alone. Rising cortisol levels block the release of CRF and ACTH (Guilliams & Edwards, 2010), which assists in returning the HPA axis to a level of homeostasis. Given the association between cortisol and ACTH, some research uses cortisol/ACTH ratios and can provide information about adrenal responsivity or functioning. Though not previously studied within AUD, this has been used in studies of health in veterans and in PTSD (de Kloet et al., 2006; Golier et al., 2007). This may be an important variable for understanding the role of cortisol within the broader physiological system.
Additionally, though beyond the scope of this paper, researchers can capitalize on the knowledge of the genetic contribution to AUD risk. Two genes most commonly associated with increased risk for developing alcoholism are associated with alcohol metabolism, alcohol dehydrogenase 1B class 1 (ADH1B) and mitochondrial aldehyde dehydrogenase 2 (ALDH2; Hurley & Edenberg, 2012). Genetic predisposition in conjunction with prolonged stress can drastically increase problematic alcohol usage. Further, cortisol sensitivity or resistance is hereditary (Lamberts, 1996), therefore considering genetics, shared environment, and their interaction in these associations could help elucidate the role of family history. Evaluating genetic predisposition as well as measures of chronic stress could give clearer results as to the role of combat exposure in the development of alcohol use disorders.
The extant literature offers tentative evidence for cortisol as a biomarker of AUD, one that may be particularly relevant for Veterans, with the potential to extend to those at high risk for repeated traumatization and sustained elevated stress, such as firefighters or first responders (Kim et al., 2018) and victims of military sexual trauma and intimate partner violence (Devries et al., 2014); populations that have been found to be at greater risk for alcohol use (Golding, 1999; Jones, 2017). Additional empirical study in veterans will help clarify its incremental contribution to vulnerability and inform clinical practice.
Clinical Implications
Providing evidence that cortisol is a biomarker of both the risk of engaging in problematic alcohol use and/or the relapse to problematic alcohol use would permit a novel treatment target for AUD among veterans that may bolster understanding of mechanisms within pharmaco- and psychotherapies (Swift & Aston, 2015).
As mentioned previously, pharmacotherapy is still underutilized for AUD within veterans (Rubinsky et al., 2015), and there are varying data on the efficacy of these treatments (Litten et al., 2016). One such contributor to the variability on treatment efficacy may be the impact that these medications have on glucocorticoids, such as cortisol. Naltrexone is an approved medication for AUDs that targets alcohol craving and has been shown to help with reducing drinking for some individuals (O’Malley et al., 1996). Research suggests that naltrexone activates the HPA axis and stimulates ACTH and cortisol responses compared to a placebo, as well as basal cortisol levels, and is associated with decreases in craving and alcohol self-administration (O’Malley et al., 2002). This suggests that naltrexone could be helpful for individuals by reducing the positively reinforcing aspects, however, it may be less helpful for individuals with withdrawal pathophysiology. Prazosin is a noradrenergic compound typically used for high blood pressure that has some impact on HPA axis and has been found to reduce stress-related alcohol cravings, as well as reducing basal cortisol responses and increasing stress-induced cortisol responses (Fox et al., 2012). Randomized controlled trials of prazosin for alcohol use disorder show promising efficacy as compared to placebo, particularly for individuals consuming high numbers of drinks per week (Simpson et al., 2018; Wilcox et al., 2018). Additionally, some preliminary evidence suggests that targeting progesterone, which increases the brain neurosteroid allopregnanolone, may have some efficacy for modifying cortisol and alcohol cravings in individuals with substance dependence (Milivojevic et al., 2016). Taken together, the literature suggests that these medications may uniquely impact alcohol craving and its reinforcing qualities based on an individual’s cortisol profile (i.e., higher basal/tonic levels or blunted reactivity). Therefore, it is possible that integrating stress and cortisol profile may help inform for whom and which intervention would be most helpful. Future research should examine how pharmacotherapies that target corticosteroids or other HPA-related factors may help with alcohol use and relapse by targeting stress-related physiology.
Considering the relevance of this research to psychotherapeutic interventions, some research has posited that the cognitive and affective mechanisms of alcohol use and alcohol use disorder, could be targeted with interventions, such as those that focus on purposeful attention (i.e., mindfulness; Garland et al., 2011). Given the role of stress and cortisol in problematic alcohol use, it is important to consider that some evidence suggests that cortisol levels across sessions differ between veterans who respond to PTSD treatment and those who do not (Rauch et al., 2017). This may be one group that could benefit from addressing dysregulated cortisol and PTSD to reduce AUD. However, it is also important to consider how reducing alcohol use among those with PTSD may lead to short-term increases in emotional distress and dysregulate the HPA axis, which could increase symptoms and thereby increase craving due to the negatively reinforcing qualities of alcohol. This is highlighted in recent research supporting both self-medication (i.e., alcohol is used to cope with PTSD symptoms and sleep) and mutual maintenance theories for the relationship between alcohol use and PTSD (Possemato et al., 2015). Thus, addressing alcohol use in those with PTSD may require a targeted and dual focus. Some research on treatment of those who are dually diagnosed has found efficacy for behavioral interventions reducing PTSD symptoms, alcohol consumption or both. Two treatments addressing both substance use and PTSD, one focused on exposure therapy (Integrated Prolonged Exposure; COPE) and the other focused on coping (Integrated Coping Skills; Seeking Safety) demonstrated comparable declines in heavy drinking in veterans with PTSD and alcohol use disorder, though greater declines in PTSD symptoms were observed in integrated prolonged exposure compared to integrated coping skills (Norman et al., 2019). Similarly, Cognitive Processing Therapy has also been successful in reducing PTSD symptoms among veterans with a dual diagnosis although the effects on drinking outcomes were not measured (Kaysen et al., 2014). Pharmacotherapy also has some support for reducing alcohol use among veterans and civilians with these dual diagnoses (Petrakis et al., 2016; Ralevski et al., 2014), particularly when combined with behavioral treatment (Foa et al., 2013). Foa and colleagues compared the effects of prolonged exposure (PE; a behavioral intervention) versus supportive counseling when combined with naltrexone versus placebo in treating PTSD and AUD symptoms in a dual diagnosis sample of veterans and civilians. All four groups (i.e., PE + placebo, PE + naltrexone, Supportive + placebo, and Supportive + naltrexone) showed declines in both alcohol use and PTSD symptoms, with naltrexone showing superior effects over placebo for less days drinking (Foa et al., 2013). Taken together, this may suggest that treating both PTSD symptoms and AUD in individuals with comorbidity is crucial but also feasible and effective. These outcomes are consistent with recent research supporting a bidirectional and mutual maintenance model of PTSD and AUD among treatment seeking veterans with a dual diagnosis (Tripp et al., 2020), with slightly larger effects for PTSD symptoms predicting alcohol use. This may indicate that treatment of PTSD among those with dual diagnosis is critically important for addressing problematic alcohol use. Consideration of cortisol may further elucidate a subgroup of veterans for which certain therapies may be more beneficial.
Stress is a robust factor that prompts motivation for alcohol, with cortisol as a common stress biomarker. As such, targeting stress may be an important area of focus. Stress reactivity, measured by changes in cortisol, has also been shown to be modifiable with behavioral treatment for PTSD (i.e., expressive writing; Smyth, Hockemeyer, & Tulloch, 2008). This lends credence to the targeting of symptoms to reduce cortisol, though this has notably not yet been extended to alcohol use and AUD. If support is found, there may also be potential for prevention prior to stress or trauma exposure. Cognitive-behavioral stress management training in two, 7-hour sessions has been shown to reduce the magnitude of cortisol responses to acute stress paradigms (Gaab et al., 2003). It is possible that trainings such as these could be adapted for pre-deployment or post-deployment to prepare members to cope with oncoming stress and perhaps modulate the degree of change in baseline cortisol level.
Conclusion
In summary, alcohol use and AUDs are heterogeneous with an increased prevalence in veterans and variable treatment effectiveness that are likely multifactorial. Thus, uncovering different endophenotypes may be helpful in better understanding differences in vulnerability, severity, and treatment response. Though future research is needed to inform the clinical implications of this framework, a long-term goal may be to help clarify effectiveness of different treatments for different subgroups of individuals. As future research elucidates the neurobiology of associations proposed here by integrating biomarkers into behavioral and genetic understandings of AUD, targeted pharmacological, psychological and behavioral interventions could be used to address dysregulation and reduce vulnerability to problematic alcohol use (Litten et al., 2015).
Given the multiple interconnected pieces of the model proposed here, targeting PFC function, improving HPA axis functioning or targeting cortisol more directly may all help improve resilience to developing AUD and the treatment or relapse prevention of AUDs. An important consideration is that individual differences in alcohol tolerance and sedation predict vulnerability and may therefore be an important factor in treatment implications. Additionally, pharmacotherapy for AUD may impact veterans differently based on their cortisol profiles and may inform the variability in efficacy. Furthermore, considering how individual differences in comorbid health presentations, including insomnia, depression and PTSD, could impact the function and reinforcement of alcohol motivation and thus modulate responses to treatment. Baine and Sinha (2017) propose that a personalized medicine approach that may help to build more effective intervention tailored to the individual risk factors of the person.
Acknowledgements
The authors would like to acknowledge Abigail Herschbach for her assistance in formatting and technical editing.
Funding
Dr. Szabo’s work on this study was supported in part by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Central Texas Veterans Healthcare System, and the VISN 17 Center of Excellence for Research on Returning War Veterans. This work was supported in part by Career Development Award Number IK1 RX003122 from the United States (U.S.) Department of Veterans Affairs Rehabilitation Research and Development (RR&D) Service. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Disclosures
All authors report no financial disclosures with commercial interests.
Data Availability Statement
There is no raw data involved in the preparation of this manuscript.
References
- Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, & Sinha R (2019). Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addiction Biology, 24(5), 1096–1108. doi: 10.1111/adb.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, & Sinha R (2017). Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology, 122, 136–147. doi: 10.1016/j.neuropharm.2017.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blosnich JR, Dichter ME, Cerulli C, Batten SV, & Bossarte RM (2014). Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry, 71(9), 1041–1048. doi: 10.1001/jamapsychiatry.2014.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA (1996). Posttraumatic stress disorder, exposure to combat, and lower plasma cortisol among Vietnam Veterans: Findings and clinical implications. Journal of Consulting and Clinical Psychology, 64(1), 191–201. doi: 10.1037/0022-006X.64.1.191 [DOI] [PubMed] [Google Scholar]
- Bray RM, Brown JM, & Williams J (2013). Trends in binge and heavy drinking, alcohol-related problems, and combat exposure in the U.S. military. Substance Use and Misuse, 48(10), 799–810. doi: 10.3109/10826084.2013.796990 [DOI] [PubMed] [Google Scholar]
- Bridevaux IP, Bradley KA, Bryson CL, McDonell MB, & Fihn SD (2004). Alcohol screening results in elderly male veterans: Association with health status and mortality. Journal of the American Geriatrics Society, 52(9), 1510–1517. doi: 10.1111/j.1532-5415.2004.52414.x [DOI] [PubMed] [Google Scholar]
- Brkic S, Söderpalm B, & Gordh AS (2016). High cortisol responders to stress show increased sedation to alcohol compared to low cortisol responders: An alcohol dose–response study. Pharmacology Biochemistry and Behavior, 143, 67–72. doi: 10.1016/j.pbb.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Browne T, Iversen A, Hull L, Workman L, Barker C, Horn O, Jones M, Murphy D, Greenberg N, Rona R, Hotopf M, Wessely S, & Fear NT (2008). How do experiences in Iraq affect alcohol use among male UK armed forces personnel? Occupational and Environmental Medicine, 65(9), 628–633. doi: 10.1136/oem.2007.036830 [DOI] [PubMed] [Google Scholar]
- Calhoun PS, Elter JR, Jones ER, Kudler H, & Straits-Tröster K (2008). Hazardous alcohol use and receipt of risk-reduction counseling among U.S. veterans of the wars in Iraq and Afghanistan. Journal of Clinical Psychiatry, 69(11), 1686–1693. doi: 10.4088/JCP.v69n1103 [DOI] [PubMed] [Google Scholar]
- CDC. (2018). Excessive Drinking is Draining the U.S. Economy.
- Chida Y, & Steptoe A (2009). Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology, 80(3), 265–278. doi: 10.1016/j.biopsycho.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Cucciare MA, Darrow M, & Weingardt KR (2011). Characterizing binge drinking among U.S. military veterans receiving a brief alcohol intervention. Addictive Behaviors, 36(4), 362–367. doi: 10.1016/j.addbeh.2010.12.014 [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, & Westenberg HGM (2006). Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Research, 40(6), 550–567. doi: 10.1016/j.jpsychires.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Devries KM, Child JC, Bacchus LJ, Mak J, Falder G, Graham K, Watts C, & Heise L (2014). Intimate partner violence victimization and alcohol consumption in women: A systematic review and meta-analysis. Addiction, 109(3). doi: 10.1111/add.12393 [DOI] [PubMed] [Google Scholar]
- Dick DM, & Bierut LJ (2006). The genetics of alcohol dependence. Current Psychiatry Reports, 8(2), 151–157. doi: 10.1007/s11920-006-0015-1 [DOI] [PubMed] [Google Scholar]
- Dvorak RD, Sargent EM, Kilwein TM, Stevenson BL, Kuvaas NJ, & Williams TJ (2014). Alcohol use and alcohol-related consequences: Associations with emotion regulation difficulties. American Journal of Drug and Alcohol Abuse, 40(2), 125–130. doi: 10.3109/00952990.2013.877920 [DOI] [PubMed] [Google Scholar]
- Enoch MA, & Goldman D (2001). The genetics of alcoholism and alcohol abuse. Current Psychiatry Reports, 3(2), 144–151. doi: 10.1007/s11920-001-0012-3 [DOI] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, & Higley JD (2000). Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcoholism: Clinical and Experimental Research, 24(5), 644–650. doi: 10.1111/j.1530-0277.2000.tb02035.x [DOI] [PubMed] [Google Scholar]
- Feige B, Gann H, Brueck R, Hornyak M, Litsch S, Hohagen F, & Riemann D (2006). Effects of alcohol on polysomnographically recorded sleep in healthy subjects. Alcoholism: Clinical and Experimental Research, 30(9). doi: 10.1111/j.1530-0277.2006.00184.x [DOI] [PubMed] [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Oslin D, O’Brien CP, Imms P, Riggs DS, & Volpicelli J (2013). Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: A randomized clinical trial. JAMA: The Journal of the American Medical Association, 310(5), 488–495. doi: 10.1001/jama.2013.8268 [DOI] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, & Sinha R (2012). Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: Preliminary findings. Alcoholism: Clinical and Experimental Research, 36(2), 351–360. doi: 10.1111/j.1530-0277.2011.01628.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuehrlein BS, Mota N, Arias AJ, Trevisan LA, Kachadourian LK, Krystal JH, Southwick SM, & Pietrzak RH (2016). The burden of alcohol use disorders in US military veterans: Results from the national health and resilience in veterans study. Addiction, 111(10). doi: 10.1111/add.13423 [DOI] [PubMed] [Google Scholar]
- Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, Elbogen E, & Beckham JC (2015). The prevalence of posttraumatic stress disorder in operation enduring freedom/operation Iraqi freedom (OEF/OIF) veterans: A meta-analysis. J Anxiety Disord, 31, 98–107. doi: 10.1016/j.janxdis.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Gaab J, Blättler N, Menzi T, Pabst B, Stoyer S, & Ehlert U (2003). Randomized controlled evaluation of the effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinology, 28(6), 767–779. doi: 10.1016/S0306-4530(02)00069-0 [DOI] [PubMed] [Google Scholar]
- Gaffey AE, Walsh EC, Ladd CO, Hoks RM, & Abercrombie HC (2019). Alterations in systemic and cognitive glucocorticoid sensitivity in depression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(3), 310–320. doi: 10.1016/j.bpsc.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Ma S, Statnikov A, Yehuda R, & Shalev AY (2017). Utilization of machine learning for prediction of post-traumatic stress: A re-examination of cortisol in the prediction and pathways to non-remitting PTSD. Translational Psychiatry, 7(3). doi: 10.1038/tp.2017.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Boettiger CA, & Howard MO (2011). Targeting cognitive-affective risk mechanisms in stress-precipitated alcohol dependence: An integrated, biopsychosocial model of automaticity, allostasis, and addiction. Medical Hypotheses, 76(5), 745–754. doi: 10.1016/j.mehy.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding JM (1999). Intimate partner violence as a risk factor for mental disorders: A meta-analysis. Journal of Family Violence, 14(2), 99–132. doi: 10.1023/A:1022079418229 [DOI] [Google Scholar]
- Golier JA, Schmeidler J, Legge J, & Yehuda R (2007). Twenty-four hour plasma cortisol and adrenocorticotropic hormone in Gulf War Veterans: Relationships to posttraumatic stress disorder and health symptoms. Biological Psychiatry, 62(10), 1175–1178. doi: 10.1016/j.biopsych.2007.04.027 [DOI] [PubMed] [Google Scholar]
- Gottesman II, & Gould TD (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry, 160(4), 636–645. doi: 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- Guilliams TG, & Edwards L (2010). Chronic stress and the HPA axis. The Standard, 9(2), 1–12. [Google Scholar]
- Hansen ÅM, Thomsen JF, Kaergaard A, Kolstad HA, Kaerlev L, Mors O, Rugulies R, Bonde JP, Andersen JH, & Mikkelsen S (2012). Salivary cortisol and sleep problems among civil servants. Psychoneuroendocrinology, 37(7), 1086–1095. doi: 10.1016/j.psyneuen.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Haskell SG, Gordon KS, Mattocks K, Duggal M, Erdos J, Justice A, & Brandt CA (2010). Gender differences in rates of depression, PTSD, pain, obesity, and military sexual trauma among Connecticut war veterans of Iraq and Afghanistan. Journal of Women’s Health (2002), 19(2). doi: 10.1089/jwh.2008.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins EJ, Grossbard J, Benbow J, Nacev V, & Kivlahan DR (2012). Evidence-based screening, diagnosis, and treatment of substance use disorders among veterans and military service personnel. Military Medicine, 177(8), 29–38. doi: 10.7205/milmed-d-12-00125 [DOI] [PubMed] [Google Scholar]
- Heim C, & Binder EB (2012). Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology, 233(1), 102–111. doi: 10.1016/j.expneurol.2011.10.032 [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grüsser SM, Grace AA, & Wrase J (2009). Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addiction Biology, 14(1), 108–118. doi: 10.1111/j.1369-1600.2008.00136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes EA, & Bourne C (2008). Inducing and modulating intrusive emotional memories: A review of the trauma film paradigm. ActaPsychologica, 127(3), 553–566. doi: 10.1016/j.actpsy.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Hurley TD, & Edenberg HJ (2012). Genes encoding enzymes involved in ethanol metabolism. Alcohol Research, 34(3), 339–344. [PMC free article] [PubMed] [Google Scholar]
- Imel ZE, Wampold BE, Miller SD, & Fleming RR (2008). Distinctions without a difference: Direct comparisons of psychotherapies for alcohol use disorders. Psychology of Addictive Behaviors, 22(4), 533–543. doi: 10.1037/a0013171 [DOI] [PubMed] [Google Scholar]
- Jakupcak M, Tull MT, McDermott MJ, Kaysen D, Hunt S, & Simpson T (2010). PTSD symptom clusters in relationship to alcohol misuse among Iraq and Afghanistan war veterans seeking post-deployment VA health care. Addictive Behaviors, 35(9), 840–843. doi: 10.1016/j.addbeh.2010.03.023 [DOI] [PubMed] [Google Scholar]
- Jones S (2017). Describing the mental health profile of first responders: A systematic review. Journal of the American Psychiatric Nurses Association, 23(3), 200–214. doi: 10.1177/1078390317695266 [DOI] [PubMed] [Google Scholar]
- Kaysen D, Schumm J, Pedersen ER, Seim RW, Bedard-Gilligan M, & Chard K (2014). Cognitive Processing Therapy for veterans with comorbid PTSD and alcohol use disorders. Addictive Behaviors, 39(2), 420–427. doi: 10.1016/j.addbeh.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Park H, & Kim JH (2018). The mediation effect of PTSD, perceived job stress and resilience on the relationship between trauma exposure and the development of depression and alcohol use problems in Korean firefighters: A cross-sectional study. Journal of Affective Disorders, 229, 450–455. doi: 10.1016/j.jad.2017.12.055 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The “Trier social stress test” - A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 39(2), 420–427. doi: 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, & Zitman FG (2012). Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: A meta-analysis. Psychoneuroendocrinology, 37(3), 317–331. doi: 10.1016/j.psyneuen.2011.07.003 [DOI] [PubMed] [Google Scholar]
- Kohn R, Saxena S, Levav I, & Saraceno B (2004). The treatment gap in mental health care. Bulletin of the World Health Organization, 82(11), 858–866. doi:/S0042–96862004001100011 [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2013). Negative reinforcement in drug addiction: The darkness within. Current Opinion in Neurobiology, 23(4), 559–563. doi: 10.1016/j.conb.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, & Kirschbaum C (2007). Ten years of research with the trier social stress test - revisited In Harmon-Jones E & Winkielman P (Eds.), Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. Guilford Press. [Google Scholar]
- Kwako LE, Bickel WK, & Goldman D (2018). Addiction Biomarkers: Dimensional Approaches to Understanding Addiction. Trends in Molecular Medicine, 39(4), 579–584. doi: 10.1016/j.molmed.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts SWJ (1996). The glucocorticoid insensitivity syndrome. Hormone Research in Paediatrics, 45, 2–4. doi: 10.1159/000184815 [DOI] [PubMed] [Google Scholar]
- Li C-SR, & Sinha R (2008). Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience and Biobehavioral Reviews, 32(3), 581–597. doi: 10.1016/j.neubiorev.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, & Koob GF (2015). Heterogeneity of alcohol use disorder: Understanding mechanisms to advance personalized treatment. Alcoholism: Clinical and Experimental Research, 39(4), 579–584. doi: 10.1111/acer.12669 [DOI] [PubMed] [Google Scholar]
- Litten RZ, Wilford BB, Falk DE, Ryan ML, & Fertig JB (2016). Potential medications for the treatment of alcohol use disorder: An evaluation of clinical efficacy and safety. Substance Abuse. doi: 10.1080/08897077.2015.1133472 [DOI] [PubMed] [Google Scholar]
- Mason JW, Wang S, Yehuda R, Lubin H, Johnson D, Bremner JD, Charney D, & Southwick S (2002). Marked lability in urinary cortisol levels in subgroups of combat veterans with posttraumatic stress disorder during an intensive exposure treatment program. Psychosomatic Medicine, 64(2), 238–246. doi: 10.1097/00006842-200203000-00006 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, & Gray JD (2016). Stress effects on eeuronal etructure: Hippocampus, emygdala, and prefrontal cortex. Neuropsychopharmacology, 41, 3–3. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor BA, Murphy KM, Albano DL, & Ceballos RM (2016). Stress, cortisol, and B lymphocytes: A novel approach to understanding academic stress and immune function. Stress, 19(2), 185–191. doi: 10.3109/10253890.2015.1127913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child and Adolescent Psychiatry, 54(9), 753–762. doi: 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, De Vries GJ, Gersons BPR, & Olff M (2007). Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. British Journal of Psychiatry, 191(5), 387–392. doi: 10.1192/bjp.bp.106.024877 [DOI] [PubMed] [Google Scholar]
- Milivojevic V, Fox HC, Sofuoglu M, Covault J, & Sinha R (2016). Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology, 65, 44–53. doi: 10.1016/j.psyneuen.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V, & Sinha R (2018). Central and peripheral biomarkers of stress response for addiction risk and relapse vulnerability. Trends in Molecular Medicine, 24(2), 173–186. doi: 10.1016/j.molmed.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. (2000). Health risks and benefits of alcohol consumption. The Journal of the National Institute on Alcohol Abuse and Alcoholism. doi: 10.1016/S0268-0033(02)00186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SB, Haller M, Hamblen JL, Southwick SM, & Pietrzak RH (2018). The burden of co-occurring alcohol use disorder and PTSD in U.S. military veterans: Comorbidities, functioning, and suicidality. Psychology of Addictive Behaviors, 32(2), 224–229. doi: 10.1037/adb0000348 [DOI] [PubMed] [Google Scholar]
- Norman SB, Trim R, Haller M, Davis BC, Myers US, Colvonen PJ, Blanes E, Lyons R, Siegel EY, Angkaw AC, Norman GJ, & Mayes T (2019). Efficacy of integrated exposure therapy vs integrated coping skills therapy for comorbid posttraumatic stress disorder and alcohol use disorder: A randomized clinical trial. JAMA Psychiatry, 76(8), 791–799. doi: 10.1001/jamapsychiatry.2019.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, & Rounsaville B (1996). Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Archives of General Psychiatry, 53(3), 217–224. doi: 10.1001/archpsyc.1996.01830030039007 [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, & Kreek M (2002). Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology, 160(1), 19–29. doi: 10.1007/s002130100919 [DOI] [PubMed] [Google Scholar]
- Orazem RJ, Frazier PA, Schnurr PP, Oleson HE, Carlson KF, Litz BT, & Sayer NA (2017). Identity adjustment among Afghanistan and Iraq war veterans with reintegration difficulty. Psychological Trauma: Theory, Research, Practice, and Policy, 9(1), 4–11. doi: 10.1037/tra0000225 [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Desai N, Gueorguieva R, Arias A, O’Brien E, Jane JS, Sevarino K, Southwick S, & Ralevski E (2016). Prazosin for veterans with posttraumatic stress disorder and comorbid alcohol dependence: A clinical trial. Alcoholism: Clinical and Experimental Research, 40(1), 178–186. doi: 10.1111/acer.12926 [DOI] [PubMed] [Google Scholar]
- Possemato K, Maisto SA, Wade M, Barrie K, McKenzie S, Lantinga LJ, & Ouimette P (2015). Ecological momentary assessment of PTSD symptoms and alcohol use in combat veterans. Psychology of Addictive Behaviors, 29(4), 894–905. doi: 10.1037/adb0000129 [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, & Morrison JH (2004). Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience, 125(1), 1–6. doi: 10.1016/j.neuroscience.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Radley Jason J., & Morrison JH (2005). Repeated stress and structural plasticity in the brain. Ageing Research Reviews, 4(2), 271–287. doi: 10.1016/j.arr.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Ralevski E, Olivera-Figueroa LA, & Petrakis I (2014). PTSD and comorbid AUD: A review of pharmacological and alternative treatment options. Substance Abuse and Rehabilitation, 5, 25–36. doi: 10.2147/SAR.S37399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SAM, King AP, Liberzon I, & Sripada RK (2017). Changes in salivary cortisol during psychotherapy for posttraumatic stress disorder: A pilot study in 30 veterans. Journal of Clinical Psychiatry, 78(5), 599–603. doi: 10.4088/JCP.15m10596 [DOI] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, & Monti PM (2010). Subjective responses to alcohol consumption as endophenotypes: Advancing behavioral genetics in etiological and treatment models of alcoholism. Substance Use and Misuse, 45(11), 1742–1765. doi: 10.3109/10826084.2010.482427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GLG, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, Roerecke M, Room R, Samokhvalov AV, & Taylor B (2010). The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction, 105(5), 817–843. doi: 10.1111/j.1360-0443.2010.02899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Taylor B, & Room R (2006). Global burden of disease from alcohol, illicit drugs and tobacco. Drug and Alcohol Review, 25(6), 503–513. doi: 10.1080/09595230600944453 [DOI] [PubMed] [Google Scholar]
- Rubinsky AD, Chen C, Batki SL, Williams EC, & Harris AHS (2015). Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. Journal of Psychiatric Research, 69, 150–157. doi: 10.1016/j.jpsychires.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Sadeh N, Wolf EJ, Logue MW, Lusk J, Hayes JP, McGlinchey RE, Milberg WP, Stone A, Schichman SA, & Miller MW (2016). Polygenic risk for externalizing psychopathology and executive dysfunction in trauma-exposed veterans. Clinical Psychological Science, 4(3), 545–558. doi: 10.1177/2167702615613310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, & Nordstrom MA (2008). Cortisol inhibits neuroplasticity induction in human motor cortex. Journal of Neuroscience, 28(33), 8285–8293. doi: 10.1523/jneurosci.1963-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Gottesman II, & Dick DM (2015). Endophenotypes for alcohol use disorder: An update on the field. Current Addiction Reports, 2, 76–90. doi: 10.1007/s40429-015-0046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM (2000). Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry, 57(10), 925–935. doi: 10.1001/archpsyc.57.10.925 [DOI] [PubMed] [Google Scholar]
- Schepis TS, Rao U, Yadav H, & Adinoff B (2011). The limbic-hypothalamic-pituitary-adrenal axis and the development of alcohol use disorders in youth. Alcoholism: Clinical and Experimental Research, 35(4), 595–605. doi: 10.1111/j.1530-0277.2010.01380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Swendsen J, & Auriacombe M (2015). Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug and Alcohol Dependence, 148, 1–20. doi: 10.1016/j.drugalcdep.2014.12.024 [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Stappenbeck C, Malte CA, Lyons R, Tell D, Millard SP, & Raskind M (2018). Double-blind randomized clinical trial of prazosin for alcohol use disorder. American Journal of Psychiatry, 175(12), 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, & Vale WW (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, Hockemeyer JR, & Tulloch H (2008). Expressive writing and post-traumatic stress disorder: Effects on trauma symptoms, mood states, and cortisol reactivity. British Journal of Health Psychology, 13, 85–93. doi: 10.1348/135910707X250866 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Poon L, Papadopoulos AS, Kumari V, & Cleare AJ (2014). Long term effects of childhood trauma on cortisol stress reactivity in adulthood and relationship to the occurrence of depression. Psychoneuroendocrinology, 50, 289–299. doi: 10.1016/j.psyneuen.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Swift RM, & Aston ER (2015). Pharmacotherapy for alcohol use disorder: Current and emerging therapies. Harvard Review of Psychiatry, 23(2), 122–133. doi: 10.1097/HRP.0000000000000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawa EA, Hall SD, & Lohoff FW (2016). Overview of the genetics of alcohol use disorder. Alcohol and Alcoholism, 51(5), 507–514. doi: 10.1093/alcalc/agw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeters J, Lancaster C, Brown D, & Back S (2017). Substance use disorders in military veterans: Prevalence and treatment challenges. Substance Abuse and Rehabilitation, 8, 69–77. doi: 10.2147/sar.s116720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann S, Schönfeld S, Behrendt S, Heinrich A, Höfler M, Siegel S, Zimmermann P, & Wittchen HU (2015). Predictors of changes in daily alcohol consumption in the aftermath of military deployment. Drug and Alcohol Dependence. doi: 10.1016/j.drugalcdep.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Trautmann Sebastian, Muehlhan M, Kirschbaum C, Wittchen HU, Höfler M, Stalder T, & Steudte-Schmiedgen S (2018). Biological stress indicators as risk markers for increased alcohol use following traumatic experiences. Addiction Biology, 23(1), 281–290. doi: 10.1111/adb.12487 [DOI] [PubMed] [Google Scholar]
- Tripp JC, Worley MJ, Straus E, Angkaw AC, Trim RS, & Norman SB (2020). Bidirectional relationship of posttraumatic stress disorder (PTSD) symptom severity and alcohol use over the course of integrated treatment. Psychology of Addictive Behaviors. doi: 10.1037/adb0000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs. (2019). Substance Use Treatment.
- Van Voorhees EE, Dennis MF, Calhoun PS, & Beckham JC (2014). Association of DHEA, DHEAS, and cortisol with childhood trauma exposure and post-traumatic stress disorder. International Clinical Psychopharmacology, 29(1), 56–62. doi: 10.1097/YIC.0b013e328364ecd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, & Kendler KS (2015). The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychological Medicine, 45(5), 1061–1072. doi: 10.1017/S0033291714002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Tonigan JS, Bogenschutz MP, Clifford J, Bigelow R, & Simpson T (2018). A randomized, placebo-controlled, clinical trial of prazosin for the treatment of alcohol use disorder. Journal of Addiction Medicine, 12(5), 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch MA, & Prescott CA (2011). The influence of gene-environment interactions on alcohol consumption and alcohol use disorders: A comprehensive review. Clinical Psychology Review, 31(5), 800–816. doi: 10.1016/j.cpr.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamorski M, & Britt TW (2010). The psychology of transition: Adapting to home after deployment. In Deployment psychology: Evidence-based strategies to promote mental health in the military. doi: 10.1037/12300-006 [DOI] [Google Scholar]


