Abstract
Enolase inhibition is a potential therapeutic strategy currently being investigated for treatment of spinal cord injury (SCI) as it reduces pro-inflammatory cytokines and chemokines, alters metabolic factors, and reduces gliosis in acute SCI. Herein, the role of enolase in SCI has been examined to better understand the effects of this enzyme on inflammation, metabolic hormones, glial cell activation, and neuroprotection under these shorter injury conditions. Immunohistochemical analyses of inflammatory markers vimentin, Cox-2, and caspase-1 indicated that enolase inhibition attenuated the elevated levels of inflammation seen following SCI. Iba1, GFAP, NFP, and CSPG staining indicated that enolase inhibition with prolonged administration of ENOblock reduced microglia/astrocyte activation and lead to enhanced neuroprotection in SCI. An analysis of metabolic hormones revealed that ENOblock treatment significantly upregulated plasma concentrations of peptide YY, glucagon-like peptide 1, glucose-dependent insulinotropic peptide, glucagon, and insulin hormones as compared to vehicle-treated controls (Mann-Whitney, p≤ 0.05). ENOblock did not have a significant effect on plasma concentrations of pancreatic polypeptide. Interestingly, ENOblock treatment inhibited chondroitin sulfate proteoglycan (CSPG), which is produced by activated glia and serves to block regrowth of axons across the lesion site following injury. An increased level of NeuN and MBP with reduced caspase-1 was detected in SCI tissues after ENOblock treatment, suggesting preservation of myelin and induction of neuroprotection. ENOblock also induced improved motor function in SCI rats, indicating a role for enolase in modulating inflammatory and metabolic factors in SCI with important implications for clinical consideration.
Keywords: Spinal cord injury, enolase, metabolic factors, gliosis, inflammation, neuroprotection
1. Introduction
Spinal cord injury (SCI) is a debilitating condition with life-long consequences that affects an estimated 285,000 people in the United States (Center, 2017). SCI has no FDA-approved treatment, even though methylprednisolone is controversially used off-label in many instances of both acute and chronic SCI (Center, 2017; Polcyn et al., 2017a). As such, research into effective treatment options and alternatives for affected individuals is urgently needed. Although the primary trauma in SCI, the initial mechanical insult to neurons at the site of injury of the CNS, is considered to be irreversible, the secondary injury mechanisms of SCI pathology provide windows of opportunity for researchers to discern effectors of favorable change to injury progression and recovery in stages in SCI. These secondary mechanisms or associated factors include hypoxia, ischemia, glutamate excitotoxicity, and inflammation that can exacerbate the injury by leading to further neuroinflammation, neurodegeneration, and neuronal cell death (Cox et al., 2015a; Haque et al., 2018).
Current strategies to mitigate neuroinflammation and promote neuroprotection in CNS injuries including SCI involve preventing secondary factors by limiting pro-inflammatory cytokine/chemokine (Cox et al., 2015b; Garcia et al., 2016; Knerlich-Lukoschus and Held-Feindt, 2015; Mukhamedshina et al., 2017), ROS (Gwak et al., 2013; Visavadiya et al., 2016), and NO production (Visavadiya et al., 2016). Furthermore, preventing or reducing glial scar formation (Moeendarbary et al., 2017; Yuan and He, 2013), promoting neuro-regeneration, and replacing the injured spinal cord with functional tissue are thought to be essential steps for recovery or functional improvement after SCI (Orlandin et al., 2017; Varma et al., 2013). One target for potential treatment has been dysregulation of macrophage activation towards a prolonged inflammatory phase that impairs wound healing after SCI (Gensel and Zhang, 2015). Formation of the glial scar from the secretion of chondroitin sulfate proteoglycans (CSPG) and other inhibitory extracellular matrix proteins by reactive astrocytes is another potential treatment target as it impedes axonal regeneration and functional recovery after SCI (Okada et al., 2018; Orlandin et al., 2017). Treatment with chondroitinase to degrade CSPG at the glial scar has been shown to support axonal regeneration and functional recovery after SCI (Bradbury et al., 2002; Okada et al., 2018; Patil et al., 2018). Additionally, STAT3, which promotes the migration of reactive astrocytes to the lesion site, has been identified as an important regulator of astrogliosis, and reduction in STAT3 led to reduced migration of reactive astrocytes and a wider injury area in SCI (Okada et al., 2006). However, the precise mechanisms for functional recovery via reactive astrocyte migration and glial scar attenuation in neuronal injury remain unclear (Okada et al., 2018). Cell transplantation of Schwann cells, neural stem cells, neural progenitor cells, olfactory ensheathing cells, OPCs, and mesenchymal stem cells have been explored as potential means to attenuate inflammatory factors and to promote neuroprotection and function. While other areas required for improvement of function include immunomodulation, axon regeneration, and/or myelin preservation/synthesis in injured spinal cord, the mechanisms of action for above cell types in reducing inflammation and promoting neural repair in SCI are not well understood. Therefore, several of these obstacles have prevented their roles in recovery of function, and minimize the therapeutic implementation (Assinck et al., 2017; Samantaray et al., 2016; Sribnick et al., 2006; Sribnick et al., 2010).
Our laboratory has identified Enoblock (AP-111-a4, Adooq Biosciences), an inhibitor of the multifunctional glycolytic enzyme, enolase, as a potential treatment strategy for mitigating the detrimental effects of secondary injury mechanisms in acute SCI in rats, (Haque et al., 2017b) though its specific mechanisms and effects on metabolic hormones and inflammatory mediators are not yet fully known. Under normal conditions, enolase is abundantly expressed in the cytosol and contributes to glucose metabolism by catalyzing the conversion of 2-phosphoglycerate to phosphoenolpyruvate (Hafner et al., 2012; Haque et al., 2017b; Haque et al., 2016; Polcyn et al., 2017a; Vizin and Kos, 2015). During inflammation, enolase can migrate to the cell surface to enhance antigen presentation for host cell invasion via plasmin activation and extracellular matrix degradation. This cell surface expression of enolase can also trigger the production of reactive oxygen species (ROS), nitric oxide (NO), and pro-inflammatory cytokines (TNF-α, IL-1β, IFN-γ, and TGF-β) and chemokines (MIP-1α and MCP-1) to augment neurodegenerative response (Haque et al., 2018; Haque et al., 2016). Neuron specific enolase (NSE, γ-enolase), one of three distinct enolase isoforms, is localized primarily in neurons and cells of neuroendocrine origin. With roles in hypoxia and ischemia, NSE has been identified as a potential biomarker of functional damage to neurons in SCI and several other conditions including traumatic brain injury, glioblastoma, diabetic peripheral neuropathy, small cell lung cancer, diffuse large B-cell lymphoma, and cardiac arrest (Chao et al., 2013; Haque et al., 2017b; Haque et al., 2018; Haque et al., 2016; Polcyn et al., 2017b; Schmidt et al., 2014; Thelin et al., 2016; Varma et al., 2013; Vizin and Kos, 2015; Wang et al., 2012). The role of enolase in SCI and the effects of ENOblock treatment on inflammation and gliosis were previously investigated in acute SCI (Haque et al., 2017b). Upregulation of NSE expression and activity in rat serum and tissue were found following SCI, which are intimately linked to an increase in pro-inflammatory cytokine and chemokine expression, alteration of key metabolic factors, and elevated MMP-9 expression in SCI tissue (Haque et al., 2017b). Further, this increase is attenuated with enolase inhibition by ENOblock (100μg/kg). These findings are significant because they indicate that ENOblock treatment reduces neuroinflammation associated with secondary mechanisms of injury may thus lead to improved outcome in SCI following treatment.
An analysis of the metabolic factors c-peptide, amylin, and leptin in acute SCI showed that there is a connection between reduced enolase/NSE activity and preferential regulation of certain metabolic hormones towards anti-inflammatory response in acute SCI (Haque et al., 2017b). C-peptide in particular has predominantly anti-inflammatory activity and has been found to be significantly elevated following ENOblock treatment. Amylin and leptin, two hormones that are markedly elevated during inflammation in a variety of conditions, including SCI, were attenuated with ENOblock treatment. These findings indicate a role for enolase in regulating metabolic hormones during inflammation in acute SCI conditions and raise a question if prolonged administration of ENOblock affects other enzymes/hormones involved in glucose metabolism and reduces inflammation in SCI.
The present study focuses on the effects of enolase/NSE inhibition via ENOblock treatment to address how enolase inhibition affects both metabolic and inflammatory profiles in SCI. For metabolic factors, enzymes such as insulin, glucagon, glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotrophic polypeptide (GIP), pancreatic polypeptide (PP), and peptide tyrosine tyrosine (PYY) were examined following injury and treatment with ENOblock. In addition, this study focuses on how changes in metabolic factors relate to inflammation and gliosis in SCI, and whether ENOblock treatment promotes neuroprotection in SCI. Our study suggests that enolase inhibition with ENOblock modulates both metabolic and inflammatory mediators, and reduces gliosis in SCI, inducing neuroprotection and improved functional outcome.
2. Materials and methods
2.1. General
All results were generated in blind-coded experiments, in which the persons who collected data were unaware of the testing groups or treatment conditions. Proteins/markers expression levels in immunohistochemistry, western blotting or arrays were quantified individually in duplicates or triplicates in the designated areas of spinal cord samples from animals.
2.2. Induction of SCI and treatment with ENOblock
Adult male Sprague-Dawley rats (200–250 g) were used for the induction of SCI and treatment with ENOblock. While gender-related differences may be found in SCI where females are favored in terms of tissue preservation and locomotor recovery (Datto et al., 2015), no significant differences between males and females could be seen in SCI regarding pain and localization, onset, distribution, factors affecting pain, number of painful body regions, pain descriptors, ratings of pain intensities, or in pain and life satisfaction. In this scenario, potential new therapeutics can be designed to improve recovery for the male population following SCI which could be used to augment the neuroprotective privilege in females for enhanced outcomes. Thus, male rats were used in this study. Rats were anesthetized with ketamine (80 mg/kg)/xylazine (10 mg/kg), and a laminectomy was performed at T10. A T-10 laminectomy was performed to expose the spinal cord. After anesthetizing the animal and preparing the surgical site, a midline incision was made on the back over the spinal processes and a T-10 laminectomy is performed. SCI induction was performed as previously described (Haque et al., 2017b; Perot et al., 1987). Briefly, spinal contusion injury was induced at T10 of the spinal cord and the injured area was divided into three sections, numbered S2, S3, and S4. These are in house designations based on our laboratory experiences. The S3 section is the lesion section, S2 is rostral to the lesion, and S4 is caudal area to the lesion and is partially damaged. This study is mainly focused on the analysis of S3 lesion section where initial injury occurred. The spine was immobilized with a stereotactic device, and the injury was induced via the method of Perot: dropping a constant weight (5g) from a height of 8cm onto an impounder (0.3cm in diameter) gently placed on the spinal cord. ENOblock (AP-111-a4, Adooq Bioscience, 100μg/kg) was administered systemically via intravenous tail vein injection at four time points: 15min, 24h, and 3d post-injury. Vehicle-treated animals received the same volume of vehicle (100μL). Sham animals underwent a T10 laminectomy. Wounds were closed using 5–0 absorbable suture (for interior wound closure). Exterior wound closure was performed with either 4–0 ethilon suturing or with application of staples 5–8mm apart (EZ-Clip wound clips). The surgical site was monitored, and based on the rate of healing sutures are removed under isoflurane anesthesia.
After surgery, animals were placed on a warming pad until awake. Food was placed on the floor of the cage until the animals have recovered enough function to reach. Long-stemmed water bottles were provided for ease of access to water. Animals were checked twice daily until sacrifice. Animals were inspected visually in the cage to check for abnormal behaviors (head pressing, piloerection, hunching) then handled gently to for a closer physical inspection, including a bladder check. If the animal were not recovering appropriately, the monitoring amplified commensurate with the animal’s health needs. Bladders were monitored and expressed twice daily. Animals that develop urine scald were bathed with warm water and gently dried. Vaseline was applied to the affected area. Urine was expressed onto a clear glass dish in order to visually check for cloudiness. Animals were monitored for weight loss, porphyrin staining, piloerection, hunching, and other abnormal behaviors (head pressing). Animals that develop these symptoms were treated with antibiotics or analgesics, given soft food, or placed on a heating, depending on the symptoms. Blood and tissue samples were collected 7d post-injury. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the US Department of Health and Human Services (National Institutes of Health, Bethesda, MD, USA) and were approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina under the protocol ARC #1254
2.3. Cytokine, chemokine, and metabolic arrays
At 7d post-injury, rats were sacrificed by decapitation and blood was collected in EDTA treated tubes. Plasma samples were then isolated from the blood sample by centrifugation at 1200 rpm for 5 min following separation with Ficoll and stored at −80°C. Plasma samples from sham operated, SCI-vehicle treated, and SCI-ENOblock treated rats were shipped in dry ice to Eve Technologies and analyzed via the Rat Cytokine/Chemokine Array (Chandran et al., 2018; Cox et al., 2015b; Haque et al., 2017a), and Mouse/Metabolic Array (MRDMET) Discovery Assays (Haque et al., 2017a), which determine levels of the following metabolic hormones: amylin, c-peptide 2, GIP, GLP-1, ghrelin, glucagon, insulin, leptin, PP, and PYY. Results were analyzed by Graphpad Prism software.
2.4. Immunohistochemistry
Spinal cord tissue segments (S3, injury section) from the injury site were fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) overnight(Haque et al., 2017a). Tissue was then embedded in paraffin, sectioned, and mounted onto slides for analysis. Following epitope retrieval, non-specific binding sites were blocked with the serum of the secondary antibody host for 1h at room temperature (Samantaray et al., 2015). Tissues were incubated with vimentin (1:500, Abcam ab92547), Iba1 (1:250, Abcam ab5076), GFAP (1:250, Santa Cruz sc-9065), Cox-2 (1:100, Abcam ab15191), caspase-1 (1:200, Abcam ab74279), CSPG (1:200, Sigma C8035), or NFP (1:1000, Sigma Aldrich N4142) antibodies overnight at room temperature. Sections were then incubated with Texas Red® Goat Anti-Rabbit IgG Antibody (Vector Laboratories, Inc., Burlingame, CA), DyLight® 488 Anti-Goat IgG (H+L) (Vector Laboratories, Inc., Burlingame, CA), Texas Red® Anti-Mouse IgM Antibody (Vector Laboratories, Inc., Burlingame, CA), or Fluorescein Anti-Rabbit IgG (Vector Laboratories, Inc., Burlingame, CA) for 1h in the dark. Slides were mounted with 1 drop of Invitrogen™ ProLong™ Gold Antifade Mountant with DAPI (Thermo Fisher Scientific) and coverslipped. After staining, SCI tissues were viewed under a fluorescence microscope with representative images taken at 20× magnification.
2.5. Western blot analysis
Spinal cord samples were homogenized in a standard homogenizing buffer (50 mM Tris-HCl, pH 7.4; 5 mM EGTA; 1 mM phenylmethylsulfonyl fluoride) on ice plus protease inhibitor as described (Haque et al., 2007; Haque et al., 2002; O’Donnell et al., 2004). Equal protein concentrations from designated samples were separated on a 4–12% Bis/Tris NuPage gel (Invitrogen, Grand Island, NY) (God et al., 2015; Haque et al., 2017a; Hathaway-Schrader et al., 2018). Proteins were transferred onto a nitrocellulose membrane (Pierce, Rockford IL), and probed with caspase-1 (1:500, Santa Cruz, sc-622), NeuN (1:100, Proteintech, Catalog # 26975–1-AP), and MBP (1:1000, Millipore, MAB384) antibodies. The secondary antibodies used were horseradish peroxidase conjugated anti-mouse (1:2000, Santa Cruz, sc-2005), and anti-rabbit (1:4000, Santa Cruz, sc-2004). Monoclonal antibody for β-actin (1:1000, Santa Cruz, sc-81178) was used as a protein loading control. Relative protein expression was assessed using Image J software (National Institutes of Health, Bethesda, MD) and expressed as relative density for each sample (Goldstein et al., 2008; Radwan et al., 2012; Zhao et al., 2011).
2.6. Basso, Beattie and Bresnahan (BBB) motor score
The functional outcome was tested in each group of rats using the BBB rating scale on 24 hours and weekly thereafter for 3 weeks. This scale used 21 points measuring hind limb movement, gait coordination, toe clearance, trunk stability and tail movement. At grade 0, there was no hind limb movement while grade 21 was normal. For this testing, the animal was placed in an open field (small plastic swimming pool) and observed for 5 minutes. DigiGait treadmill testing was done prior to surgery and at the end of the experiment before sacrifice. The treadmill was part of the DigiGait system that digitizes and analyzes the animal’s gait. Rats were initially placed on the treadmill and allowed to explore and acclimate for several minutes. The treadmill was started at a low speed (10cm/s) for a few seconds, then it was stopped and the animal was allowed to recover. This assay was repeated several times until the animal began to move when the treadmill was started. The speed was increased slowly to 30–40cm/s. If the animal began to panic or could not keep up, the treadmill was stopped and, after a rest period, it was started again. The animal was removed and given a treat. This was done twice the day before the surgery/sacrifice and twice on the day of the surgery/sacrifice. Occasionally an animal that was more resistant to learning required an extra day of training. DigiGait data was recorded at 30 and 40cm/s, and analyzed as described.
2.7. Statistical analysis
Statistical analyses were performed using Microsoft Excel and GraphPad Prism (version 6.0) Software. The immunoreactive bands obtained from Western blotting and the immunoreactive pixels of the immunohistochemistry data were analyzed with ImageJ software (U.S. National Institutes of Health, Bethesda, MD). Mann-Whitney U test was performed for all metabolic hormone statistical significance calculations. Two-tailed paired t-test and One-way ANOVA with Bonferroni test for multiple comparisons were used to determine statistical significance for all immunohistochemical analyses. One-way ANOVA with Fisher’s post-hoc test was used for BBB. Data were expressed as mean ± SEM or mean+/SDEV (n=3–8). A p-value < 0.05 was determined to be statistically significant for all calculations.
3. Results
Recently, enolase inhibition with ENOblock treatment of acute SCI in rats was shown to significantly decrease levels of pro-inflammatory cytokines/chemokines (TNF-α, IL-1β, IL-6, IP-10, and MIP-1α), alter metabolic hormones (amylin, leptin, and c-peptide), and reduce gliosis (evidenced by decreased MMP-9 expression) in serum and tissue samples (Haque et al., 2017b, Haque et al., 2018). An analysis of the metabolic factors c-peptide, amylin, and leptin revealed a connection between reduced enolase/NSE activity and alteration of metabolic hormones with roles in inflammation to favor anti-inflammatory activity. This connection between inflammation, metabolic factors, and gliosis in association with ENOblock treatment of acute SCI in rats led us to question (1) whether this anti-inflammatory activity could also be observed after prolonged treatment with ENOblock in SCI, (2) the extent of enolase/NSE’s effects on other metabolic hormones, and (3) how this glycolytic enzyme functions in neuroinflammation and neuronal protection.
3.1. Enolase inhibition by ENOblock treatment reduces neuroinflammation in SCI in rats
In order to evaluate the association between ENOblock treatment and neuroinflammation in SCI, immunohistochemistry was performed on the lesion site S3 which contains both white and grey matter as described in the methods. Immunochemical staining for Vimentin, Cox-2, and caspase-1 of SCI tissues from sham-operated, vehicle-treated, and ENO-treated (100μL at 15min, 24h, and 3d) rats harvested 7d post-injury indicate an increase in inflammatory markers following injury (Figure 1A). Analysis of cell fluorescence intensity indicated that the inflammatory markers (Vimentin, Cox-2, and caspase-1) observed in vehicle treated rats are significantly attenuated with ENOblock treatment (Figure 1B). This finding aligns with our earlier results from acute SCI (Haque et al., 2017b), showing that prolonged administration of ENOblock diminishes inflammation in acute to subacute (day 7) state of inflammation in SCI rats.
Fig. 1.
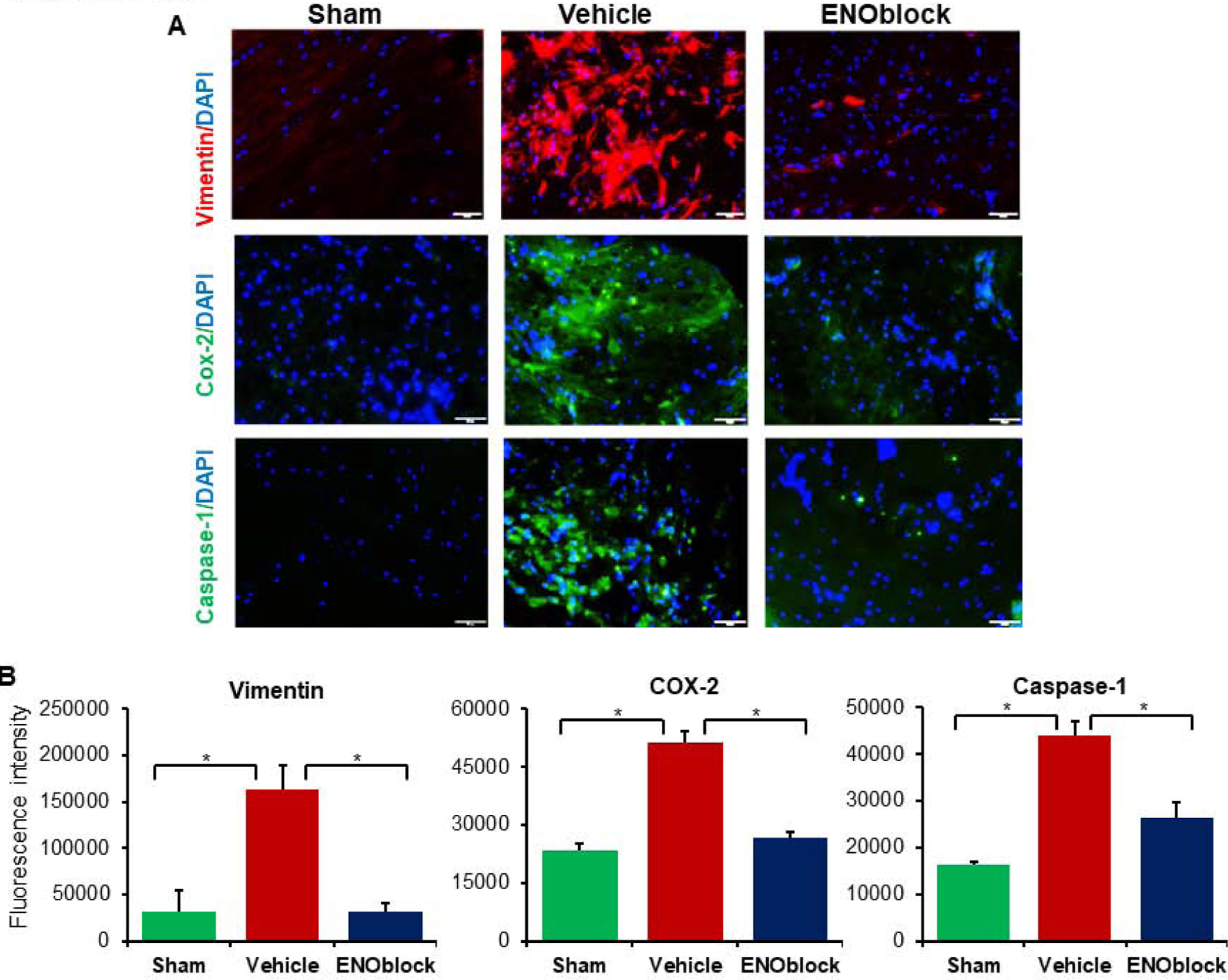
ENOblock treatment significantly reduces inflammation in SCI in male Sprague Dawley rats. The section shown in the figure is taken from the lesion site S3 which contains both white and grey matter as described in the methods. (A) Vimentin (red) and DAPI (blue) nuclear staining of SCI tissue at the injury site 7d post injury is shown in sham-operated, vehicle-treated, and ENOblock-treated rats. Representative images were taken at 20× magnification. (B, left panel) Fluorescence intensity from three representative areas show increased vimentin expression in vehicle treated tissue is significantly attenuated with ENOblock treatment (paired t-test, two-tailed, *p < 0.05). (A-B) Similarly, Cox-2 (green) and caspase-1 (green) staining of SCI tissue samples showed that ENOblock treatment significantly reduced these protein expression levels in SCI tissues. DAPI (blue) was used for nuclear staining (A) in both cases. Fluorescence intensity from three representative areas of immunohistochemistry showed increased Cox-2 and caspase-1 expression in vehicle treated tissue which is significantly attenuated with ENOblock treatment (paired t-test, two-tailed, *p < 0.05, **p<0.01; n=3–6), bar=100μM.
In order to evaluate the association between ENOblock treatment and neuroinflammation in SCI, immunohistochemistry experiments were also conducted on a distant spinal cord section (S4, caudal area to the lesion) where the damage could be reversible. Immunochemical staining for Cox-2, and caspase-1 on S4 section from vehicle-treated and ENO-treated rats indicated an increase in inflammatory markers after injury (Supplemental Figure, S1). Analysis of relative fluorescence intensity showed that the inflammatory markers Cox-2 and caspase-1 were significantly decreased by prolonged administration of ENOblock in SCI.
3.2. ENOblock alters key metabolic enzymes following SCI
Though primarily a glycolytic enzyme, enolase has multisystemic effects, the full implications of which are not yet fully known. Following observation of the impact of ENOblock treatment on neuroinflammation in SCI conditions, we sought to gain a greater understanding of the metabolic effects of ENOblock in SCI and to explore potential mechanistic avenues for the anti-inflammatory effects observed with ENOblock treatment, as several metabolic hormones have been shown to correspond to varying inflammatory effects. Plasma samples from sham-operated, vehicle treated, and ENOblock treated SD rats were sent in dry ice to Eve Technologies. An analysis of the samples using Eve Tech’s Mouse, Rat Metabolic Array (MRDMET) Discovery Assay revealed notable alterations in several key metabolic enzymes in association with ENOblock treatment as compared to vehicle-treated rats that are discussed herein.
3.2.1. Anorectic hormones
Pancreatic Polypeptide (PP) and Peptide Tyrosine Tyrosine (PYY) are anorectic gut hormones belonging to the PP fold peptide family (Holzer et al., 2012; Karra et al., 2009). As a part of the PP-fold family of enzymes, PP and PYY inhibit cyclic AMP formation through interactions between their receptors and adenylyl cyclase. Figure 2A shows the results from an analysis of pancreatic polypeptide (PP) concentration in plasma from ENOblock-treated (ENO-treated) SCI rats as compared to vehicle-treated controls. PP is a 36 amino acid amidated peptide that is released from PP cells in the pancreatic islets of Langerhans in a response to meal ingestion in an amount that is based on the caloric intake (Simpson et al., 2012). PP acts at the Y4 receptor (Y4R) to delay gastric emptying and decrease food intake. PP is also involved in energy expenditure, as increased PP levels in mice from repeated administration increased oxygen consumption and the discharge rate of adrenal gland sympathetic efferent nerves (Asakawa et al., 2003). PP hormone levels were slightly increased with ENOblock treatment, though not significantly (Figure 2A, p=0.5887). The median concentrations of PP in SCI-vehicle treated rats compared to SCI-ENO treated rats were 130.3 and 131.6 pg/ml respectively. However, PYY levels were significantly upregulated by enolase inhibition with ENOblock when compared to vehicle-treated rats (Figure 2B, p=0.0022). PYY peptide is another anorectic hormone that is comprised of 36 amino acids and is released from enteroendocrine L-cells in the distal gut after a meal (Simpson et al., 2012). PYY acts as an “ileal brake” to slow the movement of food from the stomach to the small intestine based on the level of nutrients. The truncated form of PYY, PYY3–36, selectively binds to the Y2 receptor (Y2R), which is located primarily in the CNS, to inhibit the formation of cyclic AMP (cAMP). PYY3–36 activity also reduces calcium signaling and therefore interferes with neurotransmitter vesicle exocytosis. The median concentrations of PYY in SCI-vehicle treated rats compared to SCI-ENO treated rats were 63.81 and 137.8 pg/ml respectively. This result is interesting because PP and PYY are thought to have similar functions, but the differential effect of enolase inhibition on these hormones indicates a specific role for PYY associated with enolase pathways.
Fig. 2.
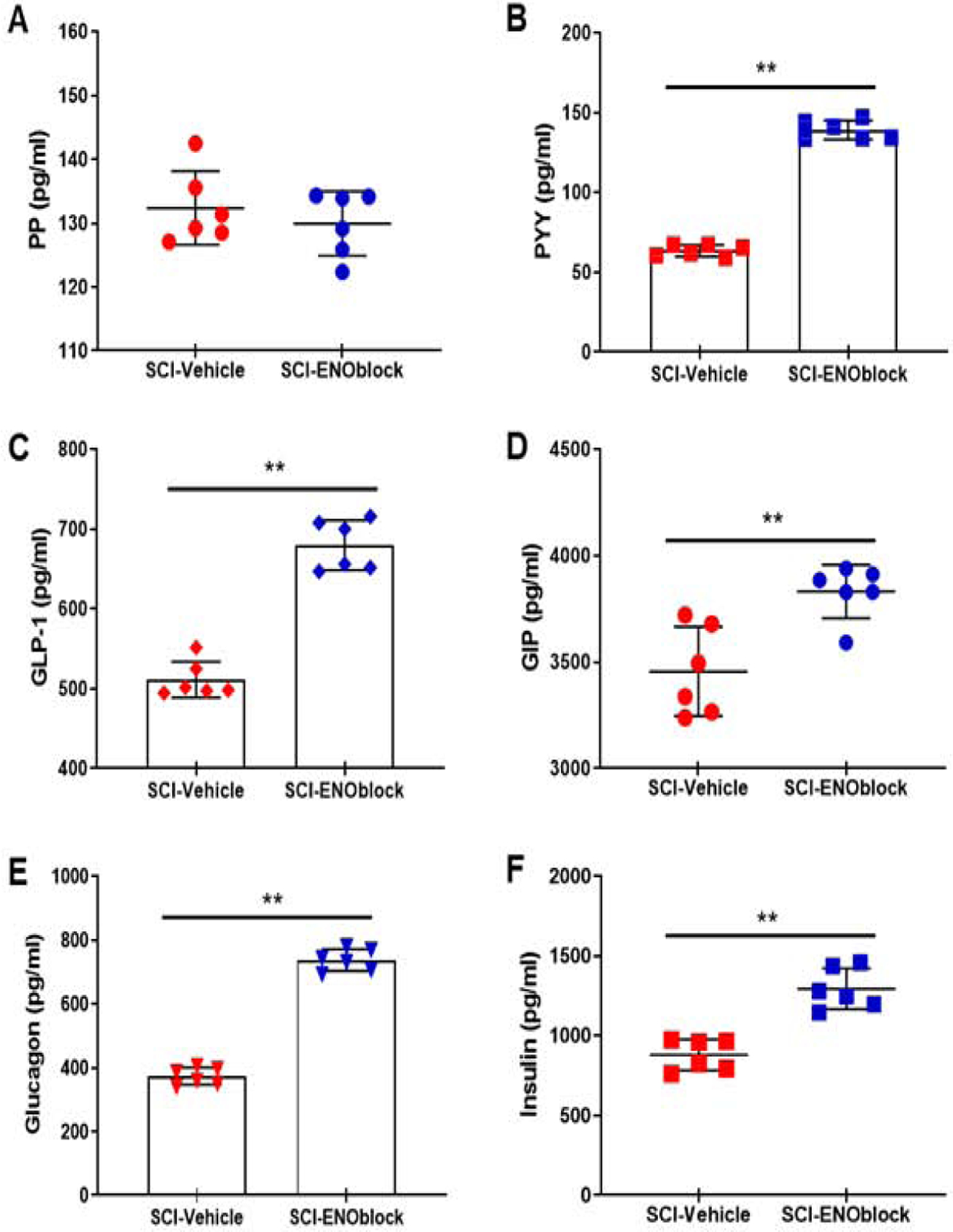
Effects of ENOblock treatment on several key metabolic factors after SCI in rats. SCI rats were treated with vehicle alone or ENOblock (100 μg/kg given 15min, 24h, and 3d post-injury via intravenous tail vein injection), and blood samples were obtained at sacrifice, 7d post-injury. Sham operated rats (T10 laminectomy) were used as controls. Samples were analyzed by using Eve Tech’s Mouse, Rat Metabolic Array (MRDMET) Discovery Assay. (A-B) Pancreatic polypeptide (PP) and peptide tyrosine tyrosine (PYY). PP concentrations in plasma samples were not significantly changed by ENOblock treatment. Median concentrations in SCI-vehicle and SCI-ENOblock treated groups were 130.3 and 131.6 pg/ml respectively; the distributions in the two groups did not differ significantly (Mann–Whitney U = 14, n1 = n2 = 6,). PYY levels were significantly increased in ENO-treated rats as compared to vehicle treated rats. Median concentrations in SCI-vehicle and SCI-ENOblock treated groups were 63.81 and 137.8 pg/ml respectively; the distributions in the two groups differed significantly (Mann–Whitney U = 0, n1 = n2 = 6, **p < 0.01 two-tailed). (C-D) Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotrophic polypeptide (GIP). Plasma levels of GLP-1 in ENO-treated SCI rat samples were significantly increased as compared to vehicle treated rats. Median concentrations in SCI-vehicle and SCI-ENOblock treated groups were 500.1 and 678.3 pg/ml respectively; the distributions in the two groups differed significantly (Mann–Whitney U = 0, n1 = n2 = 6, **p < 0.01 two-tailed). ENOblock treatment increased GIP levels after SCI in rats. Median concentrations in SCI-vehicle and SCI-ENOblock treated groups were 3416 and 3858 pg/ml respectively; the distributions in the two groups differed significantly (Mann–Whitney U =2, n1 = n2 = 6, **p < 0.01 two-tailed). (E-F) Glucagon and insulin. ENOblock treatment increased glucagon levels significantly as compared with vehicle treated SCI rats. Median concentrations in SCI-vehicle and SCI-ENOblock treated groups were 374.3 and 737.3 pg/ml respectively; the distributions in the two groups differed significantly (Mann–Whitney U = 0, n1 = n2 = 6, **p < 0.01 two-tailed). (B) ENOblock treatment significantly increased insulin levels as compared with vehicle treated SCI rats. Median concentrations in SCI-vehicle and SCI-ENOblock treated groups were 893.2 and 1264 pg/ml respectively; the distributions in the two groups differed significantly (Mann–Whitney U = 0, n1 = n2 = 6, **p < 0.01 two-tailed).
3.2.2. Incretins
GLP-1 is a 30 amino acid peptide that when bound to its receptor, activates adenylate cyclase to increase IP3 levels and gene expression, enhances β-cell proliferation in the pancreas, and facilitates insulin secretion to lower blood glucose levels (Holscher, 2014). GLP-1 receptors (GLP-1R) are found on neurons in the brains of rodents and humans, and they have also been found on microglia and astrocytes in mice (Holscher, 2014; Spielman, 2014). Our metabolic profile of ENOblock treated rats compared to vehicle treated rats showed a significant increase in GLP-1 concentration with ENOblock treatment (Figure 2C, p=0.0022). The median concentrations of GLP-1 in SCI-vehicle treated rats compared to SCI-ENO treated rats were 500.1 and 678.3 pg/ml respectively.
GIP is a 42 amino acid enzyme that enhances insulin secretion by activating pancreatic islets and promotes pancreatic β-cell growth, differentiation, and survival (Holscher, 2014). GIP analogs affect memory formation, preserve spatial learning against amyloid impairment, and protect synapses against Aβ insult. GIP receptors have been found on neuronal cells but not on microglia or astrocytes (Spielman, 2014). In a mouse model of AD, however, the GIP analog D-Ala2-GIP reduced astrogliosis and had neuroprotective effects (Holscher, 2014). Analysis of the plasma sample from ENO-treated and vehicle treated rats indicated a significant increase in GIP levels associated with ENOblock treatment (Figure 2D, p=0.0087). The median concentrations of GIP in SCI-vehicle treated rats compared to SCI-ENO treated rats were 3416 and 3858 pg/ml respectively. In peripheral inflammation, GIP is pro-inflammatory and induces the secretion of IL-6 and IL-1β from adipose tissue (Spielman, 2014). The greater increase in GLP-1 plasma concentration as compared to GIP concentration associated with ENOblock treatment observed in the metabolic profile from this study further supports the higher prevalence of anti-inflammatory cytokines/chemokines observed previously in ENOblock treated SCI rats (Haque et al., 2017b).
3.2.3. Glucagon and insulin
Glucagon is a 29 amino acid enzyme that mainly functions to protect organisms against hypoglycemia (of the CNS especially) by increasing circulating levels of glucose and inhibiting glycolysis and gluconeogenesis in the liver (Insuela and Carvalho, 2017). Activation of glucagon receptors can lead to an increase in intracellular cAMP and decrease the production of Th2 cytokines, TNF-α, and NK cell activity. Our analysis of the ENO-treated rat plasma indicated a significant increase in glucagon levels as compared to the vehicle-treated levels (Figure 2E, p=0.0022). The median concentrations of glucagon in SCI-vehicle treated rats compared to SCI-ENO treated rats were 374.3 and 737.3 pg/ml respectively. Counterintuitively, ENOblock treatment also corresponded to significantly increased levels of insulin (Figure 2F, p=0.0022). The median concentrations of insulin in SCI-vehicle treated rats compared to SCI-ENO treated rats were 893.2 and 1264 pg/ml respectively. Insulin typically functions in glucose storage while glucagon functions in glycogen breakdown (Insuela and Carvalho, 2017; Spielman, 2014). However, in the CNS it has been shown that low insulin levels correspond to pro-inflammatory conditions while high insulin levels promote anti-inflammatory effects (Spielman, 2014). This result aligns with the previously observed reduction of cytokine/chemokine levels and upregulation of amylin levels in association with ENOblock treatment (as amylin and insulin are typically co-secreted) and indicates the anti-inflammatory effects of enolase inhibition in SCI (Haque et al., 2017b; Polcyn et al., 2017a).
3.3. ENOblock treatment attenuates gliosis and supports neuroprotection in SCI
Enolase/NSE inhibition with ENOblock was recently shown to lead to reduced gliosis via attenuation of microglial and astroglial activity in acute SCI in rats (Haque et al., 2017b). This decrease in gliosis was associated with reduced levels of pro-inflammatory cytokines/chemokines and the metabolic factor c-peptide. To elucidate the effects of ENOblock on gliosis and neuroprotection in SCI, several indicative markers of these processes were evaluated through immunohistochemistry of spinal cord tissue from SCI rats.
3.3.1. Effect on microglia and astrocytes
Iba1 staining of SCI tissue from sham-operated, vehicle-treated, and ENO-treated (100μL at 15min, 24h, and 3d post-injury) rats harvested 7d post-injury indicate a significant (p<0.01) increase in microglial activation following injury that is attenuated with ENOblock treatment (Figure 3, A and B). Glial fibrillary acidic protein (GFAP) staining indicated a significant (p<0.01) increase in astrocyte expression following SCI (Figure 3, C and D). A significant reduction in astrocyte expression was noted when the fluorescence intensity was analyzed by the One-way ANOVA. These findings align with our earlier results from an acute SCI and suggest that ENOblock treatment reduces gliosis in SCI in rats.
Fig. 3.
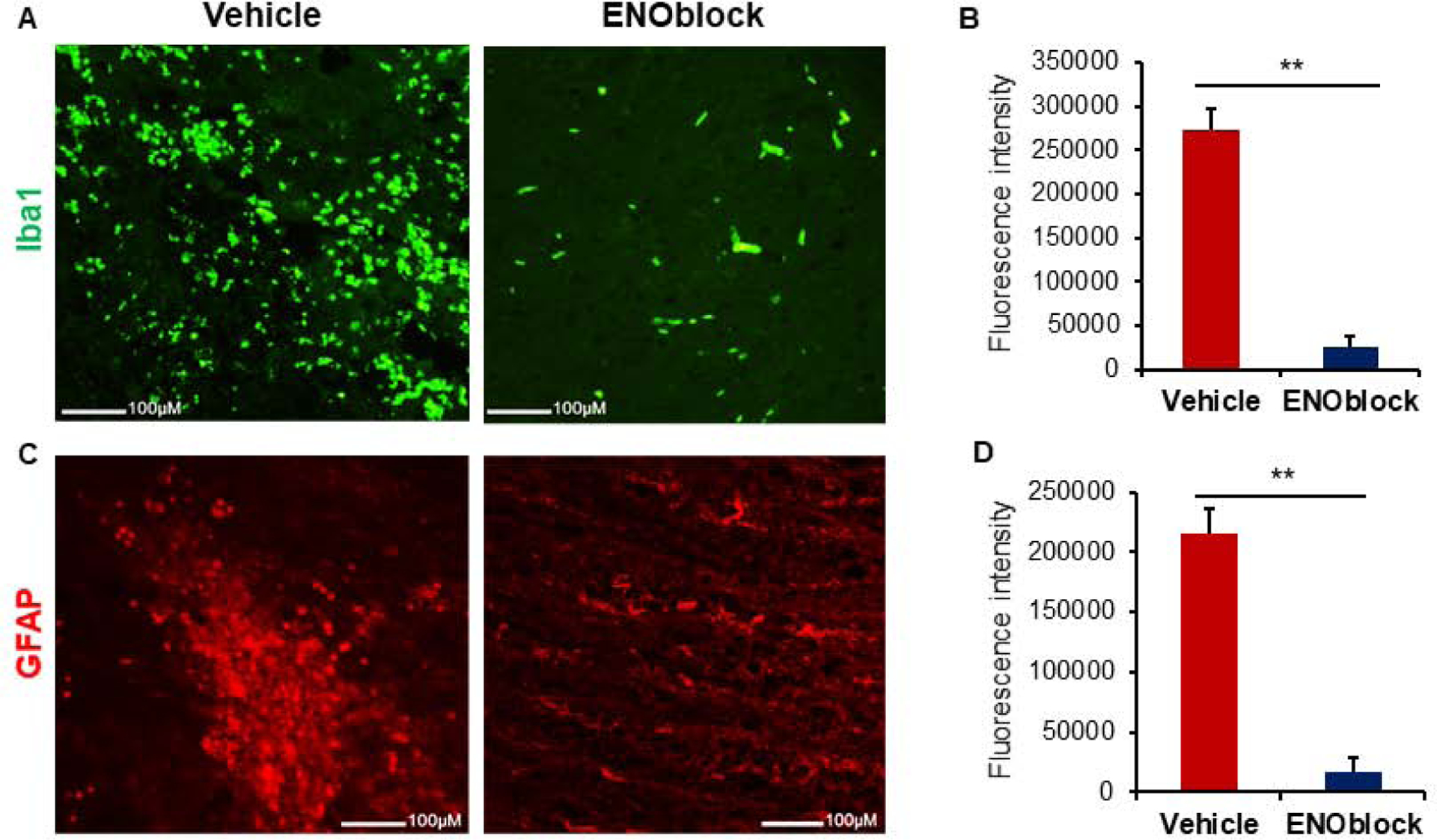
ENOblock treatment reduces gliosis in SCI. (A) Ionized calcium binding adaptor molecule 1 (Iba1, green) staining of SCI tissue at the injury site shows a reduction in microglia with ENOblock treatment as compared to vehicle treated rats. Representative images were taken at 10× (upper panel) and 20× (lower panel) magnification. (B) Mean fluorescence intensity from three representative areas of 20× images shows significantly increased Iba1 expression in vehicle treated tissue that is reduced with ENOblock treatment (One-way ANOVA, **p < 0.01; n=3–4). (C) Glial fibrillary acidic protein (GFAP, green) staining of SCI tissue at the injury site shows a reduction in astrocytes with ENOblock treatment as compared to vehicle treated rats. Panel shows representative images taken by fluorescence microscopy at 10× (upper panel) and 20× (lower panel) magnification. (D) Mean fluorescence intensity from three representative areas shows significantly increased GFAP expression in vehicle treated tissue. There is also a significant reduction of astrocytes from ENOblock treatment as compared to vehicle treated controls (One-way ANOVA, **p < 0.01; n=4–5).
3.4. ENOblock treatment and neuroprotection
Neurofilament protein (NFP) staining of SCI tissue from sham-operated, vehicle-treated, and ENO-treated (100μL at 15min, 24h, and 3d post-injury) rats harvested 7d post-injury indicate a significant (p=0.002) decrease in neuronal cytoskeleton following injury (Figure 4, A and B). This decrease is significantly attenuated with prolonged ENOblock treatment. An increased expression of NFP was detected by IHC with the recovery of neuronal cytoskeleton in ENOblock treated rats as compared to vehicle treated controls, indicating that enolase inhibition favors neuroprotection in SCI (Figure 4B). Chondroitin sulfate proteoglycan (CSPG) expression was significantly (p=0.031) increased following SCI (Figure 4, C and D). This increase was markedly attenuated by ENOblock treatment which indicates possible degradation of the glial scar, a major obstacle to functional and axonal recovery in SCI, in association with enolase inhibition.
Fig. 4.
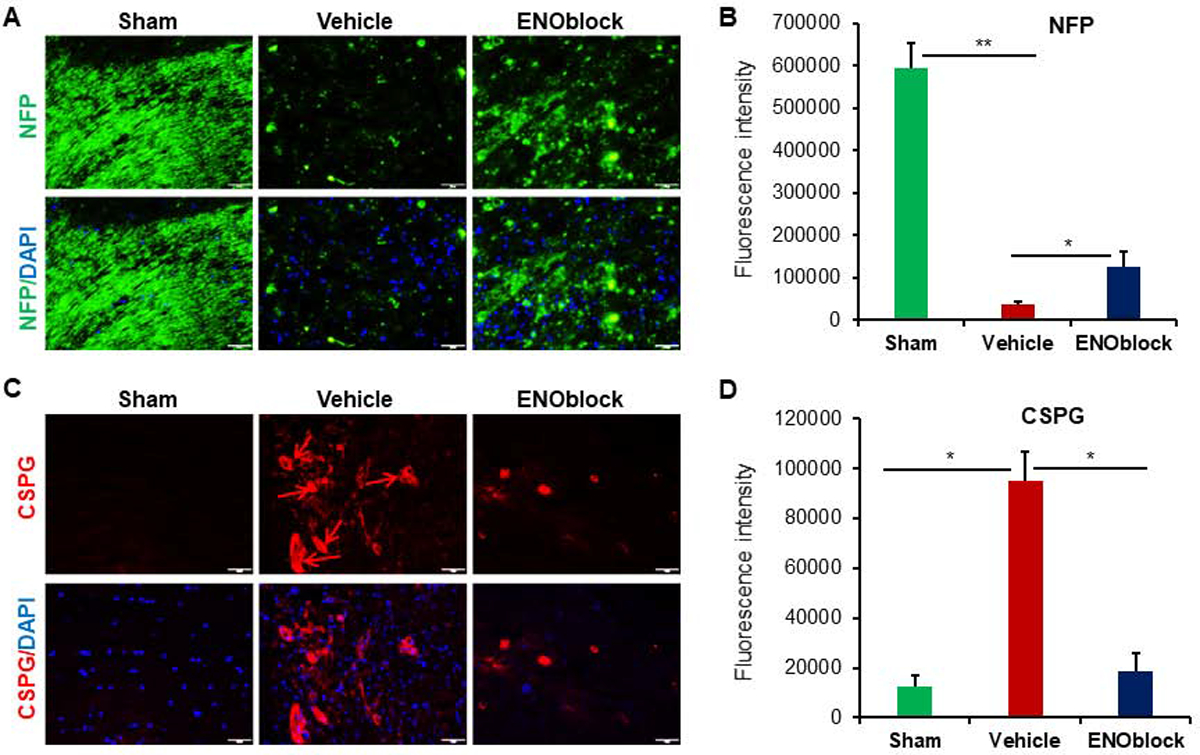
ENOblock treatment induces neuroprotection in SCI. (A) Neurofilament protein (NFP, green) and DAPI (blue) nuclear staining of SCI tissue at the injury site. Panel shows representative images taken at 20× magnification. (B) Mean fluorescence intensity from three representative areas shows significantly decreased NFP expression in vehicle treated tissue. There is also a significant increase in NFP expression indicating the recovery with ENOblock treatment as compared to vehicle treated controls (paired t-test, two-tailed, *p < 0.05, **p<0.01; n=3–5). (C) Chondroitin sulfate proteoglycan (CSPG, red; arrows) and DAPI (blue) nuclear staining of SCI tissue at the injury site. Representative images were taken at 20× magnification. (D) Mean fluorescence intensity shows a significant increase in CSPG in vehicle treated rats that is significantly reduced with ENOblock treatment (paired t-test, two-tailed, *p < 0.05; n=4–5).
The influence of ENOblock was also tested after spinal cord contusion by western blot analysis. Active form of caspase-1 protein was significantly attenuated by ENOblock (Figure 5A), suggesting a reduction of inflammation in SCI. Remyelination in the spinal cord may also correlate with increased expression of the neuronal nuclear protein NeuN. Indeed, NeuN expression was significantly increased after ENOblock treatment (Figure 5B), suggesting a possible improvement in neuronal survival after SCI. Secondary inflammation after SCI often induce demyelination and protection of myelin impacts the recovery of neurological function. Thus, the effects of ENObloc on MBP protein expression in injured spinal cord were also tested, which showed increased levels of MBP in treated rats (Figure 5C), suggesting that ENOblock protects myelin in injured spinal cord.
Fig. 5.
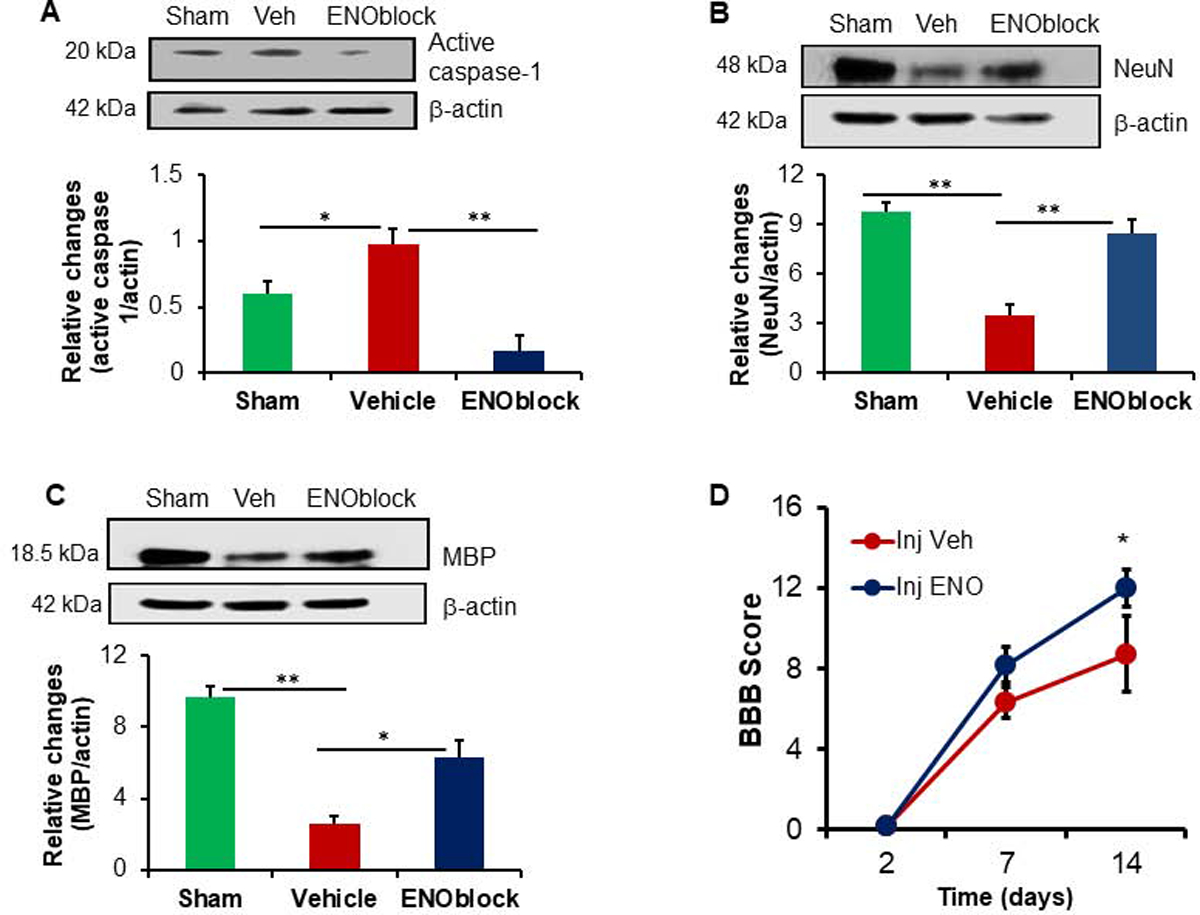
ENOblock treatment decreases active caspase-1, increases NeuN and MBP, and improves functional outcome in SCI rats. Spinal cord samples from sham, vehicle and ENOblock treated animals were subjected to western blotting for active forms of caspase-1 (A), NeuN (B), and MBP (C) as shown in the upper panels. Densitometric analysis (A, lower panel) showed that active caspase-1 was elevated in injured rats, which was significantly downregulated following ENOblock treatment. By contrast, NeuN (B, lower panel) and MBP (C, lower panel) proteins were significantly downregulated after SCI, and that ENOblock treatment protected these proteins in SCI rats. (One-way ANOVA, *p<0.05, **p<0.01; n=3–4). (D) Assessment of BBB scores in the SCI rats. An improved BBB score is found in the ENOblock treated group (Injection + ENOblock), as compared with vehicle treated group (Injection + Vehicle) on Day 14. Sham animals, scored 21 at each time point, are not displayed. Data suggest that there is a significant improvement in functional outcome in ENOblock treated rats following SCI. (One-way ANOVA with Fisher’s post-hoc test, n=5–8, *p<0.05).
3.5. ENOblock treatment improves motor function in SCI rats
To investigate whether reduced CSPG as well as increased NFP by ENOblock correlated with improved motor function, rats treated with either vehicle alone or ENOblock after SCI. BBB scores were recorded as described in the methods until sacrifice. BBB scores recorded in appropriate controls were compared with the ENOblock treated group, which showed a significant functional improvement in ENOblock treated rats at Day 14 (Figure 5D). While all other parameters in the PRM treated rats were analyzed at Day 7, BBB scores were monitored on Day 2, Day 7 and Day 14 because the marked differences in functional outcomes between control and treatment groups are commonly detected around Day 14 or later. Results showed that an improved BBB score was observed in ENOblock treated rats at Day 14, further indicating the importance of enolase inhibition in SCI.
4. Discussion
In our analysis of the effects of enolase inhibition on metabolic factors, inflammation, and gliosis in SCI, we have shown for the first time that ENOblock treatment reduces inflammation following injury, significantly increases levels of Cox-2, caspase-1, vimentin, insulin, glucagon, GLP-1, GIP, and PYY hormones, reduces activation of microglia and astrocytes, and supports neuroprotection. The data presented herein supports our hypothesis that enolase/NSE inhibition via ENOblock treatment in SCI (injured section, S-3) increases the concentration of metabolic hormones with anti-inflammatory effects in plasma and attenuates gliosis. Interestingly, ENOblock treatment also attenuated the effects of SCI at a distant site (S-4 section) significantly diminished inflammatory markers Cox-2 and caspase-1. Enolase/NSE has been identified as a marker of functional damage to neurons in several neurodegenerative conditions including SCI, TBI, and cardiac arrest (Li et al., 2014; Martens et al., 1998; Pfeifer et al., 2005; Thelin et al., 2016). NSE is associated with upregulation of pro-inflammatory chemokines/cytokines and gliosis in acute SCI, and NSE inhibition via ENOblock has been implicated as a potential therapeutic strategy for reducing these secondary SCI mechanisms (Cox et al., 2015a; Hafner et al., 2013; Haque et al., 2017b; Haque et al., 2016; Li et al., 2014; Polcyn et al., 2017a, b). The current study builds upon our previous studies on SCI to demonstrate that prolonged administration of ENOblock alters key metabolic and inflammatory factors, glial scar formation, and contributes to improved neuroprotection and motor function.
In glucose metabolism, enolase catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate (Haque et al., 2017b). Incoming nutrients trigger the secretion of incretins, GLP-1 and GIP, from intestinal cells to promote the production and secretion of insulin from pancreatic β cells (Spielman, 2014). Insulin then promotes glucose storage in muscles, liver, and adipose tissue as glycogen. Glucagon activity runs counter to insulin by triggering the breakdown of glycogen into glucose in the liver and its subsequent release into the bloodstream (Insuela and Carvalho, 2017). Cyclic AMP (cAMP) signaling mediates upregulation of insulin secretion, but cAMP formation is inhibited by anorectic hormones, PP and PYY, which are secreted in response to anticipation of a meal or mechanical stimulation from food in the gastrointestinal (GI) tract to communicate satiety to the CNS and control food intake (Gheni et al., 2014; Simpson et al., 2012). In the CNS, insulin activates PI3K/MAPK pathways with both neuroprotective and pro-inflammatory effects, and it has recently been shown that insulin and incretins may inhibit pathological processes in several neuroinflammatory CNS diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and schizophrenia through anti-apoptotic and growth-regulating signaling cascades (Spielman, 2014). Additionally, glucagon and GLP-1 have been shown to have anti-inflammatory properties in type 2 diabetes mellitus (T2DM) (Insuela and Carvalho, 2017). While GIP receptors have only been found on neurons, GLP-1 receptors (GLP-1R) are found on neurons in the brains of rodents and humans and on microglia and astrocytes in mice (Holscher, 2014; Spielman, 2014).
Insulin has been focused on as a potential treatment target for several neurodegenerative diseases based on the presence of insulin dysfunction in many CNS disorders, the observed alleviation of glutamate-induced cytotoxic damage to SH-SY5Y neuroblastoma cells with insulin treatment, and the link between intracerebroventricular insulin infusion and enhanced learning and memory in a dose-dependent manner in rodents (Haj-ali et al., 2009; Nampoothiri et al., 2014; Spielman, 2014). Insulin can cross the BBB to effect neurons, microglia, and astrocytes via an active transport mechanism (Spielman, 2014). It has roles in cellular growth and differentiation, promoting myelination via its receptor (IGF), enhanced memory formation, and activation of glycogen synthesis. In monocytes, insulin negatively regulates nuclear factor kappa B (NFκB) which reduces the production of pro-inflammatory cytokines and generation of reactive oxygen species (ROS) – both pathological processes involved in secondary injury mechanisms of SCI. The increased insulin observed in association with ENOblock treatment indicates that enolase inhibition could lead to insulin upregulation to result in a reduction of cytotoxic damage from excess glutamate following injury. This connection could have important clinical implications, particularly myelination in SCI, and should be explored further in future studies.
GLP-1 is able to cross the blood-brain barrier, and three GLP-1 mimetics are currently used for treatment of type 2 diabetes mellitus (T2DM) and are in clinical trials for Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Holscher, 2014). Insulin desensitization is believed to underly the neurodegenerative process of AD and treatment with liraglutide, a GLP-1 analog, can have neuroprotective effects. These effects are mediated by PKB/Akt and MAPK/ERK pathways as well as liraglutide stimulation of the GLP-1R, which leads to an increase in cAMP signaling, and can result in activation of calcium channels, cell survival, inhibition of apoptosis, and cell growth and repair. As mentioned in the analysis of Figure 3A, the significant increase in GLP-1 levels in ENOblock treated rats as compared to vehicle treated controls indicates an anti-inflammatory role for enolase inhibition (p=0.0022). In peripheral inflammation, GLP-1 reduces macrophage infiltration into adipose tissue and inhibits the production of IL-6, TNF-α, and MCP-1 from adipocytes for anti-inflammatory effect (Spielman, 2014). Glucagon and GLP-1 are almost 50% homologous, but GLP-1 mainly functions to reduce blood glucose while glucagon acts to increase blood glucose (Insuela and Carvalho, 2017). Due to their physiologically opposed roles, it would be expected that the increase in GLP-1 levels would correspond to a decrease in glucagon levels. However, our analysis of the ENO-treated rat plasma indicated a significant increase in glucagon levels as compared to the vehicle-treated levels (Figure 4A, p=0.0022).
Anorectic hormones are metabolic enzymes that are secreted in response to anticipation of a meal or mechanical stimulation from food in the gastrointestinal (GI) tract (Holzer et al., 2012; Simpson et al., 2012). Upon secretion, these enzymes can activate signaling pathways along the gut-brain axis to communicate satiety and terminate eating. The control of food intake involves communication between the gut and brain (CNS), which can occur via the vagus nerve as a neuroanatomical link. Some anorectic hormones can also bind to receptors in the hypothalamus and brainstem via the blood-brain barrier (BBB). Pancreatic polypeptide (PP) and peptide tyrosine tyrosine (PYY) are two such anorectic enzymes which were remarkably altered following ENOblock treatment. Upregulation of PYY would suggest an inhibition of cAMP and therefore decrease in incretin activity and insulin secretion. Thus, the observed increase in glucagon could help to counteract the cAMP inhibition from upregulated PYY. Potential explanations for this discrepancy should be investigated further in future studies.
In this paper, multisystemic beneficial effects of ENOblock treatment have been shown in a rat SCI model. The observations made in this study indicate a significant impact of ENOblock treatment on metabolic enzymes which should be explored further for clinical consideration of potential effects on metabolism, weight maintenance, and appetite in treatment of SCI. While systemic administration of ENOblock treatment has significant effects on metabolic hormones, a more targeted delivery approach to the injury site might reduce these effects. It is also important to note that enolase inhibition reduced activation of microglia and astrocytes, reduction of CSPG level, and increased neurofilament protein expression in SCI in support of neuronal survival and neuroprotection. The attenuation of CSPG expression indicating promotion of axonal regeneration/regrowth and increased expression of axonal neurofilaments protein suggesting preservation of axon at the injury site predicts their important role for improvement of function following SCI. Their functional roles may be further studied elaborately at the cellular and molecular level in chronic (4–6 weeks) SCI. Nonetheless, these current results indicate a neuroprotective role for ENOblock that favors neuronal cell survival in SCI and should be studied for further mechanistic understanding. It would be interesting to investigate the extent of neuroprotection associated with ENOblock treatment and compare this to functional recovery data to determine the related changes. Additional studies should also focus on analyzing the effects of ENOblock treatment on human primary microglia and astrocytes to determine whether a similar reduction in gliosis can be observed across species and advance this therapeutic further towards clinical evaluation. This new discovery of the benefits of ENOblock treatment on neuroinflammation, gliosis, metabolic hormones, and neuroprotection within the 7d window following spinal cord injury reinforces the importance of early intervention in SCI to enhance treatment efficacy and indicates the potential for ENOblock treatment as an effective inhibitor of several factors involved in the secondary injury mechanisms of SCI. Because demyelination is one of the most important pathological factors of SCI, restoring myelin by ENOblock may contribute to the level of locomotor recovery.
Supplementary Material
Fig. S1. ENOblock treatment significantly inhibits inflammation in SCI. (A) Cox-2 (green) and DAPI (blue) nuclear staining of SCI tissue at the injury site 7d post injury were shown in vehicle-treated and ENOblock-treated rats. Representative images were taken at 20X magnification. (B) Fluorescence intensity from three representative areas showed Cox-2 expression was significantly reduced by ENOblock-treatment (paired t-test, two-tailed, *p < 0.05). (C) Caspase-1 (green) staining of SCI tissue. DAPI (blue) was used for nuclear staining. (D) Fluorescence intensity from three representative areas of immunohistochemistry showed that caspase-1 expression was significantly attenuated by ENOblock treatment (paired t-test, two-tailed, *p < 0.05; n=3–6), bar=100μM.
Highlights.
Enolase expression and activity is increased after spinal cord injury (SCI) in rats, promoting inflammation and damage
Elevation of enolase upregulates the production of inflammatory cytokines and chemokines, and metabolic factors in SCI
Enolase inhibition reduces pro-inflammatory cytokines and chemokines, alters metabolic factors, attenuating gliosis in SCI.
Enolase inhibitor (ENOblock) treatment attenuates chondroitin sulfate proteoglycan (CSPG) formation and inflammation, preserving myelination in SCI
Inhibition of enolase activation enhances neuroprotection and improves motor function in SCI.
Acknowledgements
This study was made possible by grant from the South Carolina Spinal Cord Injury Research Funds (SCIRF#2016 I-03 and SCIRF #2018 I-01) to AH. Contents do not necessarily represent the policy of the SCIRF and do not imply endorsement by the funding agency. This work was conducted in a facility constructed with support from the National Institutes of Health, Grant Number C06 RR015455. This work was also supported by grants from the Ralph H. Johnson Veterans Administration Medical Center (1I01BX002349-01, 2I01 BX001262-05, 1I01 BX004269-01, and NIH-NINDS R01 NS62327) to NLB. We thank Ms. Margaret Romano of Histology and Immunohistochemistry Laboratory at the Department of Pathology and Laboratory Medicine at MUSC for her technical assistance with the immunohistochemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no financial conflicts of interest.
References
- Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M, Fujino MA, Niijima A, Meguid MM, Kasuga M, 2003. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology 124, 1325–1336. [DOI] [PubMed] [Google Scholar]
- Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W, 2017. Cell transplantation therapy for spinal cord injury. Nat Neurosci 20, 637–647. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB, 2002. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640. [DOI] [PubMed] [Google Scholar]
- Center, N.S.C.I.S., 2017. Facts and Figures at a Glance. University of Alabama at Birmingham, Birmingham AL. [Google Scholar]
- Chandran R, Capone M, Matzelle D, Polcyn R, Kau E, Haque A, Banik NL, 2018. Distinct Cytokine and Chemokine Expression in Plasma and Calpeptin-Treated PBMCs of a Relapsing-Remitting Multiple Sclerosis Patient: A Case Report. Neurochem Res 43, 2224–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C, Williams SG, Xu L, Chen J, Wallner LP, Porter KR, Jacobsen SJ, 2013. Statin therapy is not associated with prostate cancer recurrence among patients who underwent radiation therapy. Cancer letters 335, 214–218. [DOI] [PubMed] [Google Scholar]
- Cox A, Varma A, Banik N, 2015a. Recent advances in the pharmacologic treatment of spinal cord injury. Metab Brain Dis 30, 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A, Varma A, Barry J, Vertegel A, Banik N, 2015b. Nanoparticle Estrogen in Rat Spinal Cord Injury Elicits Rapid Anti-Inflammatory Effects in Plasma, Cerebrospinal Fluid, and Tissue. Journal of neurotrauma 32, 1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto JP, Yang J, Dietrich WD, Pearse DD, 2015. Does being female provide a neuroprotective advantage following spinal cord injury? Neural regeneration research 10, 1533–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E, Aguilar-Cevallos J, Silva-Garcia R, Ibarra A, 2016. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediators of inflammation 2016, 9476020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Zhang B, 2015. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res 1619, 1–11. [DOI] [PubMed] [Google Scholar]
- Gheni G, Ogura M, Iwasaki M, Yokoi N, Minami K, Nakayama Y, Harada K, Hastoy B, Wu X, Takahashi H, Kimura K, Matsubara T, Hoshikawa R, Hatano N, Sugawara K, Shibasaki T, Inagaki N, Bamba T, Mizoguchi A, Fukusaki E, Rorsman P, Seino S, 2014. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep 9, 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- God JM, Cameron C, Figueroa J, Amria S, Hossain A, Kempkes B, Bornkamm GW, Stuart RK, Blum JS, Haque A, 2015. Elevation of c-MYC disrupts HLA class II-mediated immune recognition of human B cell tumors. Journal of immunology 194, 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein OG, Hajiaghamohseni LM, Amria S, Sundaram K, Reddy SV, Haque A, 2008. Gamma-IFN-inducible-lysosomal thiol reductase modulates acidic proteases and HLA class II antigen processing in melanoma. Cancer immunology, immunotherapy : CII 57, 1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hassler SE, Hulsebosch CE, 2013. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain 154, 1699–1708. [DOI] [PubMed] [Google Scholar]
- Hafner A, Glavan G, Obermajer N, Zivin M, Schliebs R, Kos J, 2013. Neuroprotective role of gamma-enolase in microglia in a mouse model of Alzheimer’s disease is regulated by cathepsin X. Aging Cell 12, 604–614. [DOI] [PubMed] [Google Scholar]
- Hafner A, Obermajer N, Kos J, 2012. gamma-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. The Biochemical journal 443, 439–450. [DOI] [PubMed] [Google Scholar]
- Haj-ali V, Mohaddes G, Babri SH, 2009. Intracerebroventricular insulin improves spatial learning and memory in male Wistar rats. Behav Neurosci 123, 1309–1314. [DOI] [PubMed] [Google Scholar]
- Haque A, Capone M, Matzelle D, Cox A, Banik NL, 2017a. Targeting Enolase in Reducing Secondary Damage in Acute Spinal Cord Injury in Rats. Neurochemical research 42, 2777–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Capone M, Matzelle D, Cox A, Banik NL, 2017b. Targeting Enolase in Reducing Secondary Damage in Acute Spinal Cord Injury in Rats. Neurochem Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Das A, Hajiaghamohseni LM, Younger A, Banik NL, Ray SK, 2007. Induction of apoptosis and immune response by all-trans retinoic acid plus interferon-gamma in human malignant glioblastoma T98G and U87MG cells. Cancer immunology, immunotherapy : CII 56, 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Polcyn R, Matzelle D, Banik NL, 2018. New Insights into the Role of Neuron-Specific Enolase in Neuro-Inflammation, Neurodegeneration, and Neuroprotection. Brain sciences 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Ray SK, Cox A, Banik NL, 2016. Neuron specific enolase: a promising therapeutic target in acute spinal cord injury. Metabolic brain disease 31, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque MA, Li P, Jackson SK, Zarour HM, Hawes JW, Phan UT, Maric M, Cresswell P, Blum JS, 2002. Absence of gamma-interferon-inducible lysosomal thiol reductase in melanomas disrupts T cell recognition of select immunodominant epitopes. The Journal of experimental medicine 195, 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway-Schrader JD, Doonan BP, Hossain A, Radwan FFY, Zhang L, Haque A, 2018. Autophagy-dependent crosstalk between GILT and PAX-3 influences radiation sensitivity of human melanoma cells. Journal of cellular biochemistry 119, 2212–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C, 2014. The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer’s disease. Alzheimers Dement 10, S47–54. [DOI] [PubMed] [Google Scholar]
- Holzer P, Reichmann F, Farzi A, 2012. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 46, 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insuela DBR, Carvalho VF, 2017. Glucagon and glucagon-like peptide-1 as novel anti-inflammatory and immunomodulatory compounds. Eur J Pharmacol 812, 64–72. [DOI] [PubMed] [Google Scholar]
- Karra E, Chandarana K, Batterham RL, 2009. The role of peptide YY in appetite regulation and obesity. The Journal of physiology 587, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, Held-Feindt J, 2015. Chemokine-ligands/receptors: multiplayers in traumatic spinal cord injury. Mediators of inflammation 2015, 486758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wen H, Yan Z, Ding T, Long L, Qin H, Wang H, Zhang F, 2014. Temporal-spatial expression of ENOLASE after acute spinal cord injury in adult rats. Neuroscience research 79, 76–82. [DOI] [PubMed] [Google Scholar]
- Martens P, Raabe A, Johnsson P, 1998. Serum S-100 and neuron-specific enolase for prediction of regaining consciousness after global cerebral ischemia. Stroke; a journal of cerebral circulation 29, 2363–2366. [DOI] [PubMed] [Google Scholar]
- Moeendarbary E, Weber IP, Sheridan GK, Koser DE, Soleman S, Haenzi B, Bradbury EJ, Fawcett J, Franze K, 2017. The soft mechanical signature of glial scars in the central nervous system. Nature communications 8, 14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhamedshina YO, Akhmetzyanova ER, Martynova EV, Khaiboullina SF, Galieva LR, Rizvanov AA, 2017. Systemic and Local Cytokine Profile following Spinal Cord Injury in Rats: A Multiplex Analysis. Frontiers in neurology 8, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nampoothiri M, Reddy ND, John J, Kumar N, Kutty Nampurath G, Rao Chamallamudi M, 2014. Insulin blocks glutamate-induced neurotoxicity in differentiated SH-SY5Y neuronal cells. Behav Neurol 2014, 674164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell PW, Haque A, Klemsz MJ, Kaplan MH, Blum JS, 2004. Cutting edge: induction of the antigen-processing enzyme IFN-gamma-inducible lysosomal thiol reductase in melanoma cells Is STAT1-dependent but CIITA-independent. Journal of immunology 173, 731–735. [DOI] [PubMed] [Google Scholar]
- Okada S, Hara M, Kobayakawa K, Matsumoto Y, Nakashima Y, 2018. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res 126, 39–43. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H, 2006. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12, 829–834. [DOI] [PubMed] [Google Scholar]
- Orlandin JR, Ambrosio CE, Lara VM, 2017. Glial scar-modulation as therapeutic tool in spinal cord injury in animal models. Acta cirurgica brasileira 32, 168–174. [DOI] [PubMed] [Google Scholar]
- Patil N, Truong V, Holmberg MH, Lavoie NS, McCoy MR, Dutton JR, Holmberg EG, Parr AM, 2018. Safety and Efficacy of Rose Bengal Derivatives for Glial Scar Ablation in Chronic Spinal Cord Injury. Journal of neurotrauma 35, 1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perot PL Jr., Lee WA, Hsu CY, Hogan EL, Cox RD, Gross AJ, 1987. Therapeutic model for experimental spinal cord injury in the rat: I. Mortality and motor deficit. Cent Nerv Syst Trauma 4, 149–159. [DOI] [PubMed] [Google Scholar]
- Pfeifer R, Borner A, Krack A, Sigusch HH, Surber R, Figulla HR, 2005. Outcome after cardiac arrest: predictive values and limitations of the neuroproteins neuron-specific enolase and protein S-100 and the Glasgow Coma Scale. Resuscitation 65, 49–55. [DOI] [PubMed] [Google Scholar]
- Polcyn R, Capone M, Hossain A, Matzelle D, Banik NL, Haque A, 2017a. Enolase and Acute Spinal Cord Injury. Journal of clinical & cellular immunology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcyn R, Capone M, Hossain A, Matzelle D, Banik NL, Haque A, 2017b. Neuron specific enolase is a potential target for regulating neuronal cell survival and death: implications in neurodegeneration and regeneration. Neuroimmunology and neuroinflammation 4, 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan FF, Zhang L, Hossain A, Doonan BP, God JM, Haque A, 2012. Mechanisms regulating enhanced human leukocyte antigen class II-mediated CD4 + T cell recognition of human B-cell lymphoma by resveratrol. Leuk Lymphoma 53, 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Das A, Matzelle DC, Yu SP, Wei L, Varma A, Ray SK, Banik NL, 2016. Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. Journal of neurochemistry 137, 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Knaryan VH, Shields DC, Cox AA, Haque A, Banik NL, 2015. Inhibition of Calpain Activation Protects MPTP-Induced Nigral and Spinal Cord Neurodegeneration, Reduces Inflammation, and Improves Gait Dynamics in Mice. Molecular neurobiology 52, 1054–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FM, Mergl R, Stach B, Jahn I, Gertz HJ, Schonknecht P, 2014. Elevated levels of cerebrospinal fluid neuron-specific enolase (NSE) in Alzheimer’s disease. Neurosci Lett 570, 81–85. [DOI] [PubMed] [Google Scholar]
- Simpson K, Parker J, Plumer J, Bloom S, 2012. CCK, PYY and PP: the control of energy balance. Handb Exp Pharmacol, 209–230. [DOI] [PubMed] [Google Scholar]
- Spielman LKA, 2014. The role of insulin and incretins in neuroinflammation and neurodegeneration. Immunoendocrinology 1, e391. [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL, 2006. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. Journal of neuroscience research 84, 1064–1075. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, Banik NL, 2010. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. Journal of neuroscience research 88, 1738–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin EP, Jeppsson E, Frostell A, Svensson M, Mondello S, Bellander BM, Nelson DW, 2016. Utility of neuron-specific enolase in traumatic brain injury; relations to S100B levels, outcome, and extracranial injury severity. Crit Care 20, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma AK, Das A, Wallace G.t., Barry J, Vertegel AA, Ray SK, Banik NL, 2013. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res 38, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visavadiya NP, Patel SP, VanRooyen JL, Sullivan PG, Rabchevsky AG, 2016. Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox biology 8, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizin T, Kos J, 2015. Gamma-enolase: a well-known tumour marker, with a less-known role in cancer. Radiol Oncol 49, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu P, Chen X, Geng Q, Lu Y, 2012. Serum neuron-specific enolase is correlated with clinical outcome of patients with non-germinal center B cell-like subtype of diffuse large B-cell lymphoma treated with rituximab-based immunochemotherapy. Med Oncol 29, 2153–2158. [DOI] [PubMed] [Google Scholar]
- Yuan YM, He C, 2013. The glial scar in spinal cord injury and repair. Neuroscience bulletin 29, 421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Amria S, Hossain A, Sundaram K, Komlosi P, Nagarkatti M, Haque A, 2011. Enhancement of HLA class II-restricted CD4+ T cell recognition of human melanoma cells following treatment with bryostatin-1. Cellular immunology 271, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. ENOblock treatment significantly inhibits inflammation in SCI. (A) Cox-2 (green) and DAPI (blue) nuclear staining of SCI tissue at the injury site 7d post injury were shown in vehicle-treated and ENOblock-treated rats. Representative images were taken at 20X magnification. (B) Fluorescence intensity from three representative areas showed Cox-2 expression was significantly reduced by ENOblock-treatment (paired t-test, two-tailed, *p < 0.05). (C) Caspase-1 (green) staining of SCI tissue. DAPI (blue) was used for nuclear staining. (D) Fluorescence intensity from three representative areas of immunohistochemistry showed that caspase-1 expression was significantly attenuated by ENOblock treatment (paired t-test, two-tailed, *p < 0.05; n=3–6), bar=100μM.


