Abstract
Epithelial morphogenesis relies on constituent cells’ ability to finely tune their mechanical properties. Resulting elastic-like and viscous-like behaviors arise from mechanochemical signaling coordinated spatiotemporally at cell–cell interfaces. Direct measurement of junction rheology can mechanistically dissect mechanical deformations and their molecular origins. However, the physical basis of junction viscoelasticity has only recently become experimentally tractable. Pioneering studies have uncovered exciting findings on the nature of contractile forces and junction deformations, inspiring a fundamentally new way of understanding morphogenesis. Here, we discuss novel techniques that directly test junctional mechanics and describe the relevant Vertex Models, and adaptations thereof, capturing these data. We then present the concept of adaptive tissue viscoelasticity, revealed by optogenetic junction manipulation. Finally, we offer future perspectives on this rapidly evolving field describing the material basis of tissue morphogenesis.
Introduction
Morphogenesis is defined as the suite of underlying biological processes orchestrating the dynamic formation of macroscopic shapes in biological matter. Macroscopic tissue movements ultimately arise from the mesoscopic properties of cell–cell junctions, coordinated in both space and time. Such developmental mechanisms occur simultaneously or in sequence, involving the interplay between cell shape and mechanical forces, as well as genetic and molecular cues. The result is a cascade of interrelated events spanning the molecular, cellular, and tissue scales, all of which combine to generate organismal shape. Accordingly, a holistic view of embryogenesis necessitates the unification of both junction mechanics and molecular genetics. Developmental biology is therefore an increasingly interdisciplinary science, applying both biology and physics to investigate the contribution of mechanical forces and their underlying mechanochemical regulation at cell–cell interfaces.
Live cell imaging has produced comprehensive information on stable junction deformations observed in epithelial morphogenesis. Morphogenetic events involve highly dynamic Rho activity that signals to downstream actomyosin necessary in generating mechanical force [1]. To date, most studies have relied on the visualization of Rho, its downstream effectors, and junctional components, to correlate cell shape changes with junction deformations [1–7]. Pulsatile medioapical Rho and non-muscle myosin II (NMII) oscillate and flow to junctional interfaces on the order of minutes, followed by several minutes rest [1]. The result of these pulsatile behaviors is a junctional ratchet whereby junctions contract at a rate of about 1–2 micrometers a minute, stabilize, and then briefly expand before the next round of contractions [1,2,6,8–10]. Fluorescence recovery after photobleaching (FRAP) experiments of NMII turnover have revealed that NMII is stabilized at the cortex in regions of increased mechanical tension, offering a positive feedback loop in the generation of stable contractions within the junctional ratchet [5]. Additionally, NMII’s phosphorylation state ensures progressive interface shortening [6]. These data have led to the idea that spatiotemporal increases in mechanical tension mediate stable junction deformations [11••]. However, this hypothesis has been difficult to directly test at local intercellular junctions.
Much of the literature has used genetic or pharmacological means to globally inhibit tissue tension, completely disrupting any junctional deformations entirely. These perturbations are slow in timescale and broad in effect, often destroying any spatiotemporal feedbacks of the actomyosin network’s response. As such, the contributions of local force production to junction deformations remain unclear. New techniques are therefore necessary to directly modulate the spatiotemporal, tensional changes that drive morphogenetic processes. Here, we review the relevant literature and summarize the technological advancements exploring junctional mechanics. We first describe the canonical Vertex model, which has provided a simple mathematical framework for understanding tissue mechanical deformation. Next, we examine more advanced models and methods that have directly modulated junction lengths, including optical tweezers, rheological stretching, and optogenetics. We then describe a new phenomenon emerging in the data describing feedbacks between strain and tension, giving rise to the concept of adaptive mechanics of epithelial cell junctions.
Canonical vertex models of epithelial cell junctions
Vertex models of epithelial tissues characterize the mechanical properties of adherent cells with the following parameters: a tension at cell–cell interfaces arising from cortical actomyosin coupled to adhesive structures, a bulk tension that arises from medial actomyosin, and an area elasticity that counteracts changes in cell area (Figure 1a,b) [12]. Cell shapes are thus generated by the competition between elasticity and tension, further specified by the variables describing the area elastic modulus, edge tension, and bulk tension (Figure 1b)[13]. Each cell is defined by a polygon with cell-cell junctions represented by rectilinear edges connecting the tricellular vertices (Figure 1a,b) [13,14]. Vertices can then move in response to forces due to growth, interfacial tension, and pressure within each cell [14]. The positions of these vertices, the connections between them, and the resulting forces between vertices completely determine the shape and mechanics of the epithelial cell sheet [15].
Figure 1.
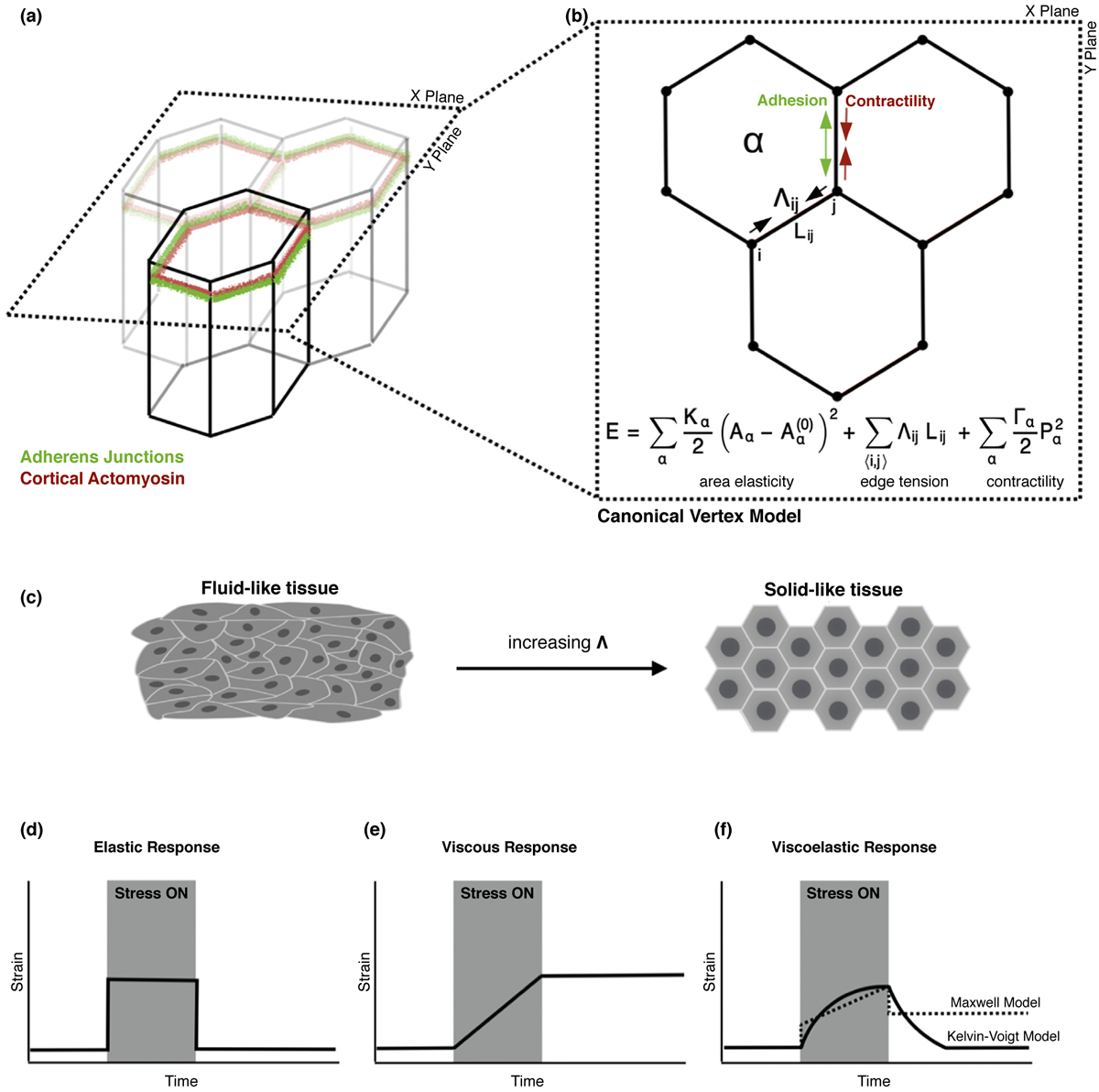
Mechanical models of epithelial tissues and viscoelasticity. (a) Schematic of a confluent epithelial monolayer. Cells are connected at their interfaces by adherens junctions, while the cytoskeleton produces contractile forces. (b) Schematic of the 2D vertex model, which models the apical network of junctions as linear edges, and each cell as a polygon. The mechanical energy of the tissue has contributions from cell area elasticity, interfacial tension given by the balance between contractility and cell–cell adhesion which aims to expand the junction, and the contractility of the cell perimeter. (c) Interfacial tension regulates tissue viscoelasticity. At low tension, the tissue behaves like a fluid with zero shear modulus and cells take irregular shapes. At high tension, the tissue behaves like a jammed solid, with non-zero shear modulus and regular polygonal shapes. (d) In elastic materials, strain is proportional to stress. During applied stress (grey region) the material maintains a constant strain. After applied stress is removed, the material returns to its original shape. (e) In viscous materials, strain rate is proportional to stress. The material remains at rest after stress is removed and remains permanently deformed. (f) Viscoelastic materials display both elastic and viscous properties. A Kelvin-Voigt material (solid line) behaves like a viscous fluid at short times but elastically at long timescales, returning to its original shape after stress. A Maxwell material (dashed line) behaves elastically at short times and viscously at long, remaining permanently deformed after stress.
This vertex model can give rise to tissue properties with elastic or fluid-like behaviors depending on the relative magnitudes of interfacial tension (Λ) and contractility [13,16]. Increasing Λ leads to a solid-like tissue (Figure 1c, right), characterized by non-zero shear modulus, isotropic cell shapes, and persistent cell motion; tissues in a fluid-like state have vanishing shear modulus, anisotropic cell shapes, and randomized cell motions with frequent neighbor exchanges (Figure 1c, left) [16–18]. In solid-state tissues, vertex models predict a purely elastic response of cell-cell junctions upon applied stress, which we term as applied tension (Λa). That is, once stress is applied the junction will deform and once applied stress is removed the junction will recoil back to its initial length (Figures 1d and 3 b). For a fluid tissue, the junctions will exhibit no recoil and the tissue will remain permanently deformed (Figures 1e and 3 b) [19••]. While standard implementations of the vertex model are widely used, little has been done in the way of experimentally testing these junctional responses to mechanical force.
Figure 3.
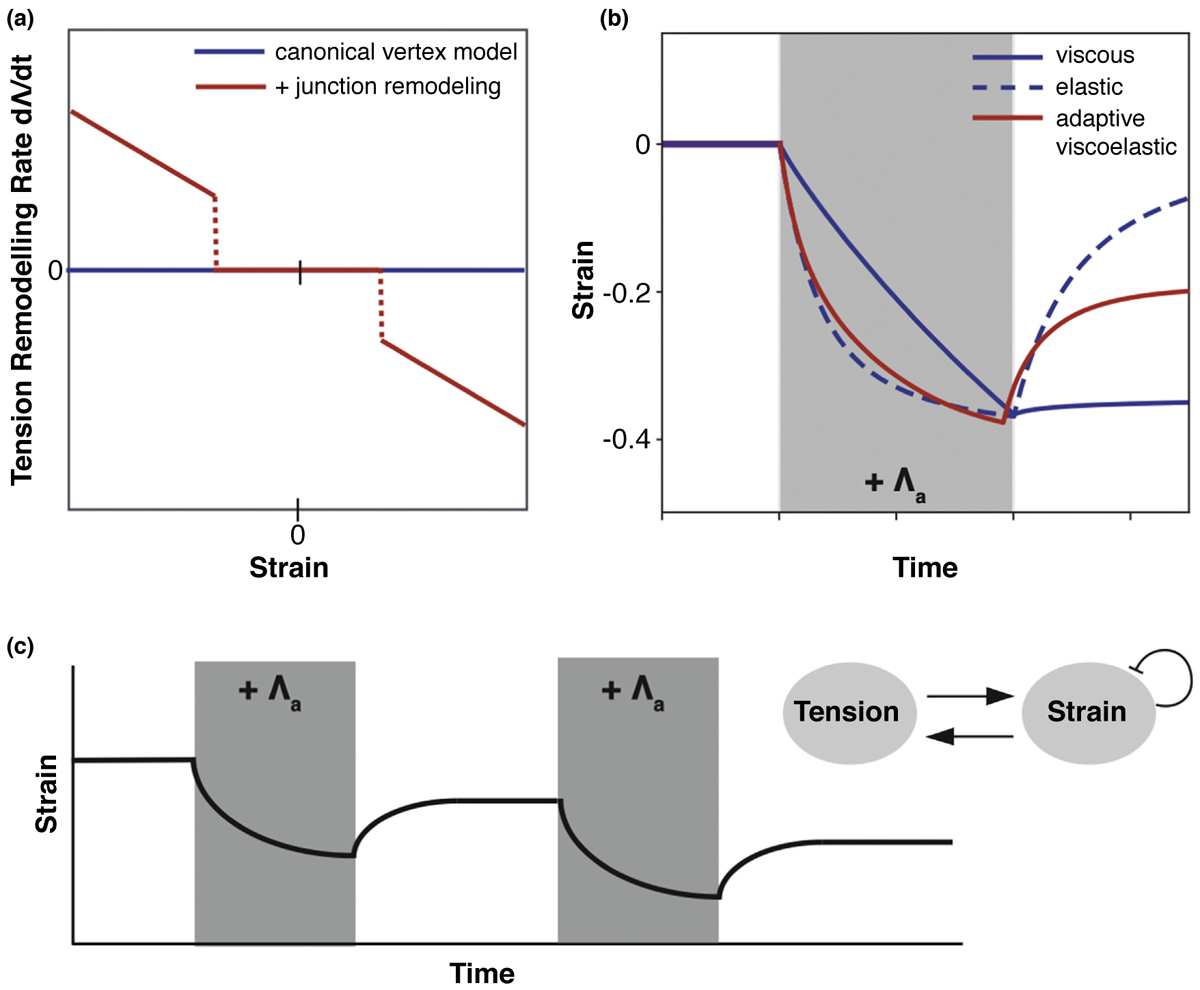
Active tension remodeling enables adaptive viscoelasticity in the vertex model. (a) Tension remodeling rate against junctional strain. In the canonical vertex model, tension remains constant. With junction remodeling, tension changes above a critical value of junction strain. (b) Junction strain over time in response to an applied tension, Λa (grey box). The canonical vertex model displays either viscous or elastic responses, if the tissue is in the fluid or solid phases respectively. With tension remodeling, the applied stress induces a strain on the junction which triggers tension remodeling. After stress removal, the junction tension remains increased and the junction is permanently shortened. (c) (Inset) Schematic of tension-strain feedback loop. Strain triggers remodeling of junctional tension. Tension induces strain on the junction, which is continuously relaxed, limiting the amount of remodeling under long contractions. (Main) Repeated contractions can further remodel the junction, past the single contraction limit.
Passive viscoelasticity in modeling cell–cell junctions
There is increasing experimental evidence for viscoelastic relaxation of junctions (Figure 1f) [11••,19••,20••,21••,22••,23]. For instance, cell–cell adhesion dynamics are implicated in epithelial stress dissipation. Work in the Drosophila wing disc has shown that there is an eight-hour delay between changes in endogenous stress and junction elongation, indicating a viscoelastic behavior. A Kelvin-Voigt material, a material that is viscous at short timescales but elastic at long timescales, was used to model the observed relationship between tissue stress and junction elongation(Figure 1f,solidline).It was found that mechanical stress-induced E-cadherin turnover sets tissue viscoelasticity, suggesting membrane and junctional remodeling tune cellular material properties [20••]. Here, E-cadherin was shown to stabilize junctions under low stress. When E-cadherin turnover is high, the junctions deform more rapidly under stress and show a shorter delay between stress and cell elongation. This is in line with recent work documenting how endocytosis regulates junction remodeling and elasticity [8,22••].
Local mechanical measurements of junctions have also revealed viscoelasticity resulting from actomyosin turnover. Using optical tweezers to locally manipulate junctions revealed important timescales in the mechanics of junction deformability in the Drosophila embryo [24]. Here, transient morphogenetic forces generated stable junction length changes if they lasted longer than a minute [11••]. Depending on the timescale of tweezing, junctions behaved like a Maxwell material: elastic at short timescales and fluid at longer timescales [11••] (Figure 1f, dashed lines). These behaviors were dependent on actomyosin to control dissipative mechanics, as embryo treatment with Cytochalasin D, which slows actomyosin turnover rates, resulted in an increased dissipation time-scale. Viscoelasticity seems to be a conserved feature of junctions, as work in the Chick embryo also shows viscoelastic deformations of cell–cell junctions dependent on NMII activity [23]. Together these data suggest that the turnover of actomyosin and adhesive structures tune stress dissipation necessary for junctional viscoelasticity, although they suggest a more passive viscoelastic model of cell–cell junctions.
Active viscoelasticity in modeling cell–cell junctions
Exciting new findings have uncovered a new model for junctional viscoelasticity, which we term as active viscoelasticity, in which cells and tissues respond to strain by changing their contractile tension [19••,20••,21••,22••,25••]. Certain feedback mechanisms therefore modulate the junction’s active tension and passive viscoelastic properties to confer junction length. What arose from these data were new and interesting junctional phenotypes dependent upon nature of the feedback in the system. As it became clearer that active viscoelasticity relied on the feedback between strain and tension, new methods and models have emerged to carefully dissect junction mechanics using quantitative and computational tools.
Recently, the Active Tension Network (ATN) model was proposed, which assumes that junctional tension dominates tissue dynamics. Within this model lies the concept of active tension remodeling, where elongated junctions increase NMII recruitment, and contracted junctions decrease NMII levels, thereby regulating tensional homeostasis [25••]. The ATN is consistent with recent work examining junction length changes within Drosophila Dorsal Closure (DC) [26••]. Junction length during DC is an active process, maintained by the coordinated action of mechanosensitive actomyosin localization and a straightness-dependent junctional removal system. Consistent with ATN predictions, squeezing embryos to stretch cell–cell junctions relocalized NMII from the medioapical pool to the cell–cell interfaces to drive subsequent remodeling. Here, junctional NMII levels were proportional to the amount to which the cell perimeters were stretched. Together these data suggest that NMII can ‘sense’ junction length, further indicating feedbacks between junction strain and tension.
Rheological studies on suspended epithelia have illustrated a similar active junctional viscoelasticity [27]. In these experiments, the monolayer experienced a period of strain before a period of relaxation. Here, stress dissipated in the cells within the monolayer, with the length of the tissue permanently elongating and buckling once strain was removed. Stepwise applications of stress showed that single cells and monolayers switched from a fluid-like behavior at second timescales to a solid-like behavior at the minute timescale. That both mono-layer and single cell rheology displayed similar features suggested a mechanism by which adhesive structures allowed the monolayer to behave like a single cell with its rheology controlled by actomyosin turnover [21••]. The resulting junctional relaxation period was successfully captured using a viscoelastic model, but in which the timescale for relaxation was strain dependent, as larger strains slowed remodeling rates. One interpretation is that as junctions were stretched further, cell area increased and thus actin concentrations in the cell decreased, reducing the rate of remodeling. Similar tissue stretching experiments using the Drosophila wing disc showed rapid, mechanosensitive changes to tissue stiffness and elasticity that were also dependent on actin turnover[28].
Optogenetics strategies to test adaptive junction mechanics
Cellular shape change is coincident with spatial variations in RhoA-mediated actomyosin contractility occurring at the junctional and medial subcellular zones [1,2,29–31]. Light-mediated protein dimerization in distinct subcellular compartments can spatially activate endogenous RhoA to test contractile zones’ function in regulating cell-cell junction viscoelasticity and deformability [22••]. Optogenetics is an emerging and powerful method that controls protein localization with focused light [32]. The principle behind these two component systems is simple: tether the photosensitive domain to the plasma membrane where RhoA sits inactive and photorecruit a RhoA-specific activator, or GEF, for local Rho activation (Figure 2a). A number of two-component optogenetic systems exist to test subcellular Rho-mediated contractility [32]. These include that of the iLID, TULIP, and CRY-2/CBN systems, which have been employed to localize RhoA in dividing, non-adherent, and adherent cells in culture, and more recently in tissue both in culture and in vivo [19••,22••,33–38].
Figure 2.
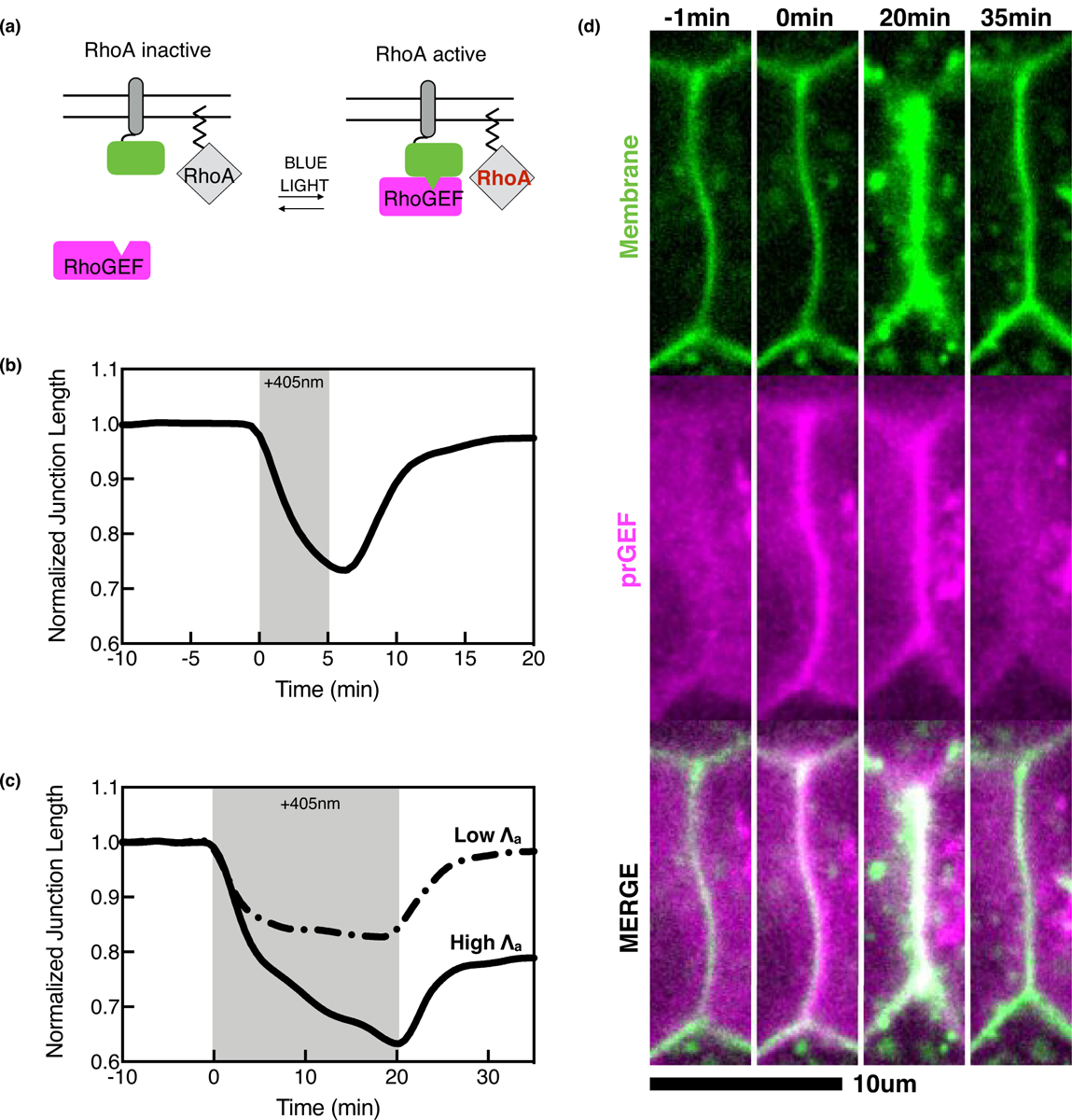
Optogenetic activation induces a stress dependence for epithelial cell junction deformations. (a) Schematic of the generalized optogenetic system. The photosensitive domain (green) is tethered to the membrane next to inactive RhoA. Blue light causes a conformational change in the photosensitive domain, recruiting an engineered, photorecruitable RhoGEF (prGEF, magenta) to the membrane to locally activate RhoA. (b) Average normalized junction length changes over time for a 5-minute optogenetic activation (indicated by +405 nm, grey box) (c) Average normalized junction length changes for 20-minute optogenetic activations with low and high applied tension, Λa. With high Λa, the junctions display irreversible junction deformations (solid line) and low Λa display reversible deformations (dashed line) after optogenetic activation (grey box). (d) Representative images of an optogenetic experiment with a 20-minute activation at high Λa shown in C (solid black line). The prGEF (magenta) is recruited junctional membrane (green) and causes irreversible junction length changes.
Recent work used subcellular optogenetics to probe junction viscoelasticity [19••,22••]. Using cultured epithelia expressing the TULIP optogenetic system, RhoA was exogenously activated at cell–cell junctions to directly increase tension by an amount, Λa (Figure 2d). Here, junctions exhibited a stress dependence for irreversible deformations. Junctions behaved elastically at short timescales while at longer timescales, the junctions exhibited a surprising viscoelastic response in which the junctions exhibited a new equilibrium length upon RhoA removal (Figure 2b,c solid line, 2d). By titrating the light to reduce the initial strain values and Λa, the junctions behaved akin to an elastic material, suggesting a thresholded viscoelastic response of intercellular junctions (Figure 2c, dashed line).
Using the canonical Vertex model for epithelium, or treating junctions as Kelvin-Voigt or Maxwell materials, failed to capture the experimental junctional response in this system [19••,22••] (Figure 3b, blue lines). To account for the experimental data, two key adaptive mechanisms were necessary in modeling junctional mechanics: continuous junctional strain relaxation and thresholded tension remodeling (Figure 3a,b red line). Tension remodeling only above a critical strain threshold enabled irreversible deformation for sufficiently strong or sustained activation of force. Such a strain threshold allowed for a filter of small amplitude fluctuations in intracellular force production, mechanically buffering the system. Continuous strain relaxation at junctions allowed the system to gradually lose memory of its mechanical deformation. One consequence of this system was that long contractions only remodeled junctions up to a limit, as strain relaxation prevented further remodeling. As a result, pulsatile contractions with periods of quiescence enabled large-scale irreversible deformations via junctional ratcheting, thus recapitulating a mysterious phenomenon commonly seen in development (Figure 3c) [8,9,10,39,40]. Optogenetics data support the existence of a mechanical feedback between junctional strain and tension, elucidating a robust mechanism to direct morphogenesis, while strain relaxation via actomyosin modulation regulates tensional homeostasis (Figure 3a). Together, these findings on junctional viscoelasticity point to a new, emerging phenomenon: adaptive junction mechanics.
Future perspectives
While the models and methods described here provide for much information on adaptive viscoelasticity, the picture is far from complete. The canonical view of the zonula adherens illustrates a circumferential actomyosin belt attached to a corresponding ring of E-cadherin adhesions, the coupling of which drives cell shape changes [31,41]. The molecular apparatus coupling adhesion and contractility may underlie junction viscoelasticity. Indeed, p120 catenin was implicated in regulating viscoelasticity by modulating the turnover of adhesive complexes [20••]. It is therefore interesting to speculate as to the molecular basis of mesoscopic junctional behaviors that translate into large-scale tissue viscoelasticity. Further work is necessary to dissect the contributions of these molecular components and their feedbacks.
Current mechanical models consider individual junctions and their edge tensions to be mechanically homogenous along their length. While this assumes functional and molecular symmetry among all junctional zones, recent work has shown that bicellular borders act as independent contractile units separate from tricellular vertices [42,43]. Even along a single bicellular interface, molecular assemblies of adhesive structures exist as puncta [44,45]. The functional output of this junctional heterogeneity is asymmetric contraction. Here, vertices exhibit sliding behavior, where a motile vertex contracts into a non-motile vertex to facilitate junction shortening [43,44]. Further work is needed to decipher how these functional units are assembled and coordinated to respond to contractile forces during development.
To add to this heterogeneity, force production along these junctions is not uniform; junctions experience very localized medioapical actomyosin flows to the bicellular interfaces and tricellular vertices [1,26••,43]. Medioapical flows to bicellular junctions is correlated with their deformations [1,2]. Elevated contractility at tricellular vertices stimulates E-cadherin recruitment, there by restricting vertex deformations to stabilize the junctional ratchet [43]. These data suggest that epithelial junctions represent a highly heterogeneous medium comprises distinct contractile units, perhaps borne by differences in the adhesive complexes and their contractile flows. A challenge for the future will be to identify the contributions of spatial variations in force production to junction shortening and remodeling.
These new discoveries, aided by ever-advancing experimental techniques, will provide increasing information for physical models, thereby generating a mutual feedback loop between the two disciplines. The union of physics and biology will therefore create exciting new avenues for which to examine adaptive mechanics and its underlying mechanochemical regulation.
Acknowledgements
KEC acknowledges an HHMI Gilliam Fellowship, National Academies of Sciences Ford Foundation Fellowship, and N.I.H. training grant GM007183. MLG acknowledges funding from NIH RO1 GM104032. This work was partially supported by the UChicago MRSEC, which is funded by the National Science Foundation under award number DMR-1420709. MFS is supported by an EPSRC funded PhD studentship at UCL. MFS was supported in part by the Heising-Simons Foundation, the Simons Foundation, and National Science Foundation Grant No. NSF PHY-1748958. SB acknowledges funding from the Royal Society (URF/R1/180187) and HFSP (RGY0073/2018).
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•• of outstanding interest
- 1.Rauzi M, Lenne P-F, Lecuit T: Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 2010, 468:1110–1114 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- 2.Munjal A, Philippe J-M, Munro E, Lecuit T: A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 2015, 524:351–355 10.1038/nature14603. [DOI] [PubMed] [Google Scholar]
- 3.Kerridge S, Munjal A, Philippe JM, Jha A, de las Bayonas AG, Saurin AJ, Lecuit T: Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis. Nat Cell Biol 2016, 18:261–270 10.1038/ncb3302. [DOI] [PubMed] [Google Scholar]
- 4.de M. Simôes S, Mainieri A, Zallen JA: Rho GTPase and Shroom direct planar polarized actomyosin contractility during convergent extension. J Cell Biol 2014, 204:575–589 10.1083/jcb.201307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Gonzalez R, de M. Simoes S, Röper J-C, Eaton S, Zallen JA: Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell 2009, 17:736–743 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasza KE, Farrell DL, Zallen JA: Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc Natl Acad Sci U S A 2014, 111:11732–11737 10.1073/pnas.1400520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu JC, Fernandez-Gonzalez R: Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila. eLife 2016, 5 10.7554/eLife.10757. e10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jewett CE, Vanderleest TE, Miao H, Xie Y, Madhu R, Loerke D, Blankenship JT: Planar polarized Rab35 functions as an oscillatory ratchet during cell intercalation in the Drosophila epithelium. Nat Commun 2017, 8:476 10.1038/s41467-017-00553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solon J, Kaya-Çopur A, Colombelli J, Brunner D: Pulsed forces timed by a Ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 2009, 137:1331–1342 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 10.Mason FM, Tworoger M, Martin AC: Apical domain polarization localizes actin–myosin activity to drive ratchet-like apical constriction. Nat Cell Biol 2013, 15:926–936 10.1038/ncb2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Clément R, Dehapiot B, Collinet C, Lecuit T, Lenne P-F: Viscoelastic dissipation stabilizes cell shape changes during tissue morphogenesis. Curr Biol 2017, 27:3132–3142.e4 10.1016/j.cub.2017.09.005 [DOI] [PubMed] [Google Scholar]; This paper studies the dynamics of junction length resulting from pulsatile activity of myosin during Drosophila embryogenesis. They found that the junction behaves like a Maxwell material. During increases in myosin concentration, the junction deforms elastically with stress being dissipated over time, allowing long contractions to irreversibly shorten the junction. They also use optical tweezers to examine the timescales of stable junction deformations.
- 12.Alt S, Ganguly P, Salbreux G: Vertex models: from cell mechanics to tissue morphogenesis. Philos Trans R Soc B 2017, 372 10.1098/rstb.2015.0520 20150520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farhadifar R, Röper J-C, Aigouy B, Eaton S, Jülicher F: The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr Biol 2007, 17:2095–2104 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher AG, Osterfield M, Baker RE, Shvartsman SY: Vertex models of epithelial morphogenesis. Biophys J 2014, 106:2291–2304 10.1016/j.bpj.2013.11.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loerke D, Blankenship JT: Viscoelastic voyages – biophysical perspectives on cell intercalation during Drosophila gastrulation. Semin Cell Dev Biol 2020, 100:212–222 10.1016/j.semcdb.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi D, Lopez JH, Schwarz JM, Manning ML: A density-independent rigidity transition in biological tissues. Nat Phys 2015, 11:1074–1079 10.1038/nphys3471. [DOI] [Google Scholar]
- 17.Merkel M, Manning ML: A geometrically controlled rigidity transition in a model for confluent 3D tissues. New J Phys 2018, 20 10.1088/1367-2630/aaaa13 022002. [DOI] [Google Scholar]
- 18.Tetley RJ, Staddon MF, Heller D, Hoppe A, Banerjee S, Mao Y: Tissue fluidity promotes epithelial wound healing. Nat Phys 2019, 15:1195–1203 10.1038/s41567-019-0618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.••.Staddon MF, Cavanaugh KE, Munro EM, Gardel ML, Banerjee S: Mechanosensitive junction remodeling promotes robust epithelial morphogenesis. Biophys J 2019, 117:1739–1750 10.1016/j.bpj.2019.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper combines modeling and optogenetics to examine junction viscoelasticity. Here, low stress, or short contractions, failed to change junction length after optogenetic activations. High stress for long times generated permanent shortening. Two components were incorporated into the Vertex Model to recapitulate experimental data. First, when junctional strain crosses a critical value, tension remodeling is triggered, resulting in permanent length changes. Second, strain is continuously relaxed at junctions, removing mechanical memory.
- 20••.Iyer KV, Piscitello-Gómez R, Paijmans J, Jülicher F, Eaton S: Epithelial viscoelasticity is regulated by mechanosensitive e-cadherin turnover. Curr Biol 2019, 29:578–591.e5 10.1016/j.cub.2019.01.021 [DOI] [PubMed] [Google Scholar]; This paper examines endogenous tissue stretch during Drosophila wing disc development. It was found that tissue viscoelasticity is regulated by E-cadherin endocytosis, mediated by mechanosensitive p120 catenin localization. These data are successfully captured by a Kelvin-Voigt material to describe its viscoelasticity.
- 21.••.Khalilgharibi N et al. : Stress relaxation in epithelial monolayers is controlled by the actomyosin cortex. Nat Phys 2019, 15:839–847 10.1038/s41567-019-0516-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper uses monolayers stretched and suspended by two tweezers. Here, adherens junctions remain stable on the time scale of applied strain, thus remodeling was driven by the actomyosin cortex. This work is successfully recapitulated using a viscoelastic model in which the relaxation is strain dependent, suggesting feedbacks between strain and tension.
- 22.••.Cavanaugh KE, Staddon MF, Munro E, Banerjee S, Gardel ML: RhoA mediates epithelial cell shape changes via mechanosensitive endocytosis. Dev Cell 2020, 52:152–166.e5 10.1016/j.devcel.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper combines modeling and optogenetics to examine junction deformability in model Caco-2 epithelial tissues. They find a stress dependent for irreversible deformations. Short contractions, or long contractions with low amplitude of RhoA, fail to permanently deform the junction. High amplitude signal for long durations generates stable junction deformations, suggesting a thresholded viscoelastic response. To model these data, they found that junction length is determined by strain relaxation and a thresholded tension remodeling, the combination of which successfully generates junction length changes and ratcheting behaviors. Underlying this remodeling is mechanosensitive endocytic turnover of junctional components to stabilize junction contractions.
- 23.Ferro V, Chuai M, McGloin D, Weijer CJ: Measurement of junctional tension in epithelial cells at the onset of primitive streak formation in the chick embryo via non-destructive optical manipulation. Development 2020, 147 10.1242/dev.175109 dev175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bambardekar K, Clément R, Blanc O, Chardès C, Lenne P-F: Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proc Natl Acad Sci U S A 2015, 112:1416–1421 10.1073/pnas.1418732112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.••.Noll N, Mani M, Heemskerk I, Streichan SJ, Shraiman BI: Active tension network model suggests an exotic mechanical state realized in epithelial tissues. Nat Phys 2017, 13:1221–1226 10.1038/nphys4219 [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper proposes an Active Tension Network model, describing the dominance of junctional tension in tissue dynamics. They predict myosin localization depending on the length of the junction. They also describe how mechanical equilibrium is defined by the relative angles of connected junctions, allowing angle preserving, or “isogonal” transformations to the tissue, which can result in large changes in apical areas. Such deformations can be observed in apical constriction of cells during formation of the ventral furrow in Drosophila.
- 26.••.Sumi A, Hayes P, D’Angelo A, Colombelli J, Salbreux G, Dierkes K, Solon J: Adherens junction length during tissue contraction is controlled by the mechanosensitive activity of actomyosin and junctional recycling. Dev Cell 2018, 47:453–463.e3 10.1016/j.devcel.2018.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper examines junction length modulation during Drosophila Dorsal Closure. They find that myosin localization is mechanosensitive, with increased junctional stretch relocalizing myosin from the medioapical pool to the junctional interfaces. Myosin localization controls junction length by modulating its remodeling due to a straightness-dependent mechanism mediated by endocytosis.
- 27.Bonfanti A, Fouchard J, Khalilgharibi N, Charras G, Kabla A: A unified rheological model for cells and cellularised materials. R Soc Open Sci 2020, 7 10.1098/rsos.190920 190920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duda M, Khalilgharibi NJ, Tozluoglu M, Yuen AC, Carpi N, Bove A, Piel M, Charras G, Baum B, Mao Y: Polarization of Myosin II refines tissue material properties to buffer mechanical stress. Dev Cell 2019, 48:245–260.e7 10.1016/j.devcel.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heisenberg C-P, Bellaïche Y: Forces in tissue morphogenesis and patterning. Cell 2013, 153:948–962 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Siang LC, Fernandez-Gonzalez R, Feng JJ: Modeling cell intercalation during Drosophila germband extension. Phys Biol 2018, 15 10.1088/1478-3975/aad865 066008. [DOI] [PubMed] [Google Scholar]
- 31.Sawyer JK, Choi W, Jung K-C, He L, Harris NJ, Peifer M: A contractile actomyosin network linked to adherens junctions by Canoe/afadin helps drive convergent extension. MBoC 2011, 22:2491–2508 10.1091/mbc.e11-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanaugh KE, Oakes PW, Gardel ML: Optogenetic control of RhoA to probe subcellular mechanochemical circuitry. Curr Protoc Cell Biol 2020, 86:e102 10.1002/cpcb.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner E, Glotzer M: Local RhoA activation induces cytokinetic furrows independent of spindle position and cell cycle stage. J Cell Biol 2016, 213:641–649 10.1083/jcb.201603025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakes PW, Wagner E, Brand CA, Probst D, Linke M, Schwarz US, Glotzer M, Gardel ML: Optogenetic control of RhoA reveals zyxin-mediated elasticity of stress fibres. Nat Commun 2017, 8:15817 10.1038/ncomms15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meshik X, O’Neill PR, Gautam N: Physical plasma membrane perturbation using subcellular optogenetics drives integrin-activated cell migration. ACS Synth Biol 2019, 8:498–510 10.1021/acssynbio.8b00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izquierdo E, Quinkler T, Renzis SD: Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nat Commun 2018, 9:1–13 10.1038/s41467-018-04754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valon L, Marín-Llauradó A, Wyatt T, Charras G, Trepat X: Optogenetic control of cellular forces and mechanotransduction. Nat Commun 2017, 8:14396 10.1038/ncomms14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Neill PR, Castillo-Badillo JA, Meshik X, Kalyanaraman V, Melgarejo K, Gautam N: Membrane flow drives an adhesion-independent amoeboid cell migration mode. Dev Cell 2018, 46:9–22.e4 10.1016/j.devcel.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mason FM, Xie S, Vasquez CG, Tworoger M, Martin AC: RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J Cell Biol 2016, 214:603–617 10.1083/jcb.201603077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razzell W, Wood W, Martin P: Recapitulation of morphogenetic cell shape changes enables wound re-epithelialisation. Development 2014, 141:1814–1820 10.1242/dev.107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roh-Johnson M, Shemer G, Higgins CD, McClellan JH, Werts AD, Tulu US, Gao L, Betzig E, Kiehart DP, Goldstein B: Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science 2012, 335:1232–1235 10.1126/science.1217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi W, Acharya BR, Peyret G, Fardin MA, Mege RM, Ladoux B, Yap AS, Fanning AA, Peifer M: Remodeling the zonula adherens in response to tension and the role of afadin in this response. J Cell Biol 2016, 213:243–260 10.1083/jcb.201506115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderleest TE, Smits CM, Xie Y, Jewett CE, Blankenship JT, Loerke D: Vertex sliding drives intercalation by radial coupling of adhesion and actomyosin networks during Drosophila germband extension. eLife 2018, 7 10.7554/eLife.34586. e34586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huebner RJ, Malmi-Kakkada AN, Sarikaya S, Weng S, Thirumalai D, Wallingford JB: Cadherin clustering controls heterogeneous, asymmetric junction dynamics during vertebrate axis elongation. bioRxiv 2020. 10.1101/2020.02.11.944033. 2020.02.11.944033. [DOI] [Google Scholar]
- 45.Cavey M, Rauzi M, Lenne P-F, Lecuit T: A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 2008, 453:751–756 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]


