Abstract
This review provides a summary of the symposium entitled “Role of Non-Genetic Risk Factors in Exacerbating Alcohol-Related Organ Damage” was held at the at the 42nd Annual Meeting of the Research Society on Alcoholism. The goals of the symposium were to provide newer insights into the role of non-genetic factors including specific external factors, notably infectious agents or lifestyle factors that synergistically act to exacerbate alcohol pathogenicity to generate more dramatic downstream biological defects. This summary of the symposium will benefit junior/senior basic scientists and clinicians’ currently investigating/treating alcohol-induced organ pathology as well as undergraduate, graduate and post-graduate students and fellows.
Keywords: alcohol; infectious agents; HIV, HBV, smoking; neutrophil extracellular traps
1. Introduction
Alcohol is known to promote a wide range of organ damages. By affecting various tissues and organs of our body, it causes significant comorbid consequences (Souza-Smith et al., 2016). Primary mechanisms of alcohol-related pathology include increased oxidative stress, inflammation, posttranslational modifications of vitally important proteins, altered protein synthesis and degradation disorders, dysregulation of lipid metabolism and impaired signaling (Osna & Kharbanda, 2016). These changes are related to alcohol metabolism and mainly affect ethanol-metabolizing cells or cells with high sensitivity to ethanol metabolites (Osna, Donohue & Kharbanda, 2017). While alcohol by itself is a potent damaging factor, its effects can be potentiated by secondary insults (so called “second hits”), which further promote detrimental consequences of alcohol abuse followed by the progression to end-stage disease. Extensive research in recent years has shown that alcohol-induced alterations in the gut microbiota and increased intestinal permeability to luminal antigens/microbes are potent second hits promoting progressive organ injury (reviewed in (Arab, Arrese & Shah, 2020; Avila et al., 2020; Cassard, Gerard & Perlemuter, 2017; Forsyth, Voigt, Burgess, Swanson & Keshavarzian, 2015; Forsyth, Voigt & Keshavarzian, 2014; Gorky & Schwaber, 2016; Massey, Beier, Ritzenthaler, Roman & Arteel, 2015; Meroni, Longo & Dongiovanni, 2019; Ridlon, Kang, Hylemon & Bajaj, 2015; Tang, Forsyth & Keshavarzian, 2014; Temko et al., 2017; Vassallo et al., 2015; World Health Organization, 2018)). Other important second hits are bacterial and viral infections that lead to the synergistic activation of oxidative stress both by pathogens and alcohol metabolism. In fact, virus by itself may induce oxidative stress in permissive cells and hijack anti-viral protection by innate immunity. Alcohol metabolism also suppresses innate immunity leading to increased viral load and spread of infection, thereby potentiating oxidative stress (Boule & Kovacs, 2017). Further scenarios depend on cytopathogenic properties of the virus: cytopathogenic viruses can kill the cells directly (the example is human immunodeficiency virus (HIV) in immune cells) (Costin, 2007), whereas non-cytopathogenic viruses (such as hepatitis C (Irshad, Gupta & Irshad, 2019) and hepatitis B (Tseng & Huang, 2017)) require activation of adaptive immunity to clear infected cells. Alcohol metabolism suppresses innate and adaptive immune responses and elimination of infected cells, and the accumulation of virus in these cells also increases oxidative stress, triggers dysregulation of signal transduction pathways and worsens the pathology (Ganesan et al., 2019; Osna, Ganesan & Kharbanda, 2015).
In patients with alcohol use disorder, infection with any hepatotropic virus promotes the progression of alcoholic liver disease as the liver damage is more severe in alcohol-abusing patients with chronic viral hepatitis compared with chronically infected patients without alcohol use disorder (Novo-Veleiro et al., 2016; Serfaty, 2016; Shoreibah, Anand & Singal, 2014; Yuan, Govindarajan, Arakawa & Yu, 2004). Further, alcohol as a second hit exacerbates inflammation and pro-fibrotic changes in virus-infected hepatocytes (Ganesan, Poluektova, Enweluzo, Kharbanda & Osna, 2018; Ganesan, Poluektova, Kharbanda & Osna, 2018). Regardless of the primary cause of liver injury, the progression to end-stage liver disease is faster when triggered by a second hit and promotes progressive liver injury, thereby increasing end-stage outcomes, including cirrhosis and hepatocellular carcinoma (Novo-Veleiro et al., 2016). In addition to viral infections, bacterial infections potentiate alcohol-related organ damage as evident from gut microbiota studies and sepsis (Bajaj, 2019; Gustot et al., 2017)
In the frame of this symposium, in addition to addressing the role of alcohol in progression of HIV and hepatitis B virus (HBV)-infections, we also discussed other insults representing non-genetic risk factors causing exacerbation of alcohol-related organ damage, which are beyond the effects of pathogens such as the role of smoking in the development of alcoholic pancreatitis. Thus, here, we will overview the roles of pathogen-related (HIV, HBV, sepsis) and pathogen-unrelated (smoking) factors in progression of alcohol-induced organ damage.
The session was comprised of the following presentations:
Alcohol and smoking in progression and severity of chronic pancreatitis.
Alcohol binge results in abnormal neutrophil extracellular traps formation and impaired macrophage efferocytosis in mice
Mechanisms underlying risk for comorbidities associated with at-risk alcohol consumption: Metabolic instability in persons living with HIV (PLWH).
Possible mechanism of alcohol induced persistence of HBV pathogenesis
2. Summary of The Presentations at the Symposium
2.1. Alcohol and smoking in progression and severity of chronic pancreatitis
The exocrine compartment of the pancreas consisting of acinar and ductal cells, is involved in synthesis and secretion of proteolytic enzymes (Shih, Wang & Sander, 2013). Any injury to the acinar cells leads to premature activation of proteolytic enzyme that initiate a cascade of events leading to auto-digestion and inflammatory response defined as pancreatitis (Lankisch, Apte & Banks, 2015). This pancreatic injury leads to either acute (AP) or chronic pancreatitis (CP) based on the extent and duration of the inflammatory response (Kleeff et al., 2017; Lankisch, Apte & Banks, 2015). Acute pancreatitis is a self-limiting disease, and the inflammation typically resolves within 1–2 weeks (Lankisch, Apte & Banks, 2015). However, recurrent AP leads to CP, characterized by inflammation, progressive and irreversible fibrosis, deposition of extracellular matrix (ECM), and exocrine and endocrine insufficiencies (Kleeff et al., 2017; Yang & Forsmark, 2017). The multiple genetic and environmental factors, including mutations (PRSS1, CTRC, and SPINK1), alcohol consumption, and tobacco smoking, have been associated with pathobiology and clinical presentation of CP (Kleeff et al., 2017). Further, the inflammation in the pancreas leads to the activation of pancreatic stellate cells (PSC), which proliferate and contributes to fibrosis and deposit ECM proteins like collagens-I, III, IV, fibronectin, and laminin (Apte, Pirola & Wilson, 2015; Apte, Wilson & Korsten, 1997).
Alcohol and smoking abuse predispose patients to recurrent pancreatic injuries (Apte, Wilson & Korsten, 1997; Singhvi & Yadav, 2018). Alcohol increases the fragility of zymogen granules and facilitates the pre-mature activation of proteolytic enzymes in the acinar cells (Apte, Wilson & Korsten, 1997). Previous studies illustrated that smoking promotes inflammation and activates PSC, as evident by increased expression of smooth muscle actin (α-SMA) (Kumar et al., 2015). However, the precise molecular mechanism of alcohol and smoke exposure-mediated progression of CP remains elusive. Studies have demonstrated that both pancreatic acinar and stellate cells metabolize alcohol by oxidative and non-oxidative pathways (Vonlaufen, Wilson, Pirola & Apte, 2007), resulting in the formation of acetaldehyde and reactive oxygen radicals, which further react with biomolecules to generate additional chemically reactive species, especially malondialdehyde (MDA), 4-hydroxy-2-nonenal (4HNE), and hybrid MDAacetaldehyde-protein (MAA) adducts (Kharbanda, Todero, Shubert, Sorrell & Tuma, 2001; Tuma & Casey, 2003). Aldehyde protein adducts have been implicated in tissue destruction and increased fibrosis. Aldehydes bind to lysine, histidine, and cysteine residues to generate adducts with collagen, tubulin, and lipoproteins modulating their structural and functional characteristics (Casini et al., 2000). MAA-adducts can initiate an inflammatory and fibrogenic response by activating hepatic stellate cells and the alveolar epithelial cells and macrophage (McCaskill et al., 2011; Wyatt et al., 2012). The ability of these adducts to affect the biology of pancreatic acinar and stellate cells, two important cell types involved in the pathobiology of CP, was examined. The MDA-collagen adducts were generated by incubating collagen-coated plates with 2, 3 or 4 mM MDA in 0.1M phosphate buffer (pH 7.2), 2mM diethylenetriaminepentaacetic acid, and 2 mM Phytic acid at 37°C for 3 days. MAA-collagen adducts were generated by incubating collagen-coated plates with 1.0 mM acetaldehyde in the presence of 2, 3 or 4 mM MDA in 0.1 M phosphate buffer (pH 7.2), 2 mM diethylenetriaminepentaacetic acid, and 2 mM phytic acid at 37°C for 3 days to generate MAA adducts at a 1:2, 1:3 and 1:4 molar ratio of Ach: MDA. Similarly, HNE-collagen adducts were generated by incubating collagen-coated plates with 20, 30 or 40μg HNE in PBS (pH 7.2) at 37°C for 3 days to generate increasing concentrations of collagen-HNE adducts. The adduction protocols are detailed in (Kharbanda, Shubert, Wyatt, Sorrell & Tuma, 2002; Kharbanda, Todero, Shubert, Sorrell & Tuma, 2001; Shearn, Backos, Orlicky, Smathers-McCullough & Petersen, 2014; Shearn et al., 2013; Tuma, Thiele, Xu, Klassen & Sorrell, 1996).
Pancreatic acinar and stellate cells were cultured for 48 h on the plates that were pre-exposed to MDA/MAA/HNE as detailed above, thus being exposed to respective collagen;aldehyde adducts. The verification that there was consistent adduct formation was verified by running controls of soluble antigens which revealed less than 5% variability between batches of the adducted plates.
The viability of pancreatic acinar and stellate cells in the presence of adducts was analyzed by MTT assay. Briefly, 5 X 103 cells per well were seeded on MDA, MAA and HNE adducted plates and allowed to grow for 48 h followed by MTT assay. MDA adducts did not alter the viability of either acinar or PSCs at any collagen:MDA molar concentrations. The MAA-collagen adducts significantly reduced the viability of the acinar (p<0.05) and PSC (p<0.001) grown on all collagen-MAA adducted plates generated using 1:2, 1:3, and 1:4 of Ach: MDA molar concentrations. The HNE-adducts decreased the viability of the PSC (p<0.01), however, acinar cells were affected only at the highest collagen:HNE molar concentration adduct generated. Collectively, these data suggest that the presence of the aldehyde adducts on the ECM proteins could predispose the acinar and PSC to exhibit an exaggerated response to the subsequent exposure to alcohol and smoke, thereby promoting pancreatic damage and inflammation. To test this hypothesis, pancreatic acinar and stellate cells grown on collagen-aldehyde adducted plates were treated for 48 h with alcohol (EtOH, 50mmol/L) and cigarette smoke condensate (CSC, 20μg/ml) and analyzed by western blotting. Exposure to EtOH and CSC resulted in a higher cleavage of PARP in acinar cells grown on MAA and the HNE-adducted collagen plates. In addition, the presence of the aldehyde-adducts increased the endoplasmic reticulum stress as demonstrated by a higher expression of C/EBP homologous protein (CHOP) in the acinar cells exposed to either EtOH or CSC alone, or in combination. The presence of higher levels of CHOP, which have been shown to significantly reduce the viability of the acinar cells by regulating transcription to promote apoptosis, suggests that the aldehyde-adducted collagen may significantly contribute to the severity and the progression of the CP. The effect of exposure of PSCs to CSC and EtOH on the expression of the ECM proteins was also examined. Both CSC and EtOH exposure alone and in combination upregulated the expression of ECM proteins, including fibronectin in these cells.
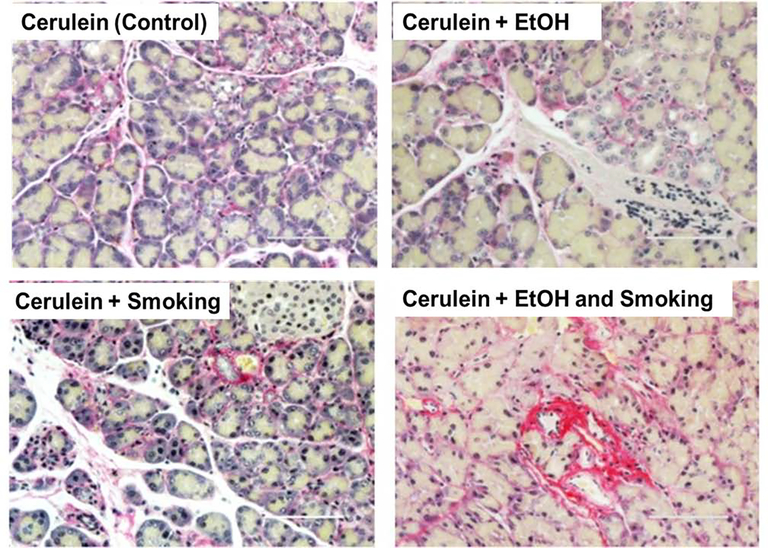
To evaluate the role of alcohol and smoking in recurring AP and its progression to CP under in vivo conditions, isocaloric Lieber-Decarli control or ethanol diet (Control diet: protein 151kcal/l, fat 359 kcal/l, carbohydrate 490kcal/l; Ethanol diet: protein 151 kcal/l, fat 359 kcal/l, carbohydrate 135 kcal/l and ethanol 355 kcal/l) was fed to the CP murine model along with the smoke exposure, using cigarette (University of Kentucky Reference Cigarette, 3R4F, Lexington, KY) smoke (Teague TE-10C, Davis, CA,) for 6 weeks (Xue et al., 2016). At the experimental end point, tissue from the respected groups were stained with hematoxylin and eosin as well as subjected to fibrotic markers analysis by immunohistochemistry for determining the disease severity. The alcohol and smoke exposure together aggravate cerulein-induced pancreatic inflammation and cause extensive damage to pancreatic parenchyma along with higher ECM deposition (Fig. 1).
Figure 1:
Picro Sirius Red staining demonstrating the higher deposition of extracellular matrix protein (collagen) in presence of combined alcohol (EtOH) and smoke exposure in murine model of chronic pancreatitis. Bar scale = 100 μm.
Thus, data presented reveals that the alcohol- and smoking co-exposure-dependent formation of adducted collagen may significantly contribute to the severity and the progression of CP. Both cigarette smoke-extract and ethanol exposure alone and in combination aggravate inflammation and upregulate the expression of ECM proteins, including fibronectin.
2.2. Alcohol binge results in abnormal neutrophil extracellular traps formation and impaired macrophage efferocytosis in mice
Liver inflammation and steatosis are the characteristics of alcoholic liver diseases (ALD) (Osna & Kharbanda, 2016; Szabo & Mandrekar, 2010). Excessive alcohol consumption induces gut “leakiness” that increases translocation of gut derived pathogen-associated molecules (PAMPs) including endotoxin to liver (Szabo, 2015). These PAMPs and danger-associated molecules from damaged hepatocytes recruit and activate immune cells to the liver, which leads to massive inflammation (Nagy, 2015; Szabo, 2015; Szabo, Petrasek & Bala, 2012). Neutrophils, as the first line of defense mechanism against invading pathogens, rapidly infiltrate into the liver upon pathogen-derived as well as sterile danger signals (Jaeschke, 2002). Among various neutrophil functions, neutrophils release sticky web-like decondensed chromatin mixed with several granular proteins, called neutrophil extracellular traps (NETs) (Brinkmann et al., 2004). NETs release is an efficient anti-microbial strategy to prevent spread of pathogens. However, uncontrolled NETs production or delayed clearance of NETs by macrophages often cause secondary tissue damage since it contains various DAMPs and sustains the inflammation (Farrera & Fadeel, 2013). Neutrophil increase in the liver has been reported in ALD (Bertola, Park & Gao, 2013; Mookerjee et al., 2007; Xu, Huang, Zhang & Wang, 2014), however, it still remains unclear how neutrophils contribute to the pathogenesis. To understand this phenomenon, the following study was conducted: human peripheral blood neutrophils were isolated from healthy subjects and incubated with 50mM ethanol for 4 hours to test in vitro NETs formation. NETs production was quantified with enzymatic activity from NETs-associated neutrophil elastase. For in vivo study, 10–12-week-old wild-type female mice received a daily oral gavage of alcohol at 5mg/kg body weight or calorie-matched sugar for three days as previously described (Bukong et al., 2018). On the last day, mice received a single intraperitoneal (i.p.) injection of lipopolysaccharide (LPS; 0.05mg/kg body weight) or saline. The mice were sacrificed 12 hours and 15 hours after the injection. Neutrophil recruitment into the liver was measured by immunofluorescence (IF) staining with neutrophil elastase (NE). NETs formation was determined by double IF staining and Western blotting against citrullinated histone H3. Serum aspartate aminotransferase (AST) and cytokines were measured by ELISA. Unpaired student ttest was used for statistical analysis.
Ex vivo, acute alcohol (50mM) treatment significantly increased spontaneous NETs formation in human primary neutrophils. Interestingly, phorbol 12-myristate 13-acetate-induced NETs production was significantly reduced in neutrophils exposed to alcohol compared to alcohol-naïve neutrophils (Bukong et al., 2018). We observed neutrophil infiltration, NETs formation and inflammation peak at 9–12 hours, and reduced neutrophil count and NETs at 15 hours after LPS challenge in the control group of mice that received sugar gavage. Interestingly, binge alcohol in mice resulted in a biphasic response to LPS; 12 hours after LPS injection, less neutrophil infiltration and NETs formation were detected in the liver of mice that received binge alcohol gavage compared to mice that received a sugar gavage. However, 15 hours after the LPS injection, significantly higher numbers of neutrophils and NETs formation were detected in the liver from mice with binge alcohol gavage. In line with increased neutrophil count and NETs formation, an increase in inflammatory cytokines including monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 as well as increased TUNEL positive cells in the liver were observed. These results indicated that binge alcohol delays LPS-induced neutrophil recruitment and NETs formation in the mice liver. Given the observations, a hypothesis that acute alcohol affects macrophage efferocytosis and thus, delays NETs clearance in liver was generated. RAW cells and human primary macrophages exposed to in vitro alcohol were co-cultured with PMA-induced NETs-producing (NETotic) neutrophils to test the effect of acute alcohol on macrophage efferocytosis. Macrophages treated with alcohol showed less phagocytotic capacity compared to the control group. Surface markers of M1 macrophages increased in the macrophages after efferocytosis of NETotic neutrophils. In vivo, neutrophil depletion prevented LPS-induced neutrophil recruitment and abnormal NETs formation in the liver from the mice that received binge alcohol gavage (Bruhn, Dekitani, Nielsen, Pantapalangkoor & Spellberg, 2016). Moreover, liver damage and inflammatory cytokine production indicated by increased serum AST and MCP-1 levels, respectively, were significantly alleviated upon neutrophil depletion, suggesting that neutrophils are involved in alcohol-induced liver damage through increased NETs formation.
To conclude, acute alcohol induces neutrophils to release spontaneous NETs, however, delays neutrophil infiltration and reduces NET formation to a subsequent insult. Furthermore, alcohol binge delays macrophages to clear up the NETs and NETotic neutrophils, which prolongs inflammation and exacerbates alcohol-induced liver damage in mice.
2.3. Mechanisms underlying risk for comorbidities associated with at-risk alcohol consumption: Metabolic instability in persons living with HIV (PLWH)
According to the World Health Organization, more than 50% of the population in the Americas consumes alcohol (2018), and the incidence of HIV infection is strongly associated with alcohol consumption (Lefevre et al., 1995). Among persons living with HIV (PLWH), 8‒12% report heavy or at-risk drinking (Galvan et al., 2002), and the prevalence of alcohol use disorders is 2‒3 greater than that of the general population (Conigliaro, Justice, Gordon & Bryant, 2006). Unhealthy alcohol use has significantly impacted the HIV epidemic through its effects on the risk and transmission of disease resulting from cognitive and executive function impairments. Moreover, alcohol can produce multisystemic effects that impact disease processes involved in HIV infection.
With the advent of combination antiretroviral therapy (cART), HIV infection has become a chronic disease. Age-adjusted mortality rates and life expectancy at age 20 for PLWH approximates that of the uninfected population (Marcus et al., 2016). Increased survival is complicated by a greater risk for comorbidities with progression of biological age in PLWH (Bernardino et al., 2019; Debroy et al., 2019; Hulgan et al., 2019; Nguyen et al., 2017; Noubissi, Katte & Sobngwi, 2018; Peltenburg et al., 2019; Taramasso et al., 2017; Zicari et al., 2019). The Center for Disease Control and Prevention estimates that more than 50% of PLWH in the US are 50 years of age or older (Centers for Disease Control and Prevention (CDC)). Long-term survivors of HIV have a disease course complicated by several comorbidities, including metabolic alterations, diabetes, cardiovascular disease, cancer, chronic renal disease, and osteoporosis (Burgess, Zeuli & Kasten, 2015; Carr & Cooper, 2000; Compston, 2015).
The study of the impact of unhealthy alcohol use on disease progression in the clinical setting is obscured by multiple factors, including polysubstance use, differences in adherence to ART, and limitations in tissue and blood sampling from these individuals. Significant knowledge on the interaction of alcohol with disease progression has been derived from integration of molecular, cellular, and animal studies interpreted in the context of current knowledge of human disease progression. Using the nonhuman primate (NHP) model of HIV infection, our group has identified a significant impact of chronic binge alcohol administration (CBA) on simian immunodeficiency virus (SIV) pathogenesis. Our studies show that CBA increases viral infectivity (Amedee, Nichols, Robichaux, Bagby & Nelson, 2014), decreases time to end-stage in SIV-infected macaques not treated with ART (Bagby, Zhang, Purcell, Didier & Nelson, 2006), and produces marked alterations in immune function particularly as they impact intestinal mucosal immunity (Veazey et al., 2015). Collectively, our studies have shown that alcohol increases lymphocyte turnover and decreases the total number of lymphocytes in the small intestine, while increasing the percent of gut and vaginal mucosal HIV target cells. The decrease in total lymphocyte count in the gut is accompanied by marked increases in both CD4+ and CD8+ T-cell proliferation coupled with increased T-cell death, especially in the CD8+ T-cell population (Katz et al., 2015; Marcus et al., 2016; Poonia, Nelson, Bagby & Veazey, 2006; Siggins et al., 2009; Veazey et al., 2015). These intestinal immunopathogenic alterations adversely affect mucosal barrier function producing gut barrier leak. Translocation of toxins and bacterial products into the systemic circulation system promotes immune activation, leading to a state of immune exhaustion and senescence and chronic subclinical inflammation and oxidative stress that contributes to tissue injury.
The chronic inflammatory and oxidative stress environment prevailing in the infected host increases the risk for comorbidities that form part of the geriatric syndromes, particularly metabolic dysregulation. Our studies have shown that CBA accentuates metabolic derangements in virally suppressed SIV-infected rhesus macaques. Those studies identified amplified localized skeletal muscle (SKM) inflammation, profound depletion of SKM antioxidant capacity (oxidative stress), increased SKM proteasomal activity (LeCapitaine et al., 2011), and decreased myoblast differentiation potential (Simon et al., 2017; Simon et al., 2014) as mechanisms preceding loss of SKM mass. In addition to enhanced SKM protein degradation and impaired myogenic potential, impaired anabolic signaling and decreased whole-body insulin sensitivity, despite viral suppression with ART was observed (Ford et al., 2016). Moreover, results from a frequently sampled intravenous glucose tolerance test (FSIVGTT) with a third-phase insulin infusion (modified minimal model; MINMOD) showed that CBA/SIV animals have lower insulin sensitivity (corrected for % lean body mass) at 11 months post-SIV infection, irrespective of ART. Data showed decreased disposition index (DI), an indicator of insulin resistance, and markedly reduced insulin release (impaired endocrine pancreas response) following a glucose load (Ford et al., 2016). These metabolic alterations were not associated with fasting hyperglycemia or hyperinsulinemia, suggesting they likely precede a phase of overt glucose intolerance developing with disease progression.
While the SKM metabolic alterations identified are hypothesized to be significant contributors to overall metabolic dyshomeostasis, results from subsequent studies suggested additional contribution from adipose tissue and liver as target organs affected by CBA, SIV, and ART. Our results showed that in addition to decreasing adiponectin circulating levels, CBA resulted in significant alterations in adipose tissue functional structure and increased inflammation, particularly in omental adipose tissue (OmAT) of SIV-infected, ART-treated macaques. Our results showed decreased OmAT cell size in CBA/SIV/ART animals in association with increased collagen expression and increased infiltration of mast cells and macrophages (Ford et al., 2018). These structural changes in OmAT were associated with decreased gene expression of PPARγ and EGR2 in differentiating adipose-derived stem cells, suggesting impaired metabolic capacity of adipocytes. Furthermore, our results showed an overall main effect of ART to increase the hepatic mRNA expression of gluconeogenic, but not glycolytic enzymes, particularly in the CBA animals. These findings suggest that in addition to CBA, ART exerts changes that can alter the balance of glucose metabolic capacity, favoring greater glucose production over glucose utilization.
Interestingly, as the components of frailty syndrome (Fried et al., 2001) are dissected, metabolic dysregulation surfaces as one of the main domains for alteration in the alcohol-consuming, SIV/HIV-infected host. In addition to the alterations in SKM and adipose tissue functional mass, blunted endocrine pancreas response, and upregulated hepatic gluconeogenic gene expression, marked alteration in SKM expression of genes involved in mitochondrial homeostasis was also observed (Duplanty, Simon & Molina, 2017). The relative expression of PPARγ coactivator 1 beta (PGC-1β) was significantly decreased in the SKM of CBA/SIV NHP compared to uninfected controls. CBA not only prevented the significant upregulation of mitophagy-related and anti-apoptotic gene expression but also increased expression of proapoptotic genes in SIV-infected NHP (Duplanty, Simon & Molina, 2017). These findings suggest that SIV infection disrupts mitochondrial homeostasis and when combined with CBA, results in differential expression of genes involved in apoptotic signaling. This led to the scientific premise that impaired mitochondrial homeostasis may contribute to the underlying pathophysiology of alcoholic and HIV-associated myopathy. Furthermore, using the succinate dehydrogenase activity assay, it was determined that during the asymptomatic phase of SIV infection, CBA and ART significantly decreased SKM oxidative capacity. The dysregulated mitochondrial function was confirmed by functional assays using the Seahorse technology. Maximal oxygen consumption rate of myoblasts isolated from the CBA/SIV/ART was significantly lower compared to that of myoblasts isolated from controls and sucrose (SUC)/SIV/ART macaques.
In summary, these data and published reports support the scientific premise that alcohol promotes gut leak and immune activation that synergizes with ART resulting in systemic chronic inflammation and oxidative stress that likely promotes cellular energy metabolism dyshomeostasis in control (pancreas), effector (liver and adipose), and target (musculoskeletal) organs implying that altered cellular energy homeostasis is a significant contributor to the increased risk for comorbidities in PLWH. Further studies are warranted to identify therapeutic targets to prevent or reverse metabolic dyshomeostasis in chronically infected PLWH.
2.4. Possible Mechanism of alcohol induced persistence of HBV pathogenesis
HBV and alcohol abuse are two major public health problems worldwide. Approximately 30% of the world’s population is infected with HBV. Every year, 350 million people are infected with HBV, and 887,000 people die from HBV-related diseases (2014., 2014; Ganesan et al., 2019). HBV infection and alcohol abuse synergistically progress liver disease development and cause chronic persistence of HBV-infection, but the mechanisms underlying these detrimental health effects are not fully understood. Current treatment of chronic HBV patients is limited to antiviral medications, interferon injections, and liver transplants. When chronic HBV is combined with alcohol abuse, these treatments are often largely ineffective. Elucidation of the mechanisms behind the exacerbation of HBV pathogenesis by alcohol is crucial to the synthesis of new drugs and treatment options for liver diseases in alcohol-abusing HBV patients.
In HBV infection, viral clearance is largely mediated by the adaptive immune response (Guidotti & Chisari, 2006). HBV is not a cytopathogenic virus, and the clearance of HBV-infected hepatocytes is performed by an immune response that is mediated mainly by cytotoxic T-lymphocytes (CTLs) (Isogawa & Tanaka, 2015). Alcohol prolongs HBV persistence in infected hepatocytes leading to establishment of chronic hepatitis by suppressing adaptive immune response, mainly, by evading recognition of infected cells by CTLs (Hughes, Wedemeyer & Harrison, 2011). The discernment between “self” and “non-self” by CTLs requires the presentation of viral peptides in the context of MHC class I (major histocompatibility complex I) on infected hepatocyte surface. This can be achieved by processing of viral antigens to peptides by proteasome followed by peptide loading into MHC class I in the endoplasmic reticulum. These peptide-MHC class I complexes with the help of transporters associated with antigen processing then traffic via trans-Golgi to the cell surface (Chefalo, Grandea, Van Kaer & Harding, 2003). Since hepatocyte is a primary site of both viral replication and ethanol metabolism, alcohol-induced defects in protein trafficking and proteasome function may play role in HBV peptide-MHC class complex presentation on these cells.
Many laboratories, including ours have shown that ethanol metabolism enhances the HBV viral replication (an increase in HBV RNA, HBV DNA, HBsAg and HBV core protein content) in HBV infected and transfected cells (Ganesan et al., 2019; Larkin, Clayton, Liu & Feitelson, 2001; Li et al., 2019). Alcohol metabolism interferes with an effective immune response (Osna, Ganesan & Kharbanda, 2015), partially, because of impaired presentation of antigenic peptides in the context of MHC class I (Osna et al., 2012). However, currently, it is not clear whether this is the case for HBV-infection. In light of a recent report showing that ethanol metabolite acetaldehyde suppressed the HBV peptide (18–27)-MHC class I complex display on HBV-expressing hepatocytes (Ganesan et al., 2019), follow-up studies were initiated to examine how ethanol metabolism affects the HBV peptide MHC class I presentation on hepatocytes.
HepG2.2.15 cells, stably transfected with HBV and able to replicate virus and produce viral particles, were utilized for these studies. Since HepG2.2.15 cells do not metabolize ethanol and all harmful effects on antigen presentation are attributed to ethanol metabolism, an acetaldehyde generating system (AGS) was employed to mimic ethanol metabolism for generation of Ach without transfection with alcohol-metabolizing enzymes (Ganesan et al., 2016; Ganesan et al., 2015). These cells were treated or not with AGS for 72 hours. For the final 24 hours of treatment, cells were exposed (or not) to 2 ng/ml interferon γ (IFNγ) because most of proteasome-related phenomena are IFNγ-inducible. Ach suppressed HBV core peptide FLPSDFFPSV-HLA-A2 complex presentation in hepatocytes due to impaired processing of the peptides by proteasome. As shown recently, chymotrypsin-like (ChT-L) and trypsin-like (T-L) proteasome activities were decreased in HepG2.2.15 cells exposed to AGS (in both IFNγ-treated and non-treated cells) (Ganesan et al., 2019). However, the magnitude of AGS-triggered suppression of ChT-L and T-L activities was higher in IFNγ-treated cells. Further, determining β5i-LMP7 activity (immunoproteasome, IPR activity) in IFNγ untreated and treated cells demonstrated that IFNγ induces the activation of immunoproteasome (IPR), and increased sensitivity of IFNγ-exposed cells to AGS is due to suppression of the activity and expression of IPR subunits. These findings indicate that ethanol metabolism, particularly Ach, suppresses proteasome activity and IPR expression leading to decreased HBV peptide-MHC class-I complex presentation on hepatocytes. The exact molecular mechanisms by which Ach affects antigen presentation machinery in HBV-infected cells is not known. However, our earlier studies indicated that proteasome activity can be dose-dependently modified by 3-nitrotyrosine adducts induced by peroxynitrite, whose enhanced release is triggered by ethanol metabolism. Peroxynitrite blocks proteasome function at high nitration levels (Osna, Haorah, Krutik & Donohue, 2004). It is possible that the Ach generated impairs proteasome activity via adducting to its subunit proteins, thereby contributing in suppressing HBV peptide processing and their presentation in the context of MHC class I.
It is also possible that the downregulation of immunoproteasome by Ach occurs at the level of IFNγ-induced signaling via the JAK-STAT1 pathway. In fact, we observed Ach-impaired IFNγ signaling (STAT1 phosphorylation) in HBV-expressing HepG2.2.15 cells (Ganesan et al., 2019). Thus, HBV-AGS-suppressed IFNγ signaling dysregulates the processing of HBV peptides and hence, the display of HBV peptide-MHC class I complex on cell surface (Ganesan et al., 2019).
In addition to impaired HBV peptide processing, a reduced expression of the transporter for the peptide-MHC class I complex, (TAP1) and tapasin, was observed in HepG2.2.15 cells exposed to AGS and IFNγ (Ganesan et al., 2019). This decreased protein expression of TAP1 and tapasin, due to the Ach-induced suppression of IFNγ signaling in hepatocytes, may have an impact on delivery of cleaved HBV peptide-HLA-A2 complex to the surface. Previous reports also support these observations on the role of TAPs and tapasin for expression of HBV CTL epitopes as well as IPR for IFNγ-activated MHC class I-restricted antigen presentation on hepatocytes (Chen et al., 2014; Lee et al., 1997). The changes in TAP1 and tapasin protein expression in HepG2.2.15 cells exposed to AGS may not be related to post-translational modification of these proteins via Ach-induced adduct formation since the mRNA expression of these proteins was also suppressed when cells were exposed to IFNγ (Ganesan et al., 2019).
In summary, these data suggest that in HBV-expressing hepatocytes, ethanol metabolism impairs proteasome function and IFNγ-signaling via the Jak-STAT1 pathway in hepatocytes, thereby decreasing HBV peptide cleavage by immunoproteasome and activation of protein loading complex (PLC) components, TAP and tapasin, necessary for HBV peptide-MHC class I trafficking to the membrane. Acetaldehyde-induced defects in both HBV peptide processing and PLC finally suppress the display of HBV core peptide 18–27- MHC class I (FLPSDFFPSV-HLA-A2) complex (a CTL epitope) on cell surface. All these events may limit the activation of CTLs and reduce their ability to recognize/eliminate HBV-expressing hepatocytes, thereby promoting HBV-infection pathogenesis.
3. Final Summary
Overall, this symposium provided an update on the roles of pathogen-related (HIV, HBV, sepsis) and pathogen-unrelated (smoking) factors in progression of alcohol-induced organ damage. (Fig. 2) The following summarizes the take-home message of the session’s presentations: (i) Data presented showed that the alcohol- and smoking co-exposure-dependent formation of adducted collagen may significantly contribute to the severity and the progression of CP. Both cigarette smoke-extract and ethanol exposure alone and in combination upregulate the expression of ECM proteins, including fibronectin; (ii) Alcohol induces spontaneous NETs production in human primary neutrophils, and that alcohol delays neutrophil infiltration and NETs production in response to LPS in mice liver. Further, alcohol reduces macrophage efferocytosis of NETs and NETotic neutrophils to prolong inflammation and increase liver damage in mice. Acute alcohol induces neutrophils to release spontaneous NETs, however, reduces NET formation to a subsequent insult. Furthermore, alcohol binge delays macrophage clearance of NETs and NETotic neutrophils, which exacerbates alcohol-induced liver damage in mice; (iii) In SIV/HIV-infection, alcohol induces gut leak and immune activation that synergizes with ART resulting in systemic chronic inflammation and oxidative stress that may promote cellular energy metabolism dyshomeostasis in control (pancreas), effector (liver and adipose), and target (musculoskeletal) organs. This altered cellular energy homeostasis could be a significant contributor to the increased risk for comorbidities in PLWH. Further studies are warranted to identify therapeutic targets to prevent or reverse metabolic dyshomeostasis in chronically infected PLWH; (iv) Ethanol metabolism impairs proteasome function and IFNγ-signaling via the Jak-STAT1 pathway in HBV-expressing hepatocytes, thereby decreasing HBV peptide cleavage by immunoproteasome and activation of protein loading complex (PLC) components, TAP and tapasin, necessary for HBV peptide-MHC class I trafficking to the membrane. Acetaldehyde-induced defects in both HBV peptide processing and PLC finally suppress the display of the CTL epitope, HBV core peptide 18–27- MHC class I (FLPSDFFPSV-HLA-A2) complex on cell surface. All these events may limit the activation of CTLs and reduce their ability to recognize/eliminate HBV-expressing hepatocytes, thereby promoting HBV-infection pathogenesis.
Figure 2:
Non-genetic risk factors in exacerbating alcohol-related organ damage summarized in the Symposium: Alcohol potentiated by “second hits”, such as smoking, bacterial or viral infections exacerbate disease pathogenesis and increase downstream organ injury.
HIGHLIGHT.
Alcohol potentiated by “second hits”, such as smoking, bacterial or viral infections exacerbate disease pathogenesis and increase downstream organ injury.
GRANT SUPPORT
The research reported herein this summary and presented at the RSA annual meeting was supported by Merit Review grants, BX004053 (KKK) from the U.S. Department of Veterans Affairs and the National Institutes of Health under award numbers R21 AA026428 (SK, KKK), R01 AA026723 (KKK), K01 AA026864 (MG), R01 AA027189 (NAO), R01 AA015576 (GS), R01 AA017729 (GS), R01 CA228524 (SKB), R01 AA12863 (SW), P01 CA217798 (SKB), P60 AA009803 (PM), UH2 AA026198 (PM) and UH2 AA026226 (PM).
Abbreviations
- Ach
acetaldehyde
- AGS
acetaldehyde generating system
- ALD
alcoholic liver diseases
- AP
acute pancreatitis
- AST
aspartate aminotransferase
- ART
antiretroviral therapy
- cART
combination antiretroviral therapy
- CBA
chronic binge alcohol administration
- CHOP
C/EBP homologous protein
- ChT-L
chymotrypsin-like
- CP
chronic pancreatitis
- CSC
cigarette smoke condensate
- CTLs
cytotoxic T-lymphocytes
- DI
disposition index
- ECM
extracellular matrix
- EGR2
early growth response protein 2
- EtOH
alcohol
- FSIVGTT
frequently sampled intravenous glucose tolerance test
- HBV
hepatitis B virus
- HIV
Human immunodeficiency virus
- 4HNE
4-hydroxy-2-nonenal
- IF
immunofluorescence
- IPR
immunoproteasome
- LPS
lipopolysaccharide
- MAA
MDA-acetaldehyde-protein adduct
- MCP-1
monocyte chemoattractant protein-1
- MDA
malondialdehyde
- MHC
major histocompatibility complex
- MINMOD
modified minimal model
- NE
neutrophil elastase
- NETs
neutrophil extracellular traps
- NETotic
NETs-producing
- NHP
nonhuman primate
- OmAT
omental adipose tissue
- PAMPs
pathogen-associated molecules
- PGC-1β
peroxisome proliferator-activated receptor gamma coactivator 1 beta
- PLC
protein loading complex
- PLWH
persons living with HIV
- PPARγ
peroxisome proliferator-activated receptor gamma
- PSC
pancreatic stellate cells
- SIV
simian immunodeficiency virus
- SUC
sucrose
- SKM
skeletal muscle
- α-SMA
smooth muscle actin
- TAP1
transporter for the peptide-MHC class I complex
- T-L
trypsin-like
Footnotes
Conflicts of Interest: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 2014. W. H. O. M. C. [Website Name]. 2014. Available at: http://www.who.int/mediacentre/factsheets/fs204/en/ Accessed Date Accessed, 2014 Accessed.
- Amedee AM, Nichols WA, Robichaux S, Bagby GJ, Nelson S (2014) Chronic alcohol abuse and HIV disease progression: studies with the non-human primate model, Curr HIV Res 12, 243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte M, Pirola RC, Wilson JS (2015) Pancreatic stellate cell: physiologic role, role in fibrosis and cancer, Curr Opin Gastroenterol 31, 416–423. [DOI] [PubMed] [Google Scholar]
- Apte MV, Wilson JS, Korsten MA (1997) Alcohol-related pancreatic damage: mechanisms and treatment, Alcohol Health Res World 21, 13–20. [PMC free article] [PubMed] [Google Scholar]
- Arab JP, Arrese M, Shah VH (2020) Gut microbiota in non-alcoholic fatty liver disease and alcohol-related liver disease: Current concepts and perspectives, Hepatol Res 50, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MA, Dufour JF, Gerbes AL, Zoulim F, Bataller R, Burra P, Cortez-Pinto H, Gao B, Gilmore I, Mathurin P, Moreno C, Poznyak V, Schnabl B, Szabo G, Thiele M, Thursz MR (2020) Recent advances in alcohol-related liver disease (ALD): summary of a Gut round table meeting, Gut 69, 764–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S (2006) Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease, Alcohol Clin Exp Res 30, 1781–90. [DOI] [PubMed] [Google Scholar]
- Bajaj JS (2019) Alcohol, liver disease and the gut microbiota, Nat Rev Gastroenterol Hepatol 16, 235–246. [DOI] [PubMed] [Google Scholar]
- Bernardino JI, Mocroft A, Wallet C, de Wit S, Katlama C, Reiss P, Mallon PW, Richert L, Molina JM, Knobel H, Morlat P, Babiker A, Pozniac A, Raffi F, Arribas JR, Group NATS (2019) Body composition and adipokines changes after initial treatment with darunavir-ritonavir plus either raltegravir or tenofovir disoproxil fumarate-emtricitabine: A substudy of the NEAT001/ANRS143 randomised trial, PLoS One 14, e0209911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Park O, Gao B (2013) Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin, Hepatology 58, 1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule LA, Kovacs EJ (2017) Alcohol, aging, and innate immunity, J Leukoc Biol 102, 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria, Science 303, 1532–5. [DOI] [PubMed] [Google Scholar]
- Bruhn KW, Dekitani K, Nielsen TB, Pantapalangkoor P, Spellberg B (2016) Ly6G-mediated depletion of neutrophils is dependent on macrophages, Results Immunol 6, 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukong TN, Cho Y, Iracheta-Vellve A, Saha B, Lowe P, Adejumo A, Furi I, Ambade A, Gyongyosi B, Catalano D, Kodys K, Szabo G (2018) Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use, J Hepatol 69, 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess MJ, Zeuli JD, Kasten MJ (2015) Management of HIV/AIDS in older patients-drug/drug interactions and adherence to antiretroviral therapy, HIV AIDS 7, 251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A, Cooper DA (2000) Adverse effects of antiretroviral therapy, Lancet 356, 1423–30. [DOI] [PubMed] [Google Scholar]
- Casini A, Galli A, Pignalosa P, Frulloni L, Grappone C, Milani S, Pederzoli P, Cavallini G, Surrenti C (2000) Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis, J Pathol 192, 81–89. [DOI] [PubMed] [Google Scholar]
- Cassard AM, Gerard P, Perlemuter G (2017) Microbiota, Liver Diseases, and Alcohol, Microbiol Spectr 5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). HIV among people aged 50 and older [Website Name]. Available at: https://www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed Date Accessed, Accessed.
- Chefalo PJ, Grandea AG 3rd, Van Kaer L, Harding CV (2003) Tapasin−/− and TAP1−/− macrophages are deficient in vacuolar alternate class I MHC (MHC-I) processing due to decreased MHC-I stability at phagolysosomal pH, J Immunol 170, 5825–33. [DOI] [PubMed] [Google Scholar]
- Chen X, Tang Y, Zhang Y, Zhuo M, Tang Z, Yu Y, Zang G (2014) Tapasin modification on the intracellular epitope HBcAg18–27 enhances HBV-specific CTL immune response and inhibits hepatitis B virus replication in vivo, Lab Invest 94, 478–90. [DOI] [PubMed] [Google Scholar]
- Compston J (2015) HIV infection and osteoporosis, BoneKEy Rep 4, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigliaro J, Justice AC, Gordon AJ, Bryant K (2006) Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: A conceptual model to guide the implementation of evidence-based interventions into practice, Med Care 44, S1–6. [DOI] [PubMed] [Google Scholar]
- Costin JM (2007) Cytopathic mechanisms of HIV-1, Virol J 4, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debroy P, Sim M, Erlandson KM, Falutz J, Prado CM, Brown TT, Guaraldi G, Lake JE, Modena HIVMCT (2019) Progressive increases in fat mass occur in adults living with HIV on antiretroviral therapy, but patterns differ by sex and anatomic depot, J Antimicrob Chemother. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplanty AA, Simon L, Molina PE (2017) Chronic binge alcohol-induced dysregulation of mitochondrial-related genes in skeletal muscle of simian immunodeficiency virus-infected rhesus macaques at end-stage disease, Alcohol Alcohol 52, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrera C, Fadeel B (2013) Macrophage clearance of neutrophil extracellular traps is a silent process, J Immunol 191, 2647–56. [DOI] [PubMed] [Google Scholar]
- Ford SM Jr., Simon L, Vande Stouwe C, Allerton T, Mercante DE, Byerley LO, Dufour JP, Bagby GJ, Nelson S, Molina PE (2016) Chronic binge alcohol administration impairs glucose-insulin dynamics and decreases adiponectin in asymptomatic simian immunodeficiency virus-infected macaques, Am J Physiol Regul Integr Comp Physiol 311, R888–R897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SM Jr., Simon Peter L, Berner P, Cook G, Vande Stouwe C, Dufour J, Bagby G, Nelson S, Molina PE (2018) Differential contribution of chronic binge alcohol and antiretroviral therapy to metabolic dysregulation in SIV-infected male macaques, Am J Physiol Endocrinol Metab 315, E892–e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Voigt RM, Burgess HJ, Swanson GR, Keshavarzian A (2015) Circadian rhythms, alcohol and gut interactions, Alcohol 49, 389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Voigt RM, Keshavarzian A (2014) Intestinal CYP2E1: A mediator of alcohol-induced gut leakiness, Redox Biol 3, 40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA (2001) Frailty in older adults: Evidence for a phenotype, J Gerontol A Biol Sci Med Sci 56, M146–56. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M (2002) The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: Results from the HIV Cost and Services Utilization Study, J Stud Alcohol 63, 179–86. [DOI] [PubMed] [Google Scholar]
- Ganesan M, Krutik VM, Makarov E, Mathews S, Kharbanda KK, Poluektova LY, Casey CA, Osna NA (2019) Acetaldehyde suppresses the display of HBV-MHC class I complexes on HBV-expressing hepatocytes, Am J Physiol Gastrointest Liver Physiol 317, G127–G140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan M, Natarajan SK, Zhang J, Mott JL, Poluektova LI, McVicker BL, Kharbanda KK, Tuma DJ, Osna NA (2016) Role of apoptotic hepatocytes in HCV dissemination: regulation by acetaldehyde, Am J Physiol Gastrointest Liver Physiol 310, G930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan M, Poluektova LY, Enweluzo C, Kharbanda KK, Osna NA (2018) Hepatitis C Virus-Infected Apoptotic Hepatocytes Program Macrophages and Hepatic Stellate Cells for Liver Inflammation and Fibrosis Development: Role of Ethanol as a Second Hit, Biomolecules 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan M, Poluektova LY, Kharbanda KK, Osna NA (2018) Liver as a target of human immunodeficiency virus infection, World J Gastroenterol 24, 4728–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan M, Zhang J, Bronich T, Poluektova LI, Donohue TM Jr., Tuma DJ, Kharbanda KK, Osna NA (2015) Acetaldehyde accelerates HCV-induced impairment of innate immunity by suppressing methylation reactions in liver cells, Am J Physiol Gastrointest Liver Physiol 309, G566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorky J, Schwaber J (2016) The role of the gut-brain axis in alcohol use disorders, Prog Neuropsychopharmacol Biol Psychiatry 65, 234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Chisari FV (2006) Immunobiology and pathogenesis of viral hepatitis, Annu Rev Pathol 1, 23–61. [DOI] [PubMed] [Google Scholar]
- Gustot T, Fernandez J, Szabo G, Albillos A, Louvet A, Jalan R, Moreau R, Moreno C (2017) Sepsis in alcohol-related liver disease, J Hepatol 67, 1031–1050. [DOI] [PubMed] [Google Scholar]
- Hughes SA, Wedemeyer H, Harrison PM (2011) Hepatitis delta virus, Lancet 378, 73–85. [DOI] [PubMed] [Google Scholar]
- Hulgan T, Ramsey BS, Koethe JR, Samuels DC, Gerschenson M, Libutti DE, Sax PE, Daar ES, McComsey GA, Brown TT (2019) Relationships between adipose mitochondrial function, serum adiponectin, and insulin resistance in persons with HIV after 96 weeks of antiretroviral therapy, J Acquir Immune Defic Syndr 80, 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad M, Gupta P, Irshad K (2019) Immunopathogenesis of Liver Injury During Hepatitis C Virus Infection, Viral Immunol 32, 112–120. [DOI] [PubMed] [Google Scholar]
- Isogawa M, Tanaka Y (2015) Immunobiology of hepatitis B virus infection, Hepatol Res 45, 179–89. [DOI] [PubMed] [Google Scholar]
- Jaeschke H (2002) Neutrophil-mediated tissue injury in alcoholic hepatitis, Alcohol 27, 23–7. [DOI] [PubMed] [Google Scholar]
- Katz PS, Siggins RW, Porretta C, Armstrong ML, Zea AH, Mercante DE, Parsons C, Veazey RS, Bagby GJ, Nelson S, Molina PE, Welsh DA (2015) Chronic alcohol increases CD8+ T-cell immunosenescence in simian immunodeficiency virus-infected rhesus macaques, Alcohol 49, 759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda KK, Shubert KA, Wyatt TA, Sorrell MF, Tuma DJ (2002) Effect of malondialdehyde-acetaldehyde-protein adducts on the protein kinase C-dependent secretion of urokinase-type plasminogen activator in hepatic stellate cells, Biochem Pharmacol 63, 553–562. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Todero SL, Shubert KA, Sorrell MF, Tuma DJ (2001) Malondialdehyde-acetaldehyde-protein adducts increase secretion of chemokines by rat hepatic stellate cells, Alcohol 25, 123–128. [DOI] [PubMed] [Google Scholar]
- Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, Mayerle J, Drewes AM, Rebours V, Akisik F, Munoz JED, Neoptolemos JP (2017) Chronic pancreatitis, Nat Rev Dis Primers 3, 17060. [DOI] [PubMed] [Google Scholar]
- Kumar S, Torres MP, Kaur S, Rachagani S, Joshi S, Johansson SL, Momi N, Baine MJ, Gilling CE, Smith LM, Wyatt TA, Jain M, Joshi SS, Batra SK (2015) Smoking accelerates pancreatic cancer progression by promoting differentiation of MDSCs and inducing HB-EGF expression in macrophages, Oncogene 34, 2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankisch PG, Apte M, Banks PA (2015) Acute pancreatitis, Lancet 386, 85–96. [DOI] [PubMed] [Google Scholar]
- Larkin J, Clayton MM, Liu J, Feitelson MA (2001) Chronic ethanol consumption stimulates hepatitis B virus gene expression and replication in transgenic mice, Hepatology 34, 792–7. [DOI] [PubMed] [Google Scholar]
- LeCapitaine NJ, Wang ZQ, Dufour JP, Potter BJ, Bagby GJ, Nelson S, Cefalu WT, Molina PE (2011) Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting, J Infect Dis 204, 1246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Lim JS, Lee KY, Choi YK, Choe IS, Chung TW, Kim K (1997) Peptide-specific CTL induction in HBV-seropositive PBMC by stimulation with peptides in vitro: novel epitopes identified from chronic carriers, Virus Res 50, 185–94. [DOI] [PubMed] [Google Scholar]
- Lefevre F, O’Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, Glassroth J (1995) Alcohol consumption among HIV-infected patients, J Gen Intern Med 10, 458–60. [DOI] [PubMed] [Google Scholar]
- Li ZM, Kong CY, Zhang SL, Han B, Zhang ZY, Wang LS (2019) Alcohol and HBV synergistically promote hepatic steatosis, Ann Hepatol [DOI] [PubMed] [Google Scholar]
- Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP Jr., Klein DB, Towner WJ, Horberg MA, Silverberg MJ (2016) Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care, J Acquir Immune Defic Syndr 73, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey VL, Beier JI, Ritzenthaler JD, Roman J, Arteel GE (2015) Potential Role of the Gut/Liver/Lung Axis in Alcohol-Induced Tissue Pathology, Biomolecules 5, 2477–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill ML, Kharbanda KK, Tuma DJ, Reynolds JD, DeVasure JM, Sisson JH, Wyatt TA (2011) Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke, Alcohol Clin Exp Res 35, 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni M, Longo M, Dongiovanni P (2019) Alcohol or Gut Microbiota: Who Is the Guilty?, Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R (2007) Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome, Hepatology 46, 831–40. [DOI] [PubMed] [Google Scholar]
- Nagy LE (2015) The Role of Innate Immunity in Alcoholic Liver Disease, Alcohol Res 37, 237–50. [PMC free article] [PubMed] [Google Scholar]
- Nguyen KA, Peer N, de Villiers A, Mukasa B, Matsha TE, Mills EJ, Kengne AP (2017) Metabolic syndrome in people living with human immunodeficiency virus: An assessment of the prevalence and the agreement between diagnostic criteria, Int J Endocrinol 2017, 1613657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noubissi EC, Katte JC, Sobngwi E (2018) Diabetes and HIV, Curr Diab Rep 18, 125. [DOI] [PubMed] [Google Scholar]
- Novo-Veleiro I, Alvela-Suarez L, Chamorro AJ, Gonzalez-Sarmiento R, Laso FJ, Marcos M (2016) Alcoholic liver disease and hepatitis C virus infection, World J Gastroenterol 22, 1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna NA, Bardag-Gorce F, White RL, Weinman SA, Donohue TM Jr., Kharbanda KK (2012) Ethanol and hepatitis C virus suppress peptide-MHC class I presentation in hepatocytes by altering proteasome function, Alcohol Clin Exp Res 36, 2028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna NA, Donohue TM Jr., Kharbanda KK (2017) Alcoholic Liver Disease: Pathogenesis and Current Management, Alcohol Res 38, 147–161. [PMC free article] [PubMed] [Google Scholar]
- Osna NA, Ganesan M, Kharbanda KK (2015) Hepatitis C, innate immunity and alcohol: friends or foes?, Biomolecules 5, 76–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna NA, Haorah J, Krutik VM, Donohue TM Jr. (2004) Peroxynitrite alters the catalytic activity of rodent liver proteasome in vitro and in vivo, Hepatology 40, 574–82. [DOI] [PubMed] [Google Scholar]
- Osna NA, Kharbanda KK (2016) Multi-Organ Alcohol-Related Damage: Mechanisms and Treatment, Biomolecules 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltenburg NC, Bierau J, Schippers JA, Lowe SH, Paulussen ADC, van den Bosch BJC, Leers MPG, Andrinopoulou ER, Bakker JA, Verbon A (2019) Metabolic events in HIV-infected patients using abacavir are associated with erythrocyte inosine triphosphatase activity, J Antimicrob Chemother 74, 157–164. [DOI] [PubMed] [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, Veazey RS (2006) Intestinal lymphocyte subsets and turnover are affected by chronic alcohol consumption: Implications for SIV/HIV infection, J Acquir Immune Defic Syndr 41, 537–47. [DOI] [PubMed] [Google Scholar]
- Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS (2015) Gut microbiota, cirrhosis, and alcohol regulate bile acid metabolism in the gut, Dig Dis 33, 338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfaty L (2016) Clinical Implications of Concomitant Alcohol Use, Obesity, and Viral Hepatitis, Gastroenterology 150, 1718–22. [DOI] [PubMed] [Google Scholar]
- Shearn CT, Backos DS, Orlicky DJ, Smathers-McCullough RL, Petersen DR (2014) Identification of 5’ AMP-activated kinase as a target of reactive aldehydes during chronic ingestion of high concentrations of ethanol, J Biol Chem 289, 15449–15462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearn CT, Smathers RL, Backos DS, Reigan P, Orlicky DJ, Petersen DR (2013) Increased carbonylation of the lipid phosphatase PTEN contributes to Akt2 activation in a murine model of early alcohol-induced steatosis, Free Radic Biol Med 65, 680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HP, Wang A, Sander M (2013) Pancreas organogenesis: from lineage determination to morphogenesis, Annu Rev Cell Dev Biol 29, 81–105. [DOI] [PubMed] [Google Scholar]
- Shoreibah M, Anand BS, Singal AK (2014) Alcoholic hepatitis and concomitant hepatitis C virus infection, World J Gastroenterol 20, 11929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins RW, Bagby GJ, Molina P, Dufour J, Nelson S, Zhang P (2009) Alcohol exposure impairs myeloid dendritic cell function in rhesus macaques, Alcohol Clin Exp Res 33, 1524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Ford SM Jr., Song K, Berner P, Vande Stouwe C, Nelson S, Bagby GJ, Molina PE (2017) Decreased myoblast differentiation in chronic binge alcohol-administered simian immunodeficiency virus-infected male macaques: Role of decreased miR-206, Am J Physiol Regul Integr Comp Physiol 313, R240–R250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, LeCapitaine N, Berner P, Vande Stouwe C, Mussell JC, Allerton T, Primeaux SD, Dufour J, Nelson S, Bagby GJ, Cefalu W, Molina PE (2014) Chronic binge alcohol consumption alters myogenic gene expression and reduces in vitro myogenic differentiation potential of myoblasts from rhesus macaques, Am J Physiol Regul Integr Comp Physiol 306, R837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi A, Yadav D (2018) Myths and realities about alcohol and smoking in chronic pancreatitis, Curr Opin Gastroenterol 34, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Smith FM, Lang CH, Nagy LE, Bailey SM, Parsons LH, Murray GJ (2016) Physiological processes underlying organ injury in alcohol abuse, Am J Physiol Endocrinol Metab 311, E605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G (2015) Gut-liver axis in alcoholic liver disease, Gastroenterology 148, 30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P (2010) Focus on: Alcohol and the liver, Alcohol Res Health 33, 87–96. [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Petrasek J, Bala S (2012) Innate immunity and alcoholic liver disease, Dig Dis 30 Suppl 1, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Forsyth CB, Keshavarzian A (2014) The role of miRNAs in alcohol-induced endotoxemia, dysfunction of mucosal immunity, and gut leakiness, Alcohol Clin Exp Res 38, 2331–4. [DOI] [PubMed] [Google Scholar]
- Taramasso L, Ricci E, Menzaghi B, Orofino G, Passerini S, Madeddu G, Martinelli CV, De Socio GV, Squillace N, Rusconi S, Bonfanti P, Di Biagio A, Group CS (2017) Weight gain: A possible side effect of all antiretrovirals, Open Forum Infect Dis 4, ofx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temko JE, Bouhlal S, Farokhnia M, Lee MR, Cryan JF, Leggio L (2017) The Microbiota, the Gut and the Brain in Eating and Alcohol Use Disorders: A ‘Menage a Trois’?, Alcohol Alcohol 52, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng TC, Huang LR (2017) Immunopathogenesis of Hepatitis B Virus, J Infect Dis 216, S765–S770. [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Casey CA (2003) Dangerous byproducts of alcohol breakdown--focus on adducts, Alcohol Res Health 27, 285–290. [PMC free article] [PubMed] [Google Scholar]
- Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF (1996) Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration, Hepatology 23, 872–880. [DOI] [PubMed] [Google Scholar]
- Vassallo G, Mirijello A, Ferrulli A, Antonelli M, Landolfi R, Gasbarrini A, Addolorato G (2015) Review article: Alcohol and gut microbiota - the possible role of gut microbiota modulation in the treatment of alcoholic liver disease, Aliment Pharmacol Ther 41, 917–27. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Amedee A, Wang X, Bernice Kaack M, Porretta C, Dufour J, Welsh D, Happel K, Pahar B, Molina PE, Nelson S, Bagby GJ (2015) Chronic binge alcohol administration increases intestinal t-cell proliferation and turnover in rhesus macaques, Alcohol Clin Exp Res 39, 1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlaufen A, Wilson JS, Pirola RC, Apte MV (2007) Role of alcohol metabolism in chronic pancreatitis, Alcohol Res Health 30, 48–54. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018) Global Status Report on Alcohol and Health 2018. World Health Organization, Geneva. [Google Scholar]
- Wyatt TA, Kharbanda KK, McCaskill ML, Tuma DJ, Yanov D, DeVasure J, Sisson JH (2012) Malondialdehyde-acetaldehyde-adducted protein inhalation causes lung injury, Alcohol 46, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Huang H, Zhang Z, Wang FS (2014) The role of neutrophils in the development of liver diseases, Cell Mol Immunol 11, 224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Zhao Q, Sharma V, Nguyen LP, Lee YN, Pham KL, Edderkaoui M, Pandol SJ, Park W, Habtezion A (2016) Aryl Hydrocarbon Receptor Ligands in Cigarette Smoke Induce Production of Interleukin-22 to Promote Pancreatic Fibrosis in Models of Chronic Pancreatitis, Gastroenterology 151, 1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Forsmark CE (2017) Chronic pancreatitis, Curr Opin Gastroenterol 33, 396–403. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Govindarajan S, Arakawa K, Yu MC (2004) Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S, Cancer 101, 1009–17. [DOI] [PubMed] [Google Scholar]
- Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, Rocca S, Zangari P, Manno EC, Palma P (2019) Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART, Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]