Abstract
Objective:
The present study compared wave I amplitude of auditory brainstem responses (ABRs), a potential indicator of cochlear synaptopathy, among musicians and non-musicians with normal audiograms.
Design:
Noise exposure background (NEB) was evaluated using an online questionnaire. Two-channel ABRs were recorded from the left ear using click stimuli. One channel utilized an ipsilateral tiptrode, and another channel utilized an ipsilateral mastoid electrode. ABRs were collected at 90, 75, and 60 dBnHL. A mixed model was used to analyze the effect of group, electrodes, and stimulus levels on ABR wave I amplitude.
Study sample:
75 collegiate students with normal hearing participated in the study and were grouped into a non-music major group (n=25), a brass major group (n=25), and a voice major group (n=25).
Results:
The NEB was negatively associated with the action potential (AP) and ABR wave I amplitude for click intensity levels at 75 dBnHL. The mean amplitude of the ABR wave I was not significantly different between the three groups.
Conclusion:
The weak negative association of AP and ABR wave I amplitude with NEB cannot be solely attributed to evidence of cochlear synaptopathy in humans as the possibility of hair cell damage cannot be ruled out. Future research should investigate the effects of reduced cochlear output on the supra-threshold speech processing abilities of student musicians.
Keywords: Brass majors, Voice majors, Non-musicians, Synaptopathy, Hearing thresholds, Auditory Brainstem Response (ABR), Amplitude, Noise exposure
1. Introduction:
Noise-induced hearing loss (NIHL) remains one of the leading occupational disorders despite the Occupational Safety and Health Administration (OSHA) implementing standards for hearing protection and public health awareness campaigns. It is estimated that more than 500 million individuals worldwide are at risk of developing NIHL due to excessive noise exposure (Nelson et al., 2005). Recent studies have shown that noise can induce changes in the peripheral auditory system that can alter the sound-evoked output of the auditory nerve independent of hair cell damage and changes in hearing thresholds (e.g., Kujawa & Liberman, 2009). This type of peripheral hearing loss could lead to impaired speech perception, and therefore, it has been referred to as “hidden hearing loss” (HHL) to reflect that the dysfunction is not revealed by the standard hearing test (Schaette & McAlpine, 2011).
Studies based on animal models have revealed that intense noise exposures can inflict irreversible damage to the synaptic connections between the inner hair cells (IHSs) of the cochlea and auditory nerve fibers in animals (Kujawa and Liberman, 2009; Lin et al., 2011; Furman et al., 2013). These studies evaluated the auditory functions before and after a complete recovery from TTS using auditory brainstem responses (ABR) and distortion-product otoacoustic emissions (DPOAE). These studies showed abrupt and permanent loss of up to 50% of afferent nerve terminal connections between IHCs and auditory nerve fibers. The ABR and DPOAE thresholds showed full recovery to pre-noise exposure levels. However, predominantly at high stimulus levels, ABR wave I amplitude was smaller in noise-exposed animals than the controls and the pre-exposure baseline. This phenomenon of loss of synaptic ribbons and nerve fibers post-TTS without affecting hearing thresholds is referred to as noise-induced cochlear synaptopathy. This noise-induced cochlear synaptopathy mostly affects low-spontaneous rate auditory nerve fibers, the ones that encode supra-threshold sounds (Furman et al., 2013). With noise-induced cochlear synaptopathy, fibers with high-spontaneous rates encoding near-threshold sounds remain unaffected, thus making this pathology insensitive to audiograms. However, it is predicted that noise-induced cochlear synaptopathy may manifest as auditory processing difficulties in challenging listening environments in the absence of clinically elevated behavioral hearing thresholds leading to HHL (Kujawa and Liberman, 2009; Furman et al., 2013).
Recent studies have shown that demyelination of auditory nerve can cause HHL independent of cochlear synaptopathy (e.g., Tagoe et al., 2014; Wan and Corfas, 2017; Kohrman et al., 2020). Myelination of the peripheral auditory system is essential for normal conduction of action potential and synchronized transmission of neural impulses. Proper growth and maintenance of auditory nerve myelination by Schwann cells are crucial for auditory processing fidelity. Defects in myelination have been associated with hearing loss in both humans and animals (Long et al., 2018). Schwann cells are important for the formation of nodes of Ranvier, the exclusive regions along myelinated fibers at which voltage-gated sodium and potassium channels are clustered for the regeneration of action potentials and the fast, synchronized transmission of electrical signals (Rasband and Peles, 2015). Wan and Corfas (2017) demonstrated that ablation of Schwann cells via genetic means causes a near-total loss of auditory nerve myelin within one week. This demyelination does not change auditory thresholds yet induces a permanent HHL evident in the form of significantly reduced suprathreshold ABR wave I amplitude and increased ABR wave I latency. In conclusion, the literature indicates that suprathreshold ABR wave amplitude and latency could be affected by noise exposure that may lead to HHL in humans. The confirmatory diagnosis of the cochlear synaptopathy includes the evaluation of the cochlear tissues. These invasive evaluations are not feasible for human studies. Therefore, the studies investigating cochlear synaptopathy in humans rely on the electrophysiological correlates that are sensitive to identify damage to the cochlear synaptic junctions. It is not yet clear to what extent these animal findings translate to humans, and the exact nature of any supra-threshold perceptual consequences of cochlear synaptopathy is now subject to intense scientific investigation (Le Prell and Clavier, 2017).
The first direct investigation of cochlear synaptopathy in humans was published by Stamper and Johnson (2015a), who obtained negative correlation coefficients between the amplitude of wave I of the ABR and noise exposure background (NEB). NEB was defined as the amount of noise exposure an individual has encountered in daily life. It was estimated using a validated questionnaire that inquired about the frequency and duration of noise exposure in ‘routine’ (e.g., home, travel, etc.) and ‘episodic’ activities (e.g., power tools, attending sporting events, etc.). The authors observed that their experimental group with high NEB contained a majority of male participants that might be a potential confound for showing weaker ABRs. Their re-analysis found a decrease in ABR wave I amplitude as a function of NEB for females, but no such relation was observed for males (Stamper and Johnson, 2015b). In a similar investigation, Liberman et al. (2016) found that individuals with high NEB had elevated behavioral hearing thresholds at ultra-high frequencies, elevated summating potential (SP; reflecting hair cell function) and compound action potential (AP; equivalent to wave I of ABR, reflecting auditory nerve function) ratio, poor performance on word recognition in noise and heightened reaction to sounds. Similarly, Bramhall et al. (2017) found evidence of lower ABR wave I amplitude in young military veterans with normal audiograms, and Grose et al. (2017) reported a reduced ratio of wave I and V amplitude in individuals with a history of frequent attendance at loud music events. On the other hand, some studies testing young adults (≤ 35 years) found no association between noise exposure and reduction in ABR wave I amplitude or subsequent supra-threshold deficits in speech-in-noise tasks (Fulbright et al., 2017; Grinn et al., 2017; Guest et al., 2017; Prendergast et al., 2017; Yeend et al., 2017).
Collegiate student musicians are a population with high risk for NIHL because they are exposed to intense sound levels during individual practice and group rehearsals on a daily basis (e.g., Barlow, 2010; Gopal et al., 2013; Washnik et al., 2016; Tufts and Skoe, 2018). Music students are exposed to higher sound exposure levels than their non-musicians counterparts largely due to participation in music activities and noisier social activities (Tufts & Skoe, 2018). Washnik et al. (2016) measured noise dose in collegiate student musicians and found that almost half of the student musicians exceeded a 100% daily noise dose limit on at least one of two measurement days. Similarly, Tufts and Skoe (2018) conducted continuous weeklong noise dosimetry measurements on student musicians and non-musicians. They reported that 47% of the student musician days exceeds 100% noise dose, while only 10% of the non-musician students’ days exceed 100% noise dose. The results showed that the higher cumulative noise exposure among student musicians was not only due to music activities but also due to the higher participation by student musicians in noisier social activities. Student musicians are likely to experience frequent episodes of TTS due to music practice and ensemble rehearsals (Gopal et al., 2013), which might increase their risk for acquiring cochlear synaptopathy, and subsequently to HHL (e.g., Wang and Ren, 2012). The ability to process signals in the presence of background noise is crucial for student musicians while performing in large ensembles. Therefore, noise-induced cochlear synaptopathy causing HHL in student musicians might further affect their music career.
The objective of the present study was to examine the relationship between a history of noise exposure and suprathreshold ABR measures in a sample of musicians and non-musicians with normal audiograms. We hypothesized that: (1) the suprathreshold ABR wave I amplitude, a potential indicator of cochlear synaptopathy in humans, would exhibit inverse relationship with noise exposure, (2) musicians would exhibit reduced ABR wave I amplitude compared to non-musicians, and (3) musicians would exhibit significantly prolonged ABR wave I latency compared to non-musicians.
Materials & Methods
2.1. Participants
The study was approved by the Institutional Review Board (IRB) at the University of North Carolina at Greensboro (IRB number - 16–0174). A recruitment flyer was distributed to undergraduate student musicians in the School of Music at the University of North Carolina at Greensboro. Potential participants were instructed to contact the investigators for arranging the laboratory session and were compensated with a $20 gift card at the end of the testing session. The sample of this study consisted of 25 non-musician students (23 females, 2 males), 25 brass major students (8 females, 17 males), and 25 voice major students (21 females, 4 males), with European descent and within the age range of 18–30 years. These students were grouped into three subgroups: a non-music major group, a brass major group, and a voice major group. All the participants from the brass major and voice major groups were classical music majors. Participants with European descent were included, as previous research suggests that people from European descent are more susceptible to NIHL than African Americans (Henselman et al., 1995; Ishii and Talbott, 1998; Jerger et al., 1986). All 75 participants passed the inclusion criteria.
2.2. Noise exposure questionnaire
Before testing the participants, the noise exposure history of each participant was assessed using an online noise exposure-screening questionnaire. This questionnaire was a modified version of the questionnaire developed by Johnson et al. (2017). Participants were asked to submit their responses to the questionnaire (Appendix A) at least one week before reporting for the testing session. The purpose of the questionnaire was to estimate noise exposure background (NEB). The first part of the questionnaire deals with sound exposure in the last year other than live music exposure. It was divided into nine sections, which deal with different types of noise exposure via power tools, heavy equipment, firearms, aircraft, music through headphones and speakers. The second part of the questionnaire was comprised of nine questions that were specific to musical instruments played by the participant. The questionnaire included questions about the frequency and duration of noise exposure. The responses were elicited using a forced-choice method. Responses were rated categorically to calculate the overall noise dose. The noise dose for each specific area of high noise exposure was calculated using the responses. Time spent in day-to-day activities performed in quiet environments was calculated by subtracting overall time spent in activities, with high acoustic exposure from 8760 hours (365 days/year × 24 hours/day). The responses from the questionnaire were further used to calculate the activity-specific noise dose and overall noise dose, which was reported as LAeq8760h. Here, “L” represents sound pressure level measured in dB, “A” presents use of an A-weighted frequency response; “eq” represents the sound pressure level (in dB) equivalent to the total sound energy over a given period of time; and “8760h” represents the total duration of the noise exposure in hours over 1 year (365 days/year × 24 hours/day). LAeq8760h was derived from the questionnaire data using the 3-dB exchange rate for calculation of the time/level relation. Details of the questionnaire can be found in Stamper and Johnson (2015a) and Johnson et al. (2017).
Participants reporting exposure to power tools, heavy equipment, aircraft, motorized vehicle, firecrackers, and firearm shooting on a monthly, weekly, or daily basis were not included. Non-musician participants who reported pursuing minor in music or playing any instrument, including voice, were excluded from the non-musician group. Potential participants were contacted through email to schedule a testing session. Participants were also informed to refrain from loud sound exposure at least 12 hours before reporting to the clinic for tests through email. Before beginning the testing session, the participants were asked to confirm that they had avoided loud sound exposure as requested. Participants who reported high noise exposure in the last 12 hours were rescheduled for the testing session. The testing session was comprised of consenting procedures, a case history, an otoscopy, an audiometric and immittance evaluation, and a suprathreshold ABR test.
2.3. Audiometric procedures
All audiometric measures described were collected in a sound-treated booth meeting ANSI standards (ANSI S3.1–1999). Audiometric thresholds were obtained at 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz (GSI-61, Eden Prairie, MN) with ER-3A insert receivers (Etymotic Research. Inc, Elk Grove Village, IL), using the modified Hughson-Westlake procedure. The hearing thresholds at 3000, 4000, and 6000 Hz were averaged to calculate the pure tone average (PTA346). Normal hearing sensitivity of participants was defined as pure tone behavioral thresholds of ≤ 15 dB HL for the octave and the inter-octave frequencies between 0.5 to 8 kHz. Normal middle ear function was defined as 226 Hz type A tympanogram. Participants with normal otoscopic findings, and with a tympanometric compliance value ranging from 0.33 to 1.75 cc, an ear canal volume ranging from 0.8 to 1.8 cc, and middle-ear pressure ranging from −50 daPa to 25 daPa in both ears were included in the study.
2.4. Electrophysiological measures
Auditory evoked potential testing was done using a commercial system (Smart EP, Intelligent Hearing Systems, Miami, FL) in a single-walled sound-treated room. ABRs were simultaneously measured using a two-channel electrode montage with a mastoid-placed electrode and a tiptrode inserted in the left ear canal. The non-inverting electrode and ground electrode was placed on the participant’s vertex (Cz) and low forehead (Fpz), while the inverting electrode from channel 1 and channel 2 (tiptrode) were placed on the mastoid of the left ear and left ear canal, respectively. These areas were prepped using an alcohol wipe and a Nuprep skin prep gel for effectively reducing the inter-electrode impedance values. Impedance values at each electrode site were monitored to remain < 3 kOhms with an inter-impedance value < 2 kOhms. These impedance values were monitored throughout the testing procedure. These impedance values were monitored throughout the testing procedure.
ABR stimuli were presented with rarefaction polarity at a rate of 11.3/sec using insert earphones (ER-3A, St. Paul, MN). ABR responses were obtained using 100 µ sec click stimuli. Presentation level began at 90 dBnHL (95.7 ±0.3 dB SPL, calibration in an IEC-711 ear simulator) and decreased in 15 dB steps to 60 dBnHL. At each stimulus presentation level, two replications of 2000 sweeps were collected for analysis. Recording parameters included a gain of 100,000 and band-pass filtering from 30 Hz to 1500 Hz. The artifact rejection threshold was set at 31 µV. ABRs were collected with a sampling frequency of 40000 Hz, a pre-stimulus window of −12.8 ms, and a post-stimulus window of 12.8 ms.
2.5. Electrophysiological waveform analysis
After ABR testing of each participant, the two replications at each intensity level were averaged, and the averaged waveforms were used for analyzing the ABR I, III, and V waveforms. The ABR wave I, III, and V amplitudes obtained with the mastoid electrode were calculated from the voltage difference between the identified positive peak and the following trough. Similarly, the ABR wave III and V amplitudes obtained with the tiptrode electrode were calculated from the voltage difference between the identified positive peak and the following trough. The ABR waveform elicited with tiptrode were used to measure AP amplitude, which was defined as being baseline-peak rather than peak-to-trough to be consistent with the previous studies (e.g., Liberman et al., 2016). AP amplitude was measured for all intensity levels and served as an electrophysiological index for auditory nerve physiology. Two audiologists separately identified the waveforms. Any disagreement pertaining to the peak identification between two audiologists were resolved by reviewing the data together.
2.6. Statistical Analysis
The statistical analyses were performed using IBM SPSS (version 25.0; SPSS, INC). A linear regression model was utilized for quantifying the relationship between NEB and measures of auditory nerve physiology (i.e., ABR wave I and AP amplitude). AP and ABR wave I amplitude were included as continuous dependent variables and NEB and gender as independent variables to quantify the influence of NEB on the dependent variable while controlling the effect of gender. A repeated measure mixed model was utilized to compare ABR wave I, III, and V amplitude and latency between musicians and non-musicians. The mixed model analyses were used to evaluate the effects of gender, groups, intensity levels, and electrode placement. The repeated measures mixed model analyses were conducted using SAS version 7.13 (SAS Institute, Cary, NC, USA).
2. Results:
3.1. Descriptive statistics
We recruited 75 young adults (mean age = 21.07, range = 18 to 29 years). The sample contained 52 females and 23 males. The average of hearing thresholds at 3, 4, and 6 kHz was calculated because the impact of noise exposure is higher on these frequencies than the other frequencies (McBride & Williams, 2001). PTA346 was not statistically different among the experimental groups (F(2, 72) = 0.338, p = 0.714) . The mean and standard error of hearing thresholds among three different groups are shown in Fig. 1. A Tukey test revealed that the mean NEB was significantly different between the groups. The mean NEB was higher for brass majors compared to the mean non-musicians (mean (brass)-mean (non-musician) = 8.37 LAeq8760h, p <10–8). Similarly, the mean NEB for voice majors was higher than non-musicians (mean (vocal)-mean (non-musician) = 7.12 LAeq8760h, p < 10–8). The mean NEB for brass majors was higher than for voice majors but was not statistically significant (mean difference = −1.24 LAeq8760h, p = 0.06). The mean NEB of non-musicians, brass majors, and voice majors groups were 68.18, 76.55, and 75.30 LAeq8760h, respectively. Fig. 2 presents NEB data as a function of experimental groups. The mean differences in NEB across the experimental groups were attributed to our sampling scheme (details in methods). Results revealed no statistically significant linear association between NEB and PTA346 (r(73)=−0.012, p = 0.91).
Figure 1.
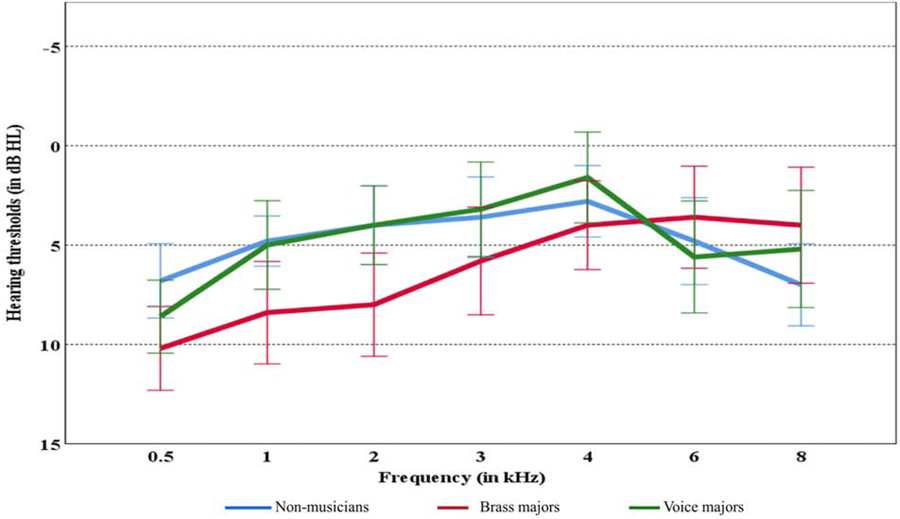
Mean hearing thresholds of left ear with standard error according to the groups
Figure 2.
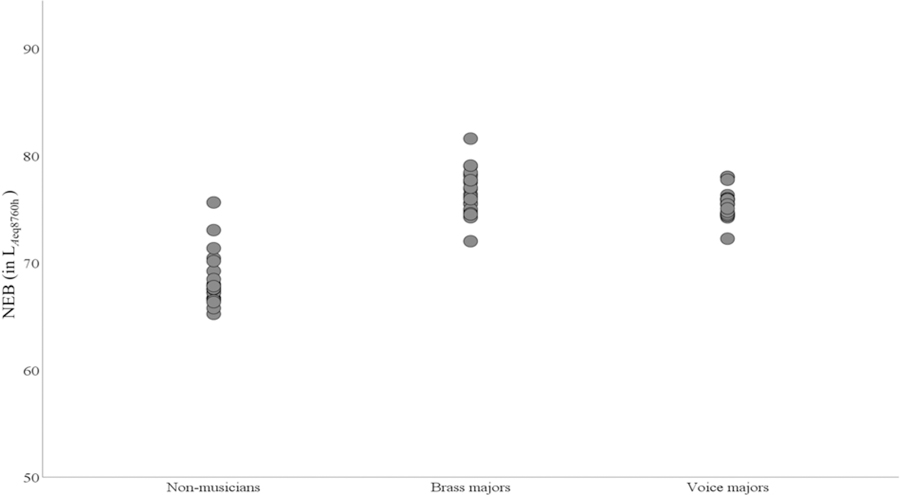
Simple scatter plot of NEB as a function of group
3.2. The relationship between NEB and electrophysiological measures of the auditory nerve:
Table 1 shows means and standard deviations for amplitudes of ABR wave I, III, and V obtained at 90, 75, and 60 dBnHL with the tiptrode and the mastoid according to gender. The grand averaged ABR waveforms at different intensity levels, and placement are shown in Fig. 3 and Fig. 4 for females and males, respectively. As shown in Table 1 and Fig. 3 and Fig. 4, wave I amplitude is larger for tiptrode than mastoid placement. The wave I amplitude is highest at 90 the dBnHL intensity level and decreases with a decrease in click levels.
Table 1.
The average amplitude of waves I, III, and V (nV) for each electrode placement, intensity level, and groups. The standard deviations are listed in parentheses.
| Gender: Female | |||||
|---|---|---|---|---|---|
| Wave I | Wave III | Wave V | |||
| Electrode Placement | Intensity | Groups | Mean (SD) | Mean (SD) | Mean (SD) |
| Mastoid | 90 | Non-musicians | 428.26(152.60) | 266.95(184.67) | 913.04(297.20) |
| Brass majors | 382.50(130.24) | 235.0(154.82) | 883.75(243.42) | ||
| Voice majors | 355.71(136.73) | 278.57(184.66) | 883.80(249.68) | ||
| 75 | Non-musicians | 363.48(110.56) | 221.73(126.55) | 612.60(223.67) | |
| Brass majors | 283.75(80.87) | 257.5(123.72) | 592.50(243.76) | ||
| Voice majors | 300.95(105.82) | 230.47(121.71) | 540.95(165.01) | ||
| 60 | Non-musicians | 121.30(65.03) | 73.47(51.22) | 357.39(142.03) | |
| Brass majors | 50.0(40.70) | 71.25(73.18) | 397.50(156.54) | ||
| Voice majors | 117.62(83.06) | 86.66(80.02) | 373.80(139.94) | ||
| Tiptrode | 90 | Non-musicians | 719.13(233.17) | 233.04(195.87) | 829.13(271.74) |
| Brass majors | 685.0(270.18) | 207.5(153.78) | 911.25(272.05) | ||
| Voice majors | 596.19(215.32) | 216.19(155.93) | 779.52(187.70) | ||
| 75 | Non-musicians | 620.43(186.85) | 199.13(122.02) | 529.56(204.32) | |
| Brass majors | 512.50(158.54) | 232.50(173.18) | 593.75(239.28) | ||
| Voice majors | 517.14(164.86) | 224.76(118.85) | 489.52(138.29) | ||
| 60 | Non musicians | 188.26(92.91) | 86.08(57.02) | 296.95(124.51) | |
| Brass majors | 113.75(59.98) | 86.25(57.55) | 361.25(127.21) | ||
| Voice majors | 159.52(99.17) | 103.8082.61) | 313.33(115.77) | ||
| Gender: Male | |||||
| Mastoid | 90 | Non-musicians | 310.00(42.42) | 310.00(42.42) | 890.00(14.14) |
| Brass majors | 312.35(97.50) | 247.05(163.04) | 758.82(260.83) | ||
| Voice majors | 285.00(47.95) | 345.00(116.18) | 820.00(225.83) | ||
| 75 | Non-musicians | 280.00(14.14) | 40.00(56.56) | 670.00(141.42) | |
| Brass majors | 242.94(97.64) | 147.64(87.21) | 515.88(196.34) | ||
| Voice majors | 210.00(77.02) | 187.50(55.60) | 497.50(156.92) | ||
| 60 | Non-musicians | 80.00(28.28) | 40.00(42.42) | 540.00(113.13) | |
| Brass majors | 66.47(42.85) | 49.41(59.42) | 318.23(101.13) | ||
| Voice majors | 45.00(12.91) | 42.50(27.53) | 475.00(205.50) | ||
| Tiptrode | 90 | Non-musicians | 665.00(35.35) | 330.00(84.85) | 755.00(120.20) |
| Brass majors | 592.35(157.54) | 230.58(196.06) | 730.58(247.90) | ||
| Voice majors | 517.50(120.38) | 350.00(135.15) | 780.00(201.99) | ||
| 75 | Non-musicians | 500.00(70.71) | 50.00(42.42) | 640.00(155.56) | |
| Brass majors | 485.88(194.93) | 150.00(96.43) | 474.11(196.05) | ||
| Voice majors | 395.00(160.10) | 175.00(50.66) | 502.50(148.63) | ||
| 60 | Non musicians | 150.00(84.85) | 85.00(106.06) | 440.00(113.13 | |
| Brass majors | 115.88(78.66) | 61.76(68.30) | 305.29(100.50) | ||
| Voice majors | 65.00(56.86) | 55.00(19.14) | 382.50(106.26) | ||
Mean and SDs are shown for ABR amplitude (nV) of wave I, III, and V obtained at 90, 75, and 60 dBnHL
Figure 3.
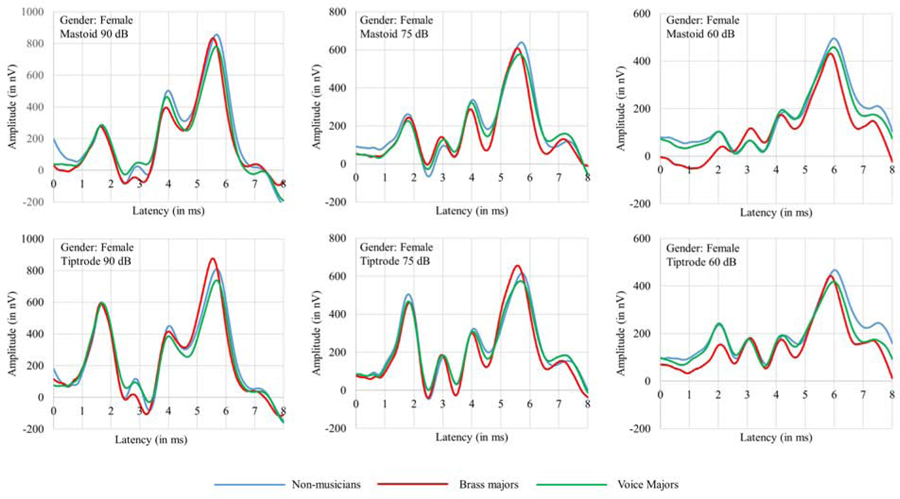
Grand average ABR waveforms of female participants of all the three groups collected with tiptrode and the mastoid electrode at 90, 75, and 60 dB nHL. The X-axis corresponds to latency and the Y-axis corresponds to ABR wave amplitude measured in nV.
Figure 4.
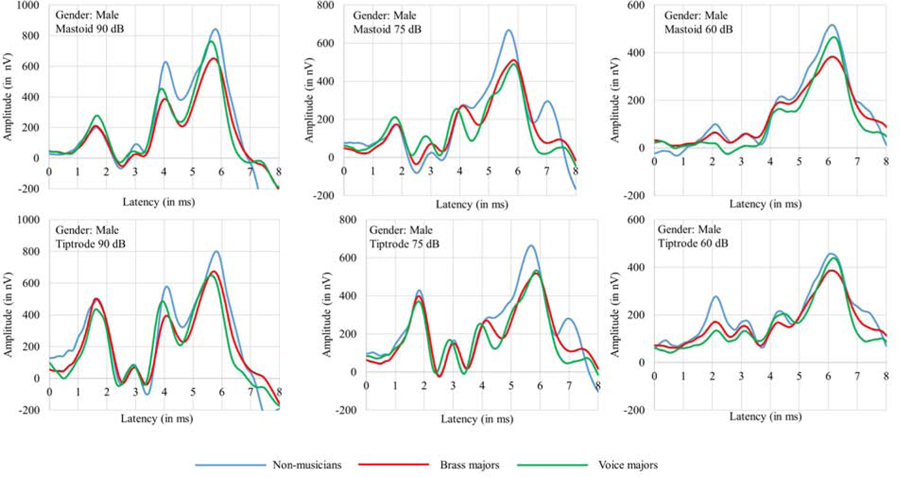
Grand average ABR waveforms of male participants of all the three groups collected with tiptrode and the mastoid electrode at 90, 75, and 60 dB nHL. The X-axis corresponds to latency and the Y-axis corresponds to ABR wave amplitude measured in nV.
Table 2 presents the results of the regression analyses. The threshold of the statistical significance was defined by p-value <0.05. The relationship between NEB and the amplitude of AP and between NEB and ABR Wave I was investigated while controlling the effects of potential confounder, gender. NEB revealed a significant association with AP and wave I for click intensity levels at 75 dBnHL. The adjusted R2 values for the models ranged from 0.04 to 0.19, indicating that a small portion of the variance in the dependent variables was exclusively attributed to NEB. Figure 5 displays the gender-wise scatter plots between NEB and amplitude of AP and wave I amplitude at 90, 75, and 60 dBnHL. Figure 5 highlights a consistent trend of a negative relationship between NEB and amplitude of AP and wave I at 90 and 75 dBnHL in females. Figure 5 further shows that there are only two and four male participants in the non-musicians and voice major groups, respectively. The lower number of male participants in these two groups is likely to compromise the statistical power for detecting the statistically significant relationship between NEB and amplitude of AP and Wave I in males.
Table 2.
Results of the regression analyses listing predictors for wave I and AP amplitudes (nV)
| Intensity: 90 dBnHL | Intensity: 75 dBnHL | Intensity: 60 dBnHL | |||||
|---|---|---|---|---|---|---|---|
| AP | Wave I | AP | Wave I | AP | Wave I | ||
| NEB | β value | −8.55+ | −3.61 | −12.39** | −6.79* | −1.32 | −1.98 |
| p value | 0.07 | 0.36 | 0.001 | 0.03 | 0.52 | 0.32 | |
| Gender | β value | −77.85+ | −70.76+ | −28.37 | −59.64* | −32.37+ | −37.38* |
| p value | 0.06 | 0.05 | 0.37 | 0.03 | 0.08 | 0.04 | |
| Model | Adj R2 | 0.11** | 0.07* | 0.19* | 0.16** | 0.04+ | 0.08* |
| p value | 0.004 | 0.02 | 0.0001 | 0.001 | 0.07 | 0.017 | |
p<0.1
p<0.05
p<0.01
p<0.001;
unstandardized coefficients β and adjusted R2 values are listed.
Figure 5.
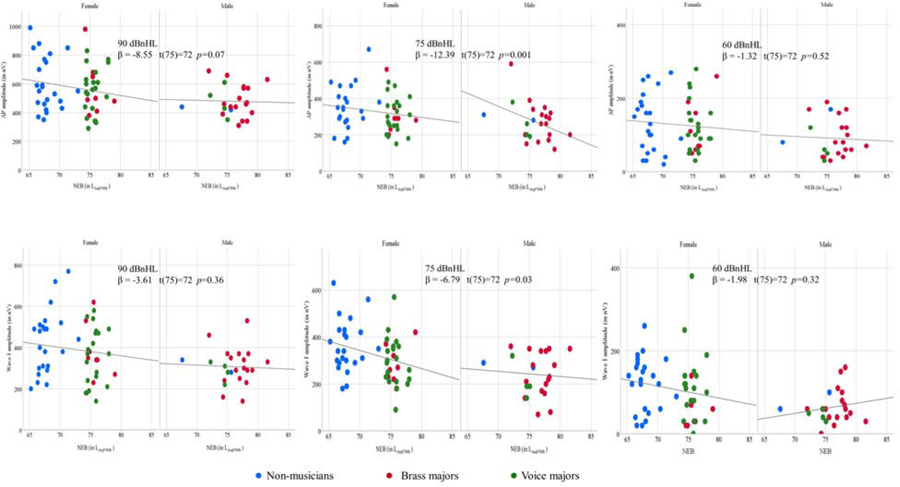
Scatter plots for NEB and AP amplitude (top row) and NEB and ABR wave I amplitude (bottom row) for females and males. Linear regression lines were inserted to show the predictive relationship. AP amplitude (top row) recorded with tiptrode and ABR wave I amplitude (bottom row) recorded with the mastoid electrode. The stimulus level is denoted in each panel (90 to 60 dB nHL). Regression results are shown in each panel.
3.3. Comparison of suprathreshold ABR measures among non-musicians, brass-majors, and voice-majors:
The ABR wave I and AP amplitude data were nested at three levels (i.e., at the level of intensity [90, 75, 60 dB], reference electrode placement [mastoid, tiptrode], and group [non-musicians, brass-majors, voice majors]) to evaluate the effects of groups, placement, and intensity levels while controlling for the effect of gender. The repeated-measures ANOVA with the mixed-model design was used to study the differences between the groups. Table 3, which summarizes the repeated measure ANOVA analysis, shows the main effects for gender, groups, intensity, and placement. The main effects for group (F(2, 69)= 1.222; p = 0.3018) and gender (F(1,69)=3.71: p=0.0583) were not statistically significant. This finding shows that the amplitude of ABR wave I was not significantly different between the three groups. The main effect for placement (F(1, 216)=670.79; p <0.0001) and intensity (F(2,144)=339.57; p <0.0001) were statistically significant.
Table 3.
Summary of repeated measure ANOVA analysis: main effects for gender, groups, intensity, and placement on ABR wave I amplitude.
| Effect | Num DF | Den DF | F Value | Pr > F |
|---|---|---|---|---|
| Gender | 1 | 69 | 3.71 | 0.0583 |
| Groups | 2 | 69 | 1.22 | 0.3018 |
| Intensity | 2 | 144 | 339.57 | <.0001 |
| Placement | 1 | 216 | 670.79 | <.0001 |
The amplitude of ABR wave III was analyzed using repeated-measures ANOVA to study the difference between the groups. The main effect for the group was not statistically significant for wave III amplitude (F(2,71)=0.32; p=0.7253). A similar analysis revealed that the main effect for groups was not statistically significant for wave V amplitude (F(2,71)=0.19; p=0.8293). The latencies of ABR wave I, III, and V were also studied using the repeated measures ANOVA with mixed model design. The main effect for group for the latencies of wave I (F(2,71)=0.47; p=0.6279), wave III (F(2,71)=0.44; p=0.6438), and wave V (F(2,71)=0.54; p=0.5823) were not statistically significant.
4. Discussion:
The results of this study support the relationship between NEB and the ABR wave I and AP amplitude, however, the fact that this trend was observed only at AP and ABR wave I elicited using 75 dBnHL clicks and not at 90 dBnHL clicks highlights that the relationship between these two variables must be interpreted with caution. It appears that the relationship between NEB and AP amplitude was consistent with the cochlear synaptopathy literature for female participants (Figure 5). This observation may be attributed to the higher number of female participants in our sample and inadequate representation of male participants with low NEB (Figure 5). We obtained no significant difference in ABR wave I among non-musicians, brass-majors, and voice-majors. Besides, we found no evidence of an association between NEB and suprathreshold ABR wave I, III, and V latency. In conclusion, our results suggest that a weak negative association of AP and ABR wave I amplitude with NEB cannot be solely attributed to evidence of cochlear synaptopathy in humans as the possibility of hair cell damage cannot be ruled out.
4.1. The relationship between NEB and electrophysiological measures of cochlear activity
The result of the present study showed a weak negative relationship between NEB and amplitude of AP and ABR wave I at 75 dBnHL. As evident from Figure 5, the relationship between NEB and amplitude of AP and wave I is more consistent for females than males. Our findings suggest that high NEB might cause a reduction in cochlear output. AP and ABR wave I amplitude could not achieve the threshold of statistical significance at 90 dBnHL (achieved p-value = 0.07; p-value threshold = 0.05). High variability in AP and ABR wave I amplitudes might be a factor contributing to the non-significant finding. We observed that the standard error (SE) of a mean for AP amplitude elicited with 90 dBnHL clicks (SE90 = 18.15 nV) was higher than the SE of a mean for AP amplitude elicited with 75 dBnHL (SE75 = 12.28 nV), and 60 dBnHL (SE60 = 7.94 nV) clicks. A similar pattern was observed for ABR wave I amplitude (SE90 = 15.54 nV, SE75 = 12.68 nV, SE60 = 7.83 nV). The higher variability in ABR wave I amplitude and AP elicited using 90 dBnHL might lead to reduced statistical power contributing to the null findings. The negative trend between NEB and suprathreshold ABR wave I amplitude might be influenced by cochlear synaptopathy in combination with cochlear hair cells dysfunction. Animal studies have shown that IHC ribbon synapses are more sensitive to noise-induced insult than cochlear hair cells (e.g., Kujawa and Liberman, 2009; Lin et al., 2011; Furman et al., 2013). Therefore, it is likely that the reduced suprathreshold ABR wave I amplitude is influenced by the noise-induced damage to IHC cochlear synaptic ribbons.
4.2. Influence of gender on electrophysiological measures of cochlear activities
We found no reliable association between NEB and wave I amplitude in males. One of the attributing factors for this finding is that the low-NEB region is not sufficiently represented in the study sample by male participants, as shown in Figure. 5. Another attributing factor is the uneven gender distribution in the study sample. The number of male participants (n=23) was less than half of the number of female participants (n=52). The numbers of male participants in non-musicians and voice major groups were two and four, respectively. Gender differences in ABR latencies and amplitudes have been reported in the literature (Jerger and Hall, 1980; Mitchell et al., 1989; Picton et al., 1981). Typically, males exhibit smaller amplitudes for ABR waves I, III, and V. One possible explanation for these sex differences is that males tend to have larger head sizes compared to females (Allison et al., 1983; Dempsey et al., 1986). The gender effect on ABR measures remained significant even after controlling the head size (Trune et al. 1988). Dehan and Jerger (1990) suggested that a combination of hormonal factors and head size are accountable for gender differences. Don et al. (1994) argued that gender differences in amplitude and latency are due to faster cochlear response times and increased synchrony in females that arise as a result of the shorter cochlear length (Sato et al., 1991). Further research is required to investigate the relationship between NEB and wave I amplitude in normal hearing males.
4.2. Comparison of NEB and cochlear output among the experimental groups
We observed that musicians significantly exhibit higher NEB compared to non-musicians (Figure. 2). Our finding suggests that the noisy lifestyle of young musicians may be responsible for exposure to high noise/music levels explaining the group difference in NEB (e.g., Washnik et al., 2016; Tufts and Skoe, 2018). The present study found no significant difference in ABR wave I amplitude and latency between the three groups. This observation is congruous with some previous studies with different study populations (e.g., Prendergast et al., 2017; Fulbright et al., 2017; Grinn et al., 2017; Guest et al., 2017; Yeend et al., 2017; Bhatt & Wang, 2019). The first possible explanation is related to the noise exposure questionnaire used in this study (Johnson et al., 2017). This questionnaire quantifies noise exposure over the last one year. As shown in Figure 2, there is a notable overlap of NEB values between the groups. Hence, it is likely that some listeners may have been classified as low NEB when, in fact, earlier high NEB already may have caused synaptopathy. Some listeners might have had high exposure to impulse noises that were not evaluated by the questionnaire for calculating NEB scores. The CBA/Caj mice in which noise-induced cochlear synaptopathy was first observed (e.g., Kujawa and Liberman, 2009) were raised in a laboratory setting enabling the researchers to control noise exposure efficiently. These animal experiments ruled out other forms of hearing deficit using the post-mortem cochlear synapse and cell counts. The noise exposure in human experiments cannot be controlled as efficiently as animal studies. Non-invasive human studies do not have access to cochlear tissues for histological investigation. Therefore, the no significant group difference in AP and ABR wave I amplitudes in our study cannot rule out the possibility of cochlear synaptopathy in musicians.
A second explanation for no significant difference in ABR wave I amplitude between the groups is the possibility that humans are more resistant to noise-induced synaptic damage. In a recent investigation, it was shown that macaque monkeys are more resistant to cochlear synaptopathy than rodents (Valero et al., 2017), resulting in hypothesizing that humans are less vulnerable to noise-induced synaptopathy than rodents (Dobie and Humes, 2017). Given the high sound levels required to produce acoustic trauma resulting in significant TTS, bordering on a permanent elevation in hearing thresholds, there may be few human exposures that will result in the substantial reductions in ABR wave I seen in the original mouse study (Kujawa and Liberman, 2009). In addition, the direct comparison of protein components from human and mouse excitatory synapses showed that the mouse and human postsynaptic density was comprised of around 1556 and 1461 proteins, respectively (Bayés et al., 2012). More than 70% of human and mouse postsynaptic density proteins were overlapping. Importantly, humans showed a significant abundance of some families of key postsynaptic density proteins, including glutamatergic neurotransmitter receptors and adaptor proteins. The higher abundance of such protein contents associated with neural plasticity may provide increased synaptic plasticity to humans compared to rodents (Bayés et al., 2012). Humans also have considerable inter-individual variability in human synaptic proteins compared to laboratory rodents (Pinto et al., 2015), suggesting that there may be interactive effects between genetic susceptibility and environmental factors similar to noise-induced hearing loss (e.g., Abreu-Silva et al., 2011; Kowalski et al., 2014; Sliwinska-Kowalska and Pawelczyk, 2013; Bhatt et al., 2016; Bhatt et al., 2020).
A third explanation could be attributed to the higher variability of auditory evoked potentials in humans than in rodents. The coefficient of variation in wave I amplitude in Prendergast et al. (2018) study was 25% in the low noise exposure group, which may suggest a large degree of variability compared to the effect being measured. Head size and geometry might contribute to the inter-subject variability and reduced statistical power for the detection of differences in human auditory electrophysiological measures (Mitchell et al., 1989; Don et al., 1994). These factors might account, at least to some extent, for not observing a statistically significant difference for AP and ABR wave I amplitude among the experimental groups.
4.3. Experimental caveats
The present study was limited by its survey design to estimate NEB. Although NEB was estimated using a validated survey tool, measurements using a comprehensive battery of noise dosimetry would yield greater precision. The questionnaire did not include an exhaustive list of noise exposure areas and did not account for the use of ear protection in the process of calculating the NEB score. Even though there is no widely accepted “gold standard” for evaluating cochlear synaptopathy in humans, it can be argued that electrophysiological protocols other than the one employed by the present study might be more sensitive in identifying cochlear synaptopathy in humans (e.g., Mehraei et al., 2016). The gender distribution was not even among the groups. The number of male participants was less than half of the females in the non-musician and voice major groups. Although every participant in this study was requested to refrain from loud sound exposure at least 12 hours before reporting to the clinic for tests, there is a possibility that some of the student musicians may have developed TTS due to exposure to loud events in the last 24 hours. The duration of complete recovery ranges from a few minutes to a few weeks (Kujawa & Liberman, 2009; Ward, 1970). Hence, it is likely that some participants might not have had a complete recovery from TTS, which may affect ABR test results. Our findings should not be generalized beyond individuals with European ancestry.
Conclusion:
To summarize, the result of this study showed a weak relationship between NEB and amplitude of AP and wave I at 75 dBnHL but not at 90 or 60 dBnHL. However, on comparing wave I amplitude between the groups, we found no significant difference between the groups. The unbalanced gender distribution among the study groups affected the results. The readers are cautioned regarding deducing firm conclusions from the results of this study. Future human studies on noise-induced synaptopathy in normal-hearing individuals should consider gender as a potentially confounding variable.
Supplementary Material
Highlights.
NEB was negatively associated with the action potential (AP) and ABR wave I amplitude for click intensity levels at 75 dB nHL.
Brass major, voice major, and non musicians presented similar wave I amplitude
Acknowledgments
The research reported in the publication was partially supported by the National Institute on Deafness and Other Communication Disorders Grant R21DC016704-01A1. The authors thank Dr. Tiffany Johnson for providing the noise exposure questionnaire. The authors thank anonymous reviewers for their constructive feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abreu-Silva RS, Rincon D, Horimoto ARVR, Sguillar AP, Ricardo LAC, Kimura L, Batissoco AC, De Mello Auricchio MTB, Otto PA, Mingroni-Netto RC, 2011. The search of a genetic basis for noise-induced hearing loss (NIHL). Ann. Hum. Biol 10.3109/03014460.2010.513774 [DOI] [PubMed]
- Allison T, Wood C, Goff WR 1983. Brain stem auditory, pattern-reversal visual, and short-latency somatosensory evoked potentials: Latencies in relation to age, sex, and brain and body size. Electroencephalogr Clin Neurophysiol 55, 619–36. 10.1016/0013-4694(83)90272-9 [DOI] [PubMed] [Google Scholar]
- Barlow C, 2010. Potential hazard of hearing damage to students in undergraduate popular music courses. Med. Probl. Perform. Art 25, 175–182. [PubMed] [Google Scholar]
- Bayés À, Collins MO, Croning MDR, van de Lagemaat LN, Choudhary JS, Grant SGN, 2012. Comparative Study of Human and Mouse Postsynaptic Proteomes Finds High Compositional Conservation and Abundance Differences for Key Synaptic Proteins. PLoS One 10.1371/journal.pone.0046683 [DOI] [PMC free article] [PubMed]
- Bhatt I, Phillips S, Richter S, Tucker D, Lundgren K, Morehouse R, Henrich V, 2016. A polymorphism in human estrogen-related receptor beta (ESRRβ) predicts audiometric temporary threshold shift. Int. J. Audiol 55, 571–579. 10.1080/14992027.2016.1192693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt IS, Wang J, 2019. Evaluation of dichotic listening performance in normal-hearing, noise-exposed young females. Hear. Res 10.1016/j.heares.2019.05.008 [DOI] [PubMed]
- Bhatt IS, Dias R, Washnik N, Wang J, Guthrie O, Skelton M, Lane J, Wilder J, 2020. Association Analysis of Candidate Gene Polymorphisms and Audiometric Measures of Noise-Induced Hearing Loss in Young Musicians. Otol. Neurotol 10.1097/MAO.0000000000002615 [DOI] [PMC free article] [PubMed]
- Bramhall NF, Konrad-Martin D, McMillan GP, Griest SE, 2017. Auditory Brainstem Response Altered in Humans with Noise Exposure Despite Normal Outer Hair Cell Function. Ear Hear 10.1097/AUD.0000000000000370 [DOI] [PMC free article] [PubMed]
- Dehan CP, Jerger JJ 1990. Analysis of gender differences in the auditory brainstem response. Laryngoscope. 100, 18–24. 10.1288/00005537-199001000-00005 [DOI] [PubMed] [Google Scholar]
- Dempsey J, Censoprano E, Mazor M 1986. Relationship between head size and latency of the auditory brainstem response. Int J Audiol 25, 258–262. [PubMed] [Google Scholar]
- Dobie RA, Humes LE, 2017. Commentary on the regulatory implications of noise-induced cochlear neuropathy. Int. J. Audiol 10.1080/14992027.2016.1255359 [DOI] [PubMed]
- Don M, Ponton CW, Eggermont JJ, Masuda A, 1994. Auditory brainstem response (ABR) peak amplitude variability reflects individual differences in cochlear response times. J. Acoust. Soc. Am 10.1121/1.410608 [DOI] [PubMed]
- Fulbright ANC, Le Prell CG, Griffiths SK, Lobarinas E, 2017. Effects of Recreational Noise on Threshold and Suprathreshold Measures of Auditory Function. Semin. Hear 10.1055/s-0037-1606325 [DOI] [PMC free article] [PubMed]
- Furman AC, Kujawa SG, Liberman MC, 2013. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol 110, 577–586. 10.1152/jn.00164.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal KV, Chesky K, Beschoner E. a, Nelson PD, Stewart BJ, 2013. Auditory risk assessment of college music students in jazz band-based instructional activity. Noise Health 15, 246–52. 10.4103/1463-1741.113520 [DOI] [PubMed] [Google Scholar]
- Grinn SK, Wiseman KB, Baker JA, Le Prell CG, 2017. Hidden hearing loss? No effect of common recreational noise exposure on cochlear nerve response amplitude in humans. Front. Neurosci 10.3389/fnins.2017.00465 [DOI] [PMC free article] [PubMed]
- Grose JH, Buss E, Hall JW, 2017. Loud Music Exposure and Cochlear Synaptopathy in Young Adults: Isolated Auditory Brainstem Response Effects but No Perceptual Consequences. Trends Hear 21, 1–18. 10.1177/2331216517737417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Prendergast G, Howe S, Plack CJ, 2017. Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hear. Res 344, 265–274. 10.1016/j.heares.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henselman LW, Henderson D, Shadoan J, Subramaniam M, Saunders S, Ohlin D, 1995. Effects of noise exposure, race, and years of service on hearing in U.S. army soldiers. Ear Hear 10.1097/00003446-199508000-00005 [DOI] [PubMed]
- Ishii E, Talbott E, 1998. Race/ethnicity differences in the prevalence of noise-induced hearing loss in a group of metal fabricating workers. J. Occup. Environ. Med 40, 661–666. 10.1097/00043764-199808000-00001 [DOI] [PubMed] [Google Scholar]
- Jerger J, Hall J, 1980. Effects of Age and Sex on Auditory Brainstem Response. Arch. Otolaryngol 10.1001/archotol.1980.00790310011003 [DOI] [PubMed]
- Jerger J, Jerger S, Pepe P, Miller R, 1986. Race difference in susceptibility to noise-induced hearing loss. Am. J. Otol [PubMed]
- Johnson TA, Cooper S, Stamper GC, Chertoff M, 2017. Noise exposure questionnaire: A tool for quantifying annual noise exposure. J. Am. Acad. Audiol 10.3766/jaaa.15070 [DOI] [PMC free article] [PubMed]
- Kohrman DC, Wan G, Cassinotti L, Corfas G, 2020. Hidden hearing loss: A disorder with multiple etiologies and mechanisms. Cold Spring Harb. Perspect. Med 10.1101/cshperspect.a035493 [DOI] [PMC free article] [PubMed]
- Kowalski TJ, Pawelczyk M, Rajkowska E, Dudarewicz A, Sliwinska-Kowalska M, 2014. Genetic variants of CDH23 associated with noise-induced hearing loss. Otol. Neurotol 10.1097/MAO.0b013e3182a00332 [DOI] [PubMed]
- Kujawa SG, Liberman MC, 2009. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci 29, 14077–14085. 10.1523/jneurosci.2845-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF, 2016. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One 10.1371/journal.pone.0162726 [DOI] [PMC free article] [PubMed]
- Le Prell CG, Clavier OH, 2017. Effects of noise on speech recognition: Challenges for communication by service members. Hear. Res 349, 76–89. 10.1016/j.heares.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC, 2011. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. JARO - J. Assoc. Res. Otolaryngol 12, 605–616. 10.1007/s10162-011-0277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P, Wan G, Roberts MT, Corfas G, 2018. Myelin development, plasticity, and pathology in the auditory system. Dev. Neurobiol 10.1002/dneu.22538 [DOI] [PMC free article] [PubMed]
- McBride DI, Williams S, 2001. Audiometric notch as a sign of noise induced hearing loss. Occup. Environ. Med 10.1136/oem.58.1.46 [DOI] [PMC free article] [PubMed]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG, 2016. Auditory Brainstem Response Latency in Noise as a Marker of Cochlear Synaptopathy. J. Neurosci 10.1523/JNEUROSCI.4460-15.2016 [DOI] [PMC free article] [PubMed]
- Mitchell C, Phillips DS, Trune DR, 1989. Variables affecting the auditory brainstem response: Audiogram, age, gender and head size. Hear. Res 10.1016/0378-5955(89)90101-9 [DOI] [PubMed]
- Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M, 2005. The global burden of occupational noise-induced hearing loss. Am. J. Ind. Med 10.1002/ajim.20223 [DOI] [PubMed]
- Picton TW, Stapells DR, Campbell KB, 1981. Auditory evoked potentials from the human cochlea and brainstem. J. Otolaryngol. Suppl 1, 1–41. [PubMed] [Google Scholar]
- Pinto JGA, Jones DG, Kate Williams C, Murphy KM, 2015. Characterizing synaptic protein development in human visual cortex enables alignment of synaptic age with rat visual cortex. Front. Neural Circuits 10.3389/fncir.2015.00003 [DOI] [PMC free article] [PubMed]
- Prendergast G, Guest H, Munro KJ, Kluk K, Léger A, Hall DA, Heinz MG, Plack CJ, 2017. Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hear. Res 10.1016/j.heares.2016.10.028 [DOI] [PMC free article] [PubMed]
- Rasband MN, Peles E, 2016. The nodes of Ranvier: Molecular assembly and maintenance. Cold Spring Harb. Perspect. Biol 10.1101/cshperspect.a020495 [DOI] [PMC free article] [PubMed]
- Schaette R, McAlpine D, 2011. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J. Neurosci 10.1523/JNEUROSCI.2156-11.2011 [DOI] [PMC free article] [PubMed]
- Sato H, Sando I, Takahashi H, 1991. Sexual dimorphism and development of the human cochlea: Computer 3-d measurement. Acta Otolaryngol 10.3109/00016489109100753 [DOI] [PubMed]
- Sliwinska-Kowalska M, Pawelczyk M, 2013. Contribution of genetic factors to noise-induced hearing loss: A human studies review. Mutat. Res. - Rev. Mutat. Res 10.1016/j.mrrev.2012.11.001 [DOI] [PubMed]
- Stamper GC, Johnson TA, 2015a. Auditory function in normal-hearing, noise-exposed human ears. Ear Hear 36, 172–184. 10.1097/AUD.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper GC, Johnson TA, 2015b. Letter to the editor: examination of potential sex influences in Stamper, G. C., & Johnson, T.A. (2015). Auditory function in normal-hearing, noise-exposed human ears, ear hear, 36, 172–184. Ear Hear 36, 738e740 10.1097/AUD.0000000000000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagoe T, Barker M, Jones A, Allcock N, Hamann M, 2014. Auditory nerve perinodal dysmyelination in noise-induced hearing loss. J. Neurosci 10.1523/JNEUROSCI.3977-13.2014 [DOI] [PMC free article] [PubMed]
- Trune DR, Mitchell C, Phillips DS, 1988. The relative importance of head size, gender and age on the auditory brainstem response. Hear. Res 10.1016/0378-5955(88)90088-3 [DOI] [PubMed]
- Tufts JB, Skoe E, 2018. Examining the noisy life of the college musician: weeklong noise dosimetry of music and non-music activities. Int. J. Audiol 57, S20–S27. 10.1080/14992027.2017.1405289 [DOI] [PubMed] [Google Scholar]
- Valero MD, Burton JA, Hauser SN, Hackett TA, Ramachandran R, Liberman MC, 2017. Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta). Hear. Res 353, 213–223. 10.1016/j.heares.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G, Corfas G, 2017. Transient auditory nerve demyelination as a new mechanism for hidden hearing loss. Nat. Commun 10.1038/ncomms14487 [DOI] [PMC free article] [PubMed]
- Wang Y, Ren C, 2012. Effects of repeated “benign” noise exposures in young cba mice: Shedding light on age-related hearing loss. JARO - J. Assoc. Res. Otolaryngol 13, 505–515. 10.1007/s10162-012-0329-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WD, 1970. Temporary Threshold Shift and Damage‐Risk Criteria for Intermittent Noise Exposures. J. Acoust. Soc. Am 10.1121/1.1912172 [DOI] [PubMed]
- Washnik N, Phillips S, Teglas S, 2016. Student′s music exposure: Full-day personal dose measurements. Noise Heal 18, 98 10.4103/1463-1741.178510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeend I, Beach EF, Sharma M, Dillon H, 2017. The effects of noise exposure and musical training on suprathreshold auditory processing and speech perception in noise. Hear. Res 353, 224–236. 10.1016/j.heares.2017.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


