INTRODUCTION
Approximately 38% of U.S. adults are obese.1 By 2030, nearly 50% are projected to have at least class 1 obesity, defined as a body-mass index (BMI) of 30–35 and nearly 25% will have class 3 obesity (BMI≥40).2 This growing obesity epidemic is also reflected in the transplant candidate and recipient population. For example, from 2016–2030, the number of annual listings for nonalcoholic steatohepatitis is expected to increase by 55%.3 In 2017, 36% of all liver transplant recipients had at least class 1 obesity and 14% had class 3 obesity.4 Based on a review of the Organ Procurement and Transplant Network Standard Analytic Files (file date March 2019), 34.7% of kidney transplant recipients were obese in 2018, compared to only 25.7% in 2000. In 2016, the number of obese pancreas transplant recipients increased 29% from the year prior.5
Importantly, the available epidemiological data on obesity in transplant candidates and recipients fail to account for patients who are too obese to be considered for transplant listing. Most transplant centers endorse using BMI cutoffs for transplant listing.6–8 Indeed, current guidelines suggest various body-mass index (BMI) cutoffs depending on the organ to be transplanted, and recommend lifestyle interventions to promote weight loss to achieve a lower BMI prior to transplantation.9–12 However, significant weight loss with lifestyle interventions may not be feasible, particularly in end-stage organ disease.
Bariatric surgery in the general population is associated with a 5-year BMI reduction of 12–17 kg/m2, and significant remission rates of diabetes (92%), hypertension (75.2%), and dyslipidemia (75.8%).13 Compared to lifestyle changes, bariatric surgery is more likely to yield sustained weight loss (1% versus 18% body weight loss at 20 years), reversal of comorbidities, and 29% lower all-cause mortality.14 While longer follow-up is necessary, increasing evidence suggests that endoscopic bariatric interventions provide significant short-term weight loss as well.15,16
Increasingly, reports are being published of bariatric surgery in patients with end-stage organ diseases, with the goal of achieving weight loss that allows for transplant listing.17–20 In some cases, end-stage organ disease is reversed with significant weight loss following bariatric surgery, obviating the need for transplant.17,20,21 We performed a systematic review and meta-analysis to characterize the clinical outcomes achieved by bariatric surgery in the context of bridging patients with end-stage organ disease to listing and subsequent transplant.
METHODS
We followed the statement on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses22 and registered our review (PROSPERO Identifier: CRD42020142899). This study was exempt from institutional review board review. All authors had access to the study data and reviewed and approved the final manuscript.
Data Sources and Searches
An English language-only search strategy for PubMed/MEDLINE was developed in conjunction with an academic librarian and searched from inception to June 28, 2019 (Supplement S1). We reviewed the reference lists of eligible studies and meta-analyses to screen for additional studies. After removal of duplicate reports, titles and abstracts of the search results were independently screened for relevance by two authors (BJO, JWP). Relevant studies were screened independently in full text for inclusion. Using a standardized form, 2 reviewers worked independently to screen titles, abstracts, and full-text articles to identify potentially eligible studies. The final list of studies to be included was agreed upon by independent reviewers.
Eligibility Criteria
Included studies involved bariatric surgery performed on patients with end-stage heart, lung, liver, kidney, and/or pancreas disease with the goal of reversing end-stage organ disease and/or achieving sufficient weight loss to be eligible for solid organ transplant to cure their end-stage organ disease. We made the decision a priori to include case reports and case series as we anticipated that they would form the bulk—if not the totality—of the existing literature. We excluded studies reporting outcomes of transplanted patients with remote histories of bariatric surgery prior to onset of end-stage organ disease, as well as studies in which transplant was performed to rescue patients with complications from bariatric surgery. We excluded primary case series if secondary studies were more inclusive of patients and reported on relevant outcomes, except to supplement missing clinical data as needed. Finally, we excluded studies of endoscopic weight loss modalities (e.g., intragastric balloon placement, endoscopic gastric suturing) because of the paucity of relevant studies and insufficient sample sizes for inclusion in subgroup analyses.
Data extraction
Abstracted data included study and patient characteristics, number of participants, organ involved, etiology of end-stage organ disease, type of bariatric surgery, change in weight and/or BMI, follow-up time, achievement of listing for transplant, achievement of transplant, resolution of obesity-related comorbidities, resolution of end-stage organ disease, operative complications, hospital length of stay, and hospital readmission. The distinction between case series and cohort studies in the context of a systematic review was based on the ability of the latter to provide a measure of association for the exposure of interest, rather than just an effect measure.23 The primary outcome was achievement of listing for transplant. Secondary outcomes included achievement of transplant, weight loss, and resolution of comorbidities. Determination of listing for transplant was based on authors explicitly stating that the patients were listed or underwent transplant. Studies with heterogeneous patient populations in which the characteristics and/or results of bariatric surgery in patients with end-stage organ disease were indistinguishable from patients without end-stage organ disease were included in the qualitative synthesis only.
Quality grading of studies
To grade study quality, we assessed studies on compliance with the American Society of Metabolic and Bariatric Surgery and the Surgery for Obesity and Related Diseases bariatric surgery outcome reporting standards.24 These guidelines specifically address best practices for reporting follow-up, resolution of comorbidities, complications, and weight loss. Duration of follow-up was considered adequate to achieve short-, medium- and long-term follow-up for any duration of post-bariatric surgery follow-up less than 3 years, 3–5 years, and greater than 5 years. Adequate comorbidity outcomes reporting required sufficient information to categorize outcomes using predetermined criteria as set forth in the guidelines for diabetes mellitus (complete remission, partial remission, improvement, unchanged, recurrence), hypertension (improvement, partial remission, complete remission), dyslipidemia (improvement, remission), obstructive sleep apnea (complete remission, objective improvement, subjective improvement), and gastroesophageal reflux disease (complete objective resolution, complete subjective resolution, objective improvement, self-reported improvement) (see Supplement S2).24 Reporting of complications was divided into reporting of early (<30 days) and late (>30 days) complications. Complications were reported according to the Clavien-Dindo Classification system, which grades the severity of surgical complications based on the therapy required to treat them.25 Failure to comment on the absence of complications was not considered sufficient reporting to assume that no complications had occurred. Reporting of weight loss was graded according to four criteria, all of which are recommended for complete reporting: mean initial BMI of the cohort (initial BMI in individual case reports), change in BMI, percent of total weight loss, and percent excess BMI loss.
Data Synthesis and Analysis
Abstracted data were summarized using descriptive statistics. Pooled means and standard deviations were provided for continuous variables, and frequencies and percentages reported for dichotomous variables. Cuzick’s nonparametric test for trend was used to examine secular trends. Given the non-comparative nature of almost all of the studies in the literature, it was not feasible to report relative measures of association and therefore only proportions are reported. Analyses were performed using Stata version 13.1 (StataCorp) and Excel version 16 (Microsoft). A two-tailed P-value <0.05 was statistically significant.
RESULTS
Systematic Study Review
Excluding duplicate reports, our search strategy identified 790 records. After reviewing titles and abstracts, a total of 173 full-text articles were reviewed, which identified a total of 48 individual studies for inclusion in the systematic review (20 for heart, 3 for lung, 4 for liver, 21 for kidney, and 2 for pancreas) (Fig. 1) published between 2002 and 2019. Almost all were retrospective and observational. Not surprisingly, there were no studies of patients undergoing bariatric surgery to achieve intestinal transplant.
Figure 1.
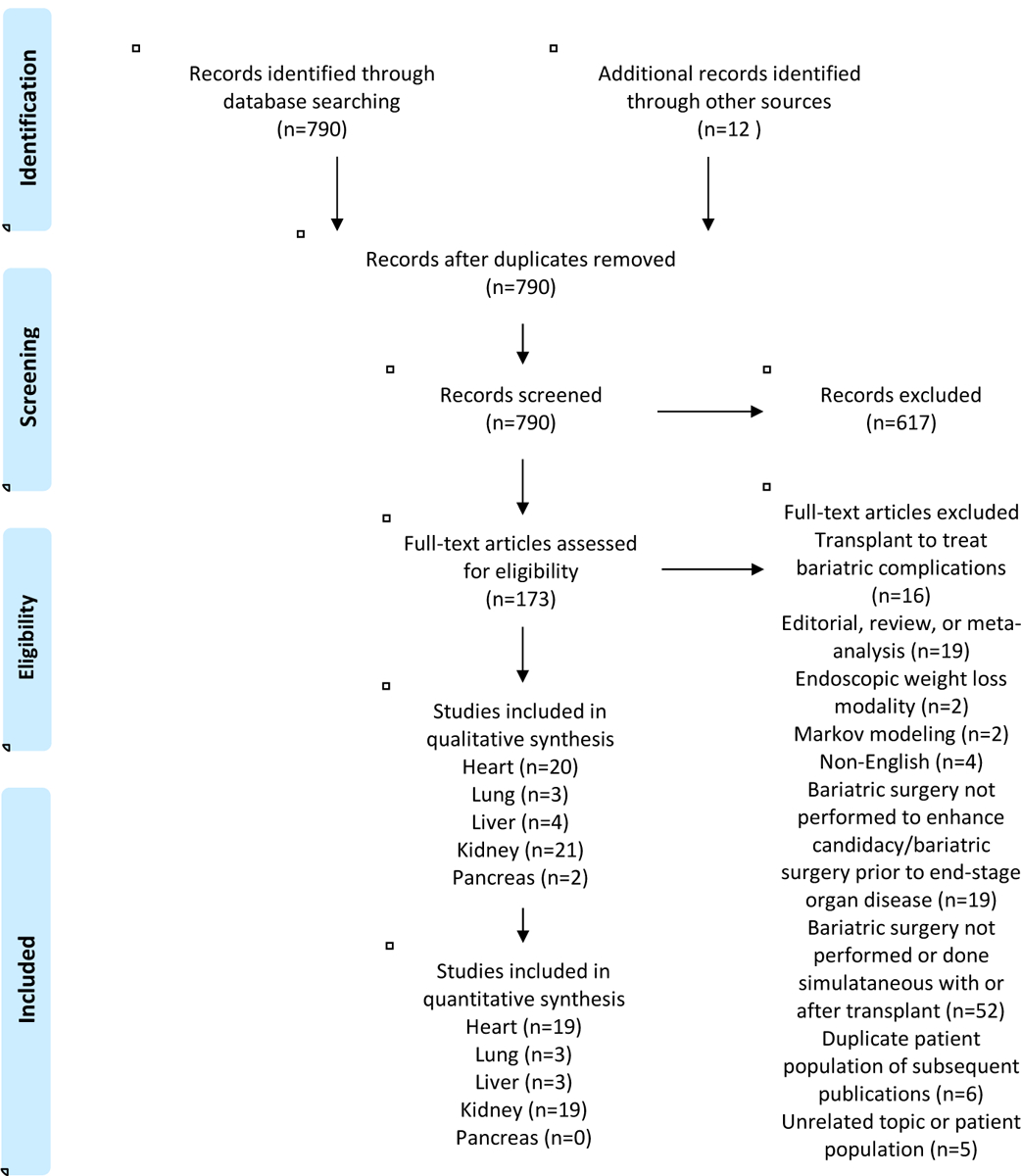
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) study selection flow diagram.
There were 13 case reports, 30 case series (totaling 293 patients; median 6.6 patients per study [interquartile range 3–11]; minimum 2, maximum 41), and 4 cohort studies. One matched cohort study compared 12 morbidly obese heart failure patients who underwent bariatric surgery to 10 matched controls who did not.26 Kim and colleagues studied the learning curve of laparoscopic sleeve gastrectomies in end-stage renal disease (ESRD) patients by comparing the outcomes of the first 25 to subsequent patients.27 Another cohort study compared outcomes of 14 ESRD patients who had Roux-en-Y gastric bypass prior to kidney transplant to 19 morbidly obese kidney transplant recipients who did not have bariatric surgery.28 Finally, Modanlou et al. used the U.S. Renal Data System to compare the outcomes of ESRD patients who had bariatric surgery before listing (n=72) to those who had it after listing (n=29) and to those who had bariatric surgery after transplant.29 One study was a prospective, single-center, non-randomized trial of 8 ESRD patients who underwent sleeve gastrectomy prior to transplant listing.30
Quality grading of studies
No study reported on all 15 recommended domains, with the median number of domains reported being 6 (interquartile range 4–7.5) (Table 1, Supplement 3a, 3b). Reporting was more robust after publication of reporting guidelines in 2015.24 There were 25 studies published prior to the release of the reporting guidelines and 23 subsequent to that. The average number of domains reported in the guideline pre-publication era was 5.0±2.0, compared to 6.8±2.6 after publication (P=0.01).
Table 1.
Study design and quality of standardized metric reporting of studies included in the systematic review and meta-analysis, by organ.
| Organ | Author | Year | Study Design | Post-Bariatric Surgery Follow- up | Resolution of Comorbidities | Complications Reporting | Weight Loss Reporting | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Short term (<3 years) | Medium- term (3–5 years) | Long term (>5 years) | DM | HTN | DL | OSA | GERD | Early | Late | Mean Initial BMI | Change in BMI | Percent of Total Weight Loss | Percent Excess BMI loss | Percent Excess Weight Loss | ||||
| Heart | Amro38 | 2015 | CR | Y | N | N | N | N | N | N | N | N | N | Y | Y | Y | N | Y |
| Caceres39 | 2013 | CR | Y | N | N | N | N | N | N | N | N | N | Y | Y | N | N | N | |
| Chaudhry40 | 2015 | CS | Y | Y | Y | N | N | N | N | N | Y | N | Y | Y | N | N | Y | |
| DeNino41 | 2013 | CR | Y | N | N | N | N | N | N | N | Y | N | Y | Y | N | N | N | |
| Gill42 | 2012 | CS | Y | N | N | N | N | N | N | N | N | N | Y | Y | N | N | N | |
| Greene43 | 2017 | CS | Y | Y | N | N | N | N | N | N | Y | Y | Y | Y | Y | N | Y | |
| Hawkins18 | 2018 | CS | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | N | Y | |
| Jeng44 | 2016 | CR | Y | Y | Y | N | N | N | N | N | Y | Y | Y | Y | N | N | N | |
| Lim45 | 2016 | CS | Y | Y | N | N | N | N | N | N | Y | N | Y* | Y | N | Y | N | |
| Lockard46 | 2013 | CS | Y | N | N | N | N | N | N | N | N | N | Y | Y | N | N | N | |
| McCloskey47 | 2007 | CS | Y | Y | Y | N | N | N | N | N | Y | N | Y | Y | N | N | Y | |
| Moulla48 | 2018 | CS | Y | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | |
| Punchai49 | 2019 | CS | Y | N | N | N | N | N | N | N | Y | Y | Y | Y | Y | N | Y | |
| Ramani26 | 2008 | CoS | Y | N | N | Y | Y | N | Y | N | Y | N | Y | Y | N | N | N | |
| Ristow21 | 2008 | CS | Y | N | N | N | N | N | Y | N | N | N | Y | Y | N | N | N | |
| Saeed50 | 2012 | CR | Y | N | N | N | N | N | N | N | N | N | Y | Y | N | N | N | |
| Samaras51 | 2012 | CS | Y | N | N | Y | N | Y | N | N | Y | N | Y | Y | N | N | N | |
| Shah52 | 2015 | CS | Y | N | N | N | N | N | N | N | Y | Y | Y | Y | N | N | N | |
| Taylor53 | 2002 | CR | Y | N | N | Y | N | N | N | N | Y | Y | Y | N | N | N | N | |
| Wikiel54 | 2014 | CS | Y | N | N | N | N | N | N | N | Y | Y | Y | Y | N | N | Y | |
| Lung | Ardila-Gatas17 | 2019 | CS | Y | Y | Y | N | N | N | N | N | Y | N | Y* | Y* | Y | N | Y |
| Martin31 | 2007 | CR | Y | N | N | N | N | N | N | N | Y | N | Y | N | N | N | N | |
| Takata32 | 2008 | CS | Y | N | N | Y | Y | N | Y | N | Y | N | Y | N | N | N | Y | |
| Liver | Garcia-Sesma | 2019 | CS | Y | Y | Y | N | N | N | N | N | Y | Y | Y | Y | N | N | Y |
| Moulla48 | 2018 | CS | Y | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | |
| Sharpton20 | 2019 | CS | Y | N | N | Y | Y | N | N | N | Y | Y | Y* | Y* | N | N | Y | |
| Taneja33 | 2013 | CR | Y | N | N | N | N | N | N | N | Y | N | Y | N | N | N | N | |
| Kidney | Adani56 | 2015 | CS/Letter to Editor | Y | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Al Sabah57 | 2017 | CR | Y | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | N | N | N | |
| Al-Bahri58 | 2017 | CS | Y | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | Y | N | Y | |
| Alexander59 | 2007 | CS | Y | Y | Y | Y | Y | Y | N | N | Y | N | N | N | N | N | N | |
| Buch60 | 2006 | CR | Y | N | N | N | N | N | N | N | N | N | N | N | N | N | N | |
| Carandina61 | 2017 | CS | Y | N | N | Y | Y | N | Y | N | Y | Y | Y | Y | N | N | Y | |
| Contreras-Villamizar62 | 2019 | CR | Y | N | N | N | N | N | N | N | Y | Y | Y | Y | N | N | N | |
| Hidalgo63 | 2012 | CS | Y | N | N | N | N | N | N | N | Y | Y | Y | Y | N | N | N | |
| Jamal64 | 2015 | CS | Y | Y | N | Y | N | N | N | N | Y | Y | Y | Y | N | N | Y | |
| Kienzl-Wagner30 | 2017 | Single-arm trial | Y | Y | Y | N | N | N | N | N | Y | Y | Y | Y | N | Y | N | |
| Kim19 | 2017 | CoS | Y | Y | N | Y | Y | N | N | N | Y | N | Y | Y | N | N | N | |
| Koshy65 | 2008 | CS | Y | Y | N | N | N | N | N | N | N | N | Y | Y | Y | N | Y | |
| Lin66 | 2013 | CS | Y | N | N | Y | N | N | N | N | Y | Y | Y | Y | N | N | Y | |
| MacLaughlin67 | 2012 | CS | Y | Y | N | Y | Y | Y | N | N | Y | Y | Y* | Y* | N | N | Y | |
| Marszalek68 | 2012 | CR | Y | N | N | N | N | N | N | N | Y | Y | Y | Y | N | N | N | |
| Modanlou29 | 2009 | CoS | Y | Y | N | N | N | N | N | N | N | N | Y | Y | N | N | Y | |
| Newcombe69 | 2005 | CS | Y | N | N | Y | N | N | N | N | N | N | Y | Y | N | N | N | |
| Proczko70 | 2013 | CS | Y | N | N | Y | Y | N | N | N | N | N | Y | Y | N | N | N | |
| Takata32 | 2008 | CS | Y | N | N | Y | Y | N | Y | N | Y | N | Y | N | N | N | Y | |
| Thomas28 | 2018 | CoS | Y | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | N | N | Y | |
| Yemeni34 | 2019 | CS | Y | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | N | Y | |
| Pancreas | Bonatti71 | 2018 | CR | Y | Y | N | N | N | N | N | N | N | N | Y | N | N | N | N |
| Gullo-Neto72 | 2014 | CS | Y | N | N | Y | N | Y | N | N | Y | Y | Y | Y | N | N | N | |
Reported median BMI rather than mean BMI
N indicates statements provided about change in comorbidities after bariatric surgery that were too vague to categorize the degree of change.
Studies in bold indicate those that were only involved in the qualitative component of the systematic review.
CR – case report; CS – case series; CoS – cohort study; DM – diabetes mellitus; HTN – hypertension; DL – dyslipidemia; OSA – obstructive sleep apnea; GERD – gastroesophageal reflux disease
No study provided complete recommended reporting of weight loss. Four studies (8.3%) provided 0 of 5 of the recommended weight loss domains, 4 (8.3%) provided 1 of 5, 19 (39.6%) provided 2 of 5, 14 (29.2%) provided 3 of 5, and 7 (14.6%) provided 4 of 5 domains. Reporting of weight loss improved after publication of reporting guidelines (2.7±1.2 versus 2.0±0.9 domains reported; P=0.03).
Overall, all studies were considered to be at high risk of bias given their study design, generally small sample sizes, retrospective nature, limited outcome reporting, and potential for publication bias (Table 1).
Meta-analysis
Trends in publications of bariatric surgery in end-stage organ disease patients
There was a single case report of an end-stage organ disease patient undergoing biliopancreatic diversion in 2002. The number of patients in published reports peaked in 2017 (n=137; P=0.009) (Fig 2). Performance of Roux-en-Y gastric bypass has generally decreased over time (P=0.014) and sleeve gastrectomy has increased (P=0.002).
Figure 2.
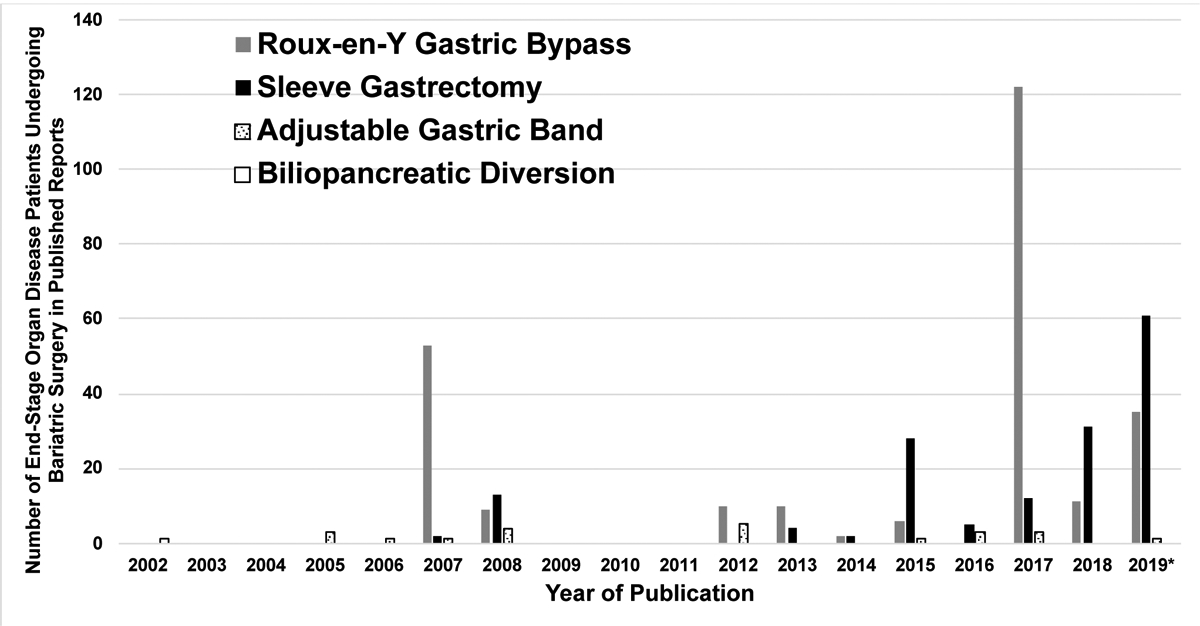
Number of end-stage organ disease patients undergoing bariatric surgery in published reports by bariatric surgery type and year.
*Data for 2019 are through June 28, 2019
Baseline characteristics of pooled populations
Heart
Of 82 patients across 19 studies, mean age was 42.9±10.7 years, and 41.5% were female (Table 2). The majority (74.4%) had non-ischemic cardiomyopathy as the etiology of their heart failure, 37.8% had a left ventricular assist device in place at the time of bariatric surgery, and 9.7% had a left ventricular assist device placed simultaneous with their bariatric surgery. Fifty percent of patients underwent Roux-en-Y gastric bypass, 36.6% underwent sleeve gastrectomy, 12.2% had an adjustable gastric band, and 1.2% had a biliopancreatic diversion.
Table 2.
Study and patient characteristics of end-stage organ disease patients undergoing bariatric surgery.
| Study | Number of latients | Age (Mean and standard deviation, except as indicated) | Male (n)/Female (n) | Etiology of End-Stage Organ Disease | Roux-en-Y Gastric Bypass (n) | Sleeve Gastrectomy (n) | Adjustable Gastric Band (n) | Bilio-pancreatic Diversion (n) | End-Stage Organ Disease-Specific Factors | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart Failure | |||||||||||||||
| NICM (n) | I CM (n) | Not Reported (n) | VAD Status Relative to Bariatric Surgery | ||||||||||||
| Pre- Bariatric Surgery (n) | Simultaneous (n) | No VAD (n) | |||||||||||||
| Amro | 1 | 34 | 1/0 | 1 | 0 | 0 | -- | -- | 1 | 0 | 0 | 1 | 0 | 0 | |
| Caceres | 1 | 56 | 0/1 | 1 | 0 | 0 | -- | -- | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Chaudhry | 6 | median 34 (range 31–66)* | 3/3 | 4 | 2 | 0 | -- | -- | 0 | 6 | 0 | 0 | 3 | 0 | 3 |
| DeNino | 1 | 24 | 0/1 | 1 | 0 | 0 | -- | -- | 1 | 0 | 0 | 1 | 0 | 0 | |
| Gill | 2 | 24, 36 | 2/0 | 0 | 0 | -- | -- | 0 | 2 | 0 | 0 | 2 | 0 | ||
| Greene | 3 | 48.7 (SD 6.1) | 3/0 | 1 | 2 | 0 | -- | -- | 3 | 0 | 0 | 0 | 3 | 0 | 0 |
| Hawkins | 11 | 43.3 (SD not reported; range 31–66)* | 6/5 | 11 | 0 | 0 | -- | -- | 11 | 0 | 0 | 0 | 11 | 0 | 0 |
| Jeng | 1 | 25 | 1/0 | 1 | 0 | 0 | -- | -- | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Lim | 7 | 44.1 (SD 8.6) | 4/3 | 1 | 0 | -- | -- | 0 | 4 | 3 | 0 | 0 | 0 | 7 | |
| Lockard | 2 | 37, unclear | 2/0 | 0 | 0 | 2 | -- | -- | 2 | 0 | 0 | 0 | 1 | 1 | 0 |
| McQoskey | 14 | 46.2 (SD 9.2) | 10/4 | 10 | 4 | 0 | -- | -- | 11 | 2 | 1 | 0 | 0 | 0 | 14 |
| Punchai | 7 | 43.6 (SD 15.0) | 3/4 | 3 | 4 | 0 | -- | -- | 0 | 7 | 0 | 0 | 7 | 0 | 0 |
| Ramani | 12 | 41 (SD 10) | 3/9 | 10 | 2 | 0 | -- | -- | 9 | 2 | 1 | 0 | 0 | 0 | 12 |
| Ristow | 2 | 35, 36 | 1/1 | 2 | 0 | 0 | -- | -- | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Saeed | 1 | 50 | 1/0 | 1 | 0 | 0 | -- | -- | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Samaras | 2 | 42, 40 | 1/1 | 2 | 0 | 0 | -- | -- | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Shah | 4 | 46.5 (SD 13.9) | 4/0 | 1 | 3 | 0 | -- | -- | 0 | 4 | 0 | 0 | 0 | 4 | 0 |
| Taylor | 1 | 57 | 0/1 | 0 | 1 | 0 | -- | -- | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Wikiel | 4 | 42.0 (SD 11.1) | 3/1 | 4 | 0 | 0 | -- | -- | 2 | 2 | 0 | 0 | 2 | 0 | 2 |
| TOTAL | 82 | 42.9 (SD 10.7) | 48 (58.5%)/34 (41.5%) | 61 (74.4%) | 19 (23.2%) | 2 (2.4%) | -- | -- | 41 (50.0%) | 30 (36.6%) | 10 (12.2%] | 1 (1.2%) | 31 (37.8%) | 8 (9.7%) | 3 (52.4%) |
| End-Stage Lung Disease | |||||||||||||||
| ILD (n) | IPF (n) | COPD (n) | |||||||||||||
| Ardila-Gatas | 25 | median 53 (IQR 42–58)* | 4/21 | 25 | 0 | 0 | -- | -- | 17 | 7 | 1 | 0 | -- | -- | -- |
| Martin | 1 | 48 | 0/1 | 1 | 0 | 0 | -- | -- | 1 | 0 | 0 | 0 | -- | -- | -- |
| Takata | 2 | 57, 59 | 2/0 | 0 | 1 | 1 | -- | -- | 0 | 2 | 0 | 0 | -- | -- | -- |
| TOTAL | 28 | 54.7 (SD 5.8) | 6 (21.4%)/22 (78.6%) | 26 (92.8%) | 1 (3.6%) | 1 (3.6%) | -- | -- | 18 (64.3%) | 9 (32.1%) | 1 (3.6%) | 0 (0%) | -- | -- | -- |
| Cirrhosis | |||||||||||||||
| HCV (n) | NASH (n) | Alcohol (n) | HBV (n) | Other (n) | Child’s Score | ||||||||||
| Class A (n) | Class (n) | Class C (n) | |||||||||||||
| Garcia-Sesma | 8 | 53.6 (8.1) | 2/6 | 2 | 5 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 6 | 2 | 0 |
| Sharpton | 32 | median 55 (IQR 50–61)* | 9/23 | 15 | 10 | 3 | 2 | 2 | 0 | 32 | 0 | 0 | 15 | 17 | 0 |
| Taneja | 1 | 29 | 1/0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| TOTAL | 41 | 50.9 (SD 11.2) | 12 (29.3%)/29 (70.7%) | 17 (41.5%) | 16 (39.0%) | 4 (9.7%) | 2 (4.9%) | 2 (4.9%) | 0 (0%) | 41 (100%) | 0 (0%) | 0 (0%) | 21 (51.2%) | 20 (48.8%) | 0 (0%) |
| Chronic Kidney Disease/End-Stage Renal Disease | |||||||||||||||
| Diabetes (n) | HTN (n) | FSGS (n) | GN (n) | Other/Unknown (n) | Desease Severity | ||||||||||
| CKD | ESRD | -- | |||||||||||||
| Adani | 3 | NR | NR | NR | NR | NR | NR | NR | 3 | 0 | 0 | 0 | NR | NR | -- |
| Al Sabah | 1 | 52 | 1/0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | -- |
| Al Bahri | 16 | 55.1 (SD 6.5) | 10/6 | 2*** | 1 | 1 | 1 | 12 | 3 | 0 | 0 | 16 | -- | ||
| Alexander | 41 | 44.4 (SD not reported)* | NR | NR | NR | NR | NR | NR | 41 | 0 | 0 | 0 | 32** | 0 | -- |
| Buch | 1 | 59 | 0/1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | -- |
| Carandina | 9 | 53.2 (SD 5.5) | 1/8 | 5 | 3 | 1 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 9 | -- |
| Contreras-Villamizar | 1 | 44 | 1/0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | -- |
| Jamal | 21 | 50.6 (SD 10.3) | 12/9 | 11 | 8 | 0 | 0 | 2 | 2 | 18 | 1 | 0 | 0 | 21 | -- |
| Kienzl-W | 8 | 48 (SD 13) | 3/5 | 3 | 0 | 0 | 1 | 4 | 8 | 0 | 0 | 0 | 0 | 8 | -- |
| Kim | 100 | median 50 (IQR 43.8 – 58.3)* | 41/59 | NR | NR | NR | NR | NR | 100 | 0 | 0 | 0 | 0 | 100 | -- |
| Koshy | 3 | 40.7 (11.9) | 2/1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 2 | -- |
| Lin | 6 | NR | NR | NR | NR | NR | NR | NR | 6 | 0 | 0 | 0 | 6 | 0 | -- |
| MacLaughlin | 9 | 46.1 (SD 7.0) | 3/6 | 1 | 3 | 2 | 0 | 3 | 9 | 0 | 0 | 0 | 4 | 5 | -- |
| Marszalek | 1 | 55 | 0/1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | -- |
| Newcombe | 3 | 43.7 (19.1) | 3/0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 1 | 2 | -- |
| Proczko | 3 | 55.0 (SD 6.0) | 1/2 | 2 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | -- |
| Takata | 7 | 45.9 (SD 6.8) | 0/7 | 0 | 0 | 0 | 0 | 7 | 0 | 7 | 0 | 0 | 0 | 7 | -- |
| Thomas | 31 | 45 (SD 2.2) | 14/17 | 16 | 9 | 0 | 0 | 6 | 0 | 31 | 0 | 0 | 1 | 30 | -- |
| Yemeni | 24 | 54 (SD 3.1) | 16/8 | 15 | 0 | 2 | 7 | 17 | 7 | 0 | 0 | 0 | 24 | -- | |
| TOTAL | 288 | 49.7 (SD 7.4) | 108 (45.4%)/130 (54.6%) | 59 (46.8%) | 24 (19.0%) | 8 (6.3%) | 1 (0.8%) | 34 (27.0%) | 199 (69.1%) | 78 (27.1%) | 11 (3.8%) | 0 (0%) | 45 (16.3%) | 231 (83.7%) | -- |
Not included in calculation for group mean and standard deviation
Study reports 32 patients with CKD, but does not provide information on the remaining 9.
One of the patients was classified as having both diabetic nephropathy and hypertensive kidney disease SD – standard deviation; NICM – non-ischemic cardiomyopathy; ICM – ischemic cardiomyopathy; VAD – ventricular assist device; IQR – interquartile range; ILD – interstitial lung disease; IPF – idiopathic pulmonary fibrosis; COPD – chronic obstructive pulmonary disease; NASH – non-alcoholic steatohepatitis; HCV – hepatitis C virus; HBV – hepatitis B virus; CKD – chronic kidney disease; ESRD – end-stage renal disease; HTN – hypertension; FSGS – focal segmental glomerulosclerosis; NR – not reported
Lung
Of 28 patients across 3 studies, mean age was 54.7±5.8 years and 78.6% were female (Table 2). The majority had idiopathic pulmonary fibrosis (92.8%) as the etiology of their lung disease and 64.2%, 32.1%, and 3.6% underwent Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band placement.
Liver
Of 41 patients across 3 studies, mean age was 50.9±11.2 years, and 70.7% were female (Table 2). The etiology of liver disease was hepatitis C, nonalcoholic steatohepatitis, alcoholic liver disease, hepatitis B, and other in 41.5%, 39.0%, 9.7%, 4.9%, and 4.9% of cases. At bariatric surgery, 51.2% had Child’s Class A liver disease and 48.8% had Child’s Class B liver disease. There were no Child’s Class C patients. All patients underwent sleeve gastrectomy.
Kidney
Of 288 patients across 19 studies, the mean age was 49.7± 7.4 years, and 54.6% were female (Table 2). Amongst studies that provided the information (n=126 patients), kidney disease etiology was diabetic nephropathy, hypertensive nephropathy, focal segmental glomerulosclerosis, glomerulonephritis, and other/unknown in 46.8%, 19.0%, 6.3%, 0.8%, and 27.0% of patients, and 83.7% had end-stage renal disease (ESRD) and 16.3% had chronic kidney disease, though the degree of chronic kidney disease was not reported. Roux-en-Y gastric bypass was performed in 69.0%, sleeve gastrectomy in 27.1%, and adjustable gastric band in 3.8%.
Effect of bariatric surgery
Heart
There was a significant reduction in mean BMI from bariatric surgery to last follow-up (48.4±6.6 versus 37.1±5.8; P<0.001) (Table 3). There was an overall decrease in the severity of heart disease by New York Heart Association Functional Classification as well (Fig. 3). Of the 22 patients who had left ventricular ejection fraction reported at the time of bariatric surgery and at last follow-up (excluding patients who got transplanted), the average ejection fraction improved from 20.5%± 4.8 to 33.2%±14.4 (P<0.001) over an average of 23.9±20.6 months of follow-up. Of the 82 total patients, 40.2% (n=33) lost sufficient weight to be listed, 29.3% (n=24) achieved heart transplantation at an average of 13.9±5.4 months post-bariatric surgery, and 8.5% (n=7) had sufficient clinical improvement following bariatric surgery that they no longer needed heart transplantation. In other words, 46.3% (n=38) of end-stage heart failure patients were listed or improved clinically to the point that they no longer required transplant.
Table 3.
End-stage organ disease patients: weight loss, ability to get listed, and ability to get transplanted after bariatric surgery.
| Study | Number of Patients | BMI (kg/m2) at Bariatric Surgery (Mean and sta nda rd deviation, except as indicated) | BMI at Last Follow-up (kg/m2) (Mean and standard deviation, except as indicated) | Waitlisted (%) | Transplanted (%) | Clinical Improvement Precluding Need for Listing | Months from Bariatric Surgery to Transplant (Mean and standard deviation, except as indicated) | Months of Follow- up After Bariatric Surgery (Mean and standard deviation, except as indicated) |
|---|---|---|---|---|---|---|---|---|
| Heart Failure | ||||||||
| Amro | 1 | 50 | 44 | NR | NR | NR | NR | 6 |
| Caceres | 1 | 37.4 | 29 | 1 (100%) | 1 (100%) | NR | 10 | 22 |
| Chaudhry | 6 | 47.6 (SD 3.0)* | NR* | 4 (66.7%) | 0 (0%) | 1 of 6 | NR | median 22 (range 12–70)* |
| DeNino | 1 | 50 | 30.4 | 1 (100%) | 1 (100%) | NR | 13 | 16 |
| Gill | 2 | 46.6, 43.7 | 34.7, 38.5 | 0 (0%) | 0 (0%) | NR | NR | 5, 14 |
| Greene | 3 | 52.3 (SD 2.5) | 29.7 (SD 3.2) | 2 (66.7%) | 2 (66.7%) | 0 of 3 | 17, 24 | 43.7 (SD 12.0) |
| Hawkins | 11 | mean 45.2 (range 39–58)* | mean 33.1 (range 26–39)* | 7 (63.6%) | 4 (36.4%) | NR | median 12 (range 544)* | median 12 (range 6–39)* |
| Jeng | 1 | 40 | 32 | 1 (100%) | 1 (100%) | NR | 7 | 71 |
| Lim | 7 | 43.3 (SD 5.0) | 32.4 (SD 4.2) | 4 (57.1%) | 2 (28.6%) | 3 of 7 | NR | 21.7 (SD 16.5) |
| Lockard | 2 | 48.8, 52.2 | 38.6, 40.8 | 1 (50.0%) | 1 (50.0%) | NR | 18 | NR, 24 |
| McCloskey | 14 | 50.8 (SD 7.6) | 37.1 (SD 7.2) | 2 (14.3%) | 2 (14.3%) | NR | 6, 8 | 6.7 (SD 2.2) |
| Punchai | 7 | 44.3 (SD 6.4) | 35.0 (SD 7.9) | 3 (42.8%) | 3 (42.8%) | NR | 17.7 (SD 5.7) | 20.6 (SD 24.2) |
| Ramani | 12 | 53 (SD 7.0) | 47 (SD 4.0) | 2 (16.7%) | 1 (8.3%) | NR | NR | NR |
| Ristow | 2 | 43, 56 | 23, 37 | 2 (100%) | 0 (0%) | 2 of 2 | NR | 24, 24 |
| Saeed | 1 | 41.6 | 41 | 1 (100%) | 1 (100%) | 0 of 1 | 13 | 21 |
| Samaras | 2 | 42, 40 | 31.2, 34.7 | 1 (50.0%) | 1 (50.0%) | 1 of 2 | 13 | 12, 13 |
| Shah | 4 | 49.2(SD 5.9) | 5.5 (4.6) | 2 (50.0%) | 1 (25.0%) | NR | 9 | 7.0 (SD 2.3) |
| Taylor | 1 | 48.6 | 28.5 | 1 (100%) | 1 (100%) | NR | 19 | 25 |
| Wikiel | 4 | 47.7 (SD 4.4) | 35.3 (SD 4.1) | 3 (75.0%) | 3 (75.0%) | NR | 13.3 (SD 4.7) | 48.5 (SD 42.1) |
| TOTAL | 82 | 48.4 (SD 6.6) | 37.1 (SD 5.8) | 33 (40.2%) | 24 (29.3%) | 7 (8.5%) | 13.9 (SD 5.4) | 21.8 (SD 22.2) |
| End-Stage Lung Disease | ||||||||
| Ardila-Gatas | 25 | median 39 (IQR 37–44)* | median 30 (IQR 25–36)* | 6 (24%) | 1 (4%) | 3 (12%) | 88 | NR |
| Martin | 1 | 37 | <30* | 0 (0%)** | 0 (0%)** | 1 (100%) | N/A | 5 |
| Takata | 2 | 46 (4.5) | NR* | 2 (100%) | 1 (50%) | 0 (0%) | NR | 12.5 (SD 0.7) |
| TOTAL | 28 | 42.7 (5.4) | -- | 8 (28.6%) | 2 (7%) | 4 (14.3%) | 88 | 10 (SD 4.3) |
| Cirrhosis | ||||||||
| Reached BMI<40 kg/m2 by 6 Months | Reached BMI<40 kg/m2 by 12 Months | |||||||
| Garcia-Sesma | 8 | 6 (of 7; 85.7% | 6 (75.0%) | 2 (25.0%) | 2 (25.0%) | -- | 7, 8 | 33.2 (SD 23.6 |
| Sharpton | 32 | 13 (of 22; 59.1% | 20 (62.5%) | 21 (65.6%)* | 14 (43.7%) | 9 (28.1%) | median 22 (IQR 1488)** | |
| Taneja | 1 | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 0 (0%) | 12 | 12 |
| TOTAL | 41 | 20 (of 30; 66.7%) | 27 (65.8%) | 24 (58.5%) | 17 (41.5%) | 9 (of 33; 27.3%) | 9.0 (SD 2.6) | 30.9 (23.2) |
| Chronic Kidney Disease | ||||||||
| BMI (kg/m2) at Bariatric Surgery | BMI (kg/m2) at Last Follow-up | |||||||
| Adani | 3 | NR* | NR* | 2 (67%) | 2 (67%) | -- | NR* | NR* |
| Al Sabah | 1 | 42 | 31.5 | 1 (100%) | 1 (100%) | -- | 29 | 6 |
| Al Bahri | 16 | 47.5 (SD 7.5) | 30.9 (6.4) | 4 (25%) | 9 (56%) | -- | 53.2 (SD 16.3) | 42.0 (32.8) |
| Alexander | 41 | 48 (SD NR)* | NR* | 9 (22%) | 9 (22%) | -- | NR | NR* |
| Buch | 1 | NR* | NR* | 1 (100%) | 1 (100%) | -- | 24 | NR* |
| Carandina | 9 | 47.0 (SD 7.1) | 33.6 (7.4) | 1 (11%) | 5 (56%) | -- | 21 | 15.7 (10.6) |
| Contreras-Villamizar | 1 | 42 | 28.6 | 0 (0%) | 1 (100%) | -- | No transplant | 12 |
| Jamal | 21 | 47.1 (SD 5.5) | 35.3 (8.4) | 2 (10%) | 18 (86%) | -- | NR | 27.6 (22) |
| Kienzl-Wagner | 8 | 38.8 (SD 3.8) | 30.7 (6.0) | 7 (88%) | 8 (100%) | -- | 17.6 (SD 10.5) | 38.4 (16.8) |
| Kim | 100 | 43.4 (SD 5.9) | 36.9 (5.7) | 19 (19%) | 19 (19%) | -- | NR | NR* |
| Koshy | 3 | 40.7 (SD 4.9) | 37.2 (1.9) | 3 (100%) | 3 (100%) | -- | NR | 13.7 (1.2) |
| Lin | 6 | NRBO* | NRBO* | 1 (17%) | 6 (100%) | -- | NRBO | NR* |
| MacLaughlin | 9 | median 44.2* | median 34.7 (range 29.2 – 38.8)* | 0 (0%) | 3 (33%) | -- | No transplant | 12* |
| Marszalek | 1 | 41.5 | 29 | 1 (100%) | 1 (100%) | -- | 10 | 10 |
| Newcombe | 3 | 44.6 (SD 5.5) | 33.8 (5.0) | 3 (100%) | 3 (100%) | -- | 16.3 (SD 10.2) | 16.3 (8.3) |
| Proczko | 3 | 39.9 (SD 2.1) | 31.1 (2.4) | 1 (33%) | 3 (100%) | -- | 3 | 3 (0) |
| Takata | 7 | 49.9 (SD 5.7) | NR* | 0 (0%) | 7 (100%) | -- | No transplant | NR* |
| Thomas | 31 | 43.5 (SD 0.7) | 28.1 (SD 0.8) | 14 (67%) | 25 (81%) | -- | median 33* | NR* |
| Yemeni | 24 | 41.5 (SD 0.8) | 29 (SD 1.3) | 16 (67%) | 21 (88%) | -- | 18 (1 – 51.6)* | 47.0 (6.5) |
| TOTAL | 288 | 44.0 (5.5) | 33.7 (5.3) | 85 (30%) | 145 (50%) | -- | 24.8 (14.2) | 32.9 (21.4) |
Not included in calculation for group mean and standard deviation
SD – standard deviation; BMI – body-mass index; IQR – interquartile range; NR – not reported; NRBO – not reported by organ
Figure 3.
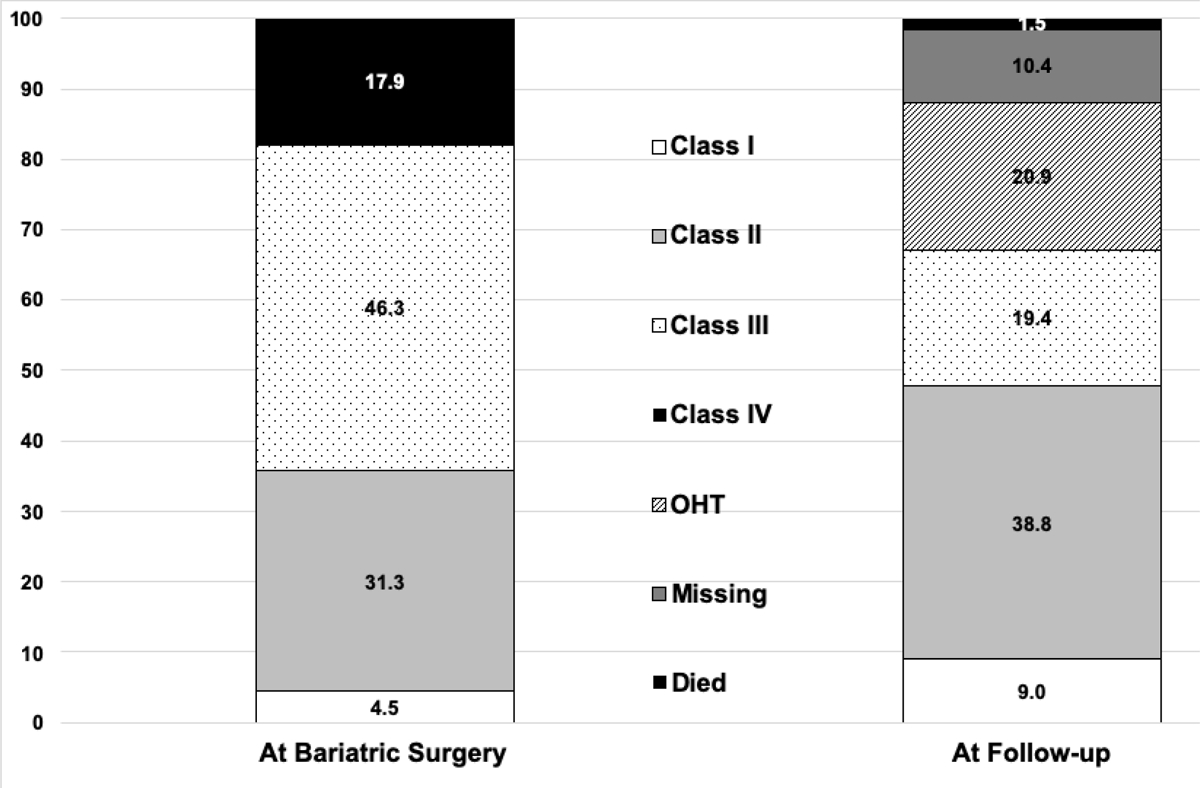
Change in New York Heart Association Functional Classification in heart failure patients from the time of bariatric surgery to last follow-up.
Lung
A summary statistic of weight or BMI loss could not be calculated for end-stage lung disease patients (Table 3). One study of 25 patients reported a change in median BMI from bariatric surgery of 39 kg/m2 (interquartile range 37–44) to 30 kg/m2 (interquartile range 25–36) at last follow-up.17 A case report stated a patient had a BMI of 37 kg/m2 at bariatric surgery to <30 kg/m2 at last follow-up.31 The third study of end-stage lung disease patients reported a BMI of 41 kg/m2 and 50 kg/m2 in two patients and they lost 50 and 73% of excess body weight at 12 and 13 months follow-up, respectively.32 Of the 28 total patients, 28.6% (n=8) were waitlisted, 7.1% (n=2) were transplanted, and 14.3% (n=4) had clinical improvement that precluded the need for listing. In other words, 42.8% (n=12) lost sufficient weight that they were listed or improved to the point that they no longer required transplant. Only one study reported change in pulmonary function tests from the time of bariatric surgery to last follow-up.17
Liver
By 6 months post-bariatric surgery, 66.7% (20 of 30) of cirrhotic patients had achieved BMI <40 kg/m2 (Table 3), a common BMI cutoff for liver transplant programs. By 12 months, 65.8% (27/41) had achieved BMI <40 kg/m2. Of the 41 patients, 58.5% achieved listing and 41.5% (17/41) were transplanted at a mean of 9.0±2.6 months post-bariatric surgery. Nine patients (21.9%) had sufficient clinical improvement following bariatric surgery that they no longer needed liver transplantation. In other words, 75.6% (31/41) of patients with cirrhosis were listed or improved clinically to the point that they no longer required transplant.
Kidney
Over an average of 32.9±21.4 months of follow-up, mean BMI decreased from 43.9±5.3 kg/m2 to 33.7±5.4 kg/m2 (P=0.003) (Table 3). Of the 288 patients, 145 were listed (50.3%), and 29.5% (85 of 288) were transplanted at a mean of 19.9+14.3 months post-bariatric surgery. No study described an occurrence of a patient stopping dialysis after weight loss, nor did any study describe an occurrence of pre-dialysis patient who had improvement in kidney function that precluded the need for dialysis.
Comorbidities
Among studies that reported sufficient information to determine evolution of diabetes after bariatric surgery, 39.6%, 10.4%, 14.2%, and 35.8% of diabetic patients had complete remission, partial remission, improvement, and no change in diabetes status after bariatric surgery (Fig. 4). Among hypertensive patients, 16.5%, 1.0%, 42.7%, and 39.8% had complete remission, partial remission, improvement, and no change after bariatric surgery. There was insufficient reporting of dyslipidemia, obstructive sleep apnea, and gastroesophageal reflux disease to pool data.
Figure 4.
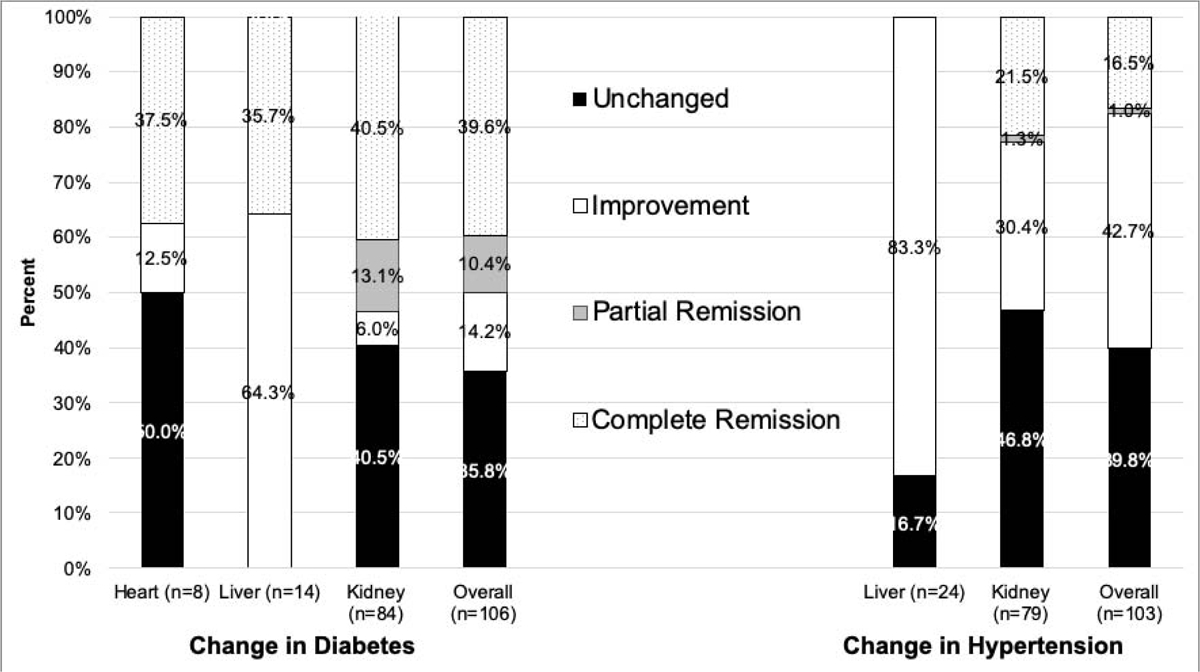
Improvement in diabetes mellitus and hypertension after bariatric surgery, by organ affected by end-stage organ disease.
Length of stay, readmissions, and complications
Heart
Mean hospital length of stay was 8.0±5.7 days (Supplement S4a). Of the 29 patients in studies reporting 30-day readmissions, there were 5 (17.2%) readmissions within 30 days. Of the 65 patients in studies that reported complications, there were 4, 5, 2, 3, and 2 Clavien-Dindo Classification class I, II, III, IVa and IVb complications. There were no class V complications.
Lung
Mean hospital length of stay was 4.0±1.0 days (Supplement S4b). None of the studies of end-stage lung disease patients reported 30-day readmissions. Of the 28 patients, there was 1 grade II and 3 grade IIIB Clavien-Dindo Classification complications.
Liver
Only one study20 reported hospital length of stay (median 3 days [IQR 2–3; range 1–6]), and no studies reported on readmissions after bariatric surgery (Supplement S4c). Of 41 patients in the three studies, there were 2 and 1 Clavien-Dindo Classification class I and IIIa complications. One study noted that a patient had progressive liver disease in the 6 months following bariatric surgery, but the authors were unable to definitively link worsening jaundice, ascites, and hepatic encephalopathy to the sleeve gastrectomy.33
Kidney
Mean hospital length of stay was 2.9±1.7 days (Supplement S4d). Of 275 patients in studies reporting complications, there were 10, 3, 3, 4, 3, 0, and 1 Clavien-Dindo Classification class I, II, IIIa, IIIb, IVa, IVb, and V complications reported.
Mortality
Across all studies, there was only one death that was directly attributable to a complication of bariatric surgery (Supplement 5). In this case, an end-stage renal disease patient died following a gastric staple line leak on post-operative day 21.34 Most of the remaining mortalities (18 of 25, 72%) were from cardiovascular and infectious etiologies, and mostly were temporally remote from bariatric surgery.
DISCUSSION
In the current study, moderate-to-low quality evidence suggests that bariatric surgery in end-stage organ disease can facilitate sufficient weight loss in appropriately selected patients to render them transplant-eligible. In heart failure, end-stage lung disease, cirrhosis, and chronic kidney disease/end-stage renal disease, 40.2%, 28.6%, 58.5%, and 50.3% of previously ineligible patients were listed for transplant after bariatric surgery. Furthermore, even in the context of end-stage organ disease, some patients were able to reverse the course of their disease with dramatic weight loss after bariatric surgery such that they no longer required a transplant, at least during the follow-up periods of the reports.
Because of variations in reporting, change in BMI after bariatric surgery was not able to be calculated for end-stage lung disease and cirrhotic patients; however, heart failure and chronic kidney disease/end-stage renal disease patients lost an average of 11 kg/m2 over an average of 22 and 33 months follow-up, respectively. This compares favorably to a 13 kg/m2 and 11.8 kg/m2 decrease in BMI at 24 and 36 months in the general bariatric population.13
Even in what is considered a high-risk population, post-bariatric surgery mortality was a rare and often remote phenomenon. Indeed, we could only identify one case in which bariatric surgery was the proximate cause of death. However, caution is required in interpreting these results as publication bias is likely. Furthermore, it is not known how much of a role bariatric surgery and the potential for ensuing nutritional deficiencies and malnutrition might play in contributing to an increased risk of mortality,35 particularly in patients who succumb to infectious causes of death—a salient consideration for potential transplant candidates who will need immunosuppressive therapy. The risk of mortality without transplant in end-stage organ disease patients is high and a survival benefit may exist for interventions that render obese patients eligible for transplant. Indeed, in the general obese population, bariatric surgery is associated with a significant survival benefit compared to usual care nonsurgical obesity management;14 however, the current state of the literature cannot answer that question for end-stage organ disease patients.
As endoscopic bariatric techniques have proliferated, studies of endoscopic sleeve gastroplasty have demonstrated 18–21% total body weight loss at two years in the general bariatric population.36,37 While more modest weight loss than is achieved with bariatric surgery, these data offer the tantalizing possibility of using endoscopic techniques in high-risk end-stage organ disease patients to allow them to achieve sufficient weight loss for transplant listing; however, further study will be needed in this population.
Obese end-stage organ disease patients face barriers to transplantation because of their weight. In addition, they face barriers to bariatric surgery from regulatory and insurance hurdles. For example, many insurance payers mandate a trial of medical weight management prior to approving bariatric surgery. This mandate may not be feasible for many patients, particularly those that are frequently hospitalized because of their end- stage organ disease. In addition, because centers that perform bariatric surgery need to maintain their Bariatric Surgery Center of Excellence designation, there may be a disincentive to perform bariatric surgery in patients perceived as being at high risk of complications and death. While the data presented here suggest that bariatric surgery can be done safely, the limitations of the included studies require further validation in higher quality studies.
Limitations of the included studies and therefore of this meta-analysis include the fact that most of the studies were case reports and uncontrolled case series with small sample sizes, with a high likelihood of publication bias. This limits external validity of bariatric surgery applied to the general end-stage organ disease population and therefore estimates obtained likely represent the best-case scenario. Furthermore, these study designs precluded the derivation of a pooled measure of association (i.e., likelihood of transplant listing for obese end-stage organ disease patients who underwent bariatric surgery compared to those who did not). Additionally, there was significant variability across studies in terms of the outcomes reported and the heterogeneous manner in which they were reported.
In conclusion, this study suggests that bariatric surgery in end-stage organ disease may help patients achieve sufficient weight loss to be eligible for transplant listing. Further high-quality studies are needed to address whether these benefits exist. If so, a number of additional questions arise, including the optimal timing and approach of surgical intervention, durability of weight loss in this population, and whether a survival benefit is achieved.
Supplementary Material
| Study | Number of patients | Age (Mean and standard deviation, except as indicated) | Male (n)/Female (n) | Etiology of End-Stage Organ Disease | Roux-en- Y Gastric Bypass (n) | Sleeve Gastrectom y (n) | Adjustabl e Gastric Band (n) | Bilio-pancreatic Diversion (n) | End-Stage Organ Disease-Specific Factors | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart Failure | |||||||||||||||
| NICM (n) | ICM (n) | Not Reported (n) | VAD Status Relative to Bariatric Surgery | ||||||||||||
| Pre-Bariatric Surgery (n) | Simultaneo us (n) | No VAD (n) | |||||||||||||
| Amro | 1 | 34 | 1/0 | 1 | 0 | 0 | -- | -- | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Caceres | 1 | 56 | 0/1 | 1 | 0 | 0 | -- | -- | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Chaudhry | 6 | median 34 (range 31–66)* | 3/3 | 4 | 2 | 0 | -- | -- | 0 | 6 | 0 | 0 | 3 | 0 | 3 |
| DeNino | 1 | 24 | 0/1 | 1 | 0 | 0 | -- | -- | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Gill | 2 | 24, 36 | 2/0 | 2 | 0 | 0 | -- | -- | 0 | 0 | 2 | 0 | 0 | 2 | 0 |
| Greene | 3 | 48.7 (SD 6.1) | 3/0 | 1 | 2 | 0 | -- | -- | 3 | 0 | 0 | 0 | 3 | 0 | 0 |
| Hawkins | 11 | 43.3 (SD not reported; range 31–66)* | 6/5 | 11 | 0 | 0 | 11 | 0 | 0 | 0 | 11 | 0 | 0 | ||
| Jeng | 1 | 25 | 1/0 | 1 | 0 | 0 | -- | -- | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Lim | 7 | 44.1 (SD 8.6) | 4/3 | 6 | 1 | 0 | -- | -- | 0 | 4 | 3 | 0 | 0 | 0 | 7 |
| Lockard | 2 | 37, unclear | 2/0 | 0 | 0 | 2 | -- | -- | 2 | 0 | 0 | 0 | 1 | 1 | 0 |
| McCloskey | 14 | 46.2 (SD 9.2) | 10/4 | 10 | 4 | 0 | -- | -- | 11 | 2 | 1 | 0 | 0 | 0 | 14 |
| Punchai | 7 | 43.6 (SD 15.0) | 3/4 | 3 | 4 | 0 | -- | -- | 0 | 7 | 0 | 0 | 7 | 0 | 0 |
| Ramani | 12 | 41 (SD 10) | 3/9 | 10 | 2 | 0 | -- | -- | 9 | 2 | 1 | 0 | 0 | 0 | 12 |
| Ristow | 2 | 35, 36 | 1/1 | 2 | 0 | 0 | -- | -- | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Saeed | 1 | 50 | 1/0 | 1 | 0 | 0 | -- | -- | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Samaras | 2 | 42, 40 | 1/1 | 2 | 0 | 0 | -- | -- | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Shah | 4 | 46.5 (SD 13.9) | 4/0 | 1 | 3 | 0 | -- | -- | 0 | 4 | 0 | 0 | 0 | 4 | 0 |
| Taylor | 1 | 57 | 0/1 | 0 | 1 | 0 | -- | -- | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Wikiel | 4 | 42.0 (SD 11.1) | 3/1 | 4 | 0 | 0 | -- | -- | 2 | 2 | 0 | 0 | 2 | 0 | 2 |
| TOTAL | 82 | 42.9 (SD 10.7) | 48 (58.5%)/34 (41.5%) | 61 (74.4%) | 19 (23.2%) | 2 (2.4%) | 41 (50.0%) | 30 (36.6%) | 10 (12.2%) | 1 (1.2%) | 31 (37.8%) | 8 (9.7%) | 43 (52.4%) | ||
| End-Stage Lung Disease | |||||||||||||||
| ILD (n) | IPF (n) | COPD (n) | |||||||||||||
| Ardila-Gatas | 25 | median 53 (IQR 42–58)* | 4/21 | 25 | 0 | 0 | -- | -- | 17 | 7 | 1 | 0 | -- | -- | -- |
| Martin | 1 | 48 | 0/1 | 1 | 0 | 0 | -- | -- | 1 | 0 | 0 | 0 | -- | -- | -- |
| Takata | 2 | 57, 59 | 2/0 | 0 | 1 | 1 | -- | -- | 0 | 2 | 0 | 0 | -- | -- | -- |
| TOTAL | 28 | 54.7 (SD 5.8) | 6 (21.4%)/22 (78.6%) | 26 (92.8%) | 1 (3.6%) | 1 (3.6%) | -- | -- | 18 (64.3%) | 9 (32.1%) | 1 (3.6%) | 0 (0%) | -- | -- | -- |
| Cirrhosis | |||||||||||||||
| HCV (n) | NASH (n) | Alcohol (n) | HBV (n) | Other (n) | Child's Score | ||||||||||
| Class A (n) | Class B (n) | Class C (n) | |||||||||||||
| Garcia-Sesma | 8 | 53.6 (8.1) | 2/6 | 2 | 5 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 6 | 2 | 0 |
| Sharpton | 32 | median 55 (IQR 50–61)* | 9/23 | 15 | 10 | 3 | 2 | 2 | 0 | 32 | 0 | 0 | 15 | 17 | 0 |
| Taneja | 1 | 29 | 1/0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| TOTAL | 41 | 50.9 (SD 11.2) | 12 (29.3%)/29 (70.7%) | 17 (41.5%) | 16 (39.0%) | 4 (9.7%) | 2 (4.9%) | 2 (4.9%) | 0 (0%) | 41 (100%) | 0 (0%) | 0 (0%) | 21 (51.2%) | 20 (48.8%) | 0 (0%) |
| Chronic Kidney Disease/End-Stage Renal Disease | |||||||||||||||
| Diabetes (n) | HTN (n) | FSGS (n) | GN (n) | Other/Unknown (n) | Disease Severity | ||||||||||
| CKD | ESRD | ||||||||||||||
| Adani | 3 | NR | NR | NR | NR | NR | NR | NR | 3 | 0 | 0 | 0 | NR | NR | -- |
| Al Sabah | 1 | 52 | 1/0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | -- |
| Al Bahri | 16 | 55.1 (SD 6.5) | 10/6 | 2*** | -- | 1 | -- | 1 | 1 | 12 | 3 | 0 | 0 | 16 | -- |
| Alexander | 41 | 44.4 (SD not reported)* | NR | NR | NR | NR | NR | NR | 41 | 0 | 0 | 0 | 32** | 0 | -- |
| Buch | 1 | 59 | 0/1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | -- |
| Carandina | 9 | 53.2 (SD 5.5) | 1/8 | 5 | 3 | 1 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 9 | -- |
| Contreras-Villamizar | 1 | 44 | 1/0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | -- |
| Jamal | 21 | 50.6 (SD 10.3) | 12/9 | 11 | 8 | 0 | 0 | 2 | 2 | 18 | 1 | 0 | 0 | 21 | -- |
| Kienzl-W | 8 | 48 (SD 13) | 3/5 | 3 | 0 | 0 | 1 | 4 | 8 | 0 | 0 | 0 | 0 | 8 | -- |
| Kim | 100 | median 50 (IQR 43.8 – 58.3)* | 41/59 | NR | NR | NR | NR | NR | 100 | 0 | 0 | 0 | 0 | 100 | -- |
| Koshy | 3 | 40.7 (11.9) | 2/1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 2 | -- |
| Lin | 6 | NR | NR | NR | NR | NR | NR | NR | 6 | 0 | 0 | 0 | 6 | 0 | -- |
| MacLaughlin | 9 | 46.1 (SD 7.0) | 3/6 | 1 | 3 | 2 | 0 | 3 | 9 | 0 | 0 | 0 | 4 | 5 | -- |
| Marszalek | 1 | 55 | 0/1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | -- |
| Newcombe | 3 | 43.7 (19.1) | 3/0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 1 | 2 | -- |
| Proczko | 3 | 55.0 (SD 6.0) | 1/2 | 2 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | -- |
| Takata | 7 | 45.9 (SD 6.8) | 0/7 | 0 | 0 | 0 | 0 | 7 | 0 | 7 | 0 | 0 | 0 | 7 | -- |
| Thomas | 31 | 45 (SD 2.2) | 14/17 | 16 | 9 | 0 | 0 | 6 | 0 | 31 | 0 | 0 | 1 | 30 | -- |
| Yemeni | 24 | 54 (SD 3.1) | 16/8 | 15 | 0 | 2 | 0 | 7 | 17 | 7 | 0 | 0 | 0 | 24 | -- |
| TOTAL | 288 | 49.7 (SD 7.4) | 108 (45.4%)/130 (54.6%) | 59 (46.8%) | 24 (19.0%) | 8 (6.3%) | 1 (0.8%) | 3 (27.0%) | 199 (69.1%) | 78 (27.1%) | 11 (3.8%) | 0 (0%) | 45 (16.3%) | 231 (83.7%) | -- |
| Study | Number of Patients | BMI (kg/m2) at Bariatric Surgery (Mean and standard deviation, except as indicated) | BMI at Last Follow-up (kg/m2) (Mean and standard deviation, except as indicated) | Waitlisted (%) | Transplanted (%) | Clinical Improvement Precluding Need for Listing | Months from Bariatric Surgery to Transplant (Mean and standard deviation, except as indicated) | Months of Follow- up After Bariatric Surgery (Mean and standard deviation, except as indicated) |
|---|---|---|---|---|---|---|---|---|
| Heart Failure | ||||||||
| Amro | 1 | 50 | 44 | NR | NR | NR | NR | 6 |
| Caceres | 1 | 37.4 | 29 | 1 (100%) | 1 (100%) | NR | 10 | 22 |
| Chaudhry | 6 | 47.6 (SD 3.0)* | NR* | 4 (66.7%) | 0 (0%) | 1 of 6 | NR | median 22 (range 12–70)* |
| DeNino | 1 | 50 | 30.4 | 1 (100%) | 1 (100%) | NR | 13 | 16 |
| Gill | 2 | 46.6, 43.7 | 34.7, 38.5 | 0 (0%) | 0 (0%) | NR | NR | 5, 14 |
| Greene | 3 | 52.3 (SD 2.5) | 29.7 (SD 3.2) | 2 (66.7%) | 2 (66.7%) | 0 of 3 | 17, 24 | 43.7 (SD 12.0) |
| Hawkins | 11 | mean 45.2 (range 39–58)* | mean 33.1 (range 26–39)* | 7 (63.6%) | 4 (36.4%) | NR | median 12 (range 5–44)* | median 12 (range 6–39)* |
| Jeng | 1 | 40 | 32 | 1 (100%) | 1 (100%) | NR | 7 | 71 |
| Lim | 7 | 43.3 (SD 5.0) | 32.4 (SD 4.2) | 4 (57.1%) | 2 (28.6%) | 3 of 7 | NR | 21.7 (SD 16.5) |
| Lockard | 2 | 48.8, 52.2 | 38.6, 40.8 | 1 (50.0%) ^ | 1 (50.0%) | NR | 18 | NR, 24 |
| McCloskey | 14 | 50.8 (SD 7.6) | 37.1 (SD 7.2) | 2 (14.3%) | 2 (14.3%) | NR | 6, 8 | 6.7 (SD 2.2) |
| Punchai | 7 | 44.3 (SD 6.4) | 35.0 (SD 7.9) | 3 (42.8%) | 3 (42.8%) | NR | 17.7 (SD 5.7) | 20.6 (SD 24.2) |
| Ramani | 12 | 53 (SD 7.0) | 47 (SD 4.0) | 2 (16.7%) | 1 (8.3%) | NR | NR | NR |
| Ristow | 2 | 43, 56 | 23, 37 | 2 (100%) | 0 (0%) | 2 of 2 | NR | 24, 24 |
| Saeed | 1 | 41.6 | 41 | 1 (100%) | 1 (100%) | 0 of 1 | 13 | 21 |
| Samaras | 2 | 42, 40 | 31.2, 34.7 | 1 (50.0%) | 1 (50.0%) | 1 of 2 | 13 | 12, 13 |
| Shah | 4 | 49.2(SD 5.9) | 5.5 (4.6) | 2 (50.0%) | 1 (25.0%) | NR | 9 | 7.0 (SD 2.3) |
| Taylor | 1 | 48.6 | 28.5 | 1 (100%) | 1 (100%) | NR | 19 | 25 |
| Wikiel | 4 | 47.7 (SD 4.4) | 35.3 (SD 4.1) | 3 (75.0%) | 3 (75.0%) | NR | 13.3 (SD 4.7) | 48.5 (SD 42.1) |
| TOTAL | 82 | 48.4 (SD 6.6) | 37.1 (SD 5.8) | 33 (40.2%) | 24 (29.3%) | 7 (8.5%) | 13.9 (SD 5.4) | 21.8 (SD 22.2) |
| End-Stage Lung Disease | ||||||||
| Ardila-Gatas | 25 | median 39 (IQR 37–44)* | median 30 (IQR 25–36)* | 6 (24%) | 1 (4%) | 3 (12%) | 88 | NR |
| Martin | 1 | 37 | <30* | 0 (0%)** | 0 (0%)** | 1 (100%) | N/A | 5 |
| Takata | 2 | 46 (4.5) | NR* | 2 (100%) | 1 (50%) | 0 (0%) | NR | 12.5 (SD 0.7) |
| TOTAL | 28 | 42.7 (5.4) | -- | 8 (28.6%) | 2 (7%) | 4 (14.3%) | 88 | 10 (SD 4.3) |
| Cirrhosis | ||||||||
| Reached BMI<40 kg/m2 by 6 Months | Reached BMI<40 kg/m2 by 12 Months | |||||||
| Garcia-Sesma | 8 | 6 (of 7; 85.7% | 6 (75.0%) | 2 (25.0%) | 2 (25.0%) | -- | 7, 8 | 33.2 (SD 23.6 |
| Sharpton | 32 | 13 (of 22; 59.1% | 20 (62.5%) | 21 (65.6%)* | 14 (43.7%) | 9 (28.1%) | median 22 (IQR 1488)** | -- |
| Taneja | 1 | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 0 (0%) | 12 | 12 |
| TOTAL | 41 | 20 (of 30; 66.7%) | 27 (65.8%) | 24 (58.5%) | 17 (41.5%) | 9 (of 33; 27.3%) | 9.0 (SD 2.6) | 30.9 (23.2) |
| Chronic Kidney Disease | ||||||||
| BMI (kg/m2) at Bariatric Surgery | BMI (kg/m2) at Last Follow-up | |||||||
| Adani | 3 | NR* | NR* | 2 (67%) | 2 (67%) | -- | NR* | NR* |
| Al Sabah | 1 | 42 | 31.5 | 1 (100%) | 1 (100%) | -- | 29 | 6 |
| Al Bahri | 16 | 47.5 (SD 7.5) | 30.9 (6.4) | 4 (25%) | 9 (56%) | -- | 53.2 (SD 16.3) | 42.0 (32.8) |
| Alexander | 41 | 48 (SD NR)* | NR* | 9 (22%) | 9 (22%) | -- | NR | NR* |
| Buch | 1 | NR* | NR* | 1 (100%) | 1 (100%) | -- | 24 | NR* |
| Carandina | 9 | 47.0 (SD 7.1) | 33.6 (7.4) | 1 (11%) | 5 (56%) | -- | 21 | 15.7 (10.6) |
| Contreras-Villamizar | 1 | 42 | 28.6 | 0 (0%) | 1 (100%) | -- | No transplant | 12 |
| Jamal | 21 | 47.1 (SD 5.5) | 35.3 (8.4) | 2 (10%) | 18 (86%) | -- | NR | 27.6 (22) |
| Kienzl-Wagner | 8 | 38.8 (SD 3.8) | 30.7 (6.0) | 7 (88%) | 8 (100%) | -- | 17.6 (SD 10.5) | 38.4 (16.8) |
| Kim | 100 | 43.4 (SD 5.9) | 36.9 (5.7) | 19 (19%) | 19 (19%) | -- | NR | NR* |
| Koshy | 3 | 40.7 (SD 4.9) | 37.2 (1.9) | 3 (100%) | 3 (100%) | -- | NR | 13.7 (1.2) |
| Lin | 6 | NRBO* | NRBO* | 1 (17%) | 6 (100%) | -- | NRBO | NR* |
| MacLaughlin | 9 | median 44.2* | median 34.7 (range 29.2 – 38.8)* | 0 (0%) | 3 (33%) | -- | No transplant | 12* |
| Marszalek | 1 | 41.5 | 29 | 1 (100%) | 1 (100%) | -- | 10 | 10 |
| Newcombe | 3 | 44.6 (SD 5.5) | 33.8 (5.0) | 3 (100%) | 3 (100%) | -- | 16.3 (SD 10.2) | 16.3 (8.3) |
| Proczko | 3 | 39.9 (SD 2.1) | 31.1 (2.4) | 1 (33%) | 3 (100%) | -- | 3 | 3 (0) |
| Takata | 7 | 49.9 (SD 5.7) | NR* | 0 (0%) | 7 (100%) | -- | No transplant | NR* |
| Thomas | 31 | 43.5 (SD 0.7) | 28.1 (SD 0.8) | 14 (67%) | 25 (81%) | -- | median 33* | NR* |
| Yemeni | 24 | 41.5 (SD 0.8) | 29 (SD 1.3) | 16 (67%) | 21 (88%) | -- | 18 (1 – 51.6)* | 47.0 (6.5) |
| TOTAL | 288 | 44.0 (5.5) | 33.7 (5.3) | 85 (30%) | 145 (50%) | -- | 24.8 (14.2) | 32.9 (21.4) |
Highlights.
Bariatrics helped obese heart and lung failure patients achieve transplant listing
Bariatric surgery improved the ejection fraction in obese heart failure patients
Bariatric surgery helped obese liver and kidney failure patients achieve listing
Some end-stage organ disease patients no longer needed transplant after weight loss
Acknowledgments
The authors would like to thank Jill Deaver, MA, MLIS, for her assistance in developing our search strategy.
The authors would like to acknowledge the following funding sources:
Grant Support: Dr. Orandi is supported by the National Center for Advancing Translational Sciences Grant/award number: 1KL2TR003097) and the Career Development Award for Clinical/Outcomes/Education Research from the Society for Surgery of the Alimentary Tract. Mr. Purvis is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant/award number: T35DK116670. Dr. Locke is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant/award number: 5R01DK113980.
Abbreviations:
- BMI
body mass index
- ESRD
end-stage renal disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no financial or personal relationships with other people or organizations that could inappropriately influence their work.
REFERENCES
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med 2019;381:2440–50. [DOI] [PubMed] [Google Scholar]
- 3.Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and nonalcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology (Baltimore, Md) 2019;70:487–95. [DOI] [PubMed] [Google Scholar]
- 4.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 Annual Data Report: Liver. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2019;19 Suppl 2:184–283. [DOI] [PubMed] [Google Scholar]
- 5.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2016 Annual Data Report: Pancreas. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2018;18 Suppl 1:114–71. [DOI] [PubMed] [Google Scholar]
- 6.Chan G, Soucisse M. Survey of Canadian Kidney Transplant Specialists on the Management of Morbid Obesity and the Transplant Waiting List. Canadian journal of kidney health and disease 2016;3:2054358116675344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pruthi R, Tonkin-Crine S, Calestani M, et al. Variation in Practice Patterns for Listing Patients for Renal Transplantation in the United Kingdom: A National Survey. Transplantation 2018;102:961–8. [DOI] [PubMed] [Google Scholar]
- 8.Pondrom S How big is too big? The AJT Report 2012;12:1663–4. [DOI] [PubMed] [Google Scholar]
- 9.Abramowicz D, Cochat P, Claas FH, et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2015;30:1790–7. [DOI] [PubMed] [Google Scholar]
- 10.Martin P, DiMartini A, Feng S, Brown R Jr., Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology (Baltimore, Md) 2014;59:1144–65. [DOI] [PubMed] [Google Scholar]
- 11.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 12.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2015;34:1–15. [DOI] [PubMed] [Google Scholar]
- 13.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA surgery 2014;149:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reges O, Greenland P, Dicker D, et al. Association of Bariatric Surgery Using Laparoscopic Banding, Roux-en-Y Gastric Bypass, or Laparoscopic Sleeve Gastrectomy vs Usual Care Obesity Management With All-Cause Mortality. Jama 2018;319:279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gys B, Plaeke P, Lamme B, et al. Endoscopic Gastric Plication for Morbid Obesity: a Systematic Review and Meta-analysis of Published Data over Time. Obesity surgery 2019;29:3021–9. [DOI] [PubMed] [Google Scholar]
- 16.Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surgical endoscopy 2018;32:2159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardila-Gatas J, Sharma G, Nor Hanipah Z, et al. Bariatric surgery in patients with interstitial lung disease. Surgical endoscopy 2019;33:1952–8. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins RB, Go K, Raymond SL, Ayzengart A, Friedman J. Laparoscopic sleeve gastrectomy in patients with heart failure and left ventricular assist devices as a bridge to transplant. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2018;14:1269–73. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Jung AD, Dhar VK, et al. Laparoscopic sleeve gastrectomy improves renal transplant candidacy and posttransplant outcomes in morbidly obese patients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2018;18:410–6. [DOI] [PubMed] [Google Scholar]
- 20.Sharpton SR, Terrault NA, Posselt AM. Outcomes of Sleeve Gastrectomy in Obese Liver Transplant Candidates. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society 2019;25:538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. Journal of cardiac failure 2008;14:198–202. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- 23.Mathes T, Pieper D. Clarifying the distinction between case series and cohort studies in systematic reviews of comparative studies: potential impact on body of evidence and workload. BMC Med Res Methodol 2017;17:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brethauer SA, Kim J, el Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2015;11:489–506. [DOI] [PubMed] [Google Scholar]
- 25.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of surgery 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramani GV, McCloskey C, Ramanathan RC, Mathier MA. Safety and efficacy of bariatric surgery in morbidly obese patients with severe systolic heart failure. Clinical cardiology 2008;31:516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y, Shi J, Freeman CM, et al. Addressing the challenges of sleeve gastrectomy in end-stage renal disease: Analysis of 100 consecutive renal failure patients. Surgery 2017;162:358–65. [DOI] [PubMed] [Google Scholar]
- 28.Thomas IA, Gaynor JJ, Joseph T, et al. Roux-en-Y gastric bypass is an effective bridge to kidney transplantation: Results from a single center. Clinical transplantation 2018;32:e13232. [DOI] [PubMed] [Google Scholar]
- 29.Modanlou KA, Muthyala U, Xiao H, et al. Bariatric surgery among kidney transplant candidates and recipients: analysis of the United States renal data system and literature review. Transplantation 2009;87:1167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kienzl-Wagner K, Weissenbacher A, Gehwolf P, Wykypiel H, Ofner D, Schneeberger S. Laparoscopic sleeve gastrectomy: gateway to kidney transplantation. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2017;13:909–15. [DOI] [PubMed] [Google Scholar]
- 31.Martin MJ, Bennett S. Pretransplant bariatric surgery: a new indication? Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2007;3:648–51. [DOI] [PubMed] [Google Scholar]
- 32.Takata MC, Campos GM, Ciovica R, et al. Laparoscopic bariatric surgery improves candidacy in morbidly obese patients awaiting transplantation. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2008;4:159–64; discussion 64–5. [DOI] [PubMed] [Google Scholar]
- 33.Taneja S, Gupta S, Wadhawan M, Goyal N. Single-lobe living donor liver transplant in a morbidly obese cirrhotic patient preceded by laparoscopic sleeve gastrectomy. Case reports in transplantation 2013;2013:279651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yemini R, Nesher E, Carmeli I, et al. Bariatric Surgery Is Efficacious and Improves Access to Transplantation for Morbidly Obese Renal Transplant Candidates. Obesity surgery 2019. [DOI] [PubMed] [Google Scholar]
- 35.Handzlik-Orlik G, Holecki M, Orlik B, Wylezol M, Dulawa J. Nutrition management of the post-bariatric surgery patient. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition 2015;30:383–92. [DOI] [PubMed] [Google Scholar]
- 36.Sharaiha RZ, Kumta NA, Saumoy M, et al. Endoscopic Sleeve Gastroplasty Significantly Reduces Body Mass Index and Metabolic Complications in Obese Patients. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2017;15:504–10. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Nava G, Sharaiha RZ, Vargas EJ, et al. Endoscopic Sleeve Gastroplasty for Obesity: a Multicenter Study of 248 Patients with 24 Months Follow-Up. Obesity surgery 2017;27:2649–55. [DOI] [PubMed] [Google Scholar]
- 38.Amro A, Murr M. A video case report of LRYGB in a patient with a left ventricular assist device. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2015;11:1406–7. [DOI] [PubMed] [Google Scholar]
- 39.Caceres M, Czer LS, Esmailian F, Ramzy D, Moriguchi J. Bariatric surgery in severe obesity and end-stage heart failure with mechanical circulatory support as a bridge to successful heart transplantation: a case report. Transplantation proceedings 2013;45:798–9. [DOI] [PubMed] [Google Scholar]
- 40.Chaudhry UI, Kanji A, Sai-Sudhakar CB, Higgins RS, Needleman BJ. Laparoscopic sleeve gastrectomy in morbidly obese patients with end-stage heart failure and left ventricular assist device: medium-term results. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2015;11:88–93. [DOI] [PubMed] [Google Scholar]
- 41.DeNino WF, Peura JL, Toole JM. Orthotopic heart transplantation after left ventricular assist device implantation and laparoscopic Roux-en-Y gastric bypass. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2013;32:377–8. [DOI] [PubMed] [Google Scholar]
- 42.Gill RS, Karmali S, Nagandran J, Frazier HO, Sherman V. Combined Ventricular Assist Device Placement With Adjustable Gastric Band (VAD-BAND): A Promising New Technique for Morbidly Obese Patients Awaiting Potential Cardiac Transplantation. Journal of clinical medicine research 2012;4:127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greene J, Tran T, Shope T. Sleeve Gastrectomy and Left Ventricular Assist Device for Heart Transplant. JSLS : Journal of the Society of Laparoendoscopic Surgeons 2017;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeng EI, Aranda JM Jr., Ahmed M, Klodell CT. Left Ventricular Assist Device and Bariatric Surgery: A Bridge to Heart Transplant by Weight and Waiting Time Reduction. Journal of cardiac surgery 2016;31:120–2. [DOI] [PubMed] [Google Scholar]
- 45.Lim CP, Fisher OM, Falkenback D, et al. Bariatric Surgery Provides a “Bridge to Transplant” for Morbidly Obese Patients with Advanced Heart Failure and May Obviate the Need for Transplantation. Obesity surgery 2016;26:486–93. [DOI] [PubMed] [Google Scholar]
- 46.Lockard KL, Allen C, Lohmann D, et al. Bariatric surgery for a patient with a HeartMate II ventricular assist device for destination therapy. Progress in transplantation (Aliso Viejo, Calif) 2013;23:28–32. [DOI] [PubMed] [Google Scholar]
- 47.McCloskey CA, Ramani GV, Mathier MA, et al. Bariatric surgery improves cardiac function in morbidly obese patients with severe cardiomyopathy. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2007;3:503–7. [DOI] [PubMed] [Google Scholar]
- 48.Moulla Y, Lyros O, Bluher M, Simon P, Dietrich A. Feasibility and Safety of Bariatric Surgery in High-Risk Patients: A Single-Center Experience. Journal of obesity 2018;2018:7498258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Punchai S, Nor Hanipah Z, Sharma G, et al. Laparoscopic Sleeve Gastrectomy in Heart Failure Patients with Left Ventricular Assist Device. Obesity surgery 2019;29:1122–9. [DOI] [PubMed] [Google Scholar]
- 50.Saeed D, Meehan K, McGee EC Jr. Bariatric surgery at the time of ventricular assist device implantation for morbidly obese patients prior to heart transplantation. Artificial organs 2012;36:450–1. [DOI] [PubMed] [Google Scholar]
- 51.Samaras K, Connolly SM, Lord RV, Macdonald P, Hayward CS. Take heart: bariatric surgery in obese patients with severe heart failure. Two case reports. Heart, lung & circulation 2012;21:847–9. [DOI] [PubMed] [Google Scholar]
- 52.Shah SK, Gregoric ID, Nathan SS, Akkanti BH, Kar B, Bajwa KS. Simultaneous left ventricular assist device placement and laparoscopic sleeve gastrectomy as a bridge to transplant for morbidly obese patients with severe heart failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2015;34:1489–91. [DOI] [PubMed] [Google Scholar]
- 53.Taylor TV, Bozkurt B, Shayani P, Lafuente J, Noon G. End-stage cardiac failure in a morbidly obese patient treated by biliopancreatic diversion and cardiac transplantation. Obesity surgery 2002;12:416–8. [DOI] [PubMed] [Google Scholar]
- 54.Wikiel KJ, McCloskey CA, Ramanathan RC. Bariatric surgery: a safe and effective conduit to cardiac transplantation. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2014;10:479–84. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Sesma A, Calvo J, Manrique A, et al. Morbidly Obese Patients Awaiting Liver Transplantation-Sleeve Gastrectomy: Safety and Efficacy From a Liver Transplant Unit Experience. Transplantation proceedings 2019;51:33–7. [DOI] [PubMed] [Google Scholar]
- 56.Adani GL, Righi E, Baccarani U, et al. Laparoscopic sleeve gastrectomy as a weight reduction strategy in obese patients after kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2015;15:1126–7. [DOI] [PubMed] [Google Scholar]
- 57.Al Sabah S, Al Haddad E. Revisional bariatric surgery in a transplant patient. International journal of surgery case reports 2017;31:86–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Bahri S, Fakhry TK, Gonzalvo JP, Murr MM. Bariatric Surgery as a Bridge to Renal Transplantation in Patients with End-Stage Renal Disease. Obesity surgery 2017;27:2951–5. [DOI] [PubMed] [Google Scholar]
- 59.Alexander JW, Goodman H. Gastric bypass in chronic renal failure and renal transplant. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition 2007;22:16–21. [DOI] [PubMed] [Google Scholar]
- 60.Buch KE, El-Sabrout R, Butt KM. Complications of laparoscopic gastric banding in renal transplant recipients: a case study. Transplantation proceedings 2006;38:3109–11. [DOI] [PubMed] [Google Scholar]
- 61.Carandina S, Genser L, Bossi M, et al. Laparoscopic Sleeve Gastrectomy in Kidney Transplant Candidates: a Case Series. Obesity surgery 2017;27:2613–8. [DOI] [PubMed] [Google Scholar]
- 62.Contreras Villamizar KM, Afanador Rubio DC, Gonzalez Gonzalez CA, Garcia Padilla PK, Rodriguez Sanchez MP. Gastric sleeve surgery in hemodialysis: A case report. International journal of surgery case reports 2019;57:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hidalgo JE, Roy M, Ramirez A, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy: a first step for rapid weight loss in morbidly obese patients requiring a second non-bariatric procedure. Obesity surgery 2012;22:555–9. [DOI] [PubMed] [Google Scholar]
- 64.Jamal MH, Corcelles R, Daigle CR, et al. Safety and effectiveness of bariatric surgery in dialysis patients and kidney transplantation candidates. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2015;11:419–23. [DOI] [PubMed] [Google Scholar]
- 65.Koshy AN, Coombes JS, Wilkinson S, Fassett RG. Laparoscopic gastric banding surgery performed in obese dialysis patients prior to kidney transplantation. American journal of kidney diseases : the official journal of the National Kidney Foundation 2008;52:e15–7. [DOI] [PubMed] [Google Scholar]
- 66.Lin MY, Tavakol MM, Sarin A, et al. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2013;9:653–8. [DOI] [PubMed] [Google Scholar]
- 67.MacLaughlin HL, Hall WL, Patel AG, Macdougall IC. Laparoscopic sleeve gastrectomy is a novel and effective treatment for obesity in patients with chronic kidney disease. Obesity surgery 2012;22:119–23. [DOI] [PubMed] [Google Scholar]
- 68.Marszalek R, Ziemianski P, Lisik W, et al. Bariatric surgery as a bridge for kidney transplantation in obese subjects. Case report. Annals of transplantation 2012;17:108–12. [DOI] [PubMed] [Google Scholar]
- 69.Newcombe V, Blanch A, Slater GH, Szold A, Fielding GA. Laparoscopic adjustable gastric banding prior to renal transplantation. Obesity surgery 2005;15:567–70. [DOI] [PubMed] [Google Scholar]
- 70.Proczko M, Kaska L, Kobiela J, Stefaniak T, Zadrozny D, Sledzinski Z. Roux-en-Y gastric bypass in dialysed morbidly obese patients as a preparation for a kidney transplantation: case series. Wideochirurgia i inne techniki maloinwazyjne = Videosurgery and other miniinvasive techniques 2013;8:174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonatti H, Iqbal N, Kling C, Melvin W, Broome J. Protracted Hypocalcemia following 3.5 Parathyroidectomy in a Kidney Pancreas Recipient with a History of Robotic-Assisted Roux-en-Y Gastric Bypass. Case reports in transplantation 2018;2018:2182083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gullo-Neto S, Padoin AV, Queiroz de Carvalho JE, et al. Metabolic surgery for the treatment of type 2 diabetes in pancreas after kidney transplant candidates. Transplantation proceedings 2014;46:1741–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


