Abstract
Parkinson’s disease (PD), a progressive neurodegenerative disorder, is associated with the destruction of dopamine neurons in the substantia nigra (SN) and the formation of Lewy bodies in basal ganglia. Risk factors for PD include aging, as well as environmental and genetic factors. Recent converging reports suggest a role for the gut microbiome and epigenetic factors in the onset and/or progression of PD. Of particular relevance and potential therapeutic targets in this regard, are histone deacetylases (HDACs), enzymes that are involved in chromatin remodeling. Butyrate, a short-chain fatty acid (FA) produced in the gut and presumably acting via several G protein-coupled receptors (GPCRs) including FA3 receptors (FA3Rs), is a well-known HDAC inhibitor that plays an important role in maintaining homeostasis of the gut-brain axis. Recently, its significance in regulation of some critical brain functions and usefulness in neurodegenerative diseases such as PD has been suggested. In this study we sought to determine whether butyrate may have protective effects against salsolionl (SALS)-induced toxicity in SH-SY5Y cells. SALS, an endogenous product of aldehyde and dopamine condensation, may be selectively toxic to dopaminergic neurons. SH-SY5Y cells, derived from human neuroblastoma cells are used as a model of these neurons. Exposure of SH-SY5Y cells for 24 h to 400 μM SALS resulted in approximately 60% cell death, which was concentration-dependently prevented by butyrate. The effects of butyrate in turn, were significantly attenuated by beta-hydroxy butyrate (BHB), a selective FA3R antagonist. Moreover, a selective FA3R agonist (AR 420626) also provided protective effects against SALS, which was totally blocked by BHB. These findings provide further support that butyrate or an agonist of FA3R may be of therapeutic potential in PD.
Keywords: Butyrate, Free Fatty Acid, FA3 Receptor, AR 420626, Parkinson’s Disease, Neurodegeneration, SH-SY5Y Cells
Introduction
The search for slowing or stopping the progression of the devastating neurodegenerative diseases in general, and Parkinson’s disease (PD) in particular, is an ongoing challenge as the etiology of these diseases remain elusive. Moreover, the complexity of neurocircuitries make it unrealistic to assume that targeting a single neurotransmitter and/or receptor may provide the sought-after “silver bullet.” However, recent advances in our understanding of the gut-brain axis and the epigenetic influences on brain function, offer new insights and potential novel targets for therapeutic intervention in PD. It is now believed that the initiation and progression of PD may be impeded through manipulation of the gut microbiota (Sampson et al. 2016: Sun and Shen 2018; Abdel-Haq et al. 2019). Specifically, it is suggested that dysbiosis or altered colonic microbiota can lead to neuroinflammation and misfolding of alpha-synuclein (α-Syn), a protein highly implicated in PD pathology (Keshavarzian et al. 2015; Sampson et al. 2016; Yang et al. 2019; Baizabal-Carvallo and Alonso-Juarez 2020). Furthermore, it has been reported that the composition of the gut microbiota and the short chain fatty acids (SCFAs) differ between patients with PD and age-matched controls (Unger et al. 2016: Sun and Shen 2018; Baizabal-Carvallo and Alonso-Juarez 2020).
Butyrate, a short chain FA, acting as an energy source for colonic epithelial cells, has anti-inflammatory, enteroendocrine and epigenetic effects that not only can influence colonic and systemic health, but can also affect the brain function (Cantu-Jungles et al. 2019). Indeed, a few studies indicate beneficial effects of butyrate in animal models of PD (Laurent et al. 2013; Liu et al. 2017). Although the exact mechanism of action of butyrate in the central nervous system remains far from complete, it is known to interact with fatty acid receptor 3 (FA3R), which is a G protein coupled receptor. FA3R also known as GPR41, and FA2R also known as GPR43, are related members of a homologous family of orphan G protein-coupled receptors that are tandemly encoded at a single chromosomal locus in both humans and mice. Moreover, GPR41 is related to GPR43 (52% similarity; 43% identity) and are activated by similar ligands but with differing specificity. Thus, acetate preferentially activates FA2R, whereas butyrate preferentially activates FA3R, which is also the one predominantly expressed in enteric neurons of the submucosal and myenteric ganglia, and in several of the postganglionic sympathetic and sensory neurons, both in autonomic and somatic peripheral nervous system (Brown et al. 2003; de Clercq et al. 2016; Kaji et al. 2018; Falomir-Lockhart et al. 2019).
It is well known that PD, characterized by movement disorders, is the result of damage or destruction of dopaminergic neurons in the substantia nigra. Although, as eluded earlier, the causes of PD are unknown, some atypical cases seem to have a genetic origin. However, these familial cases account for a rather small percentage of incidences as the majority of cases are sporadic and hence, of unknown etiology (Healy et al. 2004; Morris, 2005; Tizabi et al. 2019). Recent advances in PD pathology suggest that the neuronal degeneration in this disease likely involves several cellular and molecular events including: proapoptotic mechanisms, oxidative stress and microglia-mediated inflammation (Hurley and Tizabi 2013; Maiti et al. 2017; Reglodi et al. 2017; Ho 2019) as well as gut microbial dysbiosis (Sampson et al. 2016; Yang et al. 2019; Baizabal-Carvallo and Alonso-Juarez 2020).
SH-SY5Y cells, human neuroblastoma-derived cells, express high levels of dopaminergic activity and are used extensively as a cellular model to study mechanism(s) of toxicity and protection in nigral dopaminergic neurons (Storch et al. 2002; Maruyama et al. 2004; Naoi et al. 2004; Copeland et al. 2007; Kovalevich and Langford, 2013). Salsolinol (SALS), an endogenous neuromodulator in dopaminergic cells, is formed during the metabolism of dopamine and is the condensation product of dopamine and aldehydes (Mravec, 2006). Dysregulation of SALS, especially its (R) enantiomer in the brain, is thought to contribute to the pathogenesis of idiopathic of PD (Antkiewicz-Michaluk, 2002; Xicoy et al. 2017; Kurnik-Łucka et al. 2018). Because of its selective toxicity to dopaminergic cells, we and others have used SALS-induced toxicity in SH-SY5Y cells to screen potential neuroprotectants, specifically applicable to PD (Copeland et al. 2007; Qualls et al. 2014; Xicoy et al. 2017; Getachew et al. 2019).
In this study, using the SH-SY5Y cell line, we investigated potential protection of butyrate against SALS-induced toxicity as well as possible involvement of FA3Rs in butyrate’s action.
Materials and Methods
Butyrate, beta-hydroxy butyrate (BHB), a selective FA3R antagonist (Kimura et al. 2011; Ulven 2012; Inoue et al. 2014), SALS, and other analytical reagents including 3,(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay kit were purchased from Sigma Chemical Company (Sigma-Aldrich, St. Louis, MO). AR 420626 (AR), chemical name: N-(2,5-Dichlorophenyl)-4-(furan-2-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide, a FA3 receptor agonist (Hudson et al. 2014; Bolognini et al. 2016; Kaji et al. 2018), was purchase form Bio Techne Corp-Tocris (Minneapolis, MN). The SH-SY5Y human neuroblastoma cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA).
As reported in detail previously (Manavalan et al. 2017; Getachew et al. 2018, 2019), SH-SY5Y cells were cultured in a 1:1 mixture of Dulbeccos Modified Eagle Medium (DMEM) and Ham’s F12 supplemented with 10 % fetal bovine serum, penicillin/streptomycin (100 IU/ml), and gentamicin (50ug/ml) at 37° C in 95% O2/5% CO2 humidified incubator. The cells (un-differentiated) were trypsinized when confluent and plated in 96 well plates (1.2 × 104 cells/well). Cells were allowed to adhere to bottom surface for 24 h. Then, fresh media containing SALS (400 μM) or various concentrations of butyrate or AR with and without butyrate antagonist were added to the carefully aspirated wells. Butyrate or AR were added one hour prior to SALS and butyrate antagonist, in term was added one hour prior to butyrate or AR. In all cases, the control group consisted of cells that were maintained in media alone and without any drug treatment. All treatments were carried out for 24 h and the effects on cell viability were determined following the 24 h incubation. Each cell viability study was run in sextuplicate (i.e., 6 replicates) and a minimum of 4 assays were conducted for each experimental manipulation.
Determination of cell viability was done by MTT colorimetric assay according to the manufacturer’s protocol as described previously (Manavalan et al. 2017; Getachew et al. 2018, 2019). Briefly, the yellow MTT tetrazolium salt (0.5 mg/ml) was dissolved in phosphate-buffered saline (PBS) with 10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). 30 μl of MTT was added to each well and incubated for 3 h at 37° C. The live cells cause a reduction of the yellow salt to insoluble purple formazan crystals. The wells were then aspirated, and 50 μl of dimethyl sulfoxide (DMSO) was added to the wells to solubilize the crystals, the plates were then placed in a shaker for an hour and read spectrophotometrically at 570 nm with a background of 630 nm in a plate reader. Cell viability was determined by subtracting the test results from the background and is presented as a percentage of the control.
Data is expressed as mean ± standard error of the mean (SEM). Statistical differences within and between treatment groups were determined by one-way analysis of variance (ANOVA) followed by post-hoc Newman–Keuls Multiple comparison test, where P < 0.05 was considered statistically significant. Data were analyzed using Graphpad Prism 6 (Graphpad Software, Inc., San Diego, CA).
Results
Figure 1 depicts the effect of various concentrations of butyrate (0.01 – 20 μM) against SALS-induced toxicity in SH-SY5Y cells. For SALS, we used a concentration of 400 μM because we have consistently observed over 60% toxicity during the 24 h exposure with this concentration (Copeland et al. 2007; Getachew et al. 2018, 2019). As seen, there was a concentration-dependent protection by butyrate against SALS toxicity with full protection at 20 μM butyrate [F(4,20)=15.2, p<0.01]. Butyrate by itself, at any concentration, did not affect the cell viability (data not shown)
Fig. 1.
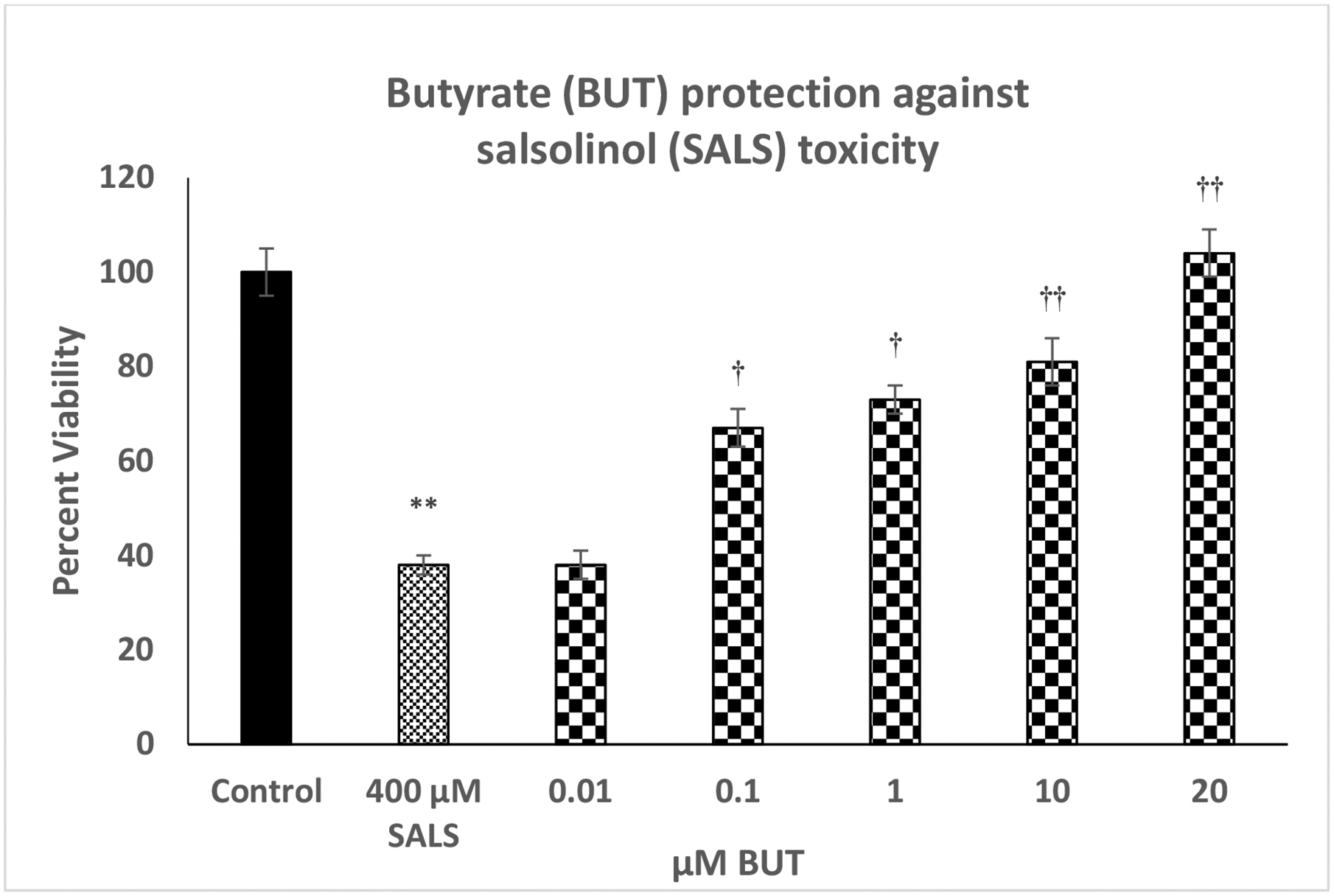
Effect of various concentrations of butyrate (BUT) against salsolinol (SALS)-induced toxicity. Cells were treated with SALS with and without BUT for 24 h and cell viability was determined by MTT. BUT was added 1 h before SALS. Values are mean ± SEM. *p<0.05, **p<0.01 compared to control. N = 5 per treatment
Figure 2 depicts the effect of various concentrations of AR (0.01 – 20 μM) against SALS-induced toxicity. Here also, there was a concentration-dependent protection by AR against SALS-induced toxicity. Full protection was achieved at 20 μM AR [F(4,20)=14.1, p<0.01]. AR by itself, at any concentration, did not affect the cell viability (data not shown).
Fig. 2.
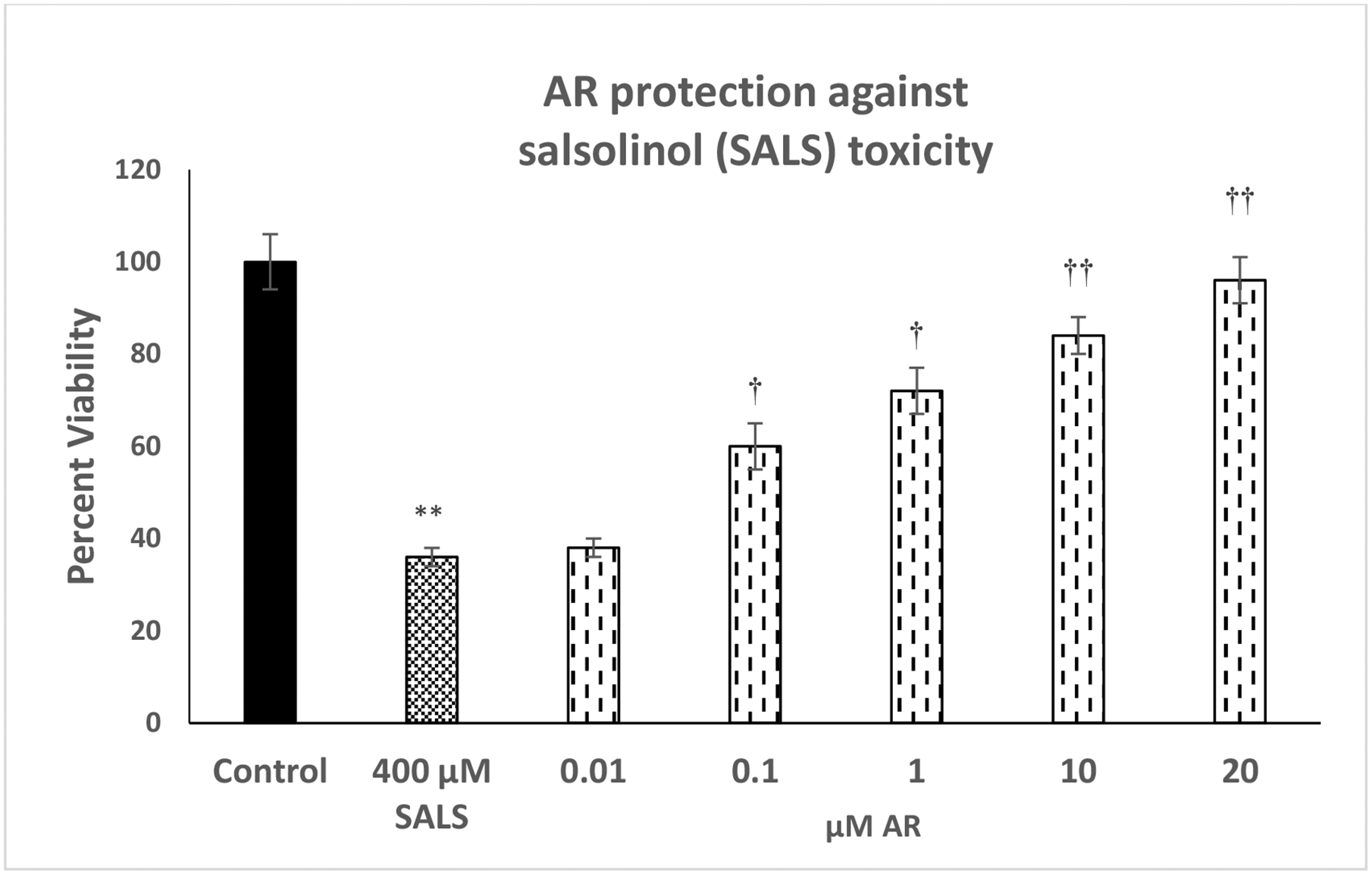
Effect of various concentrations of AR, a selective FA3R agonist, against salsolinol (SALS)-induced toxicity. Cells were treated with SALS with and without AR for 24 h and cell viability was determined by MTT. AR was added 1 h before SALS. Values are mean ± SEM. *p<0.05, **p<0.01 compared to control. N = 5 per treatment
Figure 3 depicts effect of various concentrations of FA3R antagonist (BHB) on protective effects of butyrate against SALS-induced toxicity. We used the highest concentration of butyrate (20 μM) as this concentration was fully protective against SALS toxicity. As seen, there was concentration-dependent attenuation of butyrate effect by BHB [F(4,20)=18.8, p<0.01]. The highest concentration of BHB (60 μM) reduced the protective effects of butyrate by approximately 40% (p<0.01). BHB did not have any effect of its own on cell viability at any of the concentrations used (data not shown).
Fig. 3.
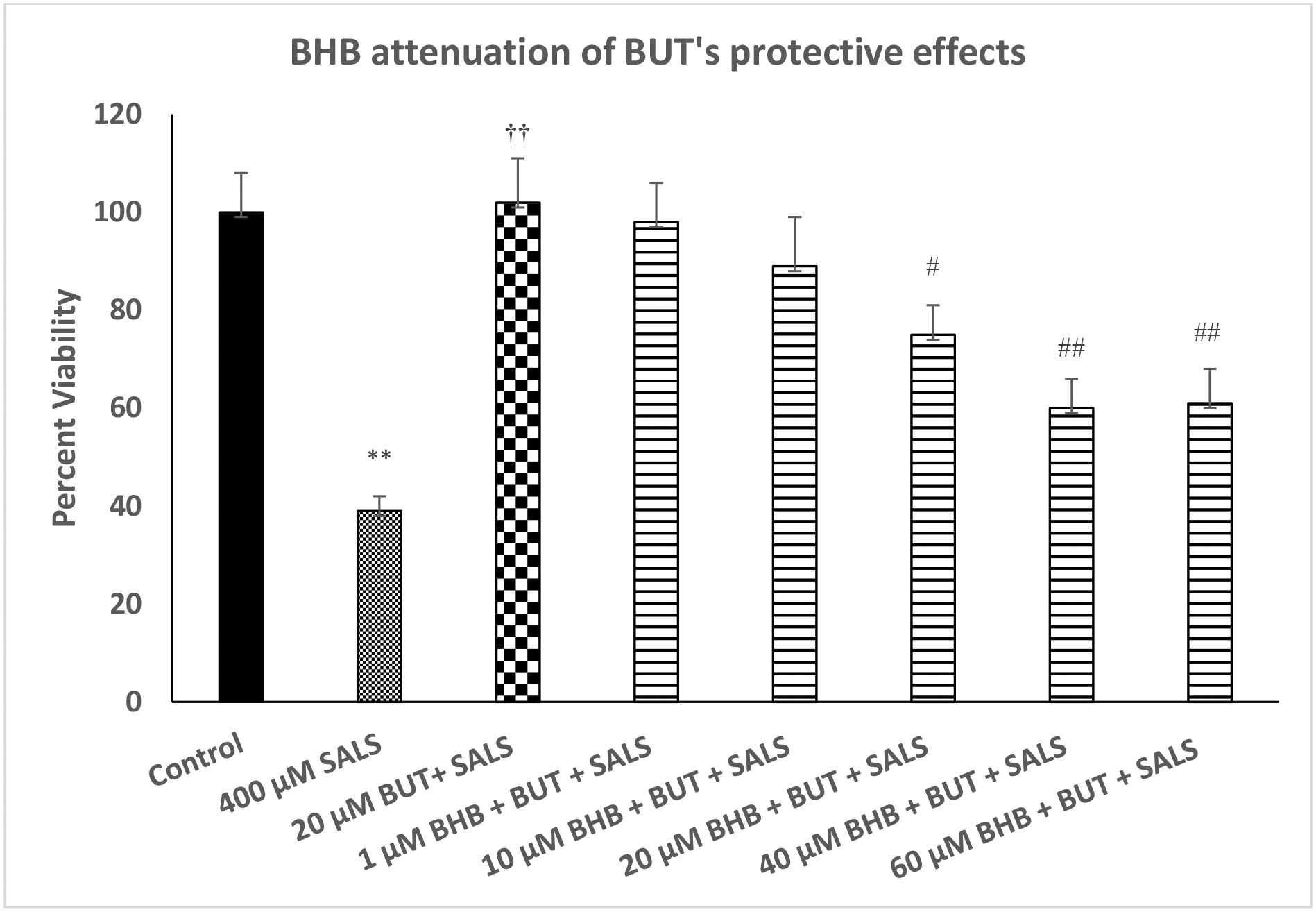
Effect of various concentrations of beta-hydroxy butyrate (BHB), a FA3R antagonist, on protective effects of butyrate (BUT) against salsilinol (SALS)-induced toxicity. Cells were treated with SALS, BUT and BHB for 24 h and cell viability was determined by MTT. BHB was added 1 h before BUT, which was added 1 h before SALS. Values are mean ± SEM. **p<0.01 compared to control. ††p<0.01 compared to SALS, #p<0.05, ##p<0.01 compared to BUT + SALS. N = 4–5 per treatment
Figure 4 depicts effect of various concentrations of BHB on protective effects of AR against SALS-induced toxicity. We used the highest concentration of AR (20 μM) as this concentration was fully protective against SALS-induced toxicity. As seen, there was a concentration-dependent attenuation of AR protection by BHB [F(4,20)=13.6, p<0.01]. In this case, however, the highest concentration of BHB (60 μM) fully blocked the effects of AR (p<0.01).
Fig. 4.
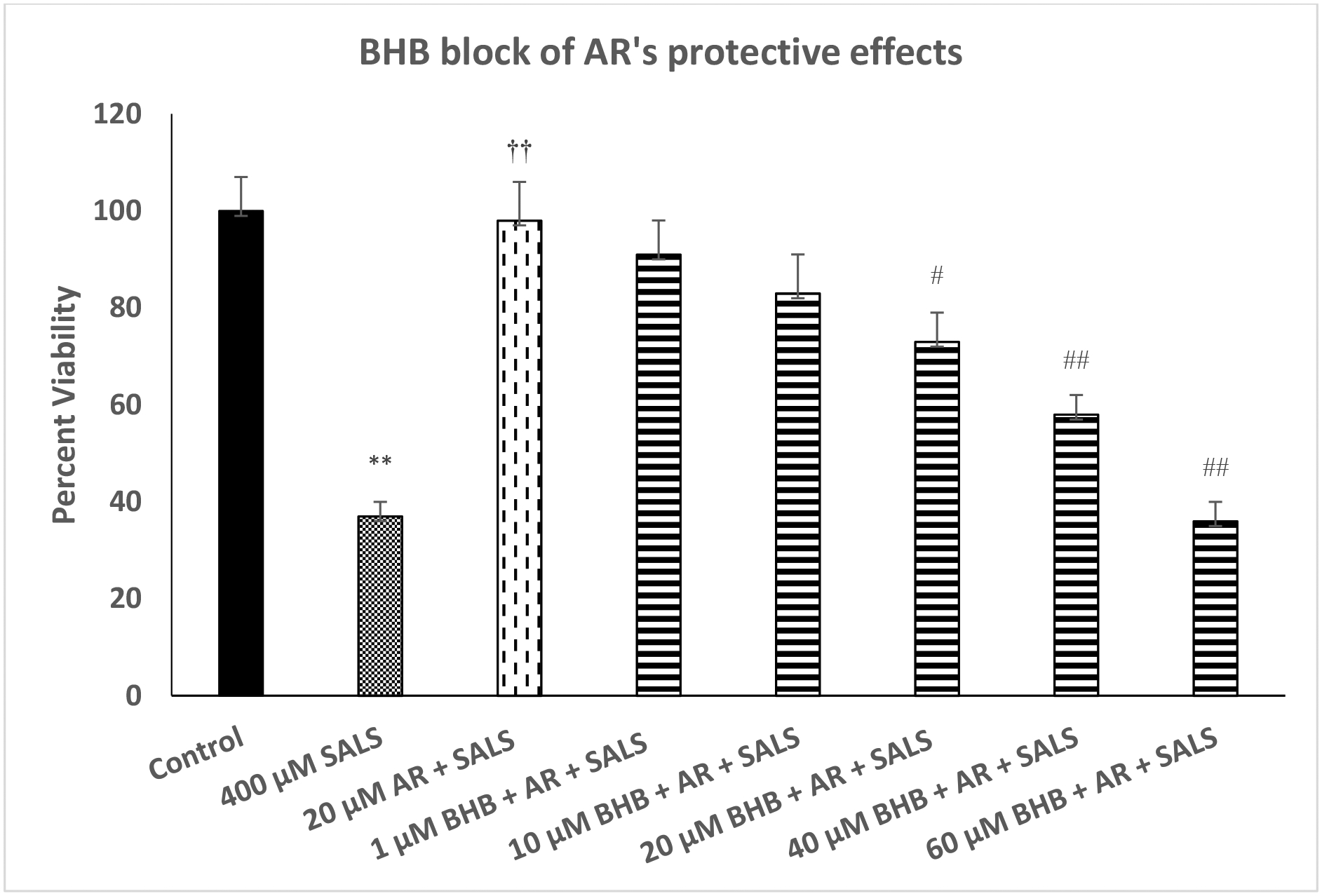
Effect of various concentrations of beta-hydroxy butyrate (BHB), a FA3R antagonist, on protective effects of AR against salsilino (SALS) iduced toxicity. Cells were treated with SALS, AR and BHB for 24 h and cell viability was determined by MTT. BHB was added 1 h before AR, which was added 1 h before SALS. Values are mean ± SEM. **p<0.01 compared to control. ††p<0.01 compared to SALS, #p<0.05, ##p<0.01 compared to AR + SALS. N = 4–5 per treatment
Discussion
The results of this study show for the first time that butyrate as well as AR, an FA3R agonist can protect against salsolinol-induced toxicity in neuroblastoma-derived dopaminergic cells. Moreover, partial effect of BUT, and whole effect of AR could be blocked by BHB, a selective FA3R antagonist. These findings support potential therapeutic efficacy of butyrate or an FA3R agonist in PD.
Although the etiology of sporadic PD remains to be fully understood, substantial evidence implicates dysbiosis or disturbance in gut microbiota as an important contributor to its pathophysiology (Sampson et al. 2016: Sun and Shen 2018; Abdel-Haq et al. 2019; Cantu-Jungles et al. 2019; Baizabal-Carvallo and Alonso-Juarez 2020). Indeed, it has been reported that PD is associated with hyperpermeability of the colonic epithelium (Forsyth et al. 2011). Moreover, as indicated above dysbiosis or altered colonic microbiota can lead to neuroinflammation and misfolding of α-Syn, a protein highly implicated in PD pathology (Keshavarzian et al. 2015). Several microbiota have been associated with PD. Thus, lower abundance of Lachnospiraceae family members and Faecalibacterium as well as low production of several SCFAs including butyrate, have been observed in patients with PD (Cantu-Jungles et al. 2019). Interestingly, administration of butyrate can shift the balance of gut flora in favor of beneficial microbiota and hence normalization of dysbiosis (Zhou et al. 2017). Moreover, beneficial effects of butyrate in animal models of PD have been reported (Laurent et al. 2013; Liu et al. 2017).
Although several SCFAs with significant importance in maintaining the colonic epithelial cells exist, butyrate has attracted particular interest not only due to its beneficial effects on cellular metabolism, but also because of its role in modulating immune and inflammatory responses as well as its potential usefulness in neurodegenerative diseases (Laurent et al 2013; Liu et al. 2018, Cantu-Jungles et al. 2019). Several mechanisms have been implicated in butyrate’s action. These include acting as an HDAC inhibitor, activation of Nrf2/HO-1 axis, stimulation of glucagon like peptide-1 and binding to several specific G protein–coupled receptors (GPCRs) such as FA2R and FA3R (Funakohi-Tago et al. 2018; Liu et al. 2017, Cantu-Jungles et al. 2019). In terms of the latter effect, it is noteworthy that butyrate preferentially activates FA3R, which is primarily expressed on neuronal cells including enteric, sympathetic and sensory neurons (Nøhr et al. 2013; Tough et al. 2018; Falomir-Lockhart et al, 2019).
Since BHB, a selective FA3R antagonist, only partially blocked the protective effects of butyrate, it may be concluded that other mechanism(s), in addition to its activation of FA3R are involved in butyrate’s neuroprotective effects. Interestingly, non-FA3R mediated effects of butyrate have also been observed in other conditions, for example, protection against diet-induced obesity (Lin et al. 2012). However, the fact that AR, a selective FA3R agonist can also exert protective effects against SALS indicates that activation of this receptor alone might be sufficient to induce neuroprotection, particularly of relevance to PD. This contention is based on the results where BHB, a selective FA3R antagonist can totally block the protective effects of AR.
Beneficial effects of butyrate in PD are further evidenced by its attenuation of motor impairments induced by 6-hydroxydopamine, rotenone or MPTP in rat models of PD (Srivastav et al. 2019). In addition, butyrate may confer neuroprotection against dopamine cell death, DNA damage, oxidative stress and protein aggregation induced by lipopolysaccharide (LPS), via HDAC inhibition (Srivastav et al. 2019). It is of relevance to note that Sampson et al (2016), using mice overexpressing αSyn and germ-free mice, which are devoid of gut microbiota, conclude that gut microbiota is required for motor deficits, microglia activation, and αSyn pathology. However, in the same study they also report that colonization of αSyn-overexpressing mice with microbiota from PD patients enhances physical impairments compared to microbiota transplants from healthy human donors, thus, strongly suggesting that abnormality in the gut bacteria transferred from PD patients contribute to movement disorders in mice (Sampson et al. 2016). Collectively, abundance of reports indicate beneficial effects of SCFA in general and butyrate in particular (Lanza et al., 2019; Srivastav et al 2019; Nuzum et al 2020).
In addition, it is now well recognized that butyrate not only may have neuroprotective effects but may also offer protection against several other diseases including graft rejection, inflammatory bowel disease, colorectal cancer and diabetes, all of which carry an inflammatory component (Tikhonova 2017; Alrafas et al. 2019; Nielson et al. 2019). In addition, butyrate was recently advocated for treatment of obesity and sleep disorders (Nielson et al. 2019; Szentirmai et al. 2019). However, butyrate’s poor pharmacologic properties such as short half-life and first-pass hepatic clearance pose some limitation on its therapeutic efficacy (Witt et al. 2003; Sampathkumar et al. 2006; Yoo and Jones, 2006; Ghosh et al. 2012). Therefore, it would be of significant interest to determine whether AR or any selective FA3R agonist, with a better pharmacologic profile than butyrate, may also have similar protection in myriad of diseases affected by butyrate. In this regard it was recently reported that selective FA3 agonist may be a potential therapeutic candidate for NSAID-induced enteropathy (Said et al. 2017).
In summary, the findings support the potential use of butyrate or a selective FA3R agonist in PD.
Supplementary Material
Acknowledgement:
Supported by NIH/NIAAA R03AA022479 (YT), NIA/NIH 1R25AG047843-01 (ABC)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK (2019) Microbiome-microglia connections via the gut-brain axis. J Exp Med 216(1):41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrafas HR, Busbee PB, Nagarkatti M, Nagarkatti PS (2019) Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J Leukoc Biol 106(2):467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L (2002) Endogenous risk factors in Parkinson’s disease: dopamine and tetrahydroisoquinolines. Pol J pharmcol 54:567–572. [PubMed] [Google Scholar]
- Baizabal-Carvallo JF, and Alonso-Juarez M (2020) The link between gut dysbiosis and neuroinflammation in Parkinson’s disease. Neuroscience 432:160–173. [DOI] [PubMed] [Google Scholar]
- Bolognini D, Tobin AB, Milligan G, Moss CE (2016) The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol 89(3):388–98. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. (2003) The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278:11312–9. [DOI] [PubMed] [Google Scholar]
- Cantu-Jungles TM, Rasmussen HE, Hamaker BR (2019) Potential of prebiotic butyrogenic fibers in Parkinson’s disease. Front Neurol 10:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland RL Jr, Das JR, Kanaan YM, Taylor RE, Tizabi Y (2007) Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Res 12(1):61–9. [DOI] [PubMed] [Google Scholar]
- Falomir-Lockhart LJ, Cavazzutti GF, Giménez E, Toscani AM (2019) Fatty acid signaling mechanisms in neural cells: fatty acid receptors. Front Cell Neurosci 24;13:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A (2001) Role of snail activation in alcohol-induced iNOS-mediated disruption of intestinal epithelial cell permeability. Alcohol Clin Exp Res 35(9):1635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakohi-Tago M, Sakata T, Fujiwara S, Sakakura A, Sugai T, Tago K, et al. (2018) Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-1 axis in SH-SY5Y cells. Eur J Pharmacol 834:246–256. [DOI] [PubMed] [Google Scholar]
- Getachew B, Csoka AB, Aschner M, Tizabi Y (2019) Nicotine protects against manganese and iron-induced toxicity in SH-SY5Y cells: Implication for Parkinson’s disease. Neurochem Int 124:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Hudson T, Heinbockel T, Csoka AB, Tizabi Y (2018) Protective effects of donepezil against alcohol-induced toxicity in cell culture: role of caspase-3. Neurotox Res 34(3):757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SK, Perrine SP, Williams RM, Faller DV (2012) Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents. Blood 119(4):1008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DG, Abou-Sleiman PM, Wood NW (2004) PINK, PANK, or PARK? A clinicians’ guide to familial parkinsonism. Lancet Neurol 3:652–662. [DOI] [PubMed] [Google Scholar]
- Ho MS (2019) Microglia in Parkinson’s disease. Adv Exp Med Biol 2019;1175:335–353. [DOI] [PubMed] [Google Scholar]
- Hudson BD, Christiansen E, Murdoch H, Jenkins L, Hansen AH, Madsen O, et al. (2014) Complex pharmacology of novel allosteric free fatty acid 3 receptor ligands. Mol Pharmacol 86(2):200–10. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Tizabi Y (2013) Neuroinflammation, neurodegeneration, and depression. Neurotox Res 23(2):131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D, Tsujimoto G, and Kimura I (2014) Regulation of energy homeostasis by GPR41. Front Endocrinol (Lausanne) 5, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Akiba Y, Furuyama T, Adelson DW, Iwamoto K, Watanabe M, et al. (2018) Free fatty acid receptor 3 activation suppresses neurogenic motility in rat proximal colon. Neurogastroenterol Motil 30(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, et al. (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–60. [DOI] [PubMed] [Google Scholar]
- Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A 108:8030–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnik-Łucka M, Panula P, Bugajski A, Gil K (2018) Salsolinol: an unintelligible and double-faced molecule-lessons learned from in vivo and in vitro experiments. Neurotox Res 33(2):485–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M, Campolo M, Casili G, Filippone A, Paterniti I, Cuzzocrea S, et al. (2019) Sodium butyrate exerts neuroprotective effects in spinal cord injury. Mol Neurobiology 56(6):3937–3947. [DOI] [PubMed] [Google Scholar]
- Laurent C, Burnouf S, Ferry B, Batalha VL, Coelho JE, Baqi Y, et al. (2016) A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol Psychiatry 21(1):97–107. [DOI] [PubMed] [Google Scholar]
- Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, et al. (2012) Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7(4):e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X (2018) Butyrate: A double-edged sword for health? Adv Nutr 9(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang F, Liu S, Du J, Hu X, Xiong J, et al. (2017) Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci 381:176–181 [DOI] [PubMed] [Google Scholar]
- Maiti P, Manna J, Dunbar GL (2017) Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl Neurodegener 6, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan S, Getachew B, Manaye KF, Khundmiri SJ, Csoka AB, McKinley R, et al. (2017) PACAP protects against ethanol and nicotine toxicity in SH-SY5Y cells: implications for drinking-smoking comorbidity. Neurotox Res 32(1):8–13. [DOI] [PubMed] [Google Scholar]
- Maruyama W, Yi H, Takahashi T, Shimazu S, Ohde H, Yoneda F, et al. (2004) Neuroprotective function of R-(−)-1-(benzofuran-2-yl)-2-propylaminopentane, [R-(−)-BPAP], against apoptosis induced by N-methyl(R)salsolinol, an endogenous dopaminergic neurotoxin, in human dopaminergic neuroblastoma SH-SY5Y cells. Life Sci 75:107–117. [DOI] [PubMed] [Google Scholar]
- Morris HR (2005) Genetics of Parkinson’s disease. Ann Med 37(2):86–96. [DOI] [PubMed] [Google Scholar]
- Mravec B (2006) Salsolinol, a derivate of dopamine, is a possible modulator of catecholaminergic transmission: a review of recent developments. Physiological research/Academia Scientiarum Bohemoslovaca 55:353–364. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Nagy GM (2004) Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains. Neurotoxicology 25:193–204. [DOI] [PubMed] [Google Scholar]
- Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. (2013) GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology154(10):3552–64. [DOI] [PubMed] [Google Scholar]
- Nuzum ND, Loughman A, Szymlek-Gay EA, Hendy A, Teo WP, Macpherson H (2020) Gut microbiota differences between healthy older adults and individuals with Parkinson’s disease: A systematic review. Neurosci Biobehav Rev 112:227–241. [DOI] [PubMed] [Google Scholar]
- Qualls Z, Brown D, Ramlochansingh C, Hurley LL, Tizabi Y (2014) Protective Effects of Curcumin Against Rotenone and Salsolinol Induced Toxicity: Implications for Parkinson’s Disease. Neurotox Res 25(1): 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Renaud J, Tamas A, Tizabi Y, Socías SB, Del-Bel E, et al. (2017) Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol 155:120–148. [DOI] [PubMed] [Google Scholar]
- Said H, Akiba Y, Narimatsu K, Maruta K, Kuri A, Iwamoto KI, et al. (2017) FFA3 activation stimulates duodenal bicarbonate secretion and prevents NSAID-induced enteropathy via the GLP-2 pathway in rats. Dig Dis Sci 62(8):1944–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar SG, Jones MB, Meledeo MA, Campbell CT, Choi SS, Hida K, et al. (2006) Targeting glycosylation pathways and the cell cycle: sugar-dependent activity of butyrate-carbohydrate cancer prodrugs. Chem Biol 13(12):1265–75. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167:1469–80.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastav S, Neupane S, Bhurtel S, Katila N, Maharjan S, Choi H, et al. (2019) Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J Nutr Biochem 69:73–86. [DOI] [PubMed] [Google Scholar]
- Storch A, Ott S, Hwang YI, Ortmann R, Hein A, Frenzel S, et al. (2002) Selective dopaminergic neurotoxicity of isoquinoline derivatives related to Parkinson’s disease: studies using heterologous expression systems of the dopamine transporter. Biochem Pharmacol 63:909–920. [DOI] [PubMed] [Google Scholar]
- Sun MF, Shen YQ (2018) Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res Rev 45:53–61. [DOI] [PubMed] [Google Scholar]
- Szentirmai É, Millican NS, Massie AR, Kapás L (2019) Butyrate, a metabolite of intestinal bacteria, enhances sleep. Sci Rep 9, 7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonova IG (2017) Application of GPCR structures for modelling of free fatty acid receptors. Handb Exp Pharmacol 236:57–77. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Getachew B, Csoka AB, Manaye KF, Copeland RL (2019) Novel targets for parkinsonism-depression comorbidity. Prog Mol Biol Transl Sci 167:1–24. [DOI] [PubMed] [Google Scholar]
- Tough IR, Forbes S, Cox HM (2018) Signaling of free fatty acid receptors 2 and 3 differs in colonic mucosa following selective agonism or coagonism by luminal propionate. Neurogastroenterol Motil 30(12):e13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulven T (2012) Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne) 3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger MM, Spiegel J, Unger MM, Spiegel J, Dillmann KU, Grundmann D, et al. (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72. [DOI] [PubMed] [Google Scholar]
- Witt O, Monkemeyer S, Rönndahl G, Erdlenbruch B, Reinhardt D, Kanbach K, et al. (2003) Induction of fetal hemoglobin expression by the histone deacetylase inhibitor apicidin. Blood 101(5):2001–7. [DOI] [PubMed] [Google Scholar]
- Xicoy H, Wieringa B, Martens GJ (2017) The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener 12(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhao D, Ali Shah SZ, Lai M, Zhang X, et al. (2019) The role of the gut microbiota in the pathogenesis of Parkinson’s disease. Front Neurol 10:1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo CB, Jones PA (2006) Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov 5(1):37–50. [DOI] [PubMed] [Google Scholar]
- Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG (2017) Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep 8;7(1):1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


