Abstract
Background and purpose
Rapamycin is a clinically approved mammalian target of rapamycin inhibitor that has been shown to be neuroprotective in animal models of stroke. However, the mechanism of rapamycin-induced neuroprotection is still being explored. Our aims were to determine if rapamycin improved leptomeningeal collateral perfusion, to determine if this is through endothelial nitric oxide synthase (eNOS)-mediated vessel dilation and to determine if rapamycin increases immediate post-reperfusion blood flow.
Methods
Wistar and spontaneously hypertensive rats (SHR) (~14 weeks old, n = 22 and n = 15, respectively) were subjected to ischemia by middle cerebral artery occlusion (MCAo, 90 and 120 min, respectively) with or without treatment with rapamycin at 30 minutes post-stroke. Changes in MCA and collateral perfusion territories were measured by dual-site laser Doppler. Reactivity to rapamycin was studied using isolated and pressurized leptomeningeal anastomoses (LMA). Brain injury was measured histologically or with triphenyltetrazolium chloride staining.
Results
In Wistar rats, rapamycin, increased collateral perfusion (43 ± 17%), increased reperfusion cerebral blood flow (CBF) (16 ± 8%) and significantly reduced infarct volume (35 ± 6 vs. 63 ± 8mm3, p < 0.05). Rapamycin dilated LMA by 80 ± 9%, which was abolished by nitric oxide synthase inhibition. In SHRs, rapamycin increased collateral perfusion by 32 ± 25%, reperfusion CBF by 44 ± 16%, without reducing acute infarct volume 2 h post- reperfusion. Reperfusion CBF was a stronger predictor of brain damage than collateral perfusion in both Wistar and SHRs.
Conclusions
Rapamycin increased collateral perfusion and reperfusion CBF in both Wistar and co-morbid SHRs that appeared to be mediated by enhancing eNOS activation. These findings suggest that rapamycin may be an effective acute therapy for increasing collateral flow and as an adjunct therapy to thrombolysis or thrombectomy to improve reperfusion blood flow.
Keywords: Collateral Circulation, eNOS, Experimental Stroke, mTOR, Rapamycin
Graphical Abstract
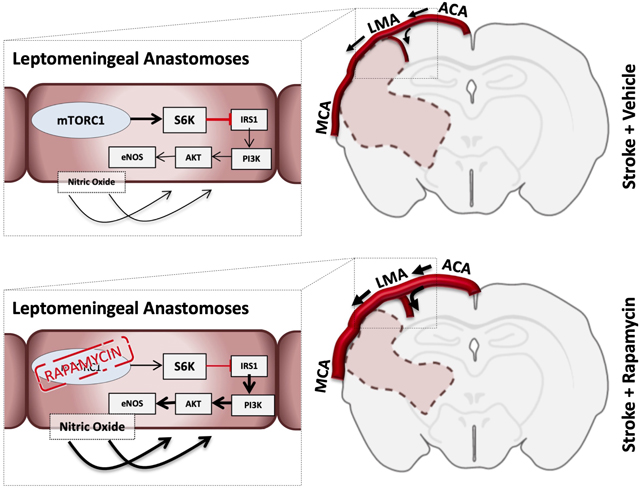
During middle cerebral artery Occlusion (MCAo) in both Wistar and Spontaneously Hypertensive rats (SHR), leptomeningeal collateral perfusion was increased in a nitric oxide (NO) dependent manner that was associated with a reduction in infarct volume and improvement of neurobehavioral scores in Wistar rats but not SHR. Rapamycin also increased reperfusion cerebral blood flow (CBF) in both Wistar and SHR. Reperfusion CBF was a stronger predictor of infarct volume than collateral CBF.
Introduction
Stroke is a leading cause of death and adult disability worldwide.1 Despite multiple drugs showing promise in preclinical studies, none have translated to clinical practice.2 Amplifying the brain’s intrinsic cytoprotective pathways is a promising avenue for developing new treatments for stroke. Mammalian Target of Rapamycin Complex 1 (mTORC1) has been shown to play a role in neuronal degeneration following global ischemia.3 In states of sufficient energy supply, mTORC1 is activated; signaling anabolic cellular processes such as protein synthesis and cell proliferation.4 Low cellular energy, such as in cerebral ischemia, induces TSC1 (Hamartin) and TSC2 (Tuberin), subsequently down-regulating mTORC1.5 Pharmacological inhibition of mTORC1 with the FDA approved anti-rejection medication, rapamycin, has been shown to be a promising brain cytoprotectant in experimental models of stroke.6 However, the mechanisms of rapamycin-induced brain cytoprotection are still be being explored.7
The success of revascularisation therapy in stroke has led stroke researchers to reconsider the important role of the cerebral perfusion in acute stroke therapy.8 Rapamycin has been shown to improve cerebral blood flow (CBF) in Alzheimer’s Disease (AD) mouse models via increased activation of endothelial nitric oxide synthase (eNOS).9 In the context of ischemic stroke, the degree of perfusion through leptomeningeal collateral anastomoses (LMAs) is one of the strongest predictors of penumbral volume, eligibility for revascularization therapies and ultimately stroke outcome.10 Unfortunately, not all patients have adequate collateral perfusion and these patients progress to infarction rapidly and may be ineligible for revascularization therapy or not benefit if they receive this therapy.11
Collateral therapeutics is an emerging area of investigation to determine if interventions can increase collateral flow in the acute setting and potentially improve patient eligibility for revascularization therapy and improve stroke outcome.12 Hypertension is the leading modifiable risk factor for stroke13 and is associated with poor LMA blood flow and worse stroke outcomes in human14 and animal stroke.15 LMAs from hypertensive animals have been shown to be more vasoconstricted than normotensive LMAs,16 thus they have more space to dilate and may benefit more from collateral enhancement therapy. Therefore, it is imperative that potential collateral therapies show efficacy in both normal and hypertensive animals.
Given the evidence of rapamycin’s cerebrovascular effect in other neurological diseases we wanted to determine whether leptomeningeal collateral perfusion was affected by rapamycin, the potential contribution of the eNOS pathway to this process, and the effects of rapamycin on reperfusion CBF in normotensive and hypertensive rats.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
Wistar rats (male, 12–14 weeks old, n = 22) and Spontaneously Hypertensive Rats (SHR, male, 12–14 weeks old, n = 15) were used in this study. All procedures conformed to the Animal (Scientific Procedures) Act 1986 (UK), the National Institutes of Health guidelines for care and use of laboratory animals and were approved by the University of Oxford Animal Ethics Committee, the Home Office (UK) and the Institutional Animal Care and Use Committee at the University of Vermont. The studies were conducted and the manuscript prepared in accordance with the ARRIVE guidelines.17 Detailed animal and experimental descriptions are available in the online-only Data Supplement, please see https://www.ahajournals.org/journal/str.
Model of Transient Focal Ischemia and Treatment
A proximal middle cerebral artery occlusion (MCAo) filament model was used. Rats underwent MCAo using the silicone-tipped intraluminal thread occlusion method, as previously described.18, 19
Multisite Laser Doppler Flowmetry (LDF)
Multisite laser Doppler probes (Oxford Optronix, Oxford, UK) were used to measure changes in the MCA and collateral (LMA) perfusion territories, as described previously (Figure 1A).20 Probe locations are based off cerebrovascular casting studies21, 22 and have been validated using magnetic resonance imaging23 and hydrogen clearance.24
Figure 1. Multisite laser Doppler Flowmetry for measurement of collateral perfusion.
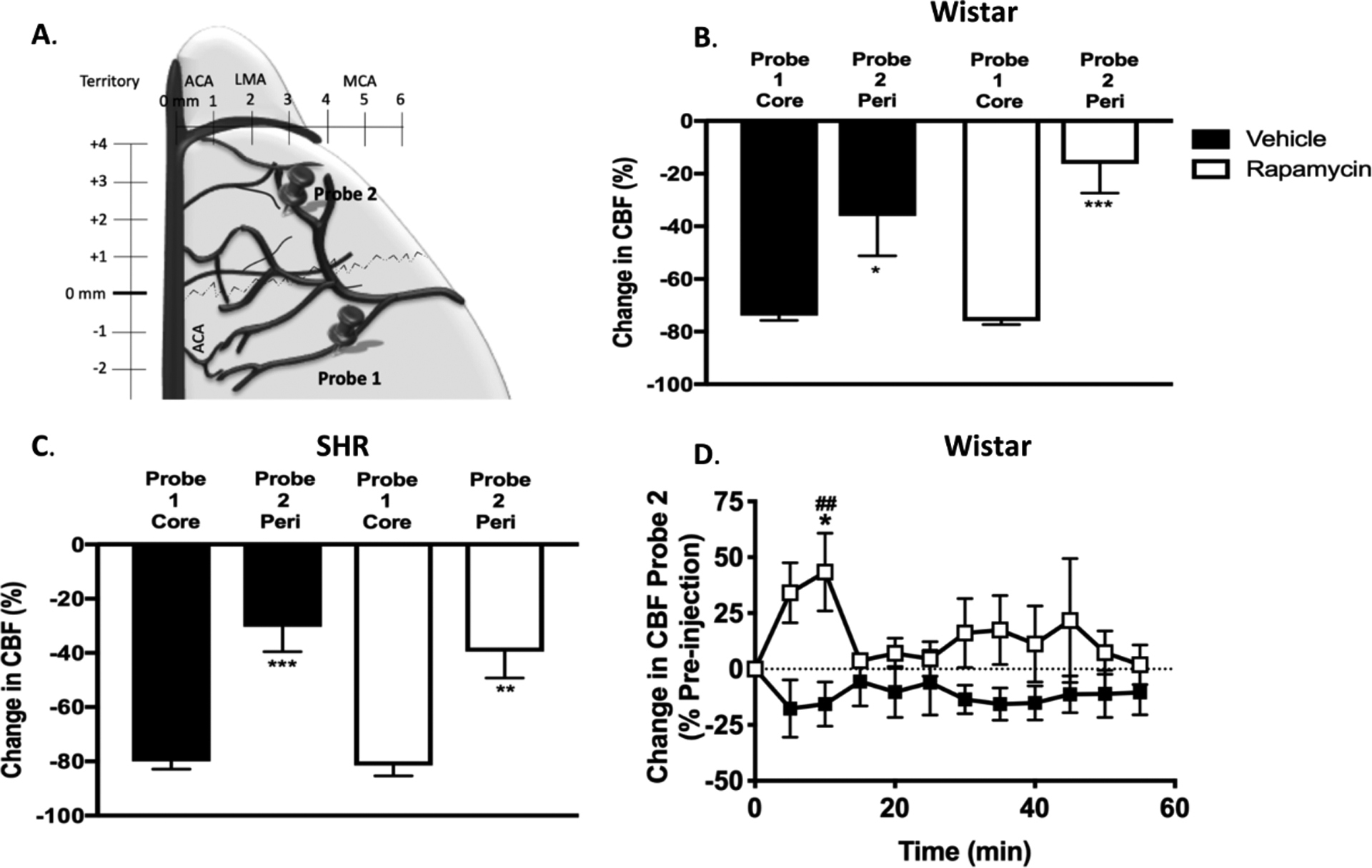
A. Schematic showing the location of the dual-site laser Doppler probes. B and C. Graphs comparing initial drop in cerebral blood flow (CBF) from baseline before filament insertion between MCA core (Probe 1) and peri-infarct (Probe 2) collateral CBF in Wistar rats and SHRs, respectively. Drop in collateral CBF (probe 2) was significantly less than that of the core MCA territory (probe 1) demonstrating that each probe was measuring different hemodynamic areas. *p < 0.05, **p < 0.01, *** p < 0.001 vs. probe 1 group, unpaired t-tests. D. Change in Leptomeningeal Anastomoses (LMA) CBF in Wistar rats calculated as % change from prior to treatment (30min after middle cerebral artery occlusion (MCAo). RM 2-Way ANOVA: F (1, 11) = 3.429, p = 0.09 for treatment, F (11, 121) = 0.5054, p > 0.05 for time, F (11, 121) = 1.463, p > 0.05 for interaction. *p < 0.05 vs. vehicle, Sidak’s post-test. ## p < 0.01 vs. pre-injection baseline in rapamycin group, Dunnett’s post-test.
MCAo was confirmed by >70% decrease in LDF signal from baseline in Probe 1. We selected > 70% drop from baseline CBF based on the infarct threshold of 20–30% of baseline CBF previously reported in normotensive and hypertensive rats.25, 26 In Wistar rats, LMA (probe 2) and MCA (probe 1) perfusion were recorded throughout the whole experiment. Changes in LMA perfusion are expressed as a % of pre-rapamycin administration at 30 min post-MCAo (baseline). Animals were randomized by sealed numbered envelope to receive vehicle (<5% ethanol in saline, n = 5) or rapamycin (Sigma Aldrich, 250 μg/kg, n=8), administered by a surgeon blind to treatment allocation. LMA recordings continued until thread withdrawal (reperfusion) at 90 min post-MCAo and reperfusion core-MCA territory CBF (probe 1, from here on referred to as reperfusion CBF) was monitored for a further 40 min.
Following 24 hours of recovery, the adhesive removal test was used to assess somatosensory deficits following stroke.27 Animals were also tested for stroke-induced neurologic deficits (methods in online-only Data Supplement).28 Brains were then taken for infarct volume analysis (Supplementary Figure I A).
In SHRs, administration of rapamycin, and recordings of LMA (probe 2) and MCA (probe 1) perfusion were conducted using the same protocol as Wistar rats, except LMA recordings continued until reperfusion at 120 min post-MCAo and reperfusion CBF was monitored for a further 120 min. SHR have more severe strokes, which can have a negative impact on animal welfare during recovery. As the primary outcome of this study was collateral perfusion, which could be assessed during the stroke under anesthesia these experiments were non-recovery with acute infarct volume assessment. Brains were taken for infarct volume analysis at 120 min post-reperfusion (Supplementary Figure I B).
Histological Analysis of Brain Damage
In Wistar rats, infarct volume was calculated at 24 h after stroke onset using coronal sections stained with hematoxylin and eosin.29 In SHRs, infarct volume was determined by 2,3,5-triphenyltetrazolium chloride (TTC) staining at 4 h post MCAo. Volumes were then corrected for oedema using the formula: corrected infarct volume (mm3) = infarct volume x (contralateral volume/ ipsilateral volume).30 LDF and histological analyses were performed by investigators blind to treatment allocation.
Reactivity of Isolated LMAs
Reactivity of isolated LMAs to rapamycin was determined using the method previously in Chan et al.16, 31 Detailed description of this method and calculation of % tone and % reactivity of isolated LMAs can be found in in the online-only Data Supplement, please see https://www.ahajournals.org/journal/str
Excluded Animals
Wistar: Four animals were excluded from vehicle-treated group: 2 due to insufficient LDF drop during filament insertion, 1 due to filament-induced haemorrhage and 1 due to lack of final infarct. SHR: Three animals were excluded: 1 rapamycin and 2 vehicle, due to experimental complications. Isolated LMAs: One animal was excluded because of insufficient tone development.
Statistical Analysis
Statistical tests were performed using Graphpad Prism 7.0 (La Jolla, USA). D’Agostino and Pearson omnibus normality tests were performed on all data. Appropriate statistical tests were used based on the normality of the data. Detailed description of statistical tests and sample size calculations for LMA perfusion are available in the online-only Data Supplement. Data are presented as mean ± standard error of the mean (SEM).
Results
Multi-site LDF probes were measuring different hemodynamic regions
MCAo was confirmed by a 70% reduction in MCA perfusion in LDF probe 1 (core/MCA). Figure 1 A and B shows that the drop in peri-infarct/LMA perfusion (probe 2) was significantly less than core flow (probe 1), demonstrating that the two probes were measuring different hemodynamic regions in both Wistar and SHR, respectively. Values are available in the online-only Data Supplement.
Rapamycin improved LMA perfusion, reperfusion CBF, infarct volume and neurological deficits in Wistar rats
Rapamycin significantly increased LMA perfusion (collateral flow) during MCAo (Probe 2) at 10 min post-drug administration, relative to vehicle and immediate pre-injection baseline (rapamycin: 43.4 ± 17.4 vs. vehicle: −15.6 ± 9.9 % of pre-injection baseline, p < 0.05, Figure 1D), demonstrating rapamycin treatment increased collateral flow. Rapamycin significantly increased reperfusion CBF (Probe 1). Reperfusion CBF was increased at 35 min, relative to vehicle and immediate post-reperfusion baseline (rapamycin: 16.2 ± 8.4 vs. vehicle: −20.9 ± 13.6% of immediate post-reperfusion baseline, p < 0.01, Figure 2A).
Figure 2. Wistar rats reperfusion cerebral blood flow and stroke outcome analysis.
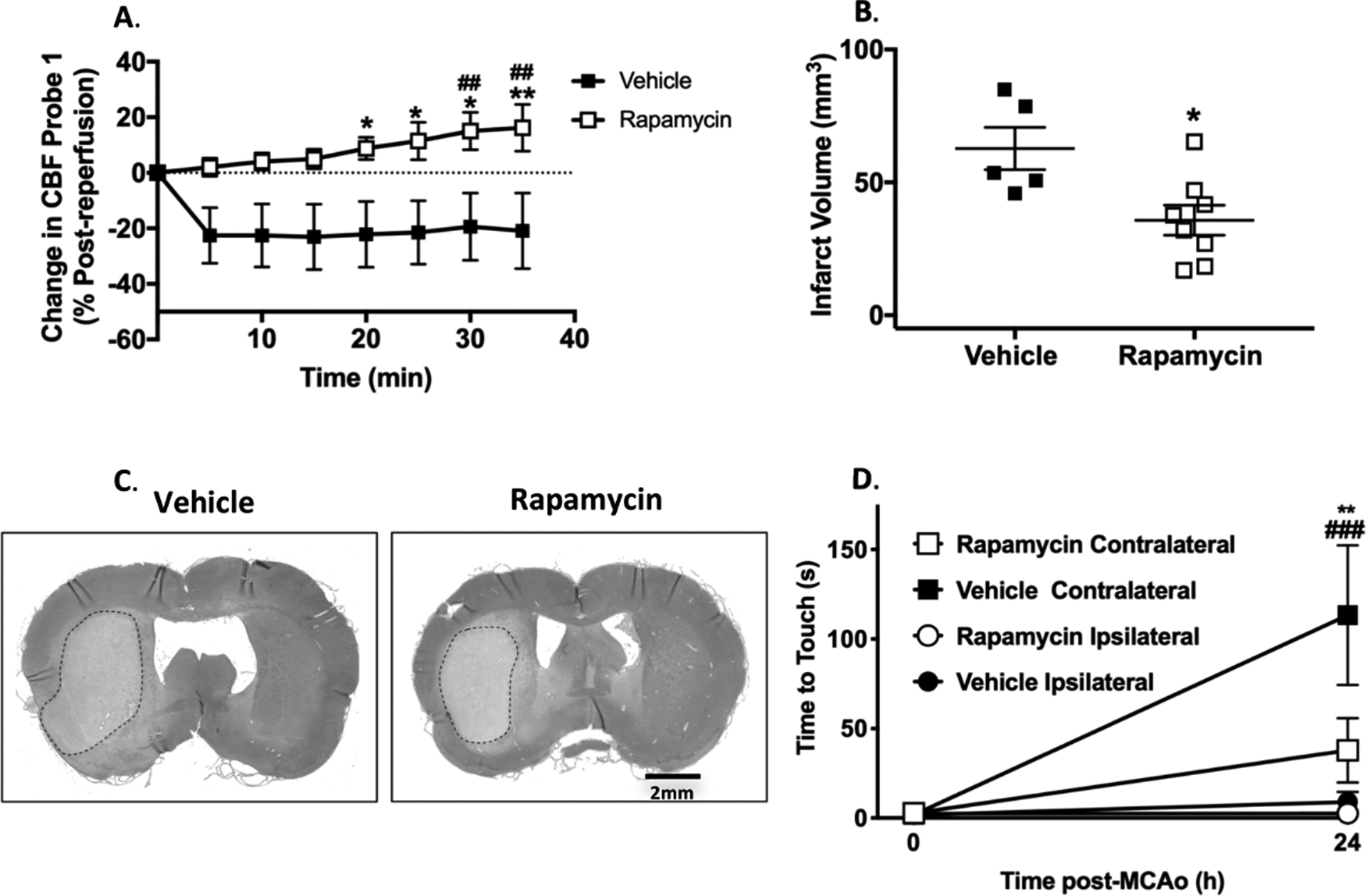
A. Change in middle cerebral artery (MCA) cerebral blood flow calculated as % change post- reperfusion (95 min post-MCAo). RM 2-Way ANOVA: F (1, 64) = 49.11, p < 0.0001 for treatment, F (7, 64) = 0.4979, p = 0.83 for time, F (7, 64) = 1.15, p = 0.35 for interaction. *p < 0.05, ** p < 0.01 vs. vehicle, Sidak’s post-test. ## p < 0.01 vs. post-reperfusion baseline in rapamycin group, Dunnett’s post-test. B. Infarct volume at 24 h post-MCAo. *p < 0.05 vs. vehicle, unpaired t-test. C. Representative histological hematoxylin and eosin stains for infarct volume. D. Time to touch in adhesive removal test at pre-MCAo baseline and 24 h after MCAo. RM 2-Way ANOVA: F (3, 22) = 6.253, p < 0.001 for treatment, F (1, 22) = 16.32, p < 0.01 for time, F (3, 22) = 6.375, p < 0.01 for interaction. **p < 0.01 rapamycin contralateral vs. vehicle contralateral, Tukey’s post-test. ### p < 0.001 vs. pre-MCAo baseline in vehicle group, Sidak’s post-test.
Rapamycin significantly reduced infarct volume at 24 h (rapamycin: 35.7 ± 5.6 mm3 vs. vehicle: 62.7 ± 7.9 mm3, p < 0.05, Figure 2B and C). There was significantly less neurological deficit at 24 h in animals treated with rapamycin (time-to-touch on contralateral forepaw = 37.8 ± 18.0 s in the rapamycin group vs. 113.3 ± 39.0 s in the vehicle group, p < 0.05). There was no significant change in time-to-touch on the ipsilateral forepaw between 0 and 24 h in either group (Figure 2D). Rapamycin showed a trend towards improved neuroscores (Rapamycin: median score of 2, range 1–3 vs. Vehicle: median score of 4, range 2–5 out of a max score of 6, Supplementary Figure II).
Rapamycin improved LMA perfusion and reperfusion CBF but not early infarct volume in SHRs
There were no significant differences in physiological variables in SHRs (mean arterial pressure, pO2, pCO2 and pH) between the rapamycin and vehicle groups prior to injection, 10 min after treatment and 60 min post-reperfusion (Table 1).
Table 1.
Physiological Parameters
| SHR | Pre-injection | 10min post-injection | Reperfusion | |||
|---|---|---|---|---|---|---|
| Vehicle | Rapamycin | Vehicle | Rapamycin | Vehicle | Rapamycin | |
| MAP (mm Hg) | 177 ± 8.2 | 180.3 ± 7.3 | 174.5 ± 9.6 | 184.5 ± 4.5 | 134.8 ± 4.4 | 141.5 ± 7.9 |
| pO2 (mm Hg) | 128.4 ± 5.4 | 140.4 ± 3.1 | 131.2 ± 4 | 135 ± 3.9 | 135.9 ± 2.5 | 140.4 ± 4.7 |
| pCO2 (mm Hg) | 38.4 ± 1.4 | 39.7 ± 1.5 | 38.2 ± 1.1 | 39.5 ± 2.2 | 40.8 ± 3.3 | 47.3 ± 0.4 |
| pH | 7.44 ± 0.008 | 7.43 ± 0.01 | 7.41 ± 0.01 | 7.38 ± 0.008 | 7.35 ± 0.005 | 7.34 ± 0.007 |
Abbreviations: MAP, mean arterial pressure; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen.
Rapamycin significantly increased LMA perfusion (collateral flow) during MCAo (Probe 2) relative to pre-injection baseline at 45, 75 and 80 min post-drug administration (80 min: 31.8 ± 24.7 % pre-injection baseline, p < 0.01, Figure 3A). Rapamycin significantly increased reperfusion CBF (Probe 1). Reperfusion CBF was increased relative to vehicle at 70 min post-reperfusion (rapamycin: 43.7 ± 16.1 vs. vehicle: - 7.2 ± 9.2 % of post-reperfusion baseline, p < 0.05, Figure 3B). Rapamycin also significantly increased reperfusion CBF relative to baseline between 5 and 85 min post-reperfusion (65 min: 47.5 ± 13.4 % post-reperfusion baseline, p < 0.0001, Figure 3B). Rapamycin did not reduce final infarct volume (rapamycin: 76.3 ± 11.2 mm3 vs. vehicle: 76.4 ± 16 mm3, p > 0.05, Figure 3C and D).
Figure 3. Spontaneously Hypertensive Rats (SHRs) studies.
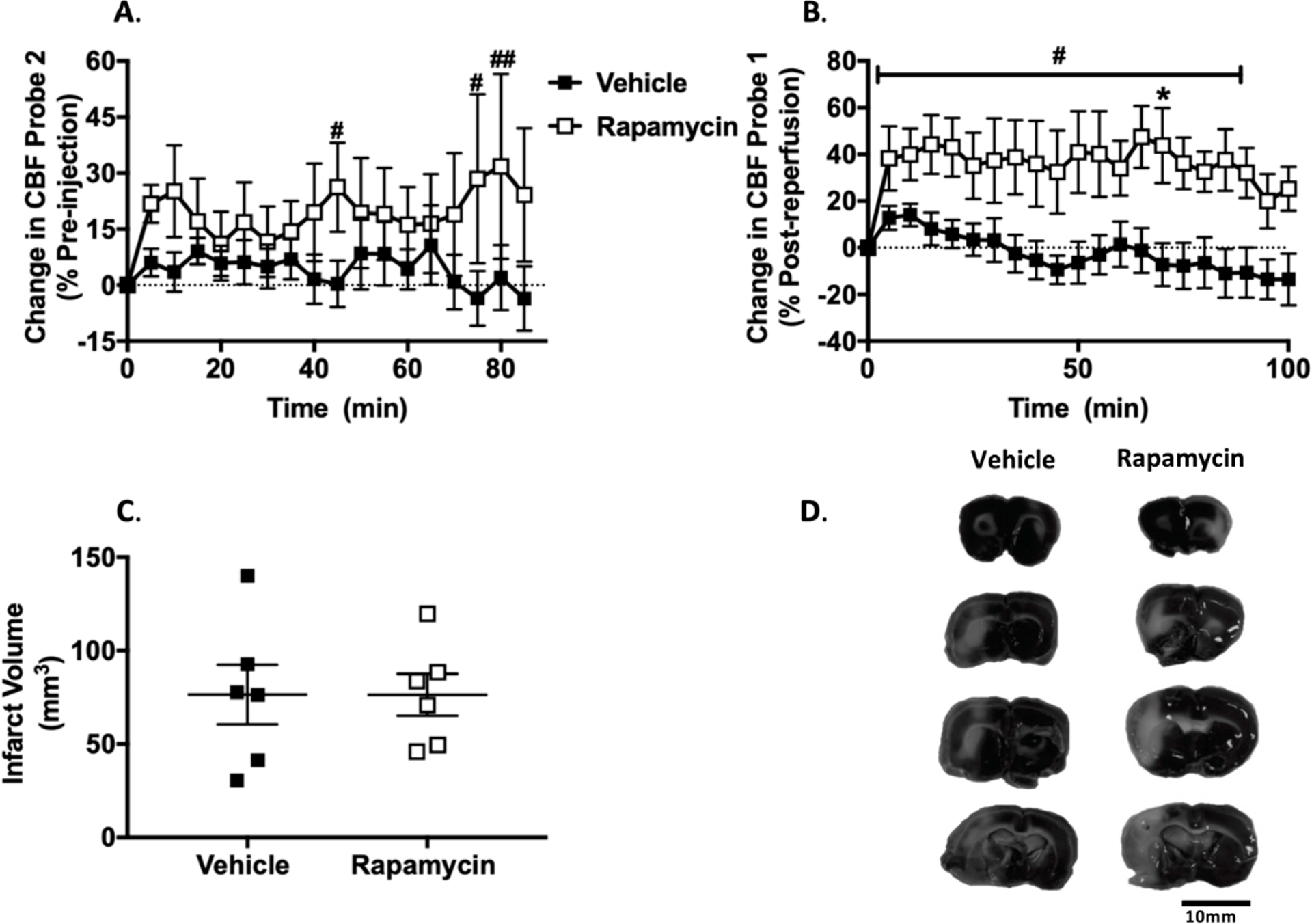
A. Change in leptomeningeal anastomoses cerebral blood flow (CBF) calculated as % change from prior to treatment at 30min after middle cerebral artery occlusion (MCAo). RM 2-Way ANOVA: F (1, 10) = 1.403, p > 0.05 for treatment, F (17, 170) = 0.6585, p > 0.05 for time, F (17, 170) = 1.102, p > 0.05 for interaction. # p < 0.05, ## p < 0.01 vs. pre-injection baseline in rapamycin group, Dunnett’s post-test. B. Change in MCA CBF calculated as % change from reperfusion (125 min post-MCAo). RM 2-Way ANOVA: F (1, 10) = 8.433, p < 0.05 for treatment, F (20, 200) = 2.113, p < 0.01 for time, F (20, 200) = 1.202, p > 0.05 for interaction.* p < 0.05 vs. vehicle, RM 2-Way ANOVA, Sidak’s post-test. # p < 0.05 vs. post-reperfusion baseline in rapamycin group, RM 2-Way ANOVA, Dunnett’s post-test. C. Infarct volume at 4 h post-MCAo. D. Representative TTC stains.
Reperfusion CBF is a stronger predictor of outcome than LMA perfusion in Wistar and SHR
In Wistar rats, average LMA perfusion during experimental stroke was not significantly correlated with final infarct volume (r2 = 0.007, p > 0.05, Figure 4A). Average reperfusion CBF was significantly inversely correlated with final infarct volume (r2 = 0.4298, p < 0.05, Figure 4B).
Figure 4. Relationship between changes in leptomeningeal anastomoses (LMA) cerebral blood flow (CBF), reperfusion CBF and final infarct volume.
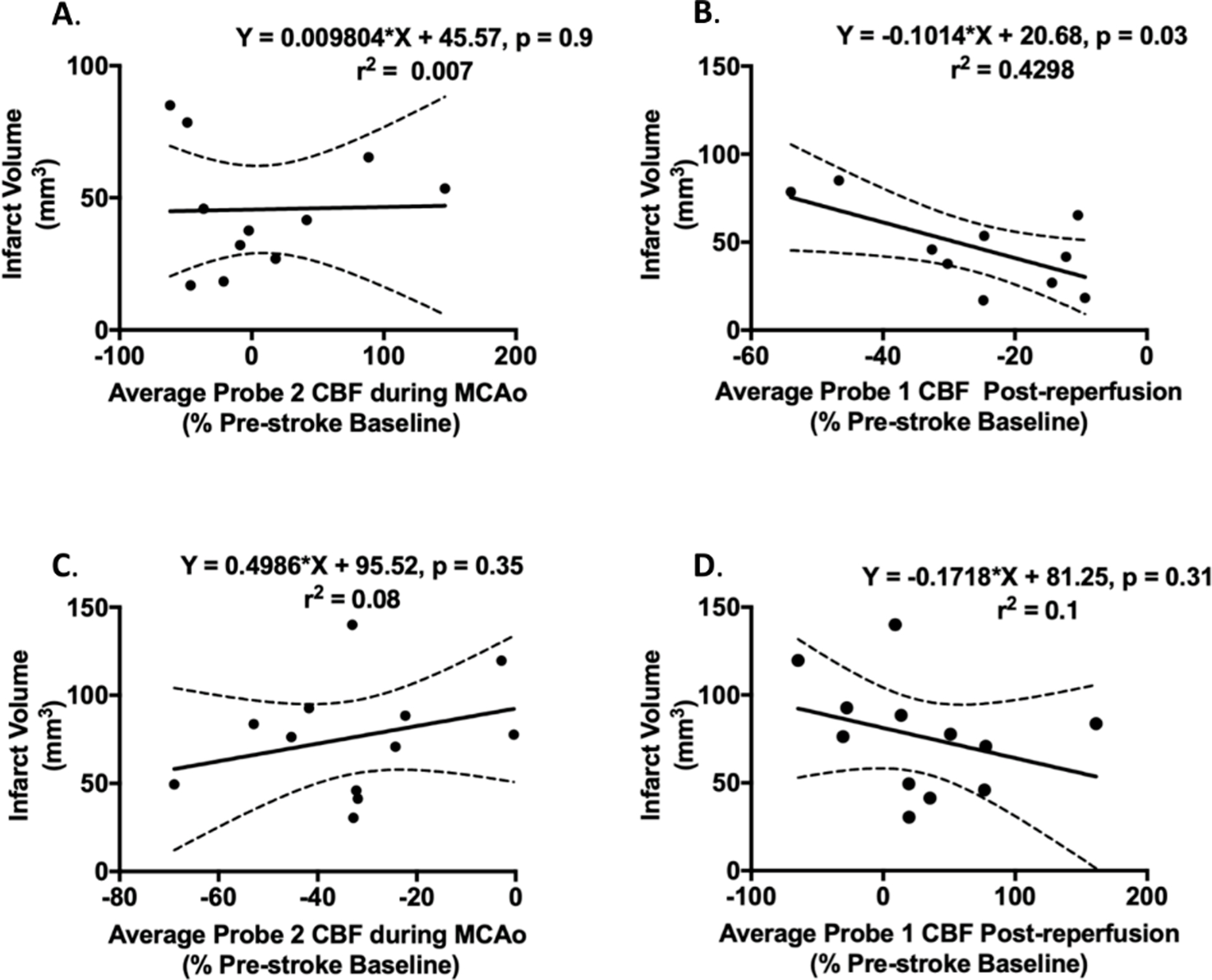
A-B. Wistar rats. A. Correlation between average LMA perfusion during middle cerebral artery occlusion (MCAo) and 24 hour infarct volume. There was a non-significant inverse correlation found between LMA blood flow and final infarct volume. B. Correlation between average reperfusion CBF and 24 hour infarct volume. There was a significant inverse correlation found between reperfusion CBF and infarct volume. C-D. SHRs. C. Correlation between average LMA perfusion during MCAo and 4 hour infarct volume. There was no significant correlation between LMA CBF and final infarct volume in SHRs. D. Correlation between Average reperfusion CBF and 4 hour infarct volume. There was no significant correlation between reperfusion CBF and infarct volume in SHRs.
In SHRs, average LMA perfusion during experimental stroke was not significantly correlated with final infarct volume (r2 = 0.08, p > 0.05, Figure 4C). Average reperfusion CBF was not-significantly inversely correlated with final infarct volume (r2 = 0.1, p > 0.05, Figure 4D).
Rapamycin dilation of isolated collateral vessels is dependent on NOS
Isolated LMA active lumen diameters were 35 ± 5 μm and % tone was 15.2 ± 3.4% at 40mm Hg. Rapamycin caused a dose-dependent vasodilation of LMAs (Figure 5 A and C). Percent reactivity to 10−6 mol/L rapamycin was 79.7 ± 9.4 % and 7.3 ± 3.6% in the presence of L-NAME, respectively (p < 0.01, Figure 5 B and C).
Figure 5. Isolated leptomeningeal anastomoses (LMAs) from Wistar rats.
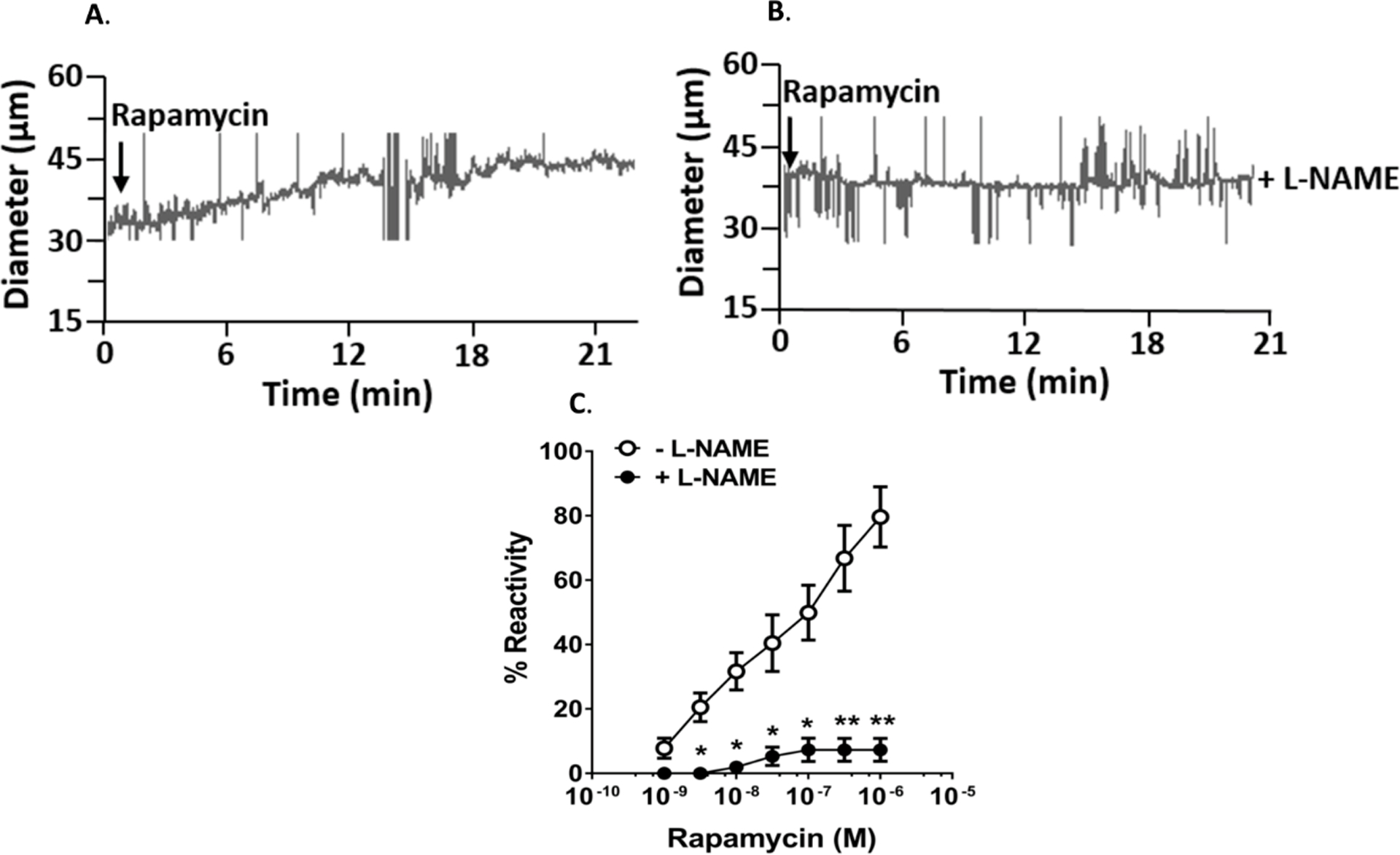
A and B. Representative tracing of changes in diameter of isolated and pressurized LMAs in response to rapamycin and rapamycin in the presence of L-NAME, respectively. C. Graph showing percent reactivity of isolated LMA to rapamycin in the absence and presence of L-NAME. Addition of rapamycin caused concentration-dependent vasodilation that was prevented by nitric oxide inhibition with L-NAME. * p < 0.05, **p < 0.001 vs. LMAs without L-NAME treatment.
Discussion
Improving collateral perfusion during vessel occlusion and microcirculatory perfusion following vessel re-opening are attractive therapeutic targets to improve penumbral survival and stroke outcome. In the current study we found that the clinically approved mTORC1 inhibitor, rapamycin, improved collateral perfusion in both normotensive Wistar rats and hypertensive SHRs, the latter being known to have poor baseline collateral perfusion. Rapamycin appeared to enhance collateral perfusion by an eNOS-dependent mechanism, as demonstrated in isolated and pressurized LMAs. We also found that rapamycin improved reperfusion CBF immediately after stroke in Wistar rats and SHRs and that this was a stronger predictor of final infarct volume stroke outcome. We have also shown that rapamycin reduced upper limb somatosensory impairments in rodents. This is important from a translational perspective, as homologous upper limb somatosensory impairments are also common in stroke patients.
Rapamycin significantly increased collateral perfusion in Wistar rats. Our results are in agreement with those of Wang et al.32, who showed that intraperitoneal administration of 10mg/kg of rapamycin immediately after MCAo in Sprague Dawley rats significantly increased the diameter of deep brain collaterals between the terminal branches of the posterior cerebral artery and MCA. They also reported that the same dose and timing of rapamycin administration in C57BL/6 mice resulted in a modest but significant improvement MCA territory perfusion between 10 and 60 min after MCAo, measured by Laser Speckle Contrast Imaging. Although these findings provide evidence of rapamycin enhancing blood flow to the core MCA territory via deep brain collaterals, we have shown that rapamycin also enhances perfusion to the peri-infarct territory via superficial leptomeningeal collaterals between the ACA and MCA. It is tempting to conclude that rapamycin enhanced penumbral perfusion and limited the size of the core infarction, however we did not measure penumbral volume or its progression over time. The improved infarct volume and neurological function seen at 24 hours may have been a combination of improved penumbral perfusion as well as rapamycin’s many other reported brain cytoprotective effects, including but not limited to, induction of autophagy, anti-inflammatory effects and reduced blood brain barrier disruption.7
It is estimated that over 50% of stroke patients have hypertension.33 Hypertension is associated with poor collateral blood flow and worse stroke outcomes in human14 and animal models of stroke.15 Therefore, hypertensive patients may have the most to gain from collateral therapeutics, if these vessels were amenable to therapies. Early animal studies revealed that SHRs have smaller diameter collateral vessels than normotensive Wistar Kyoto rats.21 This was thought to be due to remodeling of the vessels due to hypertension34 and that these vessels were unlikely to respond to acute therapeutic intervention. However, recent studies by the Cipolla group have demonstrated that LMAs from SHRs have increased vasoconstriction, such as significantly higher myogenic tone, compared to LMAs from normotensive Wistar Kyoto rats and that hypertensive collateral vessels dilate to various vasoactive mediators.16, 20, 31 This indicates greater vasoconstriction (not remodeling) of collateral vessels compared to normotensive animals, and that they are amenable to pharmacological vasodilation.
Our results further confirm the ability to enhance collateral perfusion in hypertensive animals, as rapamycin caused a variable but significant increase in collateral perfusion in SHR. The variability of SHR collateral perfusion and smaller effect size compared to Wistar collateral perfusion was surprising, given that SHR collaterals have greater tone under physiological intravascular pressure,16 thus we anticipated SHR collaterals having greater potential to dilate in response to rapamycin. It is possible that SHR collaterals were less responsive to nitric oxide (NO)-mediated vasodilation, which may explain this discrepancy.16 Rapamycin may have increased eNOS activation and NO production in the endothelium, as suggested by our Wistar results; however, the smooth muscle cells of the LMAs from young SHR may have been less responsive to NO, as has been shown previously.18 Interestingly, SHR LMAs appear to regain their sensitivity to NO during ageing16 and therefore rapamycin may be more effective in enhancing LMA in aged SHR. However, determination of the effect of rapamycin on LMA perfusion in aged SHR and the reactivity of young and aged isolated SHR LMA to rapamycin was beyond the scope of the current study and will be the focus of future work and publications.
We did, however, show that rapamycin dilated isolated collaterals from Wistar rats and the non-specific NOS inhibitor L-NAME blocked this dilation. The role of NOS inhibition in stroke is controversial, with NOS inhibitors showing conflicting effects on infarct volume and cerebral blood flow.35 This may be due to the lack of NOS isoform specificity of drugs such L-NAME. Targeted disruption of eNOS in vivo (eNOS knock-out mice) has shown the importance of eNOS in maintaining collateral perfusion during stroke.36 An advantage of our approach of utilizing L-NAME in isolated vessels, rather than in vivo, is that the isolated vessels are removed from the brain and are free from peri-vascular innervation (intrinsic innervation) and thus neuronal NOS.16 Therefore, the inhibitory effect of L-NAME on rapamycin induced dilation strongly suggest that rapamycin dilates collateral vessels via activation of eNOS rather than any other NOS isoforms.
This is in line with previous studies showing that rapamycin can increase eNOS phosphorylation and expression in mouse aorta37 and can relax aortic rings.38 Rapamycin has also been shown to increase eNOS expression in areas of low shear stress in carotid arteries of mice,39 as well as increase eNOS expression and phosphorylation in the brains of human amyloid precursor protein (AD) mice, leading to cortical vessel dilation, improved CBF and improved cognitive function in these animals.9 A possible mechanism for rapamycin-induced activation of eNOS is by rapamycin reducing mTORC1’s feedback inhibition of AKT, thus allowing AKT to phosphorylate eNOS and increase NO production.39
Structural and functional differences in pial collateral circulations have been studied in both male and female adult Wistar rats. It has been shown that LMAs from both sexes had similar myogenic tone and reactivity to pressure, similar vascular function, and similar numbers of LMAs.40 However, it is unknown if there are sex differences in collateral function during chronic hypertension. We recognize that the use of only one sex is a limitation of the current study; however, inclusion of both males and females with treatment was outside the scope of the current study. Future studies will determine the generalizability of our findings to female normotensive and hypertensive rats as well as aged animals.
In the current study, vehicle treated animals showed a decline in reperfusion CBF in the core-MCA territory in both Wistar and SHR, which has been reported in a number of previous studies.20, 24, 31, 41, 42 We show that rapamycin treatment prevented the decline in reperfusion CBF. We are aware of only one previous in vivo stroke study that has measured reperfusion CBF following rapamycin treatment. Although this study reported a non-significant increase in CBF at 2 hours post reperfusion in male Fischer 344 rats, this was associated with an increase in infarct volume measured at the same time.43 This discrepancy may be explained by the chronic (three days) high-dose administration (20 mg/kg) of rapamycin used in this study, which may have led to toxic off target effects in other neurovascular cells.6, 7
Decline in reperfusion CBF has been attributed to pericyte or smooth muscle constriction following ischemia.44, 45 Rapamycin may be reducing pericyte constriction through a Rho-A dependent pathway.46 Alternatively, rapamycin may be enhancing eNOS phosphorylation/activity and thus increasing smooth muscle cell relaxation on penetrating arterioles47 or directly reduce calcium sensitivity in smooth muscle cells to reduce vasoconstriction of penetrating arterioles.44 Direct evidence of either mechanism is yet to be obtained and warrants further investigation.
Unlike previous studies, we did not show a significant relationship between LMA perfusion and brain injury in either Wistar rats and SHRs.20, 23 However, we found that the degree of reperfusion CBF was significantly inversely correlated with final infarct volume in Wistar rats and was a stronger predictor of outcome compared to collateral perfusion in SHR. This is in line with previous studies that have shown that reperfusion CBF is a significant predictor of stroke outcome.41, 42 Our findings strongly suggest that rapamycin may be useful being administered to prevent impaired microvascular perfusion or the so-called “no-reflow” phenomenon in patients eligible for thrombolysis or thrombectomy.
Conclusions
Rapamycin significantly enhanced collateral perfusion and reperfusion CBF during experimental stroke. Isolated collateral experiments demonstrated that this increase in perfusion was potentially driven by rapamycin dilating collaterals. Rapamycin’s vasodilatory effect was largely inhibited by L-NAME, suggesting that rapamycin dilated LMAs through a mechanism involving eNOS. Rapamycin also improved MCA perfusion following stroke and this was a stronger predictor of stroke outcome. Therefore, our findings suggest that rapamycin may be an effective acute collateral enhancing therapy as well as an intervention to improve reperfusion CBF to improve stroke outcome.
Supplementary Material
Acknowledgements:
DJB, YC and AMB contributed to the study conception and design. Material preparation, data collection and analysis were performed by DJB, ZL, AMS and MJC. The first draft of the manuscript was written by DJB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Sources of funding
DJB, YC and AMB were funded by the Medical Research Council UK (MR/M022757/1). ZL was funded by a American Heart Association Pre-doctoral Fellowship (19PRE34430175). The research, authorship, and/or publication of this article was funded by a Medical Research Council UK Research Grant (MR/M022757/1 to AMB) and National Institute of Health National Institute of Neurological Disorders and Stroke grant R01 (NS093289 to MJC).
Non-standard Abbreviations and Acronyms
- AD
Alzheimer’s Disease
- CBF
Cerebral Blood Flow
- eNOS
Endothelial Nitric Oxide Synthase
- LMA
Leptomeningeal Anastomosis
- L-NAME
Nω-nitro-L-arginine methyl ester
- MCAo
Middle Cerebral Artery Occlusion
- mTORC1
Mammalian Target of Rapamycin Complex 1
- NO
Nitric Oxide
- SHR
Spontaneously Hypertensive Rat
- TSC 1
Tuberosclerosis 1 (hamartin)
- TSC 2
Tuberosclerosis 2 (tuberin)
- TTC
2,3,5-triphenyltetrazolium chloride
Footnotes
Disclosures
AMB is a senior medical science advisor and co-founder of Brainomix, a company that develops electronic ASPECTS (e-ASPECTS), an automated method to evaluate ASPECTS in stroke patients. All other authors declare no conflict of interest.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics-2019 update a report from the american heart association. Circulation. 2019;139:E56–E528 [DOI] [PubMed] [Google Scholar]
- 2.O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477 [DOI] [PubMed] [Google Scholar]
- 3.Papadakis M, Hadley G, Xilouri M, Hoyte LC, Nagel S, McMenamin MM, Tsaknakis G, Watt SM, Drakesmith CW, Chen R, et al. Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat Med. 2013;19:351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Manning BD. The tsc1-tsc2 complex: A molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates tsc1/2-mtor signaling and tumor suppression through redd1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beard DJ, Hadley G, Thurley N, Howells DW, Sutherland BA, Buchan AM. The effect of rapamycin treatment on cerebral ischemia: A systematic review and meta-analysis of animal model studies. Int J Stroke. 2019;14:137–145 [DOI] [PubMed] [Google Scholar]
- 7.Hadley G, Beard DJ, Couch Y, Neuhaus AA, Adriaanse BA, DeLuca GC, Sutherland BA, Buchan AM. Rapamycin in ischemic stroke: Old drug, new tricks? J Cereb Blood Flow Metab. 2019;39:20–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balami JS, Sutherland BA, Edmunds LD, Grunwald IQ, Neuhaus AA, Hadley G, Karbalai H, Metcalf KA, DeLuca GC, Buchan AM. A systematic review and meta-analysis of randomized controlled trials of endovascular thrombectomy compared with best medical treatment for acute ischemic stroke. Int J Stroke. 2015;10:1168–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, et al. Chronic rapamycin restores brain vascular integrity and function through no synthase activation and improves memory in symptomatic mice modeling alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33:1412–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vagal A, Aviv R, Sucharew H, Reddy M, Hou Q, Michel P, Jovin T, Tomsick T, Wintermark M, Khatri P. Collateral clock is more important than time clock for tissue fate. Stroke. 2018;49:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng XY, Fang H, Leung TWH, Mao C, Xu YM, Miao ZR, Liu LP, Wong KSL, Liebeskind DS. Impact of collateral status on successful revascularization in endovascular treatment: A systematic review and meta-analysis. Cerebrovasc Dis. 2016;41:27–34 [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg MD. The cerebral collateral circulation: Relevance to pathophysiology and treatment of stroke. Neuropharmacology. 2018;134:280–292 [DOI] [PubMed] [Google Scholar]
- 13.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): A case-control study. Lancet. 2010;376:112–123 [DOI] [PubMed] [Google Scholar]
- 14.Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, Hill MD, Demchuk AM, Damani Z, Cho KH, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013;74:241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyle P Different susceptibilities to cerebral infarction in spontaneously hypertensive (shr) and normotensive sprague-dawley rats. Stroke. 1986;17:520–525 [DOI] [PubMed] [Google Scholar]
- 16.Chan SL, Sweet JG, Bishop N, Cipolla MJ. Pial collateral reactivity during hypertension and aging understanding the function of collaterals for stroke therapy. Stroke. 2016;47:1618–U1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments-the arrive guidelines. J Cerebr Blood F Met. 2011;31:991–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, Hogan L, Howells DW. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006;155:285–290 [DOI] [PubMed] [Google Scholar]
- 19.McLeod DD, Beard DJ, Parsons MW, Levi CR, Calford MB, Spratt NJ. Inadvertent occlusion of the anterior choroidal artery explains infarct variability in the middle cerebral artery thread occlusion stroke model. PloS one. 2013;8:e75779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cipolla MJ, Linfante I, Abuchowski A, Jubin R, Chan SL. Pharmacologically increasing collateral perfusion during acute stroke using a carboxyhemoglobin gas transfer agent (sanguinate) in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2018;38:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyle P Dorsal cerebral collaterals of stroke-prone spontaneously hypertensive rats (shrsp) and wistar kyoto rats (wky). Anat Rec. 1987;218:40–44 [DOI] [PubMed] [Google Scholar]
- 22.Coyle P, Jokelainen PT. Dorsal cerebral arterial collaterals of the rat. Anat Rec. 1982;203:397–404 [DOI] [PubMed] [Google Scholar]
- 23.Cuccione E, Versace A, Cho TH, Carone D, Berner LP, Ong E, Rousseau D, Cai R, Monza L, Ferrarese C, et al. Multi-site laser doppler flowmetry for assessing collateral flow in experimental ischemic stroke: Validation of outcome prediction with acute mri. J Cereb Blood Flow Metab. 2017;37:2159–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cipolla MJ, Sweet JG, Chan SL. Effect of hypertension and peroxynitrite decomposition with fetmpyp on cbf and stroke outcome. J Cereb Blood Flow Metab. 2017;37:1276–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardutzky J, Shen Q, Henninger N, Bouley J, Duong TQ, Fisher M. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke. 2005;36:2000–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacewicz M, Tanabe J, Pulsinelli WA. The cbf threshold and dynamics for focal cerebral infarction in spontaneously hypertensive rats. J Cerebr Blood F Met. 1992;12:359–370 [DOI] [PubMed] [Google Scholar]
- 27.Bouet V et al. A master key to assess stroke consequences across species: The adhesive removal test Balestrino M, eds. In: Advances in the preclinical study of ischemic stroke. Croatia: InTech; 2012. [Google Scholar]
- 28.Rewell SS, Fernandez JA, Cox SF, Spratt NJ, Hogan L, Aleksoska E, van Raay L, Liberatore GT, Batchelor PE, Howells DW. Inducing stroke in aged, hypertensive, diabetic rats. J Cereb Blood Flow Metab. 2010;30:729–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLeod DD, Parsons MW, Levi CR, Beautement S, Buxton D, Roworth B, Spratt NJ. Establishing a rodent stroke perfusion computed tomography model. Int J Stroke. 2011;6:284–289 [DOI] [PubMed] [Google Scholar]
- 30.Nouraee C, Fisher M, Di Napoli M, Salazar P, Farr TD, Jafarli A, Divani AA. A brief review of edema-adjusted infarct volume measurement techniques for rodent focal cerebral ischemia models with practical recommendations. J Vasc Interv Neurol. 2019;10:38–45 [PMC free article] [PubMed] [Google Scholar]
- 31.Chan SL, Bishop N, Li Z, Cipolla MJ. Inhibition of pai (plasminogen activator inhibitor)-1 improves brain collateral perfusion and injury after acute ischemic stroke in aged hypertensive rats. Stroke. 2018;49:1969–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JX, Lin XJ, Mu ZH, Shen FX, Zhang LY, Xie Q, Tang YH, Wang YT, Zhang ZJ, Yang GY. Rapamycin increases collateral circulation in rodent brain after focal ischemia as detected by multiple modality dynamic imaging. Theranostics. 2019;9:4923–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appleton JP, Sprigg N, Bath PM. Blood pressure management in acute stroke. Stroke Vasc Neurol. 2016;1:72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willmot M, Gibson C, Gray L, Murphy S, Bath P. Nitric oxide synthase inhibitors in experimental ischemic stroke and their effects on infarct size and cerebral blood flow: A systematic review. Free Radical Bio Med. 2005;39:412–425 [DOI] [PubMed] [Google Scholar]
- 36.Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-l-arginine. J Cereb Blood Flow Metab. 1996;16:981–987 [DOI] [PubMed] [Google Scholar]
- 37.Naoum JJ, Zhang S, Woodside KJ, Song W, Guo Q, Belalcazar LM, Hunter GC. Aortic enos expression and phosphorylation in apo-e knockout mice: Differing effects of rapamycin and simvastatin. Surgery. 2004;136:323–328 [DOI] [PubMed] [Google Scholar]
- 38.Corbin F, Blaise GA, Parent M, Chen H, Daloze PM. Effect of rapamycin on rat aortic ring vasomotion. J Cardiovasc Pharmacol. 1994;24:813–817 [DOI] [PubMed] [Google Scholar]
- 39.Cheng C, Tempel D, Oostlander A, Helderman F, Gijsen F, Wentzel J, van Haperen R, Haitsma DB, Serruys PW, van der Steen AF, et al. Rapamycin modulates the enos vs. Shear stress relationship. Cardiovasc Res. 2008;78:123–129 [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Tremble SM, Cipolla MJ. Implications for understanding ischemic stroke as a sexually dimorphic disease: The role of pial collateral circulations. Am J Physiol Heart Circ Physiol. 2018;315:H1703–H1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burrows FE, Bray N, Denes A, Allan SM, Schiessl I. Delayed reperfusion deficits after experimental stroke account for increased pathophysiology. J Cereb Blood Flow Metab. 2015;35:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutherland BA, Buchan AM. Alteplase treatment does not increase brain injury after mechanical middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 2013;33:e1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chi OZ, Barsoum S, Vega-Cotto NM, Jacinto E, Liu X, Mellender SJ, Weiss HR. Effects of rapamycin on cerebral oxygen supply and consumption during reperfusion after cerebral ischemia. Neuroscience. 2016;316:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cipolla MJ, Chan SL, Sweet J, Tavares MJ, Gokina N, Brayden JE. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke. 2014;45:2425–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, Zhou H, Han X, Huang S. Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing rhoa expression and activity. J Biol Chem. 2010;285:38362–38373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palomares SM, Cipolla MJ. Vascular protection following cerebral ischemia and reperfusion. J Neurol Neurophysiol. 2011;2011:S1–004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


