Hypertension is a complex polygenic disease caused by a combination of genetic and environmental factors. Rat models serve as tools to dissect and prioritize genetic factors as candidate genes causing hypertension[1]. One such candidate gene prioritized through systematic linkage and substitution mapping is Secreted Phosphoprotein 2 (Spp2) [2]. A non-synonymous G/T single nucleotide polymorphism (SNP) between the Dahl Salt-Sensitive (S) rats and Spontaneously Hypertensive Rats (SHR) at the Spp2 locus was prioritized as a candidate quantitative trait nucleotide responsible for the reduction in blood pressure (BP) and bone mass observed in the S.SHR congenic strain spanning the Spp2 locus[2]. Hence, we hypothesized that CRISPR/Cas9 precision-engineering-guided replacement of the G allele at the Spp2 locus with a T allele would lower BP and bone mass of the S rat.
Methods
Animals
All animal procedures and protocols performed in this study were approved by the University of Toledo Institutional Animal Care and Use Committee. The procedure for the generation of a CRISPR/Cas9 targeted knock-in rat model has been previously described [3]. The non-founder S rat is a control derived from generating the knock-in. The nonfounder and the Spp2 knock-in rat strains were from stocks maintained at the University of Toledo animal facility. nonfounder and knock-in were not litter mates but were concomitantly bred for experimental procedures. Rats were weaned at 28 to 30 days of age and fed a low-salt diet (0.3% NaCl, TD 7034, Harlan Teklad). High-salt diet (2% NaCl, TD 94217, Harlan Teklad) was started at 6 weeks of age for all experiments.
BP Measurement
BP was recorded and analyzed using equipment from Data Sciences International, as previously described[4].
Microcomputed Tomography analysis
Trabecular bone architecture was evaluated in proximal tibia by assessing total volume (TV), bone volume (BV), BV to TV ratio, connectivity, Structure Model Index, trabecular number, trabecular thickness and trabecular spacing using micro CT. Image acquisition and analysis were conducted using μCT 35 system and Evaluation Program V6.5–3 (Scanco Medical AG, Bruettisellen, Switzerland) according to recommended guidelines[5].
Results and Discussion
Using CRISPR/Cas9 precision-engineering technology, a novel knock-in rat strain was generated, whereby the G nucleotide of the S rat was replaced with the T nucleotide of the SHR rat at the location, Rnor 6.0, Chr9:95506318 (Figure [A]). This non-synonymous SNP between the S and SHR rat is located in exon 4 of the Spp2 locus. The G/T polymorphism translates to an amino acid change from Alanine in the S rat to Serine in the Spp2 knock-in rat at amino acid 125 of Spp2 (Figure [A]). Radiotelemetry was performed with Spp2 knock-in rats using S rat as control. Systolic BP of the Spp2 knock-in male rats was significantly lower, 192 ± 4 mmHg, compared with that of the S rats, 211 ± 7 mmHg, P=0.026 (Figure [B] left). However, there was no change in systolic BP of the Spp2 knock-in female rats, 190 ± 5 mmHg, compared with S rats,183 ± 4 mmHg, P=0.268 (Figure [B] right). These data indicate that the T to G non-synonymous SNP in the Spp2 locus exhibits sexual dimorphism because it lowers systolic BP only in male rats.
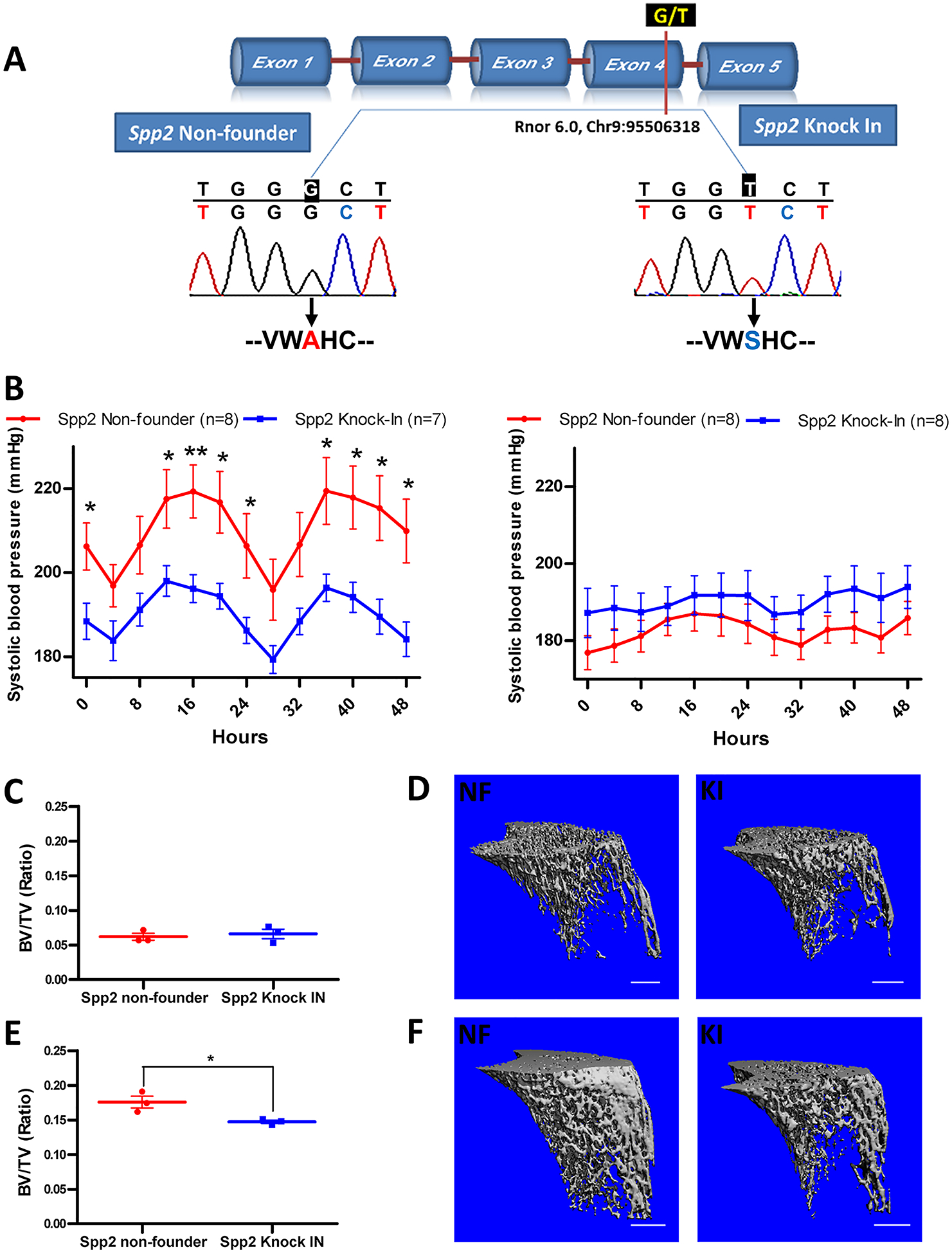
Single nucleotide polymorphism in Spp2 leads to lower systolic blood pressure in male and lower bone volume in female rats. A) DNA sequencing results from the PCR products for both Spp12 non-founder and knock-in at the location Chr9:95506318 (Rnor 6.0) within exon 4 of Spp2. B) Radiotelemetry systolic BP measurements of Spp2 non-founder (N=8) vs. Spp2 knock-in (N=7) males on the left and Spp2 non-founder (N=8) vs. Spp2 knock-in (N=8) females on the right. Spp2 non-founder- red and Spp2 knock-in- blue. *P<0.05, **P<0.01. Proximal tibia trabecular bone BV/TV from Spp2 non-founder vs. Spp2 knock-in (N=3) in males (C) and females (E) *P< 0.05. 3D renderings of trabecular bone in proximal tibia from Spp2 non-founder and knock-in (N=3) in males (D) and females (F). Bar on micro CT renderings represents 1 mm.
We performed micro CT analysis to observe differences in bone microarchitecture and bone mass that was previously shown in the male rat of the S.SHR congenic strain spanning the Spp2 locus [2]. Comparison of trabecular bone between nonfounder and knock-in males showed that Spp2 knock-in had zero effect on any of the structural parameters resulting in no effect in BV to TV ratio, P=0.657, (Figure [C]). There was also no observable structural difference between nonfounder and knock-in (Figure [D]). Analysis of trabecular bone in nonfounder and knock-in females showed that knock-in was associated with significant reduction of bone size and bone mass resulting in lower BV to TV ratio, P=0.033, (Figure [E]) stemming from lower trabecular thickness and increased trabecular spacing which is seen in (Figure [F]). Moreover, increase in structure model index in knock-in females indicated that knock-in results in a shift from plate- to rod-like trabeculae that is linked to lower bone quality.
Trabecular bone structure in nonfounder males and females differ significantly that sets “background” bone quality at significantly different start points with regard to the downstream effect of the knock-in. In females, TV is smaller by 40% but bone mass is 60% higher resulting in 3-fold higher BV to TV ratio, as compared with males. Higher bone mass in females results from higher trabecular thickness and reduced trabecular spacing. Moreover, female bone shows 5-fold higher connectivity and significantly lower structure model index indicating higher number of plates versus rods. This means that trabecular bone in females, although smaller in size, is superior with regard to the microarchitecture, and in consequence presenting higher bone quality. These differences suggest that mechanisms regulating bone growth and structure are more robust in nonfounder females, and possibly more sensitive to the effect of Spp2 knock-in in this particular rat model.
A discrepancy between the current null effect of the Knock-In on bone health in males and dramatic effect previously observed with male congenic rats suggests that the congenic segment around the Spp2 locus harbors other polymorphisms which operate in males to regulate bone physiology. A potential limitation of our study is a lack of temporal analysis, whereby, it is not clear whether the effects on bone precede the BP effect or vice versa. Taken together these data provide conclusive evidence for a SNP within the Spp2 gene as a quantitative trait nucleotide (QTN) responsible for the sex-dependent inheritance of BP and bone health.
Sources of Funding
Funding for this work to BJ from the NHLBI/NIH (HL1430820) is gratefully acknowledged.
Footnotes
Disclosures
None
References
- 1.Padmanabhan S, Joe B. Towards precision medicine for hypertension: a review of genomic, epigenomic, and microbiomic effects on blood pressure in experimental rat models and humans. Physiol Rev, 2017. 97(4): p. 1469–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nie Y, Kumarasamy S, Waghulde H, Cheng X, Mell B, Czernik PJ, Lecka-Czernik B, and Joe B, High-resolution mapping of a novel rat blood pressure locus on chromosome 9 to a region containing the Spp2 gene and colocalization of a QTL for bone mass. Physiol Genomics, 2016. 48(6): p. 409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng X, Waghulde H, Mell B, Morgan EE, Pruett-Miller SM, and Joe B, Positional cloning of quantitative trait nucleotides for blood pressure and cardiac QT-interval by targeted CRISPR/Cas9 editing of a novel long non-coding RNA. PLoS Genet, 2017. 13(8): p. e1006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saad Y, Garrett MR, Manickavasagam E, Yerga-Woolwine S, Farms P, Radecki T, and Joe B, Fine-mapping and comprehensive transcript analysis reveals nonsynonymous variants within a novel 1.17 Mb blood pressure QTL region on rat chromosome 10. Genomics, 2007. 89(3): p. 343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, and Muller R, Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res, 2010. 25(7): p. 1468–86. [DOI] [PubMed] [Google Scholar]


