Abstract
Although diabetes mellitus (DM) has been established as a risk factor for developing atrial fibrillation (AF) and is a known risk factor for stroke, it is unclear whether the presence or duration of DM is the primary adverse influence on the clinical course of AF. We retrospectively analyzed patients diagnosed with incident AF to examine the impact of DM on ischemic stroke and all-cause mortality. The diagnosis of DM was established by ICD-9 codes and review of medical records. To account for the significant differences in baseline characteristics of patients with and without diabetes, we matched 909 AF patients with DM with 909 AF patients without DM using propensity score matching based on 26 baseline variables. Cox regression analysis was used to identify independent factors associated with ischemic stroke and mortality. The mean age of the propensity matched cohort was 74 ± 11.5 years and 55.4% were male. Over a median follow-up period of 5.4 years (maximum 23.9 years), cumulative survival was significantly lower for patients with DM than those without DM; Log-rank P<.001. In the propensity-matched comparison, the risk of mortality was significantly higher in the DM group compared with the non-DM group [Hazard ratio (HR) 1.25; 95% confidence interval (CI) 1.12–1.69; P<.001]. Likewise, patients with DM had a higher risk of stroke (HR 1.32; 95% CI 1.02–1.69; P=0.03). Duration of DM was not associated with increased risk for stroke or mortality. In conclusion, the comorbidity of DM represents an independent predictor of reduced survival and further highlights the excess risk of thromboembolism in patients with AF.
Keywords: Atrial fibrillation, diabetes mellitus, survival, ischemic stroke
INTRODUCTION
Diabetes mellitus (DM) is a recognized risk factor for cardiovascular (CV) diseases and is strongly associated with atrial fibrillation (AF).1–4 The association between DM and AF is likely multifactorial, but oxidative stress and pro-inflammatory changes and consequent atrial remodeling have been suggested to initiate and maintain AF in diabetic patients.5,6 Contemporary evidence has shown that the co-existence of DM may complicate the clinical course and outcome of patients with AF,7,8 along with substantially increased risks of ischemic stroke.9–11 More recently, the duration of diabetes has also been advocated as a significant predictor of stroke risk in AF patients.9,12 However, to date, the prognostic impact of concomitant DM and AF on survival, whether complimentary or supplementary, remain mostly unknown. While patients with both DM and AF have higher mortality compared to those with DM alone,13 it remains unclear whether the presence of both conditions is uniquely associated with an elevated risk of mortality compared to those with AF alone without DM. Prior studies evaluating this question were limited by short follow-up duration, lack of information on important clinical variables such as the cause of death, duration of DM, glycemic control, and their influence on survival of patients with both DM and AF.7,10 Therefore, using a community-based cohort of patients with incident AF, we investigated the impact of comorbid DM on the risks of mortality and ischemic stroke.
METHODS
The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards and waived the requirement for informed consent. This observational, retrospective cohort study was conducted in Olmsted County, Minnesota, which has an estimated 2010 population of 144,248 residents, 85.7% of whom are Caucasian. Extensive details about the Olmsted County population have been reported elsewhere.14 Briefly, the population-based resources of the Rochester Epidemiology Project link and provide access to the medical records of all Olmsted County residents from all Olmsted County medical care providers, ensuring complete ascertainment of most medical conditions. We identified all Olmsted County residents who were diagnosed with or documented to have AF for the first time from 1980 to 2010. All AF events were verified as the first documented AF episode and confirmed by a 12-lead electrocardiogram, event monitor, Holter monitor, or cardiac implantable electronic devices. Patients with atrial flutter alone, without any evidence of AF, were not included in the study population. Patients with post-operative or post-procedure AF that did not recur within 30 days after the index operation or procedure were also not included. DM was initially identified by ICD-9-CM codes from the underlying AF population and ascertained by review of the medical records. In general, patients were considered to have DM if they had a documented history of DM prior to AF diagnosis or were being treated with insulin or oral hypoglycemic medications or had qualifying laboratory glucose values consistent with DM15. The date these individuals first met the criteria was defined as the date they reported having been first assigned a diagnosis of DM.
The primary outcome of interest was all-cause mortality. The secondary outcome was ischemic stroke, which was defined as an episode of focal neurological dysfunction diagnosed as an ischemic stroke or transient ischemic attack by neuroimaging assessment or clinical diagnosis. Patients were followed from their AF diagnosis until December 31, 2017 for deaths. Mortality and cause of death were ascertained by post-mortem review of the medical records, death certificates, autopsy reports, obituary notices, and query of the Social Security Death Index using the patient’s social security number. The cause of death was classified as cardiovascular or non-cardiovascular. Cardiovascular was defined as death due to ischemic heart disease, cardiomyopathy, cardiac arrest, stroke, or arrhythmias.
Categorical variables were expressed as numbers and percentages and compared with Pearson chi square or Fisher exact tests as appropriate. Continuous variables were reported as mean ± standard deviation (SD) or median (interquartile range [IQR]) for non-normally distributed data and were compared with a 2-sided unpaired Student t test or Wilcoxon rank sum test, as appropriate. The risk of death and ischemic stroke associated with diabetes was estimated by means of time-dependent Cox proportional-hazards models. The covariates included in the multivariable models were selected based on statistical evidence of a univariate association or known association with DM and these outcomes. Variables with complete or ≥80% complete data were entered stepwise into a multivariate Cox proportional hazards model to assess independent predictive value. Deaths were compared between AF patients with and without preexisting DM, as defined in the original cohort. The associated risk for each outcome was expressed as Hazard ratio (HR) with corresponding 95% confidence interval (CI). The event-free survival curve was plotted via the Kaplan-Meier method, with the statistical significance determined by the log-rank test. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). All p values were based on 2-sided tests and were considered statistically significant at p < 0.05.
Given that group assignment was nonrandom, confounding and selection biases were accounted for through a propensity score matching technique based on 26 baseline characteristics (Figure 1). Propensity score matching analysis was used to predict the probability of having a diagnosis of DM. We used propensity scores to balance baseline characteristics between DM and non-DM groups, reducing potential selection bias. Logistic regression models were iteratively assessed to determine the balance of baseline covariate proportions between study groups in the matched samples. The best and final model was the one that balanced covariates between study groups, as determined by standardized differences <0.10. After calculation of propensity scores, a greedy matching algorithm was used to identify one DM patient for each non-DM patient in the respective matched samples.
Figure 1. Balance Plot showing standardized differences of baseline variables before and after propensity matching.
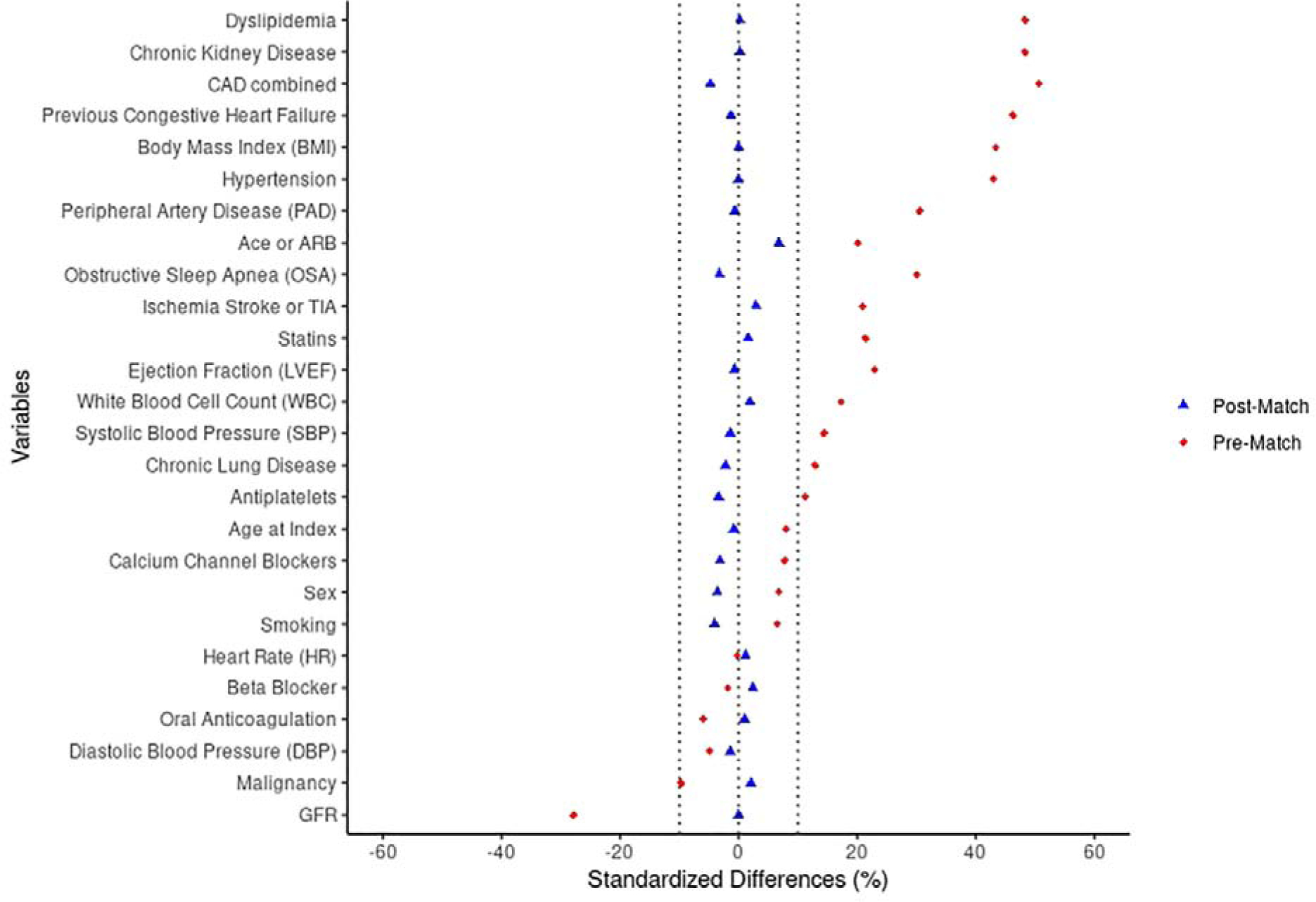
Abbreviations: CAD: Coronary artery disease; GFR: Glomerular filtration rate
RESULTS
The initial study cohort included 6077 patients with AF among which 1832 (30.1%) had preexisting DM. From this initial cohort, we identified a propensity score matched cohort of 1818 patients (909 with DM and 909 without DM). The two groups were matched based on 26 baseline variables following which, the two groups were balanced in terms of age, sex and comorbidities as shown in Figure 1. The mean age of the propensity matched cohort was 74 (± 11.5) years; 55.4 % were male and the mean CHA2DS2-VASc score was 4.7 (±2.1). Baseline characteristics of the matched cohort are shown in Table 1.
Table 1. Baseline characteristics of propensity-score matched cohort of atrial fibrillation stratified by diabetes.
| Characteristic | Diabetes Mellitus | Overall (n=1818) | P-value | |
|---|---|---|---|---|
| NO (n=909) | YES (n=909) | |||
| Age, mean (SD) (in years) | 74.1 (12.2) | 74.0 (10.7) | 74.0(11.5) | .29 |
| Men | 512(56.3%) | 495 (54.5%) | 1007 (55.4%) | .42 |
| Body mass index (kg/m2) | 30.2 (7.7) | 30.2 (6.7) | 30.2 (7.2) | .22 |
| Heart rate (beats per min) | 109.3 (32.4) | 108.9(31.7) | 109.1 (32.0) | .86 |
| Systolic Blood pressure (mmHg) | 131.6(22.1) | 131.3(21.7) | 131.4(21.9) | .70 |
| Diastolic Blood pressure (mmHg) | 71.4(14.5) | 71.2(14.1) | 71.3(14.3) | .88 |
| Hypertension | 818(90.0%) | 818(90.0%) | 1636 (90.0%) | 1.00 |
| Dyslipidemia | 689 (75.8%) | 690(75.9%) | 1379 (75.9%) | .96 |
| Coronary Artery Disease | 591 (65.0%) | 570 (62.7%) | 1161 (63.9%) | .30 |
| Heart failure | 294 (32.3%) | 288(31.7%) | 582 (32.0%) | .76 |
| Prior Stroke | 179 (19.7%) | 201 (22.1%) | 380 (20.9%) | .20 |
| Peripheral Arterial Disease | 234(25.7%) | 231 (25.4%) | 465 (25.6%) | .87 |
| Smoker (past or present) | 290(31.9%) | 273 (30.0%) | 563(31.0%) | .39 |
| Chronic lung disease | 358 (39.4%) | 348 (38.3%) | 706 (38.8%) | .63 |
| Obstructive sleep apnea | 284(31.2%) | 270 (29.7%) | 554 (30.5%) | .47 |
| Chronic Renal Disease | 360 (39.6%) | 361 (39.7%) | 721 (39.7%) | .96 |
| Malignancy | 331 (36.4%) | 340 (37.4%) | 671 (36.9%) | .66 |
| White blood cell count (1000 per mm3) | 9.4 (5.4) | 9.5 (5.3) | 9.4 (5.3) | .57 |
| Glomerular filtration rate (ml/min/1.73m2) | 55.9 (26.3) | 55.9 (27.5) | 55.9 (26.9) | .97 |
| LV ejection fraction (%) | 53.8(15.1) | 53.7 (15.4) | 53.8(15.3) | .89 |
| Statins | 515(56.7%) | 523(57.5%) | 1038(57.1%) | .70 |
| Oral Anticoagulation (Warfarin or NOAC) | 482(53.0%) | 486(53.5%) | 968 (53.2%) | .85 |
| Beta-Blocker | 625(68.8%) | 635(69.9%) | 1260 (69.3%) | .61 |
| Calcium Channel-blocker | 452(49.7%) | 437(48.1%) | 889 (48.9%) | .48 |
| ACE-I or ARB | 581(63.9%) | 610(67.1%) | 1191 (65.5%) | .15 |
| Antiplatelets | 251(27.6%) | 237(26.1%) | 488 (26.8%) | .45 |
Represented as Number (percentage) or mean (standard deviation)
Abbreviations: LV: left ventricle; ACE-I: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker
Footnote: Dyslipidemia was defined as total cholesterol >200 mg/dl or low-density cholesterol levels >100 mg/dl or high-density cholesterol levels <60 mg/dl
In the matched cohort, during a median follow up period of 5.4 years (IQR 1.4–9.6), a total of 1373 (75.5%) patients died from all causes. Figure 2 illustrates the cumulative survival for the propensity score-matched groups over a maximum follow-up of 24 years. Overall, cumulative survival was significantly lower for the DM than Non-DM cohort, Log-rank P<.001. The 5-year patient survival rate in the DM group was 49.3% and 55.2% in the non-DM group (Log-rank P<.001). The risk of mortality was significantly higher in the DM group than the non-DM group, (HR 1.25; 95% CI, 1.12–1.39; P<.001). Other variables associated with increased risk of mortality were hypertension, coronary artery disease, previous history of heart failure, prior episodes of stroke or TIA, smoking, chronic lung disease, chronic kidney disease, and higher CHA2DS2-VASc score. Higher HbA1C levels were also associated with increased risk of mortality (HR 1.07; 95% CI 1.04–1.10; P<.001) while duration of diabetes did not have any association in the DM group (P=0.32). In addition, utilization of rate control agents such as beta-blockers and calcium channel blockers, and antiarrhythmics was protective against all-cause mortality in the propensity matched cohort (Table 2). We also evaluated causes of death in these patients. There were no differences in the proportion of cardiovascular (30.5% vs 29.5%) and non-cardiovascular (46.5% vs 44.0%) deaths between the DM and the non-DM group. Congestive heart failure (14%) was the most common cause of death followed by malignancy (12%), myocardial infarction (12%), and sudden cardiac death (9%).
Figure 2.
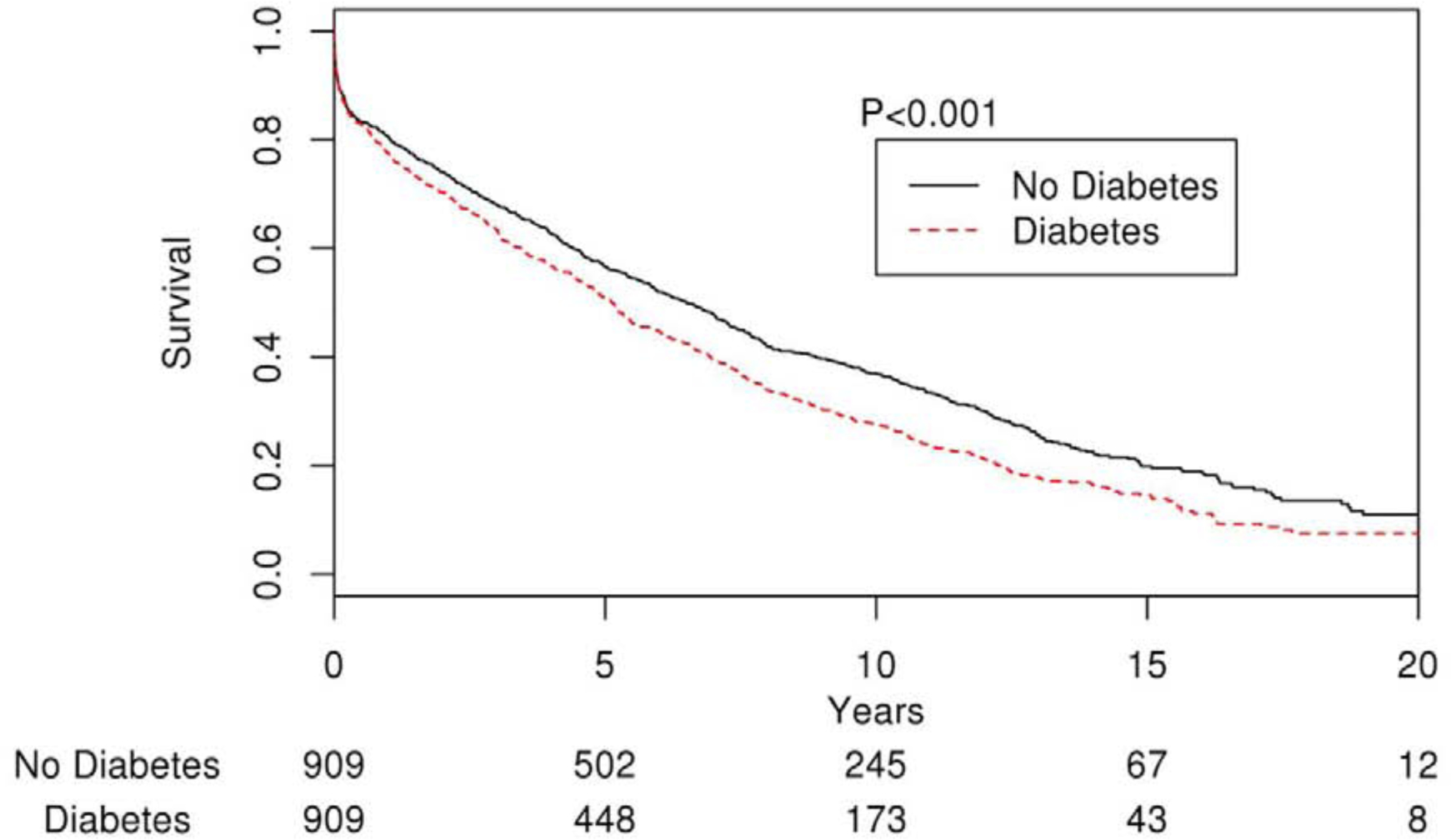
Kaplan-Meier curve showing survival differences between AF patients with and without diabetes
Table 2. Propensity-score matched Univariate Associations with Mortality.
| Variable | Hazard Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Diabetes mellitus | 1.25 | 1.12 – 1.39 | <.001 |
| Men | 0.81 | 0.73 – 0.90 | <.001 |
| Age | 1.07 | 1.06 – 1.07 | <.001 |
| Body mass index | 0.96 | 0.95 – 0.97 | <.001 |
| Systolic blood pressure | 1.00 | 0.99 – 1.00 | 0.12 |
| Diastolic blood pressure | 0.99 | 0.99 – 0.99 | <.001 |
| Mean arterial pressure | 0.99 | 0.99 – 0.99 | 0.02 |
| Hypertension | 1.43 | 1.18 – 1.73 | <.001 |
| Dyslipidemia | 0.79 | 0.70 – 0.90 | <.001 |
| Coronary Artery Disease | 1.55 | 1.39 – 1.74 | <.001 |
| Congestive heart failure | 2.20 | 1.97 – 2.46 | <.001 |
| Cardiomyopathy (Ischemic, idiopathic, hypertrophic, dilated, tachycardia-induced) | 1.47 | 1.27 – 1.69 | <.001 |
| Previous valve surgery | 1.29 | 0.94 – 1.79 | 0.12 |
| Prior Stroke or TIA | 1.89 | 1.67 – 2.14 | <.001 |
| Prior GI bleeding | 1.43 | 1.25 – 1.64 | <.001 |
| Peripheral Arterial Disease | 1.54 | 1.37 – 1.73 | <.001 |
| Smoking (past or present) | 1.34 | 1.20 – 1.49 | <.001 |
| Chronic lung disease | 1.64 | 1.47 – 1.83 | <.001 |
| Obstructive sleep apnea | 0.90 | 0.80 – 1.01 | 0.09 |
| Chronic Renal Disease | 1.95 | 1.75 – 2.17 | <.001 |
| Dialysis | 2.69 | 2.09 – 3.47 | <.001 |
| Thyroid disease | 1.20 | 1.04 – 1.38 | 0.01 |
| Malignancy | 1.63 | 1.46 – 1.82 | <.001 |
| Insulin | 0.86 | 0.74 – 1.00 | 0.06 |
| Oral hypoglycemic agents | 0.61 | 0.53 – 0.70 | <.001 |
| Statins | 0.45 | 0.40 – 0.50 | <.001 |
| Oral Anticoagulation (Warfarin or NOAC) | 0.49 | 0.44 – 0.54 | <.001 |
| beta-blocker | 0.47 | 0.42 – 0.52 | <.001 |
| Calcium Channel-blocker | 0.69 | 0.62 – 0.77 | <.001 |
| Digoxin | 1.09 | 0.97 – 1.23 | 0.15 |
| ACE-I or ARB | 0.62 | 0.55 – 0.69 | <.001 |
| Diuretics | 0.85 | 0.76 – 0.96 | 0.01 |
| Antiplatelets | 1.03 | 0.92 – 1.16 | 0.58 |
| Antiarrhythmics | 0.49 | 0.41 – 0.58 | <.001 |
| CHA2DS2-VASc score | 1.34 | 1.30 – 1.37 | <.001 |
| White blood cell count | 1.03 | 1.03 – 1.04 | <.001 |
| Creatinine | 1.26 | 1.21 – 1.31 | <.001 |
| Glomerular filtration rate | 0.99 | 0.99 – 0.99 | <.001 |
| Thyroid stimulating hormone | 1.02 | 1.00 – 1.04 | 0.01 |
| Cardiac index | 1.10 | 1.01 – 1.19 | 0.02 |
| LA volume index | 1.02 | 1.01 – 1.02 | <.001 |
| E/e’ ratio | 1.04 | 1.03 – 1.05 | <.001 |
| RV systolic pressure | 1.03 | 1.03 – 1.04 | <.001 |
| Right atrial pressure | 1.09 | 1.07 – 1.10 | <.001 |
| LV ejection fraction | 0.98 | 0.98 – 0.99 | <.001 |
| HbA1c | 1.07 | 1.04 – 1.11 | <.001 |
Abbreviations:LA: left atrial; LV: left ventricle; RV: right ventricle
Among the 1818 patients in the propensity score matched cohort, 243 (13.4%) developed stroke during follow-up. The overall annualized stroke rate was 2.3% and increased progressively according to CHA2DS2-VASc score. The annual risks of ischemic stroke for CHA2DS2-VASc score 0 or 1 was <1%, 1.9% for score 2 and 7.9% for score 9. Compared with the non-DM group, the DM cohort had a significantly higher risk of stroke (HR 1.32; 95% CI, 1.02–1.69; P=0.03) (Figure 3). Older age, female sex, hypertension, prior history of stroke and higher CHA2DS2-VASc score were all associated with increased risk of stroke in these patients (Table 3). Duration of diabetes was not associated with the risk of stroke (P= 0.52).
Figure 3.
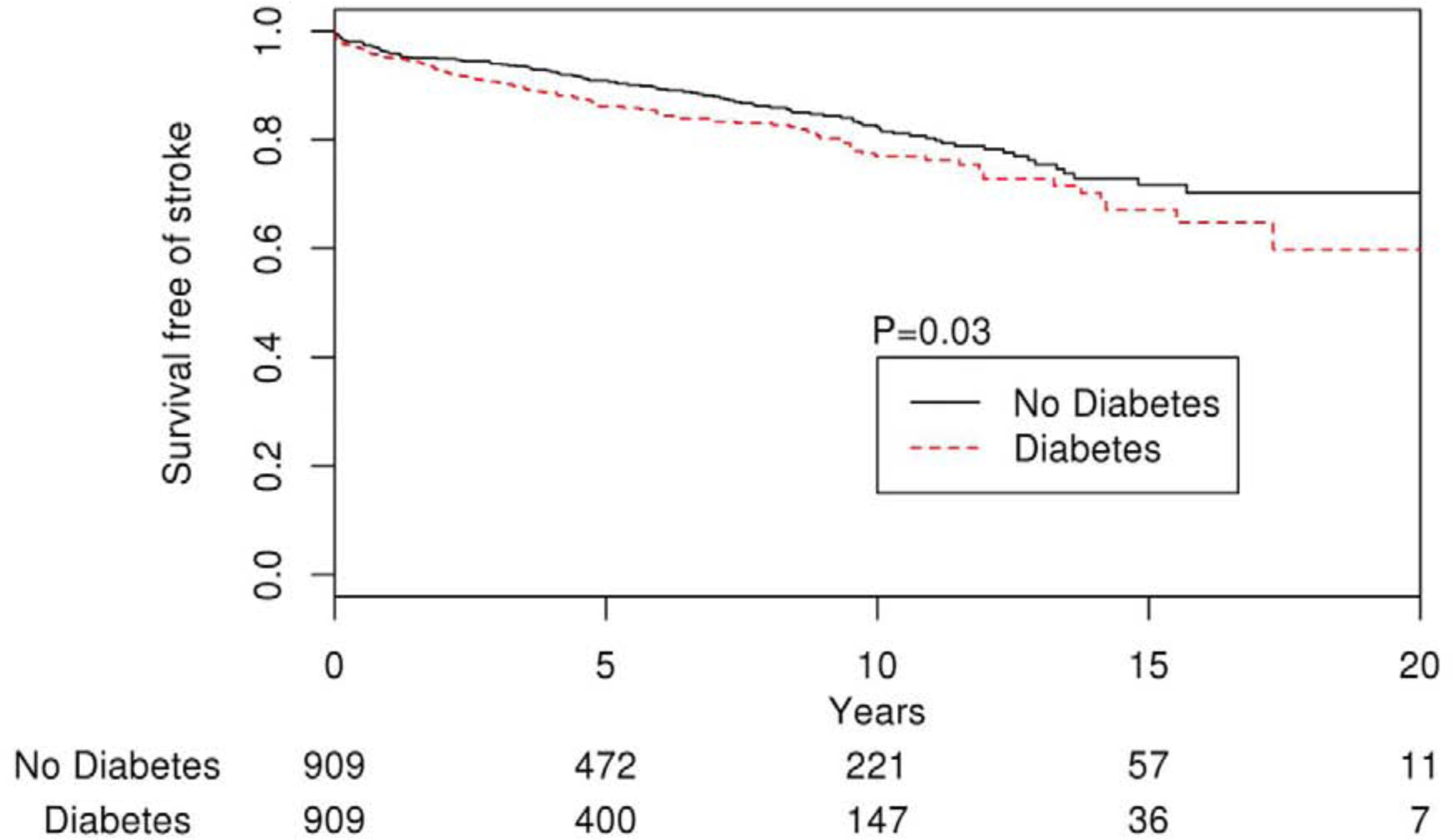
Kaplan-Meier curve showing stroke-free survival in AF patients with and without diabetes
Table 3. Propensity-score matched Univariate Associations with Stroke.
| Variable | Hazard Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Diabetes mellitus | 1.32 | 1.02 – 1.70 | 0.03 |
| Men | 0.57 | 0.44 – 0.74 | <.001 |
| Age | 1.04 | 1.03 – 1.06 | <.001 |
| Body mass index | 0.97 | 0.95 – 0.99 | 0.001 |
| Systolic Blood pressure | 1.01 | 1.00 – 1.02 | <.001 |
| Diastolic Blood pressure | 0.99 | 0.99 – 1.01 | 0.564 |
| Mean arterial pressure | 1.01 | 0.99 – 1.01 | 0.18 |
| Hypertension | 2.25 | 1.31 – 3.87 | 0.003 |
| Dyslipidemia | 0.93 | 0.69 – 1.25 | 0.62 |
| Coronary Artery Disease | 1.06 | 0.82 – 1.37 | 0.65 |
| Congestive heart failure | 1.22 | 0.91 – 1.63 | 0.18 |
| Cardiomyopathy (Ischemic, idiopathic, hypertrophic, dilated, tachycardia-induced) | 0.66 | 0.42 – 1.03 | 0.07 |
| Previous valve surgery | 1.95 | 0.94 – 4.08 | 0.07 |
| Prior Stroke or TIA | 2.00 | 1.49 – 2.68 | <.001 |
| Prior GI bleeding | 0.94 | 0.65 – 1.36 | 0.74 |
| Peripheral Arterial Disease | 1.28 | 0.96 – 1.71 | 0.09 |
| Smoker (past or present) | 0.81 | 0.61 – 1.09 | 0.16 |
| Chronic lung disease | 0.96 | 0.73 – 1.26 | 0.75 |
| Obstructive sleep apnea | 0.85 | 0.64 – 1.13 | 0.25 |
| Chronic Renal Disease | 1.13 | 0.86 – 1.48 | 0.38 |
| Dialysis | 0.67 | 0.21 – 2.11 | 0.50 |
| Thyroid disease | 1.25 | 0.90 – 1.75 | 0.18 |
| Malignancy | 1.02 | 0.78 – 1.34 | 0.87 |
| Insulin | 1.14 | 0.81 – 1.60 | 0.44 |
| Oral hypoglycemic agents | 1.02 | 0.77 – 1.36 | 0.86 |
| Statins | 0.88 | 0.67 – 1.15 | 0.34 |
| Oral Anticoagulation (Warfarin or NOAC) | 1.01 | 0.78 – 1.32 | 0.93 |
| beta-Blocker | 1.08 | 0.78 – 1.49 | 0.63 |
| Calcium Channel-blocker | 1.29 | 1.00 – 1.67 | 0.05 |
| Digoxin | 1.06 | 0.80 – 1.40 | 0.70 |
| ACE-I or ARB | 1.15 | 0.85 – 1.54 | 0.36 |
| Diuretics | 1.46 | 1.05 – 2.02 | 0.02 |
| Antiplatelets | 1.40 | 1.08 – 1.83 | 0.01 |
| Antiarrhythmics | 0.96 | 0.70 – 1.32 | 0.83 |
| CHA2DS2-VASc score | 1.20 | 1.13 – 1.28 | <.001 |
| White blood cell count | 0.98 | 0.95 – 1.02 | 0.35 |
| Creatinine | 0.92 | 0.75 – 1.12 | 0.39 |
| Glomerular filtration rate | 1.00 | 0.99 – 1.01 | 0.73 |
| Thyroid stimulating hormone | 0.97 | 0.92 – 1.02 | 0.27 |
| Cardiac index | 0.97 | 0.79 – 1.18 | 0.74 |
| LA volume index | 1.01 | 0.99 – 1.02 | 0.07 |
| E/e’ ratio | 1.05 | 1.03 – 1.06 | <.001 |
| RV systolic pressure | 1.02 | 1.01 – 1.03 | 0.002 |
| Right atrial pressure | 1.05 | 1.01 – 1.08 | 0.005 |
| LV ejection fraction | 1.00 | 0.99 – 1.01 | 0.98 |
| HbA1c | 1.04 | 0.96 – 1.13 | 0.34 |
Abbreviations: LA: left atrial; LV: left ventricle; RV: right ventricle
DISCUSSION
In this propensity matched analysis with 24 years of follow up, we examined the impact of DM on long-term survival of patients with incident AF. Overall, the prevalence of DM in our cohort was 30%. DM was significantly associated with reduced survival of patients with AF even after accounting for other comorbidities. Overall, preexisting DM conferred a 25% increased risk of all-cause mortality. We also found that patients with both DM and AF had significantly higher risk of stroke compared to patients with AF without DM. The presence of DM in patients with AF was associated with a 32% increased risk of stroke. Duration of DM did not have any association with all-cause mortality or the risk of stroke. The use of beta-blockers, calcium channel blockers and antiarrhythmics was associated with lower all-cause mortality, suggesting that the management of comorbidities could play a role in reducing excess mortality in patients with AF.
Our findings are consistent with previous studies, which also showed that DM among patients with AF is associated with an increased risk of all-cause mortality.7,10 In a post-hoc analysis of the ORBIT-AF registry, diabetic patients had higher risk of mortality compared with those without DM. In contrast to our population-based study, data on the causes of death in that study were not available.7 Analysis of the causes of death in our study showed no significant difference in the hazard of CV mortality between those with and without DM, suggesting that individual CV risk factors may affect AF patients similarly irrespective of associated DM. Moreover, the broad adherence to guideline-directed medical therapy to control CV risk factors in the modern era could also attenuate the impact of DM on CVD risk. Indeed, a recent study by Raghavan et al. showed that DM-related mortality was attenuated by cardiovascular risk factor control.16
The impact of glycemic control and DM duration on survival in patients with AF has not been fully investigated. In our study, we found that higher HbA1c values were associated with an increased risk of mortality. This finding is consistent with earlier studies showing that poor glycemic control is associated with persistence and recurrence of AF, potentially affecting survival.16,17 The effect of DM on cardiac electrophysiology affecting structural remodeling along with the metabolic changes have been implicated in increasing the CV risk in these patients.18,19 It has also been suggested that the masking effect of DM on symptom presentation in patients with AF may delay timely identification and treatment of AF, thereby resulting in poor outcome.20 Conversely, improved glycemic control appears to favorably influence outcomes in patient with DM.21 We found that the use of oral hypoglycemic agents reduced the risk of mortality possibly due to better AF outcomes with the use of these drugs.21–23
Although several contemporary studies have shown no difference in the risk of thromboembolic events in AF patients with and without DM,24,7 several others have consistently reported DM as a risk factor for ischemic stroke in patients with AF.9,12,25 Although the annualized stroke rates generally increases with higher CHA2DS2-VASc score, the rates observed in our cohort were slightly lower than those observed by Lip GY et al. using the Birmingham 2009 schema.11 The difference is likely due to the use of anticoagulation in our group (53%). More recently, two large studies evaluating the influence of DM and glycemic control in AF patients reported an increased risk of thromboembolic events in AF patients with longer duration of DM.9,12 Additionally, older studies, along with a more recent study from Danish registries, reported a higher thromboembolic risk with increasing levels of HbA1c.26–29 In contrast, despite the confirmed association between DM and stroke in our cohort, neither the duration of DM nor HbA1c levels had any impact on risk of stroke in the matched cohort. The ATRIA study likewise found no association of glycemic control measured by HbA1c with the risk of stroke in patients with AF.12 Taken together, our results suggest that improvement in DM care in the United States,16 are reflected in improvements in DM-related mortality, supported by the attenuation of the association between DM and mortality after accounting for risk factor levels and treatment. The present study showed that DM has an essential impact on survival and stroke risk. Therefore, improved treatment of DM and CV risk control may play a key role in reducing DM-associated morbidity and mortality in patients with AF.
The major strength of our study is its large population-based cohort, which allowed full ascertainment of mortality, thereby minimizing misclassification. The use of propensity matching to balance the probability of having a diagnosis of DM on a large number of covariates also reduced potential selection bias in our study. The diagnosis of AF and DM were ascertained through multiple sources including review of medical records, verifying the presence of respective management strategies along with ICD codes to increase the accuracy. The availability of duration of diabetes prior to AF helped us study the effect of duration of diabetes on outcomes. Our study also has some limitations study including its retrospective design, although propensity matching was used to minimize confounding. Ascertainment of DM was based on clinical data. Hence, the presence of diabetes could have been underestimated despite the additional use of laboratory criteria.
In conclusion, the presence of DM and poor glycemic control were both associated with increased risk of mortality in patients with concurrent AF. Improved treatment of DM and CV risk control appear to confer a significant protective effect on survival. Future prospective studies are needed to confirm the reproducibility of our findings.
SOURCES OF FUNDING
Dr. Rowlens Melduni is supported by National Institutes of Health (NIH) K01 (HL 135288).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
All authors have not reported any relationships relevant to the contents of this paper to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: The framingham heart study. JAMA 1994;271:840–844. [PubMed] [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular disease: The Framingham study. JAMA 1979;241:2035–2038. [DOI] [PubMed] [Google Scholar]
- 3.The Emerging Risk Factors C. Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA 2014;311:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Movahed M-R, Hashemzadeh M, Mazen Jamal M. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. International Journal of Cardiology 2005;105:315–318. [DOI] [PubMed] [Google Scholar]
- 5.Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol 2017;16:120–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plitt A, McGuire DK, Giugliano RP. Atrial Fibrillation, Type 2 Diabetes, and Non-Vitamin K Antagonist Oral Anticoagulants: A Review. JAMA Cardiology 2017;2:442–448. [DOI] [PubMed] [Google Scholar]
- 7.Echouffo-Tcheugui JB, Shrader P, Thomas L, Gersh BJ, Kowey PR, Mahaffey KW, Singer DE, Hylek EM, Go AS, Peterson ED, Piccini JP, Fonarow GC. Care Patterns and Outcomes in Atrial Fibrillation Patients With and Without Diabetes: ORBIT-AF Registry. Journal of the American College of Cardiology 2017;70:1325–1335. [DOI] [PubMed] [Google Scholar]
- 8.Melduni RM, Lee H-C, Bailey KR, Miller FA, Hodge DO, Seward JB, Gersh BJ, Ammash NM. Real-time physiologic biomarker for prediction of atrial fibrillation recurrence, stroke, and mortality after electrical cardioversion: A prospective observational study. American Heart Journal 2015;170:914–922. [DOI] [PubMed] [Google Scholar]
- 9.Overvad TF, Skjøth F, Lip GYH, Lane DA, Albertsen IE, Rasmussen LH, Larsen TB. Duration of Diabetes Mellitus and Risk of Thromboembolism and Bleeding in Atrial Fibrillation. Stroke 2015;46:2168–2174. [DOI] [PubMed] [Google Scholar]
- 10.Igor K, Cornelius W, Barbara S, Elisabeth H, Josef F, Claudia S. Diabetic atrial fibrillation patients: mortality and risk for stroke or embolism during a 10-year follow-up. Diabetes/Metabolism Research and Reviews 2003;19:320–328. [DOI] [PubMed] [Google Scholar]
- 11.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 12.Ashburner JM, Go AS, Chang Y, Fang MC, Fredman L, Applebaum KM, Singer DE. Effect of Diabetes and Glycemic Control on Ischemic Stroke Risk in AF Patients: ATRIA Study. Journal of the American College of Cardiology 2016;67:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, Bigger T, Cushman W, Goff D, Soliman EZ, Thomas A, Papademetriou V. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol 2014;114:1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 Suppl 1:S62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghavan S, Vassy JL, Ho YL, Song RJ, Gagnon DR, Cho K, Wilson PWF, Phillips LS. Diabetes Mellitus-Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. J Am Heart Assoc 2019;8:e011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandhu Roopinder K, Conen D, Tedrow Usha B, Fitzgerald Kathryn C, Pradhan Aruna D, Ridker Paul M, Glynn Robert J, Albert Christine M. Predisposing Factors Associated With Development of Persistent Compared With Paroxysmal Atrial Fibrillation. Journal of the American Heart Association;3:e000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y, Li H, Lan X, Chen X, Zhang A, Li Z. Mechanism of and therapeutic strategy for atrial fibrillation associated with diabetes mellitus. TheScientificWorldJournal 2013;2013:209428–209428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation 2008;117:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugishita K, Shiono E, Sugiyama T, Ashida T. Diabetes influences the cardiac symptoms related to atrial fibrillation. Circulation journal : official journal of the Japanese Circulation Society 2003;67:835–838. [DOI] [PubMed] [Google Scholar]
- 21.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation: The ARREST-AF Cohort Study. Journal of the American College of Cardiology 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Wang J, Wang G. Beneficial Effects of Pioglitazone on Retardation of Persistent Atrial Fibrillation Progression in Diabetes Mellitus Patients. International Heart Journal 2014;55:499–505. [DOI] [PubMed] [Google Scholar]
- 23.Pallisgaard JL, Lindhardt TB, Staerk L, Olesen JB, Torp-Pedersen C, Hansen ML, Gislason GH. Thiazolidinediones are associated with a decreased risk of atrial fibrillation compared with other antidiabetic treatment: a nationwide cohort study. European Heart Journal - Cardiovascular Pharmacotherapy 2016;3:140–146. [DOI] [PubMed] [Google Scholar]
- 24.Bansilal S, Bloomgarden Z, Halperin JL, Hellkamp AS, Lokhnygina Y, Patel MR, Becker RC, Breithardt G, Hacke W, Hankey GJ, Nessel CC, Singer DE, Berkowitz SD, Piccini JP, Mahaffey KW, Fox KAA. Efficacy and safety of rivaroxaban in patients with diabetes and nonvalvular atrial fibrillation: The Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF Trial). American Heart Journal 2015;170:675–682.e678. [DOI] [PubMed] [Google Scholar]
- 25.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: Analysis of pooled data from five randomized controlled trials. Archives of Internal Medicine 1994;154:1449–1457. [PubMed] [Google Scholar]
- 26.Kuusisto J, Mykkänen L, Pyörälä K, Laakso M. Non-insulin-dependent diabetes and its metabolic control are important predictors of stroke in elderly subjects. Stroke 1994;25:1157–1164. [DOI] [PubMed] [Google Scholar]
- 27.Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. The Lancet Neurology 2005;4:821–826. [DOI] [PubMed] [Google Scholar]
- 28.Saliba W, Barnett-Griness O, Elias M, Rennert G. Glycated hemoglobin and risk of first episode stroke in diabetic patients with atrial fibrillation: A cohort study. Heart Rhythm 2015;12:886–892. [DOI] [PubMed] [Google Scholar]
- 29.Fangel MV, Nielsen PB, Kristensen JK, Larsen TB, Overvad TF, Lip GYH, Jensen MB. Glycemic Status and Thromboembolic Risk in Patients With Atrial Fibrillation and Type 2 Diabetes Mellitus. Circulation: Arrhythmia and Electrophysiology 2019;12:e007030. [DOI] [PubMed] [Google Scholar]


