Abstract
The effect of various combinations of cervical arterial ligations (Combinations) on retinal blood flow (RBF) levels is not known in rats. We hypothesized: 1) No artery exists between the Circle of Willis and the eye, 2) Selective Combinations enable varying RBF levels between normal and zero, 3) In certain Combinations, the capillary bed of the head participates in supplying the eye. Twenty-six Combinations were studied in one eye of 20 Long-Evans rats under general anesthesia. RBF was quantitatively evaluated with our published imaging methods based on direct measurements of venous diameter and blood velocity from the displacement of fluorescent microspheres over time. For each Combination, one or more RBF values (runs) were measured. Data were obtained from 59 runs (2.9 ± 2.7 runs/rat). Levels of RBF ranged from normal to zero. An artery between the Circle of Willis and the eye was excluded. With some Combinations, flow traversed the capillary bed. Combinations were consolidated into five Groups based on the blood flow paths remaining after the ligations. A mixed linear model accounting for multiple measurements in the same eye demonstrated an effect of Group on RBF (P<0.0005). By major source of ocular blood supply, the trend of RBF levels was: ipsilateral carotid artery> contralateral carotid artery> ipsilateral distal internal carotid artery retrograde from Circle of Willis. The findings advanced knowledge of the sources of blood supply to the rat eye and demonstrated a method of selective cervical arterial ligations for varying RBF levels with potential to impact future retinal ischemia research.
Keywords: Ocular Blood Supply, Retinal Blood Flow, Retinal Ischemia, Animal Model, Imaging
1. Introduction:
Retinal ischemia is a major component of many clinically important, blinding conditions, such as retinal artery and vein occlusions, diabetic retinopathy,(Bresnick et al., 1975; Silva et al., 2015) sickle retinopathy(Do and Rodger, 2017) and the ocular ischemic syndrome. Much has been learned about retinal ischemic injury from animal models of retinal ischemia,(Ben-Nun et al., 1988; Blair et al., 2018; Blair et al., 2019; Buchi et al., 1991; Ernest and Archer, 1979; Hayreh et al., 1980; Karamian et al., 2019; Lafuente et al., 2002; Lelong et al., 2007; Mosinger and Olney, 1989; Otori et al., 1994; Slakter et al., 1984; Stefansson et al., 1988) but all of these models have limitations. It would be desirable to have an animal model meeting the following criteria:
Any level of retinal blood flow (RBF) from unimpeded to zero RBF, and its duration, can be set by the investigator,
RBF and other variables of interest can be measured at any time before, during, or after altering the blood flow,
The method does not require the eye to be manipulated, or cause collateral, nonischemic injurious ocular (especially retinal) effects.
We have particular interest in learning the effects of interfering with RBF over the full range from normal to zero (criterion 1). We consider it likely that the retinal injuries with various levels of partial ischemia would be different from that with total ischemia, which was studied in most previous models.(Ben-Nun et al., 1988; Blair et al., 2019; Buchi et al., 1991; Ernest and Archer, 1979; Hayreh et al., 1980; Lafuente et al., 2002; Mosinger and Olney, 1989; Otori et al., 1994; Stefansson et al., 1988) Since the ultimate objective is extrapolation of the experimental results to the clinical situation, we note that at least some blood flow usually is present when patients with retinal ischemia are examined (unpublished data, N P Blair).
Previously, we presented a model in rats to induce acute, graded retinal ischemia that was based on ligating the contralateral common carotid artery (CCAc, see Figure 1 and the Abbreviations List for abbreviations of the vessels referred to this paper) and then variably compressing the ipsilateral common carotid artery (CCA).(Blair et al., 2018) Some RBF could be measured even with complete compression of CCA, consistent with RBF measurements in the permanent bilateral common carotid occlusion (BCCAO) ischemia model.(Karamian et al., 2019) This observation also accords with only mild to moderate injury with BCCAO in other studies. (Barnett and Osborne, 1995; Block et al., 1992; Block and Sontag, 1994; Davidson et al., 2000; Guo et al., 2014; Huang et al., 2014; Lavinsky et al., 2006; Slakter et al., 1984; Stevens et al., 2002; Takamatsu et al., 1984; Yamamoto et al., 2006) These results raise the question of where the residual RBF comes from.
Figure 1:
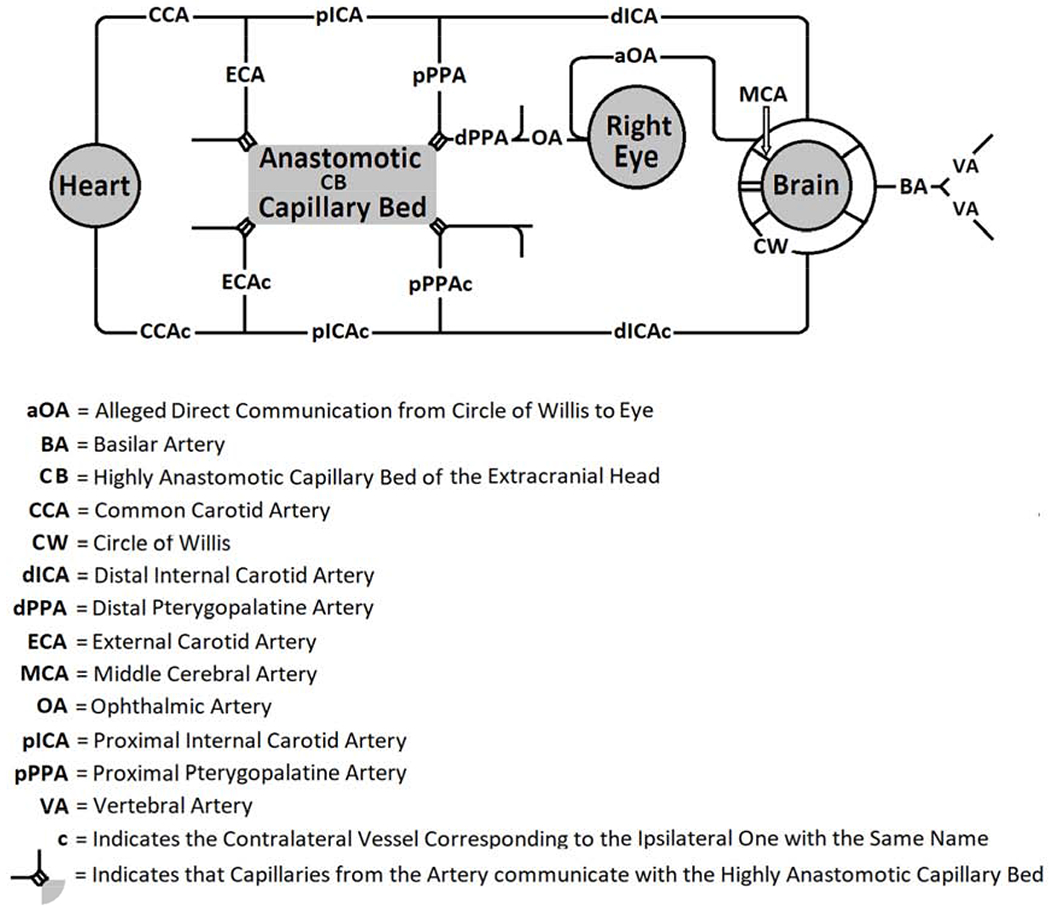
Schematic Diagram of Blood Vessels that could be involved in Supplying Blood Flow to the Eye in Rats
According to the careful work of Greene on the anatomy of the rat, the ophthalmic artery (OA) supplying the eye (Figure 1) eventually originates from the pterygopalatine artery (PPA, composed of proximal, pPPA, and distal, dPPA, parts) which branches from the proximal internal carotid artery (pICA) just before the distal internal carotid artery (dICA) penetrates the cranium via the carotid canal.(Greene, 1935) The dPPA itself bifurcates into pterygoid and palatine branches, and OA arises from the palatine branch. More recently, Shabanzadeh et al confirmed this by showing filling of the retinal vasculature after injecting Evans blue into a tube inserted into the pPPA and dPPA of the rat. (Shabanzadeh et al., 2018) This anatomy is unlike that in humans in which the OA branches from the intracranial internal carotid artery part of the Circle of Willis (CW) near the origin of the middle cerebral artery (MCA), and then enters the orbit through the optic canal on its way to the eye. Some investigators have presented diagrams of the anatomy in rodents in which a so-called OA (aOA) supplies the eye by branching intracranially from CW and communicates directly to the eye as in humans,(Allen et al., 2014; Steele et al., 2008) and others have affirmed this anatomy.(Allen et al., 2015; Allen et al., 2016; Kalesnykas et al., 2008; Li et al., 2009; Michalski et al., 2013) We have not been able to find photographs of the vessels at the base of the brain of rats that show the origin of any aOA.(Longa et al., 1989; Ma et al., 2006; Sutherland et al., 2016; Zuo et al., 2012)
If, indeed, OA does not originate intracranially, but instead is derived from pPPA, it might be possible to interfere with RBF over the full range (criterion 1) by ligating various combinations of cervical arteries (criterion 3). Retinal ischemic effects have been shown by combined ligation of two vessels, the external carotid artery (ECA) and pPPA.(Lelong et al., 2007; Ogishima et al., 2011) Also, Tamaki et al clamped pICA in mice, and found evidence of circulatory communication between ECA and dPPA.(Tamaki et al., 2006) This suggested that in the presence of certain cervical arterial ligations, blood might flow from one artery to another one by way of the capillary bed of the head (CB) and then on to the eye. We are unaware of investigations on the retinal effects of multiple combinations of cervical arterial ligations, especially ones in which actual measurement of RBF was the outcome (criterion 2).
We hypothesized that ligating various combinations of cervical arteries would allow characterization of the paths by which the rat eye can be supplied with blood and form the basis for inducing various levels of RBF in subsequent investigations. As part of this overall hypothesis, we also made the following subhypotheses: 1) There is no direct communication between CW and the eye, that is, aOA does not exist, 2) There is at least one combination of arterial ligations in which RBF will be normal, at least one in which RBF will be zero and others in which RBF will range between these two values, 3) During certain cervical arterial ligations, CB, the capillary bed, either from the ipsilateral or contralateral side, can participate in supplying the eye with blood. We also consider that to evaluate these hypotheses the physiological variable RBF, which can be measured in vivo on a ratio scale, is superior as an outcome variable compared to outcome variables in many previous studies that must be measured on ordinal or interval scales, often postmortem. Using methods we have established previously, we tested these hypotheses by measuring RBF during various combinations of cervical arterial ligations.
2. Methods:
2.1. Animals:
Twenty male Long-Evans pigmented rats (age: 14.8 ± 2.1 weeks; weight: 436 ± 41.4 g; N=19, data not available from one rat) were included in the study. All procedures were approved by the University of Southern California Institutional Animal Care and Use Committee, complied with the ARRIVE Guidelines and carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals. The rats were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (5 mg/kg) and were given additional doses to maintain anesthesia as needed. The femoral artery was cannulated and a catheter was secured in place. The right pupil was dilated with 2.5% phenylephrine and 1% tropicamide. An anterior midline incision then was made in the neck, and, using blunt dissection, the salivary glands, lymph nodes, and ventral neck muscles were retracted to expose CCA and CCAc. Care was taken to avoid injury to the vagus and sympathetic nerves. The dissection was carried superiorly on the right (ipsilateral) side past the bifurcation of CCA into ECA and pICA in order to expose and isolate the bifurcation into dICA and pPPA. dICA then courses medially and posteriorly into the carotid canal through the cranium, and pPPA then courses more laterally. The same dissection was also performed on the contralateral side (Figure 1). Ligations with 5 – O silk sutures were applied to various arteries, as described below. The incision was covered with gauze soaked with saline solution. The rats were placed on an animal holder that incorporated copper tubing perfused with heated water to maintain the body temperature. A glass cover slip with 2.5% hypromellose ophthalmic demulcent solution (HUB Pharmaceuticals, Plymouth, MI) was applied to the cornea to minimize its refractive power and prevent dehydration for imaging. As described elsewhere,(Wanek et al., 2011) 2-μm polystyrene fluorescent microspheres (Invitrogen, Grand Island, NY) were injected through the catheter for RBF imaging. The time required to perform the procedures to expose the cervical vessels was about 20 minutes. The time needed for each blood flow measurement was less than 10 minutes. Ligating each additional vessel took around 10 minutes.
2.2. Imaging:
RBF was measured in the right eye using our previously reported imaging system.(Wanek et al., 2011) Red-free retinal images were analyzed to determine retinal venous diameter for each major vein (DVind). Image sequences that displayed the position of individual fluorescent microspheres were analyzed to provide measurements of blood velocity (Vind) in each major vein. RBF was calculated by the product of Vind, π and (DVind)2/4, summed over all major veins.
2.3. Cervical Arterial Ligations:
Figure 2 indicates schematically the vascular paths proposed to supply the eye of the rat. We divided the PPA into pPPA and dPPA based on where a ligature could be placed. Because of the short distance between the origin of pPPA from pICA and the point at which it goes around the tympanic bulla (see Figure 210 in Greene (Greene, 1935)), surgical access by way of the cervical incision only permits ligation of pPPA very near its origin. The following arteries on the ipsilateral (right) side and their corresponding arteries on the contralateral (left) side (designated by the suffix “c”) could be accessed from the cervical incision: CCA, pICA, ECA, dICA, and pPPA. After exposure of the cervical arteries, one artery was ligated, and RBF was measured in the right eye. Each such measurement is referred to as a “run”. After the measurement, another artery was ligated, leaving any previous arterial ligations in place. RBF was then measured again. This was repeated several times in each rat. There was never more than one run with a given combination of ligated cervical arteries in any rat. Since the phrase “combination of ligated cervical arteries” is so unwieldy, for the purposes of this study, we will use the term “Combination” to have this meaning instead (See Table 1). We note that when RBF was too low to be measured because of the scarcity or lack of observed microspheres in the circulation, this was associated with the presence of extremely narrowed arteries and/or segmented blood columns and a pale optic nerve head. In some instances we have also included RBF from rats treated with the same procedures who had sham surgery (n=8) or acute permanent BCCAO (n=10) in another study that had different objectives.(Karamian et al., 2019)
Figure 2:
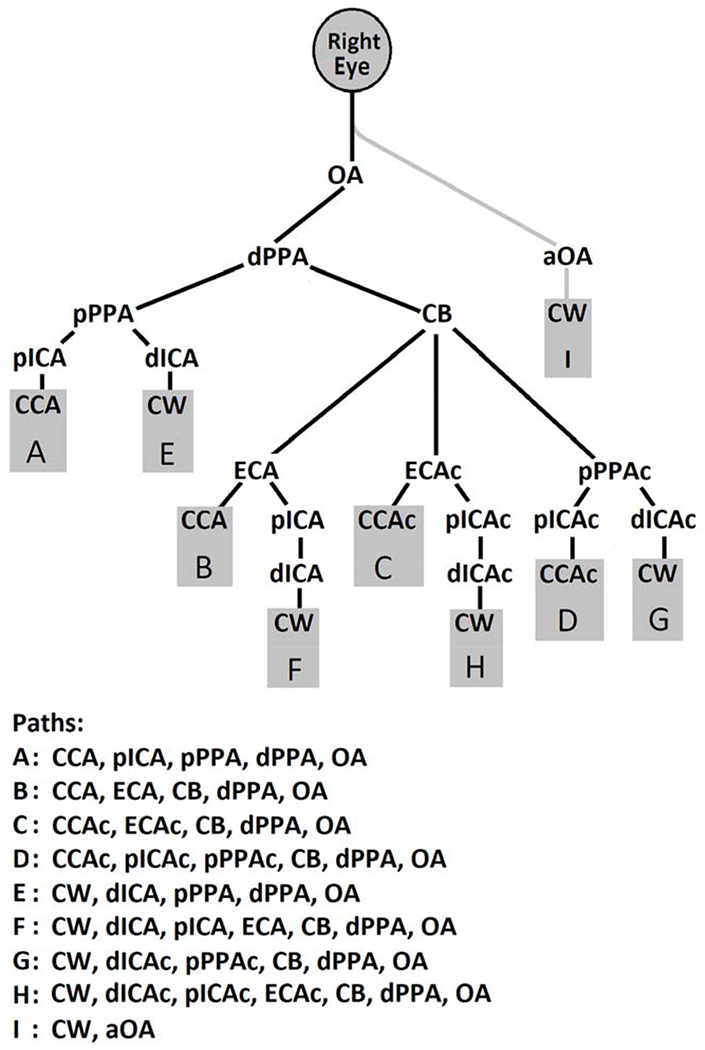
Proposed Paths of Blood Flow to the Eye in Rats
Abbreviations refer to blood vessels as indicated in Figure 1. Single letters refer to blood vessel paths to the eye. Note that the final common path is the ophthalmic artery, and blood flow gains access to the eye (and retina) only by way of it. The only possible exception is the alleged ophthalmic artery (path shown in gray) whose existence has been asserted but was not substantiated in the current study. Also, note that blood flow to the eye must be derived orthograde from either the ipsilateral or contralateral common carotid artery or retrograde from the Circle of Willis by either the ipsilateral or contralateral distal internal carotid artery.
Table 1.
Mean and SD of Retinal Blood Flow after Various Cervical Arterial Ligations and the Unobstructed Paths to the Eye in Rats
| Combination Number | Vessels Ligated | N | Retinal Blood Flow (μL/min) | Unobstructed Paths to Eye | Unobstructed Paths Group | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| 1 | CCAc, dICA | 3 | 8.5 | 1.9 | A, B, G, H, I | 1 |
| 2 | CCA, pPPA | 4 | 7.2 | 2.4 | C, D, F, G, H, I | 3 |
| 3 | dICA, pICA | 2 | 7.2 | 1.6 | B, C, D, G, H, I | 2 |
| 4 | pPPA | 10 | 6.6 | 2.9 | B, C, D, F, G, H, I | 2 |
| 5 | CCAc, pPPA | 4 | 5.3 | 1.8 | B, F, G, H, I | 2 |
| 6 | CCA | 3 | 4.8 | 0.0 | C, D, E, F, G, H, I | 3 |
| 7 | CCA, dICA | 2 | 4.3 | 0.5 | C, D, G, H, I | 3 |
| 8 | CCA, dICA, pPPA | 2 | 2.9 | 0.8 | C, D, G, H, I | 3 |
| 9 | CCA, dICA, ECA, pPPA | 4 | 2.8 | 0.5 | C, D, G, H, I | 3 |
| 10 | CCA, CCAc | 11* | 2.2 | 1.4 | E, F, G, H, I | 4 |
| 11 | dICA, ECA, pPPA | 2 | 1.8 | 1.4 | C, D, G, H, I | 3 |
| 12 | CCA, CCAc, dICA | 6 | 0.0 | 0.0 | G, H, I | 5 |
| 13 | CCA, CCAc, pPPA | 3 | 0.0 | 0.0 | F, G, H, I | 5 |
Column 1 contains the Combination Number (Combination is the abbreviation for [combination of cervical arterial ligations]) that was assigned after arranging the rows in descending order according to the values for Mean Retinal Blood Flow (RBF) in Column 4. The abbreviations in column 2 correspond to the blood vessels as shown in Figure 1. Column 3 indicates the number of runs. RBF was measured once, that is, there was one run, in a given eye for each of several Combinations. Thus, RBF values of runs obtained from an individual eye appear in several rows, but each row contains no more than one run from a given eye. Column 6 displays the RBF paths (according to Figure 2) that contain no ligated vessels and therefore can contribute to the blood supply of the eye. Column 7 presents the unobstructed paths group (Group) in which the Combination in the same row belongs. Combinations were consolidated retrospectively into Groups based on the capacity of the unobstructed paths (indicated in Column 6) to supply the eye according to Figure 2 after ligation of the cervical arteries. Group 1 consisted of any combination of unobstructed paths that included Path A. Group 2 included any combination of unobstructed paths containing Path B, but not A. Group 3 comprised any combination of unobstructed paths having Path C or D, but not Paths A or B. Group 4 included any combination of unobstructed paths with Path E, but not A, B, C or D. Group 5 contained any combination of unobstructed paths which excluded Paths A, B, C, D or E but included F, G, H or I.
indicates that 10 runs having ligation of CCA and CCAc (Combination 10) are included from Karamian et al.(Karamian et al., 2019) In 13 additional Combinations there was only one run. Since the value when there is only one run is a poor indicator of the true mean of a Combination, these values were not included in this Table. Thus, the total of runs in this Table is 56.
2.4. Statistical Analysis:
RBF runs were categorized retrospectively according to 5 unobstructed paths groups (Groups), on the basis of which paths (Figure 2) were still available after the cervical arterial ligations and the level of RBF through them (See Section 3). A mixed linear model that accounted for multiple measurements in the same eye was generated to determine the effect of Group on RBF with animal considered as a random variable. Pairwise comparisons between Groups were performed using Bonferroni adjustments for multiple comparisons. In general, the study was not designed to make fine distinctions between individual Combinations because the numbers were too small. Thus, the ranking of Combinations in Table 1 must be considered tentative. Statistical analysis of data was performed using SSPS Statistics Software (Version 24 IBM Armonk, New York)
3. RESULTS:
All blood flow paths to the eye come orthograde from either CCA or CCAc or retrograde from CW into either dICA or dICAc. CW, which supplies the brain, itself is derived from the basilar artery (BA) and the two vertebral arteries (VA) that supply it. If aOA exists, it also is derived from CW. Figure 2 shows all possible paths, and they are designated by letters. In this figure blood flow paths converge at the eye and all inputs to each bifurcation are delineated extending upstream to all of the possible origins of blood to the eye noted above. Note that one possible segment in the sequence of vessels going to the eye is CB, which is assumed to be highly anastomotic and can eventually connect to dPPA and from there to the eye. Since arteries from both sides contribute to CB, depending on which arteries are ligated, it is possible for blood originating from either the ipsilateral or contralateral side to contribute to the blood supply of the right eye. Study of the cervical arteries and what structures they supply is in the domain of gross anatomy, and, accordingly, we refer to blood supply to the eye, rather than to the retina. This clearly implies that these vessels also supply the retina, but we will refer explicitly to the retina when we are specifically referring to the blood supply to it. We do not mean to imply that referring to the blood supply to the eye excludes supply by the same vessels to extraocular structures or to intraocular structures other than the retina.
In the present study, RBF data were obtained from 59 runs of combinations of ligated cervical arteries. Although there are 1024 possible Combinations using the five ipsilateral and five contralateral surgically accessible cervical arteries, we chose 26 Combinations that we thought would be most revealing of the blood supply to the eye. Data were obtained on 2.9 ± 2.7 (mean and SD; range: 1-7) Combinations per rat. There was only one run with a given Combination in any rat. Table 1 gives mean and SD of RBF in descending order that were observed with 13 Combinations (46 runs), excluding 13 additional Combinations in which there was only one run. Since the value when there is only one run is a poor indicator of the true mean RBF of a Combination, these values were not included. Table 1 also includes additional values for Combination 10 in 10 rats from our previous study(Karamian et al., 2019) performed with exactly the same procedures as in the present study. Thus, 56 runs are presented in Table 1.
Overall, as might be expected, there was a tendency for RBF to be lower when more vessels were ligated. The highest RBF, which equaled the sham surgery RBF of 8.5 ± 2.1 μL/min, N = 8, from Karamian and coworkers,(Karamian et al., 2019) was seen when CCAc and dICA (Combination 1) were ligated. This left free flow to the retina from CCA to pICA to pPPA while simultaneously preventing any of that flow into dICA and from there to the brain.
On the other hand, RBF was low in the 25 runs in which CCA plus CCAc with or without other vessels were ligated. When only both CCA and CCAc were ligated (Combination 10), RBF was about 25% of normal. However, when dICA was added to these ligations (Combination 12), RBF was eliminated. Accordingly, retrograde flow in dICA from CW must have been the only source for RBF prior to adding ligation of dICA (Figure 2, Path E). This also indicates that the data provided no evidence for the existence of aOA, (Path I), and no blood was available to the ipsilateral eye from dICAc (Paths G and H). Otherwise, there still would be some flow to the retina after ligation of dICA was added. The only other instance in which RBF was zero was when both CCA and CCAc plus pPPA were ligated (Combination 13). In this situation the only unobstructed possible path to the eye was Path F. As opposed to Path E in which retrograde flow in dICA could pass directly into pPPA and on to the eye, in Path F blood flowing retrograde from dICA could not enter pPPA, but would have had to take the more circuitous path through pICA, ECA and CB to gain access to dPPA and then the eye. These results demonstrate that Paths F, G, H and I in Figure 2 do not contribute significantly to the blood supply of the ipsilateral retina.
Some Combinations gave unexpected RBF values, most notably Combination 4 in which pPPA was ligated near its origin. RBF was only mildly reduced, to about 78% of normal, despite this artery being the best established normal path to the eye.(Greene, 1935; Shabanzadeh et al., 2018) Since ligating pPPA eliminates flow to the eye via Paths A and E, and we have shown Paths F, G, H and I do not contribute to RBF in the ipsilateral eye, this flow must have been via some aggregate of Paths B, C and D. Figure 2 indicates that all three of these paths go through CB to get to dPPA and on to eye, indicating that CB can be a significant segment in paths to the eye.
In all 69 runs (including 10 runs of Combination 10 from Karamian and coworkers),(Karamian et al., 2019) after ligation, there were 4.9 ± 1.6 unobstructed possible paths of blood flow to the eye (not excluding Paths F, G, H and I which were just shown not to contribute in actuality to RBF of the ipsilateral eye). It seemed highly unlikely that all possible unobstructed paths contributed equally to the RBF. Instead, it seemed probable that the paths associated with higher RBF upon inspection of Table 1 would dominate the total flow. Our inspection led us to assume that the order of contribution to RBF by the unobstructed paths was A > B > C/D (C/D, our data did not permit differentiation between these) > E. Accordingly, we retrospectively consolidated the Combinations into unobstructed paths groups (Groups, as indicated in the Statistics section) based on this sequence. Thus, Group 1 consisted of any combination of unobstructed paths that included Path A, which is the path dominating supply to the eye in the normal condition. Group 2 included any combination of unobstructed paths containing Path B, but not A. Path B provides flow from CCA, but routes blood through CB. Group 3 comprised any combination of unobstructed paths having Path C or D, but not Paths A or B. In this Group blood flow is derived mainly from CCAc through CB. Group 4 included any combination of unobstructed paths with Path E, but not A, B, or C/D. In this Group no flow to the eye was derived from either carotid artery, but instead was derived from CW by way of retrograde flow through dICA to pPPA. Group 5 contained any combination of unobstructed paths which excluded Paths A, B, C/D or E but included F, G, H or I. We have already shown that none of the latter paths provided significant RBF to the right eye.
For the five Groups the linear mixed model gave estimated marginal means and SEM values for RBF that accounted for multiple measurements in the same eye. These are shown in Table 2. The model showed a significant effect of Group on RBF (P < 0.0005). The results of pairwise comparisons are given in Table 3. The main unobstructed paths to the eye in the four Groups having substantial RBF (A, B, C/D, and E) are depicted in Figure 3.
Table 2.
Mean and SEM of Retinal Blood Flow in Five Groups Based on the Unobstructed Paths to the Eye after Various Cervical Arterial Ligations in Rats
| Unobstructed Paths Group | Unobstructed Paths | N | Retinal Blood Flow (μL/min) | |
|---|---|---|---|---|
| Estimated Marginal Mean | SEM | |||
| 1 | A and others | 4 | 7.6 | 0.9 |
| 2 | B and others, but not A | 18 | 6.2 | 0.5 |
| 3 | C or D and others, but not A or B | 20 | 4.3 | 0.5 |
| 4 | E and others, but not A-D | 11* | 2.2 | 0.6 |
| 5 | F through I, but not A-E | 16 | 0.3 | 0.5 |
The paths by which the unobstructed paths groups (Groups) are defined are presented in Figure 2. N in Column 3 includes all runs in each of the Combinations (Combination is the abbreviation for [combination of cervical arterial ligations]) that fit within a given Group, including not only the 56 runs presented in Table 1, but also the 13 runs from the 13 additional Combinations in which there was only one run. Accordingly, the total N is 69. The values in Columns 4 and 5 are derived from the mixed linear model that determined the effect of Group on RBF while accounting for multiple measurements in the same eye.
indicates that 10 runs having ligation of CCA and CCAc (Combination 10, Table 1) are included from Karamian et al.(Karamian et al., 2019)
Table 3.
Probability Values for Pairwise Comparisons of Retinal Blood Flow in Five Groups Based on the Unobstructed Paths to the Eye after Various Cervical Arterial Ligations in Rats
| P Values | |||||
|---|---|---|---|---|---|
| Unobstructed Paths Group | 1 | 2 | 3 | 4 | 5 |
| 1 | 1.000 | 0.01* | <0.0005* | <0.0005* | |
| 2 | 0.013* | <0.0005* | <0.0005* | ||
| 3 | 0.07 | <0.0005* | |||
| 4 | 0.18 | ||||
| 5 | |||||
Combinations (Combination is the abbreviation for [combination of cervical arterial ligations]) were consolidated retrospectively into unobstructed paths groups (Group) based on the capacity of the unobstructed paths according to Figure 2 to supply the eye after ligation of the cervical arteries. Group 1 consisted of any combination of unobstructed paths that included Path A. Group 2 included any combination of unobstructed paths containing Path B, but not A. Group 3 comprised any combination of unobstructed paths having Path C or D, but not Paths A or B. Group 4 included any combination of unobstructed paths with Path E, but not A, B, C or D. Group 5 contained any combination of unobstructed paths which excluded Paths A, B, C, D or E but included F, G, H or I.
indicates P < 0.05, the criterion used for statistical significance. In Table 2 the value for retinal blood flow for each unobstructed paths group is given.
Figure 3:
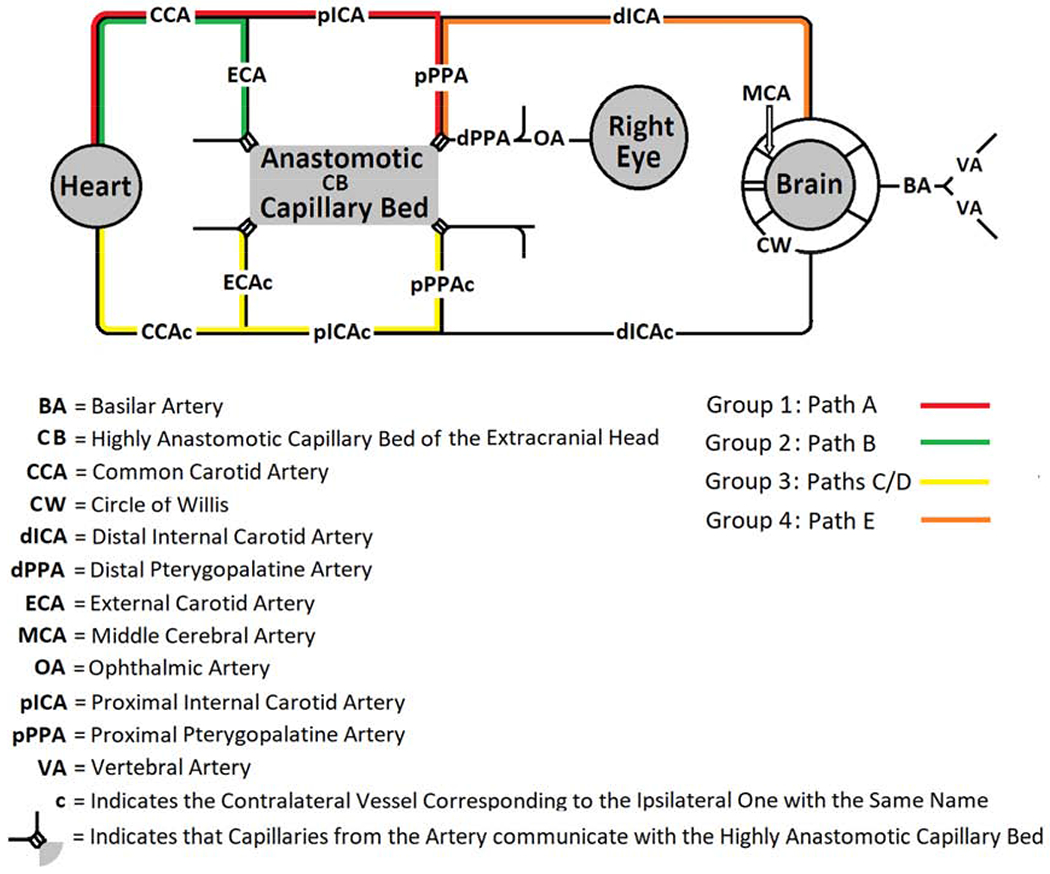
Schematic Diagram Depicting Major Paths Supplying Blood Flow to the Eye in Groups after Various Cervical Arterial Ligations in Rats
Combinations of cervical arterial ligations were consolidated retrospectively into unobstructed paths groups (Groups) based on the capacity of the unobstructed paths according to Figure 2 to supply the eye after ligation of the cervical arteries. Group 1 consisted of any combination of unobstructed paths that included Path A. Group 2 included any combination of unobstructed paths containing Path B, but not A. Group 3 comprised any combination of unobstructed paths having Path C or D, but not Paths A or B. Group 4 included any combination of unobstructed paths with Path E, but not A, B, C or D. Group 5 contained any combination of unobstructed paths which excluded Paths A, B, C, D or E but included F, G, H or I. No path is indicated for Group 5 since blood flow in this unobstructed paths group was extremely low or absent. Note that the alleged communication between the Circle of Willis and the eye has been removed from the diagram because the data did not confirm its existence.
4. DISCUSSION:
In the present study we were able to confirm the overall hypothesis that ligating various combinations of cervical blood vessels would allow characterization of the paths by which the rat eye can be supplied with blood. Also, we were able to confirm the three subhypotheses. First, there is no direct communication between CW and the eye (aOA, Path I). Some investigators have used the intra-arterial filament model of MCA occlusion to study ocular ischemia because the method also interferes with ocular blood flow.(Allen et al., 2015; Allen et al., 2014; Allen et al., 2016; Block et al., 1997; Kaja et al., 2003; Kalesnykas et al., 2008; Li et al., 2009; Michalski et al., 2013; Steele et al., 2008) In this method a filament, such as a nylon suture, is passed into pICA (usually from ECA by passing the filament retrograde through it) after temporarily clamping CCA. The filament is then advanced intra-arterially to the origin of MCA, thereby obstructing it. We propose that in this method ocular ischemia results from the combination of ligation of ECA and interference by the filament to flow into pPPA at its origin. However, ocular ischemia has been attributed previously to obstruction of the origin of aOA by the filament near the origin of MCA. In some of these reports, diagrams have been presented in which an artery (aOA) branches from CW between the entry of dICA into the cranium from the carotid canal and the origin of MCA (Path I).(Allen et al., 2014; Steele et al., 2008) In contrast, we have not been able to find clear photographs or other convincing anatomic documentation of aOA. Our blood flow measurements provide physiologic confirmation of the anatomic findings of Greene and others that dPPA is the source of blood supply to the eye.(Greene, 1935; Ogishima et al., 2011; Shabanzadeh et al., 2018) Two observations support this: 1) With ligation of CCA plus CCAc (Combination 10), RBF was reduced to about 25%. However, by adding ligation of dICA (Combination 12) RBF was eliminated. This indicates that the only blood flow to the eye with the unobstructed dICA had been retrograde to pPPA (Path E) and not through aOA (Path I). We also note that under these conditions (ligation of CCA, CCAc and dICA), no flow came to the ipsilateral eye from dICAc, although we assume that about 25% of normal RBF was contributed to the contralateral left eye by it (dICAc). 2) The highest mean RBF was seen when CCAc and dICA were ligated (Combination 1). In this situation, there would have been plentiful blood flow from CCA and pICA to pPPA and to the eye (Path A). On the other hand, without either of these two major contributors (dICA and CCAc), essentially the only supply to CW and the brain would have to have been via BA and VA. Consequently, flow from CW through aOA, as opposed to flow via dPPA, would have to have been markedly reduced. We note that the conclusions of the studies that assume blood supply to the eye via aOA would not have been significantly different had the authors assumed supply to the eye by pPPA. (Allen et al., 2015; Allen et al., 2014; Allen et al., 2016; Kalesnykas et al., 2008; Li et al., 2009; Michalski et al., 2013; Steele et al., 2008)
Second, there are combinations of ligated cervical arteries that will make it possible to vary RBF between normal and zero by variably compressing CCA or CCAc. Of course, this requires that there be no aOA, which would be surgically inaccessible. We have described a method to induce graded retinal ischemia in which CCAc was ligated. Then a rod attached to a micromanipulator was used to compress CCA against a backstop with high precision to produce reductions of RBF. (Blair et al., 2018) However, RBF was not reduced to zero even when CCA was completely closed by the rod. This is consistent with the current results in which CCA plus CCAc were occluded (Combination 10), as well as with several reports in which permanent BCCAO was used as a model to study ocular ischemia. (Barnett and Osborne, 1995; Block et al., 1992; Block and Sontag, 1994; Davidson et al., 2000; Guo et al., 2014; Huang et al., 2014; Karamian et al., 2019; Lavinsky et al., 2006; Slakter et al., 1984; Stevens et al., 2002; Takamatsu et al., 1984; Yamamoto et al., 2006) Since this residual flow was accounted for in the present study by retrograde flow from CW through dICA to pPPA, ligating all three of these arteries (CCA, CCAc, and dICA, Combination 12) caused RBF to be reduced to zero. If CCAc and dICA are ligated, RBF should be essentially normal (Combination 1). Then, having ligated CCAc and dICA, adding variable compression on CCA should allow RBF to be adjusted to any value from normal (with no compression) to no flow (with complete compression). The resulting model then will meet all of the criteria listed in the Introduction. We also note that by ligating any of the combinations in Table 1 one can induce a partial reduction in RBF without the experimental complexity imposed by external compression to the carotid artery. For example, if one wanted to establish RBF at approximately 5.3 μL/min, about 62% of normal RBF, one could ligate CCAc and pPPA (Combination 5).
We were particularly interested in being able to induce RBF over the full range from normal to zero (criterion 1) for two reasons: 1) we considered it likely that the development of retinal injury with various levels of ischemia might be different from that with total ischemia. For example, some injurious metabolic cascades might able to proceed during partial ischemia in which some energy metabolism persists that would be unable to proceed after energy metabolism is eliminated by total ischemia. Furthermore, it is unknown how the results of various durations of total retinal ischemia, which were studied in most previous models,(Ben-Nun et al., 1988; Blair et al., 2019; Buchi et al., 1991; Ernest and Archer, 1979; Hayreh et al., 1980; Lafuente et al., 2002; Mosinger and Olney, 1989; Otori et al., 1994; Stefansson et al., 1988) correlate with those of partial ischemia of various durations and levels, 2) Studies of rats with permanent BCCAO show significant, but not severe, eventual retinal injury. This means that the processes that determine whether the tissue will survive, die or hover in a penumbra-like state comparable to an idling car are particularly prominent at levels of blood flow maintained below those present under these conditions. Because tissue in the penumbra-like state could respond to therapy, it would be of particular interest to establish a level of RBF in which the penumbra-like state would predominate.(Lipton, 1999) Study of tissue in this state would have great potential for facilitating the development of therapeutic approaches to clinical conditions with incomplete retinal ischemia.
Third, during certain cervical arterial ligations the CB can serve as a vascular segment in a path to the eye, either from the ipsilateral or contralateral side. Perhaps the most unexpected result of the current study, especially after we concluded that aOA does not exist, was that when pPPA was ligated, RBF was the fourth highest we recorded, still about 78% of normal. Furthermore, ligating dICA and pICA (Combination 3), which isolates pPPA, had the third highest RBF, about 85% of normal. Because of surgical access, pPPA can be ligated only near its branching from the pICA. Tamaki et al used synchrotron radiation microangiography to investigate the blood flow pattern in mice.(Tamaki et al., 2006) After closing pICA, they found retrograde flow derived from ECA in dPPA, eventually going toward the brain. This demonstrates that significant communications can exist between ECA and dPPA, presumably by way of anastomotic channels in CB and possibly between other micro-vessels, which, if contributing, we assume under the designation CB. In Combination 4 in which pPPA alone is ligated, our model of potential paths to the eye (Figure 2) included only pathways involving an anastomotic CB. As noted above, our results led us to exclude Paths F, G, H and I. With the pPPA ligated, Paths A and E are excluded from contributing to RBF. This means that only Paths B, C, and D could be contributing to RBF, and all pass through CB on the way to dPPA and the eye. Furthermore, Paths C and D originate from the contralateral side, and the RBF for paths C and / or D was about 51% of normal, presumably because of the longer path (and higher resistance to flow) to dPPA. Again, to eliminate RBF, closing contralateral vessels is necessary (Combinations 12 and 13), demonstrating the existence of a path (CB) from the contralateral side to the ipsilateral eye. We consider these observations to be compelling evidence that CB can contribute significantly to the RBF, including flow from the contralateral side. On the other hand, Shabanzadeh et al clamped pPPA for 30 minutes in rats.(Shabanzadeh et al., 2018) 14 days after removal of the clamp, they found significant ganglion cell loss, suggesting that RBF via CB was not sufficient to prevent this. Relating these results to ours is very difficult because of major differences in the experiments. Among these are rat strain, outcome variable, timing of outcome acquisition and the possible presence of delayed reflow after removal of the clamp.(Blair et al., 2019)
The extracranial sources of blood supply to the eye and brain are CCA, CCAc and the 2 VA, and the latter contribute to CW (as do dICA and dICAc when flow is orthograde). Since our data exclude the existence of aOA, in principle the eye can be supplied from four sources: CCA, CCAc or retrograde from CW through dICA or dICAc. We simplified the results of the 26 Combinations into 5 Groups based on their ability to supply the eye. We now can appreciate that these Groups closely relate to these four sources. In Groups 1 and 2, blood is derived from CCA. They differ by Group 1 being dominated by Path A, the normal route, and Group 2 being dominated by Path B in which flow is diverted through CB. Despite this, no statistically significant difference was seen in mean RBF between these Groups. In Group 3, RBF originated mainly from CCAc necessitating passage through CB. Furthermore, in this situation, CCA was ligated so that much of the blood flow through CCAc had to supply the brain and contralateral eye. This would have left less to be available to the ipsilateral eye, as indicated in Tables 2 and 3. In Group 4, both CCA and CCAc were ligated so that the eye had to be supplied by Path E by way of retrograde flow through dICA from CW. In this situation (Combination 10), RBF in the ipsilateral eye was only about 25% of normal. Presumably, this low RBF was because the entire brain and both eyes had to be supplied by the VA, which left little for the eyes. In Group 5, both CCA and CCAc, as well as other arteries, were ligated, and RBF was extremely low or absent. Path I was theoretically possible, but excluded based on our data. RBF by way of Path F had to start out at a low level as it did in Path E (Group 4), but it then had to pass though pICA and CB to get to the eye. RBF via Paths G and H had to be supplied from the contralateral side and by retrograde flow from CW. The low retrograde flow available from dICAc then had to traverse CB. Apparently, the added distance and resistance in Paths F, G and H left RBF at minimal or undetectable levels. Thus, the Groups fit nicely into a 2 by 2 table in which better RBF is associated with an ipsilateral and/or orthograde source of blood flow, Table 4. The only exceptions are Path F in Group 5, in which low flow had a long, circuitous path, and Path I, which was shown not to exist. We note that a Group must be based on the entire path involved and not only on which of the four sources noted above are ligated. For example, even if CCA is not ligated, RBF cannot be derived from it if ECA and pPPA are ligated.
Table 4.
Major Extracranial Sources of Retinal Blood Flow in Five Groups Based on the Unobstructed Paths to the Eye after Various Cervical Arterial Ligations in Rats
| Orthograde from Common Carotid Artery | Retrograde from Circle of Willis via Vertebral Arteries | |
|---|---|---|
| Ipsilateral | Groups 1 and 2 | Group 4 |
| Contralateral | Group 3 | Group 5 |
Combinations (Combination is the abbreviation for [combination of cervical arterial ligations]) were consolidated retrospectively into unobstructed paths groups (Groups) based on the capacity of the unobstructed paths according to Figure 2 to supply the eye after ligation of the cervical arteries. Group 1 consisted of any combination of unobstructed paths that included Path A. Group 2 included any combination of unobstructed paths containing Path B, but not A. Group 3 comprised any combination of unobstructed paths having Path C or D, but not Paths A or B. Group 4 included any combination of unobstructed paths with Path E, but not A, B, C or D. Group 5 contained any combination of unobstructed paths which excluded Paths A, B, C, D or E but included F, G, H or I.
The current study had limitations. First, it is known that there can be variability in the vascular patterns from species to species and also between strains within species, (Oliff et al., 1995), as well as sex differences, which may limit the generalizability of the results. Second, in each rat several ligations were added successively, making RBF measurements after each ligation. The interval between ligations was less than 20 minutes. Thus, any vascular readjustments that would take longer than that to develop were not represented in our results. This also meant that in some instances the duration of anesthesia may have had some affect. Third, the method interferes with both the retinal and choroidal circulations, but we only measured blood flow in the retinal circulation. Thus, this study did not provide information on the levels of choroidal blood flow and their rankings associated with the Combinations.
5. Conclusions:
By ligating various cervical arteries, the paths of blood flow to the rat eye have been clarified. In decreasing order of capacity, retinal blood flow can be derived, 1) orthograde from the ipsilateral common carotid artery either directly or indirectly through the capillary bed, 2) orthograde from the contralateral common carotid artery via the capillary bed, or 3) retrograde from the Circle of Willis through the distal internal carotid artery. The results disaffirm the existence of a direct communication between the Circle of Willis and the eye in the rat. A method to induce any level in the full range of retinal blood flows was identified. These results may prove useful in studying various levels of retinal ischemic injury and aid in developing improved therapy.
Highlights:
Various combinations of cervical ligations can clarify blood supply to the eye
There is no direct communication between the Circle of Willis and the eye
The pterygopalatine artery is the input to the ophthalmic artery
The capillary bed of the head can be a link in the ocular blood supply
By choosing the ligations, retinal blood flow can be varied from normal to zero
ACKNOWLEDGEMENT:
The authors thank James Burford for performing all animal procedures.
Funding: This study was supported by the National Eye Institute, Bethesda, MD (grants EY017918 and EY029220) and an unrestricted departmental award from Research to Prevent Blindness, New York, NY.
Abbreviations:
- aOA
Alleged Direct Communication from Circle of Willis to Eye
- BA
Basilar Artery
- BCCAO
Bilateral Common Carotid Artery Occlusion
- c
Indicates the Contralateral Vessel Corresponding to the Ipsilateral One with the Same Name
- CB
Highly Anastomotic Capillary Bed of the Extracranial Head
- CCA
Common Carotid Artery
- CW
Circle of Willis
- dICA
Distal Internal Carotid Artery
- dPPA
Distal Pterygopalatine Artery
- DVind
Venous Diameter for Each Individual Major Retinal Vein
- ECA
External Carotid Artery
- MCA
Middle Cerebral Artery
- OA
Ophthalmic Artery
- pICA
Proximal Internal Carotid Artery
- PPA
Pterygopalatine Artery
- pPPA
Proximal Pterygopalatine Artery
- RBF
Retinal Blood Flow
- VA
Vertebral Artery
- Vind
Blood Velocity in an Individual Major Retinal Vein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None.
REFERENCES:
- Allen RS, Olsen TW, Sayeed I, Cale HA, Morrison KC, Oumarbaeva Y, Lucaciu I, Boatright JH, Pardue MT, Stein DG, 2015. Progesterone treatment in two rat models of ocular ischemia. Investigative ophthalmology & visual science 56, 2880–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RS, Sayeed I, Cale HA, Morrison KC, Boatright JH, Pardue MT, Stein DG, 2014. Severity of middle cerebral artery occlusion determines retinal deficits in rats. Exp Neurol 254, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RS, Sayeed I, Oumarbaeva Y, Morrison KC, Choi PH, Pardue MT, Stein DG, 2016. Progesterone treatment shows greater protection in brain vs. retina in a rat model of middle cerebral artery occlusion: Progesterone receptor levels may play an important role. Restor Neurol Neurosci 34, 947–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NL, Osborne NN, 1995. Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Experimental eye research 61, 83–90. [DOI] [PubMed] [Google Scholar]
- Ben-Nun J, Alder VA, Cringle SJ, Constable IJ, 1988. A new method for oxygen supply to acute ischemic retina. Investigative ophthalmology & visual science 29, 298–304. [PubMed] [Google Scholar]
- Blair NP, Felder AE, Tan MR, Shahidi M, 2018. A Model for Graded Retinal Ischemia in Rats. Transl Vis Sci Technol 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair NP, Tan MR, Felder AE, Shahidi M, 2019. Retinal Oxygen Delivery, Metabolism and Extraction Fraction and Retinal Thickness Immediately Following an Interval of Ophthalmic Vessel Occlusion in Rats. Sci Rep 9, 8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block F, Grommes C, Kosinski C, Schmidt W, Schwarz M, 1997. Retinal ischemia induced by the intraluminal suture method in rats. Neurosci Lett 232, 45–48. [DOI] [PubMed] [Google Scholar]
- Block F, Schwarz M, Sontag KH, 1992. Retinal ischemia induced by occlusion of both common carotid arteries in rats as demonstrated by electroretinography. Neurosci Lett 144, 124–126. [DOI] [PubMed] [Google Scholar]
- Block F, Sontag KH, 1994. Differential effects of transient occlusion of common carotid arteries in normotensive rats on the somatosensory and visual system. Brain Res Bull 33, 589–593. [DOI] [PubMed] [Google Scholar]
- Bresnick GH, De Venecia G, Myers FL, Harris JA, Davis MD, 1975. Retinal ischemia in diabetic retinopathy. Archives of ophthalmology 93, 1300–1310. [DOI] [PubMed] [Google Scholar]
- Buchi ER, Suivaizdis I, Fu J, 1991. Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde 203, 138–147. [DOI] [PubMed] [Google Scholar]
- Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA, 2000. Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res 859, 96–103. [DOI] [PubMed] [Google Scholar]
- Do BK, Rodger DC, 2017. Sickle cell disease and the eye. Curr Opin Ophthalmol 28, 623–628. [DOI] [PubMed] [Google Scholar]
- Ernest JT, Archer DB, 1979. Vitreous body oxygen tension following experimental branch retinal vein obstruction. Investigative ophthalmology & visual science 18, 1025–1029. [PubMed] [Google Scholar]
- Greene EC, 1935. Anatomy of the Rat.
- Guo XJ, Tian XS, Ruan Z, Chen YT, Wu L, Gong Q, Wang W, Zhang HY, 2014. Dysregulation of neurotrophic and inflammatory systems accompanied by decreased CREB signaling in ischemic rat retina. Experimental eye research 125, 156–163. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Kolder HE, Weingeist TA, 1980. Central retinal artery occlusion and retinal tolerance time. Ophthalmology 87, 75–78. [DOI] [PubMed] [Google Scholar]
- Huang Y, Fan S, Li J, Wang YL, 2014. Bilateral Common Carotid Artery Occlusion in the Rat as a Model of Retinal Ischaemia. Neuroophthalmology 38, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, Yang SH, Wei J, Fujitani K, Liu R, Brun-Zinkernagel AM, Simpkins JW, Inokuchi K, Koulen P, 2003. Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Investigative ophthalmology & visual science 44, 3155–3162. [DOI] [PubMed] [Google Scholar]
- Kalesnykas G, Tuulos T, Uusitalo H, Jolkkonen J, 2008. Neurodegeneration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience 155, 937–947. [DOI] [PubMed] [Google Scholar]
- Karamian P, Burford J, Farzad S, Blair NP, Shahidi M, 2019. Alterations in Retinal Oxygen Delivery, Metabolism, and Extraction Fraction During Bilateral Common Carotid Artery Occlusion in Rats. Investigative ophthalmology & visual science 60, 3247–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente MP, Villegas-Perez MP, Selles-Navarro I, Mayor-Torroglosa S, Miralles de Imperial J, Vidal-Sanz M, 2002. Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insult. Neuroscience 109, 157–168. [DOI] [PubMed] [Google Scholar]
- Lavinsky D, Arterni NS, Achaval M, Netto CA, 2006. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 244, 199–204. [DOI] [PubMed] [Google Scholar]
- Lelong DC, Bieche I, Perez E, Bigot K, Leemput J, Laurendeau I, Vidaud M, Jais JP, Menasche M, Abitbol M, 2007. Novel mouse model of monocular amaurosis fugax. Stroke 38, 3237–3244. [DOI] [PubMed] [Google Scholar]
- Li SY, Fu ZJ, Ma H, Jang WC, So KF, Wong D, Lo AC, 2009. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Investigative ophthalmology & visual science 50, 836–843. [DOI] [PubMed] [Google Scholar]
- Lipton P, 1999. Ischemic cell death in brain neurons. Physiol Rev 79, 1431–1568. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R, 1989. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20, 84–91. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhao L, Nowak TS Jr., 2006. Selective, reversible occlusion of the middle cerebral artery in rats by an intraluminal approach. Optimized filament design and methodology. J Neurosci Methods 156, 76–83. [DOI] [PubMed] [Google Scholar]
- Michalski D, Hartig W, Krugel K, Edwards RH, Boddener M, Bohme L, Pannicke T, Reichenbach A, Grosche A, 2013. Region-specific expression of vesicular glutamate and GABA transporters under various ischaemic conditions in mouse forebrain and retina. Neuroscience 231, 328–344. [DOI] [PubMed] [Google Scholar]
- Mosinger JL, Olney JW, 1989. Photothrombosis-induced ischemic neuronal degeneration in the rat retina. Exp Neurol 105, 110–113. [DOI] [PubMed] [Google Scholar]
- Ogishima H, Nakamura S, Nakanishi T, Imai S, Kakino M, Ishizuka F, Tsuruma K, Shimazawa M, Hara H, 2011. Ligation of the pterygopalatine and external carotid arteries induces ischemic damage in the murine retina. Investigative ophthalmology & visual science 52, 9710–9720. [DOI] [PubMed] [Google Scholar]
- Oliff HS, Weber E, Eilon G, Marek P, 1995. The role of strain/vendor differences on the outcome of focal ischemia induced by intraluminal middle cerebral artery occlusion in the rat. Brain Res 675, 20–26. [DOI] [PubMed] [Google Scholar]
- Otori Y, Shimada S, Tanaka K, Ishimoto I, Tano Y, Tohyama M, 1994. Marked increase in glutamate-aspartate transporter (GLAST/GluT-1) mRNA following transient retinal ischemia. Brain Res Mol Brain Res 27, 310–314. [DOI] [PubMed] [Google Scholar]
- Shabanzadeh AP, D’Onofrio PM, Monnier PP, Koeberle PD, 2018. Neurosurgical Modeling of Retinal Ischemia-Reperfusion Injury. J Stroke Cerebrovasc Dis 27, 845–856. [DOI] [PubMed] [Google Scholar]
- Silva PS, Dela Cruz AJ, Ledesma MG, van Hemert J, Radwan A, Cavallerano JD, Aiello LM, Sun JK, Aiello LP, 2015. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 122, 2465–2472. [DOI] [PubMed] [Google Scholar]
- Slakter JS, Spertus AD, Weissman SS, Henkind P, 1984. An experimental model of carotid artery occlusive disease. American journal of ophthalmology 97, 168–172. [DOI] [PubMed] [Google Scholar]
- Steele EC Jr., Guo Q, Namura S, 2008. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke 39, 2099–2104. [DOI] [PubMed] [Google Scholar]
- Stefansson E, Wilson CA, Schoen T, Kuwabara T, 1988. Experimental ischemia induces cell mitosis in the adult rat retina. Investigative ophthalmology & visual science 29, 1050–1055. [PubMed] [Google Scholar]
- Stevens WD, Fortin T, Pappas BA, 2002. Retinal and optic nerve degeneration after chronic carotid ligation: time course and role of light exposure. Stroke 33, 1107–1112. [DOI] [PubMed] [Google Scholar]
- Sutherland BA, Neuhaus AA, Couch Y, Balami JS, DeLuca GC, Hadley G, Harris SL, Grey AN, Buchan AM, 2016. The transient intraluminal filament middle cerebral artery occlusion model as a model of endovascular thrombectomy in stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 36, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu J, Hirano A, Levy D, Henkind P, 1984. Experimental bilateral carotid artery occlusion: a study of the optic nerve in the rat. Neuropathol Appl Neurobiol 10, 423–428. [DOI] [PubMed] [Google Scholar]
- Tamaki M, Kidoguchi K, Mizobe T, Koyama J, Kondoh T, Sakurai T, Kohmura E, Yokono K, Umetani K, 2006. Carotid artery occlusion and collateral circulation in C57Black/6J mice detected by synchrotron radiation microangiography. Kobe J Med Sci 52, 111–118. [PubMed] [Google Scholar]
- Wanek J, Teng PY, Albers J, Blair NP, Shahidi M, 2011. Inner retinal metabolic rate of oxygen by oxygen tension and blood flow imaging in rat. Biomedical optics express 2, 2562–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Schmidt-Kastner R, Hamasaki DI, Yamamoto H, Parel JM, 2006. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Experimental eye research 82, 767–779. [DOI] [PubMed] [Google Scholar]
- Zuo XL, Wu P, Ji AM, 2012. Nylon filament coated with paraffin for intraluminal permanent middle cerebral artery occlusion in rats. Neurosci Lett 519, 42–46. [DOI] [PubMed] [Google Scholar]


