Abstract
Congenital anomalies of external genitalia affect approximately 1 in 125 live male births. Development of the genital tubercle, the precursor of the penis and clitoris, is regulated by the urethral plate epithelium, an endodermal signaling center. Signaling activity of the urethral plate is mediated by Sonic hedgehog (SHH), which coordinates outgrowth and patterning of the genital tubercle by controlling cell cycle kinetics and expression of downstream genes. The mechanisms that govern Shh transcription in urethral plate cells are largely unknown. Here we show that deletion of Foxa1 and Foxa2 results in persistent cloaca, an incomplete separation of urinary, genital, and anorectal tracts, and severe hypospadias, a failure of urethral tubulogenesis. Loss of Foxa2 and only one copy of Foxa1 results in urethral fistula, an additional opening of the penile urethra. Foxa1/a2 participate in an autoregulatory feedback loop with Shh, in which FOXA1 and FOXA2 positively regulate transcription of Shh in the urethra, and SHH feeds back to negatively regulate Foxa1 and Foxa2 expression. These findings reveal novel roles for Foxa genes in development of the urethral tube and in division of the embryonic cloaca.
Keywords: Foxa1, Foxa2, Shh, Cloaca, External genitalia, Urethra, Hypospadias
1. Introduction
Most vertebrates possess a cloaca, a single chamber that functions as the common outlet for the gastrointestinal and genitourinary tracts. In therian mammals, however, the cloaca is a transitory embryonic structure that becomes partitioned into a ventral genitourinary sinus, which forms the lower bladder and urethra, and a dorsal anorectal sinus, which forms the rectum and anus. Defective division of the cloaca underlies some of the most severe human anorectal malformations, such as cloacal dysgenesis, in which the cloaca is unseptated and there is no external opening, and persistent cloaca, in which the urethra, rectum, and vagina are connected and share a single outlet (Warne et al., 2011; Williams et al., 2005; Winkler et al., 2012; Zhang et al., 2017).
Initiation of external genital development is marked by the emergence of paired genital swellings on either side of cloacal membrane, where cloacal endoderm abuts ventral body wall ectoderm (Perriton et al., 2002). The paired genital swellings merge to form the genital tubercle, the embryonic precursor of the penis and clitoris. The ventral wall of the cloacal epithelium, which is endodermal in origin, is incorporated into the genital tubercle, where it forms the bilaminar urethral plate epithelium. In males, the bilaminar urethral plate undergoes tubulogenesis and is internalized to form the penile urethra. In addition to forming the penile urethra, the urethral plate epithelium is a signaling center that has polarizing activity and promotes outgrowth of the genital tubercle (Murakami and Mizuno, 1986; Perriton et al., 2002).
Signaling activity of the urethral plate is mediated by Sonic hedgehog (SHH), and loss of function mutations in Shh result in failure of genital tubercle formation, congenital absence of the phallus, and persistent cloaca (Haraguchi et al., 2001; Perriton et al., 2002). SHH sustains outgrowth of the genital tubercle by regulating the duration of the cell cycle (Seifert et al., 2010), and is required for urethral tubulogenesis (Lin et al., 2009; Miyagawa et al., 2009; Seifert et al., 2009). Although a number of downstream targets of SHH have been identified in the genital tubercle, little is known about the regulation of Shh expression in urethral epithelial cells. Transcriptional profiling of mouse genital tubercle cells identified two forkhead box genes, Foxa1 and Foxa2, with enriched expression in the urethral plate epithelium at embryonic day (E) 12.5 (Armfield et al., 2016). Based on their co-localization with Shh and evidence that FOXA1 and FOXA2 can regulate Shh transcription in other organ systems (Gao et al., 2005; Ma et al., 2019; Maier et al., 2013; Mavromatakis et al., 2011; Wan et al., 2005), we tested the hypothesis that these two transcription factors are required for development of the external genitalia in mice.
Here we report that Foxa1 and Foxa2 have essential and functionally redundant roles in external genital and cloacal morphogenesis. Although deletion of either Foxa1 or Foxa2 alone does not affect external genital development, loss of both transcription factors results in severe anogenital anomalies. Mice lacking Foxa1 and Foxa2 develop persistent cloaca, which results from incomplete separation of the genitourinary and anorectal tracts, and severe hypospadias due to failure of urethral tubulogenesis. We also find that retention of a single copy of Foxa1 in the absence of FOXA2 is sufficient for division of the cloaca but not for normal urethral tube formation. Molecular analyses of mutants revealed an autoregulatory loop between Foxa1/a2 and Shh; deletion of Foxa1 and Foxa2 leads to downregulation of Shh expression in urethral epithelial cells, whereas loss of SHH causes urethral cells to upregulate Foxa1 and Foxa2. Together these findings reveal novel roles for Foxa1 and Foxa2 in mammalian urogenital and anorectal development.
2. Materials and methods
2.1. Transgenic embryo generation and collection
Mouse strains with the Foxa1lacZ (Kaestner et al., 1999), Foxa2flox (Sund et al., 2000), Rosa26lacZ (Soriano, 1999), ShhCreERt2 (Harfe et al., 2004), and ShhC (Dassule et al., 2000) transgenes have been previously described. Timed matings were generated by crossing male mice carrying Cre and one floxed allele to females homozygous for the same floxed allele. Pregnant dams were administered tamoxifen dissolved in corn oil by oral gavage at 100 mg/kg at E10.5 to induce recombination by E11.5. Genotypes and sex were determined by standard PCR of DNA isolated from tail biopsies taken from individual embryos at euthanasia. For lineage studies, Rosa26lacZ expression, which labels cells with β-galactosidase, and deletion of conditional Foxa2 alleles were simultaneously induced by tamoxifen administration. Male mutants were analyzed for penile and cloacal defects.
2.2. Lineage tracing, histology, in situ hybridization, and quantitative real-time PCR
β-galactosidase activity as detected by X-gal staining (Hogan et al., 1994), paraffin histology with hematoxylin and eosin staining (Gredler et al., 2015), whole mount in situ hybridization (Nieto et al., 1996), and qRT-PCR (Gredler et al., 2015) were performed according to published methods.
3. Results
3.1. Foxa1 and Foxa2 are expressed in the developing cloacal and urethral epithelia
To determine the roles of Foxa1 and Foxa2 in external genital development, we began by mapping their spatial and temporal expression patterns from E10.5 (when there is a single, undivided cloacal sinus and no genital tubercle) through E14 (after division of urogenital and anorectal sinuses and before sexual differentiation of the genital tubercle). Foxa1 transcripts were evident throughout the cloacal epithelium of E10.5 embryos (Fig. 1A). At E11.5 and E12.5, Foxa1 expression was detected in a contiguous domain that included cloacal and urethral plate epithelia (Fig. 1A). The expression patterns of Foxa2 were similar to those of Foxa1 at these stages (Fig. 1B). Foxa1 and Foxa2 expression persisted in the urethral epithelium through E13.5 and E14, when the urogenital sinus has separated from the hindgut. Neither Foxa1 nor Foxa2 were detected in the genital mesenchyme or ectoderm at any stages investigated (Fig. 1A and B).
Fig. 1. Foxa1 and Foxa2 in urorectal development.
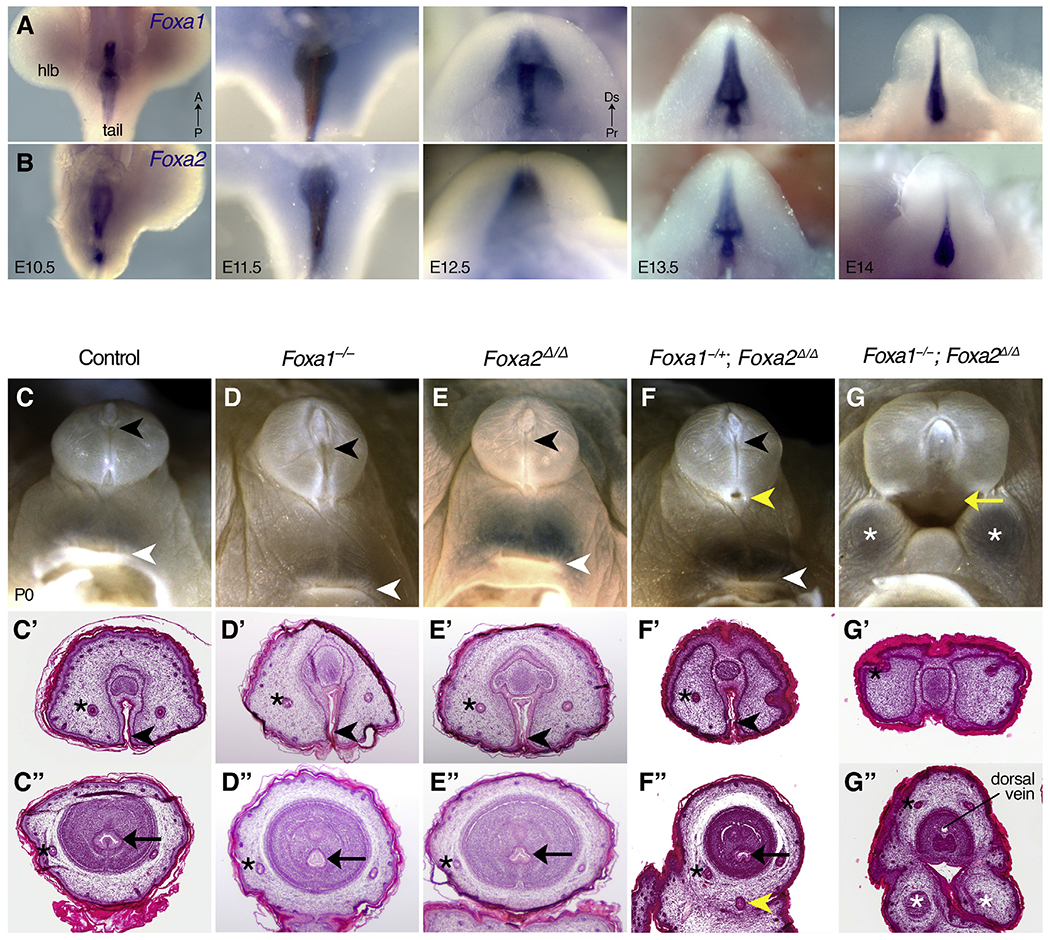
(A, B) Whole mount in situ hybridization on wild type mouse embryos shows that Foxa1 (A) and Foxa2 (B) are expressed throughout the cloacal epithelium and the urethral plate epithelium of the genital tubercle. (C-G″) Light micrographs (C–G) and histological sections through the distal (C′-G′) and proximal (C″-G″) penises of control (C), Foxa1−/− (D), Foxa2Δ/Δ (E), Foxa1−/+; Foxa2Δ/Δ (F), and Foxa1−/−; Foxa2Δ/Δ (G) newborn males show that Foxa1−/+; Foxa2Δ/Δ mice have hypospadias (yellow arrowhead in F, F″) and Foxa2−/+; Foxa2Δ/Δ mice have persistent cloaca (yellow arrow in G, G″) with bifid scrotum (white asterisks in G, G″). A distal urethral meatus (black arrowhead), internal penile urethra (black arrow), and anus (white arrowhead) are visible in each genotype except the Foxa1−/−; Foxa2Δ/Δ mutants. Preputial glands (black asterisks) are annotated for reference. Note that the lumen visible inside the Foxa1−/−; Foxa2Δ/Δ penis in (G″) is the dorsal vein. hlb: hindlimb bud.
3.2. Foxa1 and Foxa2 are required for development of the urethral tube and division of the embryonic cloaca
Based on the expression patterns of Foxa1 and Foxa2 in embryonic urorectal tissues, we asked whether these factors are required for cloacal, urethral, and/or anorectal morphogenesis. Single and compound mutants were generated using a Foxa1 null allele (Kaestner et al., 1999), designated Foxa1−, and a floxed allele of Foxa2 (Sund et al., 2000) – designated as Foxa2flox before recombination and Foxa2Δ after deletion by the tamoxifen-inducible ShhCreERt2 allele (Harfe et al., 2004) – to circumvent the early embryonic lethality of Foxa2 knockouts (Weinstein, 1994). Phenotyping was conducted at E18.5.
Penile morphology of Foxa1−/− mutants and Foxa2Δ/Δ mutants appeared normal relative to control mice, with a single urethral meatus at the distal tip of the penis, a circumferentially contiguous prepuce, and a well-developed perineum separating the external genitalia from the anus (Fig. 1C–E). Histological analysis revealed that the cellular architecture of the penis in Foxa1−/− mutants and Foxa2Δ/Δ mutants was similar to that of control mice; the urethral meatus was evident in the distal penis, and the penile shaft contained three concentric tissue layers: the prepuce surrounded the glans, which, in turn, enveloped the internal penile urethra (Fig. 1C’–E″).
In a Foxa2Δ/Δ mutant background, loss of one Foxa1 allele (Foxa1−/+; Foxa2Δ/Δ disrupted urethral tubulogenesis but not cloacal septation (n = 7). The number, position and size of the urethral openings varied in Foxa1−/+; Foxa2Δ/Δ mutants; the most severe urethral malformation was development of a secondary urethral meatus at the base of the penis, which formed in addition to the normal urethral opening at the distal tip (Fig. 1F). Histological analysis confirmed the presence of an ectopic urethral duct in the ventral prepuce (Fig. 1F”). Sagittal sections revealed that both the normal distal meatus and the ectopic urethral duct were connected to the primary urethral tube in Foxa1−/+; Foxa2Δ/Δ mutants (Fig. 2B, compare with control in Fig. 2A). Thus, the ectopic urethral opening observed on the ventral surface of the penis is the meatus of a congenital urethral fistula. In other Foxa1−/+; Foxa2Δ/Δ mutants, the urethral meatus was either normal (Figs. S1A–D) or it was expanded (Fig. S1E) or displaced (Fig. S1F) along the ventral side of the penis. Despite these urethral anomalies, a male-typical perineum and distinct anorectal canal were present in Foxa1−/+; Foxa2Δ/Δ mutants, indicating that the cloaca had undergone normal division (Fig. 2B, E). In contrast to the genital phenotype found in Foxa1−/+; Foxa2Δ/Δ mutants, conditional deletion of a single copy of Foxa2 in a Foxa1 homozygous mutant background (Foxa1−/−; Foxa2+/Δ) had no discernible effect on external genital morphology (data not shown).
Fig. 2. Defects in urethral tubulogenesis and cloacal septation in Foxa1/a2 compound mutants.
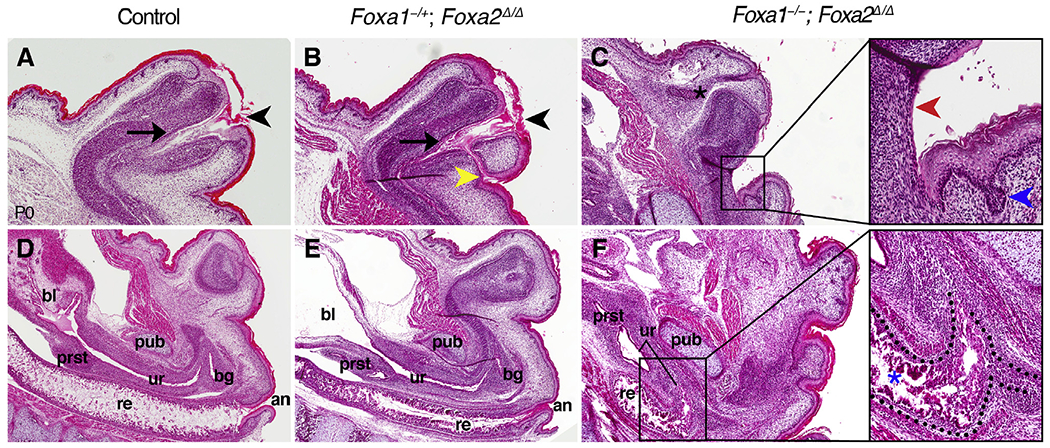
(A-C) Sagittal sections of the penis at P0 show that the penile urethra (black arrow) terminates at the definitive urethral meatus (black arrowhead) in controls (A) and Foxa1−/+; Foxa2Δ/Δ mutants (B). The ectopic urethral opening of Foxa1−/+; Foxa2Δ/Δ mutants connects to the glandar urethra (yellow arrowhead in B). In the absence of a penile urethra, Foxa1−/−; Foxa2Δ/Δ mutants (C) have a cloacal opening composed of a thin, discontinuous epithelium on the ventral side (red arrowheads, inset) and a robust, stratified, and glandular epithelium on the dorsal side (blue arrowheads, inset). Note the preputial gland (asterisk in C). (D-F) Sections through the pelvis demonstrate that the pelvic urethra is connected to the bladder (bl), prostate (prst), and bulbourethral gland (bg) but is separate from the rectum (re) and anus (an) in control (D) and Foxa1−/+; Foxa2Δ/Δ mutant (E) mice. A rectourethral fistula is visible as a connection between the urogenital and anorectal tracts at the level of the pubis (pub) in Foxa1−/−; Foxa2Δ/Δ mutants (F), and indicates failed cloacal septation. The internal common cloacal chamber of Foxa1−/−; Foxa2Δ/Δ mutants (dotted line in inset) is lined by epithelial crypts dorsally (blue asterisk) and a thin, fragmented epithelium ventrally.
Deletion of both copies of Foxa1 and Foxa2 (Foxa1−/−; Foxa2Δ/Δ) resulted in failure of penile urethra development altogether (compare control in Fig. 1A with double mutant in Fig. 1G). Agenesis of the ventral prepuce exposed an open urethral groove that extended along the entire ventral side of the penis (Fig. 1G’–G″). Foxa1−/−; Foxa2Δ/Δ mutants also developed persistent cloaca (n = 5/6), which ranged in severity from a small opening at the base of the penis to a large cloacal orifice that occurred in conjunction with hypospadias and bifid scrotum (Fig. 1G; Figs. S1G and H). Anterior to the cloacal outlet, a rectourethral fistula was evident; the pelvic urethra and the rectum connected to a single cloacal chamber at the level of the pubis (Fig. 2F).
Since cloacal or external genital anomalies were not detected in either the Foxa1 or the Foxa2 homozygous mutants, the presence of anogenital malformations in compound Foxa1/a2 mutant mice indicates that Foxa1 and Foxa2 function redundantly in urorectal development. The difference in severity of anogenital malformations seen in Foxa1−/+; Foxa2Δ/Δ versus Foxa1−/−; Foxa2Δ/Δ mutants (Foxa1−/+; Foxa2Δ/Δ mice developed urethral fistula, hypospadias, and/or persistent cloaca, whereas Foxa1−/−; Foxa2Δ/Δ mice always have persistent cloaca) demonstrates that, in the absence of Foxa2, a single Foxa1 allele can rescue cloacal division but not urethral morphogenesis.
3.3. Foxa1 and Foxa2 positively regulate Shh expression in the urethral epithelium
In other organ systems, Foxa transcription factors can function as upstream regulators of Shh and/or as downstream targets of hedgehog signaling (Gao et al., 2005;Jeong and Epstein, 2003; Maier et al., 2013; Mavromatakis et al., 2011; Wan et al., 2005). Co-expression of Foxa1, Foxa2, and Shh in cloacal and urethral epithelial cells, along with the similarity of cloacal and external genital defects observed in Foxa1/a2 mutants and Shh conditional mutants (Lin et al., 2009; Miyagawa et al., 2009; Seifert et al., 2009), led us to hypothesize that the Foxa1/a2 mutant phenotypes could be the result of disrupted Shh signaling. To test this hypothesis, we examined Shh expression in Foxa1/a2 mutant external genitalia by in situ hybridization and quantitative real-time PCR (qRT-PCR). At E13.5, Shh transcripts were detected in the urethral epithelium of control, Foxa1−/− and Foxa2Δ/Δ mice, but Shh staining in the urethra was weaker in Foxa1−/+; Foxa2Δ/Δ mice and was extremely faint in Foxa1−/−; Foxa2Δ/Δ mutants (Fig. 3A). Expression of Ptch1 (a transcriptional target of Shh signaling and a marker of SHH-responding cells) in genital mesenchyme adjacent to the urethra indicated that although hedgehog signaling may be diminished in Foxa1/a2 compound mutants, it was not completely eliminated (Fig. 3B). Note that Shh and Ptch1 expression appeared normal in the nascent preputial glands (Fig. 3A and B), which are not sites of Foxa1 or Foxa2 expression.
Fig. 3. Regulatory interactions between Foxa1/a2 and Shh in the genital tubercle.
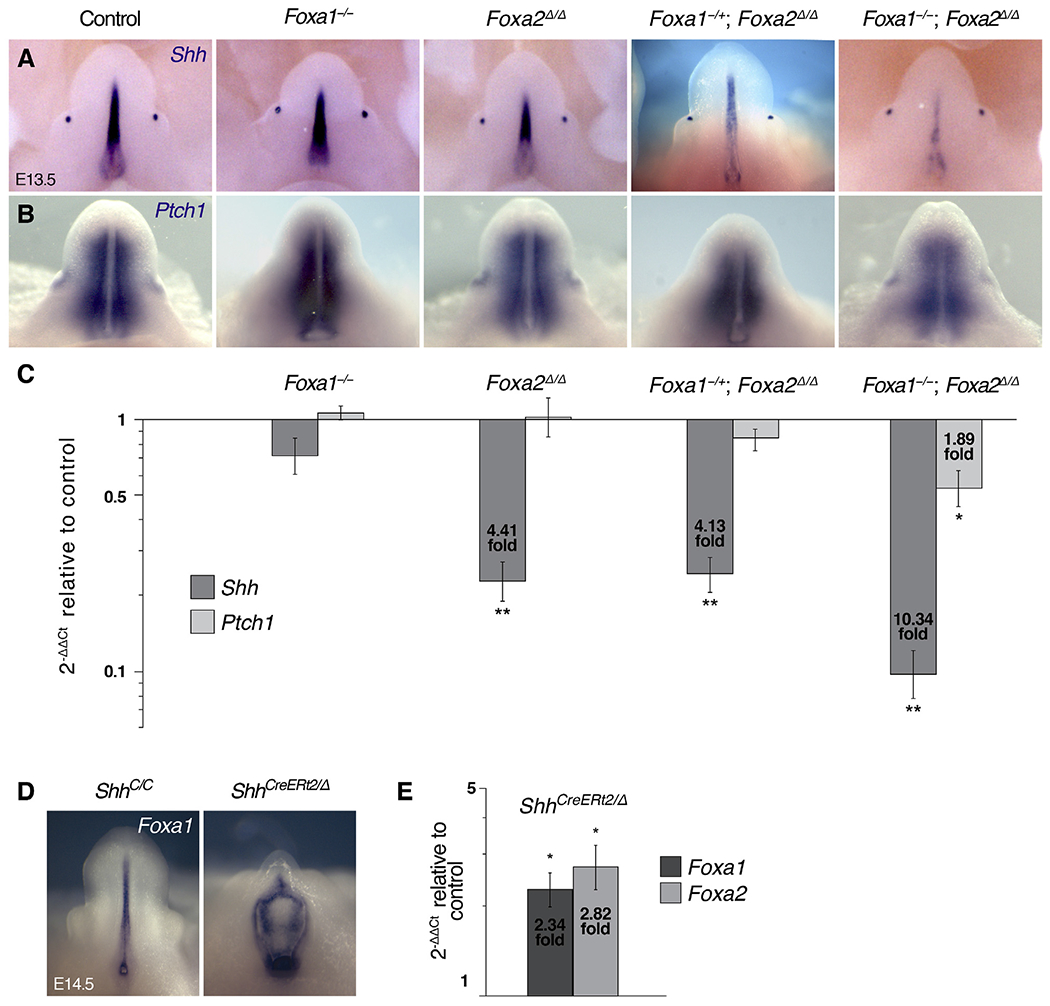
(A, B) Whole mount in situ hybridization on E13.5 single and compound Foxa1/a2 mutants showing expression of Shh (A) in the urethral epithelium and Ptch1 (B) in the genital mesenchyme and adjacent to the preputial glands. (C) qRT-PCR performed on E14.5 whole genital tubercles shows significant reductions in Shh transcript levels in Foxa1−/−; Foxa2Δ/Δ (10.34 fold), Foxa1−/+; Foxa2Δ/Δ (4.13 fold), and Foxa2Δ/Δ (4.41 fold) mutants, and decreased Ptch1 in Foxa1−/−; Foxa2Δ/Δ mutants (1.89 fold). (D) Whole mount in situ hybridization on E14.5 ShhCreERT2/Δ embryos shows abundant Foxa1 transcript in the exposed urethral epithelium of control (ShhC/C) and shhCreERT2/Δ mutant genital tubercles. (E) qRT-PCR analysis of Foxa1 and Foxa2 performed on E14.5 whole genital tubercles shows significantly increased levels of Foxa1 (2.34 fold) and Foxa2 (2.82 fold) in ShhCreERT2/Δ embryos relative to controls.
Quantitative analysis of Shh and Ptch1 mRNA in Foxa1/a2 mutant genital tubercles at E14.5 showed that Foxa1−/−; Foxa2Δ/Δ mutants underwent the most severe reduction in Shh expression (10.34 fold decrease) and that Ptch1 expression was also significantly diminished (1.89 fold decrease, Fig. 3C). Shh expression levels were also significantly reduced in Foxa2Δ/Δ (4.41 fold decrease) and Foxa1−/+; Foxa2Δ/Δ mutants (4.13 fold decrease), although no significant changes in Ptch1 were detected in these allele combinations (Fig. 3C). Neither Shh nor Ptch1 expression levels were significantly affected in Foxa1−/− mutants (Fig. 3C). These data indicate that FOXA1 and FOXA2 are positive regulators of Shh transcription in urethral epithelial cells.
To determine whether SHH feeds back to regulate Foxa1 and/or Foxa2, we conditionally deleted Shh at E10.5 and examined Foxa1 and Foxa2 in the genital tubercles of ShhCreERT2/Δ mutants at E14.5. Foxa1 expression was detected in the urethral and cloacal epithelia of controls and ShhCreERT2/Δ mutants by in situ hybridization (Fig. 3D), and qRT-PCR revealed significant increases in both Foxa1 (2.7 fold) and Foxa2 (3.0 fold) transcripts in the Shh mutant genital tubercles compared to controls (Fig. 3E). These results show that loss of SHH results in upregulation of Foxa1 and Foxa2 in the genital tubercle, suggesting that SHH is a negative regulator of Foxa1 and Foxa2 in during external genital development.
3.4. The persistent cloaca in Foxa1/a2 double mutants comprises two distinct epithelia
Given the failure of cloacal division in Foxa1−/−; Foxa2Δ/Δ mutants, we asked whether the epithelial lining of the persistent cloacal duct showed evidence of urethral or anorectal differentiation. In newborn controls, the penile urethra is a complex, stratified epithelium (Fig. 4A), the pelvic urethra is a transitional epithelium (Fig. 4B, top inset), and the anus is a stratified, squamous epithelium with extensive cornified keratinocytes in the stratum corneum (Fig. 4B, bottom inset). Sagittal sections through Foxa1−/−; Foxa2Δ/Δ mutants showed that the ventral wall of the cloacal opening (at the base of the penis) was lined by a thin, discontinuous epithelium, whereas the dorsal side of the cloaca was lined by a robust, stratified, and glandular epithelium (Fig. 2C and inset). The dorsal side of the common cloacal chamber had epithelial crypts that resembled the rectal epithelium of control mice, and the ventral side of the chamber was lined by a fragmented, poorly organized epithelium (Fig. 2G).
Fig. 4. Epithelial differentiation and cell lineage in the Foxa1−/−; Foxa2Δ/Δ cloaca.
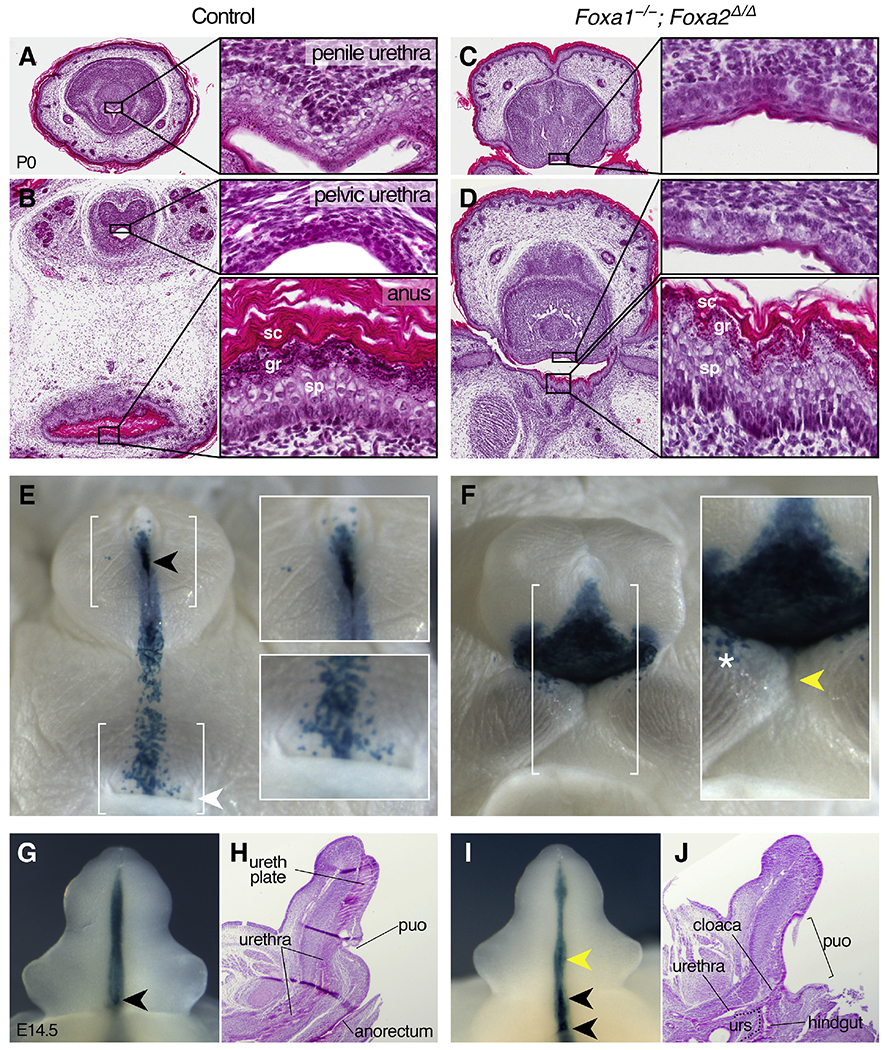
(A-D) Transverse sections of male control and Foxa1−/−; Foxa2Δ/Δ mutant urorectal epithelia at P0. In controls, the penile urethra (A) is a complex, stratified epithelium with columnar basal cells, round and vacuolar intermediate cells, intermittent keratohyalin granules, and minimal keratin fibers. (B) The control pelvic urethra is a transitional epithelium composed of densely packed cuboidal and rounded cells (top inset), and the anus is a stratified, squamous epithelium with elongate, cylindrical intermediate cells in the spinous layer (sp), abundant keratohyalin granules in the granular layer (gr), and extensive keratin in the stratum corneum (sc, bottom inset). In Foxa1−/−; Foxa2Δ/Δ mutants, both the ventral surface of the penis (C) and the ventral wall of the cloacal cavity (D, top inset) are lined by stratified, pseudocolumnar epithelia that do not resemble any region of the control urethral epithelia. The dorsal side of the cloacal duct (adjacent to the tail) in Foxa1−/−; Foxa2Δ/Δ mutants (D, bottom inset) is lined by a stratified, squamous epithelium that resembles the control anus. (E, F) Newborn X-gal stained, ShhCreERT2/+; Rosa26lacZ control and ShhCreERT2/+; Foxa1−/−; Foxa2Δ/Δ; Rosa26lacZ external genitalia reveal β-gal-positive cells in the urethral meatus (black arrowhead and top inset in E), along the perineal raphe, and on the ventral side of the anus (white arrowhead and bottom inset in E) in controls. Foxa1−/−; Foxa2Δ/Δ mutants have a triangular sheet of β-gal-positive cells on the ventral penile surface (F), isolated blue cells on the surface epithelium adjacent to the base of the penis (white asterisk), and βgal-negative cells on the dorsal side of the cloacal opening (yellow arrowhead). (G, I) X-gal staining of ShhCreERT2/+; Rosa26lacZ control and Foxa1−/−; Foxa2Δ/Δ external genitalia at E14.5 shows the presence of the endodermal (β-gal-positive) urethral plate and proximal urethral opening (black arrowheads). Note the stretched proximal urethral opening and ectopic urethral opening (yellow arrowhead in I). (H, J) Sagittal sections of E14.5 control and Foxa1−/−; Foxa2Δ/Δ external genitalia show failed cloacal septation in mutants. The urorectal septum (urs) is outlined in J. ureth plate: urethral plate, puo: proximal urethral opening/urethral duct.
In Foxa1−/−; Foxa2Δ/Δ mutants, both the ventral surface of the penis and the ventral wall of the cloacal cavity of were lined by stratified, pseudocolumnar epithelia that did not closely resemble any region of the control urethral epithelia (Fig. 4C and D, and top inset in D). However, the dorsal side of the cloacal duct (adjacent to the tail) had a prominent granular layer, keratinized stratum corneum, and columnar basal cells (Fig. 4D, bottom inset), similar to the anal epithelium of controls. Thus, although division of the embryonic cloaca and differentiation of ventral cloacal cells into urethral epithelium requires at least one functional Foxa1 allele, differentiation of the dorsal cloacal epithelium into rectal tissue can occur in the absence of Foxa1/a2 and does not require division of the cloaca.
Features of the Foxa1−/−; Foxa2Δ/Δ mutant cloacae, including polarized distribution of subepithelial glands and extensive maturation of the dorsal epithelium, prompted us to investigate the lineage of dorsal and ventral cloacal epithelia in Foxa1/a2 mutants. We used the Rosa26lacZ reporter (R26R, Soriano, 1999) to examine the contribution of ShhCreERT2-expressing endodermal cells to the open cloaca of Foxa1−/−; Foxa2Δ/Δ mutants. In control mice at P0, the ventral surface of the penis was largely unstained, with the exception of β-gal-positive cells in the urethral meatus and along the penile raphe (Fig. 4E). A stripe of β-gal--positive epithelial cells between the base of the penis and the anus marked the junction of the urorectal septum and the perineum; however, the lateral and dorsal sides of the anal orifice were β-gal-negative (Fig. 4E, insets). In Foxa1−/−; Foxa2Δ/Δ mutants, a triangular sheet of β-gal-positive tissue lined the sinus on the ventral penile surface (Fig. 4F). Isolated blue cells were detected on the surface epithelium immediately adjacent to the base of the penis, although it was unclear if these cells were remnants of perineum or urethral progenitor cells that had migrated into the ectodermal epithelium (Fig. 4F). While the ventral side of the Foxa1−/−; Foxa2Δ/Δ cloacal orifice (on the surface of the penis) was β-gal-positive, the dorsal side of the opening (on the tail side) was β-gal-negative, indicating that it is not derived from ShhCreERT2-expressing endoderm (Fig. 4F, inset). These data suggest that the epithelial lining of the external cloacal opening of Foxa1−/−; Foxa2Δ/Δ mutants is of mixed embryonic origin.
3.5. Cloacal division requires Foxa1 and Foxa2 activity at mid-gestational stages
Cell lineage and histological analysis also provided insight into the ontogeny of the urorectal defects in Foxa1−/−; Foxa2Δ/Δ mice. In control genital tubercles at E14.5, a ventral stripe of β-gal-positive cells marked the endodermal urethral plate, and the proximal urethral opening was visible as a β-gal-positive orifice (Fig. 4G). Foxa1−/−; Foxa2Δ/Δ mutant genital tubercles showed a similar ventral stripe of β-gal-positive cells, but an ectopic opening was evident at the level of the preputial swellings, and the proximal urethral opening extended from the base of the genital tubercle to the labioscrotal swellings (Fig. 4I). Histological sections of E14.5 control embryos revealed distinct urogenital and anorectal orifices, and the proximal urethral opening (urethral duct) was situated at the base of the phallus, where the internal pelvic urethra was connected to the penile urethral plate (Fig. 4H). By contrast, in Foxa1−/−; Foxa2Δ/Δ mutants, the urogenital and anorectal sinuses converged into a common duct, which emptied into a single cloacal chamber that extended from the ventral side of the phallus to the tail (Fig. 4J). The position of this large cloacal sinus corresponds to the ectopic opening that was seen in X-gal stained double mutants (compare Fig. 4F with 4J). Persistence of a common urorectal (cloacal) chamber in Foxa1−/−; Foxa2Δ/Δ embryos is consistent with a role for Foxa1 and Foxa2 in division of the embryonic cloaca into separate genitourinary and anorectal sinuses.
4. Discussion
Our data implicate Foxa1 and Foxa2 in development of the urethral tube and division of the cloaca and show that Foxa1 and Foxa2 regulate Shh transcription and respond to SHH in the genital tubercle. The secondary opening of the urethra in Foxa1−/−; Foxa2Δ/Δ mutants resembles a human malformation known as congenital urethral fistula (Karnak et al., 1995; Mukhopadhyay et al., 2011; Ritchey et al., 1994). Previous studies have proposed several mechanisms for displacement of the ventrally tethered urethral plate to form the internal urethral tube, including growth of the urorectal septum mesenchyme into the genital tubercle (Seifert et al., 2008) and lateral-to-medial growth of the genital mesenchyme (Hynes and Fraher, 2004). In Foxa1−/−; Foxa2Δ/Δ males, the presence of an ectopic urethral opening in conjunction with a distal urethral meatus suggests that urethral internalization is at least partially driven by lateral-medial tissue movements, since proximodistal migration of the urorectal septum alone cannot account for the presence of genital mesenchyme between the ectopic and normal urethral opening.
Embryonic cloacal epithelium lacks morphological distinction along the dorsoventral axis, indicating that differentiation of the cloacal endoderm into functional urethral and rectal epithelia occurs after physical separation of the two tracts. The relationship between structural division of the cloaca and differentiation of appropriate tissue types on the dorsal (anorectal) and ventral (genitourinary) sides is poorly understood. Despite absence of distinct urogenital and anorectal orifices in Foxa1−/−; Foxa2Δ/Δ mutants, we found evidence for dorsoventral differentiation of the external cloacal opening. Given the dissimilarity between the cloacal epithelial cells of mutants and the pelvic urethral, penile urethral, anal, and rectal epithelia of normal mice, we conclude that the ventral cloacal epithelium of Foxa1−/−; Foxa2Δ/Δ mutants resembles the indeterminate epithelium that has been described for abnormal cloacal chambers in both mice and humans (Runck et al., 2014). The proctodeum, which normally forms the ectodermal component of the anus (Morgan, 1936; Yamaguchi et al., 2008), differentiates into properly patterned anal epithelium in Foxa1−/−; Foxa2Δ/Δ mutants, despite its connection to an undivided cloaca. Thus, in contrast to the endodermally-derived rectal epithelium, differentiation of the ectodermally-derived epithelium of the posterior anal canal does not require FOXA1/A2, nor is it dependent upon cloacal division or differentiation of the adjacent endodermal lineage. While it is possible that deletion of Foxa2 could lead to downregulation of the Shh allele that contains cre, and diminished cre would affect later activation of the reporter in the endodermal lineage, absence of LacZ mosaicism suggests that the early activation of LacZ in cloacal endoderm labels the entire lineage. Therefore, we interpret the absence of LacZ-positive cells in the dorsal cloacal epithelium of Foxa1−/−; Foxa2Δ/Δ mutants, together with its structural similarity to the control anus, as evidence that these cells have the same identity as the ectodermally-derived posterior anal canal. An ectodermal origin could account for the ability of these cells to differentiate appropriately when Foxa1 and Foxa2 are deleted from the endodermal lineage.
Regulatory interactions among FOXA transcription factors and the hedgehog signaling pathway are abundant in organogenesis. Positive regulation of Shh by FOXA1/A2 proteins has been reported in the notochord (Maier et al., 2013), brain (Mavromatakis et al., 2011), and lung (Wan et al., 2005), whereas FOXA1 negatively regulates Shh in the developing prostate (Gao et al., 2005). We find that deletion of Foxa1 and Foxa2 causes significant reduction of Shh transcription in the urethral epithelium of the genital tubercle, indicating that FOXA1 and FOXA2 act upstream of Shh to promote its expression. Reciprocal signaling was recently reported in antler chondrocytes, where SHH acts upstream as a positive regulator of Foxa gene expression (Ma et al., 2019). By contrast, our finding that deletion of Shh from the urethral plate epithelium results in upregulation of Foxa1 and Foxa2 suggests that SHH negatively regulates expression of Foxa genes in the genital tubercle. This could reflect a mechanism that maintains a steady state of Shh expression in the genital tubercle, in which SHH controls the activity of own transcriptional activators. Accordingly, a reduction in SHH signaling triggers upregulation of Foxa1 and Foxa2 to increase transcription of Shh. It is noteworthy that Ptch1 has been detected in genital tubercle mesenchyme adjacent to the urethral epithelium, but not in the urethral epithelium itself (Perriton et al., 2002). Thus, it is unlikely that Foxa1 and Foxa2 are direct targets of the SHH signal transduction pathway. Instead, the negative regulation of Foxa1/a2 by SHH could reflect a relay mechanism that is mediated by adjacent mesenchymal cells.
Our finding that Foxa2 deletion results in significantly diminished Shh expression but does not disrupt anorectal or external genital development suggests that even low levels of Shh transcript are sufficient to drive cloacal division and urethral tubulogenesis. It is intriguing that removal of one Foxa1 allele in a Foxa2 null background disrupts the Hh pathway in a manner that is quantitatively similar to that observed after loss Foxa2Δ/Δ alone, yet only the former perturbs urethral tubulogenesis. These data suggest that Shh is not the only FOXA target with a role in urethral tubulogenesis.
The discovery that loss of Foxa2 results in a marked reduction in Shh mRNA but loss of Foxa1 has no significant effect on Shh transcription indicates that Foxa1 and Foxa2 are not interchangeable in the context of genital development. Non-equivalence of FOXA1 and FOXA2 is further demonstrated by the development of a urethral malformation in Foxa1−/+; Foxa2Δ/Δ mutants but not in Foxa1−/−; Foxa2+/Δ mutants. which could reflect the difference in the ability of FOXA1 and FOXA2 to regulate Shh. These results are consistent with previous reports that FOXA2 is a more potent regulator of pancreas development than FOXA1, and that these proteins have different binding affinities for various cis-regulatory elements and promoters (Gao et al., 2008; Lai et al., 1991).
Cloacal septation is a sexually monomorphic process that occurs in both male and female embryos, whereas urethral tubulogenesis is a sexually dimorphic process mediated by sex hormones (Glenister, 1954; Seifert et al., 2009; Yucel et al., 2003; Zheng et al., 2015). FOXA1/A2 have been shown to interact with hormone receptors in multiple contexts; for example, sexual dimorphism of hepatocellular carcinoma is driven by differential regulation of Foxa1/a2 and their targets by estrogen and androgen (Li et al., 2012). Our results demonstrate that Foxa1 and Foxa2 are required both for early/sexually monomorphic and late/sexually dimorphic anogenital development. Finally, this study raises the possibility that division of the cloaca into separate urogenital and anorectal tracts and specification of the cell types that comprise these organs might be controlled by distinct developmental processes.
Supplementary Material
Acknowledgments
We thank Emily Merton, Rikesh Patel, Brittany Maillet, Stacey Gray, and Shelby Frantz for assistance and Klaus Kaestner for providing the Foxa1− and Foxa2flox mouse lines. M.L.G. was supported by a UF Alumni Fellowship. S.E.P. was supported by a Ruth L. Kirschstein National Research Service Award (F32 HD062164). This study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK110408) and the National Institute of Environmental Health Sciences (R01-ES017099) to M.J.C.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ydbio.2020.06.009.
References
- Armfield BA, Seifert AW, Zheng Z, Merton EM, Rock JR, Lopez MC, Baker HV, Cohn MJ, 2016. Molecular characterization of the genital organizer: gene expression profile of the mouse urethral plate epithelium. J. Urol 196, 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP, 2000. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, 4775–4785. [DOI] [PubMed] [Google Scholar]
- Gao N, Ishii K, Mirosevich J, Kuwajima S, Oppenheimer SR, Roberts RL, Jiang M, Yu X, Shappell SB, Caprioli RM, Stoffel M, Hayward SW, Matusik RJ, 2005. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development 132, 3431–3443. [DOI] [PubMed] [Google Scholar]
- Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH, 2008. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 22, 3435–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenister TW, 1954. The origin and fate of the urethral plate in man. J. Anat 88, 413–425. [PMC free article] [PubMed] [Google Scholar]
- Gredler ML, Seifert AW, Cohn MJ, 2015. Tissue-specific roles of Fgfr2 in development of the external genitalia. Development 142, 2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G, 2001. Unique functions of Sonic hedgehog signaling during external genitalia development. Development 128, 4241–4250. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ, 2004. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517–528. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E, 1994. Manipulating the Mouse Embryo: A Laboratory Manual, 2 ed. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- Hynes PJ, Fraher JP, 2004. The development of the male genitourinary system: III. The formation of the spongiose and glandar urethra. Br. J. Plast. Surg 57 (3), 203–214. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Epstein DJ, 2003. Distinct regulators of Shh transcription in the floor plate and notochord indicate separate origins for these tissues in the mouse node. Development 130, 3891–3902. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Katz J, Liu Y, Drucker DJ, Schtz G, 1999. Inactivation of the winged helix transcription factor HNF3$\alpha$ affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev. 13, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnak I, Tanyel FC, Hisnmez A, 1995. Congenital urethrocutaneous fistula: a case report and literature review, with a nomenclature proposal. J. Pediatr. Surg 30. [DOI] [PubMed] [Google Scholar]
- Lai E, Prezioso VR, Tao WF, Chen WS, Darnell JE Jr., 1991. Hepatocyte nuclear factor 3 alpha belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes Dev. 5, 416–427. [DOI] [PubMed] [Google Scholar]
- Li Z, Tuteja G, Schug J, Kaestner KH, 2012. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell 148, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L, 2009. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development 136, 3959–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Duan CC, Yang ZQ, Ding JL, Liu S, Yue ZP, Guo B, 2019. Crosstalk between Activin A and Shh signaling contributes to the proliferation and differentiation of antler chondrocytes. Bone 123, 176–188. [DOI] [PubMed] [Google Scholar]
- Maier J.a., Lo Y, Harfe BD, 2013. Foxa1 and Foxa2 are required for formation of the intervertebral discs. PloS One 8, e55528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavromatakis YE, Lin W, Metzakopian E, Ferri ALM, Yan CH, Sasaki H, Whisett J, Ang SL, 2011. Foxa1 and Foxa2 positively and negatively regulate Shh signalling to specify ventral midbrain progenitor identity. Mech. Dev 128, 90–103. [DOI] [PubMed] [Google Scholar]
- Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, Nakahara C, Suzuki K, Matsumaru D, Kaneko T, Matsuo I, Yang L, Taketo MM, Iguchi T, Evans SM, Yamada G, 2009. Dosage-dependent hedgehog signals integrated with Wnt/betacatenin signaling regulate external genitalia formation as an appendicular program. Development 136, 3969–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CN, 1936. The surgical anatomy of the anal canal and rectum. Postgrad. Med 12, 287–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay B, Mandal K, Barman S, Shukla R, 2011. Congenital penile urethrocutaneous fistula: a rare anomaly and review of literature. Urol. Ann 3, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami R, Mizuno T, 1986. Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. J. Embryol. Exp. Morphol 92, 133–143. [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG, 1996. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 51, 219–235. [DOI] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ, 2002. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev. Biol 247, 26–46. [DOI] [PubMed] [Google Scholar]
- Ritchey ML, Sinha A, Argueso L, 1994. Congenital fistula of the penile urethra. J. Urol 151, 1061–1062. [DOI] [PubMed] [Google Scholar]
- Runck LA, Method A, Bischoff A, Levitt M, Pena A, Collins MH, Gupta A, Shanmukhappa S, Wells JM, Guasch G, 2014. Defining the molecular pathologies in cloaca malformation: similarities between mouse and human. DMM Dis. Model. Mechan 7, 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Bouldin CM, Choi KS, Harfe BD, Cohn MJ, 2009. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development 136, 3949–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, Cohn MJ, 2008. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev. Biol 318 (1), 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Zheng Z, Ormerod BK, Cohn MJ, 2010. Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat. Commun 1, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P, 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet 21, 70–71. [DOI] [PubMed] [Google Scholar]
- Sund NJ, Ang SL, Sackett SD, Shen W, Daigle N, Magnuson M.a., Kaestner KH, 2000. Hepatocyte nuclear factor 3beta (Foxa2) is dispensable for maintaining the differentiated state of the adult hepatocyte. Mol. Cell Biol 20, 5175–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett J.a., 2005. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J. Biol. Chem 280, 13809–13816. [DOI] [PubMed] [Google Scholar]
- Warne SA, Hiorns MP, Curry J, Mushtaq I, 2011. Understanding cloacal anomalies. Arch. Dis. Child 96, 1072–1076. [DOI] [PubMed] [Google Scholar]
- Weinstein D.C.a., 1994. The winged-helix transcription factor HNF-3B is required for notochord development in the mouse embryo. Cell 78, 575–588. [DOI] [PubMed] [Google Scholar]
- Williams D.H.t., Fitchev P, Policarpio-Nicolas ML, Wang E, Brannigan RE, Crawford SE, 2005. Urorectal septum malformation sequence. Urology 66, 657. [DOI] [PubMed] [Google Scholar]
- Winkler NS, Kennedy AM, Woodward PJ, 2012. Cloacal malformation: embryology, anatomy, and prenatal imaging features. J. Ultrasound Med. : Off. J. Am. Inst. Ultrasound Med 31, 1843–1855. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Kiyokawa J, Akita K, 2008. Developmental processes and ectodermal contribution to the anal canal in mice. Ann. Anat 190, 119–128. [DOI] [PubMed] [Google Scholar]
- Yucel S, Cavalcanti AG, Desouza A, Wang Z, Baskin LS, 2003. The effect of oestrogen and testosterone on the urethral seam of the developing male mouse genital tubercle. BJU Int. 92, 1016–1021. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lee C, Lawson LY, Svete LJ, McIntyre LM, Harfe BD, 2017. SHH protein variance in the limb bud is constrained by feedback regulation and correlates with altered digit patterning. G3 (Bethesda) 7, 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Armfield BA, Cohn MJ, 2015. Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proc. Natl. Acad. Sci. Unit. States Am 112, E7194–E7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


