Introduction
Optimal cognitive function is of great importance for the lives of people with or without clinical cognitive impairment. Alzheimer’s disease (AD), is the most common cause of dementia and was first described more than 100 years ago (1). Today, AD burden is substantial with a prevalence of 40.2 cases per 1000 individuals over 60 years old (2). Mild cognitive impairment (MCI), especially its amnestic form, is often a transitory clinical entity that corresponds to the prodromal phase of AD (3). Despite decades of intense efforts and despite targeting multiple presumed pathogenic processes, no disease modifying treatment has been identified for MCI or AD (4). The only four currently approved medications are either acetylcholinesterase inhibitors enhancing cholinergic transmission (donepezil, rivastigmine, galantamine) or NMDA antagonist (memantine) and their effects are merely symptomatic. In this context, numerous natural products such as herbs, vitamins, antioxidants or naturally occurring compounds found in foods have been examined for their possible therapeutic use in MCI/AD (5-7) and, occasionally, for cognitive enhancement in healthy individuals (7-9).
The dried stigma of the plant Crocus Sativus L. is called saffron and is used in medicine, cosmetics and coloring industries (10). In animal studies, saffron was efficacious against various symptoms and disease processes including anxiety, insomnia, hyperglycemia, atherosclerosis, Parkinson’s disease, cancer and morphine withdrawal syndrome (11). In addition, human studies have suggested that saffron may have therapeutic effects in depression and AD, with a relatively favorable safety profile (11). In the present systematic review, we aim to elucidate the evidence for effects of oral saffron intake on cognitive function in cognitively impaired and non-impaired individuals (Table 1).
Table 1.
PICOS presentation
| Participants | Healthy or Mild Cognitive Impairment/Alzheimer’s Disease (MCI/AD) patients |
| Interventions | Oral saffron (Crocus L. Sativus) administration |
| Comparator | Placebo or any approved anti-AD drug or nothing |
| Outcome | Cognitive performance on standardized tests before/after the intervention |
| Study Design | Randomized controlled trials (RCTs) or cross-over RCTs |
Methods
For the conduction of the present systematic review, we adopted the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (12).
Eligibility criteria
In order to be included in the systematic review, a study had to follow the next eligibility criteria: (a) designed as randomized controlled trial (RCT) or cross-over RCT; (b) published in any language up to 11/12/2018.; (c) included humans only; (d) subjects were given saffron orally and compared to either placebo or any approved anti-AD drug (with no limitation in the dosing scheme or duration of intervention) or nothing; and (e) cognitive performance was evaluated through objective standardized tests before and after the intervention.
Information sources
We searched for relevant published studies, according to our eligibility criteria, in the next electronic databases: Medline, Science Direct and Cochrane Central Register of Controlled Trials.
Search
The following search query was followed for the identification of studies: “(saffron OR Crocus Sativus) AND (Alzheimer OR dementia OR cognitive OR cognition OR memory)”
Study selection
Eligible studies were identified by two reviewers (CV, NC), who searched independently based in the inclusion criteria. Any conflicts between the results of reviewers were solved with consensus and the addition of a third reviewer (KK).
Data collection process and data items
Two reviewers extracted data independently (CV, NC). Any conflicts between the results were solved with consensus and the addition of a third reviewer (KK). Collected data items included: title, first author, year of publication, ID, journal, country of origin, study type, study duration, number of patients assigned to saffron and placebo/drug of comparison, dosage of saffron/placebo/drug of comparison, baseline participants’ characteristics (gender, age, education), cognitive tests performed and their scores and finally, side effects.
Risk of bias in individual studies
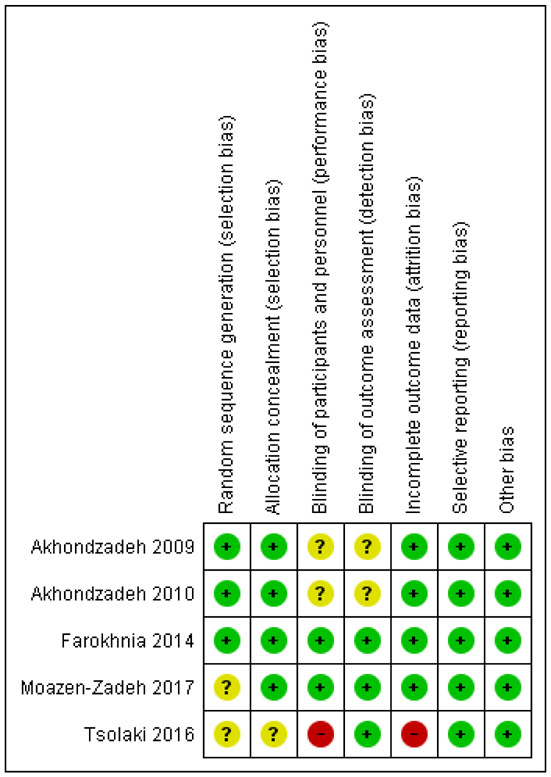
Two reviewers assessed the risk of bias (ROB) independently (KK, PGM) by using the Cochrane Collaboration’s tool for assessing ROB (13). The following possible domains of ROB were evaluated: random sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias). A study was characterized as “low risk”, only if all domains of possible ROB were “low risk”. If at least one domain was “unclear risk”, the study was deemed as “unclear risk”. If at least one domain was “high risk”, the study was deemed as “high risk”.
Results
Search results
The search yielded 200 possibly eligible studies. After duplications were removed and after screening of title and abstract, seven articles remained for full text assessment (figure 1). Of these, two articles were excluded for specific reasons (figure 1). Thus, five articles were finally included in qualitative synthesis (figure 1).
Fig. 1.
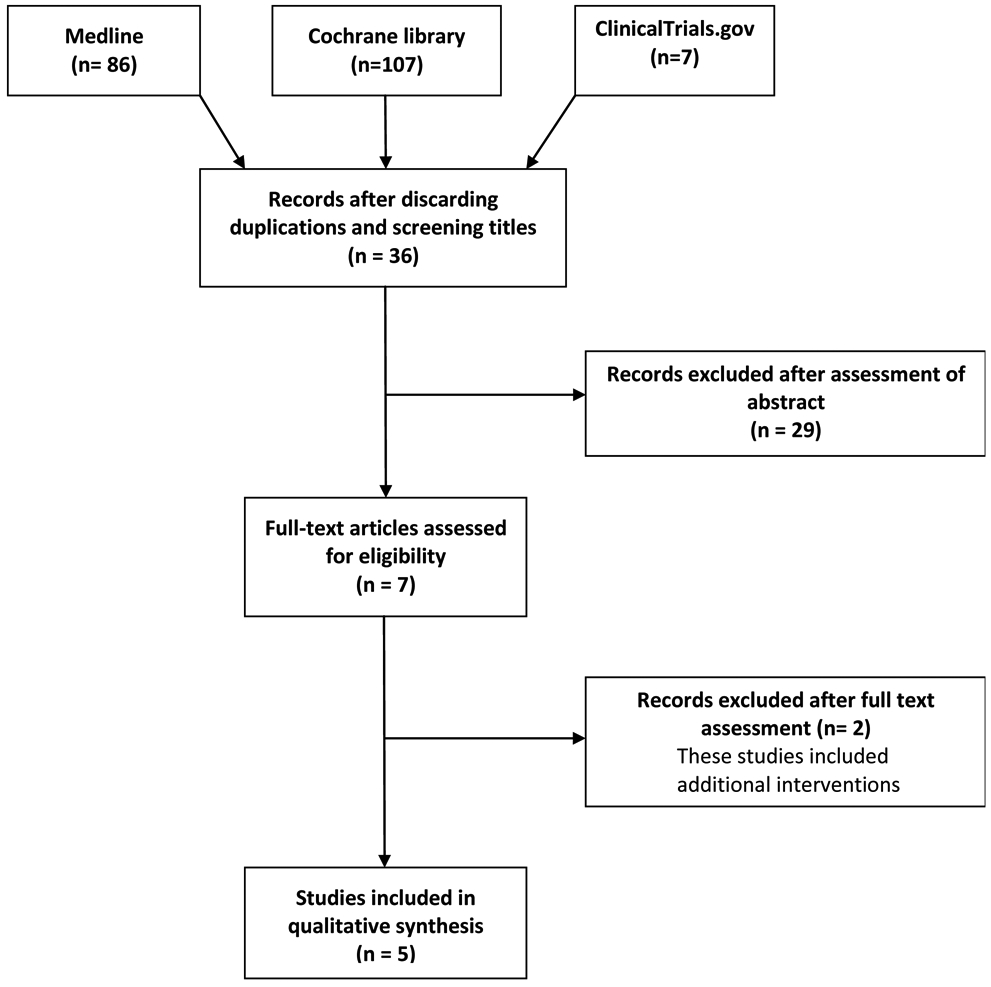
flow diagram of studies selection
Studies ’ and patients ’ characteristics
The five included studies were all designed as randomized controlled trials (RCTs) and had a total of 325 participants. In four studies subjects had a diagnosis of AD or MCI and in one study subjects were not cognitively impaired (table 1). Saffron intake was compared either with placebo or another drug for AD or nothing (table 1). Various cognitive batteries were applied for cognitive assessment (table 1). Details about the characteristics of the studies and their participants are depicted in tables 2 and 3, respectively.
Table 2.
Characteristics of included studies
| First author and year |
Countr y |
Stud y type |
Treatmen t duration (weeks) |
Patients with |
Total n of patient s in study |
Daily saffro n dose |
Daily comparato r dose |
Cognitiv e tasks used |
|---|---|---|---|---|---|---|---|---|
| Akhondzade h, 2010 (14) | Iran | RCT | 22 | Mild/moderate AD | 54 | 30 mg | 10 mg (donepezil) | ADAS-Cog, CDRS-SB |
| Akhondzade h, 2010’ (15) | Iran | RCT | 16 | Probable AD | 46 | 30 mg | 30 mg (placebo) | ADAS-Cog, CDRS-SB |
| Farokhnia, 2014 (16) | Iran | RCT | 48 | Moderate / severe AD | 68 | 30 mg | 20 mg (memantine) | SCIRS, MMSE FAST |
| Tsolaki, 2016 (18) | Greece | RCT | 48 | aMCI | 102 | N/R | N/R | MoCA, MMSE |
| Moazen-Zadeh, 2018 (17) | Iran | RCT | 12 | CABG | 55 | 30 mg | 30 mg (placebo) | WMS-R, MMSE |
ADAS-Cog: AD assessment scale-cognitive subscale; CDRS-SB: Clinical Dementia Rating Scale- Sums of Boxes: SCIRS: Severe Cognitive Impairment Rating Scale; FAST: Functional Assessment Staging; CABG: Coronary Artery Bypass Surgery; WMS-R: Wechsler Memory Scale-Revised MMSE: Mini Mental State Examination; aMCI: amnestic Mild Cognitive Impairment; MoCA: Montreal Cognitive Assessment
Table 3.
Demographic characteristics of patients in each study
| First author year |
Gender (m/f) | Age (years) |
Education (diploma/no diploma)* or (years)** |
|---|---|---|---|
| Akhondzadeh, 2010 (14) | 14/13 (saffron) 15/12 (donepezil) |
72.70±6.20 (saffron) 73.85±4.63 (donepezil) |
11/16 (saffron)* 12/15 (donepezil)* |
| Akhondzadeh, 2010’ (15) | 13/10 (saffron) 12/11 (placebo) |
72.65 ± 3.89 (saffron) 73.13 ± 4.70 (placebo) |
11/12 (saffron)* 10/13 (placebo)* |
| Farokhnia, 2014 (16) | 21/13 (saffron) 18/16 (memantine) |
77.73 ± 8.05 (saffron) 77.47±7.99 (memantine) |
5.88 ± 4.63 (saffron)** 5.91 ± 4.84 (memantine)** |
| Tsolaki, 2016 (18) | 5/12 (saffron) 4/14 (control) |
71.47 ± 6.73 (saffron) 69.72 ± 7.33 (control) |
8.17 ± 4.91 (saffron)** 10.1 ± 4.00 (control)** |
| Moazen-Zadeh, 2018 (17) | 20/2 (saffron) 21/2 (placebo) |
58.14 ± 4.43 (saffron) 56.61 ± 5.60 (placebo) |
18/4 (saffron)* 19/4 (placebo)* |
Age is reported as mean ± SD in most studies; Education is reported as mean ± SD in Farokhnia and Tsolaki studies.
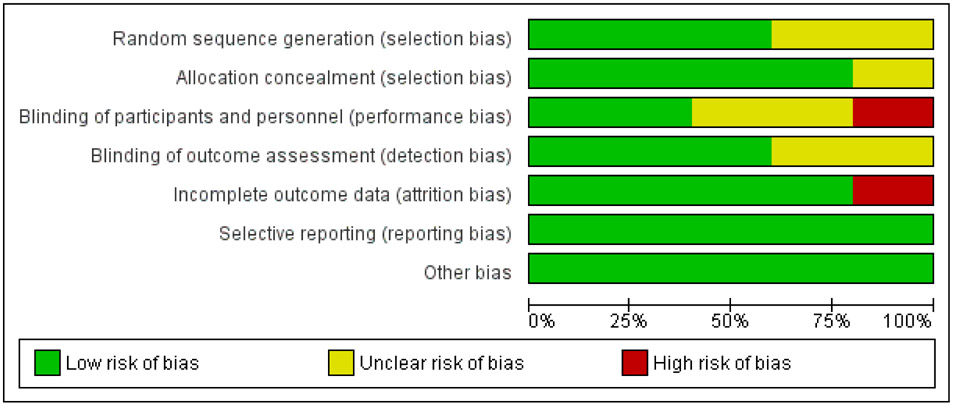
Risk of bias
Three studies were deemed as “unclear” for ROB, one study as “low” for ROB and one study as “high” for ROB. Details about the ROB assessment are shown in figures 2 and 3.
Fig. 2.
Risk of bias graph authors’ judgements about each risk of bias item, presented as percentage across all included studies
Fig. 3.
Risk of bias summary
Results of individual studies
Safety
Four out of five studies reported adverse events (14-17). Compared to another drug or placebo, saffron administration showed no significant difference in adverse events like nausea, dizziness, mouth dryness, fatigue, hypomania, agitation and confusion (14-17). Of note, in a study that compared saffron to donepezil, donepezil group had significantly higher incidence of vomiting (15).
Cognitive performance assessment
Scores on ADAS-cog (Alzheimer's Disease Assessment Scale-cognitive subscale) were assessed in two studies (14, 15). Score change was similar when saffron was compared to donepezil in the first study and better for saffron when it was compared to placebo, in the second study (14, 15). Mini Mental State Examination (MMSE) was assessed in three studies (16-18). In the first study, MMSE score change was significantly higher in the saffron group when compared to the no treatment group (18). Score change did not differ in the second study in which saffron was compared to memantine (16). No differences in MMSE score change was identified in the third study, which was the only study that enrolled non-demented participants and saffron was compared to placebo (17). More details about cognitive outcomes regarding the above and additional tests are shown in table 4.
Table 4.
Cognitive and functional status outcomes
| First author and year |
Comparison | Cognitive task |
Change of scores from baseline to endpoint |
|---|---|---|---|
| Akhondzadeh, 2010 (14) | Saffron vs donepezil | ADAS-Cog CDRS-SB |
NS difference in score change between two groups (t = 0.18, df = 52, p = 0.85) NS difference in score change between two groups (t = 0.21, df = 52, p = 0.83) |
| Akhondzadeh, 2010’ (15) | Saffron vs placebo | ADAS-Cog CDRS-SB |
Significantly better score change in saffron group (t = 17.27, d.f = 44, p < 0.0001) Significantly better score change in saffron group (t = 12.06, d.f = 44, p < 0.0001) |
| Farokhnia, 2014 (16) | Saffron vs memantine | SCIRS MMSE FAST |
NS difference in score change between two groups (t = 0.87, df = 66, p = 0.38) NS difference in score change between two groups (t = −1.07, df = 66, P = 0.28) NS difference in score change between two groups (t = −0.15, df = 66, p = 0.87) |
| Tsolaki, 2016 (18) | Saffron vs control | MoCA MMSE FRSSD |
NS difference in score between two groups (p = 0.62) Sig. positive change in saffron group compared no treatment group (p =0.02) NS difference in score change between two groups (p = 0.67) |
| Moazen-Zadeh, 2018 (17) | Saffron vs placebo | WMS-R MMSE |
NS difference in score change between two groups (t = −0.09, df = 43, p = 0.93) NS difference in score change between two groups (t= 0.39, df = 29.01, p = 0.69) |
ADAS-Cog: AD assessment scale-cognitive subscale; CDRS-SB: Clinical Dementia Rating Scale- Sums of Boxes; SCIRS: Severe Cognitive Impairment Rating Scale; FAST: Functional Assessment Staging; WMS-R: Wechsler Memory Scale-Revised; MMSE: Mini Mental State Examination;;MoCA: Montreal Cognitive Assessment; NS: Non-significant; FRSSD: Functional Rating Scale of Symptoms of Dementia
Assessment of functional status
Scores on Clinical Dementia Rating scale-sum of boxes (CDR-SB) were assessed in two studies (14, 15). Results showed equal efficacy of saffron with donepezil and superiority of saffron against placebo (14, 15). Other functional assessment scales used were FAST (Functional Assessment Staging) and FRSSD (Functional Rating Scale of Symptoms of Dementia) (16, 18). In one study in which saffron was compared to memantine, FAST score changes did not differ significantly (16). In another study, saffron resulted in no significant change on FRSSD score (18). Details about functional status assessments are found in table 4.
Discussion
In this systematic review of RCTs, we found evidence that oral intake of saffron by patients with AD, may have similar efficacy with common anti-AD drugs (such as donepezil and memantine) on cognitive function and functional status. Furthermore, saffron was superior to placebo. Additionally, saffron was shown to be safe, since the saffron group had no more side effects than the comparison group (either anti-AD drug or placebo). However, we emphasize that despite results may be deemed encouraging, they should not be overinterpreted. The degree of uncertainty regarding these conclusions is high since several studies upon which we based our conclusions, were of unknown risk of bias and one was of high risk of bias.
All the currently approved treatments for AD (cholinesterase inhibitors and NMDA receptor antagonist memantine) are merely symptomatic and do not modify the course of the disease. Disappointingly, numerous other agents, whether repurposed drugs (such as statins, NSAIDs, omega-3 fatty acids) (19-21) or novel compounds have failed as disease modifying agents in clinical trials urging the field to re-examine its underlying assumptions about disease pathophysiology (4). Several herbal therapies have already shown both efficacy and safety against mental diseases like major depression, premenstrual syndrome, anxiety, sleep disorders and may potentially be effective against cognitive impairment (22, 23). Ingestion of certain vegetables, fruits and spices which contain hormetic phytochemicals such as capsaicin, curcumin, resveratrol and sulforaphane, results in expression of cytoprotective proteins (growth factors, mitochondrial proteins, antioxidant enzymes and protein chaperones) that reduce neurodegenerative damage and thus could possibly have therapeutic action (24-26).
Saffron is a product of Crocus L. Sativus, which is a perennial bulb widely cultivated in Iran, India and Mediterranean countries (mostly Greece) (11). It has been used since ancient years for medicinal and cosmetic purposes (11). There is evidence from animal studies that saffron has antidiabetic, anti-inflammatory, anti- atherosclerotic, antitumor, immunomodulatory and antioxidant effects (11, 27, 28). In addition, saffron has four potential pathophysiologic actions that may partially explain why it could be effective in AD. First, it may inhibit glutamate excitotoxicity and neuronal death through NMDA receptor antagonism similar to memantine (29). Second, it has a relative inhibitory effect on acetylo-cholinesterase in the same way as homonymous AD drugs do (30). Third, there is some evidence that it may inhibit deposition of beta-amyloid fibrils, which is one of the pathological hallmarks of AD (31). Fourth, it has antioxidant properties which may further establish saffron’s beneficial effects against oxidative stress that is known to occur in the AD brain (31, 32). The described mechanisms lend some external validity and biological plausibility to the RCT findings. Moreover, the magnitude of saffron’s effect in AD may not be negligible. In two studies, saffron had same efficacy as donepezil and memantine, perhaps, because saffron shares similar mechanisms of actions with these drugs (15, 16).
Three studies compared saffron to placebo and results were conflicting (14, 17, 18). In the first study, saffron was found to be superior to placebo, which supports its claim as an anti-AD treatment (14). In two other studies (second, third), saffron showed no benefit against placebo except for one cognitive task (17, 18). Unfortunately, the second study was of low methodological quality (high ROB study) and therefore could not be used as a basis to confirm the null hypothesis (18). In the third study, participants were non-demented; therefore, the failure of saffron to improve their cognitive scores could have been due to a ceiling effect (17).
Depression is common among MCI/AD patients, affecting half of all patients (33, 34). It results in higher morbidity and mortality among patients and a higher occurrence of depression among caregivers (35). Rivastigmine has shown some promising effects on depressive symptoms of AD patients, but further studies are needed to confirm that (36, 37). Regarding the use of anti-depressants for depression in AD, the evidence is not supportive; their efficacy is uncertain and there is a potential of side effects (38, 39). Therefore, natural compounds could be potentially useful in the treatment of AD depression (35). Saffron, has anti-depressive properties and has been shown to be superior to placebo and equal to an SSRI and a TCA in various RCTs (40-42). However, SSRIs may cause serotonin syndrome, agitation, insomnia, sexual dysfunction, dizziness and other symptoms, while TCAs are responsible for anticholinergic effects (therefore, they are generally avoided in AD) and cardiac arrhythmias (35). Saffron is associated with no known side effects and as such, it could be a better choice for depression in AD patients. The combination of putative cognitive-enhancing and anti-depressive effects of saffron make it a reasonable treatment option for AD patients.
Limitations and strengths
The finding that a natural compound (saffron) is likely as efficacious as common drugs against AD is promising. However, our results should be interpreted with caution for several reasons. First, this systematic review included only five studies, and, of those five, three were deemed as of unknown ROB while one as of high ROB. In addition, a meta-analysis was not feasible mainly because i) the comparison group was different in each study (in some studies the comparator was placebo, while in other studies it was donepezil or memantine or was not described adequately) ii) the cognitive scales varied greatly from study to study. Finally, four of five studies took place in Iran, a fact that results in non-generalizable conclusions.
Conclusion
In the present systematic review, we examined the effects of oral saffron intake on cognitive function. Saffron showed similar efficacy in improving cognitive scores as common anti-AD drugs. The incidence of side effects was similar in the saffron and comparison groups. However, these findings should be interpreted cautiously since there was potential risk of bias in many of the included studies. On the other hand, saffron has been reported to have additional anti-depressive properties, which are of interest given the increased incidence of depression in AD. Taken together, these findings and background evidence should motivate future RCTs to explore the potential properties of this herb as an alternative or adjunct treatment for MCI/AD. Such trials would require larger sample sizes and inclusion of a sufficient number of patients with high-probability AD.
Footnotes
Ethical issues
On behalf of all authors, the corresponding author states that there is no conflict of interest. Also, there was no need for informed consent before conducting the present study, since it was a systematic review of literature. The present study was supported in part by the Intramural Program of the National Institute on Aging, National Institutes of Health.
References
- 1.Keuck L Slicing the cortex to study mental illness: Alois Alzheimer's pictures of equivalence. Progress in brain research. 2017;233:25–51. [DOI] [PubMed] [Google Scholar]
- 2.Fiest KM, Roberts JI, Maxwell CJ, Hogan DB, Smith EE, Frolkis A, et al. The Prevalence and Incidence of Dementia Due to Alzheimer's Disease: a Systematic Review and Meta-Analysis. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2016;43 Suppl 1:S51–82. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–92. [DOI] [PubMed] [Google Scholar]
- 4.Becker RE, Kapogiannis D, Greig NH. Does traumatic brain injury hold the key to the Alzheimer's disease puzzle? Alzheimers Dement. 2018;14(4):431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauthier S, Schlaefke S. Efficacy and tolerability of Ginkgo biloba extract EGb 761(R) in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clinical interventions in aging. 2014;9:2065–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang M, Xu DD, Zhang Y, Liu X, Hoeven R, Cho WC. A systematic review on natural medicines for the prevention and treatment of Alzheimer's disease with meta-analyses of intervention effect of ginkgo. The American journal of Chinese medicine. 2014;42(3):505–21. [DOI] [PubMed] [Google Scholar]
- 7.Enderami A, Zarghami M, Darvishi-Khezri H. The effects and potential mechanisms of folic acid on cognitive function: a comprehensive review. Neurol Sci. 2018;39(10):1667–75. [DOI] [PubMed] [Google Scholar]
- 8.Chen N, Yang M, Zhou M, Xiao J, Guo J, He L. L-carnitine for cognitive enhancement in people without cognitive impairment. The Cochrane database of systematic reviews. 2017;3:CD009374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avgerinos KI, Spyrou N, Bougioukas KI, Kapogiannis D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp Gerontol. 2018;108:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bathaie SZ, Mousavi SZ. New applications and mechanisms of action of saffron and its important ingredients. Critical reviews in food science and nutrition. 2010;50(8):761–86. [DOI] [PubMed] [Google Scholar]
- 11.Christodoulou E, Kadoglou NP, Kostomitsopoulos N, Valsami G. Saffron: a natural product with potential pharmaceutical applications. The Journal of pharmacy and pharmacology. 2015;67(12):1634–49. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhondzadeh S, Sabet MS, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. Saffron in the treatment of patients with mild to moderate Alzheimer's disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther. 2010;35(5):581–8. [DOI] [PubMed] [Google Scholar]
- 15.Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer's disease. Psychopharmacology (Berl). 2010;207(4):637–43. [DOI] [PubMed] [Google Scholar]
- 16.Farokhnia M, Shafiee Sabet M, Iranpour N, Gougol A, Yekehtaz H, Alimardani R, et al. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer's disease: a double-blind randomized clinical trial. Human psychopharmacology. 2014;29(4):351–9. [DOI] [PubMed] [Google Scholar]
- 17.Moazen-Zadeh E, Abbasi SH, Safi-Aghdam H, Shahmansouri N, Arjmandi-Beglar A, Hajhosseinn Talasaz A, et al. Effects of Saffron on Cognition, Anxiety, and Depression in Patients Undergoing Coronary Artery Bypass Grafting: A Randomized Double-Blind Placebo-Controlled Trial. J Altern Complement Med. 2018;24(4):361–8. [DOI] [PubMed] [Google Scholar]
- 18.Tsolaki M, Karathanasi E, Lazarou I, Dovas K, Verykouki E, Karacostas A, et al. Efficacy and Safety of Crocus sativus L. in Patients with Mild Cognitive Impairment: One Year Single-Blind Randomized, with Parallel Groups, Clinical Trial. Journal of Alzheimer's disease : JAD. 2016;54(1):129–33. [DOI] [PubMed] [Google Scholar]
- 19.Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77(6):556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Group ADC, Bentham P, Gray R, Sellwood E, Hills R, Crome P, et al. Aspirin in Alzheimer's disease (AD2000): a randomised open-label trial. Lancet Neurol. 2008;7(1):41–9. [DOI] [PubMed] [Google Scholar]
- 21.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63(10):1402–8. [DOI] [PubMed] [Google Scholar]
- 22.Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85–91. [DOI] [PubMed] [Google Scholar]
- 23.Sarris J Herbal medicines in the treatment of psychiatric disorders: a systematic review. Phytother Res. 2007;21(8):703–16. [DOI] [PubMed] [Google Scholar]
- 24.Mattson MP. Hormesis defined. Ageing research reviews. 2008;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattson MP. Dietary factors, hormesis and health. Ageing research reviews. 2008;7(1):43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabrese V, Cornelius C, Trovato A, Cavallaro M, Mancuso C, Di Rienzo L, et al. The hormetic role of dietary antioxidants in free radical-related diseases. Current pharmaceutical design. 2010;16(7):877–83. [DOI] [PubMed] [Google Scholar]
- 27.Premkumar K, Thirunavukkarasu C, Abraham SK, Santhiya ST, Ramesh A. Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum Exp Toxicol. 2006;25(2):79–84. [DOI] [PubMed] [Google Scholar]
- 28.Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocus sativus Linn.) on genotoxins-induced oxidative stress in Swiss albino mice. Phytother Res. 2003;17(6):614–7. [DOI] [PubMed] [Google Scholar]
- 29.Berger F, Hensel A, Nieber K. Saffron extract and trans-crocetin inhibit glutamatergic synaptic transmission in rat cortical brain slices. Neuroscience. 2011;180:238–47. [DOI] [PubMed] [Google Scholar]
- 30.Geromichalos GD, Lamari FN, Papandreou MA, Trafalis DT, Margarity M, Papageorgiou A, et al. Saffron as a source of novel acetylcholinesterase inhibitors: molecular docking and in vitro enzymatic studies. J Agric Food Chem. 2012;60(24):6131–8. [DOI] [PubMed] [Google Scholar]
- 31.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, et al. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;54(23):8762–8. [DOI] [PubMed] [Google Scholar]
- 32.Montoro P, Maldini M, Luciani L, Tuberoso CI, Congiu F, Pizza C. Radical scavenging activity and LC-MS metabolic profiling of petals, stamens, and flowers of Crocus sativus L. J Food Sci. 2012;77(8):C893–900. [DOI] [PubMed] [Google Scholar]
- 33.Lyketsos CG, Olin J. Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry. 2002;52(3):243–52. [DOI] [PubMed] [Google Scholar]
- 34.Scaricamazza E, Colonna I, Sancesario GM, Assogna F, Orfei MD, Franchini F, et al. Neuropsychiatric symptoms differently affect mild cognitive impairment and Alzheimer's disease patients: a retrospective observational study. Neurol Sci. 2019. [DOI] [PubMed] [Google Scholar]
- 35.Starkstein SE, Mizrahi R, Power BD. Depression in Alzheimer’s disease: phenomenology, clinical correlates and treatment. Int Rev Psychiatry. 2008;20(4):382–8. [DOI] [PubMed] [Google Scholar]
- 36.Spalletta G, Caltagirone C, Padovani A, Sorbi S, Attar M, Colombo D, et al. Cognitive and affective changes in mild to moderate Alzheimer's disease patients undergoing switch of cholinesterase inhibitors: a 6-month observational study. PLoS One. 2014;9(2):e89216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spalletta G, Gianni W, Giubilei F, Casini AR, Sancesario G, Caltagirone C, et al. Rivastigmine patch ameliorates depression in mild AD: preliminary evidence from a 6-month open-label observational study. Alzheimer Dis Assoc Disord. 2013;27(3):289–91. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):403–11. [DOI] [PubMed] [Google Scholar]
- 39.Dudas R, Malouf R, McCleery J, Dening T. Antidepressants for treating depression in dementia. Cochrane Database Syst Rev. 2018;8:CD003944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi AH, Khalighi-Cigaroudi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement Altern Med. 2004;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19(2):148–51. [DOI] [PubMed] [Google Scholar]
- 42.Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281–4. [DOI] [PubMed] [Google Scholar]