Abstract
We examined baseline and longitudinal associations between plasma neurofilament light (NfL) and total tau (t-tau), and the clinical presentation of Alzheimer’s disease (AD). 579 participants (238, normal cognition [NC]; 185, mild cognitive impairment [MCI]; 156, AD dementia) had baseline blood draws; 82% had follow-up evaluations. Plasma samples were analyzed for NfL and t-tau using Simoa technology. Baseline plasma NfL was higher in AD dementia compared to MCI (standardized mean difference=0.55, 95% CI: 0.37-0.73) and NC (standardized mean difference=0.68, 95% CI: 0.49-0.88), corresponded to CDR scores (OR=1.94, 95% CI: 1.35-2.79]), and correlated with all neuropsychological tests (r’s=0.13-0.42). Longitudinally, NfL did not predict diagnostic conversion, but predicted decline on 3/10 neuropsychological tests. Baseline plasma t-tau was higher in AD dementia compared to NC with a small effect (standardized mean difference=0.33, 95% CI: 0.10-0.57) but not MCI. T-tau did not statistically significant predict any longitudinal outcomes. Plasma NfL may be useful for the detection of AD dementia and monitoring of disease progression. In contrast, there was minimal evidence in support of plasma t-tau.
Keywords: Plasma biomarkers, Neurofilament Light, Total Tau, Alzheimer’s disease
1. BACKGROUND
Early and accurate detection of Alzheimer’s disease (AD) is critical for timely diagnosis and implementation of preventative strategies and therapeutic interventions. The National Institute on Aging and Alzheimer’s Association (NIA-AA) Research Framework recommends AD to be defined based on its underlying pathophysiologic processes using in vivo biomarkers (Jack et al., 2018). These biomarkers can allow for the preclinical detection of pathological changes prior to onset of observable clinical symptoms (Blennow & Zetterberg, 2018; Dubois et al., 2014; Dubois et al., 2016). AD biomarkers are classified into markers of amyloid (“A”), tau (“T”), and neurodegeneration (“N”). The amyloid and tau biomarkers have been traditionally assessed via analysis of cerebrospinal fluid (CSF) and PET imaging of amyloid and phosphorylated tau (p-tau). The “N” biomarkers are non-specific markers of neurodegeneration often observed in AD, such as hippocampal atrophy on magnetic resonance imaging (MRI), fluoro-deoxyglucose (FDG) PET, as well as CSF measurements of neurofilament light (NfL) and total tau (t-tau). These biomarkers may be associated with AD neuropathologic changes in addition to other neurodegenerative and non-neurodegenerative pathologies.
PET and CSF are considered the gold standard in vivo AD biomarkers because they provide a direct window into the central nervous system (CNS) environment. The high expense and perceived invasiveness of these procedures limits their potential feasibility for large-scale research studies and clinical utility (Menendez-Gonzalez, 2014). In recent years, ultrasensitive methods (e.g., single molecule array [Simoa]) have been developed that now make it possible to detect proteins in blood that might accurately reflect the pathological processes in the CNS in a cost-effective and non-invasive manner (Zetterberg & Blennow, 2018). With these advancements, growing literature has focused on the use of blood-based AD biomarkers (Zetterberg & Blennow, 2018). This includes plasma measurements of “A” biomarkers including plasma β-amyloid, specifically the ratio between Aβ42 and Aβ40 as the “A” biomarker using immunoprecipitation-mass spectrometry (Nakamura et al., 2018; Schindler et al., 2019) or immunoassays (Albani et al., 2019; Nabers et al., 2018; Verberk et al., 2018) and the “T” biomarker of p-tau using different immunoassay platforms (Mielke et al., 2018; Tatebe et al., 2017; Yang et al., 2018). These measurements have demonstrated promise as viable biomarkers that correlate with clinical disease progression and amyloid PET.
There have been advancements in the study of blood-based “N” biomarkers of NfL and t-tau. These plasma measurements correlate with disease severity in AD dementia and other biomarkers of AD neuropathologic changes (Ashton et al., 2019; Chiu et al., 2014; Fossati et al., 2019; Lewczuk et al., 2018; Lin et al., 2018; Mattsson et al., 2017; Mattsson et al., 2019; Mattsson et al., 2016; Mielke et al., 2017; Mielke et al., 2018; Pase et al., 2019; Preische et al., 2019; Sanchez-Valle et al., 2018; Yang et al., 2018; Zetterberg et al., 2013; Zhou et al., 2017). NfL is a structural neuronal protein that is released into the surrounding CSF following axonal or neuronal damage in many conditions. Elevations in CSF NfL are observed not only in individuals with AD dementia, but also in frontotemporal lobar degeneration (FTLD), Huntington disease, and other neurological conditions (Bacioglu et al., 2016; Blennow, 2004; Bridel et al., 2019; Olsson et al., 2016; Skillback, Farahmand, et al., 2014; Zetterberg et al., 2016). Blood-based measurements of NfL (in plasma or serum) may be a valuable biomarker of neurodegeneration in AD. For example, NfL has been shown to be elevated in individuals with AD dementia compared to MCI and normal cognition (NC) (Lin et al., 2018; Mattsson et al., 2017; Mattsson et al., 2019; Zhou et al., 2017); is associated with concurrent CSF measurements of AD pathology (Mattsson et al., 2017; Mattsson et al., 2019; Sanchez-Valle et al., 2018); correlates with neuropsychological test performance (Lin et al., 2018; Mattsson et al., 2017; Preische et al., 2019; Sanchez-Valle et al., 2018; Zhou et al., 2017) and structural neuroimaging indices (Mattsson et al., 2019); can predict cognitive changes (Preische et al., 2019); and is associated with AD neuropathologic changes at autopsy including neurofibrillary tangles (Ashton et al., 2019). Yet, research has been equivocal. While NfL can discriminate between AD dementia, MCI, and NC individuals in some samples, there is substantial overlap between diagnostic groups (Mattsson et al., 2017; Zhou et al., 2017). Moreover, one study (Lin et al., 2018) did not observe significant differences between MCI and NC. Zhou et al. (2017) also noted substantial positive correlations with age (r’s > 0.3) in all three groups and commented that NfL may not be valuable for the clinical diagnosis of AD. Rather, plasma NfL may be best suited for monitoring disease progression and tracking the effectiveness of disease-modifying treatments in clinical research (Mattsson et al., 2019; Zhou et al., 2017).
T-tau is also a non-specific index of neurodegeneration. The tau protein is involved with stabilization of microtubules in the neuronal cytoskeleton and is released into the surrounding CSF during cell death and neurodegeneration. Tau may be released by neurons impacted by amyloid pathology (Maia et al., 2013; Sato et al., 2018). CSF t-tau elevations can be observed with several neurological conditions including AD, Lewy body dementia, FTLD, stroke, vascular dementia, and Creutzfeldt-Jacob disease (Diniz et al., 2008; Hesse et al., 2000; Olsson et al., 2016; Parnetti et al., 2008; Ritchie et al., 2017; Skillback, Rosen, et al., 2014; van Harten et al., 2011). Plasma levels of t-tau have been shown to be elevated in individuals with AD dementia (Chiu et al., 2014; Fossati et al., 2019; Mattsson et al., 2016; Mielke et al., 2018; Yang et al., 2018; Zetterberg et al., 2013); are predictive of subsequent cognitive decline (Mielke et al., 2017; Pase et al., 2019); and correlate with cognitive functioning (Chiu et al., 2014; Fossati et al., 2019; Mattsson et al., 2016), structural neuroimaging indices (Chiu et al., 2014; Fossati et al., 2019; Mattsson et al., 2016; Mielke et al., 2018), and amyloid PET (Mielke et al., 2018). However, it is unclear how meaningful and sensitive a biomarker it may be. For example, t-tau has not discriminated between NC and MCI in multiple studies, where elevated levels were only observed in AD dementia, with considerable overlap between groups (Mattsson et al., 2016; Mielke et al., 2018; Zetterberg et al., 2013). The predictive utility also appears limited, as plasma t-tau has not significantly predicted conversion from MCI to dementia (Mielke et al., 2017; Zetterberg et al., 2013). There have also been concerns regarding the convergent validity with other measures of AD pathology, as plasma t-tau has demonstrated small or non-significant correlations with CSF t-tau (Fossati et al., 2019; Mattsson et al., 2016; Zetterberg et al., 2013).
Given the ambiguous findings for plasma measurements of both NfL and t-tau, further investigation is crucial to validate these measurements as in vivo biomarkers for AD. The objective of this study was to examine the utility of plasma NfL and t-tau in the detection of cognitive decline in a sample of participants with normal cognition and cognitive impairment due to AD. We leveraged the Boston University (BU) Alzheimer’s Disease Center (ADC) Clinical Core registry data set and biosample repository to examine the ability of plasma NfL and t-tau to predict diagnostic conversion, as well as neuropsychological test performance over time. We hypothesized that plasma NfL would successfully discriminate between individuals with NC, MCI, and AD dementia while t-tau would only be elevated in individuals with dementia, consistent with previous research findings. Longitudinally, we hypothesized that plasma NfL would have superior predictive validity compared to t-tau in predicting odds for conversion to MCI and AD dementia and declines on neuropsychological testing. For individuals with multiple measurements, we hypothesized that increases in both biomarkers would correspond with the clinical progression of AD dementia.
METHODS
2.1. Participants and Design
The sample included participants from the BU ADC Clinical Core Registry. The BU ADC is one of ~30 centers funded by the NIA and provides data to the National Alzheimer’s Coordinating Center (NACC) to promote collaborative research on AD (Beekly et al., 2004; Morris et al., 2006; Weintraub et al., 2009). A description of the BU ADC Clinical Core Registry, including participant recruitment and eligibility criteria, is provided elsewhere (Ashendorf et al., 2009; Gavett et al., 2012; Jefferson et al., 2007). The BU ADC longitudinally follows older adults with and without cognitive impairment to facilitate the study of AD and related dementias. Inclusion criteria includes community dwelling, English speaking older adults who have adequate hearing and visual acuity. Participants are excluded if they had a history of serious mental illness (e.g., schizophrenia, bipolar disorder), non-neurodegenerative disease neurological illness (e.g., multiple sclerosis, brain tumor), and/or other disorders that preclude the ability of the participant to participate in study procedures.
As part of the BU ADC protocol, participants complete an annual evaluation that consists of a clinical and medical interview, neurological examination, neuropsychological testing, and measures of functional independence. Beginning in 2008, all BU ADC participants (i.e., existing individuals in the registry and new participants) completed one voluntary blood draw during a study visit. Starting in 2015, all participants were asked to complete blood draws as part of all annual study visits. All participants had data from at least one blood draw available for analysis, and many participants had data from multiple study visits. All study visits in the current analyses took place between 2008 and 2018. Participants were eligible for inclusion in the current study if at least one plasma sample was available from a study visit in which they received a designation of NC, MCI due to AD, or AD dementia. We focus on AD as a suspected etiology because there are very few other suspected etiologies in the BU ADC Clinical Core (only 17 participants in this sample had cognitive impairment due to non-AD neurological conditions) and because our goal was to determine the utility and validity of these plasma biomarkers specifically in the setting of AD. All BU ADC data collection procedures were approved by the BU Medical Center Institutional Review Board. Participants (or their Legally Authorized Representatives) provided written informed consent to participate in the study.
2.2. Plasma Biomarker Analyses
Non-fasting blood samples were collected for all participants. Blood was collected into plastic dipotassium EDTA tubes, and processed according to standard procedures, with plasma aliquoted and frozen at −80°C. Frozen plasma aliquots were shipped on dry ice to the University of Gothenburg (Sweden) for batch analysis. Plasma NfL concentration was measured using an ultrasensitive in-house Simoa method on an HD-1 Analyzer (Quanterix, Billerica, Massachusetts), as previously described in detail (Gisslen et al., 2016). This in-house Simoa assay is very similar to commercial Simoa methods (only minor variations) and this same method was previously found to have a high correlation (r = .89) with CSF NfL in a sample of patients with HIV infections (Gisslen et al., 2016). The LLOQ was 1.9 pg/mL, with a dynamic range of 1.9-1800 pg/mL. T-tau concentrations were measured using the commercially available Tau 2.0 kit and the HD-1 analyzer (Quanterix, Billerica, Massachusetts) with an LLOQ of 0.061 pg/mL and a dynamic range of 0.061-360 pg/mL. The measurements were performed by board-certified laboratory technicians who were blinded to clinical data. All samples were detectable with an average coefficient of variation (CV) of 4%.
2.3. Diagnostic Procedures
Cognitive diagnoses (i.e., MCI; cognitively impaired, not MCI; dementia; or NC, reflecting a lack of cognitive diagnosis) are made for all participants enrolled in the BU ADC during multidisciplinary diagnostic consensus conferences that are comprised of neurologists and neuropsychologists, as well as geriatricians and/or psychiatrists. Consensus diagnosis is adjudicated following presentation and discussion of all examination and test findings (including review of structural MRI, if available), as well as social, family, and medical history. Established criteria are used for MCI (Winblad et al., 2004) and AD dementia (McKhann et al., 1984) diagnoses. For participants who progressed to MCI and/or dementia at any point during the study, only those with a primary suspected etiology of AD were included in analyses (for reasons discussed above). Note that there were two follow-up study visits in the entire sample where “cognitively impaired, not MCI” was listed as the clinical diagnosis but the suspected etiology was AD and therefore these study visits were not excluded; neither was a baseline or final study visit and these visits did not impact the baseline diagnostic discrimination analyses. Participants who performed within the normal range on all neuropsychological tests were classified as having NC.
2.4. Dementia Severity
All participants received a global rating from the CDR® Dementia Staging Instrument to assess dementia severity at each visit (Hughes et al., 1982; Morris, 1993). The CDR stages dementia severity through the assessment of several factors including memory, orientation, judgment/problem solving, community affairs, home and hobbies, and personal care. Each domain is rated on a scale from 0 (no impairment) to 3 (severe impairment) and an algorithm is used to calculate a global severity rating designated as: 0 (no dementia), 0.5 (questionable dementia/MCI), 1.0 (mild dementia), 2.0 (moderate dementia), and 3.0 (severe dementia).
2.5. Neuropsychological Testing
Participants completed a battery of common neuropsychological tests at each study visit. Many of these are NACC-UDS tests (Beekly et al., 2004; Weintraub et al., 2009) and include the Mini-Mental State Examination (MMSE), 30-item short form Boston Naming Test (BNT), Semantic Fluency (Animals and Vegetables), Wechsler Adult Intelligence Scale-IV Digit Span (WAIS-IV DS), and Wechsler Memory Scale, Revised Logical Memory Immediate Recall (LM-IA) and Delayed Recall (LM-IIA). In addition to standard NACC-UDS tests, the Neuropsychological Assessment Battery (NAB) List Learning Test is administered as part of the BU ADC Clinical Core Registry protocol.
2.6. Statistical Analyses
The study visit with the first plasma sample served as the baseline visit, with all subsequent visits being follow-up visits. Descriptive analyses compared the three groups on demographic covariates at baseline. Statistical analyses were conducted to determine the cross-sectional and longitudinal relationship between plasma NfL and t-tau with diagnostic status and conversion, CDR score, and neuropsychological test performance. Analyses examined each biomarker (e.g., NfL and t-tau), separately. Models also evaluated both biomarker measurements to determine the incremental validity in discriminating between participants groups at baseline compared to either biomarker alone. The specific statistical models conducted based on the outcomes examined included:
Diagnostic status and conversion:
For cross-sectional analyses, ANCOVA models compared plasma NfL and t-tau levels between individuals classified as NC, MCI, or AD dementia at baseline, accounting for relevant covariates. Receiver operating characteristic (ROC) analyses were conducted between each diagnostic pairwise comparison using logistic regression to determine the ability of the biomarkers to discriminate between groups, using the area under the curve (AUC) statistic. Level of discrimination accuracy was interpreted using guidelines set forth by Hosmer and Lemeshow (2000). These analyses were conducted in a two-step approach. The first step examined demographic and APOE covariates (covariate selection described below), followed by the second step that included the biomarkers along with the covariates. Three models were conducted for each pairwise group comparison: 1) only plasma NfL, 2) only plasma t-tau, and 3) a combined model with both NfL and t-tau. For longitudinal analyses, logistic regression models tested whether baseline plasma t-tau and NfL concentrations predicted increased odds for conversion from baseline NC to MCI or AD dementia. This was repeated for individuals with MCI at baseline to determine if baseline biomarker levels could discriminate between individuals with stable MCI and those who progressed to AD dementia. For the subset of participants who had multiple biomarker measurements, we evaluated whether individuals who experienced a change in diagnosis across study visits experienced a corresponding change in biomarker measurements using logistic regression modelling.
CDR Score:
Ordinal logistic regression models examined if the biomarkers could differentiate between dementia severity levels (as measured by CDR ratings) for individuals diagnosed with AD dementia at baseline. For individuals with dementia at baseline who had follow-up data available, ordinal logistic regression models were conducted to determine whether each biomarker predicted CDR rating at the final study visit. We also examined whether changes in biomarker levels over time were associated with changes in CDR rating for participants who had multiple biomarker measurements available.
Neuropsychological Test Scores:
Partial Pearson correlations were used to examine the association between plasma biomarkers and performance on all neuropsychological tests at baseline across the entire sample, accounting for demographic covariates. Generalized linear models estimated via generalized estimating equations (GEE) tested whether NfL and t-tau levels at baseline could predict subsequent changes in neuropsychological test performance based on the interaction effect between the biomarker level and time since baseline. The GEE models included all study visits and used an autoregressive (AR1) correlation structure. Only individuals classified as having NC or MCI at baseline were included in these analyses to avoid restricted range (i.e., floor effects) among individuals with AD dementia.
Age, education, race (white vs. other), sex, and APOE ε4 allele status (carriers vs. non-carriers) were included as covariates in all models. These covariates were a priori selected based on their known associations with cognitive and brain aging outcomes, in general, and AD dementia, in particular. For longitudinal models, the follow-up interval (time between the baseline and final study visits in years) and the baseline level of the outcome variable were included as additional covariates. To reduce the risk of Type I error, all p-values were false discovery rate (FDR) adjusted using the Benjamini-Hochberg Procedure (Benjamini & Hochberg, 1995). FDR is a common procedure that is used to reduce the rate of Type I (false positive errors) when conducting multiple statistical analyses and to maintain overall false positive rate at 5%. FDR adjusts the threshold for statistical significance based on the number of models conducted within each group of statistical analyses (specified in the table legends). Statistical significance was defined as an FDR-adjusted alpha level less than 0.05. All p-values presented are FDR-adjusted.
3. RESULTS
3.1. Participants
Demographics and clinical characteristics for the sample are shown in Table 1. A total of 579 individuals were included in this sample, including 238 with NC at baseline, 185 with MCI due to AD, and 156 with AD dementia. A total of 638 individuals in the BU ADC Clinical Core Registry had blood draws available, but 59 were excluded due to not having qualifying diagnoses at the time of the draw (14 missing; 28 cognitively impaired, not MCI; and 17 with cognitive impairment due to non-AD disorders).
Table 1.
Participant demographics at baseline. Significant findings are baser on three-group ANOVAs or Chi-square tests of independence, with further two-groups post hoc comparisons for significant findings at an alpha level of p<0.05.
| NC (n = 238) | MCI (n = 185) | AD dementia (n = 156) | Between-group differences | |
|---|---|---|---|---|
| Age (SD) | 72.38 (7.69) | 74.99 (7.24) | 76.74 (8.12) | NC < MCI < AD |
| Sex | 62.6% Female | 58.4% Female | 44.2% Female | (NC = MCI) > AD |
| Race | 89.5% White, 9.7% AA, 0.8% Asian |
75.1% White, 23.8% AA, 1.1% Asian |
91.0% White, 7.7% AA, 1.3% Asian |
(NC = AD) > MCI* |
| Education (SD) | 16.56 (2.54) | 15.51 (2.74) | 14.95 (2.95) | NC > (MCI = AD) |
| MMSE (SD) | 29.39 (0.91) | 28.20 (1.67) | 21.11 (6.17) | NC > MCI > AD |
| APOE ε4 carrier status | 77/235 (32.8%) | 59/181 (32.6%) | 88/153 (57.5%) | (NC = MCI) < AD |
| NfL (pg/mL) | 15.33 (10.47) | 17.77 (10.25) | 26.49 (17.30) | NC < MCI < AD |
| t-tau (pg/mL) | 3.22 (2.73) | 3.30 (2.39) | 3.73 (3.01) | not significant |
| # with follow-up | 206 (86.6%) | 150 (81.1%) | 120 (76.9%) | NC > AD |
| Years follow-up (SD) | 5.62 (2.64) | 5.10 (2.78) | 3.20 (2.06) | (NC = MCI) > AD |
AA=African American.
Higher proportion of White participants in NC and AD groups compared to MCI.
The range of values (NfL: 1.9-179 pg/mL; t-tau: 0.4-74.8) fell within the dynamic ranges for the assays. The distributions for both biomarkers were highly positively skewed. To minimize the influence of outlier values, the natural logs of the values for both biomarkers were utilized for all remaining analyses. Shapiro-Wilk testing confirmed how these analyses resulted in the data better conforming to a normal distribution. For t-tau, the raw values had a W-value of 0.537 with p < .001. The transformed data had a W-value much closer to 1 (0.966), indicating a more normal distribution; this value was still significant (p < .001), which reflects a minor deviation from normality in the context of a large sample size. Likewise, for NfL, the W-value increased from 0.771 (p < .001) to 0.992 (p = .003) following the log transformation. All scores were then transformed into standardized scores (i.e., z-scores) to facilitate interpretation of the coefficients in statistical analyses. The two biomarkers were not significantly correlated with each other (using the log-transformed values) either across the entire sample (r = .07, p = .078) or within the participant groups of NC (r = −.04, p = .521), MCI (r = .13, p = .074), or AD dementia (r = .05, p = .550).
For participants with NC at baseline, 49 of them converted to MCI at a subsequent study visit, including 10 who progressed to AD dementia. Twenty-four participants with MCI at baseline subsequently progressed to AD dementia. Among the 196 participants who had biomarker data from multiple study visits, 115 participants had NC at baseline, 56 were diagnosed with MCI, and 25 had AD dementia. The mean follow-up interval was 5.09 years (SD = 2.66 years). Among participants with NC at baseline, 20 had a diagnosis of MCI at a subsequent measurement, and 4 had AD dementia (an insufficient number of participants for analyses). Among participants with MCI at baseline, 8 progressed to a diagnosis of AD dementia at a subsequent biomarker measurement, while 48 remained stable at MCI (n = 27) or reverted to NC (n = 21).
3.2. Outcome 1: Diagnostic Status and Conversion
3.2.1. NfL
Baseline NfL and Diagnostic Group Comparisons
Results from the ANCOVA model (Table 2, Figure 1) demonstrated that individuals with AD dementia had statistically significantly higher levels of standardized log-transformed plasma NfL compared to NC (mean adjusted difference [mean diff.] = 0.68, 95% CI: [0.49, 0.88], p < .001) and MCI (mean diff. = 0.55, 95% CI: [0.37, 0.73], p < .001). Plasma NfL did not significantly discriminate between NC and MCI (mean diff. = 0.10, 95% CI: [−0.05, 0.26], p = .226). The AUC based on ROC analyses was also conducted using pairwise comparisons (Figure 2). For the comparison between NC and AD dementia, the AUC model including NfL with demographic and APOE covariates fell in the range of “excellent discrimination” at 0.83, according to interpretation guidelines set forth by Hosmer and Lemeshow (2000), and improved upon a baseline model including only the demographic and APOE covariates (AUC = 0.79). For the comparison between MCI and AD dementia, the AUC was in the range of “acceptable discrimination” at 0.78 (compared to 0.72 for the demographic model). The AUC for the model comparing NC and MCI fell below the range of “acceptable discrimination” at 0.69 and did not improve upon the model including only demographic and APOE covariates.
Table 2.
Post hoc comparisons between participant groups at baseline based on log transformed NfL and t-tau levels (converted to z-scores to facilitate interpretation). All analyses other than AUV are based on ANCOVA models controlling for age, sex, education, race, and APOE ε4 carrier status. Omnibus group effects were significant for both biomarkers.
| Adjusted Difference | 95% CI | F | p-value | |
|---|---|---|---|---|
| 2.1 NC (n = 238) vs MCI (n = 185) | ||||
| Baseline NfL | 0.10 | [−0.05, 0.26] | 1.74 | .226 |
| Baseline t-tau | 0.07 | [−0.13,0.27] | 0.45 | .501 |
| 2.2 NC (n = 238) vs AD (n = 156) | ||||
| Baseline NfL | 0.68 | [0.49, 0.88] | 47.80 | < .001 |
| Baseline t-tau | 0.33 | [0.10, 0.57] | 8.07 | .010 |
| 2.3 MCI (n = 185) vs AD (n = 156) | ||||
| Baseline NfL | 0.55 | [0.37, 0.73] | 33.70 | < .001 |
| Baseline t-tau | 0.21 | [−0.03, 0.44] | 3.01 | .126 |
p < .05 after correction for false discovery rate based on six analyses.
Figure 1.
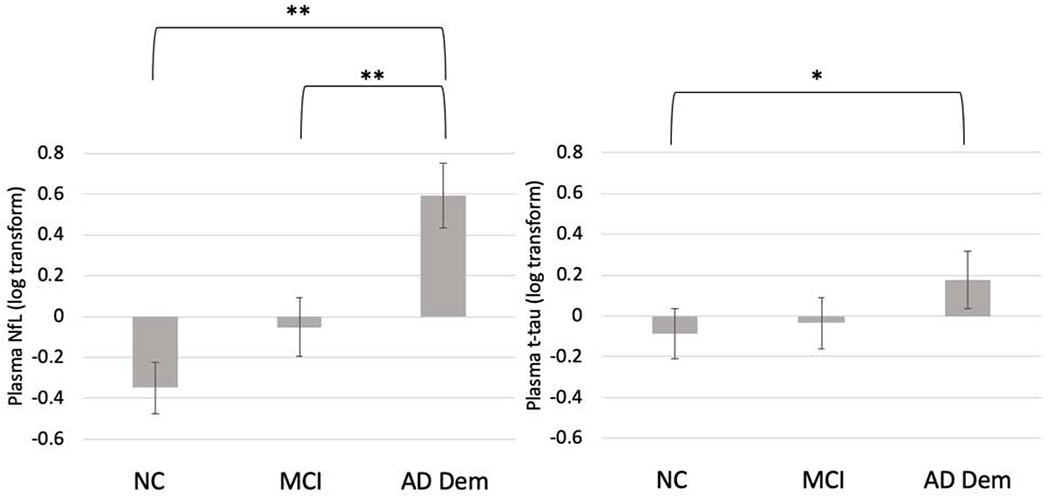
Mean plasma biomarker levels at baseline, separated by consensus diagnostic groups. Error bars represent the 95% confidence intervals. Values are shown as standardized log-transformed scores. *p < .01, *p < .001 based on results from ANCOVA models and post-hoc comparisons (shown in Table 2) accounting for demographic covariates and APOE ε4 carrier status.
Figure 2.
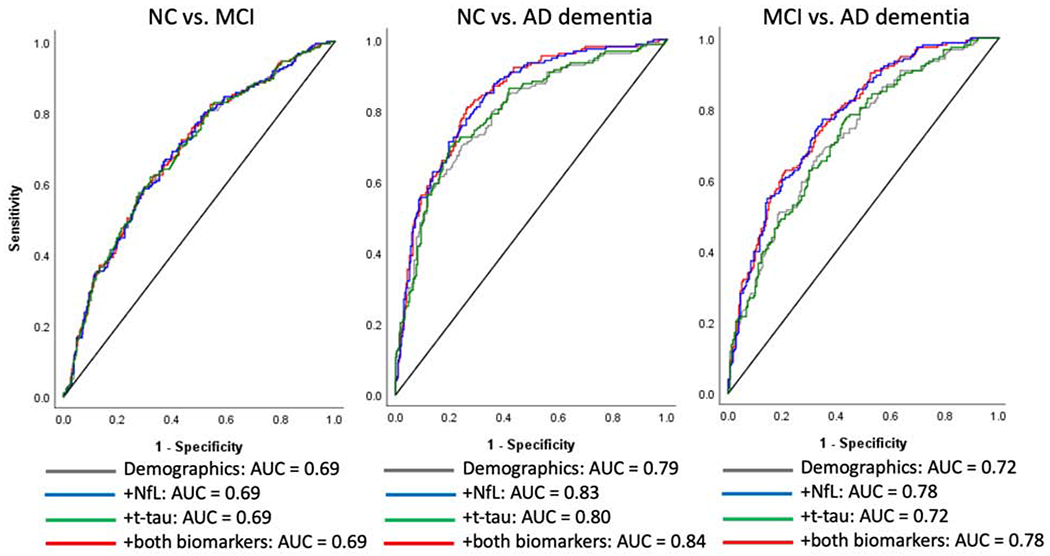
Receiver operating characteristic (ROC) curves for plasma NfL and t-tau comparing the area under the curve (AUC) for diagnostic comparisons at baseline, including models for plasma NfL, t-tau, and both biomarkers combined. Each graph displays a baseline model including only demographic covariates (age, sex, education, and race) and APOE ε4 carrier status, and models adding plasma NfL, t-tau, and simultaneously including both biomarkers.
NfL and Diagnostic Change
There were no statistically significant effects between log-transformed baseline NfL and odds for progression from NC to MCI or from MCI to AD dementia (Models 3.1 and 3.2 in Table 3). Similarly, there were no statistically significant associations between changes in NfL and conversion from NC to MCI or from MCI to AD dementia. See Models 3.3 and 3.4 in Table 3.
Table 3.
Binary and ordinal logistic regression models demonstrating the effect of log-transformed plasma NfL and t-tau in predicting subsequent cognitive change at the final study visit (3.1 and 3.2), and examining the correspondence between changes in biomarker level and corresponding diagnostic change over time (3.3 and 3.4). Odds ratios are based on the standardized values of log-transformed NfL or t-tau at baseline and represent the odds of having a more severe cognitive diagnosis for each SD increase in the biomarker at baseline (3.1 and 3.2) or biomarker change score across study visits (3.3 and 3.4). Age, sex, education, race, APOE ε4 carrier status, and the time from baseline to the final study visit are included as covariates in each model. p-values are adjusted based on a false discovery rate correction for eight comparisons.
| OR | 95% CI | Wald Z | p-value | |
|---|---|---|---|---|
| 3.1 Predicting NC to MCI/AD at Final Visit (n = 190) | ||||
| Baseline NfL | 1.37 | [0.92, 2.05] | 1.55 | .856 |
| Baseline t-tau | 0.74 | [0.51, 1.07] | 1.61 | .484 |
| 3.2 Predicting MCI to AD at Final Visit (n = 77) | ||||
| Baseline NfL | 0.64 | [0.28, 1.46] | 1.06 | .773 |
| Baseline t-tau | 0.83 | [0.44, 1.57] | 0.57 | .648 |
| 3.3 Biomarker Change: NC to MCI (n = 111) | ||||
| NfL Change Score | 1.20 | [0.50, 2.90] | 0.41 | .684 |
| t-tau Change Score | 1.15 | [0.82, 1.62] | 0.80 | .680 |
| 3.4 Biomarker Change: MCI to AD (n = 34) | ||||
| NfL Change Score | 0.43 | [0.06, 3.31] | 0.81 | .838 |
| t-tau Change Score | 0.76 | [0.37, 1.53] | 0.77 | .585 |
3.2.2. T-tau
Baseline T-tau and Diagnostic Group Comparisons
Table 2 and Figure 1 display the ability of standardized log-transformed plasma t-tau to discriminate between diagnostic groups at baseline after adjusting for relevant covariates. Plasma t-tau was significantly higher in participants with AD dementia at baseline compared to NC (mean diff. = 0.33, 95% CI: [0.10, 0.57], p = .010), but not significantly higher compared to MCI (mean diff. = 0.21, 95% CI: [−0.03, 0.44], p = .126). Plasma t-tau did not significantly differ between NC and MCI (mean diff. = 0.07, 95% CI: [−0.13, 0.27], p = .501). ROC analysis (Figure 2) revealed that the addition of t-tau to demographic and APOE covariates minimally improved discrimination between NC and AD dementia, improving the model from an AUC of 0.79 to 0.80; the overall model fit fell in the range of “excellent discrimination.” The addition of t-tau did not improve the AUC compared to demographic and APOE covariates alone in models examining the comparisons between NC and MCI (overall AUC = 0.69, falling below the range of “acceptable discrimination”) or between MCI and AD dementia (AUC = 0.72, “acceptable discrimination”).
Baseline T-tau and Diagnostic Change
Longitudinally, baseline plasma t-tau levels did not assist with prediction of future diagnostic changes among individuals with NC or MCI at baseline (Models 3.1 and 3.2 in Table 3). For participants with multiple biomarker measurements, changes in plasma t-tau levels over time did not correspond with changes in clinical status. The increase in t-tau did not significantly differ between individuals who progressed from NC to MCI compared to individuals who remained stable at NC, or between individuals with MCI who progressed to AD dementia compared to those who remained stable at MCI. Refer to Models 3.3-3.4 in Table 3.
3.2.3. Combination of both biomarkers
Additional ROC analyses tested whether inclusion of both biomarkers could improve diagnostic discrimination beyond each variable alone; these are displayed in Figure 2. The addition of t-tau did not significantly improve upon models with just NfL for the discrimination between NC and MCI (overall AUC = 0.69, below the range of “acceptable discrimination”) or between MCI and AD dementia (AUC = 0.78; “acceptable discrimination”). T-tau significantly discriminated NC and AD dementia groups (p = 0.049), but the AUC improved minimally compared to the model with NfL only, from 0.83 to 0.84, “excellent discrimination.”
3.3. Outcome 2: CDR levels
3.3.1. NfL
Among the 156 individuals diagnosed with AD dementia at baseline, 42 had a CDR score of 0.5, 76 had a CDR of 1, 26 had a CDR of 2, and 12 had a CDR score of 3. Higher levels of NfL were significantly associated (p < .001) with more severe dementia in an ordinal logistic regression model (Table 4). Each SD increase corresponded to a 94% increased risk of having a higher CDR level. However, baseline NfL did not significantly contribute to prediction of CDR rating at the final study visit among individuals diagnosed with dementia at baseline (p = .252). Likewise, for the 24 participants with dementia at baseline and NfL measurements available from multiple study visits, there was not a statistically significant relationship (p = .909) between changes in NfL over time and corresponding changes in CDR rating after accounting for the baseline value (Table 4).
Table 4.
Ordinal logistic regression models demonstrating the effect of plasma NfL and t-tau in discriminating between dementia severity levels at baseline (as measured by CDR rating; 4.1), predicting subsequent changes in CDR among individuals with dementia based on baseline NfL and t-tau (4.2), and examining the correspondence between biomarker change scores and CDR level over time (4.3). Odds ratios are based on the standardized values of log-transformed NfL or t-tau at baseline (i.e., z-scores) and represent the odds of having a more severe cognitive diagnosis rating for each SD increase in biomarker at baseline (4.1 and 4.2) or increase in biomarker between visits (4.3). Age, sex, education, race, and APOE ε4 carrier status are included as covariates in models 4.1 and 4.2. Only age, time since baseline, and baseline CDR values were used as covariates in 4.3 due to limited sample size (n = 24). p-values are adjusted based on a false discovery rate correction for six comparisons.
| OR | 95% CI | Wald Z | p-value | |
|---|---|---|---|---|
| 4.1 AD Dementia: Baseline CDR (n = 156) | ||||
| Baseline NfL | 1.94 | [1.35, 2.79] | 3.55 | < .001 |
| Baseline t-tau | 1.31 | [0.98, 1.76] | 1.80 | .207 |
| 4.2 CDR Rating at Final Visit (n = 116) | ||||
| Baseline NfL | 1.48 | [0.90, 2.44] | 1.53 | .252 |
| Baseline t-tau | 0.89 | [0.59, 1.35] | 0.54 | .704 |
| 4.3 Biomarker Change: CDR Ratings (n = 24) | ||||
| NfL Change Score | 1.13 | [0.14, 9.12] | 0.11 | .909 |
| t-tau Change Score | 0.34 | [0.04, 2.82] | 1.00 | .474 |
3.3.2. T-tau
Among participants with dementia at baseline, higher plasma t-tau was not significantly associated (p = .207) with more severe dementia on the CDR after accounting for relevant covariates. Total tau at baseline also did not significantly assist with the prediction of CDR rating the final study visit (p = .704), nor were changes in t-tau associated with corresponding changes in CDR across multiple study visits (p = .474; Table 4).
3.4. Outcome 3: Neuropsychological testing
Table 5 displays a partial correlation matrix examining the relationships between plasma NfL and log-transformed t-tau and neuropsychological test performance at baseline across the entire sample (N = 579). Higher concentrations of NfL were associated with worse performance across all tests. The absolute values of the correlation coefficients were 0.30 or greater for all tests other than DS (both forward and backward) and the BNT. Across the entire sample, t-tau was significantly correlated with only the MMSE (p = .041). The magnitudes of all correlations were smaller than those observed for plasma NfL.
Table 5.
Matrix displaying partial correlations between plasma log-transformed NfL and t-tau neuropsychological test performance at baseline across the entire sample (N = 579). Raw scores were used for all tests. Age, sex, education, race, and APOE ε4 carrier status are included as covariates.
| Test | NfL | t-tau |
|---|---|---|
| 1. MMSE | −0.36* | −0.13* |
| 2. Animals | −0.42* | −0.07 |
| 3. Vegetables | −0.36* | −0.05 |
| 4. Digits Forward | −0.13* | −0.04 |
| 5. Digits Backward | −0.24* | −0.06 |
| 6. BNT | −0.30* | −0.11 |
| 7. Logical Memory II | −0.35* | −0.07 |
| 8. NAB Trials 1-3 | −0.39* | −0.09 |
| 9. NAB Short Delay | −0.36* | −0.10 |
| 10. NAB Long Delay | −0.35* | −0.09 |
p < .05 based on false discovery rate for 10 comparisons for each biomarker.
Table 6 displays the results of GEE models demonstrating the relationships between baseline plasma NfL and performance on neuropsychological testing at follow-up visits. Higher values of NfL at baseline were significantly associated with worse performance on the MMSE, Vegetable Fluency, and Logical Memory II at subsequent study visits. In contrast, baseline t-tau was not significantly associated with subsequent performance on any neuropsychological test.
Table 6.
Results from generalized estimating equations predicting the relationship between baseline log-transformed plasma NfL and t-tau and subsequent change on neuropsychological testing in individuals diagnosed with NC or MCI at baseline who had longitudinal data available (n = 356). Coefficients represent the raw score difference for each SD increase of the biomarker; negative coefficients indicate worse cognitive performance with higher levels of each biomarker. Age at baseline, time since baseline, and the baseline score on the neuropsychological measure were included as covariates in all models. All study visits were included in these models. p-values are adjusted based on a false discovery rate correction for 10 comparisons for each biomarker.
| NfL | t-tau | |||||
|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | |
| 1. MMSE | −0.19 | 0.07 | .013 | −0.04 | 0.07 | .944 |
| 2. Animal Fluency | −0.12 | 0.22 | .729 | −0.19 | 0.27 | .789 |
| 3. Vegetable Fluency | −0.58 | 0.16 | < .001 | 0.21 | 0.17 | .999 |
| 4. Digits Forward | −0.02 | 0.04 | .786 | −0.03 | 0.04 | .719 |
| 5. Digits Backward | 0.06 | 0.06 | .564 | 0.02 | 0.05 | .703 |
| 6. BNT | −0.22 | 0.11 | .118 | 0.09 | 0.09 | .999 |
| 7. Logical Memory II | −0.67 | 0.21 | .005 | 0.09 | 0.17 | .672 |
| 8. NAB Trials 1-3 | −0.06 | 0.25 | .806 | 0.35 | 026 | .999 |
| 9. NAB Short Delay | −0.04 | 0.16 | .860 | 0.11 | 0.12 | .872 |
| 10. NAB Long Delay | −0.12 | 0.05 | .760 | 0.06 | 0.12 | .751 |
4. DISCUSSION
The current study evaluated the associations between plasma NfL and t-tau with the clinical presentation of AD in a longitudinal sample of 579 community-dwelling older adults from the BU ADC. At baseline, higher plasma NfL levels accurately discriminated participants with AD dementia from both NC and MCI with moderate effect sizes, as well as corresponded to greater dementia severity. Plasma NfL also significantly correlated with worse performance on all 10 neuropsychological tests examined, with moderate effect sizes for 8 of the tests (r’s ≥ 0.3). Baseline NfL did not predict diagnostic conversion, but higher baseline levels of NfL predicted decline on the MMSE and measures of verbal fluency and episodic memory. The effect sizes for prediction of decline were small (e.g., a decrease of 0.19 MMSE points per year for each SD increase in NfL). Plasma t-tau levels significantly discriminated between individuals with NC and AD dementia at baseline with a small effect size, but not between NC and MCI or between MCI and AD dementia. There were no statistically significant associations between t-tau and dementia severity or cognitive decline, and a statistically significant but small correlation coefficient was only found with the MMSE and none of the other neuropsychological tests. T-tau provided a statistically significant but not clinically meaningful improvement in discriminating between NC and AD dementia in a combined model with NfL. All results were independent of age, education, race, sex, and APOE ε4 allele status. These results support plasma NfL as a viable in vivo biomarker for the detection of AD dementia and monitoring of disease progression. In contrast, there was minimal evidence in support of plasma t-tau.
The results for NfL are consistent with previous research showing plasma NfL levels are elevated in AD dementia (Lin et al., 2018; Mattsson et al., 2017; Mattsson et al., 2019; Zhou et al., 2017) and are inversely associated with cognitive functioning (Lin et al., 2018; Mattsson et al., 2017; Preische et al., 2019; Sanchez-Valle et al., 2018; Zhou et al., 2017). In our sample, increased NfL at baseline was associated with worse performance on all neuropsychological tests administered and predicted declines on measures of global cognitive functioning, category fluency, and memory recall. Increased NfL is not only associated with AD but also with other neurological disorders, including vascular dementia, FTLD, amyotrophic lateral sclerosis, progressive supranuclear palsy, Huntington disease, TBI, multiple sclerosis, and Guillian-Barré syndrome (Bridel et al., 2019; Canto et al., 2019; Donker Kaat et al., 2018; Gaiottino et al., 2013; Shahim et al., 2016; Skillback, Farahmand, et al., 2014). NfL is therefore a non-specific biomarker of pathophysiological processes in the CNS that cause axonal and/or neuronal injury. This is notable in the context of the present sample of participants with AD dementia because AD dementia and neurodegeneration in AD can be result of mixed neuropathologies, including those that that affect the white matter, especially cerebrovascular disease (Alosco et al., 2018; Arvanitakis et al., 2016; Boyle et al., 2018; Lee et al., 2016; Snyder et al., 2015; Sweeney et al., 2019). In addition to neurodegeneration associated with AD neuropathologic changes, plasma NfL may also capture the co-morbid cerebrovascular and other white matter pathologies that frequently accompany AD. Regardless of the etiology, plasma NfL could be a useful marker to detect and monitor neurodegeneration, as well as track the effectiveness of disease-modifying treatments in AD and similar settings. Although neurodegeneration and axonal damage are observed throughout the course of AD, these become prominent with aging and disease progression (Jack et al., 2018). This may partially explain why the increased levels of plasma NfL were not observable until later in the clinical disease process (i.e., dementia), with no significant differences between NC and MCI (see Table 2 and Figure 1).
Research has indicated that increases in serum NfL can be observed prior to the onset of clinical symptoms in individuals with deterministic AD genetic mutations (Preische et al., 2019; Weston et al., 2017). Clinical trials have also indicated that NfL measurements may be effective for monitoring effects on neurodegeneration by disease-modifying therapeutics in AD (Mattsson et al., 2019; Zhou et al., 2017) and other disorders including multiple sclerosis (Novakova et al., 2017; Piehl et al., 2018) and spinal muscular atrophy (Olsson et al., 2019). In our study, there was not a significant relationship between longitudinal changes in diagnostic status and corresponding changes the plasma NfL. However, these analyses were notably limited by sample size and statistically under-powered.
Plasma t-tau was increased in individuals with AD dementia compared to NC. However, the effect size was small and there was no evidence to suggest that t-tau could predict longitudinal cognitive changes. Our finding that plasma t-tau is elevated in individuals in AD dementia but is unable to discriminate between NC and MCI is consistent with previous findings (Mattsson et al., 2016; Mielke et al., 2018; Zetterberg et al., 2013), as is the finding that t-tau does not predict diagnostic conversion (Mielke et al., 2017; Zetterberg et al., 2013). T-tau, like NfL, is a non-specific and general index of neurodegeneration that is elevated not only in AD, but also with exposure to repetitive head impacts (Alosco et al., 2017), Lewy body disease (Parnetti et al., 2008), and frontotemporal dementia (Sjogren et al., 2000). In our dataset, plasma t-tau and NfL appeared to be independent of each other, as no significant correlations were observed across the sample or within each group. Plasma t-tau may be less sensitive to detecting underlying AD-related neurodegeneration, particularly early in the clinical course of disease as observed in our sample and others (Mattsson et al., 2016; Yang et al., 2018; Zetterberg et al., 2013).
Despite our discouraging findings for plasma t-tau, other research supports its value. For example, Pase et al. (2019) found that plasma t-tau demonstrated similar predictive validity compared to CSF t-tau for determining risk for incipient dementia. Another study (Mielke et al., 2017) found that plasma t-tau significantly predicted declines on cognitive testing in a manner independent from amyloid PET imaging. Thus, plasma t-tau may still have a valuable role for determining risk and monitoring the clinical course of AD and continued research is needed.
A limitation of our findings was that we did not have data available to examine the convergence between plasma biomarkers and direct measures of CNS pathology, such as post-mortem AD neuropathologic changes, CSF measurements, or PET imaging, nor did we have an independent validation cohort available for further replication and confirmation of our findings. The lack of measures of direct CNS pathology has important implications. Approximately 17% of individuals who receive a clinical diagnosis of AD dementia do not have AD neuropathologic changes at autopsy (Beach et al., 2012). Therefore, there is potential for misclassification of AD in this sample given it was measured via clinical diagnoses. It precludes the ability to directly test whether the plasma biomarkers are capturing CNS pathological processes; it is indeed still unclear to what extent these plasma biomarkers truly represent CNS pathology (Mattsson et al., 2016; Zetterberg et al., 2013). Several previous studies using Simoa technology have evaluated the convergent validity between plasma biomarkers and other indices of AD neuropathological changes. Plasma NfL has been found to strongly correlate with CSF NfL (Gisslen et al., 2016; Mattsson et al., 2017) and is associated with a higher burden of neurofibrillary tangles and NfL in the medial temporal gyrus at autopsy (Ashton et al., 2019). Moreover, longitudinal increases in plasma NfL are associated with concurrent changes in CSF measurements of t-tau, p-tau and Aβ42, hippocampal atrophy, and FDG PET uptake (Mattsson et al., 2019). For plasma t-tau, the evidence is less convincing, as previous studies have shown weak correlations with CSF measurements (Fossati et al., 2019; Mattsson et al., 2016; Zetterberg et al., 2013) and it is unclear to what extent plasma t-tau truly reflects underlying CNS pathology. Future studies could attempt to further validate these biomarkers using these more specific indices of AD neuropathology and other types of pathologies (e.g., white matter alterations, cerebrovascular vascular disease).
The current study also did not examine plasma biomarkers of AD neuropathological changes, such as p-tau. Two recent studies compared plasma levels of p-tau and t-tau and observed that p-tau had stronger associations with amyloid and tau PET (Mielke et al., 2018) and provided superior discrimination between patients with MCI and AD dementia compared to t-tau (Yang et al., 2018). Another limitation is that the participants were volunteers in a longitudinal research study and the findings are likely more representative of a clinic-based population than the general population. The sample was also focused on the target population of AD and thus the results are unlikely to generalize to non-AD samples. As described in the Results, only 17 participants with a plasma sample in the group had non-AD cognitive impairment. Lastly, there was a relatively small number of individuals who progressed to MCI and/or dementia diagnoses at post-baseline measurements and there was a limited number of participants with multiple biomarker measurements. Thus, some of the null findings regarding the changes in biomarker level analyses could potentially represent Type II errors.
In conclusion, the current study examined plasma NfL and t-tau measurements as potential biomarkers of AD-related neurodegeneration in a large longitudinal sample of community-dwelling individuals. Overall, the results showed that plasma NfL and t-tau elevations were not observed until participants had dementia. Plasma NfL was more sensitive to cognitive functioning and prediction of cognitive decline and may be a more promising biomarker of neurodegeneration in AD compared to t-tau.
HIGHLIGHTS.
Baseline plasma NfL was higher in AD dementia compared to MCI and normal cognition
Baseline plasma NfL was associated with baseline cognitive test score and predicted subsequent decline, but did not predict diagnostic conversion
Baseline plasma t-tau was higher in AD dementia compared to MCI and normal cognition
There were no statistically significant effect for baseline plasma t-tau on cognitive decline or diagnostic conversion
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (P30AG013846; U01NS093334, U01NS086659-01; RF1AG05416; K23NS102399), the Department of Veterans’ Affairs (I01-CX001038). This publication was also supported by a Pilot Grant from the Boston University Alzheimer’s Disease Center (AG013846), as well as the National Center for Advancing Translational Sciences, National Institutes of Health, through BU-CTSI Grant Number 1UL1TR001430. The content is solely the responsibility of the authors. There is no sponsor.
Abbreviations:
- AD
Alzheimer’s disease
- ADC
Alzheimer’s Disease Center
- BU
Boston University
- BNT
Boston Naming Test
- LM-IA
Logical Memory Immediate Recall
- LM-IIA
Logical Memory Delayed Recall
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- NAB
Neuropsychological Assessment Battery
- NACC
National Alzheimer’s Coordinating Center
- NC
normal cognition
- NfL
neurofilament light
- t-tau
total tau
- UDS
Uniform Data Set
- WAIS
Wechsler Adult Intelligence Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interests: Robert A. Stern is a member of the Mackey-White Committee of the NFL Players Association. He is a paid consultant to Biogen (Cambridge, MA, USA) and Eli Lilly (Indianapolis, IN, USA). He receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA) and is a member of the Board of Directors of King-Devick Technologies (Chicago, IL, USA). Michael L. Alosco has received honorarium as a Scientific Advisor for Corino Therapeutics, Inc. There are no other possible conflicts of interest by other co-authors on this manuscript. For the remaining authors, there are no conflicts of interest to declare.
None of the data contained in the manuscript have not been previously published or are currently submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging. All authors have reviewed the contents of the manuscript being submitted, approve of its contents, and validate the accuracy of the data. All aspects of the study were approved by the human subjects review board and adhered to ethical guidelines.
References
- Albani D, Marizzoni M, Ferrari C, Fusco F, Boeri L, Raimondi I, Jovicich J, Babiloni C, Soricelli A, Lizio R, Galluzzi S, Cavaliere L, Didic M, Schonknecht P, Molinuevo JL, Nobili F, Parnetti L, Payoux P, Bocchio L, Salvatore M, Rossini PM, Tsolaki M, Visser PJ, Richardson JC, Wiltfang J, Bordet R, Blin O, Forloni G, Frisoni GB, PharmaCog C Plasma Abeta42 as a Biomarker of Prodromal Alzheimer’s Disease Progression in Patients with Amnestic Mild Cognitive Impairment: Evidence from the PharmaCog/E-ADNI Study. J Alzheimers Dis 2019; 69: 37–48. doi: 10.3233/JAD-180321 [DOI] [PubMed] [Google Scholar]
- Alosco ML, Sugarman MA, Besser LM, Tripodis Y, Martin B, Palmisano JN, Kowall NW, Au R, Mez J, DeCarli C, Stein TD, McKee AC, Killiany RJ, Stern RA. A Clinicopathological Investigation of White Matter Hyperintensities and Alzheimer’s Disease Neuropathology. J Alzheimers Dis 2018; 63: 1347–1360. doi: 10.3233/JAD-180017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Tripodis Y, Jarnagin J, Baugh CM, Martin B, Chaisson CE, Estochen N, Song L, Cantu RC, Jeromin A, Stern RA. Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimers Dement (Amst) 2017; 7: 33–40. doi: 10.1016/j.dadm.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 2016; 15: 934–943. doi: 10.1016/S1474-4422(16)30029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashendorf L, Jefferson AL, Green RC, Stern RA. Test-retest stability on the WRAT-3 reading subtest in geriatric cognitive evaluations. J Clin Exp Neuropsychol 2009; 31: 605–610. doi: 10.1080/13803390802375557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton NJ, Leuzy A, Lim YM, Troakes C, Hortobagyi T, Hoglund K, Aarsland D, Lovestone S, Scholl M, Blennow K, Zetterberg H, Hye A Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun 2019; 7: 5. doi: 10.1186/s40478-018-0649-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, Shimshek DR, Neumann U, Kahle PJ, Staufenbiel M, Neumann M, Maetzler W, Kuhle J, Jucker M Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron 2016; 91: 494–496. doi: 10.1016/j.neuron.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Beach TG, Monsell SE, Phillips LE, Kukull W Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol 2012; 71: 266–273. doi: 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA, Centers NI-AsD. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 2004; 18: 270–277. [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 1995; 57: 289–300. [Google Scholar]
- Blennow K Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx 2004; 1: 213–225. doi: 10.1602/neurorx.1.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med 2018; 284: 643–663. doi: 10.1111/joim.12816 [DOI] [PubMed] [Google Scholar]
- Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 2018; 83: 74–83. doi: 10.1002/ana.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the NFLG, Alvarez-Cermeno JC, Andreasson U, Axelsson M, Backstrom DC, Bartos A, Bjerke M, Blennow K, Boxer A, Brundin L, Burman J, Christensen T, Fialova L, Forsgren L, Frederiksen JL, Gisslen M, Gray E, Gunnarsson M, Hall S, Hansson O, Herbert MK, Jakobsson J, Jessen-Krut J, Janelidze S, Johannsson G, Jonsson M, Kappos L, Khademi M, Khalil M, Kuhle J, Landen M, Leinonen V, Logroscino G, Lu CH, Lycke J, Magdalinou NK, Malaspina A, Mattsson N, Meeter LH, Mehta SR, Modvig S, Olsson T, Paterson RW, Perez-Santiago J, Piehl F, Pijnenburg YAL, Pyykko OT, Ragnarsson O, Rojas JC, Romme Christensen J, Sandberg L, Scherling CS, Schott JM, Sellebjerg FT, Simone IL, Skillback T, Stilund M, Sundstrom P, Svenningsson A, Tortelli R, Tortorella C, Trentini A, Troiano M, Turner MR, van Swieten JC, Vagberg M, Verbeek MM, Villar LM, Visser PJ, Wallin A, Weiss A, Wikkelso C, Wild EJ. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol 2019. doi: 10.1001/jamaneurol.2019.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto E, Barro C, Zhao C, Caillier SJ, Michalak Z, Bove R, Tomic D, Santaniello A, Haring DA, Hollenbach J, Henry RG, Cree BAC, Kappos L, Leppert D, Hauser SL, Benkert P, Oksenberg JR, Kuhle J Association Between Serum Neurofilament Light Chain Levels and Long-term Disease Course Among Patients With Multiple Sclerosis Followed up for 12 Years. JAMA Neurol 2019. doi: 10.1001/jamaneurol.2019.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu MJ, Chen YF, Chen TF, Yang SY, Yang FP, Tseng TW, Chieh JJ, Chen JC, Tzen KY, Hua MS, Horng HE. Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer’s disease. Hum Brain Mapp 2014; 35: 3132–3142. doi: 10.1002/hbm.22390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Pinto JA Junior, Forlenza OV. Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? A systematic review and meta-analysis of the literature. World J Biol Psychiatry 2008; 9: 172–182. doi: 10.1080/15622970701535502 [DOI] [PubMed] [Google Scholar]
- Donker Kaat L, Meeter LH, Chiu WZ, Melhem S, Boon AJW, Blennow K, Zetterberg H, van Swieten JC. Serum neurofilament light chain in progressive supranuclear palsy. Parkinsonism Relat Disord 2018; 56: 98–101. doi: 10.1016/j.parkreldis.2018.06.018 [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014; 13: 614–629. doi: 10.1016/S1474-4422(14)70090-0 [DOI] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR Jr., Proceedings of the Meeting of the International Working, G, the American Alzheimer’s Association on “The Preclinical State of, AD, July, Washington Dc, USA. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 2016; 12: 292–323. doi: 10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati S, Ramos Cejudo J, Debure L, Pirraglia E, Sone JY, Li Y, Chen J, Butler T, Zetterberg H, Blennow K, de Leon MJ. Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer’s disease. Alzheimers Dement (Amst) 2019; 11: 483–492. doi: 10.1016/j.dadm.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, Bestwick JP, Monsch AU, Regeniter A, Lindberg RL, Kappos L, Leppert D, Petzold A, Giovannoni G, Kuhle J Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 2013; 8: e75091. doi: 10.1371/journal.pone.0075091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett BE, Lou KR, Daneshvar DH, Green RC, Jefferson AL, Stern RA. Diagnostic accuracy statistics for seven Neuropsychological Assessment Battery (NAB) test variables in the diagnosis of Alzheimer’s disease. Appl Neuropsychol Adult 2012; 19: 108–115. doi: 10.1080/09084282.2011.643947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslen M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, Blennow K, Zetterberg H Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 2016; 3: 135–140. doi: 10.1016/j.ebiom.2015.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse C, Rosengren L, Vanmechelen E, Vanderstichele H, Jensen C, Davidsson P, Blennow K Cerebrospinal fluid markers for Alzheimer’s disease evaluated after acute ischemic stroke. J Alzheimers Dis 2000; 2: 199–206. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S Applied Logistic Regression. John Wiley & Sons; New York: 2000. [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140: 566–572. doi: 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14: 535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Wong S, Gracer TS, Ozonoff A, Green RC, Stern RA. Geriatric performance on an abbreviated version of the Boston naming test. Appl Neuropsychol 2007; 14: 215–223. doi: 10.1080/09084280701509166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TL, Marcus DS, Fagan AM, Goate A, Fox NC, Cairns NJ, Holtzman DM, Buckles V, Ghetti B, McDade E, Martins RN, Saykin AJ, Masters CL, Ringman JM, Ryan NS, Forster S, Laske C, Schofield PR, Sperling RA, Salloway S, Correia S, Jack C Jr., Weiner M, Bateman RJ, Morris JC, Mayeux R, Brickman AM, Dominantly Inherited Alzheimer, N. White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann Neurol 2016; 79: 929–939. doi: 10.1002/ana.24647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, Maler JM, Kornhuber J, Blennow K, Zetterberg H Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s Research & Therapy 2018; 10. doi: 10.1186/s13195-018-0404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Lee WJ, Wang SJ, Fuh JL. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 2018; 8: 17368. doi: 10.1038/s41598-018-35766-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia LF, Kaeser SA, Reichwald J, Hruscha M, Martus P, Staufenbiel M, Jucker M Changes in amyloid-beta and Tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci Transl Med 2013; 5: 194re192. doi: 10.1126/scitranslmed.3006446 [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging, I. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 2017; 74: 557–566. doi: 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 2019; 76: 791–799. doi: 10.1001/jamaneurol.2019.0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, Palmqvist S, Baker D, Tan Hehir CA, Jeromin A, Hanlon D, Song L, Shaw LM, Trojanowski JQ, Weiner MW, Hansson O, Blennow K, Investigators A. Plasma tau in Alzheimer disease. Neurology 2016; 87: 1827–1835. doi: 10.1212/WNL.0000000000003246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Drachman D, Folstein MF, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group. Neurology 1984; 34: 939–944. [DOI] [PubMed] [Google Scholar]
- Menendez-Gonzalez M Routine lumbar puncture for the early diagnosis of Alzheimer’s disease. Is it safe? Front Aging Neurosci 2014; 6: 65. doi: 10.3389/fnagi.2014.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Hagen CE, Wennberg AMV, Airey DC, Savica R, Knopman DS, Machulda MM, Roberts RO, Jack CR Jr., Petersen RC, Dage JL. Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in the Mayo Clinic Study on Aging. JAMA Neurol 2017. doi: 10.1001/jamaneurol.2017.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, Airey DC, Knopman DS, Roberts RO, Machulda MM, Jack CR Jr., Petersen RC, Dage JL. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 2018; 14: 989–997. doi: 10.1016/j.jalz.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006; 20: 210–216. doi: 10.1097/01.wad.0000213865.09806.92 [DOI] [PubMed] [Google Scholar]
- Nabers A, Perna L, Lange J, Mons U, Schartner J, Guldenhaupt J, Saum KU, Janelidze S, Holleczek B, Rujescu D, Hansson O, Gerwert K, Brenner H Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol Med 2018; 10. doi: 10.15252/emmm.201708763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, Fowler C, Li QX, Martins R, Rowe C, Tomita T, Matsuzaki K, Ishii K, Ishii K, Arahata Y, Iwamoto S, Ito K, Tanaka K, Masters CL, Yanagisawa K High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018; 554: 249–254. doi: 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- Novakova L, Axelsson M, Khademi M, Zetterberg H, Blennow K, Malmestrom C, Piehl F, Olsson T, Lycke J Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult Scler 2017; 23: 62–71. doi: 10.1177/1352458516639384 [DOI] [PubMed] [Google Scholar]
- Olsson B, Alberg L, Cullen NC, Michael E, Wahlgren L, Kroksmark AK, Rostasy K, Blennow K, Zetterberg H, Tulinius M NFL is a marker of treatment response in children with SMA treated with nusinersen. J Neurol 2019; 266: 2129–2136. doi: 10.1007/s00415-019-09389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, Holtta M, Rosen C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 2016; 15: 673–684. doi: 10.1016/S1474-4422(16)00070-3 [DOI] [PubMed] [Google Scholar]
- Parnetti L, Tiraboschi P, Lanari A, Peducci M, Padiglioni C, D’Amore C, Pierguidi L, Tambasco N, Rossi A, Calabresi P Cerebrospinal fluid biomarkers in Parkinson’s disease with dementia and dementia with Lewy bodies. Biol Psychiatry 2008; 64: 850–855. doi: 10.1016/j.biopsych.2008.02.016 [DOI] [PubMed] [Google Scholar]
- Pase MP, Beiser AS, Himali JJ, Satizabal CL, Aparicio HJ, DeCarli C, Chene G, Dufouil C, Seshadri S Assessment of Plasma Total Tau Level as a Predictive Biomarker for Dementia and Related Endophenotypes. JAMA Neurol 2019. doi: 10.1001/jamaneurol.2018.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl F, Kockum I, Khademi M, Blennow K, Lycke J, Zetterberg H, Olsson T Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler 2018; 24: 1046–1054. doi: 10.1177/1352458517715132 [DOI] [PubMed] [Google Scholar]
- Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, Graber S, Kuder-Buletta E, LaFougere C, Laske C, Voglein J, Levin J, Masters CL, Martins R, Schofield PR, Rossor MN, Graff-Radford NR, Salloway S, Ghetti B, Ringman JM, Noble JM, Chhatwal J, Goate AM, Benzinger TLS, Morris JC, Bateman RJ, Wang G, Fagan AM, McDade EM, Gordon BA, Jucker M, Dominantly Inherited Alzheimer, N. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 2019; 25: 277–283. doi: 10.1038/s41591-018-0304-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie C, Smailagic N, Noel-Storr AH, Ukoumunne O, Ladds EC, Martin S CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 2017; 3: CD010803. doi: 10.1002/14651858.CD010803.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Valle R, Heslegrave A, Foiani MS, Bosch B, Antonell A, Balasa M, Llado A, Zetterberg H, Fox NC. Serum neurofilament light levels correlate with severity measures and neurodegeneration markers in autosomal dominant Alzheimer’s disease. Alzheimers Res Ther 2018; 10: 113. doi: 10.1186/s13195-018-0439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Barthelemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, Sullivan M, Crisp MJ, Kasten T, Kirmess KM, Kanaan NM, Yarasheski KE, Baker-Nigh A, Benzinger TLS, Miller TM, Karch CM, Bateman RJ. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron 2018; 98: 861–864. doi: 10.1016/j.neuron.2018.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Holtzman DM, Morris JC, Benzinger TLS, Xiong C, Fagan AM, Bateman RJ. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019. doi: 10.1212/WNL.0000000000008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahim P, Gren M, Liman V, Andreasson U, Norgren N, Tegner Y, Mattsson N, Andreasen N, Ost M, Zetterberg H, Nellgard B, Blennow K Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 2016; 6: 36791. doi: 10.1038/srep36791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren M, Minthon L, Davidsson P, Granerus AK, Clarberg A, Vanderstichele H, Vanmechelen E, Wallin A, Blennow K CSF levels of tau, beta-amyloid(1-42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J Neural Transm (Vienna) 2000; 107: 563–579. doi: 10.1007/s007020070079 [DOI] [PubMed] [Google Scholar]
- Skillback T, Farahmand B, Bartlett JW, Rosen C, Mattsson N, Nagga K, Kilander L, Religa D, Wimo A, Winblad B, Rosengren L, Schott JM, Blennow K, Eriksdotter M, Zetterberg H CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 2014; 83: 1945–1953. doi: 10.1212/WNL.0000000000001015 [DOI] [PubMed] [Google Scholar]
- Skillback T, Rosen C, Asztely F, Mattsson N, Blennow K, Zetterberg H Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol 2014; 71: 476–483. doi: 10.1001/jamaneurol.2013.6455 [DOI] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement 2015; 11: 710–717. doi: 10.1016/j.jalz.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJJ, Jacobs RE, Doubal FN, Ramirez J, Black SE, Nedergaard M, Benveniste H, Dichgans M, Iadecola C, Love S, Bath PM, Markus HS, Salman RA, Allan SM, Quinn TJ, Kalaria RN, Werring DJ, Carare RO, Touyz RM, Williams SCR, Moskowitz MA, Katusic ZS, Lutz SE, Lazarov O, Minshall RD, Rehman J, Davis TP, Wellington CL, Gonzalez HM, Yuan C, Lockhart SN, Hughes TM, Chen CLH, Sachdev P, O’Brien JT, Skoog I, Pantoni L, Gustafson DR, Biessels GJ, Wallin A, Smith EE, Mok V, Wong A, Passmore P, Barkof F, Muller M, Breteler MMB, Roman GC, Hamel E, Seshadri S, Gottesman RF, van Buchem MA, Arvanitakis Z, Schneider JA, Drewes LR, Hachinski V, Finch CE, Toga AW, Wardlaw JM, Zlokovic BV. Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement 2019; 15: 158–167. doi: 10.1016/j.jalz.2018.07.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, Kondo M, Allsop D, Tokuda T Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener 2017; 12: 63. doi: 10.1186/s13024-017-0206-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harten AC, Kester MI, Visser PJ, Blankenstein MA, Pijnenburg YA, van der Flier WM, Scheltens P Tau and p-tau as CSF biomarkers in dementia: a meta-analysis. Clin Chem Lab Med 2011; 49: 353–366. doi: 10.1515/CCLM.2011.086 [DOI] [PubMed] [Google Scholar]
- Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM, Scheltens P, Teunissen CE, van der Flier WM. Plasma Amyloid as Prescreener for the Earliest Alzheimer Pathological Changes. Ann Neurol 2018; 84: 648–658. doi: 10.1002/ana.25334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009; 23: 91–101. doi: 10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston PSJ, Poole T, Ryan NS, Nair A, Liang Y, Macpherson K, Druyeh R, Malone IB, Ahsan RL, Pemberton H, Klimova J, Mead S, Blennow K, Rossor MN, Schott JM, Zetterberg H, Fox NC. Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology 2017; 89: 2167–2175. doi: 10.1212/WNL.0000000000004667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240–246. doi: 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- Yang CC, Chiu MJ, Chen TF, Chang HL, Liu BH, Yang SY. Assay of Plasma Phosphorylated Tau Protein (Threonine 181) and Total Tau Protein in Early-Stage Alzheimer’s Disease. J Alzheimers Dis 2018; 61: 1323–1332. doi: 10.3233/JAD-170810 [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Blennow K From Cerebrospinal Fluid to Blood: The Third Wave of Fluid Biomarkers for Alzheimer’s Disease. J Alzheimers Dis 2018; 64: S271–S279. doi: 10.3233/JAD-179926 [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Skillback T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K, Alzheimer’s Disease Neuroimaging, I. Association of Cerebrospinal Fluid Neurofilament Light Concentration With Alzheimer Disease Progression. JAMA Neurol 2016; 73: 60–67. doi: 10.1001/jamaneurol.2015.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, Hansson O Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther 2013; 5: 9. doi: 10.1186/alzrt163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhang J, Ye F, Xu G, Su H, Su Y, Zhang X, Alzheimer’s Disease Neuroimaging, I. Plasma neurofilament light chain levels in Alzheimer’s disease. Neurosci Lett 2017; 650: 60–64. doi: 10.1016/j.neulet.2017.04.027 [DOI] [PubMed] [Google Scholar]


