Abstract
Objectives:
The role of topical anti-infectives in acute exacerbations of chronic rhinosinusitis is controversial. Povidone-iodine is an anti-bacterial and anti-viral that is affordable and available over-the-counter and may demonstrate advantages over mupirocin as a sinus irrigation therapy. The objective was to compare povidone-iodine or mupirocin versus saline sinus irrigations for sinusitis exacerbations in post-surgery subjects as well as to assess tolerability of povidone-iodine sinus irrigations.
Materials and Methods:
This was a prospective single-blinded (clinician only) randomized controlled trial. Subjects were post-surgery with acute exacerbations of chronic rhinosinusitis and gram-positive bacteria on culture. They received povidone-iodine, mupirocin, or saline sinus irrigations, twice daily for 30 days. Outcomes were post-treatment culture negativity (primary) and Sinonasal Outcome Test-20 and Lund-Kennedy endoscopic score change (secondary).
Results:
Of the 62 subjects analyzed, post-treatment culture negativity rate was higher in the MUP (14/20, 70%) group compared to the PI (9/21, 43%) and SAL (9/19, 47%) groups, although this was not significant (p=0.28). Povidone-iodine sinus irrigations at the 1% concentration were very well-tolerated, similar to saline irrigations. There were no significant differences in Sinonasal Outcome Test-20 score (povidone-iodine −0.3 [−0.6, 0.05] vs. mupirocin −0.3 [−0.7, 0.05] vs. saline −0.4 [−0.8, 0.05]; p=0.74) or Lund-Kennedy endoscopic score (povidone-iodine −3.5 [−7, −0.5] vs. mupirocin −2 [−4, 2] vs. saline −3 [−5, 0]; p=0.35) change. No serious adverse effects were reported.
Conclusions:
In patients who have had prior sinus surgery with acute exacerbations of CRS and gram-positive bacteria on culture, mupirocin sinus irrigations achieved a better post-treatment culture “control” rate compared to saline and povidone-iodine. In addition, 1% povidone-iodine solution was well-tolerated as a sinus irrigation and may represent a feasible method for temporarily disinfecting the sinonasal cavity of bacteria and viruses such as COVID-19.
Keywords: Sinusitis, Microbiota, Anti-Infective Agents, Therapeutic Irrigation, Sino-Nasal Outcome Test, Endoscopy, Quality of Life
1. INTRODUCTION
Sinusitis is common, affecting 14% of adults, with a subset of these patients developing chronic rhinosinusitis (CRS).[1] Even with sinus surgery and medical therapy, some patients with CRS continue to have acute exacerbations.[2–4] An acute exacerbation of CRS has not been strictly defined, but existing definitions involve symptom worsening, with purulent discharge on endoscopy indicative of an infectious trigger.[5] In these patients requiring frequent systemic therapy for symptom and disease control, topical high-volume irrigation administers a greater concentration of drug directly to the site of infection and reduces the potential for adverse effects. In particular, culture-directed topical anti-infectives may be an option if the trigger is infectious, as evidenced by purulent discharge on endoscopy.[4,6]
In the CRS population, gram-positive bacteria, notably Staphylococcus aureus, are the most commonly identified and persistent organisms by standard culture, likely related to their propensity for biofilm formation, intracellular residence, and superantigen production.[2,7,8] Previous studies have focused on the adjunctive use of mupirocin irrigations to target Staphylococcus aureus and have demonstrated significant decreases in re-culture rates. Unfortunately, these benefits ultimately have relatively high microbiological failure rates.[2,3,8,9] Factors that have limited mupirocin irrigation use include concerns regarding resistance development, unknown effectiveness, and lack of insurance coverage for compounded medications.[10]
The bactericidal activity of povidone-iodine is well-established, demonstrating a bell-shaped curve for killing effect that peaks at 1% povidone-iodine.[11,12] It has also been shown to rapidly inactivate a number of upper respiratory viruses, including severe acute respiratory distress syndrome, Middle East respiratory syndrome coronavirus, rotavirus, and influenza virus A subtype H1N1 at concentrations as low at 0.23%.[13–15] In the clinical setting, varying concentrations up to 10% povidone-iodine have been studied and demonstrated efficacy with minimal side effects, mainly related to uses in hand disinfection, surgical skin preparation, and wound irrigation.[16,17] There is very limited data on the toxicity profile of povidone-iodine in sinonasal epithelial cells specifically. Recent in vitro data has demonstrated that it is not ciliotoxic at the 0.5% concentration but is at the 5% concentration.[15,18] Thus far, only one clinical study has been done investigating nasal povidone-iodine, delivered as a swab at the 5% concentration, but the primary endpoint was surgical site infections. The only side effect related to povidone-iodine application was a vasovagal reaction during swab administration.[19]
Given the efficacy shown with povidone-iodine in other uses, lack of any known resistance development, and its affordability and availability over-the-counter, it has potential as a novel anti-infective sinus irrigation therapy. Povidone-iodine may also be a useful delivery method for temporarily disinfecting the sinonasal cavity for viruses such as COVID-19, which demonstrates a high viral load in the sinonasal mucosa.[20,21] Therefore, the aim of this study was to determine, in subjects who have had prior sinus surgery, the tolerability and effectiveness of povidone-iodine or mupirocin versus saline sinus irrigations in the treatment of acute exacerbations of CRS with gram-positive bacteria on culture.
2. MATERIALS AND METHODS
2.1. Participants
This study was a prospective single-blinded (clinician only) randomized controlled trial (RCT) conducted at the University of Washington Medical Center. Our local institutional review board granted ethics approval. Patients were approached for this study between October 2014 and October 2015. Eligible patients were post-sinus surgery with patent sinus ostia on endoscopic examination, and had ongoing signs and symptoms of CRS (as defined by the American Academy of Otolaryngology – Head and Neck Surgery clinical practice guidelines).[22] Patients meeting these criteria then had an endoscopically-collected sinus culture and were enrolled if the culture grew out Staphylococcus or Streptococcus species. Exclusion criteria were <18 years of age, terminal illness, significant immune dysfunction, severe or emergent complications from CRS or presence of a sinus tumor, unwillingness to stop other topical anti-infective sinus irrigations if already receiving them, and factors associated with a potential risk of adverse effects with povidone-iodine (iodine sensitivity, thyroid disease that necessitated a low iodine diet, or renal disease).[16]
2.2. Randomization and Intervention
Subjects were block randomized in sets of 6 to receive 1 of the following 3 treatments: 1% povidone-iodine (PI), 0.05% mupirocin (MUP), or saline (SAL) control sinus irrigations. The 1% povidone-iodine concentration has peak bactericidal activity and is also below ciliotoxic levels.[11,12,15] The 0.05% mupirocin concentration is a common formulation, both in our clinical practice and at other institutions.[2,3,9] An electronic random number generator (Sealed Envelope Ltd; London, UK) was used to create a blocked randomization list. A member of the research team (VSL) was informed of the next assigned irrigation treatment on the list at the time of enrollment.
All supplies and instructions to make the assigned irrigation treatment were provided to the subject (Table 1). Subjects irrigated with ½ of the bottle twice daily for 30 days. They were also informed to use previously boiled or distilled water, to clean the rinse bottle at the end of each day, and to use a new bottle after 15 days in order to reduce the risk of bacterial contamination.
Table 1.
Recipe for Irrigation Treatments.
| Treatment | Supplies | Instructions |
|---|---|---|
| 1% Povidone-Iodine | 2 rinse bottles1 Buffered salt packet1 10% povidone-iodine solution2 3 mL syringe |
Mix 2.4 mL 10% povidone-iodine solution (measured using syringe) with buffered salt packet and 240mL of water4 in rinse bottle |
| 0.05% Mupirocin | 2 rinse bottles1 Buffered salt packet1 125 mg mupirocin capsule3 |
Mix contents of 125 mg mupirocin capsule with buffered salt packet and 240 mL of water4 in rinse bottle |
| Saline Control | 2 rinse bottles1 Buffered salt packet1 |
Mix buffered salt packet with 240 mL of water4 in rinse bottle |
NeilMed Pharmaceuticals (Santa Rosa, CA).
Betadine®, Purdue Products L.P. (Stamford, CT).
Compounding pharmacy.
Previously boiled or distilled.
Each subject also was offered a medical treatment regimen for active CRS, consisting of a culture-directed oral antibiotic for up to 3 weeks, and/or oral steroids depending on the presence of polyps/inflammation for up to 3 weeks, and/or high-volume topical steroid sinus irrigations (budesonide 0.5 mg/2 mL vial or 0.6 mg/2 mL capsule, ½ bottle to each nasal cavity twice daily) depending on the presence of polyps/inflammation for 30 days. Per hospital infectious disease recommendations, a 4% chlorhexidine gluconate (HIBICLENS®; Mölnlycke Health Care; Norcross, Georgia) body wash for 3 days was recommended to minimize bacterial cross-contamination from skin to sinus.
2.3. Outcomes
The primary outcome was post-treatment culture negativity, defined as “negative” if the pathogen(s) targeted on pre-treatment culture (Staphylococcus, Streptococcus, or both) was (were) absent. Using a 30-degree rigid endoscope for visualization, a sterile alligator forceps and culture swab (Becton, Dickinson and Company, Franklin Lakes, NJ) or Xomed Sinus Secretion Collector (Medtronic-Xomed, Jacksonville, FL) were used to collect cultures. To assess tolerability, discomfort associated with the irrigations was measured using a visual analog scale (VAS, continuous scale comprised of a 100 mm horizontal line bounded by no pain [0] to worst imaginable pain [100]). Additional secondary outcomes assessed were the Sinonasal Outcome Test-20 score (SNOT-20, consisting of 20 items, each scored from 0–5; total score recorded as the average of all items, 0–5) and Lund-Kennedy endoscopic score (consisting of 10 items, each scored from 0–2; total score recorded as the sum of all items, 0–20) changes from baseline.[23,24]
2.4. Sample Size
The randomized controlled study comparing mupirocin and saline sinus irrigations published by Jervis-Bardy et al. (2012) found post-treatment culture positivity of 11% and 100% respectively, a nearly 90% difference between the groups.2 More conservatively, we powered our study to detect a 45% difference between the PI or MUP and SAL control groups. Assuming a power of 0.80, significance level of 0.05, and 10% loss to follow-up rate based on our clinic’s experience, our total target enrollment was 54 subjects, or 18 subjects in each group.
2.5. Data Collection
The clinic visit at time of enrollment was the pre-treatment time point. Descriptive characteristics, including demographics (age, sex, and race), Lund-Mackay CT score (for the scan closest to the treatment period), relevant comorbidities (nasal polyposis, asthma, inhalant allergies, aspirin sensitivity, cystic fibrosis, immunodeficiency, vasculitis, smoking, and depression), concurrent therapies (oral antibiotics or steroids and topical steroid sinus irrigations), and recent sinus surgery (within 6 weeks prior to enrollment), as well as SNOT-20 scores, were recorded.[25] A nasal endoscopy was performed and an endoscopically-collected sinus culture obtained. A member of the research team blinded to the assigned irrigation treatment graded the Lund-Kennedy endoscopic score (GED).
Subjects returned to the clinic after 30 days of treatment. Again, a nasal endoscopy was performed and an endoscopically-collected sinus culture obtained. A member of the research team blinded to the assigned irrigation treatment reviewed the endoscopic exam and completed the Lund-Kennedy endoscopic score (GED). Subjects also filled out the SNOT-20 questionnaire and VAS form.
2.6. Statistical Analysis
Statistical analyses were performed using Stata/IC 13.1 software (StataCorp LP, College Station, TX). Distribution and summary statistics were evaluated for descriptive characteristics and VAS scores. Data were examined for normality prior to hypothesis testing. To assess for inadequate randomization of known confounders and the need for adjusted analyses of outcome data, a chi-squared test for binary variables and an ANOVA test for continuous variables were performed evaluating differences in descriptive characteristics among treatment groups. A global p-value <0.2 was considered significant and the criterion to perform adjusted analyses.
For post-treatment culture negativity rate, logistic regression analysis was used to perform adjusted comparisons among the treatment groups. For the VAS score and SNOT-20 score and Lund-Kennedy endoscopic score changes from baseline, linear regression analyses were used to perform adjusted comparisons among the treatment groups. An intention-to-treat analysis was performed primarily, and a per-protocol analysis was performed secondarily. Median and interquartile range are presented unless otherwise specified. A global p-value less than 0.05 was considered significant and the criterion to perform post hoc individual comparisons among the treatment groups.
3. RESULTS
3.1. Study Overview
A total of 65 subjects were randomized (Figure 1). In the PI group (n=22), 1 subject was excluded from the analysis due to loss to follow-up. In the MUP group (n=22), no subjects were excluded from the analysis. In the SAL group (n=21), 2 subjects were excluded from the analysis due to loss to follow-up. One subject discontinued treatment in the PI group due to staining of linens, crossing over to the SAL group. Two subjects discontinued treatment in the MUP group due to lack of insurance coverage for compounded medications and therefore were unable to afford the treatment, crossing over to the SAL group. The study ended because the targeted sample size was achieved.
Figure 1. Participant Flow.
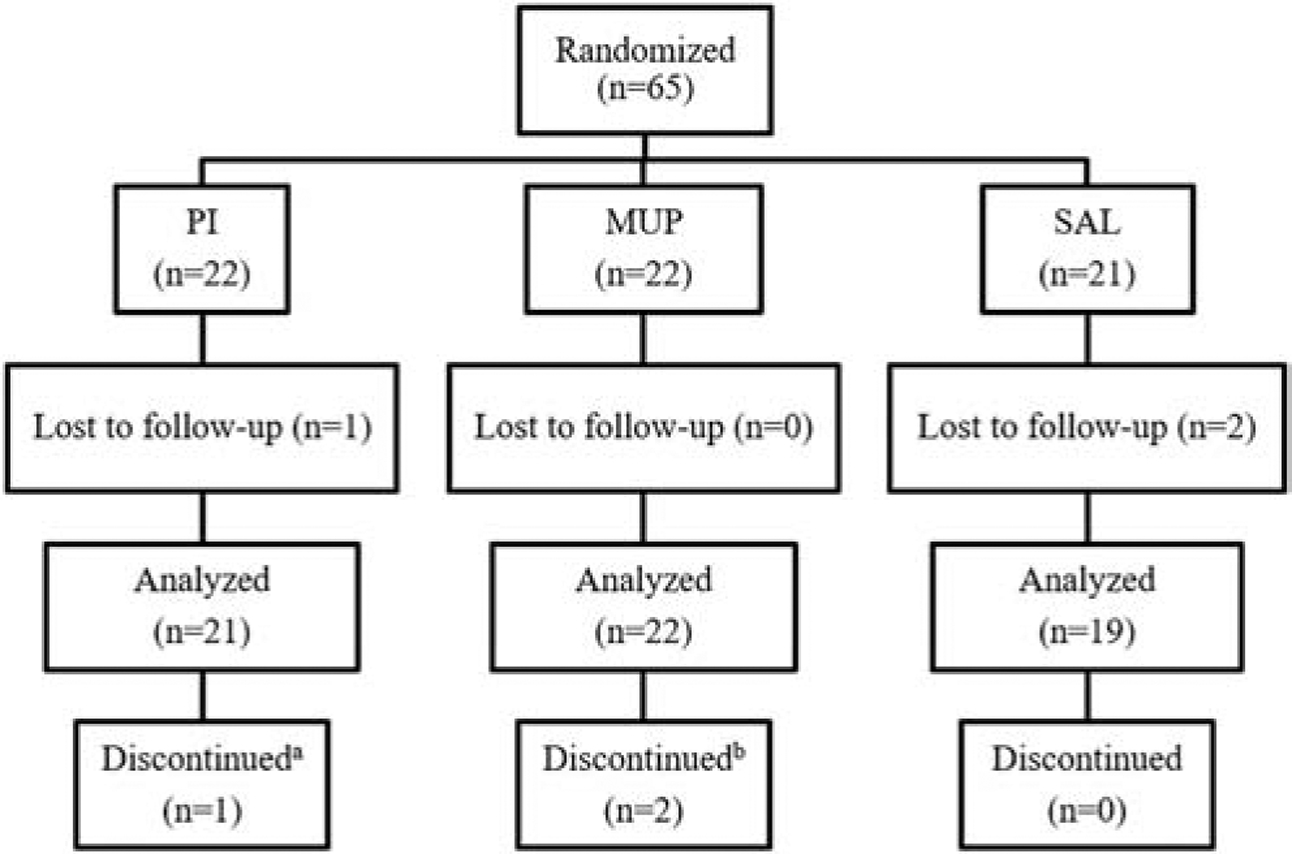
PI = povidone-iodine, MUP = mupirocin, SAL = saline.
3.2. Descriptive Characteristics
A total of 62 subjects were included in the analysis. Comorbidities are depicted in Table 2 and represent characteristics consistent with challenging CRS. There were significant differences among the treatment groups in age and the number of subjects with nasal polyposis, vasculitis, smoking, depression, and on concurrent oral steroids. These characteristics were therefore adjusted for in the analyses of the outcome data.
Table 2.
Descriptive Characteristics (n=62).
| PI (n=21) | MUP (n=22) | SAL (n=19) | p-value | |
|---|---|---|---|---|
| Age (years) | 44 (36–60) | 48 (36–58) | 58 (52–62) | 0.13* |
| Female | 7 (33%) | 9 (41%) | 11 (58%) | 0.28 |
| Caucasian | 19 (90%) | 20 (91%) | 16 (84%) | 0.76 |
| Lund-Mackay CT score1 | 11 (8–15) | 11 (7–16) | 11 (6–19) | 0.97 |
| Nasal polyposis | 15 (71%) | 10 (45%) | 9 (47%) | 0.17* |
| Asthma | 11 (52%) | 10 (45%) | 11 (58%) | 0.73 |
| Inhalant allergies | 16 (76%) | 14 (64%) | 14 (74%) | 0.63 |
| Aspirin sensitivity | 5 (24%) | 2 (9%) | 2 (11%) | 0.33 |
| Cystic fibrosis | 1 (5%) | 4 (18%) | 1 (5%) | 0.24 |
| Immunodeficiency | 3 (14%) | 1 (5%) | 0 (0%) | 0.17 |
| Vasculitis | 3 (14%) | 0 (0%) | 3 (16%) | 0.16* |
| Smoking | 0 (0%) | 4 (18%) | 1 (5%) | 0.08* |
| Depression | 1 (5%) | 6 (27%) | 3 (16%) | 0.13* |
| Oral antibiotics | 10 (48%) | 11 (50%) | 10 (53%) | 0.95 |
| Oral steroids | 4 (19%) | 4 (18%) | 9 (47%) | 0.06* |
| Topical steroids | 18 (86%) | 15 (68%) | 15 (79%) | 0.38 |
| Recent sinus surgery | 10 (48%) | 6 (27%) | 7 (37%) | 0.39 |
| Time to follow-up | 45 (32–72) | 49 (42–59) | 36 (28–52) | N/A |
PI = povidone-iodine, MUP = mupirocin, SAL = saline.
Median (interquartile range) or number (%) of patients is presented.
Radiologic grading of sinus systems, consisting of 6 items for each nasal cavity, each scored from 0–2; total score recorded as the sum of all items, 0–24.
indicates global significant differences among groups at the 0.2 level.
3.3. Outcome Measures
3.3.1. Primary Outcome Measure
A higher post-treatment culture negativity rate is a better response. Post-treatment culture negativity rate was higher in the MUP (14/20, 70%) group compared to the PI (9/21, 43%) and SAL (9/19, 47%) groups. An adjusted logistic regression analysis, however, found no significant differences among the treatment groups (global p-value = 0.28; Figure 2).
Figure 2. Bar plot comparison of post-treatment culture negativity (p=0.28).
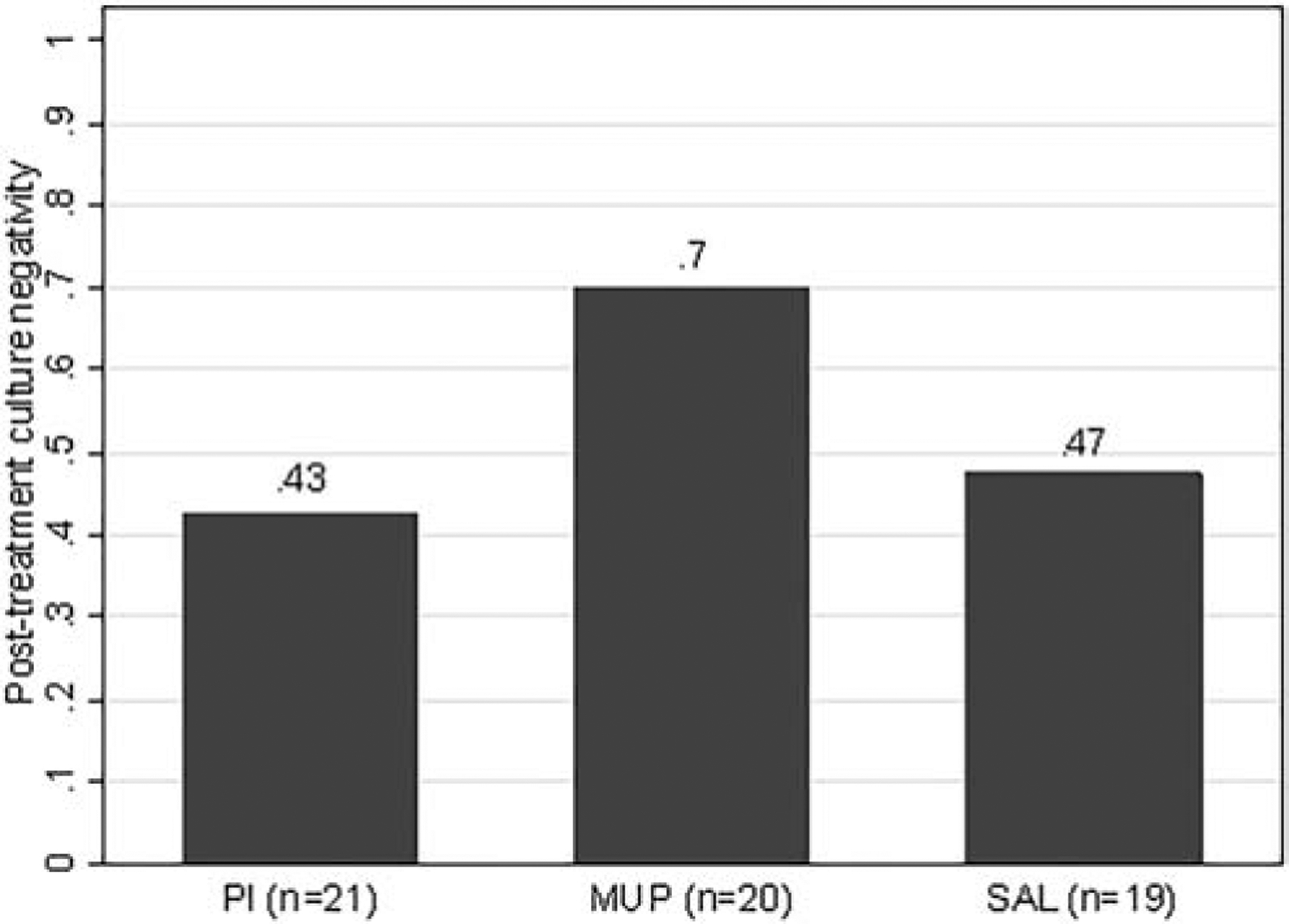
PI = povidone-iodine, MUP = mupirocin, SAL = saline.
Proportion of negative post-treatment cultures is provided for each treatment group above the respective bar.
Missing outcome data due to difficulty coordinating clinic logistics for data collection: post-treatment culture in MUP group (n=2).
3.3.2. Tolerability and Adverse Effects
Median VAS scores (measured in millimeters out of 100) were low in all treatment groups (PI 9mm [2, 15] vs. MUP 2mm [0, 7] vs. SAL 4mm [0, 10]), indicating all irrigations were well-tolerated, including PI at the 1% concentration. No serious adverse effects, such as bronchospasm, serum toxicity, nephrotoxicity, or ototoxicity, were reported with any of the irrigation treatments.
3.3.3. SNOT-20 and Lund-Kennedy Endoscopic Scores
A negative SNOT-20 score change represents an improvement, and the more negative the value, the better the response. A change of −0.8 is considered a clinically significant improvement.[24] SNOT-20 score change from baseline improved similarly in the PI (−0.3 [−0.6, 0.05]), MUP (−0.3 [−0.7, 0.05]), and SAL (−0.4 [−0.8, 0.05]) groups, with no group achieving a clinically significant improvement. An adjusted linear regression analysis found no significant differences among the treatment groups (global p-value = 0.74; Figure 3).
Figure 3. Box plot comparison of SNOT-20 score change from baseline (p=0.74).
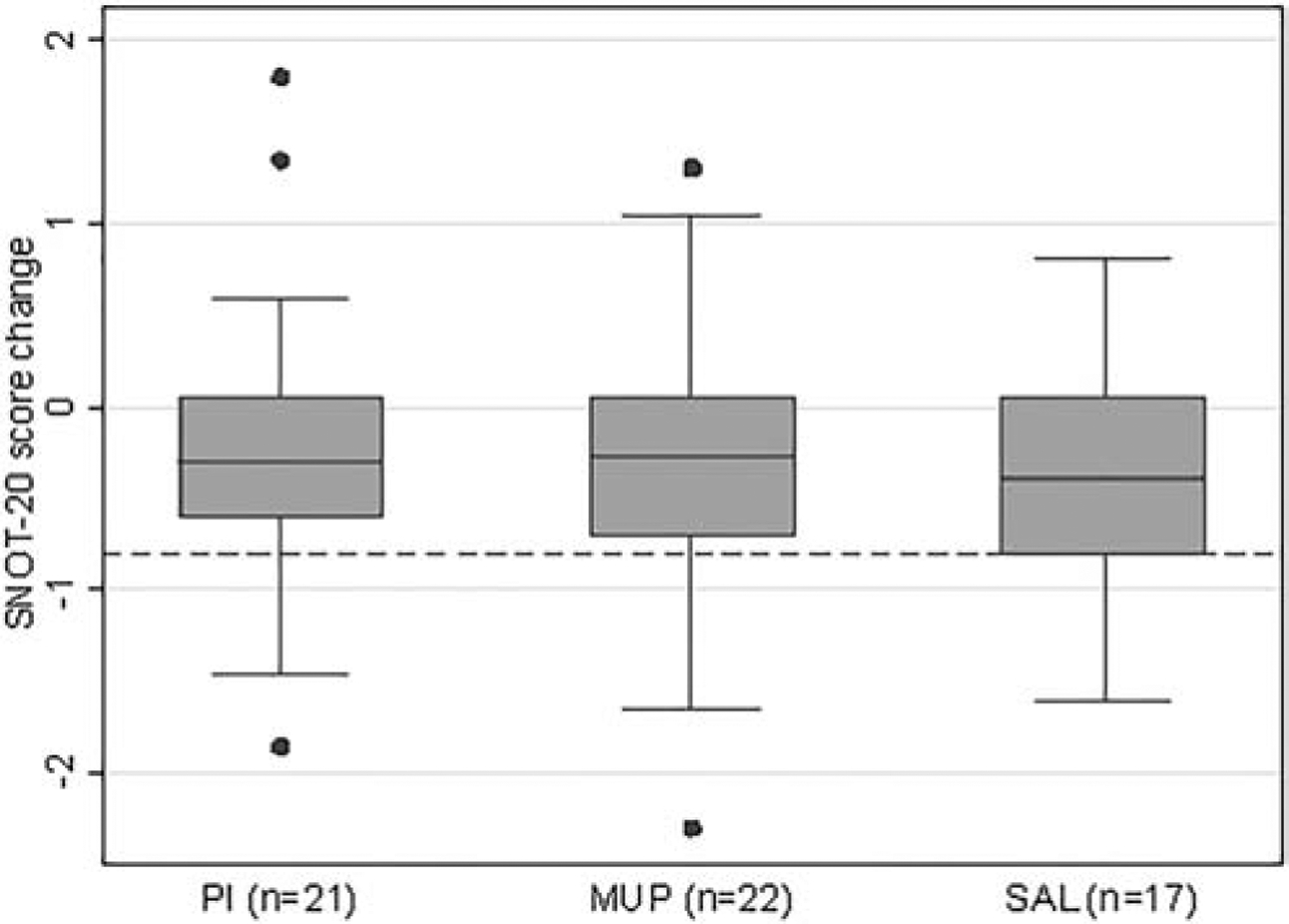
SNOT-20 = Sinonasal Outcome Test-20, PI = povidone-iodine, MUP = mupirocin, SAL = saline.
Dotted line indicates SNOT-20 score change of −0.8, considered a clinically significant improvement.
Missing outcome data due to difficulty coordinating clinic logistics for data collection: pre-treatment SNOT-20 score in SAL group (n=2).
A Lund-Kennedy endoscopic score change below 0 is an improvement, and a more negative value is a better response. Lund-Kennedy endoscopic score change from baseline improved slightly more in the PI (−3.5 [−7, −0.5]) compared to the MUP (−2 [−4, 2]), and SAL (−3 [−5, 0]) groups. An adjusted linear regression analysis found no significant differences among the treatment groups (global p-value = 0.35; Figure 4).
Figure 4. Box plot comparison of Lund-Kennedy endoscopic score change from baseline (p=0.35).
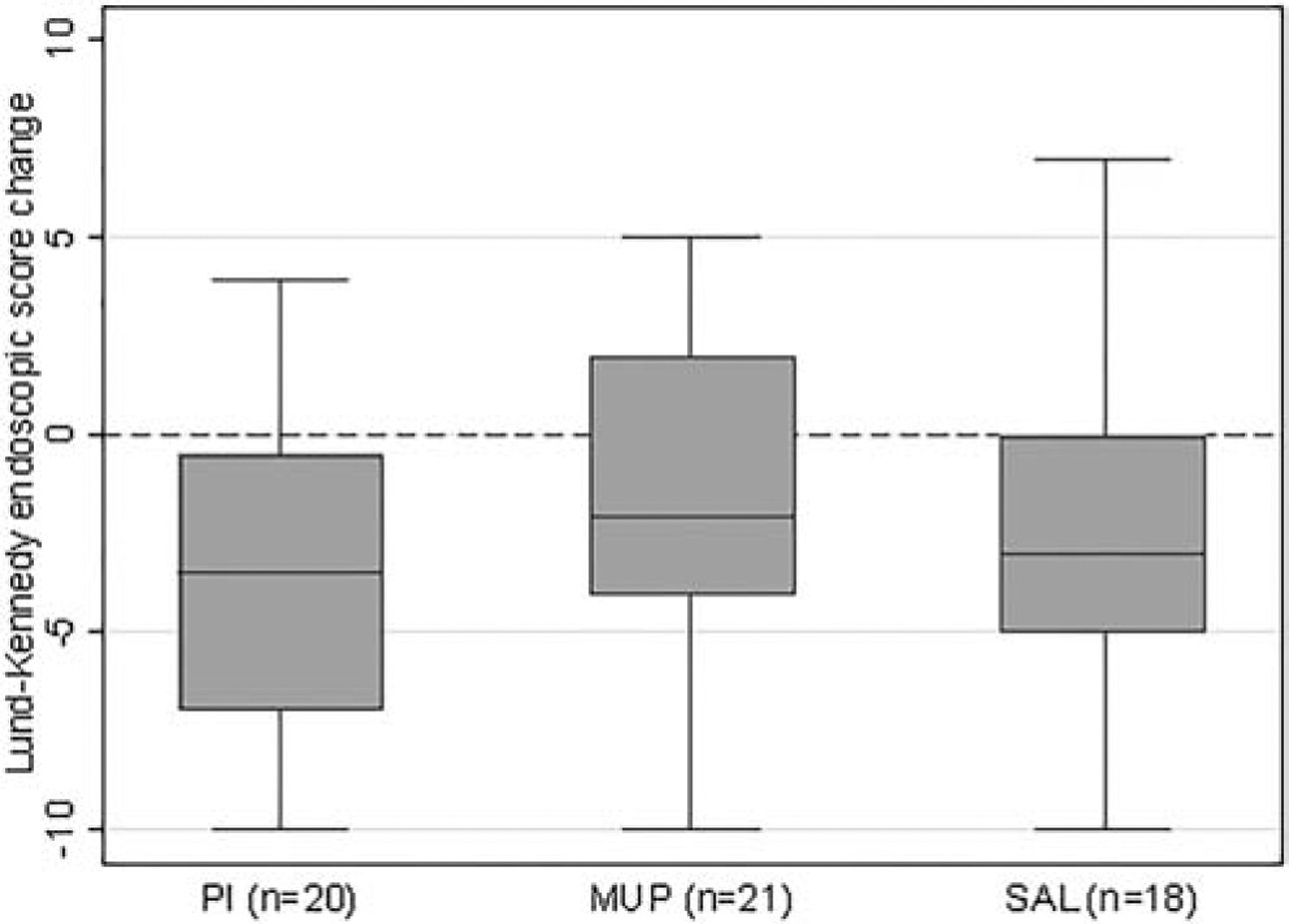
PI = povidone-iodine, MUP = mupirocin, SAL = saline.
Dotted line indicates LK endoscopic score change of 0, below which is considered an improvement.
Missing outcome data due to difficulty coordinating clinic logistics for data collection: pre-treatment Lund-Kennedy endoscopic score in PI (n=1), MUP (n=1), and SAL (n=1) groups.
Given that none of the global tests for comparisons among the treatment groups were significant, no post-hoc comparisons were conducted. A secondary per-protocol analysis showed similar results.
3.3.4. Subgroup Analysis for No Oral Antibiotics or Steroids
We were particularly interested in the impact of PI or MUP alone in active CRS after sinus surgery. There were enrollment issues that prevented a pure MUP or PI versus SAL study if oral antibiotics or steroids were not also offered as those treatments may be considered standard of care therapy for active CRS. There was, however, a population of subjects who chose not to receive oral antibiotic or oral steroid therapy (n=28; PI n=10, MUP n=11, SAL n=7). We decided a priori to perform an exploratory secondary adjusted analysis of this subgroup adjusting for the same factors.
Post-treatment culture negativity was higher in the MUP (7/10, 70%) group compared to the PI (3/10, 30%) and SAL (4/7, 57%) groups, but these differences were not significant (p=0.56). SNOT-20 score change from baseline improved similarly in the PI (−0.6 [−1.5, −0.3]) and MUP (−0.5 [−0.8, −0.1]) compared to the SAL (−0.7 [−1.4, 0.05]) groups, and these differences were not significant (p=0.72). Lund-Kennedy endoscopic score change from baseline improved more in the PI (−5 [−8, −1]) compared to the MUP (−2 [−4, 2]), and SAL (−3 [−6, −2]) groups, and these differences were closer to significance compared to the initial analysis but still did not reach it (p=0.15). Overall, results were similar to the initial analysis with the entire sample size.
4. DISCUSSION
In post-sinus surgery subjects with acute exacerbations of CRS and gram-positive bacteria on culture, our study did not show any significant differences in post-treatment culture negativity rate among the povidone-iodine, mupirocin, or saline groups. From a microbiological standpoint, mupirocin was perhaps more promising, trending towards better post-treatment culture negativity, implying some level of microbiological “control,” although this was not statistically superior. The saline group in our study also performed surprisingly well, achieving a 47% post-treatment culture negativity rate. This is quite notable taking into consideration that these subjects represented the “worst of the worst” in the sense that they had acute exacerbations of CRS despite prior surgical and medical therapies. At face value, the conclusion drawn from this performance may be that a subset of patients improve with a regimen of oral antibiotics or steroids and saline irrigations, supporting previous data that has shown saline irrigations improve symptoms of CRS.[26]
Our study also sought to evaluate a novel topical therapy, povidone-iodine sinus irrigations. Although a similar result was achieved in the saline group, the povidone-iodine group did achieve 43% post-treatment culture negativity, suggesting that a subset of patients do achieve microbiological “control” with this technique. Importantly, this is the only study to have demonstrated the tolerability of povidone-iodine as a sinus irrigation formula. In the context of the current COVID-19 pandemic, this is particularly relevant as povidone-iodine is virucidal, and as a sinus irrigation, may have potential as a topical treatment, delivered directly to the areas of highest viral load while avoiding systemic side effects.[6,13,14,20,27] It is also important to note, however, that we did not assess ciliotoxicity or ciliary beat frequency in response to povidone-iodine, mupirocin, or saline irrigations. This represents an area for future research. Nonetheless, our study concentrations were well below previously demonstrated ciliotoxicity levels, but the data is limited to testing of substantially higher concentrations.[15,28]
There were also no significant differences in the other outcome measures, SNOT-20 score, which evaluates patient-reported quality of life, and the Lund-Kennedy endoscopic score, which quantifies the severity of nasal endoscopic findings, among the treatment groups. These results suggest that with regard to patient-reported quality of life and endoscopic findings, mupirocin and povidone-iodine do not have substantial benefit compared to saline sinus irrigations. It is also worth noting that although the SNOT-20 score did show a small overall median improvement in all treatment groups, this was not clinically significant. Potential reasons for the failure to achieve clinical significance in any treatment group include that symptom improvement in particular may be slow and difficult to achieve.
This study has a number of limitations. First, it was underpowered. The power analysis was based on the only other randomized trial by Jervis Bardy et al. (2012), which found a nearly 90% difference in post-treatment culture negativity between the mupirocin and saline groups.[2] Although we were more conservative in our targeted difference of 45%, this was not conservative enough given the high post-treatment culture negativity rate in the saline group. We did not continue enrolling after reaching our target sample size because this would be based on a post-hoc power analysis. The differences found in our study can serve as a basis to better power future studies. The previous randomized trial also used an equivalent concentration of mupirocin and dosing protocol, twice daily for 30 days, but doubled the volume, which may also have contributed to the smaller difference observed in our study.[2] We used a lower volume because our experience has shown that the willingness of patients to pay for a one-month trial of a topical treatment noticeably decreases when out of pocket costs exceed $100. Doubling the volume would have increased the out of pocket cost from $90 to $180. Future studies should explore optimal volume as well as concentration, frequency, and duration regimens.
There may also be unknown confounders that were not adjusted for in the analysis. It was also impossible to blind subjects to the irrigation treatment because povidone-iodine solution is much different in appearance and smell than mupirocin solution, and this may have biased the self-reported SNOT-20 score. In addition, as a tertiary rhinology practice, there was a high proportion of subjects with nasal polyposis, asthma, inhalant allergies, aspirin sensitivity, an immunodeficiency, or a vasculitis in our study, which may influence the generalizability of the results, although there would be a greater benefit expected in a less comorbid population. Future studies should also incorporate longer follow-up intervals to assess the durability of results.
5. CONCLUSIONS
In patients who have had prior sinus surgery with acute exacerbations of CRS and gram-positive bacteria on culture, this randomized trial showed mupirocin sinus irrigations were the most promising, achieving a better post-treatment culture “control” rate compared to saline and povidone-iodine, though not statistically different. In addition, 1% povidone-iodine solution was well-tolerated as a sinus irrigation and may represent a feasible method for temporarily disinfecting the sinonasal cavity of bacteria and viruses such as COVID-19.
ACKNOWLEDGEMENTS
We would like to acknowledge Carolyn Bea, our research coordinator, for her work in obtaining IRB approval for this study, and the University of Washington Otolaryngology Outcomes Research Group, for valuable feedback.
FUNDING
Dr. Lee was supported by the National Institutes of Health National Institute on Deafness and Other Communication Disorders T32 DC000018 Research Training Grant. The funding source had no involvement in the project.
ABBREVIATIONS
- CRS
Chronic rhinosinusitis
- PI
Povidone-iodine
- MUP
Mupirocin
- SAL
Saline
- SNOT-20
Sinonasal Outcome Test-20 score
- VAS
Visual analog scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented as a podium presentation on May 19, 2016 at the ARS at Combined Otolaryngology Spring Meetings (COSM), in Chicago, IL.
DECLARATIONS OF COMPETING INTEREST
None.
REFERENCES
- [1].Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult sinusitis executive summary. Otolaryngol Head Neck Surg. 2015;152(4):598–609. 10.1177/0194599815574247. [DOI] [PubMed] [Google Scholar]
- [2].Jervis-Bardy J, Boase S, Psaltis A, Foreman A, Wormald PJ. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope. 2012;122(10):2148–2153. 10.1002/lary.23486. [DOI] [PubMed] [Google Scholar]
- [3].Jervis-Bardy J, Wormald PJ. Microbiological outcomes following mupirocin nasal washes for symptomatic, staphylococcus aureus-positive chronic rhinosinusitis following endoscopic sinus surgery. Int Forum Allergy Rhinol. 2012;2(2):111–115. 10.1002/alr.20106. [DOI] [PubMed] [Google Scholar]
- [4].Orlandi RR, Smith TL, Marple BF, et al. Update on evidence-based reviews with recommendations in adult chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4 Suppl 1:S1–S15. 10.1002/alr.21344. [DOI] [PubMed] [Google Scholar]
- [5].Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6 Suppl 1:22 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- [6].Rudmik L, Hoy M, Schlosser RJ, et al. Topical therapies in the management of chronic rhinosinusitis: An evidence-based review with recommendations. Int Forum Allergy Rhinol. 2013;3(4):281–298. 10.1002/alr.21096. [DOI] [PubMed] [Google Scholar]
- [7].Mahdavinia M, Keshavarzian A, Tobin MC, Landay AL, Schleimer RP. A comprehensive review of the nasal microbiome in chronic rhinosinusitis (CRS). Clin Exp Allergy. 2016;46(1):21–41. 10.1111/cea.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Solares CA, Batra PS, Hall GS, Citardi MJ. Treatment of chronic rhinosinusitis exacerbations due to methicillin-resistant staphylococcus aureus with mupirocin irrigations. Am J Otolaryngol. 2006;27(3):161–165. https://doi.org/S0196-0709(05)00175-4. [DOI] [PubMed] [Google Scholar]
- [9].Uren B, Psaltis A, Wormald PJ. Nasal lavage with mupirocin for the treatment of surgically recalcitrant chronic rhinosinusitis. Laryngoscope. 2008;118(9):1677–1680. 10.1097/MLG.0b013e31817aec47. [DOI] [PubMed] [Google Scholar]
- [10].van Rijen M, Bonten M, Wenzel R, Kluytmans J. Mupirocin ointment for preventing staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev. 2008;(4):CD006216 10.1002/14651858.CD006216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Durani P, Leaper D. Povidone-iodine: Use in hand disinfection, skin preparation and antiseptic irrigation. Int Wound J. 2008;5(3):376–387. 10.1111/j.1742-481X.2007.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Greenstein G Povidone-iodine’s effects and role in the management of periodontal diseases: A review. J Periodontol. 1999;70(11):1397–1405. 10.1902/jop.1999.70.11.1397. [DOI] [PubMed] [Google Scholar]
- [13].Eggers M, Eickmann M, Zorn J. Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus ankara (MVA). Infect Dis Ther. 2015;4(4):491–501. 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eggers M, Koburger-Janssen T, Eickmann M, Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect Dis Ther. 2018;7(2):249–259. 10.1007/s40121-018-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim JH, Rimmer J, Mrad N, Ahmadzada S, Harvey RJ. Betadine has a ciliotoxic effect on ciliated human respiratory cells. J Laryngol Otol. 2015;129 Suppl 1:45 10.1017/S0022215114002746. [DOI] [PubMed] [Google Scholar]
- [16].Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: An evidence-based review. Can J Surg. 2007;50(6):473–481. [PMC free article] [PubMed] [Google Scholar]
- [17].Li B, Nentwich MM, Hoffmann LE, et al. Comparison of the efficacy of povidone-iodine 1.0%, 5.0%, and 10.0% irrigation combined with topical levofloxacin 0.3% as preoperative prophylaxis in cataract surgery. J Cataract Refract Surg. 2013;39(7):994–1001. 10.1016/j.jcrs.2013.02.039. [DOI] [PubMed] [Google Scholar]
- [18].Ramezanpour M, Smith JLP, Psaltis AJ, Wormald PJ, Vreugde S. In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells. Int Forum Allergy Rhinol. 2020. 10.1002/alr.22575. [DOI] [PubMed] [Google Scholar]
- [19].Phillips M, Rosenberg A, Shopsin B, et al. Preventing surgical site infections: A randomized, open-label trial of nasal mupirocin ointment and nasal povidone-iodine solution. Infect Control Hosp Epidemiol. 2014;35(7):826–832. 10.1086/676872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mady LJ, Kubik MW, Baddour K, Snyderman CH, Rowan NR. Consideration of povidone-iodine as a public health intervention for COVID-19: Utilization as “personal protective equipment” for frontline providers exposed in high-risk head and neck and skull base oncology care. Oral Oncol. 2020:104724. doi: S1368–8375(20)30160–3 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moein ST, Hashemian SMR, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: A biomarker for COVID-19. Int Forum Allergy Rhinol. 2020. 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 Suppl):1 https://doi.org/S0194-5998(07)01449-0. [DOI] [PubMed] [Google Scholar]
- [23].Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2):35 https://doi.org/a83514. [DOI] [PubMed] [Google Scholar]
- [24].Piccirillo JF, Merritt MG, Richards ML. Psychometric and clinimetric validity of the 20-item sino-nasal outcome test (SNOT-20). Otolaryngol Head Neck Surg. 2002;126(1):41–47. https://doi.org/S0194599802759597. [DOI] [PubMed] [Google Scholar]
- [25].Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- [26].Pynnonen MA, Mukerji SS, Kim HM, Adams ME, Terrell JE. Nasal saline for chronic sinonasal symptoms: A randomized controlled trial. Arch Otolaryngol Head Neck Surg. 2007;133(11):1115–1120. https://doi.org/133/11/1115. [DOI] [PubMed] [Google Scholar]
- [27].Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212 Suppl 1:119–123. https://doi.org/89211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Birk R, Aderhold C, Wenzel A, et al. Mupirocin reduces ciliary beat frequency of human nasal epithelial cells. Eur Arch Otorhinolaryngol. 2016;273(12):4335–4341. 10.1007/s00405-016-4161-8. [DOI] [PubMed] [Google Scholar]


