Abstract
Iron-containing proteins rely on the incorporation of a set of iron cofactors for activity. The cofactors must be synthesized or assembled from raw materials located within the cell. The chemical nature of this pool of raw material - referred to as the labile iron pool – has become clearer with the identification of micro- and macro-molecules that coordinate iron within the cell. These molecules function as a buffer system for the management of intracellular iron and are the focus of this review, with emphasis on the major iron chaperone protein coordinating the labile iron pool: poly C-binding protein 1.
Iron cofactors
Cells use iron in the form of cofactors that are stably incorporated into enzymes and regulatory proteins. These cofactors consist of heme, iron-sulfur clusters, and mono-and di-nuclear iron centers. In the case of heme synthesis, the protoporphyrin IX scaffold is synthesized from succinyl CoA and glycine in a series of 8 steps and culminates in the insertion of iron by the enzyme ferrochelatase. This final step occurs in the mitochondrial matrix at the inner membrane. Heme is subsequently distributed throughout the cell to be incorporated into hemoproteins. The system by which heme is distributed requires that heme be transported both across membranes and through aqueous compartments. The components of this system are not fully known, but several have been identified (see ref. Hamza, this issue).
Iron-sulfur clusters are assembled in both the mitochondrial matrix and the cytosol, with several of the core components of the assembly apparatus exhibiting isoforms that localize to both compartments (Rouault and Maio, 2017). Systems that allow for mitochondrial export of sulfur species or simple iron-sulfur species used in cytosolic iron-sulfur cluster assembly have been identified. Proteins involved in the delivery of cytosolic [2Fe-2S] and [4Fe-4S] to apoenzymes have been identified, as well (see ref. Lill, this issue), although, again, the components and mechanistic details of these systems, including the source of iron, are not fully known.
The iron cofactors found in mono- and di-nuclear iron centers require neither synthesis nor assembly and the kinetic lability of Fe(II) is sufficient to metallate apoenzymes without the requirement of a distribution system (Hider and Kong, 2013). Yet cells have evolved factors controlling the chemical reactivity and distribution of iron in the cytosol.
The labile iron pool
The “labile iron pool” (LIP) is the term that describes the chemically reactive, kinetically exchangeable pool of iron that is used as the raw material for iron cofactor synthesis, assembly, and insertion (Fig. 1) (Esposito et al., 2002; Hider and Kong, 2013; Petrat et al., 2002a). The iron that is incorporated into cytosolic iron-sulfur clusters and the iron used to directly metallate mono- and di-nuclear iron centers is pulled from the cytosolic LIP. Similarly, the iron used within mitochondria is acquired from the LIP, although mechanisms to directly transfer iron from the endo-lysosomal compartment to mitochondria may exist (Hamdi et al., 2016). A substantial amount of theoretical and empiric evidence indicates that the LIP is comprised of iron in the ferrous, 2+ state, [Fe(II)] (Chakrabarti et al., 2015; Kozlov et al., 1992; Williams, 1982). The LIP is also thought to be coordinated by a polydisperse buffer system consisting of both micro- and macro-molecules. The identities of these molecules and how they coordinate iron were largely restricted to evidence from computer simulation and modeling of candidate compounds. More recent empiric evidence makes the character of the LIP clearer.
Figure 1. Molecular composition of cytosolic labile iron pool (LIP).
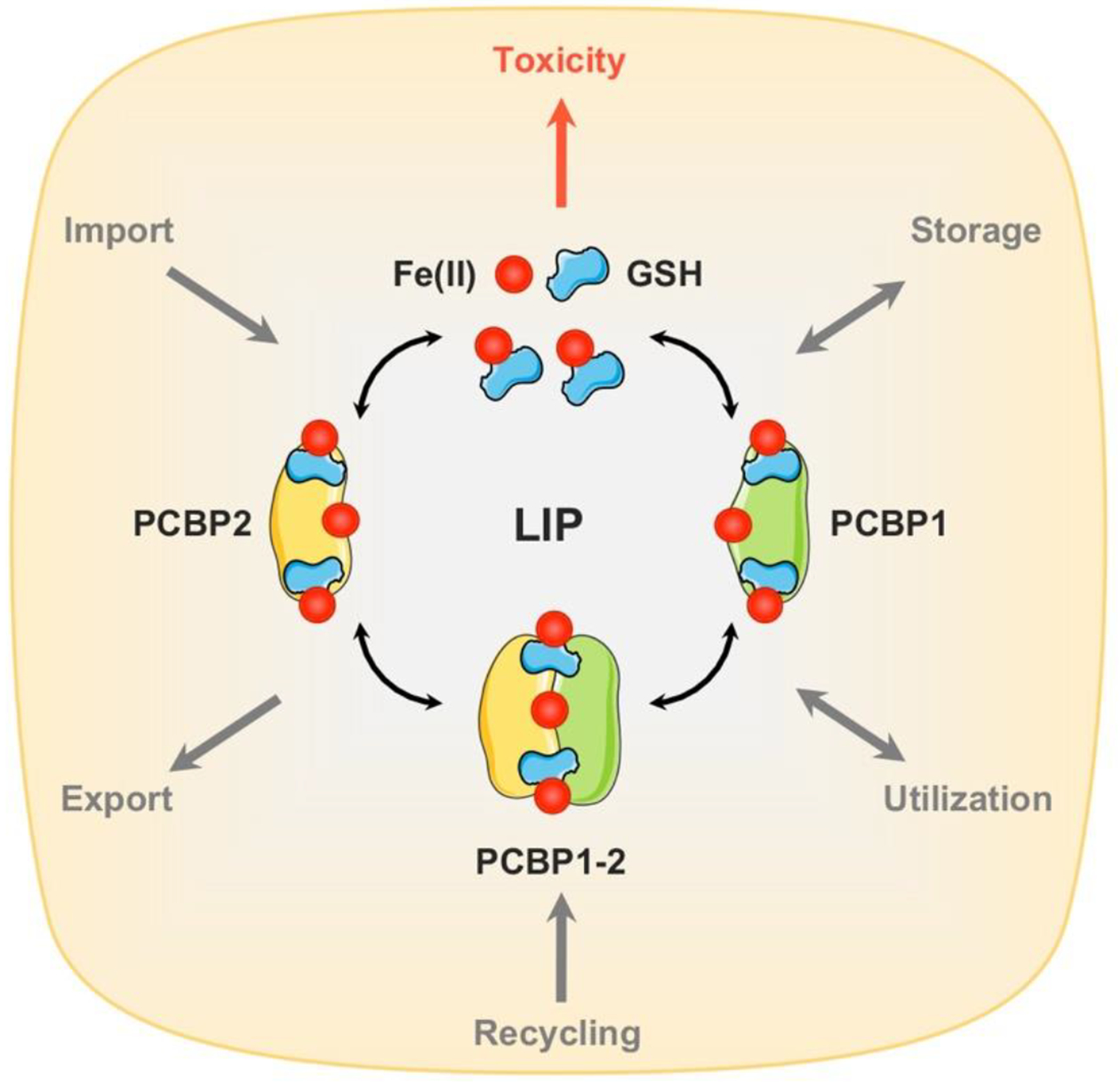
Iron uptake systems release Fe(II) into the cytosol. Cytosolic Fe(II) is coordinated by reduced glutathione (GSH) to form the cytosolic LIP. Under physiological conditions, a significant proportion (>90%) of LIP may be coordinated by iron chaperones, PCBPs. Structurally, PCBPs contain three tandem K-homology domains (KH1–3) that can bind GSH coordinated Fe(II) with high affinity in a 1:1 ratio. PCBP1 and PCBP2 are the major iron chaperones that play integral roles in intracellular iron trafficking. PCBP1 and PCBP2 can bind to each other in cells, potentially transferring iron between them. Unchaperoned free Fe(II) reacts with cellular oxidants to produce cytotoxic hydroxyl radicals via the Fenton reaction.
Many small organic compounds found in cells can potentially function as iron ligands in vitro. The limiting factors within cells, however, are the concentration of the ligand and its affinity for Fe(II) vs. Fe(III). Using selective Fe(II) vs. Fe(III) chelators, the LIP has been measured and found to consist almost entirely of Fe(II) (Breuer et al., 1995; Petrat et al., 2002b). Studies using Mossbauer spectroscopy confirm these measurements and they are consistent with the prevalence of reduced forms of physiologic reductants (e. g., NADPH) in the cytosol (Chakrabarti et al., 2015). Of the cellular ligands with significant affinity for Fe(II), only one is present in cells at a sufficiently high concentration to support complex formation: reduced glutathione (GSH). Speciation plots developed in silico suggest that at pH 7.0 and at the physiologic cytosolic concentrations of GSH (2–10 mM) and Fe(II) (0.5–2 μM), >95% of cytosolic iron is predicted to be in the form of Fe(II)-GS. Non-enzymatic, in vitro reactions of either Fe(III) or Fe(II) salts with GSH at the pH range of 3–7 yield Fe(II)-GS complexes. Thus, high concentrations of GSH may promote the depletion of Fe(III) in the cytosol by solubilizing Fe(II) at pH 7.0, forming Fe(II)-GS complexes that facilitate iron mobilization. (Hamed and Silver, 1983; Hider and Kong, 2011). Coordination of Fe(II) by GSH could serve to limit the oxidation and reactivity of Fe(II). It also accounts for the capacity of cells to discriminate between Fe(II) and Mn(II). Both metals are present in the cytosol, although iron is more abundant than Mn, and both can be coordinated in transition metal binding sites dominated by O- and N- ligands. Mn(II) exhibits a much lower affinity for GSH, however, and is not predicted to form Mn-GS complexes in the cytosol. Thus, trafficking of iron as a Fe(II)-GS complex allows cells to selectively exclude Mn from iron-binding centers.
Empiric data that support the formation of Fe(II)-GS complexes in the cytosol can be found in two sets of studies. In the first, investigators measured the LIP by using a ratiometric, Fe(II)-reactivity-based fluorescent sensor termed FIP-1 (FRET Iron Probe 1). Previous studies measuring the LIP used fluorescent iron chelators to estimate the chelator-accessible pool (Ma et al., 2015). The FIP-1 sensor measures the capacity of “free” Fe(II) within cells to react with and cleave an iron-sensitive endoperoxide linker (Aron et al., 2018). Cleavage of the linker impairs the transfer of a FRET signal and alters the emission of fluorophores attached to the linker. In in vitro and cell-based studies, FIP-1 responds to changes in intracellular iron caused by supplementation of the medium with iron salts or with cell-impermeable iron chelators. However, when cells were treated with a GSH-depleting agent (35MEW28, known as Erastin-2), cleavage of the sensor rapidly increased, indicating an increase in the chemical reactivity of the LIP due to the loss of GSH in the cytosol (Aron et al., 2016). Although the authors of this study suggested that GSH depletion stimulated an influx of iron to the cytosol, an alternative interpretation posits that the loss of GSH leads to an increase in the chemical reactivity of uncoordinated Fe(II). This increase in reactivity supports the role of GSH in directly coordinating iron. A second set of studies confirms the role of GSH in coordinating iron when both ligands are bound to the iron chaperone poly r(C)-binding protein 1 (PCBP1).
The PCBP family of iron chaperones
Metallochaperones are macromolecules that bind metal ions and transfer them, through direct protein-protein interactions, to the active sites of target metalloenzymes (Capdevila et al., 2017; Robinson and Winge, 2010). Chaperone proteins delivering a variety of transition metals have been described in both prokaryotes and eukaryotes. In mammalian cells, the sole iron chaperones identified are the multifunctional adaptor proteins PCBP1 and PCBP2 (Philpott and Jadhav, 2019). PCBP1 and PCBP2, also named hnRNP E1 and E2 and α-CP1 and 2, were initially characterized as RNA-binding proteins detected in heterogeneous nuclear ribonucleoprotein complexes (hnRNPs) (Dreyfuss et al., 1988; Geuens et al., 2016). PCBP1 and 2 preferentially bind to cytosine-rich sequences in pol II transcripts and affect subsequent steps in mRNA processing, stability, and translation by facilitating the interaction of the RNA with other proteins (Chaudhury et al., 2010; Makeyev and Liebhaber, 2002; Ostareck-Lederer and Ostareck, 2004). They can also bind to single-stranded DNA and to other proteins, affecting the fates of both. Structurally, PCBPs are comprised of three discrete hnRNP K-homology domains, with two amino-terminal domains separated from the third, carboxy-terminal, domain by a poorly conserved, unstructured linker domain. Each KH domain functions as an RNA-binding module and adjacent C-rich RNA motifs can be bound by adjacent KH domains, conferring additional stability and some degree of specificity to the interactions.
In a genetic screen of a human liver cDNA library conducted in baker’s yeast expressing human ferritins, PCBP1 was identified as a human protein that bound iron and enhanced the loading of iron into ferritin, the human iron storage protein (Shi et al., 2008). Subsequent studies demonstrated that PCBP1 directly binds ferritins in vitro, in yeast cells, and in human cells. Furthermore, human and murine cells lacking PCBP1 exhibit defects in ferritin iron storage. This iron chaperone activity exhibited by PCBP1 was also found, to a weaker extent, in the highly-homologous PCBP2 and in heterologously-expressed PCBP3 and PCBP4, which are other members of the PCBP family (Leidgens et al., 2013).
Initial in vitro studies of iron binding by PCBP1 indicated that it coordinated Fe(II) in a 3:1 stoichiometric ratio, with the highest affinity site exhibiting a dissociation constant in the low micromolar range, similar to the estimated concentrations of the LIP. A recent study examined the iron-binding activity of PCBP1 in cells and found that GSH was necessary for the stable coordination of iron (Patel et al., 2019). Iron-bound PCBP1 could be isolated from cells labeled with radioisotopes of iron, but only when GSH was included in buffers. Structure-function analyses of PCBP1 suggested that the first and third KH domains of PCBP1 could function independently as iron chaperone domains in cells. An in silico docking analysis of GSH on the crystal structure of the KH3 domain suggested a potential binding site for GSH that was distinct from the previously identified RNA binding site (Fig. 2). Examination of the structure around the potential GSH site revealed conserved amino acid residues with thiol and carboxylate side chains that could serve as potential iron ligands. Mutational analysis of the KH3 domain revealed that conserved cysteine and glutamate residues were needed for iron coordination and conserved arginine, asparagine, and glutamate residues were necessary for both GSH and iron binding. In in vitro studies using purified recombinant KH3, GSH was bound with an apparent Kd of 400 μM and the affinity of GSH increased 5-fold to 80 μM in the presence of Fe(II). In a separate set of studies, KH3 bound Fe(II) with an affinity of 4.2 μM, which increased to 0.8 μM in the presence of 5 mM GSH. Given the measured concentrations of PCBP1 (5 μM), GSH (2–10 mM), and Fe(II) (0.5–2 μM) in the cytosol (Esposito et al., 2002; Hider and Kong, 2011), these affinities suggest that KH3-Fe(II)-GS complexes will form in cells under physiological conditions and supports the coordination of Fe(II)-GS in the PCBP1 and KH3 complexes isolated from cells. Future studies will determine whether the first and second KH domains of PCBP1 bind iron and GSH in an analogous manner. Conservation of these KH3 residues in PCBP2 suggests a similar coordination of Fe(II) and GSH, but this has not been empirically determined. Given the multivalent binding potential of PCBP1, these studies suggest that much of the cytosolic LIP is coordinated as a Fe(II)-GS complex on PCBP1 (and possibly PCBP2). These modeling and kinetic studies indicate that the Fe(II)-GS cluster on KH3 is surface accessible and kinetically labile. This feature is also consistent with the function of PCBP1 as an iron chaperone, as the iron should not be bound too tightly to facilitate the critical associative transfer between proteins.
Figure 2. Structural and functional details of PCBP1 as RNA or iron chaperone.
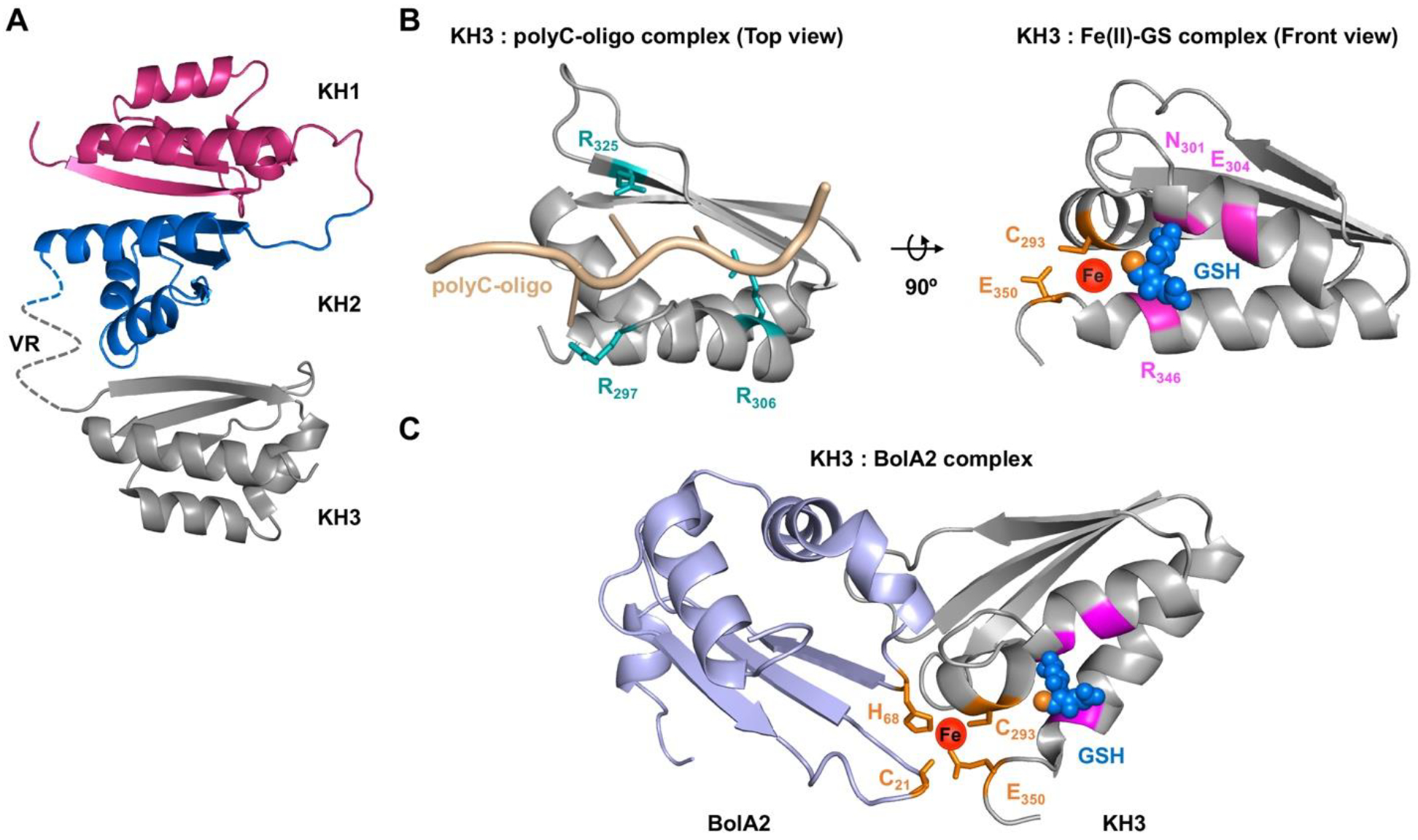
A, A complete projected structure of PCBP1 based on K-homology (KH) domains 1 and 2 (PDB 2JZX) and domain 3 (PDB 1WVN) linked together via unstructured variable region (VR). B, Structural comparison of PCBP1 KH3 domain in complex with RNA or iron. Left: a homology structure of poly C-oligo bound form of KH3 domain (PDB 2P2R) illustrating ligands for cytosine binding (teal sticks). Right: a ribbon structure of KH3 domain (PDB 1WVN) illustrating ligands for GSH binding (magenta) and iron binding (orange sticks), coordinating the iron (red sphere) and GSH (blue spheres, thiol in orange). C, A model for electrostatic interaction between BolA2 (PDB 1V9J, light blue) and KH3 domain (PDB 1WVN, grey) presenting ligands for iron binding (orange sticks), coordinating the iron (red sphere) and GSH (blue spheres, thiol in orange). Models were generated using Swiss-model for homology modeling, HADDOCK 2.2 protein docking platform and PyMOL version 2.3.
Although the residues involved in ligating oligonucleotides and Fe(II)-GS on the KH3 domain of PCBP1 are on different surfaces of the protomer, binding one ligand may influence the coordination of the other. The cysteine residue of KH3 that is involved in coordination of Fe(II)-GS was previously identified as a site of homocysteinylation of PCBP1, a modification that occurs in placental cells in response to prolonged folate deficiency (Antony et al., 2004; Tang et al., 2017; Tang et al., 2011). Homocysteine modification of PCBP1 cysteine 293 enhances both binding to and translation of folate receptor mRNA. Addition of iron to in vitro assays of RNA binding in buffers containing GSH or homocysteine demonstrated dose-dependent inhibition of RNA binding by PCBP1. Thus, binding Fe(II)-GS on PCBP1 may influence interactions with RNA transcripts and vice versa.
Interactions of PCBPs with non-heme iron enzymes in cytosol
In contrast to the Ni and Cu chaperones that have been studied in prokaryotic and eukaryotic systems, PCBP1 does not appear to exhibit high-affinity interactions with target apoproteins. Moreover, the Ni and Cu chaperones exhibit a high specificity for their targets that PCBP1 does not. Because mammalian cells express a large number of iron enzymes, cells are unlikely to rely on an equally large number of different, highly-specific, iron chaperones. Thus, the interactions of PCBPs with target enzymes are likely to exhibit broad specificity and lower affinity. The interaction between ferritin and PCBP1 in yeast cells was dependent on iron, as it was directly detected only when Fe(II) was present in buffers used for affinity capture (Shi et al., 2008). In vitro interactions between recombinant purified Fe-PCBP1 or Fe-PCBP2 and equine spleen ferritin exhibited greater affinity by isothermal titration calorimetry than iron binding to ferritin in the absence of PCBPs, but these studies were conducted in buffers without GSH, which likely affected the stability of the Fe-PCBP complexes (Leidgens et al., 2013). In a study conducted in murine proerythroblasts, the binding of PCBP1 to ferritin was enhanced when cells initiated the iron uptake that accompanies hemoglobinization, but was inhibited in late erythroid development, an effect that could be recapitulated in vitro with excess iron (Ryu et al., 2018). Thus, the binding of PCBP1 to ferritin appears to be regulated by iron and may be mediated by a bridging iron ligand, but mechanistically this is not yet clear (Fig. 3a). Of note, although ferritin H chain functions as a monooxygenase with a dinuclear iron center, this active site is inaccessible to macromolecules, such as iron chaperones, because it is located on the interior surface of ferritin in its mature heterooligomeric state (Arosio et al., 2017). For this reason, PCBP1 cannot be delivering iron directly to the enzymatic active site but instead is likely releasing iron to the carboxylate residues that line the pore of 3-fold symmetry that leads to the interior. This mechanism of associative transfer makes the role of a bridging iron ligand more likely.
Figure 3. Iron chaperone-mediated handling of cytosolic labile iron pool.
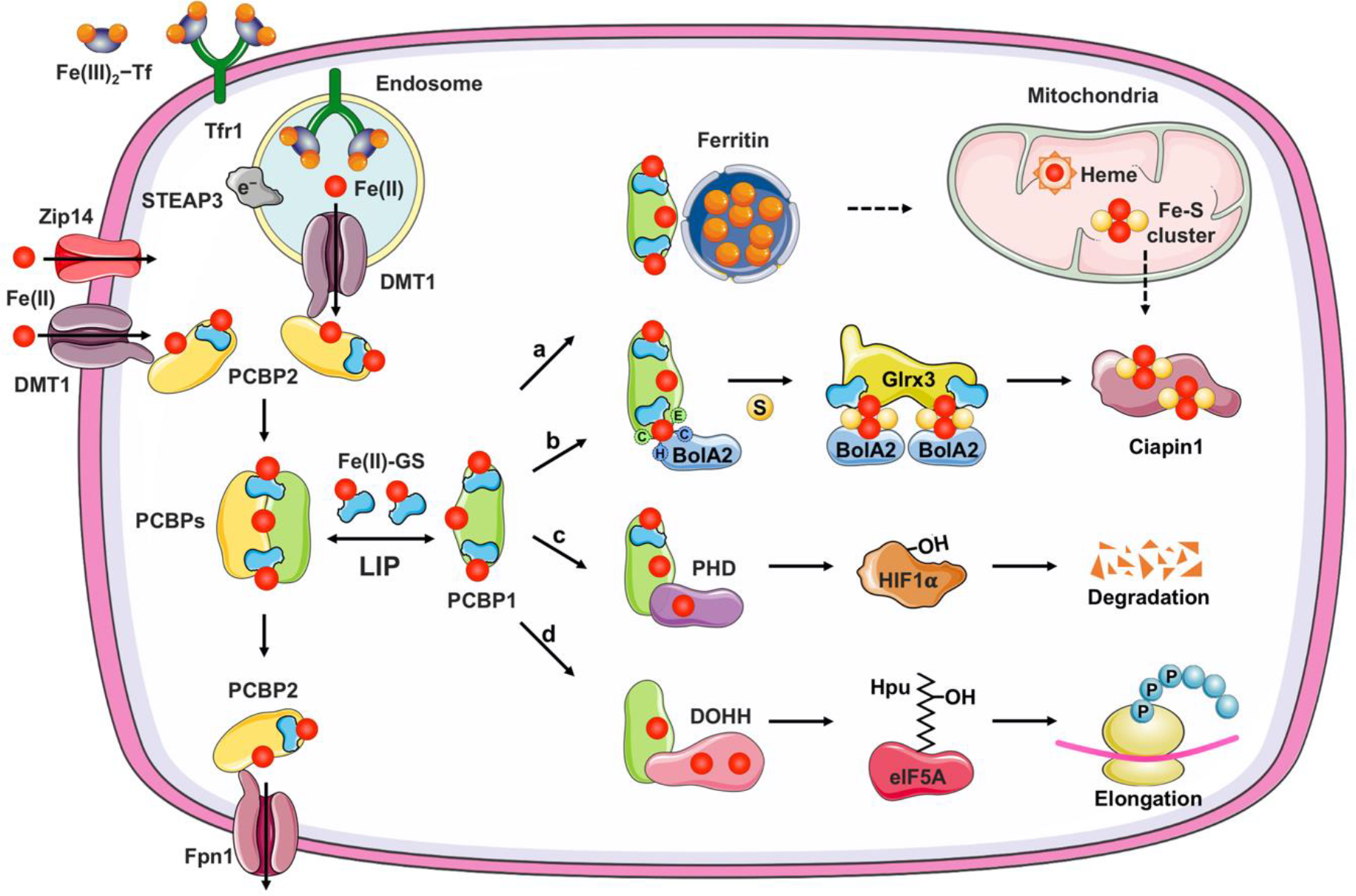
Divalent metal transporter 1 (DMT1) and Zip14, located on plasma membrane or endocytic vesicles, imports non-transferrin-bound or transferrin-bound iron, respectively. Iron enters the cytosolic labile iron pool (LIP) as Fe(II) and is largely coordinated by reduced GSH and bound to iron chaperones, PCBPs. PCBP1 and PCBP2 efficiently distribute iron for storage, non-heme iron cofactor assembly, or efflux through ferroportin (Fpn1). a, PCBP1 (and to a lesser extent, PCBP2) facilitates iron sequestration into ferritin, which can be delivered to mitochondria by unknown mechanism for heme or Fe-S cluster synthesis. b, PCBP1 form a complex with cytosolic BolA2 via a bridging Fe-GS ligand. Iron provided by PCBP1-BolA2 complex can be combined with sulfur (S) compound, with the help of other cytosolic Fe-S cluster assembly proteins and facilitate the [2Fe-2S] cluster formation on BolA2-Glrx3 distribution system. c, PCBP1 delivers iron to mononuclear iron enzyme prolyl hydroxylase (PHD2), which regulates the degradation of hypoxia inducible factor 1-α (HIF1α). d, PCBP1 also metallates the dinuclear iron enzyme deoxyhypusine hydroxylase (DOHH), which catalyzes the hydroxylation of hypusine (Hpu) on eukaryotic translation initiation factor 5A (elF5A) to promote translation of polyproline motifs.
PCBP1 can deliver iron to non-heme enzymes with mono- and di-nuclear iron centers (Fig. 3c,d) (Philpott et al., 2017). This activity was initially observed in the prolyl hydroxylases (PHDs) that regulate the degradation of hypoxia inducible factor 1-α in cells (Nandal et al., 2011). Cells from which PCBP1 is depleted exhibit loss of the iron cofactors from PHD2 and concomitant loss of prolyl hydroxylase activity, reduction of hydroxylated HIF1-α, and accumulation of active HIF1-α. Similarly, PCBP1-depleted cells exhibit loss of activity of the related asparagyl hydroxylase and PCBP1 was found to directly interact with both of these mononuclear iron enzymes. A recent publication also indicates that acireductone dioxygenase 1, a mono-nuclear iron enzyme involved in the methionine salvage pathway, is also metallated by PCBP1 in cells (Bae et al., 2020).
Deoxyhypusine hydroxylase (DOHH) is a di-nuclear iron enzyme that is structurally unrelated to PHDs or ferritins, but also depends on PCBP1 for efficient metalation of its peroxo-bridged, di-nuclear iron center (Frey et al., 2014). Cells lacking PCBP1 or PCBP2, but not wild type HEK cells, lost DOHH activity when exposed to low levels of iron chelators. Iron cofactors were selectively lost from DOHH under these conditions and could be restored in vitro with iron or with cell lysates containing PCBP1. Again, PCBP1 demonstrated iron-dependent binding to DOHH in cells by coimmunoprecipitation. Because ferritin and the non-heme iron enzymes targeted by PCBP1 are structurally dissimilar, we have proposed that the interactions are largely mediated through bridging iron ligands that may include GSH. Additional electrostatic interactions, for example, cannot be ruled out, but may not be primarily responsible for complex formation.
Interaction of PCBP1 with cytosolic Fe-S assembly machinery through BolA2
A proteomics analysis of the PCBP1 interactome indicated that PCBP1 was capable of binding many different protein components in the cell, including ferritins, other PCBPs, and some non-heme iron enzymes. Inspection of the interactome for possible co-chaperone, rather than client, proteins revealed BolA2 as a candidate (Fig. 3b) (Patel et al., 2019). BolA2 homologues have been characterized in budding and fission yeast as components of a cytosolic/nuclear [2Fe-2S] carrier complex that includes the cytosolic monothiol glutaredoxin, Grx3 (Banci et al., 2015b; Frey et al., 2016; Haunhorst et al., 2013; Li et al., 2012; Muhlenhoff et al., 2010). In yeast this complex is proposed to transfer [2Fe-2S] clusters to iron-regulatory transcription factors, which directly affect their activity (Encinar del Dedo et al., 2015; Jacques et al., 2014; Kumanovics et al., 2008; Li et al., 2009). In mammalian cells, BolA2-Glrx3 complexes also function as [2Fe-2S] chaperones; the only known recipient of these clusters in mammalian cells is Ciapin 1, an early-acting component of the cytosolic [4Fe-4S] assembly system that contains itself either a pair of [2Fe-2S] clusters or a [2Fe-2S] and a [4Fe-4S] cluster (Banci et al., 2015a; Banci et al., 2015b; Frey et al., 2016; Haunhorst et al., 2013; Li et al., 2012; Muhlenhoff et al., 2010). Whether other apoproteins receive clusters from BolA2-Glrx3 has not been determined.
PCBP1 forms a complex with BolA2 in mammalian cells. This complex requires both Fe(II) and GSH for stability, but in vivo and in vitro evidence does not suggest that the complex coordinates an Fe-S cluster (Fig. 3b) (Patel et al., 2019). Conserved histidine and cysteine residues on BolA2 that serve as iron ligands in the [2Fe-2S] complex with Glrx3 are also required for iron coordination and complex formation with PCBP1. BolA2 does not appear to stably bind iron in cells without either PCBP1 or Glrx3. Furthermore, PCBP1-[Fe-GS]-BolA2 complex formation appears to facilitate the formation of the [2Fe-2S] complex on BolA2-Glrx3. Although both the KH1 and the KH3 domains of PCBP1 can interact with BolA2, only the KH3 domain is necessary and sufficient to bind BolA2 and to facilitate assembly of a [2Fe-2S] complex on BolA2-Glrx3. The capacity of a KH3-[Fe-GS]-BolA2 complex to facilitate this Fe-S formation suggests that this complex is an intermediate in Glrx3-BolA2 complex assembly and may be the source of iron for the bridging [2Fe-2S] cluster. Alternatively, PCBP1-[Fe-GS]-BolA2 could function to repair a partially disassembled cluster on Glrx3. Cells lacking PCBP1 have the capacity to assemble [2Fe-2S] on BolA2-Glrx3, but only when iron is supplemented in growth medium. Cells lacking PCBP2 exhibit enhanced capacity to form BolA2-[2Fe-2S]-Glrx3, suggesting that PCBP1 and PCBP2 may be in competition for binding the same pool of cellular iron, but only PCBP1 donates bound iron to BolA2-Glrx3. Mammalian cells likely express partially or fully redundant systems for Fe-S assembly; nevertheless, PCBP1 appears to facilitate iron entry into the cytosolic Fe-S assembly machinery through its binding with BolA2.
Murine models of PCBP1 deficiency
Many of the iron defects associated with PCBP1 deficiency in cultured cells can be reversed with supplemental iron; therefore, the importance of the iron chaperone to cellular physiology is not entirely clear from these studies. Animal studies, however, make clear the importance of the PCBP1 iron chaperone to cellular iron management. Constitutive deletion of either PCBP1 or PCBP2 in mice results in early embryonic lethality, confirming that both proteins are essential to early embryonic development and are not functionally redundant, despite their similarity (Ghanem et al., 2016). In a mouse model of inducible PCBP1 deficiency, mice developed microcytic anemia that was mechanistically traced to defects in the intracellular delivery of iron to ferritin. Bone marrow cells deficient in PCBP1 had reduced iron storage in ferritin, which impeded delivery of iron to mitochondria for heme synthesis and reduced hemoglobinization of developing red blood cells. Mice with PCBP1 deficiency and anemia activated compensatory erythropoietic systems that included transcription of the regulators erythropoietin and erythroferrone and repression of the iron-regulatory hormone hepcidin (Ryu et al., 2017). These studies highlighted the importance of ferritin iron storage in managing the tremendous influx of iron that is necessary during terminal erythroid differentiation.
A mouse model of hepatic PCBP1 deletion reveals further roles of the iron chaperone in managing cellular iron (Protchenko et al., 2020). Similar to erythropoietic cells, the liver is highly specialized in the management and utilization of iron. Dietary iron and splenic iron secreted from macrophages enter the liver directly from the portal circulation. Because liver cells have to deal with significant fluctuations in plasma iron from the portal circulation, safe handling and storage of the reactive LIP is very important for liver function. Liver diseases, such as NAFLD and NASH are associated with elevation of liver iron (Nelson et al., 2012). Mouse studies also demonstrate that excess liver iron contributes to oxidative stress, steatosis, and liver damage (Folgueras et al., 2018; Handa et al., 2016).
Deletion of PCBP1 in hepatocytes and cholangiocytes of the liver was associated with the development of hepatic steatosis and accelerated hepatocyte death (Protchenko et al., 2020). While iron stores, ferritin, and the activities of some iron enzymes were reduced in the livers of these animals, an iron-supplemented diet did not improve the steatosis and hepatocyte death. Instead, an iron-deficient diet improved the liver disease, which implicated iron toxicity as the underlying cause. PCBP1-deleted livers exhibited activation of the Nrf2-dependent oxidative-stress response pathway. Direct and indirect measurements of elevated levels of oxidatively-damaged lipids, as well as the capacity of vitamin E, a lipophilic antioxidant, to impede the development of steatosis indicated that unchaperoned iron was catalyzing the formation of reactive oxygen species in the PCBP1-deleted livers.
The deleterious effects of unchaperoned iron could also be measured in primary hepatocytes isolated from the PCBP1-deleted livers. These hepatocytes exhibited enhanced sensitivity to supplemental iron, which could be blocked with vitamin E. They also exhibited increased oxidation of a lipophilic redox sensor (Bodipy C11) and increased cleavage of the chemically-reactive iron sensor FIP-1. Furthermore, dietary iron overload in wild-type mice produced a very similar phenotype of steatosis, Nrf2 activation, increased lipid peroxidation, and increased iron reactivity in hepatocytes (Fig. 4). Although measurement of increased activity of the LIP in iron-loaded wild type hepatocytes was expected, it was surprising to find increases in the LIP of PCBP1-deleted hepatocytes, as the cells were, overall, iron depleted.
Figure 4. Increased labile iron pool triggers steatosis in mice.

(A) Livers from iron-loaded PCBP1fl/fl mice resemble livers from PCBP1Δhep mice on a normal iron diet. Mice were fed normal (50 ppm) or 2% carbonyl iron (20,000 ppm) diets for 16–18 days before analysis. (B) Increased labile iron pool in PCBP1Δhep and iron-overloaded PCBP1fl/fl primary hepatocytes visualized by FIP-1 staining. FIP-1, a ratiometric, fluorescent iron sensor that detects kinetically-labile, chemically-reactive, ferrous iron in cells. Intact FIP-1 undergoes fluorescence resonance energy transfer (FRET) from fluorophores attached to the linker. Fe(II)-triggered cleavage of endoperoxide linker separates fluorophores, prevents FRET, and allows ratiometric iron measurement. Schematic of labile iron Fe(II) detection using FIP-1, by the ratio of green/FRET fluorescence (top) and FIP-1 fluorescence in primary hepatocytes (bottom).
These studies revealed the role of PCBP1 as a true chaperone for iron. Not only does the chaperone enhance the cell’s capacity to direct iron to storage and utilization sites, but it reduces the chemical reactivity of Fe(II), preventing the formation of damaging reactive oxygen species (Fig. 5). In a species that relies on cytosolic Fe to activate hundreds of different iron enzymes, the capacity to manage iron in a chemically less reactive form offers a powerful selective advantage in maintaining cells in an aerobic environment.
Figure 5. PCBP1 protects the liver from unchaperoned reactive iron.

Reactivity of unchaperoned iron in PCBP1-depleted hepatocytes causes increased lipid peroxidation. Then, the accumulation of lipid peroxidation products damages the liver, alters lipid metabolism, and results in liver steatosis. Reactivity of iron pool and lipid peroxidation were visualized using the FIP-1 iron sensor and BODIPY C11. Morphological changes in the livers were detected by hematoxylin and eosin (H&E) staining of liver sections.
The iron-mediated oxidative damage to cellular lipids and the enhanced hepatocyte cell death observed in the livers of mice with PCBP1 deletion or iron overload is suggestive of ferroptosis. Ferroptosis is the term describing a non-apoptotic cell death pathway characterized by elevated levels of iron-mediated lipid peroxidation that leads to loss of membrane integrity, enhanced cell permeability, and death (Bayir et al., 2020; Dixon and Stockwell, 2019; Stoyanovsky et al., 2019). Both the bulk peroxidation of polyunsaturated fatty acyl-containing phospholipids and the targeted, enzymatic lipoxidation of arachidonyl- and adrenoyl-phosphotidylethanolamine have been implicated in this process. In some cultured cells and animal tissues, drugs that deplete intracellular glutathione or inhibit the glutathione peroxidase Gpx4 promote ferroptosis, while iron depletion or lipophilic antioxidants, such as vitamin E and coenzyme Q, will inhibit ferroptosis. Although multiple types of cell death likely account for the reduced viability of hepatocytes in PCBP1-deleted livers, cell death is due, at least in part, to the oxidative damage caused by unchaperoned iron. While the role of GSH as an intracellular ligand for iron has not received attention in studies of ferroptosis, the PCBP1-Fe(II)-GS complex is clearly important for controlling the chemical reactivity of the cytosolic LIP and for holding ferroptosis in check.
Specific roles for PCBP2 in membrane-associated iron transfers
Cells and tissues lacking PCBP1 exhibit iron trafficking defects despite the continued activity of PCBP2. Although PCBP1 is 83% identical to PCBP2, the two iron chaperones are not functionally redundant and have some specific activities not shared by the paralog. Depletion of either PCBP1 or PCBP2 in cultured cells reduced the metalation of non-heme enzymes; however, only PCBP1 is directly detected in protein-protein interactions with the client iron proteins. Studies specifically focused on PCBP2-interacting proteins suggest that membrane-associated proteins may be specific targets. In vitro studies carried out in hepatocyte-derived cells indicate that PCBP2 can interact with DMT1 and ferroportin via the KH2 domain. In overexpression systems, PCBP2 facilitated iron import and efflux, respectively, through these transporters (Yanatori et al., 2016; Yanatori et al., 2014). Furthermore, the membrane-associated heme oxygenases, HO-1 and HO-2, can transfer the iron released from heme to PCBP2 (Yanatori et al., 2017). These studies were recently reviewed in detail (Yanatori et al., 2020). The activities of PCBP2 may be affected by iron transporter expression, intracellular iron levels, or cell type. In a murine model of in vitro erythroid development, PCBP2 depletion was associated with increased transferrin-iron accumulation (mediated by DMT1), increased iron transfer to ferritin, and increased heme and hemoglobin synthesis (Ryu et al., 2017). In contrast, HEK293 cells depleted of PCBP2 did not exhibit significant changes in total cellular iron, although a small reduction in mitochondrial iron was observed (Frey et al., 2014). Furthermore, HEK293 cells depleted of PCBP2 exhibit enhanced formation of Glrx3-[2Fe-2S]-BolA2 complexes, which are dependent on PCBP1 (Patel et al., 2019). PCBP2 iron chaperone activity likely impacts the level of the LIP that is available for coordination by PCBP1 and may possibly be involved in the direct exchange of iron with PCBP1. How the two chaperones work together to maintain cellular iron balance warrants further investigation.
Conclusions
Our conceptualization of the intracellular pools of iron used by the cell for iron cofactor assembly has rapidly changed with new experimental evidence. The labile iron in the cytosol is largely coordinated by reduced GSH and bound to an iron chaperone of the PCBP family. Coordination of the LIP by PCBP1 enables the cell to direct iron into storage in ferritin, to efficiently deliver iron to non-heme iron enzymes, and to make iron available to the Fe-S cluster assembly/repair system. Coordination of Fe(II)-GS by PCBP1 reduces the chemical reactivity of the LIP, thereby protecting cells from the potentially toxic effects of reactive oxygen species and allowing them to use this redox-active metal in the presence of oxygen and other pro-oxidants. Yet many questions remain to be resolved. How are the chaperone activities of PCBP1 coordinated with PCBP2? Is Fe(II)-GS freely transferred between the two chaperones? Is the PCBP1-Fe(II)-GS-BolA2 complex strictly an intermediate in the assembly of [2Fe-2S] on Glrx3-BolA2 or is this complex involved in other iron trafficking or cofactor assembly processes? PCBP iron chaperones do not appear to be required for delivery of iron to mitochondria, so how is iron efficiently directed to this organelle? PCBPs are multifunctional adapter proteins that have important roles in RNA processing and transcription. Does PCBP1 play entirely separate roles in iron and RNA metabolism or are these activities linked in some processes? Does the appearance of PCBP1 in the mammalian radiation suggest that animals have benefitted from directly linking iron coordination and nucleic acid binding in the same protein? We look forward to addressing these questions in future studies.
Highlights.
PCBP1 coordinates iron from the cytosolic labile iron pool as a Fe(II)-glutathione complex.
PCBP1 delivers iron to ferritin and non-heme iron enzymes.
PCBP1 forms a Fe(II)-glutathione complex with BolA2 for use in iron-sulfur cluster assembly.
In murine liver, PCBP1 protects hepatocytes from iron-mediated oxidative damage.
Acknowledgements
We thank the members of the Genetics and Metabolism Section of the Liver Diseases Branch of NIDDK, NIH for their helpful discussions and ideas. The authors are supported by the intramural research program of NIDDK, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antony A, Tang YS, Khan RA, Biju MP, Xiao X, Li QJ, Sun XL, Jayaram HN, and Stabler SP (2004). Translational upregulation of folate receptors is mediated by homocysteine via RNA-heterogeneous nuclear ribonucleoprotein E1 interactions. J Clin Invest 113, 285–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AT, Loehr MO, Bogena J, and Chang CJ (2016). An Endoperoxide Reactivity-Based FRET Probe for Ratiometric Fluorescence Imaging of Labile Iron Pools in Living Cells. J Am Chem Soc 138, 14338–14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AT, Reeves AG, and Chang CJ (2018). Activity-based sensing fluorescent probes for iron in biological systems. Curr Opin Chem Biol 43, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosio P, Elia L, and Poli M (2017). Ferritin, cellular iron storage and regulation. IUBMB Life 69, 414–422. [DOI] [PubMed] [Google Scholar]
- Bae DH, Lane DJR, Siafakas AR, Sutak R, Paluncic J, Huang MLH, Jansson PJ, Rahmanto YS, and Richardson DR (2020). Acireductone dioxygenase 1 (ADI1) is regulated by cellular iron by a mechanism involving the iron chaperone, PCBP1, with PCBP2 acting as a potential co-chaperone. Biochim Biophys Acta Mol Basis Dis, 165844. [DOI] [PubMed] [Google Scholar]
- Banci L, Camponeschi F, Ciofi-Baffoni S, and Muzzioli R (2015a). Elucidating the Molecular Function of Human BOLA2 in GRX3-Dependent Anamorsin Maturation Pathway. J Am Chem Soc 137, 16133–16143. [DOI] [PubMed] [Google Scholar]
- Banci L, Ciofi-Baffoni S, Gajda K, Muzzioli R, Peruzzini R, and Winkelmann J (2015b). N-terminal domains mediate [2Fe-2S] cluster transfer from glutaredoxin-3 to anamorsin. Nat Chem Biol 11, 772–778. [DOI] [PubMed] [Google Scholar]
- Bayir H, Anthonymuthu TS, Tyurina YY, Patel SJ, Amoscato AA, Lamade AM, Yang Q, Vladimirov GK, Philpott CC, and Kagan VE (2020). Achieving Life through Death: Redox Biology of Lipid Peroxidation in Ferroptosis. Cell Chem Biol 27, 387–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer W, Epsztejn S, and Cabantchik ZI (1995). Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J Biol Chem 270, 24209–24215. [DOI] [PubMed] [Google Scholar]
- Capdevila DA, Edmonds KA, and Giedroc DP (2017). Metallochaperones and metalloregulation in bacteria. Essays Biochem 61, 177–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti M, Cockrell AL, Park J, McCormick SP, Lindahl LS, and Lindahl PA (2015). Speciation of iron in mouse liver during development, iron deficiency, IRP2 deletion and inflammatory hepatitis. Metallomics 7, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A, Chander P, and Howe PH (2010). Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1’s multifunctional regulatory roles. RNA 16, 1449–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, and Stockwell BR (2019). The Hallmarks of Ferroptosis. 3, 35–54. [Google Scholar]
- Dreyfuss G, Philipson L, and Mattaj IW (1988). Ribonucleoprotein particles in cellular processes. J Cell Biol 106, 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinar del Dedo J, Gabrielli N, Carmona M, Ayte J, and Hidalgo E (2015). A cascade of iron-containing proteins governs the genetic iron starvation response to promote iron uptake and inhibit iron storage in fission yeast. PLoS Genet 11, e1005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito BP, Epsztejn S, Breuer W, and Cabantchik ZI (2002). A review of fluorescence methods for assessing labile iron in cells and biological fluids. Anal Biochem 304, 1–18. [DOI] [PubMed] [Google Scholar]
- Folgueras AR, Freitas-Rodriguez S, Ramsay AJ, Garabaya C, Rodriguez F, Velasco G, and Lopez-Otin C (2018). Matriptase-2 deficiency protects from obesity by modulating iron homeostasis. Nat Commun 9, 1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey AG, Nandal A, Park JH, Smith PM, Yabe T, Ryu MS, Ghosh MC, Lee J, Rouault TA, Park MH, et al. (2014). Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proc Natl Acad Sci U S A 111, 8031–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey AG, Palenchar DJ, Wildemann JD, and Philpott CC (2016). A Glutaredoxin.BolA Complex Serves as an Iron-Sulfur Cluster Chaperone for the Cytosolic Cluster Assembly Machinery. J Biol Chem 291, 22344–22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuens T, Bouhy D, and Timmerman V (2016). The hnRNP family: insights into their role in health and disease. Hum Genet 135, 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem LR, Kromer A, Silverman IM, Chatterji P, Traxler E, Penzo-Mendez A, Weiss MJ, Stanger BZ, and Liebhaber SA (2016). The Poly(C) Binding Protein Pcbp2 and Its Retrotransposed Derivative Pcbp1 Are Independently Essential to Mouse Development. Mol Cell Biol 36, 304–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi A, Roshan TM, Kahawita TM, Mason AB, Sheftel AD, and Ponka P (2016). Erythroid cell mitochondria receive endosomal iron by a “kiss-and-run” mechanism. Biochim Biophys Acta 1863, 2859–2867. [DOI] [PubMed] [Google Scholar]
- Hamed M, and Silver J (1983). Studies on the reactions of ferric iron with glutathione and some related thiols. Part II. Complex formation in the pH range three to seven. Inorganica Chimica Acta 80, 115–122. [Google Scholar]
- Handa P, Morgan-Stevenson V, Maliken BD, Nelson JE, Washington S, Westerman M, Yeh MM, and Kowdley KV (2016). Iron overload results in hepatic oxidative stress, immune cell activation, and hepatocellular ballooning injury, leading to nonalcoholic steatohepatitis in genetically obese mice. Am J Physiol Gastrointest Liver Physiol 310, G117–127. [DOI] [PubMed] [Google Scholar]
- Haunhorst P, Hanschmann EM, Brautigam L, Stehling O, Hoffmann B, Muhlenhoff U, Lill R, Berndt C, and Lillig CH (2013). Crucial function of vertebrate glutaredoxin 3 (PICOT) in iron homeostasis and hemoglobin maturation. Mol Biol Cell 24, 1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider RC, and Kong X (2013). Iron speciation in the cytosol: an overview. Dalton Trans 42, 3220–3229. [DOI] [PubMed] [Google Scholar]
- Hider RC, and Kong XL (2011). Glutathione: a key component of the cytoplasmic labile iron pool. Biometals 24, 1179–1187. [DOI] [PubMed] [Google Scholar]
- Jacques JF, Mercier A, Brault A, Mourer T, and Labbe S (2014). Fra2 is a co-regulator of Fep1 inhibition in response to iron starvation. PLoS One 9, e98959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AV, Yegorov D, Vladimirov YA, and Azizova OA (1992). Intracellular free iron in liver tissue and liver homogenate: studies with electron paramagnetic resonance on the formation of paramagnetic complexes with desferal and nitric oxide. Free Radic Biol Med 13, 9–16. [DOI] [PubMed] [Google Scholar]
- Kumanovics A, Chen OS, Li L, Bagley D, Adkins EM, Lin H, Dingra NN, Outten CE, Keller G, Winge D, et al. (2008). Identification of FRA1 and FRA2 as genes involved in regulating the yeast iron regulon in response to decreased mitochondrial iron-sulfur cluster synthesis. J Biol Chem 283, 10276–10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidgens S, Bullough KZ, Shi H, Li F, Shakoury-Elizeh M, Yabe T, Subramanian P, Hsu E, Natarajan N, Nandal A, et al. (2013). Each member of the poly-r(C)-binding protein 1 (PCBP) family exhibits iron chaperone activity toward ferritin. J Biol Chem 288, 17791–17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mapolelo DT, Dingra NN, Naik SG, Lees NS, Hoffman BM, Riggs-Gelasco PJ, Huynh BH, Johnson MK, and Outten CE (2009). The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation. Biochemistry 48, 9569–9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mapolelo DT, Randeniya S, Johnson MK, and Outten CE (2012). Human glutaredoxin 3 forms [2Fe-2S]-bridged complexes with human BolA2. Biochemistry 51, 1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Abbate V, and Hider RC (2015). Iron-sensitive fluorescent probes: monitoring intracellular iron pools. Metallomics 7, 212–222. [DOI] [PubMed] [Google Scholar]
- Makeyev AV, and Liebhaber SA (2002). The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA 8, 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlenhoff U, Molik S, Godoy JR, Uzarska MA, Richter N, Seubert A, Zhang Y, Stubbe J, Pierrel F, Herrero E, et al. (2010). Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab 12, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandal A, Ruiz JC, Subramanian P, Ghimire-Rijal S, Sinnamon RA, Stemmler TL, Bruick RK, and Philpott CC (2011). Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab 14, 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JE, Klintworth H, and Kowdley KV (2012). Iron metabolism in Nonalcoholic Fatty Liver Disease. Curr Gastroenterol Rep 14, 8–16. [DOI] [PubMed] [Google Scholar]
- Ostareck-Lederer A, and Ostareck DH (2004). Control of mRNA translation and stability in haematopoietic cells: the function of hnRNPs K and E1/E2. Biol Cell 96, 407–411. [DOI] [PubMed] [Google Scholar]
- Patel SJ, Frey AG, Palenchar DJ, Achar S, Bullough KZ, Vashisht A, Wohlschlegel JA, and Philpott CC (2019). A PCBP1-BolA2 chaperone complex delivers iron for cytosolic [2Fe-2S] cluster assembly. Nat Chem Biol 15, 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrat F, de Groot H, Sustmann R, and Rauen U (2002a). The chelatable iron pool in living cells: a methodically defined quantity. Biol Chem 383, 489–502. [DOI] [PubMed] [Google Scholar]
- Petrat F, Weisheit D, Lensen M, de Groot H, Sustmann R, and Rauen U (2002b). Selective determination of mitochondrial chelatable iron in viable cells with a new fluorescent sensor. Biochem J 362, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott CC, and Jadhav S (2019). The ins and outs of iron: Escorting iron through the mammalian cytosol. Free Radic Biol Med 133, 112–117. [DOI] [PubMed] [Google Scholar]
- Philpott CC, Ryu MS, Frey A, and Patel S (2017). Cytosolic iron chaperones: Proteins delivering iron cofactors in the cytosol of mammalian cells. J Biol Chem 292, 12764–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protchenko O, Baratz E, Jadhav S, Li F, Shakoury-Elizeh M, Gavrilova O, Ghosh MC, Cox JE, Maschek JA, Tyurin VA, et al. (2020). Iron chaperone PCBP1 protects murine liver from lipid peroxidation and steatosis. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NJ, and Winge DR (2010). Copper metallochaperones. Annu Rev Biochem 79, 537–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, and Maio N (2017). Biogenesis and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J Biol Chem 292, 12744–12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MS, Duck KA, and Philpott CC (2018). Ferritin iron regulators, PCBP1 and NCOA4, respond to cellular iron status in developing red cells. Blood Cells Mol Dis 69, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MS, Zhang D, Protchenko O, Shakoury-Elizeh M, and Philpott CC (2017). PCBP1 and NCOA4 regulate erythroid iron storage and heme biosynthesis. J Clin Invest 127, 1786–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Bencze KZ, Stemmler TL, and Philpott CC (2008). A cytosolic iron chaperone that delivers iron to ferritin. Science 320, 1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanovsky DA, Tyurina YY, Shrivastava I, Bahar I, Tyurin VA, Protchenko O, Jadhav S, Bolevich SB, Kozlov AV, Vladimirov YA, et al. (2019). Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic Biol Med 133, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YS, Khan RA, Xiao S, Hansen DK, Stabler SP, Kusumanchi P, Jayaram HN, and Antony AC (2017). Evidence Favoring a Positive Feedback Loop for Physiologic Auto Upregulation of hnRNP-E1 during Prolonged Folate Deficiency in Human Placental Cells. J Nutr 147, 482–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YS, Khan RA, Zhang Y, Xiao S, Wang M, Hansen DK, Jayaram HN, and Antony A (2011). Incrimination of heterogeneous nuclear ribonucleoprotein E1 (hnRNP-E1) as a candidate sensor of physiological folate deficiency. J Biol Chem 286, 39100–39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ (1982). Free manganese (II) and iron (II) cations can act as intracellular cell controls. FEBS Lett 140, 3–10. [DOI] [PubMed] [Google Scholar]
- Yanatori I, Richardson DR, Imada K, and Kishi F (2016). Iron Export through the Transporter Ferroportin 1 Is Modulated by the Iron Chaperone PCBP2. J Biol Chem 291, 17303–17318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanatori I, Richardson DR, Toyokuni S, and Kishi F (2017). The iron chaperone poly(rC)-binding protein 2 forms a metabolon with the heme oxygenase 1/cytochrome P450 reductase complex for heme catabolism and iron transfer. J Biol Chem 292, 13205–13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanatori I, Richardson DR, Toyokuni S, and Kishi F (2020). The new role of poly (rC)-binding proteins as Iron transport chaperones: Proteins that could couple with inter-organelle interactions to safely traffic iron. Biochim Biophys Acta Gen Subj, 129685. [DOI] [PubMed] [Google Scholar]
- Yanatori I, Yasui Y, Tabuchi M, and Kishi F (2014). Chaperone protein involved in transmembrane transport of iron. Biochem J 462, 25–37. [DOI] [PubMed] [Google Scholar]


