Abstract
The present study aimed to evaluate the structure, survival and development of isolated caprine (secondary-SEC and early antral-EANT) follicles, after vitrification in the presence of synthetic polymers and in vitro culture. Additionally, transzonal projections (TZPs) and p450 aromatase enzyme were evaluated. After isolation, SEC and EANT follicles were in vitro cultured for six days or vitrified. After one week, SEC and EANT follicles were warmed and also in vitro cultured for six days. Data revealed that the percentage of morphologically normal follicles was similar between fresh and vitrified follicles in both follicular categories and antrum formation rate was similar between fresh and vitrified SEC follicles. Fluorescence by calcein-AM did not show difference between fresh and vitrified (SEC and EANT) follicles, however, the trypan blue test showed low viability for vitrified follicles. The integrity of TZPs was not affected between fresh and vitrified SEC follicles, however, in vitrified EANT follicles, there were signs of TZPs loss. Regarding steroidogenic function, it was observed a positive staining for p450 aromatase enzyme in fresh and vitrified SEC and EANT follicles. It was concluded that SEC follicles seem to be more resistant to vitrification than EANT follicles, as shown by the trypan blue test and TZPs assay. Future studies may confirm this hypothesis, in order to consolidate the use of SEC and EANT follicles as an alternative to ovary cryopreservation.
Keywords: Cryopreservation, Ovarian follicles, TZPs, Endocrine function, Goat
1. Introduction
Cryopreservation is a great ally of assisted reproduction techniques in humans, making a positive contribution in clinic therapies in women who wish to restore their reproductive function after oncologic treatments [1], especially through ovarian tissue cryopreservation (OTCP). With the OTCP aid, by slow freezing [2] as well as vitrification [3,4], more than a hundred births were recording after transplantation of thawed tissue in humans [5]. Notwithstanding these significant advances, there still are great challenges related ischemia and reperfusion, as well as malignant cells reintroduction after grafting of ovarian tissue [6,7], particularly for cancer patients. Fortunately, the usage of preantral (secondary) and early antral follicles isolated from ovarian tissue could be a viable alternative for these patients [8,9].
These follicles are located close to the ovarian cortex surface and could be mechanically removed from the ovary without compromising their structure (secondary [10] e early antral [11]). After recovering, secondary and early antral follicles could be frozen or vitrified and stored for undetermined period. Subsequently, follicles could be in vitro cultured for full growth and oocyte maturation, functioning then as a source of potentially fertilizable oocyte [12]. In a previous study performed with caprine species, by our team, isolated secondary follicles were capable of growing and forming antral cavity, after vitrification and in vitro culture [13]. Nonetheless, these follicles were vitrified enclosed in the ovarian cortex, whose extracellular matrix composed by different cell types, represents a barrier for optimal performance of cryoprotectant agents [14,15] and for adequate preservation. On the other hand, although OTCP is more challenging than isolated cell structures, the cryopreservation of isolated ovarian follicles also involves some factors, which must be carefully considered. Among these factors we can highlight: (1) the absence of ovarian stroma that acts supporting follicles; (2) the contact of cryoprotectants and (3) osmotic forces that act directly on the follicles. These factors could cause damage to transzonal projections (TZPs) present among granulosa cells and the oocyte, jeopardizing follicle development and hormone function [16,17].
In this study, we vitrified caprine secondary and early antral follicles, as translational model for human species, using a vitrification protocol previously employed for vitrifying isolated non-human primate follicles, associating intracellular to extracellular cryoprotectants as synthetic polymers, in the vitrification solution [18,19]. Synthetic polymers are molecules similar to ice-binding proteins (IBP) found in living beings of cool temperature environments [18], which when added to vitrification solution, they could reduce ice crystal formation. Previous studies showed the benefits of these polymers for cryopreservation of rabbit [20] embryos, swine [21] and equine [22] oocytes, and ovarian preantral follicles in non-human primates [18-20] and bovine [23] species.
In order to preserve female fertility, the present study aimed to compare the capacity of survival, growth, formation, potential endocrine function (presence of p450-aromatase) and TZPs integrity between secondary and early antral follicles, after vitrification, followed by in vitro culture.
2. Materials and methods
2.1. Ethical approval and chemicals
This study was approved and performed under the guidelines of the Ethics Committee for Animal Use of the State University of Ceará (4999537/2018).
Ethylene glycol and glycerol (cryoprotectants) were obtained from Dinâmica Química and Sigma Chemical co. (St. Louis, MO, EUA), respectively. The culture medium and other chemicals were obtained from Sigma Chemical (unless stated otherwise).
2.2. Collection of ovaries and experimental design
Ovaries (n = 80) from forty mixed-breed goats (1–3 years of age) were collected at a local abattoir. Immediately after slaughter the ovaries were recovered and washed once in 70% ethanol and then washed twice in buffered minimum essential medium (MEM) supplemented with HEPES and antibiotics (100-mg/mL penicillin and 100-mg/mL streptomycin). Ovaries were immediately transported to the laboratory in MEM at 4 °C within 1 h of collection [24].
Once at the laboratory, ovaries were submitted to isolation of preantral (secondary) and early antral follicles, as following described. Both secondary (SEC) and early antral (EANT) follicles were randomly distributed into two groups, i.e. fresh and vitrified. Fresh follicles were immediately in vitro cultured for 6 days, whilst the remaining follicles were vitrified and stored in liquid nitrogen (LN2) for 7 days. After cryostorage period follicles (SEC and EANT) were warmed and immediately in vitro cultured for 6 days. During and at the end of the culture, the following parameters were evaluated: morphological appearance, follicular development (antrum formation and growth) and viability. Furthermore, the presence and integrity of transzonal projections (TZPs) were evaluated on days 2 (D2) and 6 (D6) of culture by immunofluorescent staining. On D6 the activity of aromatase enzyme was also evaluated by immunohistochemistry staining. Each treatment was repeated five times.
2.3. Follicular isolation
Slices (1–2 mm thick) of ovarian cortex were initially obtained under sterile conditions using a surgical blade and then placed in holding medium consisting of MEM–HEPES with antibiotics (penicillin and streptomycin, both at 100 μg/mL). Secondary and early antral follicles visualized in ovarian cortical slices were selected, considering morphology and diameter, and then were isolated mechanically by microdissection using 26-gauge (26-G) needles under a stereomicroscope (SMZ 645 Nikon, Tokyo, Japan). Secondary follicles (250 μm in diameter, surrounded by at least two granulosa cell layers, without antral cavity and visible oocyte) [25] and early antral follicles (350 μm in diameter with visible antral cavity) [11] were in vitro cultured or vitrified and stored in LN2 for 7 days.
2.4. Cryoprotectants exposure, vitrification, warming and cryoprotectants removal procedures
The vitrification/warming was performed according to the protocol described by Ting et al. [19], with slight modifications. Briefly, follicles of each category (secondary and early antral) were successively exposed to the intracellular cryoprotectants in three different equilibration solutions (ES), presenting a common base consisted by MEM-HEPES, 15% fetal calf serum (FCS), and 1 μl/mL ascorbic acid, added by intracellular cryoprotectants: a) 5% glycerol (ES1); b) 15% glycerol and 15% ethylene glycol (ES2) and c) 30% glycerol and 30% ethylene glycol (ES3). The exposure of follicles to the equilibration solutions (ES1, ES2 and ES3) was performed at room temperature for 5, 3 and 2 minutes, respectively. After last exposure, follicles were transferred to vitrification solution (VS) composed by 50% of ES added to extracellular cryoprotectants, i.e. synthetic polymers (20% Supercool X-1000, 40% Supercoool Z-1000 and 20% Polyvinylpyrrolidone – PVP).
For the vitrification procedure itself, groups of 5 follicles (SEC or EANT) were transferred to 0.25 mL straws (MINITUBE BRASIL). The straws were filled with VS-air-follicles-air-VS and were finally heat sealed. Subsequently, straws were exposed to LN2 vapor for 2 min and then were directly plunged into LN2 and stored until warming procedure.
After one week of storage, straws containing follicles were warmed at room temperature for 5 s and were subsequently submerged into a 45 °C water bath for 30 s, until the VS had completely warmed to a liquid state. Follicles were recovered from straws and submitted to removal, by using four different successive washes (3 minutes each), in different washing solutions consisting of MEM-HEPES added by decreasing concentrations of sucrose (i.e., 1 M, 0.75 M, 0.5 M and 0.25 M). Thereafter follicles were washed twice in MEM-HEPES supplemented with 100 μg/mL penicillin and streptomycin, 15% FCS for 5 minutes. Finally, follicles were in vitro cultured, according to the appropriate protocol for each follicular category.
2.5. In vitro culture of secondary and early antral follicles
Fresh and vitrified/warm secondary follicles were individually cultured in 96-well ultra-low attachment microplates (96-Well, Corning Incorporated, Corning, Kennebunk – ME, EUA) containing 250 μL of medium per well [26]. The culture medium consisted of α-MEM (Sigma - M8650, pH 7,2–7,4) supplemented with 3 mg/mL bovine serum albumin (BSA), 10 μg/mL recombinant human insulin, 5.5 μg/mL transferrin, 5 ng/mL selenium, 2 mM glutamine, 2 mM hypoxanthine and 50 μg/mL ascorbic acid [25].
Fresh and vitrified/warm early antral follicles were also individually cultured, in 100 μL-drops of culture medium under mineral oil in Petri dishes (60 × 15 mm, Corning Incorporated, Corning, EUA). The culture medium for this follicular category consisted of α-MEM (Sigma – M8650, pH 7,2–7,4) supplemented with 3 mg/mL BSA, 10 ng/mL recombinant human insulin, 5.5 μg/mL transferrin, 5 ng/mL selenium, 2 mM glutamine, 2 mM hypoxanthine, 50 μg/mL ascorbic acid, and 50 ng/mL bovine growth hormone as described by Cadenas et al. [11]
For both follicular categories, the culture was carried out at 38.5 °C in 7.5% CO2 in air for 6 days and the medium was replaced partially every other day.
2.6. Morphological evaluation and in vitro follicular development
Fresh or vitrified secondary and early antral follicles before and after in vitro culture were classified as normal (intact basement membrane and without oocyte extrusion, bright and homogeneous granulosa cells and homogeneous cytoplasm of the oocyte) or degenerated (rupture of basement membrane and extruded oocyte, dark oocyte and granulosa cells associated with a reduced follicular diameter, at the end of culture).
For growth evaluation, follicular diameter was measured using an ocular micrometer attached to a stereomicroscope on D0 and D6 of culture. Thus, the mean of two perpendicular measures of each follicle was calculated to determine follicular diameter. The daily follicular growth rate (DFGR) was calculated using the formula shown below. Antrum formation was defined by the presence of a visible translucent cavity among granulosa cell layers.
DFGR – Daily follicular growth rate
NF – Normal follicles
2.7. Viability assays
At the end of the culture period, fresh or vitrified follicles were randomly distributed for viability assay by fluorescence or vital stain test, establishing a percentage of viable follicles in each assessment. For fluorescence analysis, a total of 92 secondary follicles (fresh: 45 and vitrified: 47) and 82 early antral follicles (fresh: 41 and vitrified: 41) were evaluated. Follicles were incubated at room temperature during 30 minutes in 100 μL of PBS supplemented with 4 μM calcein-AM and 2 μM ethidium homodimer-1, (Molecular Probes - LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells - L3224, Invitrogen, Karlsruhe, Germany) and fixed in 0.5% glutaraldehyde. Then follicles were immediately examined by fluorescence microscopy (Eclipse 80i; Nikon, Tokyo, Japan) and classified as viable when cytoplasm was stained in green with calcein-AM and non-viable when chromatin was stained in red with ethidium. The fluorescence emission of both calcein-AM (green) and ethidium homodimer-1 (red) was measured at 488 and 568 nm, respectively [13].
For the trypan blue dye exclusion test, 95 secondary follicles (fresh: 45 and vitrified: 50) and 87 early antral follicles (fresh: 46 and vitrified: 41) were incubated for 2 minutes at room temperature in 0.4 % trypan blue solution (Sigma Chemical co.). Evaluation of trypan blue staining was carried out under a stereomicroscope and follicles were classified as viable when unstained and non-viable when stained with trypan blue [27].
2.8. Analysis to visualize the transzonal projections (TZPs)
Fresh and vitrified follicles (secondary and early antral) in day 2 and day 6 were fixed in 4% paraformaldehyde (PAF) for up 1 h at room temperature. Then follicles were transferred to a fixation solution consisted of PBS added by 0.1% BSA and 0.1% Tween20 for 8–12 h at 4 °C. Briefly, follicles were transferred to a blocking solution composed of PBS added by 0.5% BSA, 0.2% sodium azide, 1% milk powder, 10% goat serum, 1% donkey serum, 0.1 M glycine and 0.1% Triton X for 1 h under agitation and protected from light. Subsequently, follicles were incubated for 2 h at room temperature with Alexa 488 Phalloidin antibody (Invitrogen, cat# T6199 – dilution 1:50) under agitation and protected from light. After the incubation period, follicles were submitted to three rinses in blocking solution, for 5 minutes each. Finally, follicles were placed in 60-well slides (μ-Slide Angiogenesis IbiTreat, Ibidi GmbH – Germany) with DAPI (ABCAM - 104139) and examined by confocal laser scanning microscopy (Zeiss LSM 700META, Weimer, Germany).
2.9. Immunohistochemistry for aromatase
Secondary and early antral follicles (fresh or vitrified) were fixed in 4% PAF for 1 h, dehydrated in alcohol, cleared in xylene, embedded in paraffin wax and serially sectioned at 7 μm. Sections were mounted on positively charged slides and processed by immunohistochemistry for aromatase staining, expressing steroid activity. The antigen retrieval was performed by incubating slides in a retrieval buffer for 20 min at 98 °C (low pH). For blocking endogenous peroxidase activity, 10% H2O2 in methanol was used. Subsequently, slides were incubated for 30 minutes with rabbit polyclonal anti-aromatase (1:200 – AB18995). Then slides were incubated for 30 minutes with anti-rabbit IgG secondary antibody (1:200 – ab97046; abcam, Cambridge, UK), followed by incubation in the avidin-biotin complex (ABC; Vector Laboratories, Burlingame, CA, USA), which interacts with diaminobenzidine chromagen solution (DAB; Dako, inc, USA). The slides were later counterstained with hematoxylin and Scott’s solution. Negative controls were obtained by omission of the primary antibodies. Immunostaining was evaluated based on the presence, or not, of positive staining for aromatase.
2.10. Statistical analysis
Statistical analyses were performed using Sigma Plot 11.0 (Systat Software Inc., EUA). Variables presented as percentage were evaluated by chi-square or Fischer’s exact tests. The comparison of mean values was evaluated by one-way ANOVA, followed by Student-Newman-Keuls post-hoc test or t-test, when appropriate. Data are presented as mean ( ± s.e.m.) and percentage and the statistical significance was defined as P < 0.05 (two-sided).
3. Results
3.1. Follicular morphological features after vitrification and in vitro culture
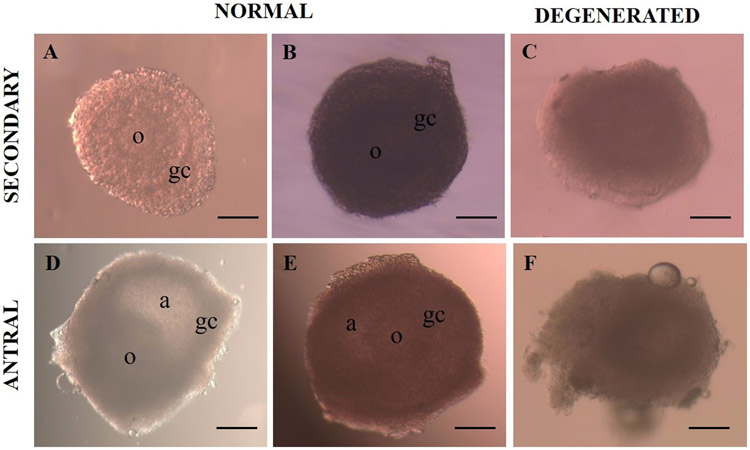
Regarding morphology (visual analysis), in Fig. 1, we could see normal (A, B, D e E) and degenerated (C and F) follicles. Table 1 shows the percentage of normal follicles, follicular diameter and antrum formation (SEC follicles). Although a significant reduction was observed in the morphologically normal SEC and EANT follicles at the end of the in vitro culture period (day 6), there was no significant difference between fresh and vitrified secondary follicles.
Fig. 1.
Representative images of secondary and early antral follicles. Morphologically normal, translucent follicles, with intact membrane and bright and homogenous surrounding granulosa cells (A, B, D and E). Degenerated follicles, with irregular shape, dark oocyte and granulosa cells, integrity loss and diameter reduction (C and E). a: antrum, o: oocyte, gc: granulosa cells (original magnification 10×). Scale bar = 200 μm.
Table 1.
Percentage of normal and degenerated follicles, antrum formation and follicular diameter of vitrified and non-vitrified in vitro cultured secondary and early antral follicles.
| Follicular category | Follicles (n) | Normal follicles (%) | Degenerated follicles (%) | Antrum formation (%) | Follicle diameter (μm) | Growth rate (%) |
|---|---|---|---|---|---|---|
| Fresh SEC | 90 | 91 (82/90)A | 9 (8/90) | 95 (79/90) | 335 ± 97A | 15 ± 12A |
| Vitrified SEC | 97 | 84 (82/97)AB | 15 (15/97) | 90 (89/97) | 259 ± 58B | 8 ± 7B |
| Fresh EANT | 87 | 78 (68/87)B | 22 (19/87) | – | 352 ± 58A | 8 ± 7BC |
| Vitified EANT | 82 | 78 (64/82)B | 22 (18/82) | – | 314 ± 45A | 3 ± 2C |
Upper case letter differ in the same column (P < 0.05).
All data are expressed as mean ± standard error of the mean.
Abbreviations: SEC, Secondary; EANT, early antral.
After 4 days of culture, 61% of secondary follicles (fresh and vitrified) showed a small antral cavity (data not shown), and subsequently, it was observed at the end of culture (D6) that the rate of antrum formation increased to 95% in fresh follicles and 89% in vitrified follicles, with no significant difference between groups (P < 0.05). At day 6 of in vitro culture, we observed that the diameter of vitrified secondary follicles was lower (P < 0.05) than fresh follicles diameter. Similar results were observed for follicular growth rate between fresh and vitrified secondary follicles. As expected, at the end of culture, the follicular diameter of early antral follicles was higher (P < 0.05) than diameter of vitrified secondary follicles. Nonetheless, the growth rate of vitrified secondary follicles was higher than vitrified early antral follicles (P < 0.05).
3.2. Follicular viability after in vitro culture
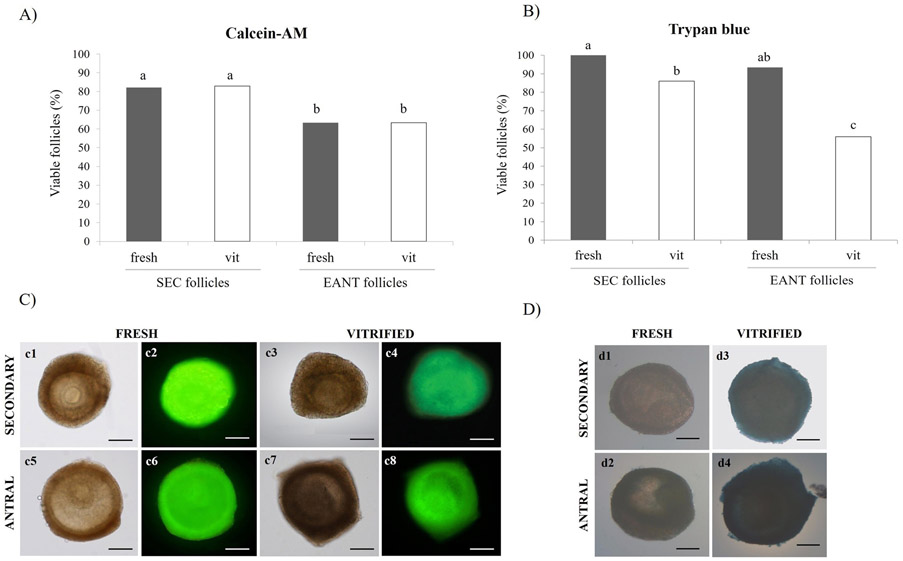
A total of 187 and 169 secondary and early antral follicles (fresh or vitrified) was evaluated for follicular viability after six days of in vitro culture, respectively. Fig. 2 shows graphics and representative images of follicular viability for calcein-AM and ethidium homodimer-1, as well as for trypan blue dye. Regarding viability by calcein and ethidium, it was demonstrated that follicular viability did not differ (P > 0.05) between fresh and vitrified follicles in both follicular categories (secondary and early antral). On the other hand, the percentage of viable SEC follicles (fresh or vitrified) was higher (P < 0.05) than percentage of viable EANT follicles.
Fig. 2.
Viability of fresh and vitrified secondary and early antral follicles after in vitro culture. Percentage of viable follicles per treatment in both categories evaluated by (A) Calcein-AM and (B) Trypan blue. (C) Representative images of secondary (c1 and c3) and early antral (c5 and c7) follicles in light field and after fluorescent staining by calcein-AM (secondary: c2 and c4) and (early antral: c6 and c8). Representative images of fresh and vitrified follicles, viable (d1 and d2) e non-viable (d3 and d4), evaluated by trypan blue, after six days of in vitro culture. a,b,c Within a column (P < 0.05). Scale bar = 200 μm.
With relation to viability evaluated by trypan blue dye test, the percentage of fresh viable follicles was higher than vitrified follicles in both follicular categories (SEC and EANT). Furthermore, the viability of vitrified SEC follicles was higher (P < 0.05) than viability of vitrified EANT follicles.
3.3. Structural analysis of transzonal projections (TZPs)
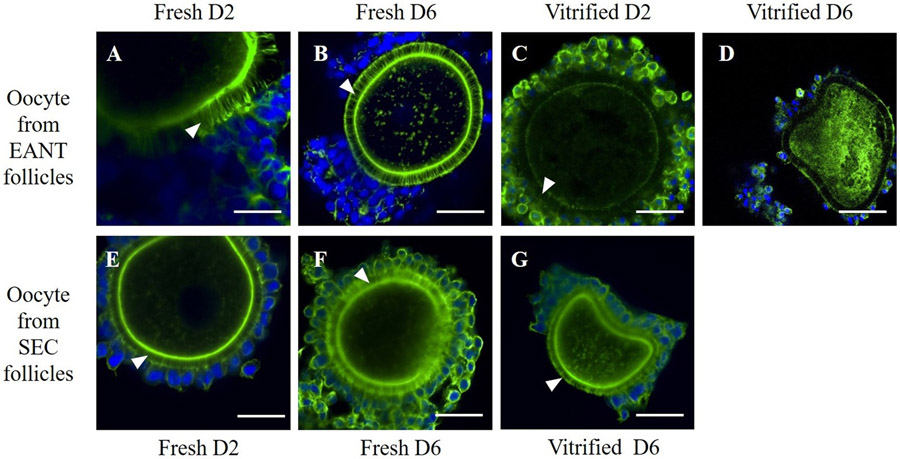
Considering that TZPs play an important role in information exchange among granulosa cells and among these cells and oocyte, the integrity of these structures was evaluated after vitrification/warning and in vitro culture. Our images suggested that TZPs were present and intact in fresh (secondary and early antral) and vitrified (only secondary) follicles (Fig. 3). However, vitrified early antral follicles seem to show a lower fluorescent intensity for the structures, suggesting the loss of TZPs in some segments of these follicles.
Fig. 3.
Confocal microscopy images depicting trans-zonal projections (TZPs) in oocytes of goat secondary and early antral follicles. COCs from D2 (a) and D6 (b) fresh EANT follicles, COCs from D2 (c) and D6 (d) vitrified EANT follicles (d), COCs from D2 (e) and D6 (f) fresh SEC follicles and (g) COCs from vitrified D6 SEC follicles. White arrow indicates the TZPs. Scale bar = 50 μm.
3.4. Activity of enzyme p450 aromatase
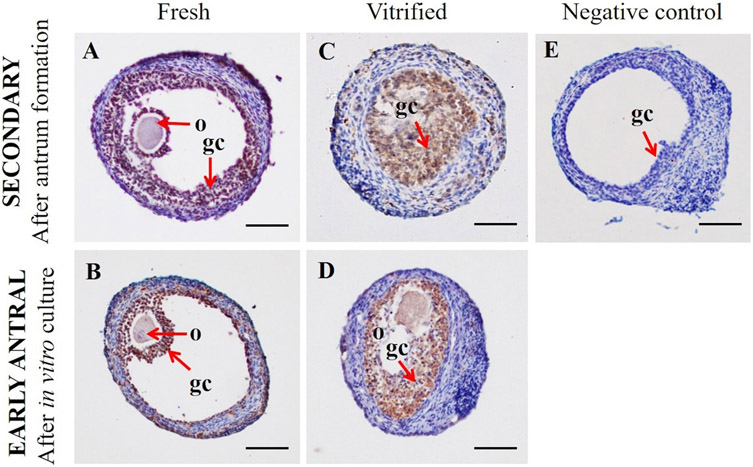
We used immunohistochemistry assay for evaluating p450 aromatase enzyme activity on granulosa cells of fresh or vitrified ovarian follicles (Fig. 4). Immunostaining was weak, moderate or strong, based on staining intensity. Data showed strong staining on granulosa cells for both category of follicles (fresh and vitrified) after six days of in vitro culture. As expected, negative control (Fig. 4E), obtained by primary antibody omission, did not show staining for the p450 enzyme.
Fig. 4.
Immunohistochemical staining for p450 aromatase in (A) fresh secondary follicles (B) fresh early antral follicles (C) vitrified secondary follicles (D) vitrified early antral follicles (E) negative control. (o) oocyte and; (gc) granulosa cells. Scale bar = 100 μm.
4. Discussion
The cryopreservation of follicles (secondary and early antral) isolated from the ovarian environment in order to preserve female gamete from toxic effects caused by chemo- and/or radio-therapies could be a promising strategy for reproductive function restoration for women submitted to treatments against cancer. Nevertheless, due to ethical, legal and religious issues, as well as lack of necessary material for investigation, several studies involving cryopreservation and in vitro culture of ovarian follicles have been performed in different animal species (caprine [13], ovine [28], mouse [29], and non-human primate [18,19]) as translational models for human species. For this purpose, in the present study, we used caprine ovarian follicles, considering not only the similarity of structure and ovarian organization, but also aspects related to folliculogenesis [30,31] between the two species.
The data showed that at the end of the culture period (6 days), the percentage of morphologically normal follicles between fresh and vitrified follicles did not differ in both follicular categories. Similar results were related in previous studies either for secondary follicles (non-human primates [19], ovine [32], and caprine [13]), as for early antral follicles [33]. It is known that the cryopreservation process causes follicular injuries, however, according to our results and to other’s authors findings [34], depending on severity grade, these injuries could not be visualized by light field optic microscopy. This means that follicles that present normal aspects analyzed by stereomicroscope could not have been viable when submitted to a viability test.
Our results showed that a great percentage of fresh (95%) or vitrified (90%) secondary follicles were capable of growing and forming an antral cavity after culture in a similar way. These results also are in agreement with previous studies performed by our team in ovine [32] and caprine [13] species. The formation of antral cavity in fresh secondary follicles isolated from ovarian tissue and in vitro culture has been a common event in the caprine species [35-37,25]. This suggests that the responsible mechanism for this feature was not probably blocked in vitro and could be influenced by components (amino acids, proteins, growth factors and hormones) present in culture medium [38,39]. Considering this statement as true, we also supposed that this follicle function was not really affected by the applied vitrification procedure.
The diameter and in vitro daily follicular growth rate of vitrified secondary follicles were lower than fresh follicles. Previous studies also revealed that vitrified follicles exhibited lower growth rates [40]. This could be due to stress conditions in which follicles are submitted during vitrification, like cryoprotectant exposure, low temperatures, as well as solution effect caused by the osmotic pressure of different vitrification solutions and removal [41]. Nevertheless, vitrified early antral follicles maintained mean diameter and growth rate similar to fresh follicles. In this case, the increase of follicular diameter could have been attributed only by antral cavity expansion, since this feature was not negatively affected in both follicles, fresh or vitrified. In addition, the growth rate of vitrified secondary follicles was higher than early antral follicles also vitrified. We suggest that this result is due to the fact that secondary follicles are less developed and present a lower number of cells and are less metabolically active [42-44], giving them better resistance to vitrification process, compared to early antral follicles.
According to fluorescent staining with calcein-AM, independent to the follicular category, viability was similar between fresh and vitrified follicles. Calcein-AM is a marker capable of being transported through the cellular membrane in live cells. After transport in the cell, intracellular esterases remove the acetomethoxy group and the molecule binds to the calcium in the cell interior, resulting in green fluorescence. As dead cells lose active esterases, only live cells are stained [45]. Based on this assay, we state that the vitrification process also did not affect the activity of this enzyme, in both follicular categories.
On the other hand, the trypan blue dye test shows that the viability of vitrified follicles was lower than fresh follicles in both categories. Knowing that this method is adopted to evaluate membrane rupture, we could suggest that this damage could have occurred due to the mechanic stress suffered by follicles during concentration gradient generation that naturally takes place during the steps of addition and removal of cryoprotectant agents [46]. Several researchers have reported the reduction of follicle viability after vitrification procedure [47,33,48], that could be due to osmotic and toxic effects exerted by cryoprotectant exposures, leading to membrane rupture and consequently follicular death. In addition, some crucial elements such as the correct balance of solutions, time and exposure procedures to cryoprotectants, temperatures and the use of different devices are essential to reduce possible damage caused during cryopreservation. [49,50].
For a more accurate evaluation of the follicles, we also assayed TZPs. The TZPs are transmembrane extensions that originate in granulosa cells of ovarian follicles and reach the oocyte surface through the zona pellucida. In the oocyte, these projections form gap junctions and adherent that allow the exchange of information between two cells [51]. TZPs are essential structures for the exchange of paracrine factors (hormones, growth factors, transcription factors, second messengers and nutrients) [52]. Thus, maintaining the integrity of these structures is essential to guarantee the follicular development process.
In the present study, confocal microscopy analyses showed that TZPs were present and intact in both fresh and vitrified follicles for both categories. Nevertheless, it was observed that in vitrified early antral follicles, the TZPs were less intense, not only at day 6, but already from day 2 of in vitro culture. This finding suggests that TZPs could not be restored throughout the culture period, which could be due to either the vitrification protocol or the in vitro culture system that is not yet fully suitable for these follicles. Barret et al. [53] also observed a reduction on TZP quantity in human ovarian follicles cryopreserved and cultured for 2 and 6 days, compared to non-cryopreserved follicles.
Besides morphological and structural aspects, we also evaluated follicular functionality, by immunohistochemistry of p450 aromatase enzyme in granulosa cells and in oocyte of fresh and vitrified follicles. Previous studies reported that gene expression of p450 aromatase is intimately related to the production of high levels of estradiol in caprine secondary follicles [39]. The p450 aromatase is a steroidogenic enzyme that catalyzes androgens to estrogens [54], indicating normal follicular endocrine function. The results of the present study indicated the presence of this enzyme in both granulosa cells and oocytes of secondary and early antral follicles (fresh and vitrified), suggesting that even after vitrification process, follicles maintained their steroidogenic function.
In summary, this study showed that secondary and early antral follicles isolated from caprine ovarian stroma were capable of surviving and growing after vitrification and in vitro culture for a short period. Although we are not yet able of conducting full in vitro development of preantral follicles, secondary follicles seem to better resist than antral follicles, to the stress caused by vitrification and in vitro culture conditions, as it was revealed by viability assay with trypan blue. Although vitrified early antral follicles have shown fragility in the TZPs, our data are quite encouraging, since these structures seem to be maintained in secondary follicles (fresh and vitrified), additionally, evidence of steroidogenic capacity was maintained in both follicular categories.
We can conclude that SEC follicles seem to be more resistant to vitrification than EANT follicles, as shown by the trypan blue test and TZPs assay. Future studies may confirm this hypothesis, in order to consolidate the use of SEC and EANT follicles as an alternative for preserving fertility.
Acknowledgments
The authors are grateful to Central Analítica UFC/CT-INFRA/MCTI-SISNANO/Pró- Equipamentos, UFC Department of Physics for the technical assistance.
Funding
Funding for this work was provided by National Council of Technological and Scientific Development (CNPq: 437458/2018-0). Everton Pimentel Ferreira Lopes is a recipient of a grant from CNPq. Ana Paula Ribeiro Rodrigues is the recipient of a grant (Number of the process: 308.071/2016-6) from CNPq. This work was also supported by a Fulbright Visiting Professor Scholarship to Ana Paula Ribeiro Rodrigues, NIH R01HD083930 to M.B. Zelinski and NIH P51OD011092 to the Oregon National Primate Research Center.
Abbreviations:
- SEC
secondary
- EANT
early antral
- TZPs
transzonal projections
- OTCP
ovarian tissue cryopreservation
Footnotes
Declaration of Competing Interest
None.
References
- [1].Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 2013;99:1523–33. 10.1016/j.fertnstert.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee S, Ryu KJ, Kim B, Kang D, Kim YY, Kim T. Comparison between slow freezing and vitrification for human ovarian tissue cryopreservation and xenotransplantation. Int J Mol Sci 2019;20:3346 10.3390/ijms20133346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proceed Nat Acad Sci 2013;43:17474–9. 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod 2015;30:608–15. 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- [5].Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet 2015;32:1167–70. 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dolmans MM, Marinescu C, Saussoy P, Langendonckt AV, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 2010;116:2908–14. 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- [7].Rosendahl M, Andersen MT, Ralfkiaer E, Kjeldsen L, Andersen MTK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil Steril 2010;94:2186–90. 10.1016/j.fertnstert.2009.11.032. [DOI] [PubMed] [Google Scholar]
- [8].Bus A, Langbeen A, Martin B, Leroy JLMR, Bols PEJ. Is the pre-antral ovarian follicle the ‘holy grail’ for female fertility preservation? Anim Reprod Sci 2019;207:119–30. 10.1016/j.anireprosci.2019.05.017. [DOI] [PubMed] [Google Scholar]
- [9].Santos RR, Amorim C, Cecconi S, Fassbender M, Imhof M, Lornage J, et al. Cryopreservation of ovarian tissue: an emergin technology for female germline preservation of endangered species and breeds. Ani Reprod Sci 2010;122:151–63. 10.1016/j.anireprosci.2010.08.010. [DOI] [PubMed] [Google Scholar]
- [10].Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, et al. A bio-prosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun 2017;8:1–10. 10.1038/ncomms15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cadenas J, Leiva-Revilla J, Vieira LA, Apolloni LB, Aguiar FLN, Alves BG, et al. Caprine ovarian follicle requirements differ between preantral and early antral stages after IVC in medium supplemented with GH and VEGF alone or in combination. Theriogenology 2017;87:321–32. 10.1016/j.theriogenology.2016.09.008. [DOI] [PubMed] [Google Scholar]
- [12].Rossi G, Nisio VD, Macchiarelli G, Nottola SA, Halvaei I, Santis L, et al. Technologies for the production of fertilizable mammalian oocytes. Appl Sci 2019;9:1536–53. 10.3390/app9081536. [DOI] [Google Scholar]
- [13].Leal ÉSS, Vieira LA, Sá NAR, Silva GM, Lunardi FO, Ferreira ACA, et al. In vitro growth and development of isolated secondary follicles from vitrified caprine ovarian cortex. Reprod Fertil Dev 2017;32:359–70. 10.1071/RD16487. [DOI] [PubMed] [Google Scholar]
- [14].Zhou XM, Qiao WT, Zhang XL, Liu Z, Gao D. Physical modeling of flow boiling in microchannels and its induced vitrification of biomaterials. Int J Heat Mass Tran 2015;83:659–64. 10.1016/j.ijheatmasstransfer.2014.12.063. [DOI] [Google Scholar]
- [15].Devismita D, Kumar A. Effect of cryoprotectant on optimal cooling rate during cryopreservation. Cryobiology 2015;70:53–9. 10.1016/j.cryobiol.2014.12.002. [DOI] [PubMed] [Google Scholar]
- [16].VandeVoort CA, Shirley CR, Hill DL, Leibo SP. Effects of cryoprotectants and cryopreservation on germinal vesicle-stage cumulus–oocyte complexes of rhesus monkeys. Fertil Steril 2008;90:805–16. 10.1016/j.fertnstert.2007.06.105. [DOI] [PubMed] [Google Scholar]
- [17].Appeltant R, Somfai T, Santos EC, Dang-Nguyen TQ, Nagai T, Kikuchi K. Effects of vitrification of cumulus-enclosed porcine oocytes at the germinal vesicle stage on cumulus expansion, nuclear progression and cytoplasmic maturation. Reprod Fertil Dev 2017;29:2419–29. 10.1071/RD16386. [DOI] [PubMed] [Google Scholar]
- [18].Ting AY, Yeoman RR, Lawson MS, Zelinski MB. Synthetic polymers improve vitrification outcomes of macaque ovarian tissue as assessed by histological integrity and the in vitro development of secondary follicles. Hum Reprod 2012;18:1199–216. 10.1016/j.cryobiol.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, et al. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod 2013;28:1267–79. 10.1093/humrep/det032. Epub 2013 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marco-Jiménez F, Jimenez-Trigos E, Lavara R, Vicente JS. Generation of live off-spring from vitrified embryos with synthetic polymers SuperColl X-1000 and SuperCool Z-1000. Cryo Lett 2014;35:286–92. [PubMed] [Google Scholar]
- [21].Marco-Jiménez F, Casares-Crespo L, Vicente JS. Porcine oocyte vitrification in optimized low toxicity solution with open pulled straws. Zygote 2012;22:1–9. 10.1017/S0967199412000524. [DOI] [PubMed] [Google Scholar]
- [22].Curcio BR, Gastal MO, Pereira GR, Corcini CD, Landim-Alvarenga FC, Barros SS, et al. Ultrastructural morphology and nuclear maturation rates of immature equine oocytes vitrified with different solutions and exposure times. J Equine Vet Sci 2014;34:632–40. 10.1016/j.jevs.2013.12.002. [DOI] [Google Scholar]
- [23].Shahsavari MH, Moghaddam G, Kia HD, Rodrigues APR. Effects of new synthetic cryoprotectant agents on histological characteristics of various classes of vitrified bovine pre-antral follicles. Vet Res Forum 2019;10:9–16. 10.30466/vrf.2019.34306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chaves RN, Martins FS, Saraiva MVA, Celestino JJH, Lopes CAP, Correia JC, et al. Chilling ovarian fragments during transportation improves viability and growth of goat preantral follicles cultured in vitro. Reprod Fertil Dev 2008;20:640–7. 10.1071/RD07195. [DOI] [PubMed] [Google Scholar]
- [25].Ferreira ACC, Cadenas J, Sá NAR, Correia HHV, Guerreiro DD, Lobo CH, et al. In vitro culture of isolated preantral and antral follicles of goats using human recombinant FSH: concentration dependent and stage-specific effect. Anim Reprod Sci 2018;196:120–9. 10.1016/j.anireprosci.2018.07.004. Epub 2018 Jul 17. [DOI] [PubMed] [Google Scholar]
- [26].Xu J, Xu F, Lawson MS, Tkachenko OY, Ting AY, Kahl CA, et al. Anti-Mullerian hormone is a survival factor and promotes the growth of rhesus macaque preantral follicles during matrix-free culture. Biol Reprod 2018;98:197–207. 10.1093/biolre/iox181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Celestino JJH, Santos RR, Lopes CA, Martins FS, Matos MH, Melo MA, et al. Preservation of bovine preantral follicle viability and ultra-structure after cooling and freezing of ovarian tissue. Anim Reprod Sci 2008;108:309–18. 10.1016/j.anireprosci.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [28].Lunardi FO, Aguiar FL, Apolloni LB, Duarte ABG, Sá NAR, Leal ESS, et al. Sheep isolated secondary follicles are able to produce metaphase II oocytes after vitrification and long-term in vitro growth. Biopreserv Biobank 2017;15:321–31. 10.1089/bio.2016.0098. Epub 2017 Apr 10. [DOI] [PubMed] [Google Scholar]
- [29].Lee J, Kim EJ, Kong HS, Youm HW, Kim SK, Lee JR, et al. Establishment of an improved vitrification protocol by combinations of vitrification medium for isolated mouse ovarian follicles. Theriogenology 2018;121:97–103. 10.1016/j.theriogenology.2018.07.022. [DOI] [PubMed] [Google Scholar]
- [30].Lucci CM, Silva RV, Carvalho CA, Figueiredo JR, Baó N. Light microscopical and ultrastructural characterization of goat preantral follicles. Small Rumin Res 2001;41:61–9. 10.1016/S0921-4488(01)00196-1. [DOI] [PubMed] [Google Scholar]
- [31].Santos RR, Rodrigues APR, Costa SHF, Silva JRV, Matos MHT, Lucci CM, et al. Histological and ultrastructural analysis of cryopreserved sheep preantral follicles. Anim Reprod Sci 2006;91:249–63. 10.1016/j.anireprosci.2005.04.013. [DOI] [PubMed] [Google Scholar]
- [32].Lunardi FO, Chaves RN, Lima LF, Araújo VR, Brito IR, Souza CE, et al. Vitrified sheep isolated secondary follicles are able to grow and form antrum after a short period of in vitro culture. Cell Tissue Res 2015;362:241–51. 10.1007/s00441-015-2181-0. [DOI] [PubMed] [Google Scholar]
- [33].Nikiforov D, Russo V, Nardinocchi D, Bernabo N, Mattioli M, Barboni B. Innovative multi-protectoral approach increases survival rate after vitrification of ovarian tissue and isolated follicles with improved results in comparison with conventional method. J Ovarian Res 2018; 11:65 10.1186/s13048-018-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oktem O, Alper E, Balaban B, Palaoglu E, Peker K, Karakaya C, et al. Vitrified human ovaries have fewer primordial follicles and produce less antimullerian hormone than slow-frozen ovaries. Fertil Steril 2011;95:2661–4. 10.1016/j.fertnstert.2010.12.057. [DOI] [PubMed] [Google Scholar]
- [35].Araújo VR, Silva GM, Duarte ABG, Magalhães DM, Almeida AP, Gonçalves RFB, et al. Vascular endothelial growth factor-A 165 (VEGF-A 165) stimulates the in vitro development and oocyte competence of goat preantral follicles. Cell Tissue Res 2011;346:273–81. 10.1007/s00441-011-1251-1. [DOI] [PubMed] [Google Scholar]
- [36].Silva CMG, Castro SV, Faustino LR, Rodrigues GQ, Brito IR, Rossetto R, et al. Activin-A promotes the development of goat isolated secondary follicles in vitro. Zygote 2015;23:41–52. 10.1017/S0967199413000294. [DOI] [PubMed] [Google Scholar]
- [37].Sá NAR, Araújo VR, Correia HHV, Ferreira ACA, Guerreiro DD, Sampaio AM, et al. Anethole improves the in vitro development of isolated caprine secondary follicles. Theriogenology 2017;89:226–34. 10.1016/j.theriogenology.2015.12.014. [DOI] [PubMed] [Google Scholar]
- [38].Saraiva MVA, Celestino JJH, Araújo VR, Chaves RN, Almeida AP, Lima-Verde IB, et al. Expression of follicle-stimulating hormone receptor (FSHR) in goat ovarian follicles and the impact of sequential culture medium on in vitro development of caprine preantral follicles. Zygote 2011;19:205–14. 10.1017/S0967199410000511. [DOI] [PubMed] [Google Scholar]
- [39].Chaves RN, Duarte ABG, Rodrigues GQ, Celestino JJ, Silva GM, Lopes CA, et al. The effects of insulin and follicle-stimulatinghormone (FSH) during in vitro development of ovarian goat preantralfollicles and the relative mRNA expression for insulin and FSH receptors and cytochrome P450 aromatase in cultured follicles. Biol Reprod 2012;3:1–11. 10.1095/biolreprod.112.099010. [DOI] [PubMed] [Google Scholar]
- [40].Wang TR, Yan J, Lu CL, Xia X, Yin TL, Zhi X, et al. Human single follicle growth in vitro from cryopreserved ovarian tissue after slow freezing or vitrification. Hum Reprod 2016;31:763–73. 10.1093/humrep/dew005. [DOI] [PubMed] [Google Scholar]
- [41].Vajta G, Holm P, Kawayama M, Booth PJ, Jacobsen H, Greve T. Open pulled Straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev 1998;51:53–8. . [DOI] [PubMed] [Google Scholar]
- [42].Smitz JE, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction 2002;123:185–202. 10.1530/rep.0.1230185. [DOI] [PubMed] [Google Scholar]
- [43].Oktay K, Newton H, Aubard Y, Osama SMB, Gosden RG. Cryopreservation of immature human oocytes and ovarian tissue: an emerging technology? Fertil Steril 1998;69:1–7. 10.1016/S0015-0282(97)00207-0. [DOI] [PubMed] [Google Scholar]
- [44].Shaw JM, Oranratnachai A, Trounson AO. Fundamental cryobiology of mammalian oocytes and ovarian tissue. Theriogenology 2000;53:59–72. 10.1016/S0093-691X(99)00240-X. [DOI] [PubMed] [Google Scholar]
- [45].Lopes CAP, Santos RR, Celestino JJH, Melo MAP, Chaves RN, Campello CC, et al. Short-term preservation of canine preantral follicles: effects of temperature, medium and time. Anim Reprod Sci 2009;115:201–14. 10.1016/j.anireprosci.2008.12.016. [DOI] [PubMed] [Google Scholar]
- [46].Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Lett 2004;25:375–88. [PubMed] [Google Scholar]
- [47].Sadr SZ, Fatehi R, Maroufizadeh S, Amorim CA, Ebrahimi B. Utilizing fibrin-alginate and Matrigel-alginate for mouse follicle development in three-dimensional culture systems. Biopreserv Biobank 2018;16:120–7. 10.1089/bio.2017.0087. [DOI] [PubMed] [Google Scholar]
- [48].Hatami S, Zavareh S, Salehnia M, Lashkarbolouki T, Ghorbanian MT, Karimi L. The impact of alpha lipoic acid on developmental competence of mouse vitrified preantral follicles in comparison to those isolated from vitrified ovaries. Iran J Reprod Med 2014;12:1–57. [PMC free article] [PubMed] [Google Scholar]
- [49].Arav A, Natan Y, Kalo D, Komsky-Elbaz A, Roth Z, Levi-Setti PE, et al. A new, simple, automatic vitrification device: preliminary results with murine and bovine oocytes and embryos. J Assist Reprod Genet 2018;35:1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ledda S, Bogliolo L, Succu S, Ariu F, Bebbere D, Leoni GG, et al. Oocyte cryopreservation: oocyte assessment and strategies for improving survival. Rep Fert Dev 2007;19:13–23. 10.1071/rd06126. [DOI] [PubMed] [Google Scholar]
- [51].Jaffe LA, Egbert JR. Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle. Annu Rev Physiol 2017;79:237–60. 10.1146/annurev-physiol-022516-034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Albertini DF, Combelles CMH, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction 2001;121:647–53. 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- [53].Barrett SL, Shea LD, Woodruff TK. Noninvasive index of cryorecovery and growth potential for human follicles in vitro. Biol Reprod 2010;82(6):1180–9. 10.1095/biolreprod.109.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kandiel MM, Watanabe G, Taya K. Ovarian expression ofinhibin-subunits, 3 β-hydroxysteroid dehydrogenase, andcytochrome P450 aromatase during the estrous cycle and pregnancyof shiba goats (Capra hircus). Exp Anim 2010;59:605–14. 10.1538/expanim.59.605. [DOI] [PubMed] [Google Scholar]