Abstract
Objective:
Despite the losses commonly associated with aging, older adults seem to possess particularly preserved emotional regulation. To further understand this phenomenon, the authors examined longitudinal trajectories between age, depressive symptoms, brain structure, and cognition.
Methods:
Seven hundred and sixteen functionally intact older adults (age M = 67.9, 56.8% female), followed longitudinally (visit range: 1–13, M = 2.5), completed cognitive testing and the Geriatric Depression Scale (GDS). A subset (N = 327) underwent 3T brain MRI. Mixed-effects linear regression models were conducted controlling for sex, education, and total intracranial volume.
Results:
There was a significant interaction between age and time on GDS, such that GDS improved with increasing age over time, but attenuated around age 71 (age*time b = 0.10, p <0.001). Fractional anisotropy (FA) and mean diffusivity interacted with age to predict longitudinal changes in GDS (FA: b = −0.02, p = 0.01; MD: b = 0.03, p = 0.007), such that age-related benefits on GDS were attenuated in those with declining FA. Executive function (EF) and processing speed also interacted with age to predict longitudinal changes in GDS (EF: b = −0.04, p = 0.03; speed: b = 0.04, p = 0.04). Again, the positive effect of age on GDS attenuated in those with worsening EF and speed. There were no associations with memory, semantic fluency, or gray matter (p values >0.05).
Conclusion:
EF, processing speed, and white matter integrity moderated the longitudinal relationship between age and mood. Previous studies demonstrate the link between positivity and better cognitive control, leading to improved mood in older adults. Our results are not only consistent, but establish a potential neurobiological correlate. Future research further exploring biological mechanisms driving psychological processes may have important therapeutic implications.
Keywords: Mood, white matter microstructural, integrity, executive function, processing speed
OBJECTIVE
It is widely acknowledged that aging is associated with more losses than gains, from physical and cognitive health to social losses. Despite adverse experiences and increased life stressors, older adults demonstrate remarkable emotional resilience.1,2 While a number of theories exist offering possible insights into why emotional regulation is preserved in older adults, one of the most prevalent is the socioemotional selectivity theory. The socioemotional selectivity theory posits that individuals, when faced with time horizon constraints, mentally restructure motivational priorities such that emotionally gratifying experiences and relationships supersede novel experiences or knowledge-gathering activities.3 This is thought to apply to those who are acutely aware of mortality and end-of-life issues, including people of all ages with limited time (i.e., terminal illness).3
While the biological mechanisms driving this shift in motivational thought are less clear, two competing models attempt to bridge this gap. The aging brain model suggests, based on observational studies of patients with amygdalar lesions, that age-related functional degeneration in the amygdala leads to the attentiveness to positive over negative stimuli in older adults.4 In support, one study found that in older adults, functional response to negative emotional stimuli in the amygdala measured by fMRI was diminished compared to their younger counterparts, while amygdalar response to positive stimuli was comparable.5 Others propose a cognitive control model, which argues that compared to younger adults, older adults recruit more cognitive resources to regulate emotion as a result of their evolving motivations.6 Thus, exposure to emotional stimuli should elicit greater activation of the prefrontal cortex (PFC) in older adults, as the PFC is tied to cognitive control of emotion.6 This theory has garnered support from studies demonstrating increased activation of the PFC coupled with decreased activity in the amygdala, as well as increased functional connectivity between the two regions in older compared to younger adults when presented with emotionally valent stimuli.7,8
In the field of psychiatry, there has long been a push to find defined regions of the brain to explain psychiatric disorders such as major depressive disorder (MDD). However, the evidence has fallen short of definitively linking MDD to the gray matter (GM) volume of any one brain region.9 Consequently, the focus shifted to network connectivity, resulting in the discovery of white matter damage as a leading suspect in the neurobiology of psychiatric disorders. The presence of white matter hyperintensities has been implicated in suicidality and mood disorders, specifically in the elderly.10,11 White matter changes are also an established hallmark of “typical” aging and correlate with changes in cognition, specifically executive function (EF) and speed. Despite these changes, there is evidence that mood improves with age. The nuanced role of white matter integrity in mood and its cognitive correlates has not been thoroughly investigated in a typically aging population.
The current study aims to further characterize the longitudinal cognitive and neurobiological correlates of preserved emotional regulation in older adults. While there are a number of studies linking cognitive control to improved mood in older adults, most are cross-sectional in design.12,13 Second, while white matter disease has been linked to late-life depression, as well as a variety of psychiatric disorders, studies investigating positivity in aging have primarily focused on the amygdala and the PFC without commenting on white matter integrity, despite its role in late-life mood disorders.6 Utilizing a large cohort of deeply phenotyped typically aging older adults, we examined the associations between changes in mood, cognitive outcomes, and global brain structure over time. We hypothesized that older age would be associated with improving mood; due to the literature on cognitive control and positivity, we also expected measures of EF and white matter integrity to moderate the relationship between age and mood.
METHODS
Participants
Participants included community-dwelling older adults40–99 recruited from the Hillblom Aging Network, an observational, longitudinal study at the University of California, San Francisco (UCSF), Memory and Aging Center. All participants were reviewed by a multidisciplinary panel consisting of a board-certified neuropsychologist and neurologist and were determined to be neurologically and cognitively within normative standards. Each subject underwent baseline and follow-up cognitive testing and informant interviews (Clinical Dementia Rating Scale) to assess functionality. All subjects included in this sample received a Clinical Dementia Rating Scale = 0, demonstrating an absence of functional impairments. Exclusion criteria included major memory concerns or diagnosed neurodegenerative condition, active substance abuse, active neoplastic disease, history of significant stroke, current evidence or history in the past 2 years of epilepsy, and/or current evidence of any major psychiatric disorders. Longitudinal data from 716 participants were included in the final data-set (Table 1). A subset of 327 participants underwent neuroimaging studies. All subjects provided written informed consent, and the UCSF Committee on Human Research approved the study protocol.
TABLE 1.
Baseline Study Participant Demographic and Clinical Characterization.
| N | 716 |
|---|---|
| Average # of study visits | 2.5 (range: 1, 13) |
| N with structural DTI | 365 |
| Average # imaging visits | 1.6 (range: 1, 5) |
| Demographics | Mean (SD) or Percent (%) |
|---|---|
| Age, years | 67.9 (8.6) |
| Sex, % (N) female | 56.8% (407) |
| Education, years | 17.6 (3.9) |
| Race/ethnicity | 94.3% White |
| 4% Asian (Chinese, Japanese, Filipino) | |
| 0.4% Asian Indian | |
| 0.2% Black | |
| 1.1% Other/Refuse to state |
| Baseline Mood Assessment | Median (IQR) (range) |
|---|---|
| Geriatric Depression Scale (GDS) | 2 (0, 1, 5) (0–24) |
| GDS Subscales (Weighted Averages) | Mean (SD) |
|---|---|
| Dysphoria | 0.097(0.16) |
| Negative mood | 0.091 (0.18) |
| Apathy | 0.160(0.21) |
| Hopelessness | 0.052 (0.16) |
| Social withdrawal | 0.092 (0.15) |
| Decreased concentration | 0.310(0.15) |
| Cognitive impairment | 0.092 (0.19) |
| Baseline Cognition | Mean (SD) (range) or Median (IQR) |
|---|---|
| Clinical Dementia Rating (CDR) | 0 |
| Mini-Mental State Exam (MMSE) | 29 (1.1) (25, 30) |
DTI: Diffuse Tensor Imaging; GDS: Geriatric Depression Scale; CDR: Clinical Dementia Rating; MMSE: Mini-Mental State Exam.
Mood Assessment
The 30-item Geriatric Depression Scale (GDS) is a validated measure of depressive symptoms geared specifically toward a geriatric population. The GDS is a self-reported scale consisting of 30 Yes/No questions, 20 of which indicate the presence of depressive symptoms when answered positively while the remaining 10 indicate the presence of depressive symptoms when answered negatively.14 Each question receives zero or one point, with one point denoting the presence of a depressive symptom. A score of 0–10 is considered normal/mild, 11–20 demonstrates moderate depressive symptoms, and 21–30 indicates severe depressive symptoms.
Neuropsychological Battery
Executive functions:
EFs were evaluated using a composite score developed from five neuropsychological measures. These tasks evaluated working memory (Digit Span backward), response inhibition (Stroop Color Naming Inhibition), set shifting (Modified Trail Making Test), and generativity (D-word fluency, DKEFS Design Fluency, condition 1).15
Memory:
Memory was evaluated using a composite score of two measures - the California Verbal Learning Test−Second Edition16 and free recall of the Benson Complex Figure after a 10 minute delay. Metrics employed from the California Verbal Learning Test− Second Edition included total learning, immediate recall, delayed recall, and discrimination.
Processing speed:
Speed was measured using a composite score consisting of five computer-based tests evaluating spatial reaction time developed by Kerchner and colleagues17 (lines, search, dots, abstract matching 1, abstract matching 2). In lines, participants were confronted with two parallel, offset lines and instructed to choose the longer line. During dots, participants saw three dots and were instructed to choose the dot closest to the target central dot. In search, participants were presented with an array of shapes and asked to indicate if the target shape was present. During the abstract matching tasks, participants were asked to compare two choice arrays to a sample array and determine the best match. In abstract matching 1, participants were asked to consider shape, color, and number of items. Abstract matching 2 echoed abstract matching 1, but added a level of complexity by asking participants to additionally consider orientation.
Imaging
A subset (N = 327) of subjects underwent brain MR imaging within 6 months of completing the GDS and comprehensive neuropsychological evaluation. Participants completed a safety screening form and were screened for metal before entering the scanner suite.
MRI acquisition:
All MRI data were acquired at the UCSF Neuroscience Imaging Center on a Siemens TIM Trio 3-Tesla scanner. A 3D T1-weighted MPRAGE sequence was acquired with sagittal slice orientation, slice thickness = 1.0 mm, slices per slab = 160, voxel size = 1.0 × 1.0 × 1.0 mm3, matrix = 240 × 256, TR = 2,300 ms, TE = 2.98 ms, TI = 900 ms, and flip angle = 9°. Diffusion Tensor Imaging (DTI) data were acquired using the following parameters: TR/TE 8,200/86 ms; B = 0 image and 64 directions at B = 2,000 s/mm2; FOV 220 × 220 mm2 and 2.2 mm thick slices; matrix 100 × 100 with 60 slices yielding 2.2 mm3 isotropic voxels/(TR/TE 8,000/109 ms; B = 0 image and 64 directions at B = 2,000 s/mm2; FOV 220 × 220 mm2 and 2.2 mm thick slices; matrix 100 × 100 with 55 slices yielding 2.2 mm3 isotropic voxels).
Volumetric processing:
Before any preprocessing, all T1-weighted images were visually inspected for quality control. Images with excessive motion or image artifact were excluded. T1-weighted images underwent bias field correction using N3 algorithm, and segmentation was performed using SPM12 (Wellcome Trust Center for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm) unified segmentation.32 A group template was generated by nonlinear and rigid-body registration using Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL).31 Subjects’ native space gray and white matter were modulated and normalized to group template space using inter-subject transformations. For statistical purposes, linear and nonlinear transformations between DARTEL’s space and International Consortium of Brain Mapping (ICBM) space34 were applied to the modulated gray and white matter segmentations. The images were then smoothed using a Gaussian kernel with 4»mm full width half maximum. Each subject’s segmentation was carefully inspected to ensure robustness of the process. Regional and global volumes were calculated by transforming a standard space atlas33 to ICBM space and summing all modulated GM voxels within each region.
Diffusion processing:
Diffusion images were processed using FSL36 and Dipy35 utilities. The diffusion direction images were co-registered to the b = 0 image using FSL MCFLIRT.37 Background voxels not considered as brain tissue were then masked out of the diffusion volumes by applying a median Otsu function.39 Gradient direction eddy current and distortion correction were applied to the realigned images. Diffusion tensors were fitted using Dipy35 with a nonlinear least-squares approach. Fractional anisotropy (FA) derived from the fitted tensor was reconstructed in the native space for quality control. A group template was created through iterative linear and nonlinear registration using DTI-TK software,40 which maximizes the alignment of white matter structures and minimizes interpolation of the diffusion images. Deformations were applied to bring each subject to group template space and to the standard space atlas ICBM-DTI-8138 to extract tracts of interest. Mean FA and MD were calculated from scalar images masked with white matter.
Statistical Methods
First, we examined the longitudinal relationship between age and mood by conducting a linear mixed-effects (LME) model examining the relationship between age (time-varying) and GDS scores over time, adjusting for sex and education. All LME models allowed for subject-specific estimation of intercepts and slopes. Additionally, to allow for differing mood trajectories by age, we examined the interaction between age and time (years in study) on longitudinal GDS scores, adjusting for sex and education, again using LME.
Next, we examined neural (DTI FA and MD, select GM ROIs) and cognitive (EFs, speed, memory, language) factors that may moderate the longitudinal relationship between age and mood. To do so, we decomposed each moderator into within- (i.e., changes from average) and between- (i.e., average) subject components in order to associate purely within- or between-subject changes in the moderator with changes in GDS scores and to avoid estimation bias resulting from incorrectly assuming common within- and between-subject effects following guidelines from Neuhaus et al.29,30 Final LME models therefore included the interaction between baseline age by within-person changes in neural/cognitive moderator of interest on longitudinal GDS scores, adjusting for sex, education, and between-subject (average) levels of the moderator. Again, all LME modeled subject-specific slopes and intercepts. For visualization purposes (Figs. 1–3), we selected the sample-specific median to probe and plot significant interactions.
FIGURE 1.
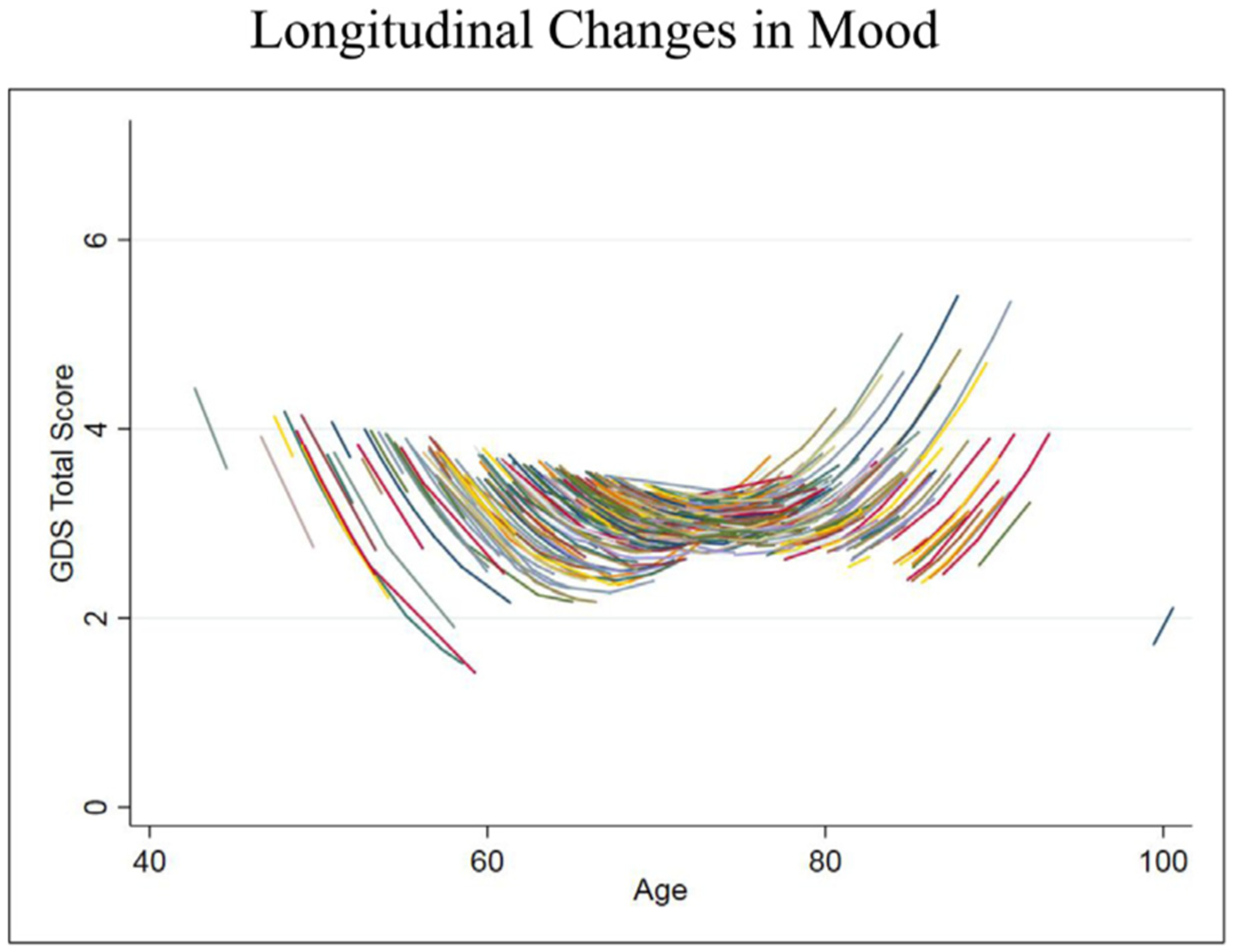
Longitudinal relationship between aging and Geriatric Depression Scale (GDS) scores. GDS is scored on a scale of 0–30, with higher scores indicating more depressive symptoms. Estimates were derived from the LME model.
FIGURE 3.
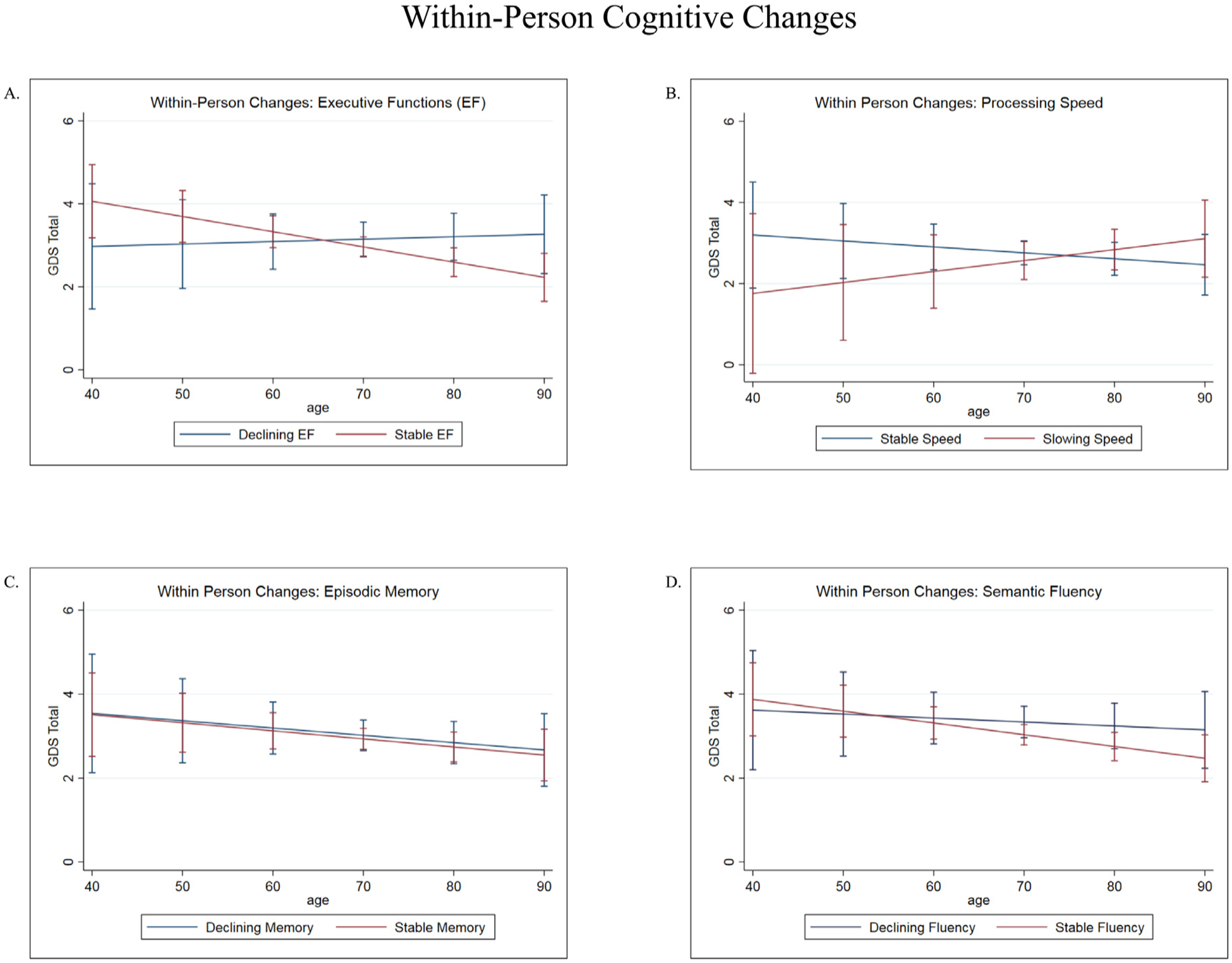
[A] Executive function (EF) interacted with age to predict changes in GDS over time. In those with stable EF, mood symptoms continued to improve over time. Mood symptoms worsened in those with declining EF. Error bars indicate 95% CIs. [B] Processing speed interacted with age to predict changes in GDS over time. In those with stable speed, mood symptoms continued to improve over time. Mood symptoms worsened in those with slowing speed. Error bars indicate 95% CIs. [C] No significant associations between memory, age, and mood. Error bars indicate 95% CIs. [D] No significant associations between semantic fluency, age, and mood. Error bars indicate 95% CIs.
RESULTS
Older Age Is Associated With Positive Mood Outcomes
Our initial model showed a negative association between time-varying age and total GDS scores, but did not reach significance (Table 2, Model 1: b = −0.03, z = −1.90, p = 0.06). However, there was a significant interaction between time-varying age and time, such that the negative relationship between age and total GDS attenuated and appeared to reverse around age 71 (Table 2, Model 2: b = 0.10, z = 5.80, p <0.001; Fig. 1).
TABLE 2.
Results Summary.
| Outcome: Geriatric Depression Scale (GDS) | Unstd. Beta | 95% CI | Z | P Value |
|---|---|---|---|---|
| Model 1: Age and GDS | ||||
| N = 716 (1–13, avg 2.5), df = 3 | ||||
| Sex | 0.15 | −0.36, 0.60 | 0.50 | 0.62 |
| Education | 0.003 | −0.06, 0.06 | −0.02 | 0.98 |
| Age | −0.03 | −0.05, 0.001 | −1.90 | 0.06 |
| Model 2: Age × Time and GDS | ||||
| N = 716 (1–13, avg 2.5), df = 5 | ||||
| Sex | 0.30 | −0.19, 0.80 | 0.33 | 0.23 |
| Education | 0.03 | −0.02, 0.08 | 1.54 | 0.22 |
| Age | −0.02 | −0.68, 0.21 | −2.86 | <0.001 |
| Time | −0.02 | −0.06, 0.02 | −5.73 | 0.41 |
| Age × Time | 0.10 | 0.06, 0.14 | 5.80 | <0.001 |
| Model 3: Age × Fractional anisotropy and GDS | ||||
| N = 359 (1–5 visits, avg 1.6), df=7 | ||||
| Sex | −0.54 | −1.21, 0.12 | −1.60 | 0.11 |
| Education | −0.02 | −0.11, 0.06 | −0.56 | 0.57 |
| Age | −0.04 | −0.08, 0.009 | −1.56 | 0.12 |
| Within-person FA Z-score | 1.70 | 0.282, 3.13 | 2.35 | 0.02 |
| Between-person FA Z-score | −1.30 | −3.73, 1.12 | −1.05 | 0.29 |
| Within-person FA Z-score × age | −0.02 | −0.04, −0.005 | −2.48 | 0.01 |
| Between-person FA Z-score × age | 0.01 | −0.02, 0.05 | 0.72 | 0.47 |
| Model 4: Age × Mean Diffusivity and GDS | ||||
| N = 359 (1–5 visits, avg 1.6), df=7 | ||||
| Sex | −0.62 | −1.301, 0.07 | −1.76 | 0.08 |
| Education | −0.02 | −0.10, 0.06 | −0.43 | 0.66 |
| Age | −0.04 | −0.09, 0.007 | −1.68 | 0.09 |
| Within-person MD Z-score | −1.90 | −3.27, −0.52 | −2.71 | 0.007 |
| Between-person MD Z-score | 0.84 | −1.74, 3.41 | 0.64 | 0.52 |
| Within-person MD Z-score × age | 0.03 | 0.007, 0.05 | 2.70 | 0.007 |
| Between-person MD Z-score × age | −0.006 | −0.04, 0.03 | −0.34 | 0.73 |
| Model 5: Age × Executive Function and GDS | ||||
| N = 716 (1–13, avg 2.5), df=7 | ||||
| Sex | 0.09 | −0.39, 0.56 | 0.36 | 0.72 |
| Education | 0.01 | −0.05, 0.07 | 0.22 | 0.83 |
| Age | −0.04 | −0.06, −0.01 | −2.47 | 0.01 |
| Within-person EF | 2.80 | 0.01, 5.57 | 1.97 | 0.049 |
| Between-person EF | −0.16 | −3.27, 2.94 | −0.10 | 0.92 |
| Within-person EF × age | −0.04 | −0.08, −0.004 | −2.17 | 0.03 |
| Between-person EF × age | −0.01 | −0.05, 0.04 | −0.29 | 0.77 |
| Model 6: Age × Processing Speed and GDS | ||||
| N = 402 (1–5 visits, avg 1.7), df = 7 | ||||
| Sex | −0.43 | −0.97, 0.11 | −1.55 | 0.12 |
| Education | −0.13 | −0.26, 0.01 | −1.88 | 0.06 |
| Age | −0.01 | −0.09, 0.07 | −0.26 | 0.80 |
| Within-person PS | −3.11 | −6.15, −0.06 | −2.00 | 0.046 |
| Between-person PS | 0.42 | −1.76, 2.61 | 0.38 | 0.71 |
| Within-person PS × age | 0.04 | 0.002, 0.08 | 2.04 | 0.04 |
| Between-person PS × age | −0.002 | −0.03, 0.03 | −0.11 | 0.91 |
GDS: Geriatric Depression Scale; EF: executive function; PS: processing speed; FA: fractional anisotropy; GMV: gray matter volume.
Changes in Measures of White Matter Microstructural Integrity Moderate the Relationship Between Age and Mood
Subsequent analyses investigated neurobiological factors that may moderate the relationship between baseline age and mood. We found a significant interaction between within-person FA changes and age on the GDS (Table 2, Model 3: b = −0.02, z = −2.48, p = 0.01), such that the negative relationship between age and depressive symptoms became disproportionately more positive (older age, worsening mood) in individuals with declining FA, adjusting for sex, education and between-subject (average) change in FA (Fig. 2A). To confirm this relationship, we also examined the moderating effect of within-person MD changes and age on GDS scores, and found consistent results (Table 2, Model 4: b = 0.03, z = 2.70, p = 0.007).
FIGURE 2.
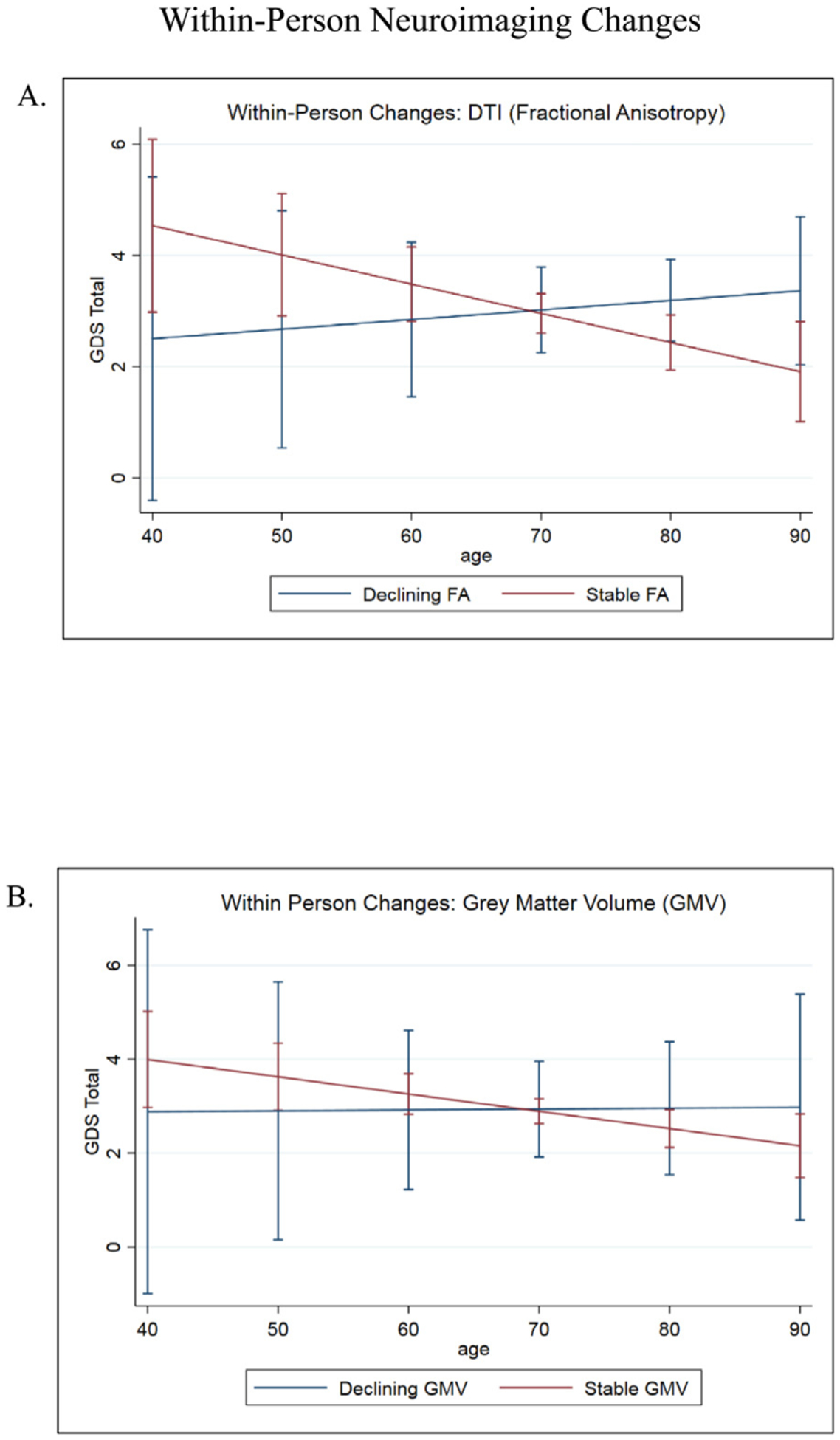
[A] FA interacted with age to predict changes in GDS over time. In those with stable FA values, mood symptoms continued to improve over time. Mood symptoms worsened in those with declining FA values. Error bars indicate 95% CIs. [B] No significant associations between gray matter regions of interest, age, and mood. GM ROIs included medial temporal lobes, dorsolateral prefrontal cortex, amygdala, subcortical network, salience network, and executive network. Error bars indicate 95% CIs.
Grey Matter Regions of Interest and Network Volumes Did not Significantly Moderate Age-related Mood Outcomes
We also explored the relationship between age, mood, and other GM ROIs. Within-person changes in total brain GM, medial temporal lobe, subcortical network, amygdalar, and dorsal lateral prefrontal cortex volumes were not significantly associated with GDS score trajectories, nor did they interact with age to moderate the relationship between age and mood (p values >0.05; Fig. 2B). All models involving GM ROIs controlled for sex, education, and total intracranial volume (TIV).
Executive Functions (EF) and Spatial Processing Speed Moderate the Relationship Between Age and Mood
Lastly, we examined possible cognitive moderators of mood in older adults. We found a significant interaction between within-person changes in EF and age (Table 2, Model 5: b = −0.04 z = −2.17, p = 0.03), as well as within-person changes in speed and age (Table 2, Model 6: b = 0.04, z = 2.04, p = 0.04). These interactions indicated that the initial negative association between age and depressive symptoms attenuated and became more positive in individuals with worsening EF (Fig. 3A) or speed over time (Fig. 3B). There were no main effects or age-related interactions with measures of memory or semantic fluency on the GDS (p values >0.05; Fig. 3C and D).
Due to the skewed nature of our GDS scores, we repeated all of the above analyses using log+1 transformed GDS values. The results were consistently in the same, expected directions.
DISCUSSION
We investigated how mood changes with age, and the moderating influence of neurobiology and cognition. To this aim, we found a negative association between age and worsening mood that notably attenuated and appeared to reverse in the oldest ages (>71 years). This relationship was moderated by white matter microstructural integrity and performances in EFs and processing speed. Our analyses indicated that mood appears to improve with age, but only to a point (<71 years). To maintain increasingly positive mood into very old age, white matter microstructural integrity, EFs, and processing speed may also need to be preserved over time.
While we did not directly measure the “positivity effect,” the concept provides important context for understanding our results. The “positivity effect” is defined as an age-related phenomenon in which older adults more selectively attend to positive over negative stimuli.18 Of particular interest to the current study, the positivity effect in memory and attention has been linked to better mood in older adults. In a study investigating the effects of varying mood states on attentional preferences of positive or negative faces between younger and older adults, Isaacowitz et al.12 found there was no significant difference in attentional preferences between the two age groups when subjects were induced into neutral or positive mood states. Conversely, when both groups were in a negative mood state, older adults illustrated a preference for positive faces while young adults favored negative faces. Circling back to the cognitive control theory, these findings support the idea that older adults could be recruiting more cognitive resources to use positivity, either consciously or unconsciously, as a way to improve mood. Our findings are consistent in that they show that better EF and speed improve mood as age increases.
In our investigation of the neurobiological correlates of these age-associated changes in mood, we found that white matter microstructure, but not GM, significantly moderated the relationship between age and longitudinal mood trajectories. Specifically, older adults with better white matter microstructure continued to show improving mood with age, while those with declining white matter demonstrated declines in mood at the oldest ages. The relationship between white matter integrity and depression is well-documented throughout the lifespan. Significant differences in white matter as indexed via FA have been noted even between asymptomatic groups of children with and without a family history of depression, such that children with a family history of depression have lower FA compared to those without a family history.19 This study emphasizes the role of white matter integrity as a biological basis for changes in mood. We know that family history is a risk factor for depression, and the above study shows that children with a family history of depression have fundamentally different white matter microstructure compared to children without a family history, implicating white matter integrity long before mood symptom onset. Similarly, in adults aged 30–65, researchers found FA to be significantly decreased in regions of the brain specific to mood, such as the right uncinate fasciculus, in patients with MDD compared to normal controls.20 Additionally, depression is a common comorbidity of a variety of age-related neurologic diseases that affect the white matter, including Alzheimer disease, Parkinson disease, and Huntington’s disease.21,22 For instance, in Parkinson disease, lower FA, globally and in mood-specific regions such as left uncinate fasciculus, thalamus, and left superior longitudinal fasciculus, have been found to correlate significantly with symptoms of depression.21–23 Teodorczuk et al.24 illustrated that baseline severity of white matter changes predicted increases in depression up to 3 years from initial evaluation, supporting the vascular depression hypothesis and suggesting that white matter changes may play an early or even causal role in the development of late-life depression. Other recent studies further show consistent evidence that alterations in white matter structure also predict the onset and severity of late-life depressive symptoms.25,26 It is clear from the literature that white matter microstructure is important for mood across the lifespan and across diagnoses. Our findings are consistent with those noted here in that changes in white matter microstructure interact with age to predict changes in mood, even within our healthy and functionally intact cohort.
In addition to probing the neurobiology related to positive mood in aging, we aimed to explore cognitive correlates linked to this effect. Our models showed parallel results in processing speed and EFs, whereby worsening EFs and slower speed independently interacted with age to attenuate the positive effect of age on mood in older adults longitudinally. Conversely, stable EFs and speed over time appeared to have a protective effect on mood in older adults. It is well established that decreased EFs and slowed processing speed are important cognitive comorbidities found in late-life depression.27 Isaacowitz et al.12 determined that executive control was a key moderating factor in the functional regulation of negative mood. Mather and Knight13 similarly illustrated a strong link between positivity and increased cognitive control. Their experimental paradigm examined the ability of younger and older adults to recall emotionally valent stimuli. They showed that older adults who demonstrated preserved cognitive control tended to recall greater positive over negative stimuli, a finding that closely mirrors our own initial interaction. Interestingly, older adults attending fully to the task continued to recall positive over negative stimuli, whereas the effect completely reversed in older adults with divided attention. Young adults tended to recall negative over positive stimuli regardless of cognitive control or attentional abilities. This finding was replicated by Joubert et al.,28 and Knight et al.29 used an eye-tracking attentional task to illustrate an analogous result.29 We know from neuroimaging studies that emotional regulation requires significant cognitive control30 and our own findings support the notion that increased EFs interact with age to preserve positive mood. Taken together, we can hypothesize that cognitive control may govern mood through older adults’ refined ability to employ the positivity effect, meaning that they use their increased EFs to recall positive stimuli over negative stimuli.
However, our results do not indicate causation, and it is most likely that the mechanism(s) of these brain-mood relationships are bidirectional. It is reasonable to hypothesize that mood and cognition are independent outcomes of shared network connectivity in white matter tracts and thus, when white matter integrity is compromised, both mood and cognition are negatively but independently affected. It is also possible that severe, sustained depressive mood disturbs important white matter tracts in the brain, perhaps as a result of a neuroimmune, metabolic, or another disrupted molecular signaling response. Alternatively, regarding cognitive mechanisms, there is evidence to suggest that decreases in EF produce ruminative symptoms, which in turn can directly lead to depressive moods.31 Given our current design, the mechanisms responsible for these associations cannot be disentangled, and more experimental research is necessary in characterizing the relationship between mood, age, white matter integrity, and cognition.
To explore the clinical and theoretical impact of these models, we further interpreted relative effect sizes based on our sample. In models examining age along, each one-year increase in age was associated with a 0.04 point decrease on the GDS (GDS scale range: 0–30 points). When FA was the factored into the model, the relationship between age and GDS became 0.02 standard deviations (SDs), such that at each 1 SD increase in white matter integrity (FA), a one year increase in age was related to ~50% steeper decreases in GDS. A slightly larger effect was observed in our MD model, such that for each 1 SD decrease in MD, each year of increasing age was associated with 75% steeper decreases in GDS. Regarding cognition, for each 1 SD increase in executive functioning or processing speed, a one year increase in age was associated with 100% or 400% steeper decrease in GDS symptoms, respectively. In all, when the relationship between age and GDS is moderated by any one of these factors, age-related depressive symptoms improved by 75%−400%. A 1 SD change on brain structure or cognitive metrics is commonly considered as meaningful clinical change; our data therefore suggest that clinically meaningful change in brain structure or cognition appears to track with large effect on age-related mood symptoms. Of potential clinical significance, these late-life mood changes could act as a harbinger of future cognitive decline and prompt early intervention. Clinicians could also prescribe lifestyle changes and/or medications aimed at improving vascular health, given its importance in maintaining healthy white matter microstructure.
One major limitation of this study includes the demographic homogeneity of our cohort—our participants were largely White and highly educated. To support generalizability, the observed relationships between mood, age, white matter integrity, and cognition should be evaluated in more racially and educationally diverse groups. Second, as we ran a number of analyses, it is important to acknowledge the possibility of a Type 1 error. Nonetheless the pattern of results were in line with our a priori hypotheses and converge with existing literature, supporting their potential utility and generalizability. Additionally, the range of GDS scores was restricted in our cohort of typically aging adults, in very few participants endorsed sufficient depressive symptoms to be concerning for clinical depression. Nonetheless, our significant results despite a narrow GDS range may suggest more a nuanced phenomenon between age and mood, even within the “subclinical” threshold. This has important implications, because it supports the idea that even “typical” late-life changes in mood are associated with declines in structural integrity and declines in cognition. Additionally, the data used in our analyses were collected as part of an observational study. As such, we cannot determine what is driving these associations. Lastly, it is important to note that our analyses were performed in a clinically healthy population. It would be interesting to investigate how this concept manifests in a population affected by neurodegenerative disease, especially as recent evidence suggests psychological changes could be a prodromal symptom to cognitive decline resulting from a neurodegenerative process.32
It is clear that there is a complex interplay among age, brain structure, and cognition on late life mood. From our results, we found age and mood were associated such that older age generally coincides with improving mood to a point (~71 years old), after which the effect seems to reverse. We also demonstrated that this relationship is dependent on both white matter microstructural integrity and the specific cognitive domains of EFs and processing speed. To begin to comprehensively characterize the association between mood and measures of brain health, future studies should aim to employ large-scale, longitudinal epidemiological methodologies capturing the neurodevelopmental spectrum. The results from such studies would have far-reaching implications for physical and mental health outcomes in older adults by informing interventions across a variety of neurodegenerative and neuropsychiatric conditions.
Acknowledgments
This study was supported by the Larry L. Hillblom Foundation and the National Institutes of Health Grant: R01 AG032289.
Footnotes
References
- 1.Gurera JW, Isaacowitz DM: Emotion regulation and emotion perception in aging: a perspective on age-related differences and similarities. Prog Brain Res 2019; 247:329–351 [DOI] [PubMed] [Google Scholar]
- 2.Gooding PA, Hurst A, Johnson J, et al. : Psychological resilience in young and older adults. Int J Geriatr Psychiatry 2012; 27:262–270 [DOI] [PubMed] [Google Scholar]
- 3.Carstensen LL: The influence of a sense of time on human development. Science 2006; 312:1913–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacioppo JT, Berntson GG, Bechara A, et al. : Could an aging brain contribute to subjective well-being? The value added by a social neuroscience perspective In: Todorov A, Fiske SDP, eds. Social Neuroscience: Toward Understanding the Underpinnings of the Social Mind, New York: Oxford University Press, 2011:249–262 [Google Scholar]
- 5.Mather M, Canli T, English T, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Available at: https://doiorg/101111/j0956-7976200400662x. 2016. [DOI] [PubMed]
- 6.Nashiro K, Sakaki M, Mather M: Age differences in brain activity during emotion processing: reflections of age-related decline or increased emotion regulation? Gerontology 2012; 58:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Jacques P, Dolcos F, Cabeza R: Effects of aging on functional connectivity of the amygdala during negative evaluation: a network analysis of fMRI data. Neurobiol Aging 2010; 31:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murty VP, Sambataro F, Das S, et al. : Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J Cog Neurosc 2009; 21:1920–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen X, Reus LM, Cox SR, et al. : Subcortical volume and white matter integrity abnormalities in major depressive disorder: findings from UK Biobank imaging data. Sci Rep 2017; 7:5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grangeon MC, Seixas C, Quarantini LC, et al. : White matter hyperintensities and their association with suicidality in major affective disorders: a meta-analysis of magnetic resonance imaging studies. CNS Spectr 2010; 15:375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Firbank MJ, Teodorczuk A, van der Flier WM, et al. : Relationship between progression of brain white matter changes and late-life depression: 3-year results from the LADIS study. Br J Psychiatry 2012; 201:40–45 [DOI] [PubMed] [Google Scholar]
- 12.Isaacowitz DM, Toner K, Neupert SD: Use of gaze for real-time mood regulation: effects of age and attentional functioning. Psychol Aging 2009; 24:989–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mather M, Knight M: Goal-directed memory: the role of cognitive control in older adults’ emotional memory. Psychol Aging 2005; 20:554–570 [DOI] [PubMed] [Google Scholar]
- 14.Yesavage JA, Brink TL, Rose TL, et al. : Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17:37–49 [DOI] [PubMed] [Google Scholar]
- 15.Kramer JH, Jurik J, Sha SJ, et al. : Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 2003; 16:211–218 [DOI] [PubMed] [Google Scholar]
- 16.Delis DC, Kramer JH, Kaplan E, et al. : California Verbal Learning Test—Second Edition. Adult Version. Manual Test. San Antonio, TX: The Psychological Corporation, 2000 [Google Scholar]
- 17.Kerchner GA, Racine CA, Hale S, et al. : Cognitive processing speed in older adults: relationship with white matter integrity. PLoS One 2012; 7:e50425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mather M, Carstensen LL: Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci 2005; 9:496–502 [DOI] [PubMed] [Google Scholar]
- 19.Hung Y, Saygin ZM, Biederman J, et al. : Impaired frontal-limbic white matter maturation in children at risk for major depression. Cereb Cortex 2017; 27:4478–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang A, Leow A, Ajilore O, et al. : Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology 2012; 37:959–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang P, Xu X, Gu Q, et al. : Disrupted white matter integrity in depressed versus non-depressed Parkinson’s disease patients: a tract-based spatial statistics study. J Neurol Sci 2014; 346:145–148 [DOI] [PubMed] [Google Scholar]
- 22.Li W, Liu J, Skidmore F, et al. : White matter microstructure changes in the thalamus in Parkinson disease with depression: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol 2010; 31:1861–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu JY, Zhang Y, Wu WB, et al. : Impaired long contact white matter fibers integrity is related to depression in Parkinson’s disease. CNS Neurosci Ther 2018; 24:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teodorczuk A, Firbank MJ, Pantoni L, et al. : Relationship between baseline white-matter changes and development of late-life depressive symptoms: 3-year results from the LADIS study. Psychol Med 2010; 40:603–610 [DOI] [PubMed] [Google Scholar]
- 25.Carceller-Sindreu M, Serra-Blasco M, de Diego-Adelino J, et al. : Altered white matter volumes in first-episode depression: Evidence from cross-sectional and longitudinal voxel-based analyses. J Affect Disord 2019; 245:971–977 [DOI] [PubMed] [Google Scholar]
- 26.Shen X, Adams MJ, Ritakari TE, et al. : White Matter Microstructure and Its Relation to Longitudinal Measures of Depressive Symptoms in Mid- and Late Life. Biolog Psychiatry 2019; 86:759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sexton CE, McDermott L, Kalu UG, et al. : Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychol Med 2012; 42:1195–1202 [DOI] [PubMed] [Google Scholar]
- 28.Joubert C, Davidson PSR, Chainay H: When do older adults show a positivity effect in emotional memory? Exp Aging Res 2018; 44:455–468 [DOI] [PubMed] [Google Scholar]
- 29.Knight M, Seymour TL, Gaunt JT, et al. : Aging and goal-directed emotional attention: distraction reverses emotional biases. Emotion 2007; 7:705–714 [DOI] [PubMed] [Google Scholar]
- 30.Ochsner KN, Gross JJ: The cognitive control of emotion. Trends Cogn Sci 2005; 9:242–249 [DOI] [PubMed] [Google Scholar]
- 31.Philippot P, Agrigoroaei S: Repetitive thinking, executive functioning, and depressive mood in the elderly. Aging Ment Health 2017; 21:1192–1196 [DOI] [PubMed] [Google Scholar]
- 32.Barnes DE, Yaffe K, Byers AL, et al. : Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012; 69:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashburner J: A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38:95–113;doi: 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 34.Ashburner J, Friston KJ: Unified segmentation. Neuroimage 2005; 26:839–851;doi: 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- 35.Desikan RS, Segonne F, Fischl B, et al. : An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31:968–980;doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 36.Fonov VS, Evans AC, McKinstry RC, et al. : Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage 2009; 47:S102 10.1016/S1053-8119(09)70884-5 [DOI] [Google Scholar]
- 37.Garyfallidis E, Brett M, Amirbekian B, et al. : Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 2014; 8:8; doi: 10.3389/fninf.2014.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkinson M, Beckmann CF, Behrens TE, et al. : FSL. Neuroimage 2012; 62:782–790;doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 39.Jenkinson M, Bannister P, Brady M, et al. : Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002; 17:825–841 [DOI] [PubMed] [Google Scholar]
- 40.Mori S, Oishi K, Jiang H, et al. : Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008; 40:570–582;doi: 10.1016/j.neuroimage.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otsu N: A threshold selection method from gray-level histograms. IEEE Trans Sys Man Cyb 1979; 9:62–66;doi: 10.1109/TSMC.1979.4310076 [DOI] [Google Scholar]
- 42.Zhang H, Yushkevich PA, et al. : Unbiased white matter atlas construction using diffusion tensor images. Med Image Comput Comput Assist Interv 2007; 10(Pt 2):211–218;doi: 10.1007/978-3-540-75759-7_26 [DOI] [PubMed] [Google Scholar]


