Abstract
Asymmetric cell division (ACD) is a cellular process that forms two different cell types through a cell division and is thus critical for the development of all multicellular organisms. Not all but many of the ACD processes are mediated by proper orientation of the mitotic spindle, which segregates the fate determinants asymmetrically into daughter cells. In many cell types, the evolutionarily conserved protein complex of Gαi/AGS-family protein/NuMA-like protein appears to play critical roles in orienting the spindle and/or generating the polarized cortical forces to regulate ACD. Studies in various organisms reveal that this conserved protein complex is slightly modified in each phylum or even within species. In particular, AGS-family proteins appear to be modified with a variable number of motifs in their functional domains across taxa. This apparently creates different molecular interactions and mechanisms of ACD in each developmental program, ultimately contributing to developmental diversity across species. In this review, we discuss how a conserved ACD machinery has been modified in each phylum over the course of evolution with a major focus on the molecular evolution of AGS-family proteins and its impact on ACD regulation.
Introduction
Asymmetric cell division (ACD) is a process in which cell fate determinants are differentially distributed, generating daughter cells with distinct molecular profiles. The varying concentrations and identities of determinants present in the daughter cells lead to cell fate diversification followed by unique transcriptional and translational regulation in each lineage (Betschinger & Knoblich, 2004). During development, therefore, ACD generates cellular diversity and controls tissue formation and morphogenesis through the precise spatial arrangement of cell divisions (Culurgioni et al., 2011; Johnston et al., 2009; Lu & Johnston, 2013; Morin & Bellaiche, 2011). ACD is thus essential for the development of all multi-cellular organisms, and its variation leads to developmental diversity and, ultimately, to species diversification during the course of evolution.
ACD is widely seen in various cell types and biological contexts across taxa. For example, during embryonic development of C. elegans, the fertilized zygote divides asymmetrically for the first four cell division rounds. The first ACD produces a large anterior blastomere (AB) and a small posterior blastomere (P1) that markedly differ from each other in terms of localized cell fate determinants, cell cycle periods and cleavage patterns (Schnabel et al., 1996; Sulston et al., 1983; Watts et al., 1996). In Drosophila, embryonic neuroblast cells divide asymmetrically to produce a self-renewed neuroblast as well as a new ganglion mother cell that later gives rise to postmitotic differentiated neurons or glial cells (Bate, 1976; Doe, 2008; Doe et al., 1988; Hartenstein & Campos-Ortega, 1984; Parmentier et al., 2000). Another classical model in Drosophila includes Sensory Precursor (SOP) cells that undergo two rounds of ACD to generate four cells of distinct fates: the internal neuron and the sheath, hair and socket cells (Bate, 1978; Bodmer et al., 1989; Roegiers et al., 2001). In the sea urchin, the embryo undergoes ACD at the 16-cell stage and forms a lineage called micromeres (Boveri, 1901; Horstadius, 1928; Ransick & Davidson, 1993). Micromeres display a number of unique features compared to the rest of blastomeres in the embryo, including the smaller size, the distinct fates and the function as an organizing center to induce endomesodermal specification in the embryo (Horstadius, 1928; Okazaki, 1975; Ransick & Davidson, 1993; Yajima & Wessel, 2012).
The importance of ACD continues throughout adulthood where it sustains tissue homeostasis and promotes regeneration of damaged tissue (Culurgioni et al., 2011; Doe, 2008; Johnston et al., 2009; Lu & Johnston, 2013; Morin & Bellaiche, 2011). In mammals, one of the well-studied models is the neuronal progenitor cells in the cerebral cortex. These cells first divide symmetrically during early neurogenesis to produce two neuronal progenitor cells for expansion of the pool size but later divide asymmetrically to generate one progenitor and one differentiating neuronal cells (Cayouette & Raff, 2003; Chenn & McConnell, 1995; Haydar et al., 2003; Konno et al., 2008; Noctor et al., 2004). Similarly, another mammalian example includes basal epidermal cells that divide symmetrically early in development to expand the basal layer but switch to asymmetric divisions thereafter to generate a committed suprabasal cell and a self-renewed proliferative basal cell (Lechler & Fuchs, 2005). Overall, ACD facilitates the formation of distinct cell types in a single organism. Therefore, variations in the timing, location, and steps of ACD among organisms contribute to the establishment of a diverse set of developmental programs across taxa.
The process of ACD typically requires the fulfilment of all or some of the key steps such as the polarization of the mother cell, the segregation of polarity and cell fate determinants, and/or the proper orientation and positioning of the mitotic spindle relative to a defined polarity axis prior to cytokinesis (Gonczy, 2008; Lu & Johnston, 2013). In many cases, spindle orientation and/or positioning plays critical roles during ACD, yet some exceptions are also present where asymmetric chromosome segregation or fate determinants localization drives ACD independent of the spindle (Box 1). A number of extensive original research as well as comprehensive reviews are available that explain the molecular mechanisms of spindle orientation and ACD in various model systems (Ajduk & Zernicka-Goetz, 2016; Bergstralh et al., 2017; Cowan & Hyman, 2004; di Pietro et al., 2016; Goldstein, 2003; Gonczy, 2008; Kaltschmidt & Brand, 2002; Knoblich, 2008; Kotak, 2019; Lu & Johnston, 2013; Morin & Bellaiche, 2011; Poulson & Lechler, 2012; Rose & Gonczy, 2014; Seirin-Lee, 2020; Siller & Doe, 2009; Willard et al., 2004; Williams & Fuchs, 2013). These studies identified evolutionarily conserved molecular factors and mechanism of ACD among organisms, while ACD often appears randomly across phyla and contributes to the formation of a unique cell type within each organism. This suggests modifications of existing ACD molecules and machinery provide unique ACD regulation to each organism. In this review, we analyze this idea by focusing on the evolutionary modifications of Activator of G-protein Signaling (AGS) protein family, the polarity factors that are conserved among metazoans and known to primarily control spindle orientation in various organisms. Although AGS-family proteins typically function in both symmetric and asymmetric cell divisions, we propose, in this review, their evolutionary modifications might have played critical roles in creating a diversity of the ACD process among organisms. First, we summarize variations of AGS-family proteins present among organisms and even within phyla, and then further discuss how those modifications may have influenced their differential functions in ACD, contributing to the more nuanced and unique ACD mechanism in each organism.
Box 1. Asymmetric cell division and AGS-family protein categories.
Asymmetric cell division (ACD) is a process that leads to the formation of two daughter cells with distinct molecular composition and sometimes unequal sizes. ACD thus leads to the creation of new cell types with different fates, a process critical for the development of multicellular organisms.
ACD can occur through various means, including spindle orientation and/or positioning along a specific polarity axis, differential segregation of fate determinants, asymmetric distribution of chromosomes, or a combination of these. Notably, while spindle orientation drives ACD in many cell types, it is not always required for ACD to occur.
AGS-family proteins can be classified into four groups based on their interactions with G-protein subunits and function: Group I AGS proteins act as nonreceptor guanine nucleotide exchange factors, Group II act as guanine nucleotide dissociation inhibitors, Group III interact with Gβγ, and Group IV activate G-protein signaling with Gα16 (Blumer & Lanier, 2014).
LGN/Pins/GPSM2, part of group II, regulate mitotic spindle positioning and orientation. Of note, it typically functions both in symmetric and asymmetric cell divisions across taxa.
Variations of AGS-family proteins among organisms
AGS-family proteins activate G protein signaling in the absence of a G-protein-coupled receptor (Bernard et al., 2001; Blumer et al., 2005). Some of the first studies using yeast hybrid assays identified human AGS3 and LGN as binding partners of Gαi, providing insight into the functional role of AGS-family proteins (Cismowski et al., 1999; Mochizuki et al., 1996; Takesono et al., 1999). Some but not all of AGS-family proteins were then found to be involved in regulating cell polarity and proper spindle orientation. Among those, G-protein regulator (GPR-1/2) in C. elegans, partner of inscuteable (Pins) in Drosophila, and LGN in mammals have been extensively studied and known to regulate spindle orientation and function in ACD. Some of the others, on the other hand, were identified to have no apparent function in ACD. For example, besides GPR-1/2, C. elegans possesses an AGS3-like protein, yet this protein is involved in food-seeking behavior but not in ACD (Bernard et al., 2001; Hofler & Koelle, 2011). Similarly, in mammals, AGS3 protein is unable to regulate spindle orientation (Saadaoui et al., 2017) and is expressed specifically in the brain and testes in the rat (Blumer et al., 2002), whereas LGN regulates spindle orientation and is ubiquitously expressed in every tissue. Therefore, each of AGS-family proteins appears to undergo minor but distinct modifications, resulting in unique expression and subcellular distribution, thereby presenting separate cellular function. In order to keep the focus of this review on ACD, we will mainly discuss hereafter the AGS-family proteins that are involved in ACD.
Although many organisms appear to have multiple AGS-family proteins, both Drosophila (protostome) and the sea urchin (deuterostome) appear to have only one AGS3/LGN-like protein. Considering their evolutionary distance, AGS-family genes likely underwent independent duplication event in each phylum. Indeed, phylogenetic tree analysis shows that AGS-family proteins typically cluster within each phylum: AGS3 and LGN-like proteins of vertebrates cluster together within each protein group, yet these two branches of vertebrate proteins are closer to each other than any other branches of non-vertebrate AGS/LGN-like proteins (Fig. 1). Of note, despite being a part of the chordate phylum, C. intestinalis (ascidian) AGS clusters better with the protostomes AGS/LGN-like proteins, suggesting it may have evolved uniquely from the rest of the chordates.
Figure 1.
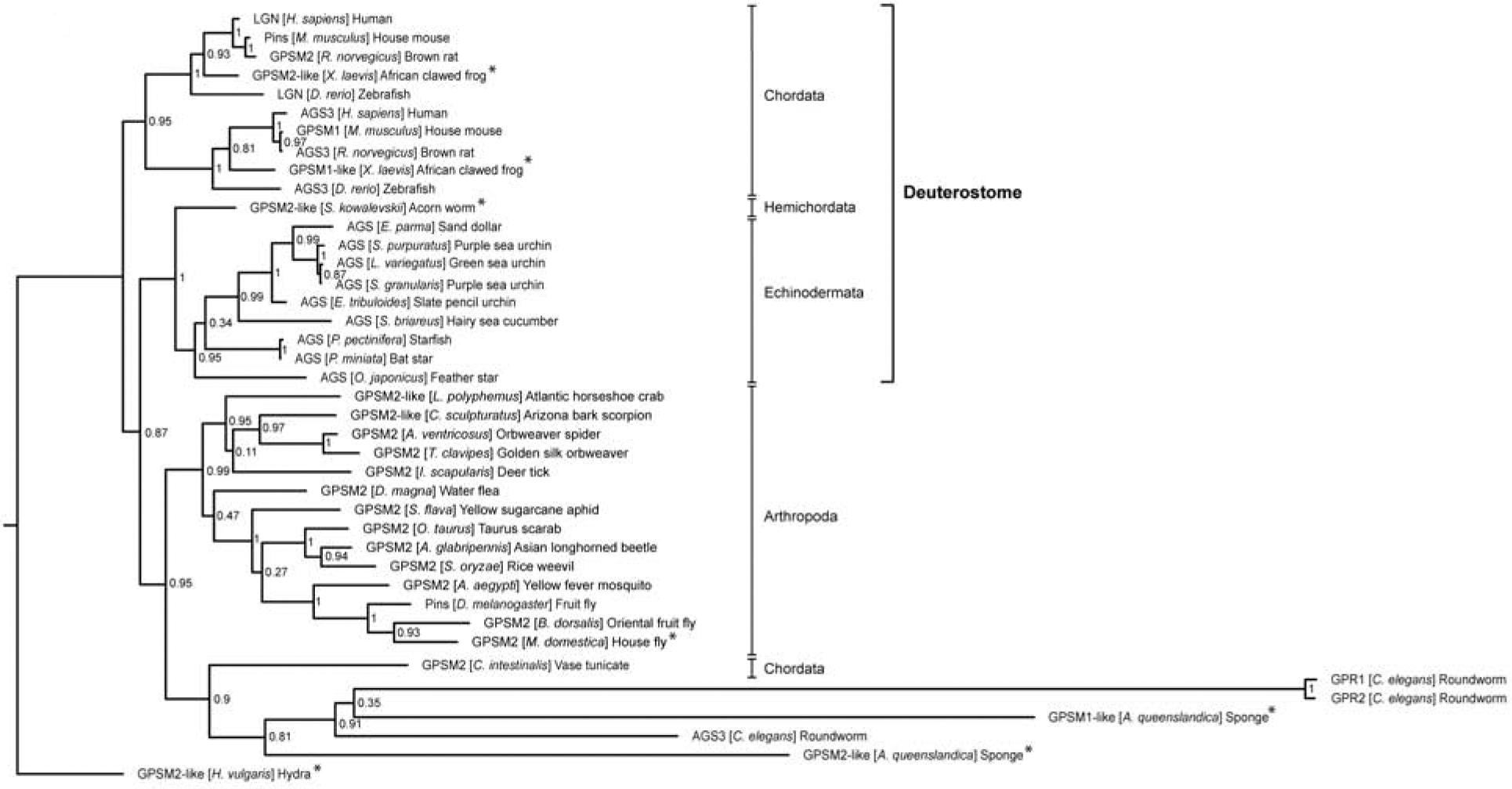
AGS-family protein phylogeny constructed using CIPRES Science Gateway V. 3.3 (http://www.phylo.org/). FASTA sequences for LGN-like proteins (also known as Pins, GPSM2, AGS, GPR-1/2) and AGS3-like proteins (also known as GPSM1) were collected. Overall, AGS-family proteins tend to cluster by phylum, with the exception of C. intestinalis (ascidian) AGS that clusters better with the protostomes AGS despite being part of the chordate phylum. Vertebrate AGS3-like proteins also cluster more closely with vertebrate LGN-like protein than with other phyla AGS-family proteins. Bootstrap values are included at each branching.
* indicate predicted-only sequences. Of note, only a partial sequence was available for the predicted Hydra [H. vulgaris] AGS sequence. Species and proteins represented include: Homo sapiens (LGN, AAN01266.1; AGS3, AAO17260.1), Mus musculus (Pins, AAL87447.1; GPSM1, NP_700459.2), Rattus norvegicus (GPSM2, NP_001178891.1; AGS3, AAF08683.1), Xenopus laevis (GPSM2-like, XP_018114183.1; GPSM1-like, XP_018088064.1), Danio rerio (LGN, AAU14175.1; AGS3, ABB60051.1), Saccoglossus kowalevskii (GPSM2-like, XP_006813223.1), Ciona intestinalis (GPSM2, XP_018672348.1), Echinarachnius parma (AGS, (Poon et al., 2019)), Strongylocentrotus purpuratus (AGS, ABC88013.1), Lytechinus variegatus (AGS, (Poon et al., 2019)), Sphaerechinus granularis (AGS, (Poon et al., 2019)), Eucidaris tribuloides (AGS, (Poon et al., 2019)), Sclerodactyla briareus (AGS, (Poon et al., 2019)), Patiria pectinifera (AGS, (Poon et al., 2019)), Patiria miniata (AGS, (Poon et al., 2019)), Oxycomanthus japonicus (AGS, (Poon et al., 2019)), Limulus Polyphemus (GPSM2-like, XP_022238444.1), Centruroides sculpturatus (GPSM2-like, XP_023234019.1), Araneus ventricosus (GPSM2, GBM12092.1), Trichonephila clavipes (GPSM2, PRD25538.1), Ixodes scapularis (GPSM2, XP_029823219.1), Daphnia magna (GPSM2, KZS10949.1), Sipha flava (GPSM2, XP_025418717.1), Onthophagus Taurus (GPSM2, XP_022906171.1), Anoplophora glabripennis (GPSM2, XP_018563552.1), Sitophilus oryzae (GPSM2, XP_030762782.1), Aedes aegypti (GPSM2, XP_021701486.1), Drosophila melanogaster (Pins, AAF64499.1), Bactrocera dorsalis (GPSM2, XP_011205309.1), Musca domestica (GPSM2, XP_005185499.1), Caenorhabditis elegans (GPR1, CCD62140.1; GPR2, CAA79548.1; AGS3, NP_741787.1), Amphimedon queenslandica (GPSM2-like, XP_019856151.1; GPSM2-like, XP_011409880.2), Hydra vulgaris (GPSM2-like, XP_012559993.1).
Structural and Functional variations of AGS-family proteins among organisms
AGS-family proteins are defined by a two-domain structure (Bernard et al., 2001). They are comprised of tetratricopeptide repeats (TPR) in the N-terminal half with a linker domain that separates the C-terminal half containing G-protein regulatory motifs (GoLoco (GL) motifs). The TPR motifs are commonly involved in protein-protein interactions whereas the GL motifs are important for interacting with the heterotrimetric G-protein subunit, Gα (Du & Macara, 2004). The number of TPR and GL motifs varies across taxa and, in some cases, even within a phylum (Fig. 2). For example, among arthropods, Drosophila Pins possesses seven TPR and three GL motifs, the horseshoe crab (L. polyphemus) possesses eight TPR and four GL motifs, and the scorpion (C. sculpturatus) possesses six TPR and three GL motifs. Vertebrate AGS-family proteins, on the other hand, vary slightly in the number of TPR motifs yet consistently have four GL motifs within a phylum. As a general trend, AGS-family proteins of the higher-order organisms appear to have, more consistently, a greater number of functional motifs. C. elegans GPR-1/2 refers to a pair of nearly identical proteins called GPR-1 and GPR-2. Their structure is being debated in the field: It is sometimes represented in the literature with no TPR (Willard et al., 2004), one TPR (Gotta et al., 2003; Siller & Doe, 2009; Werts et al., 2011), or two TPR motifs (Betschinger & Knoblich, 2004; Nguyen-Ngoc et al., 2007; Srinivasan et al., 2003). To be noted, a diagram in Fig. 2 is based on the NCBI motif search using the same threshold of 0.05 for all AGS-family proteins listed, which predicted a presence of a TPR-like motif for both GPR-1 and GPR-2 in C. elegans. In many of the AGS-family proteins, these TPR and GL motifs are connected by a linker region. Although the linker region is less conserved among organisms compared to the TPR or GL motifs, it has been reported to mediate specific protein interactions and conformational changes of AGS-family proteins (Johnston et al., 2009; Saadaoui et al., 2014). Therefore, the linker region may be also considered as the third functional domain of AGS-family proteins.
Figure 2.
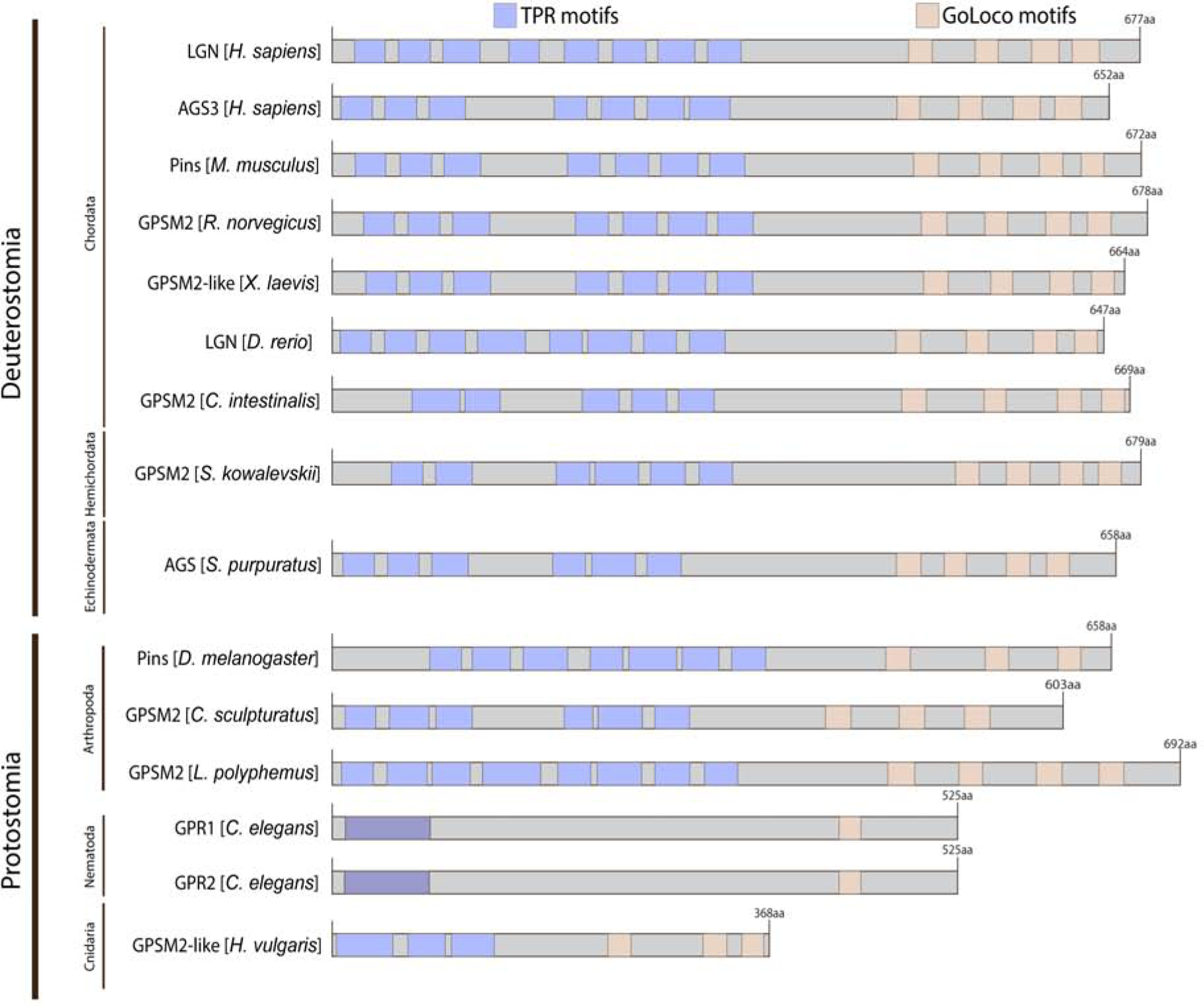
LGN-like protein structure grouped by phylum based on NCBI motif search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) using a threshold of 0.05 and CDD v3.18 database. Human AGS3 protein structure was also included for comparison. Blue squares indicate TPR motifs, beige squares indicate GL motifs, and purple squares (C. elegans) indicate debated TPR motifs. Higher-order organisms tend to have more GL or TPR motifs. This may suggest a relatively linear evolution of AGS-family protein with more functionals motifs in higher-order organisms.
The TPR motif is a widespread structural motif that is typically arranged in tandem arrays and facilitates specific interaction with a partner protein (D’Andrea & Regan, 2003; Zeytuni & Zarivach, 2012). It is a degenerate repeat of 34 amino acids with eight consensus residues that define the motif and are loosely conserved in terms of size, hydrophobicity and spacing (Blatch & Lassle, 1999; Lamb et al., 1995). A high degree of sequence diversity of the TPRs reflects the functional diversity of TPR-containing proteins (Lamb et al., 1995). In AGS-family proteins, the TPR motifs are confined within the N-terminus and the number TPR motifs ranges from one (GPR-1/2) to eight (LGN). The number and/or sequence of these TPR motifs are significantly varied among different phyla. These modifications likely help facilitate specific interactions of AGS-family proteins to their binding partners that are unique to each species.
The GL motif comprises a conserved sequence of 19 amino acids that can be found in a series of signaling proteins such as RGS12 and Purkinje cell protein-2 (Pcp-2) in addition to AGS proteins (Willard et al., 2004). GL motifs of AGS-family proteins generally interact specifically with Gαi with a preference for its GDP-loaded form, and further act as guanine dissociation inhibitors (GDI) to inhibit Gα from GTP-loading (Bernard et al., 2001; De Vries et al., 2000; Jia et al., 2012; Kopein & Katanaev, 2009; Liu et al., 2018; McCudden et al., 2005; Natochin et al., 2001; Schaefer et al., 2001; Voronina & Wessel, 2006; Willard et al., 2008). In mammals, while these GL motifs are also able to bind to Gαo with low affinity, GDI activity towards Gαo appears to be either absent or minimal (Kaushik et al., 2003; McCudden et al., 2005; Natochin et al., 2001). This suggests that strong binding by Gα is likely necessary to activate AGS proteins in mammals (Liu et al., 2018). On the other hand, Drosophila Pins binds both the GDP- and GTP-loaded forms of Gαo through its GL1 motif due to its unique lysine residue (Kopein & Katanaev, 2009; Luchtenborg et al., 2015; Nipper et al., 2007). This Pins-Gαo interaction is necessary for polarity establishment and proper ACD of neuroblasts and SOP cells. However, unlike Pins-Gαi interaction, Pins-Gαo interaction requires the activation of Tre1 GPCR in neuroblasts and Frizzled in SOP cells (Katanaev & Tomlinson, 2006; Kopein & Katanaev, 2009; Yoshiura et al., 2012). Similarly, in C. elegans, GPR-1/2 interacts with both GOA-1 (Gαo-like subunit) and GAP-16 (Gαi-like subunit) to regulate ACD in the zygote (Afshar et al., 2005; Colombo et al., 2003). Therefore, protostome AGS-family proteins appear to use two partially redundant Gαi/o subunits to modulate ACD, yet this trait is not maintained in higher-order organisms.
AGS protein acts as a conformational switch that can exist in a closed or open structure based on the intramolecular binding between the GL and TPR motifs (Fig. 3A) (Du & Macara, 2004; Johnston et al., 2009; Nipper et al., 2007; Pan et al., 2013). By default, AGS exists in an autoinhibited state when the GL motifs are interacting with the TPR domains. The binding of Gα to the GL motif region relieves the TPR-GL interaction and allows the protein to adopt an open conformation, promoting the binding of TPR motifs with their targets. Each GL motif of AGS-family proteins binds Gα, thus multiple Gα proteins may bind an AGS-family protein sequentially or simultaneously (Bernard et al., 2001; Blumer & Lanier, 2014; Jia et al., 2012; McCudden et al., 2005; Nipper et al., 2007; Smith & Prehoda, 2011). Since Gαi is myristoylated, the AGS-complex loaded with Gαi proteins is recruited and anchored to the cortex, which further recruits other factors necessary for regulating spindle orientation. AGS-complex loaded with several Gαi proteins likely facilitates the stronger recruitment and pulling forces at the cortex (Poon et al., 2019). Therefore, the timing and level of AGS activities at the cortex may be fine-controlled by the pattern of Gαi-binding. Having additional GL motifs might thus allow the AGS-family protein to facilitate the more complex spindle regulation in the higher-order organisms.
Figure 3.
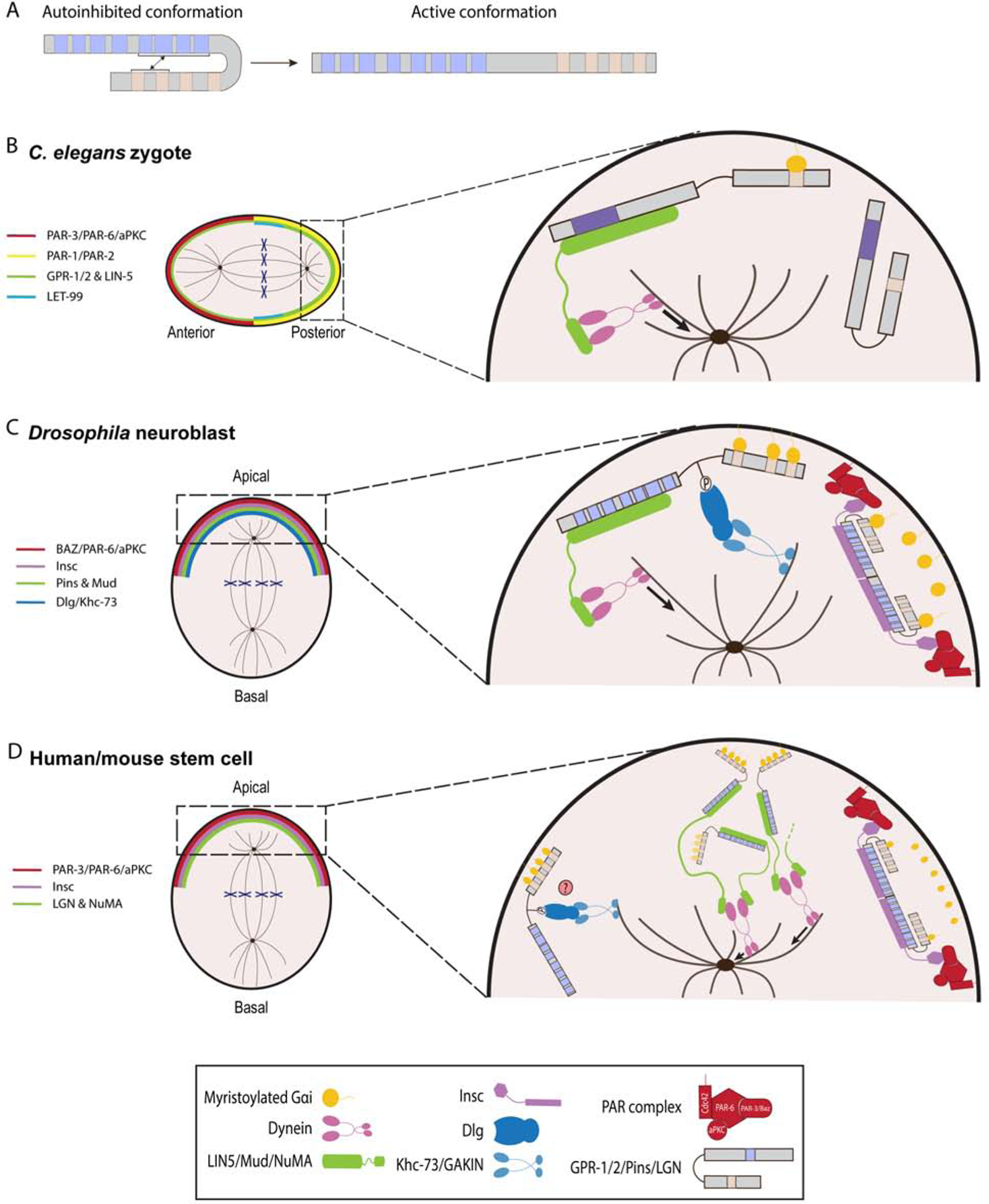
AGS-family proteins regulate spindle orientation and ACD. A, Schema depicting a transition of human LGN from a closed to an open conformation. In autoinhibited form, the GL motifs bind to the TPR motifs. B-D, Working models of AGS-family protein function in C. elegans zygote (A), Drosophila neuroblasts (B), and human/mouse stem cells (C). For each AGS-family protein, blue squares indicate TPR motifs, beige squares indicate GL motifs, and purple squares (C. elegans) indicate debated TPR motifs. A, C. elegans zygote is polarized along an anterior-posterior axis by the activity of PAR proteins complex. GPR-1/2 protein is localized to the posterior complex as LET-99 restricts GPR-1/2 to posterior-most cortex. GPR-12 is anchored at the cortex through the GL motifs that are bound to myristoylated Gαi. Pins then interacts with LIN-5 to recruit dynein and generate microtubule pulling forces on the astral microtubules. Of note, GPR-1/2 and LIN-5 are more concentrated towards the posterior cortex. B, Drosophila neuroblast is polarized along an apical-basal axis by PAR protein complex. Pins forms heterotetramers with Insc to localize to the apical cortex. Pins then recruits Mud as well as Dlg and Khc-73 proteins to orient mitotic spindle. C, Human and mouse stem cells divide asymmetrically along an apical-basal polarity axis via LGN. LGN is able to form heterotetramers with Insc for its localization and heterohexamers with NuMA, which creates a network that recruits dynein to the cortex. However, the functional interactions between such oligomers remain to be determined. The LGN/Dlg/GAKIN is predicted to be involved in spindle orientation but has yet to be shown.
Evolutionary modifications of AGS-mediated ACD
AGS-mediated ACD mechanism appears to be highly conserved across taxa with some minor modifications in each phylum. We here highlight those conserved and modified mechanisms of ACD in major model organisms (Fig. 3B–D; Table 1). In C. elegans (Fig. 3B), PAR proteins facilitate cell polarity and establish the asymmetric distribution of key proteins such as Gβγ and LET-99 to ensure GPR-1/2 enrichment at the posterior cortex where it binds to the Gα subunits GOA-1 and GPA-16 (Park & Rose, 2008; Thyagarajan et al., 2011; Tsou et al., 2002; Tsou et al., 2003a; Wu & Rose, 2007; Zwaal et al., 1996). This cortical GPR-1/2 localization appears to be also mediated by LIN-5, a large coiled-coil protein (Srinivasan et al., 2003). LIN-5 also recruits dynein-dynactin complex to the cell cortex by acting as an adaptor protein of dynein (Couwenbergs et al., 2007; Fielmich et al., 2018; Kotak et al., 2012; Nguyen-Ngoc et al., 2007). A ternary complex of Gα/GPR-1/2/LIN-5 bound to dynein then moves on the astral microtubules towards the centrosome, which generates microtubule pulling forces towards the cell cortex, facilitating ACD in the zygote (Gusnowski & Srayko, 2011; Laan et al., 2012; Lorson et al., 2000; Nguyen-Ngoc et al., 2007; Rodriguez-Garcia et al., 2018). Loss of any members in this ternary complex results in an equal division (Colombo et al., 2003; Gotta et al., 2003; Lorson et al., 2000; Srinivasan et al., 2003; Tsou et al., 2003b), but further mechanistic details of GPR-1/2 function in ACD are yet to be identified. These include how GPR-1/2 interacts with its partner proteins such as PARs, G-proteins and LIN-5, and through what motifs. These studies should reveal how GPR-1/2 regulates ACD with only a single GL and TPR motifs because AGS-family proteins in other organisms appear to require at least three GL motifs and six TPR motifs for their function in ACD as discussed below.
Table 1.
Protein factors involved in ACD in the different model organisms
| C. elegans | Drosophila | Vertebrates |
|---|---|---|
| GOA-1; GPA-16 | Gαi | Gαi 1, 2, 3 |
| GPR-1/2 | Pins | LGN (GPSM2) |
| LIN-5 | Mud | NuMA |
| - | Insc | Insc (mlnsc) |
| PAR-3 | Bazooka (Baz) | PAR-3 |
| PAR-6 | DmPAR-6 | PAR-6 |
| PKC-3 | DaPKC | aPKC, PKCζ |
| DLG-1* | Dig | Dlgl (SAP97) |
| - | Khc-73 | GAKIN(KIF13B) |
not yet tested in spindle orientation
In Drosophila neuroblasts (Fig. 3C), similar to C. elegans zygotes, PAR proteins establish a protein complex of Baz (PAR-3 homolog), PAR-6 and aPKC at the apical cortex to facilitate cell polarity (Cai et al., 2003; Petronczki & Knoblich, 2001; Schober et al., 1999; Wodarz et al., 2000; Wodarz et al., 1999). Gβγ protein also influences the asymmetric localization and stability of the PAR protein and Pins/Gαi complex yet the mechanistic details of this process are still poorly understood (Fuse et al., 2003; Izumi et al., 2004; Schaefer et al., 2001; Yu et al., 2003a). A protein complex of Pins, Gαi and the coiled-coil protein Mushroom body defect (Mud; structurally and functionally similar to LIN-5 with limited sequence homology) is then established at the apical cortex to pull the spindle pole to the apical cortex (Bowman et al., 2006; Izumi et al., 2006; Parmentier et al., 2000; Siller et al., 2006; Yu et al., 2000). Different from C. elegans, the adaptor protein Inscutable (Insc) had been considered to connect the two complexes of Baz/PAR-6/aPKC and Gαi/Pins/Mud to coordinate proper ACD (Parmentier et al., 2000; Schaefer et al., 2000; Schober et al., 1999; Wodarz et al., 2000; Yu et al., 2000). In SOP cells, for example, the absence of Insc results in separation of the two complexes with Gαi/Pins present at the anterior cortex and Baz/PAR-6/aPKC at the posterior cortex (Bellaiche et al., 2004; Bellaiche et al., 2001; Lu et al., 1999; Roegiers et al., 2001; Schaefer et al., 2001). However, recent evidence suggests that Pins cannot bind Insc and Mud simultaneously because both Insc and Mud require the entire TPR domain and compete for their binding with Pins (Bowman et al., 2006; Izumi et al., 2006; Mauser & Prehoda, 2012; Siller et al., 2006; Yu et al., 2002; Zhu et al., 2011). While Mud binding to Pins requires Gαi to relieve Pins autoinhibited conformation (Du & Macara, 2004; Nipper et al., 2007), Insc is able to bind Pins regardless of Gαi interaction (Zhu et al., 2011). Therefore, current model proposes that, during ACD, Pins first interacts with Insc for apical cortex localization, then becomes released from Insc by yet unknown mechanism, and binds Gαi, which then allows Mud-binding and dynein recruitment to facilitate ACD (Johnston et al., 2009; Siller & Doe, 2008; Wang et al., 2005; Zhu et al., 2011).
An additional mechanism of AGS that controls spindle orientation in flies involves the plus-end-directed microtubule motor protein Khc73 (kinesin heavy chain 73) and the membrane-associated Dlg (Discs large). Ser436 of Pins linker domain between the TPR and GL motifs is phosphorylated by Aurora kinase A, facilitating the binding of Dlg which further recruits kinesin Khc73 that is localized to microtubule plus ends to form a ternary complex (Bellaiche et al., 2001; Johnston et al., 2009; Siegrist & Doe, 2005, 2006). In normal process, Dlg-Khc73 functions sequentially with Insc/Pins-complex during early mitosis for astral microtubule attachment and then with Mud/Pins-complex for spindle pulling during late mitosis (Mauser & Prehoda, 2012; Siegrist & Doe, 2005). Additionally, Dlg is able to partially mediate Pins cortical localization both in symmetrically and asymmetrically dividing cells, suggesting Pins and Dlg are mutually dependent for their cortical localization (Bell et al., 2015; Bellaiche et al., 2001; Bergstralh et al., 2013; Carvalho et al., 2015; Siegrist & Doe, 2005). For instance, in insc-null mutant neuroblasts, Pins and Dlg still localize to a cortical crescent, but Pins loses its cortical localization in the double mutant of dlg and insc (Siegrist & Doe, 2005). Therefore, the PAR/Insc and Khc73/Dlg pathways are partially redundant but function independently to regulate the polarized cortical localization of Pins (Siegrist & Doe, 2005; Siller et al., 2006; Zhu et al., 2011).
In humans and mice (Fig. 3D), the similar cortical protein complex PAR-3/PAR-6/aPKC is responsible for establishing cell polarity, and then a complex of LGN/Gαi/Nuclear Mitotic Apparatus (NuMA; structurally and functionally similar to LIN-5 and Mud with limited sequence homology) is responsible for orienting the mitotic spindle along the polarity axis with the adaptor protein Insc (Culurgioni et al., 2011; Lechler & Fuchs, 2005; Takayanagi et al., 2019; Williams et al., 2014; Yuzawa et al., 2011; Zhu et al., 2011). LGN organizes a molecular complex with NuMA that actively facilitates efficient microtubule pulling forces through dynein during spindle orientation (Okumura et al., 2018; Seldin et al., 2016), which creates protein networks that favor cortical dynein-dynactin clusters (Pirovano et al., 2019). These networks further form hetero-hexameric ring-like modules where TPR domains of LGN interact with the C-terminus of dimeric NuMA. Further, LGN also dimerizes and forms heterotetramers with Insc through its TPR domain, which appears to be important for ACD regulation (Culurgioni et al., 2018). These biochemical and structural studies demonstrate the high conformational flexibility of the TPR domain as it forms different molecular complexes with its binding partners. Importantly, Drosophila Pins also dimerizes and forms a complex with Insc (Culurgioni et al., 2018) but may be less inclined to form hexamers with NuMA due to its unique sequence in the TPR domain (Pirovano et al., 2019). Additional studies are required to determine if LGN, Pins and GPR-1/2 indeed adopt similar or different conformations during ACD. Further, similar to Drosophila, mammalian Dlg1 (or called SAP97) interacts with LGN through its phosphorylated Ser401 and further with GAKIN (or called KIF13B), the vertebrate equivalent of Khc-73 (Asaba et al., 2003; Hanada et al., 2000; Yamada et al., 2007). In chick neuroepithelium, Dlg1 regulates cortical localization of LGN and spindle orientation (Saadaoui et al., 2014), suggesting a possible conserved role of Dlg1 in LGN regulation and spindle orientation of vertebrate cells, which needs to be tested further in the future. Besides regulating the interaction with Dlg, phosphorylation also modulates LGN localization. Indeed, in both Drosophila and mammalian cells, aPKC phosphorylates LGN/Pins to exclude LGN/Pins from specific cortical regions in symmetrically dividing cells (Guilgur et al., 2012; Hao et al., 2010). However, this regulation mechanism remains to be evaluated in asymmetrically dividing cells. Overall, AGS-family proteins of higher-order organisms appear to facilitate more complex and specific interactions with various binding partners, likely refining the regulation of spindle orientation and, overall, the process of ACD.
As summarized above, the general machinery for ACD regulation is conserved among major model organisms, but some key differences can be highlighted. In C. elegans embryos, Gα, GPR-1/2 and LIN-5 act downstream of the polarity cues so that their depletion does not impact the anterior-posterior polarity (Colombo et al., 2003; Gotta & Ahringer, 2001; Gotta et al., 2003; Srinivasan et al., 2003). On the other hand, in Drosophila, Gαi/Pins and the polarity factors are dependent on each other for apical complex formation, thus Gαi/Pins signaling is necessary both for establishing cell polarity and molecular segregation (Betschinger & Knoblich, 2004; Gonczy, 2008; Willard et al., 2004; Yu et al., 2005). For example, in neuroblasts, Pins is necessary for maintaining Baz localization at the apical cortex during M-phase, and its depletion releases Insc from the cortex, misorients the spindle, disrupts fate determinant segregation, and causes incomplete ACD (Cai et al., 2003; Parmentier et al., 2000; Schaefer et al., 2001; Schaefer et al., 2000; Schober et al., 1999; Wodarz et al., 1999; Yu et al., 2000). Additionally, C. elegans likely lacks the Dlg and Insc-mediated ACD mechanisms (Siegrist & Doe, 2005) that appear to be present and conserved in the higher-order organisms.
Evolutionary modifications of AGS protein and its function in ACD
The AGS-family proteins of the higher-order organisms tend to have extra TPR and GL motifs, which might provide additional molecular interactions and mechanisms to modulate spindle orientation in a more nuanced manner. In particular, the mechanism for intramolecular interactions that regulate AGS conformation appear to be critical for its ability to localize and interact with other factors such as Insc and NuMA at the cortex. Therefore, we here discuss how varying number and sequence of TPR and GL motifs of AGS could change its conformation and function in the process of ACD.
Each of GL motifs shares similar binding affinities to Gαi in vitro yet displays different activity in vivo both in Drosophila Pins and human LGN (Jia et al., 2012; McCudden et al., 2005; Nipper et al., 2007). For instance, in the closed form of Pins, GL1 is free from TPRs and has the highest affinity for Gα, while GL2 and GL3 have lower affinity due to their binding to TPRs (Nipper et al., 2007). Similarly, in human LGN, only GL1 is available for binding to Gαi when LGN is in its closed conformation (Takayanagi et al., 2019), while the GL3 and GL4 motifs of LGN are essential for TPR binding, specifically with TPR4–7 motifs (Pan et al., 2013; Takayanagi et al., 2019). Therefore, it has been proposed that Gαi binding to GL1 is needed to recruit Pins/LGN to the cortex in the closed form, which can be then activated at the cortex through additional Gαi binding to the rest of GL motifs (Nipper et al., 2007). Further, recent study in flies with in vitro approach proposes a mechanism where each of GL motifs has the same affinity and competes for Gαi binding, yet GL2 and GL3 loses this competition due to their affinity also with TPR. GL1, on the other hand, lacks an ability to bind TPR (Smith & Prehoda, 2011). Therefore, in vivo, Gαi likely first binds GL1 that is free from TPR-binding, then GL2 that has more affinity to Gαi, and lastly GL3 to fully activate Pins for Mud interaction. This multi-step mechanism allows a cell to limit Pins activity specifically at the apical cortex where and when Gαi concentration is high. On the other hand, C. elegans GPR-1/2 has a single GL motif, thus may employ more simplified activation steps. Collectively, a different number of GL motifs provides a unique mechanism of ACD regulation in each organism. Studies in various phyla will be essential to further assess this point.
Studies that introduced AGS-family gene of another species further support the hypothesis that AGS of the higher-order organisms is capable of regulating a more complex ACD process. Mouse LGN is able to substitute for Pins to rescue spindle orientation and ACD defects in Drosophila neuroblasts in the Pins-depleted background (Yu et al., 2003b). On the other hand, Drosophila Pins is incapable of localizing nor functioning in chick neuroepithelium (Saadaoui et al., 2017). Hence, despite that Pins and LGN shares the high sequence homology, minor modifications in the LGN protein appears to be critical for regulating more complex processes of ACD in vertebrates. These minor modifications not just include having more or fewer GL motifs in LGN or Pins, respectively, but also include subtle modifications in the other domains that impact the protein’s ability to facilitate specific interactions with its binding partners (Saadaoui et al., 2017). For example, LGN forms hexamers with NuMA for its proper function (Pirovano et al., 2019), which may not occur with Pins due to an inability to form such conformation with NuMA. Consistently, mouse LGN can substitute for chick LGN (Morin et al., 2007), suggesting again that AGS-family protein of the higher-order organisms can substitute for that of the more basic organisms. Further detailed analyses are needed to identify critical motifs and residues responsible for a differential function of AGS-family proteins in each organism.
Even though AGS-family proteins cluster by phylum, significant differences can be observed within a phylum, particularly for echinoderms and arthropods. The recent work in echinoderms, the basal deuterostomes, suggests that AGS evolved differently even within the same phylum and impacted introduction of ACD in the developmental program of the sea urchin. In echinoids (sea urchin, sand dollar and pencil urchin), a derived class of the echinoderms, AGS is critical for facilitating ACD at the 16-cell stage (Poon et al., 2019; Voronina & Wessel, 2006). This ACD forms a distinct cell lineage, called micromeres, which then functions as a major organizing center in the embryo. Introduction of sea urchin AGS into the sea star embryo induces micromere-like cell formation, while the same protein that lacks GL1 does not. Therefore, it is proposed that additional GL1 in the sea urchin AGS has facilitated ACD and formation of a new cell lineage in the sea urchin embryo (Poon et al., 2019). Indeed, echinoids that form micromeres appear to have an additional GL1, while other echinoderms (sea stars, feather stars) that do not undergo ACD during early embryogenesis appear to lack a complete GL1- or GL2-like motifs (Fig. 4). Exception is the sea cucumber that does not form micromeres yet appear to have both GL1 and GL2 in its AGS. These GL1 and GL2 are, however, located in close proximity to each other. This may compromise GL1 independence from TPR-binding and/or lower the Gαi-binding affinity for cortex localization, and further a lack of GL4 may loosen the autoinhibition state of AGS. On the contrary, the sand dollar that forms micromeres also lacks GL2. As observed in other models, GL2 does not appear to have significant role in autoinhibition nor in cortical recruitment, which may have led to its loss in the sand dollar, a derived subclass of the echinoids. Overall, the evolutionary modifications of AGS proteins appear to have facilitated micromere formation in echinoids, providing the developmental diversity even within the same phylum.
Figure 4.
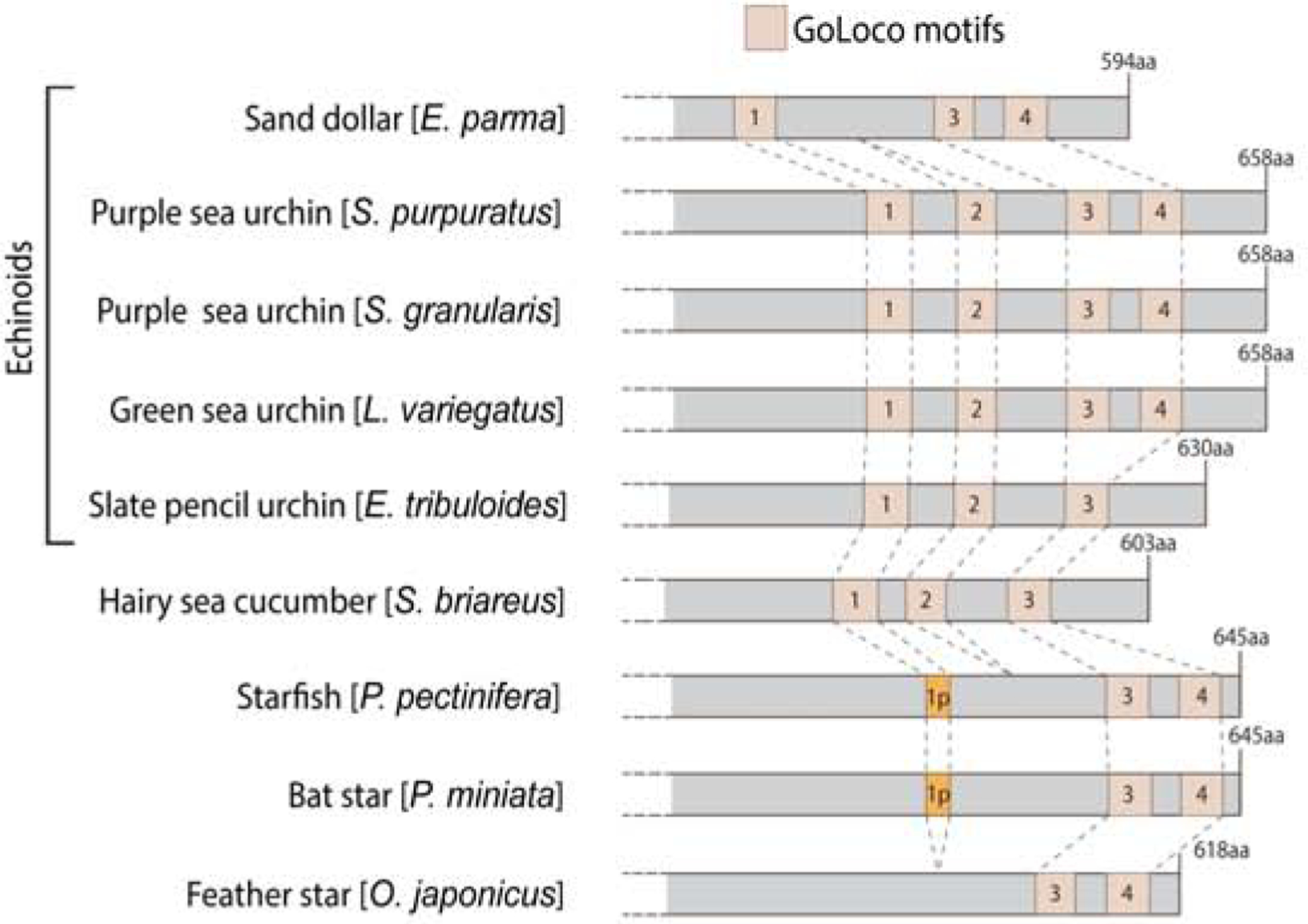
Echinoderm AGS GL domain based on NCBI motif search using a threshold of 0.02, a more stringent threshold than Fig. 2 to remove further non-specific hits. Each GL motif was labeled from 1 to 4 based on sequence similarity to S. purpuratus GL motifs. Blue squares indicate TPR motifs, beige squares indicate GL motifs, and orange squares (sea star) indicate partial GL motifs with a sequence of only 15 amino acids. Variations from Poon et al. (2019) diagram result from using different analytical conditions.
Among arthropods, similarly, a great diversity is present in the number of TPR and GL motifs. While most chelicerates (e.g. horseshoe crab, spider and tick) that are considered to preserve more ancestral characters possess seven to eight TPR and four GL motifs, scorpion, another chelicerate, only possesses six TPR and three GL motifs. Additionally, various fly species possess three GL motifs, suggesting a possibility that some arthropods such as scorpions and flies lost a GL motif. The lack of functional studies in non-fly species of arthropods in this biological process prevents further analyses or interpretations, but the observed diversity may reflect findings in echinoderms. Therefore, future comparative studies among different classes within the same phylum of arthropods are awaited to determine if/how molecular evolution of AGS-family proteins may have impacted the developmental diversity among arthropods.
Mammalian LGN and AGS3 likely arose by duplication after divergence of vertebrate (Saadaoui et al., 2017; Yu et al., 2003b), thus these two proteins share very similar sequence. Both show similar binding affinities to the partners such as NuMA and Gαi in vitro, but AGS3 is unable to mediate spindle orientation in vivo. This functional difference between LGN and AGS3 appears to be caused in part by subtle modifications in the interdomain sequence of GL motifs (Saadaoui et al., 2017), leading to differential modulation of the autoinhibition status of AGS3 and LGN. In human LGN, the interdomain of GL2 and GL3 strongly enhances the intramolecular interaction between GL and TPR domains (Takayanagi et al., 2019). However, this interdomain is relatively well conserved among organisms, even between human LGN and AGS3 (Fig. 5A). Therefore, very small residue changes and/or additional interdomain regions must account for the distinct function and localization of LGN and AGS3. Similarly, in fly Pins, the interdomain of GL2 and GL3 (the region corresponding to GL3 and GL4 of LGN) also partially enhances the GL-TPR interactions (Smith & Prehoda, 2011). This interdomain seems to be more conserved only within each phylum (Fig. 5B). In Drosophila, the sequence corresponding to this region is much longer, possibly contributing to a unique regulation of Pins autoinhibition. Therefore, while the number of conserved motifs is critical for establishing an interaction with the TPR and other partner proteins, subtle residue changes outside of these motifs may also modulate stronger or weaker molecular binding, providing further nuanced AGS regulation that contributes to unique ACD process in each cell and organism.
Figure 5.
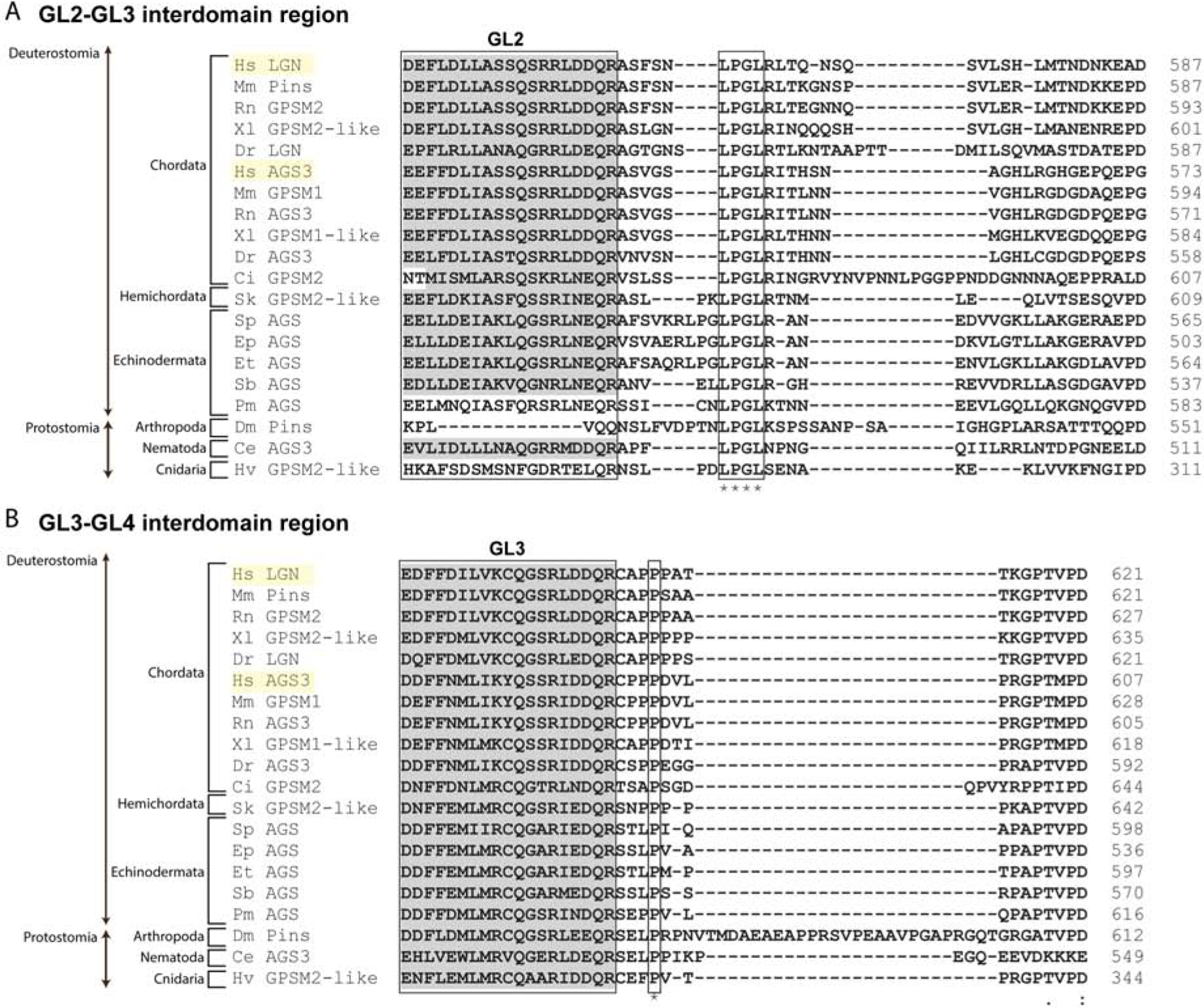
GL motif interdomain sequence alignment for LGN-like and AGS3-like proteins. A, Alignment of sequences corresponding to LGN GL2 and GL3 interdomain. A gray highlighted section represents GL2 motifs. Four residues (LPGL) are conserved across all AGS-family proteins analyzed, and a few residues at the beginning and end of the interdomain are conserved within each phylum. B, Alignment of sequences corresponding to LGN GL3 and GL4 interdomain with a highlighted section showing GL3 motifs. GL3–4 interdomain is more conserved only within phyla with only one residue (P) conserved across all proteins analyzed. Drosophila has a uniquely long interdomain sequence that may contribute to unique regulation of Pins function. Species represented include: Homo sapiens (Hs), Mus musculus (Mm), Rattus norvegicus (Rn), Xenopus laevis (Xl), Danio rerio (Dr), Ciona intestinalis (Ci), Saccoglossus kowalevskii (Sk), Strongylocentrotus purpuratus (Sp), Echinarachnius parma (Ep), Eucidaris tribuloides (Et), Sclerodactyla briareus (Sb), Patiria miniata (Pm), Drosophila melanogaster (Dm), Caenorhabditis elegans (Ce), Hydra vulgaris (Hv).
Conclusions
Studies in a number of model organisms have unraveled key players and mechanisms that drive ACD. Even though these factors and mechanisms of ACD are relatively conserved across taxa, some variations such as the introduction of Insc in ACD of the higher-order organisms and modifications of AGS-family protein across taxa are notable. In conclusion, evolutionary modifications of AGS-family protein and the ACD machinery appear to impact ACD, a process contributing to the formation of a unique cell type in each phylum or even in each species. This ultimately causes changes in the developmental program and contributes to species diversification over the course of evolution. A comparative biological study that directly addresses this point has been available only in the limited models such as echinoderms. Further studies in this direction of research using various model organisms will be essential to identify how each of the TPR and GL motifs as well as interdomain regions of each AGS-family protein is involved in its differential ACD function, and if/how that may have impacted the creation of a unique cell type in each species. Further, only recently, structural insights into the complex molecular interactions of AGS-family protein and its binding partners such as NuMA were reported. These works highlight a need for further biochemical and structural studies in different species to reveal the conserved or unique molecular mechanism of AGS regulation during ACD across organisms. Lastly, much of the work described in this review is focused on the upstream ACD mechanism in which AGS-family proteins are involved, yet their possible direct involvement in the downstream mechanisms such as the mechanisms of microtubule pulling-force regulation and of chromosome distribution also needs to be investigated in the future. For instance, a recent study in ascidians reveals that Kif2, a member of the kinesin-13 family, is responsible for depolymerizing astral microtubules and is critical for asymmetric spindle positioning (Costache et al., 2017). It will be important to test, in the near future, if/how AGS-family proteins may interact with Kif2 and contributes to the downstream ACD events.
Highlights:
The evolutionarily conserved protein complex of Gαi/AGS-family protein/NuMA-like protein plays critical roles in regulating asymmetric cell division (ACD).
Evolutionary modifications of AGS-family proteins are present with a variable number of motifs in their functional domains across taxa.
Modifications of AGS-family protein could create different molecular interactions and mechanisms of ACD in each developmental program, which may contribute to developmental diversity across species.
Acknowledgement
This work was supported by NIH R01GM126043 to MY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afshar K, Willard FS, Colombo K, Siderovski DP, & Gonczy P (2005). Cortical localization of the Galpha protein GPA-16 requires RIC-8 function during C. elegans asymmetric cell division. Development, 132(20), 4449–4459. doi: 10.1242/dev.02039 [DOI] [PubMed] [Google Scholar]
- Ajduk A, & Zernicka-Goetz M (2016). Polarity and cell division orientation in the cleavage embryo: from worm to human. Mol Hum Reprod, 22(10), 691–703. doi: 10.1093/molehr/gav068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaba N, Hanada T, Takeuchi A, & Chishti AH (2003). Direct interaction with a kinesin-related motor mediates transport of mammalian discs large tumor suppressor homologue in epithelial cells. J Biol Chem, 278(10), 8395–8400. doi: 10.1074/jbc.M210362200 [DOI] [PubMed] [Google Scholar]
- Bate CM (1976). Embryogenesis of an insect nervous system. I. A map of the thoracic and abdominal neuroblasts in Locusta migratoria. J Embryol Exp Morphol, 35(1), 107–123. [PubMed] [Google Scholar]
- Bate CM (1978). Development of Sensory Systems in Arthropods Jacobson M(Ed.), Handbook of Sensory Physiology, 9, 1–53. doi: 10.1007/978-3-642-66880-7_1 [DOI] [Google Scholar]
- Bell GP, Fletcher GC, Brain R, & Thompson BJ (2015). Aurora kinases phosphorylate Lgl to induce mitotic spindle orientation in Drosophila epithelia. Curr Biol, 25(1), 61–68. doi: 10.1016/j.cub.2014.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaiche Y, Beaudoin-Massiani O, Stuttem I, & Schweisguth F (2004). The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development, 131(2), 469–478. doi: 10.1242/dev.00928 [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, Radovic A, Woods DF, Hough CD, Parmentier ML, O’Kane CJ, … Schweisguth F (2001). The Partner of Inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell, 106(3), 355–366. doi: 10.1016/s0092-8674(01)00444-5 [DOI] [PubMed] [Google Scholar]
- Bergstralh DT, Dawney NS, & St Johnston D (2017). Spindle orientation: a question of complex positioning. Development, 144(7), 1137–1145. doi: 10.1242/dev.140764 [DOI] [PubMed] [Google Scholar]
- Bergstralh DT, Lovegrove HE, & St Johnston D (2013). Discs large links spindle orientation to apical-basal polarity in Drosophila epithelia. Curr Biol, 23(17), 1707–1712. doi: 10.1016/j.cub.2013.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard ML, Peterson YK, Chung P, Jourdan J, & Lanier SM (2001). Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J Biol Chem, 276(2), 1585–1593. doi: 10.1074/jbc.M005291200 [DOI] [PubMed] [Google Scholar]
- Betschinger J, & Knoblich JA (2004). Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol, 14(16), R674–685. doi: 10.1016/j.cub.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Blatch GL, & Lassle M (1999). The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays, 21(11), 932–939. doi: [DOI] [PubMed] [Google Scholar]
- Blumer JB, Chandler LJ, & Lanier SM (2002). Expression analysis and subcellular distribution of the two G-protein regulators AGS3 and LGN indicate distinct functionality. Localization of LGN to the midbody during cytokinesis. J Biol Chem, 277(18), 15897–15903. doi: 10.1074/jbc.M112185200 [DOI] [PubMed] [Google Scholar]
- Blumer JB, Cismowski MJ, Sato M, & Lanier SM (2005). AGS proteins: receptor-independent activators of G-protein signaling. Trends Pharmacol Sci, 26(9), 470–476. doi: 10.1016/j.tips.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Blumer JB, & Lanier SM (2014). Activators of G protein signaling exhibit broad functionality and define a distinct core signaling triad. Mol Pharmacol, 85(3), 388–396. doi: 10.1124/mol.113.090068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer R, Carretto R, & Jan YN (1989). Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron, 3(1), 21–32. doi: 10.1016/0896-6273(89)90112-8 [DOI] [PubMed] [Google Scholar]
- Boveri T (1901). Über die Polarität des Seeigeleies. In (Vol. 34, pp. 145–176). Wurzburg: Verhandl. Der. Phys. — Med. Geselsch. [Google Scholar]
- Bowman SK, Neumuller RA, Novatchkova M, Du Q, & Knoblich JA (2006). The Drosophila NuMA Homolog Mud regulates spindle orientation in asymmetric cell division. Dev Cell, 10(6), 731–742. doi: 10.1016/j.devcel.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Cai Y, Yu F, Lin S, Chia W, & Yang X (2003). Apical complex genes control mitotic spindle geometry and relative size of daughter cells in Drosophila neuroblast and pI asymmetric divisions. Cell, 112(1), 51–62. doi: 10.1016/s0092-8674(02)01170-4 [DOI] [PubMed] [Google Scholar]
- Carvalho CA, Moreira S, Ventura G, Sunkel CE, & Morais-de-Sá E (2015). Aurora A triggers Lgl cortical release during symmetric division to control planar spindle orientation. Curr Biol, 25(1), 53–60. doi: 10.1016/j.cub.2014.10.053 [DOI] [PubMed] [Google Scholar]
- Cayouette M, & Raff M (2003). The orientation of cell division influences cell-fate choice in the developing mammalian retina. Development, 130(11), 2329–2339. doi: 10.1242/dev.00446 [DOI] [PubMed] [Google Scholar]
- Chenn A, & McConnell SK (1995). Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell, 82(4), 631–641. doi: 10.1016/0092-8674(95)90035-7 [DOI] [PubMed] [Google Scholar]
- Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, Fuernkranz H, … Duzic E (1999). Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol, 17(9), 878–883. doi: 10.1038/12867 [DOI] [PubMed] [Google Scholar]
- Colombo K, Grill SW, Kimple RJ, Willard FS, Siderovski DP, & Gonczy P (2003). Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science, 300(5627), 1957–1961. doi: 10.1126/science.1084146 [DOI] [PubMed] [Google Scholar]
- Costache V, Hebras C, Pruliere G, Besnardeau L, Failla M, Copley RR, … McDougall A (2017). Kif2 localizes to a subdomain of cortical endoplasmic reticulum that drives asymmetric spindle position. Nat Commun, 8(1), 917. doi: 10.1038/s41467-017-01048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couwenbergs C, Labbe JC, Goulding M, Marty T, Bowerman B, & Gotta M (2007). Heterotrimeric G protein signaling functions with dynein to promote spindle positioning in C. elegans. J Cell Biol, 179(1), 15–22. doi: 10.1083/jcb.200707085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CR, & Hyman AA (2004). Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu Rev Cell Dev Biol, 20, 427–453. doi: 10.1146/annurev.cellbio.19.111301.113823 [DOI] [PubMed] [Google Scholar]
- Culurgioni S, Alfieri A, Pendolino V, Laddomada F, & Mapelli M (2011). Inscuteable and NuMA proteins bind competitively to Leu-Gly-Asn repeat-enriched protein (LGN) during asymmetric cell divisions. Proc Natl Acad Sci U S A, 108(52), 20998–21003. doi: 10.1073/pnas.1113077108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culurgioni S, Mari S, Bonetti P, Gallini S, Bonetto G, Brennich M, … Mapelli M (2018). Insc:LGN tetramers promote asymmetric divisions of mammary stem cells. Nat Commun, 9(1), 1025. doi: 10.1038/s41467-018-03343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea LD, & Regan L (2003). TPR proteins: the versatile helix. Trends Biochem Sci, 28(12), 655–662. doi: 10.1016/j.tibs.2003.10.007 [DOI] [PubMed] [Google Scholar]
- De Vries L, Fischer T, Tronchere H, Brothers GM, Strockbine B, Siderovski DP, & Farquhar MG (2000). Activator of G protein signaling 3 is a guanine dissociation inhibitor for Galpha i subunits. Proc Natl Acad Sci U S A, 97(26), 14364–14369. doi: 10.1073/pnas.97.26.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pietro F, Echard A, & Morin X (2016). Regulation of mitotic spindle orientation: an integrated view. EMBO Rep, 17(8), 1106–1130. doi: 10.15252/embr.201642292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ (2008). Neural stem cells: balancing self-renewal with differentiation. Development, 135(9), 1575–1587. doi: 10.1242/dev.014977 [DOI] [PubMed] [Google Scholar]
- Doe CQ, Hiromi Y, Gehring WJ, & Goodman CS (1988). Expression and function of the segmentation gene fushi tarazu during Drosophila neurogenesis. Science, 239(4836), 170–175. doi: 10.1126/science.2892267 [DOI] [PubMed] [Google Scholar]
- Du Q, & Macara IG (2004). Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell, 119(4), 503–516. doi: 10.1016/j.cell.2004.10.028 [DOI] [PubMed] [Google Scholar]
- Fielmich LE, Schmidt R, Dickinson DJ, Goldstein B, Akhmanova A, & van den Heuvel S (2018). Optogenetic dissection of mitotic spindle positioning in vivo. Elife, 7. doi: 10.7554/eLife.38198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse N, Hisata K, Katzen AL, & Matsuzaki F (2003). Heterotrimeric G proteins regulate daughter cell size asymmetry in Drosophila neuroblast divisions. Curr Biol, 13(11), 947–954. doi: 10.1016/s0960-9822(03)00334-8 [DOI] [PubMed] [Google Scholar]
- Goldstein B (2003). Asymmetric division: AGS proteins position the spindle. Curr Biol, 13(22), R879–880. doi: 10.1016/j.cub.2003.10.050 [DOI] [PubMed] [Google Scholar]
- Gonczy P (2008). Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol, 9(5), 355–366. doi: 10.1038/nrm2388 [DOI] [PubMed] [Google Scholar]
- Gotta M, & Ahringer J (2001). Distinct roles for Galpha and Gbetagamma in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nat Cell Biol, 3(3), 297–300. doi: 10.1038/35060092 [DOI] [PubMed] [Google Scholar]
- Gotta M, Dong Y, Peterson YK, Lanier SM, & Ahringer J (2003). Asymmetrically distributed C. elegans homologs of AGS3/PINS control spindle position in the early embryo. Curr Biol, 13(12), 1029–1037. doi: 10.1016/s0960-9822(03)00371-3 [DOI] [PubMed] [Google Scholar]
- Guilgur LG, Prudêncio P, Ferreira T, Pimenta-Marques AR, & Martinho RG (2012). Drosophila aPKC is required for mitotic spindle orientation during symmetric division of epithelial cells. Development, 139(3), 503–513. doi: 10.1242/dev.071027 [DOI] [PubMed] [Google Scholar]
- Gusnowski EM, & Srayko M (2011). Visualization of dynein-dependent microtubule gliding at the cell cortex: implications for spindle positioning. J Cell Biol, 194(3), 377–386. doi: 10.1083/jcb.201103128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T, Lin L, Tibaldi EV, Reinherz EL, & Chishti AH (2000). GAKIN, a novel kinesin-like protein associates with the human homologue of the Drosophila discs large tumor suppressor in T lymphocytes. J Biol Chem, 275(37), 28774–28784. doi: 10.1074/jbc.M000715200 [DOI] [PubMed] [Google Scholar]
- Hao Y, Du Q, Chen X, Zheng Z, Balsbaugh JL, Maitra S, … Macara IG (2010). Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr Biol, 20(20), 1809–1818. doi: 10.1016/j.cub.2010.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V, & Campos-Ortega JA (1984). Early neurogenesis in wild-typeDrosophila melanogaster. Wilehm Roux Arch Dev Biol, 193(5), 308–325. doi: 10.1007/bf00848159 [DOI] [PubMed] [Google Scholar]
- Haydar TF, Ang E Jr., & Rakic P (2003). Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc Natl Acad Sci U S A, 100(5), 2890–2895. doi: 10.1073/pnas.0437969100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofler C, & Koelle MR (2011). AGS-3 alters Caenorhabditis elegans behavior after food deprivation via RIC-8 activation of the neural G protein G alphao. J Neurosci, 31(32), 11553–11562. doi: 10.1523/jneurosci.2072-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstadius S (1928). Uber die determination des keimes bei echinodermen. Acta Zoologica, 9, 1–191. doi: 10.1111/j.1463-6395.1928.tb01165.x [DOI] [Google Scholar]
- Izumi Y, Ohta N, Hisata K, Raabe T, & Matsuzaki F (2006). Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat Cell Biol, 8(6), 586–593. doi: 10.1038/ncb1409 [DOI] [PubMed] [Google Scholar]
- Izumi Y, Ohta N, Itoh-Furuya A, Fuse N, & Matsuzaki F (2004). Differential functions of G protein and Baz-aPKC signaling pathways in Drosophila neuroblast asymmetric division. J Cell Biol, 164(5), 729–738. doi: 10.1083/jcb.200309162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M, Li J, Zhu J, Wen W, Zhang M, & Wang W (2012). Crystal structures of the scaffolding protein LGN reveal the general mechanism by which GoLoco binding motifs inhibit the release of GDP from Galphai. J Biol Chem, 287(44), 36766–36776. doi: 10.1074/jbc.M112.391607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Hirono K, Prehoda KE, & Doe CQ (2009). Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell, 138(6), 1150–1163. doi: 10.1016/j.cell.2009.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt JA, & Brand AH (2002). Asymmetric cell division: microtubule dynamics and spindle asymmetry. J Cell Sci, 115(Pt 11), 2257–2264. [DOI] [PubMed] [Google Scholar]
- Katanaev VL, & Tomlinson A (2006). Dual roles for the trimeric G protein Go in asymmetric cell division in Drosophila. Proc Natl Acad Sci U S A, 103(17), 6524–6529. doi: 10.1073/pnas.0601853103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik R, Yu F, Chia W, Yang X, & Bahri S (2003). Subcellular localization of LGN during mitosis: evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression. Mol Biol Cell, 14(8), 3144–3155. doi: 10.1091/mbc.e03-04-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA (2008). Mechanisms of asymmetric stem cell division. Cell, 132(4), 583–597. doi: 10.1016/j.cell.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, & Matsuzaki F (2008). Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol, 10(1), 93–101. doi: 10.1038/ncb1673 [DOI] [PubMed] [Google Scholar]
- Kopein D, & Katanaev VL (2009). Drosophila GoLoco-protein Pins is a target of Galpha(o)-mediated G protein-coupled receptor signaling. Mol Biol Cell, 20(17), 3865–3877. doi: 10.1091/mbc.E09-01-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S (2019). Mechanisms of Spindle Positioning: Lessons from Worms and Mammalian Cells. Biomolecules, 9(2). doi: 10.3390/biom9020080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S, Busso C, & Gonczy P (2012). Cortical dynein is critical for proper spindle positioning in human cells. J Cell Biol, 199(1), 97–110. doi: 10.1083/jcb.201203166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan L, Pavin N, Husson J, Romet-Lemonne G, van Duijn M, Lopez MP, … Dogterom M (2012). Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell, 148(3), 502–514. doi: 10.1016/j.cell.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JR, Tugendreich S, & Hieter P (1995). Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci, 20(7), 257–259. doi: 10.1016/s0968-0004(00)89037-4 [DOI] [PubMed] [Google Scholar]
- Lechler T, & Fuchs E (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature, 437(7056), 275–280. doi: 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Weng J, Wang D, Yang M, Jia M, & Wang W (2018). A Residue outside the Binding Site Determines the Galpha Binding Specificity of GoLoco Motifs. Biochemistry, 57(47), 6562–6569. doi: 10.1021/acs.biochem.8b00848 [DOI] [PubMed] [Google Scholar]
- Lorson MA, Horvitz HR, & van den Heuvel S (2000). LIN-5 is a novel component of the spindle apparatus required for chromosome segregation and cleavage plane specification in Caenorhabditis elegans. J Cell Biol, 148(1), 73–86. doi: 10.1083/jcb.148.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Usui T, Uemura T, Jan L, & Jan YN (1999). Flamingo controls the planar polarity of sensory bristles and asymmetric division of sensory organ precursors in Drosophila. Curr Biol, 9(21), 1247–1250. doi: 10.1016/s0960-9822(99)80505-3 [DOI] [PubMed] [Google Scholar]
- Lu MS, & Johnston CA (2013). Molecular pathways regulating mitotic spindle orientation in animal cells. Development, 140(9), 1843–1856. doi: 10.1242/dev.087627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchtenborg AM, Purvanov V, Melnik BS, Becker S, & Katanaev VL (2015). Mode of interaction of the Galphao subunit of heterotrimeric G proteins with the GoLoco1 motif of Drosophila Pins is determined by guanine nucleotides. Biosci Rep, 35(6). doi: 10.1042/bsr20150201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauser JF, & Prehoda KE (2012). Inscuteable regulates the Pins-Mud spindle orientation pathway. PLoS One, 7(1), e29611. doi: 10.1371/journal.pone.0029611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden CR, Willard FS, Kimple RJ, Johnston CA, Hains MD, Jones MB, & Siderovski DP (2005). G alpha selectivity and inhibitor function of the multiple GoLoco motif protein GPSM2/LGN. Biochim Biophys Acta, 1745(2), 254–264. doi: 10.1016/j.bbamcr.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Cho G, Wen B, & Insel PA (1996). Identification and cDNA cloning of a novel human mosaic protein, LGN, based on interaction with G alpha i2. Gene, 181(1–2), 39–43. doi: 10.1016/s0378-1119(96)00456-8 [DOI] [PubMed] [Google Scholar]
- Morin X, & Bellaiche Y (2011). Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev Cell, 21(1), 102–119. doi: 10.1016/j.devcel.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Morin X, Jaouen F, & Durbec P (2007). Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat Neurosci, 10(11), 1440–1448. doi: 10.1038/nn1984 [DOI] [PubMed] [Google Scholar]
- Natochin M, Gasimov KG, & Artemyev NO (2001). Inhibition of GDP/GTP exchange on G alpha subunits by proteins containing G-protein regulatory motifs. Biochemistry, 40(17), 5322–5328. doi: 10.1021/bi015505w [DOI] [PubMed] [Google Scholar]
- Nguyen-Ngoc T, Afshar K, & Gonczy P (2007). Coupling of cortical dynein and G alpha proteins mediates spindle positioning in Caenorhabditis elegans. Nat Cell Biol, 9(11), 1294–1302. doi: 10.1038/ncb1649 [DOI] [PubMed] [Google Scholar]
- Nipper RW, Siller KH, Smith NR, Doe CQ, & Prehoda KE (2007). Galphai generates multiple Pins activation states to link cortical polarity and spindle orientation in Drosophila neuroblasts. Proc Natl Acad Sci U S A, 104(36), 14306–14311. doi: 10.1073/pnas.0701812104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, & Kriegstein AR (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci, 7(2), 136–144. doi: 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Okazaki K (1975). Spicule formation by isolated micromeres of the sea urchin embryo. Amer Zool, 15(3), 567–581. [Google Scholar]
- Okumura M, Natsume T, Kanemaki MT, & Kiyomitsu T (2018). Dynein-Dynactin-NuMA clusters generate cortical spindle-pulling forces as a multi-arm ensemble. Elife, 7. doi: 10.7554/eLife.36559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Zhu J, Shang Y, Wei Z, Jia M, Xia C, … Zhang M (2013). An autoinhibited conformation of LGN reveals a distinct interaction mode between GoLoco motifs and TPR motifs. Structure, 21(6), 1007–1017. doi: 10.1016/j.str.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Park DH, & Rose LS (2008). Dynamic localization of LIN-5 and GPR-1/2 to cortical force generation domains during spindle positioning. Dev Biol, 315(1), 42–54. doi: 10.1016/j.ydbio.2007.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier ML, Woods D, Greig S, Phan PG, Radovic A, Bryant P, & O’Kane CJ (2000). Rapsynoid/partner of inscuteable controls asymmetric division of larval neuroblasts in Drosophila. J Neurosci, 20(14), Rc84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M, & Knoblich JA (2001). DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol, 3(1), 43–49. doi: 10.1038/35050550 [DOI] [PubMed] [Google Scholar]
- Pirovano L, Culurgioni S, Carminati M, Alfieri A, Monzani S, Cecatiello V, … Mapelli M (2019). Hexameric NuMA:LGN structures promote multivalent interactions required for planar epithelial divisions. Nat Commun, 10(1), 2208. doi: 10.1038/s41467-019-09999-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon J, Fries A, Wessel GM, & Yajima M (2019). Evolutionary modification of AGS protein contributes to formation of micromeres in sea urchins. Nat Commun, 10(1), 3779. doi: 10.1038/s41467-019-11560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson ND, & Lechler T (2012). Asymmetric cell divisions in the epidermis. Int Rev Cell Mol Biol, 295, 199–232. doi: 10.1016/b978-0-12-394306-4.00012-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransick A, & Davidson EH (1993). A complete second gut induced by transplanted micromeres in the sea urchin embryo. Science, 259(5098), 1134–1138. doi: 10.1126/science.8438164 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garcia R, Chesneau L, Pastezeur S, Roul J, Tramier M, & Pecreaux J (2018). The polarity-induced force imbalance in Caenorhabditis elegans embryos is caused by asymmetric binding rates of dynein to the cortex. Mol Biol Cell, mbcE17110653. doi: 10.1091/mbc.E17-11-0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegiers F, Younger-Shepherd S, Jan LY, & Jan YN (2001). Two types of asymmetric divisions in the Drosophila sensory organ precursor cell lineage. Nat Cell Biol, 3(1), 58–67. doi: 10.1038/35050568 [DOI] [PubMed] [Google Scholar]
- Rose L, & Gonczy P (2014). Polarity establishment, asymmetric division and segregation of fate determinants in early C. elegans embryos. WormBook, 1–43. doi: 10.1895/wormbook.1.30.2 [DOI] [PubMed] [Google Scholar]
- Saadaoui M, Konno D, Loulier K, Goiame R, Jadhav V, Mapelli M, … Morin X (2017). Loss of the canonical spindle orientation function in the Pins/LGN homolog AGS3. EMBO Rep, 18(9), 1509–1520. doi: 10.15252/embr.201643048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadaoui M, Machicoane M, di Pietro F, Etoc F, Echard A, & Morin X (2014). Dlg1 controls planar spindle orientation in the neuroepithelium through direct interaction with LGN. J Cell Biol, 206(6), 707–717. doi: 10.1083/jcb.201405060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Petronczki M, Dorner D, Forte M, & Knoblich JA (2001). Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell, 107(2), 183–194. doi: 10.1016/s0092-8674(01)00521-9 [DOI] [PubMed] [Google Scholar]
- Schaefer M, Shevchenko A, & Knoblich JA (2000). A protein complex containing Inscuteable and the Galpha-binding protein Pins orients asymmetric cell divisions in Drosophila. Curr Biol, 10(7), 353–362. doi: 10.1016/s0960-9822(00)00401-2 [DOI] [PubMed] [Google Scholar]
- Schnabel R, Weigner C, Hutter H, Feichtinger R, & Schnabel H (1996). mex-1 and the general partitioning of cell fate in the early C. elegans embryo. Mech Dev, 54(2), 133–147. doi: 10.1016/0925-4773(95)00466-1 [DOI] [PubMed] [Google Scholar]
- Schober M, Schaefer M, & Knoblich JA (1999). Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature, 402(6761), 548–551. doi: 10.1038/990135 [DOI] [PubMed] [Google Scholar]
- Seirin-Lee S (2020). Asymmetric cell division from a cell to cells: Shape, length, and location of polarity domain. Dev Growth Differ. doi: 10.1111/dgd.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin L, Muroyama A, & Lechler T (2016). NuMA-microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. Elife, 5. doi: 10.7554/eLife.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist SE, & Doe CQ (2005). Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell, 123(7), 1323–1335. doi: 10.1016/j.cell.2005.09.043 [DOI] [PubMed] [Google Scholar]
- Siegrist SE, & Doe CQ (2006). Extrinsic cues orient the cell division axis in Drosophila embryonic neuroblasts. Development, 133(3), 529–536. doi: 10.1242/dev.02211 [DOI] [PubMed] [Google Scholar]
- Siller KH, Cabernard C, & Doe CQ (2006). The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat Cell Biol, 8(6), 594–600. doi: 10.1038/ncb1412 [DOI] [PubMed] [Google Scholar]
- Siller KH, & Doe CQ (2008). Lis1/dynactin regulates metaphase spindle orientation in Drosophila neuroblasts. Dev Biol, 319(1), 1–9. doi: 10.1016/j.ydbio.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller KH, & Doe CQ (2009). Spindle orientation during asymmetric cell division. Nat Cell Biol, 11(4), 365–374. doi: 10.1038/ncb0409-365 [DOI] [PubMed] [Google Scholar]
- Smith NR, & Prehoda KE (2011). Robust spindle alignment in Drosophila neuroblasts by ultrasensitive activation of pins. Mol Cell, 43(4), 540–549. doi: 10.1016/j.molcel.2011.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan DG, Fisk RM, Xu H, & van den Heuvel S (2003). A complex of LIN-5 and GPR proteins regulates G protein signaling and spindle function in C elegans. Genes Dev, 17(10), 1225–1239. doi: 10.1101/gad.1081203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, & Thomson JN (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol, 100(1), 64–119. doi: 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Hayase J, Kamakura S, Miyano K, Chishiki K, Yuzawa S, & Sumimoto H (2019). Intramolecular interaction in LGN, an adaptor protein that regulates mitotic spindle orientation. J Biol Chem, 294(51), 19655–19666. doi: 10.1074/jbc.RA119.011457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S 3rd, … Lanier SM (1999). Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem, 274(47), 33202–33205. doi: 10.1074/jbc.274.47.33202 [DOI] [PubMed] [Google Scholar]
- Thyagarajan K, Afshar K, & Gonczy P (2011). Polarity mediates asymmetric trafficking of the Gbeta heterotrimeric G-protein subunit GPB-1 in C. elegans embryos. Development, 138(13), 2773–2782. doi: 10.1242/dev.063354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou MF, Hayashi A, DeBella LR, McGrath G, & Rose LS (2002). LET-99 determines spindle position and is asymmetrically enriched in response to PAR polarity cues in C. elegans embryos. Development, 129(19), 4469–4481. [DOI] [PubMed] [Google Scholar]
- Tsou MF, Hayashi A, & Rose LS (2003a). LET-99 opposes Galpha/GPR signaling to generate asymmetry for spindle positioning in response to PAR and MES-1/SRC-1 signaling. Development, 130(23), 5717–5730. doi: 10.1242/dev.00790 [DOI] [PubMed] [Google Scholar]
- Tsou MF, Ku W, Hayashi A, & Rose LS (2003b). PAR-dependent and geometry-dependent mechanisms of spindle positioning. J Cell Biol, 160(6), 845–855. doi: 10.1083/jcb.200209079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E, & Wessel GM (2006). Activator of G-protein signaling in asymmetric cell divisions of the sea urchin embryo. Dev Growth Differ, 48(9), 549–557. doi: 10.1111/j.1440-169X.2006.00895.x [DOI] [PubMed] [Google Scholar]
- Wang H, Ng KH, Qian H, Siderovski DP, Chia W, & Yu F (2005). Ric-8 controls Drosophila neural progenitor asymmetric division by regulating heterotrimeric G proteins. Nat Cell Biol, 7(11), 1091–1098. doi: 10.1038/ncb1317 [DOI] [PubMed] [Google Scholar]
- Watts JL, Etemad-Moghadam B, Guo S, Boyd L, Draper BW, Mello CC, … Kemphues KJ (1996). par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development, 122(10), 3133–3140. [DOI] [PubMed] [Google Scholar]
- Werts AD, Roh-Johnson M, & Goldstein B (2011). Dynamic localization of C. elegans TPR-GoLoco proteins mediates mitotic spindle orientation by extrinsic signaling. Development, 138(20), 4411–4422. doi: 10.1242/dev.070979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, Kimple RJ, & Siderovski DP (2004). Return of the GDI: the GoLoco motif in cell division. Annu Rev Biochem, 73, 925–951. doi: 10.1146/annurev.biochem.73.011303.073756 [DOI] [PubMed] [Google Scholar]
- Willard FS, Zheng Z, Guo J, Digby GJ, Kimple AJ, Conley JM, … Siderovski DP (2008). A point mutation to Galphai selectively blocks GoLoco motif binding: direct evidence for Galpha.GoLoco complexes in mitotic spindle dynamics. J Biol Chem, 283(52), 36698–36710. doi: 10.1074/jbc.M804936200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, & Fuchs E (2013). Oriented divisions, fate decisions. Curr Opin Cell Biol, 25(6), 749–758. doi: 10.1016/j.ceb.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Ratliff LA, Postiglione MP, Knoblich JA, & Fuchs E (2014). Par3-mInsc and Galphai3 cooperate to promote oriented epidermal cell divisions through LGN. Nat Cell Biol, 16(8), 758–769. doi: 10.1038/ncb3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Grimm A, & Knust E (2000). Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol, 150(6), 1361–1374. doi: 10.1083/jcb.150.6.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, & Knust E (1999). Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature, 402(6761), 544–547. doi: 10.1038/990128 [DOI] [PubMed] [Google Scholar]
- Wu JC, & Rose LS (2007). PAR-3 and PAR-1 inhibit LET-99 localization to generate a cortical band important for spindle positioning in Caenorhabditis elegans embryos. Mol Biol Cell, 18(11), 4470–4482. doi: 10.1091/mbc.e07-02-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, & Wessel GM (2012). Autonomy in specification of primordial germ cells and their passive translocation in the sea urchin. Development, 139(20), 3786–3794. doi: 10.1242/dev.082230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KH, Hanada T, & Chishti AH (2007). The effector domain of human Dlg tumor suppressor acts as a switch that relieves autoinhibition of kinesin-3 motor GAKIN/KIF13B. Biochemistry, 46(35), 10039–10045. doi: 10.1021/bi701169w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura S, Ohta N, & Matsuzaki F (2012). Tre1 GPCR signaling orients stem cell divisions in the Drosophila central nervous system. Dev Cell, 22(1), 79–91. doi: 10.1016/j.devcel.2011.10.027 [DOI] [PubMed] [Google Scholar]
- Yu F, Cai Y, Kaushik R, Yang X, & Chia W (2003a). Distinct roles of Galphai and Gbeta13F subunits of the heterotrimeric G protein complex in the mediation of Drosophila neuroblast asymmetric divisions. J Cell Biol, 162(4), 623–633. doi: 10.1083/jcb.200303174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Morin X, Cai Y, Yang X, & Chia W (2000). Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell, 100(4), 399–409. doi: 10.1016/s0092-8674(00)80676-5 [DOI] [PubMed] [Google Scholar]
- Yu F, Morin X, Kaushik R, Bahri S, Yang X, & Chia W (2003b). A mouse homologue of Drosophila pins can asymmetrically localize and substitute for pins function in Drosophila neuroblasts. J Cell Sci, 116(Pt 5), 887–896. doi: 10.1242/jcs.00297 [DOI] [PubMed] [Google Scholar]
- Yu F, Ong CT, Chia W, & Yang X (2002). Membrane targeting and asymmetric localization of Drosophila partner of inscuteable are discrete steps controlled by distinct regions of the protein. Mol Cell Biol, 22(12), 4230–4240. doi: 10.1128/mcb.22.12.4230-4240.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Wang H, Qian H, Kaushik R, Bownes M, Yang X, & Chia W (2005). Locomotion defects, together with Pins, regulates heterotrimeric G-protein signaling during Drosophila neuroblast asymmetric divisions. Genes Dev, 19(11), 1341–1353. doi: 10.1101/gad.1295505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzawa S, Kamakura S, Iwakiri Y, Hayase J, & Sumimoto H (2011). Structural basis for interaction between the conserved cell polarity proteins Inscuteable and Leu-Gly-Asn repeat-enriched protein (LGN). Proc Natl Acad Sci U S A, 108(48), 19210–19215. doi: 10.1073/pnas.1110951108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeytuni N, & Zarivach R (2012). Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure, 20(3), 397–405. doi: 10.1016/j.str.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Zhu J, Wen W, Zheng Z, Shang Y, Wei Z, Xiao Z, … Zhang M (2011). LGN/mInsc and LGN/NuMA complex structures suggest distinct functions in asymmetric cell division for the Par3/mInsc/LGN and Galphai/LGN/NuMA pathways. Mol Cell, 43(3), 418–431. doi: 10.1016/j.molcel.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal RR, Ahringer J, van Luenen HG, Rushforth A, Anderson P, & Plasterk RH (1996). G proteins are required for spatial orientation of early cell cleavages in C. elegans embryos. Cell, 86(4), 619–629. doi: 10.1016/s0092-8674(00)80135-x [DOI] [PubMed] [Google Scholar]


