Summary
Despite high-resolution crystal structures of both inactive and active G protein-coupled receptors (GPCRs), it is still not known how ligands trigger the large structural change on the intracellular side of the receptor since the conformational changes that occur within the extracellular ligand-binding region upon activation are subtle. Here, we use solid-state NMR and FTIR spectroscopy on rhodopsin to show that Trp2656.48 within the CWxP motif on transmembrane helix H6 constrains a proline hinge in the inactive state, suggesting that activation results in unraveling of the H6 backbone within this motif, a local change in dynamics that allows helix H6 to swing outward. Notably, Tyr3017.48 within activation switch 2 appears to mimic the negative allosteric sodium ion found in other family A GPCRs, a finding that is broadly relevant to the mechanism of receptor activation.
Keywords: GPCR, G protein-coupled receptor, rhodopsin, activation, retinal, A295V, congenital stationary night blindness, tryptophan, NMR, FTIR
Graphical Abstract
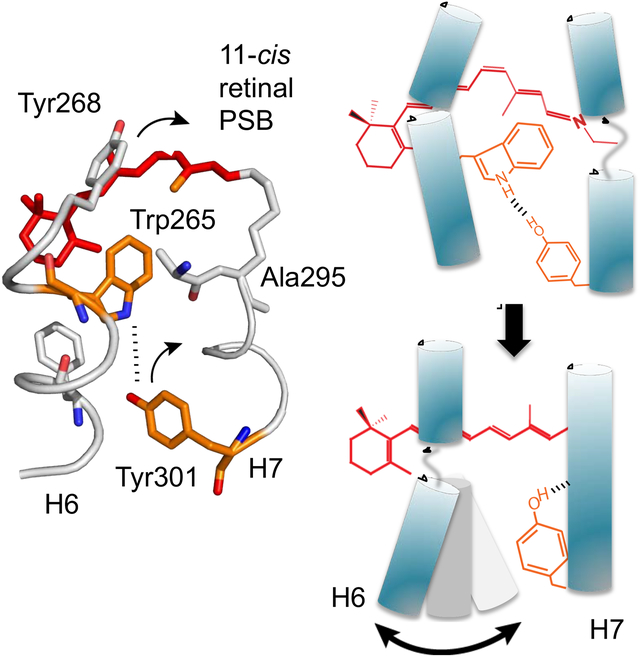
eTOC blurb:
The hallmark of GPCR activation is a large outward motion of helix H6. NMR spectroscopy was used to determine how interactions in the prototypical GPCR rhodopsin regulate the dynamics of the conserved proline kink within this helix. These findings establish the mechanism for coupling of retinal isomerization to receptor activation.
Introduction
G protein-coupled receptors (GPCRs), the largest family of cell-surface receptors, typically mediate signal transduction in response to diverse diffusible ligands (Weis and Kobilka, 2018). Rhodopsin, the light-sensing GPCR responsible for dim light vision, has evolved tightly regulated properties that prime the receptor for this unique physiological role (Smith, 2010). Unlike other family A GPCRs, rhodopsin does not recognize a diffusible ligand. Rather, the receptor is designed to detect low levels of light via a covalently bound retinal chromophore, which forms a protonated Schiff’s base (PSB) with Lys2967.43 (superscript numbers correspond to the general Ballesteros-Weinstein numbering scheme for amino acids in family A GPCRs (Ballesteros and Weinstein, 1995)). In the dark, the receptor is stabilized in an inactive conformation by the 11-cis configuration and positively charged PSB of its retinal chromophore. Upon light exposure, the 11-cis retinal isomerizes to the all-trans configuration and the PSB linkage deprotonates, converting the retinal from an inverse agonist to a full agonist. Since a single active rhodopsin molecule in rod cells can trigger a neuronal response (Hecht et al., 1942; Pugh, 2018; Rieke and Baylor, 1998), it is crucial that the entire rhodopsin population is maintained in an inactive conformation in the absence of light. Dysregulated rhodopsin activation, even at low levels, is linked to diseases, such as congenital stationary night blindness (CSNB) and autosomal dominant retinitis pigmentosa (ADRP) (Park, 2014). Conversely, many ligand-activated receptors operate with measurable levels of basal activity (Deupi and Kobilka, 2007; Seifert and Wenzel-Seifert, 2002).
Despite these differences, the high level of sequence conservation within the transmembrane (TM) helices of rhodopsin and other family A GPCRs argues that there is a common pathway for signal transduction across the TM domain of these receptors. The outcome is an outward rotation of the cytoplasmic end of TM helix H6, the structural hallmark of activation that produces the G protein binding site (Farrens et al., 1996). Of fundamental importance for this process is the conserved TM core, which spans ~2 helical turns in the middle of the TM domain (Smith, 2010). We have previously deconstructed this region into two packing clusters centered on TM helices H1, H2, H3 and H4, which are responsible for folding and structural rigidity of the TM core, and two activation switches centered on TM helices H5, H6 and H7, which are responsible for the dynamic and functional components of the core (Figure 1, Figure S1) (Sanchez-Reyes et al., 2017).
Figure 1. Activation Switches 1 and 2 in the TM core of rhodopsin. See also Figure S1.
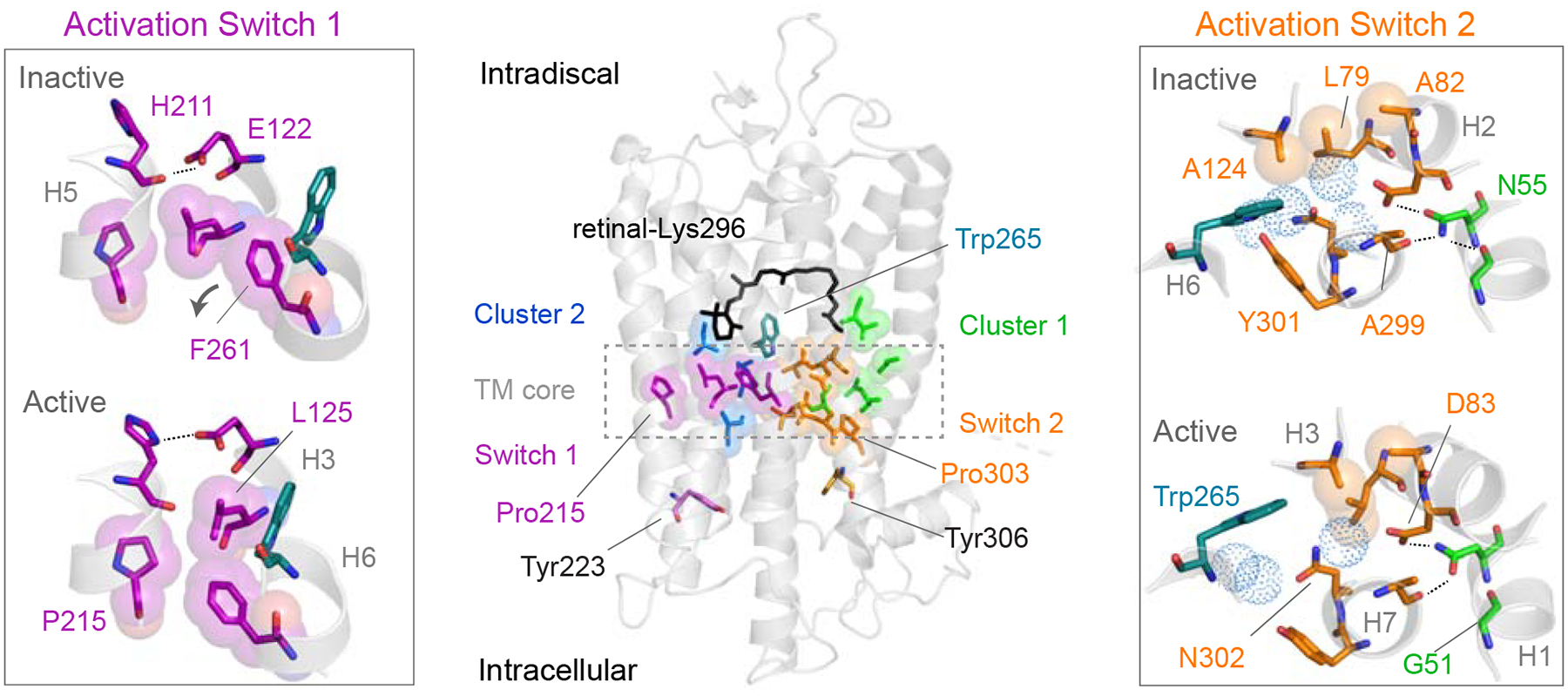
Organization of the conserved TM core of rhodopsin shows packing cluster 1 (green) and packing cluster 2 (blue) centered on helices H1 – H4 and Switch 1 (purple) and Switch 2 (orange) centered on helices H5 – H7 (Sanchez-Reyes et al., 2017). Comparison of the inactive (PDB: 1U19) and active (PDB: 3PQR) rhodopsin crystal structures reveals the details of rearrangements in the switches to the left and right of the full receptor structure (Choe et al., 2011; Okada et al., 2004). The largest change in Switch 1 involves motion of the Phe2616.44 side chain as it repacks between Leu1253.40 and Pro2155.50 when TM helix H6 rotates outward. The largest change in Switch 2 is rotation of Tyr3017.48. Models were made using PyMOL 1.8.07 and the figure was assembled using Adobe Illustrator 15.0.0.
A key conceptual element in defining the two activation switches is that they each contain a highly conserved proline. In fact, the most conserved amino acids on helices H5, H6 and H7 are prolines. Although prolines are generally associated with helix dynamics and flexibility, a defining feature of prolines is that they generate a free backbone carbonyl group on the amino acid one helical turn away. In rhodopsin, the free carbonyl groups at His2115.46 and Ala2997.46, which are associated with Pro2155.50 and Pro3037.50, respectively, behave as discrete hydrogen-bonding switches (Kimata et al., 2016b). In contrast, the free carbonyl associated with Pro2676.50 on helix H6 is oriented toward the surrounding lipid and not hydrogen-bonded, suggesting that this proline allows flexibility for the outward rotation of H6 rather than contributing directly to the activation switches. Here, we address the mechanism by which this putative flexible hinge is constrained in the dark and how these constraints are released upon photoactivation of rhodopsin.
Along with the conserved prolines, each activation switch contains conserved packing and hydrogen-bonding interactions. The first activation switch (Switch 1) includes the P5.50-I3.40-F6.44 motif, a group of hydrophobic residues on helices H3, H5 and H6 initially observed to rearrange in the β2-adrenergic receptor crystal structures (Rasmussen et al., 2011). In rhodopsin, receptor activation leads to a rearrangement of these positions as H6 rotates outward (Figure 1, Figure S1). Additionally, there are changes in rhodopsin-specific hydrogen-bonding interactions between His2115.46, Glu1223.37 and Trp1263.41 (Figure 1, Figure S1). We consider this hydrogen-bonding network to be a key part of Switch 1, where the amino acids comprising this network are specific to each subfamily within the family A GPCRs.
In this paper, we emphasize our findings on the second switch (Switch 2) and its role in controlling the H6 proline hinge. The hydrogen-bonding network that comprises Switch 2 links helices H1, H2, H6 and H7. In contrast to Switch 1, the hydrogen-bonding network within Switch 2 is highly conserved across GPCR subfamilies. At one end of this network, the indole NH of Trp2656.48 forms a water-mediated hydrogen-bond with Tyr3017.48, which in turn interacts with Asn3027.49 via structural water. Trp2656.48 undergoes a change in orientation upon activation (Chabre and Breton, 1979; Lin and Sakmar, 1996) and interaction with these groups is weakened (Patel et al., 2005). At the other end of this network, the free backbone C=O of Ala2997.46 hydrogen-bonds with Asn551.50, the most conserved residue across all family A GPCRs. Receptor activation triggers a loss of the hydrogen-bonding between Ala2997.46 and Asn551.50 (Kimata et al., 2016b) and a corresponding strengthening of hydrogen-bonds involving the Asp832.50 side chain (Fahmy et al., 1993). However, the mechanism by which these changes couple retinal isomerization to helix H6 motion is not known.
In many family A GPCRs, the hydrogen-bonding network comprising Switch 2 accommodates a Na+ ion, which acts as a negative allosteric modulator (NAM) (Katritch et al., 2014). The allosteric Na+ ion is coordinated by Asp2.50, Ser3.39, water and additional polar residues to stabilize the inactive receptor structure. Agonist binding leads to release of the sodium and conversion to an active receptor conformation. Mutation of the conserved Asp2.50 results in loss of G protein signaling (Katritch et al., 2014). However, the mechanism by which agonist binding and loss of Na+ coordination is associated with the conversion to an active receptor conformation is still not well understood. While rhodopsin and the other visual receptors do not accommodate a Na+ ion in this region, most Switch 2 residues remain conserved within the visual receptor subfamily, reflecting a well-preserved function across the family A GPCRs that is explored by these studies.
Results
Tyr3017.48 Hydrogen-bonding Changes Upon Rhodopsin Activation
One of the advantages of the visual receptors for structure-function studies is that light rapidly converts the covalently bound retinal PSB chromophore from an inverse agonist to a full agonist. After light-induced isomerization of the retinal PSB from the 11-cis to all-trans configuration, the receptor passes through a series of intermediates to form Metarhodopsin I (Meta I). Deprotonation of the PSB in Meta I generates the active Metarhodopsin II (Meta II) intermediate. Below we compare structural changes in dark, inactive rhodopsin with the light-activated Meta II state or with rhodopsin containing specific mutations that exhibit either constitutive activity (without retinal bound) or dark activity (with 11-cis retinal bound).
In crystal structures of rhodopsin and Meta II (Choe et al., 2011; Li et al., 2004; Okada et al., 2004), rotation of Tyr3017.48 represents the largest structural change in Switch 2 associated with activation. This tyrosine is highly conserved (80% identity) in the visual receptors (Isberg et al., 2014) and is located in a key position near Trp2656.48, Ser2987.45, Cys2646.47 and Asn3027.49 (Figure 2). The hydroxyl group of Tyr3017.48 forms water-mediated hydrogen-bonds with Trp2656.48 and Asn3027.49. Asn3027.49 is part of the conserved NPxxY sequence on H7, which connects Switch 2 to the intracellular side of the receptor. The large change in position of the tyrosine ring has the potential to disrupt these hydrogen-bonds and form new packing or hydrogen-bonding interactions with Ser2987.45 and Cys2646.47. Ser2987.45 is within a section of 310 helix in H7 whose conformation changes upon activation (Fritze et al., 2003; Ren et al., 2016), while Cys2646.47 is thought to mediate H6-H7 interactions (Olivella et al., 2013) (Figure S1). As a result, the role of Tyr3017.48 may be to modulate the conformation of H7 and/or the H6-H7 interface via hydrogen-bonding interactions.
Figure 2. Hydrogen bonding changes of Tyr3017.48 upon rhodopsin activation. See also Figure S2.
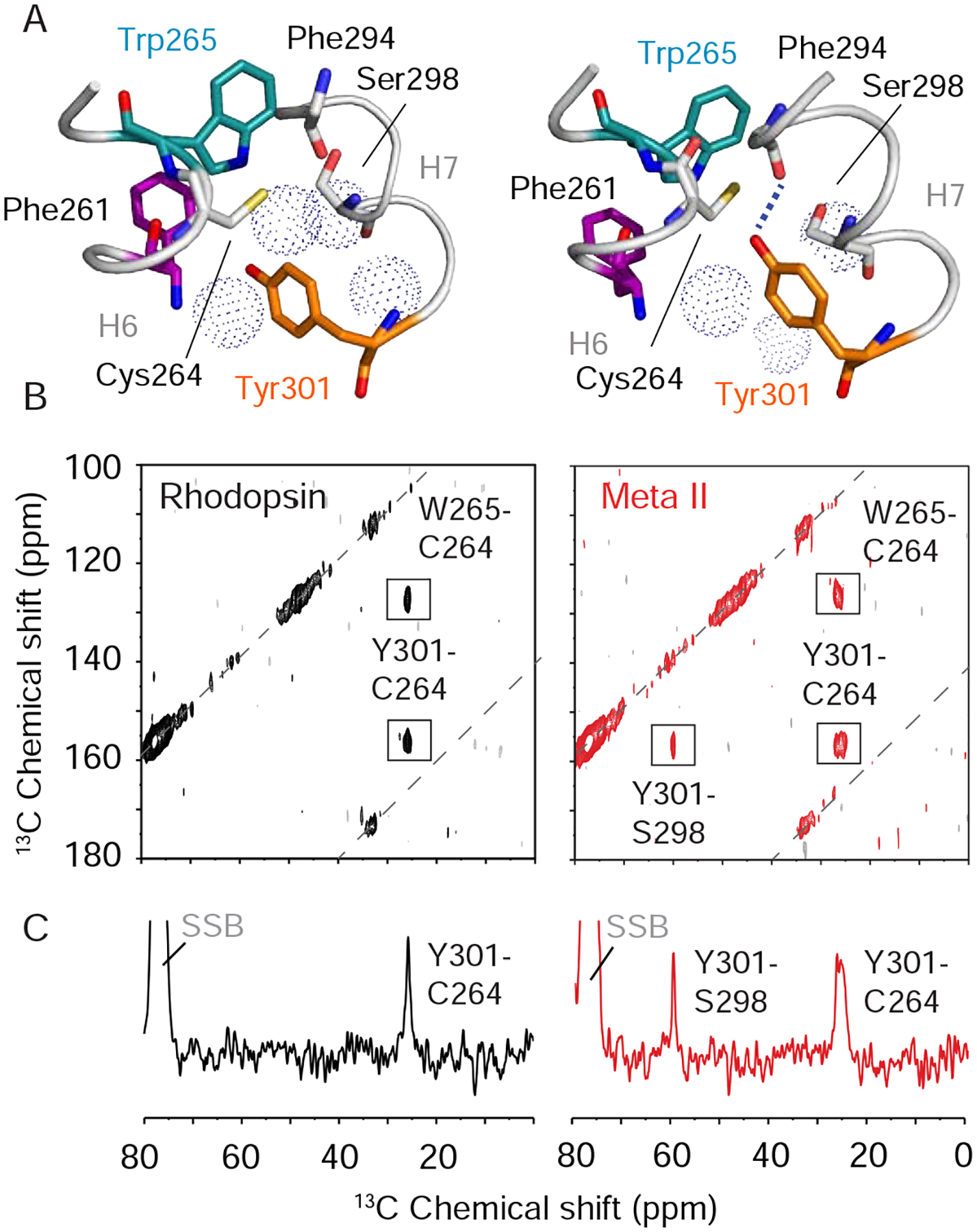
(A) Comparison of the position of Tyr3017.48 between the inactive-state (left, PDB: 1U19) and the active-state (right, PDB: 3PQR) crystal structures of rhodopsin (Choe et al., 2011; Li et al., 2004; Okada et al., 2004). Tyr3017.48 forms a water-mediated hydrogen bond with the Trp2656.48 indole NH in rhodopsin and a direct hydrogen bond with the backbone C=O of Phe2947.41 in Meta II. Rotation of the Tyr3017.48 side chain upon activation influences hydrogen-bonding interactions of the tyrosine Cζ-OH group, interactions across the H6-H7 interface and the local conformation of H7. (B) Regions of the 2D 13C NMR spectra exhibiting cross peaks for the following residue contacts in rhodopsin (black) and Meta II (red): 13Cζ-Tyr301 -13Cβ-Cys264, 13Cζ-Tyr301 – 13Cβ-Ser298 and 13Cδ1-Trp265 – 13Cβ-Cys264. (C) Rows through the 13Cζtyrosine diagonal resonance corresponding to the 13Cζ-Tyr301 – 13Cβ-Cys264 crosspeak and 13Cζ-Tyr301 – 13Cβ-Ser298 crosspeak. SSB = spinning side bands. Models were made using PyMOL 1.8.07 and the figure was assembled using Adobe Illustrator 15.0.0.
To assess the changes in hydrogen-bonding, packing and dynamics of the Tyr3017.48 side chain, two-dimensional (2D) 13C NMR spectra using dipolar assisted rotational resonance (DARR) were obtained of rhodopsin isotopically enriched with 13Cζ-tyrosine, 13Cβ-cysteine, 13Cβ-serine and 13Cδ1-tryptophan before and after light-induced conversion to the active Meta II species (Figure 2, Figure S2). The 13C labels provide site-specific probes within the protein. The 2D NMR spectra exhibit off-diagonal crosspeaks between 13C labels that are ~6 Å or less apart from each other (Crocker et al., 2004; Takegoshi et al., 2001). When the 13C-labels are in regions of the protein with defined conformations, the linewidths of individual resonances are typically between 100–150 Hz. Importantly, solid-state NMR measurements were acquired at low temperature (190 – 220 K) where dynamic processes are slowed or halted, resulting in conformational heterogeneity. For both rhodopsin and Meta II, the receptor at room temperature was rapidly cooled to low temperature where the NMR measurements were made. In regions of the protein exhibiting conformational dynamics at high temperature (e.g. room temperature), the rapid cooling will result in broadening of the NMR linewidth reflecting a distribution of chemical shifts and protein conformations.
Inspection of the rhodopsin and Meta II crystal structures reveals that Tyr3017.48 rotation brings the Cζ atom of the ring closer to the Ser2987.45 Cβ carbon in the active state (Choe et al., 2011; Li et al., 2004; Okada et al., 2004). An intense crosspeak between 13Cζ-Tyr3017.48 and 13Cβ-Ser2987.45 emerges in the 2D NMR spectrum of Meta II (Figure 2B, right panel) consistent with the large change in position of Tyr3017.48. The narrow linewidth of this crosspeak implies that the Tyr3017.48 – Ser2987.45 contact is well-defined in Meta II.
In the Meta II crystal structure (Choe et al., 2011), the Tyr3017.48 Cζ-OH hydrogen-bonds directly with the backbone C=O of Phe2947.41 across roughly two helical turns in H7. The 13Cζ-OH chemical shift of Tyr3017.48 moves downfield to 156.8 ppm indicating a stronger hydrogen-bonding interaction than in rhodopsin (Herzfeld et al., 1990). In addition, the strong crosspeak between Tyr3017.48 and 13Cβ-Ser2987.45 in Meta II is consistent with helix H7 becoming more helical in the active conformation (Figure S2). These observations suggest that the hydrogen-bonding contacts in Switch 2, which stabilize the distorted 310 helix in rhodopsin, change in Meta II to allow helix H7 to adopt a more canonical α-helix, explaining why mutations that favor a non-distorted helical conformation, such as P303A7.50, lead to hyperactivity (Fritze et al., 2003). Straightening of helix H7 likely promotes rotation of the Tyr3067.53 in the NPxxY motif into the helical bundle to stabilize the active conformation of H6 (Figure S1).
The crystal structures of rhodopsin and Meta II also reveal that the Tyr3017.48-13Cζ carbon comes closer to the Cys2646.47-13Cβ carbon within the CWxP motif of H6 (Choe et al., 2011; Li et al., 2004; Okada et al., 2004). We observe a corresponding crosspeak in both rhodopsin and Meta II for this contact. However, the crosspeak of Cys2646.47-13Cβ to Tyr3017.48-13Cζ in Meta II is considerably broader, which is indicative of heterogeneity in the CWxP motif at low temperature due to increased dynamics in Meta II in this region at high temperature. Dynamics at Cys6.47 has been implicated in promoting helix H6 flexibility in constitutively active mutants of the β2 adrenergic receptor (Ballesteros et al., 2001; Shi et al., 2002).
Constraints on Trp2656.48 Weaken Upon Rhodopsin Activation
The observed linewidths of the 13Cβ resonances of Cys2646.47 and Ser2987.45 suggest that rotation of Tyr3017.48 to form an intra-helical hydrogen-bond with the Phe2947.41 C=O leads to a more dynamic H6 conformation and a more defined H7 helical conformation. The labeling strategy described in Figure 2 also allows us to monitor the packing and hydrogen-bonding interactions of Trp2656.48, which is in a central location at the intracellular side of the retinal binding site and bridges the two switch regions of the TM core. Crystal structures of rhodopsin show that the Trp2656.48 indole ring is located within a tightly packed environment. We targeted the close packing interaction between Cys2646.47 and Trp2656.48 to address the position and conformational dynamics of Trp2656.48. The 13Cδ1-Trp and 13Cβ-Cys resonances are unambiguously assigned in Figure 2B to Trp2656.48 and Cys2646.47 since this is the only close - 13Cδ1-Trp – 13Cβ-Cys- pair within the receptor able to generate a crosspeak in the 2D NMR spectrum. In rhodopsin, this crosspeak is narrow, consistent with a well-defined, restricted conformation of the CWxP motif that spans the H6 kink (Figure 3SA, black). Following conversion to Meta II, we observe a broadening of the crosspeak (Figure 3SA, red), which we attribute to increased dynamics that arise from overall weakening of the packing constraints on Trp2656.48. Moreover, the 13Cδ1 carbon is adjacent to the indole NH nitrogen and its chemical shift is sensitive to NH hydrogen-bonding similar to the 13Cζ-OH of tyrosine. The change in chemical shift of the 13Cδ1 resonance from 128 ppm to 126 ppm is consistent with weakening of the Trp2656.48 indole NH hydrogen-bond.
Figure 3. Activation weakens constraints on Trp2656.48. See also Figure S3.
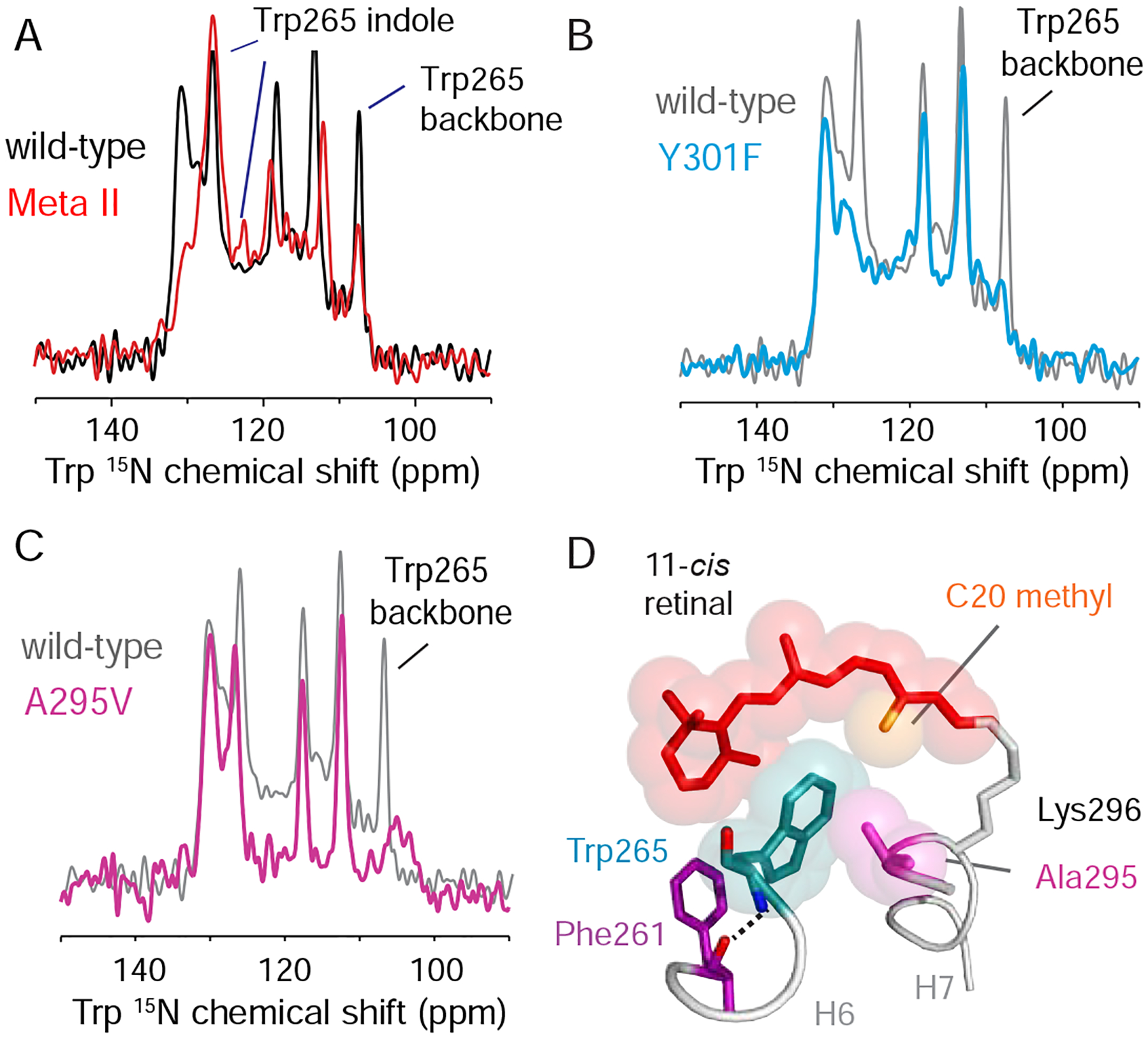
(A) One dimensional 15N NMR spectra of dark-state rhodopsin (black) and Meta II (red) containing 15N-labeled tryptophan. Resonances assigned to the Trp2656.48 indole 15N atom (127 ppm) and the Trp2656.48 backbone 15N atom (108 ppm) are indicated. Both the side chain and backbone 15NH resonances of Trp2656.48 lose intensity upon activation. (B) Dark-state 15N-tryptophan 1D spectra of wild-type rhodopsin (grey) and the Y301F7.48 mutant (cyan). A striking loss of intensity is observed in both the side chain and backbone 15NH resonances of Trp2656.48. (C) Dark-state 15N-tryptophan 1D spectra of wild-type rhodopsin (grey) and the A295V7.42 mutant (magenta). Mutation leads to loss of intensity of backbone NH resonance of Trp2656.48. (D) Packing interactions between Trp2656.48, Ala2957.42 and 11-cis retinal in the rhodopsin crystal structure (PDB: 1U19) (Okada et al., 2004). The aromatic ring of Trp2656.48 is in van der Waals contact with the C20 methyl group of the 11-cis retinal chromophore and the side chain methyl group of Ala2957.42. Models were made using PyMOL 1.8.07 and the figure was assembled using Adobe Illustrator 15.0.0.
Release of constraints on Trp2656.48 may allow it to function as a lever that triggers changes in Phe2616.44 of Switch 1. A concerted change of the side chain rotamer angles of Cys6.47, Trp6.48 and Phe6.52 upon activation was originally thought to allow the proline hinge to toggle between two conformations (Shi et al., 2002). However, comparison of the crystal structures has generally not supported this proposed rotamer toggle switch. To assess if Trp2656.48 rotates around the Cα-Cβ bond, we monitored the crosspeaks that emerge between the directly bonded 13Cα and 13Cβ atoms of Trp2656.48 in rhodopsin. The 13Cα – 13Cβ chemical shifts are sensitive to the backbone and sidechain dihedral angles (Wishart, 2011). We found that the Trp2656.48 crosspeaks have unique chemical shifts in both rhodopsin and Meta II (Figure S3C). These crosspeaks were assigned to Trp2656.48 on the basis of their absence in the W265F6.48 mutant (Figure S3D). The 13Cα chemical shift moves upfield (56 to 54 ppm) and the 13Cβ chemical shift moves slightly downfield (29 to 30 ppm) in Meta II. The change in the Trp2656.48 13Cα and 13Cβ resonances between rhodopsin and Meta II implies rotation of the Trp2656.48 side chain. Such a rotation (in conjunction with the change in position of Tyr3017.48) would be consistent with disruption of the water-mediated hydrogen-bonding interactions with Asn3027.49.
To monitor hydrogen-bonding changes at the Trp2656.48 position that accompany the spatial rearrangements presented in Figures S3A and S3B, we collected one-dimensional (1D) 15N NMR spectra on rhodopsin before and after activation (Figure 3A), which are in good agreement with those published previously in the literature (Patel et al., 2005). The rhodopsin spectrum exhibits narrow resonances (~1 ppm, 125 Hz) for both the backbone and indole 15N atoms of Trp2656.48. The resonances assigned to Trp2656.48 remain sharp following conversion to Meta II. In addition, we observe a decrease in the indole 15N chemical shift of Trp2656.48 following illumination, which was previously attributed to a weakening of the indole NH hydrogen-bond (Patel et al., 2005). Importantly, the intensities of both the Trp2656.48 indole and backbone 15NH resonances decrease markedly upon activation. The loss of intensity suggests that there is a component of the receptor exhibiting substantial dynamics at the Trp2656.48 side chain and backbone in Meta II, which give rise to conformational heterogeneity in the low temperature solid-state NMR experiments.
The Y301F7.48 Mutation Leads to Increased Dynamics of the H6 Kink in the Dark
To address if Tyr3017.48 regulates Switch 2, we measured the effects of the Y301F7.48 mutation on Trp2656.48 hydrogen-bonding using solid-state NMR and tested whether this mutation alters the inactive state (Meta I) – active state (Meta II) equilibrium using FTIR spectroscopy.
The 15N-tryptophan resonances provide a good probe of Trp2656.48 hydrogen-bonding interactions. Figure 3B presents the 15N-tryptophan spectrum of the Y301F7.48 mutant in rhodopsin overlaid with the spectrum of the wild-type receptor. Strikingly, both the Trp2656.48 indole nitrogen (127 ppm) and backbone nitrogen (108 ppm) resonances, which are intense in wild-type rhodopsin, are absent in the Y301F mutant. These data are indicative of substantial dynamics emerging at Trp2656.48 in the dark, inactive state demonstrating that Trp2656.48 interactions with Switch 2 are an important constraint on the H6 proline hinge.
To assess the overall role of Tyr3017.48 in mediating the conversion to an active receptor, we measured the equilibrium between the inactive Meta I and active Meta II states using FTIR spectroscopy. Illumination of rhodopsin in 1-palmitoyl-2-oleolyl-sn-glycero-3-phosphocholine (POPC) lipids rather than detergent results in a photoproduct equilibrium between Meta I and Meta II (Mahalingam et al., 2008). The carboxylic acid side chain vibrations of Asp832.50 and Glu1223.37 provide probes for measuring the relative amounts of each state (Figure 4A, Figure S4) (Mahalingam et al., 2008). Asp832.50 is a central conserved residue in Switch 2, while Glu1223.37 is a rhodopsin-specific residue in Switch 1 (Figure S1). In wild-type rhodopsin, a two-state equilibrium between Meta I and Meta II occurs for each of these FTIR probes that is sensitive to pH. Comparison of the equilibrium for Y301F7.48 versus the wild-type receptor reveals a shift toward Meta II (Figure 4B). This observation indicates that the hydroxyl group of Tyr3017.48 contributes to the stability of the inactive conformation.
Figure 4. Tyr3017.48 mimics the function of allosteric sodium found in other family A GPCRs. See also Figure S4.
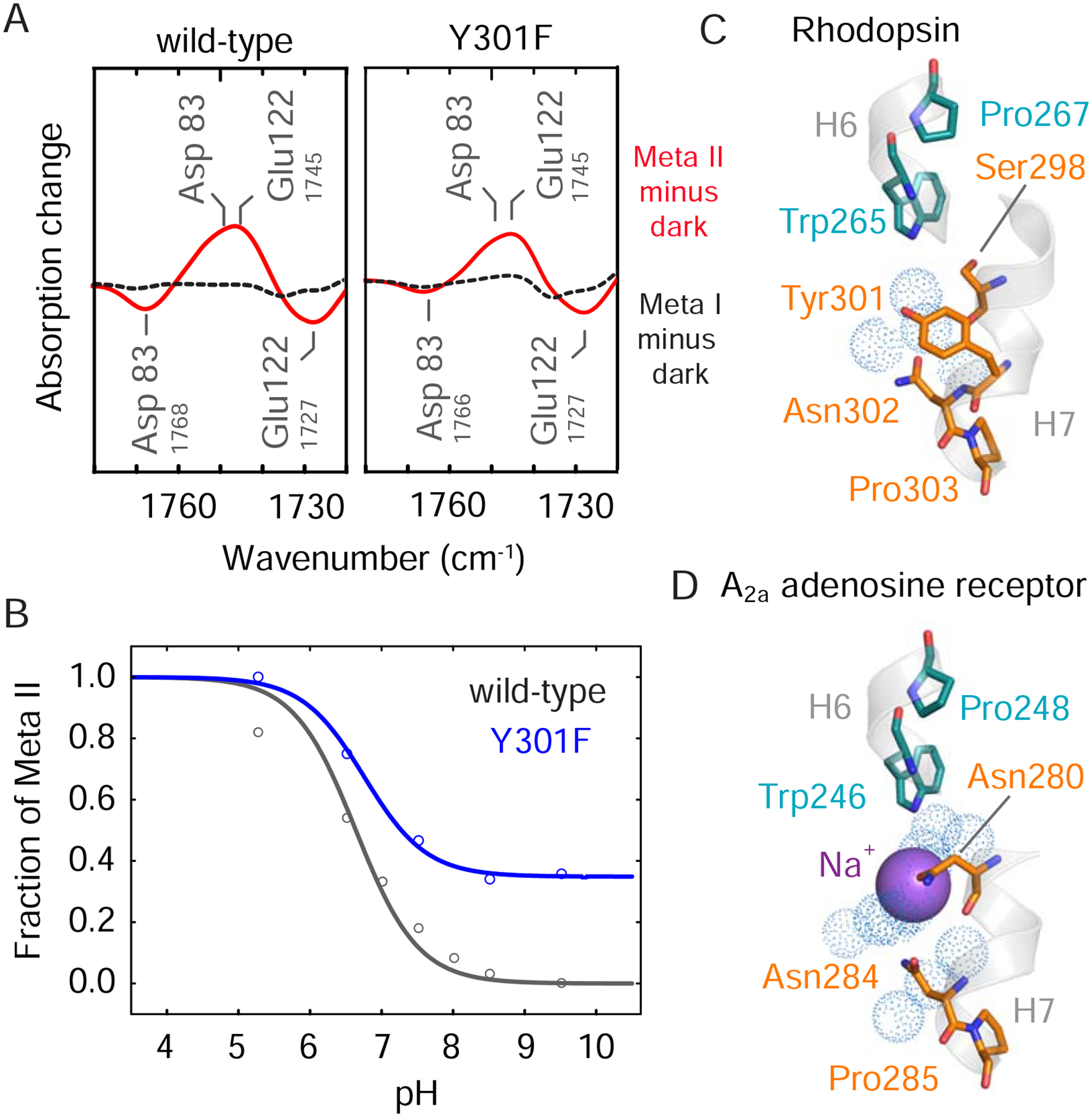
(A) FTIR difference spectra in the region of the COOH vibrations for the wild-type (left) and Y301F7.48 (right) receptors. Dark minus Meta I is represented as a black dotted curve and dark minus Meta II is represented as a solid red curve. (B) FTIR titration plot showing the fraction of Meta II as a function of pH in the wild-type receptor and the Y301F7.48 receptor. See also Figure S4. Structures of (C) rhodopsin (PDB: 1U19) and (D) the A2a adenosine receptor (PDB: 4EIY) showing Tyr3017.48 and the sodium ion, respectively, in the region of Switch 2 (Liu et al., 2012; Okada et al., 2004). Models were made using PyMOL 1.8.07 and the figure was assembled using Adobe Illustrator 15.0.0.
Tyr3017.48 appears to mimic the function of sodium present in ligand-activated receptors such as the A2a adenosine receptor (Liu et al., 2012) (Figures 4C,D). In this receptor, a water molecule bridges the Trp6.48 indole NH to a Na+ ion, which is coordinated by Asp2.50. Recent NMR data collected on the A2a adenosine receptor show that the Trp2466.48 indole 15N chemical shift is sensitive to the D52N2.50 mutation indicating that these residues are structurally coupled (Eddy et al., 2018). Our results support this conserved connection between Trp6.48 and Asp2.50. In fact, the effects of the Y301F7.48 mutation in rhodopsin extend to the Asp832.50 position at the center of Switch 2, where some of the changes that normally occur during activation are already observed in the dark state of the mutant (Figure 4A).
The CSNB Mutant A295V7.42 Leads to Increased Dynamics of the H6 Hinge in the Dark.
Congenital stationary night blindness (CSNB) is a rare, non-progressive retinopathy leading to deficient night vision. To date, four CSNB rhodopsin mutations have been identified (McAlear et al., 2010). Three of the CSNB mutants appear to directly destabilize the retinal PSB electrostatic environment and two have been successfully crystallized (G90D2.57 and T94I2.61) in active-like conformations (Singhal et al., 2016; Singhal et al., 2013). However, there have been challenges in capturing these mutants in an inactive conformation. Moreover, the mechanism responsible for CSNB remains controversial (see Figure S5).
The fourth and most recently discovered CSNB mutation (A295V7.42) is at position 7.42, which is conserved in many family A GPCRs as an amino acid with a small side chain. Although crystal structures of the A295V7.42 mutant have not been reported for either the active or inactive states of the receptor, biophysical studies have shown that this mutant has increased constitutive activity as the apo-receptor, opsin (Zeitz et al., 2008), with no detectable dark activity when bound to the inverse agonist, 11-cis retinal (Zeitz et al., 2008).
Of importance for our studies is that the side chain of Ala2957.42 packs against the indole ring of Trp2656.48 in rhodopsin. In the active state crystal structures of rhodopsin, Ala2957.42 remains in van der Waals contact with Trp2656.48. On the basis of the data described above, we tested whether the A295V7.42 mutation leads to a similar change in the dynamics of the H6 hinge as monitored by the 15N chemical shifts of Trp2656.48. The 15N-Trp NMR spectrum collected on the A295V7.42 rhodopsin mutant reveals substantial broadening of the backbone NH resonance of Trp2656.48 as observed in the Y301F7.48 mutant (Figure 3C). Thus, the presence of valine at position 2957.42 appears to destabilize the inactive conformation of the Pro2676.50 hinge by altering the packing environment of Trp2656.48. If the other CSNB mutants behave like A295V7.42, then the more dynamic nature of the H6 backbone provides an explanation for the inability to crystallize the inactive dark state of these receptors. Nevertheless, the indole 15N resonance of Trp2656.48 in the A295V7.42 mutant appears to retain intensity (Figure 3C). This observation suggests that Switch 2 has not converted to an active conformation, which would be in agreement with either the absence or low levels of dark activity (i.e. with the 11-cis retinal PSB bound to the receptor).
Discussion
The signature of an active GPCR is the large outward rotation of the cytoplasmic end of TM helix H6 (Farrens et al., 1996), which contrasts with the more subtle structural changes on the extracellular side of the receptor. A key element in coupling retinal isomerization (or ligand binding) to the motion of H6 is that the most conserved residues on TM helices H5, H6 and H7 are prolines. We have recently proposed that these prolines have unique roles in the activation of rhodopsin (Kimata et al., 2016b). The free carbonyl associated with Pro6.50 on H6 is oriented away from the TM core and does not have a hydrogen-bonding partner in the dark, inactive state of rhodopsin, suggesting that this proline is designed to function as a flexible hinge. Here, we show that this proline is indeed a hinge, and that Trp2656.48 and surrounding residues in the retinal binding pocket and Switch 2 lock the hinge in a rigid conformation in the inactive receptor.
The first step in the cascade of structural changes leading to rhodopsin activation is retinal isomerization. The Trp2656.48 side chain is located within the arc created by 11-cis retinal in the dark, which is thought to constrain motion of the indole ring and impart low basal activity (Figure 5) (Crocker et al., 2006). The dominant motion in the retinal chromophore upon 11-cis to 11-trans isomerization involves the C20 methyl group, which packs against Trp2656.48 in rhodopsin and rotates toward the extracellular surface in Meta II (Kimata et al., 2016a). The bulky β-ionone ring at one end of the retinal is sterically occluded and does not rotate upon isomerization, whereas the PSB end of the retinal is primed for rotation. The importance of the C20 methyl group is highlighted by modified retinal derivatives that have long been used as tools for interrogating how different components of the chromophore influence rhodopsin stability and activity. Early experiments showed that rhodopsin formed with retinal lacking the C20 methyl group exhibits increased dark activity (Ebrey et al., 1980). Consequently, disruption of the steric interaction between Trp2656.48 and the retinal C20 methyl group via retinal isomerization is likely the first constraint released on the pathway to rhodopsin activation.
Figure 5. Trp2656.48 is part of a string of closely packed aromatic amino acids. See also Figure S5.
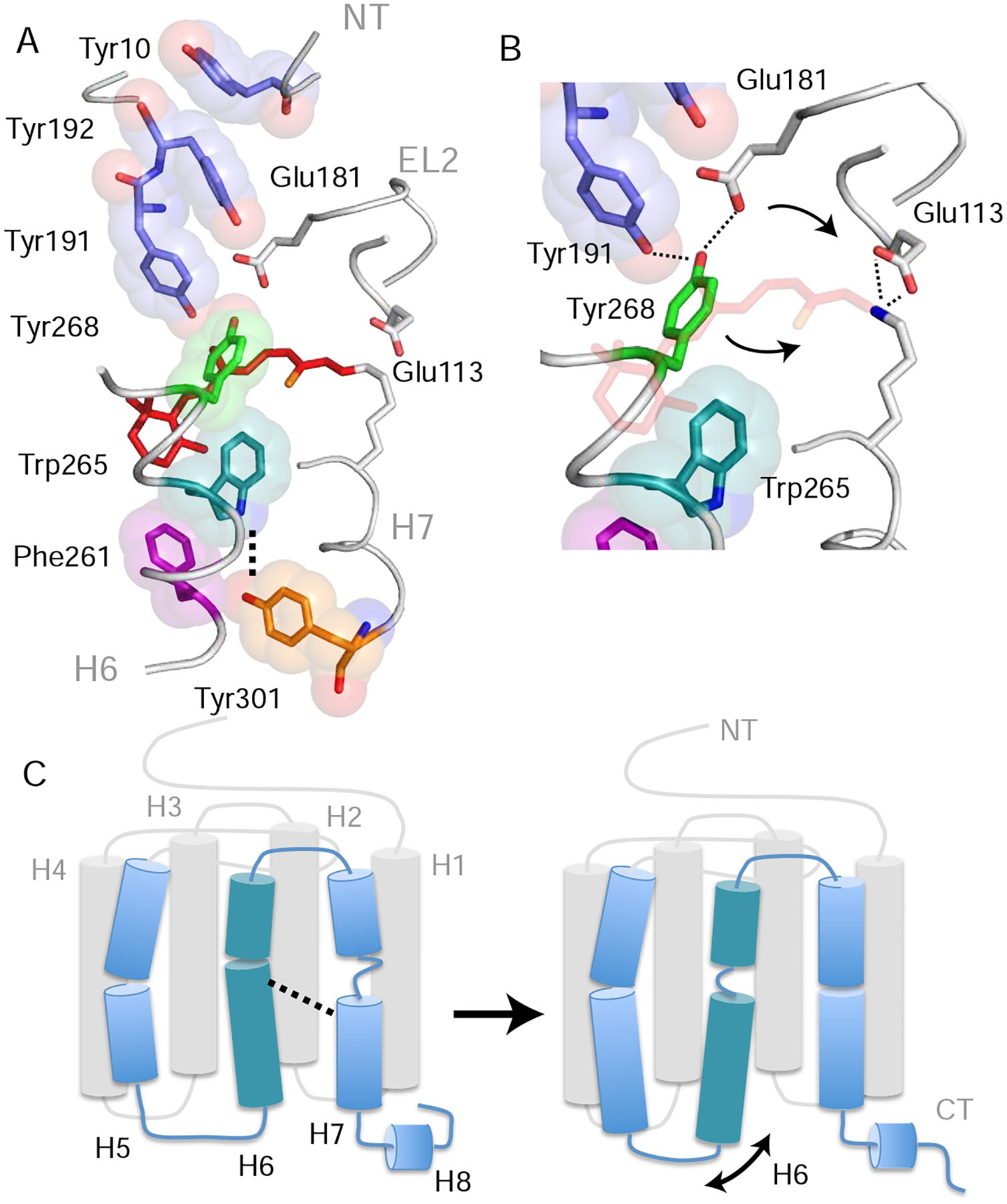
(A) Structure of rhodopsin (PDB: 1GZM) showing the packing of aromatic amino acid side chains stretching from Tyr10NT on the N-terminus to Tyr3017.48 in the TM core (Li et al., 2004). Lys2967.43 forms a positively charged PSB with the retinal that interacts with counter-ions Glu1133.28 on H3 and Glu181EL2 on EL2. Retinal isomerization and PSB deprotonation influence both the hydrogen-bonding network that includes these glutamate residues as well as Tyr191EL2, Tyr192EL2 and Tyr2686.51 (Kimata et al., 2016a). (B) Expansion of panel (A) in the region of Tyr2686.51. Tyr2686.51 is one of the most conserved residues in the visual receptor subfamily. Its position is defined by aromatic packing against Trp2656.48 and hydrogen-bonding interactions with Tyr191 EL2 and Glu181 EL2. Retinal isomerization (and conversion to Meta I) leads to a shift of the counterion from Glu1133.28 to Glu181EL2 (Yan et al., 2003) and movement of Tyr2686.51 toward the positively charged retinal PSB (Kimata et al., 2016a). (C) Summary of the overall structural changes in H6 and H7 described above. Retinal isomerization and PSB deprotonation lead to unraveling of the H6 helix in the region of Trp2656.48 and helix formation of H7 between Phe2947.41 and Tyr3017.48. The loss of helical structure in H6 facilitates an increase in dynamics of the intracellular end of the helix. Models were made using PyMOL 1.8.07 and the figure was assembled using Adobe Illustrator 15.0.0.
Trp2656.48 is also within a string of aromatic residues stretching from Tyr10NT on the N-terminus to Phe2616.44 in Switch 1 and Tyr3017.48 in Switch 2 (Figure 5A). The Trp2656.48 indole side chain stacks directly with Tyr2686.51, which is one of the most conserved positions (95% identity) in visual receptors. The side chain hydroxyl of Tyr2686.51 is intricately associated with the hydrogen-bonding network between the retinal PSB and the second extracellular loop (EL2) via hydrogen-bonds with Tyr191EL2 and Glu181EL2 (Figure 5B) (Kimata et al., 2016a). The FTIR titration curves for samples where Tyr2686.51, Tyr191EL2 and Tyr192EL2 are individually mutated to phenylalanine show a similar shift in the Meta I - Meta II equilibrium toward Meta II as observed for the Y301F7.48 mutant in Figure 5 (Kimata et al., 2016a). As a result, a change in the hydrogen-bonding network associated with EL2 appears to be coupled to the position and dynamics of Tyr2686.51 and Trp2656.48 (Yan et al., 2003). Such coupling between EL2 and Trp2656.48 is likely a common feature of family A GPCRs.
Increased dynamics of Trp2656.48 would also serve to disrupt aromatic stacking with Phe2616.44. This change in packing occurs in addition to disruption of the Trp2656.48 backbone NH hydrogen-bond with the Phe2616.44 backbone C=O. Repacking of Phe2616.44 with Pro2155.50 and Leu1253.40 is widely recognized as an important rearrangement in family A GPCR activation (Rasmussen et al., 2011). In the context of our TM core organization, we refer to this hydrophobic triad as Switch 1.
Switch 1 appears to stabilize the active state via hydrogen-bonding interactions that lock H5 in an active orientation and prolong the lifetime of Meta II. For example, His2115.46 on the extracellular end of H5 is pairwise conserved with Glu1223.37 on H3 in the dim-light rhodopsin receptors (Figure 1). Mutation of Glu1223.37 to glutamine or isoleucine results in rapid decay of Meta II (Imai et al., 1997). In a similar fashion, Tyr2235.58 at the intracellular end of H5 forms hydrogen bonding interactions with Arg1353.50 in Meta II. The Y223F5.58 mutation leads to faster decay of Meta II (Goncalves et al., 2010).
In addition to the results on Trp2656.48 and Tyr3017.48, there are several other observations that support the idea that Switch 2 may in fact be the primary activation switch, while Switch 1 modulates the ligand response. For example, Met2576.40 in rhodopsin is located adjacent to Tyr3017.48 and Asn3027.49 in Switch 2 (Figure S1). Asn3027.49 is part of the conserved NPxxY motif on TM helix H7 and Met2576.40 is one of a few residues in rhodopsin whose mutation leads to constitutive activity arguing that it stabilizes the inactive state of the receptor (Han et al., 1998).
The importance of packing interactions involving Trp2656.48 is supported by 15N-measurements on the A295V7.42 mutant (Figure 3C). A295V7.42 alters the packing environment of Trp2656.48 in the dark. We propose that the increase in constitutive activity of the A295V7.42 mutant is due to increased dynamics of the H6 proline hinge. To address whether the A295V7.42 mutation also influences the retinal PSB linkage as appears to be the case in the other CSNB mutants, solid-state NMR measurements were also collected on A295V7.42 rhodopsin labeled with 13Cε-Lys and 13Cζ-Tyr (Figure S5). It is well-known that the retinal PSB – Glu1133.28 salt-bridge is required to hold the receptor in an inactive conformation and, at least for the G90D2.57 CSNB mutation, it has been suggested that a change in this interaction is sufficient to produce a low level of activity in rhodopsin with 11-cis retinal bound, rather than in opsin (Dizhoor et al., 2008). Our studies (Figure S5) show that A295V7.42 influences the position of the retinal chromphore and tyrosines in EL2 that pack against Tyr2686.51 in rhodopsin, perhaps revealing a common element between A295V7.42 and the other CSNB mutants.
More generally, position 7.42 is conserved as a small residue in about 70% of family A GPCRs (Arakawa et al., 2011; Holst et al., 2004; Mirzadegan et al., 2003). In the C-C chemokine receptor CCR5, mutation of Gly2867.42 to phenylalanine results in hyperactive G protein signaling (Steen et al., 2013). Conversely, in the neurotensin 1 receptor, where position 7.42 is an unusually large residue (Phe3587.42), mutation to alanine causes reduced activity (Krumm et al., 2015).
Aside from extensive packing interactions, the Trp2656.48 indole NH engages in a conserved water-mediated hydrogen-bond within Switch 2. In GPCRs that bind diffusible ligands, a sodium at the center of Switch 2 acts as a NAM that is displaced upon activation. In most of these sodium-binding receptors, Trp6.48 coordinates water surrounding this ion (Katritch et al., 2014), which appears to be substituted by the Trp2656.48 – Tyr3017.48 hydrogen-bond in the visual receptors. Tyr3017.48 movement away from Switch 2 during activation is functionally analogous to expulsion of Na+ from the allosteric binding pocket in these receptors. In either situation, the surrounding Switch 2 residues have to respond to the loss of these stabilizing interactions by adopting new hydrogen-bonds. Evidence that Tyr3017.48 in rhodopsin, and likely the other visual receptors, mimics the negative allosteric sodium found in the ligand activated GPCRs is provided by the FTIR titration curves in Figure 4B, which show that the Y301F7.48 mutation favors active Meta II over the inactive Meta I state. This observation highlights the contribution of Tyr3017.48 in stabilizing the inactive conformation of rhodopsin in a manner similar to the allosteric sodium in other family A GPCRs (Figure 4).
Interestingly, the neurokinin receptors are the only other members of family A GPCRs that have tyrosine at position 7.48, which may in part explain the low basal activity common to this subfamily and the visual receptors. An additional unique feature of Switch 2 in the neurokinin receptors is that the highly conserved aspartic acid at position 2.50 is a glutamic acid. The longer Glu2.50 sidechain in the neurokinin 1 receptor was previously described as a “tethered NAM” that replaces the more common Asp2.50 – Na+ interaction (Valentin-Hansen et al., 2015). The evolutionary adaptation of the visual receptors and the neurokinin receptors relying on fixed residues as tethered NAMs rather than diffusible sodium may enforce the low basal activity characteristic of these proteins.
In summary, our results reveal a key consequence of the hydrogen-bonding changes induced in Switch 2 upon activation. Specifically, there is a disruption of the hydrogen-bond between Trp2656.48 and the Switch 2 network that leads to a dramatic change in the mobility of the H6 backbone within the conserved CWxP motif. The loss of intensity in the backbone NH resonance of Trp2656.48 is attributed to conformational dynamics, which provides flexibility for the rearrangement of Phe2616.44 and allows this region of H6 to function as a hinge for helix motion (Figure 5C). Rearrangement of water in the TM helix bundle is likely associated with an increase in H6 dynamics and formation of the active receptor conformation (Angel et al., 2009; Grossfield et al., 2008; Venkatakrishnan et al., 2019). These insights on one of the original members of the GPCR superfamily provide a foundation for understanding the mechanisms underlying inhibition and activation of the diverse and pharmaceutically important ligand-activated GPCRs.
STAR*METHODS
LEAD CONTACT
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Dr. Steven Smith, steven.o.smith@stonybrook.edu.
MATERIALS AVAILABILITY
This study did not generate new unique reagents.
DATA and CODE AVAILABILITY
NMR chemical shift data has been deposited in the BMRB (Access code 50232)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bovine rhodopsin was expressed in HEK293S cells. HEK293S cells were cultured in DMEM/F12 at 37 °C, 5 % CO2. All media was supplemented with heat-treated USDA approved 10% FBS, 2 mM L-glutamine, and 100 units/mL penicillin 100 μg/mL streptomycin. The sex of the cells is not available.
METHOD DETAILS
Construction of pACMV-tetO Expression Plasmid Containing Rod Opsin Gene Mutants
Rod opsin mutants Y301F7.48 and A295V7.42 were generated previously via site-directed mutagenesis within the pMT4 vector. Rod opsin gene insert fragments were prepared via digestion of 3 μg pMT4 DNA with EcoRI restriction enzyme at 37 °C for 2 h. Linear DNA was then purified using the Macherey-Nagel NucleoSpin® Gel and PCR Clean-up kit (Fisher Scientific). Next the 3’ overhang was end repaired using DNA PolI Large (Klenow) fragment in the presence of 10 mM dNTP at room temperature for 20 min. The reaction was stopped with 10 mM EDTA and incubation at 75 °C for 20 min followed by purification as above. Linear DNA was then digested with NotI restriction enzyme at 37 °C for 2 h. The 1 kb fragment containing the rod opsin gene was isolated via 1% agarose gel DNA electrophoresis and purified using the kit above.
The pACMV-tetO linear vector was previously prepared via digestion with KpnI restriction enzyme at 37 °C for 2 h, followed by DNA purification using a commercial kit. The 5’ overhang was then end repaired using DNA PolI and the DNA purified. DNA was then digested with NotI restriction enzyme, isolated via 1% agarose gel DNA electrophoresis, and purified as above. Finally, plasmid DNA was dephosphorylated using FastAP Antarctic Phosphatase (Thermo Fisher Scientific) at 37 °C for 1 h and then purified as above.
Ligation between pMT4 insert and pACMV-tetO linear vector was performed with T4 DNA ligase in the presence of 5% PEG with vector:insert ratio of 1:3 using the Thermo Fisher Rapid DNA Ligation kit (Thermo Fisher Scientific) (Reeves et al. 2002).
Stable Transfection of HEK293S-tetR Cells with pACMV-tetO-Rho
Following initial growth in DMEM/F12 for 24 h HEK293S-tetR cells were grown in DMEM/F12, 5 μg/mL blasticidin for 5 d at 37 °C, 5 % CO2 (all media was supplemented with heat-treated USDA approved 10% FBS, 2 mM L-glutamine, and 100 units/mL penicillin 100 μg/mL Streptomycin unless otherwise stated). Prior to transfection, 2.5×105 HEK293S-tetR cells were seeded on 10 cm cell culture dishes in 9 mL DMEM. 24 h later, with HEK293S-tetR cells at 40–45 % confluence, stable transfection via calcium phosphate precipitation was initiated. To 30 μg pACMV-tetO plasmid DNA, made up to 450 μL with milliQ water, was added 50 μL 2.5 M CaCl2 drop wise while vortex mixing. Next, 500 μL 2x N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES) buffer (50 mM BES, 250 mM NaCl, 1.5 mM Na2HPO4, pH 7.02) was added drop wise within 1 min, while under constant vortex mixing. This 1 mL transfection cocktail was then added directly to HEK293S-tetR cells in 9 mL DMEM on a 10 cm dish. Cells were incubated at 37 °C, 1 % CO2 for 24 h. Cells were washed 24 h after transfection with un-supplemented DMEM and incubated in DMEM at 37 °C, 5 % CO2 for 24 h.
Selection of HEK293S-tetR Stable Cell Lines Expressing Rhodopsin Mutants
Transfected HEK293S-tetR were treated with 1 mL trypsin, resuspended in 10 mL DMEM/F12 and diluted 1:1, 1:4, 1:9, 1:19, 1:45, 1:90 with DMEM/F12. Cells were incubated at 37 °C, 5 % CO2 for 24 h, after which media was changed for DMEM/F12, 2 mg/mL geneticin G418, 10 % conditioned media. This was replaced every 2–3 days for 19 days by which time a range of colonies of different sizes had formed.
Larger size G418 resistant colonies (2–5 mm diameter) were isolated, following careful aspiration of media, with silicon grease coated metal isolation rings. To each sealed ring 4 drops of trypsin was added and incubated for 2.5 min, before 4–6 drops of DMEM/F12, 2 mg/mL geneticin G418 was added. Cells were immediately resuspended by pipetting up and down 5 times before transferring to 1 mL DMEM/F12, 2 mg/mL geneticin G418 in a single well of a 24 well cell culture dish. Cells were grown to confluence at 37 °C, 5 % CO2 then treated with 100 μL trypsin, resuspended in 1 mL DMEM/F12, 2 mg/mL geneticin G418 and diluted 1:2 in 1 mL DMEM/F12, 2 mg/mL geneticin G418 in 3x wells of a 24 well cell culture dish. These were grown to confluence at 37 °C, 5 % CO2.
Stable cell lines displaying high levels of rod opsin expression after induction in growth medium containing tetracycline (2 μg/mL) and sodium butyrate (5 mM) were identified by dot blot analysis using the rho-1D4 antibody. These cell lines were expanded further to near confluence in three 10 cm cell culture dishes. Cells from of one of these 10 cm dishes were used to make freeze-downs and stored in liquid nitrogen. The other two dishes were fed with DMEM, one of which included tetracycline and sodium butyrate. After 48 h incubation at 37 °C, 5% CO2, cells were harvested, washed with PBS and re-suspended in 500 μL of PBS. This cell suspension was treated with 11-cis retinal (1 μL from a 10 mM stock in ethanol) and incubated for 3 h at 4 °C with mixing in the dark. These cell suspensions were solubilized using 1% n-β-D dodecyl maltopyranoside (DDM) and clarified by centrifugation. These lysates were examined by UV-vis absorbance spectroscopy before and after photo bleaching using a 150 W light source fitted with a 495 nm long- pass filter and delivered through a fiber optic cable. The HEK293S-tetR cell lines with the best growth and inducible gene expression properties were used in large-scale expression experiments.
Large-Scale Expression of Isotope-Labeled Rhodopsin
For large-scale production of rhodopsin containing stable-isotope labeled amino acids, stably transfected HEK293S cells containing the bovine opsin gene under the control of the CMVTetO/TetR promoter/repressor system were expanded under selection by 5 μg/mL blasticidin and 2 mg/mL geneticin on 15 cm plates in commercially available Dulbecco’s Modified Eagle Media (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 1% (v/v) penicillin (100 units/mL) and 1% (v/v) streptomycin (100 μg/mL). Once plates were confluent, the commercial DMEM was exchanged for modified DMEM (10% dialyzed FBS depleted of amino acids) and supplemented with the desired 13C and/or 15N isotope-labeled amino acids and remaining non-isotope labeled amino acids. About 24 h after being introduced to isotope-enriched DMEM, cells were induced with 2 μg/mL tetracycline and 5 mM sodium butyrate to express rod opsin (the apo-receptor of rhodopsin) (Reeves et al., 2002). Following 48 to 72 h of induction (depending on cell viability), HEK293S cells containing isotope enriched opsin were harvested from the plates and pelleted by centrifugation at 4k rpm for 15 min. The resulting pellet was washed with chilled phosphate buffered saline (PBS) at pH 7.2 with protease inhibitors (50 μg/mL benzamidine and 400 μM phenylmethylsulfonyl fluoride (PMSF)) and pelleted by centrifugation at 6 k rpm for 30 min. The PBS wash was repeated a total of three times.
Pigment Generation and Purification of Isotope-Labeled Rhodopsin
All of the following steps were carried out under dim red light (Kodak #2 filter). The cell pellets were incubated with 15–100 μM 11-cis retinal at 4 °C for 4 h to generate rhodopsin, which was subsequently extracted from the HEK293S cells by incubation with 1% (w/v) DDM in PBS (pH 7.2) at 4 °C overnight. The resulting lysate was clarified by centrifugation at 15 k rpm at 4 °C for 30 min in a JA 25.50 rotor in a Beckman Coulter Avanti J-26S XP centrifuge to remove insoluble components. The clarified supernatant containing rhodopsin was collected and the amount of pigment was estimated by measuring the absorbance difference at 500 nm before and after 60s of illumination by a light source containing a 495 nm long-pass filter.
Isotope-labeled rhodopsin was captured from the crude lysate by incubating with rho-1D4 antibody bound Sepharose at 4 °C overnight. The rho-1D4 antibody was coupled Sepharose beads as described by GE Healthcare manufacturer’s instructions. The following day, the resin bound to rhodopsin was washed with 50 column volumes of wash buffer (0.02% DDM in PBS pH7.2), equilibrated with 10 column volumes of equilibration buffer (0.02% DDM, 2 mM sodium phosphate pH 6.0) and eluted with elution buffer (100 μM 9-mer elution peptide (TETSQVAPA), 0.02% DDM, 2 mM sodium phosphate pH 6.0). The purity of the elution was assessed by monitoring the ratio between the absorbance peaks at 280 nm and 500 nm. The resulting elution was concentrated to a volume of 500 μL using Amicon Ultra-15 centrifugation devices with a molecular weight cutoff of 50 kDa. The volume was further reduced to 60 μL under a gentle stream of argon gas while on ice. The concentrated sample was packed into a 4 mm MAS rotor.
Solid-State NMR Spectroscopy
Solid-state NMR experiments were conducted on Bruker NMR spectrometers at static field strengths of either 500 or 600 MHz using either a two-channel or three-channel 4 mm magic angle spinning (MAS) probe at spinning rates between 7 – 10 kHz. Spectra were collected using a 2 ms contact pulse during cross polarization and SPINAL64 decoupling was used during acquisition with a 1H RF field strength between 70 – 90 kHz (Brauniger et al., 2002). All spectra were acquired at temperatures between 190 – 220K to cryo-trap rhodopsin and Meta II states as previously described (Eilers et al., 1999). Typically 2–3 k two-dimensional spectra were averaged for each data set. Data were collected and processed using Topspin 2.1.
13C spectra were externally referenced to the 13C resonance of neat tetramethylsilane (TMS) at 0 ppm at room temperature. Using TMS as the external reference, we calibrated the carbonyl resonance of solid glycine at 176.46 ppm. The chemical shift difference between 13C of the internal standard 4,4-dimethyl- 4-silapentane-1- sulfonic acid (DSS) methyl resonance in D2O relative to neat TMS is 2.01 ppm.
15N 1D spectra presented in this work consist of 20 k – 125 k scans depending on protein quantity. 15N spectra were referenced to liquid ammonia, which is 39 ppm upfield of the ammonium chloride standard used for optimization (Bertani et al., 2014).
FTIR Experiments in Phosphocholine Membranes
FTIR difference spectroscopy was performed on a Bruker Vertex 70 FTIR spectrometer equipped with a mercury–cadmium–telluride detector. Spectra were recorded using time-resolved rapid-scan FTIR methods with a spectral resolution of 4 cm−1. The wild-type and mutant rhodopsin samples were purified from HEK293S cell membranes in DDM and reconstituted at a 1:200 molar ratio into egg phosphocholine using biobeads for detergent removal. The results shown in Figure 4 are for samples in phosphocholine membranes obtained at 0 °C. Samples were prepared by drying solutions of rhodopsin between two CaF2 windows and then pre-equilibrating the sample with buffer (200 mM Bis-Tris propane or 2-(N-morpholino)ethanesulfonic acid (MES) buffer at pH 5.0 and 5.5) at the appropriate pH. Photolysis was carried out using an LED array centered at 530 nm for 1 s, and experiments were performed with an acquisition time of 12 s.
QUANTIFICATION AND STATISTICAL ANALYSIS
The 1D 15N NMR spectra were obtained with 2 k points over a spectral width of 12.5 kHz leading to a resolution of 6 Hz per point. Typical linewidths (full width half maximum) in the 15N spectra were 60–70 Hz. A sine squared window multiplication was applied to the free induction decay, which was then zero-filled to 8 k points. The 2D 13C NMR spectra were obtained with 1 k points in the F2 dimension over a spectral width of 33,333 kHz leading to a resolution of 33.3 Hz (or ~0.2 ppm) per point. The size of the F1 dimension was 64, and linear predicted to 128. A sine squared window multiplication was applied to the free induction decay, which was then zero-filled to 2 k points in F1.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-rhodopsin Rho 1D4 | Sigma Aldrich | Cat# MAB5356 |
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Blasticidin | ThermoFisher | Cat# R21001 |
| Geneticin G418 | Teknova | Cat# 100218–044 |
| Tetracycline hydrochloride | Sigma-Aldrich | Cat# T8032 |
| DMEM depleted of amino acids and CaCl2 | Atlanta Biologicals | N/A |
| L-tryptophan (15N2) | Cambridge Isotope Laboratories | Cat# NLM-800-PK |
| L-tryptophan (13C11, 15N2) | Cambridge Isotope Laboratories | Cat# CNLM-2475-H-PK |
| L-tryptophan (indole-2–13C) | Cambridge Isotope Laboratories | Cat# CLM-1543-PK |
| L-tyrosine (phenol-4–13C) | Cambridge Isotope Laboratories | Cat# CLM-622-PK |
| L-serine (3–13C) | Cambridge Isotope Laboratories | Cat # CLM-1574-PK |
| L-cysteine (3–13C) | Cambridge Isotope Laboratories | Cat# CLM-1868-PK |
| L-methionine (methyl-13C) | Cambridge Isotope Laboratories | Cat# CLM-206-PK |
| L-lysine:2HCl (6–13C) | Cambridge Isotope Laboratories | Cat# CLM-632-PK |
| Benzamidine | Sigma-Aldrich | Cat# 12072 |
| Phenylmethylsulfonyl fluoride | Sigma-Aldrich | Cat# 10837091001 |
| 11-cis retinal | National Eye Institute | N/A |
| n-β-D dodecyl maltopyranoside | Anatrace | Cat# D310 |
| Rho 1D4 peptide (TETSQVAPA) | ERI Amyloid | N/A |
| CNBr-Activated Sepharose 4B | GE Healthcare | Cat# 17043001 |
| FastAP Antarctic Phosphatase | Thermo Fisher | Cat# EF0654 |
| Critical Commercial Assays | ||
| Macherey-Nagel NucleoSpin Gel and PCR Clean-up kit | Fisher Scientific | Cat# NC0389463 |
| Thermo Fisher Rapid DNA Ligation kit | Thermo Fisher | Cat# K1422 |
| Deposited Data | ||
| 13C and 15N chemical shift assignments | This paper | BMRB: 50232 |
| Experimental Models: Cell Lines | ||
| Human: HEK293S-tetR | https://www.lgcstandards-atcc.org/products/all/CRL-1573.aspx?geo_country=gb# | Derivative of 293 (HEK-293) ATCC® CRL-1573™ |
| Experimental Models: Organisms/Strains | ||
| Oligonucleotides | ||
| A295V-F: 5’-CCATCCCGGCTTTCTTTGTCAAGACGTCTGCCG |
This paper | N/A |
| A295V-R:
5’-CGGCAGACGTCTTGACAAAGAAAGCCGGGATGG |
This paper | N/A |
| Y301F-F:
5’-GACGTCTGCCGTCTTCAACCCGGTCATC |
This paper | N/A |
| Y301F-R: 5’-GATGACCGGGTTGAAGACGGCAGACGTC |
This paper | N/A |
| Recombinant DNA | ||
| pACMVtetO-A295V | This paper | N/A |
| pACMVtetO-W265F | This paper | N/A |
| pACMVtetO-Y301F | This paper | N/A |
| pACMVtetO | https://www.pnas.org/content/99/21/13413 | N/A |
| Software and Algorithms | ||
| PyMOL 1.8.07 | Schrodinger | https://pymol.org/2/ |
| Topspin 2.1 | Bruker | https://www.bruker.com/service/support-upgrades/software-downloads/nmr/free-topspin-processing/nmr-topspin-license-for-academia.html |
| Adobe Illustrator 15.0.0 | Adobe Systems | https://www.adobe.com/products/illustrator/free-trial-download.html?gclid=EAIaIQobChMIuZSX88yT6QIVUV8NCh1WrQdfEAAYASABEgLNevD_BwE&sdid=JRSIH&mv=search&ef_id=EAIaIQobChMIuZSX88yT6QIVUV8NCh1WrQdfEAAYASABEgLNevD_BwE:G:s&s_kwcid=AL!3085!3!356518782395!e!!g!!adobe%20illustrator%20download |
| Other | ||
Highlights:
Rhodopsin activation results in unraveling of the H6 backbone within the CWxP motif.
Tyr3017.48 appears to mimic the negative allosteric sodium ion found in other GPCRs.
The A295V constitutively-active CSNB mutation unravels the H6 backbone.
Switch 2 is likely the primary activation switch in rhodopsin and other class A GPCRs.
Acknowledgements
This work was supported by the National Institutes of Health (R01 GM-129012). We thank the laboratory of Dr. Rosalie Crouch at the Medical University of South Carolina and the National Eye Institute for providing 11-cis retinal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing interests.
REFERENCES
- Angel TE, Gupta S, Jastrzebska B, Palczewski K, and Chance MR (2009). Structural waters define a functional channel mediating activation of the GPCR, rhodopsin. Proc. Natl. Acad. Sci. U S A 106, 14367–14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa M, Chakraborty R, Upadhyaya J, Eilers M, Reeves PJ, Smith SO, and Chelikani P (2011). Structural and functional roles of small group-conserved amino acids present on helix-H7 in the β2-adrenergic receptor. Biochim. Biophys. Acta. Biomembr 1808, 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SGF, Shi L, Gether U, and Javitch JA (2001). Activation of the β2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem 276, 29171–29177. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, and Weinstein H (1995). Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 25, 366–428. [Google Scholar]
- Bertani P, Raya J, and Bechinger B (2014). 15N chemical shift referencing in solid state NMR. Solid State Nucl. Magn. Reson 61–62, 15–18. [DOI] [PubMed] [Google Scholar]
- Bräuniger T, Wormald P, and Hodgkinson P (2002). Improved proton decoupling in NMR spectroscopy of crystalline solids using the SPINAL-64 sequence. Monatsh. Chem 133, 1549–1554. [Google Scholar]
- Chabre M, and Breton J (1979). Orientation of aromatic residues in rhodopsin. Rotation of one tryptophan upon the meta I to meta II transition after illumination. Photochem. Photobiol 30, 295–299. [DOI] [PubMed] [Google Scholar]
- Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, Hofmann KP, Scheerer P, and Ernst OP (2011). Crystal structure of metarhodopsin II. Nature 471, 651–655. [DOI] [PubMed] [Google Scholar]
- Crocker E, Eilers M, Ahuja S, Hornak V, Hirshfeld A, Sheves M, and Smith SO (2006). Location of Trp265 in metarhodopsin II: Implications for the activation mechanism of the visual receptor rhodopsin. J. Mol. Biol 357, 163–172. [DOI] [PubMed] [Google Scholar]
- Crocker E, Patel AB, Eilers M, Jayaraman S, Getmanova E, Reeves PJ, Ziliox M, Khorana HG, Sheves M, and Smith SO (2004). Dipolar assisted rotational resonance NMR of tryptophan and tyrosine in rhodopsin. J. Biomol. NMR 29, 11–20. [DOI] [PubMed] [Google Scholar]
- Deupi X, and Kobilka B (2007). Activation of G protein coupled receptors. Adv. Protein Chem 74 137–166. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Woodruff ML, Olshevskaya EV, Cilluffo MC, Cornwall MC, Sieving PA, and Fain GL (2008). Night blindness and the mechanism of constitutive signaling of mutant G90D rhodopsin. J. Neurosci 28, 11662–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T, Tsuda M, Sassenrath G, West JL, and Waddell WH (1980). Light activation of bovine rod phosphodiesterase by non-physiological visual pigments. FEBS Lett. 116, 217–219. [DOI] [PubMed] [Google Scholar]
- Eddy MT, Lee MY, Gao ZG, White KL, Didenko T, Horst R, Audet M, Stanczak P, McClary KM, Han GW, et al. (2018). Allosteric coupling of drug binding and intracellular signaling in the A2A adenosine receptor. Cell 172, 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M, Reeves PJ, Ying WW, Khorana HG, and Smith SO (1999). Magic angle spinning NMR of the protonated retinylidene Schiff base nitrogen in rhodopsin: Expression of 15N-lysine and 13C-glycine labeled opsin in a stable cell line. Proc. Natl. Acad. Sci. U S A 96, 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy K, Jäger F, Beck M, Zvyaga TA, Sakmar TP, and Siebert F (1993). Protonation states of membrane-embedded carboxylic acid groups in rhodopsin and metarhodopsin II: A Fourier-transform infrared spectroscopy study of site-directed mutants. Proc. Natl. Acad. Sci. U S A 90, 10206–10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrens DL, Altenbach C, Yang K, Hubbell WL, and Khorana HG (1996). Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274, 768–770. [DOI] [PubMed] [Google Scholar]
- Fritze O, Filipek S, Kuksa V, Palczewski K, Hofmann KP, and Ernst OP (2003). Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc Natl. Acad. Sci. U S A 100, 2290–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JA, South K, Ahuja S, Zaitseva E, Opefi CA, Eilers M, Vogel R, Reeves PJ, and Smith SO (2010). Highly conserved tyrosine stabilizes the active state of rhodopsin. Proc. Natl. Acad. Sci. U S A 107, 19861–19866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossfield A, Pitman MC, Feller SE, Soubias O, and Gawrisch K (2008). Internal hydration increases during activation of the G-protein-coupled receptor rhodopsin. J. Mol. Biol 381, 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Smith SO, and Sakmar TP (1998). Constitutive activation of opsin by mutation of methionine 257 on transmembrane helix 6. Biochemistry 37, 8253–8261. [DOI] [PubMed] [Google Scholar]
- Hecht S, Shlaer S, and Pirenne MH (1942). Energy, quanta, and vision. J. Gen. Physiol 25, 819–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld J, Das Gupta SK, Farrar MR, Harbison GS, McDermott AE, Pelletier SL, Raleigh DP, Smith SO, Winkel C, Lugtenburg J, et al. (1990). Solid-state 13C NMR study of tyrosine protonation in dark-adapted bacteriorhodopsin. Biochemistry 29, 5567–5574. [DOI] [PubMed] [Google Scholar]
- Holst B, Holliday ND, Bach A, Elling CE, Cox HM, and Schwartz TW (2004). Common structural basis for constitutive activity of the ghrelin receptor family. J. Biol. Chem 279, 53806–53817. [DOI] [PubMed] [Google Scholar]
- Imai H, Kojima D, Oura T, Tachibanaki S, Terakita A, and Shichida Y (1997). Single amino acid residue as a functional determinant of rod and cone visual pigments. Proc. Natl. Acad. Sci. U S A 94, 2322–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg V, Vroling B, van der Kant R, Li K, Vriend G, and Gloriam D (2014). GPCRDB: an information system for G protein-coupled receptors. Nucleic Acids Res. 42, D422–D425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, and Stevens RC (2014). Allosteric sodium in class A GPCR signaling. Trends Biochem. Sci 39, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata N, Pope A, Eilers M, Opefi CA, Ziliox M, Hirshfeld A, Zaitseva E, Vogel R, Sheves M, Reeves PJ, et al. (2016a). Retinal orientation and interactions in rhodopsin reveal a two-stage trigger mechanism for activation. Nat. Commun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata N, Pope A, Sanchez-Reyes OB, Eilers M, Opefi CA, Ziliox M, Reeves PJ, and Smith SO (2016b). Free backbone carbonyls mediate rhodopsin activation. Nat. Struct. Mol. Biol 23, 738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim B, Hofmann KP, Ernst OP, and Hubbell WL (2007). Sequence of late molecular events in the activation of rhodopsin. Prc. Natl. Acad. Sci. USA 104, 20290–20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumm BE, White JF, Shah P, and Grisshammer R (2015). Structural prerequisites for G-protein activation by the neurotensin receptor. Nat. Commun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Edwards PC, Burghammer M, Villa C, and Schertler GFX (2004). Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol 343, 1409–1438. [DOI] [PubMed] [Google Scholar]
- Lin SW, and Sakmar TP (1996). Specific tryptophan UV-absorbance changes are probes of the transition of rhodopsin to its active state. Biochemistry 35, 11149–11159. [DOI] [PubMed] [Google Scholar]
- Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, Ijzerman AP, et al. (2012). Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam M, Martinez-Mayorga K, Brown MF, and Vogel R (2008). Two protonation switches control rhodopsin activation in membranes. Proc. Natl. Acad. Sci. U S A 105, 17795–17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlear SD, Kraft TW, and Gross AK (2010). 1 Rhodopsin mutations in congenital night blindness. Adv. Exp. Med. Biol 664, 263–272. [DOI] [PubMed] [Google Scholar]
- Mirzadegan T, Benkö G, Filipek S, and Palczewski K (2003). Sequence analyses of G-protein-coupled receptors: Similarities to rhodopsin. Biochemistry 42, 2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, and Buss V (2004). The retinal conformation and its environment in rhodopsin in light of a new 2.2 Å crystal structure. J. Mol. Biol 342, 571–583. [DOI] [PubMed] [Google Scholar]
- Olivella M, Caltabiano G, and Cordomi A (2013). The role of cysteine 6.47 in class A GPCRs. BMC Struct. Biol 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PS-H (2014). Constitutively active rhodopsin and retinal disease. Adv. Pharmacol 70, 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Crocker E, Reeves PJ, Getmanova EV, Eilers M, Khorana HG, and Smith SO (2005). Changes in interhelical hydrogen bonding upon rhodopsin activation. J. Mol. Biol 347, 803–812. [DOI] [PubMed] [Google Scholar]
- Pugh EN (2018). The discovery of the ability of rod photoreceptors to signal single photons. J Gen. Physiol 150, 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SGF, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, DeVree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. (2011). Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PJ, Kim JM, and Khorana HG (2002). Structure and function in rhodopsin: A tetracycline-inducible system in stable mammalian cell lines for high-level expression of opsin mutants. Proc. Natl. Acad. Sci. U S A 99, 13413–13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Ren PX, Balusu R, and Yang XJ (2016). Transmembrane helices tilt, bend, slide, torque, and unwind between functional states of rhodopsin. Sci. Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, and Baylor DA (1998). Single-photon detection by rod cells of the retina. Rev. Mod. Phys 70, 1027–1036. [Google Scholar]
- Roth CB, Hanson MA, and Stevens RC (2008). Stabilization of the human β2-adrenergic receptor TM4-TM3-TM5 helix interface by mutagenesis of Glu122(3.41), a critical residue in GPCR structure. J. Mol. Biol 376, 1305–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Reyes OB, Cooke ALG, Tranter DB, Rashid D, Eilers M, Reeves PJ, and Smith SO (2017). G protein-coupled receptors contain two conserved packing clusters. Biophys J 112, 2315–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, and Wenzel-Seifert K (2002). Constitutive activity of G-protein-coupled receptors: Cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol 366, 381–416. [DOI] [PubMed] [Google Scholar]
- Shi L, Liapakis G, Xu R, Guarnieri F, Ballesteros JA, and Javitch JA (2002). β2 adrenergic receptor activation:Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J. Biol. Chem 277, 40989–40996. [DOI] [PubMed] [Google Scholar]
- Singhal A, Guo Y, Matkovic M, Schertler G, Deupi X, Yan ECY, and Standfuss J (2016). Structural role of the T94I rhodopsin mutation in congenital stationary night blindness. EMBO Rep. 17, 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal A, Ostermaier MK, Vishnivetskiy SA, Panneels V, Homan KT, Tesmer JJG, Veprintsev D, Deupi X, Gurevich VV, Schertler GFX, et al. (2013). Insights into congenital stationary night blindness based on the structure of G90D rhodopsin. EMBO Rep. 14, 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SO (2010). Structure and activation of the visual pigment rhodopsin. Annu. Rev. Biophys 39, 309–328. [DOI] [PubMed] [Google Scholar]
- Steen A, Thiele S, Guo D, Hansen LS, Frimurer TM, and Rosenkilde MM (2013). Biased and constitutive signaling in the CC-chemokine receptor CCR5 by manipulating the interface between transmembrane helices 6 and 7. J. Biol. Chem 288, 12511–12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegoshi K, Nakamura S, and Terao T (2001). 13C-1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem. Phys. Lett 344, 631–637. [Google Scholar]
- Valentin-Hansen L, Frimurer TM, Mokrosinski J, Holliday ND, and Schwartz TW (2015). Biased Gs versus Gq proteins and β arrestin signaling in the NK1 receptor determined by interactions in the water hydrogen bond network. J. Biol. Chem 290, 24495–24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Ma AK, Fonseca R, Latorraca NR, Kelly B, Betz RM, Asawa C, Kobilka BK, and Dror RO (2019). Diverse GPCRs exhibit conserved water networks for stabilization and activation. Proc. Natl. Acad. Sci. U S A 116, 3288–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI, and Kobilka BK (2018). The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem 87, 897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS (2011). Interpreting protein chemical shift data. Prog. Nucl. Magn. Reson. Spectrosc 58, 62–87. [DOI] [PubMed] [Google Scholar]
- Yan ECY, Kazmi MA, Ganim Z, Hou JM, Pan DH, Chang BSW, Sakmar TP, and Mathies RA (2003). Retinal counterion switch in the photoactivation of the G protein-coupled receptor rhodopsin. Proc. Natl. Acad. Sci. U S A 100, 9262–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, Gross AK, Leifert D, Kloeckener-Gruissem B, McAlear SD, Lemke J, Neidhardt J, and Berger W (2008). Identification and functional characterization of a novel rhodopsin mutation associated with autosomal dominant CSNB. Invest. Ophthalmol. Vis. Sci 49, 4105–4114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NMR chemical shift data has been deposited in the BMRB (Access code 50232)


