Abstract
Background
Vitamin K antagonists are the only approved oral anticoagulants for long-term prophylaxis against valve thrombosis and thromboembolism in patients with a mechanical heart valve. Despite the proven efficacy and safety of anticoagulation with the oral direct factor Xa inhibitor, apixaban compared with warfarin in high-risk populations including subjects with atrial fibrillation or with venous thromboembolism, it remains unknown whether patients with a mechanical heart valve can be safely managed with apixaban. The On-X® Aortic Heart Valve and On-X® Ascending Aortic Prosthesis with the Vascutek Gelweave Valsalva™ Graft may have lower rates of valve thrombosis and thromboembolism than conventional bileaflet and tilting disc valves due its unique pyrolytic carbon composition and flared inlet design.
Design
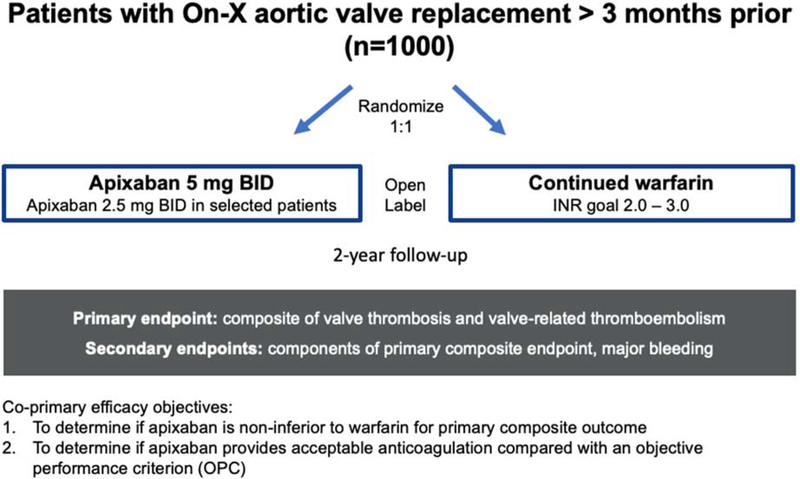
PROACT Xa is a randomized, multicenter, open-label, active-controlled trial comparing apixaban with warfarin in patients with an On-X Aortic Heart Valve or On-X Ascending Aortic Prosthesis with the Vascutek Gelweave Valsalva™ Graft. The study will randomize approximately 1,000 patients from approximately 60 sites in North America who underwent aortic valve replacement at least 3 months prior. Patients will be randomized 1:1 to receiving apixaban 5 mg twice daily or warfarin with a target International Normalized Ratio (INR) of 2.0–3.0. The last randomized participant will be followed for at least 2 years. The primary efficacy outcome is the composite of valve thrombosis and valve-related thromboembolism and the primary safety outcome is major bleeding. Assuming the primary outcome occurs in warfarin-anticoagulated patients at a rate of 1.75%/pt-yr, the study has more than 90% power to assess non-inferiority of apixaban treatment with an absolute non-inferiority margin of 1.75%/pt-yr. A second co-primary analysis is to compare the hazard rate for the apixaban arm to twice the objective performance criterion for thromboembolism and valve thrombosis, i.e. 3.4%/pt-yr.
Summary
PROACT Xa will determine whether patients with an On-X Aortic Heart Valve can be anticoagulated with apixaban as an alternative to warfarin.
Introduction
Each year, over 100 million people worldwide are diagnosed with valvular heart disease and over 300,000 require surgical valve replacement.1,2 Despite advances in device biocompatibility and durability, mechanical prosthetic valves are associated with a significant risk of thrombosis, systemic thromboembolism, and stroke. As a result, patients with a mechanical valve require lifelong anticoagulation and the only currently approved anticoagulant is with a vitamin K antagonist (VKA).3,4
While VKAs are safe and effective in patients with mechanical valves, VKAs, including warfarin, are associated with a number of significant drawbacks that impact the intended therapeutic effect and patient compliance. Due to its relative narrow therapeutic window and multiple pharmacodynamic and pharmacokinetic interactions, patients on warfarin require frequent blood draws for International Normalized Ratio (INR) monitoring and are also subject to dietary and alcohol restrictions, which can be inconvenient and anxiety-provoking.5,6 In addition, multiple studies have demonstrated that patients requiring prolonged VKA therapy for various indications, including mechanical valves, venous thromboembolism, and chronic atrial fibrillation, spend a significant amount of time with INRs outside of therapeutic range, diminishing the protective effect of warfarin against thrombosis and stroke, and increasing the risk of bleeding.7–11
Patients undergoing aortic valve replacement (AVR) have a choice between mechanical and bioprosthetic valves. Although bioprosthetic valves, unlike mechanical valves, do not require long-term anticoagulation, they are prone to structural valve deterioration necessitating subsequent reoperation, assuming the patient remains an operative candidate. Guidelines have typically recommended mechanical valves in patients less than 60 years of age due to the increased morbidity associated with redo valve procedures and the higher rates of reoperation required for degenerative bioprosthetic valves among younger compared with older patients.3,12–16 Despite these recommendations and the evidence presented in multiple randomized, matched, and risk adjusted analyses, there has been a trend in recent years of an increasing number of patients choosing bioprosthetic over mechanical aortic valves, including bioprosthetic transcatheter valves, primarily as a way of avoiding required VKA therapy.17 A recent retrospective registry analysis demonstrated an increase in the proportion of patients age 50–65 years undergoing isolated AVR with a bioprosthetic valve from 20% in 1991 to greater than 90% in 2015.18
While not approved for use in patients with prosthetic heart valves, patients requiring chronic anticoagulation therapy for other indications have reported improved satisfaction and compliance with direct oral anticoagulants (DOACs), which do not require routine lab monitoring and have fewer drug interactions.19,20 Providing an acceptable alternative to warfarin anticoagulation may increase the number of patients choosing a mechanical valve with greater durability and potentially better clinical outcomes.
On-X® Aortic Heart Valve
The On-X® Aortic Heart Valve (CryoLife, Kennesaw, GA) is a bileaflet mechanical heart valve, which consists of an orifice housing and 2 leaflets (Figure 1). The On-X Aortic Heart Valve was originally approved by the Food and Drug Administration (FDA) in 2001, with more than 260,000 implanted worldwide to date. Unique among mechanical valves, the On-X Valve orifice inflow area has a flared inlet designed to reduce flow turbulence and is composed of graphite substrate coated with On-X® Carbon, a pure unalloyed form of pyrolytic carbon. The On-X® Ascending Aortic Prosthesis (CryoLife, Kennesaw, GA) combines the On-X Valve and the Gelweave Valsalva™ Vascular Prosthesis (Vascutek Ltd., Renfrewshire, Scotland, UK). The Gelweave Valsalva™ Vascular Prosthesis is incorporated into the cuff structure of the On-X Valve to create the ascending aortic prosthesis. The On-X Ascending Aortic Prosthesis (AAP) with the Vascutek Gelweave Valsalva™ Graft was originally approved by the FDA in 2016. The On-X Aortic Heart Valve has been suggested to have lower rates of valve thrombosis and thromboembolism than conventional bileaflet and tilting disc valves and was thus selected for study in PROACT Xa.6
Figure 1.
On-X® Aortic Heart Valve top and cross-section of valve bottom, which has been suggested to have lower rates of valve thrombosis and thromboembolism than conventional bileaflet and tilting disc valves
Alternatives to standard dose warfarin
Several randomized clinical trials have been conducted testing various alternative anticoagulation strategies to standard warfarin in patients with mechanical valves. In the Prospective Randomized On-X Valve Anticoagulation Clinical Trial (PROACT), patients with at least one thromboembolic risk factor were randomized to standard warfarin therapy (INR goal 2.0–3.0) or lower dose warfarin (INR goal 1.5–2.0).21 Major bleeding events were significantly less likely in the lower INR arm (1.48% vs 3.26%/pt-yr, p=0.047), leading to subsequent approval by the FDA for this lower INR target window in patients with an On-X Aortic Heart Valve. Importantly, thromboembolic events were statistically similar in the lower INR arm (2.67% vs 1.59%/pt-yr, p=0.164) but were not analyzed using a non-inferiority approach. Also in PROACT, patients without thromboembolic risk factors undergoing mechanical AVR with an On-X Aortic Heart Valve were randomized to receive dual antiplatelet therapy (DAPT) or standard warfarin plus aspirin beginning 3 months post-surgery.22 The low-risk arm of the trial was terminated early due to an excess of cerebral thromboembolic events in the DAPT arm, demonstrating the inadequacy of antiplatelet therapy for thromboembolic prophylaxis in patients with an On-X Aortic Heart Valve.
DOACs have emerged as an efficacious and safe alternative to warfarin for patients that require prolonged anticoagulation for several indications including atrial fibrillation and deep vein thrombosis/pulmonary embolism (DVT/PE). In the Randomized, Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients after Heart Valve Replacement (RE-ALIGN) trial, the safety and efficacy of anticoagulation with a DOAC was tested in the mechanical valve population.23 Patients with a bileaflet mechanical mitral or aortic valve were randomized to the direct thrombin inhibitor, dabigatran, or standard warfarin. The trial was also terminated early due to excess thromboembolic and bleeding events in the dabigatran group. Most thromboembolic events and all strokes occurred in the arm of the trial that underwent randomization within 1 week, as opposed to at least 3 months, after valve surgery. The excess thromboembolic events seen during this early period, the majority of which involved thrombi arising from the sewing ring, likely reflects the increased thrombogenicity related to delayed endothelialization of the prosthetic valve. Additionally, all patients who experienced major bleeding were randomized in the first week following surgery and experienced pericardial effusion, illustrating the vulnerability of patients to bleeding complications in this perioperative period.
Rationale for apixaban
In prior trials comparing apixaban to warfarin for patients requiring prolonged anticoagulation, apixaban has consistently demonstrated an excellent balance of efficacy and safety. In the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial, apixaban was superior to warfarin in preventing ischemic or hemorrhagic stroke or systemic embolism in patients with atrial fibrillation and at least one additional risk factor for stroke.24,25 Major bleeding events were also significantly less likely in the apixaban arm. In a 2015 meta-analysis by Touma and colleagues examining over 24,000 patients across 5 randomized clinical trials, apixaban was associated with a lower risk of bleeding compared with warfarin among patients requiring anticoagulation for atrial fibrillation, venous thromboembolism, and after total knee replacement.26 Furthermore, the preponderance of data in the literature suggests that apixaban, as labeled for dosing in patients with atrial fibrillation, has an attractive efficacy and safety profile compared with other DOACs. Given these findings, and the multiple drawbacks associated with VKAs already discussed, a randomized clinical trial of apixaban versus warfarin in patients with the On-X Aortic Heart Valve is needed.
Methods
Study overview
PROACT Xa (ClinicalTrials.gov No. NCT04142658) is a prospective, randomized, multicenter, open-label, active (warfarin) controlled, parallel-arm clinical trial to determine if patients with an On-X Aortic Heart Valve or On-X AAP can be maintained safely and effectively on the factor Xa inhibitor apixaban. Eligible, consenting patients will be enrolled and randomized to either continue warfarin with an INR target range of 2.0–3.0 or switch to apixaban 5mg twice daily at least 3 months after AVR with an On-X Aortic Heart Valve or On-X AAP. The primary endpoint is the composite of valve thrombosis and valve-related thromboembolism. The primary objective of this trial is to determine if apixaban provides acceptable anticoagulation for patients with an On-X Aortic Heart Valve or On-X AAP for this endpoint in comparisons to both warfarin (INR target range 2.0 – 3.0) and an objective performance criterion (OPC).27
This trial will be conducted in compliance with the Declaration of Helsinki and Good Clinical Practice, as defined by the International Conference on Harmonization. Prior to patient participation, written informed consent will be obtained from each patient, and approval will be obtained from appropriate ethics committees at participating sites.
Study population
PROACT Xa will randomize approximately 1,000 patients at approximately 60 sites in North America. Briefly, eligible patients will be at least 18 years of age and have had an AVR with an On-X Aortic Heart Valve or On-X AAP at least 3 months (90 days) prior to enrollment. Patients must also be able to receive warfarin with an INR target range of 2.0–3.0 as well as aspirin 75100mg daily or have a documented contraindication to aspirin use. Patients with a mechanical valve in any position, other than the aortic valve, those who have had cardiac surgery or an ischemic/hemorrhagic stroke within the 3 months prior to enrollment, as well as those with a creatinine clearance <25 ml/min will be excluded. Full study inclusion and exclusion criteria are listed in Table 1.
Table 1.
Trial inclusion and exclusion criteria
| Inclusion criteria | |
| 1. Male or female at least 18 years of age at the time of giving informed consent. | |
| 2. Able to receive warfarin with a target INR 2.0 to 3.0 | |
| 3. Able to take low-dose aspirin at a dose of 75–100 mg daily or have a documented contraindication to aspirin use. | |
| 4. Implantation of an On-X mechanical valve in the aortic position at least 3 months (90 days) prior to enrollment. | |
| 5. If female participant of childbearing potential, including those who are less than 2 years post-menopausal, she must agree to and be able to use a highly effective method of birth control (eg, barrier contraceptives [condom or diaphragm with spermicidal gel], hormonal contraceptives [implants, injectables, combination oral contraceptives, transdermal patches, or contraceptive rings], intrauterine devices, or sexual abstinence) continuously through the study until the last study visit. | |
| 6. Able to provide written informed consent | |
| Exclusion criteria | |
| 1. Mechanical valve in any position other than aortic valve. | |
| 2. Any cardiac surgery in the 3 months (90 days) prior to enrollment. | |
| 3. Need to be on aspirin >100 mg daily or a P2Y12 inhibitor (clopidogrel, ticagrelor, prasugrel, or ticlopidine). | |
| 4. Known hypersensitivity or other contraindication to apixaban. | |
| 5. On dialysis or a creatinine clearance > 25 mL/min. | |
| 6. Experienced an ischemic stroke or intracranial hemorrhage within 3 months of screening for enrollment. | |
| 7. Active pathological bleeding at the time of screening for enrollment. | |
| 8. Active endocarditis at the time of screening for enrollment. | |
| 9. Pregnant at the time of screening for enrollment, plan to become pregnant at any point during the study, or are breast feeding at the time of screening for enrollment. | |
| 10. On concomitant combined strong P-glycoprotein (P-gp) and cytochrome P450 3A4 (CYP3A4) inducers or inhibitors. | |
| 11. History of non-compliance with recommended monthly INR testing. |
Randomization and treatment
Enrolled patients will be randomized (interactive web-based) in a 1:1 ratio to either warfarin with a target INR goal of 2.0–3.0 or apixaban (Figure 2). Apixaban will be dosed at 5 mg twice daily or 2.5 mg twice daily for patients with at least two of the following characteristics: age ≥ 80 years, weight ≤ 60 kg, or serum creatinine ≥ 1.5 mg/dL (133 micromol/L). Patients randomized to apixaban will transition from warfarin using the INR testing algorithm in Supplemental Table 1. Warfarin will be supplied in 1 and 5 mg tablets. Patients randomized to warfarin should have their dosage titrated to an INR goal of 2.0 to 3.0. Warfarin dosage required to achieve the target INR range should be per clinical oversight. Patients in the warfarin arm may continue to use their prior at-home or in-clinic INR monitoring method with close supervision by clinical sites. Sites will be provided with TTR reports for their patients throughout the trial. Patients in both arms are also recommended to take aspirin 75–100 mg daily unless they have a documented contraindication. Study warfarin and apixaban will be sourced from a designated drug distribution center. In the US, patients will receive study drug on a monthly basis via direct shipment to their home.
Figure 2.
PROACT Xa trial design
Follow-up and outcomes
Follow-up will occur monthly via phone call to assess for bleeding, thrombosis, and thromboembolic events, assess for changes in antithrombotic therapy, review concomitant medications, record INR values for patients randomized to warfarin, and to trigger filling of study drug for the following month. On the day of randomization and yearly thereafter, laboratory data including hemoglobin and serum creatinine will be collected. If no clinical results are available within 45 days before the visit, an in-person visit will be required to collect these data. Quality of life assessments with the EQ5D and Duke Anticoagulation Satisfaction Scale instruments will be performed at randomization and at 6 months, 12 months, and yearly thereafter.
Each participant will be followed until the last enrolled participant reaches 2 years. The study will end when the last enrolled participant has completed the 2-year follow-up visit and at least 800 patient-years of cumulative follow-up have been reached in each randomized arm.
The primary efficacy outcome is the composite of valve thrombosis and valve-related thromboembolism. Valve thrombosis will be defined as any thrombus not caused by infection attached to or near an implanted On-X Aortic Heart Valve or On-X AAP that occludes part of the blood flow path, interferes with valve function, or is sufficiently large to warrant treatment other than continued oral anticoagulation. Furthermore, valve thrombus found at autopsy in a participant whose cause of death was not valve related or found at operation for an unrelated indication will also be reported as valve thrombosis. Valve-related thromboembolism will be defined as any thromboembolic stroke, thromboembolic transient ischemic attack (TIA), thromboembolic myocardial infarction (MI), or arterial thromboembolism to another organ or limb, occurring after the immediate perioperative period and not associated with infection or intracardiac tumor. The primary safety outcome, major bleeding, will be defined as any episode of internal or external bleeding that causes death, hospitalization, or permanent injury (e.g., vision loss) or necessitates transfusion, pericardiocentesis or reoperation.
Secondary outcomes will include the individual components of the primary composite outcome, valve thrombosis and valve-related thromboembolism, as well as the primary composite outcome in pre-specified subgroups of patients with an On-X Aortic Heart Valve or On-X AAP. Additional secondary outcomes will include quality of life and satisfaction with anticoagulation assessed with the EQ5D and Duke Anticoagulation Satisfaction Scale instruments.
Outcomes will be adjudicated according to standard and pre-defined definitions (Supplemental Table 2) by an independent committee blinded to treatment assignment. Events to be adjudicated by the Clinical Events Committee (CEC) will include valve thrombosis or dysfunction, stroke or TIA, MI, arterial thromboembolism, bleeding, hospitalization and the reason for hospitalization, and death. Each potential event will be adjudicated by 2 physicians, and disagreements will be reviewed by a third physician.
Sample size calculation and statistical analysis
PROACT Xa is designed to evaluate 2 co-primary efficacy hypotheses: (1) apixaban is non-inferior to warfarin (INR target range 2.0–3.0) for patients with an On-X Aortic Heart Valve or On-X AAP for the primary composite outcome of valve thrombosis and valve-related thromboembolism and (2) apixaban provides acceptable anticoagulation for patients with an On-X Aortic Heart Valve or On-X AAP for the primary composite outcome of valve thrombosis and valve-related thromboembolism compared with an OPC.
With an accrual time of 1 year and a minimum follow-up period of 2 years, the assumed loss to follow-up rate for the period of 2 years is 5%, which is equivalent to a loss hazard rate of 0.026 for both the test and control groups. Assuming a one-sided alpha of 2.5%, an approximate power of 90%, an equal event rate of 1.75%/pt-yr in both warfarin and apixaban arms, and an absolute NI margin of 1.75%/pt-yr, the estimated sample size is 990, therefore this study will randomize approximately 1000 participants in a 1:1 ratio to apixaban or warfarin. This event rate is an estimate based on a review of the literature available pertaining to patients with On-X aortic devices maintained on warfarin.21,22,28–30 Since the number of historical trials to estimate the event rate in warfarin arm is relatively small, the Hartung-Knapp-Sidik-Jonkman (HKSJ) method was used to produce more robust estimates of variance of the event rate.31 The pooled event rate of warfarin was estimated as 1.75%/pt-yr with a 95% confidence interval (CI) of 1.09%/pt-yr and 2.81%/pt-yr by a random effects HKSJ meta-analysis model. We project that there will be a similar event rate with apixaban and set the non-inferiority margin for this study at 1.75%/pt-yr, equivalent to a doubling of the estimated event rate in warfarin arm.
The second co-primary analysis is to compare the primary outcome hazard rate for the apixaban arm to two times the OPC for thromboembolism and valve thrombosis or 3.4%/pt-yr (= 2 × 1.7%/pt-yr).27
A linearized event rate for each treatment group will be calculated as percentage per patient-year using the intention-to-treat (ITT) population and used for the co-primary analyses. For the first co-primary analysis, if the upper bound of the 95% CI of the difference of event rates is less than the non-inferiority margin of 1.75%/pt-yr, it will be concluded that apixaban is non-inferior to warfarin (INR target range 2.0 – 3.0) for the primary efficacy outcome in participants with an On-X Aortic Heart Valve or On-X AAP. For the second co-primary analysis, if the apixaban arm achieves at least 800 patient-years and the linearized event rate for the apixaban arm is less than two times the OPC (2 × 1.7%/pt-yr), the OPC test is passed.
If both co-primary analyses are met using the ITT population, we will conclude that apixaban is a reasonable alternative to warfarin for thromboembolic event prevention in this population. If non-inferiority for the primary efficacy outcome is established, a superiority test for the difference of event rates in the primary efficacy outcome will be performed as follows; if the upper bound of the 95% CI is less than zero, it will be concluded that apixaban is superior to warfarin (INR target range 2.0 – 3.0) for the primary efficacy outcome in participants with an On-X Aortic Heart Valve or On-X AAP.
The analysis of the primary safety outcome will be performed using an assumed major bleeding hazard rate for the warfarin arm of 3.63%/pt-yr, calculated using the event rates from the high and low-risk arms of the PROACT trial.21,22 Using the proposed sample size of 1000, a superiority test for major bleeding has 90% power to detect a difference of 2.13%/pt-yr (i.e., 3.63%/pt-yr for warfarin arm and 1.5%/pt-yr for apixaban arm).
Event rates for the secondary outcomes will be summarized by apixaban and warfarin treatment group. Prespecified subgroup analyses will stratify by age, sex, race, conduit type, time from surgery, valve size, baseline apixaban dose, and post-randomization time in therapeutic range. An additional subgroup analysis will be performed stratified by risk, with high-risk patients defined as having any of the following criteria as defined in the PROACT trial: atrial fibrillation, left ventricular ejection fraction <30%, left atrial dimension >50mm, significant vascular disease, or a history of neurological events within 1 year.22 Linearized event rates and a 2-sided 95% CI for the difference of linearized event rates will be constructed for the outcome using the methods described for the primary outcome.
Coordinating center and study approval
PROACT Xa will be coordinated by the Duke Clinical Research Institute (DCRI). Participating sites will receive approval by local or central Institutional Review Boards (IRBs), as applicable. Appropriate informed consent will be obtained by all study participants.
Role of steering and data safety monitoring committees
The PROACT Xa Steering Committee oversees the design, execution, analysis, and reporting of the study. This committee will convene regularly to address policy issues and monitor study progress and management.
An independent Data Safety Monitoring Committee (DSMC), comprised of 4 clinicians and 1 statistician, will review trial data regarding safety, efficacy, outcomes, and trial conduct at least annually after the first participant is enrolled. The committee will recommend to the steering committee and sponsor study continuation without modification, continuation with modification, or termination based on periodic review of accumulating data. No interim analysis for efficacy or futility is planned.
PROACT Xa is sponsored by CryoLife, Inc. The authors are solely responsible for the design and conduct of this study, all study analyses, and the drafting and editing of the manuscript and its final contents. A comprehensive blinding plan has been developed and both the sponsor and trial leadership will remain as blinded as feasible to outcomes during the trial. A detailed statistical analysis plan has been finalized and will be published along with the main results of the trial.
Discussion
While multiple trials have demonstrated that patients requiring chronic anticoagulation for atrial fibrillation and venous thromboembolic (VTE) disease can be safely and effectively managed with a DOAC, patients with a mechanical heart valve still require anticoagulation with a VKA. Apixaban, which has been shown to be superior to warfarin in terms of thromboembolism prophylaxis in these high-risk populations, with a lower risk of bleeding, has never been studied in the mechanical valve population. Integrating lessons learned from prior trials of alternative anticoagulation regimens in patients with mechanical valves, PROACT Xa will determine if apixaban is a reasonable alternative to warfarin for the prevention of valve thrombosis and thromboembolism in patients with an On-X Aortic Heart Valve or On-X AAP. The RE-ALIGN trial examining anticoagulation with dabigatran in mechanical valve patients demonstrated the vulnerability of mechanical valve patients to complications in the perioperative period. Patients randomized to DOAC therapy in the weeks following surgery were not only significantly more likely to experience a major bleeding event but were also much more likely to experience valve thrombosis, possibly related to delayed endothelialization and associated increased thrombogenicity of the mechanical valve sewing ring. To help mitigate the increased risk inherent to the perioperative period, patients in PROACT Xa will be enrolled at least 3 months following valve replacement. Furthermore, while 3 months is the minimum time period allowed between surgery and randomization, PROACT Xa will uniquely enroll patients several years out from valve replacement as well, enhancing the generalizability of the study population to reflect the current large pool of patients requiring lifelong anticoagulation after mechanical aortic valve replacement.
While apixaban has never been studied in humans with a mechanical heart valve, apixaban has been compared to warfarin in a heterotopic aortic valve porcine model with bileaflet mechanical aortic valve implants.32 In this preclinical study, postmortem valve thrombus weight was lowest in the apixaban infusion group (compared with no anticoagulation, oral warfarin oral, and oral apixaban). Importantly, positive findings of a study examining dabigatran for thromboprophylaxis in a porcine mechanical valve model were not predictive of dabigatran’s lack of effectiveness in RE-ALIGN, highlighting the limitations of preclinical models.33
The planned dosage of apixaban in PROACT Xa, 5mg twice daily, was selected because of its proven efficacy and safety profile as demonstrated in prior trials among patients requiring anticoagulation for chronic atrial fibrillation. In ARISTOTLE, this dosage of apixaban was associated with a 21% reduction in ischemic or hemorrhagic stroke or systemic embolism as well as a 31% reduction in major bleeding events compared with warfarin (target INR 2.0 to 3.0).24 This dosage has also been examined in VTE populations as well as in patients with atrial fibrillation and acute coronary syndrome, with consistent efficacy and safety.26,34 Thus, we believe that this dosage will be the most appropriate for examining the efficacy and safety of apixaban in patients with a mechanical aortic valve.
Patients randomized to warfarin in PROACT Xa will be managed with a target INR of 2.0–3.0. While the high-risk arm of PROACT demonstrated that a lower INR target range, 1.52.0, was safe in patients with an On-X® Aortic Heart Valve, this lower target INR range is not suitable for testing our primary efficacy hypothesis. In PROACT, the positive findings associated with the lower INR target range were driven primarily by the lower rate of bleeding in the composite endpoint. Evidence from prior trials, including ARISTOTLE, already support the use of apixaban over warfarin for bleeding and there is no reason to presume that a different outcome will be observed in mechanical valve patients months to years post-surgery. Instead, to truly determine if apixaban is indeed non-inferior to warfarin for the prevention of valve thrombosis and thromboembolism, it will need to be compared with the “gold-standard” higher warfarin INR threshold of 2.0–3.0. As such, patients enrolled in PROACT Xa must be willing to maintain their target INR at 2.0–3.0 during the course of the study. Additionally, given the guideline recommendations for concomitant VKA and aspirin use in patients with mechanical valves (2017 ACC/AHA Class I recommendation, 2017 ESC/EACTS Class IIa recommendation), patients in PROACT Xa will receive aspirin 75–100mg daily in addition to warfarin or apixaban, unless they have a documented contraindication.3,4
As PROACT Xa has been designed with two separate primary efficacy objectives, there is a possibility, although unlikely, that only one of the two objectives are met. The scenario in which non-inferiority of apixaban compared with warfarin is demonstrated without demonstrating clinical acceptability compared with the OPC could occur if the warfarin event rate is significantly higher than expected, due to poor INR control, for example. In this situation, apixaban might still be considered an acceptable alternative to warfarin depending on the explanation for the higher than expected event rate with warfarin. The opposite scenario could also occur, where apixaban’s event rate is below the OPC threshold but non-inferiority of apixaban compared with warfarin is not achieved. This could occur if the observed warfarin event rate is much lower than expected, perhaps due to low event ascertainment. While the primary efficacy objective will not have been met, these results might also may reflect and be interpreted as a clinically acceptable efficacy profile associated with apixaban.
From conception to its implementation, PROACT Xa has been designed with patientcentered and pragmatic elements, the results of which have the potential to significantly improve the care of patients with an On-X Aortic Heart Valve. Most sites identified for participation in PROACT Xa will have a robust history of implanting On-X valves. By including patients who underwent valve replacement surgery months to years prior to randomization, we hope to facilitate rapid enrollment and maximize patient eligibility as trial sites will be able to recruit participants from the group of patients they have previously implanted On-X valves, as well as those who will require AVR during the course of the study. Further, the trial has been designed to minimize the burden of required face-to-face visits when feasible. Monthly follow-up will all be conducted by telephone and annual clinical evaluations may be based upon clinical data collected in the prior 45 days. In addition, to enhance generalizability and promote pragmatism, patients randomized to the warfarin arm may continue to undergo INR monitoring using their preferable at-home or in-clinic methods with close oversight by clinical sites. Lastly, direct-topatient shipment of study drug will help decrease the workload of clinical site staff and minimize potential interruptions in anticoagulation therapy. This drug delivery paradigm will be familiar to the significant number of patients who normally receive their medications via mail-order pharmacy. If PROACT Xa supports the efficacy and safety of apixaban in patients with an On-X Aortic Heart Valve or On-X AAP, additional clinical trials will be necessary to generalize these results to patients with different types of mechanical valves, as well as to DOACs other than apixaban.
Conclusion
The PROACT Xa trial will compare apixaban with warfarin anticoagulation for the prevention of valve thrombosis and thromboembolism in patients with an On-X Aortic Heart Valve or On-X AAP. The study findings will determine if patients with an On-X Aortic Heart Valve or On-XAAP can be managed with the DOAC, apixaban, rather than warfarin.
Supplementary Material
Acknowledgements
The PROACT Xa clinical trial is being funded by CryoLife, Inc. (Kennesaw, GA). Dr. Jawitz was supported by a NIH T-32 grant 5T32HL069749.
Disclosures*: OKJ: none; TYW: research grants to the Duke Clinical Research Institute from AstraZeneca, Bristol Myers Squibb, Cryolife, Chiesi, Portola, and Regeneron, as well as consulting honoraria from AstraZeneca, Sanofi, and Cryolife; RDL: grants and personal fees from Bristol-Myers Squibb and Pfizer, personal fees from Boehringer Ingelheim and Bayer AG and grants from Amgen Inc, GlaxoSmithKline, Medtronic PLC, and Sanofi Aventis outside the submitted work; AC: none; BB: CryoLife; HK: none; KJA: research funding from Merck, Bayer, and NIH; RCB: scientific advisory board for Janssen, DSMB committees for Ionis Pharmaceuticals, Akcea Therapeutics, and Novartis, research funding from NIH and American Heart Association; EB: none; MR: none; VHT: advisor/research: CryoLife; JDP: none; MWG: consultant, CryoLife; DJ: consulting for Edwards Lifesciences, educational honoraria from LivaNova; SC: CryoLife; JHA: research Funding from Bayer, Bristol Myers Squibb, CryoLife, and XaTek and consulting or honorarium from Bayer, Bristol Myers Squibb, CryoLife, Pfizer, and XaTek; LGS: unpaid member of PARTNER Executive committee and Chairman of the Writing Committee
Footnotes
*TYW, HK, RCB, EB, MR, VHT, JDP, MWG, DJ, JHA, RDL, and LGS are members of the PROACT Xa clinical trial steering committee
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Musumeci L, Jacques N, Hego A, Nchimi A, Lancellotti P, Oury C. Prosthetic Aortic Valves: Challenges and Solutions. Front Cardiovasc Med. 2018;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li KYC. Bioprosthetic Heart Valves: Upgrading a 50-Year Old Technology. Front Cardiovasc Med. 2019;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–2791. [DOI] [PubMed] [Google Scholar]
- 5.Almeida Gde Q, Noblat Lde A, Passos LC, do Nascimento HF. Quality of life analysis of patients in chronic use of oral anticoagulant: an observational study. Health Qual Life Outcomes. 2011;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacIsaac S, Jaffer IH, Belley-Cote EP, McClure GR, Eikelboom JW, Whitlock RP. How Did We Get Here?: A Historical Review and Critical Analysis of Anticoagulation Therapy Following Mechanical Valve Replacement. Circulation. 2019;140(23):19331942. [DOI] [PubMed] [Google Scholar]
- 7.Orensky IA, Holdford DA. Predictors of noncompliance with warfarin therapy in an outpatient anticoagulation clinic. Pharmacotherapy. 2005;25(12):1801–1808. [DOI] [PubMed] [Google Scholar]
- 8.Kimmel SE, Chen Z, Price M, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Arch Intern Med. 2007;167(3):229–235. [DOI] [PubMed] [Google Scholar]
- 9.Eitz T, Schenk S, Fritzsche D, et al. International normalized ratio self-management lowers the risk of thromboembolic events after prosthetic heart valve replacement. Ann Thorac Surg. 2008;85(3):949–954; discussion 955. [DOI] [PubMed] [Google Scholar]
- 10.Meijer K, Kim YK, Schulman S. Decreasing warfarin sensitivity during the first three months after heart valve surgery: implications for dosing. Thromb Res. 2010;125(3):224229. [DOI] [PubMed] [Google Scholar]
- 11.Chen SY, Wu N, Gulseth M, et al. One-year adherence to warfarin treatment for venous thromboembolism in high-risk patients and its association with long-term risk of recurrent events. J Manag Care Pharm. 2013;19(4):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser N, Jackson V, Holzmann MJ, Franco-Cereceda A, Sartipy U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur Heart J. 2016;37(34):2658–2667. [DOI] [PubMed] [Google Scholar]
- 13.Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N Engl J Med. 2017;377(19):1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Head SJ, Celik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017;38(28):2183–2191. [DOI] [PubMed] [Google Scholar]
- 15.McClure RS, Narayanasamy N, Wiegerinck E, et al. Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: up to 17-year follow-up in 1,000 patients. Ann Thorac Surg. 2010;89(5):1410–1416. [DOI] [PubMed] [Google Scholar]
- 16.Bourguignon T, Lhommet P, El Khoury R, et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 50–65 years. Eur J Cardiothorac Surg. 2016;49(5):1462–1468. [DOI] [PubMed] [Google Scholar]
- 17.Chiang YP, Chikwe J, Moskowitz AJ, Itagaki S, Adams DH, Egorova NN. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA. 2014;312(13):1323–1329. [DOI] [PubMed] [Google Scholar]
- 18.Iribarne A, Leavitt BJ, Robich MP, et al. Tissue versus mechanical aortic valve replacement in younger patients: A multicenter analysis. J Thorac Cardiovasc Surg. 2019;158(6):1529–1538 e1522. [DOI] [PubMed] [Google Scholar]
- 19.Koretsune Y, Ikeda T, Kozuma K, et al. Patient satisfaction after switching from warfarin to apixaban in patients with nonvalvular atrial fibrillation: AGAIN study. Patient Prefer Adherence. 2017;11:1987–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilke T, Bauer S, Mueller S, Kohlmann T, Bauersachs R. Patient Preferences for Oral Anticoagulation Therapy in Atrial Fibrillation: A Systematic Literature Review. Patient. 2017;10(1):17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puskas J, Gerdisch M, Nichols D, et al. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg. 2014;147(4):1202–1210; discussion 1210–1201. [DOI] [PubMed] [Google Scholar]
- 22.Puskas JD, Gerdisch M, Nichols D, et al. Anticoagulation and Antiplatelet Strategies After On-X Mechanical Aortic Valve Replacement. J Am Coll Cardiol. 2018;71(24):2717–2726. [DOI] [PubMed] [Google Scholar]
- 23.Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–1214. [DOI] [PubMed] [Google Scholar]
- 24.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. [DOI] [PubMed] [Google Scholar]
- 25.Lopes RD, Alexander JH, Al-Khatib SM, et al. Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159(3):331–339. [DOI] [PubMed] [Google Scholar]
- 26.Touma L, Filion KB, Atallah R, Eberg M, Eisenberg MJ. A meta-analysis of randomized controlled trials of the risk of bleeding with apixaban versus vitamin K antagonists. Am J Cardiol. 2015;115(4):533–541. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Butchart EG, Borer JS, Yoganathan A, Grunkemeier GL. Clinical evaluation of new heart valve prostheses: update of objective performance criteria. Ann Thorac Surg. 2014;98(5):1865–1874. [DOI] [PubMed] [Google Scholar]
- 28.McNicholas KW, Ivey TD, Metras J, et al. North American multicenter experience with the On-X prosthetic heart valve. J Heart Valve Dis. 2006;15(1):73–78; discussion 79. [PubMed] [Google Scholar]
- 29.Tossios P, Reber D, Oustria M, et al. Single-center experience with the On-X prosthetic heart valve between 1996 and 2005. J Heart Valve Dis. 2007;16(5):551–557. [PubMed] [Google Scholar]
- 30.Chambers JB, Pomar JL, Mestres CA, Palatianos GM. Clinical event rates with the On-X bileaflet mechanical heart valve: a multicenter experience with follow-up to 12 years. J Thorac Cardiovasc Surg. 2013;145(2):420–424. [DOI] [PubMed] [Google Scholar]
- 31.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lester PA, Coleman DM, Diaz JA, et al. Apixaban Versus Warfarin for Mechanical Heart Valve Thromboprophylaxis in a Swine Aortic Heterotopic Valve Model. Arterioscler Thromb Vasc Biol. 2017;37(5):942–948. [DOI] [PubMed] [Google Scholar]
- 33.McKellar SH, Abel S, Camp CL, Suri RM, Ereth MH, Schaff HV. Effectiveness of dabigatran etexilate for thromboprophylaxis of mechanical heart valves. J Thorac Cardiovasc Surg. 2011;141(6):1410–1416. [DOI] [PubMed] [Google Scholar]
- 34.Lopes RD, Heizer G, Aronson R, et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019;380(16):1509–1524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.