This work shows that the septum of Staphylococcus warneri is composed of two layers and that the peptidoglycan on the inner surface of the double-layered septum is organized into concentric rings. Moreover, new cell cycles of S. warneri can be initiated before the previous cell cycle is complete. This work advances our knowledge about a basic structure of bacterial cell and provides information on the double-layered structure of the septum for bacteria that divide with the “popping” mechanism.
KEYWORDS: Staphylococcus, division, septum, cell wall, atomic force microscopy, cell division
ABSTRACT
Cell division of Staphylococcus adopts a “popping” mechanism that mediates extremely rapid separation of the septum. Elucidating the structure of the septum is crucial for understanding this exceptional bacterial cell division mechanism. Here, the septum structure of Staphylococcus warneri was extensively characterized using high-speed time-lapse confocal microscopy, atomic force microscopy, and electron microscopy. The cells of S. warneri divide in a fast popping manner on a millisecond timescale. Our results show that the septum is composed of two separable layers, providing a structural basis for the ultrafast daughter cell separation. The septum is formed progressively toward the center with nonuniform thickness of the septal disk in radial directions. The peptidoglycan on the inner surface of double-layered septa is organized into concentric rings, which are generated along with septum formation. Moreover, this study signifies the importance of new septum formation in initiating new cell cycles. This work unravels the structural basis underlying the popping mechanism that drives S. warneri cell division and reveals a generic structure of the bacterial cell.
IMPORTANCE This work shows that the septum of Staphylococcus warneri is composed of two layers and that the peptidoglycan on the inner surface of the double-layered septum is organized into concentric rings. Moreover, new cell cycles of S. warneri can be initiated before the previous cell cycle is complete. This work advances our knowledge about a basic structure of bacterial cell and provides information on the double-layered structure of the septum for bacteria that divide with the “popping” mechanism.
INTRODUCTION
The septum is one of the key cellular structures during the cell division of Gram-positive bacteria. A mother cell propagates by septum formation and subsequent septum splitting (1, 2). The septum structure and formation are closely related to the means of daughter cell separation during cell division. Gram-positive organisms have evolved various methods of daughter cell separation. For example, division of Bacillus subtilis cells is mediated by the well-known gradual enzymatic splitting on the septum (3–5). Unlike this slow splitting process, cell division of Staphylococcus aureus, an aggressive pathogen and model coccoid Gram-positive bacterium, occurs as an ultrafast “popping” on the septum, usually on a millisecond timescale (6, 7). The fast mechanical propagation has been found not only in Staphylococcus and its close relative genus Macrococcus but also in Actinobacteria, with diverse shapes ranging from coccoid to rod (5).
Septum separation represents a key step of bacterial cell division. Understanding the generic structure and biosynthesis process of the septum is of paramount importance for elucidating the mechanism of bacterial division. In contrast to the one-layered septum in most Gram-positive bacteria, such as Bacillus (3, 8, 9), the septum structure of Staphylococcus seemed more complicated. Transmission electron microscopy (TEM) revealed a thin densely stained line located in the middle of the septum, and this structure was named the splitting system (10). Further cryo-electron microscopy (cryo-EM) studies have led to advanced knowledge about the structure and formation of the bacterial septum (11, 12). Cryo-EM of S. aureus cells showed a low-density region sandwiched between two high-density regions in the septum, suggesting that the septum of S. aureus was composed of two layers (11). Results of scanning electron microscopy (SEM) indicated that during the separation of daughter cells, breakage of the peripheral rings of the septum was initiated by perforations and eventually led to the separation of daughter cells through the ultrafast popping mechanism (7).
Advanced knowledge of septum formation in Staphylococcus requires extensive studies on the three-dimensional structure of the whole septum, similar to research on isolated septa in B. subtilis (13, 14). In addition to the electron-microscopic studies, atomic force microscopy (AFM) has been exploited in characterizing the overall structure of isolated septa from S. aureus (15) and perforations on the peripheral rings of septa (16). The peripheral rings of septa were further demonstrated to be mechanically softer than adjacent cell walls by AFM indentation (17). Despite the previous findings, the dynamic structures of the septa during septum formation and the relationship between the septum structure and cell wall synthesis remain unclear.
Staphylococcus warneri belongs to the clinically defined Staphylococcus epidermidis group within the genus Staphylococcus (18). It is less virulent than S. aureus and can usually be detected on the surface of the human body, on animals, and in fermented food (18). Although clinical cases related to S. warneri have been increasingly reported (19–22), we still know little about the growth and division of this species. In this study, we conducted in-depth structural visualization of the septa isolated from S. warneri using AFM, which is a powerful tool in delineating the structures and spatial organization of cell wall and biological membranes with the high resolution (23–27), combined with high-speed time-lapse confocal microscopy and electron microscopy. A comprehensive analysis of the structure and formation of bacterial septa will advance our knowledge about the generic structure of the bacterial cell and the structural basis of the popping cell division.
RESULTS
S. warneri divides in a fast popping manner within milliseconds.
S. warneri is a close relative of S. aureus and a coagulase-negative Staphylococcus species (18). To examine whether S. warneri cells divide in the same ultrafast popping manner as S. aureus cells, we visualized the division process of S. warneri using high-speed time-lapse Andor Dragonfly confocal microscopy at 2.5-ms intervals (Fig. 1A to D) following previously reported procedures (5–7). A rapid increase in cell volume occurred within 2.5 ms (n = 6), which is a characteristic feature of the popping division resulting from the ultrafast separation of daughter cells (5–7). Moreover, SEM images revealed failures in the peripheral ring (Fig. 1E) and hinges between daughter cells (Fig. 1F), morphological characteristics of the popping cell division found in S. aureus (7). Our results demonstrated that S. warneri undergoes a fast popping daughter cell separation, on a millisecond timescale.
FIG 1.

Division of S. warneri cells. (A to D) Time-lapse microscopy for daughter cell separation in S. warneri. The time interval for each frame was 2.5 ms. Arrows (B and C) indicate the division event. Bars, 1 μm. (E and F) SEM observations of S. warneri cells. The arrow in panel E indicates the mechanical failure in the peripheral ring. Arrows in panel F indicate the separated daughter cells connected with hinges. Bars, 1 μm.
The septum is formed progressively toward the center with nonuniform thickness.
To explore the structural basis that mediates the popping division, we characterized the overall structure of septa isolated from the broken sacculus fragments of S. warneri using AFM (see Fig. S1 in the supplemental material). The septum comprises a peripheral ring and a septal disk (Fig. 2). The piecrust feature that has been shown on the peripheral ring in S. aureus (15) was not observed in S. warneri in the present study. This may be ascribed to the differences among Staphylococcus species. The prepared septa contain the complete septa and incomplete septa with central gaps that vary in size, representing distinct stages of septum formation. These results suggest that the septal disk is formed progressively toward the center until the center gap is sealed, which is similar to the synthesis process in Bacillus (13, 14). Analysis of the whole septa showed that the width of the annulus in the incomplete septa in every radial direction appears even (Fig. 2), indicating a simultaneous formation in all directions on the septal disk.
FIG 2.
Progression of septum formation observed with AFM. (A, D, and G) Peak force error images. (B, E, and H) Height images. (C, F, and I) Cross-section analysis of the corresponding AFM height images in the row above. Black and red arrows indicate the position and direction of the section analyses. Bars, 0.5 μm.
The thickness of the incomplete septum varies across the septal disk in radial directions, with a thinner leading edge than the lagging edge, in agreement with previous observations (10, 11). The thickness of incomplete septa in S. warneri varies at different positions of the septum, in contrast to the even thickness observed in Bacillus (14). In contrast, the thickness of the completed septum appears relatively consistent, though a thin central region was occasionally seen in the septal disk, likely representing the newly completed septum (Fig. 2G and H). Such thickness distribution suggests that the septum at the leading edge is first synthesized as a thin form; during the progress of septum formation, new cell wall materials are integrated across the developing septal surface to generate a thicker septal disk until the completed septum is formed. This dynamic synthesis process proposed based on AFM observations supports the hypothesis of septum formation suggested by thin-section TEM of S. aureus cells (28).
The septum of S. warneri comprises two structural layers.
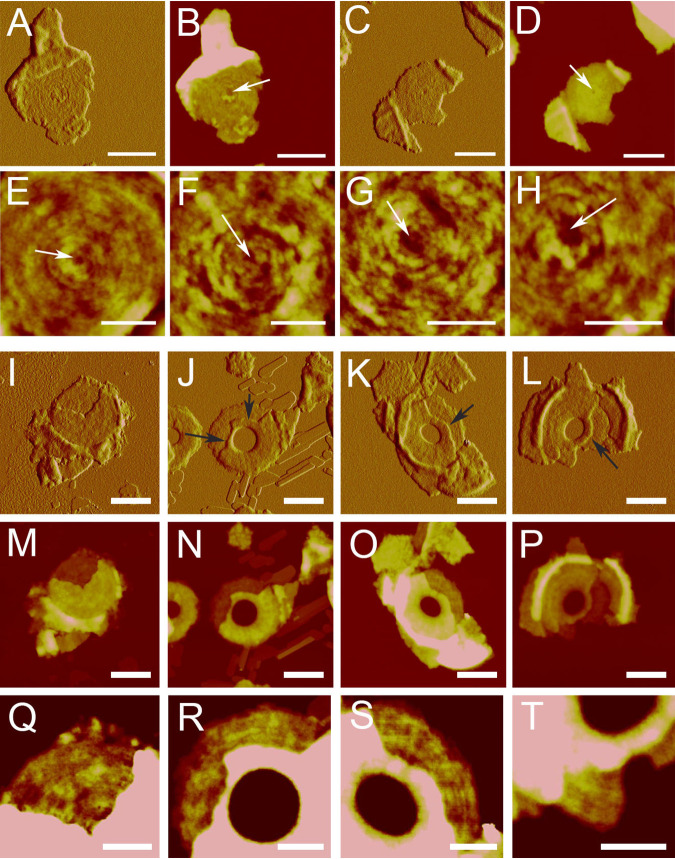
To elucidate the septal structure, broken septa were isolated and imaged by AFM. A total of 43 broken complete septa were found, and they all explicitly showed a double-layered structure (Fig. 3A to O). The upper layers of the broken complete septa were partially lost, exposing the bottom layers. The thickness of each layer (∼10 nm) is about half of the thickness of a septum (∼20 nm), demonstrating that the septum is composed of two separable layers. The formation of double-layered septa could function as a structural basis for the popping daughter cell separation in S. warneri.
FIG 3.
Broken complete and incomplete septa show a double-layered structure. (A, D, G, J, and M) Peak force error images of complete septa. White arrows indicate the central depressions. (B, E, H, K, and N) Height images of complete septa. (C, F, I, L, and O) Cross-section analysis of complete septa in their AFM height images in the row above. (P, R, T, V, and X) Peak force error images of incomplete septa. (Q, S, U, W, and Y) Cross-section analysis of incomplete septa in the corresponding AFM height images in the row above. Positions and directions for the cross-section analyses are indicated by black and red arrows. Bars, 0.5 μm.
AFM imaging of broken completed septa did not allow us to answer whether (i) the double-layered structure was formed during the formation of the septum or (ii) a completed single-layered septum was first formed and then split into two layers. To address these questions, we carried out AFM analysis on the broken incomplete septa at various stages of septal formation. The broken site of the septum where double-layered structures could be visualized occurred across the septal disk from the leading edge to the lagging edge of the incomplete septa (Fig. 3P to Y), suggesting that the double-layered structure was formed during septum formation.
Concentric-ring structures on new cell walls were formed prior to separation of the septum.
We identified the concentric rings as the characteristic structures of new cell walls in S. warneri cells (Fig. S2), in line with other Staphylococcus species in previous studies (16, 29). Similar structures have also been discerned in the cell walls of other coccoid Gram-positive organisms (30–32). Then, we imaged the isolated new cell wall fragments of S. warneri using AFM. Of the 117 isolated new cell wall fragments characterized, which have a flat disc shape with a thickness of ∼10 nm (Fig. S3), 65 fragments (56%) showed concentric-ring structures on the cell wall surfaces (Fig. 4A and B), whereas the surface features of the other fragments (44%) were less clear (Fig. 4C and D). It is likely that the featured surface and relatively featureless surface represent the two distinctly oriented sides of new cell walls. High-resolution AFM images showed that the ring structures were tightly associated, with an average bandwidth of ∼16 nm (n = 30) (Fig. 4E to H).
FIG 4.
Concentric-ring structures on the surface of new cell walls and the inner surfaces of double-layered septa. (A to D) AFM images of isolated new cell walls. (A and B) New cell wall with concentric rings. Bars, 0.5 μm. (C and D) New cell wall with unclear surface features. Bars, 0.5 μm. (A and C) Peak force error images; (B and D) height images. (E to H) High-resolution AFM height images of the concentric rings on new cell walls. Bars, 200 nm. Arrows indicate the central depressions. (I to L) Peak force error images of broken septa. Arrows indicate fractures. Bars, 0.5 μm. (M to P) Height images of the broken septa in the corresponding peak force error images in the row above. Bars, 0.5 μm. (Q to T) Zoomed-in images from the corresponding height images in the row above. Bars, 200 nm. Pink areas in the height images represent higher structures that were beyond the height range for display.
When the concentric-ring structures in new cell walls were generated remains unclear. It is known that new cell walls were formed by separation of the double-layered septum. This information was further confirmed by the fact that about half of the isolated new cell wall fragments showed concentric-ring structures while the other half did not (Fig. 4). No concentric rings were detected on the outer surface of the septum (Fig. 2; Fig. S4). However, in the broken complete septa, concentric-ring structures appeared in the exposed inner surface (Fig. 4I, M, and Q), indicating that each layer of the septum had one relatively featureless side and one featured side with concentric rings.
We next questioned whether the concentric rings were formed after the completion of septum formation or along with septum formation. AFM images of the exposed inner surface of the incomplete septa revealed the presence of concentric rings on the inner surface of the double-layered incomplete septum (Fig. 4J to L, N to P, and R to T). The results demonstrated that the concentric-ring structure was formed during septum formation.
Visualization of the concentric rings provides clues as to how peptidoglycan is organized in the septum. The concentric-ring organization of peptidoglycan could be generated with the progressive formation of the septum toward the center of the septal disk (Fig. 2). Moreover, we found that fractures on the broken septum layers often appear along the direction of the concentric rings (Fig. 4J to L, arrows), further suggesting that the concentric rings might not only be a surface feature but also reflect the intrinsic peptidoglycan organization in cell walls.
A connection between two layers of the incomplete septum at the interior leading edge.
Among the broken incomplete septa that we characterized (n = 30), partial upper layers were absent and bottom layers were exposed. In most cases (29 of 30), the broken incomplete septa showed no sign of separation of two layers at the interior edge. In some cases, the fractures were close to the interior edge, as depicted in Fig. 4J to L (arrows). However, the interior edge remained intact. This led to the assumption that the two layers are separable but the physical separation may be hard, implying a strong connection between the two layers of the incomplete septum at the interior edge, consistent with previous cryo-EM observations (11).
To verify this hypothesis, we investigated the incomplete septa that have lost their peripheral rings. Previous SEM (7) and cryo-EM (11) of the dividing Staphylococcus cells suggested that the peripheral ring functions as the site for the structural connection between septum layers. Based on this, when the peripheral ring is removed, connections between two layers at the peripheral sites should be lost. Therefore, since the two layers of the septum could be separated, as proved by our AFM observations, for the incomplete septa without peripheral rings, it is expected that two layers could be completely separated if there are no connections between them at the interior edge, and vice versa. We found a total of 17 incomplete septa whose peripheral rings were completely lost (Fig. 5A to H). Section analysis showed that their average heights were about 20 nm (Fig. 5I to L). No separation of the two layers and no separated single layers of incomplete septa were observed (Fig. 5A to H).
FIG 5.
Analysis of the connection at the interior leading edge of incomplete septa. (A to D) Peak force error images of incomplete septa that had lost peripheral rings. Bars, 0.5 μm. (E to H) Height images of the corresponding peak force error images in the row above. Bars, 0.5 μm. (I to L) Cross-section analyses of the corresponding AFM height images in the row above. Arrows indicate the position and direction of the section analyses. (M to P) Height images of new cell walls with complete belts. Bars, 0.5 μm. (Q and R) Height images of new cell walls with discontinuous belts. Bars, 0.5 μm. Arrows indicate the central depressions. (S) Structural model of an incomplete septum.
In Staphylococcus, new septa are synthesized perpendicular to the previous division plane crossing the previous new cell wall (6, 15). We visualized 48 new cell wall fragments with belt-like structures growing across the new cell walls in S. warneri (Fig. 5M to P), which may represent the initial synthesis of new septa (15). These belt-like structures do not exhibit a two-layered structure but are complete belts that are sealed at the top (Fig. 5M to P). This result further suggests that the two layers of incomplete septa could be connected at the interior leading edge.
The central depression is an inherent structure in the septum and new cell wall.
AFM images revealed the existence of the central depression in almost all the complete septa (Fig. 3A to O). One might question whether the central depression we observed is an inherent structure or resulted from the incomplete synthesis of the septum. To address this question, we analyzed the structures of the new cell walls. New cell walls are transformed from the complete septa after daughter cell separation. The possibility of incomplete synthesis of septa could be excluded by analyzing the structure of new cell walls. We found that the central depression is always present in the centers of new cell walls enclosed by the concentric rings (Fig. 4A to H and Fig. 5M to P). Moreover, the depression structures have also been seen in the centers of new cell walls in intact bacterial cells (16, 29). Therefore, it is unlikely that the central depression resulted from the incomplete synthesis of the septum, but it may represent an intrinsic structure of the septum and new cell walls. This depression feature has not been observed in previous TEM images (33–37), as identifying this structure requires proper sectioning across the center of the septum. Our AFM results provide insight into the septum structure and raise an open question of how the last step of septum synthesis is performed.
An interesting observation was the initiation of the synthesis of a new septum. When new septum synthesis was initiated, the septa always chose a path to avoid crossing the central depressions (Fig. 5M to P). Although the physiological function of the central depression remains unclear, the results indicate that the central depression is mechanically unfavorable for the synthesis of a new septum. It is possible that the central depression hampers proper localization and interactions of protein complexes that are contributing factors in septum synthesis. In some cases, cells tend to build a new septum across the center depression. However, synthesis was halted in the vicinity of center depressions (Fig. 5Q and R). No other types of discontinuous belt structures were observed in our work. These results suggest a negative effect of the center depression on new septum synthesis.
A new round of the cell cycle can be initiated before the septum is separated.
The cell cycle of S. aureus involves three phases (6). In phase 1, cells are slightly elongated without formation of the septum. Then, the cells initiate and complete the septum formation in phase 2 (Fig. 6G). In phase 3, cells with complete septa are elongated and ultimately divide into two daughter cells (6). After division, a new round of the cell cycle in Staphylococcus cells commences. We found that some S. warneri cells adopt different types of cell cycles. Of 115 completed septa, 32 have unequivocal belt-like structures (Fig. 6A to F). The thickness of these sacculus fragments is ∼20 nm, indicating that they are septa instead of new cell walls. More importantly, some broken septa with belt-like structures exhibit double-layered structures (Fig. 6A to F), suggesting that these belt-like structures were synthesized on real septa. These belt-like structures were observed only on complete septa and never on incomplete septa, probably revealing that the belt structures were synthesized after the septa were complete. Formation of such a belt structure showed initiation of the synthesis of new septum before the old septum was separated. These results indicate that a new round of the cell cycle can commence before the last one is complete (Fig. 6H).
FIG 6.
Belt structures formed on the complete septa. (A to F) Complete septa with belt structures. (A and D) Peak force error images. (B and E) Height images. (C and F) Section analyses of the images in panels B and E, respectively. Positions and directions for section analyses are indicated with black and red arrows. White arrows indicate the central depressions. Bars, 0.5 μm. (G) Model of the cell cycle in S. aureus, adapted from reference 6. (H) Model of the cell cycle in S. warneri based on this work.
DISCUSSION
The septum is a key cell wall structure that is of physiological significance for cell division of Gram-positive bacteria. Here, we conducted extensive characterization of the dynamic structures of septa from S. warneri, which divides via a fast popping mechanism on a millisecond timescale. A striking feature is that the septum is composed of two separable layers (Fig. 5S), which may lay the mechanistic foundation for bacteria like S. warneri to undergo daughter cell separation in an extremely fast popping fashion (6, 7).
The peptidoglycan on the newly synthesized cell wall of S. warneri is organized to form concentric-ring structures, in agreement with the previous finding (16, 29). Our results suggested that such structures in new cell walls may be formed before the septum is separated. We demonstrated that the concentric-ring structures appear on the inner surface of the septal peptidoglycan and could be formed with the progressive formation of the septum toward the center of the septal disk. The concentric-ring structures in septal peptidoglycan resemble the so-called splitting system observed with thin-section TEM of bacterial cells (10). The nature of the splitting system has remained elusive over the past decades. This work revealed that the peptidoglycan is organized as concentric-ring structures in the septum.
The bacterial cell cycle requires several complex cellular processes that are thought to be tightly regulated and precisely coordinated (2, 38, 39). Formation of the septum is an important process in the bacterial division and should occur in time to ensure the quality of propagation (38). We found that a portion of the S. warneri cells initiated the next round of the cell cycle before the previous one was complete. It remains unknown whether such an unusual cell cycle resulted from the failure of regulation or is an adopted strategy in response to internal or environmental variations. This work indicated that the arrangement of a cell cycle in S. warneri differs greatly at the single-cell level. Elucidating how bacterial cells enter the next round of the cell cycle is of fundamental importance for a deeper understanding of the regulatory mechanism underlying bacterial growth and division.
MATERIALS AND METHODS
Purification of sacculi.
Sacculi were purified as described previously (13, 15). Briefly, S. warneri was grown in Luria-Bertani (LB) medium at 25°C overnight with agitation. Cells were collected and boiled for 7 min. For isolating septa or broken sacculi, cells were broken by ultrasonication at approximately 200 to 400 W (5-s pulses with 5-s intervals for 99 rounds). Ultrasonication was repeated 4 or 5 times until the suspension became clear. Then, broken cells were treated by boiling in SDS (5% [wt/vol]), RNase (0.5 mg ml−1; Sigma-Aldrich Co., LLC, Shanghai, China), DNase (0.5 mg ml−1; Sigma-Aldrich Co., LLC, Shanghai, China), and pronase (2 mg ml−1; Solarbio Co., Ltd., Beijing, China). Removal of accessory polymers was achieved by incubation in 48% (vol/vol) HF at 4°C for 24 h. Purified sacculi were washed with MilliQ water six times at room temperature.
AFM.
AFM imaging was carried out by using a Multimode VIII AFM with a Nanoscope V controller (Bruker AXS, Germany). Silicon nitride cantilevers (XSC11/AL BS; NanoAndMore Corp., USA) were used for imaging under ambient conditions. AFM imaging in air condition was carried out in ScanAsyst mode. Peak force error images and height images were recorded simultaneously. The height images provide quantitative height information and can be used for analysis, whereas peak force error is obtained by subtraction of the set-point force from the deflection signal. The peak force error images show the edges of features in the height image; for soft materials, peak force error images normally provide clearer three-dimensional views than the height images (40, 41).
SEM.
Samples were fixed in glutaraldehyde and osmium tetroxide and then dehydrated by a series of increasing concentrations of ethanol (10%, 30%, 50%, 75%, 90%, 95%, and 100%). Then samples were dried with carbon dioxide in an EM CPD 300 critical-point dryer (Leica, Germany), sputter coated with gold-platinum in a 108 sputter coater (Cressington, UK) for 240 s, and examined with a Quanta FEG 250 (FEI, USA) at 10 kV.
High-speed time-lapse confocal microscopy.
Two-dimensional (2D) time-lapse imaging was performed on an Andor Dragonfly confocal system. The microscope was Leica DMi8 with a 100× (numerical aperture [NA], 1.4) oil immersion objective. Cells were imaged at room temperature. The camera was Andor Zyla 4.2 Plus USB version, and the capture speed was 400 frames per s with an array size of 512 by 512 pixels. Camera pixel size was 6.5 μm.
Supplementary Material
ACKNOWLEDGMENTS
We thank Haiyan Yu, Xiaomin Zhao, Sen Wang, and Qi Chen from State Key Laboratory of Microbial Technology of Shandong University for technical help in this work. We thank Gang Wang from Andor Technology, Ltd., for technical help in confocal microscopy observation.
This work was supported by the National Natural Science Foundation of China (31900023), National Key R&D Program of China (2018YFC1406701), Natural Science Foundation of Shandong (ZR2018ZB0211), State Key Laboratory of Microbial Technology Open Projects Fund (M2019-07), Program of Shandong Taishan Scholars (TS20090803), Young Scholars Program of Shandong University (2017WLJH22), UK Royal Society (UF120411, URF\R\180030), and Biotechnology and Biological Sciences Research Council grants (BB/M024202/1, BB/R003890/1).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
H.-N.S. and L.-N.L. designed the research; H.-N.S., L.-N.L., K.L., L.-S.Z., X.-X.Y., M.-Y.Z., and S.-M.L. performed the research; H.-N.S., X.-L.C., and L.-N.L. analyzed the data; H.-N.S., Y.-Z.Z., and L.-N.L. wrote the paper.
We declare that no competing interests exist.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Adams DW, Errington J. 2009. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol 7:642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- 2.Pinho MG, Kjos M, Veening J-W. 2013. How to get (a)round: mechanisms controlling growth and division of coccoid bacteria. Nat Rev Microbiol 11:601–614. doi: 10.1038/nrmicro3088. [DOI] [PubMed] [Google Scholar]
- 3.Koch AL, Burdett I. 1986. Normal pole formation during total inhibition of wall synthesis of Bacillus subtilis. J Gen Microbiol 132:3441–3449. doi: 10.1099/00221287-132-12-3441. [DOI] [PubMed] [Google Scholar]
- 4.Smith TJ, Blackman SA, Foster SJ. 2000. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiol 146:249–262. doi: 10.1099/00221287-146-2-249. [DOI] [PubMed] [Google Scholar]
- 5.Zhou X, Halladin DK, Theriot JA. 2016. Fast mechanically driven daughter cell separation is widespread in Actinobacteria. mBio 7:e00952-16. doi: 10.1128/mBio.00952-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro JM, Fernandes PB, Vaz F, Pereira AR, Tavares AC, Ferreira MT, Pereira PM, Veiga H, Kuru E, VanNieuwenhze MS, Brun YV, Filipe SR, Pinho MG. 2015. Cell shape dynamics during the staphylococcal cell cycle. Nat Commun 6:8055. doi: 10.1038/ncomms9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Halladin DK, Rojas ER, Koslover EF, Lee TK, Huang KC, Theriot JA. 2015. Mechanical crack propagation drives millisecond daughter cell separation in Staphylococcus aureus. Science 348:574–578. doi: 10.1126/science.aaa1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdett IDJ, Higgins ML. 1978. Study of pole assembly in Bacillus subtilis by computer reconstruction of septal growth zones seen in central, longitudinal thin sections of cells. J Bacteriol 133:959–971. doi: 10.1128/JB.133.2.959-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch AL, Kirchner G, Doyle RJ, Burdett ID. 1985. How does a Bacillus split its septum right down the middle? Ann Inst Pasteur Microbiol 136(Suppl A):91–98. doi: 10.1016/s0769-2609(85)80028-4. [DOI] [PubMed] [Google Scholar]
- 10.Giesbrecht P, Kersten T, Maidhof H, Wecke J. 1998. Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol Mol Biol Rev 62:1371–1414. doi: 10.1128/MMBR.62.4.1371-1414.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matias VRF, Beveridge TJ. 2007. Cryo-electron microscopy of cell division in Staphylococcus aureus reveals a mid-zone between nascent cross walls. Mol Microbiol 64:195–206. doi: 10.1111/j.1365-2958.2007.05634.x. [DOI] [PubMed] [Google Scholar]
- 12.Zuber B, Haenni M, Ribeiro T, Minnig K, Lopes F, Moreillon P, Dubochet J. 2006. Granular layer in the periplasmic space of gram-positive bacteria and fine structures of Enterococcus gallinarum and Streptococcus gordonii septa revealed by cryo-electron microscopy of vitreous sections. J Bacteriol 188:6652–6660. doi: 10.1128/JB.00391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayhurst EJ, Kailas L, Hobbs JK, Foster SJ. 2008. Cell wall peptidoglycan architecture in Bacillus subtilis. Proc Natl Acad Sci U S A 105:14600–14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li K, Yuan X-X, Sun H-M, Zhao L-S, Tang R, Chen Z-H, Qin Q-L, Chen X-L, Zhang Y-Z, Su H-N. 2018. Atomic force microscopy of side wall and septa peptidoglycan from Bacillus subtilis reveals an architectural remodeling during growth. Front Microbiol 9:620. doi: 10.3389/fmicb.2018.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner RD, Ratcliffe EC, Wheeler R, Golestanian R, Hobbs JK, Foster SJ. 2010. Peptidoglycan architecture can specify division planes in Staphylococcus aureus. Nat Commun 1:26. doi: 10.1038/ncomms1025. [DOI] [PubMed] [Google Scholar]
- 16.Touhami A, Jericho MH, Beveridge TJ. 2004. Atomic force microscopy of cell growth and division in Staphylococcus aureus. J Bacteriol 186:3286–3295. doi: 10.1128/JB.186.11.3286-3295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey RG, Turner RD, Mullin N, Clarke N, Foster SJ, Hobbs JK. 2014. The interplay between cell wall mechanical properties and the cell cycle in Staphylococcus aureus. Biophys J 107:2538–2545. doi: 10.1016/j.bpj.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker K, Heilmann C, Peters G. 2014. Coagulase-negative staphylococci. Clin Microbiol Rev 27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arslan F, Saltoglu N, Mete B, Mert A. 2011. Recurrent Staphylococcus warneri prosthetic valve endocarditis: a case report and review. Ann Clin Microbiol Antimicrob 10:14. doi: 10.1186/1476-0711-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaconu R, Golumbeanu E, Constantin A, Donoiu I. 2019. Native valve endocarditis with Staphylococcus warneri. BMJ Case Rep 12:e229546. doi: 10.1136/bcr-2019-229546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanuparthy A, Challa T, Meegada S, Siddamreddy S, Muppidi V. 2020. Staphylococcus warneri: skin commensal and a rare cause of urinary tract infection. Cureus 12:e8337. doi: 10.7759/cureus.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuvhenguhwa MS, Belgrave KO, Shah SU, Bayer AS, Miller LG. 2017. A case of early prosthetic valve endocarditis caused by Staphylococcus warneri in a patient presenting with congestive heart failure. Cardiol Res 8:236–240. doi: 10.14740/cr588w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller DJ, Dufrêne YF. 2011. Atomic force microscopy: a nanoscopic window on the cell surface. Trends Cell Biol 21:461–469. doi: 10.1016/j.tcb.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Turner RD, Vollmer W, Foster SJ. 2014. Different walls for rods and balls: the diversity of peptidoglycan. Mol Microbiol 91:862–874. doi: 10.1111/mmi.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L-N, Scheuring S. 2013. Investigation of photosynthetic membrane structure using atomic force microscopy. Trends Plant Sci 18:277–286. doi: 10.1016/j.tplants.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Casella S, Huang F, Mason D, Zhao G-Y, Johnson GN, Mullineaux CW, Liu L-N. 2017. Dissecting the native architecture and dynamics of cyanobacterial photosynthetic machinery. Mol Plant 10:1434–1448. doi: 10.1016/j.molp.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L-N, Duquesne K, Oesterhelt F, Sturgis JN, Scheuring S. 2011. Forces guiding assembly of light-harvesting complex 2 in native membranes. Proc Natl Acad Sci U S A 108:9455–9459. doi: 10.1073/pnas.1004205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lund VA, Wacnik K, Turner RD, Cotterell BE, Walther CG, Fenn SJ, Grein F, Wollman AJ, Leake MC, Olivier N, Cadby A, Mesnage S, Jones S, Foster SJ. 2018. Molecular coordination of Staphylococcus aureus cell division. Elife 7:e32057. doi: 10.7554/eLife.32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francius G, Domenech O, Mingeot-Leclercq MP, Dufrêne YF. 2008. Direct observation of Staphylococcus aureus cell wall digestion by lysostaphin. J Bacteriol 190:7904–7909. doi: 10.1128/JB.01116-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andre G, Kulakauskas S, Chapot-Chartier M-P, Navet B, Deghorain M, Bernard E, Hols P, Dufrêne YF. 2010. Imaging the nanoscale organization of peptidoglycan in living Lactococcus lactis cells. Nat Commun 1:27. doi: 10.1038/ncomms1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dover RS, Bitler A, Shimoni E, Trieu-Cuot P, Shai Y. 2015. Multiparametric AFM reveals turgor-responsive net-like peptidoglycan architecture in live streptococci. Nat Commun 6:7193. doi: 10.1038/ncomms8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Mei HC, Busscher HJ, Bos R, Vries J, Boonaert CJP, Dufrêne YF. 2000. Direct probing by atomic force microscopy of the cell surface softness of a fibrillated and nonfibrillated oral streptococcal strain. Biophys J 78:2668–2674. doi: 10.1016/S0006-3495(00)76810-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belley A, Neesham-Grenon E, McKay G, Arhin FF, Harris R, Beveridge T, Parr TRJ, Moeck G. 2009. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother 53:918–925. doi: 10.1128/AAC.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton-Miller JM, Shah S. 1999. Disorganization of cell division of methicillin-resistant Staphylococcus aureus by a component of tea (Camellia sinensis): a study by electron microscopy. FEMS Microbiol Lett 176:463–469. doi: 10.1111/j.1574-6968.1999.tb13698.x. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS. 2010. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother 54:3132–3142. doi: 10.1128/AAC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorian V. 1975. Some effects of subinhibitory concentrations of penicillin on the structure and division of staphylococci. Antimicrob Agents Chemother 7:864–867. doi: 10.1128/AAC.7.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugai M, Yamada S, Nakashima S, Komatsuzawa H, Matsumoto A, Oshida T, Suginaka H. 1997. Localized perforation of the cell wall by a major autolysin: atl gene products and the onset of penicillin-induced lysis of Staphylococcus aureus. J Bacteriol 179:2958–2962. doi: 10.1128/JB.179.9.2958-2962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu LJ, Errington J. 2012. Nucleoid occlusion and bacterial cell division. Nat Rev Microbiol 10:8–12. doi: 10.1038/nrmicro2671. [DOI] [PubMed] [Google Scholar]
- 39.Willis L, Huang KC. 2017. Sizing up the bacterial cell cycle. Nat Rev Microbiol 15:606–620. doi: 10.1038/nrmicro.2017.79. [DOI] [PubMed] [Google Scholar]
- 40.Dujardin A, Wolf PD, Lafont F, Dupres V. 2019. Automated multi-sample acquisition and analysis using atomic force microscopy for biomedical applications. PLoS One 14:e0213853. doi: 10.1371/journal.pone.0213853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maghsoudy-Louyeh S, Kropf M, Tittmann BR. 2018. Review of progress in atomic force microscopy. Open Neuroimag J 12:86–104. doi: 10.2174/1874440001812010086. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.