Abstract
The objective of the present study was to develop extended-release (ER) hot-melt extruded (HME) abuse-deterrent pellets of acetaminophen, a model drug, by utilizing high molecular weight polyethylene oxide (PEO) and gelling agents (xanthan gum, guar gum, and gellan gum). The HME pellets were evaluated for their abuse-deterrence (AD) potential by Category-1 laboratory in-vitro evaluation parameters, including particle size reduction (PSR), small volume extraction, dissolution, viscosity, syringeability, and injectability. Further, the pellets were investigated for resistance to physical (crushing) and thermal (oven and microwave) manipulation to evaluate the strength of the AD properties. Physical manipulation studies demonstrated that the pellets were intact, extremely hard, and resistant to PSR and manipulation to bypass ER properties. Dissolution of all intact and physically manipulated pellets led to complete drug release within 8 h, and resistance to dose-dumping in 40% ethanol was observed. The drug extraction was <50% in 10 mL of ingestible and non-ingestible solvents under static, agitation, and thermal manipulation conditions with an incubation time of 30 min. The PEO/xanthan gum-based formulation showed higher viscosity, syringe and injection forces, and lower syringeable volume in all manipulation conditions compared with plain PEO pellets. These findings supported the AD potential of PEO and xanthan gum pellets against intravenous abuse.
Keywords: Abuse-deterrent formulation, Polyethylene oxide, Gelling agent, Hot melt extrusion, Physical and thermal manipulation, Syringeability and Injectability
1. Introduction
Millions of people in the United States (US) experience chronic pain. Non-opioid (non-steroidal anti-inflammatory) and opioid drugs are used to alleviate pain. However, owing to the different mechanisms of action of opioid analgesic drugs, some patients find better pain relief with these drugs than from the non-opioid therapies; thus, opioid therapies are more effective for symptomatic relief of chronic pain (Rahman et al., 2016; Wiffen et al., 2016).
The rise in abuse of prescription opioid drugs in the US has attracted the attention of regulatory authorities, guiding focus on the design and development of robust abuse-deterrent formulations (ADFs). Depending on the route of administration chosen for abuse, the frequent nonmedical use of opioids leads to drug addiction, overdose, and sometimes death (Kinzler et al., 2019). Different ways in which prescription opioid drugs can be abused are ingestion (chewing), inhalation (snorting and smoking), injection (parenteral route of administration) by mechanical manipulation (crushing or grinding) (Gasior et al., 2016). To combat prescription opioid drug abuse, abuse-deterrent (AD) products are designed to resist or make it difficult to manipulate by various formulation approaches, such as physical/chemical barriers, agonist/antagonist combinations, aversion agent inclusion, new molecular entities, and prodrugs (FDA, 2017). Physical and chemical barriers are the most effective approaches to prevent or reduce abuse. The physical barrier approach is to make the formulation resistant to crushing, whereas a chemical barrier approach is to prevent or reduce the extraction of drug using common household solvents (Herry et al., 2013). Currently, commercially available opioid formulations with AD label claims are based on either physical/chemical barrier or antagonist/agonist combination approaches to impede the drug abuse (Gasior et al., 2016).
Extended-release (ER) opioid formulations are more desirable for potential opioid abusers owing to the higher amounts of active ingredient compared with immediate-release (IR) formulations. To achieve rapid onset of the euphoric or psychoactive effects, opioid abusers manipulate the ER opioids to bypass the ER profile, rendering it into an IR form, to seek a rapid rise in opioid blood level. Several novel ADFs have been introduced to reduce the abuse of ER opioid formulations and reduce their attractiveness to abusers (Butler et al., 2011; Maincent and Zhang, 2016). The FDA guidance document describes three categories of premarket studies recommended for the development of a new AD products, namely: Category 1-laboratory-based in vitro manipulation or chemical extraction; Category 2- pharmacokinetic; and Category 3- clinical abuse potential studies (FDA, 2015).
The FDA-approved opioid products with ADF labeling utilize polyethylene oxide (PEO), a commonly used excipient for abuse deterrence. PEO is a nonionic, water-soluble, and thermoplastic homo-polymer with a molecular weight (MW) of 100,000 to 8,000,000 Da and a melting temperature range of 65°C–70°C (Joshi et al., 2018). The PEO-based matrix formulations are prepared using HME technology because of its low melting temperature and thermoplastic nature (Ma et al., 2014; Zhang and McGinity, 1999). Hot-melt extrusion (HME) has become popular in the manufacture of ADFs. The INTAC® drug delivery platform, developed by Grünenthal GmbH, uses HME with high MW PEO to make a tamper-resistant formulation, which is less prone to abuse (Bartholomaeus et al., 2012). Owing to hydration and swelling in aqueous solvents, the higher molecular weight PEOs are used to produce highly viscous, gel-like solutions that deter abuse by injection. The major drawback with PEO is its susceptibility to thermal auto oxidative degradation, which leads to reduction in polymer molecular weight and lower viscosity of solutions (Crowley et al., 2002; Meruva and Donovan, 2019). Abusers have been known to manipulate formulations using an oven, microwave, or other heating devices, resulting in reduced viscosity of the PEO solutions, which facilitates syringeability and injectability and nullifies the intravenous (IV) AD properties of ER opioid ADF.
Owing to the restrictions and controls on access to opioid analgesics, Acetaminophen (APAP) was selected as a model drug in this study because of its high aqueous and alcoholic solubility. Given the high solubility of APAP in ethanol, it will start to dissolve or extract in alcoholic media, resulting in dose dumping and consequently nullifying the AD characteristics of the formulation. Therefore, to exhibit AD properties, an ideal formulation should withstand the influence of alcohol and remain intact under all tested manipulation conditions. PEO with MW of 7,000,000 Da was utilized in the formulation of ER pellets of APAP. The high MW and crush resistance properties of PEO have led to its use in several commercialized ADFs (Litman et al., 2018). The aqueous solutions of PEO are known to exhibit lower viscosity owing to temperature-dependent oxidative degradation (Meruva and Donovan, 2019); however, this confers susceptibility to abuse by the IV route. Hence, the addition of gelling agents (xanthan gum, guar gum, and gellan gum) was investigated with the PEO matrix to impart IV AD properties. Xanthan gum is a polysaccharide produced predominantly by Xanthomonas campestris (Bueno et al., 2013). Gellan gum is a polysaccharide gum produced by culture of the microbe Sphingomonas elodea as a fermentation product. Guar gum is a polysaccharide of galactose and mannose, obtained from the endosperms of Cyamopsis tetragonoloba (Das et al., 2015). All these gelling agents are insoluble in alcohol, and show hydration and swelling behavior in non-alcoholic and alcoholic media.
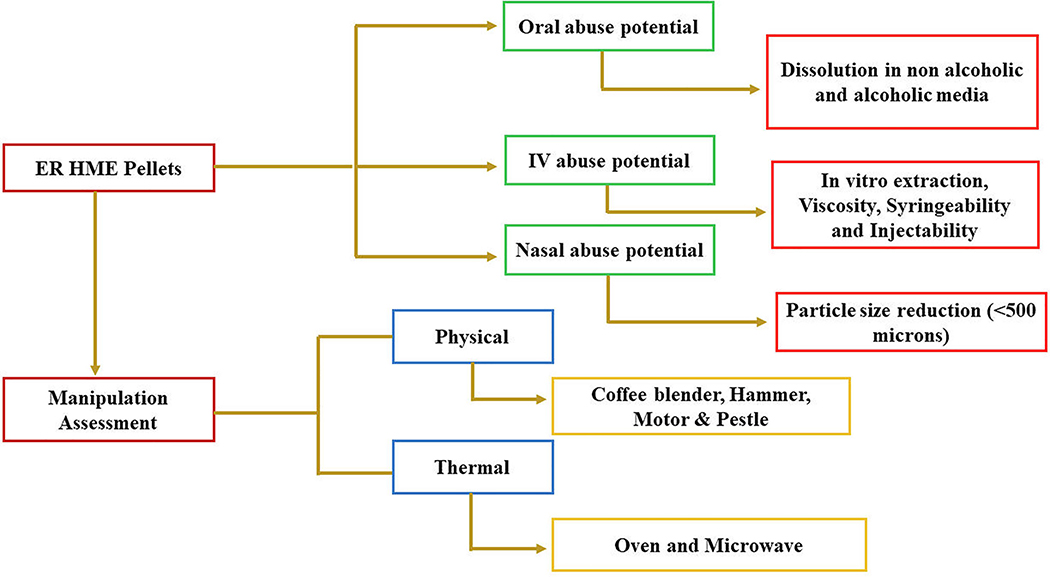
The novelty of the current work was to investigate the effect of PEO, and PEO with different gelling agents, namely xanthan gum, guar gum, and gellan gum, on the AD properties of multiple-unit ER pellets manufactured by using HME technology. Further, we aimed to investigate the effect of gelling agents on the IV AD performance and to examine an effective manipulation tool for particle size reduction (PSR). The ER pellets manufactured were evaluated for in-vitro laboratory AD characteristics. The overall evaluation of ER HME pellets under various manipulation conditions have been represented in Fig. 1.
Fig. 1.
Overview of in-vitro AD features. Evaluation of AD performance of ER HME pellets under various manipulation conditions.
2. Materials and methods
2.1. Materials
APAP selected as a model drug, was obtained from Spectrum Chemicals (New Brunswick, NJ, USA). Polyox™ WSR-303 with a MW of 7,000,000 Da was obtained from Colorcon (West Point, PA, USA). The gelling agents xanthan gum, guar gum, and gellan gum were obtained from CP Kleo (Atlanta, GA, USA), Aqua Solutions (Deer Park, TX, USA), and MP Biomedicals (Santa Ana, CA, USA), respectively. Hydrochloric acid, sodium hydroxide, and isopropyl alcohol (IPA) were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Ethanol was purchased from Decon Labs (Glennie Circle, PA, USA). All other chemicals used were analytical grade and were used as received.
2.2. Methods
2.2.1. Solubility determination
The solubility of APAP was determined in water, 0.1 N NaOH, 0.1 N HCl, and 0.1 N HCl containing 20% and 40% v/v of ethanol, and in organic solvents such as 96% ethanol and isopropyl alcohol (IPA). Excess APAP was added to a 20 mL scintillation vial, containing 10 mL of each of the aqueous and non-aqueous solvents, and was vortexed and agitated at 25°C for 48 h. After 48 h, the samples were centrifuged for 5 min at 13000 rpm to separate the undissolved drug. The supernatant solutions were suitably diluted, and the concentration of the dissolved drug was measured at a wavelength of 244 nm by using a UV-Vis spectrophotometer (Genesys 190, Thermo Scientific, NJ, USA). The test was performed in triplicate.
2.2.2. HME process
Powder mixtures of APAP with PEO, and APAP/PEO with each gelling agents (i.e., xanthan, guar gum, and gellan gum) were sieved through a USP #30 (600 μm) mesh and blended by using a Maxiblend™ V-shell blender (Globe Pharma, New Brunswick, NJ, USA) at 25 rpm for 10 min to obtain a homogeneous blend. The physical mixture was gravimetrically fed into a co-rotating twin-screw extruder (16 mm Prism EuroLab; Thermo Fisher Scientific, Waltham, MA, USA) with a length-to-diameter ratio (L/D) of 40/1. The powder blend was extruded at a temperature of 110°C using a 3 mm circular die. The screw design utilized was composed of three mixing zones along with conveying zones. The screw speed and feed rate of the extruder were set at 100 rpm and 5 gm/min, respectively. The extrudate strands obtained were cooled at ambient temperature and then pelletized into 3 mm pellets by using a bench-top pelletizer (Thermo Fisher Scientific, Waltham, MA, USA). The pellets were filled into 000 size hard gelatin capsules.
2.2.3. Differential scanning calorimetry (DSC)
The thermal properties of the drug substance, excipients, and the HME pellets were characterized by using a DSC (Discovery DSC25, TA Instruments, New Castle, DE, USA). Accurately weighed 5–8 mg of samples were sealed in the aluminum pans and equilibrated for 1 min at 25°C followed by heating from 25°C to 200°C at a ramp rate of 10°C/min, with ultra-pure nitrogen as the purge gas at a flow rate of 50 mL/min. An empty aluminum pan was used as a reference.
2.2.4. Physical manipulation and particle size reduction
The purpose of reducing particle size of an ADF is to determine the potential of the drug product to be amenable to abuse by the intranasal route (snorting). Physical manipulation studies were performed using destructive methods, including manual (hammer or pestle and mortar) and mechanical (coffee blender) tests. These are common household items used for tampering with prescription opioid medications. For PSR, the intact pellets were hit by a hammer with ten repeated strokes (Muppalaneni et al., 2016). Approximately 5 g of each formulation was weighed, and placed in a motor or coffee blender, and ground for 3 min using a pestle and 1–3 min in the coffee blender. Particle size distribution was determined after each physical manipulation technique by passing the obtained particles through a USP # 25 (500 μm) mesh, and the fraction retained on the sieve was recorded.
2.2.5. Thermal treatment
Thermal treatment was performed by heating the intact pellets in an oven (Precision, Econotherm, OH, USA) at 90°C for 30 min. After the thermal exposure, the pellets were analyzed by performing in-vitro dissolution studies.
2.2.6. Morphological characterization by scanning electron microscopy
The surface morphology of intact, physically manipulated (crushed by hammer), and thermally manipulated (oven at 90°C for 30 min) pellets was determined by using scanning electron microscopy (SEM, JSM-5610LV, JOEL, Peabody, MA, USA) with an accelerating voltage of 5 kV. The pellets were placed on the SEM stubs and adhered by using double-sided adhesive tape. Prior to imaging, the samples were sputter-coated with platinum using a Hummer 6.2 Sputter Coater (Ladd Research Industries, Williston, VT, USA) under an argon atmosphere.
2.2.7. In-vitro drug release studies
The in-vitro dissolution behavior of the intact, physically and thermally manipulated pellets were examined by using an USP type- II Apparatus (Hanson SR8-plus™; Hanson Research, Chatsworth, CA, USA) in 900 mL of 0.1 N HCl (pH 1.2, non-alcoholic) dissolution medium maintained at 37°C±0.5°C with paddle speed of 50 rpm. For each formulation, 500 mg of the pellets (equivalent to 100 mg of APAP) was transferred into the dissolution vessel. Approximately 3 mL of sample was collected at 0.5, 1, 2, 4, 6, 8 h and filtered through a 10-μm filter. The drug released was analyzed at 244 nm by using a UV spectrophotometer. All dissolution studies were performed in triplicate (n = 3). For the alcohol dose dumping studies, dissolution testing was conducted in 900 mL 0.1 N HCl with 40% v/v ethanol (alcoholic dissolution medium, equivalent to hard liquor) over a period of 8 h. The drug release profiles of intact formulations in alcoholic and non-alcoholic media and the manipulated formulations in non-alcoholic media were compared by using the f2 similarity factor (Kallakunta et al., 2018; Moore and Flanner, 1996).
| (1) |
where n represents the number of dissolution time points considered and Rt and Tt are the percentage of the drug dissolved in the reference (0.1 N HCl) and test (0.1 N HCl with 40% ethanol) formulations at time point t. The dissolution profiles were considered similar when the f2 value was in the range of 50–100, and the formulation was considered non-alcohol resistant when the f2 value was below 50.
2.2.8. Small volume in-vitro extraction studies
Intact formulations were tested for in-vitro extraction studies by using different levels of solvents, as recommended by FDA to evaluate drug extraction from ADF (FDA, 2017). The different levels of solvents employed for extraction studies were water (level-1); 40% ethanol (level-2); and 96% ethanol, IPA, 0.1 N HCl, and 0.1 N NaOH (level-3). Accurately weighed intact pellets (500 mg) equivalent to 100 mg APAP were placed into scintillation vials containing 10 mL of different solvents. The vials were vortexed for 15 s and then kept under static or agitation (1000 rpm) conditions by using a bench mixer (Benchmark Scientific, USA) for 30 min at room temperature (RT). After 30 min, the samples were withdrawn into a previously weighed vial by using a 10-mL syringe, and the volume withdrawn was calculated from the density of the solvents. The extracted samples were appropriately diluted and analyzed at 244 nm by using a UV spectrophotometer to quantify the extraction of the drug in various solvents.
Extraction studies of intact pellets were also performed in 10 mL water using an oven and a microwave (Sharp Electronics Corporation, NJ, USA) as thermal manipulation tools. Briefly, 500 mg pellets from each formulation were transferred into scintillation vials containing 10 mL water, and the vials were placed in an oven (90°C–95°C) and microwave (1500 W) for 30 min and 15 s, respectively. The extraction of drug and the volume withdrawn was similar to the process utilized for static and agitated samples.
2.2.9. Syringeability and injectability
Syringeability and injectability tests are key testing parameters for a drug product that is abused intravenously. Each 500 mg of intact pellet formulation was placed into scintillation vials containing 10 mL water, and the vials were kept under static and agitating conditions (1000 rpm) at RT for 30 min and in the oven at 90°C–95°C for 30 min for thermal manipulation. The texture analyzer (TA-XT2i, Texture Technologies, Hamilton, MA, USA) was equipped with a syringe setup (TA-270N, TA272B) and employed to evaluate the syringeability (pulling) and injectability (pushing) force exerted by each formulation. A 25-gauge needle (Becton Dickinson) equipped with a 3-mL syringe was used during the measurements. The test parameters utilized were: measurement mode compression or distance; pre-test speed of 1.0 mm/s; post-test speed of 20.0 mm/s; an automatic trigger force at 15.0 g, and the syringe plunger was set to move a distance of 30 mm each time during pushing and pulling. The target distance and compression mode were used to record the syringeability and injectability force, respectively. The data were collected and analyzed by exponent software version 6.1.5.0 (Stable Micro Systems, Godalming, UK).
2.2.10. Viscosity analysis
Viscosity of formulations was measured after manipulation of intact pellets in various conditions (i.e., agitation at 1000 rpm at RT, and at 90°C in the oven for up to 30 min). After 30 min, the viscosity of the aqueous extracts was measured by using a Brookfield cone-and-plate rheometer (LVDV-II+ Pro Viscometer, Middleboro, USA). A test sample of 0.5 mL was placed onto the middle of the plate and then equilibrated for 2 min. The measurements were performed with spindle number 40 at 6 rpm, a cone radius of 1.2 cm, at 25.0°C±0.3°C.
3. Results and discussion
3.1. Solubility
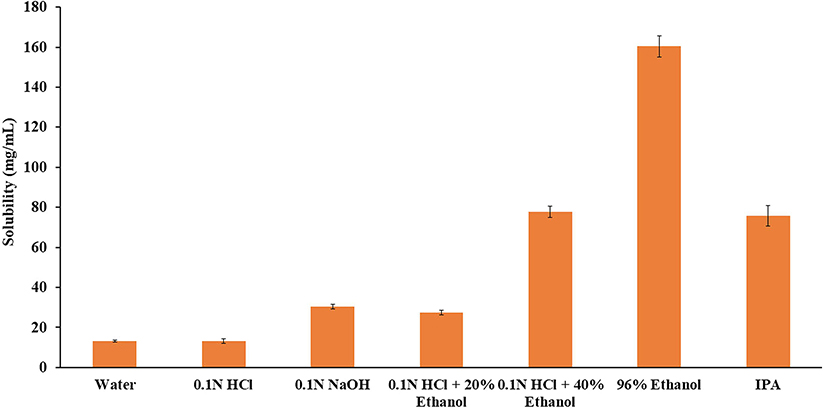
The solubility of APAP in alcoholic and non-alcoholic media could affect the in-vitro extraction and dissolution rate. The solubility data for APAP in various solvents are presented in Fig. 2, and results indicated that APAP showed high solubility in all the tested solvents. The solubility was significantly increased with a gradual increase in ethanol concentration, and was very high in 96% ethanol and IPA. In alcoholic media, the solubility of APAP was approximately 6 and 12-fold higher in 40% and 96% ethanol, respectively, compared with the non-alcoholic media, i.e., water. The saturation solubility in aqueous solvents varied from 13.11 ± 0.57 to 30.34 ± 1.18 mg/mL, with the highest solubility in 0.1 N NaOH, followed by that in 0.1 N HCl and water.
Fig. 2.
Solubility of APAP in various aqueous and alcoholic solvents. All values expressed as mean ± SD (n = 3).
3.2. HME process
The extrusion trials were performed using PEO at 80% w/w and APAP at 20% w/w. Similarly, the polymeric blends of PEO at 50% w/w were examined with three gelling agents (xanthan gum, guar gum, and gellan gum) each at 30% w/w and APAP at 20% w/w at a temperature of 110°C, screw speed of 100 rpm, and a feed rate of 5 g/min. The drug load was maintained at 20% w/w for all formulations. The process temperature (110°C) was selected on the basis of the glass transition temperature (69°C) of Polyox™ WSR 303. At this temperature, APAP and gelling agents are non-molten and are molecularly dispersed in the PEO matrix to obtain homogeneous extrudates. The obtained extrudates of PEO and the blends of PEO with individual gelling agents were cut into 3 mm pellets and were evaluated for their potential for abuse via the oral, nasal, and IV routes. The formulation composition is provided in Table 1.
Table 1.
Formulation composition of pellets prepared by HME.
| Component | Composition (wt %) |
|||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| Acetaminophen | 20.0 | 20.0 | 20.0 | 20.0 |
| PEO | 80.0 | 50.0 | 50.0 | 50.0 |
| Xanthan gum | - | 30.0 | - | - |
| Guar gum | - | - | 30.0 | - |
| Gellan gum | - | - | - | 30.0 |
3.3. Thermal characterization by DSC
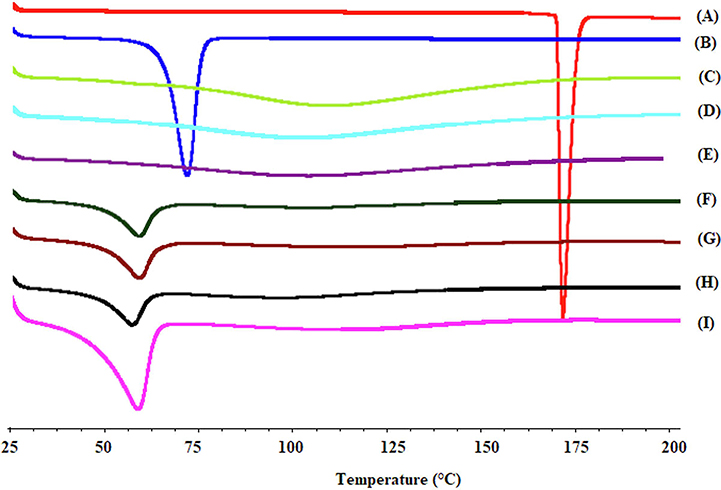
DSC thermograms of pure APAP, PEO, gelling agents, and HME pellets are shown in Fig. 3. APAP and PEO showed endothermic melting peaks at 170.2°C (Jedinger et al., 2015) and 69.85°C (Rahman et al., 2016), respectively, due to the crystalline and semi-crystalline nature of APAP and PEO. For xanthan gum, guar gum, and gellan gum, no thermal events were recorded between 25°C and 200°C, and a broad endothermic event observed at approximately 110°C, which may have been due to evaporation of moisture content (Jedinger et al., 2016; P. More et al., 2017). A thermogram of HME pellets did not show the melting peak of APAP, indicating that the drug substance was molecularly dispersed in the matrix of PEO-gelling agents. The decrease in the melting point of PEO in the thermograms of HME pellets was due to the change in crystallinity of the PEO matrix polymer upon thermal treatment and the molecular dispersion of the drug in the crystalline polymer matrix. This decrease in the degree of melting point depends on the MW and duration of the thermal treatment. These findings were in accordance with the previous literature (Huang and Dai, 2014; Rahman et al., 2016).
Fig. 3.
DSC thermograms of A) APAP, B) PEO, C) guar gum, D) xanthan gum, E) gellan gum, and the F) F4, G) F3, H) F2, and I) F1 formulations.
3.4. PSR studies
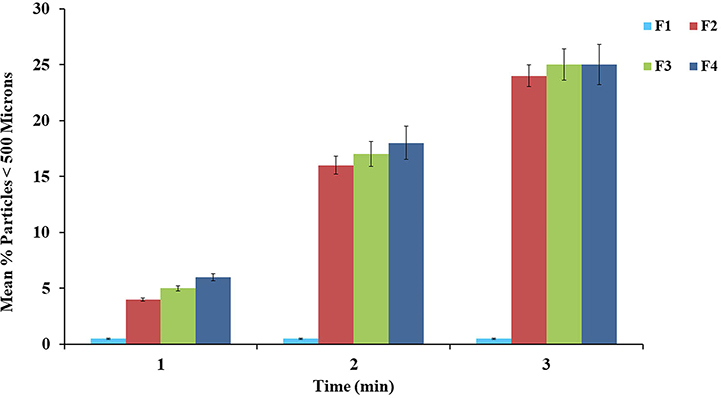
Manipulation refers to any process used to alter or destroy the integrity of the intact pellets. PSR of ADF is a common practice to increase the drug release, turn an ER formulation into IR, and to prepare a formulation amenable to abuse by the nasal or IV routes. The feasibility of PSR was evaluated by using a hammer, pestle and mortar, and coffee blender (Fig. 4). Of these, the coffee blender was identified as an effective tool for PSR. Particle sizes greater than 500 microns are difficult to abuse by snorting/insufflation and also lower the absorption through the nasal mucosa (Bartholomaeus et al., 2012; Vosburg et al., 2012). Hence, particles of <500 μm were set as the limit to determine the resistance to nasal abuse. PSR using hammer and a pestle and mortar resulted in deformation and minor fractures of the intact pellets, showing that these tools were not able to reduce the particles below 500 μm. PSR achieved by using a coffee blender (< 500 μm) is shown in Fig. 5. Approximately < 2% of particles of manipulated pellets were smaller than 500 μm for the PEO-based formulation (F1). In contrast, other formulations resulted in approximately 25% of particles smaller than 500 μm. Owing to the high percentage of PEO (80%) in the F1 formulation, the pellets were hard to reduce to particle sizes below 500 μm, and this was attributable to the crush resistance potential of high MW PEO polymers. There was no significant difference (p > 0.05) between the particle size distribution of pellets manufactured with the three gelling agents. The decrease in PSR, with an increase in PEO percentage, can be explained by the high MW and thermoplastic nature of PEO, which imparts elastic deformation and crush resistance properties to the HME pellets. Thus, the deformation properties of plain PEO-based pellets showed a decrease in the potential for PSR. In contrast, the increase in PSR was significant (p < 0.05) for all formulations containing gelling agents, which was attributable to the relatively smaller percentage of PEO, and may increase the susceptibility to PSR because of the evolution of minor fractures during physical manipulation. These results suggested that a formulation containing a high percentage of PEO was not suitable for snorting, and it was observed that formulations containing gelling agents could potentially be snorted following physical manipulation using a coffee blender. However, the particles smaller than 500 μm was less than 25% this may be equivalent to immediate dose of API in the formulation. Further, this may vary with different API’s with abuse deterrent potential.
Fig. 4.
Images of household tools used during physical manipulation attempts (F2 formulation).
Fig. 5.
Particle size distribution of HME pellets after grinding with a coffee blender for up to 3 min. All values expressed as mean ± SD (n = 3).
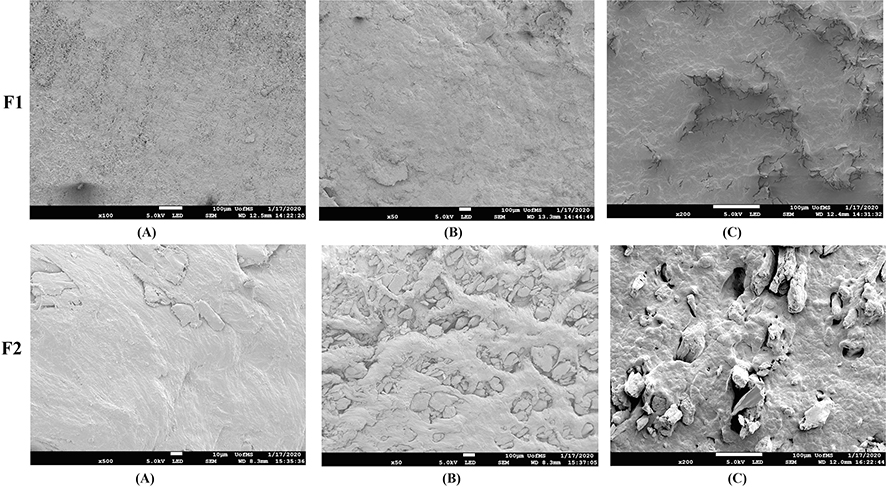
3.5. SEM imaging
SEM images of intact and manipulated pellet formulations are presented in Fig. 6. The surface of intact pellets of formulation (F1) was rough with a few cracks. The surface of physically manipulated F1 pellets showed minimal changes, with a few fragments on the surface, whereas thermally manipulated pellets showed surface cracks and fissures. These changes were the result of polymer melting and fusion due to manipulation above the melting point of PEO. The surface of the intact F2 formulation was smooth with few cracks, whereas the physically manipulated pellets showed a large number of cracks and crevices, indicating that the F2 formulation was susceptible to PSR compared with F1. The surface characteristics of the thermally manipulated F2 formulation showed more cracks and fissures than those of F1. This was probably due to presence of xanthan gum, which clearly interferes with the fusion/bridge formation of PEO and the difference in density of pellets after thermal curing. The F1 formulation contains a large amount of PEO (80%); upon cooling, the PEO matrix shrinks, leaving behind deep cracks on the surface (Dharani et al., 2019; Meruva and Donovan, 2019). However, in the F2 formulation, the PEO content was 50%, and the xanthan gum in the formulation could interfere with the fusion and densification of PEO polymer matrix, resulting in an increased number and depth of cracks. These observed microstructural changes in the F2 formulation may be due to the less dense packed structure of xanthan gum in thermally manipulated pellets. In the case of F1 formulation, these microstructural changes are less significant because of densification in the heat-cured PEO matrix.
Fig. 6.
SEM images of A) intact, B) physically manipulated, and C) thermally manipulated pellets of F1 and F2 formulations.
3.6. In-vitro drug release studies
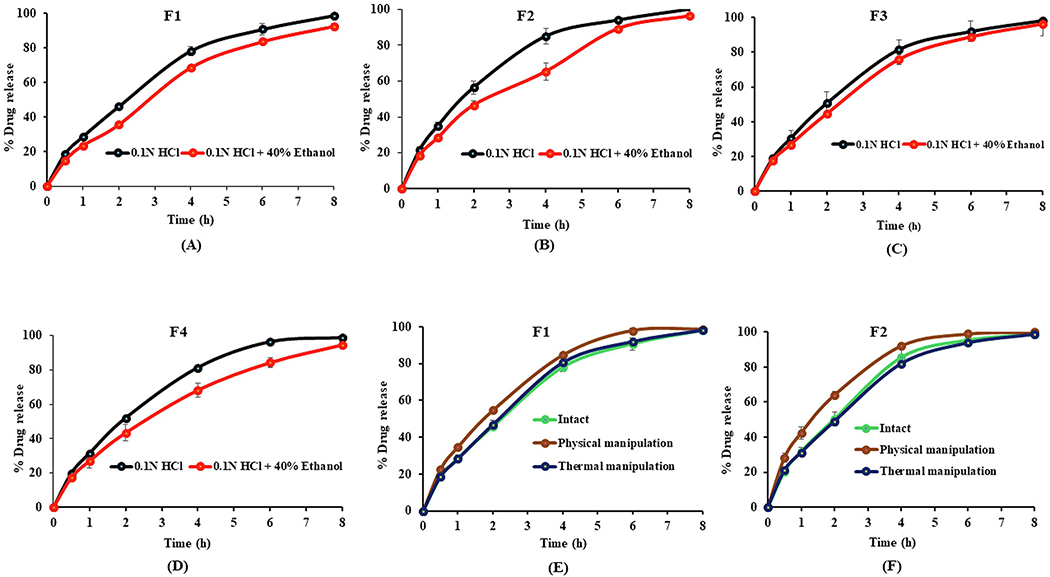
In-vitro dissolution testing of intact pellets was performed in 0.1 N HCl, and 0.1 N HCl with 40% v/v ethanol for up to 8 h. The dissolution studies in 0.1 N HCl with 40% ethanol were performed to assess the dose dumping potential of HME pellets when co-ingested with alcohol. The percentage of drug release over a period of 8 h is shown in Fig. 7A–7D. All the formulations have shown more than 90% drug release in both non-alcoholic and alcoholic media over the 8 h interval. The ER profile of the pellet formulations is due to the high MW and viscosity of the PEO matrix polymer. The mechanism of drug release from the pellets is mainly through the erosion of the polymer matrix and diffusion of the dissolved drug through a gel layer formed on the outer surface of the pellets (Zhang and McGinity, 1999). The gelling agents did not have a major impact on the ER characteristics owing to their rapid hydration and faster dissolution when in contact with aqueous media. Dissolution profile of F2, F3, and F4 formulations was not significantly (p > 0.05) different because of the similar wettability of the pellets in 0.1 N HCl and 0.1 N HCl with 40% ethanol. This indicates that the drug release was completely dependent on the PEO concentration. The dissolution rate of the intact pellets in nonalcoholic medium (0.1 N HCl) was similar to that in alcoholic medium (0.1 N HCl with 40%) indicated by an F2 value of >50, suggesting that the HME pellet formulations withstood the alcohol-induced dose dumping. This robust dissolution profile in 0.1 N HCl with 40% alcoholic medium was due to the insolubility of PEO and gelling agents.
Fig. 7.
Dissolution profiles of intact HME pellets in nonalcoholic (0.1 N HCl) and alcoholic (0.1 N HCl + 40% ethanol) media for formulations A) F1, B) F2, C) F3, D) F4 and the dissolution profiles of intact versus physically and thermally manipulated pellets in nonalcoholic medium (0.1 N HCl) for the E) F1 and F) F2 formulations. All values expressed as mean ± SD (n = 3).
The dissolution testing of physically and thermally manipulated pellets was performed in 0.1 N HCl. The percentage of in-vitro drug release of manipulated pellets compared to intact pellets is shown in Fig. 7E and 7F. Physically manipulated pellets demonstrated a relatively higher dissolution profile from those observed with the intact pellets owing to the deformation and fracture of manipulated pellets. There was no significant difference (p > 0.05) in the drug release for thermally manipulated pellets versus intact pellets owing to the hardening, densification, and increased crushing strength of the PEO matrix after thermal treatment (Meruva and Donovan, 2019). The dissolution profile of physically and thermally manipulated pellets in nonalcoholic medium (0.1 N HCl) was similar (F2 < 50) to that of intact pellets. These results demonstrated that HME pellets maintained their ER profile, even after physical and thermal manipulation.
3.7. Small volume extraction and IV injection studies
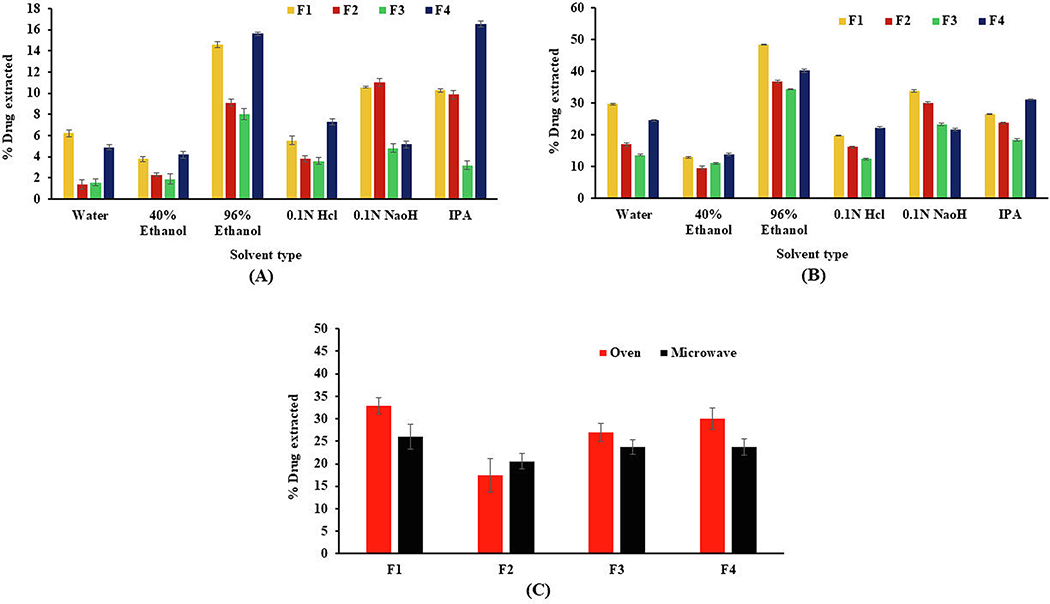
After the oral route, the most common route of abuse for ER opioid products is the IV route. To determine the feasibility of preparing HME pellets as a solution for IV abuse, in vitro extraction studies were conducted by using a small volume of potentially injectable solvents, as recommended by the FDA. Because of common availability, water, and water with 40% alcohol are the preferred first-line household solvents for small-volume extraction studies. The data from extraction studies performed on different levels of solvents are shown in Fig. 8A and 8B. For intact pellets, the mean percentage of the drug extracted after 30 min in water and 40% alcohol was in the range of 1.39% ± 0.44 to 6.22% ± 0.33, and 1.92% ± 0.49 to 4.20% ± 0.35, respectively, under static conditions compared with the 13.68% ± 0.22 to 29.75% ± 0.31 and 9.62% ± 0.50 to13.91% ± 0.31 respectively for water and 40% alcohol under agitation. There was a significant difference (p < 0.05) in the drug extraction profile in water and 96% ethanol for the F2 formulation compared with the F1 formulation under agitating and static conditions. However, this difference was relatively less in 40% ethanol under both static and agitating conditions. This was attributable to the higher viscosity and more rapid gel formation with xanthan gum when hydrated in small volumes of alcoholic and non-alcoholic media. Drug extraction studies were also conducted in level 3 solvents (reflective of different polarity and pH) to assess the ability of these solvents for preparation of drug solutions intended for IV abuse. As per the FDA draft guidance for industry on evaluation of abuse-deterrent properties of generic oral opioid products, formulations are considered without abuse deterrent properties if they are above the threshold of ≥50% drug extracted at 30 min at various temperatures and agitation conditions (FDA, 2017). The percentage of drug extracted was in the range of 8.0% ± 0.51% to 15.6% ± 0.19%, and 3.2% ± 0.38% to 16.5% ± 0.28% for 96% ethanol and IPA, respectively, in static conditions. Similarly, under agitated conditions, drug extraction in 96% ethanol and IPA was in the range of 34.33% ± 0.18 to 48.45± 0.22%, and 18.44% ± 0.41 to 31.22% ± 0.18%, respectively. The higher drug extraction in IPA and 96% ethanol was due to higher drug solubility, and the drug was extracted by the capillary action of solvents through the pores of HME pellets (Xu et al., 2019). The percentage drug extraction in 0.1 N HCl (pH 1.2) was < 7.3% and < 22.32% for static and agitating conditions, respectively. The drug extraction in 0.1 N NaOH (pH 11.5) was < 11.31% and < 33.78% under static and agitating conditions, respectively. The higher drug extraction in 0.1 N NaOH was related to the high solubility of the drug at alkaline pH compared with the solubility at acidic pH values. The lower drug extraction of the F2 formulation in water and 40% ethanol can be explained by solubility and the gelling phenomenon of xanthan gum, as it forms a rapid gelling matrix in the water and 40% alcohol compared with other formulations.
Fig. 8.
Small-volume extraction of APAP at A) static and B) agitating conditions over a 30-min interval in ingestible and non-ingestible solvents C) mean percentage of APAP extracted from thermally manipulated pellets after incubation in 10 mL water. All values expressed as mean ± SD (n = 3).
Another extraction study was performed to evaluate the effect of thermal manipulation on the extraction profile of intact pellets in 10 mL water by incubating the pellets in the oven (90 °C) or microwave (1500 W) for 30 min and 15 s, respectively. All the formulations showed < 35% drug extraction (Fig. 8 C) following both types of thermal manipulation. The drug extraction profile of F2 formulations was 15.62% and 5.43% less, respectively, for oven and microwave conditions compared with the F1 formulation. The presence of xanthan gum in the F2 formulation significantly reduced (p < 0.05) the drug extraction under both manipulation conditions compared with other formulations (F3 and F4).
The volume withdrawn from each formulation after 30 min of extraction was recorded for intact formulations under static, agitating, and thermal manipulation conditions (Table 2). The volume withdrawn from the F2 formulation was 7.0 ± 0.19, 5.8 ± 0.41, and 6.1 ± 0.59 mL under static, agitating, and thermal manipulation conditions, respectively. In contrast, the volume withdrawn for other formulations was > 8 mL under static and agitating conditions. Thermally manipulated formulations result in a relatively lower volume available for withdrawal compared with static and agitating conditions. The low drug extraction i.e., below the threshold level (≤50%) may be due to the relatively lower temperature (90–95°C) applied during thermal manipulation. Further, extraction potential of APAP may vary depending on the thermal treatment conditions such as temperature and time. The lower withdrawable volume from the F2 formulation when manipulated suggested AD properties that may deter IV abuse.
Table 2.
Volume recovered (mL) and amount of drug extracted in the recovered volume (mg) after incubation with water at room temperature under static, agitating conditions and after thermal manipulation. (mean ± SD, n = 3).
| Manipulation condition | Volume recovered (out of 10 mL) and amount of drug extracted (mg) in the recovered volume |
||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | ||
| Static | Volume recovered (mL) | 8.7 ± 0.15 | 7.0 ± 0.19 | 8.8 ± 0.11 | 8.7 ± 0.20 |
| Amount of drug extracted (mg) | 5.41 ± 0.33 | 0.97 ± 0.44 | 1.38 ± 0.30 | 4.26 ± 0.26 | |
| Agitation | Volume recovered (mL) | 8.4 ± 0.29 | 5.8 ± 0.41 | 8.0 ± 0.37 | 8.7 ± 0.26 |
| Amount of drug extracted (mg) | 24.99 ± 0.32 | 9.85 ± 0.55 | 10.95 ± 0.22 | 21.36 ± 0.29 | |
| Thermal (oven) | Volume recovered (mL) | 8.6 ± 0.49 | 6.1 ± 0.59 | 7.5 ± 0.50 | 7.9±0.28 |
| Amount of drug extracted (mg) | 28.58 ± 1.62 | 12.16 ± 3.29 | 26.06 ± 1.76 | 23.71 ± 2.09 | |
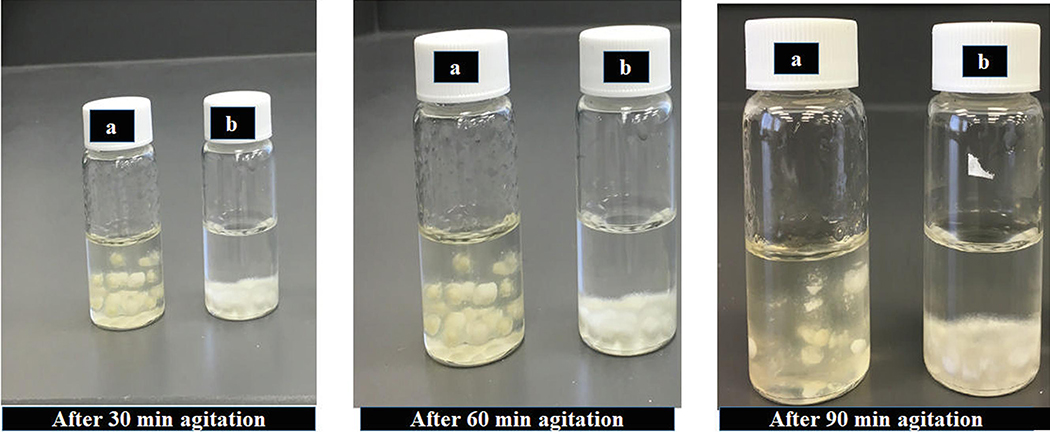
The F2 formulation continued to exhibit gelling properties for up to 90 min. At the end of 90 min, the formulation became a gel with no further volume remaining for withdrawal, whereas the F1 formulation showed the gelling property at the bottom of the vial, with < 8 mL remaining for withdrawal (Fig. 9).
Fig. 9.
Images of samples of a) F2 and b) F1 formulations after incubation in 10 mL water with agitation for up to 90 min.
3.8. Syringeability and injectability
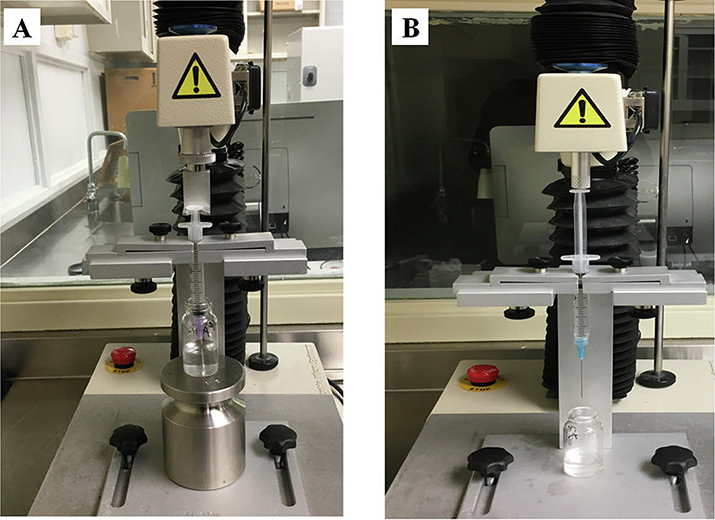
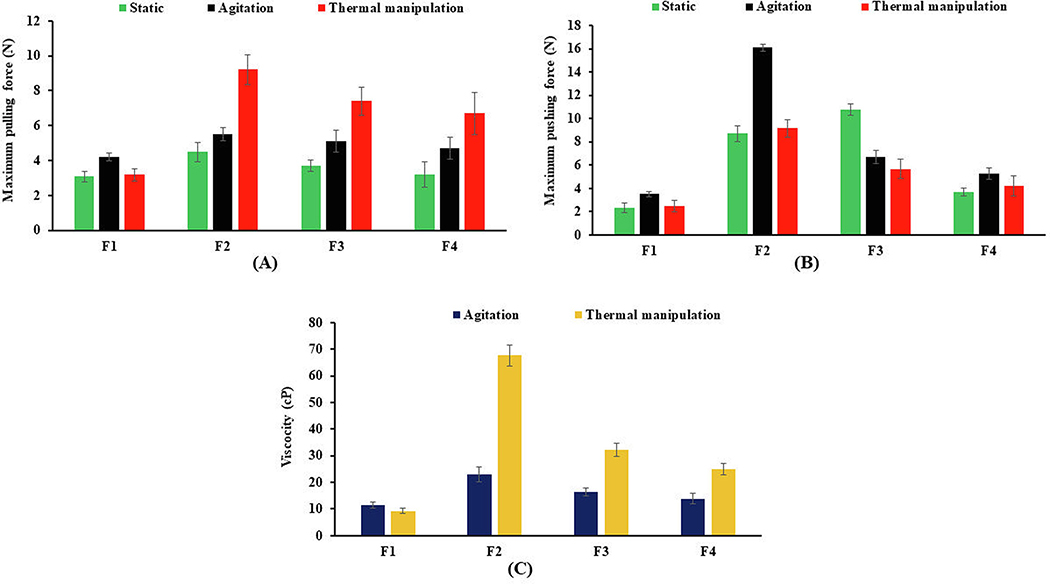
Syringeability and injectability studies were conducted to assess the AD potential of HME pellets by the IV route. The term syringeability refers to the force or pressure required to withdraw the dissolved drug or extract from a vial through hypodermic needle prior to the injection, whereas injectability refers to the amount of force or pressure required during injection (Cilurzo et al., 2011). These tests are key performance attributes of any ADF intended to provide resistance to drug abuse by the IV route. A texture analyzer equipped with syringeability and injectability accessories (Fig. 10) was used to measure the syringeability and injectability of pellet formulation using water as a control. The syringeability and injectability results of the intact and thermally manipulated formulations are shown in Fig. 11A and 11B. The pulling and pushing forces (N) of water were 1.74 and 2.59, respectively. The syringeability forces of static, agitating, and thermally manipulated pellets varied from 3.1 ± 0.3 to 4.5 ± 0.5, 4.2 ± 0.2 to 5.5 ± 0.3, and 3.2 ± 0.3 to 9.2 ± 0.8, respectively. Similarly, the injectability force was observed to be from 2.4 ± 0.4 to 10.8 ± 0.4, 3.5 ± 0.2 to 16.1 ± 0.3 and 2.5 ± 0.4 to 9.1 ± 0.7, respectively for static, agitated, and thermally manipulated pellets. The decrease in resistance to syringe (pulling) and injection (pushing) for the PEO formulation (F1) after thermal manipulation may be due to a reduction in solution viscosity, degradation, and a shortened polymer chain end-to-end distance of PEO after thermal treatment (Joshi et al., 2018). Thus, reduced pulling and pushing forces could be attributed to ease of syringreability and injectability, which led to compromising the IV abuse deterrent potential. Similar observations were reported by (Rahman et al., 2016), and they reported a significant reduction in the syringeability and injectability of thermally cured formulations prepared with Polyox™ WSR 301 and 303 when compared with the uncured formulations. Of all the studied formulations, higher pulling and pushing forces, i.e., greater resistance to syringeability and, injectability was observed for the PEO-xanthan gum pellets (F2). This could be attributable to the rapid hydration and swelling behavior of xanthan gum pellets, which form a highly viscous solution under intact and manipulated conditions. The low syringaebility and injectability of xanthan gum based formulation (F2) demonstrated greater IV abuse deterrent potential because of characteristics of both difficulty of withdrawal (syringreability) of an extracted solution and their subsequent passage (injectability) via a 25-gauge needle.
Fig. 10.
Texture analyzer setup of A) syringeability and B) injectability test.
Fig. 11.
A) Syringeability and B) injectability profiles of HME pellets using a 3-mL syringe with a 25-gauge needle. C) Mean viscosities of formulations with agitation at RT and thermal manipulation at 90°C for 30 min. All values expressed as mean ± SD (n = 3).
3.9. Viscosity measurement
The viscosity of extracted solutions provides a determination of effectiveness of formulation deterrent for parenteral abuse. The viscosity of extracted solutions prepared from intact and manipulated formulations is shown in Fig. 11C. Among all the formulations, the PEO-xanthan gum pellets (F2) produced highly viscous solutions under agitation and thermal manipulation. The viscosities of all the formulations in increasing order were as follows: F2 > F3 > F4 > F1. The higher viscosity of the F2 formulation could be attributed to the rapid hydration and swelling characteristics of xanthan gum, which forms a gel in a small volume of water. Moreover, xanthan gum has been widely used as a thickener in pharmaceutical products, and a low concentration of xanthan gum can produce highly viscous solutions with shear-thinning properties (Esposito et al., 2018). The aqueous extracts of thermally manipulated F1 formulation have lower viscosity (9.33 ± 0.90 cp) compared with extracts of intact F1 and other formulations in both agitation and thermal manipulation conditions. Thus, the reduction in solution viscosity of the F1 formulation, after thermal manipulation, was due to random chain scission of the PEO structure into smaller fragments at high temperature. Joshi et al., 2018 reported the effect of temperature and concentration of PEO on the solution viscosity. The reduction in solution viscosity was observed as the temperature increased up to 110°C and a further increase in temperature from 110°C to 150°C resulted in a sharp drop in solution viscosity. Compared with F1, the other formulations containing gelling agents have shown higher viscosities and can also produce a gel-like consistency in small-volume aqueous solutions. These results suggested that the F2 formulation is difficult to be drawn into a syringe and deter abuse by the parenteral route (IV) of administration.
4. Conclusion
Extensive laboratory-based category-1 in-vitro AD studies performed by using various manipulation methods revealed the AD characteristics of the formulation. Results demonstrated that ER pellets manufactured by the HME process provide resistance to PSR, which creates a barrier for oral and nasal routes of abuse. The in- vitro dissolution study revealed that ER properties were maintained even after the thermal and physical manipulation of pellets, indicating that dissolution was not affected by the manipulation method. The PEO/xanthan gum formulation (F2) exhibited superior viscosity and gelling properties, making it difficult to extract in different levels of solvents and thus making IV abuse less desirable. The syringeability, injectability, and viscosity measurements showed that intact and thermally manipulated xanthan gum-based formulations possessed high viscosity and gelling properties when prepared as IV solutions. Furthermore, the intact and manipulated PEO formulation showed decreased solution viscosity, syringe and injection forces when compared with other formulations, suggesting that PEO alone may not be the excipient of choice to deter the IV abuse of formulations. Thus, a combination of PEO with xanthan gum makes the formulation difficult to abuse via injection route, thus diminishing the feasibility of IV abuse. Therefore, the pellets manufactured by the HME process using PEO and gelling agents improves the AD properties of the formulation by preventing the IV abuse. However, controlled substances as model drugs should be evaluated for AD properties when prepared using the HME process.
Acknowledgments
Arun Butreddy would like to thank Randy Koch (Southern regional manager, Texture Technologies Corporation, GA, USA) for the provision of a syringeability fixture.
Funding
This project was also partially supported by Grant Number P30GM122733-01A1, funded by the National Institute of General Medical Sciences (NIGMS) a component of the National Institutes of Health (NIH) as one of its Centers of Biomedical Research Excellence (COBRE).
Abbreviations
- HME
hot melt extrusion
- ER
extended release
- PEO
polyethylene oxide
- AD
abuse deterrent
- PSR
particle size reduction
- ADFs
abuse-deterrent formulations
- IR
immediate-release
- MW
molecular weight
- IV
intravenous
- APAP
acetaminophen
- IPA
isopropyl alcohol
- DSC
differential scanning calorimetry
- SEM
scanning electron microscopy
- FDA
Food and drug administration
- Tg
glass transition temperature
- cP
centipoise
- RT
room temperature
- UV
ultraviolet
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- FDA, 2015. Guidance for Industry, Abuse-Deterrent Opioids — Evaluation and Labeling. https://www.fda.gov/downloads/Drugs/Guidances/UCM334743.pdf.
- FDA, 2017. Guidance for Industry, General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products. https://www.fda.gov/downloadsDrugs/.../Guidances/UCM492172.pdf.
- Bartholomaeus JH, Arkenau-Marić E, Galia E, 2012. Opioid extended-release tablets with improved tamper-resistant properties. Expert Opinion on Drug Delivery 9, 879–891. 10.1517/17425247.2012.698606 [DOI] [PubMed] [Google Scholar]
- Bueno VB, Bentini R, Catalani LH, Petri DFS, 2013. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydrate Polymers 92, 1091–1099. 10.1016/j.carbpol.2012.10.062 [DOI] [PubMed] [Google Scholar]
- Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH, 2011. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J 8, 1–17. 10.1186/1477-7517-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilurzo F, Selmin F, Minghetti P, Adami M, Bertoni E, Lauria S, Montanari L, 2011. Injectability Evaluation: An Open Issue. AAPS PharmSciTech 12, 604–609. 10.1208/s12249-011-9625-y [DOI] [PubMed] [Google Scholar]
- Crowley MM, Zhang F, Koleng JJ, McGinity JW, 2002. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials 23, 4241–4248. 10.1016/S0142-9612(02)00187-4 [DOI] [PubMed] [Google Scholar]
- Das N, Tripathi N, Basu S, Bose C, Maitra S, Khurana S, 2015. Progress in the development of gelling agents for improved culturability of microorganisms. Front Microbiol 6, 698 10.3389/fmicb.2015.00698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharani S, Barakh Ali SF, Afrooz H, Mohamed EM, Cook P, Khan MA, Rahman Z, 2019. Development of Methamphetamine Abuse–Deterrent Formulations Using Sucrose Acetate Isobutyrate. Journal of Pharmaceutical Sciences S0022354919308081 10.1016/j.xphs.2019.12.003 [DOI] [PubMed] [Google Scholar]
- Esposito E, Sguizzato M, Bories C, Nastruzzi C, Cortesi R, 2018. Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers 10, 160 10.3390/polym10020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Bond M, Malamut R, 2016. Routes of abuse of prescription opioid analgesics: a review and assessment of the potential impact of abuse-deterrent formulations. Postgrad Med 128, 85–96. 10.1080/00325481.2016.1120642 [DOI] [PubMed] [Google Scholar]
- Herry C, Monti A, Vauzelle-Kervroedan F, Oury P, Michel L, 2013. Reducing abuse of orally administered prescription opioids using formulation technologies. Journal of Drug Delivery Science and Technology 23, 103–110. 10.1016/S1773-2247(13)50017-7 [DOI] [Google Scholar]
- Huang Y, Dai W-G, 2014. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharmaceutica Sinica B, SI: Drug Delivery System and Pharmaceutical Technology 4, 18–25. 10.1016/j.apsb.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedinger N, Khinast J, Roblegg E, 2014. The design of controlled-release formulations resistant to alcohol-induced dose dumping--a review. Eur J Pharm Biopharm 87, 217–226. 10.1016/j.ejpb.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Jedinger N, Schrank S, Fischer JM, Breinhälter K, Khinast J, Roblegg E, 2016. Development of an Abuse- and Alcohol-Resistant Formulation Based on Hot-Melt Extrusion and Film Coating. AAPS PharmSciTech 17, 68–77. 10.1208/s12249-015-0373-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedinger N, Schrank S, Mohr S, Feichtinger A, Khinast J, Roblegg E, 2015. Alcohol dose dumping: The influence of ethanol on hot-melt extruded pellets comprising solid lipids. European Journal of Pharmaceutics and Biopharmaceutics 92, 83–95. 10.1016/j.ejpb.2015.02.022 [DOI] [PubMed] [Google Scholar]
- Joshi Y, Muppalaneni S, Omidian A, Mastropietro DJ, Omidian H, 2018. Determining Abuse Deterrence Performance of Poly (ethylene oxide) Using a Factorial Design. Adv Pharm Bull 8, 495–505. 10.15171/apb.2018.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallakunta VR, Tiwari R, Sarabu S, Bandari S, Repka MA, 2018. Effect of formulation and process variables on lipid based sustained release tablets via continuous twin screw granulation: A comparative study. European Journal of Pharmaceutical Sciences 121, 126–138. 10.1016/j.ejps.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler ER, Pantaleon C, Iverson MS, Aigner S, 2019. Syringeability of morphine ARER, a novel, abuse-deterrent, extended-release morphine formulation. The American Journal of Drug and Alcohol Abuse 45, 377–384. 10.1080/00952990.2019.1599383 [DOI] [PubMed] [Google Scholar]
- Litman RS, Pagán OH, Cicero TJ, 2018. Abuse-deterrent Opioid Formulations. Anesthes 128, 1015–1026. 10.1097/ALN.0000000000002031 [DOI] [PubMed] [Google Scholar]
- Ma L, Deng L, Chen J, 2014. Applications of poly(ethylene oxide) in controlled release tablet systems: a review. Drug Development and Industrial Pharmacy 40, 845–851. 10.3109/03639045.2013.831438 [DOI] [PubMed] [Google Scholar]
- Maincent J, Zhang F, 2016. Recent advances in abuse-deterrent technologies for the delivery of opioids. International Journal of Pharmaceutics 510, 57–72. 10.1016/j.ijpharm.2016.06.012 [DOI] [PubMed] [Google Scholar]
- Meruva S, Donovan MD, 2019. Effects of Drug-Polymer Interactions on Tablet Properties During the Development of Abuse-Deterrent Dosage Forms. AAPS PharmSciTech 20, 1–12. 10.1208/s12249-018-1221-y [DOI] [PubMed] [Google Scholar]
- Moore JW, Flanner HH, 1996. Mathematical Comparison of Dissolution Profiles. PHARMACEUTICAL TECHNOLOGY 20, 64–75. [Google Scholar]
- Muppalaneni S, Mastropietro DJ, Omidian H, 2016. Crush resistance and insufflation potential of poly(ethylene oxide)-based abuse deterrent formulations. Expert Opinion on Drug Delivery 13, 1375–1382. 10.1080/17425247.2016.1211638 [DOI] [PubMed] [Google Scholar]
- More P, M., Bhamare S, M., Bhavsar J, C., Patil O, P., Deshmukh KP, 2017. Development of novel thiolated carboxymethyl-gellan gum as potential mucoadhesive polymer: Application of DoE. Adv Mater Sci 2 10.15761/AMS.1000125 [DOI] [Google Scholar]
- Rahman Z, Yang Y, Korang-Yeboah M, Siddiqui A, Xu X, Ashraf M, Khan MA, 2016. Assessing impact of formulation and process variables on in-vitro performance of directly compressed abuse deterrent formulations. International Journal of Pharmaceutics 502, 138–150. 10.1016/j.ijpharm.2016.02.029 [DOI] [PubMed] [Google Scholar]
- Vosburg SK, Jones JD, Manubay JM, Ashworth JB, Benedek IH, Comer SD, 2012. Assessment of a formulation designed to be crush-resistant in prescription opioid abusers. Drug and Alcohol Dependence 126, 206–215. 10.1016/j.drugalcdep.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiffen PJ, Wee B, Moore RA, 2016. Oral morphine for cancer pain. Cochrane Database of Systematic Reviews. 10.1002/14651858.CD003868.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Siddiqui A, Srinivasan C, Mohammad A, Rahman Z, Korang-Yeboah M, Feng X, Khan M, Ashraf M, 2019. Evaluation of Abuse-Deterrent Characteristics of Tablets Prepared via Hot-Melt Extrusion. AAPS PharmSciTech 20, 1–11. 10.1208/s12249-019-1448-2 [DOI] [PubMed] [Google Scholar]
- Zhang F, McGinity JW, 1999. Properties of Sustained-Release Tablets Prepared by Hot-Melt Extrusion. Pharmaceutical Development and Technology 4, 241–250. 10.1081/PDT-100101358 [DOI] [PubMed] [Google Scholar]