Abstract
Despite advances in understanding the consequences of age-related episodic memory decline for future simulation, much remains unknown regarding changes in the neural underpinnings of future thinking with age. We used a repetition suppression paradigm to explore age-related changes in the neural correlates of emotional future simulation. Younger and older adults simulated positive, negative, and neutral future events either two or five times. Reductions in neural activity for events simulated five versus two times (i.e., repetition suppression) identifies brain regions responsive to the specific emotion of simulated events. Critically, older adults showed greater repetition suppression than younger adults in the temporal pole for negative simulations, and the cuneus for positive simulations. These findings suggest that older adults distance themselves from negative future possibilities by thinking about them in a more semantic way, consistent with the view that older adults down-regulate negative affect and up-regulate positive affect. More broadly this study increases our understanding of the impact of aging on the neural underpinnings of episodic future simulation.
Keywords: Episodic future thinking, Emotion, Aging
1. Introduction
Cognitive aging is associated with changes in various forms of episodic memory (for recent reviews, see Devitt & Schacter, 2016; Park & Festini, 2017). Recent research has examined the consequences of episodic memory decline for adaptive functions, such as simulating possible future experiences (Schacter, Devitt, & Addis, 2018). It is thought that episodic memory supports future simulation via the capacity to flexibly retrieve and recombine information from distinct past experiences into novel representations (Schacter & Addis, 2007a, 2007b, 2020). In support of this idea, a wealth of evidence shows that age-related declines in episodic memory are accompanied by a reduced ability to imagine specific and novel future scenarios (Addis, Musicaro, Pan, & Schacter, 2010; Addis, Wong, & Schacter, 2008; Cole, Morrison, & Conway, 2013; De Beni et al., 2013; Gaesser, Sacchetti, Addis, & Schacter, 2011; Jumentier, Barsics, & Van der Linden, 2018; Lapp & Spaniol, 2017; Madore, Gaesser, & Schacter, 2014; Zavagnin, De Beni, Borella, & Carretti, 2016; for review, see Schacter, Gaesser, & Addis, 2013). However, there is little evidence regarding the impact of aging on the neural underpinnings of episodic future simulation.
Younger adults recruit a common core brain network when remembering past events and imagining future events, comprising regions in the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), medial temporal lobe (MTL) including hippocampus, and lateral temporal and parietal regions (for a meta-analysis, see Benoit & Schacter, 2015). The few studies exploring age effects on neural activation during future thinking demonstrate that older adults also recruit the core network in response to simulating novel future episodes (Addis, Roberts, & Schacter, 2011), and planning future events (Spreng & Schacter, 2012; Viard et al., 2011). These studies also reveal several age differences. Addis et al. (2011) found that during the initial construction phase of remembering and imagining, older adults exhibit less activation than younger adults in regions linked with the retrieval of episodic detail, including the hippocampus, parahippocampus and precuneus. In contrast, during the elaboration phase older adults exhibit increased recruitment of the left lateral temporal cortex, a region linked with semantic or conceptual autobiographical information. Furthermore, activity in this region correlated with subjective ratings of detail for older, but not younger adults. These results fit well with behavioral findings that older adults are more likely to use semantic information to embellish events low in episodic detail (Devitt, Addis, & Schacter, 2017). In the related process of autobiographical planning, older adults displayed a reduced ability to modulate default and control network activity in response to different task demands (Spreng & Schacter, 2012).
While these findings begin to elucidate differences in the networks engaged during future simulation with age, much remains unknown about how age-related changes in episodic simulation are related to the functions of specific regions within the core network. One possible function of specific core network regions may be to process the emotional quality of future events (D’Argembeau, Renaud, & Van der Linden, 2011; Szpunar, Jing, Benoit, & Schacter, 2015). As such, in the current study we examine the influence of aging on the neural mechanisms involved in the simulation of emotional future events.
Aging is associated with a positivity bias in attention and memory, which manifests as a preference to focus on positive over negative information (for reviews, see Carstensen & DeLiema, 2018; Mather & Carstensen, 2005). Proponents of the socioemotional selectivity theory (SST) account for this positivity bias by arguing that as people age and their perceived time left in life diminishes, motivational changes direct an increased focus on emotion regulation to improve quality of life (Carstensen, Isaacowitz, & Charles, 1999). The cognitive control model of the age-related positivity bias further suggests that this emotion regulation is achieved through spontaneous efforts to up-regulate emotion for positive stimuli, including increased self-referential or elaborative processing, and down-regulate emotion for negative stimuli, including suppression of negative affect; processes which are dependent on frontal cortex function (Mather, 2012; Mather & Knight, 2005; Nashiro, Sakaki, & Mather, 2012).
In support of the cognitive control model, neuroimaging studies of emotional processing reveal that aging is associated with increased recruitment of regions involved in cognitive control and emotion processing, including prefrontal cortex (PFC) and anterior cingulate cortex (ACC) regions (Nashiro et al., 2012). For instance, when encoding positive stimuli, older adults exhibit increased activity throughout the PFC, particularly dorsolateral PFC (dlPFC) and ACC/ventromedial PFC (vmPFC), and increased connectivity between vmPFC, amygdala, and the hippocampus (Addis, Leclerc, Muscatell, & Kensinger, 2010; Brassen, Gamer, & Büchel, 2011; Gutchess, Kensinger, & Schacter, 2007; Kensinger & Schacter, 2008; Leclerc & Kensinger, 2008; Ritchey, Bessette-Symons, Hayes, & Cabeza, 2011; though see Leclerc & Kensinger, 2011). Older adults also show increased dlPFC activity when processing negative stimuli (Ford, Morris, & Kensinger, 2014; Murty et al., 2009), and exhibit more habituation in dlPFC activity towards baseline over consecutive viewings of negative images (Roalf, Pruis, Stevens, & Janowsky, 2011). Moreover, older adults display increased negative functional coupling between vmPFC and amygdala (Corbett, Rajah, & Duarte, 2019; Sakaki, Nga, & Mather, 2013), and between dmPFC and the hippocampus for negative stimuli (Ford & Kensinger, 2018; Ford et al., 2014). With age, dmPFC activity is associated with decreased subjective vividness for negative memories, but increased vividness for positive memories (Ford & Kensinger, 2017).
The current study aims to explore whether these age-related neural changes when processing emotional stimuli translate to future simulation. Although older adults demonstrate a positivity bias in memory, prior studies are mixed regarding whether this positivity bias extends to the future. While some have found an age-related increase in positivity when simulating future events (Devitt & Schacter, 2019; Gallo, Korthauer, McDonough, Teshale, & Johnson, 2011; García-Bajos, Migueles, & Aizpurua, 2017), others have found no age differences (Chessell, Rathbone, Souchay, Charlesworth, & Moulin, 2014; Grysman, Prabhakar, Anglin, & Hudson, 2015), or a reverse age effect (Durbin, Barber, Brown, & Mather, 2019). Moreover, the majority of evidence regarding age-related neural changes when processing emotional stimuli have focused on impersonal stimuli such as images, yet recent evidence suggests that these findings do not readily extend to autobiographical stimuli (Ford & Kensinger, 2019). As such, the impact of aging on the neural mechanisms underpinning the simulation of emotional personal future events is an open question. This topic is of particular interest, given approximately two-thirds of everyday future thoughts are emotionally charged (D’Argembeau, Renaud, & Van der Linden, 2011).
When simulating positive scenarios, younger adults recruit vmPFC and rostral ACC more than when simulating negative scenarios, implicating these regions in representing the affective quality of future events (Benoit, Szpunar, & Schacter, 2014; D’Argembeau, Xue, Lu, Van der Linden, & Bechara, 2008; Sharot, Riccardi, Raio, & Phelps, 2007). However, it is difficult to disentangle the contribution of brain regions responding to the emotional valence of simulated events from those that reflect differences in the subjective phenomenology of the events. For instance, the PCC and precuneus are also activated more for positive simulations (D’Argembeau et al., 2008; Sharot et al., 2007), which likely represents the increased subjective vividness of positive events (D’Argembeau & Van der Linden, 2004; Szpunar, Addis, & Schacter, 2012; Szpunar & Schacter, 2013).
To avoid the phenomenological confounds commonly observed between emotional events, Szpunar, Jing, Benoit, and Schacter (2015) employed a repetition suppression technique (Barron, Garvert, & Behrens, 2016), in which they modulated the frequency of exposure to an emotional future simulation. Brain regions responsive to the specific emotion of simulated events showed a repetition-related reduction, or repetition suppression, in neural activity. Szpunar and colleagues (2015) found that negative future simulations were associated with repetition suppression in bilateral pulvinar nucleus, a region involved in processing aversive events (Van Le et al., 2013; Ward, Calder, Parker, & Arend, 2007). Positive simulations were associated with repetition suppression in the orbitofrontal cortex (OFC), a region involved in processing both real and imagined rewards (Bray, Shimojo, & O’Doherty, 2010). A similar repetition suppression technique has been used to examine neural activity linked with specific components (people and locations) of a simulated event for younger adults (Szpunar, St. Jacques, Robbins, Wig, & Schacter, 2014), and to identify neural components of self-related processing (Heleven & Van Overwalle, 2019).
Given that younger and older adults display differences in the subjective experience of future simulations (Addis, Musicaro, et al., 2010; Luchetti & Sutin, 2018), we employed this repetition suppression technique to examine age-related changes in the neural underpinnings of emotional future simulation. We systematically varied the emotional valence and repetition status of simulated future events to evoke emotion selective repetition suppression in neural activity, which we compared across younger and older adults. A strength of this paradigm is that the critical comparison (repeated versus novel) is within-event types, which avoids potential confounds of phenomenological differences between positive and negative events, as well as global differences in neural activity between younger and older adults (D’Esposito, Deouell, & Gazzaley, 2003). Participants simulated positive, negative or neutral future events either two or five times across the course of the experiment.
Because aging is often accompanied by a positivity bias in memory, we expected older adults to be more sensitive than younger adults to the affect associated with positive simulation, and less sensitive to the affect associated with negative simulation. As such, we predicted age-related increases in repetition suppression (i.e., greater two > five activity) in regions previously shown to respond to positive simulation, such as the OFC, and decreases in repetition suppression (i.e., reduced two > five activity) in regions associated with negative simulation, specifically the pulvinar nucleus (Szpunar et al., 2015). We also predicted age-related increases in repetition suppression during positive simulation in the rostral ACC/vmPFC, regions implicated in affective processing of positive future events, and during negative simulation in the dlPFC, indicative of the recruitment of frontally-mediated control processes to monitor affective response. Finally, we expected repetition enhancement in bilateral ventral precuneus irrespective of emotional valence or age, reflecting increased subjective familiarity with and detail of repeatedly simulated events (Gaesser, Spreng, McLelland, Addis, & Schacter, 2013; Szpunar et al., 2015, 2014; van Mulukom, Schacter, Corballis, & Addis, 2013).
2. Methods
2.1. Participants
We recruited 23 younger adults and 25 older adults via postings around the Greater Boston area. Participants gave informed consent in a manner approved by Harvard University’s ethics board. All participants were fluent English speakers, right-handed, with no history of neurological or psychiatric impairments, and had normal or corrected-to-normal vision. One younger adult was dropped for task noncompliance, one for excessive motion, and one for task incompletion. Three older adults were dropped due to task noncompliance, one for an incidental finding, and one for excessive motion. Therefore, 20 younger adults (M age = 21.45 yr, SD = 3.33, 10 males) and 20 older adults (M age = 71.60 yr, SD = 6.07, 8 males) were in the final sample. This sample size was chosen to be similar to Szpunar et al. (2015). Years of education did not differ significantly across younger (M = 14.79, SD = 2.75) and older adults (M = 16.40, SD = 2.23, t(37) = 2.01, p = 0.052). Older adults had a mean MMSE score of 29.20 (SD = 1.32). For session one, younger adults were compensated with $10/hour and older adults were compensated with $15/hour, for session two all participants were compensated with $20/hour.
2.2. Stimuli
The cues were 108 nouns taken from Clark and Paivio’s (2004) extended norms. These nouns were divided into six lists of 18 words each, which were matched on concreteness, frequency, emotion, pleasantness and imageability. Lists of cue words cycled through conditions in a fully counterbalanced design. Each participant was randomly assigned to a counterbalanced version.
2.3. Procedure
This study comprised two sessions, 24 hr apart. Session one involved future event generation, and session two involved an MRI scan. All materials were presented on a computer using E-prime Version 3 (Psychology Software Tools, Pittsburgh, PA). See Figure 1 for an overview of the paradigm.
Figure 1.
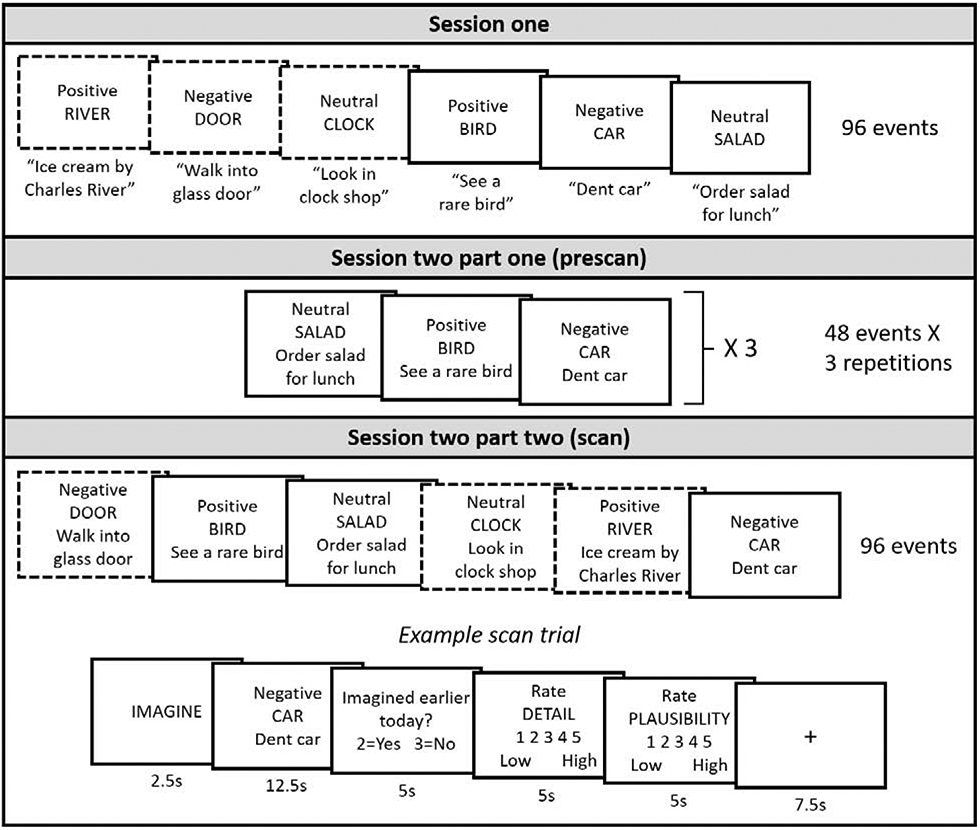
Repetition suppression paradigm. Dotted border = simulated two times, solid border = simulated five times.
Session one.
Participants simulated 36 positive, negative and neutral future events in random order (108 trials total). For each trial, participants were shown a noun cue word and an emotion word (‘positive’, ‘negative’ or ‘neutral’), and were given 12.5 s to silently imagine a personal future scenario that could take place within the next 5 years. Participants were instructed to use the cue word as inspiration to think of a future event, but their event did not have to strictly involve the cue word. This future event was to be plausible, not previously experienced by the participant, to focus on one day or less, and the emotion of the event had to be consistent with the presented emotion word. At the end of the simulation phase, participants rated their event for emotional valence on a 5-point scale (1 = strongly negative, 3 = neutral, 5 = strongly positive), then typed a brief summary description of the imagined event (a few words, self-paced). Participants first completed three practice trials aloud to ensure they understood all instructions. Session one took approximately two hours to complete, including consent, instructions, and practice.
Because older adults often have difficulty generating specific future events compared to younger adults (Addis, Roberts, & Schacter, 2011), we included a buffer in our trial numbers in Session one. Participants simulated 108 trials total in Session one, and 96 were carried forward to Session two (32 in each emotion condition). Therefore, 12 trials from Session one were dropped for each participant (4 from each emotion condition). These were either trials for which participants were unable to imagine a specific future event, or for which they provided a valence rating that was inconsistent with the emotion word. If participants generated more than 96 useable events, then 12 randomly selected trials were dropped.
Session two, part one.
The following day participants were presented with half of the events simulated in session one (16 each positive, negative and neutral). For each event, participants were presented with the associated noun cue word, emotion word, and summary description, and were given 12.5 s to silently re-simulate the future scenario that they had generated the previous day, without adding additional details. Each event was re-simulated three times over in random order (144 trials total). Participants first completed two practice trials aloud to ensure that they understood the instructions. To ensure there were no age-differences in difficulty re-simulating future scenarios, after each simulation participants rated how well they were able to re-simulate the event from the previous day (5-point scale, with a 1 indicating they were unable to re-simulate the corresponding scenario). Only 1% of all trials (across all participants) were assigned a “1” on the first re-simulation (equal numbers for younger and older adults), and half of these future scenarios received increased ratings on subsequent re-simulations, indicating that difficulty re-simulating future scenarios was not a concern in the current paradigm. To ensure that participants would remain focused on the simulation task, they were told that they would later answer some questions about their imagined events. Part one was conducted outside of the scanner, and took approximately 30 min to complete.
Session two, part two.
After a 30 min delay (during which participants practiced the fMRI task and changed into scrubs) participants were placed in the MRI scanner. During the scan, participants re-simulated all 96 of the originally generated future events (32 positive, negative and neutral), so that these events were simulated for either the second or the fifth time over the course of the study. Participants simulated events across eight scans, each 7.5 min in length2. During each scan, participants re-imagined 12 future events, two for each of the six emotion/repetition conditions (imagined two times: positive, negative, and neutral, imagined five times: positive, negative, and neutral). Simulation cues were presented in a pseudorandom order.
Each event was presented in the context of a trial that lasted 30 s, and consisted of the following sequence: (1) the word ‘imagine’ presented for 2.5 s, alerting participants that they were about to simulate a future event; (2) a simulation cue (noun cue word, emotion word, and the participants’ summary description) presented for 12.5 s, during which participants imagined the corresponding future event; (3) the question “imagined earlier today? YES or NO” presented for 5 s, during which participants decided, with a button press, whether they had simulated the future event in part one before entering the scanner (using index and middle fingers, response options counterbalanced across participants); (4) the instruction “rate detail” presented for 5 s above a 5-point rating scale, during which participants rated the detail of their simulation (1 = low, 5 = high); and (5) the instruction “rate plausibility” presented for 5 s above a 5-point rating scale, during which participants rated the plausibility of their simulation (1 = low, 5 = high). Each trial was randomly interleaved with 6, 7.5 or 9 s of fixation, to introduce temporal jitter into the experimental design.
2.4. fMRI acquisition and analysis
Images were acquired on a 3 T Siemens Prisma scanner equipped with a 32-channel head coil. Anatomic images were acquired with a magnetization-prepared rapid gradient echo sequence (matrix size of 256 × 256, 1 mm3 resolution, 176 slices). Functional images were acquired with a multiband echo-planar imaging (EPI) sequence (TR = 2000 ms, TE = 30 ms, matrix size of 136 × 136, FOV = 204, 84 slices, 1.5 mm3 resolution, multiband factor of 3, in-plane GRAPPA acceleration factor of 2). For each fMRI scan, 225 images were acquired. Slices were auto-aligned to an angle 20° towards coronal from anterior-posterior commissure alignment. To allow equilibration of tissue magnetization, each scan began with a 30 s fixation period during which dummy scans were collected. Task stimuli were projected onto a mirror and responses were made via a right-handed five-button MR-compatible response box.
fMRI data were analyzed using Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, UK). Functional data preprocessing included slice-time correction (using the first slice as a reference), two-pass spatial realignment (first to mean image within sessions and then to mean image across sessions), and normalization into Montreal Neurological Institute (MNI) space (using the TPM template supplied by SPM12; no resampling). Functional data were smoothed with a 3 mm full-width half maximum (FWHM) Gaussian kernel. The smoothed functional images of the participants who moved more than 1 voxel were repaired by an interpolation method using the Artefact Repair toolbox (http://cibsr.stanford.edu/tools/ArtRepair/ArtRepair.htm). One younger and one older adult were dropped for motion greater than 3 mm/3 degrees. Anatomic images were also normalized into MNI space.
Preprocessed data were analyzed using a general linear model. There were six events of interest, comprising trials associated with each emotion (positive, negative, and neutral) and repetition condition (two and five times). For each participant (i.e., fixed effects/first-level model) the blood oxygen level dependent (BOLD) response for each event was modeled with a boxcar function convolved with SPM12’s canonical hemodynamic response function over a 15 s window that immediately followed trial onset. An event of no interest was used to model the 15 s window including the memory judgement and detail and plausibility ratings. Six regressors representing movement-related variance (three for rigid-body translation and three for rotation), and regressors modeling each scan session were also entered into the design matrix. Temporal smoothing was conducted before estimation of the parameter estimates using a high-pass filter of 128 s.
Contrasts of two > five activity and five > two activity for each emotion were specified at the first level, and moved forward to a second-level (i.e., random effects) analysis, to assess similarities and differences in repetition suppression and enhancement across younger and older adults, for future simulation generally, and for each of the positive, negative, and neutral simulation conditions. In all analyses, an individual voxel threshold of p < .001 was used, corrected for multiple comparisons to p < .05 with a cluster extent threshold of 35 voxels (Slotnick, 2017; Slotnick, Moo, Segal, & Hart Jr., 2003). The cluster extent threshold was computed using a Monte Carlo simulation with 10,000 iterations, with an estimated spatial autocorrelation of 6 mm (i.e., the average full-width-half-maximum of each participant’s image corresponding to the standard error of the first level model, derived from the ResMS image).
Repetition suppression common to age groups and emotion conditions was assessed by comparing activity for trials simulated two > five times for both younger and older adults combined, inclusively masked with the same contrast for younger and older adults separately, to identify common age effects that are not driven solely by one group. The inclusive masks were set at a threshold of p < .01. The conjoint probability following inclusive masking approached p = .001 (Fisher, 1950). The reverse contrast (five > two simulations) was specified to identify repetition enhancement.
Repetition suppression common to age groups but selective for emotion was assessed by comparing activity for trials simulated two > five times separately for each of the positive, negative and neutral simulation conditions. To identify voxels for which effects were not shared between emotion conditions, each emotion condition was exclusively masked with the same contrast for the other two emotions at a threshold of p < .05 (e.g., two > five for negative, exclusively masked with two > five for neutral and two > five for positive, to identify regions that showed selective reductions in activity for repeated negative future simulations, but not to neutral or positive events). As with the collapsed analysis above, these contrasts were conducted for both younger and older adults, and inclusively masked with the same contrast for younger and older adults separately at a threshold of p < .01. The conjoint probability following inclusive masking approached p = .001 (Fisher, 1950). The reverse contrasts (five > two simulations) were specified to identify emotion selective repetition enhancement. Similar approaches to inclusive and exclusive masking have been used elsewhere to explore age effects (Dulas & Duarte, 2011) and emotion effects (Smith, Henson, Dolan, & Rugg, 2004).
Age differences in overall repetition suppression were identified with an interaction contrast (i.e., two > five simulations, older > younger adults). Age differences in repetition suppression selective for the emotional valence of simulation were assessed using an interaction contrast for each emotion condition separately, exclusively masked with the same contrast for the other emotions (e.g., two > five, older > younger adults for negative, exclusively masked with two > five, older > younger adults for neutral and two > five, older > younger adults for positive, to identify regions showing age-specific reductions in activity for repeated negative simulations, but not to neutral or positive events).
3. Results
3.1. Behavioral results
We examined valence ratings made during the initial simulation in session one using a 2 X 3 ANOVA, with age group (younger, older; between-subjects) and emotion condition (negative, neutral, positive; within-subjects) (see Table 1). There was a significant main effect of emotion (F(1.13, 42.85) = 650.64, p < .001, η2p = .95), with significant pairwise comparisons across all three emotion conditions (ps < .001). The main effect of group was also significant, with older adults rating events as more positive than younger adults (F(1, 38) = 5.99, p = .019, η2p = .14). These main effects were qualified by a significant group by emotion interaction (F(2, 76) = 4.48, p = .015, η2p = .11), with pairwise comparisons revealing that older adults rated negative events as less negative than younger adults (p = .011), whereas no age differences were found for neutral (p = .219) and positive events (p = .296).
Table 1.
Behavioral results collected during session one (valence rating), and during session two (vividness rating, plausibility rating, accuracy of identifying repetition status).
| Age group | Younger adults |
Older adults |
|||||
|---|---|---|---|---|---|---|---|
| Simulation valence | Negative | Positive | Neutral | Negative | Positive | Neutral | |
| Rating | Repetition | ||||||
| Valence | 1.43 (0.23) | 4.53 (0.25) | 3.06 (0.09) | 1.79 (0.55) | 4.42 (0.37) | 3.12 (0.19) | |
| Vividness | Two | 3.71 (0.50) | 3.73 (0.48) | 3.48 (0.54) | 4.12 (0.83) | 4.20 (0.75) | 3.99 (0.90) |
| Five | 4.13 (0.52) | 4.06 (0.50) | 3.90 (0.48) | 4.34 (0.70) | 4.51 (0.61) | 4.27 (0.78) | |
| Plausibility | Two | 3.30 (0.64) | 3.87 (0.51) | 3.91 (0.57) | 3.77 (0.94) | 4.03 (0.72) | 4.04 (0.85) |
| Five | 3.50 (0.72) | 3.92 (0.54) | 4.14 (0.46) | 3.84 (0.93) | 4.29 (0.62) | 4.23 (0.62) | |
| Accuracy | Two | 0.98 (0.03) | 0.98 (0.04) | 0.96 (0.08) | 0.91 (0.17) | 0.90 (0.19) | 0.89 (0.19) |
| Five | 0.95 (0.10) | 0.95 (0.08) | 0.92 (0.13) | 0.89 (0.19) | 0.90 (0.14) | 0.90 (0.14) | |
To explore vividness and plausibility ratings during the scan session, we used a 2 X 2 X 3 ANOVA, with age group, emotion condition, and repetition status (two times, five times; within-subjects). For vividness, we found a main effect of emotion (F(2, 76) = 18.46,p < .001, η2p = .33), with pairwise comparisons revealing that neutral events were rated as less vivid than both negative (p = .001) and positive events (p < .001). We also found a main effect of repetition (F(1, 38) = 23.40,p < .001, η2p = .38), with events repeated five times rated as more vivid than events repeated two times. A main effect of group was also evident (F(1, 38) = 4.77, p = .035, η2p = .11), with older adults rating events as more vivid than younger adults. For plausibility, we found a main effect of emotion (F(1.70, 64.55) = 34.61,p < .001, η2p = .48), where negative events were rated as less plausible than both neutral (p < .001) and positive events (p < .001). A main effect of repetition was also found (F(1, 38) = 15.00,p < .001, η2p = .28), with events repeated five times rated as more plausible than events repeated two times. The main effect of group was not significant (F(1, 38) = 1.79,p = .189, η2p = .05). No interactions for vividness or plausibility ratings were significant (ps > .067).
We also used a 2 X 2 X 3 ANOVA to explore the accuracy during the MRI scan of identifying future events that had been simulated immediately before entering the scanner (i.e., response to the question “imagined earlier today?”). We found a main effect of age group (F(1, 38) = 4.55, p = .039, η2p = .11), with younger adults more accurate overall than older adults. No other comparisons were significant (ps > .317).
3.2. fMRI results
3.2.1. Age similarities in repetition suppression
We first examined repetition suppression and enhancement common to both age groups, collapsed across all emotion conditions. Younger and older adults both exhibited repetition suppression (i.e., greater activity for events imagined two times versus five times) in regions commonly associated with future simulation (Benoit & Schacter, 2015) (see Table 2 and Figure 2). These regions included mPFC, lateral temporal cortex, angular gyrus, and PCC, and correspond closely to the regions identified by Szpunar et al. (2015). There was no common repetition enhancement (i.e., greater activity for events imagined five times versus two) across the age groups and emotion conditions.
Table 2.
Regions showing repetition suppression common to younger and older adults, collapsed across emotion conditions.
| MNI Coordinates |
Peak Z | k | Region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 10 | −84 | −38 | 6.06 | 723 | R cerebellum |
| −44 | 20 | 24 | 6.02 | 3020 | L inferior frontal gyrus |
| −52 | −13 | −13 | 5.69 | 224 | L middle temporal gyrus |
| 39 | 11 | 30 | 5.49 | 41 | R middle frontal gyrus |
| 36 | −88 | −8 | 5.47 | 767 | R occipital pole |
| −2 | 32 | 50 | 5.37 | 222 | L superior frontal gyrus |
| −50 | −34 | −1 | 5.33 | 104 | L posterior superior temporal gyrus |
| 3 | 23 | −20 | 5.32 | 466 | R ventromedial prefrontal cortex |
| −63 | −48 | −4 | 5.3 | 294 | L inferior temporal gyrus |
| −34 | −86 | −4 | 5.18 | 469 | L occipital pole |
| −42 | −58 | −18 | 5.15 | 98 | L fusiform gyrus |
| −51 | −68 | 28 | 5.06 | 341 | L angular gyrus |
| −56 | −26 | 2 | 4.96 | 51 | L superior temporal gyrus |
| 36 | −76 | −26 | 4.95 | 36 | R cerebellum |
| 36 | 35 | −13 | 4.94 | 130 | R orbitofrontal cortex |
| 30 | −80 | −22 | 4.9 | 123 | R cerebellum |
| −14 | 42 | 40 | 4.74 | 58 | L superior frontal gyrus |
| 52 | 32 | −10 | 4.71 | 64 | R orbitofrontal cortex |
| −2 | −52 | 16 | 4.64 | 57 | L posterior cingulate gyrus |
| −6 | 48 | 28 | 4.38 | 43 | L superior frontal gyrus |
| −4 | 22 | 59 | 4.14 | 42 | L superior frontal gyrus |
Notes. k = number of above-threshold voxels; L = left; R = right.
Figure 2.
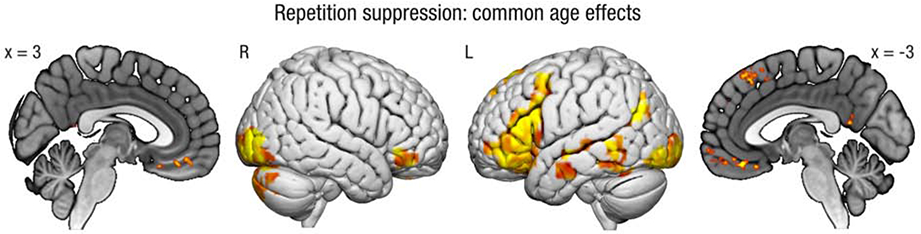
Regions showing repetition suppression (i.e., greater activity for events imagined two times versus five times) for both younger and older adults, collapsed across emotion conditions. Results projected onto surface template from Surfice, and slice template from MRIcroGL. L = left; R = right.
3.2.2. Age similarities in repetition suppression selective for simulation emotion
No regions exhibited repetition suppression or enhancement that was common to both age groups, but selective for either negative, positive, or neutral imagination.
3.2.3. Repetition status and age interaction
We next examined age differences in repetition suppression collapsed across emotion conditions (i.e., two > five, older > younger adults). A number of regions showed an interaction between repetition status and age, either reflecting greater repetition suppression for older than younger adults, or greater repetition enhancement for younger than older adults (see Table 3 and Figure 3). Key regions in this interaction included the right lateral PFC, right inferior temporal cortex, bilateral insula, left amygdala (extending into hippocampus), right lateral parietal, and bilateral lateral occipital cortex. Extraction of the parameter estimates from peak voxels revealed greater repetition suppression for older than younger adults in the left amygdala, and greater repetition enhancement for younger than older adults in the precuneus, consistent with our hypothesis that the precuneus would track the familiarity of the event. The reverse contrast examining greater repetition suppression for younger than older adults revealed no significant clusters.
Table 3.
Regions showing an interaction between repetition suppression and age collapsed across emotion conditions.
| MNI Coordinates |
Peak Z | k | Region | Interaction direction(s) |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 4 | −37 | 24 | 5.2 | 320 | R posterior cingulate gyrus | A, B |
| −2 | −19 | 26 | 4.7 | L posterior cingulate gyrus | B | |
| 14 | −62 | 35 | 4.66 | 614 | R precuneus | B |
| 58 | −52 | −14 | 4.62 | 99 | R inferior temporal gyrus | A |
| −3 | −24 | 44 | 4.62 | 59 | L posterior cingulate gyrus | A, B |
| 4 | −20 | 42 | 3.67 | R posterior cingulate gyrus | B | |
| 39 | 6 | −1 | 4.6 | 148 | R insula | A, B |
| 45 | 11 | 17 | 4.57 | 49 | R inferior frontal gyrus | A, B |
| −46 | −56 | 12 | 4.46 | 50 | L middle temporal gyrus | A, B |
| −38 | −62 | 12 | 3.59 | L inferior lateral occipital cortex | A, B | |
| 9 | 5 | 60 | 4.46 | 39 | R supplementary motor cortex | A, B |
| 6 | 14 | 62 | 3.64 | R superior frontal gyrus | A, B | |
| −44 | −80 | 14 | 4.41 | 71 | L superior lateral occipital cortex | A, B |
| 38 | 48 | 30 | 4.3 | 99 | R frontal pole | A, B |
| 32 | 42 | 34 | 4.25 | R middle frontal gyrus | B | |
| 32 | 4 | 52 | 3.76 | R middle frontal gyrus | A, B | |
| 44 | 18 | −26 | 4.3 | 80 | R temporal pole | A, B |
| 54 | 20 | 0 | 4.29 | 295 | R inferior frontal gyrus | A |
| 56 | −55 | 14 | 4.24 | 169 | R angular gyrus | A, B |
| 54 | −64 | 11 | 3.44 | R inferior lateral occipital cortex | A | |
| −38 | 5 | −2 | 4.19 | 45 | L insula | A, B |
| 22 | 11 | 58 | 4.18 | 101 | R superior frontal gyrus | A, B |
| 58 | −26 | 35 | 4.17 | 38 | R supramarginal gyrus | B |
| 39 | 26 | 42 | 4.15 | 46 | R middle frontal gyrus | A, B |
| 9 | −78 | 47 | 4.06 | 86 | R precuneus | B |
| −14 | −10 | −19 | 3.99 | 35 | L amygdala (extends into hippocampus) | A |
| 16 | −64 | 59 | 3.95 | 38 | R precuneus | A |
| 34 | −37 | 44 | 3.94 | 81 | R superior parietal lobule | B |
| 52 | −32 | 44 | 3.92 | 51 | R supramarginal gyrus | A, B |
| −15 | −58 | 40 | 3.81 | 36 | L precuneus | A, B |
| 26 | −73 | 48 | 3.8 | 75 | R superior lateral occipital cortex | A, B |
| −10 | −74 | 52 | 3.57 | 36 | L precuneus | B |
Notes. A = Greater repetition suppression for older than younger adults; B = greater repetition enhancement for younger than older adults, k = number of above-threshold voxels; L = left; R = right.
Figure 3.
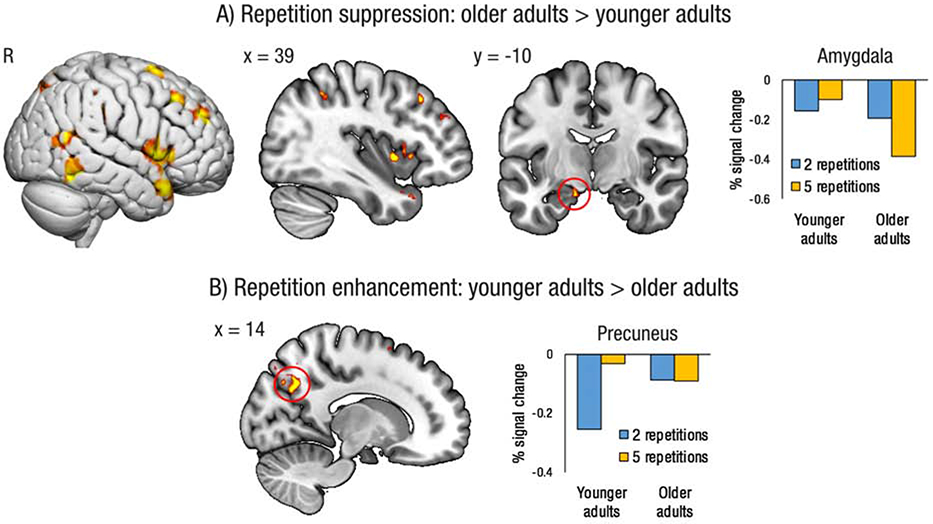
A) Regions in which older adults exhibit greater repetition suppression (i.e., greater activity for events imagined two times versus five times) than younger adults, collapsed across emotion conditions. B) Regions in which younger adults exhibit greater repetition enhancement (i.e., greater activity for events imagined five times versus two times) than older adults, collapsed across emotion conditions. Plots show percent signal chance extracted from a 3mm sphere centered on the peak voxel (amygdala xyz = −14 −10 −19; precuneus xyz = 14 −62 35). Results projected onto surface template from Surfice, and slice template from MRIcroGL. R = right.
3.2.4. Repetition status and age interaction selective for simulation emotion
Finally, we examined regions showing an interaction between repetition status and age that was selective for negative, positive, or neutral imagination (see Table 4 and Figure 4). For negative imagination, older adults exhibited greater repetition suppression than younger adults in the right temporal pole. For positive imagination, older adults exhibited repetition suppression in the cuneus, a region in which younger adults displayed repetition enhancement. There were no interaction effects selective for neutral imagination, or regions showing greater repetition suppression for younger than older adults.
Table 4.
Regions showing an interaction between repetition status and age, selective for simulation emotion.
| MNI Coordinates |
Peak Z | k | Region | Interaction direction(s) |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Negative simulation | ||||||
| 39 | 18 | −32 | 4.42 | 35 | R temporal pole | A |
| Positive simulation | ||||||
| −6 | −82 | 20 | 5.07 | 35 | L cuneus | A, B |
Notes. A = Greater repetition suppression for older than younger adults; B = greater repetition enhancement for younger than older adults; k = number of above-threshold voxels; L = left; R = Right.
Figure 4.
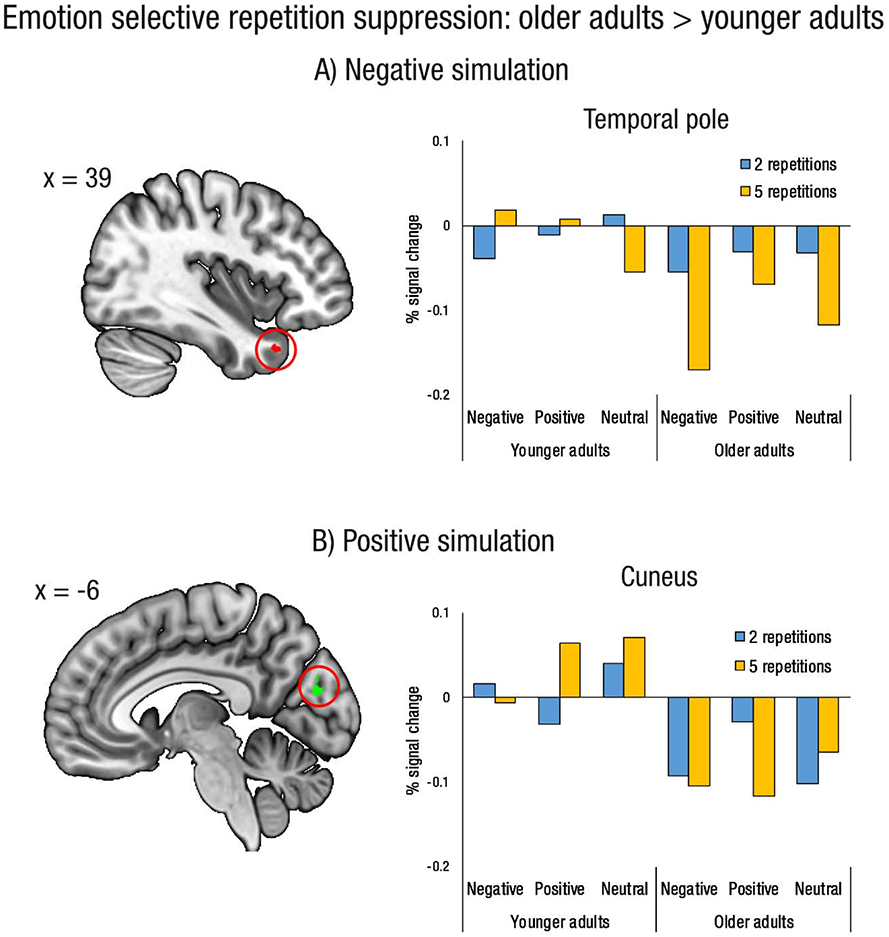
Regions in which older adults exhibit greater repetition suppression (i.e., greater activity for events imagined two times versus five times) than younger adults, selective for emotion condition. A) Repetition suppression selective for negative future simulation. B) Repetition suppression selective for positive future simulation. Plots show percent signal chance extracted from a 3mm sphere centered on the peak voxel (temporal pole xyz = 39 18 – 32; cuneus xyz = −6 −82 20). Results projected onto slice template from MRIcroGL.
3.2.5. Repetition enhancement, vividness, and plausibility
A possible explanation for repetition enhancement is that it reflects increases in the vividness or plausibility of future scenarios across repeated simulations. To evaluate this possibility, we re-ran the second-level model testing the interaction between repetition enhancement and age (collapsed across all emotions) twice, including the average difference between two versus five repetitions for vividness and for plausibility as a subject-level covariate (cf., Szpunar et al., 2015). The results from these covariates were inclusively masked with the results of the interaction between repetition enhancement and age collapsed across emotion conditions (reported in Table 2) at a threshold of p < .01. In none of the regions exhibiting repetition enhancement was activity also associated with increases in vividness or plausibility.
4. Discussion
While age differences have been found in neural activation when thinking about the future (e.g., Addis et al., 2011; Spreng & Schacter, 2012), much remains unknown about the functions of specific regions involved in episodic simulation, and how these change with age. In the current study we examined age-related changes in the neural substrates of emotional future simulation. We used a repetition suppression paradigm to identify regions sensitive to the emotional valence of future events, which we compared across younger and older adults. We found common repetition suppression (i.e., greater activity for events imagined two times versus five times) across the age groups in regions commonly associated with future simulation. Significant age differences emerged in the temporal pole during negative simulation and cuneus during positive simulation, with older adults displaying greater repetition suppression than younger adults. These results provide insight into age differences in the neural resources recruited when faced with emotional future possibilities.
4.1. Behavioral results
Consistent with an age-related positivity bias in memory and attention (see Mather & Carstensen, 2005), and in future imagination (Devitt & Schacter, 2019; Gallo et al., 2011; García-Bajos et al., 2017), older adults judged their negative future simulations as less negative than younger adults. Older adults also rated their simulations as more vivid than younger adults, in line with prior findings on subjective ratings and aging (Addis, Musicaro, et al., 2010; Addis et al., 2008; Luchetti & Sutin, 2018). For both age groups, repeated simulation increased ratings of vividness and plausibility, indicating that the repeated simulation manipulation was effective. Negative and positive events were rated as more vivid than neutral events, and negative events were rated less plausible overall. Importantly, these subjective differences across age groups and emotional valence support the use of a repetition suppression paradigm: because the main comparison in our analyses was within-subjects (i.e., two versus five simulations), between-subject differences in subjective experience are accounted for within the design.
4.2. Age similarities
Collapsed across emotion, both younger and older adults exhibited repetition suppression in regions commonly associated with future simulation, including the mPFC, lateral temporal cortex, angular gyrus, and PCC (Benoit & Schacter, 2015). These regions correspond closely with those identified by Szpunar et al. (2015) for younger adults, and are consistent with prior reports of common recruitment of a core network across age groups when simulating episodic future events (Addis et al., 2011). In contrast to the age similarities when collapsing across emotion, no regions exhibited repetition suppression common to younger and older adults that was selective for either positive, negative or neutral simulation. While Szpunar and colleagues (2015) found that younger adults displayed repetition suppression in the pulvinar nucleus for negative events, and the OFC for positive events, our results suggest that these regions do not contribute to emotional simulation with advancing age. Indeed, as we discuss in the following section, older adults exhibit repetition suppression in different regions than younger adults in service of positive and negative future simulation. However, it is also possible that methodological differences in cueing techniques (e.g., using noun cue words rather than personalized person and place details), or statistical unreliability of the original findings (which were reported at an uncorrected threshold, Szpunar et al., 2015), account for the lack of emotion-specific activity in the OFC and pulvinar nucleus. Notably, we replicated all regions reported at a corrected statistical threshold by Spzunar et al. (2015) in our analysis collapsed across emotions and age groups.
4.3. Age differences
Across all emotion conditions, older adults exhibited greater repetition suppression than younger adults in several regions previously associated with episodic simulation, including the right lateral PFC, right inferior temporal cortex, right lateral parietal, and bilateral lateral occipital cortex. Addis and colleagues (2011) found similar regions were recruited more by older than younger adults when elaborating on remembered past and imagined future events. Our results also identified increased repetition suppression on the border between the left amygdala and hippocampus with age, consistent with reports of differential emotion processing by older adults (Mather, 2004, 2012; Mather & Knight, 2005; Nashiro et al., 2012).
Two regions exhibited repetition suppression specific to age and selective for emotional valence. Older adults showed greater repetition suppression than younger adults during negative simulation in the right temporal pole, a region involved in semantic and emotion processing, with known anatomical and functional connections to limbic structures (Binder & Desai, 2011; Burianová, McIntosh, & Grady, 2010). This finding is consistent with research showing that older adults tend to recruit the temporal pole more than younger adults when simulating future events (Addis et al., 2011), although the current findings suggest that these effects may be enhanced by negative simulation. It is possible that older adults process negative future events in a more semantic way as a means of distancing themselves from the negative affect generated by the simulation, an idea supported by the age-related reduction in subjective negativity ratings for negative events. This notion is in line with recent work showing that older adults show increased activity in areas involved in emotion processing when anticipating the future presentation of negative images, which contributes to reduced subsequent memory for those images (Corbett et al., 2019). Similarly, Ford & Kensinger (2017, 2018) found increased recruitment of the mPFC in older adults during negative event retrieval, which was associated with decreased hippocampal activation and reduced subjective richness of the memory. These findings support the theory that older adults down-regulate negative affect in response to emotional stimuli (Mather, 2012; Mather & Knight, 2005; Nashiro et al., 2012), and the current results provide novel evidence that similar regulation strategies may be employed when thinking about future events, which could contribute to a more optimistic view of the future with age (Devitt & Schacter, 2019; Gallo et al., 2011; García-Bajos et al., 2017).
For positive events, older adults exhibited repetition suppression in the cuneus, a region in which younger adults exhibited repetition enhancement. The cuneus is involved in visual imagery and reactivation of visual details during autobiographical memory and future simulation (c.f. Addis, Pan, Vu, Laiser, & Schacter, 2009; Addis et al., 2011; Gilmore, Nelson, Chen, & McDermott, 2018). As such, these age differences may reflect increased recruitment of visual imagery resources by older than younger adults in service of positive simulation, as a strategy to enhance associated positive affect, further contributing to age-related increases in future optimism. However, caution must be taken with this interpretation, given younger and older adults did not differ in subjective emotion and vividness ratings for positive simulations.
As predicted, younger adults exhibited repetition enhancement (i.e., greater activity for events imagined five times versus two times) in PCC and precuneus. In a covariate analysis, we did not find evidence that regions exhibiting repetition enhancement were senstive to changes in vividness or plausibility ratings. This finding replicates those of Szpunar et al. (2015) who also found no association between repetition enhancement and vividness or plausibility in PCC or precuneus. Instead, it is possible that for younger adults, repetition enhancement in these regions tracks the familiarity of the event (Gaesser et al., 2013; Szpunar et al., 2015, 2014; van Mulukom et al., 2013). Older adults did not modulate activity in these regions with repetition, even though our behavioral measures indicated that they identified events repeated before the scan at a high rate, suggesting they were sensitive to the familiarity of each event. Similar reductions in modulation of the posteromedial cortex during encoding of face-name pairs with age has been linked with increased amyloid deposition (Vannini et al., 2012). The present findings suggest that age-related reductions in modulation extends to adaptive functions reliant on episodic memory, and calls for further exploration of the mechanism driving decreased repetition enhancement in posterior medial parietal regions with age.
A limitation of this study is that we did not collect emotion ratings during the scan due to time constraints. As such, we do not know the effect of repeated simulation on affective responses for younger or older adults. We can speculate based on prior findings that repetition would increase affect, particularly for positive events. For instance, Szpunar and Schacter (2013) found that after repeated simulation, younger adults rated positive future simulations as more positive, but emotion ratings for negative and neutral events were not influenced by repetition. Similarly, De Brigard, Szpunar, and Schacter (2013) found that upward and downward counterfactuals were rated as more positive after repeated simulation. However, to our knowledge there are no extant data on emotion ratings for repeatedly simulated events in older adults. Future research would benefit from tying possible age differences in emotion ratings across repetitions to neural changes with age.
In sum, we used a repetition suppression paradigm to explore age-related changes in the functions of specific brain regions when simulating emotional future events. Critically, we found increased repetition suppression with age in the temporal pole during negative simulation, and in the cuneus during positive simulation. These findings are in line with the view that older adults employ emotion regulation strategies to down-regulate negative affect and up-regulate positive affect, and may reflect increased recruitment of semantic resources to distance themselves from negative future events, and visual imagery resources to enhance positive affect. More broadly, these findings increase our understanding of the impact of aging on the neural underpinnings of episodic future simulation, and illuminate a potential neural mechanism contributing to age-related increases in optimism about the future.
Highlights.
Neural repetition suppression (RS) occurs for events simulated 5 vs. 2 times
We explore age-related changes in RS during emotional future simulation
Greater RS with age in temporal pole for negative and cuneus for positive simulation
Identifies potential neural mechanism supporting increased future optimism with age
Acknowledgements
We thank Ethan Harris, Susana Arango and Matthew Spence for help with participant scheduling, data collection, and analysis.
Funding
This work was supported by the National Institute on Aging [grant number R01 AG008441 to D.L.S]. D.R.A. was supported in part thanks to funding from the Canada 150 Research Chairs Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None.
Note that four older adults completed only seven scan runs (84 trials): two terminated the scan early, and two only generated sufficient usable future events during Session one for seven scan runs due to an initial misunderstanding of task instructions.
References
- Addis DR, Leclerc CM, Muscatell KA, & Kensinger EA (2010). There are age-related changes in neural connectivity during the encoding of positive, but not negative, information. Cortex, 46(4), 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Musicaro R, Pan L, & Schacter DL (2010). Episodic simulation of past and future events in older adults: Evidence from an experimental recombination task. Psychology and Aging, 25(2), 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu M, Laiser N, & Schacter DL (2009). Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia, 47, 222–238. [DOI] [PubMed] [Google Scholar]
- Addis DR, Roberts RP, & Schacter DL (2011). Age-related neural changes in autobiographical remembering and imagining. Neuropsychologia, 49(13), 3656–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, & Schacter DL (2008). Age-related changes in the episodic simulation of future events. Psychological Science, 19, 33–41. [DOI] [PubMed] [Google Scholar]
- Barron HC, Garvert MM, & Behrens TEJ (2016). Repetition suppression: a means to index neural representations using BOLD? Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1705), 20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, & Schacter DL (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Szpunar KK, & Schacter DL (2014). Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proceedings of the National Academy of Sciences, 111(46), 16550–16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, & Desai RH (2011). The neurobiology of semantic memory. Trends in Cognitive Sciences, 15(11), 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassen S, Gamer M, & Büchel C (2011). Anterior cingulate activation is related to a positivity bias and emotional stability in successful aging. Biological Psychiatry, 70(2), 131–137. [DOI] [PubMed] [Google Scholar]
- Bray S, Shimojo S, & O’Doherty JP (2010). Human medial orbitofrontal cortex is recruited during experience of imagined and real rewards. Journal of Neurophysiology, 103(5), 2506–2512. [DOI] [PubMed] [Google Scholar]
- Burianová H, McIntosh AR, & Grady CL (2010). A common functional brain network for autobiographical, episodic, and semantic memory retrieval. NeuroImage, 49(1), 865–874. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, & DeLiema M (2018). The positivity effect: a negativity bias in youth fades with age. Current Opinion in Behavioral Sciences, 19, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, & Charles ST (1999). Taking time seriously: A theory of socioemotional selectivity. American Psychologist, 54(3), 165–181. [DOI] [PubMed] [Google Scholar]
- Chessell ZJ, Rathbone CJ, Souchay C, Charlesworth L, & Moulin CJA (2014). Autobiographical memory, past and future events, and self-images in younger and older adults. Self and Identity, 13(4), 380–397. [Google Scholar]
- Clark JM, & Paivio A (2004). Extensions of the Paivio, Yuille, and Madigan (1968) norms. Behavior Research Methods, Instruments, & Computers, 36(3), 371–383. [DOI] [PubMed] [Google Scholar]
- Cole SN, Morrison CM, & Conway MA (2013). Episodic future thinking: Linking neuropsychological performance with episodic detail in young and old adults. The Quarterly Journal of Experimental Psychology, 66(9), 1687–1706. [DOI] [PubMed] [Google Scholar]
- Corbett B, Rajah MN, & Duarte A (2019). Preparing for the Worst: Evidence that Older Adults Proactively Downregulate Negative Affect. Cerebral Cortex. [DOI] [PMC free article] [PubMed]
- D’Argembeau A, Renaud O, & Van der Linden M (2011). Frequency, characteristics and functions of future-oriented thoughts in daily life. Applied Cognitive Psychology, 25(1), 96–103. [Google Scholar]
- D’Argembeau A, & Van der Linden M (2004). Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: Influence of valence and temporal distance. Consciousness and Cognition, 13(4), 844–858. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Xue G, Lu Z-L, Van der Linden M, & Bechara A (2008). Neural correlates of envisioning emotional events in the near and far future. Neuroimage, 40(1), 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, & Gazzaley A (2003). Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nature Reviews Neuroscience, 4(11), 863. [DOI] [PubMed] [Google Scholar]
- De Beni R, Borella E, Carretti B, Zavagnin M, Lazzarini L, & Milojevi G (2013). Remembering the past and imagining the future: age-related differences between young, young-old and old-old. Aging Clinical and Experimental Research, 25(1), 89–97. [DOI] [PubMed] [Google Scholar]
- Devitt AL, Addis DR, & Schacter DL (2017). Episodic and semantic content of memory and imagination: A multilevel analysis. Memory & Cognition, 45(7), 1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt AL, & Schacter DL (2016). False memories with age: neural and cognitive underpinnings. Neuropsychologia, 91, 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt AL, & Schacter DL (2019). Looking on the bright side: Aging and the impact of emotional future simulation on subsequent memory. The Journals of Gerontology: Series B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulas MR, & Duarte A (2011). The effects of aging on material-independent and material-dependent neural correlates of contextual binding. Neuroimage, 57(3), 1192–1204. [DOI] [PubMed] [Google Scholar]
- Durbin KA, Barber SJ, Brown M, & Mather M (2019). Optimism for the future in younger and older adults. The Journals of Gerontology: Series B, 74(4), 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA (1950). Statistical methods for research workers. Biological monographs and manuals. No. V. Statistical Methods for Research Workers. Biological Monographs and Manuals. No. V. [Google Scholar]
- Ford JH, & Kensinger EA (2017). Prefrontally-mediated alterations in the retrieval of negative events: Links to memory vividness across the adult lifespan. Neuropsychologia, 102, 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, & Kensinger EA (2018). Older adults use a prefrontal regulatory mechanism to reduce negative memory vividness of a highly emotional real-world event. NeuroReport, 29(13), 1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, & Kensinger EA (2019). Older adults recruit dorsomedial prefrontal cortex to decrease negativity during retrieval of emotionally complex real-world events. Neuropsychologia, 107239. [DOI] [PubMed] [Google Scholar]
- Ford JH, Morris JA, & Kensinger EA (2014). Neural recruitment and connectivity during emotional memory retrieval across the adult life span. Neurobiology of Aging, 35(12), 2770–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Sacchetti DC, Addis DR, & Schacter DL (2011). Characterizing age-related changes in remembering the past and imagining the future. Psychology and Aging, 26, 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, McLelland VC, Addis DR, & Schacter DL (2013). Imagining the future: Evidence for a hippocampal contribution to constructive processing. Hippocampus, 23(12), 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA, Korthauer LE, McDonough IM, Teshale S, & Johnson EL (2011). Age-related positivity effects and autobiographical memory detail: Evidence from a past/future source memory task. Memory, 19(6), 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bajos E, Migueles M, & Aizpurua A (2017). Age-based positivity effects in imagining and recalling future positive and negative autobiographical events. Frontiers in Psychology. [DOI] [PMC free article] [PubMed]
- Gilmore AW, Nelson SM, Chen H-Y, & McDermott KB (2018). Task-related and resting-state fMRI identify distinct networks that preferentially support remembering the past and imagining the future. Neuropsychologia, 110, 180–189. [DOI] [PubMed] [Google Scholar]
- Grysman A, Prabhakar J, Anglin SM, & Hudson JA (2015). Self-enhancement and the life script in future thinking across the lifespan. Memory, 23(5), 774–785. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, & Schacter DL (2007). Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience, 2(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Heleven E, & Van Overwalle F (2019). Neural representations of others in the medial prefrontal cortex do not depend on our knowledge about them. Social Neuroscience, 14(3), 286–299. [DOI] [PubMed] [Google Scholar]
- Jumentier S, Barsics C, & Van der Linden M (2018). Reduced specificity and enhanced subjective experience of future thinking in ageing: the influence of avoidance and emotion-regulation strategies. Memory, 26(1), 59–73. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, & Schacter DL (2008). Neural processes supporting young and older adults’ emotional memories. Journal of Cognitive Neuroscience, 20(7), 1161–1173. [DOI] [PubMed] [Google Scholar]
- Lapp LK, & Spaniol J (2017). Impact of age-relevant goals on future thinking in younger and older adults. Memory, 1–14. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, & Kensinger EA (2008). Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective, & Behavioral Neuroscience, 8(2), 153–164. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, & Kensinger EA (2011). Neural processing of emotional pictures and words: A comparison of young and older adults. Developmental Neuropsychology, 36(4), 519–538. [DOI] [PubMed] [Google Scholar]
- Luchetti M, & Sutin AR (2018). Age differences in autobiographical memory across the adult lifespan: older adults report stronger phenomenology. Memory, 26(1), 117–130. [DOI] [PubMed] [Google Scholar]
- Madore KP, Gaesser B, & Schacter DL (2014). Constructive episodic simulation: Dissociable effects of a specificity induction on remembering, imagining, and describing in young and older adults. Journal of Experimental Psychology: Learning, Memory, and Cognition, 40(3), 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M (2004). Aging and Emotional Memory In Hertel DRP (Ed.), Memory and emotion (pp. 272–307). New York, NY, US: Oxford University Press. [Google Scholar]
- Mather M (2012). The emotion paradox in the aging brain. Annals of the New York Academy of Sciences, 1251(1), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, & Carstensen LL (2005). Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences, 9(10), 496–502. [DOI] [PubMed] [Google Scholar]
- Mather M, & Knight M (2005). Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging, 20(4), 554. [DOI] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan H-Y, Callicott JH, Goldberg TE, ... Mattay VS (2009). Age-related alterations in simple declarative memory and the effect of negative stimulus valence. Journal of Cognitive Neuroscience, 21(10), 1920–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, & Mather M (2012). Age differences in brain activity during emotion processing: Reflections of age-related decline or increased emotion regulation. Gerontology, 58(2), 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, & Festini SB (2017). Theories of memory and aging: A look at the past and a glimpse of the future. The Journals of Gerontology: Series B, 72(1), 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Bessette-Symons B, Hayes SM, & Cabeza R (2011). Emotion processing in the aging brain is modulated by semantic elaboration. Neuropsychologia, 49(4), 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Pruis TA, Stevens AA, & Janowsky JS (2011). More is less: emotion induced prefrontal cortex activity habituates in aging. Neurobiology of Aging, 32(9), 1634–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Nga L, & Mather M (2013). Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults’ memory. Journal of Cognitive Neuroscience, 25(8), 1206–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, & Addis DR (2007a). On the constructive episodic simulation of past and future events. Behavioural and Brain Sciences, 30, 299–351. [Google Scholar]
- Schacter DL, & Addis DR (2007b). The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society (B), 362, 773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, & Addis DR (2020). Memory and imagination: Perspectives on constructive episodic simulation In Abraham A (Ed.), The Cambridge Handbook of the imagination (pp. 111–131). Cambridge University Press; Cambridge (England). [Google Scholar]
- Schacter DL, Devitt AL, & Addis DR (2018). Episodic future thinking and cognitive aging In Oxford Research Encyclopedia of Psychology and Aging. Oxford University Press. [Google Scholar]
- Schacter DL, Gaesser B, & Addis DR (2013). Remembering the past and imagining the future in the elderly. Gerontology, 59(2), 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, & Phelps EA (2007). Neural mechanisms mediating optimism bias. Nature, 450(7166), 102–105. [DOI] [PubMed] [Google Scholar]
- Slotnick SD (2017). Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cognitive Neuroscience, 8(3), 150–155. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, & Hart J Jr. (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research, 17(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Smith APR, Henson RNA, Dolan RJ, & Rugg MD (2004). fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage, 22(2), 868–878. [DOI] [PubMed] [Google Scholar]
- Spreng RN, & Schacter DL (2012). Default network modulation and large-scale network interactivity in healthy young and old adults. Cerebral Cortex, 22(11), 2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Addis DR, & Schacter DL (2012). Memory for emotional simulations: Remembering a rosy future. Psychological Science, 23, 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Jing HG, Benoit RG, & Schacter DL (2015). Repetition-Related Reductions in Neural Activity during Emotional Simulations of Future Events. PLOS ONE, 10(9), e0138354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, & Schacter DL (2013). Get real: Effects of repeated simulation and emotion on the perceived plausibility of future experiences. Journal of Experimental Psychology: General, 142, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, St. Jacques PL, Robbins CA, Wig GS, & Schacter DL (2014). Repetition-related reductions in neural activity reveal component processes of mental simulation. Social Cognitive and Affective Neuroscience, 9(5), 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Le Q, Isbell LA, Matsumoto J, Nguyen M, Hori E, Maior RS, ... Nishijo H (2013). Pulvinar neurons reveal neurobiological evidence of past selection for rapid detection of snakes. Proceedings of the National Academy of Sciences, 110(47), 19000–19005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mulukom V, Schacter DL, Corballis MC, & Addis DR (2013). Re-imagining the future: Repetition decreases hippocampal involvement in future simulation. PLoS ONE, 8(7), e69596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A, Chetelat G, Lebreton K, Desgranges B, Landeau B, de La Sayette V, ... Piolino P (2011). Mental time travel into the past and the future in healthy aged adults: An fMRI study. Brain and Cognition, 75, 1–9. [DOI] [PubMed] [Google Scholar]
- Ward R, Calder AJ, Parker M, & Arend I (2007). Emotion recognition following human pulvinar damage. Neuropsychologia, 45(8), 1973–1978. [DOI] [PubMed] [Google Scholar]
- Zavagnin M, De Beni R, Borella E, & Carretti B (2016). Episodic future thinking: the role of working memory and inhibition on age-related differences. Aging Clinical and Experimental Research, 28(1), 109–119. [DOI] [PubMed] [Google Scholar]


