Abstract
Neurexins (NRXNs) are cell-adhesion molecules important in the formation and remodeling of neural circuits. It has been shown that aversive environmental stimuli can affect the expression pattern of Neurexin genes (Nrxns) impacting the regulation of synaptic strength. Accumulated evidence suggests that, after chronic exposure to psychological stress, the triggered changes in gene expression and splicing patterns of Nrxns may be involved in aversive conditioning. Previously, we have demonstrated that a novel treatment using dietary phytochemicals can modulate the response to chronic variable stress (CVS) in mice. Here, we aimed to further investigate the long-term plasticity changes after CVS by focusing on the regulation of NRXNs at synapses. We found that CVS differentially triggers the region-specific gene expression of Nrxns in mice Nucleus Accumbens (NAc) and Hippocampus (HIPP). The prophylactic treatment with the combination of two phytochemicals dihydrocaffeic acid (DHCA) and Malvidin-3-O-glucoside (Mal-gluc) differentially modulated the stress-induced effects on Nrxn1 and 3 mRNA expression in these brain areas and promoted the alternative splicing of Nrxn3 in HIPP.
Overall, our data supports the prophylactic effect of dietary phytochemicals in the restoration of stress-induced plasticity changes in mouse brain. By intervening in activity-dependent plasticity at synapses, these compounds may attenuate the effects of chronic aversive conditioning. We propose that an early therapeutic intervention may help with disorders of negative affect, such as depression or post-traumatic stress disorder. Our future studies will address how DHCA/Mal-gluc might serve as a potential complement for current therapies in depression and other mood disorders.
Keywords: Dietary phytochemicals, Neurexins, Chronic variable stress, inflammation, Major Depression Disorder
1-. Introduction
Synaptic maladaptation to excessive and/or harmful environmental inputs can cause an imbalance of neural circuits involved in cognition, decision-making, anxiety or depression (Chan et al., 2018; Jafari et al., 2016; McEwen et al., 2016; Radley et al., 2005; Wang et al., 2018). In addition, there are important sex differences in how the brain responds to stressors (Hodes et al., 2015, Summer et al., 2017). Unfortunately, current therapeutics in major depressive disorder (MDD) and other mood disorders mainly target neurochemical or neurobiological mechanisms. Only a temporary relief of depression symptoms is achieved in less than 50% of MDD patients, with moderate to severe side effects. The success rate is even lower in female MDD patients (Maeng et al.,2015; Pandarakalam et al., 2018; Weissman et al., 1995). Therefore, there is an urgent need to design disease-modifying drugs that target underlying specific molecular mechanisms.
One of the key pharmacological targets being studied are Cell-Adhesion Molecules (CAMs). Neuroligins (NLGN) and their main presynaptic ligand, Neurexins (NRXNs), are CAMs that play important roles in the formation and maintenance of synaptic connections (Bang et al., 2013; Schreiner et al., 2014). The NRXN family comprises three different isoforms (NRXN1-3) that are expressed at comparable levels in different brain regions. The relative expression of NRNXs and their ligands in the synapse regulates the function of key neural circuits and determines the state of the network (Bang et al., 2013; Etherton et al., 2009; Sudorf et al., 2008). For instance, point mutations and aberrant patterns of synaptic expression of NRXN-1 and NLGN-2 have been described in cell-type specific mechanisms in the pathophysiology of neurological and psychiatric disorders (Van der Kooki et al., 2014; Liang et al., 2015; Heshmati et al., 2018; Van Spronsen et al., 2010; Owczarek et al., 2015). NLGNs and NRXNs are molecules in the synapse that undergo rapid functional changes to quickly adapt to internal and external inputs (Schreiner et al., 2014; Sudorf et al., 2008). In order to achieve these rapid plasticity changes triggered by neuronal activity the cells use different regulatory mechanisms, including the metabolism of messenger RNA (mRNA).
Alternative splicing (AS) is an example of these mechanisms. AS allows the generation of a specific splice variant of a gene to acquire a higher biodiversity of the targeted protein product. This, in turn, will have direct consequences on the cellular trafficking of the targeted protein or its signaling properties (Raj and Blencowe, 2015). In the Nrxn genes, the functional studies have mainly focused on the alternatively spliced segment 4 (SS4) on exon 20, which is highly conserved in all Nrxns pre-mRNAs (Aoto et al., 2013; Iijima et al., 2011; Ding et al., 2017). Nrxn-SS4(+) is the splicing variant containing the exon 20 at SS4, which shows only weak adhesion and binding properties.
Conversely, exon skipping SS4 in the Nrxn-SS4(−) variant, allows for strong adhesive interactions in the synapse. In mouse brain, Nrxn1-3 genes are alternatively spliced on exon 4, in a coordinated manner. Among the Nrxn genes, Nrxn3 has the most diverse expression patterns in different brain regions (Aoto et al., 2013).
In addition, epigenetic mechanisms also regulate Nrxn mRNA alternative splicing in hippocampal memory-activated neurons. Recent studies address how hippocampal formation may participate in the regulation of negative emotion. Ding, et al. demonstrated in a mouse model of aversive conditioning that activity-dependent Nrxn-SS4 inclusion was required for memory preservation. Thus, alternative splicing in the hippocampus may regulate the stability of aversive memories (Ding et al., 2017). Similar lines of research have made important links between the hippocampus and the regulation of stress hormones that affect aversive memory (Glover et al., 2015; Goosens et al., 2011; Sherin et al., 2011; Summer et al., 2017). Taken together, this evidence suggests that interventions aiming at restoring normal hippocampal function may help with disorders of negative affect, such as depression or post-traumatic stress disorder (Glover et al., 2015; Goosens et al., 2011).
Previously, we have shown that oral treatment with phytochemicals DHCA and Mal-gluc can attenuate stress effects in repeated social defeat in mice (Wang et al., 2018). Our results are in line with recent findings showing that these behavioral phenotypes are associated with the concurrence of both peripheral inflammation and changes in synaptic plasticity (Adzic et al., 2018; Cuevas et al., 2013; Golden et al., 2013; Patel et al., 2018). The prophylactic effect of the DHCA/Mal-gluc combination might be explained by a synergistic mechanism of action that simultaneously reduces high levels of inflammatory markers in the periphery, along with the normalization of gene expression patterns of key synaptic plasticity elements, like Rac1 (Cuevas et al., 2013; Wang et al., 2018). In the present study, we investigated the effects of CVS on the experience-dependent regulation of NRXNs in synapses triggered by repeated stress exposure, and the potential protective effects of prophylactic treatment with DHCA/Mal-gluc.
2-. Materials and Methods
2.1-. Animals
C57BL/6 mice were purchased from The Jackson Laboratory. Both male and female animals 8 weeks old were used for the experiments. All the animals were housed at the animal facilities , with free access to food and water and under a 12/12h cycle and following the approved protocols by the Institutional Animal Care and Use Committee of Icahn School of Medicine at Mount Sinai, IACUC-2014-0081.
2.2-. Drugs and Treatment
The phytochemicals Mal-gluc, Malvidin-3-O-glucoside (Extra- synthesis, Genay Cedex, France) and DHCA, 3-[(3,4-Dihydroxyphenyl) propionic acid] (Sigma-Aldrich) were analyzed by LC-MS. We have performed the in vivo safety, toxicology analyses following the NCCIH Product Integrity guidelines as previously reported (Wang et al., 2018). Animals were treated, through their drinking water, using a combination of DHCA and Mal-Gluc administered two weeks before and during the CVS paradigm. The dosage 5 mg/kg-BW/day DHCA and 500 ng/kg-BW/day Mal-gluc has been established elsewhere (Wang et al., 2018).
2.3-. Repeated stress induction and behavioral tests
2.3.1-. Variable Stress Paradigm.
The chronic variable stress (CVS) protocol has been replicated as previously reported (Wang et al., 2018), and animals were placed in their home cage after each session. CVS was administered 1h a day, and consisted of one of three different stressors, sequentially and in the same order: one hundred mild foot shocks for 2 s at 0.45 mA during 1 h (Med Associates, St. Albans, Vermont, USA); tail suspension during 1h; restraint stress inside a falcon tube during 1h.
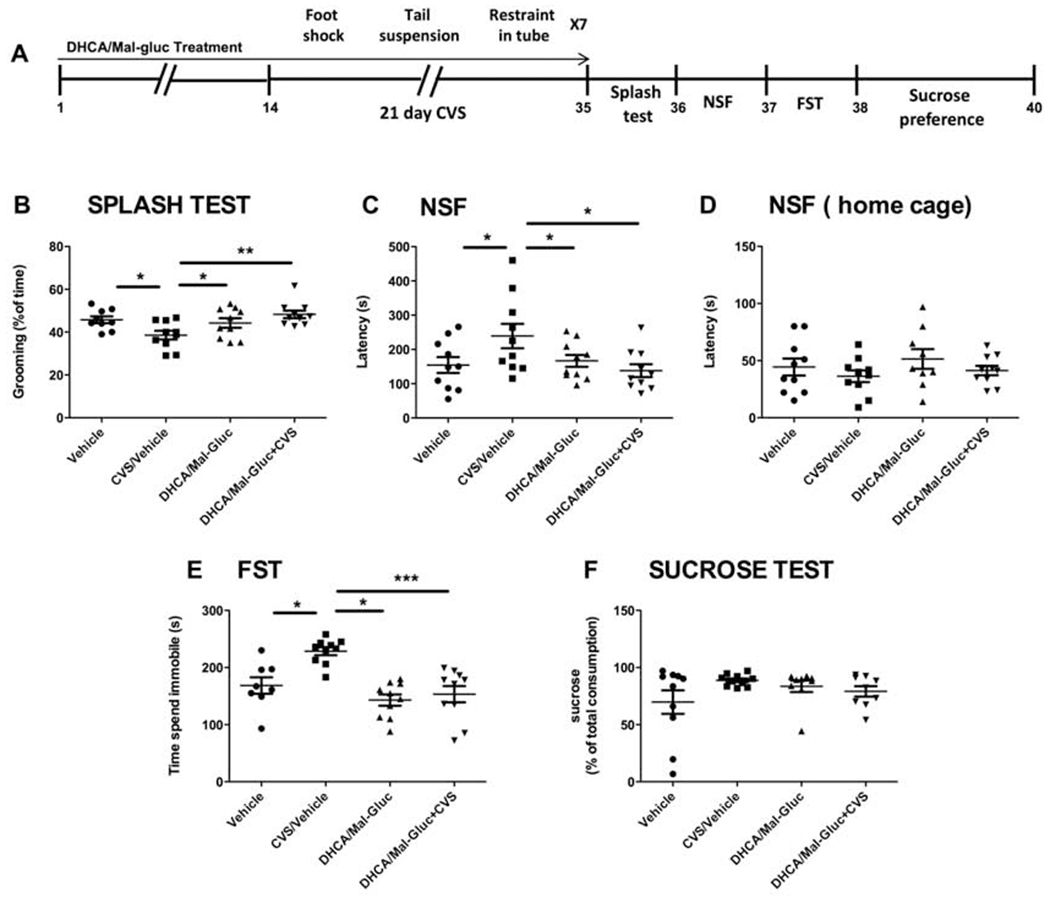
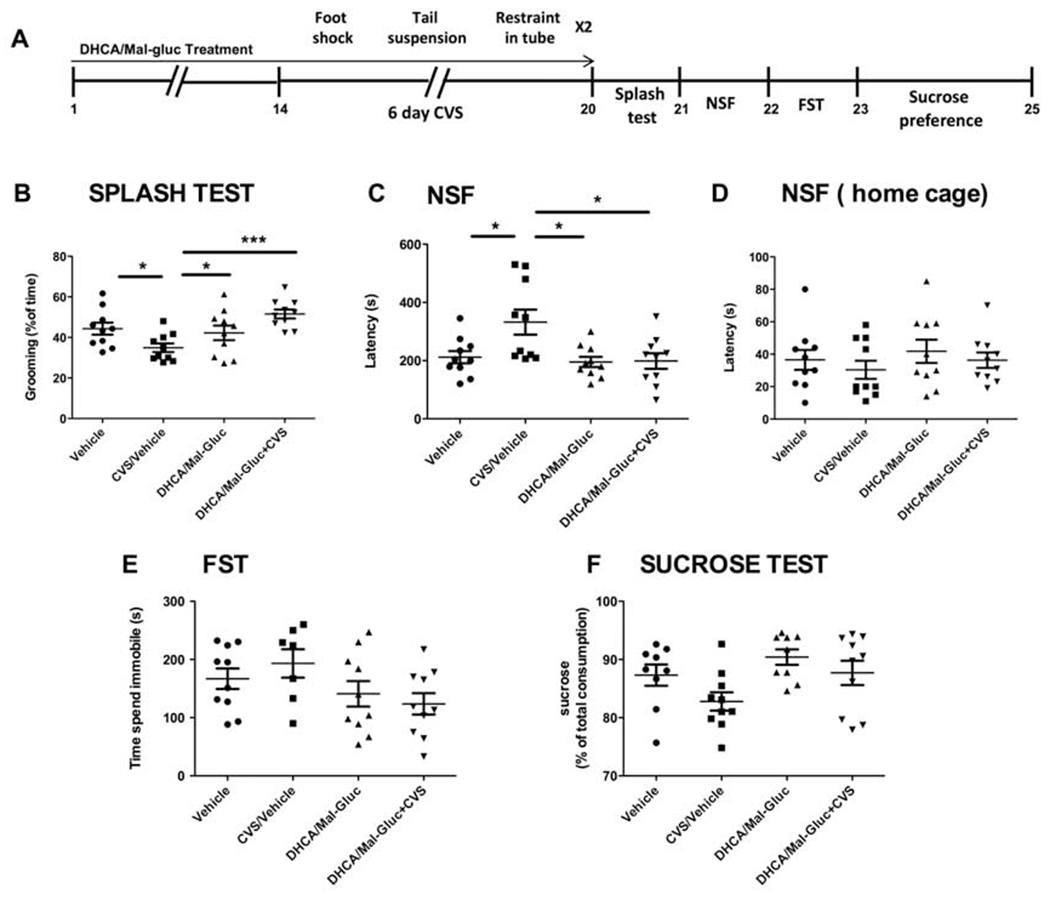
The stress paradigm for female mice consisted in 6 days of CVS whereas in male mice the protocol was 21 days long. This dual protocol has been proven to induce comparable effects in male and female mice (Hodes et al., 2015, Wang et al., 2018) due to this latter are more vulnerable to repeated stress induction (Fig.1A, Fig.2A). For the duration of the stress protocol, two parallel non-stressed groups of both, male and female mice, were kept in their home cages. These two non-stressed groups, vehicle controls and treated group of mice were housed under the same conditions.
Fig. 1-. Prophylactic treatment with phytochemicals attenuates CVS impact and increases resilience in male mice.
(A) Scheme of the variable stress paradigm for male mice. (B-F) Quantification of the behavioral assessment is shown for the four experimental groups: (B) Splash test (one-way ANOVA, F3,38 = 4.61, P = 0.008); (C) NSF test (one-way ANOVA: F3,39 = 3.23, P = 0.034); (D) NSF home cage (one-way ANOVA: F3,38= 0,933, P = 0.434); (E) FST (one-way ANOVA, F3,39 = 10.30, P < 0.0001); (F) Sucrose preference test (one-way ANOVA: F3,37 = 1.647, P = 0.1969). Results are represented as mean ± S.E.M. (*P< 0.05, **p < 0.01, ***P < 0.001, n=8-10 animals/group). NSF: Novelty suppressed feeding; FST: Force swim test; CVS: Chronic Variable Stress.
Fig. 2-. Prophylactic treatment with phytochemicals attenuates CVS impact and increases resilience in female mice.
(A) Scheme of the variable stress paradigm for female mice. (B-F) Quantification of the behavioral assessment is shown for the four experimental groups: (B) Splash test (one-way ANOVA: F3,39 = 5.33, P = 0.004); (C) NSF test (one-way ANOVA, F3,39 = 5.22, P = 0.004);(D) NSF home cage (one-way ANOVA, F3,39 = 0,6087, P = 0.6137); (E) FST (one-way ANOVA, F3,36 = 2,028, P = 0.1290) (F) Sucrose preference test (one-way ANOVA: F3,36 = 2.491, P = 0.0773). Results are represented as mean ± S.E.M.( *p < 0.05, **P < 0.01, ***P < 0.001; n=8-10 animals/group). NSF: Novelty suppressed feeding; FST: Force swim test; CVS: Chronic Variable Stress.
2.3.2-. Novelty suppressed feeding (NSF).
In the NSF test, the latency to eat is scored for up to 10 min. The previous night, animals were prevented from accessing food. On the day of the experiment, mice were brought to acclimate to the room 1h prior to the test and then placed at the corner into the testing box (50 χ 50 χ 50 cm) that contains a single pellet of food.
As soon as the animal was observed to eat, or the 15-min time limit was reached, the mouse was removed from the testing box and placed in the home cage, and the latency to eat in the familiar environment was measured.
2.3.3-. Forced swim test (FST).
Mice were placed in the room 1h prior to the test. Animals were placed in a Pyrex glass beaker filled with 2 L of water and their behavior was videotaped for 6 min An external investigator, blind to the test conditions, scored the time that mice spent immobile.
2.3.4-. Splash test.
We performed this test for attest self-neglected behavior in mice. Animals prompted to grooming behavior by spraying them in their back with a sucrose solution (10% wt./vol). Grooming frequency and latency were measured for 5 min in the home cage with no bedding.
2.3.5-. Sucrose preference testing.
Animals had unlimited access to drinking water from two different bottles, one of them filled up with 1% sucrose solution. The order of the bottles was switch after every recording point at 24, 48 and 72h. The preference for sucrose is expressed as a percentage of the sucrose consumption relative to total liquid drunken by the animal.
2.4-. RNA isolation, gene expression and Alternative Splicing Analysis.
For total RNA isolation from mice hippocampus, cortex or Nucleus Accumbens (NAc) we used RNeasy® Mini Kit (Qiagen, Valencia, CA, USA). For gene expression by quantitative RT-PCR, we used triplicates of each RNA sample and Maxima SYBR® Green master mix (Fermentas, Thermofisher Scientific) in the ABI Prism 7900HT fast real-time PCR system (SeqGen, Inc.).
The list of the custom-designed oligonucleotide primers included in the reaction for RT-PCR is presented in Table 1. We used GADPH expression level as an internal control and we normalized the data following the 2-ΔΔCt method. Fold changes were expressed as relative to non-stressed control mice.
Table 1.
Oligonucleotide sequences of primer sets for semi-quantitative PCR
| Primer set | Sequence (5’→3’) |
|---|---|
| Nrxn1-F | TAGCAAAGGTGGTGGACAG |
| Nrxn1-R | ATGTCCTCATCGTCACTGG |
| Nrxn3-F | CCTCATCCTCTATACCTGG |
| Nrxn3-R | CTGTACTTGGTTCACATTCG |
| Nrxn1 ex20-F | ACAGCTGGCCAGTTATCGAACGCT |
| Nrxn1 ex20-R | AGCTGACGCCCTTTGTCGAGTAGCCATT |
| Nrxn1 del-F | TACCCTGCAGGGCGTCA |
| Nrxn1 del-R | GCCATATTCAGAACTTTCAAGCC |
| Nrxn3 ex20-F | TACGGGCCGGTTATATTTG |
| Nrxn3 ex20-R | TGGCCAGTGAATGAGCAC |
| Nrxn3 del-F | AGAGAACTCCTGTCAATGATG |
| Nrxn3 del-R | TTAGCTGCCGGCCTGTA |
| Gapdh-F | CATGGCCTTCCGTGTTCCTA |
| Gapdh-R | GCGGCACGTCAGATCCA |
2.5-. Statistical Analysis
For comparison of four groups in both, the behavioral and biochemical analyses, one-way ANOVAs followed by Bonferroni’s comparison was used (n=8-10 animals/group). All values are expressed as mean and S.E.M. In all studies, outliers (2 SD from the mean) were excluded and the null hypothesis was rejected at the 0.05 level. All statistical analyses were performed using Prism Stat program (GraphPad Software, Inc.).
3-. Results.
3.1-. The combination of DHCA/Mal-gluc attenuates the impact of CVS in male and female mice.
As shown previously (Wang et al., 2018), two different groups of mice were treated with either vehicle or DHCA/Mal-gluc combination for 14 days before a CVS paradigm (Fig.1A, Fig.2A). The study also included two additional experimental groups of mice, housed under the same conditions but not submitted to the stress protocol ( Vehicle treated group and treatment control group). After chronic stress mice were subjected to a battery of behavioral tests to elucidate whether animals pretreated with these phytochemicals have an increased coping response when compared to untreated animals and in comparison to the non-stressed cohorts of mice. We confirmed a significantly improvement on the behavior outcome in the treated group (Fig. 1, Fig.2). These results suggest that the prophylactic administration of DHCA/Mal-gluc increased the resilience to CVS in these animals. In the splash test, non-treated stressed mice groomed significantly less when sprayed with 10% sucrose solution compared to the non-stressed control mice, whereas the treatment with DHCA/Mal-gluc completely reversed the CVS-induced self-neglect behavior (Fig.1B, Fig.2B). In the NSF, used to examine an anxiety component of stress-induced behavior, mice exhibited longer latency to feed compared to the non-stressed mice following overnight food deprivation, and the DHCA/Mal-gluc treatment significantly reduced the latency to eat (Fig.1C, Fig.2C). Lastly, the forced swim test (FST) was used to measure passive vs. active coping response. We found that male mice after CVS, spent significantly more time immobile compared to the non-stressed mice and treatment with DHCA/Mal-gluc significantly reduced the time of floating (Fig.1E). However, the stress paradigm did not impact significantly the results on the sucrose preference test (Fig. 1F, Fig. 2F) or passive coping behavior in the FST for female (Fig.2E). Here we confirmed, as previously described (Hodes et al., 2015, Wang et al., 2018), that female mice are more vulnerable to the duration of the CVS paradigm. While 21 days of stress is necessary to induce depression-like behavior in male mice, female mice are more susceptible to variable stress and express a depression-associated phenotype after only 6 days of stress (Fig.1A, Fig.2A). These differences observed in mice behavior are consistent with the experimental observation that males and females may manifest different symptoms and responses toward stress and further emphasizes the importance of testing any therapeutics in both sexes.
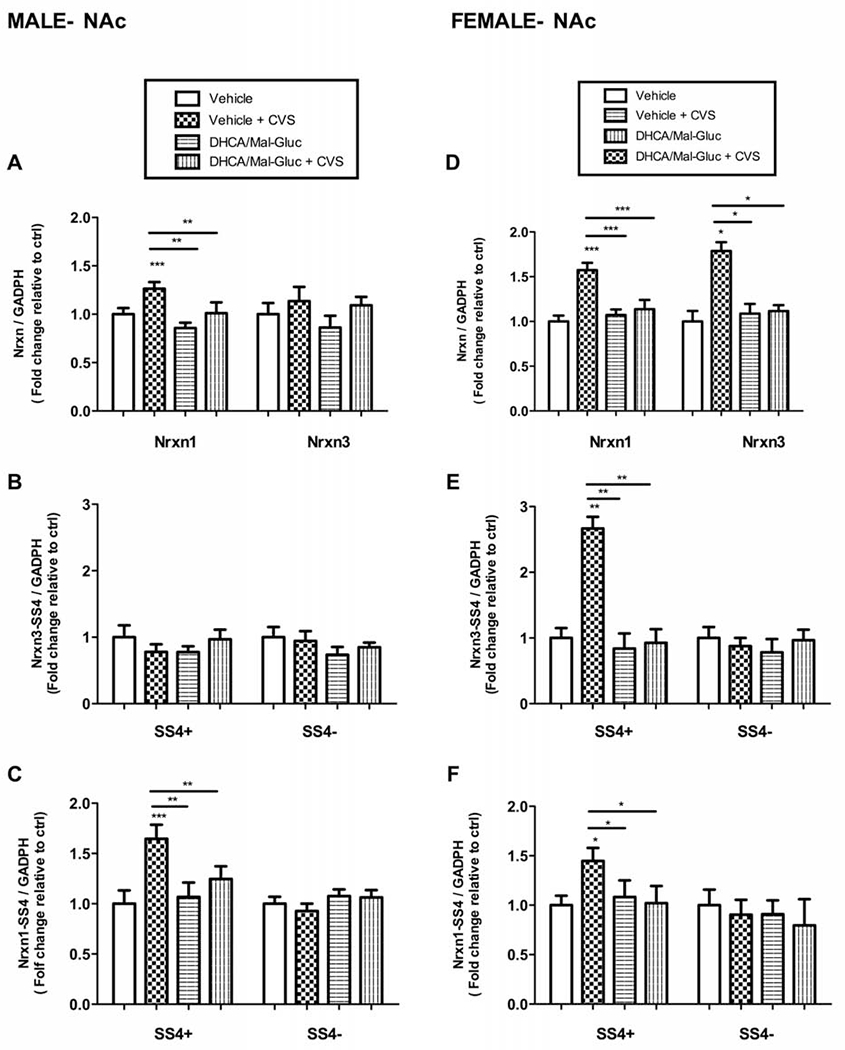
3.2-. Nrxn gene expression and alternative splicing in Nucleus Accumbens (NAc).
In male mice, we did not find any changes in the total expression of Nrxn3 or their splicing variants (Figs.3A,3B). Our results showed, instead, an increase in mRNA expression levels of the Nrxn1-SS4(+) splicing variant with a parallel increase on total Nrxn1 mRNA after CVS exposure (Figs.3A, 3C). However, we did not observe significant changes of Nrxn1-SS4(−) mRNA levels (Fig.3C). This suggests that CVS induced a total increase on gene expression of Nrxn1 rather than an alternative splicing shift. The prophylactic treatment with DHCA/Mal-gluc significantly attenuates the stress-induced total Nrxn1 and Nrxn1-SS4 (+) mRNA upregulation (Figs.3A,3C). In female mice, we found an increase in the expression levels of both total Nrxn1 and Nrxn3 mRNA (Fig.3D) parallel to the upregulation of the splicing variants Nrxn1-SS4 (+) and Nrxn3-SS4(+) after repeated stress but no significant changes were detected in the Nrxn1-SS4(−) or Nrxn3-SS4(−) splicing variants (Figs.3E,3F). Similar to the effect observed in their male counterparts, the experimental treatment rescued these effects of stress on mRNA upregulation (Figs.3D, 3E, 3F).
Fig.3-. Differential regulation of the expression of total Nrxn1 and 3 and their alternative splicing in NAc after CVS.
(A-C) Real-time PCR quantification of total Nrxn1/3 and alternative splicing variants (splicing site 4, SS4) in male stressed mice with or without DHCA treatment. Fold change of (A) Nrxn1 and Nrxn3 total mRNA (B) Nrxn3 SS4+ and SS43− mRNA and (C) Nrxn1 SS4+ and SS4− mRNA in stressed male mice with or without treatment relative to the non -stressed control mice (vehicle). (D-F) Real-time PCR quantification of total Nrxn1/3 and alternative splicing variants (splicing site 4, SS4) in female stressed mice with or without DHCA treatment. Fold change of (D) Nrxn1 and Nrxn3 total mRNA (E) Nrxn3 SS4+ and SS43− mRNA and (F) Nrxn1 SS4+ and SS4− mRNA in stressed female mice with or without treatment relative to non -stressed control mice (vehicle). Female mice, One-way ANOVA: F 3,40= 13.68 , P < 0.0001 for NRX1; F 3,36 = 3.42 , P = 0.023 for Nrxn1 SS4+; F 3, 35 = 0.44, P= 0,72 for Nrxn1 SS4−; F 3,40 = 7.45 , P = 0.0001 for Nrxn3; F 3,39 = 10.66, P < 0.0001 for Nrxn3 SS4+; F 3, 38 = 3.911, P = 0.0123 for Nrxn3 SS4−. Male mice, One-way ANOVA: F 3,36 =10.49, P <0.001 for NRX1; F 3,39 =6.253 , P= 0.0011 for Nrxn1 SS4+ ; F 3,37 = 1.72, P= 0.170 for Nrxn1 SS4−; F 3,34 =2.162, P=0.195 for Nrxn3; F 3,38 =2.062, P= 0.3915 for Nrxn3 SS4+; F 3,34= 0.91, P= 0.40 for Nrxn3 SS4−). Nrxn mRNA values were normalized vs GADPH as internal control and expressed as fold changes relative to non-treated controls (vehicle). Bar graphs represents Mean ± S.E.M. ( *P <0.05; **P <0.01; ***P <0.001; n=8-10 animals/group).
3.3-. Nrxn gene expression and alternative splicing in Hippocampus (HIPP) and prefrontal cortex (PFC)
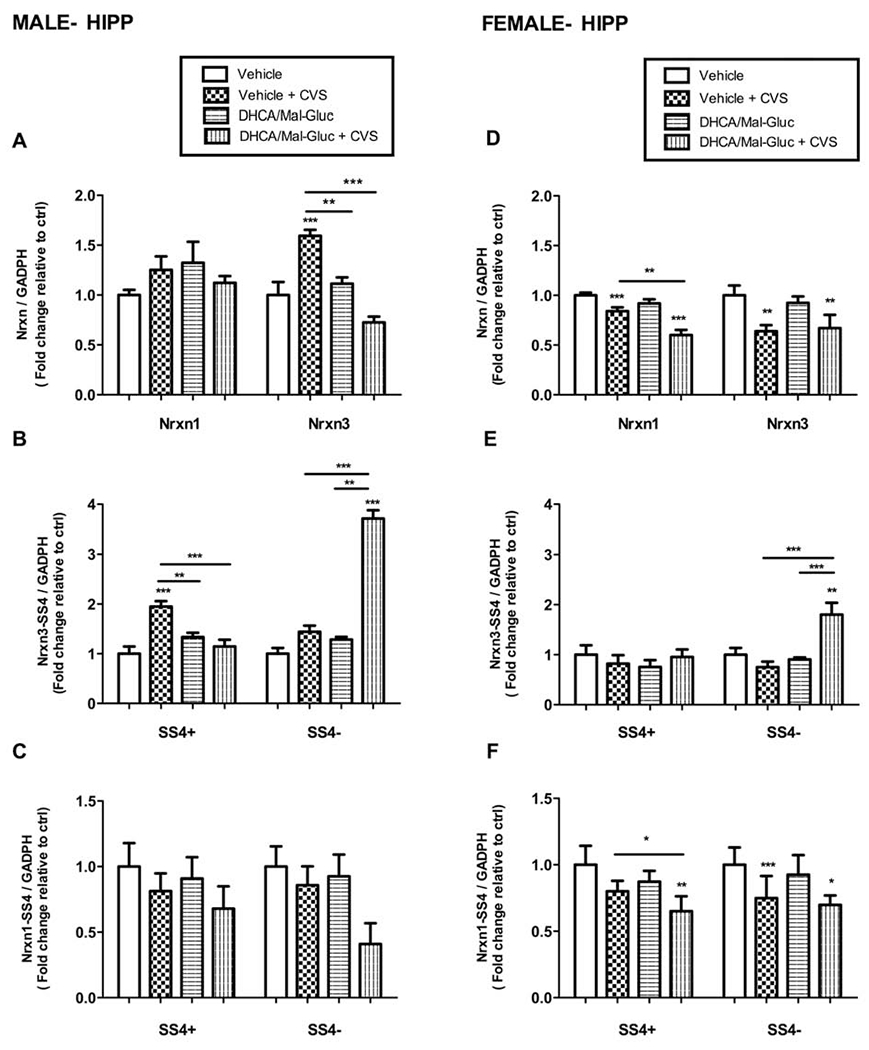
In male HIPP, the CVS protocol induced an increased expression of the Nrxn3-SS4(+) splicing variant with a parallel increase on total Nrxn3 mRNA after CVS exposure (Fig.4A, 4B). However, no significant changes were observed on total Nrxn mRNA levels or any of its splicing variants after chronic stress (Fig.4A, 4C). Similar to the results obtained in the analysis of NAc extracts, the prophylactic treatment with DHCA/Mal-gluc prevented the upregulation of Nrxn3 mRNA in male (Fig.4A). In addition, the pretreatment with phytochemicals seems to modulate a splicing shift of Nrxn3-SS4 after CVS (Fig.4B). Importantly, the total Nrxn3 mRNA levels were not altered in pretreated animals indicating a change in alternative splicing rather than a decreased mRNA stability (Fig.4A).
Fig.4-. Regulation of the expression of total Nrxn1 and 3 and alternative splicing in HIPP after Chronic Variable Stress.
(A-C) Real-time PCR quantification of total Nrxn1/3 and alternative splicing variants (splicing site 4, SS4) in the HIPP of male stressed mice with or without DHCA treatment. Fold change of (A) Nrxn1 and Nrxn3 total mRNA (B) Nrxn3 SS4+ and SS43− mRNA and (C) Nrxn1 SS4+ and SS4− mRNA in stressed male mice with or without treatment relative to the non -stressed control mice (vehicle). (D-F) Real-time PCR quantification of total Nrxn1/3 and alternative splicing variants (splicing site 4, SS4) in the HIPP of female stressed mice with or without DHCA treatment. Fold change of (D) Nrxn1 and Nrxn3 total mRNA (E) Nrxn3 SS4+ and SS43− mRNA and (F) Nrxn1 SS4+ and SS4− mRNA in stressed female mice with or without treatment relative to non -stressed control mice (vehicle). Female mice, One-way ANOVA: F 3,35 =56.11 , P < 0.0001 for NRX1; F 3,35 =18.37 , P < 0.0001 for Nrxn1 SS4+; F 3,34 = 8.89 , P= 0.0004 for Nrxn1 SS4−; F 3,37= 10.29 , P =0.0001 for Nrxn3; F 3,28 = 15.45, P < 0.0001 for Nrxn3 SS4+; F 3,40 =0.79 , P= 0.46 for Nrxn3 SS4−. Male mice, One-way ANOVA: F 3,33 = 2.34,P= 0,092 for NRX1; F 3,35 =0.716, P= 0.5528 for Nrxn1 SS4+; F 3,35= 2.453, P = 0.0902 for Nrxn1 SS4− ; F 3,29 =37.55, P < 0.0001 for Nrxn3; F 3,39= 10.74, P < 0.0001 for Nrxn3 SS4+ ; F 3,40 =49.07, P < 0.0001 for Nrxn3 SS4−). Nrxn mRNA values were normalized vs GADPH as internal control and expressed as fold changes relative to non-treated controls (vehicle). Bar graphs represents Mean ± S.E.M.( *P <0.05; **P <0.01, ***P <0.001; n=8-10 animals/group).
In female mice, the stress protocol significantly reduced the total mRNA expression of Nrxn1 and Nrxn3 in the HIPP (Fig.4D). Contrary to the observation in male, the prophylactic treatment had no effect on the stress-induced downregulation of Nrxn1 or Nrxn3 mRNA in female (Fig.4D). We also tested whether the stress induced an experience-dependent alternative splicing of Nrxn1-SS4 or Nrxn3-SS4. A similar turnover on Nrxn3-SS4 alternative splicing variants was observed in females compared to male. However, the total Nrxn3 mRNA transcripts were also reduced in female (Fig.4D). These results suggest that Nrxn3-SS4(+) mRNA destabilization may have occurred simultaneously with the activity-dependent increase of Nrxn3-SS4 (−) production in female mice HIPP (Fig.4E, 4F).
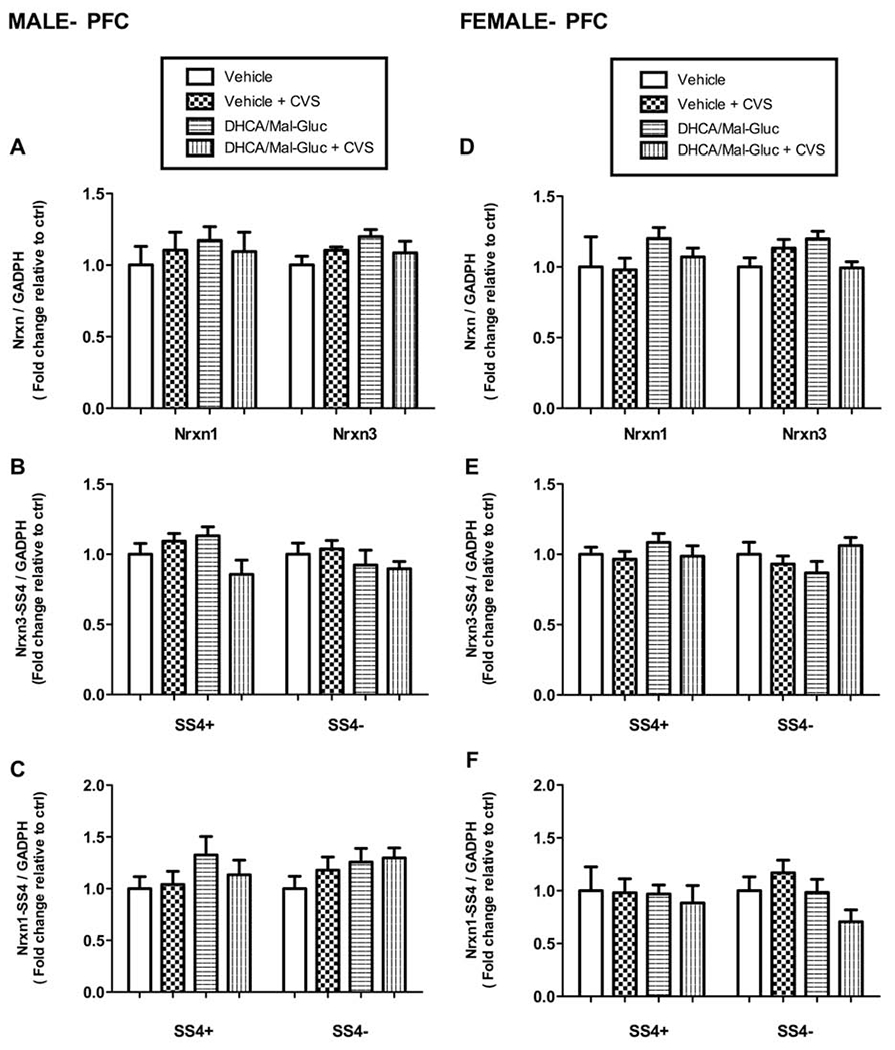
No significant changes were detected on the gene expression or alternative splicing of Nrxn1 or 3 in the PFC extracts from either male or female mice after the stress paradigm with or without treatment (Fig.5).
Fig. 5-. DHCA/Mal-gluc did not induce changes in regulation of Nrxn1 and 3 total expression or alternative splicing in PFC after CVS.
Quantitative real-time PCR for total Nrxn1/3 and alternative splicing variants (splicing site 4, SS4) for male (A-C) and female mice (D-F). No significant changes were found in total mRNA expression or alternative splicing variants (SS4) of NRXN 1 and 3 in male or female mice. Female mice, One-way ANOVA: F 3,37 = 1.59, P= 0.218 for NRX1; F 3,39= 0.318 , P=0.729 for Nrxn1 SS4+; F 3,36 = 0.84 , P= 0.439 for Nrxn1 SS4−; F 3, 40 =1.31 , P=0.28 for Nrxn3; F 3,40 = 0.12 , P= 0.87 for Nrxn3 SS4+; F 3,40 = 2.83 , P= 0.070 for Nrxn3 SS4−. Male mice, One-way ANOVA: F 3,38= 4.7, P= 0.86 for NRX1; F 3,40 = 0.88 , P=0.41 for Nrxn1 SS4+ ; F 3,40= 3.10 , P=0.056 for Nrxn1 SS4−; F 3,35= 0.623 , P= 0.603 for Nrxn3; F 3,37 =2.41, P= 0.1014 for Nrxn3 SS4+; F 3,37 =3.1 P =0.054 for Nrxn3 SS4−. Nrxn mRNA values were normalized vs GADPH as internal control and expressed as fold changes relative to non-treated controls (vehicle). Mean ± S.E.M are represented (n=8-10 animals/group).
4-. Discussion
Here we demonstrate that the combination of DHCA/Mal-gluc, two previously characterized phytochemicals, has a prophylactic effect on the behavioral phenotype triggered by CVS in mice. We show activity-dependent changes in Nrxn gene expression, in the analysis of mice brain extracts. We focused on Nrxn1 and Nrxn3 due to their differential expression pattern throughout the brain, and their higher susceptibility to regulation by activity-dependent plasticity mechanisms in the synapses. NRXNs interact with several postsynaptic ligands forming a dynamic interaction network. The overall state of this network characterizes a given synapse. Therefore, changes in the relative concentrations of NRXNs will determine the state of this network (Bang et al., 2013; Etherton et al., 2009; Sudorf et al., 2008).
We analyzed synaptic changes in NAc and HIPP after CVS. NAc is a brain area related to aversion, reward and reinforcement, and it is believed to be involved in anxiety and depressive-like disorders (Russo et al, 2013; Van Spronsen et al., 2010). The HIPP has also been described to play a role in these psychiatric disorders by interfering with the consolidation of aversive memories (Ding et al., 2017). In NAc, we observed that a shorter stress paradigm was enough to elicit more dramatic changes in Nrxn mRNA levels in female mice. Despite these differences, in both male and female mice, the stress-induced Nrxn gene expression was similarly prevented by prophylactic treatment with DHCA/Mal-gluc (Fig.3). In HIPP, the DHCA/Mal-gluc combination only prevented the stress-induced upregulation of Nrxn1 and Nrxn3 mRNA in male HIPP but mRNA was not restored by the treatment in females (Fig.4). Altogether, these data may partially explain the sex differences in stress susceptibility observed in the behavioral testing. Female mice were more vulnerable than males to the duration of the CVS paradigm. In females, a shorter CVS paradigm was sufficient to trigger comparable consequences on the depression-like behavioral phenotype (Fig. 1, Fig.2). We suggest that male and female mice may manifest different adaptive responses in synaptic plasticity mechanisms. Our results show that DHCA/Mal-gluc might intervene in the stress response by altering the dynamic interactions of NRXNs at synapses. Thus, the effects of the treatment would explain, in part, the attenuation of CVS-induced depressive behavior and anxiety in these animals.
We also extended our study to analyze the potential prophylactic effects of DHCA/Mal-gluc on alternative splicing. NRXNs are key components on the maintenance of the synaptic function. They undergo rapid regulatory changes triggered by neural response to environmental inputs, like mRNA splicing (Bang et al., 2013; Etherton et al., 2009; Sudorf et al., 2008). Recent studies have described Nrxn gene alternative splicing of the segment 4 on exon 20 (SS4) to play a role in the stability of aversive conditioning in HIPP (Ding et al., 2017). Interestingly, the prophylactic treatment with DHCA/Mal-gluc, triggered Nrxn3 alternative splicing of SS4 in both male and female mice that underwent CVS. This effect was isoform and region specific. This specificity can be partially explained by the larger expression of the Nrxn3 isoform in the HIPP, which also undergoes higher basal splicing activity in comparison with other brain areas (Aoto et al., 2013; Sudorf et al. 2008; Owczarek et al., 2015). Similarly to the alterations on the relative concentrations of NRXNs at synapses, the alternative splicing of SS4 in NRXNs is considered a pivotal regulator of the neurexin-based dynamic protein-interaction network. In the memory consolidation process, the adhesive interactions of NRXNs with synaptic receptors is especially significant. It has been previously shown that increasing the splicing variant Nrx3-SS4(−) but not Nrx3-SS4(+) facilitates anchoring sites for postsynaptic AMPA (a-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid) receptors. This affects the rapid trafficking of the receptor, promoting the repression of long-term plasticity (LTP) (Ding et al., 2017). Here we showed that the combination of DHCA/Mal-gluc modulates Nrxn3-SS4 splicing. We propose that dietary phytochemicals might intervene in aversive conditioning by repressing CVS-induced LTP. Considering recent evidence and previous works in our group, we have reasons to believe that the prophylactic effect of DHCA/Mal-gluc on Nrxn splicing might be epigenetically regulated. It has been shown that epigenetic mechanisms regulate the formation, consolidation and reconsolidation of memories (Graaf et al., 2014; Guan et al., 2009). Histone modifications repress plasticity in remote memory circuits underlying aversive conditioning .Thus, specific histone modifiers, such as HDAC2, CBP and others, regulate long-term memory formation and consolidation (Ding et al., 2017). We previously found that in both, humans and in rodents, chronic stress reduces RAS-related C3 botulinum toxin substrate 1 (Rac1) expression in the NAc. In psychiatric disorders, Rac1-mediated spine morphological changes may contribute this way to structural and functional changes in the brain (Dietz et al., 2012; Hayashi et al., 2010). We found that downregulation of Rac1 mRNA is associated with a repressive chromatin state surrounding its proximal promoter region. DHCA/Mal-gluc treatment normalizes Rac1 transcription by inhibiting histone deacetylases (HDACs), and rescues aversive conditioning after CVS (Wang et al, 2018). Similar to previous results in our group, we hypothesize that DHCA/Mal-gluc might intervene in the function of the histone modifier HDAC that, in turn, might affect downstream targets such as alternatively spliced synaptic proteins like NRXNs. Future studies will be directed to elucidate how DHCA/Mal-gluc might alter HDCA function by affecting SS4 inclusion in Nrxn mRNA.
It is important to highlight that the DHCA/Mal-gluc had no effect on PFC synapses. We attribute these results, in part, to the temporal scale on our experimental approach. The lack of plasticity changes in PFC might be a result of the study design. Our model might not allow for the appropriate time window to detect any possible long-term consequences in learning and memory. Therefore, we could not confirm the engagement of PFC in the consolidation of aversive memories. Nevertheless, we do not rule out the possibility that, this lack of effect observed in the PFC, might also be a component of the intrinsic action mechanism of these phytochemicals. These compounds might have their primary active role in rapid plastic changes triggered to adapt to environmental inputs, thus, long-term effects might not be present in other brain areas over time. Further experiments will be directed to mechanistically understand DHCA/Mal-gluc action, considering temporal and spatial components on brain plasticity induced after CVS exposure.
In summary, we showed that early intervention with the dietary phytochemicals DHCA/Mal-gluc affected NAc and HIPP brain circuits by altering experience-dependent changes in Nrxn expression, and triggered a region-specific alternative splicing of Nrxn3-SS4 in HIPP. The underlying mechanism of the latter remains unknown, but growing evidence suggests that interventions aiming to restore normal hippocampal function may help with disorders of negative affect, such as depression or post-traumatic stress disorder (Ding et al., 2017; Glover et al., 2015; Goosens, et al., 2011; Sherin et al., 2011; Sumner et al., 2017). Overall, we hypothesize that the prophylactic effect of phytochemicals, might play a role in promoting resilience behaviors by avoiding the consolidation of aversive memories. Current and future work in our laboratory will address potential sex-specific effects of phytochemicals due to dosage, administration and total duration of treatment. We will further investigate the pharmacological mechanisms of prevention and/or rescue of stress-induced aberrant synaptic remodeling and potential benefits in stress-induced short term and long-term memory processing. These studies will further elucidate the molecular mechanism by which early interventions with dietary phytochemicals might influence resilience and susceptibility to chronic stress.
Acknowledgments:
Funding was provided by the P50 AT008661-01 from the National Center for complementary and Integrative Health (NCCIH) and the Office of Dietary Supplements (ODS). In addition, J.W. holds positions in the Research and Development unit of the Basic and Biomedical Research and Training Program at the James J. Peters Veterans Affairs Medical Center. We acknowledge that the contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the views of the NCCIH, NIH, ODS or the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement: The authors declare no competing financial interests
References
- Adzic M, Brkic Z, Mitic M, Francija E, Jovicic MJ, Radulovic J, Maric NP.Therapeutic Strategies for Treatment of Inflammation-related Depression. Curr Neuropharmacol, 2018. 16(2): p. 176–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Sudhof TC. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell, 2013. 154(1): p. 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang ML and Owczarek S A matter of balance: role of neurexin and neuroligin at the synapse. Neurochem Res, 2013. 38(6): p. 1174–89. [DOI] [PubMed] [Google Scholar]
- Chan TE, Grossman YS, Bloss EB, Janssen WG, Lou W , McEwen BS, Dumitriu D and Morrison JM. Cell-Type Specific Changes in Glial Morphology and Glucocorticoid Expression During Stress and Aging in the Medial Prefrontal Cortex. Front Aging Neurosci, 2018. 10: p. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas A, Saavedra N, Salazar LA and Abdalla DSP. Modulation of immune function by polyphenols: possible contribution of epigenetic factors. Nutrients, 2013. 5(7): p. 2314–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat. Neurosci. 15, 891–896 (20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Liu S, Tian M, Zhang W, Zhu T, Li D, Wu J, Deng H, Jia Y, Xie W, Xie H, Guan JS. Activity-induced histone modifications govern Neurexin-1 mRNA splicing and memory preservation. Nat Neurosci, 2017. 20(5): p. 690–699. [DOI] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A, 2009. 106(42): p. 17998–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, and Norrholm SD. Estrogen and extinction of fear memories: implications for posttraumatic stress disorder treatment. Biol Psychiatry, 2015. 78(3): p. 178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, Kennedy PJ, Robison AJ, Gonzalez-Maeso J, Neve RL, Turecki G, Ghose S, Tamminga CA, Russo SJ. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med, 2013. 19(3): p. 337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA. Hippocampal regulation of aversive memories. Curr Opin Neurobiol, 201121(3): p. 460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 156, 261–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459, 55–60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A et al. Disrupted-in-Schizophrenia 1 (DISCI) regulates spines of the glutamate synapse via Rac1. Nat. Neurosci 13, 327–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati M, Aleyasin H, Menard C, Christoffel DJ, Flanigan ME, Pfau ML, Hodes GE, Lepack AE, Bicks LK, Takahashi A, Chandra R, Turecki G, Lobo MK, Maze I, Golden SA, Russo SJ.Cell-type-specific role for nucleus accumbens neuroligin-2 in depression and stress susceptibility. Proc Natl Acad Sci U S A, 2018. 115(5): p. 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Menard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci, 2015. 35(50): p. 16362–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T, Wu K, Witte H, Hanno-Iijima Y, Glatter T, Richard S, Scheiffele P SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell, 2011. 147(7): p. 1601–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari Z, Kolb BE and Mohajerani MH. Chronic traffic noise stress accelerates brain impairment and cognitive decline in mice. Exp Neurol, 2018. 308: p. 1–12. [DOI] [PubMed] [Google Scholar]
- Liang J, Xu W, Hsu YT, Yee AX, Chen L, Sudhof TC. Conditional neuroligin-2 knockout in adult medial prefrontal cortex links chronic changes in synaptic inhibition to cognitive impairments. Mol Psychiatry, 2015. 20(7): p. 850–9. [DOI] [PubMed] [Google Scholar]
- Maeng LY and Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav, 2015. 76: p. 106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, and Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology, 2016. 41(1): p. 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owczarek S, Bang ML, and Berezin V Neurexin-Neuroligin Synaptic Complex Regulates Schizophrenia-Related DISC1/Kal-7/Rac1 “Signalosome”. Neural Plast, 2015. 2015: p. 167308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandarakalam JP. Challenges of Treatment-resistant Depression. Psychiatr Danub, 2018. 30(3): p. 273–284. [DOI] [PubMed] [Google Scholar]
- Patel D, Anilkumar S, Chattarji S, Buwalda B Repeated social stress leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Behav Brain Res, 2018. 347: p. 314–324. [DOI] [PubMed] [Google Scholar]
- Radley JJ and Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev, 2005. 4(2): p. 271–87. [DOI] [PubMed] [Google Scholar]
- Raj B and Blencowe BJ. Alternative Splicing in the Mammalian Nervous System: Recent Insights into Mechanisms and Functional Roles. Neuron, 2015. 87(1): p. 14–27. [DOI] [PubMed] [Google Scholar]
- Russo SJ and Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci, 2013. 14(9): p. 609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner D, Nguyen TM, Russo G, Heber S, Patrignani A, Ahrne E, Scheiffele P Targeted combinatorial alternative splicing generates brain region-specific repertoires of neurexins. Neuron, 2014. 84(2): p. 386–98. [DOI] [PubMed] [Google Scholar]
- Sherin JE and Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci, 2011. 13(3): p. 263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature, 2008. 455(7215): p. 903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Hagan K, Grodstein F, Roberts AL, Harel B, Koenen KC. Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depress Anxiety, 2017. 34(4): p. 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VI, Vallejo D, and Inestrosa NC. Emerging Synaptic Molecules as Candidates in the Etiology of Neurological Disorders. Neural Plast, 2017. 2017: p. 8081758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kooij MA. Impaired hippocampal neuroligin-2 function by chronic stress or synthetic peptide treatment is linked to social deficits and increased aggression. Neuropsychopharmacology, 2014. 39(5): p. 1148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Spronsen M and Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep, 2010. 10(3): p. 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hodes GE, Zhang H, Zhang S, Zhao W, Golden SA, Bi W, Menard C, Kana V, Leboeuf M, Xie M, Bregman D, Pfau ML, Flanigan ME, Esteban-Fernandez A, Yemul S, Sharma A, Ho L, Dixon R, Merad M, Han MH, Russo SR, Pasinetti GM. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun, 2018. 9(1): p. 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM and Olfson M Depression in women: implications for health care research. Science, 1995. 269(5225): p. 799–801. [DOI] [PubMed] [Google Scholar]


