Abstract
The UDP-2,3-diacylglucosamine pyrophosphate hydrolase LpxH is essential in lipid A biosynthesis and has emerged as a promising target for the development of novel antibiotics against multidrug-resistant Gram-negative pathogens. Recently, we reported the crystal structure of Klebsiella pneumoniae LpxH in complex with 1 (AZ1), a sulfonyl piperazine LpxH inhibitor. The analysis of the LpxH-AZ1 co-crystal structure and ligand dynamics led to the design of 2 (JH-LPH-28) and 3 (JH-LPH-33) with enhanced LpxH inhibition. In order to harness our recent findings, we prepared and evaluated a series of sulfonyl piperazine analogs with modifications in the phenyl and N-acetyl groups of 3. Herein, we describe the synthesis and structure–activity relationship of sulfonyl piperazine LpxH inhibitors. We also report the structural analysis of an extended N-acyl chain analog 27b (JH-LPH-41) in complex with K. pneumoniae LpxH, revealing that 27b reaches an untapped polar pocket near the di-manganese cluster in the active site of K. pneumoniae LpxH. We expect that our findings will provide designing principles for new LpxH inhibitors and establish important frameworks for the future development of antibiotics against multidrug-resistant Gram-negative pathogens.
Keywords: antibiotics, Gram-negative bacteria, lipid A, LpxH, sulfonyl piperazine, structure–activity relationship
Graphical Abstract

1. Introduction
Nosocomial infection by multidrug-resistant Gram-negative pathogens is one of the greatest global health challenges.1,2 However, the lack of financial return on investment has resulted in pharmaceutical companies dropping out from the antibacterial field. As a consequence, no new class of antibiotics for treating Gram-negative bacteria has been approved since the 1980s.1 At the same time, widespread antibiotic resistance has emerged as a major threat to global health. In light of these issues, the Emerging Infections Network and the WHO cited the lack of both treatment options for Gram-negative bacteria and development pipelines to be the greatest areas of unmet need.1,2 Therefore, an urgent need exists for a fundamentally new class of anti-microbial therapeutics against Gram-negative pathogens, for which there is no established resistance mechanism.
Gram-negative bacteria are characterized by the presence of a unique cell wall component known as lipopolysaccharide (LPS) or lipooligosaccharide (LOS) in the bacterial outer membrane. Lipid A, a glucosamine-based phospholipid, is the hydrophobic anchor of LPS/LOS. Lipid A is also an active component of the bacterial endotoxin responsible for Gram-negative septic shock during bacterial infection. As constitutive biosynthesis of lipid A is required for bacterial viability and fitness in the human host,3–5 essential lipid A enzymes are excellent novel antibiotic targets.
Among the nine enzymes involved in lipid A biosynthesis, three functional orthologs (LpxH in β- and γ-proteobacteria,6 LpxI in α-proteobacteria,7 and LpxG in Chlamydiae8) carry out the cleavage of the pyrophosphate group of UDP-2,3-diacylglucosamine (UDP-DAGn) to form lipid X, but they never co-exist. LpxH and LpxG are unique members of the metal-dependent calcineurin-like phosphoesterase (CLP) family, though they share limited sequence similarity;8 LpxI, on the other hand, is structurally and mechanistically unrelated to LpxH and LpxG.9,10 Among these three enzymes, LpxH is most widespread, functioning in the vast majority of WHO priority Gram-negative pathogens, including Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, Haemophilius influenzae, and Neisseria gonorrhoeae. Therefore, LpxH inhibitors are ideally positioned to overcome this emerging public health crisis.
A small molecule LpxH inhibitor with a sulfonyl piperazine scaffold (referred to as AZ1 below; chemical structure shown in Figure 1) was discovered to display antibiotic activity against efflux-deficient E. coli strains.11 We prepared and biochemically evaluated a series of sulfonyl piperazine analogs, and our biochemical characterization established a preliminary structure–activity relationship (SAR) and identified the pharmacophore of this series of LpxH inhibitors.12 Building upon this work, we recently reported the first crystal structure of K. pneumoniae LpxH in complex with AZ1 (1).13 We showed that AZ1 fits into the L-shaped acyl chain-binding chamber of LpxH with its indoline ring situated adjacent to the active site, its sulfonyl group adopting a sharp kink, and its N-CF3-phenyl substituted piperazine group reaching out to the far side of the LpxH acyl chain-binding chamber. The discovery of two 19F signals of the LpxH-bound AZ1 led us to propose a model of CF3-phenyl flipping, which resulted in the design of AZ1 derivatives with the phenyl ring doubly substituted with CF3 and chloro groups, such as JH-LPH-28 and JH-LPH-33 (2 and 3, respectively; chemical structures shown in Figure 1) with enhanced potency in enzymatic assays.13 These designed compounds displayed striking improvement in antibiotic activity over AZ1 against wild-type K. pneumoniae, and co-administration with outer membrane permeability enhancers significantly sensitizes E. coli to designed LpxH inhibitors.
Figure 1.
Sulfonyl piperazine antibiotics inhibit LpxH of the Raetz pathway of lipid A biosynthesis.
Encouraged by the promising antibacterial activity of 3, we set off to further explore the SAR of 3. Herein, we describe our efforts toward the synthesis and SAR study of 2nd-generation sulfonyl piperazine analogs with modifications in the head and tail regions in order to further enhance the antibacterial activity of 3. We also report an LpxH inhibitor that occupies a polar binding pocket of K. pneumoniae LpxH, which has never been exploited before.
2. Results and discussion
2.1. Chemistry
AZ1 (1) and JH-LPH-33 (3) consist of three parts (Figure 1): the substituted phenyl group, sulfonyl piperazine linker, and N-acetyl indoline group. Following our previous report that the replacement of the m-hydrogen of the phenyl group of 1 with a m-chloro substituent significantly enhanced the LpxH inhibition and antibacterial activity of 1,13 we set out to systematically derivatize the substituents of the phenyl group in order to identify the optimal substituent pattern for LpxH inhibition. We also envisioned that an extended N-acyl chain of 1 and 3 may reach the di-manganese metal cluster in the active site of LpxH, leading to LpxH inhibitors with improved potency. We anticipated that our effort would help the understanding of the effect of various substituents on LpxH inhibition. Such information will allow structural modifications to increase potency and specificity and improve drug performance.
2.1.1. Analogs with aryl group modifications
To gain insights into the importance of the m-chloro group of 3 in LpxH inhibition and to evaluate the effect of the size at m-position of the phenyl ring of 3, we prepared aryl group analogs by replacing the m-chloro group of 3 with various functional groups, including Br, CH3, and CF3 (Scheme 1). Our previous synthesis of 313 provided the basis for the synthesis of m-bromo analog 9a, m-methyl analog 9b, and m-trifluoromethyl analog 9c (Scheme 1A). Starting from commercially available 1,3-dibromo-5-(trifluoromethyl)benzene (4a), Pd-mediated coupling of 4a with 1-Boc-piperazine (5) provided the Boc-protected N-aryl piperazine 6a. Boc deprotection of 6a by treatment with TFA followed by coupling of the resulting piperazine 7a with commercially available 1-acetyl-5-indolinesulfonyl chloride (8) in the presence of Et3N proceeded smoothly to afford the desired m-bromo analog 9a in 43% for 2 steps. The m-methyl analog 9b and the m-trifluoromethyl analog 9c were also prepared in a similar manner starting from the commercially available 1-bromo-3-methyl-5-(trifluoromethyl)benzene (4b) and 1,3-bis(trifluoromethyl)-5-bromobenzene (4c), respectively.
Scheme 1.
Synthesis of analogs with aryl group modifications.
To evaluate the effect of symmetrical substituents, we prepared the m-difluoro analog 13a and the m-dichloro analog 13b (Scheme 1B). Commercially available 1-bromo-3,5-difluorobenzene (10) was coupled to 1-Boc-piperazine (5) to afford the N-aryl piperazine 11. Boc deprotection of 11 followed by coupling of the resulting piperazine 12a to 1-acetyl-5-indolinesulfonyl chloride (8) completed the synthesis of the m-difluoro analog 13a. Similarly, the m-dichloro analog 13b was prepared by coupling commercially available 1-(3–5-dichlorophenyl)piperazine (12b) with 8.
2.1.2. Analogs with indoline modifications
We also synthesized an indole analog 17 and an N-methanesulfonyl group analog 19 by replacing the indoline and N-acetyl group of 3 with an indole group and a methanesulfonyl group, respectively. 1-(3-Chloro-5-(trifluoromethyl)phenyl)piperazine (14)13 was coupled to 1H-indole-5-sulfonyl chloride (15) to give 16 (Scheme 2A). N-Acetylation of the indole ring of 16 by treatment with Ac2O completed the synthesis of the indole analog 17. Compound 3 was converted to the N-methanesulfonyl group analog 19 by Ac deprotection under acidic conditions followed by N-methanesulfonylation of the resulting indoline.
Scheme 2.
Synthesis of analogs with indoline modifications.
2.1.3. Analogs with extended N-acyl chains
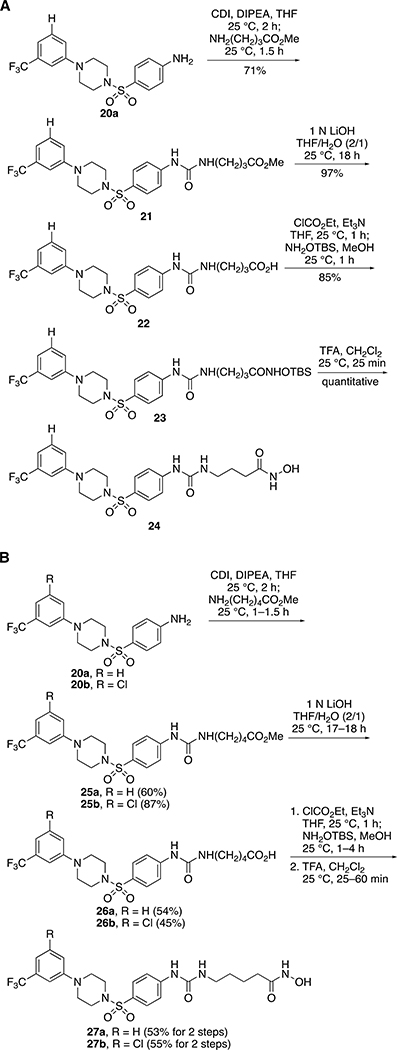
In order to explore the role of the N-acetyl group of 3 in LpxH inhibition, we prepared several sulfonyl piperazine compounds with extended N-acyl chains. Since LpxH contains two manganese metals in the active site, we hypothesized that a sulfonyl piperazine LpxH inhibitor that exploits the chelation to the di-manganese metal cluster would be more potent than the parent compounds, 1 or 3. Therefore, we capped the N-acyl group with a hydroxamic acid since hydroxamic acid is a well-characterized manganese-chelating group.14 We envisioned the hydroxamic acid group would tightly bind to the manganese metals in the LpxH active site and improve the binding affinity of LpxH inhibitors. Since the N-acetylsulfanilyl analog of 1 was active in our previous study,12 we modeled our new extended N-acyl chain analogs based on a sulfanilamide scaffold. Starting from the known 4-((4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)aniline (20a),12 we successfully introduced a urea linkage with methyl 4-aminobutanoate in the presence of CDI (Scheme 3A). To install a hydroxamic acid group, we hydrolyzed the methyl ester 21 to the corresponding carboxylic acid 22 and then coupled 22 with NH2OTBS. The final TBS deprotection of 23 by TFA successfully afforded the extended N-acyl chain hydroxamic acid analog 24. To gain insights into the effect of chain length on LpxH inhibition, we further elongated the acyl chain by replacing methyl 4-aminobutanoate with methyl 5-aminopentanoate and prepared the analogs 27a and 27b (Scheme 3B).
Scheme 3.
Synthesis of analogs with extended acyl chains.
2.2. Analysis of structure–activity relationships
2.2.1. Substituted phenyl and indoline analogs
After the completion of analog synthesis, we biochemically characterized the K. pneumoniae LpxH inhibition by these analogs at 0.1 μM using the nonradioactive, colorimetric malachite green assay that we had previously reported.12 Among the tested analogs, nearly all of the compounds show comparable or better activity than 1 (AZ1, 22% inhibition; Table 1). The extended N-acyl chain analog 27b (JH-LPH-41) showed the strongest inhibition of LpxH activity and inhibited ~64% of LpxH activity. Such an activity is only slightly worse than 3 (JH-LPH-33, 79% inhibition), but it is better than 2 (JH-LPH-28, 48% inhibition). Several other analogs, including 9a (57% inhibition), 9b (50% inhibition), 13b (59% inhibition), and 17 (48% inhibition), also showed noticeable LpxH inhibition, whereas 9c (16% inhibition), 19 (12% inhibition), and 24 (15% inhibition) showed significantly lower activity than 3, but their activities were comparable to 1 (Table 1).
Table 1.
Specific activity K. pneumoniae LpxH in the presence of sulfonyl piperazine LpxH inhibitors.
| Compounds | Structure | 0.1 μM compound | ||
|---|---|---|---|---|
| Activity (μmol/min/mg)a | Percentage Activity | Percentage Inhibition | ||
| DMSO | 298.7 ± 9.0 | 100 ± 3 | 0 | |
| 1 (AZ1) |  |
78b | 22 | |
| 2 (JH-LPH-28) |  |
52b | 48 | |
| 3 (JH-LPH-33) |  |
21b | 79 | |
| 9a |  |
128.3 ± 12 | 43 ± 4 | 57 |
| 9b |  |
148.8 ± 9.3 | 50 ± 3 | 50 |
| 9c |  |
252.1 ± 38.9 | 84 ± 13 | 16 |
| 13a |  |
182.4 ± 16.7 | 61 ± 6 | 39 |
| 13b |  |
121.7 ± 25.1 | 41 ± 8 | 59 |
| 17 |  |
156.1 ± 15.2 | 52 ± 5 | 48 |
| 19 |  |
262.7 ± 34.7 | 88 ± 12 | 12 |
| 24 | 253.3 ± 20.0 | 85 ± 7 | 15 | |
| 27a | 210.0 ± 26.3 | 70 ± 9 | 30 | |
| 27b (JH-LPH-41) | 107.6 ± 15.6 | 36 ± 5 | 64 | |
Data are mean values of three independent experiments. Errors represent standard error.
Values calculated from previously reported IC50 curves13 obtained under identical assay conditions.
The LpxH activity assay data provided several valuable insights into the SAR of sulfonyl piperazine LpxH inhibitors (Figure 2). Among the phenyl group analogs of 3 with a second m-substituent of the m-trifluoromethyl substituted phenyl ring (e.g., m-hydrogen substituted analog 1, m-fluoro substituted analog 2, m-methyl substituted analog 9b, and m-chloro substituted analog 3), there is a general trend of increasing potency following the increase in the volume of substituents (H < F < CH3 <Cl), except for m-bromo substituted analog 9a and m-trifluoromethyl substituted analog 9c, which may have become too bulky for the buried acyl chain chamber to tolerate.13
Figure 2.
SAR of phenyl and indoline substitutions.
A similar trend was also observed when both m-positions are substituted with fluoro (analog 13a), chloro (analog 13b), and trifluoromethyl (analog 9c) groups, with the dichloro substituted analog 13b displaying better activity (59% inhibition) than the difluoro substituted analog 13a (39% inhibition) and the difluoromethyl substituted analog 9c (16% inhibition).
Replacement of the indoline of 3 with indole (analog 17) slightly decreased the potency (48% inhibition for 17 vs 79% inhibition for 3). When the N-acetyl group of the indoline ring of 3 was replaced with a methanesulfonyl group (analog 19), the activity dropped significantly (12% inhibition), indicating that the N-acetyl group of 3 is critical to the LpxH inhibition.
2.2.2. Extended N-acyl chain analogs
As our previous structural analysis of LpxH in complex with 1 and 3 has shown that the active site of LpxH was not occupied by sulfonyl piperazine LpxH inhibitors such as 1 or 3,13 we synthesized analogs with extended acyl chains to test the feasibility of expanding the compound-LpxH interaction to the active site. For proof-of-concept studies, we selected the sulfanilamide scaffold and attached an extended acyl chain to the aniline nitrogen. Three compounds (24, 27a, and 27b) were synthesized containing different lengths of acyl chains with a terminal hydroxamate group designed to chelate the di-manganese cluster in the active site. The shorter N-acyl chain analog 24 showed slightly lower activity (15% inhibition) than 1 (22% inhibition), whereas extending the acyl chain of 24 by one methylene group (analog 27a, 30% inhibition) improved the potency over 1. Considering that aryl replacement of the indoline ring always results in reduced activity,12 attaching an acyl chain generally improves the potency of the compound. Combining the long acyl chain with the phenyl ring doubly substituted with trifluoromethyl and chloro groups yielded the most active compound of this series 27b (JH-LPH-41) that inhibited 64% activity of KpLpxH at 0.1 μM compound concentration. The activity of 27b is only slightly worse than 3 (79% inhibition). Despite the excellent in vitro activity of 27b, its long acyl chain with many rotatable bonds negatively impacted the antibiotic activity of 27b, yielding an unimpressive MIC of 32 μg/mL against K. pneumoniae. The MIC value 27b was still an improvement over 1 (MIC > 64 μg/mL), but is significantly worse than 3 (1.6 μg/mL).13 Such a result was attributed to the poor membrane permeability of 27b due to the highly flexible and hydrophobic nature of the extended acyl chain of 27b.
2.3. Structure of K. pneumoniae LpxH in complex with 27b (JH-LPH-41)
In order to gain a better understanding of the nature of the interaction of 27b with K. pneumoniae LpxH, we determined the co-crystal structure of the K. pneumoniae LpxH/27b complex at 1.85 Å (Figure 3, Figure S1, and Table S1). We found that, similar to previously reported sulfonyl piperazine compound structures,13 27b also occupies the hydrophobic substrate-binding chamber between the calcineurin-like phosphatase (CLP) domain and the insertion cap domain (Figure 3A). However, its N-acyl chain snakes into the active site as designed: the acyl chain picks up additional hydrophobic interactions with Y125 of the insertion cap and I171 on the loop connecting the cap back to the CLP core domain (Figure 3B). Most unexpectedly, the hydroxamate group did not chelate the di-manganese cluster as we designed; instead, its carbonyl group and N-hydroxyl group form two hydrogen bonds with the backbone amide and carbonyl group of M172 of the same loop as I171 (Figure 3B). Although these hydroxamate-containing acyl chains did not reach the metal cluster as we designed, they demonstrate the feasibility to expand molecular interactions into the active site of LpxH.
Figure 3.
Expansion of the LpxH inhibitor interaction into the active site of LpxH. (A) Side view of the KpLpxH/27b (JH-LPH-41) complex. LpxH is shown in the cartoon model, the catalytic di-manganese cluster is shown in the sphere model, and 27b is shown in the stick model. Location of the cap domain and the CLP core domain is labeled. (B) Top view of the KpLpxH/27b complex. Hydrophobic residues of LpxH (Y125 and I171) interacting with the N-acyl chain of 27b are labeled. The hydrogen bonds between the hydroxamate group of 27b and the backbone of M172 of LpxH are indicated by dashed lines.
3. Conclusion
We recently reported the first crystal structure of K. pneumoniae LpxH in complex with AZ1 (1), a sulfonyl piperazine LpxH inhibitor, and the identification of a more potent LpxH inhibitor, JH-LPH-33 (3). In order to further elaborate the SAR of these compounds, we prepared and biochemically characterized a series of sulfonyl piperazine LpxH inhibitors. Our SAR study revealed an important correlation between the compound activity and the volume of the functional groups at the meta-position of the trifluoromethyl substituted distal phenyl ring, with the compound potency increasing from H, F, CH3 and achieving maximal activity at Cl substitution. Further increases in volume with Br and CF3 substitutions resulted in reduced activity. A similar trend was observed with the symmetrical substitutions at the meta-positions of the distal phenyl ring. Moreover, our efforts resulted in the identification of 27b (JH-LPH-41) that occupies a previously untapped pocket near the di-manganese cluster in the LpxH active site. Despite having a proximal phenyl group instead of an indoline group, the acyl chain extension of 27b into the active site nearly restored the in vitro activity of 27b to that of 3 with an indoline group. These findings establish a clear SAR of the m-substituted distal phenyl ring and demonstrate the feasibility to expand drug interactions to the active site of LpxH. We anticipate our study will ultimately contribute to developing more potent and selective LpxH inhibitors for multidrug-resistant Gram-negative pathogens.
4. Materials and methods
4.1. Synthesis of sulfonyl piperazine LpxH inhibitors
General chemistry procedures
All reactions were conducted in oven-dried glassware under nitrogen or argon. Unless otherwise stated all reagents were purchased from commercial suppliers and used without further purification. All solvents were American Chemical Society (ACS) grade or better and used without further purification except tetrahydrofuran (THF), which was freshly distilled from sodium/benzophenone each time before use. Analytical thin layer chromatography (TLC) was performed with glass backed silica gel (60 Å) plates with fluorescent indication (Whatman). Visualization was accomplished by UV irradiation at 254 nm and/or by staining with p-anisaldehyde solution. Flash column chromatography was performed by using silica gel (particle size 230−400 mesh, 60 Å). All 1H spectra were recorded with a Varian 400 spectrometer. All 1H NMR δ values are given in parts per million (ppm) and are referenced to the residual solvent signals (CDCl3: δ = 7.26 ppm, CD3OD: δ = 3.31 ppm, CD3COCD3: δ = 2.05 ppm). Coupling constants (J) are given in Hertz (Hz) and multiplicities are indicated using the conventional abbreviation (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet or overlap of non-equivalent resonances, br = broad). Electrospray ionization (ESI) mass spectrometry (MS) was recorded with an Agilent 1100 series (LC/MSD trap) spectrometer in order to obtain the molecular masses of compounds. Optical rotation values were measured with a Rudolph Research Analytical (A21102 API/1W) polarimeter. The purity of final compounds used in bioassays was determined by NMR and was found to be >95%.
tert-Butyl 4-(3-bromo-5-(trifluoromethyl)phenyl)piperazine-1-carboxylate (6a). Anhydrous toluene (4.8 mL) was added to a mixture of 1,3-dibromo-5-(trifluoromethyl)benzene (4a, 500 mg, 1.6 mmol), 1-Boc-piperazine (5, 596 mg, 3.2 mmol), NaOt-Bu (307 mg, 3.2 mmol), JohnPhos (71.6 mg, 0.24 mmol), and Pd2(dba)3 (68.6 mg, 0.08 mmol). Argon (Ar) was bubbled through the reaction mixture for 15 min before the reaction mixture was heated to reflux for 14 h. The reaction mixture was concentrated in vacuo, dissolved in CH2Cl2/MeOH (1/1), and filtered through a pad of Celite. The filtrate was concentrated in vacuo and purified by column chromatography (silica gel, hexanes/EtOAc, 5/1) to afford 6a (100 mg, 14%) as a yellow solid: 1H NMR (400 MHz, CDCl3) δ 7.20 (s, 1H), 7.15 (s, 1H), 7.01 (s, 1H), 3.61–3.53 (m, 4H), 3.23–3.15 (m, 4H), 1.48 (s, 9H); HRMS (ESI) m/z 431.0545 [(M+Na)+ calcd for C16H20BrF3N2O2 431.0553].
1-(3-Bromo-5-(trifluoromethyl)phenyl)piperazine (7a). To a cooled (0 °C) solution of 6a (100 mg, 0.22 mmol) in anhydrous CH2Cl2 (1 mL) was added dropwise TFA (0.5 mL). After stirring at 25 °C for 2.5 h, the solvents were removed under reduced pressure to give 7a (100 mg) as an orange solid. Compound 7a was used in the following step without further purification: 1H NMR (400 MHz, CDCl3) δ 9.35 (br s, 1H), 7.34 (s, 1H), 7.21 (s, 1H), 7.05 (s, 1H), 3.50 (t, J = 5.1 Hz, 4H), 3.45–3.36 (m, 4H); HRMS (ESI) m/z 309.0216 [(M+H)+ calcd for C11H12BrF3N2 309.0209].
1-(5-((4-(3-Bromo-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)indolin-1-yl)ethan-1-one (9a). A solution of 7a (100 mg, 0.32 mmol) and Et3N (50 μL, 0.38 mmol) in anhydrous 1,4-dioxane (0.22 M, 1.5 mL) was heated to 60 °C. 1-Acetylindoline-5-sulfonyl chloride (8, 41.5 mg, 0.16 mmol) in anhydrous 1,4-dioxane (1 mL) was added to the reaction mixture. After stirring at 60 °C for 3 h, the reaction mixture was cooled to 25 °C. The reaction was quenched by an addition of H2O and the resulting mixture was extracted with EtOAc. The combined organic layers were washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 1/1) to afford 9a (37 mg, 43% for 2 steps) as a white solid: 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 8.5 Hz, 1H), 7.63 (d, J = 8.4 Hz, 1H), 7.57 (s, 1H), 7.21 (s, 1H), 7.09 (s, 1H), 6.95 (s, 1H), 4.16 (t, J = 8.6 Hz, 2H), 3.34–3.24 (m, 6H), 3.17–3.11 (m, 4H), 2.27 (s, 3H); HRMS (ESI) m/z 532.0517 [(M+H)+ calcd for C21H21BrF3N3O3S 532.0512].
tert-Butyl 4-(3-methyl-5-(trifluoromethyl)phenyl)piperazine-1-carboxylate (6b). To a solution of 1-bromo-3-methyl-5-(trifluoromethyl)benzene (4b, 50 mg, 0.21 mmol), 1-Boc-piperazine (5, 51 mg, 0.27 mmol), and NaOt-Bu (30 mg, 0.31 mmol) in toluene (0.63 mL) was added JohnPhos (6.3 mg, 0.02 mmol, 10 mol%), and Pd2(dba)3 (9.2 mg, 0.01 mmol, 5 mol%). Argon (Ar) was bubbled through the reaction mixture for 15 minutes and then the reaction was heated to reflux for 15 h. The reaction mixture was concentrated in vacuo, dissolved in CH2Cl2/MeOH (1/1), and filtered through a pad of Celite. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 20/1) to afford 6b (58 mg, 81%): 1H NMR (400 MHz, CDCl3) δ 6.92 (s, 2H), 6.87 (s, 1H), 3.62–3.51 (m, 4H), 3.21–3.08 (m, 4H), 2.35 (s, 3H), 1.47 (s, 9H).
1-(3-Methyl-5-(trifluoromethyl)phenyl)piperazine (7b). To a solution of 6b (58 mg, 0.17 mmol) in CH2Cl2 (0.85 mL) was added TFA (0.33 mL). The reaction mixture was stirred at 25 °C for 2 h and then was concentrated in vacuo to afford 7b (41 mg). Compound 7b was used in the following step without further purification: 1H NMR (400 MHz, CDCl3) δ 7.03 (s, 1H), 6.93 (s, 1H), 6.88 (s, 1H), 3.60–3.46 (m, 4H), 2.58–2.40 (m, 4H), 2.37 (s, 3H).
1-(5-((4-(3-Methyl-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)indolin-1-yl)ethan-1-one (9b). A solution of 7b (27 mg, 0.11 mmol) and Et3N (17 μL, 0.13 mmol) in 1,4-dioxane (1.63 mL) was heated to 60 °C. To this solution, 1-acetylindoline-5-sulfonyl chloride (8, 29 mg, 0.11 mmol) was added and the resulting mixture was stirred at 60 °C for 3 h followed by at 25 °C for 14 h. The reaction was quenched by an addition of H2O and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by column chromatography (silica gel, hexanes/EtOAc, 2/1) to afford 9b (26.3 mg, 51% for 2 steps): 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 7.9 Hz, 1H), 7.62 (d, J = 7.9 Hz, 1H), 7.56 (s, 1H), 6.93 (s, 1H), 6.85 (s, 1H), 6.82 (s, 1H), 4.14 (t, J = 8.6 Hz, 2H), 3.31–3.22 (m, 6H), 3.18–3.10 (m, 4H), 2.32 (s, 3H), 2.25 (s, 3H); HRMS (ESI) m/z 468.1554 [(M+H)+ calcd for C22H24F3N3O3S 468.1563].
tert-Butyl 4-(3,5-bis(trifluoromethyl)phenyl)piperazine-1-carboxylate (6c). Toluene (1.6 mL) was added to a mixture of 1,3-bis(trifluoromethyl)-5-bromobenzene (4c, 150 mg, 0.51 mmol), 1-Boc-piperazine (5, 123 mg, 0.66 mmol), NaOt-Bu (74 mg, 0.77 mmol), JohnPhos (15 mg, 10 mol%), and Pd2(dba)3 (28 mg, 5 mol%). Argon (Ar) was bubbled through the reaction mixture for 30 min, and the reaction mixture was refluxed for 18 h. The reaction mixture was concentrated in vacuo and the residue was dissolved in CH2Cl2/MeOH (1/1), filtered through a pad of Celite, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 20/1) to afford 6c (203 mg, quantitative): 1H NMR (400 MHz, CDCl3) δ 7.30 (s, 1H), 7.25 (s, 2H), 3.65–3.59 (m, 4H), 3.27–3.24 (m, 4H), 1.48 (s, 9H).
1-(3,5-Bis(trifluoromethyl)phenyl)piperazine (7c). To a solution of 6c (200 mg, 0.5 mmol) in CH2Cl2 (3 mL) was added TFA (1.3 mL) at 0 °C. The reaction mixture was stirred under N2 at 0 °C for 10 min and then warmed to 25 °C. The reaction mixture was stirred under N2 at 25 °C for 3.5 h. The reaction mixture was concentrated in vacuo to give 7c as an orange solid. Compound 7c was used in the following step without further purification: 1H NMR (400 MHz, CDCl3) δ 7.45 (s, 1H), 7.30 (s, 2H), 3.95 (br s, 1H), 3.59 (m, 4H), 3.46 (m, 4H).
1-(5-((4-(3,5-Bis(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)indolin-1-yl)ethan-1-one (9c). To a solution of 7c (50 mg, 0.17 mmol) in 1,4-dioxane (3 mL) was added Et3N (32 μL, 0.24 mmol) at 25 °C. 1-Acetyl-5-indoline sulfonyl chloride (8, 44 mg, 0.17 mmol) was added to the reaction mixture. The resulting mixture was stirred under N2 at 60 °C for 3 h. The reaction mixture was cooled to 25 °C and kept at the same temperature for 10 h. The reaction mixture was diluted with EtOAc. The layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 4/1) to afford 9c as a white solid (35 mg, 40% for 2 steps): 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 8.6 Hz, 1H), 7.62 (dd, J = 8.5, 1.9 Hz, 1H), 7.57 (s, 1H), 7.31 (s, 1H), 7.19 (s, 2H), 4.17 (t, J = 8.5 Hz, 2H), 3.36–3.34 (m, 4H), 3.28 (t, J = 8.8 Hz, 3H), 3.16–3.15 (m, 4H), 2.27 (s, 3H); HRMS (ESI): m/z 522.1281 [(M+H)+ calcd for C22H21F6N3O3S 522.1289].
tert-Butyl 4-(3,5-difluorophenyl)piperazine-1-carboxylate (11). Toluene (4 mL) was added to a mixture of 1-bromo-3,5-difluorobenzene (10, 250 mg, 1.3 mmol), 1-Boc-piperazine (5, 315 mg, 1.69 mmol), NaOt-Bu (187 mg, 1.95 mmol), JohnPhos (39 mg, 10 mol%), and Pd2(dba)3 (60 mg, 5 mol%). Argon (Ar) was bubbled through the reaction mixture for 30 min, and the reaction mixture was refluxed for 15 h. The reaction mixture was concentrated in vacuo and the residue was dissolved in CH2Cl2/MeOH (1/1), filtered through a pad of Celite, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 20/1) to afford 11 (436 mg, quantitative) as a solid: 1H NMR (400 MHz, CDCl3) δ 6.36 (d, J = 8.8 Hz, 2H), 6.27 (t, J = 8.8 Hz, 1H), 3.60–3.52 (m, 4H), 3.20–3.11(m, 4H), 1.48 (s, 9H).
1-(3,5-Difluorophenyl)piperazine (12a). TFA (1.4 mL) was added to a solution of 11 (207 mg, 0.69 mmol) in CH2Cl2 (3.5 mL), and the resulting mixture was stirred at 25 °C for 1 h. The reaction mixture was concentrated in vacuo to afford 12a. Compound 12a was used in the following step without further purification: 1H NMR (400 MHz, CD3OD) δ 6.61 (d, J = 10.0 Hz, 2H), 6.42 (t, J = 9.0 Hz, 1H), 3.48–3.45 (m, 4H), 3.36–3.33 (m, 4H); HRMS (ESI) m/z 199.1041 [(M+H)+ calcd for C10H12F2N2 199.1041].
1-(5-((4-(3,5-Difluorophenyl)piperazin-1-yl)sulfonyl)indolin-1-yl)ethan-1-one (13a). A solution of 12a (50 mg, 0.25 mmol) and Et3N (40 μL, 0.3 mmol) in 1,4-dioxane (3.7 mL) was heated to 60 °C and 1-acetyl-5-indolinesulfonyl chloride (8, 65 mg, 0.25 mmol) was added. After stirring at 60 °C for 3 h, the reaction mixture was cooled to 25 °C and stirred for additional 14 h. The reaction was quenched by an addition of H2O. The reaction mixture was extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 1/1) to afford 13a (48 mg, 46% for 2 steps) as a white solid: 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 8.2 Hz, 1H), 7.62 (d, J = 8.2 Hz, 1H), 7.56 (s, 1H), 6.31–6.25 (m, 3H), 4.16 (t, J = 8.5 Hz, 2H), 3.30–3.26 (m, 6H), 3.12– 3.11 (m, 4H), 2.26 (s, 3H); HRMS (ESI) m/z 422.1349 [(M+H)+ calcd for C20H21F2N3O3S 422.1345].
1-(5-((4-(3,5-Dichlorophenyl)piperazin-1-yl)sulfonyl)indolin-1-yl)ethan-1-one (13b). To a solution of commercially available 1-(3–5-dichlorophenyl)piperazine (12a, 50 mg, 0.22 mmol) in 1,4-dioxane (3.0 mL) was added Et3N (34 μL, 0.26 mmol) at 25 °C. The reaction mixture was treated with 1-acetyl-5-indoline sulfonyl chloride (8, 57 mg, 0.22 mmol) and stirred under N2 at 60 °C for 3 h. The reaction mixture was cooled to 25 °C and then stirred for an additional 20 h. The reaction mixture was diluted with EtOAc. The layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 4/1) to afford 13b as a white solid (46 mg, 47 %): 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 8.5 Hz, 1H), 7.60 (dd, J = 8.6, 1.9 Hz, 1H), 7.55 (s, 1H), 6.83 (t, J = 1.7 Hz, 1H), 6.69 (d, J = 1.8 Hz, 2H), 4.15 (t, J = 8.6 Hz, 2H), 3.27–3.24 (m, 6H), 3.13–3.10 (m, 4H), 2.26 (s, 3H); HRMS (ESI): m/z 454.0753 [(M+H)+ calcd for C20H21Cl2N3O3S 454.0759].
5-((4-(3-Chloro-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)-1H-indole (16). To a solution (60 °C) of 1H-indole-5-sulfonyl chloride (15) (50 mg, 0.23 mmol) and Et3N (72 μL, 0.55 mmol) in anhydrous 1,4-dioxane (0.22 M, 1 mL) was added 1-(3-chloro-5-(trifluoromethyl)phenyl)piperazine13 (14, 121 mg, 0.46 mmol) in anhydrous 1,4-dioxane (0.5 mL). After stirring at 60 °C for 2 h, the reaction mixture was cooled to 25 °C and stirred for an additional 18 h. The reaction was quenched by an addition of H2O and the resulting mixture was diluted with EtOAc. The combined organic layers were washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 3/1) to afford 16 (32 mg, 32%) as a white solid: 1H NMR (400 MHz, CDCl3) δ 8.58 (br s, 1H), 8.15 (s, 1H), 7.60 (d, J = 8.5 Hz, 1H), 7.52 (d, J = 8.3 Hz, 1H), 7.39–7.35 (m, 1H), 7.04 (s, 1H), 6.91 (s, 1H), 6.89 (s, 1H), 6.70 (s, 1H), 3.34–3.26 (m, 4H), 3.20–3.13 (m, 4H); HRMS (ESI) m/z 444.0760 [(M+H)+ calcd for C19H17ClF3N3O2S 444.0755].
1-(5-((4-(3-Chloro-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)-1H-indol-1-yl)ethan-1-one (17). To a solution of 16 (15 mg, 0.03 mmol), Et3N (12 μL, 0.09 mmol), and N,N-dimethyl-4-aminopyridine (1.4 mg, 0.012 mmol) in anhydrous 1,2-dichloroethane (1 mL) was added Ac2O (11.2 μL, 0.12 mmol). The resulting mixture was stirred at 80 °C for 24 h, the reaction was quenched by an addition of H2O. The mixture was extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 3/1) to afford 17 (14 mg, 96%) as a white solid: 1H NMR (400 MHz, CDCl3) δ 8.63 (d, J = 8.8 Hz, 1H), 8.05 (s, 1H), 7.75 (d, J = 9.1 Hz, 1H), 7.58 (d, J = 3.7 Hz, 1H), 7.04 (s, 1H), 6.92 (s, 1H), 6.89 (s, 1H), 6.77 (d, J = 3.7 Hz, 1H), 3.37–3.25 (m, 4H), 3.24–3.12 (m, 4H), 2.69 (s, 3H); HRMS (ESI) m/z 486.0867 [(M+H)+ calcd for C21H19ClF3N3O3S 486.0872].
5-((4-(3-Chloro-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)indoline (18). To a cooled (0 °C) solution of 313 (89 mg, 0.18 mmol) in EtOH (0.41 mL) was added c-HCl (0.2 mL). The resulting mixture was refluxed for 2 h and then ice water was added followed by 35% NH4OH. The reaction was extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo to afford 18 (80 mg). Compound 18 was used in the following step without further purification: 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 6.2 Hz, 1H), 7.41 (s, 1H), 7.03 (s, 1H), 6.93 (s, 1H), 6.90 (s, 1H), 6.57 (d, J = 8.8 Hz, 1H), 3.68 (t, J = 8.4 Hz, 2H), 3.32–3.25 (m, 4H), 3.15–3.09 (m, 4H), 3.08 (t, J = 8.5 Hz, 2H).
5-((4-(3-Chloro-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)-1-(methylsulfonyl)indoline (19). To a solution of 18 (36 mg, 0.08 mmol) in pyridine (3.5 mL) was added methanesulfonyl chloride (0.03 mL, 0.39 mmol). The reaction mixture was stirred at 25 °C for 18 h. The reaction was quenched by an addition of saturated NH4Cl solution and the organic layer was extracted with CH2Cl2. The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 2/1) to afford 19 as a white solid (29 mg, 67% for 2 steps): 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 8.5 Hz, 1H), 7.59 (s, 1H), 7.49 (d, J = 7.5 Hz, 1H), 7.06 (s, 1H), 6.94 (s, 1H), 6.91 (s, 1H), 4.08 (t, J = 8.6 Hz, 2H), 3.33–3.27 (m, 4H), 3.23 (t, J = 8.6 Hz, 2H), 3.17–3.12 (m, 4H), 2.95 (s, 3H); HRMS (ESI) m/z 524.0689 [(M+H)+ calcd for C20H21ClF3N3O4S2 524.0687].
Methyl 4-(3-(4-((4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)butanoate (21). To a solution of 4-((4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)aniline12 (20a, 103 mg, 0.26 mmol) and CDI (216 mg, 1.33 mmol) in anhydrous THF (1.3 mL) was added DIPEA (0.23 mL, 1.33 mmol). After stirring at 25 °C for 2 h, methyl 4-aminobutanoate (204 mg, 1.33 mmol) was added to the reaction mixture. After stirring at 25 °C for 1.5 h, the reaction mixture was concentrated under reduced pressure. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 1/1) to afford 21 (100 mg, 71%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 8.5 Hz, 2H), 7.56 (d, J = 8.7 Hz, 2H), 7.37–7.29 (m, 1H), 7.11 (d, J = 7.6 Hz, 1H), 7.06 (s, 1H), 7.01 (d, J = 8.6 Hz, 1H), 5.55 (br s, 1H), 3.68 (s, 2H), 3.34–3.23 (m, 4H), 3.16–3.08 (m, 4H), 2.41 (t, J = 6.8 Hz, 2H), 1.91–1.83 (m, 2H); HRMS (ESI) m/z 529.1726 [(M+H)+ calcd for C23H27F3N4O5S 529.1727].
4-(3-(4-((4-(3-(Trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)butanoic acid (22). To a solution of 21 (100 mg, 0.18 mmol) in THF/H2O (2/1, 1.8 mL) was added 1 N LiOH (0.37 mL) at 25 °C. After stirring for 18 h, the reaction was quenched by an addition of 1 N HCl, and the resulting mixture was diluted with CH2Cl2. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (silica gel, CH2Cl2/MeOH (10/1) to 100% MeCN) to afford 22 (90 mg, 97%) as a colorless oil: 1H NMR (400 MHz, CD3OD) δ 7.68 (d, J = 8.8 Hz, 2H), 7.62 (d, J = 8.7 Hz, 2H), 7.37 (dd, J = 7.8, 7.9 Hz, 1H), 7.15 (d, J = 8.6 Hz, 1H), 7.14 (s, 1H), 7.08 (d, J = 7.6 Hz, 1H), 3.28–3.22 (m, 6H), 3.14–3.09 (m, 4H), 2.35 (t, J = 7.3 Hz, 2H), 1.84–1.82 (m, 2H); HRMS (ESI) m/z 515.1570 [(M+H)+ calcd for C22H25F3N4O5S 515.1571].
N-((tert-Butyldimethylsilyl)oxy)-4-(3-(4-((4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)butanamide (23). To a solution of 22 (22 mg, 0.04 mmol) in anhydrous THF (2.86 mL) were added ethyl chloroformate (8 μL, 0.08 mmol) and Et3N (11 μL, 0.08 mmol). After stirring at 25 °C for 1 h, NH2OTBS (12.5 mg, 0.08 mmol) in anhydrous MeOH (0.66 mL) was added to the reaction mixture. After stirring at 25 °C for 1 h, the reaction mixture was concentrated in vacuo. The residue was purified by column chromatography (silica gel, CH2Cl2/MeOH, 50/1 to 10/1) to afford 23 (23 mg, 85%) as a white solid: 1H NMR (400 MHz, CD3OD) δ 7.68 (d, J = 9.3 Hz, 2H), 7.62 (d, J = 9.1 Hz, 2H), 7.37 (dd, J = 7.9, 8.0 Hz, 1H), 7.14 (d, J = 7.6 Hz, 1H), 7.13 (s, 1H), 7.08 (d, J = 7.7 Hz, 1H), 6.35–6.30 (m, 1H), 3.32–3.21 (m, 6H), 3.13–3.09 (m, 4H), 2.17 (t, J = 7.4 Hz, 2H), 1.87–1.78 (m, 2H), 0.95 (s, 9H), 0.16 (s, 6H); HRMS (ESI) m/z 644.2542 [(M+H)+ calcd for C28H40F3N5O5SSi 644.2544].
N-Hydroxy-4-(3-(4-((4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)butanamide (24). To a cooled (0 °C) solution of 23 (7.7 mg, 0.01 mmol) in anhydrous CH2Cl2 (1.7 mL) was added dropwise TFA (0.5 mL). After stirring at 25 °C for 25 min, the reaction mixture was concentrated in vacuo. The residue was purified by column chromatography (silica gel, CH2Cl2/MeOH, 10/1) to afford 24 as a white solid (7 mg, quantitative): 1H NMR (400 MHz, CD3OD) δ 7.68 (d, J = 8.7 Hz, 2H), 7.62 (d, J = 8.4 Hz, 2H), 7.38 (dd, J = 7.8, 7.8 Hz, 1H), 7.16 (d, J = 8.1 Hz, 1H), 7.14 (s, 1H), 7.09 (d, J = 7.8 Hz, 1H), 3.29–3.19 (m, 6H), 3.15–3.07 (m, 4H), 2.15 (t, J = 7.5 Hz, 2H), 1.87–1.78 (m, 2H); HRMS (ESI) m/z 530.1680 [(M+H)+ calcd for C22H26F3N5O5S 530.1680].
Methyl 5-(3-(4-((4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)pentanoate (25a). To a solution of 20a (88 mg, 0.23 mmol) and CDI (185 mg, 1.14 mmol) in THF (1.14 mL) was added DIPEA (0.2 mL, 1.14 mmol). After stirring at 25 °C for 2 h, the reaction mixture was treated with methyl 5-aminopentanoate15 (150 mg, 1.14 mmol) and allowed to stir for 1 h. The reaction mixture was concentrated in vacuo and the residue was purified by column chromatography (silica gel, hexanes/EtOAc, 1/1) to afford 25a (75 mg, 60%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 7.73 (s, 1H), 7.62 (d, J = 8.9 Hz, 2H), 7.53 (d, J = 8.7 Hz, 2H), 7.33 (dd, J = 8.2, 8.2 Hz, 1H), 7.10 (d, J = 7.7 Hz, 1H), 7.05 (s, 1H), 7.00 (d, J = 8.2 Hz, 1H), 5.60 (t, J = 5.5 Hz, 1H), 3.64 (s, 3H), 3.27–3.22 (m, 6H), 3.13–3.11 (m, 4H), 2.33 (t, J = 7.1 Hz, 2H), 1.70–1.62 (m, 2H), 1.58–1.51 (m, 2H).
5-(3-(4-((4-(3-(Trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)pentanoic acid (26a). 1 N LiOH (0.25 mL) was added to 25a (34 mg, 0.063 mmol) in THF/H2O (2/1, 0.63 mL) at 25 °C. After stirring at 25 °C for 17 h, the reaction mixture was cooled to 0 °C and acidified with 1 N HCl. After an addition of H2O, the resulting mixture was extracted with CH2Cl2. The organic layers were combined, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, CH2Cl2/MeOH, 10/1) to afford 26a (18 mg, 54%) as a white solid: 1H NMR (400 MHz, CD3OD) δ 7.67 (d, J = 9.0 Hz, 2H), 7.62 (d, J = 9.0 Hz, 2H), 7.37 (dd, J = 8.3, 8.3 Hz, 1H), 7.15 (d, J = 7.4 Hz, 1H), 7.14 (s, 1H), 7.08 (d, J = 7.6 Hz, 1H), 3.29–3.26 (m, 4H), 3.22 (t, J = 6.7 Hz, 2H), 3.11–3.10 (m, 4H), 2.34 (t, J = 8.0 Hz, 2H), 1.70–1.63 (m, 2H), 1.60–1.53 (m, 2H); HRMS (ESI) m/z 529.1733 [(M+H)+ calcd for C23H27F3N4O5S 529.1727].
N-Hydroxy-5-(3-(4-((4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)pentanamide (27a). Ethyl chloroformate (6.2 mg, 0.057 mmol) and Et3N (8 μL) were added to a solution of 26a (15 mg, 0.028 mmol) in THF (2 mL). After stirring for 1 h, the reaction mixture was treated with NH2OTBS (8.4 mg, 0.057 mmol) in MeOH (0.44 mL). After an additional 2 h, NH2OTBS (8.4 mg, 0.057 mmol) in MeOH (0.44 mL) was added and the resulting mixture was left to stir for additional 2 h. The reaction mixture was concentrated in vacuo, dissolved in CH2Cl2 (2 mL), and treated with TFA (0.17 mL). The resulting mixture was stirred at 0 °C for 1 h and then concentrated in vacuo to afford 27a (8.1 mg, 53% for 2 steps) as a solid: 1H NMR (400 MHz, CD3OD) δ 7.69 (d, J = 8.9 Hz, 2H), 7.62 (d, J = 8.9 Hz, 2H), 7.39 (dd, J = 7.9, 7.9 Hz, 1H), 7.19 (d, J = 8.3 Hz, 1H), 7.18 (s, 1H), 7.11 (d, J = 7.5 Hz, 1H), 3.32–3.30 (m, 4H), 3.22 (t, J = 6.7 Hz, 2H), 3.14–3.11 (m, 4H), 2.14 (t, J = 7.3 Hz, 2H), 1.72–1.63 (m, 2H), 1.61–1.50 (m, 2H); HRMS (ESI) m/z 544.1840 [(M+H)+calcd for C23H28F3N5O5S 544.1836].
Methyl 5-(3-(4-((4-(3-chloro-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)pentanoate (25b). To a solution of 20b (290 mg, 0.69 mmol) and CDI (561 mg, 3.46 mmol) in anhydrous THF (3.45 mL) was added DIPEA (0.6 mL, 3.46 mmol). After stirring at 25 °C for 2 h, the reaction mixture was transferred to methyl 5-aminopentanoate (114 mg, 0.87 mmol). After stirring at 25 °C for 1.5 h, the reaction mixture was concentrated under reduced pressure and the residue was purified by column chromatography (silica gel, hexanes/EtOAc, 1/1) to afford 25b (350 mg, 87%) as a white sticky solid: 1H NMR (400 MHz, CDCl3) δ 7.63 (s, 1H), 7.59 (d, J = 8.5 Hz, 2H), 7.49 (d, J = 8.2 Hz, 2H), 7.05 (s, 1H), 6.95 (s, 1H), 6.93 (s, 1H), 5.56 (br s, 1H), 3.65 (s, 3H), 3.34– 3.20 (m, 6H), 3.15–3.06 (m, 4H), 2.33 (t, J = 7.2 Hz, 2H), 1.69–1.59 (m, 2H), 1.58–1.50 (m, 2H).
5-(3-(4-((4-(3-chloro-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)pentanoic acid (26b). To a solution of 25b (34 mg, 0.05 mmol) in THF/H2O (2/1, 0.5 mL) was added 1 N LiOH (0.13 mL) at 25 °C. After stirring for 18 h, the reaction was quenched by an addition of 1 N HCl, and the resulting mixture was diluted with CH2Cl2. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (silica gel, CH2Cl2/MeOH (30/1) to 100% MeCN) to afford 26b (15 mg, 45%) as a white solid: 1H NMR (400 MHz, CD3COCD3) δ 8.60 (s, 1H), 7.75 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 8.6 Hz, 2H), 7.20 (s, 1H), 7.17 (s, 1H), 7.06 (s, 1H), 6.18 (br s, 1H), 3.50–3.41 (m, 4H), 3.27–3.24 (m, 2H), 3.15– 3.07 (m, 4H), 2.33 (t, J = 7.2 Hz, 2H), 1.67–1.64 (m, 2H), 1.59–1.56 (m, 2H); HRMS (ESI) m/z 563.1340 [(M+H)+ calcd for C23H26ClF3N4O5S 563.1337].
N-Hydroxy-5-(3-(4-((4-(3-chloro-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)-pentanamide (27b). [Coupling Reaction] To a solution of 26b (13 mg, 0.02 mmol) in anhydrous THF (1.69 mL) were added ethyl chloroformate (4.3 μL, 0.04 mmol) and Et3N (6 μL, 0.04 mmol). After stirring at 25 °C for 1 h, NH2OTBS (6 mg, 0.04 mmol) in anhydrous MeOH (0.39 mL) was added to the reaction mixture. After stirring at 25 °C for 1 h, the reaction mixture was concentrated in vacuo and purified by column chromatography (silica gel, CH2Cl2/MeOH, 30/1) to afford N-((tert-butyldimethylsilyl)oxy)-5-(3-(4-((4-(3-chloro-5-(trifluoromethyl)phenyl)piperazin-1-yl)sulfonyl)phenyl)ureido)pentanamide (13 mg) as a white solid: 1H NMR (400 MHz, CD3COCD3) δ 9.78 (br s, 1H), 8.46 (s, 1H), 7.74 (d, J = 9.2 Hz, 2H), 7.66 (d, J = 8.9 Hz, 2H), 7.20 (s, 1H), 7.17 (s, 1H), 7.06 (s, 1H), 6.04 (t, J = 5.5 Hz, 1H), 3.50–3.42 (m, 4H), 3.26–3.19 (m, 2H), 3.15–3.07 (m, 4H), 2.15–2.13 (m, 2H), 1.69–1.60 (m, 2H), 1.56–1.53 (m, 2H), 0.94 (s, 9H), 0.15 (s, 6H); HRMS (ESI) m/z 692.2313 [(M+H)+ calcd for C29H41ClF3N5O5SSi 692.2311]; [TBS Deprotection] To a cooled (0 °C) solution of the TBS protected hydroxamic acid (13 mg, 0.01 mmol) in anhydrous CH2Cl2 (2.8 mL) was added dropwise TFA (240 μL). After stirring for 25 min at 25 °C, the reaction mixture was concentrated in vacuo and purified by column chromatography (silica gel, CH2Cl2/MeOH, 10/1) to afford 27b (7 mg, 55% for 2 steps) as a white solid: 1H NMR (400 MHz, CD3COCD3) δ 10.04 (br s, 1H), 8.09 (br s, 1H), 7.74 (br s, 2H), 7.66 (br s, 2H), 7.21 (s, 1H), 7.18 (s, 1H), 7.06 (s, 1H), 3.49–3.37 (m, 4H), 3.26–3.18 (m, 2H), 3.12–3.10 (m, 4H), 2.19–2.11 (m, 2H), 1.70–1.59 (m, 2H), 1.56–1.48 (m, 2H); HRMS (ESI) m/z 578.1441 [(M+H)+ calcd for C23H27ClF3N5O5S 578.1446].
4.2. Cloning and purification of K. pneumoniae LpxH
Cloning and purification of K. pneumoniae LpxH for crystallography studies were carried out as previously reported.13 Briefly, K. pneumoniae LpxH was cloned into a modified pET21b (Novagen/Millipore Sigma) vector, yielding the LpxH fusion protein with a C-terminal TEV protease site (ENLYFQGS) and His10 tag. Vector-transformed BL21 STAR (DE3) E. coli cells (Thermo Fisher Scientific) were grown in M9 minimal medium to an OD600 of 0.5 at 37 °C, prior to being induced with 1 mM IPTG at 30 °C. After 5 h, the cells were then harvested by centrifugation. Protein purification was carried out at 4 °C. Cell pellets were lysed in a buffer containing 50 mM phosphate-citrate, 20 mM MES (pH 6.0), 600 mM NaCl, 10% sucrose, 5 mM 2-mercaptoethanol, 10 mM imidazole, and 0.1% Triton X-100 using a French press. After removing cell debris by centrifugation, a HisPur Ni-NTA column (Thermo Fisher Scientific) was pre-equilibrated with the lysis buffer, and the supernatant was loaded. Following extensive washes using a purification buffer containing 20 mM phosphate-citrate, 20 mM MES (pH 6.0), 300 mM NaCl, 5% glycerol, 5 mM 2-mercaptoethanol, and 40 mM imidazole, LpxH was eluted from the column by increasing the imidazole concentration stepwise from 40 to 400 mM. The protein sample was concentrated and further purified with size-exclusion chromatography (Superdex 200; GE Healthcare Life Sciences) in the FPLC buffer containing 20 mM MES (pH 6.0), 800 mM NaCl, 1 mM DTT, and 5% glycerol.
4.3. Co-crystallization of K. pneumoniae LpxH with a sulfonyl piperazine antibiotic 27b (JH-LPH-41)
Peak fractions containing K. pneumoniae LpxH were buffer-exchanged into a buffer containing 20 mM MES (pH 6.0), 200 mM NaCl, 1 mM DTT, and 5% glycerol. During buffer exchange, concentrated 27b solution in DMSO was added to the protein solution in a 1:1 molar ratio. The solution was then concentrated to 8 mg/mL for crystallization and additional concentrated 27b solution in DMSO was added to the protein solution in a 1:1 molar ratio (final ratio = 2:1 drug: protein, final 27b concentration = 0.54 mM, DMSO = 2%).
Protein crystals were grown using the sitting-drop vapor diffusion method at 20 °C. Each drop was prepared by mixing 1 μL of the protein solution with 1 μL of the reservoir solution (200 mM KCl, 100mM sodium citrate, 37% pentaerythritol propoxylate (5/4 PO/OH), pH 5.5). The final drop solution contained 4 mg/mL of LpxH with 0.27 mM 27b, 10 mM MES (pH 6.0), 100 mM NaCl, 100 mM KCl, 50 mM sodium citrate (pH 5.5), 18.5% pentaerythritol propoxylate (5/4 PO/OH), 0.5 mM DTT, 1% DMSO, and 2.5% glycerol. Diffraction quality protein crystals were harvested after 2 weeks and soaked with the reservoir solution additionally containing 20% glycerol, 100 μM MnCl2, 0.27 mM 27b, and 2% DMSO for cryoprotection.
4.4. Structural analysis of K. pneumoniae LpxH complexes with 27b (JH-LPH-41)
The X-ray diffraction data of the K. pneumoniae LpxH complex with 27b were collected at the Northeastern Collaborative Access Team (NECAT) 24-ID-C beamline at the Advanced Photon Source at Argonne National Laboratory. The X-ray diffraction data was processed using XDS.16 The phase information of the crystal structures of the K. pneumoniae LpxH complex was obtained by molecular replacement with the PHASER module in the PHENIX suite17 using the PDB entry 6PJ3 as the search model. Restraints of the inhibitors were generated by using eLBOW18 and edited manually. Iterative model building and refinement was carried out using COOT19 and PHENIX.17 The 2mFo-DFc omit maps were generated using PHENIX.17
4.5. Enzymatic assay for LpxH inhibition
The LpxE-coupled LpxH activity assay12 was conducted as described previously using the GB1-K. pneumoniae LpxH-His10 fusion protein.13 Briefly, two reaction mixtures were prepared that contain 20 mM Tris-HCl (pH 8.0), 0.5 mg/mL BSA, 0.02% Triton X-100, 1 mM MnCl2, 1 mM DTT, and 10% DMSO, with one additionally containing 200 μM substrate (UDP-DAGn) and the other containing both LpxH (20 ng/mL) and 0.2 μM inhibitor. The reaction mixtures were pre-incubated at 37 °C for 10 minutes before an equal volume of the LpxH mixture was added to the substrate mixture to initiate the reaction at 37 °C. The final reaction solution contains 100 μM substrate, 10 ng/mL enzyme, and 0.1 μM inhibitor. At the desired reaction time points, an aliquot of 20 μL reaction mixture was removed and added to a well in 96-well half-area plate containing 5 mM EDTA (final concentration) to quench the LpxH reaction. Purified Aquifex aeolicus LpxE was then added to a final concentration of 5 μg/mL. The plate was incubated at 37 °C for 30 minutes followed by addition of formic acid to a final concentration of 3.75 M to quench the LpxE reaction. The malachite green reagent (Sigma Aldrich, catalog MAK307) was added with a 5-fold dilution, and the solution was incubated for 30 minutes at room temperature before the absorbance at 620 nm was measured. All measurements were done in triplicates, and standard error was calculated. Percentage LpxH activities for 1, 2, and 3 at 0.1 μM were calculated from previously reported IC50 values,13 which were extracted from fitting of the dose-response curve of vi/v0 = 1/(1 + [I]/IC50) assayed under identical conditions.
Supplementary Material
Highlights.
Synthesis and biochemical evaluation of sulfonyl piperazine LpxH inhibitors
Analysis of the structure–activity relationship of sulfonyl piperazine LpxH inhibit ors
Report of the X-ray crystal structure of an extended N-acyl chain analog (JH-LPH −41) in complex with K. pneumoniae LpxH, revealing that the inhibitor reaches an untapped polar pocket near the di-manganese cluster in the active site of K. pneum oniae LpxH
7. Acknowledgments
This work was supported in part by the grants from the National Institute of Allergy and Infectious Diseases (AI139216) and National Institute of General Medical Sciences (GM115355). X-ray diffraction data were collected at the Northeastern Collaborative Access Team beam lines (24-ID-C), which is funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165). The Pilatus 6M detector on 24-ID-C beam line is funded by a NIH-ORIP HEI grant (S10 RR029205). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. C.G.W. and A.F.E were supported by the NIGMS Pharmacological Sciences Training Grant (NIH GM007105). The authors would like to thank Dr. Jinshi Zhao for insightful discussions about the LpxH inhibition assay.
Footnotes
Supplementary material
Copies of 1H NMR spectra; Data collection and statistics of the K. pneumoniae LpxH/27b complex structure and the omit (2mFo-DFc) map of 27b bound to K. pneumoniae LpxH contoured at 0.75σ.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48 (1), 1–12. 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.WHO Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics; World Health Organization, 2017. [Google Scholar]
- 3.Raetz CRH, Whitfield C (2002) Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700. 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barb AW, Zhou P (2008) Mechanism and inhibition of LpxC: an essential zinc-dependent deacetylase of bacterial lipid A synthesis. Curr. Pharm. Biotechnol. 9 (1), 9–15. 10.2174/138920108783497668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P, and Zhao J (2017) Structure, inhibition, and regulation of essential lipid A enzymes. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 1862 (11), 1424–1438. 10.1016/j.bbalip.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babinski KJ, Ribeiro AA, Raetz CR (2002) The Escherichia coli gene encoding the UDP-2,3-diacylglucosamine pyrophosphatase of lipid A biosynthesis. J. Biol. Chem. 277 (29), 25937–25946. 10.1074/jbc.M204067200. [DOI] [PubMed] [Google Scholar]
- 7.Metzger LE IV, Raetz CR (2010) An alternative route for UDP-diacylglucosamine hydrolysis in bacterial lipid A biosynthesis. Biochemistry 49 (31), 6715–6726. 10.1021/bi1008744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young HE, Zhao J, Barker JR, Guan Z, Valdivia RH, Zhou P (2016) Discovery of the elusive UDP-Diacylglucosamine hydrolase in the lipid A biosynthetic pathway in Chlamydia trachomatis. mBio. 7 (2), e00090–16. 10.1128/mBio.00090-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzger LE IV, Lee JK, Finer-Moore JS, Raetz CR, Stroud RM (2012) LpxI structures reveal how a lipid A precursor is synthesized. Nat. Struct. Mol. Biol. 19 (11), 1132–1138. 10.1038/nsmb.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzger LE IV, and Raetz CR (2009) Purification and characterization of the lipid A disaccharide synthase (LpxB) from Escherichia coli, a peripheral membrane protein. Biochemistry 48 (48), 11559–11571. 10.1021/bi901750f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nayar AS, Dougherty TJ, Ferguson KE, Granger BA, McWilliams L, Stacey C, Leach LJ, Narita S, Tokuda H, Miller AA, Brown DG, McLeod SM (2015) Novel antibacterial targets and compounds revealed by a high-throughput cell wall reporter assay. J. Bacteriol. 197 (10), 1726–1734. 10.1128/JB.02552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee M, Zhao J, Kwak SH, Cho J, Lee M, Gillespie RA, Kwon DY, Lee H, Park HJ, Wu Q, Zhou P, Hong J (2019) Structure-activity relationship of sulfonyl piperazine LpxH inhibitors analyzed by an LpxE-coupled malachite green assay. ACS Infect. Dis. 5 (4), 641–651. 10.1021/acsinfecdis.8b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho J, Lee M, Cochrane CS, Webster CG, Fenton BA, Zhao J, Hong J, Zhou P (2020) Structural basis of the UDP-diacylglucosamine pyrophosphohydrolase LpxH inhibition by sulfonyl piperazine antibiotics. Proc. Natl. Acad. Sci. U. S. A. 117 (8) 4109–4116. 10.1073/pnas.1912876117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huguet F, Melet A, Alves de Sousa R, Lieutaud A, Chevalier J, Maigre L, Deschamps P, Tomas A, Leulliot N, Pages JM, Artaud I (2012) Hydroxamic acids as potent inhibitors of Fe(II) and Mn(II) E. coli methionine aminopeptidase: biological activities and X-ray structures of oxazole hydroxamate-EcMetAP-Mn complexes. ChemMedChem. 7 (6), 1020–1030. 10.1002/cmdc.201200076. [DOI] [PubMed] [Google Scholar]
- 15.Gros L, Lorente SO, Jimenez CJ, Yardley V, Rattray L, Wharton H, Little S, Croft SL, Ruiz-Perez LM, Gonzalez-Pacanowska D, Gilbert IH (2006) Evaluation of azasterols as anti-parasitics. J. Med. Chem. 49 (20), 6094–6103. 10.1021/jm060290f. [DOI] [PubMed] [Google Scholar]
- 16.Kabsch W (2010) “Xds.” Acta Crystallographica Section D. Biol. Crystallogr. 66 (2), 125–132. 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66 (2), 213–221. 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriarty NW, Grosse-Kunstleve RW, Adams PD (2009) electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D Biol. Crystallogr. 65 (10), 1074–1080. 10.1107/S0907444909029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60 (12), 2126–2132. 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.