Summary
Background:
Allogeneic blood or marrow transplantation (alloBMT) is potentially life-saving treatment for individuals with HIV and hematologic malignancies; challenges include identifying donors and maintaining antiretroviral therapy (ART). The objectives of our study were to investigate interventions to expand donor options and to prevent ART interruptions for patients with HIV in need of alloBMT.
Methods:
We conducted a single-center, single-arm interventional trial of alloBMT in patients with HIV and high-risk hematologic malignancy in which we used post-transplantation cyclophosphamide (PTCy) as graft-versus-host-disease (GvHD) prophylaxis to expand donor options and an optimized ART strategy of avoiding pharmacoenhancers and adding subcutaneous enfuvirtide during PTCy and during oral medication intolerance. Our primary outcome was the proportion of participants who maintained ART through day 60. We measured the HIV latent reservoir (LR) using a quantitative viral outgrowth assay. ClinicalTrials.gov, NCT01836068.
Findings:
Between June 1, 2013 and August 27, 2015, nine patients who were referred for transplant provided consent. Two patients had relapsed malignancy before donor searches were initiated. Seven patients had suitable donors identified (two matched sibling, two matched unrelated, two haploidentical, and one single-antigen mismatched unrelated) and proceeded to alloBMT. All patients maintained ART through day 60 and required ART changes (median 1, range 1-3) in the first 90 days. One patient stopped ART and developed HIV rebound with grade 4 meningoencephalitis at day 146. Among six alloBMT patients with measurements, the HIV LR was not detected post-alloBMT in four patients with >95% donor chimerism, consistent with a 2·06- to 2·54 log10 reduction in the HIV LR. In two patients, with <95% donor chimerism, the HIV LR remained relatively stable.
Interpretation:
By using PTCy as GvHD prophylaxis, we successfully expanded alloBMT donor options for patients with HIV. Continuing ART with a regimen that includes enfuvirtide post-alloBMT was safe, but life-threatening viral rebound can occur with ART interruption.
Funding:
amfAR The Foundation for AIDS Research, the Johns Hopkins University Center for AIDS Research and National Cancer Institute.
Trial registration:
Introduction
Allogeneic blood or marrow transplantation (alloBMT) is an accepted approach to the treatment of hematologic malignancies in individuals with HIV.1 Identifying suitable donors and managing antiretroviral therapy during transplant are challenges. In addition, the impact of alloBMT on the long-term HIV reservoir has attracted interest as a potential curative strategy.
Identifying matched donors for people living with HIV has been a barrier as it has been for other transplant populations, particularly minority populations.2 Post-transplant cyclophosphamide (PTCy) to prevent or reduce graft-versus-host-disease (GvHD) allows the use of HLA-matched related and unrelated donors and haploidentical (haplo) donors and partially mismatched unrelated donors.3-5 In addition to curing hematologic malignancies, there was enthusiasm that alloBMT might also eradicate HIV infection. The major barrier to HIV cure is the persistence of latent HIV integrated within long-lived resting memory CD4+ T cells and perhaps other hematopoietic-derived cells.6
More than ten years ago, Timothy Ray Brown (‘the Berlin Patient’) received an alloBMT for treatment of acute myeloid leukemia (AML) from a donor who was homozygous for the genetic polymorphism CCR5Δ32 and was cured of both AML and HIV.7 Recently, a patient with Hodgkin lymphoma (‘the London Patient’) achieved long-term HIV remission following alloBMT also from a CCR5Δ32 homozygous donor.8 The 32-base pair deleted variant of CCR5 affects about 1% of Caucasians and results in a non-functional CCR5 receptor resistant to infection with the most common strains of HIV (R5).9 In these cases, alloBMT was analogous to providing the patient with gene therapy; however, limited numbers of HLA-matched CCR5Δ32 homozygous donors and HIV strains that can use the alternative cellular receptor CXCR4 (X4) have limited the applicability of this approach.10,11
The report of the Berlin patient rekindled our interest in the possibility that alloBMT with uninterrupted ART might prevent infection of donor cells which would in turn, replace all HIV-infected host cells leading to cure. Our group initially explored this concept in an patient with HIV receiving alloBMT combined with zidovudine in 1989.12 That patient died with relapsed lymphoma but had no detectable HIV by early PCR assays. We investigated the feasibility of optimizing ART to minimize drug interactions and minimize periods without ART coverage in patients with HIV undergoing alloBMT for high-risk hematologic malignancies. Here, we report a single-arm trial of patients with HIV and hematologic malignancies referred for allogeneic transplant, results of donor searches, transplant outcomes, and measurements of the latent HIV reservoir in peripheral blood. In addition, we highlight a special risk in patients with HIV undergoing alloBMT when ART is interrupted in the post-transplant period.
Methods
Study design and participants
This was a single-arm interventional trial at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (SKCCC), Baltimore, MD, USA. Individuals with HIV ≥18 years of referred for alloBMT for a standard clinical indication were eligible. The only exclusion criteria was a history of documented resistance to enfuvirtide. Patients provided written consent prior to HLA matching and/or registry searches. Any donor source (matched sibling, haploidentical relative, matched or partially mismatched unrelated) was permitted as was co-enrollment in other transplant trials. The study (NCT01836068; Appendix pages 2-24) was approved by the institutional review board, and all patients provided written informed consent in accordance with the Declaration of Helsinki. In order to protect participant confidentiality in this small cohort, we will not report age, sex, or race individually. Patients transplanted after August 2015 received transplants from multiply mismatched unrelated donors. Patients enrolled in that trial will be reported separately.
Procedures
ART regimens were optimized prior to transplant by replacing protease inhibitors and pharmacoenhancers with other antivirals based on drug resistance and ART history. Changes in ART were made in cases of drug toxicity or interactions by a multidisciplinary team that included an HIV specialist, pharmacologist, and oncologist. Conditioning regimens were guided by standard of care guidelines or by parallel transplant protocols if patients were co-enrolled.
Continuation of ART was defined as no periods >24 hours without ART. It was determined using medication administration records during inpatient hospitalization and using electronic pill bottle caps (medication event monitoring systems MEMS®) for outpatients. In October 2018, the protocol was amended to remove MEMS monitoring in response to participant feedback.
All patients received cyclophosphamide 50 mg/kg/day IV on days three and four and mycophenolate mofetil with tacrolimus or sirolimus day five after alloBMT except in patients receiving HLA-matched marrow with a myeloablative preparative regimen where no GvHD prophylaxis other than high-dose cyclophosphamide was used.13 As PTCy is associated with nausea and vomiting that might interfere with continuation of oral ART, subcutaneous enfuvirtide was administered on those days and at any time when oral ART was not tolerated. Standard antibiotic prophylaxis with a fluoroquinolone and antifungal prophylaxis were given until neutrophil recovery. Cytomegalovirus (CMV) quantitative PCR was monitored weekly through day 60 and reactivation was preemptively treated. Filgrastim was administered from day five until neutrophil recovery to ≥1,000/μL. Patients received Pneumocystis jiroveci and anti-HSV/VZV prophylaxis for one year.
Leukocyte chimerism and T-cell chimerism (sorted CD3+) from peripheral blood were determined using a set of microsatellites or short tandem repeats (ABI, AmpflSTR) to distinguish donor from recipient with a limit of detection of 1%. Plasma HIV RNA viral load was measured using COBAS AMPLICOR Monitor test version 2·0 (Roche Diagnostics, Indianapolis IN).
The HIV latent reservoir (LR) within resting memory CD4+ T-cells was measured prior to alloBMT and every 12 weeks using a quantitative viral outgrowth assay (qVOA) as previously described.14-16 Briefly, purified resting memory CD4+ T-cells from whole blood were plated at limiting-dilution with healthy donor γ-irradiated peripheral blood mononuclear cells (PBMCs). When 11x106 CD4+ T-cells or fewer were isolated, the enrichment for resting CD4+ T-cells was not performed. The cells were subjected to ex vivo activation by the T-cell mitogen, phytohaemagglutinin. Viral outgrowth was amplified by the addition of MOLT-4/CCR5+ cell line and was detected by ELISA for HIV p24 antigen in the supernatant. The MOLT-4/CCR5+ cell line is susceptible to infection by both R5- and X4-tropic viruses.
Outcomes
The primary outcome was the proportion of patients who maintained ART through day 60 post-alloBMT. Secondary outcomes included donor chimerism (week 4, 8, 12, then every 12 weeks), plasma HIV RNA (week 4, 8, 12, then every 12 weeks), frequency of resting CD4 T cells harboring infectious HIV (baseline and every 12 weeks), copies of HIV DNA per million PBMCs (every 12 weeks), the incidence and severity of GvHD, and the incidence and severity of adverse events related to enfuvirtide through end of follow-up. We do not report copies of HIV DNA per million PBMCs due to technical issues with the PCR assay which made the results unreliable.
Statistical Analysis
ART adherence:
We considered a probability of at least 20% of maintaining ART to be evidence of clinically meaningful feasibility to warrant further investigation. The study design included interim analyses for futility. Interim analyses, based on Bayesian computations, were designed to recommend stopping the study if there was 90% probability that no more than 20% of patients would continue uninterrupted ART through day 90. The Bayesian calculation assumed a priori that the chance that a patient would continue ART through day 60 was anything between 0% and 100% with equal probability.
HIV LR changes:
The frequency of cells harboring infectious virus from each sample was expressed as the frequency of infectious units per million cells (IUPM), determined by two different methods: 1) maximum-likelihood estimate of each sample individually,17 and 2) a mixed-effects Bayesian model that jointly estimates IUPM for all samples as well as treatment effects. This Bayesian joint modeling framework has previously been shown to be capable of identifying mixed effects on IUPM even when some measurements are below the limit of detection. Maximum likelihood and two-stage approaches (estimating IUPM from raw assay output prior to fitting treatment effects) can result in biases, particularly where measurements are near or below detection limits.18 Our modeling framework effectively “smooths out” apparent rapid changes caused by random sampling from a small frequency of infected cells. Since the modeling framework shares information across samples and patients, model-based estimates of IUPM can fall below the assay’s limit of detection. This limit of detection is based on number of input cells, and ranged from 0·0043 - 0·10 IUPM for each sample considered individually. For further details see Appendix Material (Appendix page 25).18 Using PyStan 2·7, the model was executed with Markov Chain Monte Carlo, four parallel chains of 10,000 iterations each were run for the samples (Appendix pages 26-35).
Trial registration: ClinicalTrials.gov, NCT01836068
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication
Results
Between June 1, 2013 and August 27, 2015, nine patients with HIV and hematologic malignancies were referred for alloBMT. Seven patients were in remission had donors identified, and proceeded to transplant. Two other patients had progressive disease and donor searches were never carried out. There were no patients referred who were appropriate for transplant for whom we were unable to identify a suitable bone marrow donor.
All seven patients received bone marrow transplants with a median of 5·9 x 106 CD34+ cells/kg dose (range 2·4 x 106 – 27·3 x 106 CD34+ cells/kg) and all successfully engrafted (Table 1). Underlying malignancies among those who received transplant were AML (n=3), diffuse large B-cell lymphoma (DLBCL) (n=2), and Hodgkin lymphoma (n=2). Donors were matched related (n=2), matched unrelated (n=3), and haplo (n=2). Patient ages at alloBMT ranged from 30 to 55 years and four patients were non-white. All patients were on ART at alloBMT with HIV RNA plasma viral load <200 copies/mL. The conditioning regimen was non-myeloablative Flu/Cy/TBI19 (n=4), non-myeloablative Flu/Bu20 (n=2), and myeloablative Flu/Bu21 (n=1).
Table 1. Patient characteristics and outcomes.
| Patient | 1 | 2 | 3 | 4 | 6 | 7 | 9 |
|---|---|---|---|---|---|---|---|
| Cancer | HL | DLBCL | AMLa | AMLb | DLBCL | HL | AMLc |
| Conditioning Type | NMA | NMA | NMA | MAC | NMA | NMA | NMA |
| Donor type | MUD | MUD | MRD | MRD | Haplo | Haplo | MMUD |
| Neutrophil >500X103/mm3 (days)d | +17 | +28 | +19 | +24 | +17 | +14 | +17 |
| PBMC Donor Chimerisme | >95% | 87% | >95% | 73% | >95% | >95% | >95% |
| CD3+ Donor Chimerismf | >95% | 74% | >95% | 5% | >95% | >95% | >95% |
| HIV diagnosis (years) | 8·9 | 22·2 | 10·5 | 12·2 | 13·6 | 10·2 | 22·2 |
| Baseline HIV plasma RNA (c/mL) | 79 | <20 | 21 | <20 | <20 | 21 | <20 |
| Baseline CD4 count (cells/μL) | 99 | 231 | 254 | 57 | 227 | 547 | 219 |
| HIV Tropism | R5 | R5 | R5 | R5/X4 | R5 | R5/X4 | R5 |
| ART Resistance Mutations | M184V | None | K103N, M184V |
None | None | None | None |
| ART changes through day 90 | 3 | 1 | 1 | 3 | 1 | 2 | 1 |
| Oncology outcomes, cause of death | Died, week 49, liver failure | Remission, week 231 | Died, week 64 GvHD |
Remission week 188 | Died, week 67, sepsis | Remission, week 155 | Remission week 128 |
Abbreviations Cancer: HL (Hodgkin lymphoma), DLBCL (Diffuse large B-cell lymphoma), AML (acute myeloid leukemia)
AML (multiply relapsed)
AML (biphenotypic acute myeloid leukemia)
AML (Flt3 AML). Donor type: MUD (Match-unrelated donor), MRD (match-related donor), Haplo (Haploidentical), MMUD (mismatched-unrelated donor). Conditioning-Type: Non-myeloablative (NMA), Myeloablative-conditioning (MAC). R5: HIV strains which use the beta-chemokine receptor CCR5 for cell entry and are able to replicate in macrophages and CD4+ T-cells. R5/X4: HIV strains which can use chemokine receptor CCR5 or CXCR4 for entry.
Engraftment defined where for 3 consecutive days with Absolute neutrophil count (ANC) >500X103/mm.
At week 26 for patient 1 and week 52 for patients 2, 3, 4, 6, 9.
At week 8 for patient 1, week 24 for patient 3, week 52 for patient 2, 4, 6, 7, 9.
Median time to neutrophil recovery over 500 X 103/mm3 was 17 days (range, 14-28) (Table 1). Five patients achieved complete donor chimerism (>95% donor DNA detected) in PBMCs and CD3+ T-cells by 24 weeks. Two patients had persistent mixed chimerism with 74% and 5% donor CD3+ T-cells at week 52 (Table 1).
Opportunistic infections were not infrequent. Three patients developed CMV viremia one month post-transplant. One had hemorrhagic cystitis with concomitant adenovirus viremia; the other two were asymptomatic. All patients responded to treatment with valganciclovir. One patient with asymptomatic CMV also developed persistent fevers 8 weeks post-transplant; an extensive microbiologic evaluation was unrevealing including negative urine and blood Histoplasma antigen tests, however due to a history of disseminated histoplasmosis 8 years prior, the patient was empirically treated with liposomal amphotericin and the fevers resolved. One patient developed cutaneous Kaposi’s sarcoma 5 months post-transplant which resolved when tacrolimus was changed to sirolimus.
Three patients developed GvHD. Patient 1 had chronic hepatitis C virus (HCV) infection at the time of transplant and developed elevated liver transaminases 6 months post-alloBMT. With HCV direct-acting antivirals obtained via a compassionate use protocol, HCV viremia was suppressed but liver function continued to deteriorate. Liver biopsy showed non-specific inflammatory changes that might have been related to HCV, GvHD, or drug-induced liver injury. There were no signs of cutaneous or gastrointestinal GvHD. The patient died with progressive liver failure. Patient 3 stopped ART and immunosuppression after discharge from the transplant intensive outpatient care program (described below). The patient developed liver GvHD and died 64 weeks post-alloBMT with liver failure. Patient 9 developed skin and gastrointestinal GvHD while on sorafenib. This resolved without sequelae when sorafenib was stopped.
Three patients died. As above, patients 1 and 3 died with liver failure. Patient 6 died at an outside hospital 67 weeks after transplant due to presumed sepsis with no specific pathogen identified and no autopsy performed.
All patients required ART changes (Table 2). The median number of changes was 1 (range 1-3). At baseline, three patients were switched off PIs to avoid drug interactions. Additional changes were made due to acute kidney injury (n=5), insurance issues (n=1), drug interaction with cidofovir (n=1), and concern for fevers possibly related to abacavir hypersensitivity despite negative HLA-B*5701 testing (n=1). All patients received enfuvirtide during PTCy therapy when nausea and vomiting are common. Patient 3 also received enfuvirtide for 6 days during a period of encephalopathy (see below). No adverse events (AEs) occurred related to short-term enfuvirtide administration.
Table 2. Antiretroviral therapy changes through day 90.
| Patient | ART changes | ART regimen | Days relative to alloBMT | Reason for change |
|---|---|---|---|---|
| 1 | 3 | TDF FTC DRV/r TDF FTC RAL MVC ABC FTC RAL MVC ABC 3TC RAL MVC |
Pre-transplant 0 +5 +25 |
• Avoid ritonavir interaction • Acute kidney injury • Insurance formulary preference |
| 2 | 1 | EFV TDF FTC EFV ABC FTC |
Pre-transplant +9 |
• Acute kidney injury |
| 3 | 1 | ABC 3TC RAL DRV/r ABC 3TC RAL MVC |
Pre-transplant 0 |
• Avoid ritonavir interaction |
| 4 | 3 | TDF FTC RAL ABC FTC RAL TDF FTC RAL ABC FTC RAL |
Pre-transplant +24 +41 +49 |
• Acute kidney injury • Renal function improved • Interaction with cidofovir |
| 6 | 1 | EFV TDF FTC EFV ABC 3TC |
Pre-transplant +81 |
• Acute kidney injury |
| 7 | 2 | TDF FTC RAL ABC 3TC RAL ETR 3TC RAL |
Pre-transplant +45 +69 |
• Acute kidney injury • Concern for ABC-related fevers |
| 9 | 1 | TDF FTC ATV TDF FTC DTG |
Pre-transplant 0 |
• Avoid atazanavir drug interaction |
Abbreviations efavirenz (EFV), etravirine (ETR), tenofovir (TDF), emtricitabine (FTC), abacavir (ABC), lamivudine (3TC), raltegravir (RAL), dolutegravir (DTG), atazanavir (ATV), ritonavir-boosted darunavir (DRV/r), maraviroc (MVC).
All seven patients maintained ART during the first 60 days. Five patients had no ART interruptions according to inpatient medication administration records and outpatient MEMS® records. Two patients did not use MEMS®. One of these (patient 9) had no ART interruptions according to inpatient records and outpatient self-report. The other (patient 3) admitted to stopping ART after day 60 for unclear reasons and developed complications, described below.
All patients who maintained ART had suppression of HIV plasma RNA at week 4, 8, 12 and every 12 weeks through end of follow-up. All measurements were <20 copies/mL, except for 2 patients who had isolated viral blips of 87 and 117 copies/mL at week 24.
Patient 3 missed clinic, laboratory, and study visits post-alloBMT. Around day 100, the patient developed fevers without a clear cause, which progressed to lethargy and confusion leading to hospital admission on day 146. Blood cultures for bacteria, fungi, and mycobacteria were negative. Cerebrospinal fluid (CSF) analysis showed 28 white blood cells (range 0 - 3 cells/μL), protein 150 (range 15-45 mg/dL), and glucose 50 mg/dL (range 50 - 80 mg/dL). CSF was negative for varicella zoster, herpes simplex, Epstein-Barr, CMV, JC, and West Nile virus by PCR; cryptococcal antigen was not detected. HIV plasma RNA was 25,518 copies/mL and 17,000 copies/mL in CSF. Aspartate transaminase and alanine transaminase were 282 (range 5 - 40 U/L) and 286 (range 7 - 56 U/L), respectively. Enfuvirtide was initiated as the patient was unable to take oral medications. After three days, mental status and liver enzymes improved. HIV genotype from plasma demonstrated no drug resistant mutations (Celera Diagnostics ViroSeq v·2·8 assay). Oral ART (maraviroc, raltegravir, abacavir, lamivudine) was restarted and he was discharged at day 159. Plasma HIV RNA was 23 copies/mL on day 190.
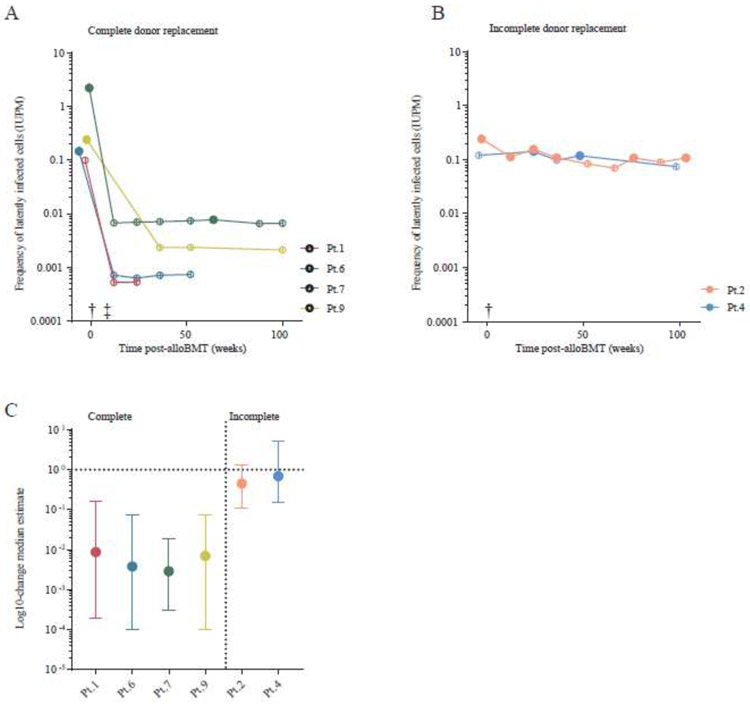
Five patients had viral outgrowth from the HIV LR at baseline (median 0·28 IUPM, range 0·18 - 2·32 IUPM) (Table 3, maximum-likelihood estimate). Two patients had an undetectable LR by qVOA at baseline. Six patients had longitudinal measurements available; patient 3 did not provide post-alloBMT samples. Among these six, four patients achieved complete donor CD3+ T-cell chimerism; the HIV LR was undetectable by qVOA at all post-alloBMT timepoints in these patients except at one time-point (week 64) for patient 7 (Figure 1A & Table 3). Among these six patients with complete donor chimerism, individual patient Bayesian model-based log-reductions in the LR were: 2·06 log10 (Patient 1), 2·43 log10 (Patient 6), 2·54 log10 (Patient 7), and 2·15 log10 (Patient 9) (Figure 1 C). Since most follow-up measurements were negative, the possibility of much larger reductions could not be excluded. Two patients with incomplete donor chimerism maintained a detectable HIV LR at multiple measurements post-alloBMT (Figure 1B & Table 3). There was weak evidence that one of these incomplete chimerism donor patients (Patient 2) achieved a small reduction in reservoir size (0·34 log10 (95% CI 0·97 log10 reduction - 0·19 log10 increase)), and no evidence of reduction in Patient 4 (95% CI 0·82 log10 reduction - 0·71 log10 increase) (Figure 1C). An alternate model that excluded the possibility of reservoir reduction in the two patients with incomplete chimerism fit the data equally well (ΔWAIC = −0·6).
Table 3. Longitudinal viral outgrowth results for participants.
Resting memory CD4+ T-cells or total CD4+ T-cells were plated in a limiting dilution format for the qVOA. The number of wells that were positive for p24 production over total wells plated are indicated. Both maximum-likelihood and Bayesian model-based IUPM estimates reported. The Bayesian model-based approach shares information across samples, “smoothing out” apparent rapid changes caused by random sampling from a small frequency of infected cells (e.g., alternation of positive and negative samples from Pt.4)
| Patient | Timepoint | Donor replacement |
CD4 T-cell plated |
Number of cells plated |
Viral outgrowth detected |
p24 positive wells /total wells |
IUPM Estimate | |
|---|---|---|---|---|---|---|---|---|
| Maximum- likelihood IUPM estimate |
Bayesian model-based IUPM estimate |
|||||||
| 1 | Baseline | - | Total | 6·24 x106 | No | 0/6 | 0·120 | 0·099 |
| Week 12 | Complete | Total | 10·55 x106 | No | 0/20 | 0·066 | 0·001 | |
| Week 24 | Complete | Resting | 7·95 x106 | No | 0/18 | 0·088 | 0·001 | |
| 2 | Baseline | - | Resting | 20·55 x106 | Yes | 5/30 | 0·280 | 0·242 |
| Week 12 | Incomplete | Resting | 5·55 x106 | Yes | 1/15 | 0·201 | 0·112 | |
| Week 24 | Incomplete | Resting | 5·55 x106 | Yes | 2/15 | 0·452 | 0·155 | |
| Week 36 | Incomplete | Resting | 12·55 x106 | Yes | 1/22 | 0·083 | 0·108 | |
| Week 52 | Incomplete | Resting | 9·25 x106 | No | 0/19 | 0·075 | 0·084 | |
| Week 64 | Incomplete | Resting | 16·05 x106 | No | 0/26 | 0·043 | 0·070 | |
| Week 76 | Incomplete | Resting | 19·95 x106 | Yes | 3/30 | 0·160 | 0·108 | |
| Week 90 | Incomplete | Resting | 3·75 x106 | No | 0/14 | 0·187 | 0·089 | |
| Week 103 | Incomplete | Resting | 23·55 x106 | Yes | 3/33 | 0·135 | 0·107 | |
| 3 | Baseline | - | Resting | 22·50 x106 | Yes | 12/32 | 0·762 | --- |
| 4 | Baseline | - | Resting | 7·55 x106 | No | 0/17 | 0·092 | 0·120 |
| Week 24 | Incomplete | Resting | 3·92 x106 | Yes | 3/14 | 0·534 | 0·141 | |
| Week 36 | Incomplete | Total | 2·55 x106 | No | 0/12 | 0·277 | 0·098 | |
| Week 48 | Incomplete | Resting | 5·55 x106 | Yes | 2/15 | 0·452 | 0·118 | |
| Week 98 | Incomplete | Resting | 7·55 x106 | No | 0/17 | 0·092 | 0·074 | |
| 6 | Baseline | - | Resting | 12·55 x106 | Yes | 2/22 | 0·179 | 0·146 |
| Week 12 | Complete | Total | 9·55 x106 | No | 0/19 | 0·073 | 0·001 | |
| Week 24 | Complete | Resting | 16·55 x106 | No | 0/26 | 0·042 | 0·001 | |
| Week 36 | Complete | Total | 7·55 x106 | No | 0/17 | 0·092 | 0·001 | |
| Week 52 | Complete | Resting | 6·90 x106 | No | 0/17 | 0·087 | 0·001 | |
| 7 | Baseline | - | Resting | 21·55 x106 | Yes | 22/31 | 2·319 | 2·199 |
| Week 12 | Complete | Total | 11·55 x106 | No | 0/21 | 0·060 | 0·007 | |
| Week 24 | Complete | Resting | 6·72 x106 | No | 0/17 | 0·104 | 0·007 | |
| Week 36 | Complete | Resting | 40·55 x106 | No | 0/50 | 0·017 | 0·007 | |
| Week 52 | Complete | Resting | 30.00 x106 | No | 0/39 | 0·023 | 0·007 | |
| Week 64 | Complete | Resting | 30·55 x106 | Yes | 1/40 | 0·033 | 0·008 | |
| Week 88 | Complete | Total | 6·55 x106 | No | 0/16 | 0·107 | 0·007 | |
| Week 100 | Complete | Resting | 14·55 x106 | No | 0/24 | 0·048 | 0·007 | |
| 9 | Baseline | - | Resting | 12·55 x106 | Yes | 3/22 | 0·232 | 0·240 |
| Week 36 | Complete | Total | 4·55 x106 | No | 0/14 | 0·154 | 0·002 | |
| Week 52 | Complete | Resting | 10·55 x106 | No | 0/22 | 0·066 | 0·002 | |
| Week 100 | Complete | Resting | 12.00 x106 | No | 0/22 | 0·070 | 0·002 | |
FIGURE 1.
Measurements of HIV persistence following alloBMT. (A) Individuals who achieved complete donor replacement post-alloBMT. (B) Individuals who did not achieve complete donor replacement post-alloBMT. The HIV latent reservoir measurement of inducible replication-competent virus by qVOA, a limiting-dilution based co-culture assay. The frequency of latently infected cells was determined by a mixed-effects Bayesian model and expressed as a frequency of infectious units per million cells (IUPM); median posterior estimates are shown. Samples where viral outgrowth was undetected are indicated with open shapes. (C) The log10-change median estimate pre- to post-transplant with 95% confidence intervals reported using a mixed-effects Bayesian model implemented in PyStan 2·7 (see Appendix, pp 26-35.). A log10-change greater than 100 (or 1) indicate an increase size in the latent reservoir size whereas values less than 1 suggest a reduction. The symbol (†) indicates when patients underwent bone marrow transplant and corresponds to the statistical model (0 to 1); and (‡) indicates the point when the patients achieved complete leukocyte donor replacement and corresponds to the statistical model (0 to 1).
Discussion
We found that although identification of a suitable donor for alloBMT has been an important obstacle, particularly for minority populations,2 we were able to identify suitable donors for every patient, including patients of African descent. The use of PTCy made less stringent donor selection criteria with regard to HLA possible.2,4,22-25 The only definitive GvHD death occurred in a patient with a fully matched sibling donor who discontinued his immunosuppression against medical advice. Thus, as is the situation with patients with sickle cell disease, the use of post-transplantation cyclophosphamide meaningfully enlarged the donor pool for patients living with HIV who require alloBMT.
Our strategy for ART during alloBMT avoided treatment interruptions and proved to be safe and feasible. In all patients, ART was continued through day 60, which is a critical period post-alloBMT during which engraftment occurs, and patients must stay locally for close monitoring. Enfuvirtide was a well-tolerated supplement to oral ART in alloBMT patients who are at high risk for missed doses, with no AEs in any of seven patients who received it for short periods. This finding is also supported by a series by Johnson and colleagues that included one patient with HIV post-alloBMT in whom enfuvirtide was used without.26 In our study, modification of ART was commonly required due to drug interactions and changes in renal or hepatic dysfunction. The most common indication for ART change was acute kidney injury prompting substitution of tenofovir disproxil fumarate (TDF) with abacavir with less potential for kidney toxicity.
Our patients did develop several opportunistic infections including possible histoplasmosis reactivation, adenovirus, CMV viremia, and Kaposi sarcoma, but these complications resolved with appropriate treatment.
We found the impact of alloBMT on the HIV LR depended on the extent of donor chimerism. In patients who achieved full donor chimerism in peripheral blood, the HIV LR as measured by qVOA from CD4+ T-cells decreased to levels below the limit of detection. In individuals who achieved complete donor replacement post-alloBMT, there was a measured 2·06 - 2·54 log10 reduction in the LR size. Conversely, the LR was easily detected in two patients who had mixed chimerism (incomplete replacement by donor cells), and no significant change was detected, including one whom had received myeloablative conditioning. These results are consistent with those recently reported by the European alloBMT IciStem Consortium in which the HIV LR is reduced in patients who achieve full donor chimerism following alloBMT.27 While these reductions in persistent HIV measurements appear promising for HIV cure strategies, there are important caveats. We are not suggesting there is any clinical benefit to reductions in the size of the HIV reservoir for the patients in our study. These patients received transplants due to life-threatening malignancies for which alloBMT is standard therapy. However the observed impact on the HIV LR might help to guide new approaches to cure.
In one patient who stopped ART, we observed a nearly fatal case of acute retroviral syndrome associated with HIV RNA rebound in the plasma and CSF at day 146 post-alloBMT with fever, liver dysfunction, and meningoencephalitis. This suggests that despite >95% donor chimerism in peripheral blood, the HIV LR persisted or that donor cells were newly infected.
Henrich and colleagues previously described two HIV+ patients who received alloBMT as treatment for refractory lymphoma.28 HIV could not be detected in peripheral blood three and five years post-alloBMT using highly sensitive assays. The HIV LR in the two patients had an estimated 2·35 log10 and 2·75 log10 reduction, which is consistent with our current report.29 A follow-up study of a carefully monitored ART interruption, also known as an analytic treatment interruption, was performed in these same patients to investigate the possibility of HIV eradication. However, HIV viral rebound occurred at 12 and 32 weeks after stopping ART, respectively; both patients had fevers and one had meningitis.30 The severity of HIV rebound in our report and in the report of Henrich may reflect the lack of donor cellular immune response to HIV. It is also interesting to note that there was symptomatic central nervous system involvement in two out of three cases of viral rebound after alloBMT. Analytic treatment interruption after alloBMT is distinct from interruption in other settings given that the donor immune system is immunologically HIV naïve and thus there are unique clinical and ethical considerations.31 Patients with HIV and their providers need to be aware of the serious risks of interrupting ART after alloBMT. We have modified our consent form to acknowledge this risk. Novel strategies to prevent and treat HIV rebound are needed in alloBMT recipients.32 Careful selection criteria and long-term follow-up are important in this patient population. More intense adherence support and frequent post-transplant monitoring may reduce morbidity and mortality.
Our study was limited by the fact that it was small. A larger cohort of alloBMT recipients with HIV is needed to confirm these measured effects. In addition, transplant-related toxicities preclude the application of this approach to patients who would not otherwise benefit from a transplant.
In conclusion, with PTCy and less stringent donor selection criteria it is possible to identify appropriate donors for most patients. It was possible to maintain ART during the intensive first two months after transplant and enfuvirtide is a well-tolerated option when nausea or vomiting interfere with oral ART. Finally, achievement of full donor chimerism has profound effects on the HIV LR but how this may translate into cure for patients without HIV-resistant donors has yet to be established.33
Supplementary Material
Acknowledgments
Role of funding
amfAR The Foundation for AIDS Research (108707-54-RKRL), the Johns Hopkins University Center for AIDS Research, (P30AI094189), and the National Cancer Institute (K23CA177321-01A1; P01CA015396; P01 CA225618-01A1) provided funding. The funder had no role in the study design, data collection, data analysis, data interpretation or writing the report.
Declaration of Interests
CMD reports grants from amfAR, The Foundation for AIDS Research (Mathilde Krim Fellow108707-54-RKRL), the National Cancer Institute (K23CA177321-01A1) and the National Institute of Allergy and Infectious Diseases (Johns Hopkins University Center for AIDS Research; P30AI094189) and has served on a grant review committee for Gilead Sciences and has received research grants paid directly to the institution from Abbvie, Merck Sharp and Dohme Corp., GlaxoSmithKline, and ViiV Healthcare outside the submitted work. RFA and RJJ reports grants from NCI P01CA015396 and P01 CA225618-01A1 . DISR reports personal fees from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA as an employee and personal fees from Accelevir Diagnostics; outside the submitted work. RKA has received research grants from pharmaceutical sponsors. CB has a patent HIV-specific T cells licensed to Mana Therapeutics. CF reports personal fees from Cipla, Merck, Mylan, ViiV Healthcare and grants from Gilead Sciences. JG is now an employee of Gilead Sciences and reports personal fees from Gilead Sciences, Theratechnologies, Merck & Co., ViiV Therapeutics, grants from Gilead, Merck, Janssen Therapeutics, Bristol-Myers Squibb, and AbbVie. NWJ reports other support from Gilead, Janssen, ADC Therapeutics, Bayer, and CALIB-R. JDS reports serving as an advisor for Gilead Sciences. ML reports non-financial support from ArticulateScience LLC, personal fees from Amgen, Menarini, Novartis, grants from Astellas, FujiFilm, and Novartis. LL receives research support from Genentech and Merck, serves on a speaker’s bureau for Merck, serves as consultant for Kyte, and is a patent holder for WindMIL Therapeutics. KAM reports personal fees from Amplyx, Cidara, Merck, and other support from MycoMed Technologies. JBM reports DSMB fees from Incyte Corporation. SM reports personal fees from Shionogi. KP reports grants from Abbvie, Agios, Astellas, and personal fees from Abbvie, Agios, Astellas, Boston Biomedical. SS reports grants from Ansun, Astellas, Cidara, F2G, Gilead, Johnson and Johnson, Merck, Shire, Scynexis, and personal fees from Ansun and Jannssen. All other authors have nothing to disclose.
Footnotes
Data Sharing Statement
The authors will share de-identified participant data that underline the results reported in this article immediately following publication and ending 36 months following article publication with researchers who provide a methodologically sound proposal. Proposals should be directed to christinedurand@jhmi.edu. To gain access, data requestors will need to sign a a data use agreement.
Trial registration: ClinicalTrials.gov, NCT0183606
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambinder RF, Wu J, Logan B, et al. Allogeneic hematopoietic cell transplant for HIV patients with hematologic malignancies: the BMT CTN-0903/AMC-080 trial. Biology of Blood and Marrow Transplantation 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolanos-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood 2012; 120(22): 4285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biology of Blood and Marrow Transplantation 2008; 14(6): 641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasamon YL, Ambinder RF, Fuchs EJ, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Advances 2017; 1(4): 288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elmariah H, Kasamon YL, Zahurak M, et al. Haploidentical Bone Marrow Transplantation with Post-Transplant Cyclophosphamide Using Non-First-Degree Related Donors. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2018; 24(5): 1099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 2012; 37(3): 377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. The New England journal of medicine 2009; 360(7): 692–8. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019; 568(7751): 244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996; 382(6593): 722–5. [DOI] [PubMed] [Google Scholar]
- 10.Duarte RF, Salgado M, Sanchez-Ortega I, et al. CCR5 Delta32 homozygous cord blood allogeneic transplantation in a patient with HIV: a case report. Lancet HIV 2015; 2(6): e236–42. [DOI] [PubMed] [Google Scholar]
- 11.Kordelas L, Verheyen J, Beelen DW, et al. Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. The New England journal of medicine 2014; 371(9): 880–2. [DOI] [PubMed] [Google Scholar]
- 12.Holland HK, Saral R, Rossi JJ, et al. Allogeneic bone marrow transplantation, zidovudine, and human immunodeficiency virus type 1 (HIV-1) infection. Studies in a patient with non-Hodgkin lymphoma. Annals of internal medicine 1989; 111(12): 973–81. [DOI] [PubMed] [Google Scholar]
- 13.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 2010; 115(16): 3224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278(5341): 1295–300. [DOI] [PubMed] [Google Scholar]
- 15.Laird GM, Eisele EE, Rabi SA, et al. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS pathogens 2013; 9(5): e1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laird GM, Rosenbloom DI, Lai J, Siliciano RF, Siliciano JD. Measuring the Frequency of Latent HIV-1 in Resting CD4(+) T Cells Using a Limiting Dilution Coculture Assay. Methods Mol Biol 2016; 1354: 239–53. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbloom DI, Elliott O, Hill AL, Henrich TJ, Siliciano JM, Siliciano RF. Designing and Interpreting Limiting Dilution Assays: General Principles and Applications to the Latent Reservoir for Human Immunodeficiency Virus-1. Open Forum Infect Dis 2015; 2(4): ofv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbloom DIS, Bacchetti P, Stone M, et al. Assessing intra-lab precision and inter-lab repeatability of outgrowth assays of HIV-1 latent reservoir size. PLoS Comput Biol 2019; 15(4): e1006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2008; 14(6): 641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimoni A, Hardan I, Shem-Tov N, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia 2007; 21(10): 2109–16. [DOI] [PubMed] [Google Scholar]
- 21.Kanakry CG, O'Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014; 32(31): 3497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood 2015; 125(19): 3024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs EJ. Haploidentical transplantation for hematologic malignancies: where do we stand? Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program 2012; 2012: 230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2010; 16(4): 482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh N, Karmali R, Rocha V, et al. Reduced-Intensity Transplantation for Lymphomas Using Haploidentical Related Donors Versus HLA-Matched Sibling Donors: A Center for International Blood and Marrow Transplant Research Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston C, Harrington R, Jain R, Schiffer J, Kiem HP, Woolfrey A. Safety and Efficacy of Combination Antiretroviral Therapy in Human Immunodeficiency Virus-Infected Adults Undergoing Autologous or Allogeneic Hematopoietic Cell Transplantation for Hematologic Malignancies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2016; 22(1): 149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salgado M, Kwon M, Gálvez C, et al. Mechanisms That Contribute to a Profound Reduction of the HIV-1 Reservoir After Allogeneic Stem Cell Transplant. Annals of internal medicine 2018. [DOI] [PubMed] [Google Scholar]
- 28.Henrich TJ, Hu Z, Li JZ, et al. Long-Term Reduction in Peripheral Blood HIV Type 1 Reservoirs Following Reduced-Intensity Conditioning Allogeneic Stem Cell Transplantation. The Journal of infectious diseases 2013; 207(11): 1694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill AL, Rosenbloom DI, Goldstein E, et al. Real-Time Predictions of Reservoir Size and Rebound Time during Antiretroviral Therapy Interruption Trials for HIV. PLoS Pathog 2016; 12(4): e1005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med 2014; 161(5): 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugarman J, Lewin SR, Henrich TJ, Rasmussen TA. Ethics of ART interruption after stem-cell transplantation. Lancet HIV 2016; 3(1): e8–e10. [DOI] [PubMed] [Google Scholar]
- 32.Patel S, Lam S, Cruz CR, et al. Functionally Active HIV-Specific T Cells that Target Gag and Nef Can Be Expanded from Virus-Naive Donors and Target a Range of Viral Epitopes: Implications for a Cure Strategy after Allogeneic Hematopoietic Stem Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2016; 22(3): 536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta K, Im A, Rahman F, Wang H, Veldkamp P. Epidemiology and Outcomes of Hematopoietic Stem Cell Transplantation in Human Immunodeficiency Virus-Positive Patients From 1998 to 2012: A Nationwide Analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2018; 67(1): 128–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.