Abstract
2-hydroxypropyl-β-cyclodextrin (HPβCD), a cholesterol chelator, is being used to treat diseases associated with abnormal cholesterol metabolism such as Niemann-Pick C1 (NPC1) However, the high doses of HPβCD needed to slow disease progression may cause hearing loss. Previous studies in mice have suggested that HPβCD ototoxicity results from selective outer hair cells (OHC) damage. However, it is unclear if HPβCD causes the same type of damage or is more or less toxic to other species such as rats, which are widely used in toxicity research.To address these issues, rats were given a subcutaneous injection of HPβCD between 500 and 4000 mg/kg. Distortion product otoacoustic emissions (DPOAE), the cochlear summating potential (SP) and compound action potential (CAP) were used to assess cochlear function followed by quantitative analysis of OHC and inner hair cell (IHC) loss. The 3000 and 4000 mg/kg doses abolished DPOAE and greatly reduced SP and CAP amplitudes. These functional deficits were associated with nearly complete loss of OHC as well as ~80% IHC loss over the basal two-thirds of the cochlea. The 2000 mg/kg dose abolished DPOAE and significantly reduced SP and CAP amplitudes at the high frequencies. These deficits were linked to OHC and IHC losses in the high-frequency region of the cochlea. Little or no damage occurred with 500 or 1000 mg/kg of HPβCD. The HPβCD-induced functional and structural deficits in rats occurred suddenly, involved damage to both IHC and OHC and was more severe than that reported in mice.
Keywords: distortion product otoacoustic emissions, inner hair cells, cyclodextrin, compound action potential, summating potential, outer hair cells, ototoxic
1.0. Introduction
Most of the drugs and chemicals known to cause hearing loss and damage to the sensory hair cells, neurons and support cells in the cochlea, have been largely identified and extensively studied over the past 100 years (Campbell and Le Prell, 2018, Manna et al., 2019, Prakash Krishnan Muthaiah et al., 2017, Roth and Salvi, 2016, Rybak, 1986). Nevertheless, considerable research continues to be conducted on these ototoxic compounds to elucidate the mechanisms by which they induce their toxic effects and to develop new methods to mitigate their ototoxic effects (Benkafadar et al., 2017, Kim et al., 2011, Kim et al., 2008, Majumder et al., 2015, Youn et al., 2015). Each year, however, thousands of new compounds are developed (Binetti et al., 2008, Schweitzer and Lu, 2018), a small percentage of which are discovered to be ototoxic depending on the dose, route of administration, or other factors such as genetics (Nguyen and Jeyakumar, 2019, Ross et al., 2009).
Cyclodextrins, a family of cyclic oligomers of glucose, are polar molecules with a hydrophilic surface and a hydrophobic core with the unique ability to enclose small hydrophobic molecules within the pore. Cyclodextrins have been used for decades in the food, pharmaceutical and cosmetic industries (Bertolla et al., 2008, Crini, 2014, Duan et al., 2013, Li and Loh, 2008, Otero-Espinar et al., 2010, Raileanu et al., 2013, Young et al., 2012) to stabilize and enhance drug delivery (di Cagno et al., 2014, Gu et al., 2005) or to enhance the solubility and stability of food (Fenyvesi et al., 2016, Gomes et al., 2014, Samperio et al., 2010). Prior testing has suggested that cyclodextrins pose little or no health risk when administered at low or moderate doses (Gould and Scott, 2005, Marttin et al., 1998, Toyoda et al., 1997, Waalkens-Berendsen and Bar, 2004, Waalkens-Berendsen et al., 1998).
Because cyclodextrins are capable of extracting cholesterol from cell membranes (Sanchez et al., 2011, Zidovetzki and Levitan, 2007), they are being evaluated as potential treatments for diseases linked to abnormal cholesterol metabolism and lipid storage such as atherosclerosis, Alzheimer’s, Krabbe and Niemann-Pick type C disease (Camilleri et al., 1994, Coisne et al., 2016, Davidson et al., 2009, Katabuchi et al., 2018, Kim et al., 2020, Ory et al., 2017, Patterson and Walkley, 2017, Quitschke et al., 2013, Wang et al., 2019). 2-hydroxypropyl-β-cyclodextrin (HPβCD), a cyclic oligosaccharide composed of seven glucose subunits, is currently being used to treat Niemann-Pick Type C1 (NPC1) disease, an early childhood, fatal neurodegenerative disorder caused by a sphingomyelinase defect that disrupts cholesterol trafficking in the brain, liver, spleen and peripheral nervous system (Berry-Kravis et al., 2018, King et al., 2014, Matsuo et al., 2013, Ory et al., 2017, Patterson and Walkley, 2017) leading to psychiatric, pulmonary, digestive and neurological disorders (Altmann et al., 2004, Berry-Kravis et al., 2018, Bonnot et al., 2019, Dike et al., 2019, Patterson et al., 2013). While HPβCD and other cyclodextrins slow the progression of NPC1, the high dose needed to delay disease progression often causes hearing loss, which spreads from high to low frequencies with increased dose or treatment duration (Berry-Kravis et al., 2018, King et al., 2014, Maarup et al., 2015, Ory et al., 2017).
Similar to humans, when cats and mice with Niemann-Pick Type C disease are treated with subcutaneous or intrathecal injections of HPβCD, they live longer and display less neurodegeneration (Liu et al., 2009, Ramirez et al., 2010, Vite et al., 2015, Ward et al., 2010). However, the high doses of cyclodextrins needed to ameliorate NPC1 generally lead to high-frequency hearing loss. Studies of ototoxicity in cats and mice indicate that HPβCD mainly damages the outer hair cells (OHC) while sparing the inner hair cells (IHC) and spiral ganglion neurons (SGN) (Cronin et al., 2015, Crumling et al., 2017, Crumling et al., 2012, Takahashi et al., 2016, Ward et al., 2010, Zhou et al., 2018). Because the electromotile OHC provide the auditory system with its exquisite sensitivity and sharp tuning, the selective destruction of OHC by HPBCD results in a significant hearing loss (Liberman et al., 2002, Ryan and Dallos, 1975). Since HPβCD is capable of removing cholesterol from other cell types, it is conceivably that it could disrupt cholesterol homeostasis in other sensory, neural or support cells in the cochlea. To test this hypothesis, we treated rat adults with various doses of HPβCD in order to develop a comprehensive dose-response function for a species widely used for toxicity testing. The rats were allowed to survive for at least eight weeks to provide sufficient time for the full range of functional deficits and cochlear histopathologies to develop. To fully characterize the pathophysiological changes, we recorded the distortion production otoacoustic emissions (DPOAE) in order to assess the functional status of the OHC. The summating potential (SP) was recorded to evaluate the functional status of the IHC, and the compound action potential (CAP) was measured to assess the neural output of the cochlea. Afterwards, we quantified the amount of OHC and IHC damage along the entire length of the cochlea in order to relate the hair cell lesions to the functional deficits.
2.0. Materials and methods
2.1. Subjects:
Forty-eight Sprague-Dawley rats, 2–5 months of age and weighing between 250–400 g (Charles River) were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee (HER05080Y) at the University at Buffalo in accordance with NIH guidelines.
2.2. DPOAE:
Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) and then placed on a heating pad during the measurements. DPOAE were measured with an Extended-Bandwidth Acoustic Probe System (ER10X, Etymotic Research, Elk Grove Village, IL) that included a probe assembly with two loudspeakers and a low-noise microphone. Custom software (MatLab 6.1) was used to generate the f1 and f2 stimuli and record the acoustic signals in the ear canal. The system was calibrated with a ½″ microphone (model 2540, Larson Davis), a microphone preamplifier (Model 2221, Larson Davis) and custom sound level calibration software (MatLab 6.1). The probe assembly was carefully inserted into the right ear canal of rat. The intensities of f1 and f2 were calibrated in the ear canal using the probe microphone. The f2/f1 ratio was set to 1.2 and the intensity of L2 was 10 dB lower than L1. F1 and f2 were digitally generated stimuli (192 kHz sampling rate, 24-bit D/A converter). Each stimulus was 90 ms in duration, presented at the rate of 5 Hz and repeated 32 times. During stimuli presentation, the acoustic signal in the ear canal was measured with the low-noise microphone, the output digitized with a sound card (RME Babyface Pro, 192 kHz sampling rate, 24 bit A/D converter) and the amplitudes of f1, f2 and 2f1-f2 computed using a fast Fourier transform (FFT). DPOAE input/output functions were constructed at f2 frequencies of 2, 4, 6, 8, 12, 16, 20, 24, 30, 35, 40 and 45 kHz by plotting the amplitude of 2f1-f2 as function of L2 intensity at each f2 frequency. L2 intensity was varied from 15 to 70 dB SPL in 5 dB SPL steps. DPOAEs were recorded before and then two days and eight weeks after HPβCD treatment.
2.3. CAP and SP:
Our procedures for recording the CAP and SP have been described in previous publications (Liu et al., 2020, Sheppard et al., 2017, Wang et al., 2016). Tone bursts (5 ms duration, 1 ms rise/fall time, cosine2-gated, 2 to 60 kHz) were generated by using Tucker-Davis Technologies hardware and software (TDT RX6 Multifunction Processor, 200 kHz sampling rate) (Sheppard et al., 2017). The tone burst stimuli were amplified and sent to a half-inch condenser microphone (ACO ½″ microphone driven in reverse) housed in speculum-like assembly that was inserted into the ear canal. The transducer was calibrated in a cavity approximating the volume of the ear canal using a ½″ microphone (model 2540, Larson Davis) and microphone preamplifier (Model 2221, Larson Davis) and the output sent to an A/D converter (RME Babyface Pro, 192 kHz sampling rate, 24 bits) connected to a personal computer. Sound levels were calculated using custom MatLab software.
Rats were anesthetized with a ketamine/xylazine cocktail (50 mg/kg, i.m./6 mg/kg, i.m.) and then placed in a custom head-holder. Body temperature was maintained at 37 °C using a temperature controlled heating blanket (Harvard Apparatus). The right cochlea was surgically opened from a ventral-lateral approach and a recording electrode (0.25 mm diameter) made of Teflon-coated gold wire (76.2 μm diameter, Cat# 751000, A-M Systems Inc.) was placed on the round window membrane. A silver-chloride ground electrode was placed in the neck muscle. The electrical signal from the round window electrode was amplified (1000X, DAM-50 amplifier, WPI), filtered (0.1 Hz - 10 kHz) and delivered to an A/D converter (100 kHz sampling rate, TDT RX6 Multifunction Processor). The signal from the round window electrode was averaged (100X) using custom data acquisition and digital filtering software (MATLAB 6.1) with filter settings optimized to exclude the cochlear microphonic potential and isolate the CAP and SP (Chen et al., 2010, Sheppard et al., 2017, Wang et al., 2016). CAP amplitude was defined as the voltage difference between the first negative peak (N1) and following positive peak (P1). The amplitude of the positive SP was measured from the baseline to its peak 5 ms after stimulus onset. The CAP and SP amplitudes were plotted as a function of stimulus intensity level to construct a CAP and SP input/output functions for each frequency. CAP threshold was defined as the sound intensity that induced a 2.5 μV CAP amplitude. CAP threshold was plotted as a function of frequency to construct a CAP-threshold audiogram.
2.4. Cochleograms:
Cochleograms showing the percentage of missing OHC and IHC were prepared following procedures described in previous publications (Ding et al., 2001, Fu et al., 2012, Sheppard et al., 2017). After completing the CAP and SP measurements, the anesthetized rats (ketamine: 50 mg/kg, i.p.; xylazine: 6 mg/kg, i.p) were decapitated and the temporal bones carefully removed. The middle ear space was opened and the cochleae immersed in 10% formalin in phosphate buffered saline (PBS) for 24 h. Afterwards, the cochleae were decalcified (Decal, Baxter Scientific Immunoreactivity Products) for 48 h and rinsed with 0.1 M phosphate buffered saline (PBS). The entire basilar membrane was carefully dissected out with the aid of a dissection microscope and stained with Harris’ hematoxylin solution. The samples were mounted as flat surface preparations in glycerin on glass slides, cover slipped and examined under a light microscope (400X). The numbers of missing IHC and OHC were evaluated in 0.24 mm intervals along the entire basilar membrane. Cell counts were entered into a custom computer program to generate a cochleogram showing the percentage of (Ding et al., 2012, Sheppard et al., 2017, Yu et al., 2011) missing IHC and OHC as a function of percent total distance from the apex of the cochlea based on laboratory norms of cell density obtained from young normal rats. Percent distance from the apex was related to frequency using a rat frequency-place map (Müller, 1991). Averaged cochleograms were computed for each treatment group as described previously (Ding et al., 2016, Ding et al., 2001, Zhang et al., 2018).
2.4. Statistics:
Because of the large number of potential statistical comparisons, we adopted a simplified, visual approach in which the 95% confidence interval (GraphPad Prism, 5.01 software) was plotted around pre-treatment measurements or control group measures; this allow readers to judge the magnitude and consistency of HPβCD effects.
3.0. Results
3.1. HPβCD dose-dependent loss of DPOAE:
DPOAE, generated by the electromotile response of the OHC, provide a non-invasive method for monitoring the functional integrity of the OHC (Salvi et al., 2016, Trautwein et al., 1996). We measured DPOAE input/output functions over a broad range of frequencies before and after treatment with various doses of HPβCD to determine which doses and frequencies were affected and the time courses of the changes. Figure 1A–F shows the mean (n=8) DPOAE input/output functions measured at 4, 8, 16, 24, 30 and 45 kHz before and two days and eight weeks after administering 4000 mg/kg of HPβCD. The shaded area around the pre-treatment DPOAE input/output function represents the 95% confidence interval. The vertical bars around the data obtained two days and eight weeks post treatment show the standard error of the mean (SEM). The pre-treatment input/output functions consist of a low-intensity, horizontal plateau followed by a monotonically increasing component. The sound pressure level at which the DPOAE amplitude starts to rise above the horizontal plateau represents the approximate DPOAE threshold, denoted by the down arrow in each panel. DPOAE thresholds at 4, 8, 16, 24, 30 and 45 kHz were approximately 35, 25, 25, 25, 35 and 40 dB SPL respectively. DPOAE amplitudes increased with L2 intensity. The maximum DPOAE levels ranged from approximately 32 dB SPL at 45 kHz to roughly 45 dB SPL at 8, 16 and 24 kHz. DPOAE input/output functions two days and eight weeks after 4000 mg/kg HPβCD consisted of flat horizontal lines at all frequencies (Fig. 1A–F). These results show that a single dose of 4000 mg/kg HPβCD abolished DPOAE at all frequencies. This elimination of DPOAE after 4000 mg/kg HPβCD occurred very rapidly, two days post exposure, and there was no sign of recovery at eight weeks post exposure. The 3000 mg/kg dose of HPβCD (Fig. 2A–F) had nearly the same effect on DPOAE as 4000 mg/kg. Pre-treatment DPOAE input/output functions showed the normal monotonic amplitude growth for L2 levels above threshold whereas the DPOAE input/output functions at two days and eight weeks post exposure were essentially horizontal lines. Thus, the 3000 mg/kg dose completely abolished DPOAE at all frequencies by two days post treatment and there was no recovery of the DPOAE response at eight weeks post treatment.
Figure 1:
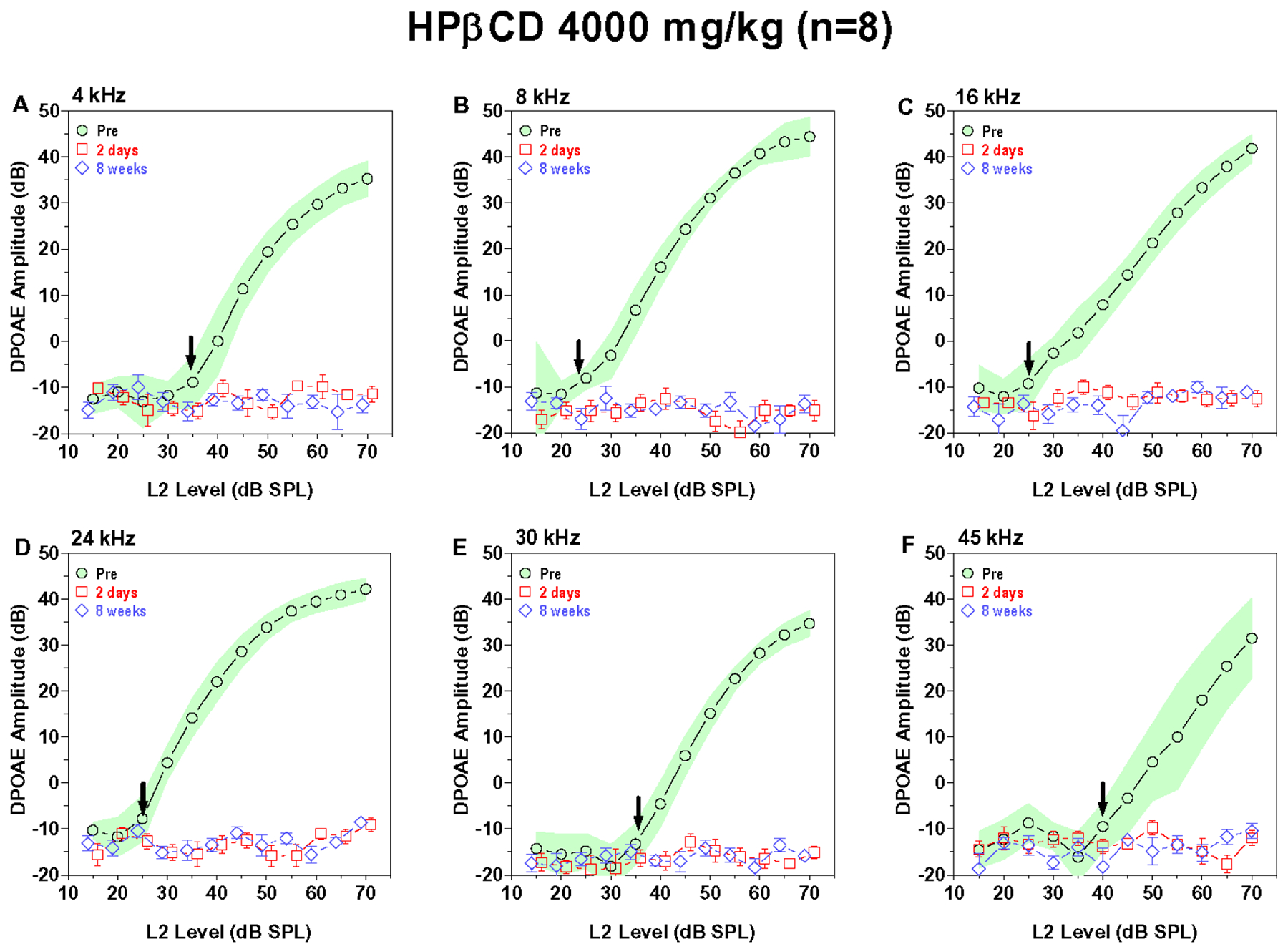
4000 mg/kg of HPBCD abolishes DPOAE responses across all frequencies. Mean (n=8) DPOAE input/output function obtained pre-treatment (shaded area: 95% confidence interval) and two days (+/−SEM) and eight weeks (+/− SEM) post treatment. Down arrow denotes approximate pre-treatment DPOAE threshold at each frequency. DPOAE input/output functions shown at f2 frequencies of (A) 4 kHz, (B) 8 kHz, (C) 16 kHz, (D) 24 kHz, (E) 30 kHz and (F) 45 kHz. DPOAE responses absent two days and eight weeks post treatment.
Figure 2:
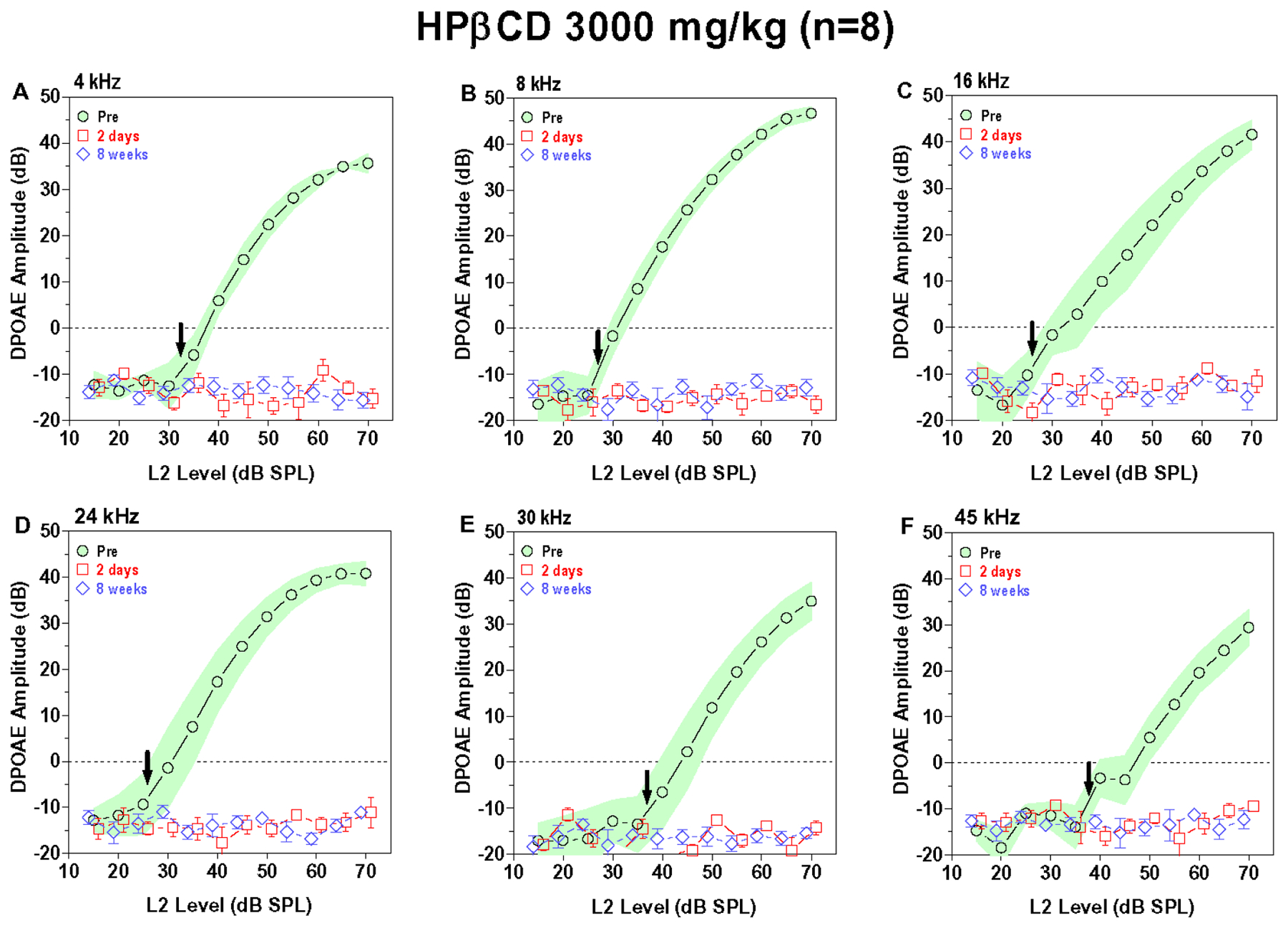
3000 mg/kg of HPβCD abolishes DPOAE responses across all frequencies. Mean (n=8) DPOAE input/output function obtained pre-treatment (shaded area: 95% confidence interval) and two days (+/−SEM) and eight weeks (+/− SEM) post treatment. Down arrow denotes approximate pre-treatment DPOAE threshold at each frequency. DPOAE input/output functions shown at f2 frequencies of (A) 4 kHz, (B) 8 kHz, (C) 16 kHz, (D) 24 kHz, (E) 30 kHz and (F) 45 kHz. DPOAE responses absent two days and eight weeks post treatment.
Lowering the dose of HPβCD to 2000 mg/kg (Fig. 3A–F) greatly reduced its toxicity. DPOAE amplitudes at 4 kHz initially decreased two days post treatment, but the input/output function completely recovered by eight weeks post treatment (Fig. 3A). DPOAE amplitudes at 8 and 16 kHz also decreased below pre-treatment values at two days post exposure. The 8 kHz and 16 kHz input/output functions had largely recovered by eight weeks post-exposure; however, some values were at the lower edge or below the 95% confidence interval (Fig. 3B–C). DPOAE amplitudes at 24 and 30 kHz were greatly reduced at two days post treatment. Both input/output functions partially recovered by eight weeks post exposure, but the amplitudes were still well below the 95% confidence interval. In contrast, DPOAE responses at 45 kHz completely absent (Fig. 3F).
Figure 3:
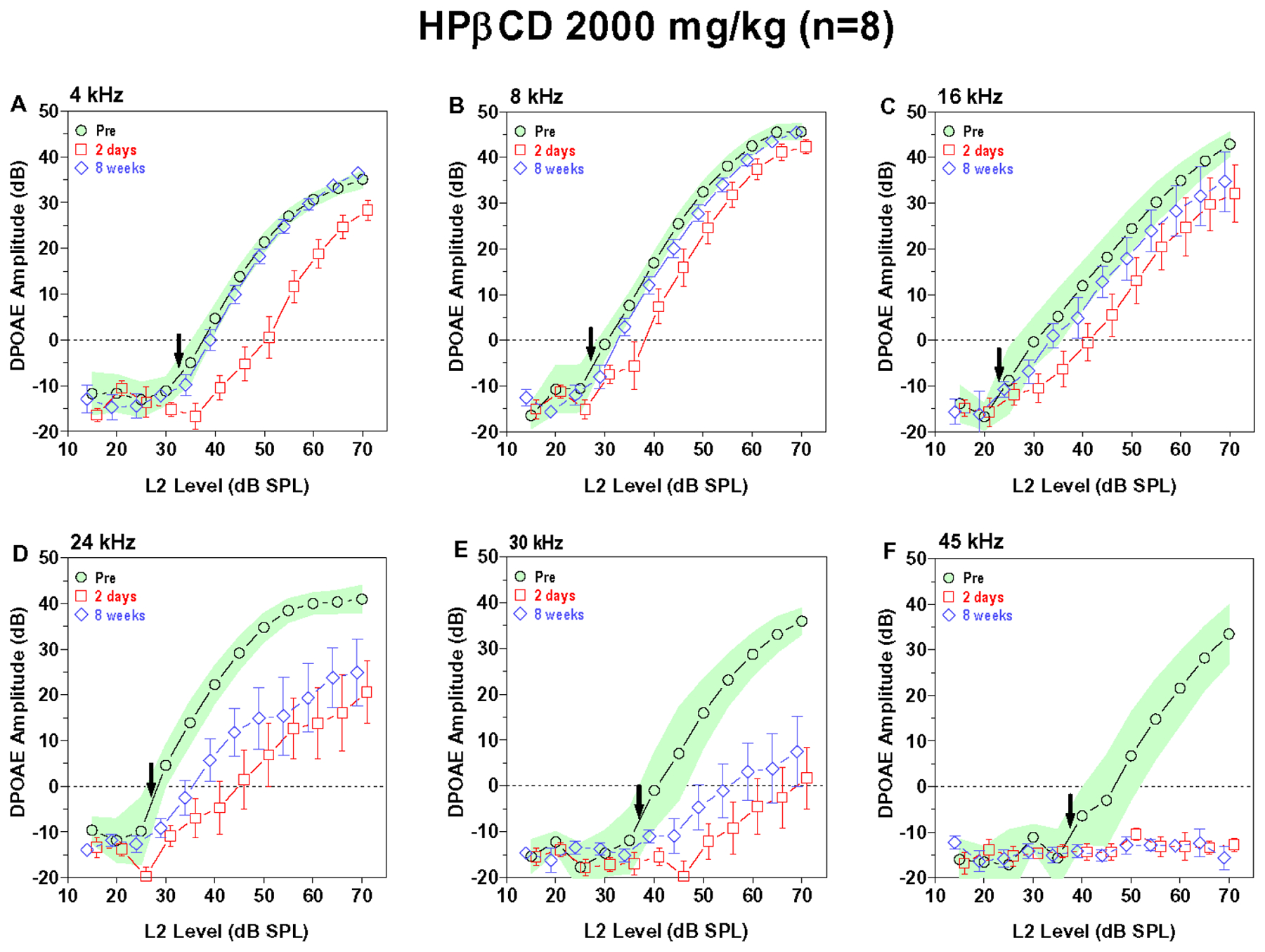
The 2000 mg/kg dose of HPβCD reduced or abolished high-frequency DPOAE. Mean (n=8) DPOAE input/output function obtained pre-treatment (shaded area: 95% confidence interval) and two days (+/−SEM) and eight weeks (+/− SEM) post treatment. Down arrow denotes approximate pre-treatment DPOAE threshold at each frequency. DPOAE input/output functions shown at f2 frequencies of (A) 4 kHz, (B) 8 kHz, (C) 16 kHz, (D) 24 kHz, (E) 30 kHz and (F) 45 kHz. (A) 4 kHz DPOAE amplitudes reduced two days post treatment; however, input/output function fully recovered by eight weeks post treatment. (B-C) 8 kHz and 16 kHz DPOAE amplitudes moderately reduced at two days post treatment; DPOAE amplitude nearly fully recovered by eight weeks post treatment. (D-E) DPOAE amplitudes greatly reduced two days post treatment and only partially recovered by eight weeks post exposure. (F) 45 kHz DPOAE abolished at two days and eight weeks post treatment.
The 1000 mg/kg dose of HPβCD had relatively little effect on DPOAE input/output functions (Figure 4A–F). There was a slight reduction DPOAE amplitudes at 45 kHz two days and eight weeks post treatment (Figure 4F) and a slight reduction in DPOAE amplitudes at 4 kHz two days post treatment. The 500 mg/kg dose of HPβCD caused also caused a slight reduction in DPOAE amplitudes at 45 kHz and 30 kHz at two days and 8-week post-treatment time, but the magnitude of these reductions were fairly negligible (Fig. 5A–F). Taken together, these results indicate that a dose of HPβCD equal to or greater than 3000 mg/kg abolishes DPOAE at all frequencies by two days post treatment whereas a dose of 1000 mg/kg or less had little or no effect. The 2000 mg/kg dose revealed important temporal- and frequency-dependent changes. The decrease in DPOAE amplitude began at the high frequencies and spread to the low frequencies. Doses of HPβCD that initially suppressed DPOAE amplitudes to a mild degree at two days post treatment largely recovered by eight weeks post treatment whereas doses that suppress DPOAE to a moderate degree only partially recovered.
Figure 4:
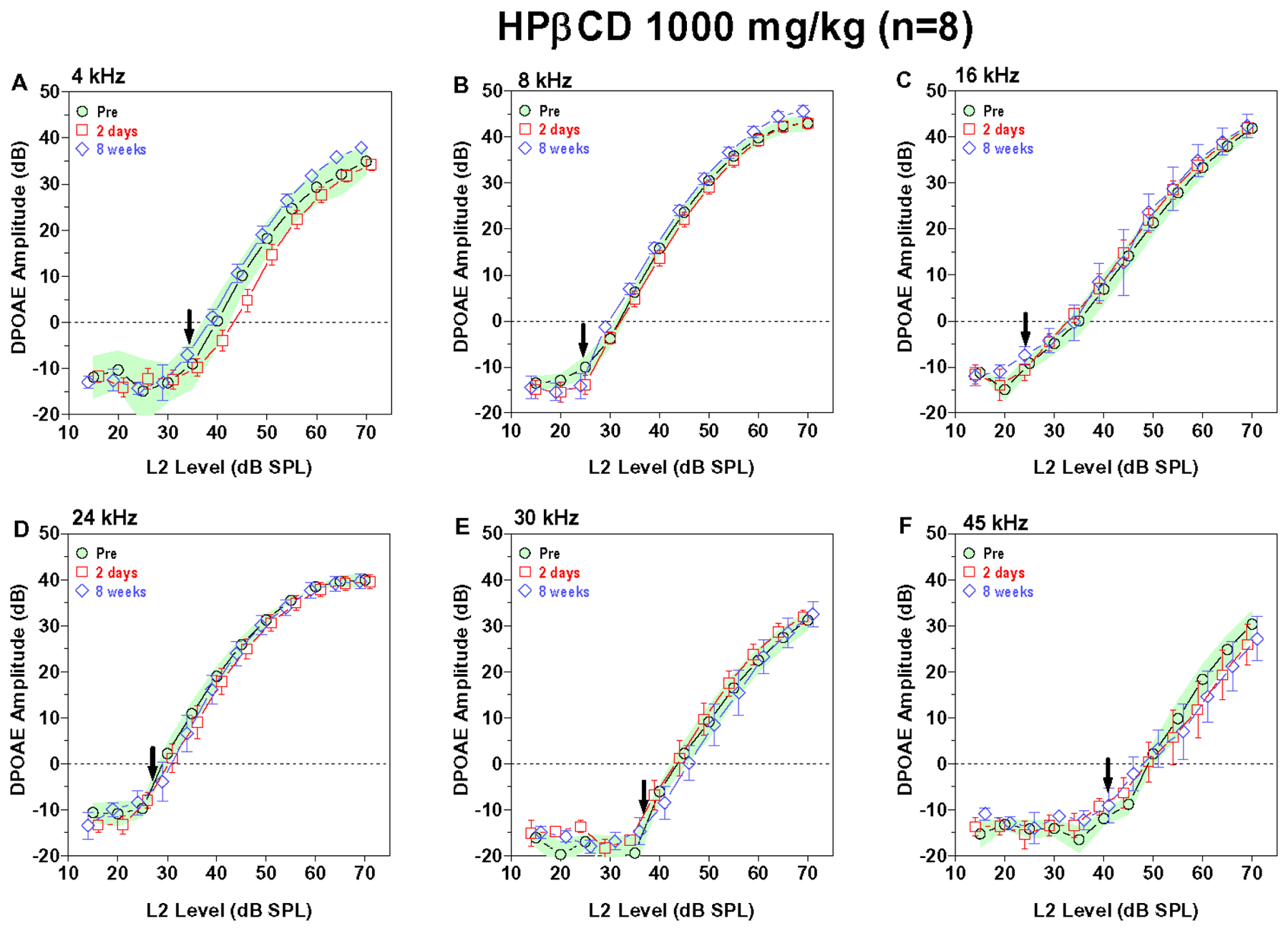
1000 mg/kg HPβCD caused a slight reduction in DPOAE amplitudes at 45 kHz. Mean (n=8) DPOAE input/output function obtained pre-treatment (shaded area: 95% confidence interval) and two days (+/−SEM) and eight weeks (+/− SEM) post treatment. DPOAE input/output functions at f2 frequencies of (A) 4 kHz, (B) 8 kHz, (C) 16 kHz, (D) 24 kHz, (E) 30 kHz and (F) 45 kHz. (F) DPOAE amplitude at 45 kHz slightly reduced two days and eight weeks post treatment. (A-E) DPOAE amplitudes at two days and eight weeks post treatment similar to pre-treatment values at 4, 8, 16, 24 and 30 kHz except for a slight reduction at 4 kHz two days post treatment.
Figure 5:
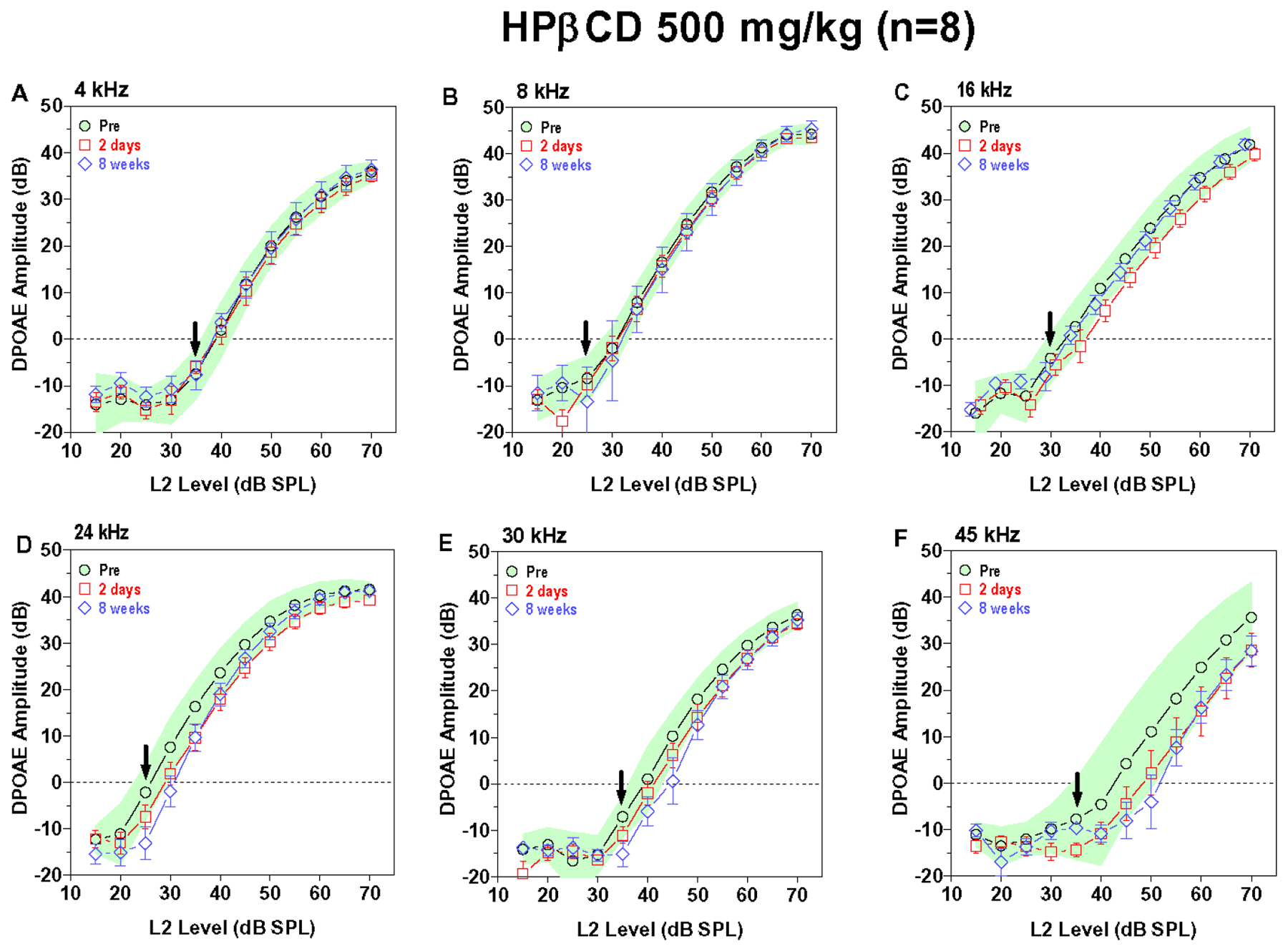
Mean (n=8) DPOAE input/output function obtained pre-treatment (shaded area: 95% confidence interval) and two days (+/−SEM) and eight weeks (+/− SEM) post treatment. DPOAE input/output functions at f2 frequencies of (A) 4 kHz, (B) 8 kHz, (C) 16 kHz, (D) 24 kHz, (E) 30 kHz and (F) 45 kHz. (E-F) DPOAE amplitudes at 24, 30 and 45 kHz within or slighly below the 95% pre-treatment confidence interval at two days and eight weeks post-treatment. (A-C) DPOAE amplitudes at 4, 8 and 16 kHz similar to pre-treatment values at two days and eight weeks post-treatment.
3.3. HPβCD dose-dependently increases CAP threshold and reduces CAP amplitudes:
The CAP represents the neural output of the cochlea relayed from the IHC through the afferent synapse to the auditory nerve fibers that project to auditory brainstem. Figure 6 shows the mean CAP thresholds (+/− 95% confidence interval, n=8) as function of frequency for the control group along with the mean CAP (+/−SEM, n=8) thresholds in the groups treated with 500, 1000, 2000, 3000 and 4000 mg/kg HPβCD at eight weeks post treatment. The CAP thresholds in the group treated with 500 mg/kg HPβCD were nearly identical to those of the control group; there was no change in sensitivity at the lowest dose of HPβCD. However, CAP thresholds in the 1000 mg/kg HPβCD group were generally 5–10 dB higher than the mean of the control group and above the 95% confidence interval at frequencies above 32 kHz. The CAP thresholds in the 2000 mg/kg group were substantially higher than the mean of the control group from 16 to 65 kHz; the thresholds in this frequency range were well above the 95% confidence interval. The thresholds in the 2000 mg/kg group were as much at 56 dB higher than the control group at 40 kHz. When the dose of HPβCD was increased to 3000 mg/kg, the mean CAP thresholds increased precipitously at the lower frequencies. Threshold ranged from 70 to 80 dB SPL from 2 to 65 kHz resulting in a flat hearing loss affecting all frequencies. When the dose of HPβCD was increased to 4000 mg/kg, CAP thresholds edged up slightly to 75 to 80 dB SPL (upper limit of acoustic system). Based on these results, it appears that critical dose of HPβCD just sufficient to cause a noticeable hearing impairment at the high frequencies was 1000 mg/kg. Doubling the dose to 2000 mg/kg resulted in high-frequency hearing impairments that extends down to 16 kHz, the most sensitive region in the rat audiogram (Kelly and Masterton, 1977). Increasing the dose of HPβCD to 3000 or 4000 mg/kg resulted in a massive hearing impairment affecting all frequencies.
Figure 6:
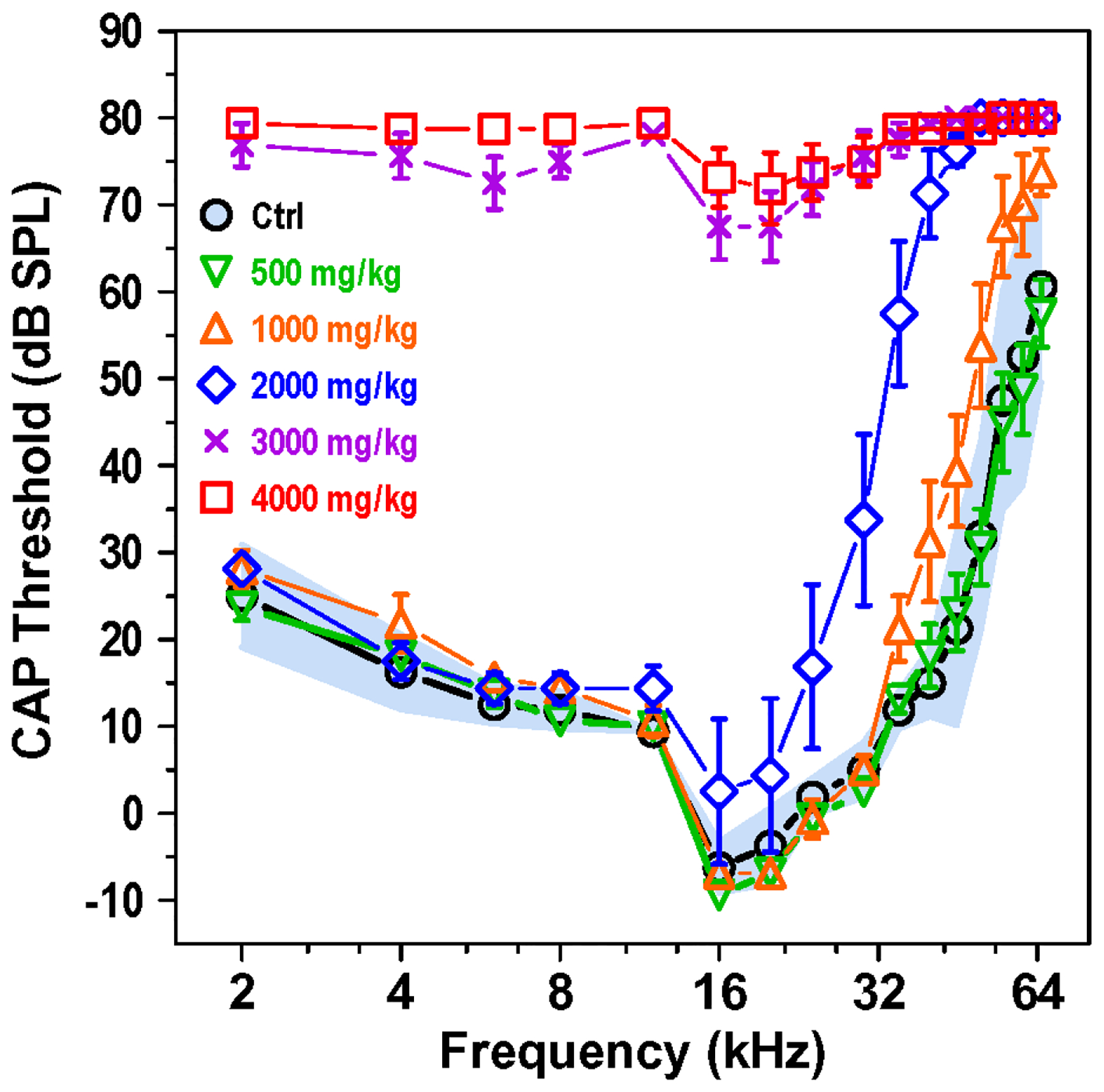
Compound action potential (CAP) thresholds rise with increasing HPβCD dose. Mean CAP thresholds for control group (Ctrl, n=8, shaded area: 95% confidence interval) from 2 to 65 kHz. Mean CAP thresholds (+/−SEM) measured eight weeks post treatment for experimental groups treated with 500, 1000, 2000, 3000 or 4000 mg/kg HPβCD. The 1000 mg/g dose of HPβCD caused a slight increase in CAP thresholds above 32 kHz. CAP thresholds increased significantly and spread to lower frequencies as the dose of HPβCD increased from 1000 to 4000 mg/kg.
CAP input/output functions provide a more comprehensive view of neural activity at suprathreshold intensities. Figure 7 shows the mean CAP input/output function (n=8; 95% confidence interval) from the control group at 4, 8, 16, 24, 30 and 45 kHz along with the mean CAP input/output functions (n=8, +/−SEM) measured eight weeks following treatment with 500, 1000, 2000, 3000 or 4000 mg/kg of HPβCD. The input/output functions in the 500 mg/kg group were nearly identical to those in the control group at all frequencies. In contrast, the mean CAP amplitudes in the 1000 mg/kg group were near or slightly below the lower edge of the 95% confidence interval of the control group at 45 kHz. The post-treatment input/output function was also shifted to the right of the control 10–15 dB. However, the mean CAP amplitudes at lower frequencies were nearly identical to those in the control group.
Figure 7:
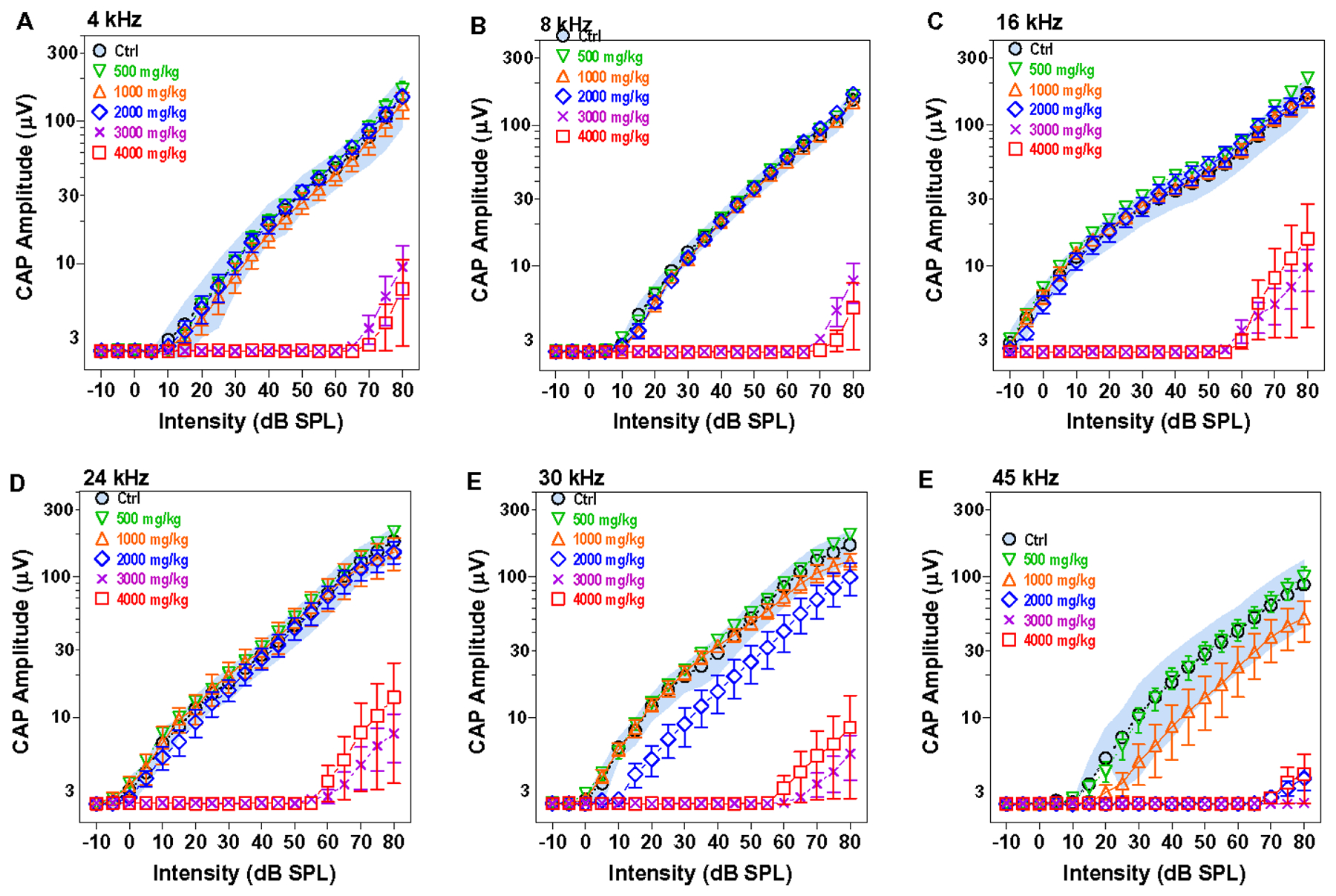
HPβCD dose-dependently suppressed CAP amplitudes starting with 1000 mg/kg. Mean (n=8, shades area 95% confidence interval) CAP input/output functions from the control group at 4, 8, 16, 24, 30 and 45 kHz compared to mean (n=8, +/−SEM) input/output function for the groups treated with 500, 1000, 2000, 3000 and 4000 HPβCD at eight weeks post treatment. CAP amplitudes at 45 kHz fall below the 95% confidence interval of the control group after treatment with 1000 mg/kg HPβCD, but CAP responses remain normal at lower frequencies. CAP amplitude reductions increase and the reductions spread to lower frequencies as the dose of HPβCD increases from 1000 to 4000 mg/kg.
When the dose of HPβCD was increased to 2000 mg/kg, the CAP amplitudes at 45 kHz were almost completely abolished. At 30 kHz, the mean CAP amplitudes were well below the 95% confidence interval of the control group and the input/output function was shifted to the right 10–15 dB. However, CAP amplitudes were nearly identical to those in the control group at 4, 8, 16 and 24 kHz.
The 3000 and 4000 mg/kg doses of HPβCD greatly reduced CAP amplitudes at all frequencies. The input/output functions at these higher doses were shifted to the right ~60–70 dB. The CAP input/output functions show a clear dose-dependent reduction in CAP amplitude starting at 1000 mg/kg. While CAP amplitudes were greatly reduced eight weeks after treatment with 3000 and 4000 mg/kg of HPβCD, weak responses could still be elicited at intensities greater than 60 or 70 dB SPL suggesting that some acoustic information was being relayed through the auditory nerve (Salvi et al., 2016, Wang et al., 1997).
3.4. HPβCD dose-dependently reduces SP amplitude:
Because the SP recorded from the round window is dominated by the IHC receptor potential generated in the high-frequency base of the cochlea (Durrant et al., 1998, Zheng et al., 1996), SP input/output functions are only shown from 16–45 kHz (Figure 8). SP amplitudes in the group treated with 500 mg/kg HPβCD were nearly identical to those in the control group at 16, 24, 30 and 45 kHz (Fig. 8A–D). SP amplitudes in the group treated with 1000 mg/kg HPβCD fell below the 95% confidence interval of controls at 45 kHz (Fig. 8D); however, the SP amplitude were essentially normal at 16, 24, and 30 kHz. When the dose of HPβCD was increased to 2000 mg/kg, SP amplitudes at 45 kHz were greatly reduced (Fig. 8D), those at 30 kHz reduced a moderate degree (Fig. 8C), whereas responses at 16 kHz and 24 kHz were normal (Fig. 8A–B). The two highest doses of HPβCD, 3000 and 4000 mg/kg caused a massive reduction in SP amplitudes at all frequencies (Fig. 8A–D). Nevertheless, a low-amplitude SP could still be evoked at intensities above 60 dB SPL. SP amplitudes were largest at 16 kHz but decreased at higher frequencies.
Figure 8:
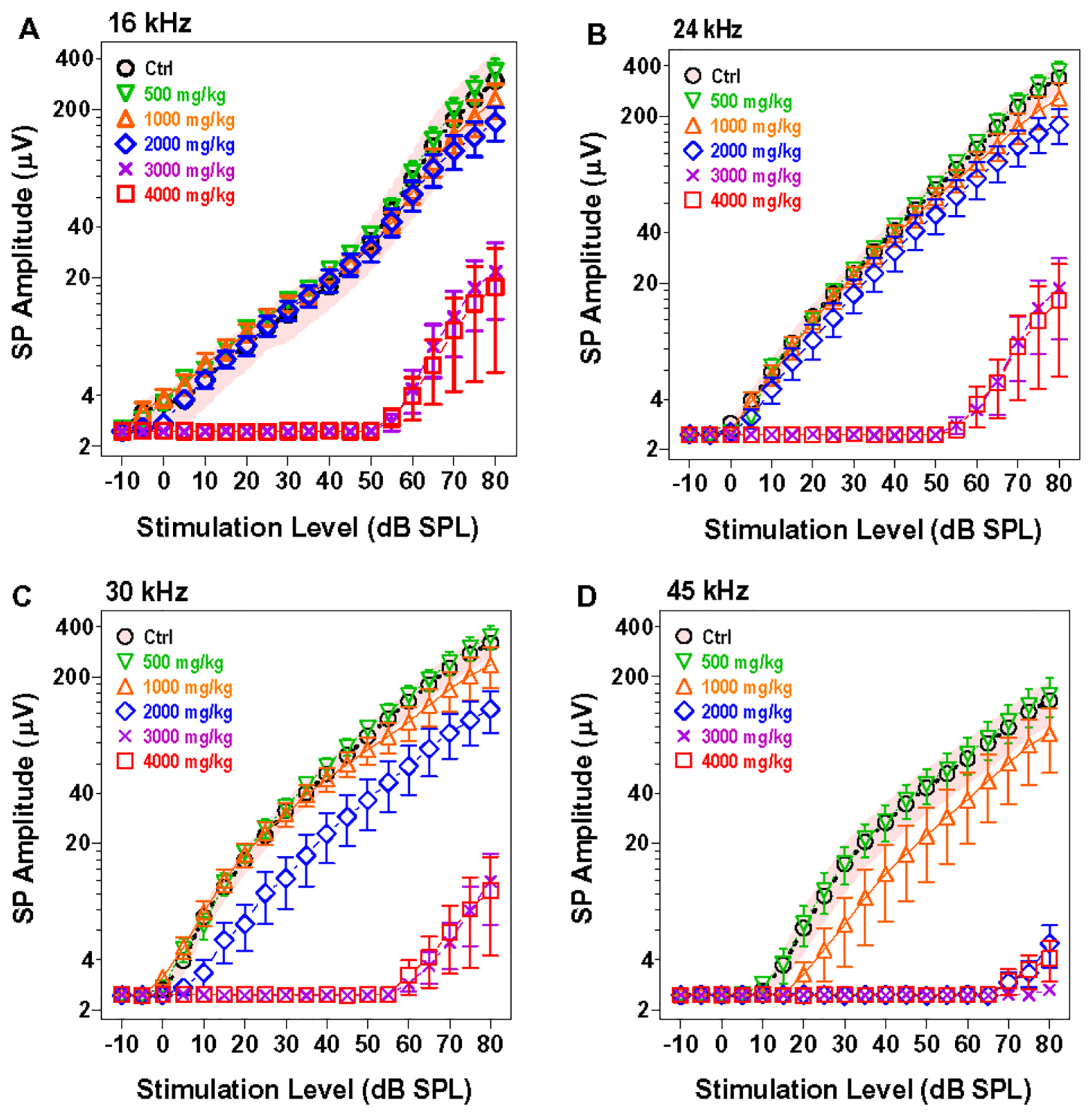
HPβCD dose dependently suppress SP amplitude. Mean (n=8, shaded area: 95% confidence interval) SP input/output function for the control group at 16, 24, 30 and 45 kHz compared to mean (n=8, +/−SEM) of groups treated with 500, 1000, 2000, 3000 and 4000 mg/kg of HPβCD. SP amplitudes decrease and SP reductions spread to lower frequencies as the dose of HPβCD increases from 1000 to 4000 mg/kg. Residual SP responses still present after treatment with 3000 and 4000 mg/kg of HPβCD.
3.4. HPβCD destroyed both IHC and OHC:
Figure 9 shows the mean cochleograms (n=6, +/−SEM) for the rats treated with 500, 1000, 2000, 3000 and 4000 mg/kg of HPβCD. The photomicrograph in Figure 10A shows a surface preparation from a rat in the control group with three orderly rows of OHC and a single row of IHC. The mean cytocochleograms in each panel of Figure 9 plots the mean percent loss of OHC and IHC as a function of percent distance from the apex of the cochlea; mean data are shown in 10% intervals. Cochlear location is related to frequency using a rat frequency-place map on the lower abscissa (Müller, 1991). There was no OHC or IHC loss in the group treated with 500 mg/kg of HPβCD (Fig. 9A) consistent with the absence of functional deficits in this group. Treatment with 1000 mg/kg of HPβCD resulted in a 21% OHC loss and 9% IHC loss at the 65 kHz location (Fig. 9B). These small OHC and IHC lesions were associated with a minor reduction in DPOAE amplitudes at 45 kHz (Fig. 4F), a small CAP threshold shift above 40 kHz (Fig. 6) and minor decreases in CAP and SP amplitudes (Fig. 7E, 8C) at 45 kHz.
Figure 9:
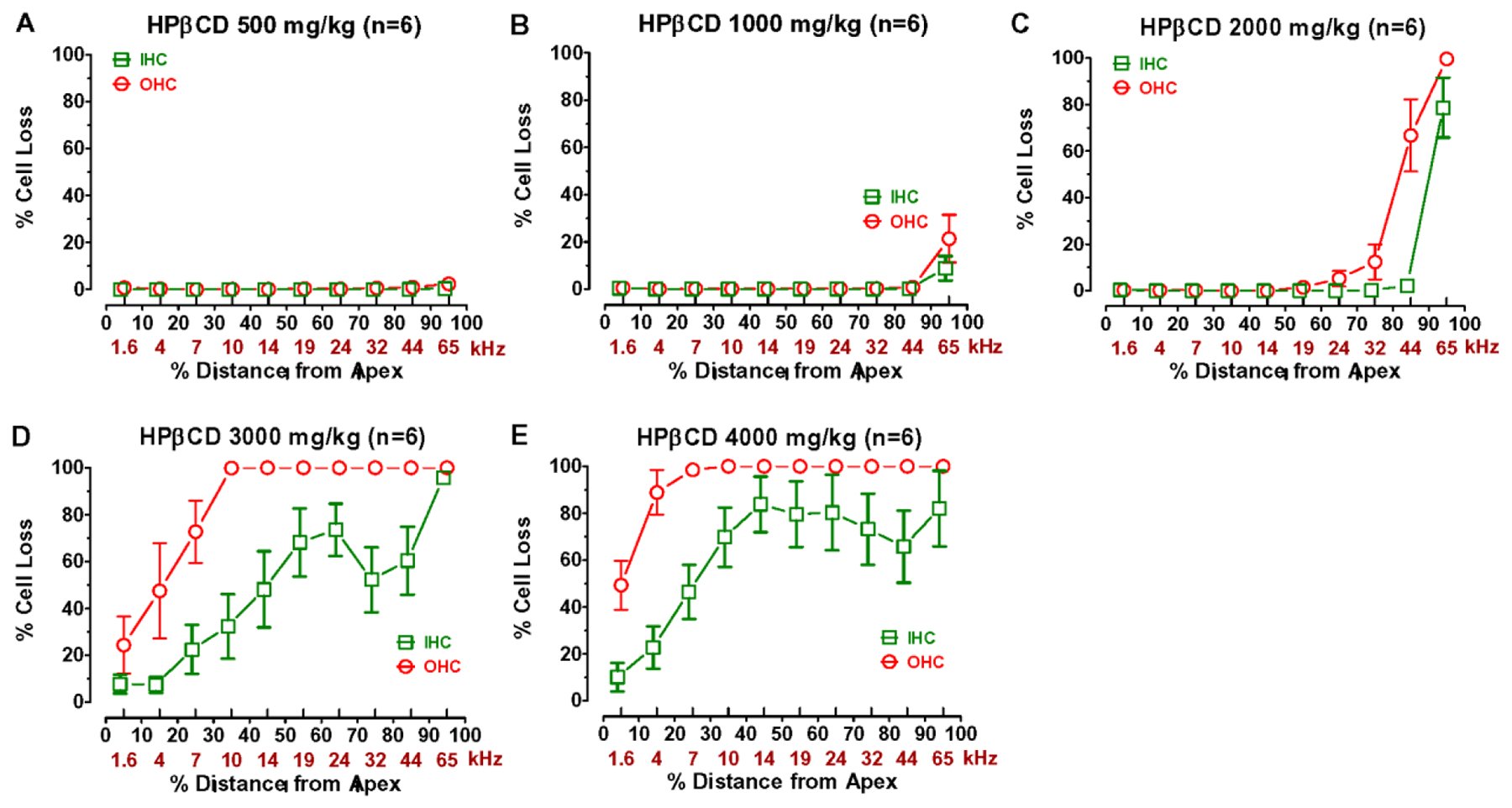
HPβCD dose dependently destroyed OHC and IHC beginning in the base and progressing towards the apex. Mean cochleograms showing the percent missing OHC and IHC as a function of percent distance from the apex of the cochlea. Cochlear location related to frequency based on rat cochlear frequency place map on lower kHz-axis as noted in Methods. Three highest doses of HPβCD destroyed IHC as well as OHC.
Figure 10:
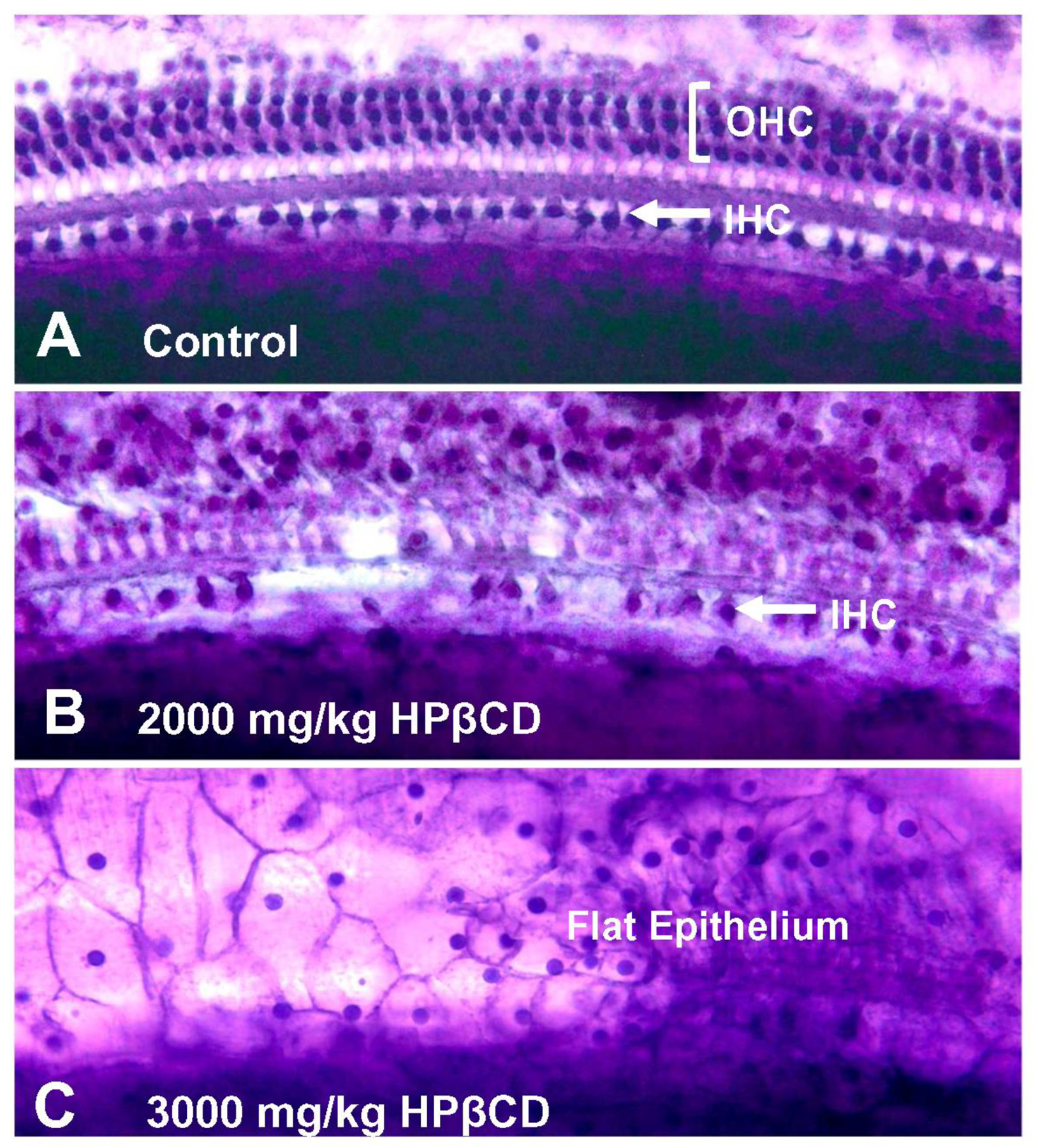
Representative photomicrographs of cochlear surface preparations stained with Harris’ hematoxylin solution. (A) Surface preparation from saline control showing nuclei in three orderly rows of outer hair cells (OHC) and a single row of inner hair cells (IHC). (B) Photomicrograph from base of cochlea of rat treated with 2000 mg/kg of HPβCD showing partial loss of IHC and absence of OHC. (C) Photomicrograph from cochlea of rat treated with 3000 mg/kg of HPβCD showing flat epithelium lined with cuboidal cell profiles and absence of any IHC or OHC.
More substantial cochlear lesions emerged following treatment with 2000 mg/kg of HPβCD (Fig. 9C). A representative photomicrograph taken from the basal turn of a rat treated with 2000 mg/kg HPβCD is shown in Figure 10B. There was an absence of OHC and missing IHC in this basal location. The mean cochleogram shows a complete absence of OHC at the 65 kHz location in the base of the cochlea; the OHC lesion decreased from 100% in the base to 5% at the 24 kHz location. The IHC lesion decreased from 79% in the base to 2% at 44 kHz region. The OHC lesion was associated with a complete loss of DPOAE at 45 kHz, a large amplitude reduction at 30 kHz, a moderate reduction at 24 kHz and a very slight reduction at 16 kHz (Fig. 3). CAP thresholds in the 2000 mg/kg group were elevated from 65 to 16 kHz while CAP amplitudes were reduced from 45 kHz to 24 kHz. The SP, which is dominated by IHC activity in the basal high-frequency region of the cochlea was greatly reduced at 45 kHz, but only reduced to a moderate degree at 30 kHz.
The cochlear lesion increased dramatically with the 3000 mg/kg dose, which destroyed virtually all the OHC and IHC in the base of the cochlea leaving behind a flat epithelium occupied by pale cells with a cuboidal shape (Figure 10C). As shown by the mean cochleogram in Figure 9D, all the OHC were missing down to the 10 kHz location after which the lesion gradually decreased to 24% at the 1.6 kHz location. Nearly all the IHC were missing in the base of the cochlea; the mean IHC loss then declined to 52% loss at the 32 kHz region, rebounded to a 75% loss at the 24 kHz location before steadily decreasing to an 8% loss at the 1.6 kHz region. The cochlear lesions in the 4000 mg/kg group were similar to the 3000 mg/kg group, but extended further towards the apex. Virtually all of the OHC were missing from the base down to the 7 kHz region after which the OHC lesion declined to 49% at the 1.6 kHz location. The IHC lesions ranged from 83% to 65% from 10–65 kHz on the cochlear frequency-place map, but then decreased to a 5% at 1.6 kHz location. The massive OHC lesions in the 3000 and 4000 mg/kg groups were associated with a complete loss of DPOAE (Fig. 1–2), very large CAP threshold shifts (Fig. 6) and large reductions in CAP (Fig. 7) and SP amplitudes (Fig. 8). However, CAP and SP responses could still be evoked at intensities above 60 dB SPL indicating that some IHC and auditory nerve were still functional.
4.0. Discussion
4.1. DPOAE abolished by massive OHC loss:
The ototoxic effects of HPβCD have been evaluated in mice and cats, but have not yet been systematically evaluated in rats, a species widely used in toxicology testing and auditory research (Mukherjea et al., 2008, Waalkens-Berendsen et al., 1998, Wu et al., 2001). Using DPOAE to assess the functional status of OHC, we found that 3000 and 4000 mg/kg of HPβCD completely and permanently abolished DPOAE at all frequencies by two days post treatment. These functional deficits were associated with a massive loss of OHC over the basal two-thirds of the cochlea eliminating the cochlear amplifier that provides the auditory system with its remarkable sensitivity and frequency selectivity (Dallos and Harris, 1978, Ryan and Dallos, 1975).
DPOAE were completely abolished at 4 kHz after treatment with 3000 mg/kg of HPβCD (Fig. 1–2); however, the mean OHC lesion associated with the 4 kHz region was only ~50%. These results suggest that even though OHC were present in this region, they may be nonfunctional because of subtle damage to the prestin motor protein in the OHC lateral wall as noted previously (Crumling et al., 2012). The OHC lateral wall is rich in cholesterol (Forge, 1991). HPβCD treatments which remove cholesterol from OHC could disrupt the function of prestin in the lateral wall and impairs electromotility.
Mice and cats treated with a single subcutaneous dose of 4000 mg/kg HPβCD failed to develop any hearing loss (Crumling et al., 2012, Ward et al., 2010); only when the dose was increased to 8000 mg/kg did significant hearing loss develop in these species. Mice treated with 8000 mg/kg of HPβCD developed significant OHC lesions. The OHC lesions only reached 100% in the basal third of the cochlea and declined to an 80% OHC loss in the middle third of the cochlea. In contrast, we observed 100% OHC loss over the basal two-thirds of the cochlea when rats were treated with only 3000 mg/kg of HPβCD. Accordingly, our results indicate that rats are more susceptible to HPβCD-induced hearing loss and OHC loss than mice or cats.
4.2. Massive IHC loss with high-dose HPβCD:
It has been reported that HPβCD only damages OHC even with doses more than 2–3 time higher than higher than those used in the current study (Cronin et al., 2015, Crumling et al., 2012, Takahashi et al., 2016, Zhou et al., 2018). We found evidence of small IHC lesions in the most basal part of the rat cochlea with HPβCD doses as low as 1000 mg/kg. The basal turn IHC reached 80% when the dose increased to 2000 mg/kg and with 4000 mg/kg, the IHC increased to 80% over the basal two-thirds of the cochlea. In contrast to studies in mice, our results clearly show that high doses of HPβCD can cause massive IHC destruction in rats (Cronin et al., 2015, Crumling et al., 2012).
The two highest doses of HPβCD resulted in CAP threshold shifts as great as 80 dB SPL in the mid frequencies. The large threshold elevation is likely due to the combined loss of OHC and IHC. The residual SP and CAP responses evoked by intensities greater than 60 dB represent the sensory and neural responses generated by the remaining IHC and auditory nerve fibers. Because the CAP and SP response at 80 dB SPL were reduced by more than 90% relative to controls (Fig. 11A–B), we speculate that the remaining IHC, support cells and auditory nerve fibers may suffer from sublethal histopathologies (Bohne et al., 2017, Kujawa and Liberman, 2015, Liberman and Kujawa, 2017).
Figure 11:
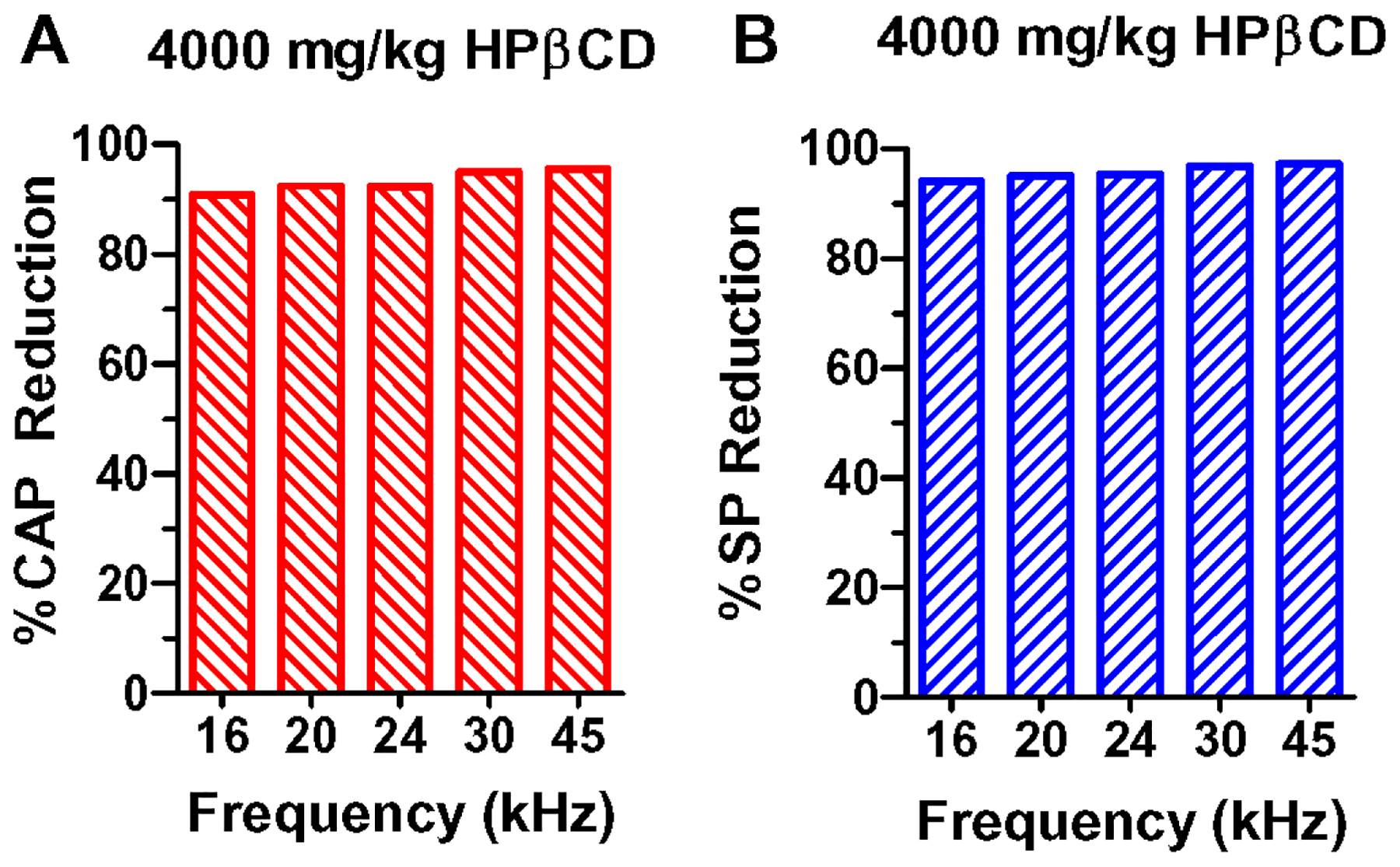
Percent reduction in (A) CAP amplitudes and (B) SP amplitudes at 80 dB SPL relative to control amplitudes at 16, 20, 24, 30 and 45 kHz. CAP and SP amplitudes reduced more than 90% of control values.
4.3. Temporal dynamics of HPβCD toxicity:
Studies in mice treated with 6000 mg/kg of HPβCD failed to reveal any damage to IHC or SGN (Cronin et al., 2015). However, the functional and structural assessments in mice were only evaluated out to 10-days post-treatment, whereas our final histological assessment was conducted eight weeks post treatment, providing additional time for the cochlear lesions to fully mature. This interpretation is supported by time-course studies of noise-induced hearing loss which revealed an extremely long period of cochlear degeneration (Bohne et al., 2017). The rate of OHC degeneration was greatest shortly after the noise exposure, but gradually slowed during the following month after the noise exposure (Bohne et al., 2017).. In contrast, IHC losses began to emerge 1–3-weeks after the noise exposure. Other support cells, with subtle histopathologies, gradually died off in the ensuing months. In places where the sensory cells and support cells degenerated after the noise exposure, the basilar membrane was covered by a flat, undifferentiated epithelium (Bohne et al., 2017). The flat, undifferentiated epithelium resulting from intense noise exposure is similar to our HPβCD results shown in Figure 10C.
4.4. Critical dose, frequency-dependent changes and recovery:
A single subcutaneous dose of 2000 mg/kg of HPβCD appears to be close to the critical level for inducing significant hearing loss and cochlear damage in the rat. Doses greater than 2000 mg/kg caused massive hair cell damage and completely abolished DPOAE, whereas lower doses had relatively little effect on functional and structural measures. The 2000 mg/kg dose revealed clear frequency-dependent changes consistent with most ototoxic drugs. The hair cell lesions and functional deficits were greatest near the high-frequency base of the cochlea and declined at lower frequencies and more apical regions of the cochlea (Forge and Schacht, 2000, Paken et al., 2019).
A clear recovery pattern was evident in the DPOAE input/output functions obtained with the 2000 mg/kg dose of HPβCD. At the low-frequencies where DPOAE were mildly suppressed two days post treatment, DPOAE had fully recovered by eight weeks, and there was no evidence OHC loss in the low-frequency region of the cochlea. These results indicate that OHC can recover from HPβCD ototoxicity if the damage is not too severe. The most likely mechanism involves re-establishing cholesterol homeostasis in OHC and other structures in the organ of Corti. On the other hand, at 24 and 30 kHz where DPOAE were depressed to a moderate degree, DPOAE only partially recovered by eight weeks post treatment. Careful inspection of the cochleograms revealed evidence of rather small OHC losses (5–12%) at these cochlear locations. These results suggest that very small OHC lesions are able to significantly reduce DPOAE. Another possibility is that the remaining OHC or support cells in these regions may be functionally impaired due to subtle histopathologies (Bohne et al., 2017). This interpretation is consistent with prior observations of abnormal prestin staining in surviving OHC from mice treated with 8000 mg/kg of HPβCD (Crumling et al., 2012). Because prestin is critical to the generation of DPOAE, a loss or disruption of this motor protein could account for the incomplete recovery of DPOAE (Liberman et al., 2002).
Limitations and future studies:
Because we did not expect HPβCD to induce IHC damage, we did not perform a detailed histological analysis of the organ of Corti or SGN. However, given the magnitude of the IHC deficits and the emergence of a flattened epithelium with undifferentiated cuboidal cells, we speculate that the 3000 and 4000 mg/kg doses of HPβCD would likely results in SGN degeneration due to the loss of trophic support from missing sensory and supporting cells (Glueckert et al., 2008, Lawner et al., 1997, McFadden et al., 2004, Miller et al., 2007). Extensive sensory and support cell damage could disrupt the tight cell junctions in the reticular lamina allowing endolymph, with its high concentration of potassium, to enter the perilymphatic spaces where it could gradually damage the remaining support cells and neurons (Bohne et al., 2017). More detailed histological studies need to be carried out to address the time course of sensory, supporting cells and neurodegeneration.
Our results illustrate the magnitude and nature of the functional and structural deficits that occur in adult male rats after a single subcutaneous dose of HPβCD. Some toxicity studies suggest that there may be gender differences and future studies should conducted to address this issue (Bellringer et al., 1995). The need for cholesterol is especially high during the development of the nervous system raising the possibility that HPβCD may be especially ototoxic during early development of the cochlea (Funfschilling et al., 2012, Tsutsui, 2012, Waalkens-Berendsen et al., 2004). Because most neurodegenerative disorders require long term treatment with HPβCD, additional studies should be performed to determine if prolonged, low-dose daily treatments or intermittent treatments with HPβCD are ototoxic. Prolonged, low-dose treatments address important toxicity issues such as drug buildup in the cochlea as well as the ability of the sensory cells to re-establish cholesterol homeostasis following HPβCD treatments.
Conclusion:
Adult rats treated with HPβCD begin to develop high-frequency cochlear hearing loss and damage to both IHC and OHC in the base of the cochlea with doses around 2000 mg/kg whereas doses of 500 and 1000 mg/kg cause little or no damage. A modest increase in the dose to 3000 mg/kg leads to a sudden, massive increase in hearing loss that results in complete loss of DPOAE and an enormous reduction in the SP and CAP. These functional deficits are associated with a nearly complete loss of OHC over most of the cochlea and roughly 80% of the IHC over the basal 70% of the cochlea. The steep dose-response function and sudden onset of HPβCD ototoxicity carries serious risk for clinical applications designed to treat life threatening diseases.
Acknowledgement:
This research was supported by NIH grant (R01DC014693)
List of Abbreviations:
- DPOAE
distortion product otoacoustic emission
- CAP
compound action potential
- HPβCD
2-hydroxypropyl-β-cyclodextrin
- IHC
inner hair cell
- NPC1
Niemann-Pick C1
- OHC
outer hair cell
- SP
summating potential
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Competing Interests:
The authors declare no competing interest related to this manuscript and research. Dr. Salvi is a consultant for Auris Medical and CilCare.
References
- Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, et al. (2004) Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science;303:1201–4. [DOI] [PubMed] [Google Scholar]
- Bellringer ME, Smith TG, Read R, Gopinath C, Olivier P (1995) beta-Cyclodextrin: 52-week toxicity studies in the rat and dog. Food and Chemical Toxicology;33:367–76. [DOI] [PubMed] [Google Scholar]
- Benkafadar N, Menardo J, Bourien J, Nouvian R, Francois F, Decaudin D, et al. (2017) Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol Med;9:7–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Chin J, Hoffmann A, Winston A, Stoner R, LaGorio L, et al. (2018) Long-Term Treatment of Niemann-Pick Type C1 Disease With Intrathecal 2-Hydroxypropyl-beta-Cyclodextrin. Pediatr Neurol;80:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolla C, Rolin S, Evrard B, Pochet L, Masereel B (2008) Synthesis and pharmacological evaluation of a new targeted drug carrier system: beta-cyclodextrin coupled to oxytocin. Bioorganic and Medicinal Chemistry Letters;18:1855–8. [DOI] [PubMed] [Google Scholar]
- Binetti R, Costamagna FM, Marcello I (2008) Exponential growth of new chemicals and evolution of information relevant to risk control. Annali dell’Istituto Superiore di Sanità;44:13–5. [PubMed] [Google Scholar]
- Bohne BA, Kimlinger M, Harding GW (2017) Time course of organ of Corti degeneration after noise exposure. Hear Res;344:158–69. [DOI] [PubMed] [Google Scholar]
- Bonnot O, Gama CS, Mengel E, Pineda M, Vanier MT, Watson L, et al. (2019) Psychiatric and neurological symptoms in patients with Niemann-Pick disease type C (NP-C): Findings from the International NPC Registry. World J Biol Psychiatry;20:310–9. [DOI] [PubMed] [Google Scholar]
- Camilleri P, Haskins NJ, Howlett DR (1994) beta-Cyclodextrin interacts with the Alzheimer amyloid beta-A4 peptide. FEBS Letters;341:256–8. [DOI] [PubMed] [Google Scholar]
- Campbell KCM, Le Prell CG (2018) Drug-Induced Ototoxicity: Diagnosis and Monitoring. Drug Safety;41:451–64. [DOI] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, et al. (2010) Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res;265:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coisne C, Tilloy S, Monflier E, Wils D, Fenart L, Gosselet F (2016) Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crini G (2014) Review: a history of cyclodextrins. Chemical Reviews;114:10940–75. [DOI] [PubMed] [Google Scholar]
- Cronin S, Lin A, Thompson K, Hoenerhoff M, Duncan RK (2015) Hearing Loss and Otopathology Following Systemic and Intracerebroventricular Delivery of 2-Hydroxypropyl-Beta-Cyclodextrin. J Assoc Res Otolaryngol;16:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumling MA, King KA, Duncan RK (2017) Cyclodextrins and Iatrogenic Hearing Loss: New Drugs with Significant Risk. Front Cell Neurosci;11:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumling MA, Liu L, Thomas PV, Benson J, Kanicki A, Kabara L, et al. (2012) Hearing loss and hair cell death in mice given the cholesterol-chelating agent hydroxypropyl-beta-cyclodextrin. PLoS One;7:e53280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Harris D (1978) Properties of auditory nerve responses in absence of outer hair cells. Journal of Neurophysiology;41:365–83. [DOI] [PubMed] [Google Scholar]
- Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, et al. (2009) Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One;4:e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Cagno M, Terndrup Nielsen T, Lambertsen Larsen K, Kuntsche J, Bauer-Brandl A (2014) beta-Cyclodextrin-dextran polymers for the solubilization of poorly soluble drugs. Int J Pharm;468:258–63. [DOI] [PubMed] [Google Scholar]
- Dike CR, Bernat J, Bishop W, DeGeeter C (2019) Niemann-Pick disease type C presenting as very early onset inflammatory bowel disease. BMJ Case Reports;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Allman BL, Salvi R (2012) Review: ototoxic characteristics of platinum antitumor drugs. Anat Rec (Hoboken);295:1851–67. [DOI] [PubMed] [Google Scholar]
- Ding D, Jiang H, Chen GD, Longo-Guess C, Muthaiah VP, Tian C, et al. (2016) N-acetyl-cysteine prevents age-related hearing loss and the progressive loss of inner hair cells in gamma-glutamyl transferase 1 deficient mice. Aging (Albany NY);8:730–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, McFadden S, Salvi RJ 2001. Cochlear hair cell densities and inner ear staining techniques. In: Willott JF, editor. The Auditory Psychobiology of the Mouse;. Boca Raton, FL: CRC Press; p. 189–204. [Google Scholar]
- Duan X, Chen S, Chen J, Wu J (2013) Enhancing the cyclodextrin production by synchronous utilization of isoamylase and alpha-CGTase. Applied Microbiology and Biotechnology;97:3467–74. [DOI] [PubMed] [Google Scholar]
- Durrant JD, Wang J, Ding DL, Salvi RJ (1998) Are inner or outer hair cells the source of summating potentials recorded from the round window? J Acoust Soc Am;104:370–7. [DOI] [PubMed] [Google Scholar]
- Fenyvesi E, Vikmon M, Szente L (2016) Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations. Critical Reviews in Food Science and Nutrition;56:1981–2004. [DOI] [PubMed] [Google Scholar]
- Forge A (1991) Structural features of the lateral walls in mammalian cochlear outer hair cells. Cell and Tissue Research;265:473–83. [DOI] [PubMed] [Google Scholar]
- Forge A, Schacht J (2000) Aminoglycoside antibiotics. Audiol Neurootol;5:3–22. [DOI] [PubMed] [Google Scholar]
- Fu Y, Ding D, Jiang H, Salvi R (2012) Ouabain-induced cochlear degeneration in rat. Neurotox Res;22:158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Jockusch WJ, Sivakumar N, Mobius W, Corthals K, Li S, et al. (2012) Critical time window of neuronal cholesterol synthesis during neurite outgrowth. J Neurosci;32:7632–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, et al. (2008) Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol;507:1602–21. [DOI] [PubMed] [Google Scholar]
- Gomes LM, Petito N, Costa VG, Falcao DQ, de Lima Araujo KG (2014) Inclusion complexes of red bell pepper pigments with beta-cyclodextrin: preparation, characterisation and application as natural colorant in yogurt. Food Chemistry;148:428–36. [DOI] [PubMed] [Google Scholar]
- Gould S, Scott RC (2005) 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology review. Food and Chemical Toxicology;43:1451–9. [DOI] [PubMed] [Google Scholar]
- Gu FG, Cui FD, Gao YL (2005) Preparation of prostaglandin E1-hydroxypropyl-beta-cyclodextrin complex and its nasal delivery in rats. Int J Pharm;290:101–8. [DOI] [PubMed] [Google Scholar]
- Katabuchi AU, Godoy V, Shil P, Moser A, Maegawa GHB (2018) Serendipitous effects of beta-cyclodextrin on murine model of Krabbe disease. Mol Genet Metab Rep;15:98–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Masterton B (1977) Auditory sensitivity of the albino rat. Journal of Comparative and Physiological Psychology;91:930–6. [DOI] [PubMed] [Google Scholar]
- Kim H, Han J, Park JH (2020) Cyclodextrin polymer improves atherosclerosis therapy and reduces ototoxicity. Journal of Controlled Release;319:77–86. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Oh GS, Lee JH, Lyu AR, Ji HM, Lee SH, et al. (2011) Cisplatin ototoxicity involves cytokines and STAT6 signaling network. Cell Research;21:944–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Jeong HJ, Myung NY, Kim MC, Lee JH, So HS, et al. (2008) The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ Health Perspect;116:854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KA, Gordon-Salant S, Yanjanin N, Zalewski C, Houser A, Porter FD, et al. (2014) Auditory phenotype of Niemann-Pick disease, type C1. Ear Hear;35:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res;330:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawner BE, Harding GW, Bohne BA (1997) Time course of nerve-fiber regeneration in the noise-damaged mammalian cochlea. Int J Dev Neurosci;15:601–17. [DOI] [PubMed] [Google Scholar]
- Li J, Loh XJ (2008) Cyclodextrin-based supramolecular architectures: syntheses, structures, and applications for drug and gene delivery. Adv Drug Deliv Rev;60:1000–17. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J (2002) Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature;419:300–4. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kujawa SG (2017) Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear Res;349:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM (2009) Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc Natl Acad Sci U S A;106:2377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li L, Chen GD, Salvi R (2020) How low must you go? Effects of low-level noise on cochlear neural response. Hear Res;392:107980. [DOI] [PubMed] [Google Scholar]
- Maarup TJ, Chen AH, Porter FD, Farhat NY, Ory DS, Sidhu R, et al. (2015) Intrathecal 2-hydroxypropyl-beta-cyclodextrin in a single patient with Niemann-Pick C1. Mol Genet Metab;116:75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder P, Duchen MR, Gale JE (2015) Cellular glutathione content in the organ of Corti and its role during ototoxicity. Front Cell Neurosci;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna S, Gray ML, Kaul VF, Wanna G (2019) Phosphodiesterase-5 (PDE-5) Inhibitors and Ototoxicity: A Systematic Review. Otol Neurotol;40:276–83. [DOI] [PubMed] [Google Scholar]
- Marttin E, Verhoef JC, Merkus FW (1998) Efficacy, safety and mechanism of cyclodextrins as absorption enhancers in nasal delivery of peptide and protein drugs. Journal of Drug Targeting;6:17–36. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Togawa M, Hirabaru K, Mochinaga S, Narita A, Adachi M, et al. (2013) Effects of cyclodextrin in two patients with Niemann-Pick Type C disease. Mol Genet Metab;108:76–81. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Salvi RJ (2004) Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res;997:40–51. [DOI] [PubMed] [Google Scholar]
- Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA (2007) Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res;85:1959–69. [DOI] [PubMed] [Google Scholar]
- Mukherjea D, Jajoo S, Whitworth C, Bunch JR, Turner JG, Rybak LP, et al. (2008) Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J Neurosci;28:13056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M (1991) Frequency representation in the rat cochlea. Hear Res;51:247–54. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Jeyakumar A (2019) Genetic susceptibility to aminoglycoside ototoxicity. Int J Pediatr Otorhinolaryngol;120:15–9. [DOI] [PubMed] [Google Scholar]
- Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L, et al. (2017) Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet;390:1758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Espinar FJ, Luzardo-Alvarez A, Blanco-Mendez J (2010) Cyclodextrins: more than pharmaceutical excipients. Mini Reviews in Medicinal Chemistry;10:715–25. [DOI] [PubMed] [Google Scholar]
- Paken J, Govender CD, Pillay M, Sewram V (2019) A Review of Cisplatin-Associated Ototoxicity. Semin Hear;40:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MC, Mengel E, Wijburg FA, Muller A, Schwierin B, Drevon H, et al. (2013) Disease and patient characteristics in NP-C patients: findings from an international disease registry. Orphanet J Rare Dis;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MC, Walkley SU (2017) Niemann-Pick disease, type C and Roscoe Brady. Mol Genet Metab;120:34–7. [DOI] [PubMed] [Google Scholar]
- Prakash Krishnan Muthaiah V, Ding D, Salvi R, Roth JA (2017) Carbaryl-induced ototoxicity in rat postnatal cochlear organotypic cultures. Environ Toxicol;32:956–69. [DOI] [PubMed] [Google Scholar]
- Quitschke WW, Steinhauff N, Rooney J (2013) The effect of cyclodextrin-solubilized curcuminoids on amyloid plaques in Alzheimer transgenic mice: brain uptake and metabolism after intravenous and subcutaneous injection. Alzheimer’s Research & Therapy;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raileanu M, Todan L, Voicescu M, Ciuculescu C, Maganu M (2013) A way for improving the stability of the essential oils in an environmental friendly formulation. Materials Science & Engineering C: Materials for Biological Applications;33:3281–8. [DOI] [PubMed] [Google Scholar]
- Ramirez CM, Liu B, Taylor AM, Repa JJ, Burns DK, Weinberg AG, et al. (2010) Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr Res;68:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CJ, Katzov-Eckert H, Dube MP, Brooks B, Rassekh SR, Barhdadi A, et al. (2009) Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet;41:1345–9. [DOI] [PubMed] [Google Scholar]
- Roth JA, Salvi R (2016) Ototoxicity of Divalent Metals. Neurotox Res;30:268–82. [DOI] [PubMed] [Google Scholar]
- Ryan A, Dallos P (1975) Effect of absence of cochlear outer hair cells on behavioural auditory threshold. Nature;253:44–6. [DOI] [PubMed] [Google Scholar]
- Rybak LP (1986) Drug ototoxicity. Annu Rev Pharmacol Toxicol;26:79–99. [DOI] [PubMed] [Google Scholar]
- Salvi R, Sun W, Ding D, Chen GD, Lobarinas E, Wang J, et al. (2016) Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain. Front Neurosci;10:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samperio C, Boyer R, Eigel WN 3rd, Holland KW, McKinney JS, O’Keefe SF, et al. (2010) Enhancement of plant essential oils’ aqueous solubility and stability using alpha and beta cyclodextrin. J Agric Food Chem;58:12950–6. [DOI] [PubMed] [Google Scholar]
- Sanchez SA, Gunther G, Tricerri MA, Gratton E (2011) Methyl-beta-cyclodextrins preferentially remove cholesterol from the liquid disordered phase in giant unilamellar vesicles. Journal of Membrane Biology;241:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer SO, Lu ZJ (2018) Pharmaceutical economics and policy : perspectives, promises, and problems. Third edition New York, NY: Oxford University Press. [Google Scholar]
- Sheppard AM, Chen GD, Manohar S, Ding D, Hu BH, Sun W, et al. (2017) Prolonged low-level noise-induced plasticity in the peripheral and central auditory system of rats. Neuroscience;359:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Homma K, Zhou Y, Nishimura S, Duan C, Chen J, et al. (2016) Susceptibility of outer hair cells to cholesterol chelator 2-hydroxypropyl-beta-cyclodextrine is prestin-dependent. Sci Rep;6:21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda K, Shoda T, Uneyama C, Takada K, Takahashi M (1997) Carcinogenicity study of beta-cyclodextrin in F344 rats. Food and Chemical Toxicology;35:331–6. [DOI] [PubMed] [Google Scholar]
- Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A (1996) Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear Res;96:71–82. [DOI] [PubMed] [Google Scholar]
- Tsutsui K (2012) Neurosteroid biosynthesis and action during cerebellar development. Cerebellum;11:414–5. [DOI] [PubMed] [Google Scholar]
- Vite CH, Bagel JH, Swain GP, Prociuk M, Sikora TU, Stein VM, et al. (2015) Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann-Pick type C1 disease. Sci Transl Med;7:276ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkens-Berendsen DH, Bar A (2004) Embryotoxicity and teratogenicity study with alpha-cyclodextrin in rats. Regulatory Toxicology and Pharmacology;39 Suppl 1:S34–9. [DOI] [PubMed] [Google Scholar]
- Waalkens-Berendsen DH, Smits-Van Prooije AE, Bar A (2004) Embryotoxicity and teratogenicity study with alpha-cyclodextrin in rabbits. Regulatory Toxicology and Pharmacology;39 Suppl 1:S40–6. [DOI] [PubMed] [Google Scholar]
- Waalkens-Berendsen DH, Verhagen FJ, Bar A (1998) Embryotoxicity and teratogenicity study with gamma-cyclodextrin in rats. Regulatory Toxicology and Pharmacology;27:166–71. [DOI] [PubMed] [Google Scholar]
- Wang D, Xiong B, Xiong F, Chen GD, Hu BH, Sun W (2016) Apical hair cell degeneration causes the increase in the amplitude of summating potential. Acta Otolaryngol;136:1255–60. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang X, Yu B, Peng X, Liu Y, Wang A, et al. (2019) Cyclodextrin Ameliorates the Progression of Atherosclerosis via Increasing High-Density Lipoprotein Cholesterol Plasma Levels and Anti-inflammatory Effects in Rabbits. Journal of Cardiovascular Pharmacology;73:334–42. [DOI] [PubMed] [Google Scholar]
- Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R (1997) Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res;107:67–82. [DOI] [PubMed] [Google Scholar]
- Ward S, O’Donnell P, Fernandez S, Vite CH (2010) 2-hydroxypropyl-beta-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr Res;68:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J (2001) Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res;158:165–78. [DOI] [PubMed] [Google Scholar]
- Youn CK, Kim J, Park JH, Do NY, Cho SI (2015) Role of autophagy in cisplatin-induced ototoxicity. Int J Pediatr Otorhinolaryngol;79:1814–9. [DOI] [PubMed] [Google Scholar]
- Young OA, Gupta RB, Sadooghy-Saraby S (2012) Effects of cyclodextrins on the flavor of goat milk and its yogurt. Journal of Food Science;77:S122–7. [DOI] [PubMed] [Google Scholar]
- Yu D, Ding D, Jiang H, Stolzberg D, Salvi R (2011) Mefloquine damage vestibular hair cells in organotypic cultures. Neurotox Res;20:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Sun H, Salvi R, Ding D (2018) Paraquat initially damages cochlear support cells leading to anoikis-like hair cell death. Hear Res;364:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XY, Wang J, Salvi RJ, Henderson D (1996) Effects of kainic acid on the cochlear potentials and distortion product otoacoustic emissions in chinchilla. Hear Res;95:161–7. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Takahashi S, Homma K, Duan C, Zheng J, Cheatham MA, et al. (2018) The susceptibility of cochlear outer hair cells to cyclodextrin is not related to their electromotile activity. Acta Neuropathol Commun;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidovetzki R, Levitan I (2007) Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta;1768:1311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


