Abstract
We previously reported that paternal preconception chronic ethanol exposure in mice imparts adult male offspring with reduced ethanol drinking preference and consumption, increased ethanol sensitivity, and attenuated stress responsivity. That same chronic ethanol exposure paradigm was later revealed to affect the sperm epigenome by altering the abundance of several small noncoding RNAs, a mechanism that mediates the intergenerational effects of numerous paternal environmental exposures. Although recent studies have revealed that the unique RNA signature of sperm is shaped during maturation in the epididymis via extracellular vesicles (EVs), formal demonstration that EVs mediate the effects of paternal preconception perturbations is lacking. Therefore, in the current study we tested the hypothesis that epididymal EV preparations are sufficient to induce intergenerational effects of paternal preconception ethanol exposure on offspring. To test this hypothesis, sperm from ethanol naïve donors were incubated with epididymal EV preparations from chronic ethanol (Ethanol EV-donor) or control-treated (Control EV-donor) mice prior to in vitro fertilization (IVF) and embryo transfer. Progeny were examined for ethanol- and stress-related behaviors in adulthood. Ethanol EV-donors imparted reduced body weight at weaning and modestly increased limited access ethanol intake to male offspring. Ethanol-EV donors also imparted increased basal anxiety-like behavior and reduced sensitivity to ethanol-induced anxiolysis to female offspring. Although Ethanol EV-donor treatment did not recapitulate the ethanol- or stress-related intergenerational effects of paternal ethanol following natural mating, these results demonstrate that coincubation of sperm with epididymal EV preparations is sufficient to impart intergenerational effects of ethanol through the male germline. This mechanism may generalize to the intergenerational effects of a wide variety of paternal preconception perturbations.
Keywords: Epigenetic inheritance, Alcohol drinking, Intergenerational, Sperm, Extracellular Vesicles, Epididymis
Introduction
While the high heritability associated with alcohol use disorder (Prescott & Kendler, 1999; Young-Wolff, Enoch, & Prescott, 2011; Ystrom, Reichborn-Kjennerud, Aggen, & Kendler, 2011) has long been exclusively attributed to genetic factors, over the past several years, many studies now suggest that familial risk may be significantly mediated by germline epigenetic mechanisms (see Rompala & Homanics, 2019 for review). For instance, previous work from our lab revealed that paternal chronic ethanol exposure reduced ethanol drinking preference and consumption, increased ethanol sensitivity, and attenuated stress responsivity in male mice on both hybrid and pure genetic backgrounds (Beeler, Nobile, & Homanics, 2019; Finegersh & Homanics, 2014; Rompala, Finegersh, & Homanics, 2016; Rompala, Finegersh, Slater, & Homanics, 2017). In addition, a plethora of studies have demonstrated intergenerational effects of paternal ethanol exposure on wide ranging phenotypes including offspring weight, learning and activity, anxiety-related behaviors, and various molecular and physiologic effects (see Finegersh, Rompala, Martin, & Homanics, 2015 for review).
While it is clear that paternal preconception ethanol exposure induces persistent effects that impact the next generation, the molecular mechanism(s) responsible for these intergenerational effects are largely unknown. Over the last five years, evidence from studies in other fields has established a causal relationship between environmentally-responsive sperm noncoding RNAs and diverse intergenerational phenotypes (Benito et al., 2018; Chen, Yan, Cao, et al., 2016; Gapp et al., 2014; Gapp et al., 2018; Rodgers, Morgan, Leu, & Bale, 2015; Sharma et al., 2016). For example, the intergenerational effects of paternal stress were partially recapitulated in mice derived from embryos injected with sperm noncoding RNAs from stressed fathers (Gapp et al., 2014; Gapp et al., 2018; Rodgers et al., 2015). Consistent with this mechanism of epigenetic inheritance, we found that chronic intermittent ethanol exposure altered several small noncoding RNAs in sperm (Rompala et al., 2018) . Such findings have prompted intense interest in understanding the biogenesis of the sperm RNA milieu and how sperm RNAs are altered in response to environmental exposures.
Following spermatogenesis in the testis, sperm are transcriptionally quiescent (Martins & Krawetz, 2007). Despite this, the composition of small noncoding RNA in sperm shifts dramatically as sperm mature during migration through the epididymis, suggesting the involvement of the extracellular luminal environment (Nixon et al., 2015). Throughout the epididymis, small (50-150 nm) membrane bound vesicles are secreted from the epithelium into the sperm-enriched lumen (Trigg, Eamens, & Nixon, 2019). These extracellular vesicles (EVs) are critical for trafficking key proteins to sperm in support of motility and capacity for fertilization (see Sullivan, 2016 for review). More recently, deep sequencing efforts have elucidated that the RNA cargo of epididymal EVs closely reflects that of mature sperm (Sharma et al., 2016). Furthermore, epididymal EVs are capable of delivering small ncRNAs to sperm in vitro (Reilly et al., 2016; Sharma et al., 2016; Sharma et al., 2018). Intriguingly, given this ability to shape the sperm small ncRNA profile, epididymal EVs may play a critical role in RNA-mediated intergenerational inheritance through the male germline (Morgan, Chan, & Bale, 2019); nevertheless, this remains to be directly tested. Therefore, in the present study, we examined the hypothesis that epididymal EV preparations are sufficient to recapitulate the intergenerational effects of paternal chronic ethanol exposure.
Materials and Methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Specific pathogen free C57BL/6J (B6) and CD-1 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were habituated to the University of Pittsburgh animal facility for at least one week prior to initiation of experiments. Mice were housed in ventilated caging (Allentown Inc., Allentown, NJ) under 12 h light/dark cycles (0700-1900) and had ad libitum access to food (irradiated 5P76 ProLab IsoPro RMH 3000, [LabDiet, St. Louis, MO]) and water.
Chronic intermittent ethanol vapor inhalation
Chronic intermittent ethanol vapor exposure was performed as previously described (Finegersh & Homanics, 2014; Rompala et al., 2016; Rompala et al., 2017). Briefly, eight-week-old male B6 mice were randomly assigned to one of two treatments: half of the mice were exposed to ethanol inhalation chambers in the home cage with water and food for five weeks from 09:00-17:00 over five consecutive day blocks with two days in between blocks. The other half of mice were assigned to the room air control group in identical chamber conditions without ethanol vapor. All animals were group-housed throughout the experiment and cages, food, and water were all changed routinely after the final exposure of each week. Blood ethanol concentration was measured after the final ethanol exposure of each week by extracting tail vein blood (≤10 μl) using heparin-coated capillary tubes (Drummond, Broomall, PA) and running plasma samples (extracted from blood by centrifugation at 2300 × g for 10 min) on an Analox Ethanol analyzer (AM1, Analox Instruments, London, UK). Tail blood was drawn from all groups to control for stress. Ethanol content in the ethanol inhalation chambers was monitored using a custom sensor generously provided by Brian McCool, PhD (Wake Forest University) and flow rates in the chambers were adjusted weekly based on blood ethanol concentration measurements made during the preceding week. Importantly, animals do not lose significant body weight (defined as >10%). In addition, the effects of ethanol vapor on lungs, heart, and liver are comparable to those associated with other chronic ethanol exposure models (Mouton et al., 2016).
Isolation of extracellular vesicles from the epididymis
EV samples were isolated from adult male mice sacrificed ~16-19 hours following the final ethanol or room air exposure during the light cycle (08:00-11:00). Briefly, after euthanasia by CO2 asphyxiation, left and right epididymides were dissected into 1.5 ml of EmbryoMax Human Tubal Fluid (HTF) (Sigma-Aldrich, St. Louis, MO) at 37 °C. Several small cuts were made in each epididymide to release the epididymal lumen including sperm and EVs into solution. The sperm-EV media was then transferred to a 1.5 ml Eppendorf tube and incubated for 20 min at 37 °C. The top 1.2 ml of supernatant was carefully collected for further processing leaving behind large tissue pieces. Next, the recovered supernatant was centrifuged at 2000 × g for 5 min to pellet the sperm. EVs were then isolated from the supernatant by filtration and ultracentrifugation. First, the EV-containing media was centrifuged at 10,000 × g for 30 min at 4 °C before being passed through a 0.2 μm nylon syringe filter. Finally, EVs were pelleted on a table top ultracentrifuge at 120,000 × g for 2 hours at 4 °C, washed once with ice cold 1.5 ml PBS to remove excess protein aggregates, centrifuged again at 120,000 × g for 2 hours at 4 °C and snap frozen on dry ice. EV concentration was quantified using the BCA protein assay.
In vitro fertilization
All media was equilibrated with mineral oil (Sigma-Aldrich) and kept at 37 °C in a 5% CO2 incubator. Six-week-old B6 oocyte donor females (habituated to the animal colony for at least one week) were superovulated by intraperitoneal (ip) injection with 5 IU pregnant mare serum gonadotropin (Sigma-Aldrich) and 5 IU human chorionic gonadotropin (hCG) (Sigma-Aldrich) 48 hr later. The following day, 10.5 hours after the hCG injection, one 10-week-old B6 donor male (habituated to the mouse colony for two weeks) was sacrificed for rapid collection of cauda epididymis into HTF. The left and right cauda epididymis were split into separate 500 μL HTF preparations and assigned to either Control-EV or Ethanol-EV donor treatment at random. Small cuts were made to release sperm into solution and sperm were incubated for 2 min. Next, the epididymal tissue was removed and the sperm suspension was centrifuged at 300 × g for 1 min. The supernatant was discarded, and the remaining sperm pellet was resuspended in 500 μL HTF. Sperm concentration was quantified with a hemocytometer and 6 × 105 sperm (in 30 μL) were coincubated with 190 μg (in 10 μL) of the epididymal EV preparation (pooled from 4 mice from the same group-housed home cage) in HTF supplemented with 1 mM ZnCl2 and adjusted to pH 6.5 at a final volume of 40 μL and incubated at 37°C in 5% CO2 for three hours. For each IVF culture, 3 oocyte donor females were sacrificed for rapid collection of oviducts into HTF media supplemented with 1 mM glutathione (GSH) (Sigma-Aldrich) to increase zona pellucida permeability. For each oocyte donor, oviducts were torn at the ampulla to release oocyte masses into solution and moved to a 300 μL HTF+GSH drop on the IVF culture dish. Finally, 20 μL of the EV-mixed sperm was added to each IVF dish and incubated for 6 hours. Following IVF, oocytes were washed in 3 different 100 μL HTF drops to remove debris and excess sperm. Presumptive zygotes were then cultured overnight. The next morning, 2-cell embryos were counted and separated from unfertilized or degenerating oocytes and cultured in KSOM media (Sigma-Aldrich) for 1-3 hours prior to transfer to pseudopregnant CD-1 foster mothers.
Embryo transfer
CD-1 females (Charles River Labs) at 8-12 weeks old were naturally mated to CD-1 vasectomized males (Charles River Labs; ~6 months of age). The following morning, females were checked for vaginal plugs; plug-positive (pseudopregnant) females were segregated to be used as recipients. 2-cell embryos (15-30 embryos per recipient) were surgically transferred to both oviducts of anesthetized recipients. Pregnant dams were maintained in single housing and were housed with pups until weaning at three weeks postnatal.
Behavioral testing
For all behavioral testing, no more than two mice of the same sex were examined per litter. For each treatment group, 8 week old offspring were split into one of two behavioral batteries with at least two weeks between each behavioral assay. Behavioral battery 1 was carried out in the following order: 1) elevated plus maze (following saline injection), 2) two-bottle free choice ethanol drinking, 3) light dark test. Behavioral battery 2: 1) elevated plus maze (following ethanol injection), 2) HPA axis responsivity to acute restraint, 3) drinking in the dark.
Elevated plus maze
Adult mice were single-housed and habituated to the test room for one hour in the home cage prior to the test trial. The elevated plus maze apparatus is fitted with two closed and open arms and both the floors and walls were made of opaque white plexiglass. Light intensity directly over the apparatus was set to 35 lux. Ten min prior to the test trial, mice received IP injections of 5% (w/vol) ethanol (1.0 g/kg) or saline (0.9% NaCl) and returned to the home cage. After 10 min, mice were placed in the center of the elevated plus maze, always positioned with the snout-end facing the same closed arm. After five min, animals were returned to the home cage. Scoring of time spent in the open and closed arms was performed automatically using LimeLight tracking software (Coulbourn Instruments, Holliston, MA).
Light dark box
The light-dark box features adjacent light and dark compartments that the test mouse can move freely between through an aperture in the dividing wall. The dark region features black plexiglass flooring and walls with a removeable cover to place the animal inside (light intensity of 2 lux). The light region has transparent flooring and walls with no roof (light intensity of 390 lux). One hour preceding the trial, single-housed mice were habituated to the test room. At the beginning of the 5-min trial, test mice were placed into the dark region of the apparatus and latency to enter and time spent in the light region were recorded with an overhead camera and scored manually. To be scored as in the light region, all four paws needed to be visible in the light region of the box.
Two-bottle free choice ethanol drinking test
Mice were single-housed for one week while habituating to two 25 ml sipper tubes filled with autoclaved water. After the one week, ethanol drinking behavior was assessed by filling one tube with ethanol. Consumption of ethanol and water was measured daily and the position of the ethanol and water tubes were rotated each day. Ethanol concentrations started at 3% (w/vol) and was increased every four days to 6,9,12, and 15% successively. Cages were changed and animals were weighted every four days.
Drinking in the dark assay
The drinking in the dark assay was performed based on published methods (Thiele, Crabbe, & Boehm, 2014). For four nights, mice were habituated to a 10-ml sipper tube filled with water that replaced their regular water bottle two hours into the animal’s dark cycle. Sipper tubes were designed by fitting ball-bearing sippers into modified 10 ml serological pipets (Corning Incorporated, Durham, NC) sawed off at the tip and securing the fit with heat-shrink and parafilm. After the final habituation trial, the 10-ml sipper tube was then filled with 20% (w/vol) ethanol and consumption was measured for two hours over three consecutive nights and finally four hours on the fourth and final night. Tail blood was collected immediately following the four-hour trial to measure BECs as described above. As a control measure, one week after the four-hour trial, saccharine consumption was examined two hours into the dark cycle in four hour trials over two consecutive days.
Acute HPA axis responsivity
Sixteen-week-old male and female mice were subjected to a 15-min restraint stress exposure. All animals were tested between 10:00-13:00 of the light cycle. Briefly, mice were removed from group-housing and restrained in conical plastic tubes with several air hole perforations near the animal’s head and an opening for the tail. After the 15-min restraint, each mouse was housed in a single novel cage in a fume hood for another 15 min. Only one mouse was tested per group-housed cage to avoid pre-stressing any test animals. Tail blood was collected at time points 0 and 30 min from the onset of restraint stress. Blood samples were centrifuged for 10 min at 2300 × g to separate plasma for measurement of corticosterone with an enzyme immunoassay (Enzo Life Sciences, Farmingdale, NY).
Transmission Electron Microscopy
Exosome microscopy was performed with a JEOL JEM-1011 transmission electron microscope using negative staining procedures at Center for Biological Imaging at the University of Pittsburgh (Pittsburgh, PA).
Statistical analysis
As we have previously observed a significant interaction between paternal ethanol exposure and offspring sex (Finegersh & Homanics, 2014; Rompala et al., 2016; Rompala et al., 2017), males and females were examined separately for all tests. For IVF experiments, unpaired student’s t-test was used to compare Control EV-donor and Ethanol EV-donor groups means for IVF efficiency, litter size, light dark box measures, open field measures, and BECs. Two-way ANOVA was used for elevated plus maze measures (factors of ethanol injection and EV-donor) and a two-way repeated measures ANOVA was used for two-bottle choice ethanol drinking, drinking in the dark, HPA axis responsivity (factors of EV-donor and trial), and body weight. Fisher’s least significant difference (LSD) post-hoc test was used to examine significant interactions from ANOVA.
Results
IVF experiments were designed to test the hypothesis that incubation of epididymal EV preparations isolated from chronic ethanol-exposed males with normal sperm (i.e., from ethanol naïve males) preceding IVF alters ethanol- and stress-related behaviors in offspring (Figures 1A and 1B). Average BECs during chronic ethanol exposure of all Ethanol EV-donor pools was ~150-175 mg/dL (Figure 1C) and there was no significant effect of chronic ethanol exposure on body weight (Figure 1D). EVs were pooled from Ethanol EV- or Control EV-donor mice (four donors per pool). There was no effect of EV-donor on IVF success rate (Figure 1E) or litter sizes (Figure 1F).
Figure 1: Examining the effects of Ethanol EVs on IVF-derived mice.
(A) Electron micrograph demonstrating enrichment of extracellular vesicles (EV) isolated from adult mouse epididymis. Arrows indicate EVs and scale bar = 100 nM. (B) Experimental design for examining the effect of EVs on intergenerational ethanol- and stress-related behaviors. After adult males were exposed to chronic ethanol or control treatment, they were sacrificed to isolate epididymal EVs. For each Ethanol EV and Control EV donor pool, EVs were pooled from four mice and incubated with sperm during capacitation immediately preceding in vitro fertilization. Fertilized oocytes were implanted in foster dams and adult progeny were phenotyped for ethanol- and stress-related behaviors. (C) Average blood ethanol concentrations (BECs) for all Ethanol (EtOH) EV donors. (D) No effect of chronic ethanol exposure on body weight of EtOH EV-donors. (E) No effect of EtOH EVs on IVF success rate. (F) No effect of EtOH EVs on litter size. Data presented as μ ± SEM. Error bars are obscured by data points in panel D. Numbers in bars denote group sizes.
Ethanol EV-donors confer reduced body weight selectively to male progeny
Analysis of IVF-derived males at weaning [postnatal day (PND) 21] and adulthood (PND 56), revealed significant effects of postnatal age (F(1, 144) = 1989, p<0.001), EV-donor (F(1, 144) = 21.03, p<0.001) and EV-donor × age (F(1, 144) = 5.66, p<0.05) (Figure 2A). Post-hoc analysis revealed a significant reduction of body weight by Ethanol EV-donor treatment vs Controls specifically at PND 21 (p<0.001). There was no effect of EV-donor treatment, but a significant effect of postnatal age (F (1, 57) = 835.0, p<0.001) and EV-donor × age (F(1, 57) = 6.85, p<0.05) on body weights in females (Figure 2B). Post-hoc test revealed no significant difference for female body weights between Control and Ethanol EV-donor groups at PND 21 or 56.
Figure 2: Ethanol EVs reduce body weight selectively in male progeny.
(A) Ethanol (EtOH)-EVs conferred reduced body weight to postnatal day (PND) 21 males vs Control EV-Donors (Ctrl-EVs). (B) There was no effect of EtOH-EVs on body weights in females. ***=p<0.001. Data presented as μ with SEM bars obscured by data points.
Ethanol EV-donors confer reduced anxiety-like behavior to female progeny
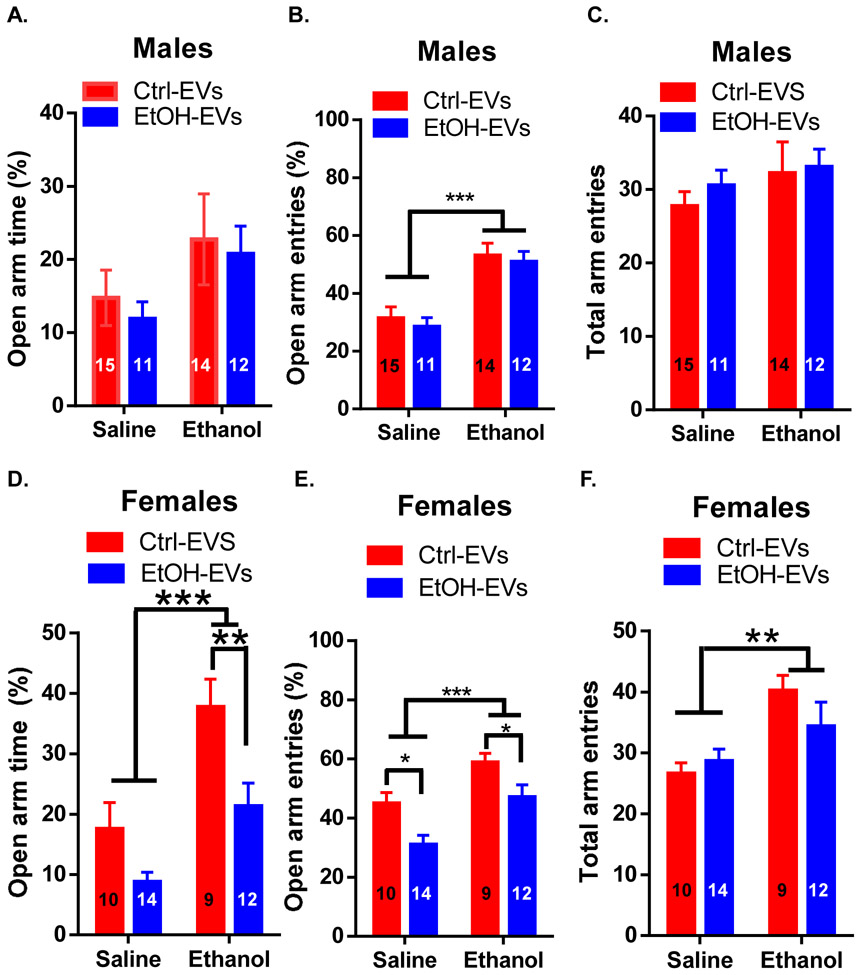
IVF-derived adult males and females were examined for basal anxiety-like behavior and ethanol-induced anxiolysis on the elevated plus maze. Basal anxiety-like behavior was assessed in mice injected with saline prior to testing. Mice injected with 1 mg/kg ethanol were assessed for ethanol-induced anxiolysis. For males, there was a trending effect of ethanol injection on open arm time (F(1, 46) = 3.75, p<0.06; Figure 3A), a significant effect of ethanol on open arm entries (F(1, 47) = 31.27, p<0.001; Figure 3B), and no effect on total arm entries (Figure 3C). There was no effect of EV-donor or EV-donor × ethanol injection on any measure in males.
Figure 3: Ethanol EVs increases anxiety-like behavior and reduce ethanol-induced anxiolysis in IVF-derived females.
(A) There was no effect of Ethanol (EtOH)-EVs on open arm time in male progeny. (B) There was a significant effect of ethanol, but not EtOH-EVs on open arm entries in male progeny. (C) There was no effect of EtOH-EVs on total arm entries in male progeny. For the female cohorts, there was a significant effect of ethanol on open arm time, open arm entries, and total arm entries in females (D-F). (D) EtOH-EVs reduced open arm time in females treated with ethanol. (E) EtOH-EVs reduced open arm entries in females treated with saline or ethanol. (F) No effect of EtOH-EVs on total arm entries in female progeny. *=p<0.05, **=p<0.01, ***=p<0.001. Data presented as μ ± SEM. Numbers in bars denote group sizes.
For females, there was a significant effect of ethanol injection on open arm time (F(1, 40) = 28.56, p<0.001; Figure 3D), open arm entries (F(1, 40) = 15.43, p<0.001; Figure 3E), and total arm entries F(1, 40) = 10.54, p<0.01; Figure 3F). In addition, there was a significant effect of EV-donor on open arm time (F(1, 40) = 10.65, p<0.01; Figure 3D) and open arm entries (F(1, 40) = 11.44, p<0.01; Figure 3E), but not total arm entries (Figure 3F). There was no interaction of EV-donor with ethanol injection on any measure for females. Post-hoc analysis revealed a significant reduction of open arm time after ethanol treatment in Ethanol EV-donor females (p<0.01) and a significant reduction in open arm entries for Ethanol EV-donor females in both saline (p<0.05) and ethanol (p<0.05) treatment groups.
To further examine basal anxiety-like behavior, males and females were tested in the light/dark transition test. Here, there was no effect of EV-donor on latency to enter the light or time spent in the light region in males (Figure 4A-B). For females, a significant increase in latency to enter the light region for Ethanol EV-donor vs Control EV-donor females (t(22) = 2.99, p<0.01; Figure 4C) was observed. There was no effect of EVs for time spent in the light region (Figure 4D).
Figure 4: Ethanol EVs confer increased anxiety-like behavior to IVF-derived females in the light-dark box transition test.
No effect of Ethanol (EtOH)-EVs on (A) latency to enter light or (B) total time spent in the light region in male progeny. (C) EtOH- EVs increased latency to enter the light region in female progeny. (D) No effect of EtOH- EVs on time in light region for female progeny. **=p<0.01. Data presented as μ ± SEM. Numbers in bars represent group sizes.
No effect of Ethanol EV-Donor on two-bottle choice ethanol drinking
In the two-bottle choice test, there was a significant effect of ethanol concentration on ethanol consumption for both male (F(4, 100) = 95.96, p<0.001) and female (F(4, 80) = 115.3, p<0.001) mice with no effect on ethanol preference or total fluid intake. There was no effect of EV-donor or EV-donor × ethanol concentration on ethanol drinking preference, ethanol consumption, or total fluid intake in IVF-derived male (Figure 5A-C) or female (Figure 5D-F) mice.
Figure 5: No effect of Ethanol EVs on two bottle-choice continuous access ethanol drinking.
No effect of Ethanol (EtOH)-EVs on male progeny for (A) EtOH preference, (B) EtOH consumption, or (C) total fluid intake in the two-bottle choice test. No effect of EtOH-EVs on female progeny for (D) EtOH preference, (E) EtOH consumption, or (F) total fluid intake. Data presented as μ ± SEM.
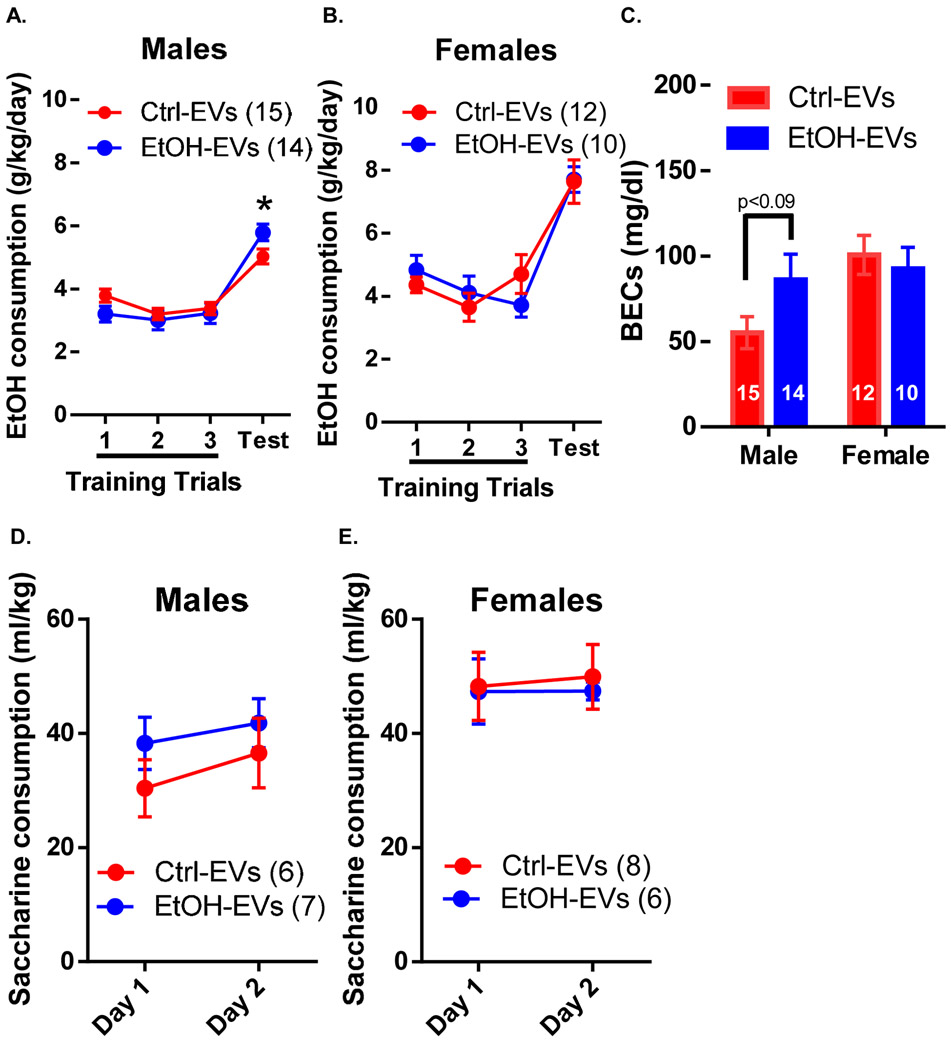
Ethanol EV-donors increase binge-like ethanol consumption in male progeny
In the limited access drinking in the dark assay, for IVF-derived males, there was no effect of EV-donor, but a significant effect of EV-donor × trial on ethanol consumption (F(3, 75) = 3.68, p<0.05; Figure 6A). Post-hoc analysis revealed significantly (p<0.05) increased ethanol consumption in Ethanol EV-donor males compared to Control EV-donor males during the four-hour test. For females, there was no effect of EV-donor or EV-donor × trial (Figure 6B). In males, there was a trending effect (p<0.09) of Ethanol EV-donor on BECs measured following the four-hour trial and no effect in females (Figure 6C). The BECs of Ethanol EV-donor males and both groups of females exceeded binge levels (>80 mg/dl) whereas Control EV-donor males did not. In addition, there was no effect of EV-donor or EV-donor × trial on saccharine consumption in males (Figure 6D) or females (Figure 6E).
Figure 6: Ethanol EVs confer increased binge-like ethanol drinking to male progeny.
(A) Ethanol (EtOH)-EVs confer increased EtOH consumption to males during the drinking the dark four-hour trial. (B) There was no effect of EtOH-EVs on EtOH consumption in female progeny. (C) There was a trending (p<0.09) increase in blood EtOH concentrations (BECs) during the four hour drinking in the dark test in EtOH-EV males, but no effect in females. There was no effect of EtOH-EVs on saccharine consumption in (D) male or (E) female progeny. *=p<0.05. Data presented as μ ± SEM.
No effect of Ethanol EV-donors on HPA axis responsivity to restraint stress
Analysis of HPA responsivity to 15 min of acute restraint stress, revealed a significant effect of stress on corticosterone levels in males (F(1, 15) = 278.6, p<0.001) and females (F(1, 17) = 73.30, p<0.001). However, there was no effect of EV-donor or EV-donor × stress interaction on corticosterone levels in males (Figure 7A) or females (Figure 7B).
Figure 7: No effect of Ethanol EVs on HPA axis responsivity to acute restraint stress.
No effect of Ethanol (EtOH)-EVs on plasma corticosterone (CORT) levels at 0 and 30 min from the onset of 15-min restraint stress (shaded bar) in (A) male or (B) female progeny. Data presented as μ ± SEM.
Discussion
In the present study, we utilized an established paternal preconception ethanol exposure model to test the hypothesis that epididymal EVs play a casual role in intergenerational ethanol-related phenotypes. Remarkably, incubating epididymal EV preparations from ethanol-treated males with sperm impacts body weight and modestly alters binge ethanol drinking in IVF-derived adult males and basal anxiety-like behavior and sensitivity to an anxiolytic dose of ethanol in IVF-derived adult females. Importantly, these results provide the first evidence establishing sufficiency of epididymal EV preparations in mediating paternally-driven intergenerational phenotypes.
The chronic ethanol exposure in the current study was previously found to affect small noncoding RNA in sperm (Rompala et al., 2018b) and have intergenerational effects on ethanol- and stress-related behaviors (Beeler et al., 2019; Finegersh & Homanics, 2014; Rompala et al., 2016; Rompala et al., 2017). In addition, a chronic binge ethanol drinking exposure has also been shown to affect sperm small noncoding RNAs (Bedi, Chang, Gibbs, Clement, & Golding, 2019) and impact metabolic and growth measures in offspring (Chang, Wang, Bedi, & Golding, 2019). Therefore, given the capacity for epididymal EVs to traffic noncoding RNAs to sperm (Reilly et al., 2016; Sharma et al., 2016; Sharma et al., 2018), the findings from the current study have broad implications for further investigations into heritable epigenetic mechanisms across paternal ethanol exposure studies.
While the exact mechanism underlying the intergenerational effects of Ethanol EV coincubation with sperm prior to IVF remains to be explored, several recent studies suggest that small noncoding RNAs may be responsible. For example, different species of sperm small noncoding RNA, such as microRNAs and tRNA-derived small RNAs, are sufficient to drive cross-generational effects of paternal environmental exposures (Chen, Yan, & Duan, 2016; Rodgers et al., 2015). Furthermore, in vitro coincubation of epididymal EVs alters the RNA profile of sperm (Reilly et al., 2016; Sharma et al., 2018). Indeed, we have previously shown that some tRNA-derived small RNAs are similarly affected in both sperm and epididymal EVs by chronic ethanol exposure (Rompala et al., 2018b). Alternatively, it is possible that ethanol-exposed EVs uniquely affected the internal and surface protein content of sperm (Martin-DeLeon, 2015). Epididymal EV-derived proteins influence immunoprotection, capacitation, and acrosomal exocytosis, all of which may conceivably affect embryonic development (Martin-DeLeon, 2015). In addition, ultracentrifugation of EV preparations may have included contaminants such as protein aggregates, ribonucleoprotein complexes, and DNA-fragments that cannot be ruled out as causal factors in the current study (Li, Kaslan, Lee, Yao, & Gao, 2017; Shurtleff et al., 2017). Thus, additional mechanistic studies are needed to determine whether the cross-generational effects of Ethanol EV preparations were specific to EV trafficking of RNA cargo to sperm.
It is critical to note that, with the exception of reduced body weight in males (Figure 2A), the observed effects of Ethanol EV preparations on the resulting progeny were inconsistent with the effects of paternal ethanol exposure on offspring following natural mating; that is, increased ethanol-induced anxiolysis, decreased ethanol drinking, and blunted HPA axis responsivity selectively in male offspring (Finegersh & Homanics, 2014; Rompala et al., 2016; Rompala et al., 2017). One likely explanation for this discrepancy is that in vitro coincubation of EV preparatons with sperm poorly models the in vivo spatial and temporal dynamics of EV/sperm interactions. Indeed, while our coincubation occurred over three hours, rodent sperm spend as long as one month between migration through and storage in the epididymis (Jones, 1999). Moreover, the epididymal EV preparations in the current study were pooled from the whole epididymis, while under biological conditions, sperm would be exposed to caput-, corpus-, and cauda-derived EVs sequentially which may be especially important given that the RNA cargo varies dramatically between EVs derived from each region (Reilly et al., 2016). It is also important to note that we utilized mature sperm that have already migrated into the epididymis and thereby have already undergone significant epididymal EV exposure endogenously. Future studies should utilize alternative methods such as intracytoplasmic injection of immature sperm from testis that were not exposed to epididymal EVs in vivo. Finally, it is notable that the EV preparations lack natural breeding-specific factors such as seminal plasma which may contribute to epigenetic mechanisms of inheritance (Watkins et al., 2018).
In summary, we report that epididymal EV preparations from ethanol-exposed mice are capable of transmitting unique intergenerational ethanol drinking and anxiety-like phenotypes to offspring. Future studies are imperative to determine if the heritable effects of epididymal EV preparations were driven by trafficking of their EV RNA cargo to sperm. Overall, the evidence strongly implicates a soma-to-germline epigenetic mechanism underlying the intergenerational effects of paternal chronic ethanol exposure. Importantly, this novel mechanism may generalize to the intergenerational effects of a wide variety of paternal preconception perturbations.
Highlights.
Epididymal EVs from EtOH-treated males increase ethanol drinking in male progeny
Epididymal EVs from EtOH-treated males increase anxiety behaviors in female progeny
Epididymal EVs may mediate paternally-driven intergenerational inheritance
Acknowledgements
This work was supported by NIH/NIAAA AA010422, AA020889 and AA024670. The experiments described were completed in partial fulfillment of GRR’s doctoral dissertation (Rompala, 2018).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedi Y, Chang RC, Gibbs R, Clement TM, & Golding MC (2019). Alterations in sperm-inherited noncoding RNAs associate with late-term fetal growth restriction induced by preconception paternal alcohol use. Reprod Toxicol, 87, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler E, Nobile ZL, & Homanics GE (2019). Paternal Preconception Every-Other-Day Ethanol Drinking Alters Behavior and Ethanol Consumption in Offspring. Brain Sci, 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito E, Kerimoglu C, Ramachandran B, Pena-Centeno T, Jain G, Stilling RM, et al. (2018). RNA-Dependent Intergenerational Inheritance of Enhanced Synaptic Plasticity after Environmental Enrichment. Cell Rep, 23(2), 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RC, Wang H, Bedi Y, & Golding MC (2019). Preconception paternal alcohol exposure exerts sex-specific effects on offspring growth and long-term metabolic programming. Epigenetics Chromatin, 12(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. (2016). Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science, 351(6271), 397–400. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yan W, & Duan E (2016). Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet, 17(12), 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh, & Homanics GE (2014). Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS One, 9(6), e99078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh A, Rompala GR, Martin DI, & Homanics GE (2015). Drinking beyond a lifetime: New and emerging insights into paternal alcohol exposure on subsequent generations. Alcohol, 49(5), 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, et al. (2014). Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci, 17(5), 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, van Steenwyk G, Germain PL, Matsushima W, Rudolph KLM, Manuella F, et al. (2018). Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RC (1999). To store or mature spermatozoa? The primary role of the epididymis. Int J Androl, 22(2), 57–67. [DOI] [PubMed] [Google Scholar]
- Li P, Kaslan M, Lee SH, Yao J, & Gao Z (2017). Progress in Exosome Isolation Techniques. Theranostics, 7(3), 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-DeLeon PA (2015). Epididymosomes: transfer of fertility-modulating proteins to the sperm surface. Asian J Androl, 17(5), 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RP, & Krawetz SA (2007). Nuclear organization of the protamine locus. Soc Reprod Fertil Suppl, 64, 1–12. [DOI] [PubMed] [Google Scholar]
- Morgan CP, Chan JC, & Bale TL (2019). Driving the Next Generation: Paternal Lifetime Experiences Transmitted via Extracellular Vesicles and Their Small RNA Cargo. Biol Psychiatry, 85(2), 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton AJ, Maxi JK, Souza-Smith F, Bagby GJ, Gilpin NW, Molina PE, et al. (2016). Alcohol Vapor Inhalation as a Model of Alcohol-Induced Organ Disease. Alcoholism-Clinical and Experimental Research, 40(8), 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B, Stanger SJ, Mihalas BP, Reilly JN, Anderson AL, Tyagi S, et al. (2015). The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol Reprod, 93(4), 91. [DOI] [PubMed] [Google Scholar]
- Prescott CA, & Kendler KS (1999). Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry, 156(1), 34–40. [DOI] [PubMed] [Google Scholar]
- Reilly JN, McLaughlin EA, Stanger SJ, Anderson AL, Hutcheon K, Church K, et al. (2016). Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci Rep, 6, 31794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, & Bale TL (2015). Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A, 112(44), 13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala G, Mounier A, Wolfe CM, Lin Q, Lefterov I, & Homanics GE (2018a). Heavy Chronic Intermittent Ethanol Exposure Alters Small Noncoding RNAs in Mouse Sperm and Epididymosomes. Front Genet, 9, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR (2018). Role of Paternal Preconception Environment in Ethanol- and Stress-Related Phenotypes. [dissertation] [Pittsburgh (PA)]: University of Pittsburgh. [Google Scholar]
- Rompala GR, Finegersh A, & Homanics GE (2016). Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol, 53, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Finegersh A, Slater M, & Homanics GE (2017). Paternal preconception alcohol exposure imparts intergenerational alcohol-related behaviors to male offspring on a pure C57BL/6J background. Alcohol, 60, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, & Homanics GE (2019). Intergenerational Effects of Alcohol: A Review of Paternal Preconception Ethanol Exposure Studies and Epigenetic Mechanisms in the Male Germline. Alcohol Clin Exp Res, 43(6), 1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Mounier A, Wolfe CM, Lin Q, Lefterov I, & Homanics GE (2018b). Heavy Chronic Intermittent Ethanol Exposure Alters Small Noncoding RNAs in Mouse Sperm and Epididymosomes. Front Genet, 9, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, et al. (2016). Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science, 351(6271), 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, et al. (2018). Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev Cell, 46(4), 481–494 e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff MJ, Yao J, Qin Y, Nottingham RM, Temoche-Diaz MM, Schekman R, et al. (2017). Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci U S A, 114(43), E8987–E8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R (2016). Epididymosomes: Role of extracellular microvesicles in sperm maturation. Front Biosci (Schol Ed), 8, 106–114. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, & Boehm SL 2nd. (2014). "Drinking in the Dark" (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci, 68, 9 49 41–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigg NA, Eamens AL, & Nixon B (2019). The contribution of epididymosomes to the sperm small RNA profile. Reproduction. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Dias I, Tsuro H, Allen D, Emes RD, Moreton J, et al. (2018). Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc Natl Acad Sci U S A, 115(40), 10064–10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch MA, & Prescott CA (2011). The influence of gene-environment interactions on alcohol consumption and alcohol use disorders: A comprehensive review. Clinical Psychology Review, 31(5), 800–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystrom E, Reichborn-Kjennerud T, Aggen SH, & Kendler KS (2011). Alcohol Dependence in Men: Reliability and Heritability. Alcoholism-Clinical and Experimental Research, 35(9), 1716–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]