Abstract
Considerable work demonstrates that Pavlovian fear conditioning depends on N-methyl-d-aspartate (NMDA) receptor-dependent plasticity within the amygdala. In addition, the bed nucleus of the stria terminalis (BNST) has also been implicated in fear conditioning, particularly in the expression of fear to poor predictors of threat. We recently found that the expression of backward (BW) fear conditioning, in which an auditory conditioned stimulus (CS) follows a footshock unconditioned stimulus (US), requires the BNST; the expression of forward (FW) fear conditioning was not disrupted by BNST inactivation. However, whether NMDA receptors within the BNST contribute to the acquisition of fear conditioning is unknown. Moreover, the central nucleus of the amygdala (CeA), which has extensive connections with the BNST, is critically involved in FW conditioning, however whether it participates in BW conditioning has not been explored. Here we test the specific hypothesis that the CeA and the BNST mediate the acquisition of FW and BW fear conditioning, respectively. Adult female and male rats were randomly assigned to receive bilateral infusions of the NMDA receptor antagonist, d,l-2-amino-5-phosphonovalerate (APV), into the CeA or BNST prior to FW or BW fear conditioning. We found that intra-CeA APV impaired the acquisition of both FW and BW conditioning, whereas intra-BNST APV produced selective deficits in BW conditioning. Moreover, APV in the BNST significantly reduced contextual freezing, whereas CeA NMDA receptor antagonism impeded early but not long-lasting contextual fear. Collectively, these data reveal that CeA and BNST NMDA receptors have unique roles in fear conditioning.
Keywords: bed nucleus of the stria terminalis, central amygdala, conditioned fear, context, rat
Introduction
Anticipating future threats is fundamental to survival—it allows animals to organize behavioral defense systems and prepare for future adversity. However, excessive worry and apprehension are core symptoms of a number of fear-related psychiatric disorders. Consequently, the brain circuits underlying fear and defensive behavior have received significant attention over the past several decades (LeDoux, 2000; Maren, 2001; Maren and Quirk, 2004; Craske et al., 2006; Johansen et al., 2011; Maren et al., 2013; Calhoon and Tye, 2015; Tovote et al., 2015; Lebow and Chen, 2016; Goode et al., 2018).
Pavlovian conditioning (Pavlov, 1927) is a powerful behavioral model for elucidating the neurobiological mechanisms underlying aversive learning and memory. In a typical experiment, rats learn to associate an innocuous conditioned stimulus (CS), such as an auditory tone, with an unavoidable and aversive unconditioned stimulus (US), such as a footshock. Fear conditioning studies have revealed that convergent sensory and nociceptive inputs within the amygdala activate N-methyl-d-aspartate (NMDA) receptors, which are critical for the induction of associative long-term potentiation (LTP) (LeDoux et al., 1990; Romanski et al., 1993; Campeau and Davis, 1995; Fanselow and LeDoux, 1999; Collins and Paré, 2000; Blair et al., 2001; Ressler and Maren, 2019). Although significant work has concentrated on NMDA-dependent plasticity within the basolateral nucleus of the amygdala (BLA), more recent work has demonstrated that NMDA receptor-dependent plasticity within the central nucleus of the amygdala (CeA) is also critical to fear learning (Samson and Paré, 2005; Goosens and Maren, 2003; Wilensky et al., 2006; Ciocchi et al., 2010; Duvarci et al., 2011; Li et al., 2013; Penzo et al., 2014, 2015). Together, these findings suggest that NMDA receptor-mediated plasticity within a distributed network of brain areas may contribute to the formation of CS-US associations during the acquisition of conditioned fear.
Although this work has been fundamental to our understanding of neural mechanisms by which the brain detects and responds to explicit threats, much less is known about how the brain encodes unpredictable threat signals that have been linked to anxiety-like behavioral states in both rodents and humans (Mineka and Hendersen, 1985; Foa et al., 1992; Grillon et al., 2004; Grupe and Nitschke, 2013; Davies and Craske, 2015). Preclinical and clinical work has shown that brain systems coordinating behavioral and physiological fear responses to predictable threats may be dissociable from those coordinating anxiety-like states evoked by uncertain or unpredictable prospective threats. Specifically, this work suggests that although the amygdala is critical for phasic fear responses to predictable threat cues, the bed nucleus of the stria terminalis (BNST) mediates sustained fear states evoked by uncertain threat (Walker and Davis, 2008; Walker et al., 2009; Davis et al., 2010; Alvarez et al., 2011). Although initial studies suggested a role for the BNST in contextual (but not cued) fear (LeDoux et al., 1988; Sullivan et al., 2004; Resstel et al., 2008; Poulos et al., 2010; Zimmerman and Maren, 2011; Hott et al., 2012, 2017; Sink et al., 2013; Davis and Walker, 2014), more recent work suggests the role of the BNST in fear conditioning may be more nuanced than previously appreciated (Waddell et al., 2006; Hammack et al., 2015; Goode et al., 2019, 2020). In a recent study from our laboratory, we found that reversible inactivation of BNST impaired the expression of fear to discrete CSs that poorly signaled when shock would occur [e.g., backward (BW) or temporally randomized]; in contrast, this manipulation had no effect on the expression of fear to forward (FW) CSs that reliably predict shock onset (Goode et al., 2019). These data suggest that the BNST may be involved in fear conditioning to temporally unpredictable threat signals (Goode and Maren, 2017).
Despite progress in our understanding of the circumstances in which the BNST is recruited to conditioned fear, very few studies have examined the molecular mechanisms that contribute to BNST-dependent aversive learning. Importantly, it is not known whether NMDA receptors within the BNST contribute to fear conditioning. To explore this question, we compared the effects of NMDA receptor antagonism in the BNST and CeA on the acquisition of FW and BW conditioning. These procedures differ in the degree to which the CS predicts when the US will occur, but equate CS modality, context exposure, the number of conditioning trials, and interstimulus intervals.
Material and Methods
Subjects
For all experiments, adult male and female Long-Evans rats (200–250 g upon arrival; Envigo; Indianapolis, IN) were used (n = 64, equal numbers of male and females per group prior to exclusions). Rats were individually housed in clear plastic cages in a climate-controlled vivarium on a fixed light/dark cycle (lights on at 7:00 a.m. and off at 9:00 p.m.). All behavioral experiments were conducted during the light phase of the cycle. All group assignments were randomized for cage position in the vivarium and male and female rats were housed together (individual, alternating cages) in the vivarium. Animals had access to standard rodent chow and water ad libitum. For five consecutive days prior to the start of surgery animals were handled by experimenters (~1 min/day). All procedures were conducted in accordance with the US National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals and were approved by the Texas A&M University Institutional Animal Care and Use Committee (IACUC).
Surgical Procedures
One week prior to behavioral testing, rats were transported to the surgical suite and anesthetized with isoflurane (5% for induction and 1–2% for maintenance) and placed into a stereotaxic instrument (Kopf Instruments). Hair was clipped from the top of the rodent’s head and povidone-iodine was applied. A small incision was made in the scalp and the skull was leveled by placing bregma and lambda in the same horizontal plane. Small holes were drilled into the skull for placement of jeweler’s screws and bilateral stainless-steel guide cannulas (8 mm for BNST; 10 mm for CeA; 26 gauge; Plastics One); the cannulas were inserted bilaterally into either the BNST or CeA. All coordinates (in mm) were relative to bregma. For the BNST, cannulas were implanted at a 10° angle (directed at the midline) at the following coordinates: anteroposterior (A/P), −0.15; mediolateral (M/L), ±2.65; dorsoventral (D/V), −6.15 from dura. For the CeA, cannulas were implanted at the following coordinates (no angle): A/P, −1.8 mm; M/L, ±3.9 mm; D/V, −7.9 mm from dura. Dental cement was used to secure the guide cannulas to the skull and stainless-steel dummies (9 mm for BNST; 11 mm for CeA; 31 gauge; Plastics One) were inserted into the guide cannulas. Topical antibiotic (Triple Antibiotic Plus; G&W Laboratories) was applied to the surgical site and one Rimadyl tablet (2 mg; Bio-Serv) was provided for post-operative pain management. Animals were given a minimum of one week to recover prior to the beginning of behavioral training.
Behavioral apparatus
Behavioral testing was conducted in two separate rooms within the laboratory each containing eight standard rodent conditioning chambers (30 × 24 × 21 cm; Med Associates), which were housed in sound attenuating cabinets. Each chamber consisted of aluminum side walls with a ceiling, rear wall, and front-hinged door made of Plexiglas. Grid floors in the chambers were composed of 19 stainless steel rods (4 mm in diameter; spaced 1.5 mm apart) that were connected to a shock source and a solid-state grid scrambler for delivery of the footshock US (Med Associates). A speaker was mounted within each chamber for delivery of the auditory CS and ventilation fans and a house light were used to generate distinct contexts as needed. Digital cameras were positioned above each conditioning chamber to record and remotely inspect behavior. Freezing behavior served as an index of conditioned fear. For unbiased measurements of freezing behavior, each chamber rested on a load-cell platform that was sensitive to cage displacement produced by each animal’s movements (Maren, 1998). Load-cell voltages ranging from −10 to +10 V were collected and digitized at 5 Hz during behavioral testing, yielding one observation every 200 ms. Load-cell voltages were converted values ranging from 0–100 with lower values indicating less cage displacement. Based on prior work, freezing bouts were defined as values of ≤ 10 for a period of 1 or more seconds (i.e., 5 observations) (Maren, 1998). For each behavioral session, freezing behavior (shown as a percentage of each period, see Results and figures for details) was calculated for the baseline (prior to CS presentation), the CS, and the intertrial interval.
Stimuli within each testing room were manipulated to generate two unique contextual settings. For “Context A”, a 15 W house light was turned on within each chamber and overhead red fluorescent room lights were turned on. Each chamber was wiped down with 3.0% acetic acid prior to each behavioral session and chamber doors remained open throughout the duration of each test. White plastic transport boxes were used to move animals to and from the vivarium and Context A. For “Context B”, the house light remained off, white overhead fluorescent lights were turned on, and a mounted ventilation fan was used in each chamber to provide constant background noise (65 dB). Chamber doors remained closed during testing and chambers were wiped down with 1% ammonium hydroxide prior to each behavioral test session. Rats were transported to and from Context B in black plastic boxes with clean sawdust bedding.
Drug infusions
Prior to behavioral testing, and in the week following surgery, animals were acclimated to the intracranial drug infusion process. Animals were transported to the infusion room from the vivarium in 5-gallon buckets and the dummies were removed from the guide cannulas and replaced with clean ones. This procedure was conducted twice, on separate days, prior to drug infusions. On the conditioning day, rats were transported to the infusion room and dummy guides were removed. Stainless steel injectors (33 gauge; 9 mm for BNST; 11mm for CeA) were connected to polyethylene tubing (PE-20; Braintree Scientific); the other end of the tubing was connected to a Hamilton syringe (10 μl; Hamilton Scientific) which was mounted on an infusion pump (KD Scientific). For all infusion procedures, the NMDA receptor antagonist, d,l-2-amino-5-phosphonovalerate (APV; Tocris Biosciences), was dissolved in physiological saline to a concentration of 10 μg/μl; saline served as a vehicle (VEH) control. This concentration of APV robustly disrupts fear conditioning when infused into the amygdala (Maren et al., 1996; Goosens and Maren, 2003). APV also produces behavioral effects when injected into the BNST, albeit in different behavioral tasks (Liu et al., 2009; Lungwitz et al., 2012; Glangetas et al., 2017). All infusions were made immediately (~10 min) prior to the start of conditioning. For all infusions, animals received bilateral infusions of 0.275 μl of APV or VEH at a rate of 0.275 μl/min. Injectors remained in the guide cannulas for 1 minute after the infusion to allow for diffusion. Once injectors were removed, clean dummies were inserted into the guides.
Behavioral Procedures and Exclusions
An overview of the behavioral procedures is shown in Figure 1. Male and female rats were randomly assigned in equal numbers (e.g., 4 males, 4 females) to receive either forward (FW) or backward (BW) conditioning and vehicle (VEH) or APV infusions into either the CeA or the BNST. Vehicle-treated animals were ultimately collapsed into a single group (VEH) insofar as there were no main effects of brain region in VEH-treated rats for any of the sessions [F’s < 1.77, p’s > 0.19]. This yielded the following factors and groups for the analyses: training procedure (FW or BW), sex (female or male), and drug treatment (VEH, CeA-APV, or BNST-APV). One animal was excluded because it became ill, and two animals had off-target cannula. This yielded the following group sizes: FW-VEH (n = 16), BW-VEH (n = 16); FW-CeA-APV (n = 7); BW-CeA-APV (n = 8); FW-BNST-APV (n = 7); BW-BNST-APV (n = 8).
Figure 1. Experimental design.
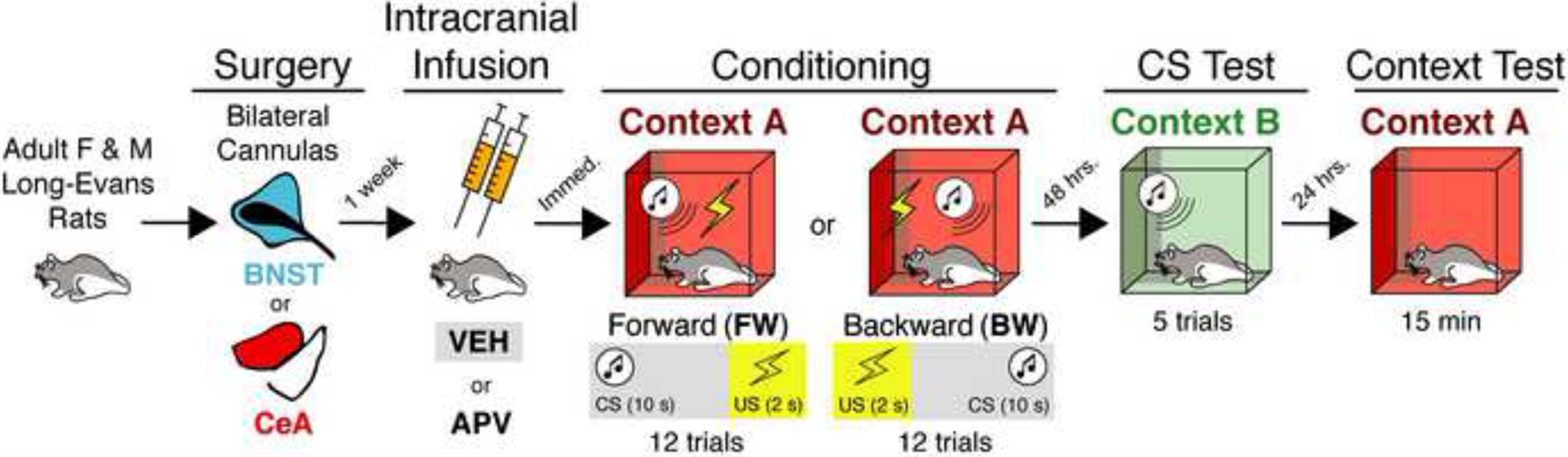
Adult male and female rats were surgically implanted with cannulas aimed at the BNST or CeA. After recovery, animals were infused with VEH or the NMDA receptor antagonist, APV, just prior to FW (CS-then-US; predictable) or BW (US-then-CS; unpredictable) auditory fear conditioning. Two days after conditioning, animals were tested off-drug to the CS in the absence of the US. One day later, animals were placed back in the conditioning context (no CS or US) to assess contextual fear conditioning.
For conditioning, FW- and BW-conditioned animals were trained in alternating squads; drug assignments and sex were counterbalanced for chamber position for all sessions. Prior to conditioning (day 1) animals were infused intracranially with APV or VEH into CeA or BNST and immediately placed into context A. For FW conditioning, after a 5-minute baseline period, rats received 12 trials in which an auditory CS (10 s, 2 kHz, 80 dB) immediately preceded an aversive footshock US (2 sec, 1 mA); each trial was separated by a 60-sec intertrial interval (ITI). Rats remained in the chamber for 1 min after the final trial (19 min total for the entire session). For BW conditioning, these parameters were identical to FW conditioning except the order of the CS and US were reversed (Goode et al., 2019).
Forty-eight hours after conditioning (day 3), all animals underwent a drug-free CS retrieval test. Rats were transported from the vivarium in squads of eight and placed into context B (drug assignment and sex were counterbalanced for chamber position) and after a 3-minute baseline period they received 5 presentations of the CS (in the absence of shock); each presentation was separated by a 60-sec interstimulus interval (ISI). Animals remained in the chamber for 1 minute after the last CS presentation (session duration was 8 min 50 sec) and were returned to their home cages after the test.
Twenty-four hours after CS retrieval (day 4) rats were again transported in squads of 8 and placed in the conditioning context (A) to assess contextual freezing in a drug-free test session (15 minutes). Rats were returned to their home cages immediately after the test.
Histological Procedures
Upon completion of the experiment, rats were overdosed with sodium pentobarbital (Fatal Plus; 100 mg/ml, 0.5 ml, i.p.) and perfused transcardially with physiological saline followed by 10% formalin. Brains were extracted and stored overnight (at 4° C) in 10% formalin after which they were transferred to a 30% sucrose-formalin solution for a minimum of 3 days. After fixation and cryoprotection, brains were flash frozen on dry ice and sections containing either CeA or BNST were collected using a cryostat (Leica Microsystems) at −20° C. Coronal sections (40 μm thick) were mounted on subbed microscope slides and stained with thionin (0.25%) for cannula tract visualization. Glass coverslips were mounted on the slides using Permount mounting medium (Fisher Scientific). Coronal sections were imaged at 10× using a Leica Microscope (MZFLIII) with Leica FireCam software. Only animals with bilateral placement of injector tips within the borders of the BNST or CeA were included in the final analyses (shown in the figures). Localization of injector tips were determined by an experimenter blind to the group assignments of the subjects.
Statistics
All behavioral data were analyzed with repeated measures ANOVA (StatView, SAS Institute) with variables of training procedure (FW or BW), sex (female or male), and drug treatment (VEH, CeA-APV, or BNST-APV) (α = 0.05). Bonferroni’s test was used for post-hoc analyses. Results are shown as means (±SEM).
Results
Backward, but not forward conditioning, requires NMDA receptors in the BNST
Immediately prior to fear conditioning, animals were infused with the NMDA antagonist APV into either the CeA (CeA-APV) or BNST (BNST-APV); saline (VEH) infusions served as the control. Rats were placed in context A where they underwent forward (FW) or backward (BW) fear conditioning procedures as described above. A summary of the behavioral design is shown in Figure 1 and representative cannula tracts and histological placements are shown in Figure 2.
Figure 2. Cannula placements.
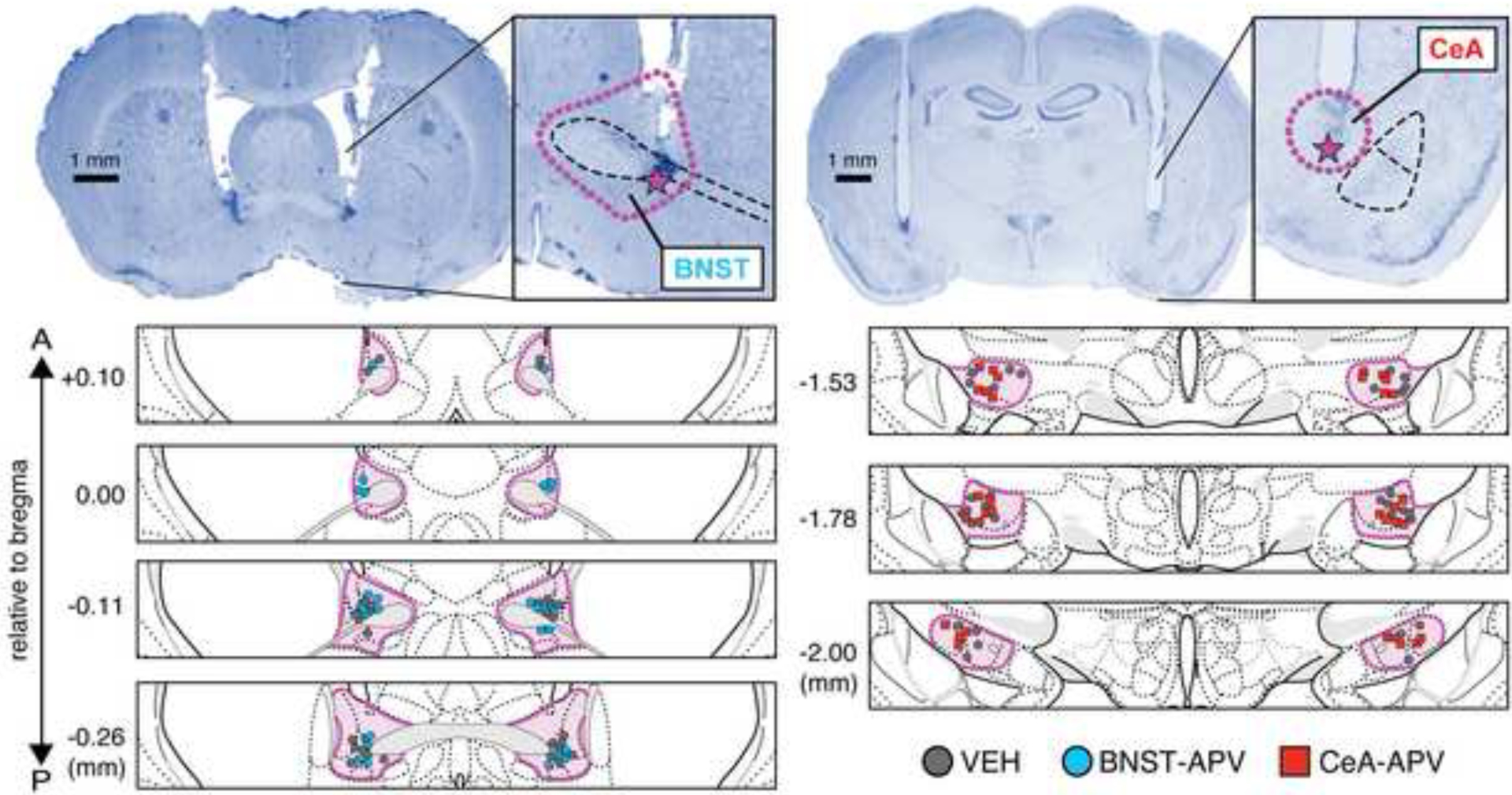
Representative photomicrographs of thionin-stained coronal sections (40 μm) with bilateral cannula placements aimed at the BNST (A) or CeA (B). Purple dotted lines indicate the approximate borders of the BNST and CeA, respectively. Purple stars denote the location of the injector tip of the cannula tract in the representative tissue. Symbols correspond to injector tips of each animal included in the final analyses. Atlas figures are adapted from (Swanson, 2018).
As shown in Figure 3, freezing behavior significantly increased throughout the conditioning session as indicated by a main effect of trial [F(4, 200) = 144.69, p < 0.0001]. A significant trial × drug interaction was detected [F(8, 200) = 4.06, p = 0.0002]; post-hoc comparisons (Bonferroni’s test) revealed that BNST-APV animals exhibited significantly more freezing than CeA-APV (p = 0.0081) or VEH animals (p = 0.0086) during the conditioning trials, independent of the FW or BW training. Additionally, the analysis revealed a main effect of sex [F(1, 50) = 5.37, p = 0.02], such that females exhibited higher levels of freezing overall; nonetheless, sex did not interact with any others in the analysis. No other main effects or interactions were detected in the ANOVA (F’s < 2.58, p’s > 0.06). Thus, both FW- and BW-conditioned rats exhibited robust freezing during conditioning, and this was not impaired by APV infusion into either the BNST or CeA, though freezing during conditioning was somewhat elevated in BNST-APV animals.
Figure 3. Intra-BNST or intra-CeA infusion of APV impairs auditory fear conditioning.
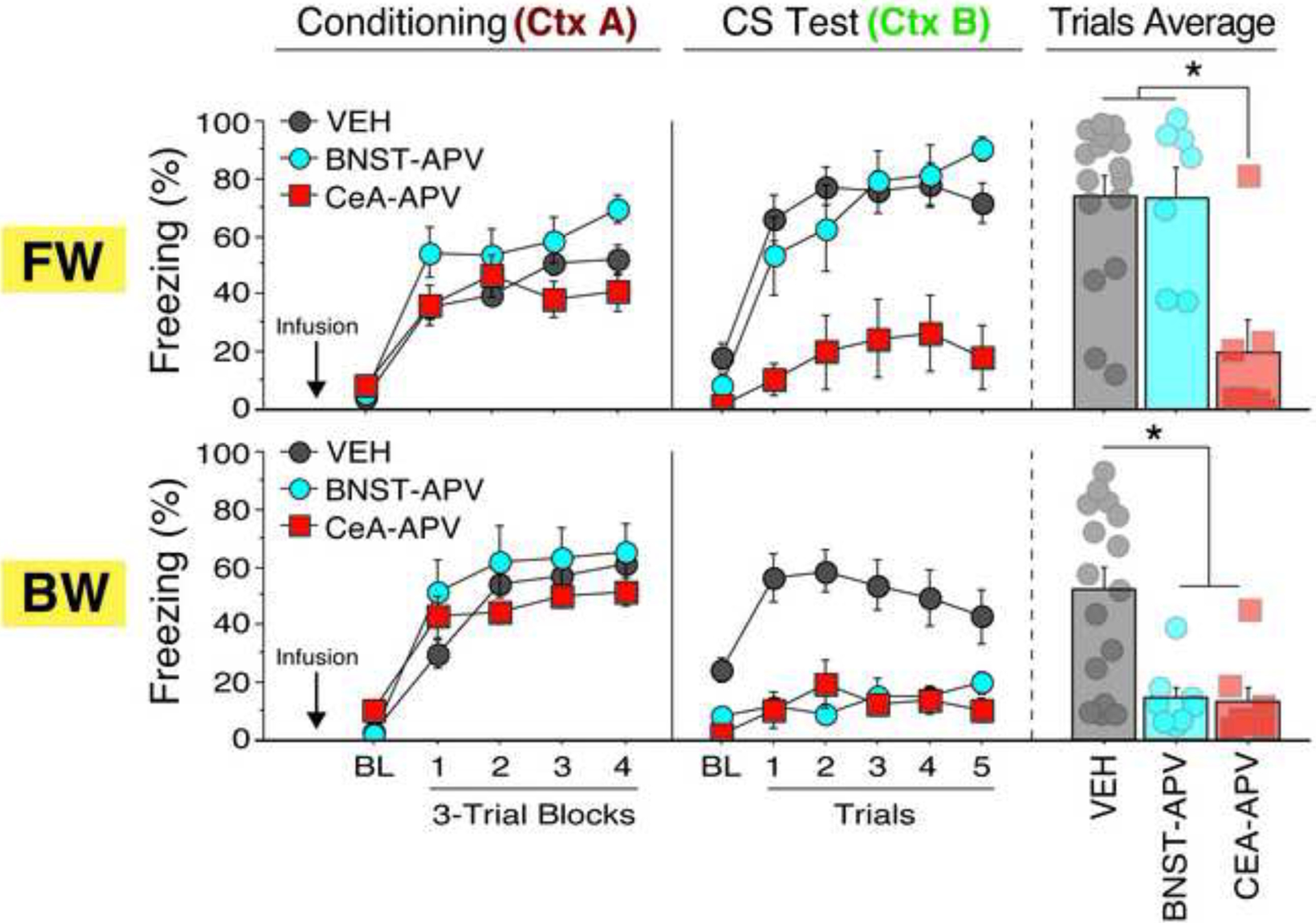
Mean percentage freezing (±SEM) during FW (top panels) or BW (bottom panels) conditioning (each trial block consisting of the three tones and their intertrial intervals), retrieval to the CS alone, and mean CS responding during test trials 1–5 (consisting of each tone and its intertrial interval). * = p < 0.05.
Forty-eight hours following conditioning, rats were placed into a novel context (B) and presented with five tone-alone presentations to assess retention of fear memory to the CS. As shown in Figure 3, intra-BNST APV selectively impaired freezing responses to the BW CS; it had no effect on freezing behavior to the FW CS. In contrast, intra-CeA APV resulted in robust impairments in freezing behavior to both the FW and BW CS. Analysis of freezing behavior across the entire session (including the baseline) revealed a main effect of trial [F(5, 250) = 43.27, p < 0.0001] (as freezing increased across the session) and a significant main effect of conditioning procedure [F(1, 50) = 13.91, p = 0.0005], such that freezing to the FW CS was higher overall than BW freezing. The ANOVA also revealed a significant main effect of drug treatment [F(2, 50) = 17.50, p < 0.0001] and a significant trial × conditioning procedure × drug interaction [F(10, 250) = 2.40, p = 0.01]. Post-hoc comparisons revealed that rats in the BNST-APV group that underwent BW conditioning showed significantly less freezing during the retention test than VEH-treated rats (p = 0.001). Conversely, rats in the BNST-APV group that underwent FW conditioning showed no difference relative to VEH-treated rats (p = 0.85). Note that these data suggest that the higher levels of freezing observed in BNST-APV animals during conditioning did not translate into higher levels of conditioning fear at recall.
In contrast to these effects, FW and BW animals that received intra-CeA APV showed significantly less freezing than FW-VEH (p < 0.0001) and BW-VEH (p = 0.001) groups, respectively. CeA-APV rats also differed from BNST-APV animals in the FW condition (p = 0.0004), but not in the BW condition (p = 0.87). To examine whether the observed effects were specific to CS-evoked freezing, we ran a separate factorial ANOVA of the post-BL trials (Figure 3). This analysis revealed a main effect of conditioning procedure [F(1, 50) = 28.93, p < 0.0001] and a main effect of drug [F(2, 50) = 8.85, p = 0.0005] and a significant conditioning procedure × drug interaction [F(2, 50) = 5.97, p = 0.005]. Post-hoc comparisons indicated that although BW conditioning was reduced by APV infusion into either the CeA (p = 0.0006) or BNST (p = 0.001), FW conditioning was only reduced by intra-CeA (p < 0.0001), but not intra-BNST (p = 0.85), APV. Lastly, there was a significant trial × sex interaction [F(5, 250) = 2.88, p = 0.02] with male rats exhibiting higher levels of freezing than females; sex did not interact with any other variables and there were no other main effects or interactions (F’s < 1.54, p’s > 0.22). Hence, the predictive relationship between the CS and the US regulates the role for BNST NMDA receptors in fear conditioning, whereas CeA NMDA receptors are involved in FW and BW fear conditioning. Moreover, APV-induced deficits on the retention of conditioned fear were not associated with a failure to express freezing during the conditioning session.
Acquisition of contextual fear requires NMDA receptors in both the CeA and BNST
Twenty-four hours after the CS retention test, rats were returned to the conditioning context (A) to examine the impact of NMDA receptor antagonism on freezing to contextual cues (Figure 4). Because we found no main effect of conditioning procedure (FW or BW) and no significant interactions between conditioning procedure and any other variable (sex or drug) in the analysis (F’s < 1.37, p’s > 0.25) we collapsed this factor for the analysis (Figure 4A). Freezing behavior was significantly reduced in rats that received intra-cranial infusion of APV relative to VEH, independent of brain region. Repeated measures ANOVA revealed a main effect of drug [F(2, 56) = 11.88, p < 0.0001], a main effect of time [F(14, 784) = 9.27, p < 0.0001], and a time × group interaction [F(28, 784) = 2.93, p < 0.0001]. No other main effects or interactions were detected (F’s < 0.87, p’s > 0.59). Post-hoc comparisons across the entire context test revealed that pre-conditioning APV infusions in the BNST significantly reduced contextual freezing relative to both VEH (p < 0.0001) and CeA-APV (p < 0.0001) rats. Conversely, CeA-APV animals did not differ across the entire session relative to VEH animals (p = 0.02). Interestingly, rats in the CeA group exhibited impairments in early, but not late, periods of the context test. To examine this further, we collapsed the session into an early period (i.e., the first 5 mins) and the remaining duration (i.e., the last 10 minutes) (Figure 4). Repeated measures ANOVA of freezing during these periods revealed a main effect of time [F(1, 56) = 40.07, p < 0.0001], main effect of drug [F(2, 56) = 12.49, p < 0.0001], and a drug × time interaction [F(2, 56) = 10.66, p < 0.0001]. No other main effects or interactions were observed (F’s < 0.78, p’s > 0.46). Post hoc comparisons showed that both BNST-APV (p < 0.0001) and CeA-APV (p = 0.0002) animals exhibited significantly less freezing than VEH-treated rats during the early time period. Conversely, freezing deficits were only observed in the BNST-APV group at the later time points relative to VEH (p < 0.0001) and CeA-APV (p = 0.0001). This pattern of deficits was similar when the FW and BW experiments are considered independently (Figure 4B). In total, these data suggest that NMDA receptors in the BNST and CeA make critical (but distinct) contributions to the acquisition of contextual fear (Figure 5).
Figure 4. Intra-BNST or intra-CeA infusion of APV impairs contextual fear conditioning.
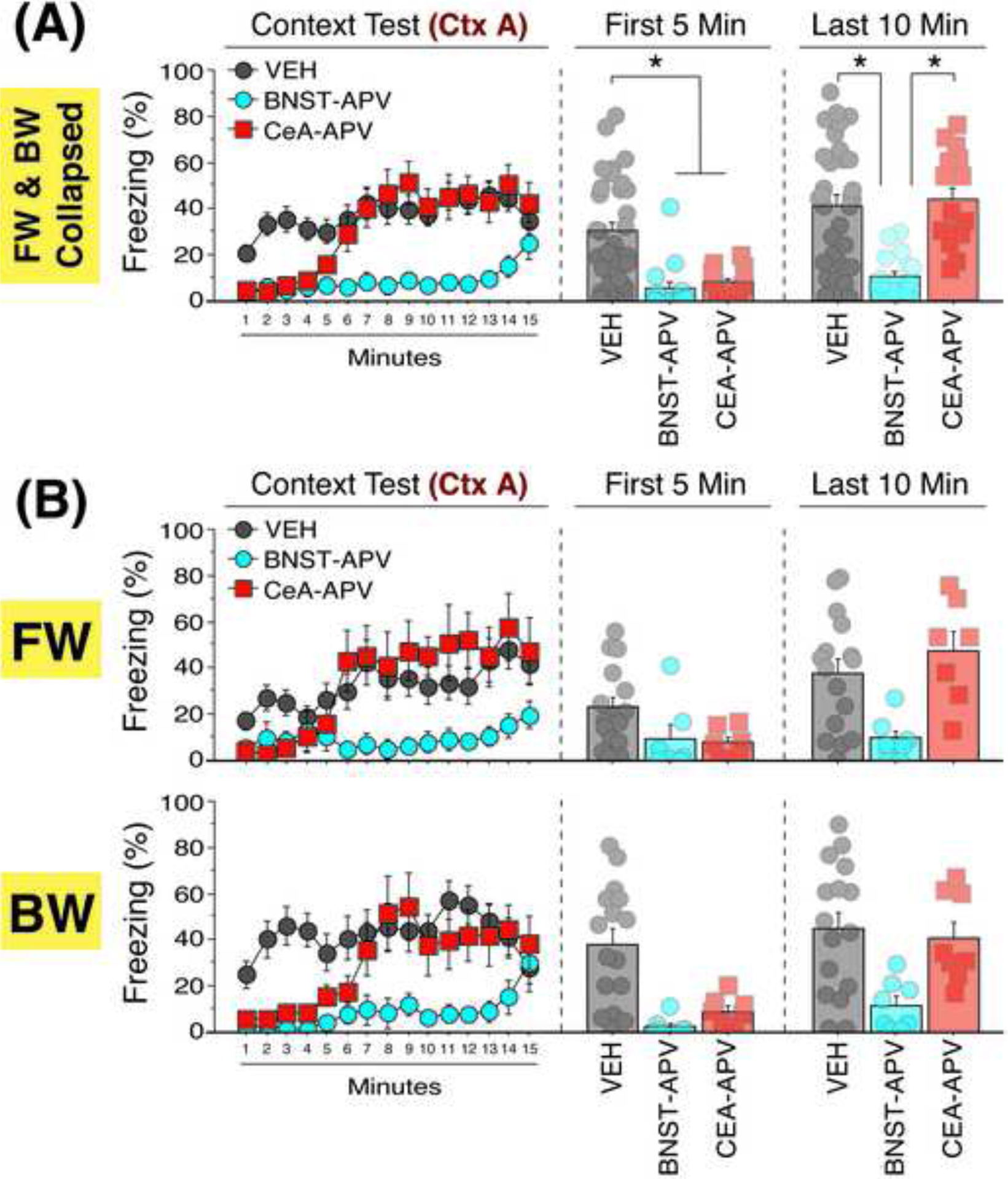
(A) Mean percentage freezing (±SEM) during each minute of retrieval to the conditioning context, collapsed across FW and BW training. Mean percentage freezing of minutes 1–5 (First 5 Min) and minutes 6–15 (Last 10 Min) are shown in the bar graphs. (B) Mean percentage freezing during each minute of retrieval to the conditioning context, split by animals trained under FW or BW conditions. * = p < 0.05.
Figure 5. Summary of the effects of intra-BNST or intra-CeA administration of APV on fear conditioning.
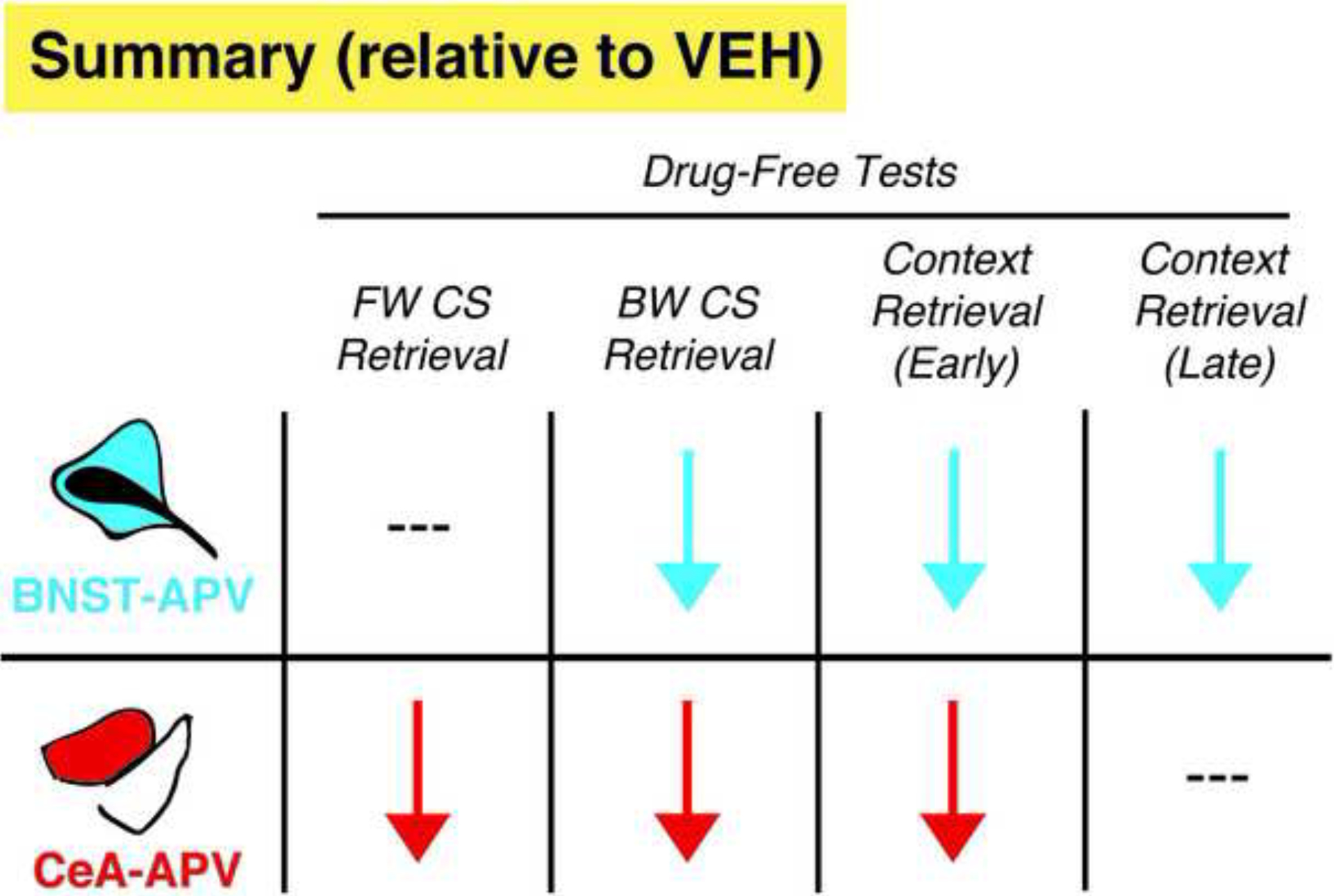
Arrows indicate significant reductions in freezing levels relative to VEH animals in the current study, whereas hash marks denote no significant changes. BNST-APV and CeA-APV animals exhibited dissociable as well as overlapping deficits in learning.
Discussion
Here we demonstrate a dissociable role for the BNST in the acquisition of conditioned freezing to unpredictable and predictable threat stimuli. Specifically, NMDA receptor antagonism in the BNST prior to fear conditioning significantly reduced freezing to an unpredictable BW CS, but not a predictable FW CS. NMDA receptor antagonism in the BNST also broadly reduced contextual freezing. Conversely, APV administration into the CeA prior to conditioning disrupted conditioned freezing to both the FW and BW CS. Although contextual freezing was also disrupted by intra-CeA APV, the impairment was restricted to early portion of the test session, whereas intra-BNST APV impaired freezing during throughout the test. Altogether these data reveal dissociable roles for NMDA receptors in the CeA and BNST in the acquisition of conditioned fear to predictable and unpredictable threats (Figure 5).
Anatomically the BNST is well positioned to integrate information from the amygdala (Krettek and Price, 1978; Sun et al., 1991; Dong et al., 2001), hippocampus (Cullinan et al., 1993), and prefrontal cortex (PFC) (McDonald et al., 1999; Hoover and Vertes, 2007), structures that provide contextual and nociceptive information that may be critical to learning-related plasticity in this region. Moreover, efferent projections to the hypothalamus and periaqueductal gray (Holstege et al., 1985; Gray and Magnuson, 1992; Nagy and Paré, 2008) position the BNST to potentially elicit or modulate defensive responses in the presence of threat. Indeed, substantial work in both humans and rodents has implicated the BNST in conditioned fear (Sullivan et al., 2004; Grillon and Morgan, 1999; Duvarci et al., 2009; Somerville et al., 2010; Alvarez et al., 2011; Zimmerman and Maren, 2011; Hott et al., 2012, 2017; Davis and Walker, 2014; Goode et al., 2015; Hammack et al., 2015; Herrmann et al., 2016; Marcinkiewcz et al., 2016; Asok et al., 2018; Luyck et al., 2018, 2020; Bjorni et al., 2020; Williams and Lattal, 2020).
In line with these data, a recent report from our lab demonstrated a role for the BNST in the expression of fear to unpredictable – but not predictable – threat signals (Goode et al., 2019). Specifically, this study demonstrated that the expression of fear to a BW (but not FW) CS is attenuated by muscimol infusions into the BNST; similar results were obtained if the CS was trained with randomized onset of the US. Based on these findings, we and others have argued that the BNST is involved in the expression of conditioned fear to threat signals that poorly predict US onset (Goode and Maren, 2017; Luyck et al., 2019; Miles and Maren, 2019). The current results extend these findings and show that NMDA receptors in the BNST are necessary for both backward fear conditioning to a discrete CS, as well contextual conditioning. These findings are also supported by recent work that observed deficits in contextual fear learning (as well in its reconditioning) after pharmacological inactivation of the BNST (Williams and Lattal, 2020) (Williams et al., 2019). Given that other studies have shown that BNST neurons exhibit experience- and NMDA-receptor-dependent plasticity (Vyas et al., 2003; Dumont et al., 2005; Kash et al., 2008a, 2008b, 2009; McElligott et al., 2010; Conrad et al., 2011; Wills et al., 2012; Haufler et al., 2013; Daldrup et al., 2016; Glangetas et al., 2017; Bjorni et al., 2020; Salimando et al., 2020), our data suggest that NMDA receptor-dependent plasticity in the BNST is critical to encoding CSs that poorly predict US onset. In line with this, a recent paper found that the spontaneous activity of BNST neurons is maximal during the period immediately after delivery of an aversive footshock during early conditioning trials, when the footshock is unexpected (Bjorni et al., 2020). Interestingly, this study found little evidence in support of a role for the BNST in cued (forward) fear conditioning. Instead the authors argued that, because responsive neurons exhibited firing rate changes during the post-shock period, when only contextual stimuli were present, these changes may be associated with contextual fear conditioning. Indeed, our current study supports this idea insofar as intra-BNST APV was shown to selectively affect the acquisition of fear to a BW CS, which occurs at the time BNST neurons exhibit the largest changes in firing rate.
In contrast to the BNST, APV administration into the CeA resulted in deficits in conditioned freezing to both the predictable FW and unpredictable BW CS. Several studies have shown that genetically distinct populations within the CeA undergo learning-dependent modifications following fear conditioning (Ciocchi et al., 2010; Duvarci et al., 2011; Li et al., 2013; Penzo et al., 2014; Fadok et al., 2017; Sanford et al., 2017) and plasticity within the BLA has been shown to rely on activity within the CeA (Yu et al., 2017). Thus, although plasticity within the CeA may be important for fear conditioning to both predictable and unpredictable threat cues, it is also possible that NMDA receptor antagonism within the CeA indirectly affects learning related plasticity in other brain regions (e.g., BNST) that, in turn, mediate dissociable forms of fear learning.
Prior research in both rodents and humans has suggested that while the CeA mediates phasic forms of fear expression, the BNST may instead control sustained fear states, which are often attributed to unpredictability (Davis et al., 2010). Indeed, there is evidence that the BNST and CeA may mediate different aspects of conditioned fear (Walker and Davis, 2008; Walker et al., 2009; Davis et al., 2010), but other studies have suggested these regions have overlapping or perhaps complementary functions (Sullivan et al., 2004; Fox et al., 2015; Gungor and Paré, 2016). The results of the current study are consistent with the proposed role of the CeA in phasic fear responses, insofar as the effects of NMDA antagonism within the CeA were restricted to the early portions of the context test. Interestingly, this freezing deficit was observed during an early part of the test (~5 min) that is similar to the length of the pre-shock baseline. As time passes in conditioned context, the uncertainty of shock onset may increase and become independent of the CeA. Given the deficit in freezing to the BW CS in the CeA-APV animals (as well as the low freezing of BNST-APV animals in the early portion of the context test), these findings also suggest that CeA-dependent plasticity is required for some aspects of BNST-dependent defensive behaviors (serving complementary roles). Nonetheless, these and other findings (Resstel et al., 2008; Mobbs et al., 2010; Choi et al., 2012; Shackman and Fox, 2016) suggest that BNST activity may not be limited to sustained responses alone, and can influence the rapid onset of defensive behaviors, at least in some cases.
Given the evidence for sexual dimorphisms in the anatomy of the BNST (Allen and Gorski, 1990; Hines et al., 1992), along with its well appreciated role in contextual fear, there has been significant interest in understanding how these neuroanatomical differences may contribute to differences in fear and anxiety. In particular, several studies have shown that males and females exhibit differences in conditioned fear to contexts, but not discrete CSs (Maren et al., 1994; Markus and Zecevic, 1997; Barker and Galea, 2010). Note that these effects may depend in part on the behavioral measure (e.g., freezing), insofar as female rats exhibit active defensive behaviors (e.g., “darting”; (Gruene et al., 2015) under some conditions. Given this, it’s possible that a lack of cued freezing in APV-infused animals in the current study reflects a change in fear response modality (e.g., darting versus freezing), as opposed to a true memory impairment. Although we did not conduct a formal analysis to examine darting behavior in the present study, we have failed to observe this behavior in current (unpublished) and past work (Maren et al., 1994), suggesting that the effects of APV in the present study were specific to an impairment in the acquisition of the cued fear memory. Although the current results suggest that NMDA receptors within the BNST and CeA play similar roles in fear conditioning in males and females, it is possible that different signaling mechanisms [e.g., neurosteroids; (Nagaya et al., 2015; Acca et al., 2017)], particularly within the BNST, may contribute to sex-related differences in fear learning.
It should be noted that there are several limitations to the current study. As a whole, the BNST is composed of several different subdivisions with unique neurochemical signatures, each of which are thought to play unique roles in fear and anxiety related behaviors (Jennings et al., 2013; Kim et al., 2013; Daniel and Rainnie, 2016; Gungor and Paré, 2016; Lebow and Chen, 2016; Giardino et al., 2018; Yamauchi et al., 2018; Xiao et al., 2020). In the present study, our histological analysis revealed that infusions sites were not restricted to any particular subregion of the BNST. Thus, our study is limited by the fact that we cannot attribute a role for NMDA receptors in the current procedures to any particular subdivision of the BNST. Additionally, although we assume that NMDA receptor antagonists influenced performance by disrupting learning-related synaptic plasticity, we cannot rule out the possibility that intracranial APV infusions impaired basal synaptic transmission (Maren and Fanselow, 1995; Maren et al., 1996). In addition, one could argue that the use of a BW conditioning procedure in the current study resulted in forward trace conditioning after the first trial, and therefore was not a truly “unpredictable” training procedure. Although a role for the BNST in trace conditioning has not been established, it may be required on the basis that trace conditioning degrades the temporal relationship between the CS and the US. Lastly, BW conditioning resulted in lower levels of conditioned freezing relative FW conditioning. Given this observation, one could argue that the BNST NMDA receptor antagonists produce impairments with procedures that produce weak, but not strong, fear conditioning. However, we and others have demonstrated that BNST lesions or inactivation reduce the expression of conditioned freezing even when those levels are high (Goode et al., 2015, 2019, 2020; Hammack et al., 2015). Based on these findings, we have argued that magnitude or duration of freezing is not predictive of BNST involvement (Goode and Maren, 2017). With regard to the CeA, future studies will need to be conducted to determine whether its participation is unique to BW conditioning, or whether it also plays a role in the acquisition of conditioned fear to truly unpredictable threat signals.
Altogether, the present results build on previous research demonstrating that an extended network of brain structures mediate different forms of fear conditioning. Moreover, these results reveal for the first time that NMDA receptors in the BNST are necessary for the acquisition of conditioned fear to unpredictable threats, including contextual fear. Although further electrophysiological studies will be needed to determine whether plasticity within the BNST does indeed mediate the learning of conditioned fear to unpredictable threats, the current results suggest that NMDA receptors within the BNST are critical for aversive learning and memory within the extended amygdala.
Supplementary Material
Highlights:
NMDA receptors in the BNST are required for the acquisition of BW, but not FW, conditioned fear.
NMDA receptors in the CeA are required for acquisition of FW and BW conditioned fear.
NMDA receptors in the CeA and BNST make distinct contributions to conditioned contextual fear.
Acknowledgments:
This work was supported by NIH grants F31MH107113 (TDG), R01MH065961 and R01MH117852 (SM), and the Brain & Behavioral Research Foundation NARSAD Distinguished Investigator grant (SM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests.
Data availability: All datasets used in the current study are available from the corresponding author upon request.
Reference List
- Acca GM, Mathew AS, Jin J, Maren S, Nagaya N (2017) Allopregnanolone induces state-dependent fear via the bed nucleus of the stria terminalis. Horm Behav 89:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LS, Gorski RA (1990) Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol 302:697–706. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C (2011) Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage 55:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Draper A, Hoffman AF, Schulkin J, Lupica CR, Rosen JB (2018) Optogenetic silencing of a corticotropin-releasing factor pathway from the central amygdala to the bed nucleus of the stria terminalis disrupts sustained fear. Mol Psychiatry 23:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Galea LAM (2010) Males show stronger contextual fear conditioning than females after context pre-exposure. Physiol Behav 99:82–90. [DOI] [PubMed] [Google Scholar]
- Bjorni M, Rovero NG, Yang ER, Holmes A, Halladay LR (2020) Phasic signaling in the bed nucleus of the stria terminalis during fear learning predicts within- and across-session cued fear expression. Learn Mem 27:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE (2001) Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem 8:229–242. [DOI] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM (2015) Resolving the neural circuits of anxiety. Nat Neurosci 18:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Davis M (1995) Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci 15:2312–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Padmala S, Pessoa L (2012) Impact of state anxiety on the interaction between threat monitoring and cognition. Neuroimage 59:1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A (2010) Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468:277–282. [DOI] [PubMed] [Google Scholar]
- Collins DR, Paré D (2000) Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−). Learn Mem 7:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Louderback KM, Gessner CP, Winder DG (2011) Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiol Behav 104:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Hermans D, Vansteenwegen D eds. (2006) Fear and learning: From basic processes to clinical implications. Washington: American Psychological Association. [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ (1993) Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol 332:1–20. [DOI] [PubMed] [Google Scholar]
- Daldrup T, Lesting J, Meuth P, Seidenbecher T, Pape H-C (2016) Neuronal correlates of sustained fear in the anterolateral part of the bed nucleus of stria terminalis. Neurobiol Learn Mem 131:137–146. [DOI] [PubMed] [Google Scholar]
- Daniel SE, Rainnie DG (2016) Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology 41:103–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CD, Craske MG (2015) Psychophysiological responses to unpredictable threat: effects of cue and temporal unpredictability. Emotion 15:195–200. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL (2014) Role of bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Struct Funct 219:1969–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C (2010) Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35:105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW (2001) Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 38:192–246. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT (2005) Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci 8:413–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Paré D (2009) The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci 29:10357–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Paré D (2011) Central amygdala activity during fear conditioning. J Neurosci 31:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Müller C, Kovacevic A, Tovote P, Lüthi A (2017) A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542:96–100. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE (1999) Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23:229–232. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO (1992) Uncontrollability and unpredictability in posttraumatic stress disorder: an animal model. Psychol Bull 112:218–238. [DOI] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp DPM, Fudge JL, Kalin NH (2015) Extending the amygdala in theories of threat processing. Trends Neurosci 38:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li S-B, Malenka RC, de Lecea L (2018) Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat Neurosci 21:1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glangetas C, Massi L, Fois GR, Jalabert M, Girard D, Diana M, Yonehara K, Roska B, Xu C, Lüthi A, Caille S, Georges F (2017) NMDA-receptor-dependent plasticity in the bed nucleus of the stria terminalis triggers long-term anxiolysis. Nat Commun 8:14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Acca GM, Maren S (2020) Threat imminence dictates the role of the bed nucleus of the stria terminalis in contextual fear. Neurobiol Learn Mem 167:107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Jin J, Maren S (2018) Neural circuits for fear relapse In: Neurobiology of abnormal emotion and motivated behaviors, pp 182–202. Elsevier. [Google Scholar]
- Goode TD, Kim JJ, Maren S (2015) Reversible inactivation of the bed nucleus of the stria terminalis prevents reinstatement but not renewal of extinguished fear. Eneuro 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Maren S (2017) Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn Mem 24:480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Ressler RL, Acca GM, Miles OW, Maren S (2019) Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S (2003) Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci 117:738–750. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ (1992) Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides 13:451–460. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J (2004) Anxious responses to predictable and unpredictable aversive events. Behav Neurosci 118:916–924. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA (1999) Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol 108:134–142. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM (2015) Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB (2013) Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 14:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, Paré D (2016) Functional heterogeneity in the bed nucleus of the stria terminalis. J Neurosci 36:8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Todd TP, Kocho-Schellenberg M, Bouton ME (2015) Role of the bed nucleus of the stria terminalis in the acquisition of contextual fear at long or short context-shock intervals. Behav Neurosci 129:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D (2013) Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem 20:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Boehme S, Becker MPI, Tupak SV, Guhn A, Schmidt B, Brinkmann L, Straube T (2016) Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum Brain Mapp 37:1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA (1992) Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res 579:321–326. [DOI] [PubMed] [Google Scholar]
- Holstege G, Meiners L, Tan K (1985) Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res 58:379–391. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212:149–179. [DOI] [PubMed] [Google Scholar]
- Hott SC, Gomes FV, Fabri DRS, Reis DG, Crestani CC, Côrrea FMA, Resstel LBM (2012) Both α1- and β1-adrenoceptors in the bed nucleus of the stria terminalis are involved in the expression of conditioned contextual fear. Br J Pharmacol 167:207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hott SC, Gomes FV, Uliana DL, Vale GT, Tirapelli CR, Resstel LBM (2017) Bed nucleus of the stria terminalis NMDA receptors and nitric oxide modulate contextual fear conditioning in rats. Neuropharmacology 112:135–143. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD (2013) Distinct extended amygdala circuits for divergent motivational states. Nature 496:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE (2011) Molecular mechanisms of fear learning and memory. Cell 147:509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, Conrad KL, Colbran RJ, Winder DG (2009) Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology 34:2420–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Matthews RT, Winder DG (2008a) Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology 33:1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG (2008b) Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci 28:13856–13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K (2013) Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL (1978) Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol 178:225–254. [DOI] [PubMed] [Google Scholar]
- Lebow MA, Chen A (2016) Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry 21:450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM (1990) The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 10:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988) Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8:2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B (2013) Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 16:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-L, Chen D-Y, Liang KC (2009) Post-training infusion of glutamate into the bed nucleus of the stria terminalis enhanced inhibitory avoidance memory: an effect involving norepinephrine. Neurobiol Learn Mem 91:456–465. [DOI] [PubMed] [Google Scholar]
- Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, Minick P, Shekhar A, Truitt WA (2012) Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav 107:726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyck K, Arckens L, Nuttin B, Luyten L (2020) It takes two: Bilateral bed nuclei of the stria terminalis mediate the expression of contextual fear, but not of moderate cued fear. Prog Neuropsychopharmacol Biol Psychiatry:109920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyck K, Goode TD, Lee Masson H, Luyten L (2019) Distinct Activity Patterns of the Human Bed Nucleus of the Stria Terminalis and Amygdala during Fear Learning. Neuropsychol Rev 29:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyck K, Nuttin B, Luyten L (2018) Electrolytic post-training lesions of the bed nucleus of the stria terminalis block startle potentiation in a cued fear conditioning procedure. Brain Struct Funct 223:1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier CE, Tipton GJ, Ramakrishnan C, Kozicz T, Deisseroth K, Thiele TE, McElligott ZA, Holmes A, Heisler LK, Kash TL (2016) Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (1998) Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci 18:3088–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2001) Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24:897–931. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS (1996) N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci 110:1365–1374. [DOI] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS (1994) Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res 661:25–34. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS (1995) Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci 15:7548–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I (2013) The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ (2004) Neuronal signalling of fear memory. Nat Rev Neurosci 5:844–852. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Zecevic M (1997) Sex differences and estrous cycle changes in hippocampus-dependent fear conditioning. Psychobiology. [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M (1999) Cortical afferents to the extended amygdala. Ann N Y Acad Sci 877:309–338. [DOI] [PubMed] [Google Scholar]
- McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, Winder DG (2010) Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc Natl Acad Sci USA 107:2271–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles OW, Maren S (2019) Role of the bed nucleus of the stria terminalis in PTSD: insights from preclinical models. Front Behav Neurosci 13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Hendersen RW (1985) Controllability and predictability in acquired motivation. Annu Rev Psychol 36:495–529. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T (2010) Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci USA 107:20582–20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Acca GM, Maren S (2015) Allopregnanolone in the bed nucleus of the stria terminalis modulates contextual fear in rats. Front Behav Neurosci 9:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy FZ, Paré D (2008) Timing of impulses from the central amygdala and bed nucleus of the stria terminalis to the brain stem. J Neurophysiol 100:3429–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov PI (1927) Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Ann Neurosci 17:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Li B (2014) Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci 34:2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, Li B (2015) The paraventricular thalamus controls a central amygdala fear circuit. Nature 519:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Ponnusamy R, Dong H-W, Fanselow MS (2010) Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proc Natl Acad Sci USA 107:14881–14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler RL, Maren S (2019) Synaptic encoding of fear memories in the amygdala. Curr Opin Neurobiol 54:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LBM, Alves FHF, Reis DG, Crestani CC, Corrêa FMA, Guimarães FS (2008) Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience 154:869–876. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE (1993) Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci 107:444–450. [DOI] [PubMed] [Google Scholar]
- Salimando GJ, Hyun M, Boyt KM, Winder DG (2020) BNST GluN2D-Containing NMDA Receptors Influence Anxiety- and Depressive-like Behaviors and ModulateCell-Specific Excitatory/Inhibitory Synaptic Balance. J Neurosci 40:3949–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RD, Paré D (2005) Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J Neurosci 25:1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, Clark M, Zweifel LS (2017) A Central Amygdala CRF Circuit Facilitates Learning about Weak Threats. Neuron 93:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS (2016) Contributions of the central extended amygdala to fear and anxiety. J Neurosci 36:8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M (2013) Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry 18:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM (2010) Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 68:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE (2004) Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience 128:7–14. [DOI] [PubMed] [Google Scholar]
- Sun N, Roberts L, Cassell MD (1991) Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Res Bull 27:651–662. [DOI] [PubMed] [Google Scholar]
- Swanson LW (2018) Brain maps 4.0-Structure of the rat brain: An open access atlas with global nervous system nomenclature ontology and flatmaps. J Comp Neurol 526:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16:317–331. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S (2003) Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res 965:290–294. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME (2006) Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci 120:324–336. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M (2008) Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct 213:29–42. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M (2009) Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry 33:1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE (2006) Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26:12387–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Kim ES, Lattal KM (2019) Behavioral and immunohistochemical characterization of rapid reconditioning following extinction of contextual fear. Learn Mem 26:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Lattal KM (2020) Involvement of the bed nucleus of the stria terminalis in initial conditioning and rapid reconditioning following extinction of contextual fear. Behav Neurosci 134:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, Delpire E, Winder DG (2012) GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci USA 109:E278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Zhou X, Wei P, Xie L, Han Y, Wang J, Cai A, Xu F, Tu J, Wang L (2020) A new GABAergic somatostatin projection from the BNST onto accumbal parvalbumin neurons controls anxiety. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi N, Takahashi D, Sugimura YK, Kato F, Amano T, Minami M (2018) Activation of the neural pathway from the dorsolateral bed nucleus of the stria terminalis to the central amygdala induces anxiety-like behaviors. Eur J Neurosci 48:3052–3061. [DOI] [PubMed] [Google Scholar]
- Yu K, Ahrens S, Zhang X, Schiff H, Ramakrishnan C, Fenno L, Deisseroth K, Zhao F, Luo M-H, Gong L, He M, Zhou P, Paninski L, Li B (2017) The central amygdala controls learning in the lateral amygdala. Nat Neurosci 20:1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S (2011) The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol Learn Mem 95:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


