Abstract
IMPORTANCE
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019, causing human coronavirus disease 2019 (COVID-19), which has now spread into a worldwide pandemic. The pulmonary manifestations of COVID-19 have been well described in the literature. Two similar human coronaviruses that cause Middle East respiratory syndrome (MERS-CoV) and severe acute respiratory syndrome (SARS-CoV-1) are known to cause disease in the central and peripheral nervous systems. Emerging evidence suggests COVID-19 has neurologic consequences as well.
OBSERVATIONS
This review serves to summarize available information regarding coronaviruses in the nervous system, identify the potential tissue targets and routes of entry of SARS-CoV-2 into the central nervous system, and describe the range of clinical neurological complications that have been reported thus far in COVID-19 and their potential pathogenesis. Viral neuroinvasion may be achieved by several routes, including transsynaptic transfer across infected neurons, entry via the olfactory nerve, infection of vascular endothelium, or leukocyte migration across the blood-brain barrier. The most common neurologic complaints in COVID-19 are anosmia, ageusia, and headache, but other diseases, such as stroke, impairment of consciousness, seizure, and encephalopathy, have also been reported.
CONCLUSIONS AND RELEVANCE
Recognition and understanding of the range of neurological disorders associated with COVID-19 may lead to improved clinical outcomes and better treatment algorithms. Further neuropathological studies will be crucial to understanding the pathogenesis of the disease in the central nervous system, and longitudinal neurologic and cognitive assessment of individuals after recovery from COVID-19 will be crucial to understand the natural history of COVID-19 in the central nervous system and monitor for any long-term neurologic sequelae.
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged from Wuhan, China, in December 2019, resulting in a severe outbreak of pneumonia1; SARS-CoV-2 causes a clinical syndrome, coronavirus disease 2019 (COVID-19), and its pulmonary manifestations have been well described. There is growing evidence of neurological complications and disease in patients with COVID-19. Two similar human coronaviruses (CoV), Middle East respiratory syndrome (MERS-CoV) and severe acute respiratory syndrome (SARS-CoV-1), have also been associated with neurological disease in rare cases. This raises the questions of whether SARS-CoV-2 is neurotropic and whether it contributes to postinfectious neurologic complications. A handful of case reports have described neurological complications in patients with COVID-19.1–4 However, it remains unknown to what extent SARS-CoV-2 damages the central nervous system (CNS) or if neurological symptoms are attributable to secondary mechanisms.
Search Strategy and Selection Criteria
References for this review were identified by searches of PubMed from April to May 2020 for articles published between 1969 and April 2020, as well as references from relevant articles. The search terms COVID-19, SARS-CoV-2, SARS-CoV, MERS-CoV, HCoV-OC43, neurotropism, neuroinvasion, and coronavirus were used. There were no language restrictions. The final list of included articles was generated on the basis of relevance to the topics covered in this review.
Neurotropic Coronaviruses
Coronaviruses (CoV) are large, enveloped, positive-sense RNA viruses divided into 3 genera: alphacoronavirus, betacoronavirus,and gammacoronavirus.5 These viruses infect humans and numerous animal species, generally causing upper or lower respiratory tract, gastrointestinal, neurological, or hepatic disease.6,7 Currently, there are 7 CoV that can infect humans, including human coronavirus (HCoV)–229E, HCoV-NL63, HCoV-HKU1, HCoV-OC43, MERS-CoV, SARS-CoV-1, and SARS-CoV-2.8 Betacoronaviruses SARS-CoV-2, SARS-CoV-1, and MERS-CoV are associated with severe disease in humans.1,3,8 Although HCoV are typically associated with respiratory tract disease, 3 HCoV have been shown to infect neurons:HCoV-229E, HCoV-OC43, and SARS-CoV-1.
HCoV-OC43
The neuroinvasive potential of HCoV-OC43hasbeenparticularlywell studied. It has been shown to thrive in neural cell in vitro cultures.9 Oligodendrocytes, astrocytes, microglia, and neurons are susceptible to acute infection with HCoV-OC43, and all except microglia support persistent infection.10 In murine models, HCoV-OC43 can invade the CNS intranasally, which is followed by a rapid spread throughout the CNS. Neuronal damage appears to be caused by direct, virus-mediated, and not immune-mediated injury.11 The CNS damage causes a range of neurological disorders in mice, including encephalitis and transient flaccid paralysis.12
In humans, history of infection with HCoV-OC43 is associated with multiple sclerosis (MS), based on the presence of viral RNA in autopsy brain tissue of donors who died with MS.13,14 In 1study,15 HCoV-OC43 RNA was also detected in the cerebrospinal fluid (CSF) in 10 of 20 living patients with MS. Although the mechanism of potential demyelination during HCoV-OC43 infection is unknown, this may be because of an adaptive immune response against HCoV-OC43 antigens that cross-react with myelin antigens. Indeed, peripheral T-cell clones in patients with MS have been shown to cross-react to both HCoV-OC43 and myelin antigens.16 In addition to demyelinating disease, there have also been pediatric case reports of children with severe immunosuppression developing encephalitis associated with HCoV-OC43 infection, with brain biopsies having positive results for HCoV-OC43 RNA on metagenomic sequencing.17,18
SARS-CoV-1
During the SARS pandemic of 2002–2003, neurological complications were reported in a subset of patients.19 A group from Taiwan reported 3 cases of axonal-variant Guillain-Barré syndrome(GBS)and 5 cases of ischemic stroke.20,21 One report22 described a patient with SARS presenting with a seizure with a positive CSF polymerase chain reaction result for SARS-CoV-1, although contamination of the CSF sample was possible. In addition, SARS-CoV-1 has been reliably detected in brain tissue specimens of autopsy donors with SARS, specifically in the cytoplasm of neurons in the cortex and hypothalamus, sometimes associated with neuronal edema and nuclear degeneration.23,24 Examination of autopsy tissue from a patient with encephalitis revealed neuronal necrosis, glial cell hyperplasia, and infiltration of monocytes and T cells.25 Additionally, virions were visualized in neurons on electron microscopy, and SARS-CoV-1 RNA was isolated from the specimen.25 In murine models, SARS-CoV-1 enters the CNS via the olfactory bulb and exhibits rapid trans synaptic spread. The infection causes significant neuronal damage and death without significant inflammatory infiltration.2
MERS-CoV
The Middle East respiratory syndrome CoV (MERS-CoV) first emerged in 2012, and since that time, approximately 2494 cases have been reported, with a 34.4% case mortality rate.26 Unlike SARS-CoV-1 and SARS-CoV-2, MERS-CoV binds to the dipeptidyl peptidase 4 receptor on cells to gain entry. Dipeptidyl peptidase 4 is widely expressed throughout the body on epithelia, vascular endothelia, and the brain.27,28 There have been several clinical case reports that suggest MERS-CoV can lead to neurological complications in humans. In a study29 of 70 patients, 6(9%) developed seizures, 9(13%) reported headache, and 18 (26%) experienced confusion. A case series30 highlighted 3 severe cases of neurological disease in MERS-CoV, including suspected acute disseminating encephalomyelitis, encephalitis, and widespread ischemic infarcts. Another case series4 highlighted neuromuscular disease in MERS-CoV, including 3 cases of GBS and a case of Bickerstaff encephalitis. However, although murine models develop CNS infection after intranasal inoculation with MERS-CoV, this virus has never been detected in the CNS of humans.28
Mouse Hepatitis Virus
Historically, the neuroinvasive potential of CoV has been illustrated through studies of the murine coronavirus mouse hepatitis virus. In mice, this virus induces a spectrum of neurological disease ranging from fatal encephalomyelitis to demyelinating disorders. Mouse hepatitis virus enters the CNS through hematogenous spread or intranasal inoculation.31,32 Once in the CNS, the virus is associated with an influx of immune cells, including CD-8 T cells, natural killer cells, and neutrophils. A significant increase in inflammatory cytokines, including interleukin 6 (IL-6), is observed in the CNS of infected mice.33
SARS-CoV-2
The SARS-CoV-2 virus shares close sequence homology to SARSCoV-1. Both viruses use spike proteins on the viral surface to bind to the angiotensin-converting enzyme 2 (ACE2) receptor on mammalian host cells, then use serine protease transmembrane protease serine 2 (TMPRSS2) to prime the spike.34 The presence of the ACE2 receptor in tissues determines viral cellular tropism in humans. In humans, ACE2 is expressed in airway epithelia, kidney cells, small intestine, lung parenchyma, and vascular endothelia throughout the body and widely throughout the CNS (Figure 1). Information about specific cellular and spatial localization within the human brain is emerging. A recent report35 (not yet peer reviewed) found that ACE2 is expressed in neurons, astrocytes, and oligodendrocytes. Expression of ACE2 was also highly concentrated in the substantia nigra, ventricles, middle temporal gyrus, posterior cingulate cortex, and olfactory bulb.35 This study35 compared human ACE2 expression with the mouse brain and demonstrated similar expression patterns. In other murine models, ACE2 expression has been identified in the motor cortex, cytoplasm of neurons, glial cells, and sympathetic pathways in the brainstem.36,37 In neuronal cell cultures, ACE2 is expressed both on the surface membrane and in the cytoplasm.38 Widespread ACE2 expression in the brain raises the concern that SARS-CoV-2, similarly to SARS-CoV-1, has the potential to infect neurons and glial cells throughout the CNS.
Figure 1. Angiotensin-Converting Enzyme 2 (ACE2) Expression in the Brain.
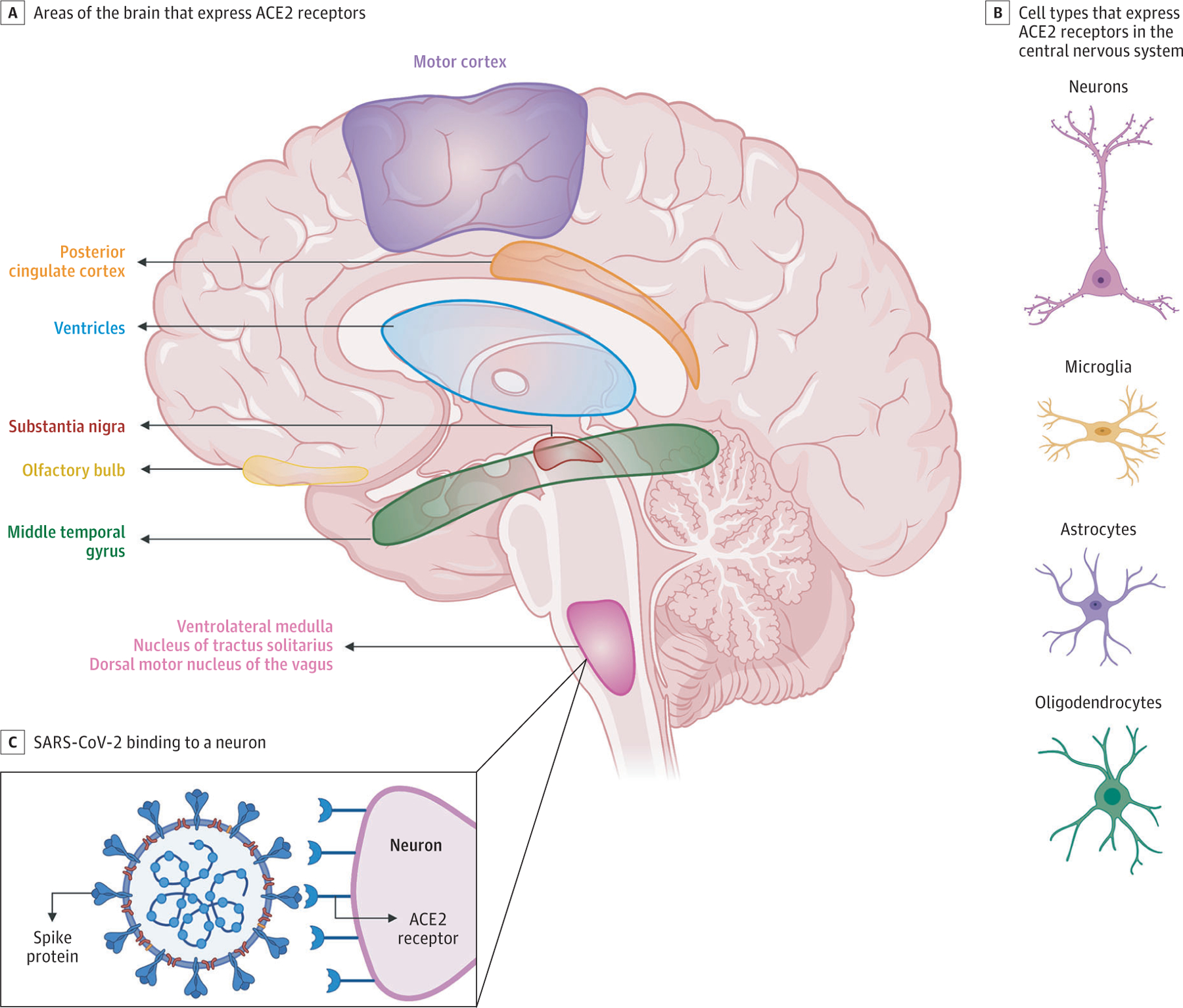
Emerging data suggest that ACE2 receptors are expressed in multiple regions of the human and mouse brain, including the motor cortex, posterior cingulate cortex, ventricles, substantia nigra, olfactory bulb, middle temporal gyrus, ventrolateral medulla, nucleus of tractus solitarius, and dorsal motor nucleus of the vagus nerve (A) and on several key cell types that make up the central nervous system, including neurons, microglia, astrocytes, and oligodendrocytes (B).35–37 C, ACE2 receptors on a medullary neuron binding to the SPIKE protein on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Potential Mechanisms of Neuroinvasion
Although there are reports of neurological complications in patients with COVID-19, it is unclear if SARS-CoV-2 is neurotropic in humans. Viral neuroinvasion could plausibly be achieved by several routes, including transsynaptic transfer across infected neurons (Figure 2), entry via the olfactory nerve (Figure 2), infection of vascular endothelium (Figure 3), or leukocyte migration across the blood-brain barrier (BBB) (Figure 3).
Figure 2. Transsynaptic Viral Spread.
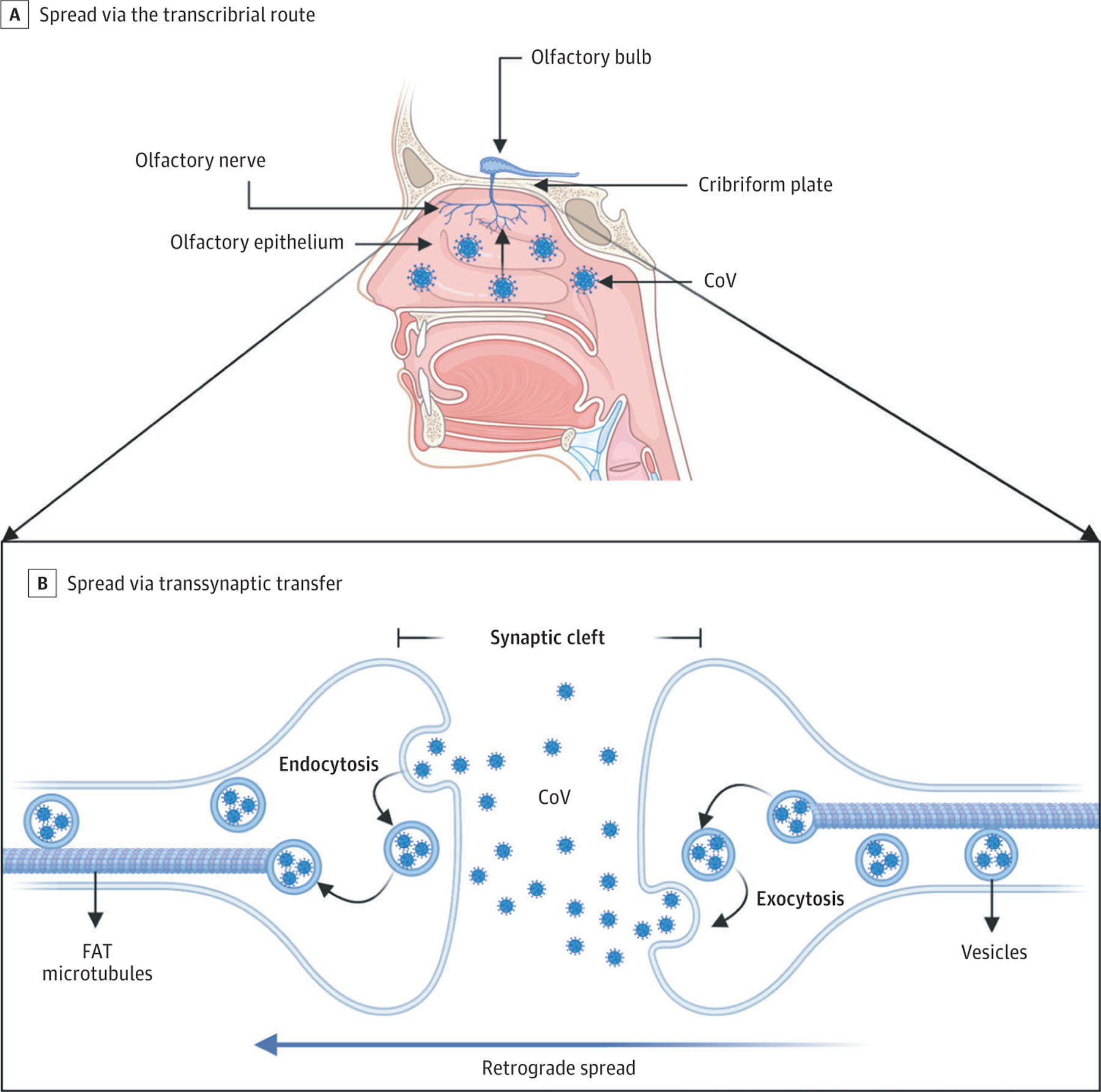
A, Coronavirus (CoV) has been shown to spread via the transcribrial route from the olfactory epithelium along the olfactory nerve to the olfactory bulb within the central nervous system. B, CoV has been shown to spread retrograde via transsynaptic transfer using an endocytosis or exocytosis mechanism and a fast axonal transport (FAT) mechanism of vesicle transport to move virus along microtubules back to neuronal cell bodies.
Figure 3. Mechanisms of Spread Across the Blood-Brain Barrier.
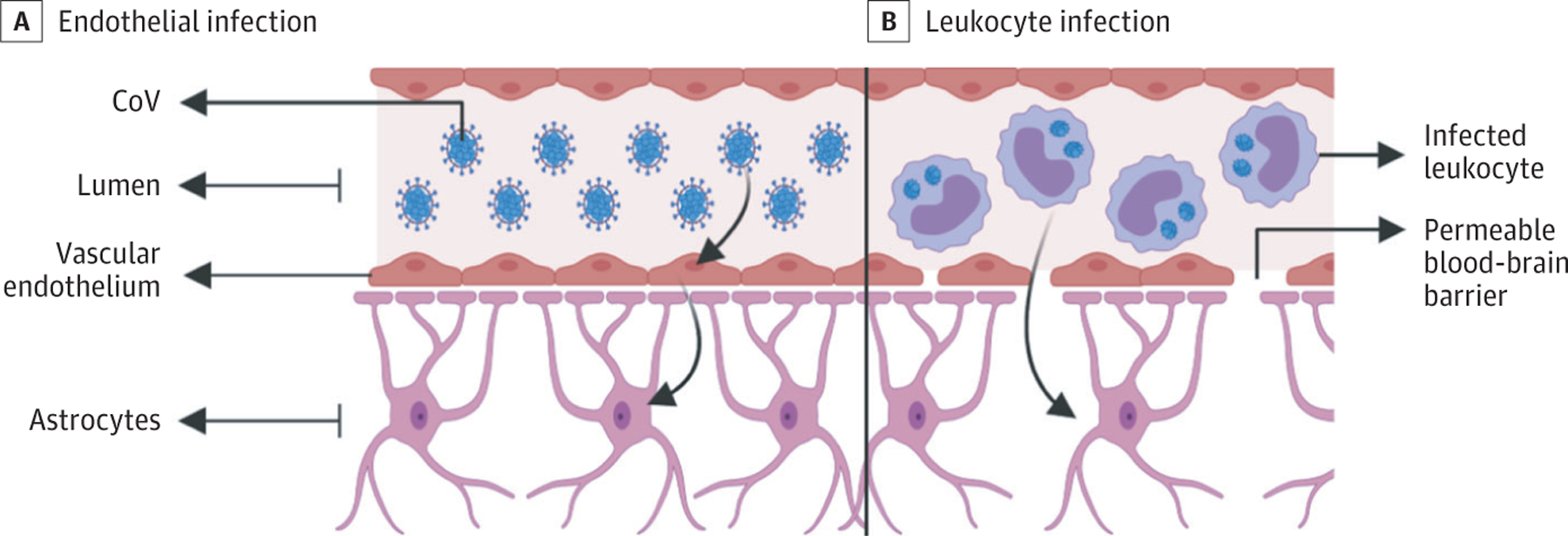
A, Infected vascular endothelial cells have been shown to spread severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to glial cells in the central nervous system. B, Known as the Trojan horse mechanism, infected leukocytes can cross the blood-brain barrier to infect the central nervous system. CoV indicates coronavirus.
Transsynaptic Spread
There is increasing evidence that human and nonhuman CoV invade peripheral nerve terminals, spread retrograde along nerve synapses, and gain access to the CNS (Figure 2).12,39 Transsynaptic transfer of virus has been demonstrated for several CoV, including HCoV-OC43, hemagglutinating encephalomyelitis virus 67(HEV67), and avian bronchitis virus.12 For example, HEV67 enters the oronasal cavity and infects the nasal mucosa, lung epithelium, and small intestine of suckling piglets and rats. It then infects the peripheral nerves and spreads retrograde to the dorsal root ganglion, ending in the medullary neurons.40 A membrane coating–mediated endocytotic or exocytotic pathway facilitates HEV67 transfer between motor cortex neurons.40 A similar, vesicle-mediated secretory pathway allows HEV67 to spread between neurons and satellite cells.41 For intracellular spread within a neuron, fast axonal transport uses axonal microtubules to move molecules retrograde or anterograde.12,42 Herpes simplex virus, HIV, and HCoV-OC43 have all been shown to use retrograde fast axonal transport to infect the cell body of neurons.42
During the COVID-19 outbreak, isolated loss of sense of smell (anosmia) and loss of sense of taste (ageusia) with or without respiratory symptoms has been reported.43 Direct entry along the olfactory nerve is another potential mechanism for SARS-CoV-2 entry to the CNS (Figure2). In a transgenic mouse model that expresses ACE2, mice inoculated with SARS-CoV-1 intranasally showed that virus invaded the CNS via a transcribrial route.44,45 The same has been demonstrated in murine MERS-CoV and HCoV-OCR43 models after in tranasal inoculation.11,28 In fact, chemically ablating the olfactory neurons protected mice from HCoV-OCR43 invasion into the CNS. It remains unclear whether SARS-CoV-2 can similarly spread to the CNS via transcribrial route. Emerging reports46,47 (not yet peer reviewed) suggest that sustentacular and stem cells in the olfactory epithelium express ACE2 and are vulnerable to SARS-CoV-2 infection, while olfactory sensory neurons do not express ACE2, suggesting SARS-CoV-2 cannot gain access to nerve cells. These preliminary findings suggest that damage to the olfactory epithelium underlies clinical anosmia, rather than neuronal injury. Further murine and autopsy studies will likely provide clarification.
Blood-Brain Barrier Spread
There are 2 possible mechanisms for SARS-CoV-2 spread across the BBB. The BBB is composed of vascular endothelium, astrocytes, pericytes, and extra cellular matrix.48 Vascular endothelial cells are joined by tight junctions and regulate the permeability of the BBB. The first mechanism is through infection of and transport across vascular endothelial cells (Figure 3). Endothelia throughout the body express ACE2 and are at risk for infection by SARS-CoV-2. An autopsy case study demonstrated the presence of SARS-CoV-2 viral particles in capillary endothelia and neurons of a frontal lobe specimen.49 Neurons were found to have viral particles packaged in dilated vesicles. Electron microscopic imaging even demonstrated endocytosis or exocytosis of viral particles across endothelial cells. Arboviruses use a similar active-transport mechanism without replication to enter endothelial cells and cross the BBB into the CNS.50 Once the virus gains access to vascular and neuronal tissue, it could begin a cycle of viral budding and further damage vascular and neuronal tissue as the virus comes into contact with ACE2 on neurons, glia, and vessels.51
The second mechanism is through infection of leukocytes that pass through the BBB, termed the Trojan horse mechanism (Figure 3).52 This mechanism is well described in HIV, in which infected immune cells pass from the blood through the BBB to infect the CNS.42,53 The SARS-CoV-1 virus has been shown to infect lymphocytes, granulocytes, monocyte derivatives, and monocytes, which all express ACE2.24,54–56 It is likely that SARS-CoV-2 infects similar cell types. It has been demonstrated that T lymphocytes allow SARS-CoV-2 infection but do not support viral replication.57 The systemic inflammation that characterizes COVID-19 likely increases the permeability of the BBB, thereby allowing infected immune cells, cytokines, and possibly virus to pass into the CNS.58
Neurologic Manifestations
Information about neurologic manifestations in patients with COVID-19 is sparse. Currently, there are a small number of published case reports and clinical studies. A systemic study in Wuhan, China,reportedneurologicfindingsin214patientshospitalizedwith COVID-19.59 Another systematic study60 in France noted neurologic symptoms in 49 of 58 patients, including confusion, encephalopathy, and corticospinal tract signs on examination, as well as leptomeningeal enhancement and perfusion abnormalities on magnetic resonance imaging (MRI).
The most common neurologic symptoms in COVID-19 are headache, anosmia, and ageusia. Other neurological findings include stroke, impairment of consciousness, coma, seizure, and encephalopathy.
Headache
Headache is one of the most common initial complaints in patients with COVID-19. In a recent case series,61 headache was a predominant complaint, along with fever, cough, sore throat, and breathlessness. Prevalence varies in different reports but can affect up to one-third of diagnosed patients.62,63 While headache is a well-described manifestation of meningitis, encephalitis, vasculitis, and intracranial hypertension, less is known about its pathophysiological connection with COVID-19. Neuroinflammatory mechanisms have been invoked in some headache syndromes via cytokines and chemokines that trigger nociceptive sensory neurons.64 Release of cytokines and chemokines by macrophages during various stages of COVID-19 infection may lead to similar mechanisms for pain.65 It is imperative to screen patients who present with headache for secondary causes if they have had a change in their headache frequency or severity, develop systemic symptoms such as a fever, or are refractory to preliminary treatments.
Anosmia and Ageusia
The prevalence of anosmia and ageusia ranges widely in the literature. In a study of patients hospitalized in Wuhan, the prevalence of hypogeusia and hyposmia was 5.6% and 5.1%, respectively,59 while 19.4% of patients in Italy had some form of chemosensory dysfunction.66 Approximately 88.5% and 88.0% of patients in Germany reported olfactory and gustatory dysfunction, respectively.67 Of patients without nasal congestion, 79.7% were hyposmic.67 Anosmia has also been noted in other respiratory infections, such as influenza.66,68 In COVID-19, anosmia is typically not accompanied by nasal swelling or rhinitis. Given the reports of anosmia presenting as an early symptom of COVID-19, dedicated testing for anosmia may offer the potential for early detection of COVID-19 infection.
Impaired Consciousness
Impairment of consciousness was reported in 37% of patients hospitalized with COVID-19 in the Mao et al59 study in Wuhan. There are several possible mechanisms of altered consciousness in patients with COVID-19, including direct infection and damage of the parenchyma, toxic-metabolic encephalopathy, seizures, or demyelinating disease.
Toxic-Metabolic Encephalopathy
The hallmark of encephalopathy is impaired attention and arousal, presenting with confusion, lethargy, delirium, or coma.69 Common risk factors that predispose patients to delirium are advanced age, underlying dementia or cognitive impairment, multiple comorbid diseases, infection, severe medical illness, poor functional baseline, and malnutrition.70 Many metabolic and endocrine derangements put patients at further risk for encephalopathy, including hyponatremia or hypernatremia, hypocalcemia or hypercalcemia, renal dysfunction, liver dysfunction, and hypoglycemia or hyperglycemia, among others. Sepsis and the subsequent inflammatory and cytokine storm can also contribute to encephalopathy with IL-6, IL-8, IL-10, and tumor necrosis factor α being implicated in states of confusion.71
Patients hospitalized with COVID-19 may exhibit numerous toxic-metabolic derangements, including cytokine storm, severe inflammation, sepsis, and renal dysfunction.65 Severe COVID-19 disease is characterized by increased IL-2, IL-6, IL-7, granulocyte–colony-stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1–α, and tumor necrosis factor α.72 Cytokine storm likely contributes significantly to toxic-metabolic encephalopathy in severe cases, along with the risk factors and metabolic derangements detailed.
Encephalitis
As discussed, MERS-CoV, SARS-CoV-1, and potentially SARS-CoV-2 can invade the CNS and potential encephalitis is a concern. However, currently there is no direct evidence of encephalitis secondary to SARS-CoV-2. A suspected case of meningoencephalitis73 in a patient with COVID-19 was reported in Japan. The patient presented with headache, fever, and seizures. An MRI showed diffusion restriction in the right temporal lobe, hippocampal atrophy, and ventriculitis, and SARS-CoV-2 RNA was detected in CSF, but it was unclear if some of the patient’s reported features could be present in the setting of seizure from other causes. A suspected case of acute necrotizing encephalopathy has also been reported,74 which is typically a peri-infectious immune-mediated syndrome, rather than direct viral encephalitis. An MRI of the patient’s brain showed hemorrhagic, rim-enhancing lesions in the bilateral thalami, medial temporal lobes, and subinsular region.74 High levels of proinflammatory cytokines in the CSF can cause breakdown and increased permeability of the BBB, which may in turn lead to viral invasion.74
Seizure
Seizures can also lead to impairment in consciousness and have been reported in other CoV infections. Additionally, subclinical seizures are reported in roughly 10% of patients with critical illness,75 and patients with primary seizure disorder are at higher risk of seizures and status epilepticus in the setting of severe infection.76 At our academic medical center, there have been a high proportion of breakthrough seizures in patients with epilepsy who have developed COVID-19.
A recent report of 304 patients diagnosed with COVID-19 only documented 2 “seizure like events,”77(p3) with no confirmed cases of new-onset seizures. The study was limited by lack of clinical testing (eg, electroencephalography, imaging) and the retrospective approach.77 A case report of a patient with no history of epilepsy who had multiple apparent tonic-clonic seizures in the setting of COVID-19 may represent an unmasked seizure disorder or the direct effect of COVID-19 in the CNS, but further study in these cases are needed.78
Stroke and Vascular Events
The Mao et al study59 reported that 5% of a hospitalized cohort in Wuhan had acute strokes. A more detailed report of the cerebrovascular disease events in this cohort revealed that 11 patients developed acute ischemic strokes, 1 had a cerebral venous sinus thrombosis, and 1 had an intracerebral hemorrhage.79 Patients who developed cerebrovascular disease were significantly older; were more likely to have severe COVID-19 disease manifestations; had more cardiovascular risk factors; and had significantly higher C-reactive protein and D-dimer levels, suggesting a hypercoagulable state.79 A study80 in New York demonstrated that young patients (younger than 50 years) developed large-vessel strokes in the setting of COVID-19, suggesting all ages are vulnerable.
The pathophysiology of increased risk of cerebrovascular disease during COVID-19 infection is likely multifactorial. Common abnormal laboratory test results in patients include elevated leukocyte count, C-reactive protein level, D-dimer level, ferritin level, and lactate dehydrogenase level.81 Severe cases are characterized by elevated inflammatory markers and hypercoagulability compared with moderate cases and with increased likelihood of stroke.59
More specific viral mechanisms may also increase risk of stroke. Infection of the vascular endothelial cells and subsequent damage to vasculature may increase the risk of ischemic and hemorrhagic infarcts. Many infections can increase the risk of stroke, often through systemic inflammation, thrombosis, or vasculitis.82,83 Autopsy in donors who had SARS-CoV-1 have demonstrated systemic vasculitis and vasculitis of venules in the brain.23
Guillain-Barré Syndrome and Peripheral Nerve Disorders
Guillain-Barré syndrome, also known as acute inflammatory demyelinating polyneuropathy (AIDP), can develop after a gastrointestinal or respiratory illness.84 This is thought to occur through a molecular mimicry mechanism in which infecting viruses likely share epitopes similar to components of peripheral nerves, which stimulates autoreactive T or B cells. The antibodies produced by the immune system to fight the virus cross-react and bind to components of the peripheral nervous system, causing neuronal dysfunction. Both AIDP and acute motor axonal neuropathy (AMAN) variants have been documented after SARS-CoV-1 infections.20 Cases of AIDP, AMAN, and Bickerstaff brainstem encephalitis have been reported in the setting of MERS-CoV.4
Reports of GBS in patients with COVID-19 are emerging. A case series85 reported 5 cases of GBS in Italy after COVID-19 infection. In 4cases,patients presented with lower-extremity weakness and paresthesias. Patients developed symptoms a mean of 5 to 10 days after onset of viral symptoms. Electromyography studies showed 2 patients had AIDP and 3 had AMAN. Additional case reports describe a patient in Iran with AMAN86 and a patient from Italy with Miller-Fisher–variant GBS.87
A clinical case of acute transverse myelitis was reported from Wuhan,88 but MRI and CSF findings were not available. The patient developed flaccid lower-extremity paralysis with loss of pinprick sensation and paresthesias below the T10 level and was successfully treated with steroids and intravenous immunoglobulin.
Possible CNS Effects of Therapies Currently in Use for COVID-19
Currently, there are numerous different medications being used to treat patients with COVID-19. Here we discuss their potential neurologic effects and/or relevance to neurologic diseases.
Chloroquine and Hydroxychloroquine
Chloroquine and hydroxychloroquine, initially developed as antimalarial drugs, work by preventing the acidification of endosomes, which interrupts cellular functions and may prevent viral entry via ACE2binding.89,90 Hydroxychloroquine inhibits SARS-CoV-2invitro, but in vivo studies are lacking, and the US Food and Drug Administration currently recommends exercising caution in using these drugs because of potential cardiotoxicity.91 Neurologic adverse effects include irritability, psychosis, peripheral neuropathy, and neuromyopathy.92,93 Hydroxychloroquine is well known to exacerbate symptoms in myasthenia gravis and has long been contraindicated for patients with this disease. It also lowers the seizure threshold and interacts with several antiepileptic drugs, including lacosamide and lamotrigine.94–96
Tocilizumab
Tocilizumab is a monoclonal antibody to the IL-6 receptor that may attenuate cytokine release in patients with severe inflammatory disease. There are limited retrospective data that suggest possible benefit.97,98 It has poor penetration into the CNS.99 Neurologic adverse effects include headache and dizziness, and there have been rare reports of multifocal cerebral thrombotic microangiopathy.100
Remdesivir
Remdesivir is a viral RNA–dependent RNA polymerase inhibitor. In vitro data have shown that it is a potent SAR-CoV-2 inhibitor, and early clinical data have shown some benefit.101 There is little noted about potential neurologic adverse effects, and clinical trials are ongoing, which will provide valuable data.
Special Considerations for Patients Under Neurological Care
Many patients under neurological care have complex conditions and comorbidities that may place them at increased risk of developing severe COVID-19 disease. Patients older than 65 years; living in a skilled nursing facility; or with comorbid lung disease, heart disease, liver disease, obesity (body mass index [calculated as weight in kilograms divided by height in meters squared] >40), diabetes, kidney disease requiring dialysis, or immunosuppression are at higher risk for severe disease in COVID-19.102
Multiple Sclerosis
Patients with MS taking disease-modifying therapies that have immunosuppressive effects may be at increased risk of developing severe COVID-19 disease. The National MS Society has released recommendations for all patients with MS, in general, to continue disease-modifying therapies.103 They advise steroids are safe to treat acute MS relapses in patients without COVID-19. If a patient is at high risk of exposure to SARS-CoV-2 and due for additional immunosuppressive therapy, the MS International Federation recommends that clinicians should weigh the risks and benefits of switching the patient to interferons, glatiramer acetate, or natalizumab.104 The National MS Society and the Consortium of MS Centers has created a patient reporting database (covims.org) for ongoing research.
Neuromuscular Disorders
Patients with neuromuscular disorders are at particular risk for deterioration with COVID-19. Many neuromuscular disorders are treated with immunosuppressive medications, which can increase the risk of developing severe COVID-19 disease. Additionally, patients with myasthenia gravis or Lambert Eaton myasthenic syndrome may have respiratory muscle weakness, which can put them at further risk for severe complications in COVID-19.105 The International Myasthenia Gravis/COVID Working Group105 recommends continuing current treatments. For those receiving immunosuppressive therapy, the group recommends extravigilant social distancing and telemedicine visits.105 As discussed, hydroxychloroquine exacerbates myasthenia gravis symptoms and is contraindicated. For patients with chronic dysimmune neuropathies, the risks and benefits of in-hospital infusions should be weighed with the risk of exposure to SARS-CoV-2 and developing severe COVID-19 disease.106
Epilepsy
Epilepsy does not increase a patient’s risk of contracting SARSCoV-2 or put patients at higher risk of severe disease.107 Nearly all antiepileptic drugs are not immunosuppressive and are safe for patients with COVID-19. Viral infections and fever may trigger seizure in patients with epilepsy.108 Clinicians should anticipate breakthrough seizures, prescribe medications for short-term management, and provide patients with a detailed plan.108
Conclusions
To date, SARS-CoV-2 has infected millions and affected billions of lives. The understanding of neurologic disease in patients with COVID-19 is evolving, and clinicians should continue to monitor patients closely for neurological disease. Early detection of neurological deficits may lead to improved clinical outcomes and better treat-mentalgorithms. Further laboratory and clinical data, including tests of CSF, brain imaging, and tests of CNS tissue, will be essential in elucidating the pathophysiology and potential for CNS injury. Lastly, longitudinal neurological assessments of patients after recovery will be crucial in understanding the natural history of COVID-19 in the CNS and monitoring for potential neurologic sequelae.
Footnotes
Conflict of Interest Disclosures:
Dr Kuruvilla reported personal fees from Allergan, Lilly, Amgen, Theranica, and Now What Marketing outside the submitted work. Dr Spudich reported grants from National Institutes of Health (the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke) outside the submitted work. Dr Farhadian reports grant K23 MH118999 from the National Institute of Health (the National Institute of Mental Health) outside the submitted work. No other disclosures were reported.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H Early lessons from the frontline of the 2019-nCoV outbreak. Lancet. 2020;395(10225):687. doi: 10.1016/S0140-6736(20)30356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau SKP, Lee P, Tsang AKL, et al. Molecular epidemiology of human coronavirus OC43 reveals evolution of different genotypes over time and recent emergence of a novel genotype due to natural recombination. J Virol. 2011;85(21):11325–11337. doi: 10.1128/JVI.05512-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass WG, Subbarao K, Murphy B, Murphy PM. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol. 2004;173(6):4030–4039. doi: 10.4049/jimmunol.173.6.4030 [DOI] [PubMed] [Google Scholar]
- 7.Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulswit RJG, Lang Y, Bakkers MJG, et al. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc Natl Acad Sci U S A. 2019;116(7):2681–2690. doi: 10.1073/pnas.1809667116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbour N, Côté G, Lachance C, Tardieu M, Cashman NR, Talbot PJ. Acute and persistent infection of human neural cell lines by human coronavirus OC43. J Virol. 1999;73(4):3338–3350. doi: 10.1128/JVI.73.4.3338-3350.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacomy H, Talbot PJ. Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology. 2003;315(1):20–33. doi: 10.1016/S0042-6822(03)00323-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubé M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol. 2018;92(17):e00404–18. doi: 10.1128/JVI.00404-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913–8921. doi: 10.1128/JVI.74.19.8913-8921.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray RS, Brown B, Brian D, Cabirac GF. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 1992;31(5):525–533. doi: 10.1002/ana.410310511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cristallo A, Gambaro F, Biamonti G, Ferrante P, Battaglia M, Cereda PM. Human coronavirus polyadenylated RNA sequences in cerebrospinal fluid from multiple sclerosis patients. New Microbiol. 1997;20(2):105–114. [PubMed] [Google Scholar]
- 16.Boucher A, Mercier G, Duquette P, Talbot PJ. Clonal T-cell cross-reactivity between myelin antigens MBP and PLP and human respiratory coronaviruses in multiple sclerosis. J Neuroimmunol. 1998;90(1):33–33. doi: 10.1016/S0165-5728(98)91373-X [DOI] [Google Scholar]
- 17.Nilsson A, Edner N, Albert J, Ternhag A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis (Lond). 2020;52(6):419–422. doi: 10.1080/23744235.2020.1729403 [DOI] [PubMed] [Google Scholar]
- 18.Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375(5):497–498. doi: 10.1056/NEJMc1509458 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Published 2003. Accessed April 23, 2020 https://www.who.int/csr/sars/country/table2004_04_21/en/
- 20.Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. 2005;14(3):113–119. [PubMed] [Google Scholar]
- 21.Tsai L-K, Hsieh S-T, Chao C-C, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61(11): 1669–1673. doi: 10.1001/archneur.61.11.1669 [DOI] [PubMed] [Google Scholar]
- 22.Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2): 342–344. doi: 10.3201/eid1002.030638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3): 282–289. doi: 10.1002/path.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41(8):1089–1096. doi: 10.1086/444461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Published 2020. Accessed April 23, 2020 https://www.who.int/emergencies/mers-cov/en/
- 27.Lambeir A-M, Durinx C, Scharpé S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40(3):209–294. doi: 10.1080/713609354 [DOI] [PubMed] [Google Scholar]
- 28.Li K, Wohlford-Lenane C, Perlman S, et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwob JE, Saha S, Youngentob SL, Jubelt B. Intranasal inoculation with the olfactory bulb line variant of mouse hepatitis virus causes extensive destruction of the olfactory bulb and accelerated turnover of neurons in the olfactory epithelium of mice. Chem Senses. 2001;26(8):937–952. doi: 10.1093/chemse/26.8.937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salimi H, Klein RS. Disruption of the blood-brain barrier during neuroinflammatory and neuroinfectious diseases In: Mitoma H, Manto M, eds. Neuroimmune Diseases: From Cells to the Living Brain. Springer International Publishing; 2019:195–234. doi: 10.1007/978-3-030-19515-1_7 [DOI] [Google Scholar]
- 33.Joseph J, Grun JL, Lublin FD, Knobler RL. Interleukin-6 induction in vitro in mouse brain endothelial cells and astrocytes by exposure to mouse hepatitis virus (MHV-4, JHM). J Neuroimmunol. 1993;42(1):47–52. doi: 10.1016/0165-5728(93)90211-G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen R, Wang K, Yu J, Chen Z, Wen C, Xu Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. Published April 9, 2020. Accessed May 18, 2020 https://www.biorxiv.org/content/10.1101/2020.04.07.030650v1 [DOI] [PMC free article] [PubMed]
- 36.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107(6):1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R373–R381. doi: 10.1152/ajpregu.00292.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao L, Haack KKV, Zucker IH. Angiotensin II regulates ACE and ACE2 in neurons through p38 mitogen-activated protein kinase and extracellular signal-regulated kinase 1/2 signaling. Am J Physiol Cell Physiol. 2013;304(11):C1073–C1079. doi: 10.1152/ajpcell.00364.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YC, Bai WZ, Hirano N, et al. Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J Comp Neurol. 2013;521(1):203–212. doi: 10.1002/cne.23171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y-X, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol. Published online February 26, 2020. doi: 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li YC, Bai WZ, Hirano N, Hayashida T, Hashikawa T. Coronavirus infection of rat dorsal root ganglia: ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res. 2012;163(2):628–635. doi: 10.1016/j.virusres.2011.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berth SH, Leopold PL, Morfini GN. Virus-induced neuronal dysfunction and degeneration. Front Biosci (Landmark Ed). 2009;14: 5239–5259. doi: 10.2741/3595 [DOI] [PubMed] [Google Scholar]
- 43.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020;ciaa330. doi: 10.1093/cid/ciaa330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuda K, Park CH, Sunden Y, et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet Pathol. 2004;41(2):101–107. doi: 10.1354/vp.41-2-101 [DOI] [PubMed] [Google Scholar]
- 45.McCray PB Jr, Pewe L, Wohlford-Lenane C, et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81(2):813–821. doi: 10.1128/JVI.02012-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brann D, Tsukahara T, Weinreb C, Logan DW, Datta SR. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. Published March 28, 2020. Accessed May 18, 2020 https://www.biorxiv.org/content/10.1101/2020.03.25.009084v2 [DOI] [PMC free article] [PubMed]
- 47.Fodoulian L, Tuberosa J, Rossier D, Landis BN, Carleton A, Rodriguez I. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. Published April 20, 2020. Accessed May 18, 2020 https://www.biorxiv.org/content/10.1101/2020.03.31.013268v1 [DOI] [PMC free article] [PubMed]
- 48.Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12(386):386. doi: 10.3389/fncel.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus–2 (SARS-CoV-2). J Med Virol. 2020. Published online April 21, 2020. doi: 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dropulić B, Masters CL. Entry of neurotropic arboviruses into the central nervous system: an in vitro study using mouse brain endothelium. J Infect Dis. 1990;161(4):685–691. doi: 10.1093/infdis/161.4.685 [DOI] [PubMed] [Google Scholar]
- 51.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 52.Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96. doi: 10.1007/978-81-322-1777-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim W-K, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003;74(5):650–656. doi: 10.1189/jlb.0503207 [DOI] [PubMed] [Google Scholar]
- 54.Spiegel M, Schneider K, Weber F, Weidmann M, Hufert FT. Interaction of severe acute respiratory syndrome-associated coronavirus with dendritic cells. J Gen Virol. 2006;87(Pt 7):1953–1960. doi: 10.1099/vir.0.81624-0 [DOI] [PubMed] [Google Scholar]
- 55.Nicholls JM, Butany J, Poon LLM, et al. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3(2):e27. doi: 10.1371/journal.pmed.0030027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trojanowicz B, Ulrich C, Kohler F, et al. Monocytic angiotensin-converting enzyme 2 relates to atherosclerosis in patients with chronic kidney disease. Nephrol Dial Transplant. 2017;32(2): 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Xu W, Hu G, et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol. 2020. doi: 10.1038/s41423-020-0424-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sankowski R, Mader S, Valdés-Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci. 2015;9(28):28. doi: 10.3389/fncel.2015.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. Published online April 10, 2020. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020. doi: 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta N, Agrawal S, Ish P, et al. Clinical and epidemiologic profile of the initial COVID-19 patients at a tertiary care centre in India. Monaldi Arch Chest Dis. 2020;90(1). doi: 10.4081/monaldi.2020.1294 [DOI] [PubMed] [Google Scholar]
- 62.Jin X, Lian J-S, Hu J-H, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borges do Nascimento IJ, Cacic N, Abdulazeem HM, et al. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020;9(4):E941. doi: 10.3390/jcm9040941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conti P, D’Ovidio C, Conti C, et al. Progression in migraine: role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur J Pharmacol. 2019;844:87–94. doi: 10.1016/j.ejphar.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 65.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020. doi: 10.1002/lary.28692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiao XF, Wang GP, Li X, Bai YH, Zheng W. Analysis of the clinical effect of olfactory training on olfactory dysfunction after upper respiratory tract infection. Acta Otolaryngol. 2019;139(7):643–646. doi: 10.1080/00016489.2019.1614224 [DOI] [PubMed] [Google Scholar]
- 69.Frontera JA. Metabolic encephalopathies in the critical care unit. Continuum (Minneap Minn). 2012; 18(3):611–639. doi: 10.1212/01.CON.0000415431.07019.c2 [DOI] [PubMed] [Google Scholar]
- 70.Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326–333. doi: 10.1093/ageing/afu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krishnan V, Leung LY, Caplan LR. A neurologist’s approach to delirium: diagnosis and management of toxic metabolic encephalopathies. Eur J Intern Med. 2014;25(2):112–116. doi: 10.1016/j.ejim.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 72.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. Published online April 6, 2020. doi: 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lau SK, Woo PC, Yip CC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44(6):2063–2071. doi: 10.1128/JCM.02614-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Li H, Fan R, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59(3):163–169. doi: 10.1159/000453066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu L, Xiong W, Liu D, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. Published online April 18, 2020. doi: 10.1111/epi.16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karimi N, Sharifi Razavi A, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828 [DOI] [Google Scholar]
- 79.Li Y, Wang M, Zhou Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electronic Journal. Published online April 2020. doi: 10.2139/ssrn.3550025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21(1):74. doi: 10.1186/s12931-020-01338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manousakis G, Jensen MB, Chacon MR, Sattin JA, Levine RL. The interface between stroke and infectious disease: infectious diseases leading to stroke and infections complicating stroke. Curr Neurol Neurosci Rep. 2009;9(1):28–34. doi: 10.1007/s11910-009-0005-x [DOI] [PubMed] [Google Scholar]
- 83.Starke RM, Chalouhi N, Ali MS, et al. The role of oxidative stress in cerebral aneurysm formation and rupture. Curr Neurovasc Res. 2013;10(3):247–255. doi: 10.2174/15672026113109990003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen TP, Taylor RS. Guillain Barre Syndrome. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 85.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020. doi: 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;S0967–5868(20)30882–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. Published online April 17, 2020. doi: 10.1212/WNL.0000000000009619 [DOI] [PubMed] [Google Scholar]
- 88.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. Published March 18, 2020. Accessed May 19, 2020 https://www.medrxiv.org/content/10.1101/2020.03.16.20035105v2 [Google Scholar]
- 89.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou D, Dai S-M, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. Published online March 20, 2020. doi: 10.1093/jac/dkaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Published online March 20, 2020. doi: 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tönnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy—a review of the literature. Immunopharmacol Immunotoxicol. 2013; 35(3):434–442. doi: 10.3109/08923973.2013.780078 [DOI] [PubMed] [Google Scholar]
- 93.Manzo C, Gareri P, Castagna A. Psychomotor agitation following treatment with hydroxychloroquine. Drug Saf Case Rep. 2017;4(1): 6. doi: 10.1007/s40800-017-0048-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prescriber’s Digital Reference. Hydroxychloroquine sulfate: drug summary. Accessed May 18, 2020 https://www.pdr.net/drug-summary/Plaquenil-hydroxychloroquine-sulfate-1911 [Google Scholar]
- 95.Fish DR, Espir ML. Convulsions associated with prophylactic antimalarial drugs: implications for people with epilepsy. BMJ. 1988;297(6647):526–527. doi: 10.1136/bmj.297.6647.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benbadis SR, Van Ness PC. Chloroquine and nonconvulsive status epilepticus. Ann Intern Med. 1996;124(6):614–615. doi: 10.7326/0003-4819-124-6-199603150-00020 [DOI] [PubMed] [Google Scholar]
- 97.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. Published online March 29, 2020. doi: 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nellan A, McCully CML, Cruz Garcia R, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018; 132(6):662–666. doi: 10.1182/blood-2018-05-846428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jewell P, Ansorge O, Kuker W, Irani SR, Zamboni G. Tocilizumab-associated multifocal cerebral thrombotic microangiopathy. Neurol Clin Pract. 2016;6(3):e24–e26. doi: 10.1212/CPJ.0000000000000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.US Centers for Disease Control and Prevention. COVID-19: people who are at higher risk for severe illness. Published 2020. Updated April 15 2020. Accessed April 20, 2020 https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html [PubMed]
- 103.National Multiple Sclerosis Society. MS treatment guidelines during coronavirus. Published 2020. Accessed April 20, 2020 https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/ms-treatment-guidelines-during-coronavirus
- 104.MS International Federation. The coronavirus and MS—global advice. Published 2020. Updated April 20 2020. Accessed April 20, 2020 http://msif.org/news/2020/02/10/the-coronavirus-and-ms-what-you-need-to-know/
- 105.Jacob S, Muppidi S, Guidon A, et al. ; International MG/COVID-19 Working Group. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803–116803. doi: 10.1016/j.jns.2020.116803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rajabally YA, Goedee HS, Attarian S, Hartung HP. Management challenges for chronic dysimmune neuropathies during the COVID-19 pandemic. Muscle Nerve. 2020. doi: 10.1002/mus.26896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Epilepsy Foundation. Concerns about COVID-19 (coronavirus) and epilepsy. Published 2020. Updated April 9, 2020. Accessed April 20, 2020 https://www.epilepsy.com/learn/covid-19-and-epilepsy
- 108.American Epilepsy Society. AES statement on COVID-19. Published 2020. Updated March 10, 2020. Accessed April 20, 2020 https://aesnet.org/about_aes/position_statements/covid-19


