Abstract
Depression is the most common psychiatric illness affecting numerous people world-wide. The currently available antidepressant treatment presents low response and remission rates. Thus, new effective antidepressants need to be developed or discovered. Aiming to give an overview of novel possible antidepressant drug targets, we summarized the molecular targets of antidepressants and the underlying neurobiology of depression. We have also addressed the multidimensional perspectives on the progress in the psychopharmacological treatment of depression and on the new potential approaches with effective drug discovery.
Keywords: Depression, antidepressant, novel targets, anxiolytics
Introduction
Major depressive disorder (MDD) is a major public health concern world-wide which can have enormous impact on the quality of life (Lam et al., 2016; WHO, 2018.). Despite the availability of a wide range of antidepressants, remission rates in MDD patients are very low (Rush et al., 2006). In fact, the remission rate for monotherapy with an antidepressant is ~50% and 20–30% of the patients even do not respond to specific antidepressants (Mrazek et al., 2014). The complex mechanisms of underlying causes of MDD and the limited knowledge about its pathogenetic risk factors have restricted the progression of psychopharmacology. Antidepressants developed on the “monoamine neurotransmitter hypothesis” are still the first-line of treatment and the hypothesis is based on the assumption of lack of monoamine neurotransmitters in MDD (Hirschfeld, 2000). As targets, antidepressants mainly inhibit the activity of serotonin (5-HT) and norepinephrine (NA) transporters. Antidepressants can block the monoamine neurotransmitter reuptake and increase their concentration in the synaptic cleft. These antidepressants include selective 5-HT reuptake inhibitors (SSRI), selective NA reuptake inhibitors (SNRI), nonselective NA/5-HT reuptake inhibitors, monoamine oxidase inhibitors (MAOI), and 5-HT receptor agonists. These antidepressants are effective only in ~60% of MDD patients, and their effects reduce depressive symptoms only after 2 to 3 weeks of treatment. Long-term treatment with these drugs also cause adverse side effects with chances of relapse after discontinuing therapy (Krishnan and Nestler, 2008).
In this review, we describe the treatment targets that are currently being tested either pre-clinically or clinically as antidepressants. We also discuss new molecular targets that can provide potential approaches for seeking effective and relatively safer antidepressant agents.
1. Antidepressants Targeting glutamate/GABA systems
Glutamate is the most abundant neurotransmitter in the human brain and is the major mediator of excitatory signals (Meldrum, 2000). It is involved in many physiological functions and participates in learning, memory and cognitive functions (McEntee and Crook, 1993). Glutamate also plays important role in the regulation of growth cones and synaptogenesis during brain development (Okubo et al., 2010). Glutamate binds to the cell surface receptors including ionotropic and metabotropic receptors and actives the intracellular signal transduction system involved in downstream tropic pathways such as CREB and BDNF to preserve neuronal viability (Martin and Finsterwald, 2011). In mammals, four families of glutamate receptors have been identified: AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors, kainate receptors, (N-methyl-D-aspartate) NMDA receptors, and metabotropic glutamate receptors. The first three families are ionotropic and when activated, they open membrane channels that allow ions to pass through (Reiner and Levitz, 2018). The metabotropic family receptors are G protein-coupled receptors. In this capacity, they exert their effects via complex second messenger system (Kandel, 2013).
Gamma-Aminobutyric Acid (GABA) is the main inhibitory neurotransmitter in the brain. In conjunction with excitatory glutamate, GABA is involved in balancing excitatory and inhibitory response, critical in proper brain functioning (Petroff, 2002). GABA binds to GABA receptors to mediate its functions. Two main types of GABA receptors exist: 1) GABAA, which are ligand-gated ion channels and 2) GABAB, which are G-protein-coupled receptors (Hayes et al., 2014). GABAA is the primary GABA receptor in the brain, which, due to its chloride ion channel activity, quickly hyperpolarizes the postsynaptic neurons, causing an inhibitory effect. GABAA receptors are localized in post-synaptic and extrasynaptic locations whereas GABAB receptors are localized pre-synaptically, post-synaptically, and potentially extrasynaptically (Hammond, 2015; Shrivastava et al., 2011).
1.1. Targeting N-methyl-d-aspartate (NMDA) receptors
NMDA receptors are ionotropic glutamate receptors. Many physiological functions have been attributed to these receptors including learning and memory, synapse development, and synaptic plasticity (Furukawa et al., 2005; Li and Tsien, 2009). NMDR receptors are tetramers and are composed of GluN1, GluN2, and GluN3. There are several splice variants of NMDA receptors: GluN1, GluN2A, GluN2B, GluN2C, GluN2D, GluN3A and GluN3B (Kristiansen et al., 2007; Loftis and Janowsky, 2003). Alterations in NMDA receptors and abnormalities in glutamatergic neurotransmission mediated by NMDR receptors have been reported in the postmortem brains of depressed subjects (Dean et al., 2016). Significantly higher expression of GluN1 and GluN2C subunits and significantly lower expression of GluN2A and GluN2B subunits have been reported in the locus coeruleus of depressed patients (Chandley et al., 2014).
Several rodent studies suggest that NMDA receptor antagonists can produce antidepressant-like response (Krystal et al., 2013). For example, non-competitive NMDA receptor antagonist MK-801 (dizocilpine), the competitive NMDA receptor antagonist CGP37849 (DL-(E)-2-amino-4-methyl-5-phosphono-3-pentonoic acid), and its (R)-enantiomer CGP40116 when given for 4–5 weeks to rats (MK-801 at the dose of 0.3 mg/kg, i.p; CGP 37849 at the dose of 5 mg/kg, i.p. and CGP40116 at the dose of 25 mg/kg, p.o.) reversed stress-induced decrease in sucrose intake (Papp and Moryl, 1994; Trullas and Skolnick, 1990). The effects were similar to tricyclic antidepressant imipramine (10 mg/kg, i.p. or p.o.). These agents did not change behavior when given to non-stressed rats. (Papp and Moryl, 1994). This suggest that these agents have no impact on their own but can be effective under pathological conditions.
Besides NMDA receptor antagonists, the antidepressant effects of competitive agonist (2-amino-7-phosphonoheptanoic acid, AP-7), a partial agonist to strychnine-insensitive glycine receptors (1-aminocylopropanecarboxylic acid, ACPC), and a non-competitive NMDA antagonists (MK-801) have also been tested. In inescapable stress model, it was reported that all the three drugs mimicked the effects of clinically effective antidepressants (Skolnick et al., 1996; Trullas and Skolnick, 1990). It was proposed that stress-induced behavioral deficits are mediated through NMDA receptor complex and that these compounds reduce depressive behavior by lowering NMDA receptor neurotransmission (Trullas and Skolnick 1990). Unlike NMDAR full antagonists, which possess antidepressant properties but are accompanied by psychotomimetic effects, NMDAR glycine-site functional partial agonist GLYX-13 produced rapid antidepressant activity via NMDAR-triggered synaptic plasticity. This compound also enhanced cognition without psychotomimetic side effects (Burgdorf et al., 2013; Moskal, J. R. et al., 2014; Moskal, Joseph R et al., 2014). Several other NMDR antagonists such as NVP-AAM077 (NR2A antagonist) (Gordillo-Salas et al., 2018; Jiménez-Sánchez et al., 2014) Ro 25–6981 (selective for NR2B receptors) (Li et al., 2011) and Eliprodil (acting at polyamine sites) (Layer et al., 1995) have been studied in various rodent models and were found to have antidepressant-like action. Other compounds with NMDA receptor antagonist properties have also been tested for their potential efficacy as anxiolytics. These include diazepam, NPC 17742 [2R,4R,5S-2-amino4,5-(1,2-cyclohexyl)-7-phosphono-heptanoic acid, phencyclidine, ACEA 1021 (5-nitro-6,7-dichloro-1,4-dihydro-2,3-quinoxalinedione, and N-nitro-L-arginine methyl ester (Tizzano et al., 2002; Wiley et al., 1995).
A growing body of evidence suggest that ketamine, originally used as anesthetic agent, can be used as an antidepressant at subanesthetic dose. Ketamine is a noncompetitive NMDA receptors antagonist, which can produce rapid and stable antidepressant action (Abdallah et al., 2015; Caddy et al., 2014; McCloud et al., 2015; Serafini et al., 2014; Tyler et al., 2017; Zhang and Hashimoto, 2019). A randomized, double-blind study was done to explore the effect of ketamine as an antidepressant in depressed patients (Berman et al., 2000). The drug’s efficacy was observed at 4 hours after 0.5 mg/kg intravenous infusion into depressed patients which lasted for 72 hours. Of the 8 patients with refractory depression, 4 had Hamilton depression scores decreased by >50% (Berman et al., 2000). Another study verified the rapid antidepressant effect of ketamine. It was found that the patients who received ketamine, significantly improved depressive symptoms, compared with the placebo 110 minutes after ketamine injection and the antidepressant effect was sustained in 35% patients within 1 week (Zarate Jr et al., 2006). More recently, it was found that ketamine can reduce the MADRS-SI scores and effectively relieve or eliminate suicidal thought within 24 hours after administration (Price et al. (2009). These reductions of suicidal ideation after ketamine treatment were substantiated in other studies (Bartoli et al., 2017; Sathyanarayana Rao and Andrade, 2017; Wilkinson et al., 2018).
Recent studies have focused on ketamine’s effects on the brain connectivity in MDD patients. It was suggested that ketamine can significantly change global signal regression in the prefrontal cortex (PFC) and cerebellum of MDD patients along with induction in glutamine release in the PFC (Abdallah et al., 2017). In 21 patients, it was also reported that ketamine can increase prefrontal glutamate-glutamine cycling in MDD patients compared to placebo, which was correlated with the Clinician-Administered Dissociative States Scale. This is the first study which provides direct evidence in humans that ketamine can increase glutamate release in the PFC (16 et al., 2018). On the contrary, a recent study, which explored global brain connectivity using rsfMRI in 28 MDD patients and 22 healthy controls at baseline, before and after ketamine treatment found a reduction in global brain connectivity in brain of MDD subjects, which was not affected by ketamine treatment (Kraus et al., 2020). More studies will be needed to examine this aspect of ketamine response.
Besides the effects of ketamine in MDD population, a recent clinical study shows that ketamine is also effective in treatment-resistant depression in a dose dependent manner (Su et al., 2017). This was confirmed by another study which compared different doses of intravenous ketamine: lower (0.1 mg/kg), standard (0.5 mg/kg), and higher (1.0 mg/kg), given to 99 treatment-resistant depressed patients. The results suggested that the standard dose (0.5 mg/kg) was better than the higher dose (1.0 mg/kg) for depressive symptom reduction. Standard dose, however, was less tolerated, caused side effects, and increased blood pressure transiently compared to lower dose (0.1 mg/kg) (Fava, M. et al., 2018). Ketamine has also been found to be effective as an adjunctive therapy in treatment-resistant depression. It was shown that a combination of imipramine or fluoxetine and ketamine not only improved the antidepressant response in an animal model of depression, but accelerated the neuroplastic events when compared with the antidepressants alone (Melo et al., 2015). This is another avenue that needs to be studied carefully and can have profound impact where ketamine or antidepressants are not effective alone.
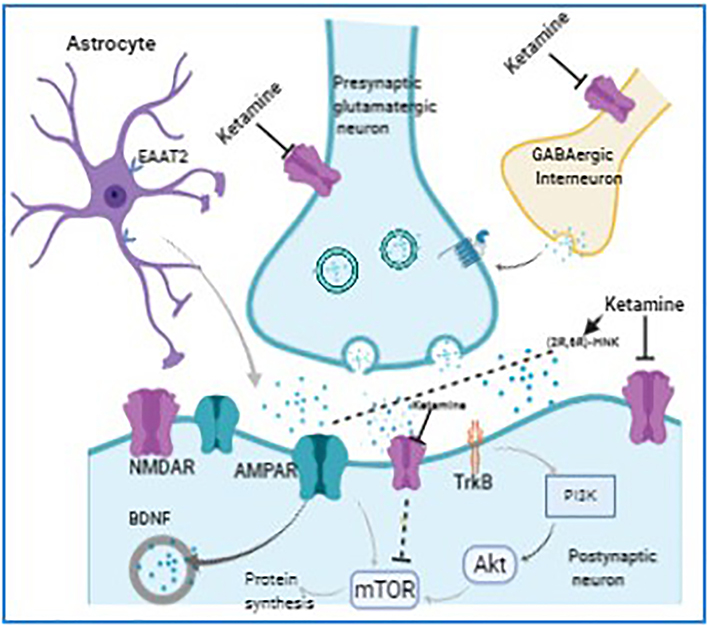
The rapid antidepressant action of ketamine have been extensively studied both at molecular and cellular levels using depression models in rodents, however, it has yet to be fully understood (Aroni et al., 2009; Xu and Lei, 2014). A schematic representation of ketamine action is provided in Fig. 1. Mechanistically, acute blockade of NMDA receptors by ketamine causes an activation of AMPA receptors, which then modulates downstream signaling systems in the limbic system to mediate antidepressant effects (Molero et al., 2018; Zanos and Gould, 2018). The downstream actions of AMPA receptors activation leads to increase in brain-derived neurotrophic factor (BDNF) levels and activation of its receptor tropomyosin receptor kinase B (TrkB). In addition, AMPA receptors stimulate mammalian target of rapamycin (mTOR) pathway, deactivation of glycogen synthase kinase 3 (GSK-3), and inhibition of the eukaryotic elongation factor 2 (eEF2) kinase phosphorylation (Bjorkholm and Monteggia, 2016; Castren and Kojima, 2017). Increase in spine density, mTOR induction, and synaptic protein expression are often associated with the antidepressant response to ketamine (Duman et al., 2012; Dwyer and Duman, 2013). In order to directly demonstrate the role of mTOR, a selective mTOR antagonist (rapamycin) was used in mice. Under this condition, ketamine not only lost dendritic formation, but it also failed to promote synaptic protein synthesis. Ketamine also lost antidepressant effects in sucrose preference, forced swimming, learned helplessness, and novel food inhibition experiments (Li et al., 2010). These results suggest that mTOR is essential in carrying out ketamine’s antidepressants’ effects. Animal studies also showed that ketamine upregulated expression of mTOR and BDNF in the hippocampus and frontal cortex of rats and decreased immobility time in the forced swimming test (FST) (Zhou, W et al., 2014). Ketamine, when given to depressed patients, not only reduced depressive symptoms but also increased BDNF levels in plasma (Duncan Jr et al., 2012; Haile et al., 2014). These findings demonstrate that induction of mTOR and BDNF expression is related to the rapid antidepressant effects produced by ketamine, however, this is not supported by all studies. For example, a study using chronic social defeat stress animal model reported that whereas antidepressant effect of S-ketamine was mediated via mTOR pathway, R-ketamine produced antidepressant effect by activating ERK signaling which was mTOR-independent (Yang et al., 2018). A recent clinical study also showed that pretreatment of depressed patients with rapamycin before intravenous administration with a single dose of ketamine (0.5 mg/kg) failed to block the antidepressant effects of ketamine, however, it prolonged ketamine’s antidepressant effects and raises questions for the role of systemic vs. local blockade of mTORC1 in the antidepressant effects of ketamine (Abdallah et al., 2020).
Fig. 1.
Possible mechanisms of action of ketamine. Mechanistically, acute blockade of NMDA receptors by ketamine causes an activation of AMPA receptors, which then modulates downstream signaling systems in the limbic system to mediate antidepressant effects. The downstream actions of AMPA receptors activation leads to increase in brain-derived neurotrophic factor (BDNF) levels and activation of its receptor tropomyosin receptor kinase B (TrkB). In addition, AMPA receptors stimulate mammalian target of rapamycin (mTOR) pathway, deactivation of glycogen synthase kinase 3 (GSK-3), and inhibition of the eukaryotic elongation factor 2 (eEF2) kinase phosphorylation.
It is well known that the half-life of ketamine is only 1 to 3 h, however, it’s antidepressant effect lasts for 3 to 7 days. Several studies have examined this aspect and speculated that the metabolites of ketamine may have a certain relationship with its antidepressant-like effects. Paul et al. (2014) found that ketamine metabolites NK and (2S,6S)-HNK were able to activate p-mTOR, suggesting that ketamine metabolites may also have antidepressant-like effects (Paul et al., 2014). Studies showed that ketamine and its metabolite NK both reduced immobility time and produced antidepressant like effects in forced swim test, while other metabolite DHNK had no similar effects (Sałat et al., 2015). Given that metabolites of ketamine are also active antidepressants in various animal models, their efficacy needs to be studied in clinical population.
Despite the notion that ketamine can produce rapid antidepressant effects, maintaining the efficacy gained by its initial treatment is critical. A recent article reported reviewed 5 studies on randomized, double-blind clinical trials focusing on repeated ketamine infusions and its prolonged action. The study included oral agents lithium and riluzole, a small negative study involving repeated ketamine infusions, and a positive yet insufficiently controlled larger study supporting infusions 2 or 3 times weekly (Papakostas, 2020). The results were inconclusive. Another recent study supported this conclusion using the fMRI scan to detect the convergent neurocircuits of depressive-like or resilient rats administrated single or repeated ketamine. The findings reported that the brain topology of repeated ketamine treatment was highly distinct from the single treatment, but resemble with the depressive-like imaging phenotype (Gass et al., 2020). This study also indicated the inefficacy of repeated ketamine to improve the network topology for depressed patients. Based on these studies, it is difficult to state whether repeated ketamine administration improves its’ antidepressant efficacy.
1.2. Targeting AMPA (Hydroxy-5-methyl-4-isoxazolepropionic acid) receptors
AMPA receptors are composed of 4 subunits: GluA1, A2, A3 and A4; all of them facilitate the functions of fast excitatory neurotransmission and participate in learning and memory (Shi et al., 1999; Song and Huganir, 2002). It has been reported that repeated stress paradigm can reduce AMPAR-mediated synaptic transmission and expression levels of glutamate receptors in the pyramidal neurons of PFC of rats (Yuen et al., 2012). On the other hand, AMPA receptors are increased in anterior cingulate cortex of depressed patients (Gibbons et al., 2012). In addition, AMPA receptor subunits, primarily the ones encoded by GRIA family, are altered in hippocampal dentatae gyrus and CA1 subfields of depressed patients (Duric et al., 2013). NMDA receptor antagonists show enhanced antidepressant-like effects by increasing AMPA receptor functions (Aleksandrova et al., 2017), which may be mediated via PI3K-Akt-mTOR signaling, by promoting the synthesis of neurotrophic factors (Zhou, W. et al., 2014). Different AMPA receptors subunits were studied in the prefrontal cortex and hippocampus of chronically administrated rats with the two different antidepressants: fluoxetine and reboxetine (Racagni and Popoli, 2008). The results demonstrate that fluoxetine mainly induces GluA2 and GluA4 while reboxetine mainly affects GluA1 and GluA3. Both drugs upregulated AMPA receptor protein levels in a time-dependent manner (Barbon et al., 2006; Heras-Sandoval et al., 2014). Another study found that chronic treatment with ketamine heightened density ratios of AMPA to NMDA receptors in the hippocampus of genetically depressed fats (Tizabi et al., 2012).
As mentioned earlier, ketamine exerts its action by blocking NMDA receptors. Interestingly, Zanos et al. (2016) published a report which suggest that ketamine exerts antidepressant-like effect in mice which can be independent of NMDAR inhibition. This happens through it’s metabolite (2S, 6S; 2R, 6R)-HNK, which activates AMPA receptors. It was shown that (2R,6R)-HNK reversed depression-like behavior in mice and did not induce ketamine-related psychotic or addictive side effects (Zanos et al., 2016). This provides a basis to test novel drugs that exert rapid antidepressant effects independent of NMDAR inhibition without the side effects associated with this inhibition.
1.3. Metabotropic Glutamate Receptors (mGluRs)
mGluRs belong to G protein-coupled receptor family. The primary functions of mGluR is to affect glutamate level. It has been demonstrated that glutamate-induced changes in synaptic plasticity is linked to the pathophysiology as well as treatment of mood disorders (Bonsi et al., 2005; Chu and Hablitz, 2000). mGluRs are primarily located pre-synaptically at glutamatergic synapse (Hinoi et al., 2001). Except mGluR1/5, mGluR activation decreases glutamate release, which may otherwise cause excitotoxic damage (Shigemoto et al., 1997). mGluR2 agonists reduce excessive glutamate release to achieve antidepressant efficacy. On the other hand, mGluR2/3 antagonists augment synaptic glutamate levels, thereby enhancing AMPA receptor transmission and firing rates (Shigemoto et al., 1997). As with ketamine, mGluR2/3 antagonists also target mTOR signaling to mediate their effects (Dwyer et al., 2013; Koike et al., 2011).
The antidepressant efficacies of mGluR2/3 antagonists and negative allosteric modulator RO4995819 have been tested in rodent depression models (Campo et al., 2011; Chaki et al., 2004b). It has been shown that mGluR antagonists can mimic ketamine-like effects (Chaki et al., 2004b; Dwyer et al., 2012). On the other hand, the safety and tolerability of positive mGluR2/3 allosteric modulators (NCT01547703 and NCT01546051) have been tested in healthy subjects, however, so far, they have not been tested in MDD patients for their clinically efficacy (A Jaso et al., 2017).
mGluR5s are expressed both pre- and post-synaptically. They are primarily involved in AMPA receptor internalization, which is a key process in regulating synaptic plasticity (Chu and Hablitz, 2000; Endoh, 2004). mGluR5s are also integrated with NMDA receptors (Lea et al., 2002). mGluR5 antagonists have been tested for their antidepressant-like effects in various rodent models and found to have positive responses (Belozertseva et al., 2007; Palucha et al., 2005). Interestingly, ketamine, which primarily acts through inhibition of NMDA receptors, was found to act through mGluR5. A study by Esterlis et al. (2018) done in 13 MDD and 13 control subjects showed a significant ketamine-induced reduction in mGluR5 availability in both MDD and control subjects measured by [11C]ABP688 binding. A significant reduction in depressive symptoms was also observed following ketamine administration in the MDD group, which was associated with the change in binding after ketamine administration. The authors speculated that glutamate released after ketamine administration moderated mGluR5 availability, which may be associated with ketamine’s antidepressant action (Esterlis et al., 2018).
Clinical trials for mGluR5 antagonist AZD2066 and the mGluR5 negative allosteric modulator basimglurant (RO4917523) have been conducted in treatment-resistant depressed patients (Quiroz et al., 2016). Of them, the effects of RO4917523 seem promising in two independent clinical trials (http://www.roche.com/irp150128-annex.pdf). On the other hand, AZD2066, given at the dose of 12–18 mg/day for six weeks was not as effective as placebo and SNRI duloxetine. In a double-blind placebo controlled study, basimglurant, when given to treatment-resistant depressed patients at the dose of 0.5 mg/day or 1.5/day as an adjunct therapy with serotonin-noradrenaline reuptake inhibitors or selective serotonin reuptake inhibitors for 9 weeks, showed significantly positive antidepressant responses (Quiroz et al., 2016). However, phase II clinical trials demonstrated a lack of efficacy. Another mGluR2 negative allosteric modulator RG1578 has been tested in MDD patients, however the results are not as promising (Dale et al., 2015).
Altogether, there seems to novel drugs that can be developed targeting mGluRs. It is promising that ketamine metabolite that acts through mGluR5 can have antidepressant efficacy without the side effects associated with NMDA receptor blockade. This and other mGluR5 modulators need to be closely monitored as future antidepressants.
1.4. Targeting γ-aminobutyric acid (GABA) receptors
γ-aminobutyric acid (GABA), as an inhibitory neurotransmitter, is involved in regulating activity in noradrenergic, dopaminergic, and serotonergic neurons (Marescaux et al., 1992). GABA receptors are of two types: ionotrpic GABAa and GABAc receptors, and metabotropic GABAb receptors. Both are members of G-protein coupled receptors (Sieghart and Sperk, 2002). A majority of preclinical antidepressant development studies have focused on GABAb receptors (Mombereau et al., 2004). In animal models of depression, GABAb receptor antagonists showed potential targets as anti-depressive agents (Slattery et al., 2005). For example, GABA antagonist CGP52432 showed antidepressant-like activity in the forced swimming test after acute, subchronic, and chronic treatment (Felice et al., 2012). Chronic treatment with CGP 52432 increased cell proliferation in hippocampus of stress-sensitive mouse strain. These effects were not present when it was given acutely or subchronically. Another study found that pharmacological inhibition of GABAB receptors stimulated neural stem/progenitor cell proliferation, whereas knockout of GABAB1 receptor subunits increased neural cell proliferation and differentiation (Giachino et al., 2014). GABAb antagonist SGS742 has been undertaken to Phase II clinical trial. In the double-blind study, SGS742 given orally for 8 weeks, significantly enhanced attention, reaction time, as well as working memory (Froestl et al., 2004). However, SGS742 also produced some potential adverse effects including hypotension, seizures and muscle weakness (Bowery, 2006). Recently, clinical trial for brexanolone, an allosteric neurosteroid modulator of synaptic and extrasynaptic GABA receptors, in postpartum depressed patients showed that Hamilton Rating Scale for Depression total score was significantly reduced compared with placebo at 60 hours (Kose and Cetin, 2017). In a clinical trial of 247 women with moderate or severe postpartum depression, brexanolone significantly reduced Hamilton Rating Scale for Depression total score compared with placebo at 60 hrs intravenous infusion (Trial 1/NCT02942004, Trial 3/NCT02614541, Trial 2/ NCT02942017) (Kose and Cetin, 2017). This is quite promising as so far as there are not many GABAergic compound that produce desired antidepressant effects.
2. Targeting Hypothalamic-Pituitary-Adrenal (HPA) Axis Pathway
2.1. Glucocorticoid receptors
Depressed patients are often accompanied with high levels of glucocorticoids (GC) (Maletic et al., 2007). The high concentration GCs lead to a significant decrease in the number and function of glucocorticoid receptors (GRs), which mediate the negative feedback inhibition of glucocorticoids, thus leading to the dysregulation of the HPA axis (Laryea et al., 2015). GR antagonists can not only inhibit the increase in glucocorticoid levels, but their long-term treatment can also increase GRs and restore HPA axis negative feedback regulation sensitivity (Schmidt and Chen, 2018). Preclinical studies have shown that a variety of GR antagonists such as Org34850 and CORT108297 show significant antidepressant response (Solomon et al., 2014; Spiga et al., 2007). Recently, CORT118335, as a GR modulator/ mineralocorticoid receptor (Mrazek et al.) antagonist, was measured for its effects on endocrine and behavior response in female rats (Nguyen et al., 2018). It directly increased the immobility and affected the secretion of ACTH and corticosterone during the forced swimming test. In another study, Mifepristone, a glucocorticoid receptor antagonist CORT108297, and imipramine, given to male rats, decreased immobility time in the FST and enhanced corticosterone response to stressor (Solomon et al., 2014). When male rats were treated concurrently with fluoxetine along with the GR antagonist (Org34850), it was found that Org34850 enhanced SSRI- induced decrease in 5-HT transporter levels in the forebrain (Johnson et al., 2009). Clinical studies have shown that patients with severe chronic depression show efficacy after taking mifepristone (RU-486; GR antagonist) for 1 week, and it’s short-term application is safe (Gallagher and Young, 2006), especially for the treatment of depression, which can also improve cognitive impairment (Donoghue et al., 2016); however, long-term treatment may cause adrenal hypofunction, liver damage, fatigue and hot flashes as well as other adverse reactions (Ragucci et al., 2017).
2.2. Targeting Corticotrophin-releasing factor-1 (CRF-1) Receptor
CRF regulates the function of the HPA axis in both underlying and stress states (Aguilera et al., 1986). There are two subtypes of CRF receptors: CRF1 and CRF2. CRF1 receptors are highly expressed in the CNS and primarily mediates stress response (Roozendaal et al., 2002; Snyder et al., 2012; Valentino and Wehby, 1988). CRF and CRF1 receptor abnormalities are one of the important mechanisms associated with major depression (Ising and Holsboer, 2007; Kehne, 2007). CRF1 receptor antagonists act as antidepressants by inhibiting the formation of thyroid hormones. Preclinically, several studies have demonstrated that CRF1 receptor antagonists show anti-depressive effects in various behavioral models (Bourke et al., 2014; Chaki et al., 2004a; Griebel et al., 2002b; Mansbach et al., 1997). Another report suggests that treatment of CRF1 receptor antagonists R121919 and DMP696 significantly decreases immobility time in the tail suspension test while the other two antagonists (antalarmin and DMP904) are not effective (Nielsen et al., 2004). The study supports the evidence that nonpeptidic CRF1 receptor antagonists may possess antidepressant-like effects (Zorrilla and Koob, 2010). Although CRF receptor antagonists are shown to be unsuccessful, positive results based on anxiety and conditional fear have shown them to be effective for treatment of anxiety and stress induced hypertension. Especially, oral administration of antalarmin (3–30 mg/kg) combined with SSRI fluoxetine has been found to have a potential for synergistic effect on immobility reduction (Ducottet et al., 2003).
Another antagonist, SRR125543, was found to have both antidepressant- and anxiolytic-like effects in immobility time in the Flinders Sensitive Line of rats as depression model (Overstreet and Griebel, 2004). CRF1 antagonist (R278995/CRA0450), when tested learned helplessness and olfactory bulbectomy models in rat, also showed dose-dependent antidepressant-like effects (Chaki et al., 2004a). The above studies support the evidence that CRF1 antagonists have antidepressant-like effects in different depressive rodent models. It is pertinent to mention that both male and female CRFR2-deficient mice show increased immobility time, indicative of depression-like behavior (Bale and Vale, 2003).
CRF1 receptor antagonist NBI-30775/R121919 was studied fir its efficacy in an open-label trial (Zobel et al., 2000). Initial results are encouraging. Another CRF1 receptor antagonist CP-316,311 was tested in a phase II placebo-controlled, double-blind, randomized study. Although, this agent was found to be safe and well tolerated but it did not show positive results in the treatment of MDD (Binneman et al., 2008). Efforts have been made for a Phase III clinical trial; however, no CRF1 antagonist has shown a definitive response (Zorrilla and Koob, 2010). CRF1 receptor antagonists are expected to be beneficial only for those patients with CRF overexpression. Thus, the utility of CRF antagonists as antidepressants in patients with major depression without HPA axis abnormalities may not be as effective. Nevertheless, if these compounds can be developed in a subpopulation of depressed subjects, it will be a major achievement (Paez-Pereda et al., 2011).
3. Targeting opioid receptors
Several animal studies show that opioid receptors can modulate depressive behavior (Lutz and Kieffer, 2013). Knockdown of δ-opioid receptors induce depression-like behavior, and δ opioid receptor agonists can ameliorate this behavior in mice (Jutkiewicz and Roques, 2012). In addition, several δ opioid receptor agonists, like UFP-512, ADL5839, and AZD2327 produce significant antidepressant-like effects in olfactory bulbectomy and other behavioral models (Saitoh and Yamada, 2012). When the kappa opioid agonist salvinorin A was injected into rats intraperitoneally, it increased the threshold of ICSS test and the immobility time of the forced swimming test (Carlezon et al., 2006; Ebner et al., 2010). It was also found that salvinorin A can reverse anhedonia and show antidepressant-like effects in chronic mild stress rats (Harden et al., 2012). Another kappa opioid receptor agonist, U-50,488, shows not only dose-independent effect but also sex-dependent effects on depressive-like behavior in rats (Russell et al., 2014). The treatment of κ opioid receptor antagonists Norbinaltorphimine (nor-BNI) significantly reduced the yielding of depression-like behavior caused by social defeat stress (McLaughlin et al., 2006). The exposure of methylphenidate to mice generally shows depression-like behavior by reducing sucrose preference and increasing immobility time in the forced-swim test. Administration of the κ opioid receptor agonist U-50488 accelerates depression-like behavior, while antagonist norBNI ameliorates this behavior (Wiley et al., 2009). Ide et al. (2010) performed behavioral tests in μ-opioid receptor (MOR) knockout mice and observed that they significantly differ from normal mice on the elevated plus maze, tail suspension, and forced swimming tests and suggested that MOR mediate the development of anxiety and depression in rodents. Buprenorphine (developmental code name ALKS-5461), as an antidepressant and anxiolytic drug, highly reacts with both μ opioid and kappa opioid receptors. By means of the NIH test and genetically modified animal model, it was found that BPN regulates MOR activity and produces antidepressant-like effects in the NIH test (Robinson et al., 2017). In addition, two phase 3, randomized, double-blind, placebo-controlled studies for buprenorphine/samidorphan as adjunctive treatment were effective in major depressed patients (Fava, Maurizio et al., 2018). ALKS-5461 was approved as the adjunctive treatment of MDD by the FDA in 2018. However, the FDA committee voted against the approval of ALKS-5461 for MDD due to lack of sufficient evidence for it’s effectiveness. More clinical data is needed to prove it’s efficacy.
4. Molecular targets associated with inflammation
Clinical studies have shown that the level of proinflammatory cytokines in peripheral blood and cerebrospinal fluid of depressed patients are significantly higher than those of healthy subjects (Black and Miller, 2015; Engler et al., 2017; Schwieler et al., 2015). Several human postmortem brain studies, including our own, also show similar changes (Wang et al., 2018). Pre-clinical studies demonstrate that antidepressants can lead to changes in the cytokine level in rodent model of depression. The two antidepressants, imipramine and fluoxetine, when administered chronically in rats alter the expression of several inflammatory genes including IFN-γ, IL-6 and IL-4 in the hypothalamus (Fazzino et al., 2009; Nguyen et al., 1998; Zhang et al., 2010). When C57BL/6 mice were treated with imipramine (15 mg/kg) and exposed to repeated social defeat stress, it diminished inflammation as well as stress-associated anxiety- and depressive-like behaviors (Ramirez and Sheridan, 2016). Moreover, SSRI, TCA and SNR1 can decrease IL-6 and TNF-α, and IFN- γ levels in depressed patients (Alboni et al., 2013). Recently, the evidence of the neuroinflammation arise from the TSPO radioligand binding studies. [18F] FEPPA was performed in the 20 depressed patients and healthy controls. It was found that uptake of the radioligand was significantly higher in the prefrontal cortex and other brain regions of MDD subjects (Setiawan et al., 2015). PET tracer uptake in the anterior cingulated cortex correlated with Hamilton Depression Rating Scale score in the depressed patients (Setiawan et al., 2015). A study on TNF-α as a target found that the TNF-α antagonist infliximab, although can improve depressive symptoms in patients who show high baseline inflammatory markers but is not effective in treatment-resistant depression (Raison et al., 2013). Anti-inflammatory agent, sirukamab, which targets pro-inflammatory interlukin-6, was found to decrease anhedonia and fatigue (Sun et al., 2017; Zhou et al., 2017). At present, this drug is undergoing clinical trial for antidepressant efficacy in depressed patients (Ionescu and Papakostas, 2017).
5. Other molecular targets
5.1. Phosphodiesterase 4
Phosphodiesterase 4 (PDE4) is a member of the PDE family and PDE4 inhibitor can inhibit cAMP hydrolysis and increase the concentration of cAMP in the cells, thus triggering the downstream pathway of cAMP-dependent protein kinase (PKA) (Spina, 2008). PKA activation can promote CREB phosphorylation in the nucleus. This in turn can lead to the synthesis of neurotrophic factors such as BDNF, which plays a critical role in antidepressant action (Gao et al., 2003). At present, several studies suggest that the administration of PDE4 inhibitors can improve emotional and cognitive behaviors (Barad et al., 1998). Fujita et al. utilized the PET technology to image 11C-(R)-rolipram, which reflects cAMP cascade activity, in the unmedicated depressed patients and healthy controls. The results demonstrated that 11C-rolipram binding and PED4 occupancies were significantly downregulated in depression patients and rescued by SSRIs treatment. Thus, a PET ligand, selectively imaging PDE4D, can be used to evaluate PDE4-selective therapeutic agents (Fujita et al., 2012). When Rolipram, as a selective PDE4 inhibitor, was given to three types of mice, depression-like behavior was found only in the PDE4 +/+ mice but not in the PDE4D−/− and PDE4D+/− mice. Simultaneously, the level of cyclic AMP was increased only in the PDE4D+/+ mice (O’Donnell and Frith, 1999; Zhang et al., 2017). Rolipram could also enhance working and reference memory functions, measured by radial-arm maze test in rat (Barad et al., 1998; Zhang et al., 2005; Zhang and O’Donnell, 2000), which was primarily related to NMDA and MEK/ERK-mediated cAMP signaling pathway (Blendy, 2006; Monti et al., 2006; Sairanen et al., 2007). Another PDE4 inhibitor chlorbipram produced antidepressants-like effects and had the cognitive enhancer ability when administrated acutely into mice, with little or no emetic potency (Zhang et al., 2013). Taken together, it can be said that PDE4 inhibitors can produce antidepressant-like effects with minimal side effects (Zhang, 2009).
5.2. Peroxisome proliferator-activated receptor
Peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins that function as transcription factors. They are comprised of PPARα, β (or δ) and γ (Berger and Moller, 2002). Once activated, PPARs can repress transcription via DNA-independent protein-protein interactions with other transcription factors. Some of these transcription factors include NFκB, STAT-1 and AP-1 (Oliveira et al., 2007). They are highly expressed in the central nervous system, especially in hippocampus (Zolezzi et al., 2017). Animal studies have shown that oral administration of PPARγ agonist rosiglitazone (6 mg/kg) can improve depressive behavior in animals, reduce plasma glucocorticoid, increase brain neurotrophic factor α−1, and promote adult hippocampus nerve regeneration (Cheng et al., 2015). The authors report that hippocampal PPARδ expression is regulated by stress such that its level is decreased by chronic mild stress and is upregulated by antidepressants. Hippocampus PPARδ overexpression or the administration of selective PPARδ agonist GW0742 (5 mg/kg, ip) can significantly improve depression in chronic mild stress-induced depressed rats and acquired helpless stress-induced mice (Briand et al., 2009). It can also increase the formation and regeneration of hippocampal neurons (Ji et al., 2016). On the other hand, down-regulated expression of PPARδ or the administration of selective PPARδ antagonist GSK0660 (1 mg/kg, ip) can induce depression in mice, reduce BDNF production, prevent hippocampal nerve regeneration, and reduce neural stem cells to neurons differentiation (Paterniti et al., 2013).
5.3. Vasopressin
Vasopressin is normally secreted by the pituitary gland and is highly expressed in the hypothalamus, pituitary and limbic brain region upon stress exposure (Insel, 2010). It plays a critical role in the circadian rhythm, neuroendocrine actin and regulation of the HPA axis (Reid and Willshaw, 1999). The level of vasopressin is significantly increased in the plasma of depressed patients (van Londen et al., 1997; van Londen et al., 1998). In addition, the SNPs located in the genome of vasopressin V1b receptor are significantly associated with depressive behavior (Ben-Efraim et al., 2013; Dempster et al., 2007). Vasopressin deficient Brattleboro rats can be used to demonstrate the CNS effects of vasopressin (Bohus and de Wied, 1999). It has been shown that male vasopressin-deficient animals have lessened depression- and partly anxiety-like behavior, whereas vasopressin deficient females show no effects on object and social memory. Stress-induced elevation in c-Fos is diminished only in females but not in males (Fodor et al., 2016). SSR149415, a selective, V(1b) receptor antagonist, show antidepressant-like properties when tested in rodent models of depression. These include forced swim test (Griebel et al., 2012; Overstreet and Griebel, 2005), the olfactory bulbectomy (Iijima and Chaki, 2007) and unpredictable chronic mild stress (Alonso et al., 2004; Griebel et al., 2002a). In 2 clinical trials in depressed patients, SSR149415 had no significant improvement compared with placebo. In another depression study, SSR149415, but not escitalopram, had significant improvement in the HDRS total score compared to placebo without any negative effects on the stress axis (Griebel et al., 2012).
5.4. Substance P
Substance P belongs to tachykinin neuropeptide family and acts as a neurotransmitter and as a neuromodulator. It is widely distributed in the mammalian CNS (Harrison and Geppetti, 2001). In humans, serotonin and substance P are co-localized in dorsal raphe nuclei and several serotonin receptors, which are targets of several antidepressants, are co-localized with substance P (Baker et al., 1991; Sergeyev et al., 1999; Ward and Dorsa, 1996). Chronic treatment to rats with the imipramine, desipramine, clomipramine, amoxapine, or mianserin reduced substance P in various brain areas such as striatum, substantia nigra, and amygdala (Schwarz and Ackenheil, 2002). Of them, imipramine and desipramine also decreased substance P in the hippocampus (Shirayama et al., 1996). The receptor for substance P, NK-1 expression is significantly increased in the brain under stressful conditions and in depressive behavior (Geracioti et al., 2006; Rupniak, 2002; Schwarz and Ackenheil, 2002). It has been shown that substance P antagonists can suppress isolation-induced vocal behavior in guinea pigs (Kramer et al., 1998). Substance P NK1 receptor antagonist MK-869 also showed robust antidepressant effects in patients with moderate to severe major depression compared to placebo (Rupniak and Kramer, 1999). Besides, it had significant anxiolytic efficacy in the depressed patients. A preclinical study found that substance P antagonist MK-869 although did not show any associations with monoamine related systems but was involve in the integration of the emotion response (Kramer et al., 1998). Substance P NK1 receptor antagonist aprepitant and paroxetine were used for randomized clinical study in multicenter trial. Interestingly, aprepitant had no antidepressant-like effect while paroxetine was effective in the remission of depression symptoms (Keller et al., 2006). The efficacy and safety of a selective NK1 antagonist L-759274 was also tested in melancholic depressed patients (Kramer et al., 2004). The improvement in total HAMD-17 was greater in depressed patients receiving L-759274 compared to placebo. Also, item 1 of HAMD-17 and Clinical Global Impressions-Improvement Scale improved significantly compared with the placebo group. Recently, a clinical trial study found that a higher dose of casopitant as NK-1 antagonist had greater efficacy on the HDRS than placebo in depressed patients (Ratti et al., 2011). However, both casopitant and paroxetine in the second trial were not different from placebo at the end point on HAMD17. More recently, overpitant, another selective NK1 antagonist, showed superior efficacy compared to placebo in two independent clinical trials in depressed patients (Ratti et al., 2013). Taken together, these studies suggest that NK-1 antagonists may be efficacious in the treatment of depressed patients.
Conclusions
Targeting the monoaminergic system has been the most successful approach to treat MDD. A significant proportion of depressed patients do not respond to currently available antidepressant treatments, all of which primarily exert their action through monoaminergic modulation. Therefore, it is imperative that depression research moves towards compounds that target non-monoaminergic molecules. In recent years, a number of novel targets have emerged that can be used to develop as antidepressants. Some of these targets have been discussed in the present review. A table (Table 1) is depicted demonstrating newer agents that are under scanner for drug development. These include agents that target NMDA receptors, mGluR receptors, CRF1 receptors, δ-opioid receptors, κ-opioid receptors, GABAB receptors, M-cholinergic receptors, IDO, CysLT1R, PDE4, PPARγ, PPARδ, and NOS. Some of the agents that act on these targets have been tested in animal models and were found to be highly effective. Some compounds have already been entered in the clinical trial stage. Interestingly, agents that target glutamate system appear to be of high value. For example, the most promising at this stage are drugs that act as NMDA receptor antagonists that have rapid antidepressant response. However, NMDA receptor antagonist ketamine has many metabolites and have several targets that exclude NMDA receptors. One of them is mGluR5. Targeting this receptor eliminates the side effects associated with NMDA receptor blockade. Ketamine is anti-suicidal and are effective in treatment-resistant depression. Thus, research on glutamate/NMDA targets holds promise, but they are in early stages and much work is needed before they are considered as viable option for treating MDD in the general population as promising results are seen in subpopulation of TRD patients.
Table 1:
Newer agents that are under scanner for drug development
| Drugs | Site of Action | Uses |
|---|---|---|
| Ketamine | NMDA receptor antagonist | Anesthesia, inhibits painful sensations, antidepressant |
| R025-6981 | Specific antagonist of NMDA receptor 2B subtype (NR2B) | Antidepressant |
| SSR125543 | CRF1 receptor antagonist | Antidepressant and anxiolytic |
| NBI-30775 and NBI34041 | CRF1 receptor antagonist | Anxiety; Depression; Irritable bowel syndrome |
| RU486 (mifepristone), Org34116, Org 34517, Org34850 | Pituitary glucocorticoid receptor (GR) antagonists | Functional impairments in the depressed brain, especially in the hippocampus |
| AZD-7268 | Delta opioid receptor agonists | Antidepressant |
| LY2456302 | κ opioid receptor antagonist | Major depressive disorder and substance use disorders including alcoholism, nicotine addiction, and illicit drug dependence |
| Mecamylamine | Nicotinic acetylcholine receptors (nAChRs) antagonist | Antidepressant |
| GSK0660 | PPARγ antagonist | Anxiolytic, antidepressant, and antiobesity effects |
| Rolipram | Selective phosphodiesterase-4 inhibitor | Autoimmune diseases, Alzheimer’s disease, cognitive enhancement, spinal cord injury, and respiratory diseases |
| Roflumilast (Daxas, Daliresp) | Selective, long-acting inhibitor of the enzyme phosphodiesterase-4 | Severe chronic obstructive pulmonary disease |
Previous studies have shown that the use of antidepressants was correlated with a high increased risks of suicidal behavior or thoughts, especially in younger population (Stone et al., 2009). In order to investigate the association between suicide bereavement, mourning, and risk of suicide in survivor, a review conducted by Pompili and colleagues indicated that emotional turmoil in suicide survivors may last a long time and, in some cases, may end with their own suicide (Pompili et al., 2013). However, the present interventions have the weak effect for people bereaved by suicide and the authors pointed out that support groups may be preferable for suicidal survivors. To evaluate biological factors associated with recent suicidal attempts in a naturalistic sample, they also found that prolactin and thyroid hormone levels were associated or even predicted in suicide attemptors in psychiatric patients (Pompili et al., 2012). These studies may help to improve the clinical treatment for suicidal survivors and decreased risk of suicidal behavior.
Since last several decades, monotherapy has been the goal for antidepressant drug development, however, it has recently been shown that adjunctive therapy can be more effective in treatment of depression. For example, ketamine along with SSRIs can be effective. Similarly, buprenorphine/samidorphan adjunctive therapy was found to be effective in MDD patients. This strategy should be further pursued in clinical studies. A critical issue in antidepressant research is the improvement in response rates. This might be overcome through critically evaluating biological mechanisms underlying the heterogeneity of depression. In this regard genetic and epigenetic studies can be highly effective. In fact, studies on non-coding RNAs are gaining strength and several microRNAs (a family of non-coding RNA) have been found to be associated with depression. Some microRNAs are associated specifically with stress response (Dwivedi, 2018). If one can distinguish microRNAs based on depression phenotypes (stress, anhedonia, etc), they can be used as biomarker for treatment response. Noncoding RNAs themselves are emerging as novel targets for drug development for a variety of diseases. There are several approaches to modulate miRNA level and develop them as potential antidepressants. Synthetic oligonucleotides mimics and packaged lentivirus that overexpress mature or pre-miRNAs can upregulate miRNA level whereas synthetic antagmirs or miRNA sponge designed by the compensating the sequence of miRNA binding sites can decrease the specific miRNA. It is easier to design the molecular targeted for miRNAs than specific proteins. To explore the potential miRNAs antidepressants, it has been shown that miR-124 antagomir directly decrease depressive-behavior in corticosterone-induced depression animal model (Wang et al., 2017). Despite many delivery systems including lipid, polymer and carbon-based carriers, the effective delivery of miRNA into the brain is still in infancy that need to be developed further. However, a recent study on the exosomal miRNA suggested that miR-139–5p antagomir when given intranasally to stressed mice caused significantly increased blood and brain level of exsomal miR-139–5p and alleviated depression-like behavior (Wei et al., 2020). This is quite promising and indicate that miRNAs can be delivered in brain for the desired effects.
Besides the above mentioned targets, several other novel agents are being tested for antidepressant response. For example, it has recently been shown that c-JUN kinase inhibition, specifically in newly generated nerve cells in the hippocampus, can alleviate anxiety and depressive behavior in mice (Mohammad et al., 2016). Certain studies are now coming up which show that non-coding RNAs that participate in regulating the expression of genes, may cause depression and they may serve as important targets for new antidepressant drug development (Dwivedi, 2017). Several epigenetic modifiers are also being considered for the development of antidepressants, although there are still concerns of the mode of delivery and non-specific effects that may be associated with these agents. Nevertheless, with the advent of new targets, the possibility of developing new and effective antidepressants is promising, which may have the potential to provide rapid and efficacious response.
Highlights.
Current novel treatment targets that are currently being tested as either pre-clinically or clinically as antidepressants.
New molecular targets that can provide potential approach for seeking effective and relatively safer antidepressant agents.
Funding Support
Funding was received from the National Institute of Mental Health (R01MH082802; R21MH081099; 1R01MH101890; R01MH100616; 1R01MH107183) to Dr. Y. Dwivedi and from National Natural Science Foundation of China (31871281) and Scientific Research Foundation for Advanced Talents of Shanghai University of Traditional Chinese Medicine to Dr. Q. Wang.
Role of the Funding Source: The funding source had no role in the study design, in the collection, analysis and interpretation of data.
Footnotes
Declarations of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A Jaso B, J Niciu M, D Iadarola N, Lally N, M Richards E, Park M, D Ballard E, C Nugent A, Machado-Vieira R, A Zarate C, 2017. Therapeutic modulation of glutamate receptors in major depressive disorder. Current neuropharmacology 15(1), 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, Sherif M, Ahn KH, D’Souza DC, Formica R, Southwick SM, Duman RS, Sanacora G, Krystal JH, 2020. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology 45(6), 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Jackowski A, Salas R, Gupta S, Sato JR, Mao X, Coplan JD, Shungu DC, Mathew SJ, 2017. The nucleus accumbens and ketamine treatment in major depressive disorder. Neuropsychopharmacology 42(8), 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Sanacora G, Duman RS, Krystal JH, 2015. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66, 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G, Wynn PC, Harwood JP, Hauger RL, Millan MA, Grewe C, Catt KJ, 1986. Receptor-mediated actions of corticotropin-releasing factor in pituitary gland and nervous system. Neuroendocrinology 43(1), 79–88. [DOI] [PubMed] [Google Scholar]
- Alboni S, Benatti C, Montanari C, Tascedda F, Brunello N, 2013. Chronic antidepressant treatments resulted in altered expression of genes involved in inflammation in the rat hypothalamus. European journal of pharmacology 721(1–3), 158–167. [DOI] [PubMed] [Google Scholar]
- Aleksandrova LR, Phillips AG, Wang YT, 2017. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. Journal of psychiatry & neuroscience: JPN 42(4), 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P, 2004. Blockade of CRF 1 or V 1b receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Molecular psychiatry 9(3), 278. [DOI] [PubMed] [Google Scholar]
- Aroni F, Iacovidou N, Dontas I, Pourzitaki C, Xanthos T, 2009. Pharmacological aspects and potential new clinical applications of ketamine: reevaluation of an old drug. J Clin Pharmacol 49(8), 957–964. [DOI] [PubMed] [Google Scholar]
- Baker K, Halliday G, Hornung J-P, Geffen L, Cotton R, 1991. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience 42(3), 757–775. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW, 2003. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. Journal of Neuroscience 23(12), 5295–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E, 1998. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A 95(25), 15020–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbon A, Popoli M, La Via L, Moraschi S, Vallini I, Tardito D, Tiraboschi E, Musazzi L, Giambelli R, Gennarelli M, Racagni G, Barlati S, 2006. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry 59(8), 713–720. [DOI] [PubMed] [Google Scholar]
- Bartoli F, Riboldi I, Crocamo C, Di Brita C, Clerici M, Carra G, 2017. Ketamine as a rapid-acting agent for suicidal ideation: A meta-analysis. Neurosci Biobehav Rev 77, 232–236. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY, 2007. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol 17(3), 172–179. [DOI] [PubMed] [Google Scholar]
- Ben-Efraim YJ, Wasserman D, Wasserman J, Sokolowski M, 2013. Family-based study of AVPR1B association and interaction with stressful life events on depression and anxiety in suicide attempts. Neuropsychopharmacology 38(8), 1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Moller DE, 2002. The mechanisms of action of PPARs. Annu Rev Med 53, 409–435. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH, 2000. Antidepressant effects of ketamine in depressed patients. Biological psychiatry 47(4), 351–354. [DOI] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T, 2008. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH 1 antagonist) in the treatment of major depression. American Journal of Psychiatry 165(5), 617–620. [DOI] [PubMed] [Google Scholar]
- Bjorkholm C, Monteggia LM, 2016. BDNF - a key transducer of antidepressant effects. Neuropharmacology 102, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C, Miller BJ, 2015. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biological psychiatry 78(1), 28–37. [DOI] [PubMed] [Google Scholar]
- Blendy JA, 2006. The role of CREB in depression and antidepressant treatment. Biological psychiatry 59(12), 1144–1150. [DOI] [PubMed] [Google Scholar]
- Bohus B, de Wied D, 1999. The vasopressin deficient Brattleboro rats: a natural knockout model used in the search for CNS effects of vasopressin, Progress in brain research. Elsevier, pp. 555–573. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, De Persis C, Centonze D, Bernardi G, Calabresi P, Pisani A, 2005. Modulatory action of metabotropic glutamate receptor (mGluR) 5 on mGluR1 function in striatal cholinergic interneurons. Neuropharmacology 49 Suppl 1, 104–113. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Glasper ER, Neigh GN, 2014. SSRI or CRF antagonism partially ameliorate depressive-like behavior after adolescent social defeat. Behavioural brain research 270, 295–299. [DOI] [PubMed] [Google Scholar]
- Bowery NG, 2006. GABAB receptor: a site of therapeutic benefit. Current opinion in pharmacology 6(1), 37–43. [DOI] [PubMed] [Google Scholar]
- Briand F, Naik SU, Fuki I, Millar JS, Macphee C, Walker M, Billheimer J, Rothblat G, Rader DJ, 2009. Both the Peroxisome Proliferator-Activated Receptor δ Agonist, GW0742, and Ezetimibe Promote Reverse Cholesterol Transport in Mice by Reducing Intestinal Reabsorption of HDL-Derived Cholesterol. Clinical and translational science 2(2), 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR, 2013. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 38(5), 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK, 2014. Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol 4(2), 75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo B, Kalinichev M, Lambeng N, Yacoubi ME, Royer-Urios I, Schneider M, Legrand C, Parron D, Girard F, Bessif A, 2011. Characterization of an mGluR2/3 negative allosteric modulator in rodent models of depression. Journal of neurogenetics 25(4), 152–166. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY-W, Cohen BM, 2006. Depressive-like effects of the κ-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. Journal of Pharmacology and Experimental Therapeutics 316(1), 440–447. [DOI] [PubMed] [Google Scholar]
- Castren E, Kojima M, 2017. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis 97(Pt B), 119–126. [DOI] [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, Vinken P, Ashton D, Langlois X, Steckler T, 2004a. Anxiolytic-and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. European journal of pharmacology 485(1–3), 145–158. [DOI] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, Yoshimizu T, Yasuhara A, Sakagami K, Okuyama S, 2004b. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 46(4), 457–467. [DOI] [PubMed] [Google Scholar]
- Chandley MJ, Szebeni A, Szebeni K, Crawford JD, Stockmeier CA, Turecki G, Kostrzewa RM, Ordway GA, 2014. Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. International Journal of Neuropsychopharmacology 17(10), 1569–1578. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Rodriguiz RM, Murthy SR, Senatorov V, Thouennon E, Cawley NX, Aryal DK, Ahn S, Lecka-Czernik B, Wetsel WC, 2015. Neurotrophic factor-α1 prevents stress-induced depression through enhancement of neurogenesis and is activated by rosiglitazone. Molecular psychiatry 20(6), 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Hablitz JJ, 2000. Quisqualate induces an inward current via mGluR activation in neocortical pyramidal neurons. Brain Res 879(1–2), 88–92. [DOI] [PubMed] [Google Scholar]
- Dale E, Bang-Andersen B, Sánchez C, 2015. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochemical pharmacology 95(2), 81–97. [DOI] [PubMed] [Google Scholar]
- Dean B, Gibbons AS, Boer S, Uezato A, Meador-Woodruff J, Scarr E, McCullumsmith RE, 2016. Changes in cortical N-methyl-D-aspartate receptors and post-synaptic density protein 95 in schizophrenia, mood disorders and suicide. Australian & New Zealand Journal of Psychiatry 50(3), 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster EL, Burcescu I, Wigg K, Kiss E, Baji I, Gadoros J, Tamas Z, Kennedy JL, Vetro A, Kovacs M, 2007. Evidence of an association between the vasopressin V1b receptor gene (AVPR1B) and childhood-onset mood disorders. Archives of general psychiatry 64(10), 1189–1195. [DOI] [PubMed] [Google Scholar]
- Donoghue K, Rose A, Coulton S, Milward J, Reed K, Drummond C, Little H, 2016. Double-blind, 12 month follow-up, placebo-controlled trial of mifepristone on cognition in alcoholics: the MIFCOG trial protocol. Bmc Psychiatry 16(1), 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducottet C, Griebel G, Belzung C, 2003. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry 27(4), 625–631. [DOI] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu R-J, Duric V, Aghajanian G, 2012. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62(1), 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC Jr, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, Yuan P, Brutsche N, Manji HK, Tononi G, 2012. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. International Journal of Neuropsychopharmacology 16(2), 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L, Duman RS, 2013. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. International Journal of Neuropsychopharmacology 16(1), 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, 2017. microRNA-124: a putative therapeutic target and biomarker for major depression. Taylor & Francis. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, 2018. MicroRNAs in depression and suicide: Recent insights and future perspectives. J Affect Disord 240, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Duman RS, 2013. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biological psychiatry 73(12), 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, Duman RS, 2012. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. International journal of neuropsychopharmacology 15(4), 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, Duman RS, 2013. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. Journal of molecular psychiatry 1(1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH, 2010. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology 210(2), 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh T, 2004. Characterization of modulatory effects of postsynaptic metabotropic glutamate receptors on calcium currents in rat nucleus tractus solitarius. Brain Res 1024(1–2), 212–224. [DOI] [PubMed] [Google Scholar]
- Engler H, Brendt P, Wischermann J, Wegner A, Röhling R, Schoemberg T, Meyer U, Gold R, Peters J, Benson S, 2017. Selective increase of cerebrospinal fluid IL-6 during experimental systemic inflammation in humans: association with depressive symptoms. Molecular psychiatry 22(10), 1448. [DOI] [PubMed] [Google Scholar]
- Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, Yang J, Pittenger C, Sanacora G, Krystal JH, Parsey RV, Carson RE, DeLorenzo C, 2018. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [(11)C]ABP688 and PET imaging study in depression. Mol Psychiatry 23(4), 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI, 2018. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatmentresistant depression (TRD). Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Thase ME, Trivedi MH, Ehrich E, Martin WF, Memisoglu A, Nangia N, Stanford AD, Yu M, Pathak S, 2018. Opioid system modulation with buprenorphine/samidorphan combination for major depressive disorder: two randomized controlled studies. Mol Psychiatry 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzino F, Urbina M, Cedeño N, Lima L, 2009. Fluoxetine treatment to rats modifies serotonin transporter and cAMP in lymphocytes, CD4+ and CD8+ subpopulations and interleukins 2 and 4. International Immunopharmacology 9(4), 463–467. [DOI] [PubMed] [Google Scholar]
- Felice D, O’Leary OF, Pizzo RC, Cryan JF, 2012. Blockade of the GABAB receptor increases neurogenesis in the ventral but not dorsal adult hippocampus: relevance to antidepressant action. Neuropharmacology 63(8), 1380–1388. [DOI] [PubMed] [Google Scholar]
- Fodor A, Kovács KB, Balázsfi D, Klausz B, Pintér O, Demeter K, Daviu N, Rabasa C, Rotllant D, Nadal R, 2016. Depressive-and anxiety-like behaviors and stress-related neuronal activation in vasopressin-deficient female Brattleboro rats. Physiology & behavior 158, 100–111. [DOI] [PubMed] [Google Scholar]
- Froestl W, Gallagher M, Jenkins H, Madrid A, Melcher T, Teichman S, Mondadori CG, Pearlman R, 2004. SGS742: the first GABAB receptor antagonist in clinical trials. Biochemical pharmacology 68(8), 1479–1487. [DOI] [PubMed] [Google Scholar]
- Fujita M, Hines CS, Zoghbi SS, Mallinger AG, Dickstein LP, Liow J-S, Zhang Y, Pike VW, Drevets WC, Innis RB, 2012. Downregulation of brain phosphodiesterase type IV measured with 11C-(R)-rolipram positron emission tomography in major depressive disorder. Biological psychiatry 72(7), 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E, 2005. Subunit arrangement and function in NMDA receptors. Nature 438(7065), 185–192. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Young AH, 2006. Mifepristone (RU-486) treatment for depression and psychosis: a review of the therapeutic implications. Neuropsychiatric disease and treatment 2(1), 33. [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Nikulina E, Mellado W, Filbin MT, 2003. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinasedependent inhibition of phosphodiesterase. J Neurosci 23(37), 11770–11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass N, Becker R, Reinwald J, Cosa-Linan A, Sack M, Weber-Fahr W, Vollmayr B, Sartorius A, 2020. The influence of ketamine’s repeated treatment on brain topology does not suggest an antidepressant efficacy. Translational psychiatry 10(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geracioti TD Jr., Carpenter LL, Owens MJ, Baker DG, Ekhator NN, Horn PS, Strawn JR, Sanacora G, Kinkead B, Price LH, Nemeroff CB, 2006. Elevated cerebrospinal fluid substance p concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry 163(4), 637–643. [DOI] [PubMed] [Google Scholar]
- Giachino C, Barz M, Tchorz JS, Tome M, Gassmann M, Bischofberger J, Bettler B, Taylor V, 2014. GABA suppresses neurogenesis in the adult hippocampus through GABAB receptors. Development 141(1), 83–90. [DOI] [PubMed] [Google Scholar]
- Gibbons AS, Brooks L, Scarr E, Dean B, 2012. AMPA receptor expression is increased post-mortem samples of the anterior cingulate from subjects with major depressive disorder. Journal of affective disorders 136(3), 1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo-Salas M, Pilar-Cuéllar F, Auberson YP, Adell A, 2018. Signaling pathways responsible for the rapid antidepressant-like effects of a GluN2A-preferring NMDA receptor antagonist. Translational psychiatry 8(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Beeské S, Stahl SM, 2012. The Vasopressin V1b Receptor Antagonist SSR149415 in the Treatment of Major Depressive and Generalized Anxiety Disorders: Results From 4 Randomized, Double-Blind, Placebo-Controlled Studiese. Journal of Clinical Psychiatry 73(11), 1403. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand J-P, Soubrié P, 2002a. Anxiolytic-and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proceedings of the National Academy of Sciences 99(9), 6370–6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand J-P, Soubrié P, 2002b. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl) ethyl] 5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor1 receptor antagonist. II. Characterization in rodent models of stress-related disorders. Journal of Pharmacology and Experimental Therapeutics 301(1), 333–345. [DOI] [PubMed] [Google Scholar]
- Haile C, Murrough J, Iosifescu D, Chang L, Al Jurdi R, Foulkes A, Iqbal S, Mahoney III J, De La Garza R, Charney D, 2014. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. International Journal of Neuropsychopharmacology 17(2), 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, 2015. Chapter 9 - The ionotropic GABAA receptor, in: Hammond C (Ed.) Cellular and Molecular Neurophysiology (Fourth Edition). Academic Press, Boston, pp. 199–219. [Google Scholar]
- Harden MT, Smith SE, Niehoff JA, McCurdy CR, Taylor GT, 2012. Antidepressive effects of the κ-opioid receptor agonist salvinorin A in a rat model of anhedonia. Behavioural pharmacology 23(7), 710–715. [DOI] [PubMed] [Google Scholar]
- Harrison S, Geppetti P, 2001. Substance p. Int J Biochem Cell Biol 33(6), 555–576. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Jupp B, Sawiak SJ, Merlo E, Caprioli D, Dalley JW, 2014. Brain γ-aminobutyric acid: a neglected role in impulsivity. European Journal of Neuroscience 39(11), 1921–1932. [DOI] [PubMed] [Google Scholar]
- Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J, Pedraza-Chaverri J, 2014. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26(12), 2694–2701. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Ogita K, Takeuchi Y, Ohashi H, Maruyama T, Yoneda Y, 2001. Characterization with [3H]quisqualate of group I metabotropic glutamate receptor subtype in rat central and peripheral excitable tissues. Neurochem Int 38(3), 277–285. [DOI] [PubMed] [Google Scholar]
- Hirschfeld R, 2000. History and evolution of the monoamine hypothesis of depression. The Journal of clinical psychiatry. [PubMed] [Google Scholar]
- Ide S, Fujiwara S, Fujiwara M, Sora I, Ikeda K, Minami M, Uhl GR, Ishihara K, 2010. Antidepressant-Like Effect of Venlafaxine Is Abolished in μ-Opioid Receptor–Knockout Mice. Journal of pharmacological sciences, 1008090431–1008090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Chaki S, 2007. An arginine vasopressin V1b antagonist, SSR149415 elicits antidepressant-like effects in an olfactory bulbectomy model. Progress in Neuro-Psychopharmacology and Biological Psychiatry 31(3), 622–627. [DOI] [PubMed] [Google Scholar]
- Insel TR, 2010. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65(6), 768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu D, Papakostas G, 2017. Experimental medication treatment approaches for depression. Translational psychiatry 7(3), e1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Holsboer F, 2007. CRH-sub-1 receptor antagonists for the treatment of depression and anxiety. Exp Clin Psychopharmacol 15(6), 519–528. [DOI] [PubMed] [Google Scholar]
- Ji M-J, Yu X-B, Mei Z-L, An Y-Q, Tang S-S, Hu M, Long Y, Miao M-X, Hu Q-H, Sun H-B, 2016. Hippocampal PPARδ overexpression or activation represses stress-induced depressive behaviors and enhances neurogenesis. International Journal of Neuro-psychopharmacology 19(1), pyv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Sánchez L, Campa L, Auberson YP, Adell A, 2014. The role of GluN2A and GluN2B subunits on the effects of NMDA receptor antagonists in modeling schizophrenia and treating refractory depression. Neuropsychopharmacology 39(11), 2673–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, Ingram CD, Grant EJ, Craighead M, Gartside SE, 2009. Glucocorticoid receptor antagonism augments fluoxetine-induced downregulation of the 5-HT transporter. Neuropsychopharmacology 34(2), 399. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Roques BP, 2012. Endogenous opioids as physiological antidepressants: complementary role of delta receptors and dopamine. Neuropsychopharmacology 37(1), 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, 2013. Principles of neural science, 5th ed. McGraw-Hill, New York. [Google Scholar]
- Kehne JH, 2007. The CRF1 receptor, a novel target for the treatment of depression, anxiety, and stress-related disorders. CNS Neurol Disord Drug Targets 6(3), 163–182. [DOI] [PubMed] [Google Scholar]
- Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, Hargreaves R, Hietala J, Lines C, Beebe K, 2006. Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biological psychiatry 59(3), 216–223. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S, 2011. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 61(8), 1419–1423. [DOI] [PubMed] [Google Scholar]
- Kose S, Cetin M, 2017. Brexanolone: an allosteric modulator of GABA-A receptors in the rapid treatment of postpartum depression. Taylor & Francis. [Google Scholar]
- Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ, Reines SA, Liu G, Snavely D, Wyatt-Knowles E, 1998. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 281(5383), 1640–1645. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Winokur A, Kelsey J, Preskorn SH, Rothschild AJ, Snavely D, Ghosh K, Ball WA, Reines SA, Munjack D, 2004. Demonstration of the efficacy and safety of a novel substance P (NK 1) receptor antagonist in major depression. Neuropsychopharmacology 29(2), 385. [DOI] [PubMed] [Google Scholar]
- Kraus C, Mkrtchian A, Kadriu B, Nugent AC, Zarate CA Jr., Evans JW, 2020. Evaluating global brain connectivity as an imaging marker for depression: influence of preprocessing strategies and placebo-controlled ketamine treatment. Neuropsychopharmacology 45(6), 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ, 2008. The molecular neurobiology of depression. Nature 455(7215), 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH, 2007. NMDA receptors and schizophrenia. Current opinion in pharmacology 7(1), 48–55. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Duman RS, 2013. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biological psychiatry 73(12), 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CA, Sherbourne C, Tang L, Belin TR, Williams P, Young-Brinn A, Miranda J, Wells KB, 2016. The impact of community engagement on health, social, and utilization outcomes in depressed, impoverished populations: secondary findings from a randomized trial. The Journal of the American Board of Family Medicine 29(3), 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laryea G, Muglia L, Arnett M, Muglia LJ, 2015. Dissection of glucocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis by gene targeting in mice. Front Neuroendocrinol 36, 150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer RT, Popik P, Olds T, Skolnick P, 1995. Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715). Pharmacology Biochemistry and Behavior 52(3), 621–627. [DOI] [PubMed] [Google Scholar]
- Lea PM, Custer SJ, Vicini S, Faden AI, 2002. Neuronal and glial mGluR5 modulation prevents stretch-induced enhancement of NMDA receptor current. Pharmacol Biochem Behav 73(2), 287–298. [DOI] [PubMed] [Google Scholar]
- Li F, Tsien JZ, 2009. Memory and the NMDA receptors. The New England journal of medicine 361(3), 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, Li X-Y, Aghajanian G, Duman RS, 2010. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994), 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, Li X-Y, Aghajanian G, Duman RS, 2011. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological psychiatry 69(8), 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A, 2003. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacology & therapeutics 97(1), 55–85. [DOI] [PubMed] [Google Scholar]
- Lutz P-E, Kieffer BL, 2013. Opioid receptors: distinct roles in mood disorders. Trends in neurosciences 36(3), 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J, 2007. Neurobiology of depression: an integrated view of key findings. Int J Clin Pract 61(12), 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Brooks EN, Chen YL, 1997. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. European journal of pharmacology 323(1), 21–26. [DOI] [PubMed] [Google Scholar]
- Marescaux C, Vergnes M, Bernasconi R, 1992. Generalized non-convulsive epilepsy: focus on GABA-B receptors. J Neural Transm Suppl 35, 1–198. [PubMed] [Google Scholar]
- Martin JL, Finsterwald C, 2011. Cooperation between BDNF and glutamate in the regulation of synaptic transmission and neuronal development. Commun Integr Biol 4(1), 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud TL, Caddy C, Jochim J, Rendell JM, Diamond PR, Shuttleworth C, Brett D, Amit BH, McShane R, Hamadi L, Hawton K, Cipriani A, 2015. Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database Syst Rev(9), CD011611. [DOI] [PubMed] [Google Scholar]
- McEntee WJ, Crook TH, 1993. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology (Berl) 111(4), 391–401. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C, 2006. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 31(6), 1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS, 2000. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130(4S Suppl), 1007S–1015S. [DOI] [PubMed] [Google Scholar]
- Melo A, Kokras N, Dalla C, Ferreira C, Ventura-Silva AP, Sousa N, Pego JM, 2015. The positive effect on ketamine as a priming adjuvant in antidepressant treatment. Transl Psychiatry 5, e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad H, Marchisella F, Ortega-Martinez S, Hollos P, Eerola K, Komulainen E, Kulesskaya N, Freemantle E, Fagerholm V, Savontous E, 2016. JNK1 controls adult hippocampal neurogenesis and imposes cell-autonomous control of anxiety behaviour from the neurogenic niche. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sanchez E, Gutierrez-Rojas L, Meana JJ, 2018. Antidepressant Efficacy and Tolerability of Ketamine and Esketamine: A Critical Review. CNS Drugs 32(5), 411–420. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF, 2004. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology 29(6), 1050–1062. [DOI] [PubMed] [Google Scholar]
- Monti B, Berteotti C, Contestabile A, 2006. Subchronic rolipram delivery activates hippocampal CREB and arc, enhances retention and slows down extinction of conditioned fear. Neuropsychopharmacology 31(2), 278. [DOI] [PubMed] [Google Scholar]
- Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, Leander JD, 2014. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert opinion on investigational drugs 23(2), 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, Leander JD, 2014. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs 23(2), 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek DA, Hornberger JC, Altar CA, Degtiar I, 2014. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatric services 65(8), 977–987. [DOI] [PubMed] [Google Scholar]
- Nguyen ET, Caldwell JL, Streicher J, Ghisays V, Balmer NJ, Estrada CM, Solomon MB, 2018. Differential effects of imipramine and CORT118335 (Glucocorticoid receptor modulator/mineralocorticoid receptor antagonist) on brain-endocrine stress responses and depression-like behavior in female rats. Behavioural brain research 336, 99–110. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF, 1998. Exposure to acute stress induces brain interleukin-1β protein in the rat. Journal of Neuroscience 18(6), 2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DM, Carey GJ, Gold LH, 2004. Antidepressant-like activity of corticotropin-releasing factor type-1 receptor antagonists in mice. European journal of pharmacology 499(1–2), 135–146. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Frith S, 1999. Behavioral effects of family-selective inhibitors of cyclic nucleotide phosphodiesterases. Pharmacology Biochemistry and Behavior 63(1), 185–192. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Sekiya H, Namiki S, Sakamoto H, Iinuma S, Yamasaki M, Watanabe M, Hirose K, Iino M, 2010. Imaging extrasynaptic glutamate dynamics in the brain. Proc Natl Acad Sci U S A 107(14), 6526–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AC, Bertollo CM, Rocha LT, Nascimento EB Jr., Costa KA, Coelho MM, 2007. Antinociceptive and antiedematogenic activities of fenofibrate, an agonist of PPAR alpha, and pioglitazone, an agonist of PPAR gamma. Eur J Pharmacol 561(1–3), 194–201. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G, 2004. Antidepressant-like effects of CRF1 receptor antagonist SSR125543 in an animal model of depression. European journal of pharmacology 497(1), 49–53. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G, 2005. Antidepressant-like effects of the vasopressin V1b receptor antagonist SSR149415 in the Flinders Sensitive Line rat. Pharmacology Biochemistry and Behavior 82(1), 223–227. [DOI] [PubMed] [Google Scholar]
- Paez-Pereda M, Hausch F, Holsboer F, 2011. Corticotropin releasing factor receptor antagonists for major depressive disorder. Expert opinion on investigational drugs 20(4), 519–535. [DOI] [PubMed] [Google Scholar]
- Palucha A, Branski P, Szewczyk B, Wieronska JM, Klak K, Pilc A, 2005. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav 81(4), 901–906. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, 2020. Maintaining Rapid Antidepressant Effects Following Ketamine Infusion: A Major Unmet Need. The Journal of Clinical Psychiatry 81(2). [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E, 1994. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. European journal of pharmacology 263(1–2), 1–7. [DOI] [PubMed] [Google Scholar]
- Paterniti I, Impellizzeri D, Crupi R, Morabito R, Campolo M, Esposito E, Cuzzocrea S, 2013. Molecular evidence for the involvement of PPAR-δ and PPAR-γ in anti-inflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma. Journal of neuroinflammation 10(1), 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, O’Loughlin K, Torjman MC, Bernier M, Wainer IW, 2014. (R, S)-Ketamine metabolites (R, S)-norketamine and (2S, 6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology: The Journal of the American Society of Anesthesiologists 121(1), 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff OAC, 2002. Book Review: GABA and Glutamate in the Human Brain. The Neuroscientist 8(6), 562–573. [DOI] [PubMed] [Google Scholar]
- Pompili M, Gibiino S, Innamorati M, Serafini G, Del Casale A, De Risio L, Palermo M, Montebovi F, Campi S, De Luca V, Sher L, Tatarelli R, Biondi M, Duval F, Serretti A, Girardi P, 2012. Prolactin and thyroid hormone levels are associated with suicide attempts in psychiatric patients. Psychiatry Res 200(2–3), 389–394. [DOI] [PubMed] [Google Scholar]
- Pompili M, Shrivastava A, Serafini G, Innamorati M, Milelli M, Erbuto D, Ricci F, Lamis DA, Scocco P, Amore M, Lester D, Girardi P, 2013. Bereavement after the suicide of a significant other. Indian J Psychiatry 55(3), 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ, 2009. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biological psychiatry 66(5), 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz JA, Tamburri P, Deptula D, Banken L, Beyer U, Rabbia M, Parkar N, Fontoura P, Santarelli L, 2016. Efficacy and safety of basimglurant as adjunctive therapy for major depression: a randomized clinical trial. JAMA psychiatry 73(7), 675–684. [DOI] [PubMed] [Google Scholar]
- Racagni G, Popoli M, 2008. Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin Neurosci 10(4), 385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragucci E, Nguyen D, Lamerson M, Moraitis AG, 2017. Effects of Mifepristone on Nonalcoholic Fatty Liver Disease in a Patient with a Cortisol-Secreting Adrenal Adenoma. Case Rep Endocrinol 2017, 6161348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH, 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry 70(1), 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, Sheridan JF, 2016. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety-and depressive-like behaviors. Brain, behavior, and immunity 57, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratti E, Bellew K, Bettica P, Bryson H, Zamuner S, Archer G, Squassante L, Bye A, Trist D, Krishnan KR, 2011. Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. Journal of clinical psychopharmacology 31(6), 727–733. [DOI] [PubMed] [Google Scholar]
- Ratti E, Bettica P, Alexander R, Archer G, Carpenter D, Evoniuk G, Gomeni R, Lawson E, Lopez M, Millns H, 2013. Full central neurokinin-1 receptor blockade is required for efficacy in depression: evidence from orvepitant clinical studies. Journal of psychopharmacology 27(5), 424–434. [DOI] [PubMed] [Google Scholar]
- Reid A, Willshaw D, 1999. Modeling prefrontal cortex delay cells: the role of dopamine in schizophrenia. Prog Brain Res 121, 351–373. [DOI] [PubMed] [Google Scholar]
- Reiner A, Levitz J, 2018. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 98(6), 1080–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SA, Erickson RL, Browne CA, Lucki I, 2017. A role for the mu opioid receptor in the antidepressant effects of buprenorphine. Behav Brain Res 319, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ, 2002. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A 99(21), 13908–13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupniak NM, 2002. New insights into the antidepressant actions of substance P (NK1 receptor) antagonists. Can J Physiol Pharmacol 80(5), 489–494. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Kramer MS, 1999. Discovery of the antidepressant and anti-emetic efficacy of substance P receptor (NK1) antagonists. Trends in Pharmacological Sciences 20(12), 485–490. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, 2006. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 31(9), 1841. [DOI] [PubMed] [Google Scholar]
- Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, Chartoff EH, 2014. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry 76(3), 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, O’leary O, Knuuttila J, Castren E, 2007. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience 144(1), 368–374. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yamada M, 2012. Antidepressant-like effects of δ opioid receptor agonists in animal models. Current neuropharmacology 10(3), 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sałat K, Siwek A, Starowicz G, Librowski T, Nowak G, Drabik U, Gajdosz R, Popik P, 2015. Antidepressant-like effects of ketamine, norketamine and dehydronorketamine in forced swim test: role of activity at NMDA receptor. Neuropharmacology 99, 301–307. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana Rao TS, Andrade C, 2017. A possible role for ketamine in suicide prevention in emergency and mainstream psychiatry. Indian J Psychiatry 59(3), 259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Chen A, 2018. Stress at its best: the 1st Munich Winter Conference On Stress. Stress 21(5), 382–383. [DOI] [PubMed] [Google Scholar]
- Schwarz MJ, Ackenheil M, 2002. The role of substance P in depression: therapeutic implications. Dialogues Clin Neurosci 4(1), 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, Finn A, Bhat M, Samuelsson M, Lundberg K, 2015. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia—significance for activation of the kynurenine pathway. Journal of psychiatry & neuroscience: JPN 40(2), 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini G, Howland RH, Rovedi F, Girardi P, Amore M, 2014. The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol 12(5), 444–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyev V, Hökfelt T, Hurd Y, 1999. Serotonin and substance P co-exist in dorsal raphe neurons of the human brain. Neuroreport 10(18), 3967–3970. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, 2015. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA psychiatry 72(3), 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R, 1999. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284(5421), 1811–1816. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N, 1997. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 17(19), 7503–7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Mitsushio H, Takashima M, Ichikawa H, Takahashi K, 1996. Reduction of substance P after chronic antidepressants treatment in the striatum, substantia nigra and amygdala of the rat. Brain research 739(1–2), 70–78. [DOI] [PubMed] [Google Scholar]
- Shrivastava AN, Triller A, Sieghart W, 2011. GABA(A) Receptors: Post-Synaptic Co-Localization and Cross-Talk with Other Receptors. Front Cell Neurosci 5, 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G, 2002. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem 2(8), 795–816. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Layer R, Popik P, Nowak G, Paul I, Trullas R, 1996. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry 29(01), 23–26. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Desrayaud S, Cryan JF, 2005. GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J Pharmacol Exp Ther 312(1), 290–296. [DOI] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ, 2012. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology 37(2), 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Wulsin AC, Rice T, Wick D, Myers B, McKlveen J, Flak JN, Ulrich-Lai Y, Herman JP, 2014. The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Hormones and behavior 65(4), 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Huganir RL, 2002. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25(11), 578–588. [DOI] [PubMed] [Google Scholar]
- Spiga F, Harrison L, Wood S, Atkinson H, MacSweeney C, Thomson F, Craighead M, Grassie M, Lightman S, 2007. Effect of the glucocorticoid receptor antagonist Org 34850 on basal and stress-induced corticosterone secretion. Journal of neuroendocrinology 19(11), 891–900. [DOI] [PubMed] [Google Scholar]
- Spina D, 2008. PDE4 inhibitors: current status. Br J Pharmacol 155(3), 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, Hammad TA, Temple R, Rochester G, 2009. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ 339, b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T-P, Chen M-H, Li C-T, Lin W-C, Hong C-J, Gueorguieva R, Tu P-C, Bai Y-M, Cheng C-M, Krystal JH, 2017. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology 42(13), 2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wittenberg G, Chen G, Drevets W, Hsu B, Curran M, 2017. 300. Improvement in Measures of Depressed Mood and Anhedonia in Two Randomized, Placebo-Controlled Phase III Studies of Sirukumab, a Human Anti-Interleukin-6 Antibody, in Patients with Rheumatoid Arthritis. Biological Psychiatry 81(10), S123. [Google Scholar]
- Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L, 2012. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar–Kyoto rats. Neuroscience 213, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano JP, Griffey KI, Schoepp DD, 2002. The anxiolytic action of mGlu2/3 receptor agonist, LY354740, in the fear-potentiated startle model in rats is mechanistically distinct from diazepam. Pharmacology Biochemistry and Behavior 73(2), 367–374. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P, 1990. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. European journal of pharmacology 185(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Tyler MW, Yourish HB, Ionescu DF, Haggarty SJ, 2017. Classics in Chemical Neuroscience: Ketamine. ACS Chem Neurosci 8(6), 1122–1134. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG, 1988. Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus ceruleus during hemodynamic stress. Neuroendocrinology 48(6), 674–677. [DOI] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, De Wied D, 1997. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology 17(4), 284. [DOI] [PubMed] [Google Scholar]
- van Londen L, Kerkhof GA, van den Berg F, Goekoop JG, Zwinderman KH, Frankhuijzen-Sierevogel AC, Wiegant VM, de Wied D, 1998. Plasma arginine vasopressin and motor activity in major depression. Biological psychiatry 43(3), 196–204. [DOI] [PubMed] [Google Scholar]
- Wang Q, Roy B, Turecki G, Shelton RC, Dwivedi Y, 2018. Role of Complex Epigenetic Switching in Tumor Necrosis Factor-α Upregulation in the Prefrontal Cortex of Suicide Subjects. American Journal of Psychiatry, appi. ajp. 2017.16070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Mu RH, Li CF, Dong SQ, Geng D, Liu Q, Yi LT, 2017. microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Prog Neuropsychopharmacol Biol Psychiatry 79(Pt B), 417–425. [DOI] [PubMed] [Google Scholar]
- Ward RP, Dorsa DM, 1996. Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. Journal of Comparative Neurology 370(3), 405–414. [DOI] [PubMed] [Google Scholar]
- Wei ZX, Xie GJ, Mao X, Zou XP, Liao YJ, Liu QS, Wang H, Cheng Y, 2020. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139–5p-regulated neurogenesis. Neuropsychopharmacology 45(6), 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2018. WHO depression fact sheet number 369. .
- Wiley JL, Cristello AF, Balster RL, 1995. Effects of site-selective NMDA receptor antagonists in an elevated plus-maze model of anxiety in mice. European journal of pharmacology 294(1), 101–107. [DOI] [PubMed] [Google Scholar]
- Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Guzmán CAB, 2009. κ-Opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology 34(5), 1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA Jr., Sanacora G, 2018. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am J Psychiatry 175(2), 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lei H, 2014. Ketamine-an update on its clinical uses and abuses. CNS Neurosci Ther 20(12), 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K, 2018. Mechanistic Target of Rapamycin-Independent Antidepressant Effects of (R)-Ketamine in a Social Defeat Stress Model. Biol Psychiatry 83(1), 18–28. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z, 2012. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73(5), 962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD, 2018. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23(4), 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, 2016. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533(7604), 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, Charney DS, 2006. A double-blind, placebo-controlled study of memantine in the treatment of major depression. American Journal of Psychiatry 163(1), 153–155. [DOI] [PubMed] [Google Scholar]
- Zhang C, Xu Y, Zhang HT, Gurney ME, O’Donnell JM, 2017. Comparison of the Pharmacological Profiles of Selective PDE4B and PDE4D Inhibitors in the Central Nervous System. Scientific reports 7, 40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-T, Huang Y, Mishler K, Roerig SC, O’Donnell JM, 2005. Interaction between the antidepressant-like behavioral effects of beta adrenergic agonists and the cyclic AMP PDE inhibitor rolipram in rats. Psychopharmacology 182(1), 104–115. [DOI] [PubMed] [Google Scholar]
- Zhang H-T, O’Donnell JM, 2000. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology 150(3), 311–316. [DOI] [PubMed] [Google Scholar]
- Zhang HT, 2009. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des 15(14), 1688–1698. [DOI] [PubMed] [Google Scholar]
- Zhang K, Hashimoto K, 2019. An update on ketamine and its two enantiomers as rapid-acting antidepressants. Expert Rev Neurother 19(1), 83–92. [DOI] [PubMed] [Google Scholar]
- Zhang MZ, Zhou ZZ, Yuan X, Cheng YF, Bi BT, Gong MF, Chen YP, Xu JP, 2013. Chlorbipram: a novel PDE4 inhibitor with improved safety as a potential antidepressant and cognitive enhancer. Eur J Pharmacol 721(1–3), 56–63. [DOI] [PubMed] [Google Scholar]
- Zhang T, Chen Y, Liu H, Zhou Z, Zhai Y, Yang J, 2010. Chronic unpredictable stress accelerates atherosclerosis through promoting inflammation in apolipoprotein E knockout mice. Thrombosis research 126(5), 386–392. [DOI] [PubMed] [Google Scholar]
- Zhou AJ, Lee Y, Salvadore G, Hsu B, Fonseka TM, Kennedy SH, McIntyre RS, 2017. Sirukumab: a potential treatment for mood disorders? Advances in therapy 34(1), 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li X-M, Zhou Z-Q, Yang J-J, 2014. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. European Psychiatry 29(7), 419–423. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ, 2014. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29(7), 419–423. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Künzel HE, Ackl N, Sonntag A, Ising M, Holsboer F, 2000. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. Journal of psychiatric research 34(3), 171–181. [DOI] [PubMed] [Google Scholar]
- Zolezzi JM, Santos MJ, Bastias-Candia S, Pinto C, Godoy JA, Inestrosa NC, 2017. PPARs in the central nervous system: roles in neurodegeneration and neuroinflammation. Biol Rev Camb Philos Soc 92(4), 2046–2069. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF, 2010. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today 15(9–10), 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]