Abstract
Background:
The Ca2+/calmodulin-dependent phosphatase calcineurin is a key regulator of cardiac myocyte hypertrophy in disease. An unexplained paradox is how the Aβ isoform of calcineurin (CaNAβ) is required for induction of pathological myocyte hypertrophy, despite calcineurin Aα expression in the same cells. In addition, it is unclear how the pleiotropic second messenger Ca2+ drives excitation-contraction coupling, while not stimulating hypertrophy via calcineurin in the normal heart. Elucidation of the mechanisms conferring this selectively in calcineurin signaling should reveal new strategies for targeting the phosphatase in disease.
Methods:
Primary adult rat ventricular myocytes were studied for morphology and intracellular signaling. New Forster Resonance Energy Transfer (FRET) reporters were used to assay Ca2+ and calcineurin activity in living cells. Conditional gene deletion and adeno-associated virus (AAV)-mediated gene delivery in the mouse were used to study calcineurin signaling following transverse aortic constriction in vivo.
Results:
Cdc42-interacting protein (CIP4/TRIP10) was identified as a new polyproline domain-dependent scaffold for CaNAβ2 by yeast-2-hybrid screen. Cardiac myocyte-specific CIP4 gene deletion in mice attenuated pressure overload-induced pathological cardiac remodeling and heart failure. Accordingly, blockade of CaNAβ polyproline-dependent anchoring using a competing peptide inhibited concentric hypertrophy in cultured myocytes, while disruption of anchoring in vivo using an AAV gene therapy vector inhibited cardiac hypertrophy and improved systolic function after pressure overload. Live cell FRET biosensor imaging of cultured myocytes revealed that Ca2+ levels and calcineurin activity associated with the CIP4 compartment were increased by neurohormonal stimulation, but minimally by pacing. Conversely, Ca2+ levels and calcineurin activity detected by non-localized FRET sensors were induced by pacing and minimally by neurohormonal stimulation, providing functional evidence for differential intracellular compartmentation of Ca2+ and calcineurin signal transduction.
Conclusions:
These results support a structural model for Ca2+ and CaNAβ compartmentation in cells based upon an isoform-specific mechanism for calcineurin protein-protein interaction and localization. This mechanism provides an explanation for the specific role of CaNAβ in hypertrophy and its selective activation under conditions of pathologic stress. Disruption of CaNAβ polyproline-dependent anchoring constitutes a rational strategy for therapeutic targeting of CaNAβ-specific signaling responsible for pathological cardiac remodeling in cardiovascular disease deserving of further pre-clinical investigation.
Keywords: cardiac hypertrophy, calcium, calcineurin, CIP4, compartmentation
Introduction
The Ca2+/calmodulin-dependent serine/threonine phosphatase calcineurin (PP2B; PPP3) is a ubiquitous pleiotropic signaling enzyme.1 Calcineurin is a heterodimer of catalytic (CaNA) and regulatory (CaNB) subunits. In mammals, there are three genes PPP3C A-C encoding CaNA α, β and γ isoforms. In addition, CaNAβ is expressed as the alternatively-spliced isoforms CaNAβ1 and CaNAβ2, the latter usually expressed at much higher levels and sharing a C-terminal domain structure similar to CaNAα (Figure 1A).2 Studies using mice with calcineurin Aα and Aβ genetic deletion have revealed isoform-specific functions in the heart, T-cells, platelets, kidney, pancreas, and skeletal muscle,3-8 including a requirement for CaNAβ in the induction of pathological cardiac hypertrophy.3 Some of these isoform-specific functions may be attributable to differences in expression or substrate specificity.7, 9 However, it remains unclear why calcineurin isoforms have a non-redundant function in tissues such as the adult heart, in which CaNAα and CaNAβ are normally present at similar protein levels and in which relevant substrates can be dephosphorylated with similar efficiency by both isoforms.3, 9 In addition, it is unclear how in an excitable cell type such as the cardiac myocyte, activation of calcineurin, that has a relatively high affinity for Ca2+/calmodulin, is selectively associated with stress conditions independently of the elevation in cytosolic Ca2+ with each contractile cycle (from 0.1 μmol/L in diastole to ~ 1 μmol/L free Ca2+ in systole).1, 10
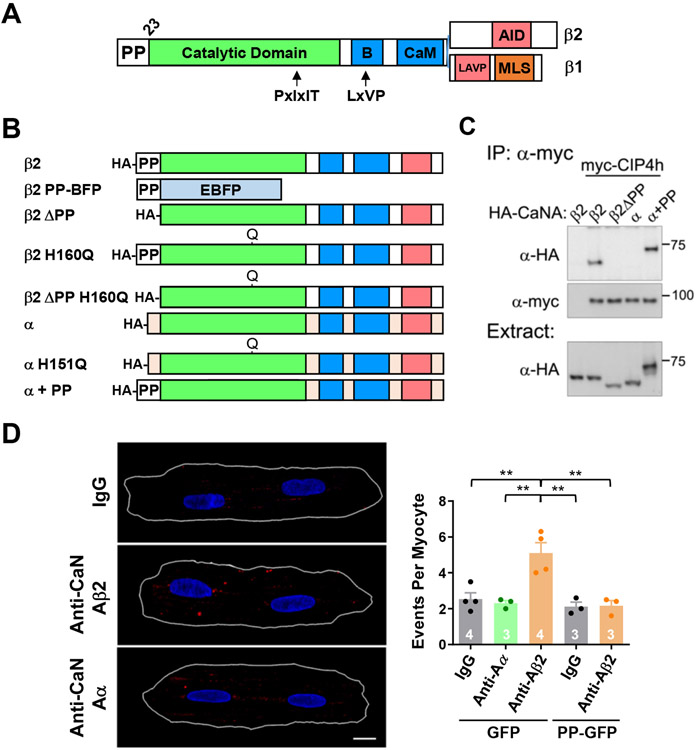
Figure 1. CIP4 binds calcineurin Aβ in adult cardiac myocytes.
A. CaNAβ structure showing alternatively-spliced C-termini. B – B-subunit binding domain; CaM – calmodulin-binding domain; LAVP inhibitory sequence; AID – autoinhibitory domain; MLS – membrane localization sequence.2 Arrows indicate binding sites for PxIxIT and LxVP linear motifs present in calcineurin substrates and previously identified calcineurin scaffolds. B. HA-tagged CaNA recombinant proteins. C. Co-immunoprecipitation with α-myc tag antibody of myc-tagged CIP4h with HA-tagged CaNA proteins expressed in COS-7 cells. n = 3. D. Detection of endogenous CaNA-CIP4 complexes (large red spots) by proximity ligation assay with mouse CIP4 and rabbit CaNAα and CaNAβ2 antibodies in adult rat ventricular myocytes infected with adenovirus for GFP or PP-GFP. Images shown for GFP-infected myocytes: PLA signal in red, Hoechst-stained nuclei in blue and the cells outlined in white. Scale bar - 10 μm. Data passed Anderson-Darling (A2*) normality test and were analyzed by 1-way ANOVA with Tukey post-hoc testing, n as indicated. ** p ≤ 0.01.
Specificity in signaling enzyme action can be conferred by differential binding to scaffold proteins that target the enzyme to discrete compartments within the cell, where, as part of a “signalosome,” the signaling enzyme can be physically associated with relevant upstream activators and target substrates (including potentially the scaffold itself).1 Calcineurin is known to associate with scaffolds that contain PxIxIT and LxVP short linear motifs.1 However, these motifs are not calcineurin isoform specific. The pro-hypertrophic activity of CaNAβ identified by catalytic domain exon deletion is apparently due to the Aβ2 isoform.3, 11 The most prominent structural difference between CaNAβ2 and other CaNA isoforms is the unique CaNAβ 23 amino acid, N-terminal proline-rich domain (Figure 1B and Figure IA in the Data Supplement). We now report that the Aβ polyproline domain constitutes a scaffold anchoring domain, including conferring isoform-specific calcineurin association with the membrane and cytoskeletal bound protein Cdc42-interacting protein 4 (CIP4; TRIP10). Like CaNAβ,3 CIP4 is required for pressure overload-induced hypertrophy and heart failure in mice. In addition, expression of a CaNAβ polyproline domain peptide to disrupt CaNAβ-specific anchoring inhibits myocyte hypertrophy and improves cardiac function during pressure overload. In conjunction with data obtained using CIP4-localized Forster Resonance Energy Transfer (FRET) reporters for Ca2+ and calcineurin activity, these results imply that CaNAβ2 is localized to distinct compartments within the cell where CaNAβ2 may be activated by select upstream stimuli. Identification of this mechanism for CaNAβ-specific protein-protein interaction and compartmentation provides a strategy for isoform-specific phosphatase inhibition useful for targeting CaNAβ signaling in disease.
Methods
Complete detailed methods are provided in the Online Data Supplement. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animal Models.
All in vivo research was performed under the supervision of the Institutional Animal Care and Use Committee at the University of Miami. All mice used in this project were C57BL/6. The conditional “floxed” CIP4 (TRIP10) mouse designed to delete CIP4 exons 2 and 3 and introduce a frameshift in exon 4 was generated by the University of Cincinnati Gene Targeted Mouse Service Core by ES cell line homologous recombination and blastocyst injection (Figure IIA in the Data Supplement). After mating to a Flp recombinase transgenic mouse, CIP4f/+ mice were mated to either B6.C-Tg(CMV-cre)1Cng/j (strain 006054) or B6.FVB(129)-A1cfTg(Myh6-cre/Esr1*)1Jmk/J (strain 005657)12 mice (The Jackson Laboratory) to provide constitutive global and tamoxifen inducible, cardiac myocyte-specific gene deletion, respectively. To induce gene knock-out, 8 week old CIP4f/f;Tg(Myh6-cre/Esr1*) “CIP4 CKO” mice were fed tamoxifen-laden chow (125 mg tamoxifen/kg chow, Harlan Teklad) for 1 week before restoration of normal chow for 1 week before use in experimentation. All control Tg(Myh6-cre/Esr1*) “MCM” and CIP4f/f mice were similarly treated with tamoxifen before study. For AAV studies, 2-3 day old wildtype mice were injected intraperitoneally with 1011 viral genomes AAV in 20 μl saline. Mice were subjected to transverse aortic constriction survival surgery as described in the Online Data Supplement.
Adult rat ventricular myocyte isolation and culture:
2-3 month old Sprague-Dawley rats were used to isolate ventricular myocytes. Myocytes were infected with adenovirus for recombinant protein or shRNA expression 1.5 hours after plating. For morphologic assays, adrenergic agonists were added on the second day in culture, with immunocytochemistry performed after 24 hours of stimulation as previously described.13 FRET imaging was performed 2 days after adenoviral transduction.
Statistics.
Statistics were computed using Graphpad Prism 7 and 8. n refers to the number of individual mice or individual myocyte preparations. All data are expressed as mean ± s.e.m. Repeated symbols are used as follows: single - p ≤ 0.05; double - p ≤ 0.01; triple - p ≤ 0.001. Anderson-Darling (A2*) test was performed to test for normality. If the data passed the normality test, two-sample t-test was used for simple pairwise comparisons, and 1-way ANOVA for the analysis of experiments with more than two groups; if normality testing failed, then the data were analyzed by non-parametric Mann-Whitney test or Kruskal-Wallis Test followed by Dunn’s Post-hoc testing, respectively. ANOVA was performed with matching for experiments involving biological replicates based upon separate myocyte preparations. 2-way ANOVA was used for experiments with 2-factor design. All datasets involving multiple comparisons for which p-values are provided were significant by ANOVA or Kruskal-Wallis testing, α = 0.05. p-values for experiments involving multiple comparisons were obtained by Tukey or Dunn’s post-hoc testing, albeit p-values for not all comparisons are indicated in the figures and tables. Log-rank (Mantel-Cox) test was used to analyze Kaplan-Meier survival curves.
Results
We hypothesized that the CaNAβ polyproline (PP) domain constitutes a protein-protein interaction motif that would confer specific CaNAβ function. To identify candidate CaNAβ-specific scaffolds, we performed a yeast 2-hybrid screen using the CaNAβ PP domain as bait and a human heart cDNA library (Figure IB in the Data Supplement). cDNAs for proteins associated with regulation of the cytoskeleton formed the majority of those isolated in this screen, including 4 clones for the membrane-associated protein CIP4 (Table I in the Data Supplement).
All 4 CIP4 clones isolated in the yeast 2-hybrid included the C-terminal CIP4 SH3 domain (Figure IC in the Data Supplement), consistent with the binding of PP sequences by SH3 domains,14 albeit further mapping of the CIP4 CaNAβ-binding domain has not been pursued. The binding of CaNAβ to CIP4 was validated by co-immunoprecipitation of HA-tagged CaNAβ wildtype and mutant proteins with myc-tagged CIP4h (Figure 1B,C), the alternatively-spliced isoform of CIP4 expressed in the cardiac myocyte (see Figure IIC in the Data Supplement discussed below). HA-tagged CaNAβ2, but not CaNAβ2 ΔPP lacking the N-terminal PP domain, nor CaNAα bound CIP4h. Notably, a CaNAα fusion protein in which the CaNAα-specific N-terminal sequence was replaced with the CaNAβ PP domain (CaNAα+PP) co-immunoprecipitated with CIP4h, demonstrating that the CaNAβ polyproline domain constitutes an independent, transferable protein-protein interaction domain. Proximity ligation assay (PLA) of endogenous proteins in adult rat ventricular myocytes (Figure 1D) using validated antibodies (Figure ID in the Data Supplement ) showed the existence of CIP4h-CaNAβ complexes in myocytes. Significant PLA signal was obtained using CIP4 antibody with CaNAβ2, but not CaNAα or control IgG antibodies, nor following expression of a CaNAβ2 small hairpin RNA (shRNA, Figure IE in the Data Supplement). Importantly, co-expression of a CaNAβ PP domain - green fluorescent protein (GFP) fusion protein (PP-GFP) blocked the binding of the scaffold and phosphatase in myocytes (Figure 1D).
To test the hypothesis that CIP4 is required for the induction of adult cardiac myocyte hypertrophy, we generated a conditional CIP4 null mouse allele (Figure II in the Data Supplement). Cardiac myocyte-specific CIP4 knock-out (CKO) mice were obtained by crossing CIP4f/f mice with the tamoxifen inducible Tg(Myh6-cre/Esr1*) line (designated hereafter MCM).12 Following tamoxifen administration, CIP4h protein expression was diminished >95% in the hearts of CIP4 CKO mice, while CIP4a expression was not significantly reduced, demonstrating that CIP4h is the predominant alternatively-spliced isoform in the adult cardiac myocyte (Figure IIC in the Data Supplement).
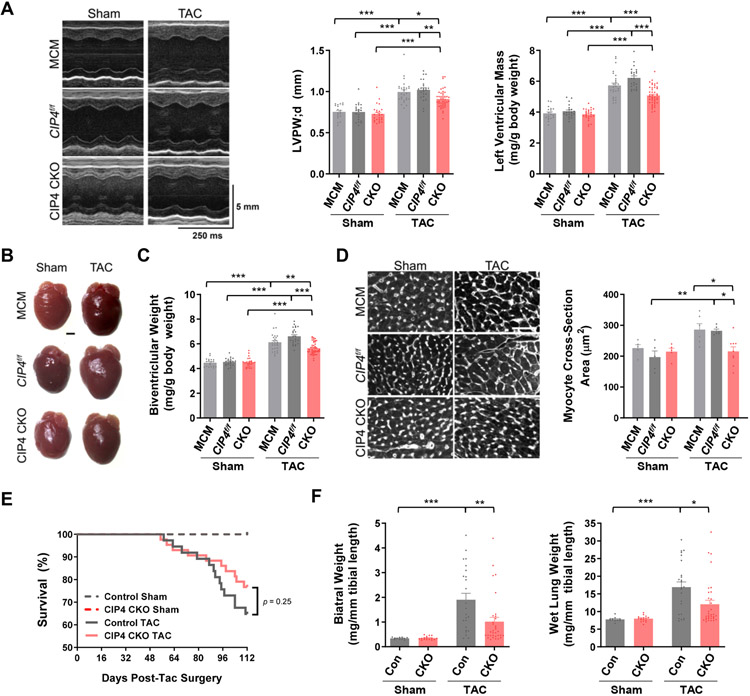
To test whether CIP4h was required for hypertrophy in vivo, CIP4 CKO and control MCM and CIP4f/f mice were subjected to two weeks of pressure overload by transverse aortic constriction (TAC) survival surgery. Echocardiography revealed that the increase in left ventricular (LV) posterior wall thickness (LVPW;d) induced by TAC was significantly decreased by CIP4 CKO (Figure 2A; Table II in the Data Supplement). The pressure overload-induced increase in indexed LV mass was similarly reduced by CIP4 CKO (Figure 2A), as confirmed by gravimetric measurement (Figure 2B,C). Histology showed that CIP4 CKO prevented the TAC-induced increase in cardiac myocyte cross-section area (Figure 2D). In addition, assay of mRNA levels for genes associated with pathological cardiac remodeling and/or activated by calcineurin-dependent nuclear factor of activated T-cells (NFATc) and myocyte enhancer factor 2 (MEF2) transcription factors showed that, for example, the increased natriuretic peptide gene expression (Nppa and Nppb) associated with cardiac stress was attenuated by CIP4 CKO (Table III in the Data Supplement). Taken together, these data showed that CIP4h cardiac myocyte-specific knock-out conferred an anti-hypertrophic phenotype similar to CaNAβ global gene deletion.3
Figure 2. CIP4h in the cardiac myocyte contributes to the development of pathological cardiac hypertrophy and heart failure.
A-D. CIP4 CKO mice were subjected to two weeks of pressure overload in mice. A. Representative M-mode echocardiography for CIP4 CKO and control CIP4f/f and MCM mice at 2 weeks after transverse aortic constriction (TAC) or sham survival surgery (Table II in the Data Supplement). Graphs show left ventricular posterior wall thickness in diastole and indexed calculated left ventricular mass. B. Representative heart images. Scale bar − 1 mm. C. Indexed gravimetric biventricular weight. D. Myocyte cross section area determined by wheat germ agglutinin staining. Scale bar − 50 μm. E,F. CIP4 CKO and control MCM mice were subjected to 16 weeks of pressure overload (Table IV in the Data Supplement). E. Kaplan-Meier survival curves for mortality of CIP4 CKO-TAC (n = 43), control mice-TAC (n = 37), CIP4 CKO-sham (n = 16), and control mice-sham (n = 11) following TAC surgery analyzed by Log-rank (Mantel-Cox) test. F. Indexed biatrial and wet lung weights 16 weeks post-TAC. Bars indicate mean ± s.e.m. for all graphs. All data except panel E were analyzed by 2-way ANOVA with Tukey post-hoc testing. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Additional parameters measured following two weeks of pressure overload suggested that CIP4 CKO would be beneficial for the prevention of heart failure. Atrial hypertrophy (indexed biatrial weight), an early indicator of diastolic dysfunction, was reduced, and Sarco/Endoplasmic Reticulum Ca2+-ATPase 2 (SERCA2, Atp2a2) and phospholamban (Pln) gene expression important for Ca2+ handling were improved by CIP4 CKO (Tables II and III in the Data Supplement). To test whether CIP4 CKO would in fact prevent heart failure, additional mice were subjected to pressure overload for 16 weeks (Figure 1E; Figure III and Table IV in the Data Supplement). In addition to atrial hypertrophy, pulmonary edema, a cardinal marker of heart failure, was significantly reduced by CIP4 CKO, as indicated by measurement of wet lung weight (Figure 1F).
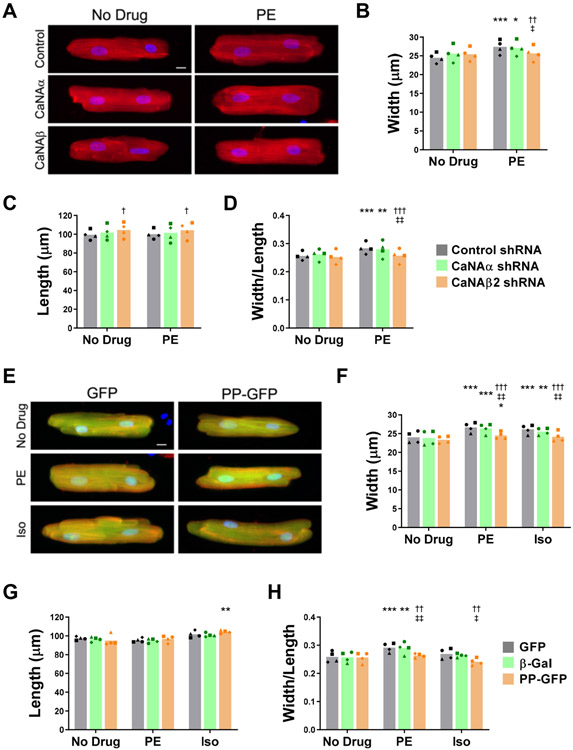
Disruption of enzyme anchoring by scaffold proteins is a strategy for inhibiting intracellular signaling in lieu of the often non-specific targeting of enzyme active sites.15 To begin testing the efficacy of a PP disruptor as an inhibitor of CaNAβ-dependent remodeling, we first validated the adult rat ventricular myocyte as an in vitro model for CaNAβ-dependent hypertrophy. Myocytes stimulated with the α-adrenergic agonist phenylephrine (PE) increased in width 10-12%, with no increase in length (Figure 3A-D), which compares favorably to the selective increase of 17-21% in width of mouse myocytes in vivo following two weeks of pressure overload.13, 16 Infection with adenovirus expressing CaNAα and CaNAβ2 shRNA (Figure ID in the Data Supplement) was used to deplete myocytes of each isoform. CaNAβ2 depletion inhibited the PE-induced myocytes growth in width (Figure 3A,B, Figure IVA in the Data Supplement). Width/length is a measure of concentricity, and CaNAβ2 depletion inhibited the PE-induced increase in that ratio completely (Figure 3D). Notably, CaNAα shRNA had no significant effect on myocyte morphology in the presence or absence of agonist. In contrast to PE, the β-adrenergic agonist isoproterenol (Iso) induces a relatively symmetric myocytes growth, resulting in no significant change in width/length ratio (Figure IVB-E in the Data Supplement, similar to the effects of chronic Iso infusion in vivo.13 CaNAβ2, but not CaNAα shRNA inhibited the Iso-induced myocyte growth in width, without affecting the Iso-induced growth in length. These results showing isoform-specific, cell autonomous CaNAβ2 function were in contrast to the reported similar inhibition of neonatal myocyte hypertrophy in vitro by CaNAα and CaNAβ shRNA,17 but support the dominant role of CaNAβ in adult cardiac myocyte hypertrophy suggested by characterization of the global CaNAβ knock-out mouse.3 To test the effect of CaNAβ PP anchoring disruption on myocyte hypertrophy, the CaNAβ PP-GFP fusion protein was (diffusely) expressed in these cells at a level sufficient to preclude CaNAβ PP-specific anchoring (Cf. Figure 1D). While not affecting baseline morphology, expression of the PP anchoring disruptor significantly inhibited >80% the myocyte growth in width induced by PE and Iso when compared to expression of control GFP and β-galactosidase (β-Gal) proteins (Figure 3E-H). Notably, myocytes expressing the PP anchoring disruptor were not significantly different in length from those expressing either control protein regardless of culture condition (Figure 3G). Accordingly, expression of the PP fusion peptide resulted in a decreased width/length ratio for both α- and β-adrenergic stimulated myocytes (Figure 3H). Taken together, these results suggest that PP-anchored CaNAβ is required for adrenergic-induced concentric adult cardiac myocyte hypertrophy.
Figure 3. A polyproline domain-anchoring disruptor inhibits adult rat ventricular myocyte hypertrophy in vitro.
A-D. Myocytes were infected with adenovirus expressing CaNAα, CaNAβ2, or control shRNA and then stimulated for 24 hours with 20 μmol/L phenylephrine (PE) before immunocytochemistry using α-actinin antibodies (red) and Hoechst nuclear stain (blue). A. Representative myocyte images. Scale bar − 10 μm. B-D. Bars and symbols indicate average mean and means of independent experiments using different myocyte preparations, respectively, for width (Cf. Figure IVA in Data Supplement), length, and width/length ratio. n = 4 independent experiments. *p-value vs. no drug control for the same shRNA. †vs. control shRNA under the same treatment condition; ‡vs. CaNAα shRNA under same the treatment condition. E-H. Myocytes were infected with adenovirus expressing GFP, β-Gal, or a CaNAβ PP-GFP fusion protein and then stimulated for 24 hours with 20 μmol/L PE or 10 μmol/L isoproterenol (Iso). E. Representative myocyte images stained as in A. Scale bar − 10 μm. F-H. Bars and symbols indicate average mean and means of independent experiments using different myocyte preparations, respectively, for width (Cf. Figure IVF in Data Supplement ), length, and width/length ratio. n = 4 independent experiments. *p-value vs. no drug control for the same protein. †vs. GFP under the same treatment condition; ‡vs. β-gal under the same treatment condition. All data were analyzed by 2-way ANOVA for matched data with Tukey post-hoc testing. Repeated symbols are used as follows: single - p ≤ 0.05; double - p ≤ 0.01; triple - p ≤ 0.001.
The potential therapeutic efficacy of CaNAβ PP anchoring disruption was studied in vivo using an adeno-associated virus serotype 9 (AAV) gene therapy vector engineered to direct PP-GFP expression under the control of the cardiac myocyte-specific troponin T promoter (AAV9.PP-GFP, Figure 3A; Figure VA in the Data Supplement).18 Anchoring disruptor delivery was validated by inhibited detection of endogenous CIP4-CaNAβ complexes by proximity ligation assay (Figure VB in the Data Supplement). Mice injected as neonates with AAV9.GFP or AAV9.PP-GFP were randomized and subjected to two-week TAC or sham survival surgery. The control AAV9.GFP mice in this experiment responded to pressure overload with both ventricular and atrial hypertrophy, as well as prominent ventricular dilatation and systolic dysfunction (but not heart failure, Table V in the Data Supplement). The TAC-induced systolic dysfunction was prevented by PP anchoring disruption, including normalization of LV volumes and ejection fraction (Figure 4B,C). In addition, AAV9.PP-GFP significantly reduced TAC-induced ventricular hypertrophy and improved remodeling-associated changes in LV gene expression, including reduced atrial natriuretic peptide and restored SERC2a and phospholamban expression (Figure 4D-F, Table VI in the Data Supplement). Taken together with the in vitro results, these findings are consistent with a model in which CaNAβ anchoring through its PP domain is required for myocyte autonomous signaling regulating pathological cardiac hypertrophy.
Figure 4. AAV-mediated expression of a CaNAβ anchoring disruptor peptide prevents cardiac dysfunction in vivo during pressure overload.
A. AAV were serotype 9 and expressed the myc and His6-tagged PP-GFP fusion peptide or GFP under the control of the cardiac myocyte-specific cardiac troponin T promoter (cTnT).18 Wildtype C57BL/6 mice, injected as neonates with AAV9.PP-GFP or AAV9.GFP, were subjected to transverse aortic constriction (TAC) from 8-10 weeks of age. B. M-mode echocardiography of TAC and sham-operated mice (Table V in Data Supplement). C. Left ventricular volume in systole; left ventricular ejection fraction; and indexed calculated left ventricular mass derived from M-mode data. PP on graphs refers to PP-GFP. D. Representative mouse heart images. Scale bar − 1 mm. E. Indexed gravimetric biventricular weight. Bars indicate mean ± s.e.m. for all graphs. F. mRNA levels for select genes detected by Nanostring Assay (Table VI in Data Supplement). All data were analyzed by 2-way ANOVA with Tukey post-hoc testing. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
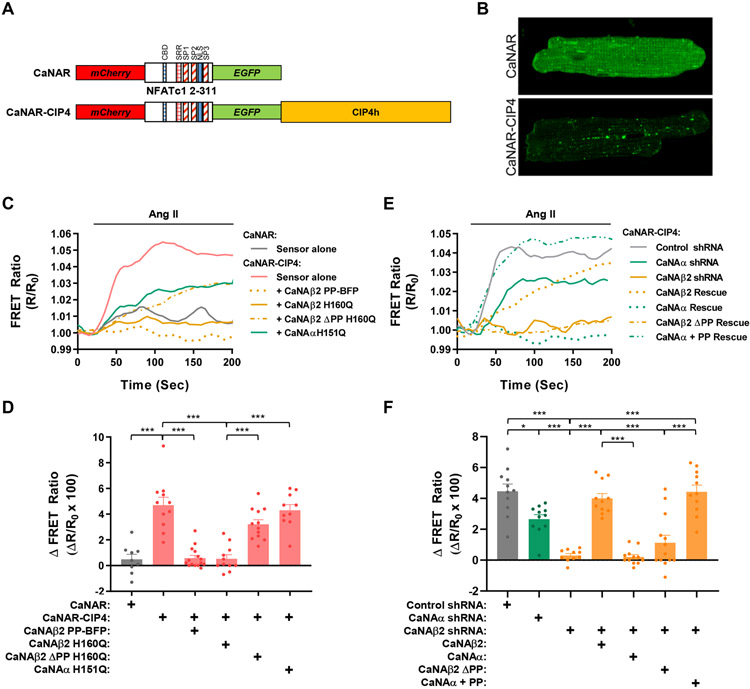
We propose that CIP4h facilitates CaNAβ-selective hypertrophic signaling by forming a discrete signaling compartment. To test this hypothesis, we generated an adenovirus that expresses a FRET-based calcineurin activity reporter - CIP4h fusion protein (CaNAR-CIP4) useful for probing calcineurin activity in a CIP4h compartment and that has a dynamic range similar to the non-localized parent CaNAR sensor (Figure 5A, Figure VI in the Data Supplement). When expressed in myocytes, the CaNAR-CIP4 sensor was present in a non-sarcomeric, vesicular and faintly streaked pattern throughout the myocyte (Figure 5B), distinct from the diffuse pattern for the parent CaNAR vector and consistent with the endosomal pattern of CIP4 staining in other cell types.19 CaNAR-CIP4 FRET signal was elevated ~4-5% in response to acute stimulation with phenylephrine and isoproterenol (Figure VII in the Data Supplement), as well as angiotensin II (Ang II), another hypertrophic inducer of calcineurin activity in these cells (Figure 5C-F). Activation of the CaNAR-CIP4 biosensor by the hypertrophic agonists was in marked contrast to the minimal effect of the agonists on the non-localized parent CaNAR sensor. Importantly, Ang II-induced CaNAR-CIP4 FRET signal was inhibited by expression of either a PP domain – blue fluorescent protein anchoring disruptor (CaNAβ2 PP-BFP, Figure 5C,D) or a catalytically inactive, dominant negative CaNAβ2 H160Q mutant protein (CaNAβ2 H160Q),20 while insignificantly affected by expression of a CaNAβ2 H160Q mutant lacking the PP targeting domain (CaNAβ2 ΔPP H160Q) or a catalytically inactive, dominant negative CaNAα H151Q mutant protein (CaNAα H151Q).21 In addition, Ang II-induced CaNAR-CIP4 FRET signal was inhibited by expression of CaNAβ2 shRNA (Figure 5E,F), confirming the specificity of the sensor. The CaNAR-CIP4 signal was partially inhibited (40%) by the CaNAα shRNA. However, the effect of the CaNAα shRNA was significantly less than that of the CaNAβ2 shRNA and was likely due to the partial (~20%) inhibition of CaNAβ2 expression by CaNAα shRNA (Figure ID in the Data Supplement), as expected by the known regulation of CaNAβ expression by calcineurin-NFAT signaling.22 The specificity of the CaNAR-CIP4 signal was further confirmed by rescue of the CaNAβ2 shRNA inhibition by expression of recombinant CaNAβ2, but not by expression of CaNAα or a CaNAβ2 mutant lacking the PP domain (CaNAβ ΔPP). Notably, rescue of CaNAβ2 shRNA with the CaNAβ PP - CaNAα fusion protein (CaNAα + PP) conferred activation of the CaNAR-CIP4 sensor. Together, these results demonstrate that CIP4h directs PP domain-anchored CaNAβ2 to a compartment at which CaNAβ2 may be selectively activated in response to hypertrophic stimuli.
Figure 5. Polyproline-dependent compartmentation of CaNAβ activity.
A. Design of EGFP-mCherry calcineurin activity FRET sensors. CBD - CaN-binding PxIxIT domain; SRR, SP1, SP2 and SP3 - serine-rich domains subject to calcineurin dephosphorylation; NLS, nuclear localization signal (apparently inactive in sensor). B. Myocytes were infected with adenovirus expressing CaNAR or CaNAR-CIP4 FRET sensors and imaged by confocal microscopy. EGFP channels shown. C,D. Myocytes expressing CaNAR-CIP4 with or without CaNA mutant proteins or CaNAR alone were stimulated with Ang II (100 nmol/L) to induce calcineurin activity. Representative tracings and amplitude change for FRET ratio (R/R0) are shown. E,F. Myocytes expressing CaNAR-CIP4, shRNA, and CaNA mutant proteins were stimulated with Ang II (100 nmol/L). Bar graphs show results from individual tracings and mean ± s.e.m. Data passed Anderson-Darling (A2*) normality test and were analyzed by 1-way ANOVA with Tukey post-hoc testing. *p ≤ 0.05; ***p ≤ 0.001.
The presence of a CaNAβ2-specific CIP4 compartment detectable using the CaNAR-CIP4 sensor suggested that there was a Ca2+ compartment at CIP4 that functioned independently of cytosolic Ca2+. To detect this putative Ca2+ compartment, the genetically-encoded, ratiometric Ca2+ FRET sensor Cameleon23 was fused to the N-terminus of CIP4 (Cam-CIP4, Figure 6A, Figure VIII in the Data Supplement). In response to Ang II infusion, the signal was demonstrably greater for the Cam-CIP4 sensor than for the parent, non-localized Cameleon sensor (Cam, Figure 6B). In contrast, pacing the myocytes at 1 Hz, that induced myocyte beating, activated the parent Cameleon sensor much greater than the CIP4-localized sensor (Figure 6C). These results imply that CIP4 is located within a Ca2+ compartment that can respond to neurohormonal hypertrophic stimuli independently of the general cytosolic compartment sensitive to Ca2+ involved in excitation-contraction coupling. Pacing also activated the diffusely localized CaNAR sensor, but not CaNAR-CIP4 (Figure 6C), showing, further, that separate, distinctly regulated pools of CaNAβ2 and other calcineurin holoenzymes exist within the cell. Expression of a PP-BFP anchoring disruptor did not increase pacing-induced cytosolic CaNAR signal, implying that PP-anchored calcineurin represents a smaller pool of calcineurin than that normally present in the cytosolic compartment and consistent with the lack of effects by PP anchoring disruption in untreated myocytes and unstressed mice.
Figure 6. CIP4-dependent Ca2+ compartmentation.
A. Ca2+ FRET biosensors. D3cpv Cameleon (Cam) contains ECFP, mutant calmodulin (mCaM) and CaM-binding peptide from smooth-muscle myosin light-chain kinase, and cp-Venus.39 B. Myocytes expressing sensors were stimulated with Ang II (100 nmol/L). Sensor responses compared by Mann-Whitney Test. C. Myocytes expressing sensors and PP-BFP were paced at 1 Hz. Representative tracings and amplitude change for FRET ratio (R/R0) are shown. Bar graphs show results from individual tracings and mean ± s.e.m. Cam and Cam-CIP4 were compared by Mann-Whitney Test. Data on graph on right were analyzed by Kruskal-Wallis Test followed by Dunn’s Post-hoc testing. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; n.s. – not significant.
Discussion
The cardiac response to chronic stress involves the activation of a signal transduction network that induces non-mitotic growth of the myocyte (hypertrophy) and that in disease promotes altered ventricular shape and myocyte metabolism, myocardial apoptosis, and interstitial fibrosis contributing to the development of heart failure. Calcineurin plays a central role in the regulation of this pathological remodeling,10, 24 such that CaNAβ gene deletion attenuated the cardiac hypertrophy induced by pressure overload and neurohormonal stimulation in vivo.3 While the CaNAβ knock-out mouse conferred global CaNAβ loss, shRNA expression in adult myocytes performed here demonstrates myocyte-autonomous CaNAβ2 function in concentric myocyte hypertrophy. It is paradoxical that CaNAβ2 would be a regulator of pathological hypertrophy in a cell type in which cytosolic Ca2+ presumably exceeds the level necessary for calcineurin activation with each heart beat.1, 10 Multiple mechanisms have been proposed for the restriction of hypertrophic calcineurin signaling to stress-related states, including altered diastolic Ca2+ levels, summation of enhanced cytosolic Ca2+ transients, and the existence of Ca2+ compartments isolated from cytosolic Ca2+ controlling excitation-contraction coupling.1, 10, 24 While electrophysiological data support the latter hypothesis,10, 24 it has not been clear that such compartments containing CaNAβ2 exist. In this study we present evidence for CaNAβ2-specific compartmentation through the binding of scaffolds by the unique CaNAβ N-terminal PP domain, including data supporting a key role of this anchoring mechanism in the induction of pathological cardiac hypertrophy.
One of the candidate CaNAβ-specific scaffolds identified in our initial protein-protein interaction screen was CIP4. CIP4 was previously shown by us to contribute via an undefined mechanism to the hypertrophic growth of neonatal ventricular myocytes in vitro.25 However, because neonatal myocytes have significant differences in Ca2+ and calcineurin signaling from adult ventricular myocytes,1 exhibit a lack of CaNAβ-selective hypertrophy,17 and express both CIP4a and CIP4h,25 it was unclear whether CIP4h would be required for adult myocyte hypertrophic signaling. The relevance of CIP4h to adult remodeling is now demonstrated by cardiac myocyte-specific conditional gene deletion, such that CIP4h ablation significantly attenuated the pathological response to pressure overload, both cardiac hypertrophy two weeks post-TAC and heart failure as assessed by wet lung weight 16 weeks post-TAC.
Using live cell FRET imaging we demonstrate that CIP4h-compartmentalized CaNAβ2 activation in cells can occur independently of other Ca2+-dependent processes and other calcineurin isoforms, including other pools of calcineurin that might be soluble within the cytosol or targeted by scaffold proteins containing PxIxIT or LxVP short linear motifs.1 Notably, Ca2+ levels and calcineurin activity in the CIP4h compartment were preferentially induced by neurohormonal stimuli associated with pathological hypertrophy, while, conversely, Ca2+ levels and calcineurin activity detected by untargeted sensors were preferentially induced by pacing that induced myocyte beating. This compartmentation of neurohormonal-stimulated Ca2+ and CaNAβ2 signaling provides a mechanism by which CaNAβ2 can be preferentially activated by pathological stimuli and, although not explicitly tested here, by which a pool of CaNAβ2 required for pathological remodeling might be insulated from stimuli for physiological hypertrophy, as is generally observed for CaNAβ.26
Our data support a role of PP-anchored CaNAβ2 in the regulation of pathological concentric hypertrophy. While functionally significant, the inhibition of pressure overload-induced cardiac hypertrophy in vivo by CIP4 CKO and AAV9.PP-GFP does not, however, appear to be as complete as that reported for CaNAβ gene deletion.3 Although it is problematic to compare results across different mouse lines in different laboratories and between global and cell type-specific targeting, we expect that there is also CIP4h- and PP-independent CaNAβ myocyte signaling important for regulation of myocyte mass, including a broader role for CaNAβ isoenzymes in modulating the overall extent of cardiac hypertrophy under diverse pathological conditions.27 Firstly, CIP4 was only one of several candidate CaNAβ PP-dependent scaffolds potentially contributing to the regulation of myocyte growth, such that CIP4 CKO would be expected to have a more limited palliative effect on remodeling than the anchoring disruptor peptide. Secondly, PP-anchoring is likely to be more relevant to CaNAβ2 than CaNAβ1, confounding comparison with the CaNAβ knock-out mouse that ablates both isoforms.3 While it is formally possible that the CaNAβ1 isoform is also bound to scaffold proteins by the PP domain, CaNAβ1 is targeted to the Golgi through its unique C-terminal Membrane Localization Sequence (MLS, Figure 1A),28 thereby conferring additional specificity in compartmentation and differing function to this isoform. CaNAβ1 expression is associated with inhibited myocyte hypertrophy and improved cardiac function after myocardial infarction.11 Our in vivo results presumably reflect inhibition of CaNAβ2 activity that is CIP4h- or PP-dependent, potentially without interfering with the palliative effects of CaNAβ1 signaling. Taken together, one would not expect CIP4 CKO, PP anchoring disruption, and CaNAβ knock-out to share the identical in vivo phenotype throughout the progression of pressure overload disease from initial concentric hypertrophy to predominantly eccentric hypertrophy and heart failure with reduced ejection fraction. What the in vitro and in vivo datasets do support is a model in which PP-anchored CaNAβ is required for the induction of concentric myocyte hypertrophy and that is in part permissive for the action of other signaling pathways, including other calcineurin-dependent processes, contributing to overall pathological cardiac remodeling.
A limitation of this study is that we were unable to define how CIP4h-CaNAβ2 complexes induce concentric hypertrophy, an issue that will require future investigation. While there are many calcineurin substrates in the myocyte,1, 29 the calcineurin substrates most commonly associated with pathological remodeling are NFATc (nuclear factor-activated T-cells) transcription factors.26 We assayed mRNA levels for the NFAT-regulated gene RCAN1.41 in both CIP4 CKO and AAV9.PP-GFP mice by both Nanostring assay and qRT-PCR (data now shown). While RCAN1.4 mRNA tended to be increased in control TAC mice, inhibition of this induction by CIP4 CKO was not significant, and PP-GFP did not oppose RCAN1.4 expression at all (Table VI in Data Supplement). Using Nanostring, we also assayed the expression of several other known NFATc and MEF2 gene targets, MEF2 also being a calcineurin substrate.30 While the MEF2 targets Nppa and Pln and the MEF2 and NFAT target Atp2a2 were normalized in expression by both CIP4 CKO and PP-GFP expression,31-33 multiple other genes regulated by MEF2 and NFAT were not significantly altered in expression in our study (Tables III and VI in Data Supplement).
Calcineurin-catalyzed dephosphorylation promotes NFATc translocation from the cytosol to the nucleus, such that NFATc immunocytochemistry is an alternative assay for activation of the transcription factor.1 Paradoxically, we found that nuclear translocation of NFATc3, an NFATc family member required for pathological cardiac hypertrophy,1 was minimally induced by phenylephrine and isoproterenol in adult rat myocytes, whether the agonists were applied acutely or as for promotion of hypertrophy (data not shown). Our results were similar to those published by the Blatter laboratory in which they found that phenylephrine minimally and angiotensin II and endothelin-1 not at all induced NFATc3 nuclear translocation in adult rabbit and cat ventricular myocytes.34 Interestingly, the Blatter, Houser, and Molkentin laboratories have reported that NFATc3 nuclear translocation was rapid and robust in adult rabbit ventricular myocytes exhibiting spontaneous Ca2+ transients and increased diastolic Ca2+I and in paced adult feline ventricular myocytes.27, 34, 35 As Ca2+ and calcineurin activity were elevated in the CIP4h compartment by hypertrophic agonists, but not pacing, and the opposite in the cytosol, we suggest that NFATc3 is unlikely to be a direct effector for CIP4h bound-CaNAβ2. Instead, we propose that NFATc and MEF2 transcription factors are regulated by calcineurin in other compartments, including that detected by the unlocalized sensors and that at perinuclear mAKAPβ signalosomes that we previously showed to bind CaNAβ, MEF2, and NFATc and to be required for pathological cardiac hypertrophy.16, 30
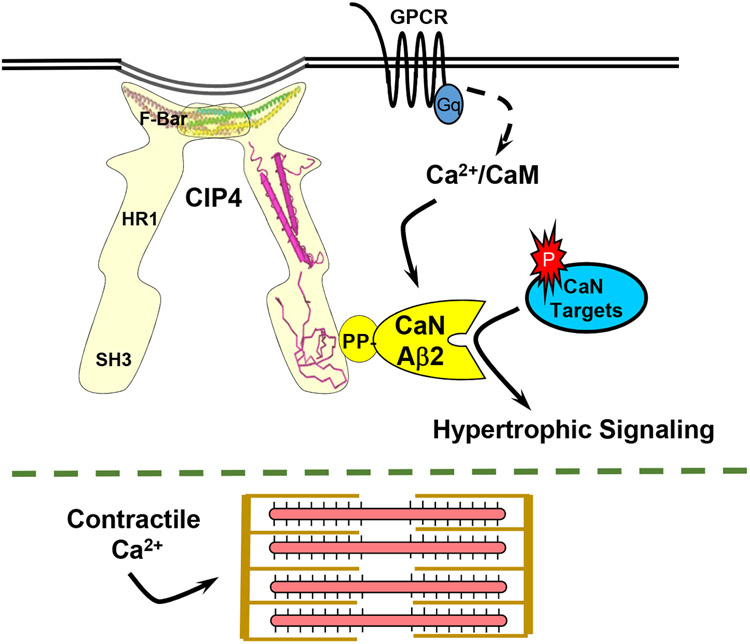
Instead of regulating directly CaNAβ-dependent transcription factors, it is likely that CIP4h brings CaNAβ near to relevant upstream plasma membrane receptors and ion channels, potentially conferring crosstalk with Rho family signaling pathways and direct regulation of the cytoskeleton, while contributing to the induction of pathological hypertrophy. Through association with endosomes, plasma membrane tubules, and the actin cytoskeleton, CIP4 is a modular scaffold protein involved in the regulation of cell surface receptor endocytosis, vesicle trafficking, membrane tubulation, and dynamic remodeling of the cytoskeleton.36 CIP4 contains a N-terminal Fes-CIP4 homology [FCH] – Bin/Amphiphysin/Rvs (F-BAR) domain that binds both cytoskeletal proteins and membrane phospholipids, mediating CIP4 oligomerization while inducing a shallow curvature to membranes (Figure 7 and Figure IB in the Data Supplement).37, 38 CIP4 also has an HR1 domain that binds active, GTP-bound Rho family members and a C-terminal Src Homology 3 (SH3) domain that binds regulators of the actin cytoskeleton and small GTPase signaling. Future research will characterize further the CIP4 signaling compartment and its downstream effectors in the cardiac myocyte, including whether ion channels previously implicated in hypertrophic signaling are associated with CIP4h-CaNAβ signalosomes.10, 24
Figure 7. Model for CIP4-dependent compartmentation of Ca2+ and calcineurin signaling that allows separate regulation of hypertrophic signaling and excitation-contraction coupling.
G-protein coupled receptors (GPCR) increase intracellular Ca2+, activating the Ca2+/calmodulin-dependent phosphatase CaNAβ. CIP4 oligomers associate via the CIP4 F-BAR domain with membranes or the cytoskeleton. Through the SH3 domain, CIP4 can serve as a scaffold for CaNAβ, resulting in the activation of that calcineurin isoform at selective intracellular sites. PDB IDs for CIP4 structures are 2EFK, 2KE4, and 2CT4.
Inhibition of calcineurin protein-protein interactions have been achieved using peptides mimicking the PxIxIT and LxVP motifs, but like the immunophilin calcineurin inhibitors cyclosporin A and FK506, these peptides bind sites conserved among CaNA isoforms, complicating potential therapeutic intervention.1, 15 Our results show that a peptide comprising the CaNAβ PP domain can constitute an anchoring disruptor with selectivity for the subset of CaNAβ signaling complexes defined by interaction with the PP domain. Importantly, disruption of PP-anchoring inhibited pathological cardiac remodeling in vivo, while not affecting baseline cardiac structure or function. These data imply that, in the absence of gross overexpression of a constitutively active calcineurin mutant,1 proper localization of CaNAβ2 through PP-bound scaffolds is critical for signaling promoting pathological hypertrophy (Figure 7). Whether PP-based anchoring disruptors might be effective in treating human cardiovascular disease will require further in vivo investigation, especially given known protective CaNAβ signaling in ischemic heart disease.26 In the meantime, these results establish this new anchoring mechanism as a potential target for intervention in the diverse set of diseases in the aforementioned organ systems in which CaNAβ activation has been implicated.3-8
Supplementary Material
Clinical Perspective.
What is New:
A new mechanism for calcineurin Aβ catalytic subunit compartmentation by polyproline-dependent binding to scaffold proteins is described.
CIP4 is identified as a scaffold protein for calcineurin Aβ2, such that CIP4 gene deletion and inhibition of calcineurin Aβ polyproline-dependent anchoring in mice attenuates pathological cardiac remodeling.
Cellular data are provided for a CIP4-calcineurin compartment that is preferentially responsive to hypertrophic neurohormonal stimuli.
What Are the Clinical Implications?
Calcineurin has long been implicated in the induction of pathological cardiac remodeling, but has been therapeutically intractable due to calcineurin pleiotropy.
The identification of a calcineurin Aβ-specific anchoring mechanism provides a potential strategy for the specific targeting of calcineurin Aβ in disease, including for the prevention of cardiac remodeling and heart failure.
Acknowledgements
The authors thank Zoharit Cozacov and Dominica Passariello for their technical assistance in this project. M.S.K. performed the initial yeast-2-hybrid screen, provided overall supervision for this project, and wrote the manuscript with the assistance of the co-authors. X.L. performed in vivo and ex vivo experimentation with the assistance of E.C.M., C.L.P., K.H., Q.Y, Y.L., and J.L. J.L. contributed to the conceptualization of the project and performed in vitro experimentation with the assistance of H.T and F.R. Live cell imaging experiments were performed by V.O.N. and A.F.
Sources of Funding
This work was supported in part by NIH Grants R01 HL126825, HL126950, and HL146111 (M.S.K.), NIH Grant T32 HL094274 (Q.Y.), the NHLBI Gene Therapy Resource Program, the Florida Biomedical Research Program (J.L.), the American Heart Association (X.L.), and the Gertraud und Heinz Rose-Stiftung (V.O.N.).
Non-standard Abbreviations and Acronyms:
- AAV
adeno-associated virus
- Ang II
angiotensin II
- CaN
calcineurin
- CaNAR
calcineurin activity reporter
- CIP4
Cdc42-interacting protein 4
- F-BAR
Fes-CIP4 homology – Bin/Amphiphysin/Rvs domain
- FRET
Forster Resonance Energy Transfer
- Iso
isoproterenol
- MEF2
myocyte enhancer factor 2
- MCM
MerCreMer
- NFAT
nuclear factor of activated T-cells
- PE
phenylephrine
- PLA
Proximity ligation assay
- PP
CaNAβ polyproline domain
- SH3
Src Homology 3
- TAC
transverse aortic constriction
Footnotes
Disclosures
The authors declare no competing financial interests.
References
- 1.Parra V, Rothermel BA. Calcineurin signaling in the heart: The importance of time and place. J Mol Cell Cardiol. 2017;103:121–136. doi: 10.1016/j.yjmcc.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond R, Ly N, Cyert MS. The unique C terminus of the calcineurin isoform CNAβ1 confers non-canonical regulation of enzyme activity by Ca2+ and calmodulin. J Biol Chem. 2017;292:16709–16721. doi: 10.1074/jbc.M117.795146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in Calcineurin Aβ -deficient mice. Proc Natl Acad Sci U S A. 2002;99:4586–4591. doi: 10.1073/pnas.072647999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manicassamy S, Gupta S, Huang Z, Molkentin JD, Shang W, Sun Z. Requirement of calcineurin Aβ for the survival of naive T cells. J Immunol. 2008;180:106–112. doi: 10.4049/jimmunol.180.1.106 [DOI] [PubMed] [Google Scholar]
- 5.Khatlani T, Pradhan S, Da Q, Gushiken FC, Bergeron AL, Langlois KW, Molkentin JD, Rumbaut RE, Vijayan KV. The β isoform of the catalytic subunit of protein phosphatase 2B restrains platelet function by suppressing outside-in αII bβ3 integrin signaling. J Thromb Haemost. 2014;12:2089–2101. doi: 10.1111/jth.12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy RN, Knotts TL, Roberts BR, Molkentin JD, Price SR, Gooch JL. Calcineurin Aβ is required for hypertrophy but not matrix expansion in the diabetic kidney. J Cell Mol Med. 2011;15:414–422. doi: 10.1111/j.1582-4934.2009.00910.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muili KA, Ahmad M, Orabi AI, Mahmood SM, Shah AU, Molkentin JD, Husain SZ. Pharmacological and genetic inhibition of calcineurin protects against carbachol-induced pathological zymogen activation and acinar cell injury. Am J Physiol Gastrointest Liver Physiol. 2012;302:G898–905. doi: 10.1152/ajpgi.00545.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons SA, Wilkins BJ, Bueno OF, Molkentin JD. Altered skeletal muscle phenotypes in calcineurin Aα and Aβ gene-targeted mice. Mol Cell Biol. 2003;23:4331–4343. doi: 10.1128/mcb.23.12.4331-4343.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilka S, Erdmann F, Migdoll A, Fischer G, Weiwad M. The proline-rich N-terminal sequence of calcineurin Aβ determines substrate binding. Biochemistry. 2009;48:1900–1910. doi: 10.1021/bi8019355 [DOI] [PubMed] [Google Scholar]
- 10.Dewenter M, von der Lieth A, Katus HA, Backs J. Calcium Signaling and Transcriptional Regulation in Cardiomyocytes. Circ Res. 2017;121:1000–1020. doi: 10.1161/CIRCRESAHA.117.310355 [DOI] [PubMed] [Google Scholar]
- 11.Felkin LE, Narita T, Germack R, Shintani Y, Takahashi K, Sarathchandra P, Lopez-Olaneta MM, Gomez-Salinero JM, Suzuki K, Barton PJ, Rosenthal N, Lara-Pezzi E. Calcineurin splicing variant calcineurin Aβ1 improves cardiac function after myocardial infarction without inducing hypertrophy. Circulation. 2011;123:2838–2847. doi: 10.1161/CIRCULATIONAHA.110.012211 [DOI] [PubMed] [Google Scholar]
- 12.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687 [DOI] [PubMed] [Google Scholar]
- 13.Li J, Kritzer MD, Michel JJ, Le A, Thakur H, Gayanilo M, Passariello CL, Negro A, Danial JB, Oskouei B, Sanders M, Hare JM, Hanauer A, Dodge-Kafka K, Kapiloff MS. Anchored p90 ribosomal S6 kinase 3 is required for cardiac myocyte hypertrophy. Circ Res. 2013;112:128–139. doi: 10.1161/CIRCRESAHA.112.276162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurochkina N, Guha U. SH3 domains: modules of protein-protein interactions. Biophys Rev. 2013;5:29–39. doi: 10.1007/s12551-012-0081-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nygren PJ, Scott JD. Therapeutic strategies for anchored kinases and phosphatases: exploiting short linear motifs and intrinsic disorder. Frontiers in pharmacology. 2015;6:158. doi: 10.3389/fphar.2015.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kritzer MD, Li J, Passariello CL, Gayanilo M, Thakur H, Dayan J, Dodge-Kafka K, Kapiloff MS. The scaffold protein muscle A-kinase anchoring protein β orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circulation Heart failure. 2014;7:663–672. doi: 10.1161/CIRCHEARTFAILURE.114.001266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad AM, Inesi G. Regulation and rate limiting mechanisms of Ca2+ ATPase (SERCA2) expression in cardiac myocytes. Mol Cell Biochem. 2012;361:85–96. doi: 10.1007/s11010-011-1092-y [DOI] [PubMed] [Google Scholar]
- 18.Prasad KM, Xu Y, Yang Z, Acton ST, French BA. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene Ther. 2011;18:43–52. doi: 10.1038/gt.2010.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Troglio F, Mukhopadhyay A, Everingham S, Kwok E, Scita G, Craig AW. F-BAR-containing adaptor CIP4 localizes to early endosomes and regulates Epidermal Growth Factor Receptor trafficking and downregulation. Cell Signal. 2009;21:1686–1697. doi: 10.1016/j.cellsig.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 20.Zhu W, Zou Y, Shiojima I, Kudoh S, Aikawa R, Hayashi D, Mizukami M, Toko H, Shibasaki F, Yazaki Y, Nagai R, Komuro I. Ca2+/calmodulin-dependent kinase II and calcineurin play critical roles in endothelin-1-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:15239–15245. doi: 10.1074/jbc.275.20.15239 [DOI] [PubMed] [Google Scholar]
- 21.Mertz P, Yu L, Sikkink R, Rusnak F. Kinetic and spectroscopic analyses of mutants of a conserved histidine in the metallophosphatases calcineurin and lambda protein phosphatase. J Biol Chem. 1997;272:21296–21302. doi: 10.1074/jbc.272.34.21296 [DOI] [PubMed] [Google Scholar]
- 22.Oka T, Dai YS, Molkentin JD. Regulation of calcineurin through transcriptional induction of the calcineurin A β promoter in vitro and in vivo. Mol Cell Biol. 2005;25:6649–6659. doi: 10.1128/MCB.25.15.6649-6659.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc. 2006;1:1057–1065. doi: 10.1038/nprot.2006.172 [DOI] [PubMed] [Google Scholar]
- 24.Heineke J, Ritter O. Cardiomyocyte calcineurin signaling in subcellular domains: from the sarcolemma to the nucleus and beyond. J Mol Cell Cardiol. 2012;52:62–73. doi: 10.1016/j.yjmcc.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 25.Rusconi F, Thakur H, Li J, Kapiloff MS. CIP4 is required for the hypertrophic growth of neonatal cardiac myocytes. J Biomed Sci. 2013;20:56. doi: 10.1186/1423-0127-20-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molkentin JD. Parsing good versus bad signaling pathways in the heart: role of calcineurin-nuclear factor of activated T-cells. Circ Res. 2013;113:16–19. doi: 10.1161/CIRCRESAHA.113.301667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis J, Davis LC, Correll RN, Makarewich CA, Schwanekamp JA, Moussavi-Harami F, Wang D, York AJ, Wu H, Houser SR, Seidman CE, Seidman JG, Regnier M, Metzger JM, Wu JC, Molkentin JD. A Tension-Based Model Distinguishes Hypertrophic versus Dilated Cardiomyopathy. Cell. 2016;165:1147–1159. doi: 10.1016/j.cell.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Salinero JM, Lopez-Olaneta MM, Ortiz-Sanchez P, Larrasa-Alonso J, Gatto A, Felkin LE, Barton PJR, Navarro-Lerida I, Angel Del Pozo M, Garcia-Pavia P, Sundararaman B, Giovinazo G, Yeo GW, Lara-Pezzi E. The Calcineurin Variant CnAβ1 Controls Mouse Embryonic Stem Cell Differentiation by Directing mTORC2 Membrane Localization and Activation. Cell Chem Biol. 2016;23:1372–1382. doi: 10.1016/j.chembiol.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Tandan S, Hill JA. Calcineurin-dependent ion channel regulation in heart. Trends Cardiovasc Med. 2014;24:14–22. doi: 10.1016/j.tcm.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Aponte Paris S, Thakur H, Kapiloff MS, Dodge-Kafka KL. Muscle A-kinase-anchoring protein-β-bound calcineurin toggles active and repressive transcriptional complexes of myocyte enhancer factor 2D. J Biol Chem. 2019;294:2543–2554. doi: 10.1074/jbc.RA118.005465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morin S, Charron F, Robitaille L, Nemer M. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J. 2000;19:2046–2055. doi: 10.1093/emboj/19.9.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlasblom R, Muller A, Musters RJ, Zuidwijk MJ, Van Hardeveld C, Paulus WJ, Simonides WS. Contractile arrest reveals calcium-dependent stimulation of SERCA2a mRNA expression in cultured ventricular cardiomyocytes. Cardiovasc Res. 2004;63:537–544. doi: 10.1016/j.cardiores.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 33.Baskin KK, Makarewich CA, DeLeon SM, Ye W, Chen B, Beetz N, Schrewe H, Bassel-Duby R, Olson EN. MED12 regulates a transcriptional network of calcium-handling genes in the heart. JCI Insight. 2017;2(14):e91920. doi: 10.1172/jci.insight.91920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinne A, Kapur N, Molkentin JD, Pogwizd SM, Bers DM, Banach K, Blatter LA. Isoform- and tissue-specific regulation of the Ca2+-sensitive transcription factor NFAT in cardiac myocytes and heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H2001–2009. doi: 10.1152/ajpheart.01072.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ Res. 2009;105:316–325. doi: 10.1161/CIRCRESAHA.109.194035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Xiong X, Zhao X, Yang X, Wang H. F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases. J Hematol Oncol. 2015;8:47. doi: 10.1186/s13045-015-0144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salzer U, Kostan J, Djinovic-Carugo K. Deciphering the BAR code of membrane modulators. Cell Mol Life Sci. 2017;74:2413–2438. doi: 10.1007/s00018-017-2478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daumke O, Roux A, Haucke V. BAR domain scaffolds in dynamin-mediated membrane fission. Cell. 2014;156:882–892. doi: 10.1016/j.cell.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 39.Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 40.Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci. 2005;118:5637–5646. doi: 10.1242/jcs.02675 [DOI] [PubMed] [Google Scholar]
- 41.Hollenberg SM, Sternglanz R, Cheng PF, Weintraub H. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol Cell Biol. 1995;15:3813–3822. doi: 10.1128/mcb.15.7.3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Negro A, Lopez J, Bauman AL, Henson E, Dodge-Kafka K, Kapiloff MS. The mAKAPβ scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J Mol Cell Cardiol. 2010;48:387–394. doi: 10.1016/j.yjmcc.2009.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ai HW, Shaner NC, Cheng Z, Tsien RY, Campbell RE. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry. 2007;46:5904–5910. doi: 10.1021/bi700199g [DOI] [PubMed] [Google Scholar]
- 44.Prasad AM, Inesi G. Silencing calcineurin A subunit reduces SERCA2 expression in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;300:H173–180. doi: 10.1152/ajpheart.00841.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman RH, Zhang J. Visualization of phosphatase activity in living cells with a FRET-based calcineurin activity sensor. Mol Biosyst. 2008;4:496–501. doi: 10.1039/b720034j [DOI] [PubMed] [Google Scholar]
- 46.Lunde IG, Kvaloy H, Austbo B, Christensen G, Carlson CR. Angiotensin II and norepinephrine activate specific calcineurin-dependent NFAT transcription factor isoforms in cardiomyocytes. J Appl Physiol (1985). 2011;111:1278–1289. doi: 10.1152/japplphysiol.01383.2010 [DOI] [PubMed] [Google Scholar]
- 47.Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N. Advanced methods of microscope control using muManager software. J Biol Methods. 2014;1doi: 10.14440/jbm.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprenger JU, Perera RK, Gotz KR, Nikolaev VO. FRET microscopy for real-time monitoring of signaling events in live cells using unimolecular biosensors. J Vis Exp. 2012:e4081. doi: 10.3791/4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bagchi S, Fredriksson R, Wallen-Mackenzie A. In Situ Proximity Ligation Assay (PLA). Methods Mol Biol. 2015;1318:149–159. doi: 10.1007/978-1-4939-2742-5_15 [DOI] [PubMed] [Google Scholar]
- 50.Feng Y, Hartig SM, Bechill JE, Blanchard EG, Caudell E, Corey SJ. The Cdc42-interacting protein-4 (CIP4) gene knock-out mouse reveals delayed and decreased endocytosis. J Biol Chem. 2010;285:4348–4354. doi: 10.1074/jbc.M109.041038 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.