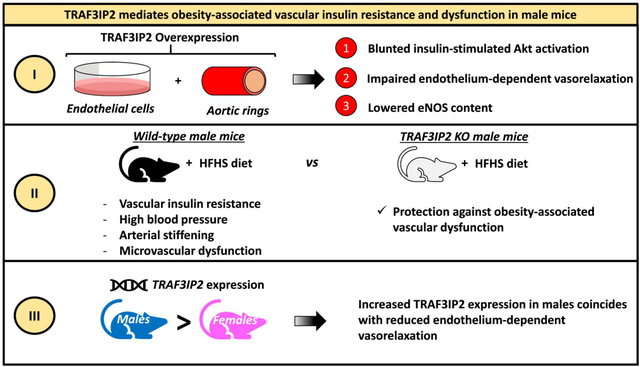
Abstract
Insulin resistance in the vasculature is a characteristic feature of obesity and contributes to the pathogenesis of vascular dysfunction and disease. However, the molecular mechanisms underlying obesity-associated vascular insulin resistance and dysfunction remain poorly understood. We hypothesized that TRAF3 Interacting Protein 2 (TRAF3IP2), a pro-inflammatory adaptor molecule known to activate pathological stress pathways and implicated in cardiovascular diseases, plays a causal role in obesity-associated vascular insulin resistance and dysfunction. We tested this hypothesis by employing genetic-manipulation in endothelial cells in vitro, in isolated arteries ex vivo, and diet-induced obesity in a mouse model of TRAF3IP2 ablation in vivo. We show that ectopic expression of TRAF3IP2 blunts insulin signaling in endothelial cells and diminishes endothelium-dependent vasorelaxation in isolated aortic rings. Further, 16 weeks of high fat/high sucrose (HFHS) feeding impaired glucose tolerance, aortic insulin-induced vasorelaxation, and hindlimb post-occlusive reactive hyperemia, while increasing blood pressure and arterial stiffness in wild-type male mice. Notably, TRAF3IP2 ablation protected mice from such HFHS feeding-induced metabolic and vascular defects. Interestingly, wild-type female mice expressed markedly reduced levels of TRAF3IP2 mRNA independent of diet and were protected against HFHS diet-induced vascular dysfunction. These data indicate that TRAF3IP2 plays a causal role in vascular insulin resistance and dysfunction. Specifically, the present findings highlight a sexual dimorphic role of TRAF3IP2 in vascular control and identify it as a promising therapeutic target in vasculometabolic derangements associated with obesity, particularly in males.
Keywords: blood pressure, arterial stiffness, Akt, endothelial function, sex differences
Graphical Abstract
INTRODUCTION
Obesity is a leading independent risk factor for cardiovascular diseases (CVD) and mortality.1–3 It is also strongly associated with insulin resistance and endothelial dysfunction.4, 5 Insulin, in addition to its role in cellular glucose uptake, increases nitric oxide production from endothelial cells through the phosphatidylinositol 3 kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) pathway.6 Notably, mounting evidence supports the notion that vascular insulin resistance manifests early during obesity7–11 and contributes causally to the development and progression of vascular dysfunction and disease.12–17 In this regard, data in humans indicate that impaired insulin signaling in endothelial cells is associated with reduced flow-mediated dilation18, 19 and increased arterial stiffness.20 Similarly, loss of insulin signaling in endothelial cells impairs endothelium-dependent relaxation and promotes atherosclerosis in ApoE-null mice.21 Conversely, restoring endothelial insulin signaling alone prevents impaired endothelium-dependent vasorelaxation in a genetic mouse model of insulin resistance;22 while selective activation of the insulin receptor-PI3K-Akt signaling pathway protects against atherosclerosis development.23 Despite the indisputable recognition that vascular insulin resistance contributes to the pathogenesis of CVD, the molecular mechanisms underlying vascular insulin resistance in obesity remain largely unknown. A better understanding of such molecular events will help us identify newer therapeutic targets for the treatment of obesity- and insulin resistance-associated vascular diseases.
TRAF3 Interacting Protein 2 (TRAF3IP2) is a pro-inflammatory cytoplasmic adaptor protein that has been identified as a candidate molecule implicated in endothelial dysfunction,24 atherosclerosis,25 and pathological stress pathways in vascular cells.26–28 Therefore, we hypothesized that TRAF3IP2 is a key molecular determinant in the development of vascular insulin resistance and dysfunction in obesity. Specifically, using loss-of-function and gain-of-function complementary in vitro and in vivo experimental approaches, we tested the hypotheses that i) adenoviral overexpression of TRAF3IP2 in cultured endothelial cells and isolated arteries leads to impaired insulin signaling and depressed endothelium-dependent vasorelaxation, respectively; and ii) TRAF3IP2 deletion protects mice against obesity-associated vascular insulin resistance and dysfunction.
METHODS
[Extended methods can be found in the online data supplement; please see http://hyper.ahajournals.org]. The following references29–44 are found in the online data supplement only.
Ethics and approvals
All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia. The University of Missouri is accredited by AAALAC International. The data to support the findings of this study can be made available by the corresponding authors on reasonable request.
Experimental protocols
Experimental protocol 1: Adenoviral overexpression of TRAF3IP2 in cultured human endothelial cells and isolated aortic rings from wild-type mice
Human skeletal muscle microvascular endothelial cells (#H-6220; Cell Biologics; Chicago, IL), human aortic endothelial cells (#CC-2535; Lonza; Allendale, NJ), and isolated aortic rings from 3–4 month-old wild-type (WT) male mice (n=11, C57BL/6NTac background; #B6-M, TACONIC, Rensselaer, NY) were used in this protocol. Endothelial cells and isolated aortic rings were cultured in VascuLife® EnGS culture medium under standard cell culture conditions (i.e., 37°C and 5% CO2) as previously described.31, 45 Endothelial cells and aortic rings were transfected with custom generated (Vector Biolabs; Malvern, PA) adenovirus expressing full length human TRAF3IP2 (Ad-GFP-h-TRAF3IP2-MycDDK, 5.7×108 PFU) or mouse TRAF3IP2 (Ad-m-TRAF3IP2, 1.6×108 PFU). Adenovirus expressing green fluorescent protein (GFP, Ad-CMV-GFP; 1.1 ×108 PFU) served as a control. Cells at 80–90% confluency were transduced with respective adenoviruses in VascuLife® medium containing 0.5% FBS for ~18 hours. Following adenoviral incubation, the medium was replaced with fresh medium lacking adenovirus and cultured for an additional 30 hours. Endothelial cells were then treated with or without insulin (100nM for 30 minutes), washed with cold sterile PBS, and lysed in RIPA buffer (R0278, Millipore Sigma, St. Louis, MO) containing appropriate protease and phosphatase inhibitors. Cell homogenates were then flash frozen for subsequent Western blotting. Skeletal muscle microvascular endothelial cells were selected as our model of choice for insulin signaling experiments due to their high responsiveness to insulin. Aortic rings were incubated in 400μL basal VascuLife® media (1% FBS, no additional growth factors added) with respective adenovirus for ~18 hours. The culture medium was then replaced with 500μL basal VascuLife® medium (1% FBS) without adenovirus for the remaining 30-hour incubation. The aortic rings were then separated from the media and used immediately to measure vasomotor function or flash frozen.
Experimental protocol 2: TRAF3IP2 knockout (KO) and WT mice fed a high fat/high sucrose (HFHS) diet
Both male and female homozygous TRAF3IP2-KO (KO) mice were bred in-house as previously described.46 Homozygous TRAF3IP2-KO mice were originally generated by Dr. Siebenlist at TACONIC (Rensselaer, NY) on a C57BL/6NTac background. Age and sex matched WT (C57BL/6NTac; #B6-M) mice were purchased from TACONIC. Thus, it should be acknowledged that WT mice were not littermates generated by crossing heterozygous KO mice. At five weeks of age, mice were randomized to be fed a chow diet (Picolab 5053) or a HFHS diet (#F1850, Bio-Serv, Flemington, NJ) for the subsequent 16 weeks. This created four experimental groups per sex (n=8–12 mice/group). Samples of the chow and HFHS diets were analyzed for caloric density and macronutrient composition by an independent laboratory (Association of Animal Feed Control Officials Guaranteed Analysis [AAFCO]; EMSL Analytical, Inc., Cinnaminson, NJ); chow diet: 3.00 kcal/g, 59.9% carbohydrate and 14.8% fat kcals/g; HFHS diet: 5.06 kcal/g, 25.0% carbohydrate and 60.1% fat kcals/g. Body weights were measured weekly. Food intake was determined by weighing food in and out over a three-day period at four weeks of the study intervention. Glucose tolerance testing was conducted 14 weeks after diet assignation. Blood pressure and hindlimb post-occlusive reactive hyperemia (superficial femoral artery) were assessed two weeks prior to euthanasia. At sacrifice (21 weeks of age), the aorta was excised and used for ex vivo assessment of vasomotor function via wire myography, aortic stiffness via atomic force microscopy (AFM), or protein expression via Western blotting. The excised femoral artery was used for mechanical testing. Terminal blood collection was performed by cardiac puncture.
Statistical analyses
GraphPad Prism (version 8.0, GraphPad Prism Software, La Jolla, CA) was used for statistical analysis. Statistical comparisons were performed by t-test, univariate or multivariate analysis of variance (ANOVA), as appropriate, followed by the Fisher-LSD test for pre-planned pairwise comparisons (e.g., effect of diet within genotype and effect of genotype within diet). Values are expressed as mean ± standard error of mean (SEM). A P-value less than 0.05 was considered significant.
RESULTS
TRAF3IP2 overexpression impairs insulin signaling in endothelial cells and blunts endothelium-dependent relaxation in isolated arteries
As displayed in Figure 1A, insulin treatment induced Akt activation (i.e., Akt phosphorylation at Ser473/total Akt) in both control and TRAF3IP2-overexpressing cells; however, the level of Akt activation was significantly less in TRAF3IP2-overexpressing cells (P<0.05). Total Akt expression was not different between the treatments. Further, as shown in Figure 1B–C, adenoviral overexpression of TRAF3IP2 in isolated aortic rings blunted ACh-induced relaxation relative to control GFP expressing rings (P<0.05). In comparison, SNP-induced relaxation was not different between the treatment conditions (P>0.05). The suppression in ACh-induced aortic vasorelaxation caused by TRAF3IP2 overexpression was in alignment with the downregulation of eNOS in aortic endothelial cells (P<0.05), as displayed in Figure 1D.
Figure 1. TRAF3IP2 overexpression impairs insulin signaling in endothelial cells and blunts endothelium-dependent relaxation in isolated arteries.
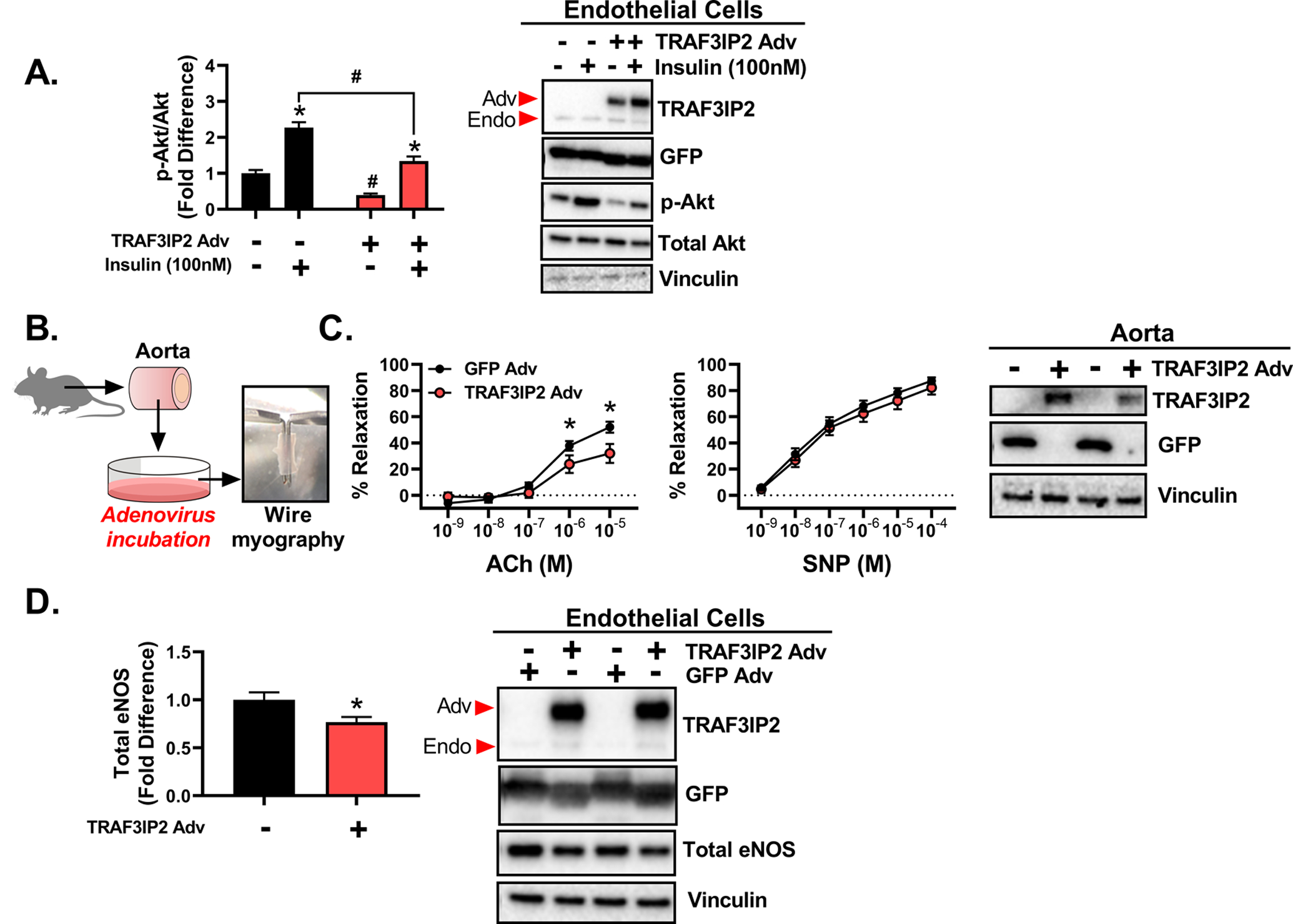
Human skeletal muscle microvascular endothelial cells and naïve isolated aortas from wild-type male mice were cultured and transduced with adenovirus (Adv) to overexpress either green fluorescent protein (GFP) or TRAF3IP2, and then treated with or without 100nM insulin for 30 minutes. A) Ratio of phosphorylated Akt (Ser473, active site) to total Akt levels in cultured endothelial cells under basal conditions and following insulin stimulation; n=5–6/treatment. B) Diagram of isolated aorta treatment and utilization. C) Endothelium-dependent (acetylcholine, ACh) and -independent (sodium nitroprusside, SNP) vasorelaxation responses of isolated aortic rings following adenoviral transduction; n=11 aortic rings/group. D) Basal levels of total eNOS content in cultured human aortic endothelial cells following overexpression of GFP or TRAF3IP2; n= 5–6/treatment. Representative Western blot images of successful TRAF3IP2 and GFP overexpression are displayed; Adv arrow indicates adenoviral TRAF3IP2 overexpression band, Endo arrow indicates endogenous TRAF3IP2 band. All values are expressed as mean ± SEM. *P<0.05 vs insulin or adenoviral treatment, #P<0.05 vs GFP Adv.
TRAF3IP2 deletion protects against obesity-associated glucose intolerance in male mice
A representative PCR image of successful TRAF3IP2 deletion in KO mice is depicted in Figure 2B. Lack of TRAF3IP2 mRNA expression was also confirmed in the heart (Figure 2B). Notably, WT male mice expressed ~35-fold more TRAF3IP2 mRNA than WT females (P<0.05), independent of diet. Sixteen weeks of HFHS feeding led to significant increases in body weight in both male and female mice relative to chow-fed counterparts (P<0.05, Figures 2C, 2E). Male and female KO mice exhibited greater weight gain from baseline (% change) relative to WT mice, independent of diet (main effect of genotype, P<0.05). Final body weights are shown in Table S1. HFHS-fed male and female WT mice consumed 23.4% and 19.9% more calories per day than chow-fed counterparts (main effect of diet, P<0.05). HFHS-fed male and female KO mice consumed 21.3% and 18.8% more calories than chow-fed KO mice (main effect of diet, P<0.05). Energy intake was not significantly affected by genotype in either males or females, irrespective of the diet (P>0.05). However, HFHS feeding increased fasting insulin concentrations (P<0.05, Figures 2D, 2F) and impaired glucose tolerance in male and female WT mice compared to chow-fed mice (P<0.05, Figures 2D, 2F). Moreover, TRAF3IP2 deletion (KO) ameliorated HFHS-induced glucose intolerance only in males (P<0.05, Figure 2D). Fasting plasma glucose concentrations were increased with HFHS feeding in both males and females, whereas HbA1c was only increased in HFHS-fed males (P<0.05, Table S1).
Figure 2. TRAF3IP2 deletion protects male mice against obesity-associated glucose intolerance.
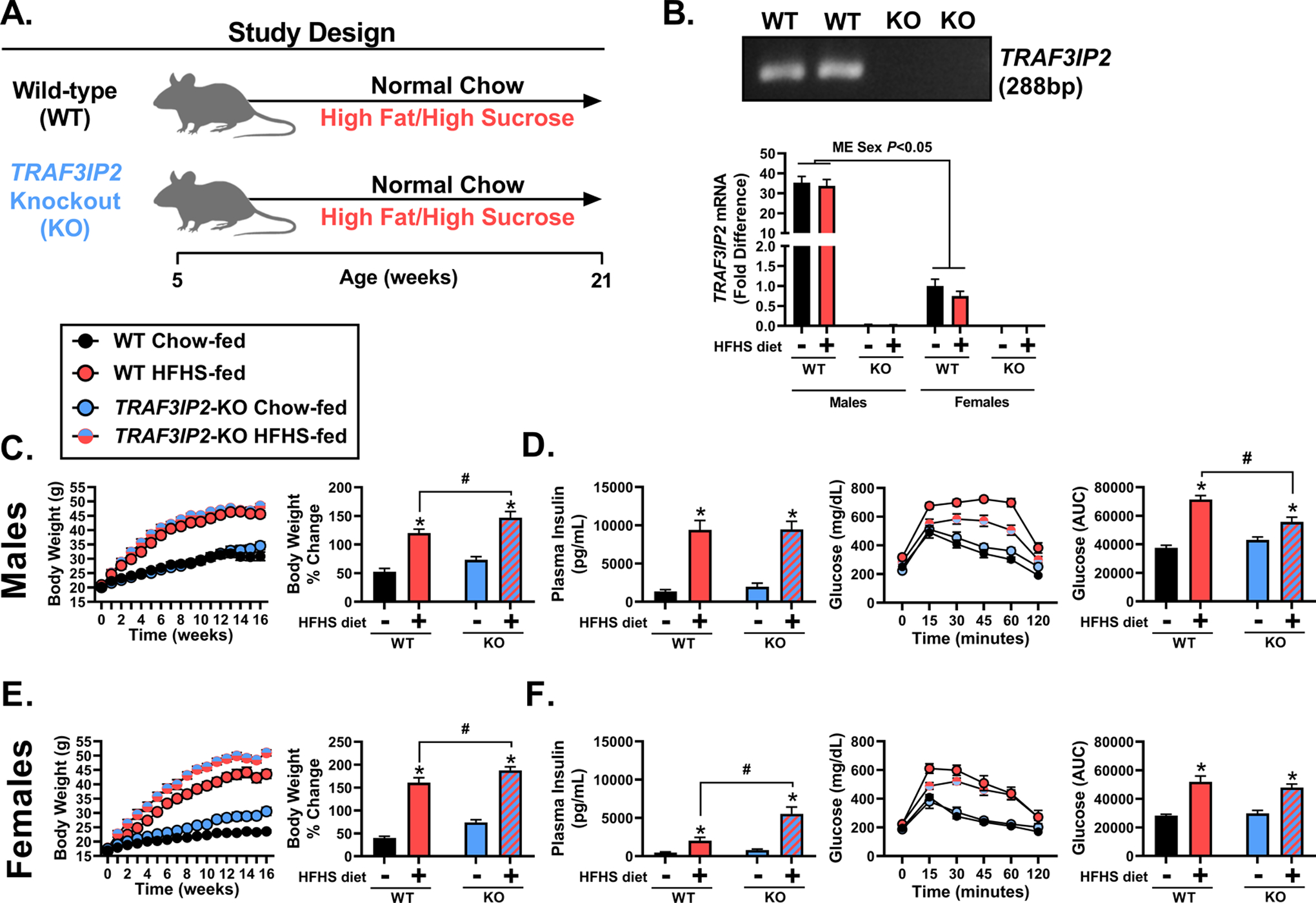
A) Graphical depiction of the study design. Male and female wild-type (WT) or TRAF3IP2-knockout (KO) mice were fed either a normal chow or high fat/high sucrose (HFHS) diet for 16 weeks. B) Representative image of PCR gel depicting ablation of TRAF3IP2 gene (indicated at 288 base pairs [bp]) in KO mice and qPCR determination of TRAF3IP2 mRNA transcripts in heart homogenates from male and female WT or KO mice; n=8–11/group. C) Changes in body weight over time and as percent change from baseline in male mice; n=8–10/group. D) Fasting plasma insulin in male mice; n=8–10/group; Glycemic excursions over time during a glucose tolerance test (GTT) and glucose area under the curve (AUC) in male mice; n=8–10/group. E) Changes in body weight over time and as percent change from baseline in female mice; n=10–12/group. F) Fasting plasma insulin in female mice; n=10–12/group; Glycemic excursions over time during a GTT and glucose AUC in female mice; n=10–12/group. All values are expressed as mean ± SEM. *P<0.05 vs chow-fed counterpart (within genotype); #P<0.05 vs WT counterpart (within diet); main effect (ME) of sex included in panel C.
TRAF3IP2 deletion protects against obesity-associated impairments in insulin-induced vasorelaxation in male mice
As shown in Figure 3A, HFHS feeding significantly blunted insulin-induced aortic vasorelaxation in male WT (P<0.05), but not KO, mice (P>0.05). ACh-induced aortic vasorelaxation was not significantly affected by diet (P>0.05); however, it was greater in HFHS-fed male KO mice relative to WT counterparts (P<0.05). HFHS feeding modestly reduced SNP-induced aortic vasorelaxation in male WT (P<0.05), but not KO, mice (P>0.05). SNP-induced relaxation was increased in HFHS-fed male KO mice relative to HFHS-fed WT mice (P<0.05). Furthermore, male HFHS-fed WT mice exhibited hyperresponsiveness to phenylephrine, an α1-adrenergic receptor vasoconstrictor, compared to chow-fed counterparts (P<0.05, Figure 3B). Notably, this dietary effect was absent in KO mice (P>0.05). Aortic Akt activation (i.e. Ser473 phosphorylation) and eNOS content were not affected by HFHS feeding in male WT mice (P>0.05). However, male KO mice fed HFHS exhibited a heightened Akt activation, which coincided with the induction of total eNOS content, relative to all other groups (P<0.05, Figure 3C).
Figure 3. TRAF3IP2 deletion protects male mice against obesity-associated impairments in insulin-induced vasorelaxation.
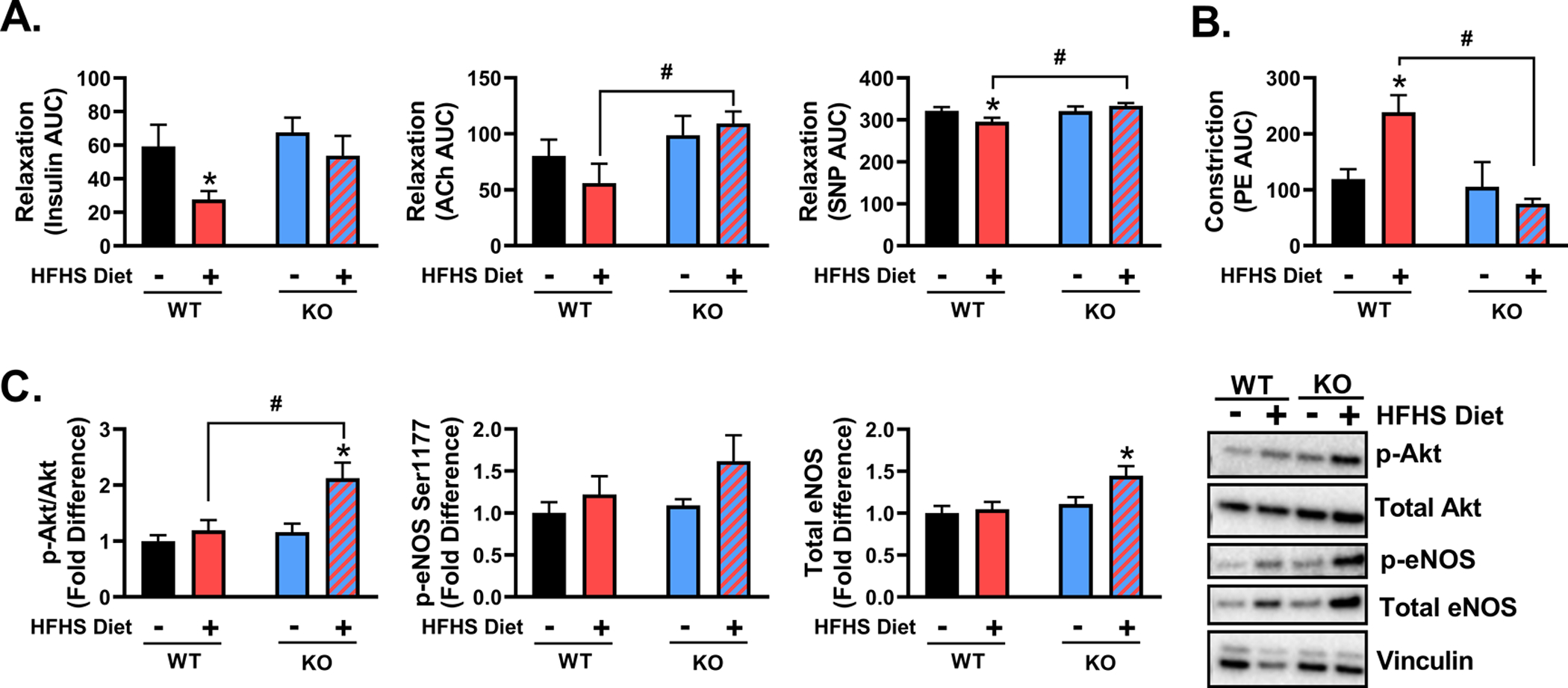
A) Aortic vasorelaxation responses to insulin, acetylcholine (ACh), and sodium nitroprusside (SNP) following preconstriction with U-46619 (20nM) in male wild-type (WT) and TRAF3IP2 knockout (KO) mice fed either a chow or high fat/high sucrose (HFHS) diet for 16 weeks; n=8–10/group. B) Aortic vasoconstriction responses to phenylephrine (PE). C) Determination of Akt activation (p-Akt Ser473/total Akt), eNOS activation (p-Ser1177), and total eNOS in aortic rings from WT and KO mice; representative Western blot images are displayed; n=7–10/group. All values are expressed as mean ± SEM. *P<0.05 vs chow-fed counterpart (within genotype); #P<0.05 vs WT counterpart (within diet).
Vasomotor reactivity data in females are summarized in Figure S1A–B. Contrary to findings in males, both WT and KO female mice were protected against HFHS feeding-associated impairments in aortic vasorelaxation responses to insulin. Similarly, ACh- and SNP-induced aortic vasorelaxation were not influenced by diet or genotype in female mice. Phenylephrine-induced aortic vasoconstriction was also not affected by diet in female WT or KO mice. However, female chow-fed KO mice exhibited hyporeactivity to phenylephrine relative to chow-fed WT mice (P<0.05).
TRAF3IP2 deletion protects against obesity-associated high blood pressure, arterial stiffening and impairments in hindlimb post-occlusive reactive hyperemia in male mice
As depicted in Figure 4A, HFHS feeding increased blood pressure in male WT (P<0.05), but not KO, mice (P>0.05). Male KO mice were also protected against HFHS feeding-induced aortic endothelial cortical stiffening as assessed by AFM (P<0.05, Figure 4B) and exhibited reduced femoral artery stiffness as determined by a rightward shift in the strain-stress curve (P<0.05, Figure 4C). In male WT, but not male KO mice, HFHS feeding suppressed hindlimb post-occlusive reactive hyperemia (P<0.05, Figure 4D–F). However, female HFHS-fed KO mice did exhibit increased blood pressure relative to chow-fed counterparts (P<0.05, Figure S1C).
Figure 4. TRAF3IP2 deletion protects male mice against obesity-associated high blood pressure, arterial stiffening and impairments in hindlimb post-occlusive reactive hyperemia.
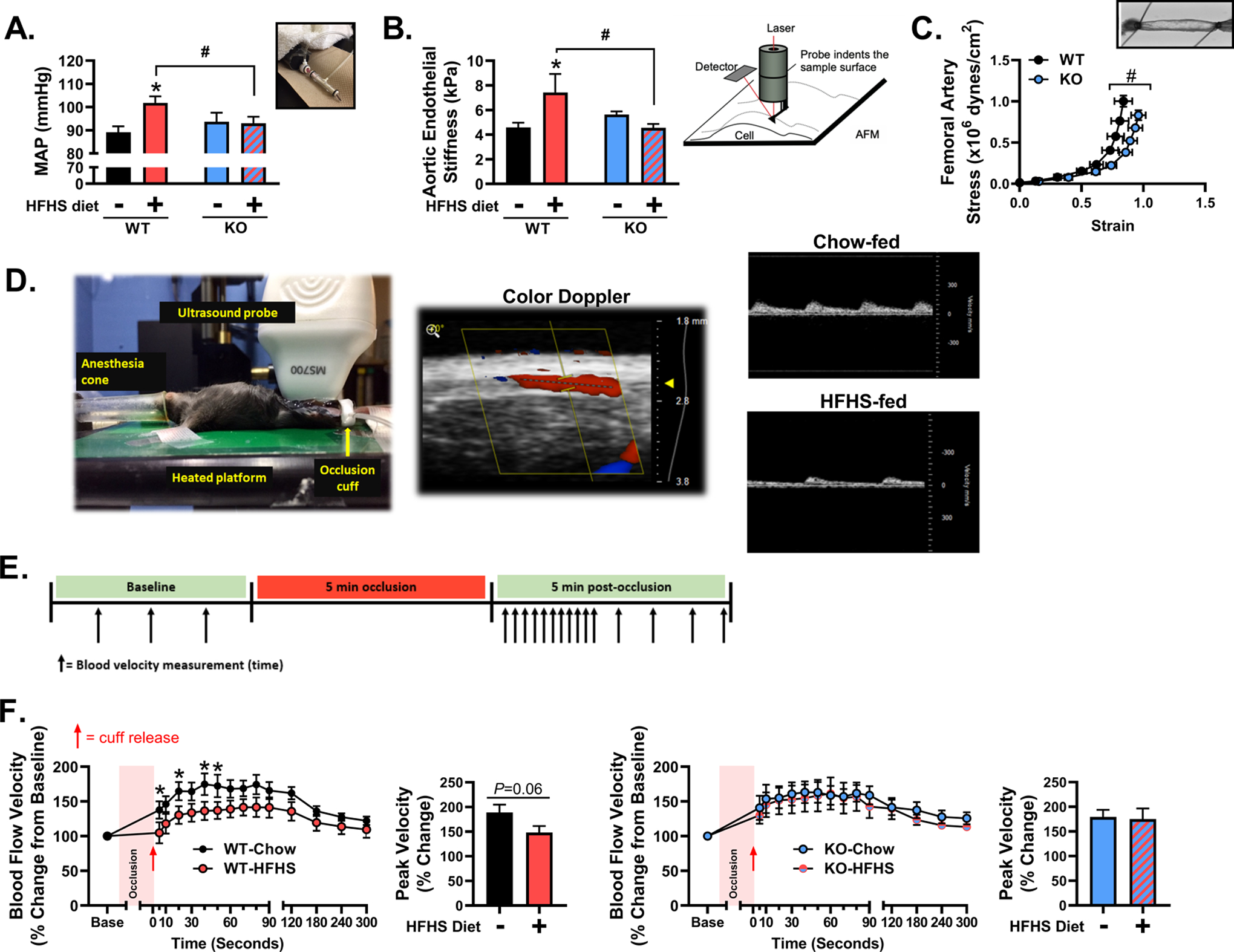
A) Mean arterial blood pressure as determined by tail-cuff plethysmography in wild-type (WT) and TRAF3IP2 knockout (KO) mice fed either a chow or high fat/high sucrose (HFHS) diet; n=8–10/group. B) Aortic endothelial cortical stiffness as assessed by atomic force microscopy (AFM) and graphical representation of instrumental setup; n=6–8/group. C) Femoral artery stiffness as determined by strain-stress analysis in arteries pressurized under passive conditions; n=18–19/group (diets collapsed). D) Depiction of ultrasonography set up for the assessment of post-occlusive reactive hyperemia in the superficial femoral artery in mice. Representative images of peak blood velocity during reactive hyperemia in chow-fed and HFHS-fed mice are displayed. E) Graphical representation of the reactive hyperemia protocol and assessment of blood velocity over time. Percent (%) change of femoral artery blood velocity over time during reactive hyperemia in F) WT and KO mice fed either a chow or HFHS diet and peak blood velocity during reactive hyperemia; n=8–10/group. All values are expressed as mean ± SEM. *P<0.05 vs chow-fed counterpart (within genotype); #P<0.05 vs WT counterpart (within diet).
Proteomic analysis of aortas from male mice
Proteomic analysis of aortas from male WT and KO mice detected 1890 proteins. Comparisons between the WT chow-fed and WT HFHS-fed groups identified 119 proteins that were differentially expressed (Figure 5A and Table S2). Additional comparisons between HFHS-fed WT and KO groups identified 143 proteins that were differentially expressed (Figure 5A and Table S3). Of note, Akt1 was increased (1.7-fold, P=0.038) in aortas from HFHS-fed KO mice relative to HFHS-fed WT mice. Using K-means Clustering within the HFHS-fed KO vs WT comparison, four protein network clusters were identified and consisted of 28 total proteins (Figure S2). In addition, 12 select statistically significant proteins with potential cardiovascular relevance were identified from the quantitative proteomic analysis. Accordingly, 40 candidate proteins (listed in Table S4) were input into the STRING resource software and the resulting protein network diagram is displayed in Figure 5B.
Figure 5. Proteomic analysis and network interactions of proteins identified in aortic tissues from male mice.
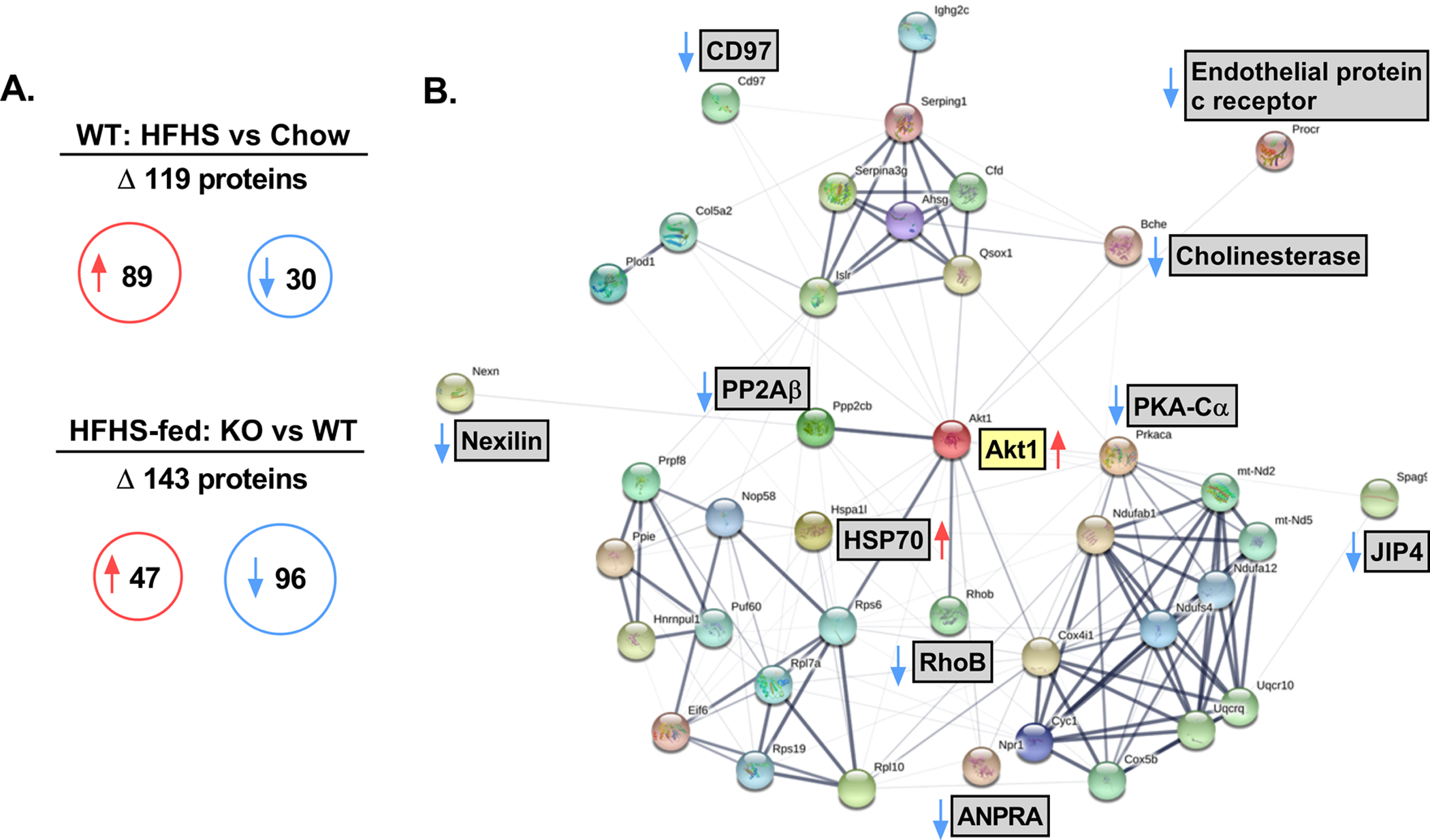
A) Quantitative proteomic analysis of differentially expressed proteins between male chow-fed TRAF3IP2 knockout (KO) vs wild-type (WT) and high fat/high sucrose (HFHS)-fed KO and WT mice; n=8–10 samples analyzed/group. In summary, 1890 proteins were identified with 119 proteins differentially expressed between chow and HFHS-fed WT mice (89 proteins increased, 30 proteins decreased) and 143 proteins differentially expressed between HFHS-fed KO and WT mice (47 proteins increased, 96 proteins decreased). B) Generated protein network interactions from 40 target proteins of interest identified in the quantitative proteomic analysis between HFHS-fed KO and HFHS-fed WT mice. Thicker bands indicate increased strength of data to support the observed protein-protein interactions as generated per the STRING resource software. Red arrows indicate increased protein expression and blue arrow indicate decreased protein expression in HFHS-fed KO mice relative to HFHS-fed WT mice. Gene names are located next to each protein node. Full protein/gene details can be found in Table S4. Noted abbreviations: HSP70, heat shock protein 70; RhoB, Rho-related GTP-binding protein RhoB; PP2Aβ, Serine/threonine-protein phosphatase 2A catalytic subunit beta isoform; PKA-Cα, protein kinase A catalytic subunit α; ANPRA, Atrial natriuretic peptide receptor 1; JIP4, C-Jun-amino-terminal kinase-interacting protein 4.
DISCUSSION
Insulin resistance in the vasculature is a distinguishing feature of obesity and contributes to the pathogenesis of vascular dysfunction and disease. However, the molecular mechanisms underlying vascular insulin resistance and dysfunction in obesity have remained largely unknown. Here, for the first time, we provide evidence that the pro-inflammatory adaptor molecule TRAF3IP2 plays a causal role in obesity-associated vascular insulin resistance and dysfunction, specifically in male mice.
We report that TRAF3IP2 overexpression by adenoviral transduction blunts insulin signaling (i.e., Akt activation) in cultured endothelial cells and depresses endothelium-dependent vasorelaxation in isolated aortic rings. Further, we found that male TRAF3IP2-KO mice are protected against HFHS diet-induced glucose intolerance and vascular insulin resistance, despite greater weight gain, relative to WT counterparts. These findings expand upon our prior work indicating that TRAF3IP2 mediates atherosclerotic plaque development in ApoE-null mice25 and the impairment in ACh-induced vasorelaxation caused by acute oxidized LDL exposure.24 Vascular insulin resistance represents an early event in obesity7–11 and a causal factor in the pathogenesis of CVD.12–17 Thus, the identification of TRAF3IP2 as a novel mediator of vascular insulin resistance is of utmost clinical importance and may be key to identifying newer strategies for the prevention and treatment of vascular diseases associated with obesity and insulin resistance.
It is well established that diet-induced obesity is associated with increases in blood pressure47, 48 and vascular stiffness;47–50 yet, the molecular mechanisms underlying these vascular derangements in obesity have yet to be fully discerned. Importantly, arterial stiffening is a significant risk factor for the development of hypertension51, 52 with previous work indicating that diet-induced arterial stiffening may in fact precede the onset of overt hypertension.47 Here, in agreement with our hypothesis, we show that TRAF3IP2-KO male mice are protected against HFHS diet-induced increases in blood pressure and arterial stiffness. Furthermore, we report that HFHS diet-induced aortic hyperreactivity to phenylephrine, an α1-adrenergic receptor agonist, was prevented by the loss of TRAF3IP2. Such attenuated α1-adrenergic receptor-mediated vasoconstriction in TRAF3IP2-deficient male mice may contribute to their normotensive phenotype despite obesity.53–55 In addition, given the known relationships between insulin resistance, arterial stiffness and hypertension, the reduced blood pressure and arterial stiffness in male HFHS-fed KO mice may also be attributed to preserved vascular insulin sensitivity.14, 56–58 Collectively, our findings for the first time support an important role for TRAF3IP2 in blood pressure control and arterial stiffening in the setting of obesity.
Growing evidence indicates that perturbations in microvascular function are linked to the development of insulin resistance, hypertension, and arterial stiffening in obesity.59–62 One of the most widely utilized assessments of resistance vessel function in humans is post-occlusive reactive hyperemia via Doppler ultrasound.63 In fact, impaired reactive hyperemia is considered an independent predictor of adverse cardiovascular events and a well-established surrogate marker of CVD risk.36, 63–67 Although the assessment of post-occlusive reactive hyperemia in small animal models has been challenging, due to their reduced vascular dimensions, advancements in ultrasound technology have made visualization and detection of conduit arteries in rodents possible. Indeed, two reports have validated the in vivo assessment of post-occlusive reactive hyperemia in mice34 and rats.35 Consistent with our hypothesis, we found that loss of TRAF3IP2 protects against HFHS diet-induced impairments in hindlimb reactive hyperemia, underscoring a hitherto unidentified novel role for TRAF3IP2 in microvascular control. We have previously demonstrated that TRAF3IP2 is a mediator of endothelin-1 production,27 a vasoconstrictor peptide primarily released from endothelial cells and a known regulator of reactive hyperemia.68 Since obesity exacerbates endothelin-1 actions,69–71 it is plausible that TRAF3IP2 ablation protects against HFHS diet-induced impairments in hindlimb reactive hyperemia by limiting microvascular endothelin-1 production/function. However, further research is warranted to better understand the relationship between TRAF3IP2 and endothelin-1 in microvascular control.
An additional important finding of the current investigation is that TRAF3IP2 regulates Akt activation and expression. Notably, TRAF3IP2 is a known regulator of stress-activated protein kinases and inflammatory signaling (i.e., IKK/NF-κB and JNK/AP-1 signaling) in various cell types,72–74 including vascular tissues.25–28 Namely, we have previously shown that overexpression of TRAF3IP2 increases IKK/NF-κB and JNK/AP-1 activation in endothelial cells,27 while silencing of TRAF3IP2 limits high glucose- and IL-17-induced inflammation in endothelial26, 27 and vascular smooth muscle cells,28 respectively. However, whether TRAF3IP2 regulates Akt, an important molecular determinant of insulin signaling, endothelium-dependent function, and overall vascular control75–79 has yet to be determined. Here we provide the first evidence that overexpression of TRAF3IP2 limits insulin-induced Akt activation in endothelial cells and that genetic ablation of TRAF3IP2 enhances Akt activation in hyperinsulinemic male mice (i.e., mice fed HFHS). In addition, the aortic proteomic analysis revealed an increase in Akt1 expression, the dominant Akt isoform active in cardiovascular tissues,80–82 in male TRAF3IP2-KO mice fed HFHS. Therefore, given the known roles of Akt in metabolic and vascular control, it is plausible that increases in vascular Akt activation and expression mediate vascular protection against obesity in TRAF3IP2-deficient mice. Stated differently, it appears that the loss of TRAF3IP2 unrestricts Akt signaling in the setting of hyperinsulinemia and obesity. Because prolonged insulin stimulation and signaling through Akt leads to increased eNOS expression,83–85 it is not surprising that increased Akt activation was accompanied with an induction of total eNOS content in aortic homogenates of hyperinsulinemic/obese mice lacking TRAF3IP2, likely further contributing to their overall preserved vascular function. These findings are highly significant in that selective activation of the PI3K/Akt/eNOS branch of the insulin signaling pathway is associated with improved vascular outcomes in a model of obesity and metabolic syndrome.23
To further identify additional potential adaptive molecular mechanisms resulting from the loss of TRAF3IP2 in male HFHS-fed mice, we utilized the STRING resource software to identify potential protein-protein interaction networks among 40 candidate proteins selected from the proteomic analysis. Interestingly, the STRING resource software generated an Akt1-centric network diagram (Figure 5B). This observation indicates that many of the differentially expressed proteins resulting from TRAF3IP2 ablation interact with Akt, thus further supporting the notion that increased Akt signaling may be a mechanism by which loss of TRAF3IP2 protects against obesity-associated vascular dysfunction. In this regard, heat shock protein 70 (HSP70), Rho-related GTP-binding protein RhoB, protein kinase A catalytic subunit α (PKA-Cα), identified in Figure 5B, are known transducers of Akt activation and sequestration in vascular tissue86–88 and are likewise implicated in vascular health and function.89, 90 Nevertheless, more research is warranted to delineate the molecular mechanisms by which TRAF3IP2 directly or indirectly regulate Akt activation and signaling.
Another important observation of this current investigation is that female mice exhibited a largely protected vascular phenotype following 16 weeks of HFHS feeding. Notably, this protection coincided with the finding that female mice displayed a ~35-fold lower TRAF3IP2 mRNA expression compared to males. Thus, it is likely that the apparent vascular protection in our female WT mice might be due to low levels of TRAF3IP2 expression. Along similar lines, given the low basal expression of TRAF3IP2 in females, it is not surprising that genetic ablation of TRAF3IP2 appears to be less consequential to their vascular function. To further explore the impact of sex and TRAF3IP2 on vasomotor function, we conducted a secondary analysis directly comparing endothelium-dependent and -independent relaxation responses between males and females. As shown in Figure 6, WT males (high TRAF3IP2 expressors) exhibit lower endothelium-dependent relaxation relative to females (low TRAF3IP2 expressors), while endothelium-independent relaxation remains similar between sexes. Notably, this sex difference in endothelial function is abolished in mice lacking TRAF3IP2, further underscoring the role of TRAF3IP2 in mediating sex-differences in vascular function. Future research should explore the relationship between sex and TRAF3IP2 throughout the lifespan since it is known that women exhibit increased susceptibility to CVD with age.91 Importantly, these findings add to the emergent literature supporting the notion that sex should be deemed as a key variable when identifying the molecular mechanisms underlying CVD.
Figure 6. Wild-type (WT) male mice exhibit lower endothelium-dependent vasorelaxation relative to females.
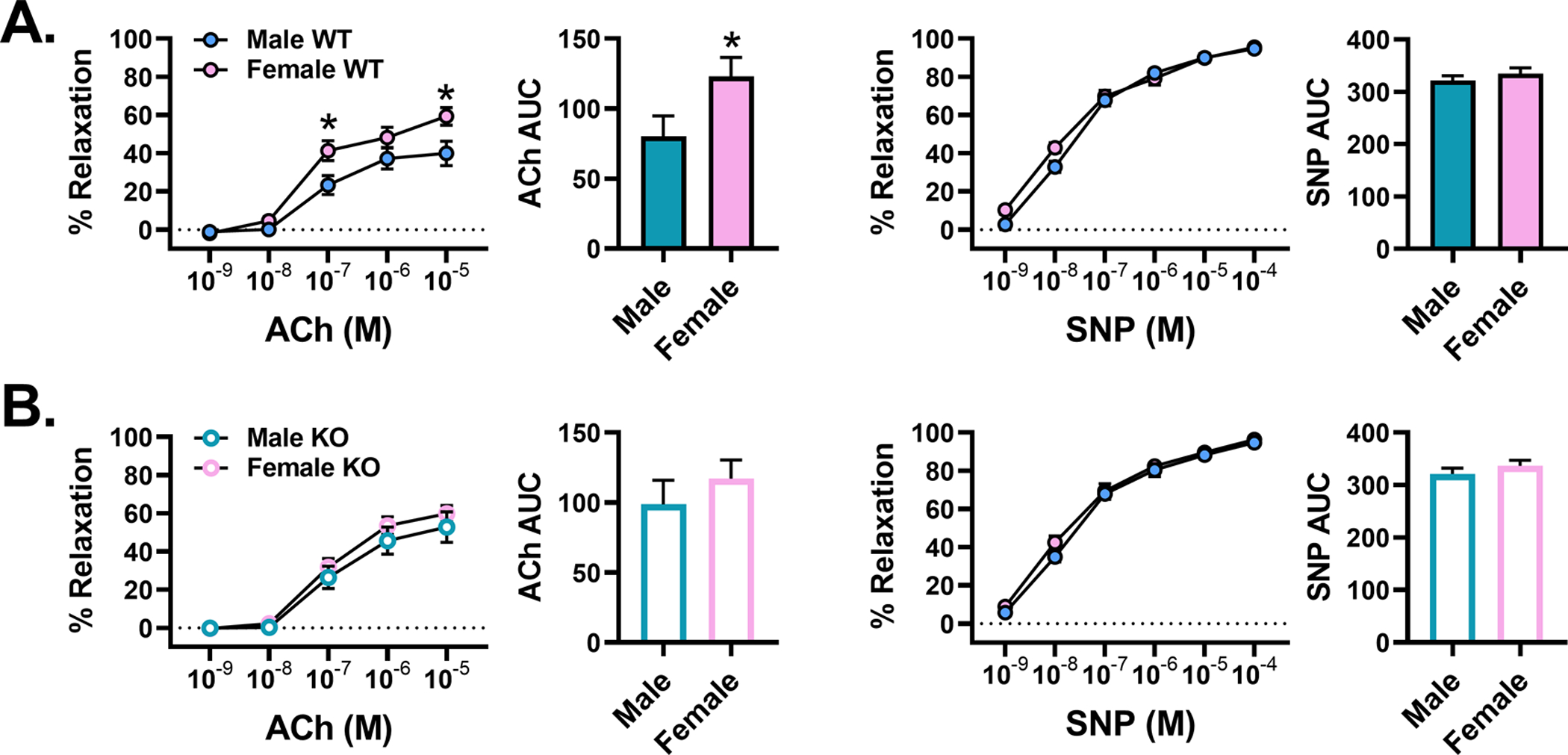
Comparison of aortic vasorelaxation responses to acetylcholine (ACh; endothelium-dependent) and sodium nitroprusside (SNP; endothelium-independent) between male and female A) WT and B) KO mice fed chow; n=8–12/group. All values are expressed as mean ± SEM. *P<0.05 vs males.
There are some limitations to our work that should be given consideration. While our HFHS diet produced the intended metabolic and vascular effects, as also recently reported by others,48 we acknowledge that the use of the standard grain-based chow diet is not the most appropriate control diet against the employed HFHS, which is a purified ingredient diet. Accordingly, future work would benefit from utilizing a matching low-fat purified ingredient diet as the control to the present HFHS diet administered. Indeed, previous reports have found that matching and controlling for purified ingredients and micronutrients between low-fat and high-fat diets can influence differences in metabolic and health outcomes in rodents and thus the interpretation of the findings.92–95 Further, consideration should be given to our glucose administration during the GTT and the use of tail-cuff plethysmography. In the present study, the glucose load utilized during the GTT may have overestimated the level of glucose intolerance in HFHS-fed mice since these mice were given a greater glucose load based on body weight. Notably, mice lacking TRAF3IP2 and fed a HFHS diet tended to be heavier than WT counterparts. Interestingly, they still displayed better glucose disposal. Along these lines, the protective metabolic phenotype displayed by this mouse model should be further interrogated by incorporating detailed measures of body composition as well as determining energy expenditure and spontaneous physical activity using metabolic cages. Lastly, although we found significant blood pressure differences between male HFHS-fed WT and KO mice using a tail-cuff blood pressure method that has been previously validated,96 future research should confirm these findings using telemetry-based methodologies.
Perspectives
Insulin resistance in the vasculature is a characteristic feature of obesity and contributes to the pathogenesis of vascular dysfunction and disease. Findings from the present study demonstrate that TRAF3IP2, a pro-inflammatory adaptor molecule, plays a causal role in obesity-associated vascular insulin resistance and dysfunction. Moreover, our studies highlight a sexual dimorphic role of TRAF3IP2 in vascular control and identify it as a promising therapeutic target in vasculometabolic derangements associated with obesity, particularly in males.
Supplementary Material
Novelty and Significance.
What is New?
Ectopic expression of TRAF3IP2 blunts insulin signaling (i.e., Akt activation) in cultured endothelial cells and depresses endothelium-dependent vasorelaxation in isolated aortic rings.
TRAF3IP2 deletion protects against obesity-associated impairments in glucose tolerance and insulin-induced vasorelaxation in male mice.
TRAF3IP2 deletion protects against obesity-associated high blood pressure, arterial stiffening and impairments in hindlimb post-occlusive reactive hyperemia in male mice.
TRAF3IP2 regulates Akt activation and expression.
What is Relevant?
Vascular insulin resistance contributes to the pathogenesis of vascular diseases.
Here we demonstrate that TRAF3IP2 mediates obesity-associated vascular insulin resistance and dysfunction in male mice.
Summary
TRAF3IP2 plays a causal role in obesity-associated vascular insulin resistance and dysfunction. Moreover, our findings highlight a sexual dimorphic role of TRAF3IP2 in vascular control and identify it as a promising therapeutic target in vasculometabolic derangements associated with obesity, particularly in males.
Acknowledgements
We would like to thank Pei Liu and the University of Missouri Charles W. Gehrke Proteomics Core for their assistance with the proteomics analysis. We would also like to acknowledge James Graham and the staff at the University of California - Davis (UC Davis) MMPC, supported by U24 DK092993, for their assistance with the mouse plasma analysis.
Sources of Funding
This project was supported by the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) grant UL1TR002345, as well as NIH R01 HL137769 (JP), R01 HL088105 (LM-L), R01 HL142770 (CM-A), the University of Missouri School of Medicine Program Project Planning Grant (LM-L), and the University of Missouri Department of Nutrition and Exercise Physiology Schade Research Fund (ZIG and JP). BC is a Research Career Scientist (IK6BX004016) and his work is supported by the VA ORD-BLRD Service Award I01-BX004220. US is supported by the NIH Intramural funding. This work was also supported by the use of resources and facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
Abbreviations:
- TRAF3IP2
TRAF3 Interacting Protein 2
- PI3K
phosphatidylinositol 3 kinase
- eNOS
endothelial nitric oxide synthase
- HFHS
high fat/high sucrose
- WT
wild-type
- KO
knockout
Footnotes
Disclosures: None.
REFERENCES
- 1.Hubert HB, Feinleib M, McNamara PM and Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD and Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS data brief. 2017:1–8. [PubMed] [Google Scholar]
- 3.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr. and Tomaselli GF. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G and Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. The Journal of clinical investigation. 1996;97:2601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L and Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Q, Gao F and Ma XL. Insulin says NO to cardiovascular disease. Cardiovascular research. 2011;89:516–24. [DOI] [PubMed] [Google Scholar]
- 7.Eringa EC, Stehouwer CD, Roos MH, Westerhof N and Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. American journal of physiology Endocrinology and metabolism. 2007;293:E1134–9. [DOI] [PubMed] [Google Scholar]
- 8.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A and Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katakam PV, Tulbert CD, Snipes JA, Erdos B, Miller AW and Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. American journal of physiology Heart and circulatory physiology. 2005;288:H854–60. [DOI] [PubMed] [Google Scholar]
- 10.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD and Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. American journal of physiology Heart and circulatory physiology. 2006;291:H1780–7. [DOI] [PubMed] [Google Scholar]
- 11.Olver TD, Grunewald ZI, Jurrissen TJ, MacPherson REK, LeBlanc PJ, Schnurbusch TR, Czajkowski AM, Laughlin MH, Rector RS, Bender SB, Walters EM, Emter CA and Padilla J. Microvascular insulin resistance in skeletal muscle and brain occurs early in the development of juvenile obesity in pigs. American journal of physiology Regulatory, integrative and comparative physiology. 2018;314:R252–r264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King GL, Park K and Li Q. Selective Insulin Resistance and the Development of Cardiovascular Diseases in Diabetes: The 2015 Edwin Bierman Award Lecture. Diabetes. 2016;65:1462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanter JE and Bornfeldt KE. Evidence stacks up that endothelial insulin resistance is a culprit in atherosclerosis. Circ Res. 2013;113:352–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JA, Montagnani M, Koh KK and Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904. [DOI] [PubMed] [Google Scholar]
- 15.Mather KJ, Steinberg HO and Baron AD. Insulin resistance in the vasculature. The Journal of clinical investigation. 2013;123:1003–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muniyappa R and Quon MJ. Insulin action and insulin resistance in vascular endothelium. Current opinion in clinical nutrition and metabolic care. 2007;10:523–30. [DOI] [PubMed] [Google Scholar]
- 17.Muniyappa R and Sowers JR. Role of insulin resistance in endothelial dysfunction. Reviews in endocrine & metabolic disorders. 2013;14:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA and Hamburg NM. Protein kinase C-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breton-Romero R, Feng B, Holbrook M, Farb MG, Fetterman JL, Linder EA, Berk BD, Masaki N, Weisbrod RM, Inagaki E, Gokce N, Fuster JJ, Walsh K and Hamburg NM. Endothelial Dysfunction in Human Diabetes Is Mediated by Wnt5a-JNK Signaling. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masaki N, Ido Y, Yamada T, Yamashita Y, Toya T, Takase B, Hamburg NM and Adachi T. Endothelial Insulin Resistance of Freshly Isolated Arterial Endothelial Cells From Radial Sheaths in Patients With Suspected Coronary Artery Disease. Journal of the American Heart Association. 2019;8:e010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall’Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR and King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell metabolism. 2010;11:379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sengupta A, Patel PA, Yuldasheva NY, Mughal RS, Galloway S, Viswambharan H, Walker AMN, Aziz A, Smith J, Ali N, Mercer BN, Imrie H, Sukumar P, Wheatcroft SB, Kearney MT and Cubbon RM. Endothelial Insulin Receptor Restoration Rescues Vascular Function in Male Insulin Receptor Haploinsufficient Mice. Endocrinology. 2018;159:2917–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanter JE, Kramer F, Barnhart S, Duggan JM, Shimizu-Albergine M, Kothari V, Chait A, Bouman SD, Hamerman JA, Hansen BF, Olsen GS and Bornfeldt KE. A Novel Strategy to Prevent Advanced Atherosclerosis and Lower Blood Glucose in a Mouse Model of Metabolic Syndrome. Diabetes. 2018;67:946–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valente AJ, Irimpen AM, Siebenlist U and Chandrasekar B. OxLDL induces endothelial dysfunction and death via TRAF3IP2: inhibition by HDL3 and AMPK activators. Free radical biology & medicine. 2014;70:117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamuri S, Higashi Y, Sukhanov S, Siddesha JM, Delafontaine P, Siebenlist U and Chandrasekar B. TRAF3IP2 mediates atherosclerotic plaque development and vulnerability in ApoE(−/−) mice. Atherosclerosis. 2016;252:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkatesan B, Valente AJ, Das NA, Carpenter AJ, Yoshida T, Delafontaine JL, Siebenlist U and Chandrasekar B. CIKS (Act1 or TRAF3IP2) mediates high glucose-induced endothelial dysfunction. Cellular signalling. 2013;25:359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padilla J, Carpenter AJ, Das NA, Kandikattu HK, Lopez-Ongil S, Martinez-Lemus LA, Siebenlist U, DeMarco VG and Chandrasekar B. TRAF3IP2 mediates high glucose-induced endothelin-1 production as well as endothelin-1-induced inflammation in endothelial cells. American journal of physiology Heart and circulatory physiology. 2018;314:H52–h64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mummidi S, Das NA, Carpenter AJ, Yoshida T, Yariswamy M, Mostany R, Izadpanah R, Higashi Y, Sukhanov S, Noda M, Siebenlist U, Rector RS and Chandrasekar B. RECK suppresses interleukin-17/TRAF3IP2-mediated MMP-13 activation and human aortic smooth muscle cell migration and proliferation. Journal of cellular physiology. 2019;234(12):22242–22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jurrissen TJ, Grunewald ZI, Woodford ML, Winn NC, Ball JR, Smith TN, Wheeler A, Rawlings AL, Staveley-O’Carroll KF, Ji Y, Fay WP, Paradis P, Schiffrin EL, Vieira-Potter VJ, Fadel PJ, Martinez-Lemus LA and Padilla J. Overproduction of endothelin-1 impairs glucose tolerance but does not promote visceral adipose tissue inflammation or limit metabolic adaptations to exercise. American journal of physiology Endocrinology and metabolism. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunewald ZI, Winn NC, Gastecki ML, Woodford ML, Ball JR, Hansen SA, Sacks HS, Vieira-Potter VJ and Padilla J. Removal of interscapular brown adipose tissue increases aortic stiffness despite normal systemic glucose metabolism in mice. American journal of physiology Regulatory, integrative and comparative physiology. 2018;314:R584–r597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grunewald ZI, Jurrissen TJ, Woodford ML, Ramirez-Perez FI, Park LK, Pettit-Mee R, Ghiarone T, Brown SM, Morales-Quinones M, Ball JR, Staveley-O’Carroll KF, Aroor AR, Fadel PJ, Paradis P, Schiffrin EL, Bender SB, Martinez-Lemus LA and Padilla J. Chronic Elevation of Endothelin-1 Alone May Not Be Sufficient to Impair Endothelium-Dependent Relaxation. Hypertension. 2019;74:1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Sur SH, Mistlberger RE and Morris M. Circadian blood pressure and heart rate rhythms in mice. The American journal of physiology. 1999;276:R500–4. [DOI] [PubMed] [Google Scholar]
- 33.Carlson SH and Wyss JM. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension. 2000;35:E1–5. [DOI] [PubMed] [Google Scholar]
- 34.Schuler D, Sansone R, Freudenberger T, Rodriguez-Mateos A, Weber G, Momma TY, Goy C, Altschmied J, Haendeler J, Fischer JW, Kelm M and Heiss C. Measurement of endothelium-dependent vasodilation in mice--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2651–7. [DOI] [PubMed] [Google Scholar]
- 35.Machin DR, Leary ME, He Y, Shiu YT, Tanaka H and Donato AJ. Ultrasound Assessment of Flow-Mediated Dilation of the Brachial and Superficial Femoral Arteries in Rats. Journal of visualized experiments : JoVE. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S and Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–9. [DOI] [PubMed] [Google Scholar]
- 37.Paine NJ, Hinderliter AL, Blumenthal JA, Adams KF Jr., Sueta CA, Chang PP, O’Connor CM and Sherwood A. Reactive hyperemia is associated with adverse clinical outcomes in heart failure. American heart journal. 2016;178:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winn NC, Jurrissen TJ, Grunewald ZI, Cunningham RP, Woodford ML, Kanaley JA, Lubahn DB, Manrique-Acevedo C, Rector RS, Vieira-Potter VJ and Padilla J. Estrogen receptor-α signaling maintains immunometabolic function in males and is obligatory for exercise-induced amelioration of nonalcoholic fatty liver. American journal of physiology Endocrinology and metabolism. 2019;316:E156–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A and Sowers JR. Low-Dose Mineralocorticoid Receptor Blockade Prevents Western Diet-Induced Arterial Stiffening in Female Mice. Hypertension. 2015;66:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, Sun Z, Meininger GA and Muthuchamy M. Application of atomic force microscopy measurements on cardiovascular cells. Methods in molecular biology (Clifton, NJ). 2012;843:229–44. [DOI] [PubMed] [Google Scholar]
- 41.Padilla J, Woodford ML, Lastra-Gonzalez G, Martinez-Diaz V, Fujie S, Yang Y, Lising AMC, Ramirez-Perez FI, Aroor AR, Morales-Quinones M, Ghiarone T, Whaley-Connell A, Martinez-Lemus LA, Hill MA and Manrique-Acevedo C. Sexual Dimorphism in Obesity-Associated Endothelial ENaC Activity and Stiffening in Mice. Endocrinology. 2019;160:2918–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ and Sowers JR. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circulation research. 2016;118:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padilla J, Ramirez-Perez FI, Habibi J, Bostick B, Aroor AR, Hayden MR, Jia G, Garro M, DeMarco VG, Manrique C, Booth FW, Martinez-Lemus LA and Sowers JR. Regular Exercise Reduces Endothelial Cortical Stiffness in Western Diet-Fed Female Mice. Hypertension. 2016;68:1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ and Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic acids research. 2019;47:D607–d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh LK, Ghiarone T, Olver TD, Medina-Hernandez A, Edwards JC, Thorne PK, Emter CA, Lindner JR, Manrique-Acevedo C, Martinez-Lemus LA and Padilla J. Increased endothelial shear stress improves insulin-stimulated vasodilatation in skeletal muscle. The Journal of physiology. 2019;597:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, Chariot A, Garcia-Perganeda A, Leonardi A, Paun A, Chen A, Ren NY, Wang H and Siebenlist U. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. Journal of immunology (Baltimore, Md : 1950). 2009;182:1617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA and Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhatta A, Yao L, Xu Z, Toque HA, Chen J, Atawia RT, Fouda AY, Bagi Z, Lucas R, Caldwell RB and Caldwell RW. Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovascular research. 2017;113:1664–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aroor AR, Jia G, Habibi J, Sun Z, Ramirez-Perez FI, Brady B, Chen D, Martinez-Lemus LA, Manrique C, Nistala R, Whaley-Connell AT, Demarco VG, Meininger GA and Sowers JR. Uric acid promotes vascular stiffness, maladaptive inflammatory responses and proteinuria in western diet fed mice. Metabolism: clinical and experimental. 2017;74:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez-Lemus LA, Aroor AR, Ramirez-Perez FI, Jia G, Habibi J, DeMarco VG, Barron B, Whaley-Connell A, Nistala R and Sowers JR. Amiloride Improves Endothelial Function and Reduces Vascular Stiffness in Female Mice Fed a Western Diet. Frontiers in physiology. 2017;8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Safar ME. Arterial stiffness as a risk factor for clinical hypertension. Nature reviews Cardiology. 2018;15:97–105. [DOI] [PubMed] [Google Scholar]
- 52.Zheng X, Jin C, Liu Y, Zhang J, Zhu Y, Kan S, Wu Y, Ruan C, Lin L, Yang X, Zhao X and Wu S. Arterial Stiffness as a Predictor of Clinical Hypertension. Journal of clinical hypertension (Greenwich, Conn). 2015;17:582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Battault S, Meziat C, Nascimento A, Braud L, Gayrard S, Legros C, De Nardi F, Drai J, Cazorla O, Thireau J, Meyer G and Reboul C. Vascular endothelial function masks increased sympathetic vasopressor activity in rats with metabolic syndrome. American journal of physiology Heart and circulatory physiology. 2018;314:H497–h507. [DOI] [PubMed] [Google Scholar]
- 54.Egan B, Panis R, Hinderliter A, Schork N and Julius S. Mechanism of increased alpha adrenergic vasoconstriction in human essential hypertension. The Journal of clinical investigation. 1987;80:812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agapitov AV, Correia ML, Sinkey CA and Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension. 2008;52:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aroor AR, Jia G and Sowers JR. Cellular mechanisms underlying obesity-induced arterial stiffness. American journal of physiology Regulatory, integrative and comparative physiology. 2018;314:R387–r398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abe H, Yamada N, Kamata K, Kuwaki T, Shimada M, Osuga J, Shionoiri F, Yahagi N, Kadowaki T, Tamemoto H, Ishibashi S, Yazaki Y and Makuuchi M. Hypertension, hypertriglyceridemia, and impaired endothelium-dependent vascular relaxation in mice lacking insulin receptor substrate-1. The Journal of clinical investigation. 1998;101:1784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heise T, Magnusson K, Heinemann L and Sawicki PT. Insulin resistance and the effect of insulin on blood pressure in essential hypertension. Hypertension. 1998;32:243–8. [DOI] [PubMed] [Google Scholar]
- 59.Climie RE, van Sloten TT, Bruno RM, Taddei S, Empana JP, Stehouwer CDA, Sharman JE, Boutouyrie P and Laurent S. Macrovasculature and Microvasculature at the Crossroads Between Type 2 Diabetes Mellitus and Hypertension. Hypertension. 2019;73:1138–1149. [DOI] [PubMed] [Google Scholar]
- 60.Stefanadis C, Vlachopoulos C, Karayannacos P, Boudoulas H, Stratos C, Filippides T, Agapitos M and Toutouzas P. Effect of vasa vasorum flow on structure and function of the aorta in experimental animals. Circulation. 1995;91:2669–78. [DOI] [PubMed] [Google Scholar]
- 61.Karaca U, Schram MT, Houben AJ, Muris DM and Stehouwer CD. Microvascular dysfunction as a link between obesity, insulin resistance and hypertension. Diabetes research and clinical practice. 2014;103:382–7. [DOI] [PubMed] [Google Scholar]
- 62.De Boer MP, Meijer RI, Wijnstok NJ, Jonk AM, Houben AJ, Stehouwer CD, Smulders YM, Eringa EC and Serne EH. Microvascular dysfunction: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Microcirculation (New York, NY : 1994). 2012;19:5–18. [DOI] [PubMed] [Google Scholar]
- 63.Limberg JK, Casey DP, Trinity JD, Nicholson WT, Wray DW, Tschakovsky ME, Green DJ, Hellsten Y, Fadel PJ, Joyner MJ and Padilla J. Assessment of resistance vessel function in human skeletal muscle: guidelines for experimental design, Doppler ultrasound, and pharmacology. American journal of physiology Heart and circulatory physiology. 2020;318:H301–h325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr., Keyes MJ, Levy D, Vasan RS and Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–9. [DOI] [PubMed] [Google Scholar]
- 65.Philpott AC, Lonn E, Title LM, Verma S, Buithieu J, Charbonneau F and Anderson TJ. Comparison of new measures of vascular function to flow mediated dilatation as a measure of cardiovascular risk factors. The American journal of cardiology. 2009;103:1610–5. [DOI] [PubMed] [Google Scholar]
- 66.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF Jr., Gokce N and Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D and Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–8. [DOI] [PubMed] [Google Scholar]
- 68.Nishiyama SK, Zhao J, Wray DW and Richardson RS. Vascular function and endothelin-1: tipping the balance between vasodilation and vasoconstriction. Journal of applied physiology (Bethesda, Md : 1985). 2017;122:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferri C, Bellini C, Desideri G, Baldoncini R, Properzi G, Santucci A and De Mattia G. Circulating endothelin-1 levels in obese patients with the metabolic syndrome. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 1997;105 Suppl 2:38–40. [DOI] [PubMed] [Google Scholar]
- 70.Reynolds LJ, Credeur DP, Manrique C, Padilla J, Fadel PJ and Thyfault JP. Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. Journal of applied physiology (Bethesda, Md : 1985). 2017;122:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weil BR, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL and DeSouza CA. Enhanced endothelin-1 system activity with overweight and obesity. American journal of physiology Heart and circulatory physiology. 2011;301:H689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X, Commane M, Nie H, Hua X, Chatterjee-Kishore M, Wald D, Haag M and Stark GR. Act1, an NF-kappa B-activating protein. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T and Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nature immunology. 2007;8:247–56. [DOI] [PubMed] [Google Scholar]
- 74.Leonardi A, Chariot A, Claudio E, Cunningham K and Siebenlist U. CIKS, a connection to Ikappa B kinase and stress-activated protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iaccarino G, Ciccarelli M, Sorriento D, Cipolletta E, Cerullo V, Iovino GL, Paudice A, Elia A, Santulli G, Campanile A, Arcucci O, Pastore L, Salvatore F, Condorelli G and Trimarco B. AKT participates in endothelial dysfunction in hypertension. Circulation. 2004;109:2587–93. [DOI] [PubMed] [Google Scholar]
- 76.Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, Sessa WC and Walsh K. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. The Journal of clinical investigation. 2000;106:493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee MY, Gamez-Mendez A, Zhang J, Zhuang Z, Vinyard DJ, Kraehling J, Velazquez H, Brudvig GW, Kyriakides TR, Simons M and Sessa WC. Endothelial Cell Autonomous Role of Akt1: Regulation of Vascular Tone and Ischemia-Induced Arteriogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2018;38:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T and Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension. 2004;44:956–62. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ and Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell metabolism. 2007;6:446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K and Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. The Journal of clinical investigation. 2005;115:2119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, Kyriakides TR, Morales-Ruiz M and Sessa WC. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiojima I and Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circulation research. 2002;90:1243–50. [DOI] [PubMed] [Google Scholar]
- 83.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ and Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. The Journal of biological chemistry. 2002;277:1794–9. [DOI] [PubMed] [Google Scholar]
- 84.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ and King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000;101:676–81. [DOI] [PubMed] [Google Scholar]
- 85.Fisslthaler B, Benzing T, Busse R and Fleming I. Insulin enhances the expression of the endothelial nitric oxide synthase in native endothelial cells: a dual role for Akt and AP-1. Nitric oxide : biology and chemistry. 2003;8:253–61. [DOI] [PubMed] [Google Scholar]
- 86.Adini I, Rabinovitz I, Sun JF, Prendergast GC and Benjamin LE. RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes & development. 2003;17:2721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shiota M, Kusakabe H, Izumi Y, Hikita Y, Nakao T, Funae Y, Miura K and Iwao H. Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:491–7. [DOI] [PubMed] [Google Scholar]
- 88.Lee JW, Chen H, Pullikotil P and Quon MJ. Protein kinase A-alpha directly phosphorylates FoxO1 in vascular endothelial cells to regulate expression of vascular cellular adhesion molecule-1 mRNA. The Journal of biological chemistry. 2011;286:6423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Molina MN, Ferder L and Manucha W. Emerging Role of Nitric Oxide and Heat Shock Proteins in Insulin Resistance. Current hypertension reports. 2016;18:1. [DOI] [PubMed] [Google Scholar]
- 90.Vega FM and Ridley AJ. The RhoB small GTPase in physiology and disease. Small GTPases. 2018;9:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia M, Mulvagh SL, Merz CNB, Buring JE and Manson JE. Cardiovascular Disease in Women: Clinical Perspectives. Circulation research. 2016;118:1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pellizzon MA and Ricci MR. Choice of Laboratory Rodent Diet May Confound Data Interpretation and Reproducibility. Current developments in nutrition. 2020;4:nzaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pellizzon MA and Ricci MR. Effects of Rodent Diet Choice and Fiber Type on Data Interpretation of Gut Microbiome and Metabolic Disease Research. Current protocols in toxicology. 2018:e55. [DOI] [PubMed] [Google Scholar]
- 94.Engber D What models eat. Nature medicine. 2018;24:692–695. [DOI] [PubMed] [Google Scholar]
- 95.Warden CH and Fisler JS. Comparisons of diets used in animal models of high-fat feeding. Cell metabolism. 2008;7:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L and DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. American journal of hypertension. 2008;21:1288–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


