Abstract
During ocular development, periocular neural crest cells (pNC) migrate into the region between the lens and presumptive corneal epithelium to form the corneal endothelium and stromal keratocytes. Although defects in neural crest cell development are associated with ocular dysgenesis, very little is known about the molecular mechanisms involved in this process. This study focuses on the corneal endothelium, a monolayer of specialized cells that are essential for maintaining normal hydration and transparency of the cornea. In avians, corneal endothelial cells are first to be specified from the pNC during their migration into the presumptive corneal region. To investigate the signals required for formation of the corneal endothelium, we utilized orthotopic and heterotopic injections of dissociated quail pNC into chick ocular regions. We find that pNC are multipotent and that the nascent cornea is competent to induce differentiation of ectopically injected pNC into corneal endothelium. Injected pNC downregulate expression of multipotency transcription factors and upregulate genes that are consistent with ontogenesis of the chick corneal endothelium. Importantly, we showed that TGFβ2 is expressed by the nascent lens and the corneal endothelium, and that TGFβ signaling plays a critical role in changing the molecular signature of pNC in vitro. Collectively, our results demonstrate the significance of the ocular environmental cues towards pNC differentiation, and have potential implications for clinical application of stem cells in the anterior segment.
Keywords: Periocular neural crest, corneal development, corneal endothelium, TGFβ2
INTRODUCTION
The corneal endothelium is a monolayer that overlays the inner-most surface of the cornea and interfaces with the aqueous humor of the anterior chamber. Corneal endothelial cells contain Na+/K+-ATPase ionic pumps and aquaporin1 (AQP1) water channels that play a critical role in maintaining proper hydration and transparency of the cornea (Reneker et al., 2000; Huang et al., 2003; Fischbarg et al., 2006; Thiagarajah and Verkman, 2002). Defects in the corneal endothelium such as Fuchs endothelial dystrophy account for the majority of corneal transplants performed in the United States (Al-Yousuf et al., 2004). Previous regenerative studies using corneal endothelial-like cells derived from human embryonic stem cells (Zhang et al., 2014), or induced cornea-derived precursors (Hatou et al., 2012) demonstrated the feasibility to generate corneal endothelial cells in vitro and their ability to incorporate into wounded corneal endothelium and prevent stromal edema and clouding. While much has been learned about the potential for neural crest-like cells to generate the corneal endothelium, the mechanisms by which periocular neural crest cells (pNC) transdifferentiate into corneal endothelial cells have not been extensively investigated.
During ocular development, the corneal endothelium develops from pNC that migrate between the presumptive corneal epithelium and lens. In avians, reptiles, and primates, this process occurs as the first wave of pNC migration, which results in the formation of a monolayer that consequently forms the corneal endothelium (Hay and Revel, 1969; Hay, 1980). In rodents, cats, cows, and pigs, the corneal endothelium forms by differentiation of the innermost layer of the presumptive corneal mesenchyme (Dublin, 1970; Pei and Rhodin, 1970; Hay, 1980). Earlier studies in chick embryos showed that interactions between the lens vesicle and the pNC are critical for the proper formation of the corneal endothelium. Lens ablation prior to corneal development resulted in aberrant migration of the periocular mesenchyme into the presumptive cornea environment and malformation of the corneal endothelium (Genis-Galvez, 1966; Beebe and Coats, 2000). We later identified that SEMA3A/NRP1 signaling plays an important role in regulating pNC migration during corneal development, given that pharmacological inhibition of SEMA3A signaling from the lens caused similar defects in pNC migration and corneal malformation (Lwigale and Bronner-Fraser, 2009). Combined, these studies suggested that pNC differentiation into corneal cells is regulated spatially and temporally by signals from the developing ocular environment.
Studies utilizing knockout mice provided additional insight into the molecular mechanisms involved in pNC development. These studies showed that upon migration into the periocular region, pNC respond to retinoic acid (RA) signaling from the optic cup (Matt et al., 2005; Mic et al., 2004; Molotkov et al., 2006), Wnt signaling from the optic cup and presumptive corneal epithelium (Jin et al., 2002; Fujimura, 2016), and TGFβ signaling from the lens vesicle (Ittner et al., 2005; Saika et al., 2001; Flügel-Koch et al., 2002). Combined, these signaling events regulate the expression of transcription factors such as Pitx2 and Foxc1 by the pNC (Gage et al., 2005, 2005; Ittner et al., 2005), which have been shown to play a critical role in the development of the anterior segment. Both Pitx2 and Foxc1 knockout mice have severe ocular defects (Kume et al., 1998; Lu et al., 1999; Evans and Gage, 2005) and phenocopy Axenfeld-Rieger syndrome, a human anterior segment disorder (ASD) that is characterized by multiple defects in neural crest-derived ocular tissues including the cornea (Semina et al., 1996; Tümer and Buch-Holm, 2009). Peters anomaly is another ASD in which corneal development is disrupted through defective function of transcription factors such as Pitx3 and Foxe3, which are expressed in the lens during ocular development (Semina et al., 1998; Blixt et al., 2000; Ormestad et al., 2002). The above studies identified the major signaling pathways and some of the associated transcription factors involved in early ocular development. However, despite the adverse malformations in the anterior segment, the pNC in these mutants migrate and in most cases, differentiate into appropriate ocular tissues. Thus, it still remains unclear how pNC differentiate into specific ocular cell types including the corneal endothelium and keratocytes.
In this study, we focused on the formation of the avian corneal endothelium, which serves as an excellent model for studying early differentiation of pNC during ocular development, given that it is derived from the initial cell migration into the presumptive corneal region. In addition, these migratory pNC form a distinct monolayer that is useful for assaying cell differentiation. We demonstrate that the nascent corneal environment influences pNC differentiation and identify novel changes in gene expression during normal and perturbed development. Our findings suggest that TGFβ signaling in the nascent corneal environment plays a critical role in controlling pNC differentiation by regulating the expression of some multipotency transcription factors and corneal endothelial genes.
METHODS
Animals
Fertilized White Leghorn chick (Gallus gallus domesticus) and quail (Coturnix coturnix japonica) eggs were obtained from commercial sources (Texas A&M and Ozark Egg Company, respectively). All eggs were incubated at 38°C under humidified conditions. Chick eggs were incubated for 3, 5, and 7 days to obtain embryonic day (E)3, E5 and E7. Quail eggs were incubated for 60 hours to obtain E2.5 embryos from which periocular mesenchyme was dissected. Chick embryos were prepared for cell injections at different timepoints as previously described by (Spurlin and Lwigale, 2013). All procedures on the embryos were carried out following protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Rice University.
Isolation and microinjection of pNC
Quail embryos were collected at E2.5 and placed in Ringer’s solution. The anterior eyes including the surrounding periocular mesenchyme were dissected and incubated in 1.5 μg/ml dispase (Worthington) for 5–10 minutes at 38°C. Following dispase treatment, the anterior eyes were rinsed in Ringer’s solution with 0.1% w/v bovine serum albumin (0.1% BSA) to inactivate the enzyme. The surface ectoderm, the lens vesicles, and the optic cups were physically separated using fine forceps. Periocular mesenchyme pooled from approximately 50 anterior eyes was mechanically dissociated by gentle trituration in 10 μl of Ringer’s solution. Dissociated cells were concentrated into a pellet and then resuspended in 5 μl of Ringer’s solution. The cells were filled in a pulled glass needle and microinjected using a Picospritzer III (Parker Hannifin) into the desired ocular regions of E3 and E5 chick embryos at approximately 3×104 and 1.5×105 cells, respectively. Injected chick eggs were sealed and re-incubated, then collected for further analysis at E5 and E7.
Lens Ablations
Lens ablations were performed on E3 chick embryos as previously described (Beebe and Coats, 2000; Lwigale and Bronner-Fraser, 2007). Briefly, eggs were windowed and the vitelline membrane and amnion were dissected from the head region to expose the right eye. An incision was made in the surface ectoderm in the ventral region of the eye between the lens and optic cup, and the lens vesicles were gently removed through the slit using fine forceps. The eggs were sealed and re-incubated for 2 days, then collected at E5 for gene expression analysis. The left unablated eyes served as control.
Collection of tissues for RNA isolation
Periocular mesenchyme was isolated from E2.5 chick embryos as described above and pooled in a 1.5 ml Eppendorf tube for RNA isolation. To obtain the monolayers of corneal endothelium, anterior eyes were dissected from E5 chick embryos and the lenses were removed and discarded. The presumptive corneas consisting of the corneal endothelium and epithelium were dissected from the surrounding mesenchyme and optic cup, then incubated in 1.5 μg/ml dispase for 5–10 minutes at 38°C. Following dispase treatment, the presumptive corneas were rinsed in 0.1% BSA, and the epithelial layers were removed using fine forceps. The isolated corneal endothelium layers were pooled in a 1.5 ml Eppendorf tube for RNA isolation.
Primary culture of dissociated pNC
Dissociated E2.5 chick pNC were initially cultured in complete media consisting of Dubeccòs modified eagle medium (DMEM, Corning) + 100 μg/ml Penicillin-Streptomycin (Gibco) + 10% fetal bovine serum (FBS, Gibco) at a density of 2.1×104 cells per cm2 in 6-well plates, and allowed to attach for two hours. Cell cultures were rinsed with minimal DMEM (DMEM), then cultured for 18 hours in DMEM alone (control), or supplemented with 50 ng/μl active recombinant human (rh) TGFβ2 (Abcam). At the end of the cell culture, plates were rinsed twice with phosphate buffered saline (PBS, Gibco) prior to RNA isolation.
Quantitative RT-PCR (qRT-PCR)
Total RNA from chick E2.5 pNC, E5 corneal endothelium, or primary cell cultures were extracted using RNAeasy MicroKit (Qiagen) in accordance with the manufacturer’s protocol. The cDNA was reverse transcribed from 100 ng of RNA using Super Script III Reverse transcriptase kit (Invitrogen). Real-time PCR was performed in triplicate using Power SYBR Green PCR Master Mix (Applied Biosystems) on StepOnePlus Real-Time PCR System (Applied Biosystems). GAPDH transcript was used to normalize all genes. Fold change was calculated as the ΔΔCt relative to mRNA expression by pNC or untreated cell cultures. Primer sequences used for qRT-PCR are listed in Supplementary Table 1.
Section in situ hybridization
Decapitated E3 chick heads or enucleated eyeballs from E5 and E7 embryos were fixed overnight at 4°C in modified Carnoy’s solution (60% ethanol, 30% formaldehyde, and 10% glacial acetic acid). Samples were embedded in paraffin blocks and sectioned at 10–12 μm. Section in situ hybridization procedures were performed as previously described (Etchevers et al., 2001). Riboprobes for (HEY1, MSX2, LMO4, PITX2, RALDH2, RHOB, SNAI1, TGFβ2, and WNT9A) were synthesized using cDNA sequences reported in NCBI. Primer sequences for generating the probes are listed in Supplementary Table 2. Sections from embryos injected with quail pNC were further immunostained with anti-QCPN antibody after in situ hybridization.
Immunofluorescence staining
Whole heads or enucleated eyeballs were fixed overnight in 4% paraformaldehyde at 4°C. Fluorescent immunostaining was performed on wholemount heads and trimmed corneas, or on 8–10 μm cryosections as described (Lwigale et al., 2005). The mouse anti-QCPN antibody (IgG1, Hybridoma Bank, Iowa City, IA. [DHSB]) was used at 1:1 dilution to label quail nuclei. The rat anti-N-cadherin antibody (IgG1, DHSB) was used at 1:3 dilution to label the corneal endothelium. Signals were visualized using the following secondary antibodies at 1:200 dilution: Alexa Fluor 488 goat anti-mouse IgG1, Alexa Fluor 594 goat anti-mouse IgG1, and Alexa Fluor 488 goat anti-rat IgG. Sections were counterstained with 4,6-Diamidino-2-phenylindole (DAPI) to label all nuclei.
Imaging
Fluorescent and brightfield images of all wholemount and sectioned samples were acquired with a Zeiss Axiocam camera mounted on an AxioImager2 fluorescent microscope with ApoTome (Carl Zeiss AG, Oberkochen, Germany). Image processing was performed using Adobe Photoshop and Image J software.
RESULTS
Orthotopically transplanted quail pNC migrate and differentiate into corneal endothelium
Unlike most quail/chick chimera studies that track neural crest cell differentiation after xenotransplantation of dorsal neural tubes, this study utilizes dissociated quail pNC injected into various ocular regions of developing chick embryos, and tracked at different stages of ocular development using a quail nuclei-specific antigen (QCPN). This approach allowed us to perform precise cell grafts into the ocular region without disruption of the adjacent optic cup, lens vesicle, or the overlaying ectoderm. Given that this is the first time such cell grafts have been performed in the ocular environment, we initially examined whether the isolation, dissociation, and injection procedures (Fig.1A) affected the ability of injected pNC to migrate and differentiate. These experiments involved orthotopic injections of dissociated E2.5 quail pNC into the temporal region of E3 chick eyes (n = 5; Fig. 1B and 1C). Injected embryos were re-incubated and examined at E5, which corresponds with the formation of the corneal endothelium (Creuzet et al., 2005; Lwigale et al., 2005). At this time there was robust displacement of QCPN-positive cells into the periocular and presumptive corneal regions (Fig. 1D). Further analysis of the QCPN-positive cells localized in the corneal region indicated that they were positive for the corneal endothelial marker N-cadherin, and displayed expression levels noticeably similar to the endogenous (QCPN-negative) chick corneal endothelial cells (Fig. 1E). We found that 96% of QCPN-positive cells were NCAD-positive (Supplementary Fig. 3G). In addition, we observed from transverse sections through the eye that the QCPN-positive cells had migrated into the cornea region and formed a monolayer between the epithelium and lens (Fig. 1F), confirming that the injected pNC cells exhibited the characteristic behavior of the first wave of cell migration into the presumptive corneal region. Furthermore, we observed in embryos collected at later stages that such injections resulted in contribution of QCPN-positive cells to the various neural crest derived ocular tissues including the stromal keratocytes, iris stroma, and pericytes in the ocular blood vessels (Supplementary Fig. 1, Creuzet et al., 2005; Lwigale et al., 2005). Similar injections into the periocular region with GFP-labeled chick fibroblasts (DF-1 cells) showed limited migration from the periocular region into the presumptive corneal region (Supplementary Fig. 2), suggesting that pNC have specific characteristics that are required for proper migration and formation of the corneal endothelium. Combined, our results indicate that pNC cells are not disrupted by the isolation and injection procedures, and that they migrate and differentiate into the corneal endothelium within normal developmental timeframe. Therefore, the quail-chick pNC graft is an excellent model for testing the differentiation potential of pNC into the corneal endothelium under different spatiotemporal conditions.
Figure 1. Differentiation of E2.5 quail pNC following orthotopic graft into chick.
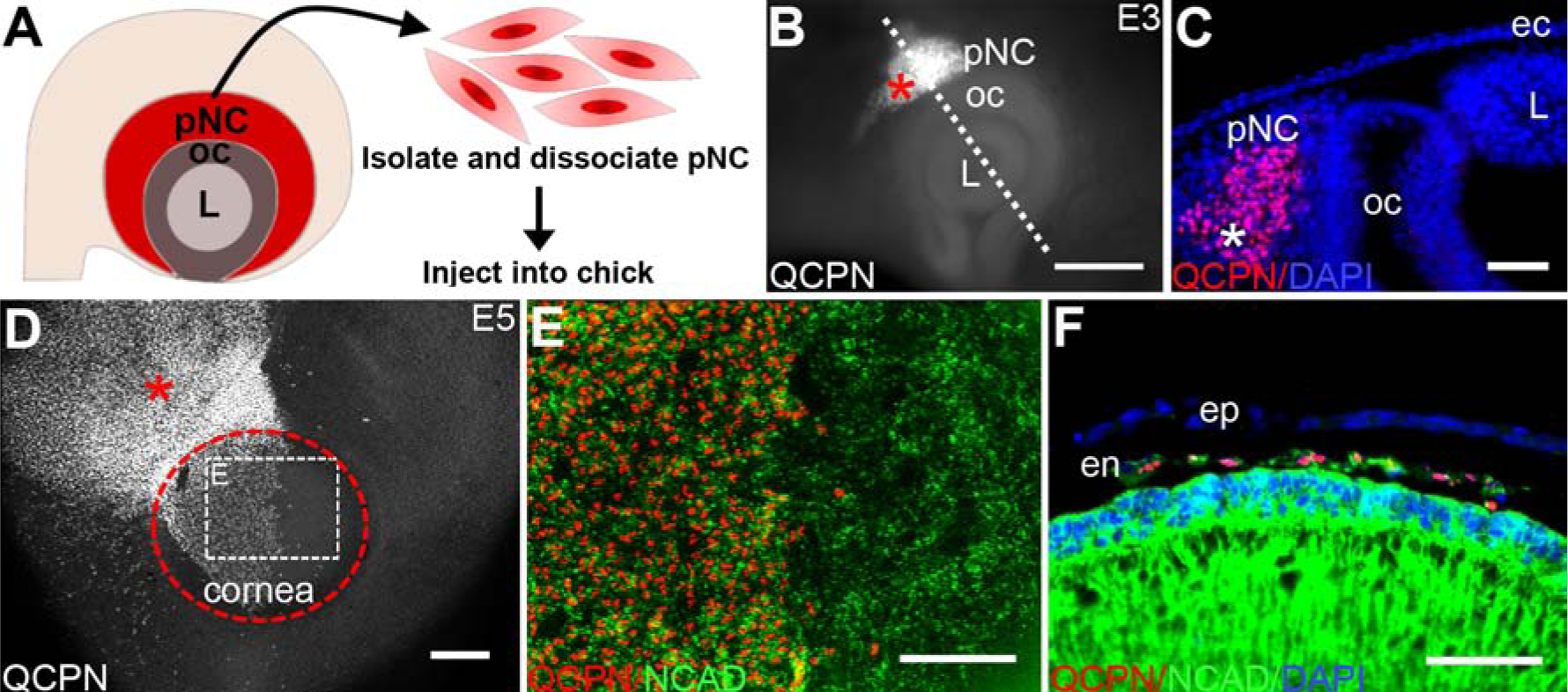
(A) Schematic overview of the isolation and dissociation procedures for obtaining quail pNC. (B) Wholemount immunostaining of an E3 chick eye showing QCPN-positive injected quail pNC (asterisk) in the periocular region. Dotted line represents the plane of cross-section in (C) showing localization of quail pNC (asterisk) within the periocular mesenchyme. (D) Wholemount immunostaining of an E5 chick eye showing the extent of migration QCPN-positive injected pNC (asterisk) in the periocular region and cornea (delineated by the dotted circle). (E) High magnification of selected region in (D) showing expression of NCAD by QCPN-positive cells and endogenous chick corneal endothelium. (F) Cross-section though the anterior eye of E5 injected embryo showing a monolayer of QCPN-positive cells co-expressing NCAD. ec, ectoderm; en, corneal endothelium; ep, corneal epithelium; L, lens; oc, optic cup. Scale bars represent 100 μm (B, D, E, F); 50 μm (C).
The ability to induce pNC differentiation into corneal endothelium is maintained by the presumptive corneal region during early ocular development
Disruption of the timing of the first wave of pNC migration during avian ocular development, which normally occurs at about E4.5, causes malformation of the corneal endothelium (Beebe and Coats, 2000; Lwigale and Bronner-Fraser, 2009). To determine whether the environmental cues required for the formation of the corneal endothelium are tightly regulated, we injected pNC into in the presumptive corneal region at different time points during early ocular development. The first set of experiments involved injections between the ectoderm and lens vesicle of E3 chick embryos, prior to endogenous pNC migration (n = 5; Fig. 2A and 2B, asterisks). Analysis of the injected eyes one day preceding the normal formation of the corneal endothelium at E4, showed precocious expression of N-cadherin by approximately 60% of the QCPN-positive cells whereas the un-injected control cornea showed no expression at this time (Fig. 2C and 2D, Supplementary Fig. 3). By E5, there was robust coverage of the entire corneal region by QCPN-positive cells also positive for N-Cadherin (n = 5; Fig. 2E and 2F). Cross sections through injected eyes showed that the QCPN- and N-cadherin-positive cells organized to form a monolayer between the epithelium and lens (Fig. 2G). Suggesting that the presumptive corneal environment is conducive for the proper differentiation of pNC into corneal endothelium between E3–E4.
Figure 2. pNC injected directly into the presumptive corneal region at different timepoints of development differentiate into corneal endothelial cells.
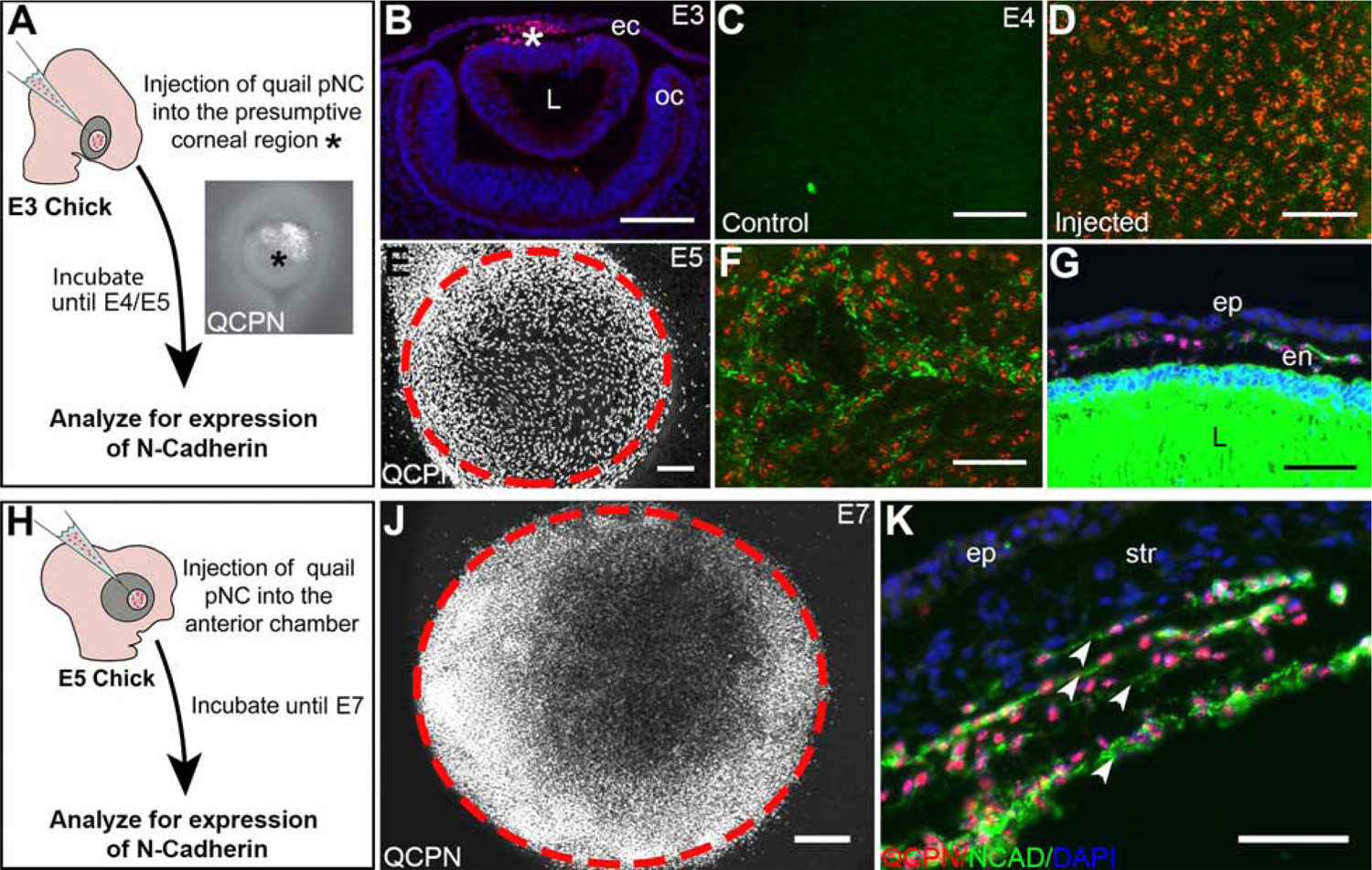
(A) Schematic overview of the injection of pNC into E3 presumptive corneal region (asterisk) and further experimental procedures. (B) cross-section through E3 eye, showing QCPN-positive pNC (asterisk) in the presumptive corneal region between the ectoderm and lens. (C) Wholemount immunostaining of an E4 chick eye showing no NCAD expression in the presumptive corneal region prior to the pNC migration comparing to (D) Wholemount immunostaining of an E4 chick eye showing the NCAD expression surrounding some of the QCPN-positive cells 1 day post-injection at E3. (E) Wholemount immunostaining of an E5 chick eye showing the localization QCPN-positive pNC in the cornea (delineated by the dotted circle) and surrounding region 2 days post injection at E3. (F) Injected pNC stain positive for NCAD at E5. (G) Cross-section of an E5 eye showing that the injected pNC form an NCAD-positive monolayered corneal endothelium. (H) Schematic overview of the injection of the pNC into E5 presumptive cornea and further experimental procedures. (J) Wholemount immunostaining of an E7 chick eye showing the localization QCPN-positive pNC in the cornea (delineated by the cells and dotted circle) following 2 days post injection at E5. (K) Cross-section through E7 eye, showing that injected cells form multiple layers of QCPN- and NCAD-positive cells (arrowheads). ec, ectoderm; en, corneal endothelium; ep, corneal epithelium; L, lens; oc, optic cup; str, corneal stroma. Scale bars represent 100 μm (B,E); 50 μm (C, D, F, G, J, K).
In the second set of experiments, we tested the ability of the corneal environment to induce injected pNC after the formation of the endogenous corneal endothelium. In this case, E5 chick eyes were injected with quail pNC then examined at E7 (n = 5; Fig. 2H and 2J). Analysis of E7 wholemount corneas indicated that almost all the QCPN-positive cells (99%) stained positive for N-cadherin (n = 5; Supplementary Fig. 3). Transverse sections through the injected eyes revealed that the QCPN-positive cells were arranged in supernumerary N-cadherin-positive monolayers, and each resembled the endogenous corneal endothelium (Fig. 2K, arrowheads). Therefore, the E5 presumptive corneal environment retains the ability to induce E3 pNC to form the corneal endothelium. Combined, our results indicate that the presumptive corneal environment can induce differentiation of injected undifferentiated E2.5 quail pNC to form corneal endothelium at multiple time points during ocular development between E3 and E7.
pNC-specific genes are downregulated following ectopic injection into the presumptive corneal region
Cranial neural crest cells express several genes during their migration into the various embryonic locations (Simoes-Costa et al., 2014). Our recent transcriptomic analysis of gene expression during corneal development identified that several of these genes including LMO4, MSX2, and SNAI1 where expressed during neural crest cell aggregation in the periocular region, and that some non-neural crest genes such as HEY1 were also expressed by pNC (Bi and Lwigale, 2019; Ma and Lwigale, 2019). To determine whether pNC change gene expression during differentiation into corneal endothelium, we first examined the expression of the above transcription factors by performing in situ hybridization on corneal sections at E3 and E5. Our results confirmed that HEY1, LMO4, MSX2, and SNAI1 were all strongly expressed in the periocular region at E3 (Fig. 3A, 3D, 3G, and 3J). In contrast, these genes were undetectable in the corneal endothelium at E5 (Fig. 3B, 3E, 3H, and 3K; arrowheads), although their expression is maintained in the periocular region (arrows). These results indicate that the above genes are downregulated during pNC differentiation into corneal endothelial cells.
Figure 3. Expression of pNC-related genes during normal development of the corneal endothelium and by the injected pNC.
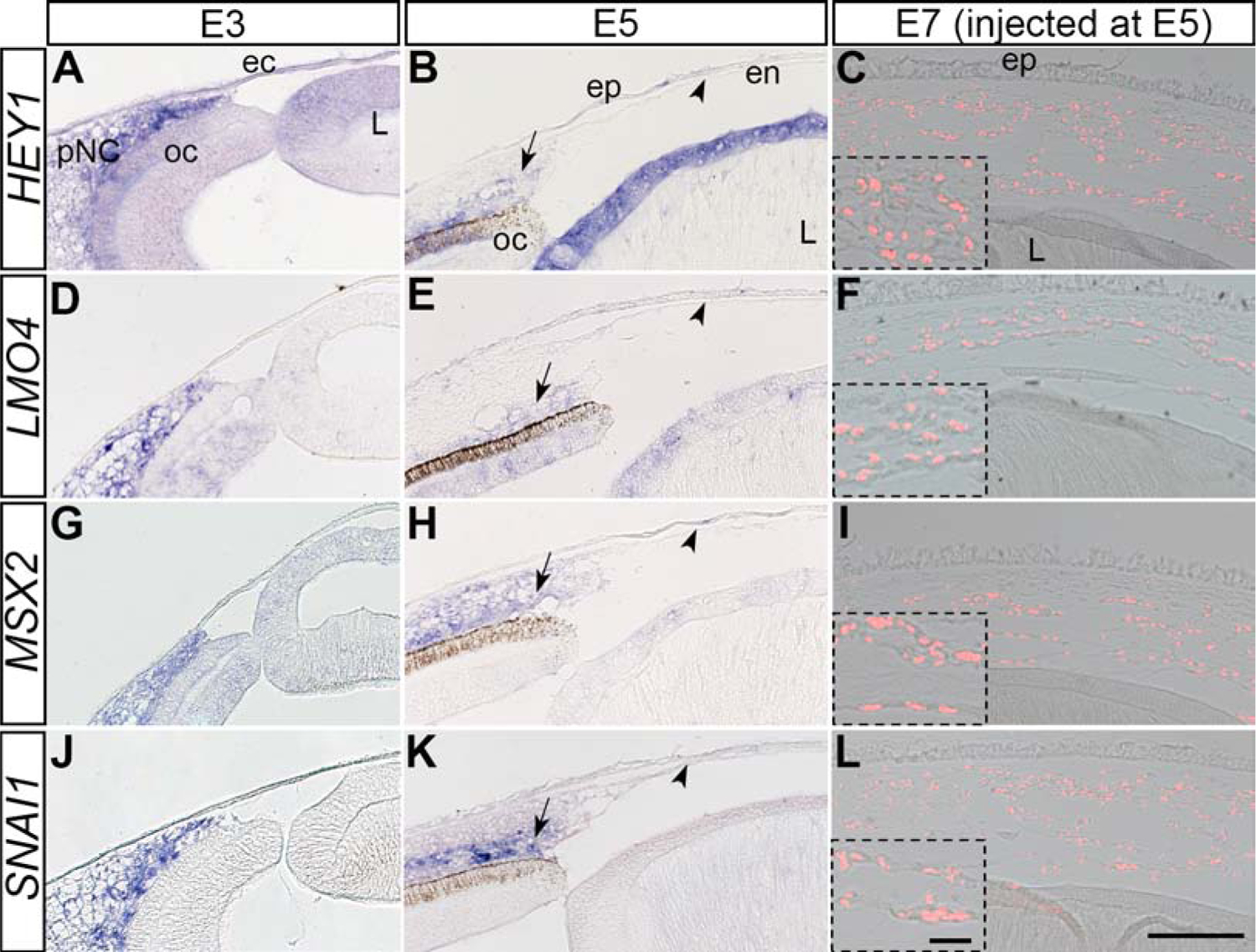
Section in situ hybridization was performed on E3, E5, and E7 injected eyes. (A, D, G, J) E3 chick eyes showing the localization of HEY1, LMO4, MSX2 and SNAI1 in the periocular region. (B, E, H, K) E5 chick eyes showing no detection of HEY1, LMO4, MSX2 and SNAI1 expression by the corneal endothelium (arrowheads), despite their expression in other ocular regions. (C, F, I, L) E7 chick eyes showing no detection of HEY1, LMO4, MSX2 and SNAI1 expression by QCPN-positive cells 2 days post injection. Inserts are higher magnifications of selected regions. ec, ectoderm; en, corneal endothelium; ep, corneal epithelium; L, lens; oc, optic cup; str, corneal stroma. Scale bars represent 100 μm (A-L) and the scale bars for the inserts represent 20 μm (C, F, I, L).
Given that E2.5 pNC injected into the presumptive corneal region at E5 transformed into N-cadherin-positive corneal endothelium, we investigated whether such injections caused similar downregulation in the expression of pNC genes. Our results reveal that the QCPN-positive pNC cells downregulated expression of HEY1, LMO4, MSX2, and SNAI1 following direct injection into the presumptive corneal region (Fig. 3C, 3F, 3I, 3L, and insets) although their expression was maintained in other ocular tissues at E7 (Supplementary Fig. 4). Combined, these results suggest a molecular transformation of pNC during early corneal development, and that the signals responsible for downregulation of transcription factors expressed by migratory cranial neural crest and pNC may reside within the presumptive corneal region.
pNC grafted into the presumptive corneal region upregulate early corneal endothelial genes
Next, we wanted to determine whether downregulation of the pNC genes by the injected cells corresponds with upregulation of corneal endothelial genes. In our recent study, we showed that several genes including (RALDH2, RHOB, TGFβ2, and WNT9A) were upregulated during formation of the chick corneal endothelium (Bi and Lwigale, 2019). Our section in situ hybridization results showed that none of the above genes were detectable in the periocular region at E3 (Fig. 4A, 4G, and 4J) except for RHOB, which was diffusely expressed in the mesenchyme, optic cup and lens (Fig. 4D). We confirmed that all the above genes were strongly expressed by the corneal endothelium at E5 (Fig. 4B, 4E, 4H, and 4K; arrowheads). Likewise, we observed induced expression of all the above genes in QCPN-positive pNC that were injected into the presumptive corneal region at E5 and analyzed at E7 (Fig. 4C, 4F, 4I, 4L, and insets). Thus, the presumptive corneal environment at E5 plays a role in inducing a molecular shift in gene expression of grafted E2.5 pNC towards the corneal endothelium lineage.
Figure 4. Expression of corneal endothelium genes during normal ocular development and by the injected pNC.
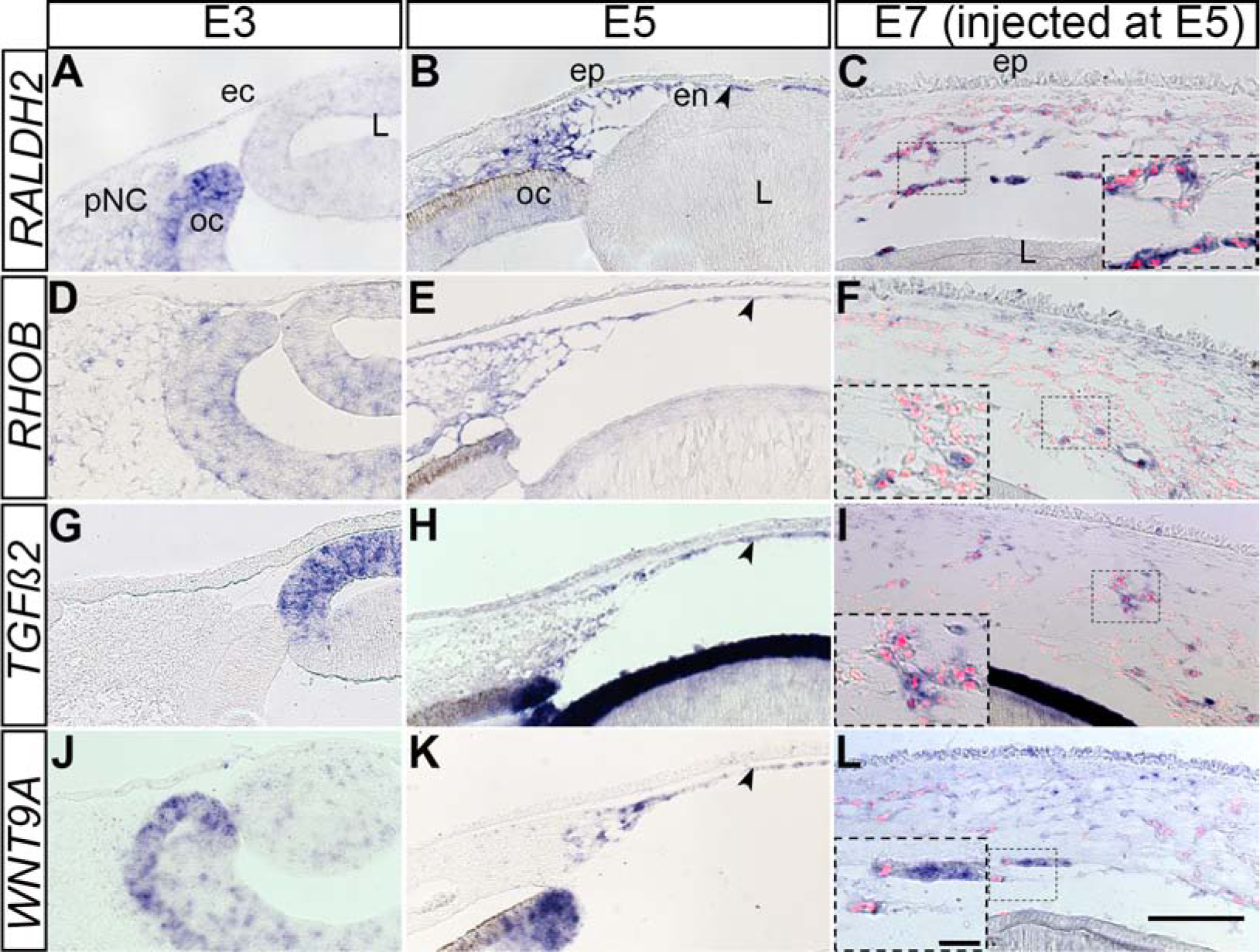
Section in situ hybridization was performed on E3, E5, and E7 injected eyes. (A, D, G, J) E3 chick eyes showing little or no expression of RALDH2, RHOB, TGFβ2 and WNT9A in the periocular region. (B, E, H, K) E5 chick eyes robust expression of RALDH2, RHOB, TGFβ2 and WNT9A by the corneal endothelium (arrowheads). (C, F, I, L) E7 chick eyes showing strong expression of expression of RALDH2, RHOB, TGFβ2 and WNT9A by QCPN-positive cells 2 days post injection. Inserts are higher magnifications of selected regions. ec, ectoderm; en, corneal endothelium; ep, corneal epithelium; L, lens; oc, optic cup; str, corneal stroma. Scale bars represent 100 μm (A-L) and the scale bars for the inserts represent 20 μm (C, F, I, L).
Role of the lens in directing pNC differentiation into corneal endothelium
During formation of the corneal endothelium, migratory pNC are localized in close proximity to the lens epithelium. Previous studies showed that lens ablation in chick prior to formation of the cornea resulted in precocious migration of pNC into the presumptive corneal region, which disrupted their differentiation into corneal endothelium and stromal keratocytes (Beebe and Coats, 2000; Lwigale and Bronner-Fraser, 2009). These observations imply that signals from the lens are critical for pNC migration and differentiation. To determine whether lens signals are required for the changes in the molecular signature of the injected pNC (Fig. 3 and Fig. 4), we performed in situ hybridization on corneal sections of malformed E5 eyes from which the lens vesicles had been ablated at E3. First, we examined the expression of pNC genes and observed that with the exception of HEY1, which was undetectable (Fig. 5A), LMO4, MSX2, and SNAI1 were all expressed in the ectopic mesenchyme of the malformed cornea (Fig. 5B, 5C, and 5D). This result indicates that downregulation of HEY1 during corneal development is independent of signals from the lens. Ectopic expression of LMO4, MSX2, and SNAI1 indicate that their downregulation in the cornea is dependent on signals from the lens.
Figure 5. Expression patterns of pNC- and corneal endothelium-related genes in the mesenchyme of malformed corneas following lens-ablation.
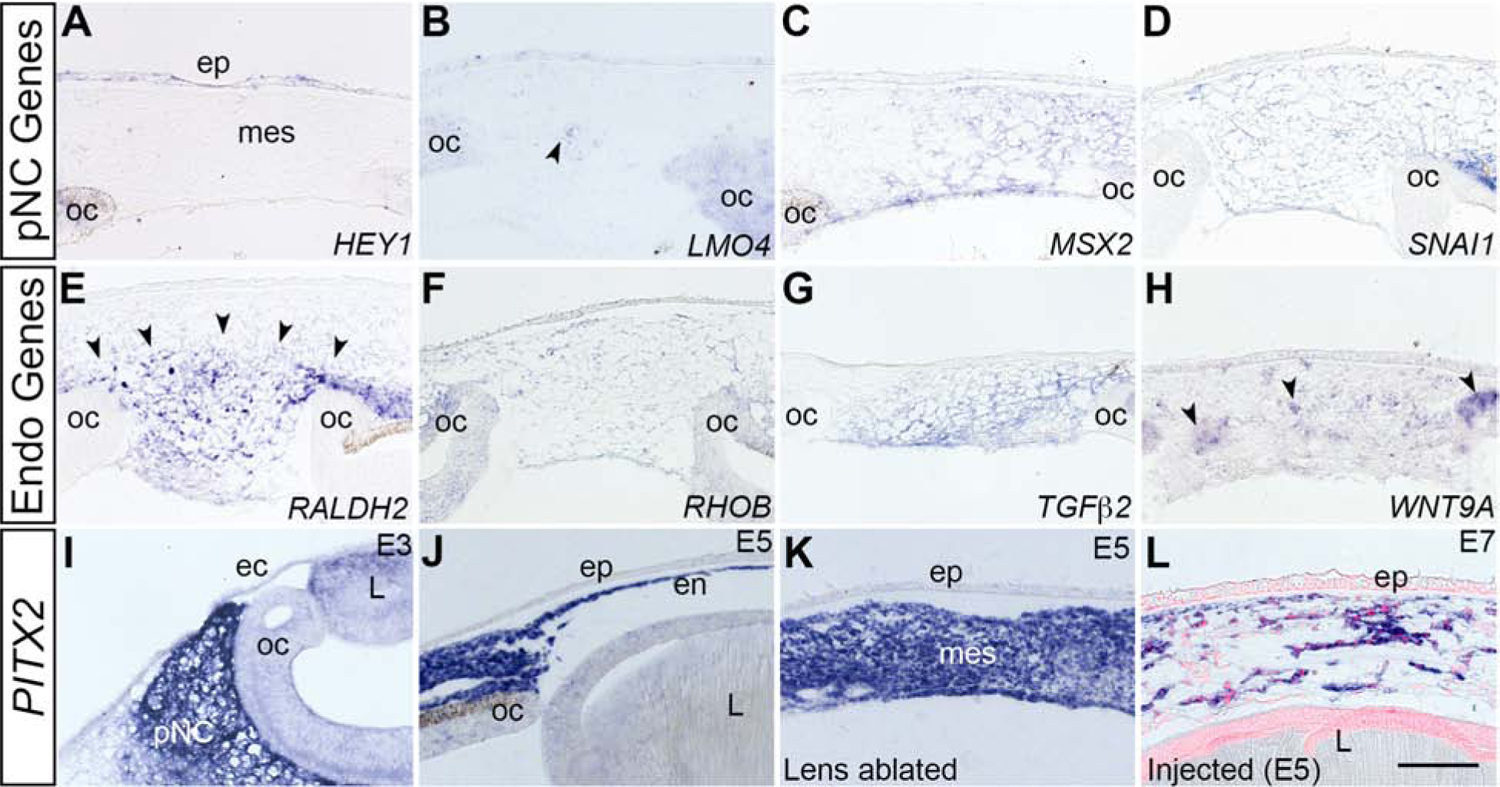
Lens ablations were performed at E3 and section in situ hybridization was performed at E5. (A-D) pNC-related genes show different patterns of expression in mesenchyme: (A) HEY1 is not detected. (B) LMO4 is expressed by a few cells (arrowheads). (C-D) MSX2 and SNAI1 are robustly expressed. (E-H) Corneal endothelium-related genes (RALDH2, RHOB, TGFβ2 and WNT9A) are all expressed in mesenchyme (RALDH2, arrowheads), (WNT9A, arrowheads), (I-L) PITX2 shows expression in the pNC, the endothelium, the ablated mesenchyme and in the injected cells in stages E3–E7. ec, surface ectoderm; ep, corneal epithelium, L, lens; mes, mesenchyme; oc, optic cup; pNC, periocular neural crest cells; str, stroma. Scale bar represents 100 μm (A-H).
Next, we analyzed the expression of corneal endothelial genes following lens ablation. To our surprise, we observed different patterns of expression of all genes in the ectopic corneal mesenchyme at E5 (Fig. 5E–5H). RALDH2 was broadly expressed by the ectopic mesenchyme that filled the void where the lens would normally reside (Fig. 5E; arrowheads). RHOB and TGFβ2 were diffusely expressed throughout the mesenchyme (Fig. 5F and 5G), whereas WNT9A was expressed in clusters of cells spread throughout the mesenchyme (Fig. 5H; arrowheads). We also analyzed the expression of PITX2, a transcription factor that was previously shown to be robustly expressed during mouse ocular development, and its absence causes sever ocular defects (Gage et al., 1999; Lu et al., 1999). Our results show that similar to mouse, PITX2 is strongly expressed in the chick pNC (Fig. 5I), and it is maintained during development of the corneal endothelium (Fig. 5J) and stromal keratocytes (Supplementary Fig. 5). Interestingly, analysis of corneal sections from E5 lens ablated eyes revealed that robust expression of PITX2 was maintained in the mesenchyme of the malformed corneas (Fig. 5K). We also observed that expression of PITX2 was maintained by the QCPN-positive cells injected as E2.5 pNC into the presumptive corneal region at E5, and examined at E7 (Fig. 5L). Our results indicate that lens ablation causes severe defects in morphogenesis of the corneal endothelium and disrupts the expression of corneal endothelial genes, but that the expression of PITX2 is unperturbed despite these defects. Since the corneal endothelial genes are also expressed in the periocular region at E5 (Fig. 4B, 4E, 4H, and 4K), and that considerably more cells are positive than what would normally form a monolayer, it is likely that their expression is induced in the pNC by signals from the optic cup prior to their ectopic migration into the malformed corneas.
Quantitative evaluation of pNC and corneal endothelial genes.
Our section in situ hybridization results showed localized expression of sets genes in the periocular mesenchyme and during development of the corneal endothelium (Fig. 3 and Fig. 4). Thus, we first undertook qPCR analysis to quantify the in vivo expression of pNC and corneal endothelial genes. Our results revealed that genes associated with the pNC (MSX2, SNAI1) were decreased, whereas those associated with corneal endothelial differentiation (RALDH2, TGFβ2, WNT9A, NCAD, and AQP1) were increased (Fig. 6A). We also observed that the genes that were expressed at both stages of neural crest development (RHOB and PITX2) showed the least increase in the corneal endothelium.
Figure 6. Molecular mechanisms of pNC differentiation into the corneal endothelium.
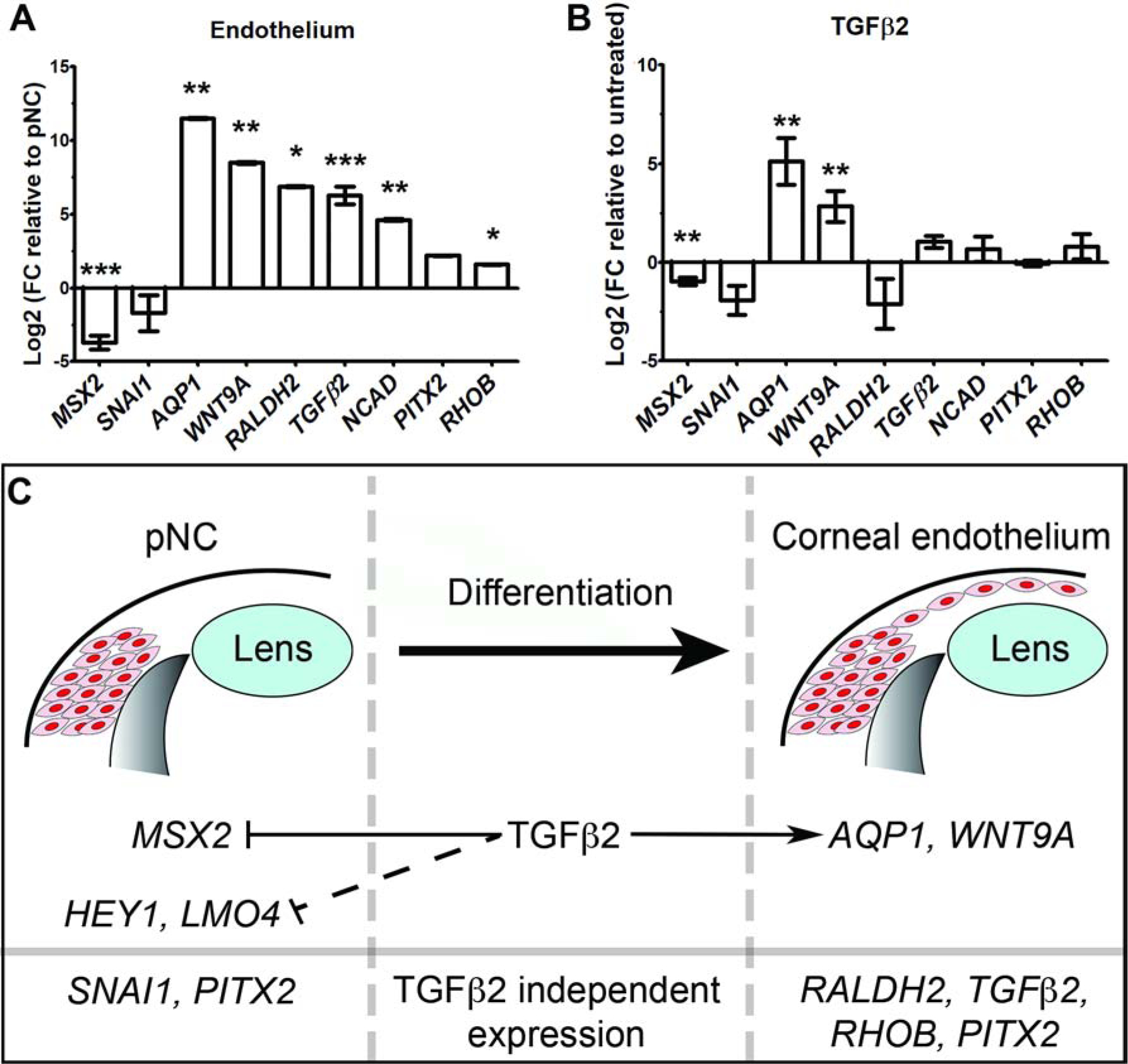
(A-B) Comparative qPCR analysis of pNC and endothelial mRNA expression under different conditions. GAPDH was used as reference gene. Independent sample replicates n=3. *P<0.05, ** P<0.01, *** P<0.001 (Welch`s two-tailed t-test). Data represented as mean ± s.d. (A) Fold change (FC) of corneal endothelial genes compared to E2.5 pNC (baseline). (B) Fold change in gene expression of TGFβ2-treated pNC compared to untreated control culture (baseline). (C) Schematic representation of the regulation of pNC and corneal endothelial genes by TGFβ2 signaling.
Given that disruption of TGFβ signaling from the lens prevents formation of the corneal endothelium in mouse (Saika et al., 2001; Flugel-Koch et al., 2002), and that TGFβ2 is vividly expressed by the lens epithelium and during development of the corneal endothelium (Fig. 4G and 4F), we examined the its role in the changes of gene expression observed during pNC differentiation. Our qPCR analysis of pNC cultured with or without TGFβ2 showed significant reduction in the expression of MSX2 and increase in AQP1 and WNT9A (Fig. 6B). Expression of SNAI1, RALDH2, TGFβ2, NCAD, PITX2, and RHOB remained mostly unchanged. These results suggest that TGFβ2 signaling is required for downregulation of some multipotency genes and specification pNC towards a corneal endothelial lineage.
DISCUSSION
During early development, a population of cranial neural crest cells migrates into the periocular region and subsequently give rise to various ocular anlage. Up to now, the molecular mechanisms involved in pNC differentiation are not clearly understood despite severe ocular malformation associated with cranial neural crest defects. Given that signals within the environment of the developing embryo play a critical role in altering neural crest cell fate (Anderson, 1997; Dorsky et al., 2000), we investigated the ability of the nascent corneal microenvironment to induce pNC differentiation, focusing on the formation of the avian corneal endothelium. Here, we have shown that pNC injected into the presumptive corneal region are induced to express N-cadherin and assemble into monolayers at various timepoints during ocular development. We found that during this process, injected pNC downregulate expression of transcription factors of the neural crest gene regulatory network (GRN) and upregulate genes that are consistent with ontogenesis of the chick corneal endothelium. Ocular expression of these genes in the presumptive cornea was disrupted in the absence of the lens. TGFβ2 was identified as a potential environmental cue that regulates the expression of some pNC and endothelial genes. From these data we concluded that pNC maintain the multipotential characteristics of migratory neural crest following their aggregation in the periocular region. These characteristics are lost during differentiation into corneal endothelial cells in response to TGFβ2 signaling.
Previous studies involving orthotopic grafts of quail dorsal neural tubes into stage-matched chick embryos showed that neural crest cells migrate into various cranial locations including the periocular region, where they give rise to multiple ocular tissues (Johnston et al., 1979; Creuzet et al., 2005; Lwigale et al., 2004; 2005). Using closely related approaches, we and others have demonstrated the potential of injected cells to differentiate into several neural crest-derived tissues. These experiments involved grafting of neural crest cells derived from human embryonic stem cells (Curchoe et al., 2010) or maintained in culture as “crestospheres” (Kerosuo et al., 2015), as well as injection of dissociated neural crest-derived corneal keratocytes (Lwigale et al., 2005). In this study, our approach involved injection of dissociated pNC into different ocular regions at various developmental timepoints. To our knowledge, this is the first time that the stem cell properties of dissociated pNC has been tested in the developing ocular environment. Therefore, we first established whether orthotopically injected pNC maintain their potential to contribute to various ocular tissues. Our analysis revealed that injected pNC are migratory, and capable of colonizing and contributing to characteristic ocular tissues including the corneal endothelium. These results also indicate that the E3 pNC are not intrinsically determined to give rise to a specific ocular tissue. Given that pNC express majority of the genes linked to cranial migratory neural crest (Simoes-Costa et al., 2014; Bi and Lwigale, 2019), and that their descendants isolated from the corneal stroma are capable of differentiating into various neural crest derivatives when grafted into the migratory path of cranial neural crest (Lwigale et al., 2005), it is very likely that they are multipotent and capable of differentiating into other tissues if they were grafted into other cranial regions.
In avians, the first tissue to differentiate from the periocular mesenchyme is the corneal endothelium, which is characterized by the formation of a monolayer of N-cadherin-positive cells between the lens vesicle and the presumptive corneal epithelium (Hay and Revel, 1969; Bard et al., 1975; Noden 1978; Beebe and Coats, 2000; Lwigale and Bronner-Fraser, 2009). Using N-cadherin as an early marker of pNC specification into corneal endothelial cells, we show that pNC injected directly into the presumptive cornea differentiate into monolayers of corneal endothelium. Our results also indicate that environmental cues that drive differentiation of pNC into corneal endothelium are present in the chick eye between E3 and E7. This process involves downregulation of neural crest GRN transcription factors LMO4, MSX2, and SNAI1, which are similarly downregulated when pNC are directly injected into the presumptive cornea. Sustained expression of these genes in the malformed lens-ablated corneas indicates that signals from the lens are involved in downregulation. However, we cannot rule out the possibility that their expression is maintained in the periocular region by signals from the optic cup, and their presence in the malformed corneal region is partly due to ectopic migration of pNC caused by the lens ablation. Previous studies showed that LMO4, MSX2, and SNAI1 function in maintaining multipotency of neural crest cells. LMO4 acts as a cofactor for SNAIL during early neural crest development (Ochoa et al., 2012; Ferronha et al., 2013). Similar downregulation of LMO4 expression was observed during neural crest differentiation in the mandibular process (Kenny et al., 1998). MSX2 is required for proper proliferation and differentiation of neural crest-derived cranial osteoblasts and teeth mesenchyme (Hu et al., 2001; Satokata et al., 2000; Han et al., 2007). Thus, lens-derived signals play an important role in the initial step of pNC differentiation by repressing the expression of genes required for maintaining multipotency.
We also observed downregulation of HEY1, a downstream effector of Notch signaling that is involved in maintaining neural precursor cells in the central nervous system (Sakamoto et al., 2003). Notch signaling is required for the survival of undifferentiated neural crest cells in vitro (Nikopoulos et al., 2009; Morrison et al., 2000), and it plays a role during cardiac neural crest cell differentiation (High et al., 2007). Expression of HEY1 by pNC could be involved in their contribution to vascular pericytes (Fischer et al., 2004; Sainson and Harris, 2008; Garcia-Quintans et al., 2016) or inhibition of their differentiation towards the ocular muscle lineage (Buas et al., 2010). Our lens-ablation results suggest that signals from the lens contribute to the induction or maintenance of HEY1 expression in the periocular region.
We found that the first wave of pNC migration into the presumptive corneal region coincided with the upregulation of RALDH2, RHOB, TGFβ2, WNT9A, and that these genes were induced when pNC were directly injected into the presumptive cornea. Suggesting that they are involved in the initial transformation of pNC into corneal endothelial cells. RALDH2 indicates potential RA signaling, which may be autocrine since several receptors including RARβ, RAYγ, and RXRα are expressed in the periocular mesenchyme during ocular development (Mori et al., 2001). Raldh2 mutant mice show abnormal distribution of cranial neural crest cells, but they are embryonic lethal prior to corneal development (Mic et al., 2004; Ribes, 2006). However, several studies have shown that RA signaling is important for proper formation of lens, optic cup and neural crest-derived ocular tissues (Matt et al., 2005). Expression of RHOB could indicate its involvement in cell adhesion and barrier formation required for mesenchyme-to-endothelial transformation (Liu et al., 2001; Adini et al., 2003; Wojciak-Stothard et al., 2012). WNT9A has been implicated in both canonical (Spater et al., 2006; Matsumoto et al., 2008; Grainger et al., 2016) and PCP pathways (Rochard et al., 2016). In the chick corneal endothelium, it is likely that this process is via the PCP pathway, since there is no nuclear localization of β-catenin despite its expression in the cell membranes at this time (unpublished observation).
We show that TGFβ2 is strongly expressed in the lens and corneal endothelium, and that its expression is upregulated in the injected pNC. Indicating that the lens induces TGFβ2 expression in the corneal endothelium. TGFβ signaling promotes neural crest cell specification into non-neural lineages that express smooth muscle actin in vitro (Shah et al 1996). Conditional knockout of TGFβ signaling in neural crest cells caused malformation of cranial bones, cardiovascular defects, and absence of smooth muscle actin (Wurdak et al., 2005). Specific disruption of TGFβ2 signaling causes similar developmental defects including severe malformation of the eye and cornea (Sanford et al. 1997). Whereas targeted knockout of Tgfβr2 in the neural crest cells (Ittner et al., 2005) or TGFβ2 in the lens (Saika et al., 2001) cause severe malformation of the cornea and absence of the corneal endothelium. Similar to mouse, TGFβ signaling is active in chick pNC during corneal development. Our recent analysis of gene expression during ocular development indicated that TGFBR1 and TGFBR2, as well as the downstream SMAD2 and SMAD3 are expressed during pNC differentiation into corneal cells (Bi and Lwigale, 2019). It is logical that similar mechanisms are involved TGFβ-mediated differentiation of pNC in chick and mouse, but the downstream targets during cell differentiation are not well understood. Here we show that TGFβ2 is upstream of MSX2, AQP1, and WNT9A during pNC differentiation. Developmental defects in mesoderm-derived supraoccipital bones in Tgfβr2 mutants were linked to downregulation of Msx2 (Hosokawa et al. 2007). However, in this study we discovered that TGFβ2 signaling downregulates MSX2 in pNC. Previous studies showed that Msx2 expression in cranial neural crest cells is mediated by BMP signaling (Brugger et al., 2004; Liu et al., 2005). This raises the possibility is that downregulation of MSX2 expression in the pNC could be due to direct response to TGFβ2, or indirectly via suppression of BMP signaling. Another study using Tgfβr2 mutants showed downregulation of Wnt9a expression during limb development (Spagnoli et al., 2007), suggesting that TGFβ signaling upregulates WNT9A. Our results link TGFβ signaling with AQP1 during ocular development and they are supported by an in vitro study that reported increased levels of AQP1 expression by alveolar epithelial cells following treatment with TGFβ (Galan-Cobo et al., 2018). Although AQP1 is important for the proper function of the adult cornea (Thiagarajah and Verkman, 2002), its role during early development remains unclear. A recent study indicated that AQP1 plays a role in cranial neural crest cell migration via FAK activity, increased turnover of integrinβ1, and degradation of the extracellular matrix (McLennan et al., 2019). There is a possibility that upregulation of AQP1 during the initial wave of pNC migration into the presumptive cornea may involve some of these activities.
Our current model (Fig. 6C) provides a foundation for understanding the molecular mechanisms involved in pNC differentiation into corneal endothelium. Our data suggest an important role of TGFβ2 in the downregulation of multipotency genes and upregulation of corneal endothelial genes. The genes that appear not to be directly regulated by TGFβ2 (PITX2, NCAD, RHOB) may require other growth factors from the optic cup and presumptive corneal epithelium. Both RA and TGFβ signaling are upstream of PITX2 during ocular development (Kumar and Duester, 2010; Ittner et al., 2005). PITX2 upregulates NCAD during gut morphogenesis (Kurpios et al., 2008; Plageman et al., 2011). This is unlikely the case in the chick cornea, since lens ablation abolishes N-cadherin expression (Beebe and Coats, 2000; Lwigale and Bronner-Fraser, 2009) although PITX2 is not affected. It would be of great interest to determine the ocular signals that regulate N-cadherin since its expression is consistent with the segregation of pNC that form the corneal endothelium from the periocular mesenchyme. It has been shown that integrin-mediated expression of Twist1 induces N-cadherin expression in human breast epithelial cells (Alexander et al., 2006). Similar process could be driven by a combination of signals from the extracellular matrix and Twist1 expressed in the periocular mesenchyme during corneal development (Bi and Lwigale, 2019; Ma and Lwigale, 2019).
Supplementary Material
KEY RESOURCES TABLE
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Mouse anti-quail cell antibody | DHSB Hybridoma Bank, Iowa City, IA. | QCPN |
| Rat anti-N-cadherin antibody | DHSB Hybridoma Bank, Iowa City, IA. | MNCD2 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dispase | Worthington | LS02104 |
| Dubecco’s modified eagle medium | Corning | 10-013-CV |
| Penicillin-Streptomycin | Gibco | 15140122 |
| Fetal bovine serum | Gibco | 26140079 |
| Recombinant human TGFβ2 | Abcam | Ab84070 |
| Oligonucleotides | ||
| Primers for qRT-PCR, see Table 1 | This paper; Shiau and Bronner-Fraser, 2010; Carré et al., 2011. | |
| Primers for generating riboprobes for in situ hybridization, see Table 2 | This paper; Bi and Lwigale, 2019. | |
Highlights.
Neural crest cells remain multipotent following their aggregation in the periocular region.
Presumptive corneal environment is sufficient to promote de novo formation of the corneal endothelium from injected periocular neural crest (pNC).
TGFβ2 is required for downregulation of some multipotency genes and promotes specification of pNC towards corneal endothelial lineage.
ACKNOWLEDGEMENTS
We would like to thank members of Lwigale lab for the helpful discussions and suggestions on this project. We would also like to thank Saad Ehsan for assistance with some of the experiments and the Warmflash lab for assistance with qPCR. This work was funded by National Institutes of Health Grant R21 EY027048 (to P.Y.L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
No competing interests to declare.
REFERENCES
- 1.Adini I, Rabinovitz I, Sun JF, Prendergast GC, and Benjamin LE, 2003. RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev. 17, 2721–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander NR, Tran NL, Rekapally H, Summers CE, Glackin C, and Heimark RL, 2006. N-cadherin Gene Expression in Prostate Carcinoma Is Modulated by Integrin-Dependent Nuclear Translocation of Twist1. Cancer Res. 66, 3365–3369. [DOI] [PubMed] [Google Scholar]
- 3.Al-Yousuf N, Mavrikakis I, Mavrikakis E, and Daya SM, 2004. Penetrating keratoplasty: indications over a 10 year period. Br. J. Ophthalmol 88, 998–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson DJ, 1997. Cellular and Molecular Biology of Neural Crest Lineage Determination. Trends Genet. 13, 276–280. [DOI] [PubMed] [Google Scholar]
- 5.Bard JBL, Hay ED, and Meller SM, 1975. Formation of the Endothelium of the Avian Cornea: A Study of Cell Movement in Vivo. Dev. Biol 42, 334–361. [DOI] [PubMed] [Google Scholar]
- 6.Beebe DC, and Coats JM, 2000. The Lens Organizes the Anterior Segment: Specification of Neural Crest Cell Differentiation in the Avian Eye. Dev. Biol 220, 424–431. [DOI] [PubMed] [Google Scholar]
- 7.Bi L, and Lwigale P, 2019. Transcriptomic analysis of differential gene expression during chick periocular neural crest differentiation into corneal cells. Dev. Dyn 248, 583–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blixt Å, Mahlapuu M, Aitola M, Pelto-Huikko M, and Carlsson P, 2000. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 14, 245–254. [PMC free article] [PubMed] [Google Scholar]
- 9.Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting M-C, Cho JY-M, Dobias SL, Yi SE, Lyons K, Bell JR, Arora K, Warrior R, and Maxson R, 2004. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development 131, 5153–5165. [DOI] [PubMed] [Google Scholar]
- 10.Buas MF, and Kadesch T, 2010. Regulation of skeletal myogenesis by Notch. Exp. Cell Res 316, 3028–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carré G-A, Couty I, Hennequet-Antier C, and Govoroun MS, 2011. Gene Expression Profiling Reveals New Potential Players of Gonad Differentiation in the Chicken Embryo. PLoS ONE 6, e23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creuzet S, Couly G, and Douarin NM, 2005. Patterning the neural crest derivatives during development of the vertebrate head: insights from avian studies. J. Anat 207, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curchoe CL, Maurer J, McKeown SJ, Cattarossi G, Cimadamore F, Nilbratt M, Snyder EY, Bronner-Fraser M, and Terskikh AV, 2010. Early Acquisition of Neural Crest Competence During hESCs Neuralization. PLoS ONE 5, e13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dublin I, 1970. [Comparative embryologic studies of the early development of the cornea and the pupillary membrane in reptiles, birds and mammals]. Acta Anat. (Basel) 76, 381–408. [PubMed] [Google Scholar]
- 15.Dorsky RI, Moon RT, and Raible DW, 2000. Environmental signals and cell fate specification in premigratory neural crest. BioEssays 22, 708–716. [DOI] [PubMed] [Google Scholar]
- 16.Etchevers HC, Vincent C, Le Douarin NM, and Couly GF, 2001. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128, 1059–1068. [DOI] [PubMed] [Google Scholar]
- 17.Evans AL, and Gage PJ, 2005. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum. Mol. Genet 14, 3347–3359. [DOI] [PubMed] [Google Scholar]
- 18.Ferronha T, Rabadan MA, Gil-Guinon E, Le Dreau G, de Torres C, and Marti E, 2013. LMO4 is an Essential Cofactor in the Snail2-Mediated Epithelial-to-Mesenchymal Transition of Neuroblastoma and Neural Crest Cells. J. Neurosci 33, 2773–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischbarg J, Diecke FPJ, Iserovich P, and Rubashkin A, 2006. The Role of the Tight Junction in Paracellular Fluid Transport across Corneal Endothelium. Electro-osmosis as a Driving Force. J. Membr. Biol 210, 117–130. [DOI] [PubMed] [Google Scholar]
- 20.Fischer A, Schumacher N, Maier M, Sendtner M, and Gessler M, 2004. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 18, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flügel-Koch C, Ohlmann A, Piatigorsky J, and Tamm ER, 2002. Disruption of anterior segment development by TGF-β1 overexpression in the eyes of transgenic mice. Dev. Dyn 225, 111–125. [DOI] [PubMed] [Google Scholar]
- 22.Fujimura N, 2016. WNT/β-Catenin Signaling in Vertebrate Eye Development. Front. Cell Dev. Biol 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gage PJ, Suh H, and Camper SA, 1999. Dosage requirement of Pitx2 for multiple organs. Development 126, 4643–4651. [DOI] [PubMed] [Google Scholar]
- 24.Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, and Thuret G, 2016. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 134, 167–173. [DOI] [PubMed] [Google Scholar]
- 25.Galán-Cobo A, Arellano-Orden E, Sánchez Silva R, López-Campos JL, Gutiérrez Rivera C, Gómez Izquierdo L, Suárez-Luna N, Molina-Molina M, Rodríguez Portal JA, and Echevarría M, 2018. The Expression of AQP1 IS Modified in Lung of Patients With Idiopathic Pulmonary Fibrosis: Addressing a Possible New Target. Front. Mol. Biosci 5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Quintans N, Sánchez-Ramos C, Prieto I, Tierrez A, Arza E, Alfranca A, Redondo JM, and Monsalve M, 2016. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1α-deficient mice. Angiogenesis 19, 217–228. [DOI] [PubMed] [Google Scholar]
- 27.Genis-Galvez JM, 1966. Role of the Lens in the Morphogenesis of the Iris and Cornea. Nature 210, 209–210. [DOI] [PubMed] [Google Scholar]
- 28.Grainger S, Richter J, Palazón RE, Pouget C, Lonquich B, Wirth S, Grassme KS, Herzog W, Swift MR, Weinstein BM, Traver D, and Willert K, 2016. Wnt9a Is Required for the Aortic Amplification of Nascent Hematopoietic Stem Cells. Cell Rep. 17, 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J, Ishii M, Bringas P, Maas RL, Maxson RE, and Chai Y, 2007. Concerted action of Msx1 and Msx2 in regulating cranial neural crest cell differentiation during frontal bone development. Mech. Dev 124, 729–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatou S, Yoshida S, Higa K, Miyashita H, Inagaki E, Okano H, Tsubota K, and Shimmura S, 2013. Functional Corneal Endothelium Derived from Corneal Stroma Stem Cells of Neural Crest Origin by Retinoic Acid and Wnt/β-Catenin Signaling. Stem Cells Dev. 22, 828–839. [DOI] [PubMed] [Google Scholar]
- 31.Hay ED, 1980. Development of the Vertebrate Cornea. Int. Rev.Cytol 63, 263–322. [DOI] [PubMed] [Google Scholar]
- 32.Hay ED, Revel JP, 1969. Fine structure of the developing avian cornea. Karger, Basel. Monographs in Developmental Biology 1, 1–144. [PubMed] [Google Scholar]
- 33.High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, and Epstein JA, 2007. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J. Clin. Invest 117, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosokawa R, Urata M, Han J, Zehnaly A, Bringas P, Nonaka K, and Chai Y, 2007. TGF-β mediated Msx2 expression controls occipital somites-derived caudal region of skull development. Dev. Biol 310, 140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu G, Lee H, Price SM, Shen MM, and Abate-Shen C, 2001. Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development 128, 2373–2384. [DOI] [PubMed] [Google Scholar]
- 36.Huang B, Blanco G, Mercer RW, Fleming T, and Pepose JS, 2003. Human Corneal Endothelial Cell Expression of Na+, K+-Adenosine Triphosphatase Isoforms. Arch. Ophthalmol 121, 840–845. [DOI] [PubMed] [Google Scholar]
- 37.Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, Hjalt TA, Suter U, Karlsson S, Hafezi F, Born W, and Sommer L, 2005. Compound developmental eye disorders following inactivation of TGFβ signaling in neural-crest stem cells. J. Biol 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin E-J, Burrus LW, and Erickson CA, 2002. The expression patterns of Wnts and their antagonists during avian eye development. Mech. Dev 116, 173–176. [DOI] [PubMed] [Google Scholar]
- 39.Johnston MC, Noden DM, Hazelton RD, Coulombre JL, and Coulombre AJ, 1979. Origins of avian ocular and periocular tissues. Exp. Eye Res 29, 27–43. [DOI] [PubMed] [Google Scholar]
- 40.Kenny DA, Jurata LW, Saga Y, and Gill GN, 1998. Identification and characterization of LMO4, an LMO gene with a novel pattern of expression during embryogenesis. Proc. Natl. Acad. Sci 95, 11257–11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerosuo L, Nie S, Bajpai R, and Bronner ME, 2015. Crestospheres: Long-Term Maintenance of Multipotent, Premigratory Neural Crest Stem Cells. Stem Cell Rep. 5, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar S and Duester G 2010. Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2. Dev Biol 340:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kume T, Deng K, and Hogan BLM, 2000. Forkhead genes in kidney and ureter development. Development 127, 1387–1395. [DOI] [PubMed] [Google Scholar]
- 44.Kurpios NA, Ibanes M, Davis NM, Lui W, Katz T, Martin JF, Belmonte JCI, and Tabin CJ, 2008. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc. Natl. Acad. Sci 105, 8499–8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu A-X, Rane N, Liu J-P, and Prendergast GC, 2001. RhoB Is Dispensable for Mouse Development, but It Modifies Susceptibility to Tumor Formation as Well as Cell Adhesion and Growth Factor Signaling in Transformed Cells. Mol. Cell. Biol 21, 6906–6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, and Martin JF, 2005. Threshold-specific requirements for Bmp4 in mandibular development. Dev. Biol 283, 282–293. [DOI] [PubMed] [Google Scholar]
- 47.Lu M-F, Pressman C, Dyer R, Johnson RL, and Martin JF, 1999. Function of Rieger syndrome gene in left–right asymmetry and craniofacial development. Nature 401, 276–278. [DOI] [PubMed] [Google Scholar]
- 48.Lwigale PY, Conrad GW, Bronner-Fraser M, 2004. Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis. Development 131, 1979–1991. [DOI] [PubMed] [Google Scholar]
- 49.Lwigale PY, and Bronner-Fraser M (2007). Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev. Biol 306, 750–759. [DOI] [PubMed] [Google Scholar]
- 50.Lwigale PY, and Bronner-Fraser M (2009). Semaphorin3A/neuropilin-1 signaling acts as a molecular switch regulating neural crest migration during cornea development. Dev. Biol 336, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lwigale PY, Cressy PA, and Bronner-Fraser M (2005). Corneal keratocytes retain neural crest progenitor cell properties. Dev. Biol 288, 284–293. [DOI] [PubMed] [Google Scholar]
- 52.Ma J, and Lwigale P, 2019. Transformation of the Transcriptomic Profile of Mouse Periocular Mesenchyme During Formation of the Embryonic Cornea. Investig. Opthalmology Vis. Sci 59, 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLennan R, McKinney MC, Teddy JM, Morrison JA, Kasemeier-Kulesa JC, Ridenour DA, Manthe CA, Giniunaite R, Robinson M, Baker RE, Maini PK, and Kulesa PM, online published 2019. Neural crest cells bulldoze through the microenvironment using Aquaporin-1 to stabilize filopodia. Development. doi: 10.1242/dev.185231. [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto K, Miki R, Nakayama M, Tatsumi N, and Yokouchi Y, 2008. Wnt9a secreted from the walls of hepatic sinusoids is essential for morphogenesis, proliferation, and glycogen accumulation of chick hepatic epithelium. Dev. Biol 319, 234–247. [DOI] [PubMed] [Google Scholar]
- 55.Matt N, Dupé V, Garnier J-M, Dennefeld C, Chambon P, Mark M, and Ghyselinck NB, 2005. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 132, 4789–4800. [DOI] [PubMed] [Google Scholar]
- 56.Mic FA, Molotkov A, Molotkova N, and Duester G, 2004. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev. Dyn 231, 270–277. [DOI] [PubMed] [Google Scholar]
- 57.Molotkov A, Molotkova N, and Duester G, 2006. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133, 1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori M, Ghyselinck NB, Chambon P, and Mark M, 2001. Systematic Immunolocalization of Retinoid Receptors in Developing and Adult Mouse Eyes. 42, 1312–1318. [PubMed] [Google Scholar]
- 59.Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, and Anderson DJ, 2000. Transient Notch Activation Initiates an Irreversible Switch from Neurogenesis to Gliogenesis by Neural Crest Stem Cells. Cell 101, 499–510. [DOI] [PubMed] [Google Scholar]
- 60.Nikopoulos GN, Duarte M, Kubu CJ, Bellum S, Friesel R, Maciag T, Prudovsky I, and Verdi JM, 2007. Soluble Jagged1 Attenuates Lateral Inhibition, Allowing for the Clonal Expansion of Neural Crest Stem Cells. Stem Cells 25, 3133–3142. [DOI] [PubMed] [Google Scholar]
- 61.Noden DM, 1978. The Control of Avian Cephalic Neural Crest Cytodifferentiation. Dev. Biol 67, 296–312. [DOI] [PubMed] [Google Scholar]
- 62.Ochoa SD, Salvador S, and LaBonne C, 2012. The LIM adaptor protein LMO4 is an essential regulator of neural crest development. Dev. Biol 361, 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ormestad M, Blixt Å, Churchill A, Martinsson T, Enerback S, and Carlsson P (2002). Foxe3 Haploinsufficiency in Mice: A Model for Peters’ Anomaly. 43, 1350–1357. [PubMed] [Google Scholar]
- 64.Pei YF, and Rhodin JAG, 1970. The prenatal development of the mouse eye. Anat. Rec 168, 105–125. [DOI] [PubMed] [Google Scholar]
- 65.Plageman TF, Zacharias AL, Gage PJ, and Lang RA, 2011. Shroom3 and a Pitx2-N-cadherin pathway function cooperatively to generate asymmetric cell shape changes during gut morphogenesis. Dev. Biol 357, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reneker LW, Silversides DW, Xu L, and Overbeek PA, 2000. Formation of corneal endothelium is essential for anterior segment development – a transgenic mouse model of anterior segment dysgenesis. Development 127, 533–542.10631174 [Google Scholar]
- 67.Ribes V, Wang Z, Dollé P, and Niederreither K, 2006. Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development 133, 351–361. [DOI] [PubMed] [Google Scholar]
- 68.Rochard L, Monica SD, Ling ITC, Kong Y, Roberson S, Harland R, Halpern M, and Liao EC, 2016. Roles of Wnt pathway genes wls, wnt9a, wnt5b, frzb and gpc4 in regulating convergent-extension during zebrafish palate morphogenesis. Development 143, 2541–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saika S, Saika S, Liu C-Y, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW-C, and Kao WW-Y, 2001. TGFβ2 in Corneal Morphogenesis during Mouse Embryonic Development. Dev. Biol 240, 419–432. [DOI] [PubMed] [Google Scholar]
- 70.Sainson RCA, and Harris AL, 2008. Regulation of angiogenesis by homotypic and heterotypic notch signalling in endothelial cells and pericytes: from basic research to potential therapies. Angiogenesis 11, 41–51. [DOI] [PubMed] [Google Scholar]
- 71.Sakamoto M, Hirata H, Ohtsuka T, Bessho Y, and Kageyama R, 2003. The Basic Helix-Loop-Helix Genes Hesr1/Hey1 and Hesr2/Hey2 Regulate Maintenance of Neural Precursor Cells in the Brain. J. Biol. Chem 278, 44808–44815. [DOI] [PubMed] [Google Scholar]
- 72.Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, and Maas R, 2000. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet 24, 391–395. [DOI] [PubMed] [Google Scholar]
- 73.Semina EV, Reiter R, Leysens NJ, Alward WLM, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, and Murray JC, 1996. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat. Genet 14, 392–399. [DOI] [PubMed] [Google Scholar]
- 74.Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WLM, Reiter RS, Funkhauser C, Daack-Hirsch S, and Murray JC, 1998. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat. Genet 19, 167–170. [DOI] [PubMed] [Google Scholar]
- 75.Shah NM, Groves AK, and Anderson DJ, 1996. Alternative Neural Crest Cell Fates Are Instructively Promoted by TGFβ Superfamily Members. Cell 85, 331–343. [DOI] [PubMed] [Google Scholar]
- 76.Shiau CE, Hu N, and Bronner-Fraser M, 2010. Altering Glypican-1 levels modulates canonical Wnt signaling during trigeminal placode development. Dev. Biol 348, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simoes-Costa M, Tan-Cabugao J, Antoshechkin I, Sauka-Spengler T, and Bronner ME, 2014. Transcriptome analysis reveals novel players in the cranial neural crest gene regulatory network. Genome Res. 24, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spagnoli A, O’Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, and Moses HL, 2007. TGF-β signaling is essential for joint morphogenesis. J. Cell Biol 177, 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spater D, Hill TP, O’Sullivan RJ, Gruber M, Conner DA, and Hartmann C, 2006. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 133, 3039–3049. [DOI] [PubMed] [Google Scholar]
- 80.Spurlin J, and Lwigale P, 2013. A Technique to Increase Accessibility to Late-Stage Chick Embryos for In Ovo Manipulations: In Ovo Access to Late-stage Chick Embryo. Dev. Dyn 242, 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thiagarajah JR, and Verkman AS, 2002. Aquaporin Deletion in Mice Reduces Corneal Water Permeability and Delays Restoration of Transparency after Swelling. J. Biol. Chem 277, 19139–19144. [DOI] [PubMed] [Google Scholar]
- 82.Tümer Z, and Bach-Holm D, 2009. Axenfeld–Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur. J. Hum. Genet 17, 1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wojciak-Stothard B, Zhao L, Oliver E, Dubois O, Wu Y, Kardassis D, Vasilaki E, Huang M, Mitchell JA, Harrington LS, Prendergast GC, Wilkins MR, 2012. Role of RhoB in the Regulation of Pulmonary Endothelial and Smooth Muscle Cell Responses to Hypoxia. Circ. Res 110, 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, and Sommer L, 2005. Inactivation of TGF signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev. 19, 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang K, Pang K, and Wu X, 2014. Isolation and Transplantation of Corneal Endothelial Cell–Like Cells Derived from In-Vitro-Differentiated Human Embryonic Stem Cells. Stem Cells Dev. 23, 1340–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


