Abstract
3D cell culture in protein-based hydrogels often begins with chemical functionalization of proteins with cross-linking agents such as methacryloyl or norbornene. An important and variable characteristic of these materials is the degree of functionalization (DoF), which controls the reactivity of the protein for crosslinking and therefore impacts the mechanical properties and stability of the hydrogel. Although 1H-NMR has emerged as the most accurate technique for quantifying absolute DoF of chemically modified proteins, colorimetric techniques still dominate in actual use and may be more useful for quantifying fractional or percent DoF. In this work, we sought to develop an optimized colorimetric assay for DoF of common gelatin-based biomaterials and validate it versus NMR; along the way, we developed a set of best practices for both methods and considerations for their most appropriate use. First, the amine-reactive ninhydrin assay was optimized in terms of solvent properties, temperature, ninhydrin concentration, and range of gelatin standards. The optimized assay produced a linear response to protein concentration in a convenient, 96-well plate format, and yielded a fractional DoF similar to NMR in most cases. In comparing to NMR, we identified that DoF can be expressed as fractional or absolute, and that fractional DoF can be inaccurate if the amino acid content of the parent protein is not properly accounted for. In summary, the fractional DoF of methacryloyl- and norbornene-functionalized gelatins was quantified by an optimized colorimetric ninhydrin assay and orthogonally by 1H-NMR. These methods will be valuable for quality control analysis of protein-based hydrogels and 3D cell culture biomaterials.
Keywords: Gelatin methacryloyl, GelMA, Gelatin norbornene, GelNB, Assay development, Protein NMR, Biomaterials
Graphical Abstract
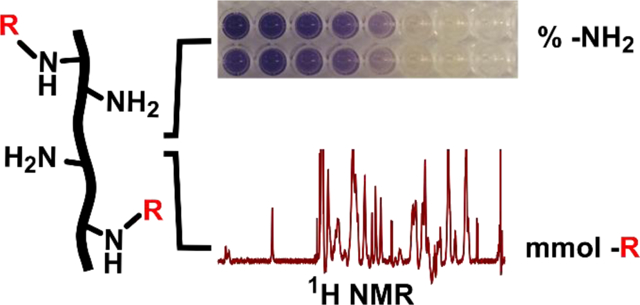
Introduction
Hydrogels composed of functionalized, protein-based polymers have been used increasingly in regenerative medicine and tissue engineering [1–3]. Applications such as controlled release and tunable 3D cell culture require precisely controlled cross-linking density, which mediates stiffness, extrudability, and shear-thinning properties. Naturally derived hydrogel materials such as gelatin are a mainstay of the field due to their low toxicity, cell-adhesion motifs, and biodegradability [2, 4]. Thus, much recent work focuses on adding chemically defined and tunable functionality to these materials [5–7].
While natural gelatin offers thermal control over gelation, inclusion of photoreactive functional groups, such as methacryloyl (GelMA) or norbornene (GelNB), provides on-demand gelation with tunable stiffness (Fig. 1a,b) [5, 6]. Claaßen et al. (2018) showed that for methacryloyl-modified gelatin, functionalization occurs primarily at lysine and hydroxylysine residues initially. As the ratio of methacryloyl (MA) to amines is increased and 100% of amines are reacted, functionalization also occurs at other residues such as threonine, serine, and tyrosine, though at low frequency compared to lysines [8, 9]. These hydrogel chemistries are rapidly gaining popularity, and studies have recently focused on improved processability and larger batch sizes [7, 10]. As interest grows in scaled-up production and novel chemical modifications of gelatin, so does the need for reproducible and simple assays for quality control and prototyping [11].
Fig. 1.
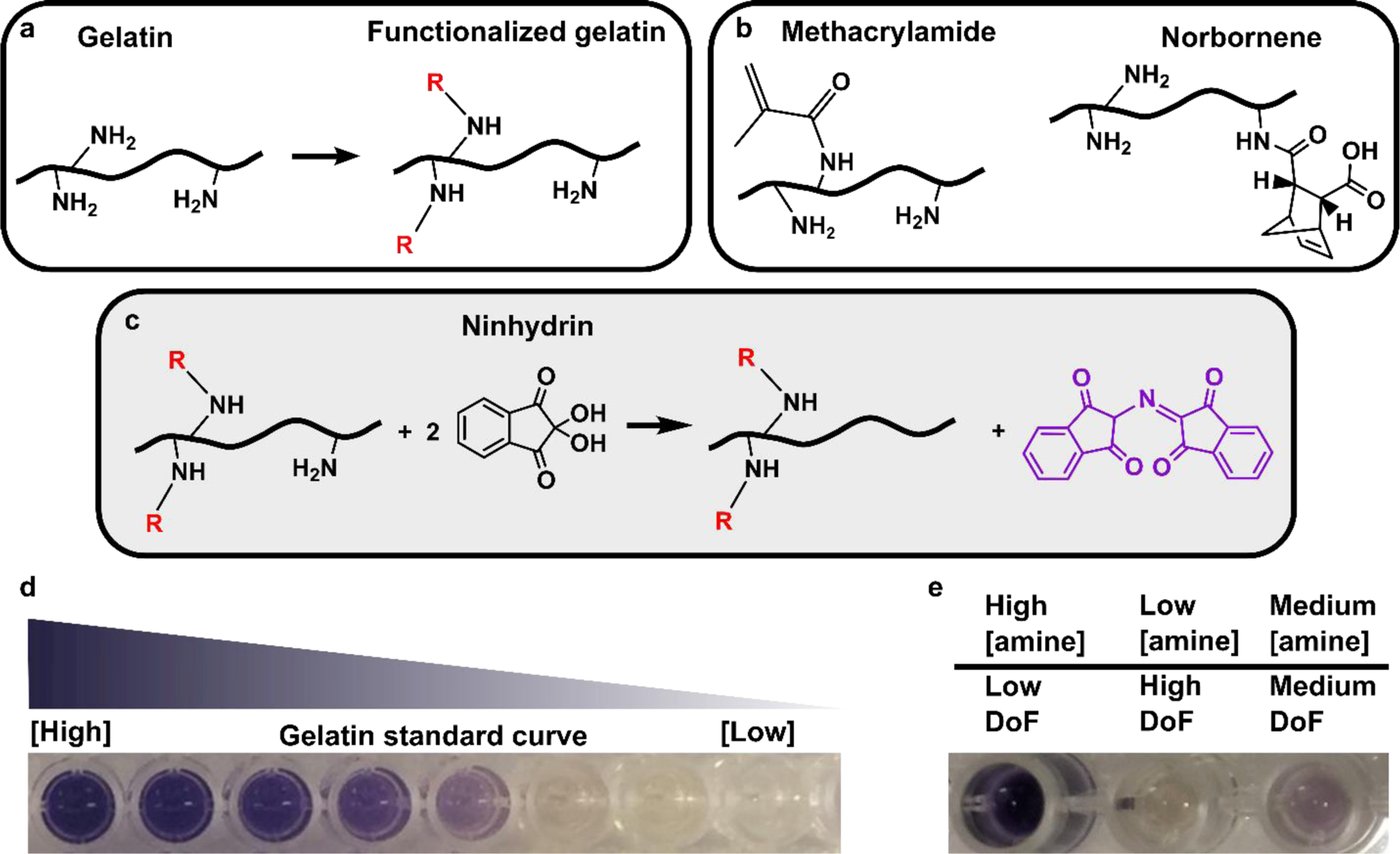
Detecting protein functionalization by loss of free amines via the colorimetric ninhydrin assay. (a) Reaction scheme for chemical functionalization of a protein by anhydrides or succinimidyl esters, which occurs primarily at free amines. (b) Structures of reaction products of amine sidechains with methacryloyl or norbornene groups. (c) Reaction scheme for the ninhydrin assay. Ninhydrin reacts with free primary amines to generate Ruhemann’s Purple, a purple-colored soluble product. (d) Photo of well-plate after running the optimized assay with a gelatin standard curve. Solutions remained clear, free of precipitation, yielded a visible color change that corresponded to free amine content in the solution. (e) Photo of well plate after analyzing functionalized gelatin samples (here, GelMA). Samples yielded color changes that inversely correlate with their respective DoF values.
During the synthesis of modified gelatin, the degree of functionalization (DoF) -- defined as the quantity or fraction of functional groups reacted -- varies with both the gelatin starting material and the reaction conditions. Both the exact amino acid content and the availability of reactive functional groups can vary between batches and sources of gelatin (Table 1), a natural product that is sourced from animal-derived collagen and extensively processed in acidic or basic conditions. This variation extends to other proteins used for chemical functionalization as well, e.g. tropoelastin [9], as well as to antibodies, in which amine content may be unknown. Beyond this variability in reactive sites, the degree of functionalization also depends strongly on reaction conditions [6, 7, 11, 12]. Quantification of the DoF is necessary for quality control of functionalized gelatin materials, because DoF influences the rate of polymerization, resulting stiffness, and mechanical stability of the hydrogel [5, 6, 13].
Table 1.
Reported lysine and amine content of bovine and porcine gelatin.
| Gelatin (species, type) | Gelatin source tissue | Lysine (mmol / g gelatin) | Amines (mmol / g gelatin | Reference |
|---|---|---|---|---|
| Bovine, type B | Skin | Not Reported | 0.35 | [10] |
| Bovine, type B | Skin | 0.28 | 0.385 | [7, 14] |
| Bovine, type B | Skin | 0.100a | Not reportedb | [15] |
| Porcine, type A | Skin | 0.245a | Not reportedb | [8] |
| Porcine, type A | Skin | 0.259 | 0.325c | [8] |
| Porcine, type A | Not Reported | 0.245a | 0.300a,c | [16] |
These data were reported as “residue / 1000 amino acids.” The values were converted to “mmol / g gelatin” by assuming an average amino acid molecular weight of 110 g/mole of amino acid.
Neither the hydroxylysine nor the total amine content was reported.
This value was determined by adding reported data for lysine and hydroxylysine.
1H-NMR and colorimetric assays have become standard methods for assessing the DoF of functionalized proteins. 1H-NMR offers direct quantification of the DoF, because the spectral peak(s) corresponding to the functional group can be easily identified and integrated. Furthermore, using an internal standard has allowed for precise determination of absolute DoF in units of moles of functional group (e.g. MA) per gram of protein [8]. However, unless the amine content is known precisely, e.g. from sequencing, the fraction of amines functionalized cannot be quantified by NMR. Furthermore, despite the accuracy of NMR methods, many biomedical researchers prefer a rapid, plate-based assay whenever possible. The continued use of colorimetric DoF assays by many research groups [9, 17, 18] indicates that there is a need for accurate and precise assays of this type, and an understanding of their limitations. A promising colorimetric approach to measuring DoF is the ninhydrin assay, which reacts primary amines with a small molecule, ninhydrin, to form Ruhemann’s Purple (Fig. 1c). This assay was developed originally for use with solutions of free amino acids or short peptides, and has been adapted to characterize collagen content within heterogenous biomaterials [19] and functionalized gelatin methacryloyl [13, 19]. By comparing to a calibration curve, the assay provides a measure of the fraction of free amines remaining after conjugation, from which a fractional DoF is determined. These protocols vary widely and often yield non-quantitative results when applied to proteins. The typical solvents and temperatures used for the ninhydrin reaction lead to protein precipitation or poor sensitivity, thus decreasing the accuracy and precision of DoF measurement. The development of a standardized ninhydrin assay for DoF of protein-based samples, particularly gelatins, would significantly advance the development of tunable biomaterials and hydrogels. While this method can detect only amine functionalization, not functionalization of other residues, amines are the primary site of functionalization by MA under typical reaction conditions [8, 9].
Here we present two advancements. First, the ninhydrin assay was optimized for work with functionalized gelatin materials using unmodified gelatin for the standard curve (Fig. 1d,e). Second, the ambiguity of the DoF expression was clarified by defining absolute and fractional DoF. With this in mind, we identified the advantages and limitations of several previously published approaches for analyzing the 1H-NMR spectrum of functionalized gelatin. Finally, the optimized ninhydrin assay was validated for accuracy against protein NMR as an effective, plate-based alternative.
Materials and methods
Reagents and solvents
Ethanol (190 proof) was obtained from Decon Labs, Inc. Phosphate-buffered saline (PBS) was prepared in house by adding 2.7 mM KCl, 13.7 mM NaCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4 to 18 MΩ Millipore water. Methacrylic anhydride, carbic anhydride, 5-Norbornene-2-carboxylic acid (endo/exo mixture), N-hydroxysuccinimide, N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide (EDC), anhydrous DMSO, ninhydrin, and Sodium trimethylsilylpropanesulfonate (DSS) were obtained from Sigma. Ninhydrin was dissolved in ethanol to the stated concentration and used within two days. DSS was used as the internal standard (δ 0.0 ppm), and D2O (Cambridge Isotope Laboratories, Inc.) was used as the solvent in 1H-NMR experiments. DSS was dissolved in D2O to at 0.25 mg/mL to make the internal standard solution.
Functionalization of gelatin
Gelatin from porcine skin, Gel Strength 300, Type A (Sigma-Aldrich) was used as the starting material for all reactions. Gelatin methacroyl (GelMA) was prepared in-house as described by Loessner et al. (2016) [13], including reaction, dialysis, and lyophilization. The ratio of methacrylic anhydride to gelatin was 13 mmol / g. Separately, three samples of GelMA were also purchased from Sigma. The Sigma GelMA was nominally 32 %, 63 %, and 70 % functionalized according to H-NMR validation by the manufacturer. The methods by which Sigma performed H-NMR analysis were proprietary.
Gelatin norbornene (GelNB) was prepared from gelatin as described by Mũnoz et al. (2014) [6] with the following exceptions: The carbic anhydride (Acros Organics) was varied from 0.4 mM – 3.7 mM to produce GelNB samples having a range of DoFs. Additional sodium hydroxide (50 % w/v) (30 – 50 mL) was needed to reach and maintain the reaction at pH 8 as the concentration of carbic anhydride was increased. The resulting product was centrifuged at 3500 x g for 3 minutes and the supernatant was dialyzed in 4 L of ultrapure water for 10 days at 40 °C with daily water changes before freezing and lyophilization.
One batch of GelNB (GelNB-NHS) was prepared using EDC/NHS conjugation chemistry as described by Hoorick et al., (2018) [7]. Briefly, norbornene carboxylic acid (1.5 equivalents) was dissolved in 500 mL of dry DMSO, followed by the addition of EDC (1 equivalent) and NHS (1.25 equivalents), degassed 3 times and left to react for 24 hours under N2 conditions. The next day, 10 g of gelatin were dissolved in 150 mL of dry DMSO under N2 and reflux conditions. Once dissolved, the 5-norborene-2succimidyl ester mixture was transferred into the gelatin flask using a transfer syringe and left to react at 50 °C for 18 hours. The solution was precipitated using 10x excess acetone, then filtered through a Büchner filter. The obtained solids were dried under vacuum for 20 hours. The product was dissolved at 2.5 % in ultrapure water by stirring overnight, pH adjusted to 7 using NaOH, and dialyzed in 4 L of ultrapure water for 24 hours at 40 °C before freezing and lyophilization.
Ninhydrin assay and DoF calculation
Lyophilized gelatin and functionalized GelMA or GelNB samples were each dissolved at 10 mg/mL in 1x PBS. To generate a standard curve, unmodified gelatin was serially-diluted in PBS from 0 – 10 mg/mL in increments of 1 mg/mL in triplicate in a 96-well plate. The functionalized gelatin samples were plated in triplicate without dilution. A 12 mM (2.2 mg/mL) solution of ninhydrin in ethanol was added to each plated sample in a 1:8 v/v ratio of ninhydrin to gelatin solution, unless otherwise noted (total volume 100 μL per well). The plate was sealed with optical sealing tape (ThermoFisher) and incubated in an oven at 70°C until a linear pattern of color development was observed (about 20 to 30 minutes). Absorbance was measured at 570 nm using a multi-modal plate reader (Clariostar). The mean absorbance for each gelatin standard was plotted to form a standard curve.
For each functionalized sample, the fraction of amines available was determined by
| (1) |
where the apparent concentration was obtained by comparison to the standard curve, and the nominal concentration was defined as the concentration at which the protein sample solution was prepared. The (percent) DoF was determined by
| (2) |
1H NMR assay and DoF calculation
Quantification of DoF in mmol -R / g gelatin was performed according to the method described by Claaßen et al. [8]. Samples were prepared for 1H-NMR by dissolving lyophilized gelatin, GelMA, or GelNB, in the internal standard solution at 20 mg/mL gelatin. This produced a known DSS to gel ratio of 0.0573 mmol DSS / g gelatin. The 1H-NMR spectra were obtained at room temperature using 5 mm diameter NMR tubes and a standard Bruker Avance III 600 MHz spectrometer for solution samples operated at 14.1 tesla. Spectra were analyzed using Mestranova software (v14.0.0). All spectra were phase adjusted and baseline corrected.
Total degree of norbornene functionalization (mmol NB) of the 5 GelNB samples was assessed by integrating the single peak at 6.0 ppm for 2 protons, corresponding to two vinyl protons of norbornene, and normalizing to the DSS peak (9 protons). The GelNB-NHS sample was assessed by integrating the four peaks appearing from 5.8 – 6.3 ppm for 4 protons, corresponding to 2 vinyl protons of endo-norbornene and 2 vinyl protons of exo-norbornene [7], and normalizing to the DSS peak (9 protons). This value was multiplied by the mmol DSS / g gel ratio to determine DoF in units of (mmol NB / g gelatin). For GelMA, total degree of methacryloylation (mmol MA) was assessed according to the method from Claaßen et al. [8]. Briefly, the two peaks at 5.5 – 5.7 ppm, corresponding to a single acrylic proton of methacrylate and a single acrylic proton of methacrylamide, were integrated. The sum of both peaks was integrated for 1 proton and normalized to the DSS peak (9 protons) to determine mmol MA. This value was multiplied by the (mmol DSS / g gel) ratio to determine DoF in units of (mmol MA / g gelatin). To estimate the fractional functionalization for comparison to the ninhydrin assay, (mmol -R / g gelatin) was divided by the reported density of amines on gelatin (0.300 mmol amines / g gelatin) (see Table 1) [14].
Results and Discussion
Optimization of ninhydrin assay conditions
In optimizing the ninhydrin assay for use with functionalized gelatins, we considered several factors based on our initial experience testing out a variety of reported procedures: choice of standards, prevention of precipitation via solvent selection, and optimization of ninhydrin concentration and temperature.
Selecting Standards:
Standards used for the ninhydrin assay with functionalized gelatins range from purified amino acids (e.g. glycine [20]) to gelatin itself [13], and the selection is not inconsequential. A glycine standard curve has the advantage of providing precisely known amine concentration, but does not account for absorbance by the protein itself (some modified proteins are yellow-tinged), nor for side reactions between ninhydrin and other amino acids present in the protein sample. In contrast, a gelatin standard curve more directly provides information of the fraction of amines reacted, which is useful when optimizing reaction conditions to obtain a desired DoF. Here, we chose to use the parent Type A porcine gelatin as the standard primarily because of the potential for side reactions between ninhydrin and the protein. The same lot and type of gelatin is used for the standards and the functionalized gelatin, which allows for direct determination of fractional functionalization.
Preventing precipitation.
The solvent conditions described in published protocols for the ninhydrin assay vary widely depending on their application. Examples include (a) dissolving amino acids in PBS and ninhydrin in ethanol [19] and (b) dissolving gelatin in water and ninhydrin in a sodium citrate / glycerol mixture [13]. Initially, we tested these and similar protocols but found that the ethanol content in (a) caused protein precipitation, and the glycerol in (b) resulted in density gradients and irreproducible color development in our hands. Other protocols for free amino acid analysis, conducted in concentrated acids, were not tested here due to their potential to hydrolyze the protein [20]. Because of the simplicity of the components of the PBS/ethanol approach, we selected this method for further optimization with gelatin. To eliminate precipitation while achieving a measurable color change, we sought to minimize the ethanol content of the mixture while maintaining a sufficient concentration of ninhydrin. For these initial tests, the mixture was heated in polypropylene tubes in a water bath at 50 °C and observed for about 15 minutes. We found that a 1:8 v/v ratio of 20 mg/mL ninhydrin solution (2.2 mg/mL final ninhydrin concentration) to 20 mg/mL gelatin solution both eliminated precipitation and yielded distinct purple color development (Table 2).
Table 2.
The concentrations and volumetric ratio of ethanolic ninhydrin solution to aqueous gelatin solution were initially adjusted to eliminate precipitation while achieving a measurable purple color change
| [Ninhydrin] in ethanol, mg/mLa | [Gelatin] in PBS, mg/mLa | VEtOH : VPBSc | Final [ninhydrin], mg/mL | Absence of Precipitation? | Purple color? |
|---|---|---|---|---|---|
| 3.5 | 3.5 | 20 : lb | 3.3 | No | No |
| 3.5 | 3.5 | 9 : 1 | 3.2 | No | No |
| 3.5 | 3.5 | 1 : 8 | 0.4 | Yes | No |
| 3.5 | 20 | 1 : 8 | 0.4 | Yes | No |
| 20 | 20 | 1 : 8 | 2.2 | Yes | Yes |
Initial concentrations
The conditions in this row were from Ma et al., (2003) [19]
VEtOH : VPBS is the volumetric ratio of ethanol to PBS after combining reagents
Ninhydrin Concentration Optimization.
Using the optimal solvent ratio (1:8 v/v EtOH:PBS), we sought to optimize the linear range of the assay by varying the concentration of ninhydrin in the final mixture. Serial dilutions of gelatin from 1–10 mg/mL were reacted in a 96-well plate at 70 °C for 30 minutes with ninhydrin at 2.2 mg/mL (12.3 mM), 4.4 mg/mL (24.7 mM), or 6.6 mg/mL (37.0 mM) (Figure 2a). We found that 2.2 mg/mL ninhydrin produced the widest linear range of absorbance. Higher ninhydrin concentrations resulted in saturated absorbance at moderate gelatin concentrations.
Fig. 2.
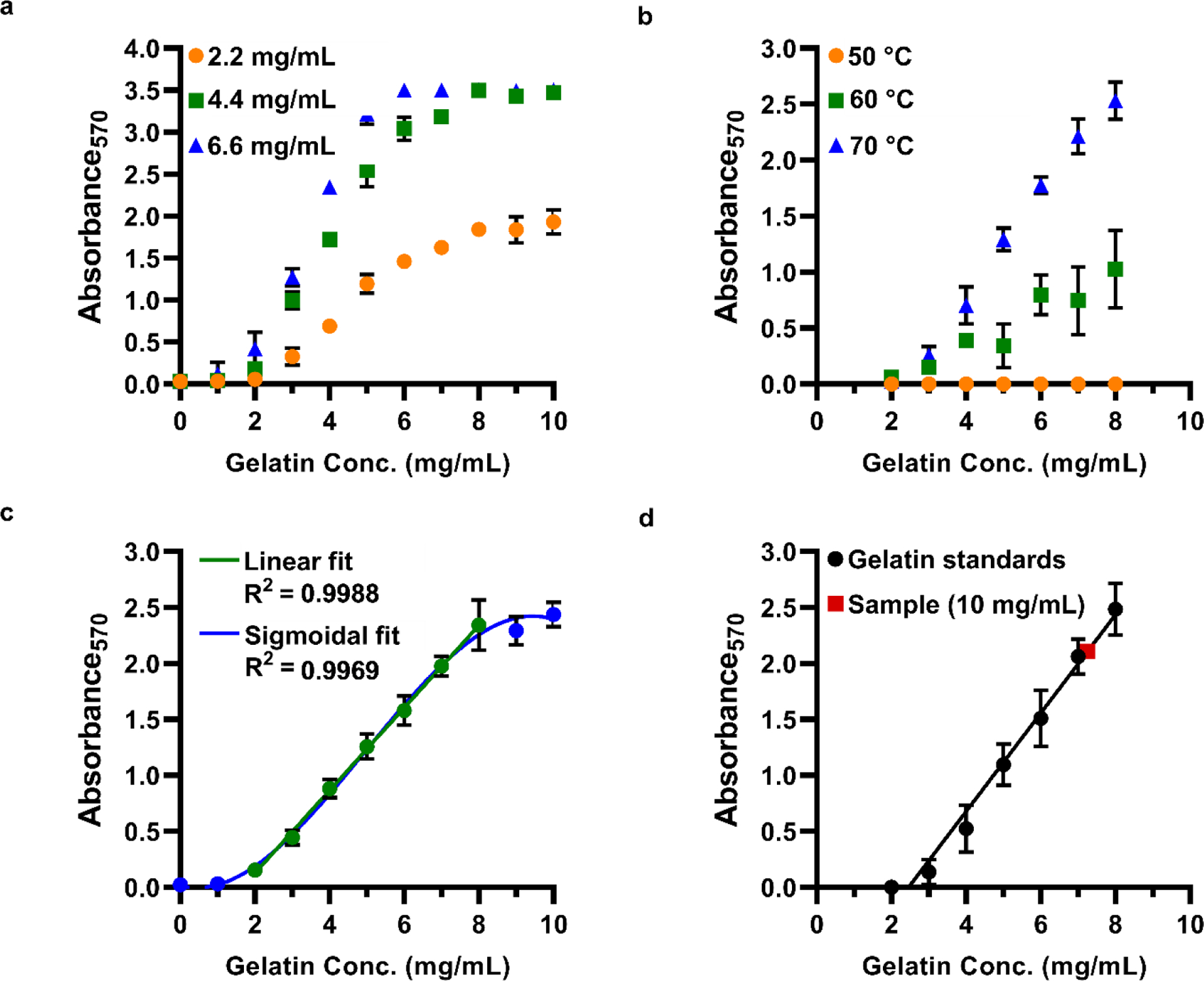
Optimization of ninhydrin assay conditions for use with functionalized gelatin in 96-well plate format. (a) Calibration curves (absorbance versus gelatin concentration) under varied ninhydrin concentrations; legend shows the final ninhydrin concentration in the reaction solution. Temperature 70 °C. (b) Calibration curves at varied reaction temperature. 2.2 mg/mL ninhydrin. (c) Expanded calibration curve, fit with a sigmoidal curve or fit in part with a linear regression. Temperature 70 °C; 2.2 mg/mL ninhydrin. (d) Determination of degree of functionalization for a representative sample of functionalized gelatin (red square). In this example, GelMA prepared at 10 mg/mL exhibited an apparent concentration of 7.3 mg/mL (DoF 27 %).
Reaction Temperature Optimization.
The ninhydrin reaction requires elevated temperatures, and the assay is frequently conducted at temperatures between 50–100 °C [13, 19]. To determine the optimal temperature for a reaction conducted in a 96-well plate format, standards (2–8 mg/mL gelatin) were prepared in the plate with 2.2 mg/mL ninhydrin, sealed, and incubated in an oven. Color development was allowed to occur for 30 minutes at temperatures ranging from 50–80 °C (Figure 2b). A reproducible linear absorbance curve was achieved at 70 °C. Temperatures lower than 70 °C resulted in larger variability, and temperatures greater than 70 °C resulted in evaporation of the samples. Similar results were observed when standards were prepared in test tubes and allowed to incubate at 70 °C for 12 minutes.
Standard Curve and Application to Functionalized Gelatin.
Finally, we applied the optimized reaction conditions and tested the dynamic range of the assay in the 96-well plate assay format. As expected, no precipitation was observed, and the reaction became visibly purple at higher gelatin concentrations. A sigmoidal absorbance curve was obtained from 0–10 mg/mL gelatin, with a linear region from 2–8 mg/mL (R2 = 0.9988) (Fig. 1d, Fig. 2c).
Functionalized gelatin samples, e.g. GelMA or GelNB, develop less purple color because fewer free amines are present. To determine the fraction of amines remaining in a sample of GelMA or GelNB, the “apparent” gelatin concentration was determined from the standard curve and normalized to the nominal concentration of the sample (Eq. 1, Materials and Methods). DoF was defined as the difference of this value from unity (Eq. 2). For example, a GelMA sample prepared at 10 mg/mL was reacted with ninhydrin and yielded an apparent concentration of 7.3 mg/mL, for a calculated DoF of 27 % (Figure 2d).
Optimizing the ninhydrin assay for intact proteins may be useful in other settings, as ninhydrin is used broadly in fields such as forensics, food science, biomedical and clinical research, and biochemistry[21]. For example, Nayuni et al., (2013) [22] showed that a clinical ninhydrin protocol was far more sensitive to free amino acids than to lysines among intact proteins, demonstrating a need for conditions that allow for color development with intact proteins.
Validation of ninhydrin assay versus 1H NMR
Having established a robust method for colorimetric analysis, we sought to validate its accuracy and limitations in comparison to 1H-NMR, the gold standard for quantification. We tested the ninhydrin assay with both GelNB and GelMA, as these are commonly used for photopatterned cell cultures [7, 13], and compared the results to DoF determined from 1H-NMR.
Interestingly, multiple methods have been demonstrated for quantifying the functional group density from 1H-NMR spectra, and there is no consensus in the literature on a single method of quantification. One elegant approach involves determining the molar concentration of a functional group (e.g. MA or NB) per gram of gelatin by integrating the functional group peak and normalizing to an internal standard [8]. This method provides an accurate measure of the absolute quantity of functional group present on gelatin (mmol -R / g gelatin), but does not provide a measure of fractional reaction completeness, or fraction of available sites functionalized. Alternatively, the concentration of the functional group may be normalized to the concentration of lysine or total amines to determine percent functionalization (fractional functionalization) [7]; the amino acid composition of the protein may be determined by sequencing or by reference to the literature [14]. Here, we adopted the latter approach to enable a comparison with the ninhydrin assay. By using literature values rather than sequencing each lot of gelatin, some bias is introduced into the fractional DoF due to slight variations in the amino acid composition (Table 1). Thus, the fractional NMR-DoF reported here may be slightly shifted from their true values.
First, we determined the DoF of six different batches of GelNB by both ninhydrin assay and NMR. Five batches of GelNB were synthesized as described by Mũnoz et al., (2014) [6], using varied feed ratios of the norbornene reagent, carbic anhydride (CA), to yield varying DoFs. The single peak at 6.04 ppm (Fig. 3a,b, “GelNB-CA”) was attributed to the two identical vinyl protons of norbornene, and is similar to what has been reported previously [20]. Full spectra of all GelNB-CA samples are provided in Fig. S1 (see Electronic Supplementary Material, ESM). To test the versatility of the method, we also synthesized a batch of GelNB-NHS, by using the EDC/NHS conjugation approach described in detail by Hoorick et al., (2018) [7]. For GelNB-NHS, four peaks were observed in 5.8 – 6.3 ppm (Fig. 3a,b) and were attributed to norbornene stereoisomers, confirming previously reported observations [7]. The results of the optimized ninhydrin assay and 1H NMR were linearly correlated with an R2 of 0.87 (Fig. 3c; ESM Table S1). As the ninhydrin assay detects functionalization only at amines, its close concordance with the NMR data suggested that norbornene functionalization occurred primarily at the amines under these conditions. In the future, 2D NMR could be used to quantify the extent of norborneneamide and possibly norborneneate functionalization under various reaction conditions [8].
Fig. 3.
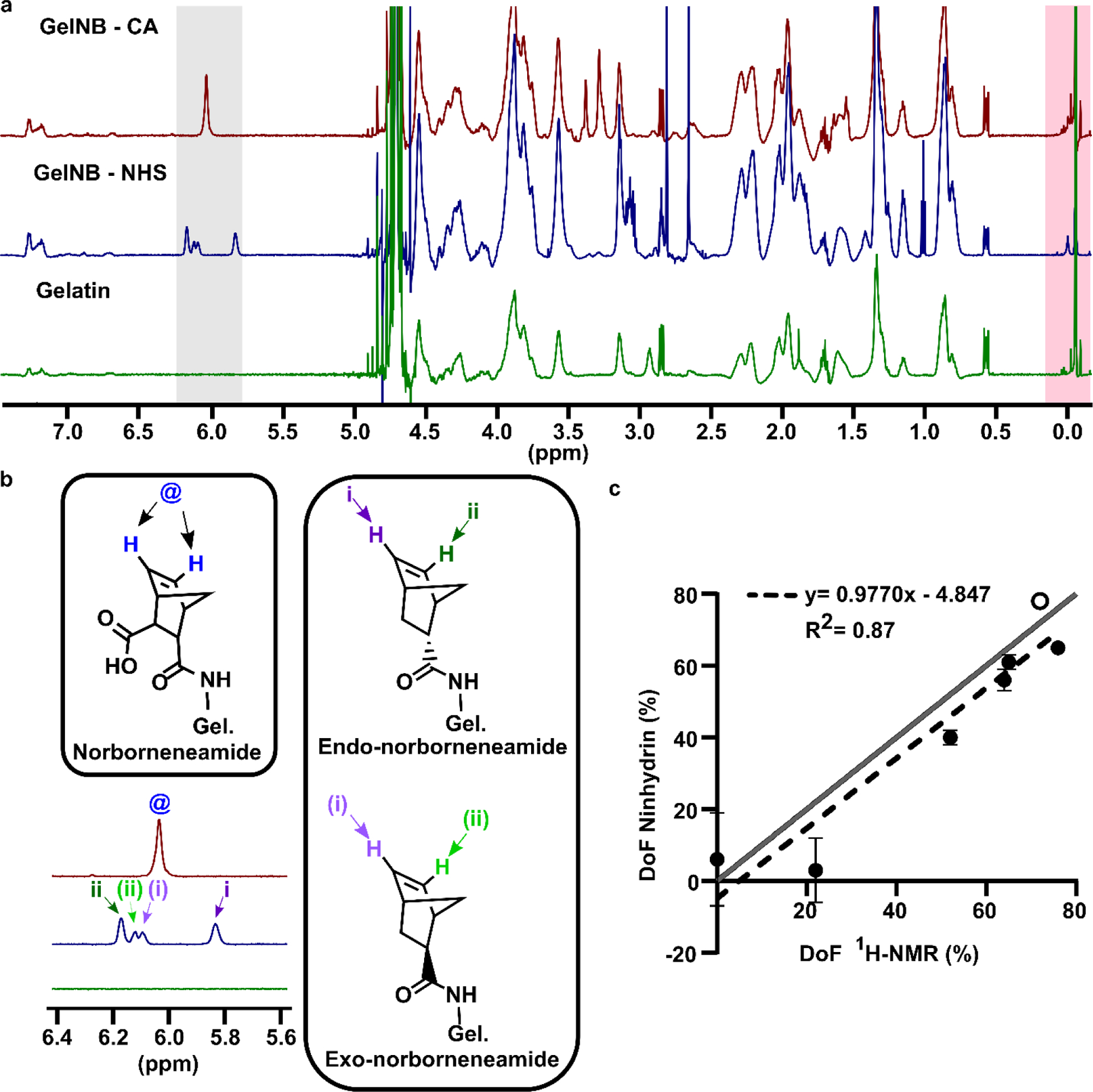
Validation of ninhydrin assay versus 1H NMR using GelNB. (a) Full NMR spectra of GelNB synthesized with carbic anhydride (GelNB-CA), GelNB synthesized via NHS conjugation (GelNB-NHS), and non-functionalized gelatin. Integration ranges for the functional group peak and internal standard are highlighted in gray and pink, respectively. (b) Structures and peak assignments for protons in the norbornene functional group in GelNB-CA and GelNB-NHS [7]. (c) Fractional DoF values determined by 1H NMR versus the optimized ninhydrin assay for five GelNB-CA samples (black dots) and one GelNB-NHS sample (open circle). Mean ± std dev of n = 3 technical replicates of ninhydrin assay; some error bars too small to see. Dotted line shows linear regression, y=0.9770x – 4.847, R2=0.87 Grey line shows the ideal scenario, y=x.
Next, we determined the DoF of one batch of GelMA synthesized in-house and three commercial preparations of GelMA, which nominally had DoF of 32 %, 63 %, and 70 % according to the vendor. The peaks for GelMA appeared at the expected chemical shifts for the acrylic groups of methacrylamide and methacrylate [8] (Fig. 4a,b). An upfield peak for the additional proton from methacrylate was not observed in these samples. While the two analysis methods yielded similar results for the medium and high-functionalized commercial GelMA (Fig. 4c) and the GelMA that was synthesized in house, they produced conflicting values for the low- functionalized commercial GelMA. Furthermore, the ninhydrin-DoF and the NMR-DoF each differed oppositely from the nominal, vendor-provided, value (ESM Table S1).
Fig. 4.
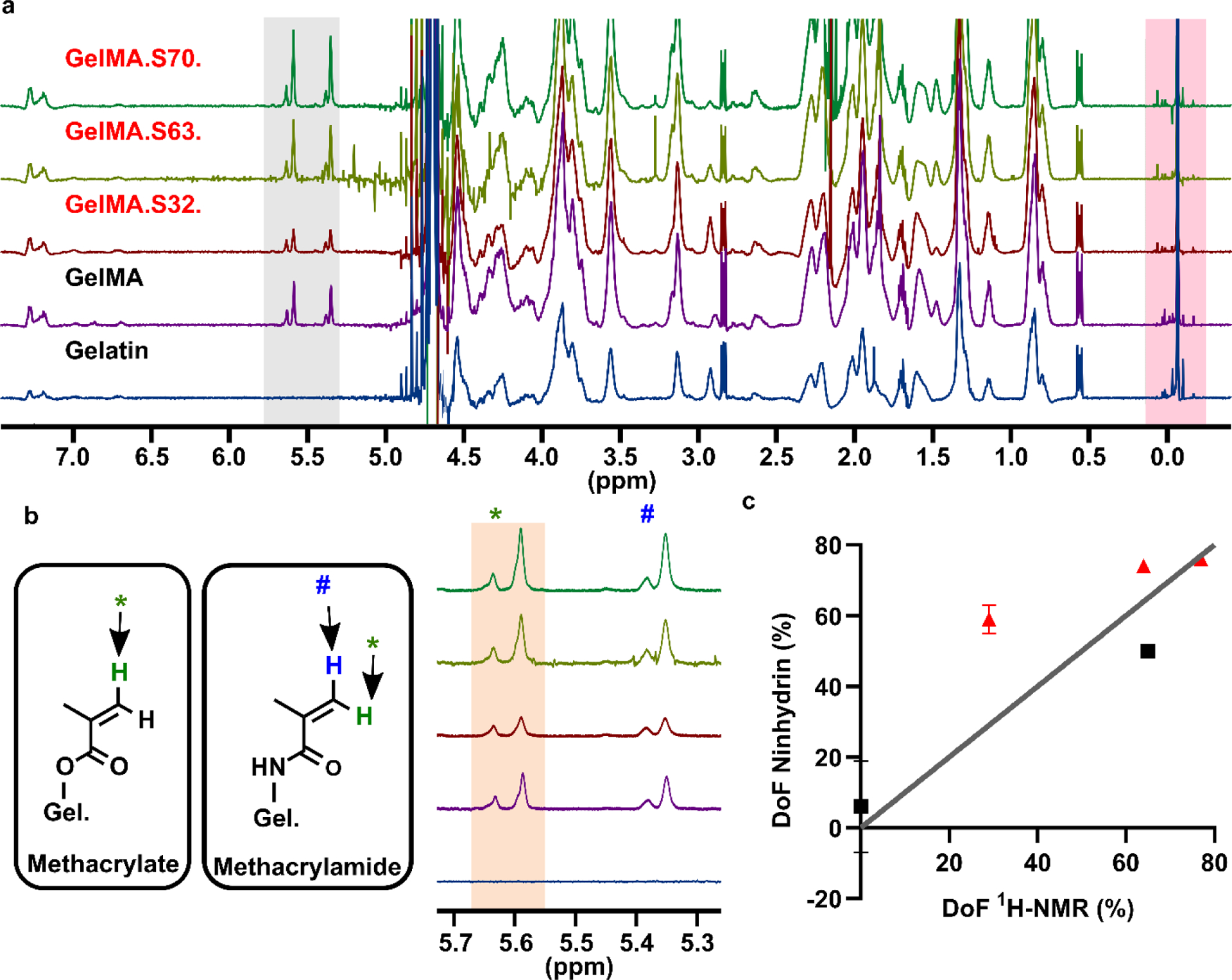
Validation of ninhydrin assay versus NMR using GelMA. (a) Full NMR spectra of non-functionalized gelatin and four GelMA samples with varying DoF. GelMA samples S32, S63, & S70 were purchased from Sigma. Peak ranges indicative of functionalization and the internal standard are highlighted in gray and pink, respectively. (b) Expanded functional group range displaying methacrylamide and methacrylate structures and peak assignments [8]. One proton of methacrylamide and of methacrylate contributes to the pair of peaks labeled as “*”. This pair of peaks was integrated (tan region) for total methacryloylation. Protons from methacrylamide (blue) and modified hydroxyproline (not shown) contribute to the pair of peaks labeled as “#”. (c) DoF values determined by 1H NMR versus the optimized ninhydrin assay. Red circles indicate samples from Sigma; black squares indicate samples prepared in house. Mean ± std dev of n = 3 technical replicates of ninhydrin assay; some error bars too small to see. Grey line shows the ideal scenario, y=x.
We note that the amine content and species of the original gelatin from which the commercial GelMA was manufactured are unknown. Based on these data, we speculate that the amine concentration may have been lower than the 0.300 mmol/g gelatin that was assumed for this analysis, which would cause the fractional DoF calculated by NMR to underestimate the true value. These results highlight the importance of knowing the amine content of each batch of gelatin when analyzing with NMR. Similarly, the ninhydrin assay was of necessity calibrated using a different lot of gelatin than the commercial GelMA was made from, which could potentially lead to over- or under-estimation of fractional DoF.
A comment on absolute versus fractional DoF
When chemically modifying proteins, two complementary metrics describe the extent of functionalization: the quantity of functional group (-R) bound to the protein (mmol -R / g protein; absolute functionalization), and the fraction of available binding sites that were functionalized (fractional or % functionalization). These metrics are used frequently and interchangeably in the literature under the general header DoF, but they provide different information. We briefly discuss each below.
A fractional DoF is essential for assessing reaction completeness while optimizing functionalization conditions. For example, as fractional DoF approaches 100%, no amount of additional reaction optimization will increase the functionalization of the protein; measuring solely the absolute mmol -R / g gelatin would not indicate this so clearly. However, to measure fractional DoF, one must either have an independent measurement of the available reaction sites on the parent protein, or the parent protein must be analyzed in parallel with the functionalized version. To this end, the ninhydrin assay described in this paper allows the user to prepare calibration standards from the parent protein; this strategy normalizes by the available reaction sites between the standards and the sample, producing an accurate fractional DoF value. Such internal normalization is not possible with 1H NMR, as the lysine peak partially overlaps with other peaks in the spectrum, so amino acid analysis or literature estimates must be used. Therefore, the ninhydrin assay may be a simpler strategy for fractional DoF. Of course, the ninhydrin assay is limited to functionalization and conjugation schemes where amines are the primary target.
Absolute functionalization, in mmol -R / g protein, may better correlate with function than fractional functionalization. For example, for GelMA and GelNB, this metric describes the number of available crosslinking sites per gram of gel and thus correlates with rheological properties [23]. A minimum absolute functionalization may be desired for the material to gel at a desired rate, while over-functionalization may sterically impede physical gelation [23]. This consideration is similar to well established guidelines for protein conjugation in other fields; e.g., antibody conjugation with fluorophores or drugs is usually best limited to a narrow range of label ratios, to optimize the function of the label without obstructing the binding epitope [24, 25]. While a ninhydrin assay with a traditional glycine standard curve could quantify absolute DoF by comparing amine content in the parent protein and the derivatized product, this analysis is limited to reactions that occur only at amines. 1H-NMR with an internal standard is best suited to measure absolute DoF, as it quantifies signature functional groups regardless of which combination of amino acids are functionalized [8]. Of course, for protein functionalization, 1H-NMR is limited to functional groups displaying peaks that are not buried within the protein’s amino acid peaks (0.5 – 5.0 ppm). For example, it is possible to measure the DoF of thiol-functionalized gelatin with the optimized ninhydrin assay but not with 1H-NMR.
Conclusion
The ninhydrin assay was optimized for use with protein-based materials to avoid precipitation and generate a quantitative and user-friendly measurement of fractional degree of functionalization. By using a gelatin standard curve, this colorimetric assay provides a fractional DoF without the need for prior knowledge of the amine content of the protein. The optimized assay was fast, inexpensive, and produced results similar to 1H-NMR for both norbornene functionalized gelatin and for most samples of methacryloyl functionalized gelatin. The assay may be suitable for other proteins as well. Considering the advantages and limitations of available methods for quantifying DoF, we recommend performing 1H-NMR with an internal standard to assess the absolute molar quantity of a functional group and performing the ninhydrin assay to quantify fractional functionalization as an indicator of reaction completeness.
Supplementary Material
Funding & Acknowledgements
This work was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) under Award Number U01EB029127 through the National Institutes of Health (NIH), with co-funding from the National Center for Advancing Translational Sciences (NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JMZ was supported in part by the Graduate Research Fellowship Program through the National Science Foundation. ANM was supported in part by a summer undergraduate research award through the Institute for Nanoscale and Quantum Scientific Advanced Research (nanoSTAR) at the University of Virginia. JOC was supported in part by the NIH-funded T32 Biotechnology Training Program at the University of Virginia. We acknowledge Andrew Kinman for testing various versions of the ninhydrin assay and Parastoo Anbaei for aiding in the synthesis of GelNB-NHS. NMR was performed at the University of Virginia Biomolecular Magnetic Resonance facility under the guidance of Dr. Jeffrey Ellena. We thank the faculty of Chemical Engineering, especially the Lampe, Caliari, and Letteri laboratories, for generous access to their lyophilization equipment.
Biographies

Jonathan M. Zatorski received his B.S. in chemistry and biology from Olivet Nazarene University in 2018. He is currently completing a PhD in Chemistry at the University of Virginia. His research focus is to develop analytical methods for characterizing complex biomaterial systems.

Alyssa N. Montalbine is completing a B.S. in chemistry with a specialization in biochemistry at the University of Virginia. Her research interests include the development of organ-on-chip microfluidic systems and related tissue engineering constructs.

Jennifer E. Ortiz-Cárdenas received her B.A. in chemistry from Binghamton University in 2015. She is currently completing her PhD in chemistry at the University of Virginia under the supervision of Dr. Rebecca R. Pompano. Her research focuses on the development of biomimetic photo-patterned 3D cultures to recreate the spatial organization of tissue on microfluidic chips.

Rebecca R. Pompano is an Assistant Professor in the Departments of Chemistry and Biomedical Engineering at the University of Virginia, and a member of the Beirne B. Carter Center for Immunology Research. Her research interests center on developing bioanalytical tools to study immunity in primary and bioengineered tissue samples, including applications in organs-on-chip and immunoengineering.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/Competing interests: The authors have no conflicts to disclose.
Availability of data and material: Raw data is available upon request.
References
- 1.Choi JR, Yong KW, Choi JY, Cowie AC. Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 2019;66:40–53 [DOI] [PubMed] [Google Scholar]
- 2.Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci-Unal G, Dokmeci MR, Peppas NA, Khademhosseini A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv Mater 2014;26:85–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ifkovits JL, Burdick JA. Review: Photopolymerizable and Degradable Biomaterials for Tissue Engineering Applications. Tissue Eng 2007;13:2369–2385 [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Rodrigues J, Tomás H. Injectable and biodegradable hydrogels: gelation , biodegradation and biomedical applications. Chem Soc Rev 2012;41:2193–2221 [DOI] [PubMed] [Google Scholar]
- 5.Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015; 73:254–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mũnoz Z, Shih H, Lin C-C. Gelatin hydrogels formed by orthogonal thiol–norbornene photochemistry for cell encapsulation. Biomater Sci 2014; 2:1063–1072 [DOI] [PubMed] [Google Scholar]
- 7.Hoorick JV, Gruber P, Markovic M, et al. Highly Reactive Thiol-Norbornene Photo-Click Hydrogels: Toward Improved Processability. Macromol Rapid Commun 2018;39:1800181. [DOI] [PubMed] [Google Scholar]
- 8.Claaßen C, Claaßen MH, Truffault V, Sewald L, Tovar GEM, Borchers K, Southan A. Quantification of Substitution of Gelatin Methacryloyl: Best Practice and Current Pitfalls. Biomacromolecules 2018;19:42–52 [DOI] [PubMed] [Google Scholar]
- 9.Yue K, Li X, Schrobback K, et al. Structural analysis of photocrosslinkable methacryloyl-modified protein derivatives. Biomaterials 2017;139:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000;1:31–38 [DOI] [PubMed] [Google Scholar]
- 11.Zhu M, Wang Y, Ferracci G, Zheng J, Cho N-J, Lee BH. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci Rep 2019;9:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015;73:254–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loessner D, Meinert C, Kaemmerer E, Martine LC, Yue K, Levett PA, Klein TJ, Melchels FPW, Khademhosseini A, Hutmacher DW. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat Protoc 2016;11:727–746 [DOI] [PubMed] [Google Scholar]
- 14.Vlierberghe SV, Fritzinger B, Martins JC, Dubruel P. Hydrogel Network Formation Revised: High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance as a Powerful Tool for Measuring Absolute Hydrogel Cross-Link Efficiencies. Appl Spectrosc 2010;64:1176–1180 [DOI] [PubMed] [Google Scholar]
- 15.Hafidz R, Yaakob CM, Amin I, Noorfaizan A. Chemical and functional properties of bovine and porcine skin gelatin. Int Food Res J 2011;18:813–817 [Google Scholar]
- 16.Karim AA, Bhat R. Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll 2009;23:563–576 [Google Scholar]
- 17.Shirahama H, Lee BH, Tan LP, Cho N-J- Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci Rep 2016;6:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldana AA, Malatto L, Rehman MAU, Boccaccini AR, Abraham GA. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features. Nanomaterials 2019;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Z, Gao C, Gong Y, Shen J. Paraffin spheres as porogen to fabricate poly(L-lactic acid) scaffolds with improved cytocompatibility for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater 2003;67B:610–617 [DOI] [PubMed] [Google Scholar]
- 20.Mario Perera M, Ayres N. Gelatin based dynamic hydrogels via thiol–norbornene reactions. Polym Chem 2017;8:6741–6749 [Google Scholar]
- 21.Friedman M Applications of the Ninhydrin Reaction for Analysis of Amino Acids, Peptides, and Proteins to Agricultural and Biomedical Sciences. J Agric Food Chem 2004;52:385–406 [DOI] [PubMed] [Google Scholar]
- 22.Nayuni NK, Cloutman-Green E, Hollis M, Hartley J, Martin S, Perrett D. Critical evaluation of ninhydrin for monitoring surgical instrument decontamination. J Hosp Infect 2013;84:97–102 [DOI] [PubMed] [Google Scholar]
- 23.Sewald L, Claaßen C, Götz T, Claaßen MH, Truffault V, Tovar GEM, Southan A, Borchers K. Beyond the Modification Degree: Impact of Raw Material on Physicochemical Properties of Gelatin Type A and Type B Methacryloyls. Macromol Biosci 2018;18:1800168. [DOI] [PubMed] [Google Scholar]
- 24.McCormack T, O’Keeffe G, Craith BM, O’Kennedy R. Assessment of the Effect of Increased Fluorophore Labelling on the Binding Ability of an Antibody. Anal Lett 1996;29:953–968 [Google Scholar]
- 25.Vira S, Mekhedov E, Humphrey G, Blank PS. Fluorescent-labeled antibodies: Balancing functionality and degree of labeling. Anal Biochem 2010;402:146–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


