Abstract
In the current era, the impact of bone marrow plasma-cell percentage (BMPC%) at diagnosis of multiple myeloma (MM) is not well described. We evaluated the prognostic impact of BMPC% ≥ 60% versus < 60% in 1426 newly diagnosed MM patients. Median progression-free and overall survival were shorter for patients with BMPC% ≥ 60%, even in a multivariate analysis that included known prognostic factors for MM.
Background:
Previous reports have suggested that a higher bone marrow plasma-cell percentage (BMPC%) is associated with worse outcomes. However, it is unknown whether BMPC% is an independent predictor because genetic information was not available at that time. Currently the impact of BMPC% at diagnosis of multiple myeloma (MM) is not well described.
Patients and Methods:
We evaluated the prognostic impact of BMPC% ≥ 60% versus < 60% in 1426 newly diagnosed MM patients. All patients had an estimation of their BMPC% at diagnosis, and the highest percentage was used. Progression-free survival (PFS) and overall survival (OS) analyses were performed by the Kaplan-Meier method. Univariate and multivariate analyses for PFS and OS using the Cox proportional hazards model were performed for age, Revised International Staging System (R-ISS) score, creatinine level, and BMPC%.
Results:
BMPC% ≥ 60% was found in 562 patients (39%), and the median PFS was shorter for these patients compared to BMPC% < 60% (22.6 vs. 32.1 months; P < .0001). Also, for OS, the median was shorter for the higher BMPC% group (53.4 vs. 75.4 months; P < .0001). On the multivariate analysis for PFS, age ≥ 65 years (hazard ratio [HR], 1.46; P < .0001), R-ISS (1-2 vs. 3) (HR, 0.49; P < .0001), and BMPC% ≥ 60% (HR, 1.23; P = .015) were predictive. On the multivariate analysis for OS, age ≥ 65 years (HR, 2.23; P < .001), R-ISS (1-2 vs. 3) (HR, 0.41; P < .0001), and BMPC% ≥ 60% (HR, 1.24; P = .02) were also predictive.
Conclusion:
BMPC% ≥ 60% at diagnosis is predictive for PFS and OS, even in a multivariate analysis that included known prognostic factors for MM.
Keywords: Outcome, Prognosis, Tumor burden
Introduction
Multiple myeloma (MM) is a heterogeneous disease, and risk stratification at diagnosis is invaluable. The International Staging System (ISS) and the Revised International Staging System (R-ISS) are currently used to stage patient disease at diagnosis.1,2 Although tumor burden was an essential component of the Durie-Salmon staging system, the impact of the bone marrow plasma cell percentage (BMPC%) at diagnosis is not well described in the current era. In a retrospective study that evaluated the histologic prognostic factors in 220 MM patients between 1970 to 1979, the median overall survival (OS) was significantly shorter in the 46 patients who had BMPC% > 50%.3 In 1983, Cavo et al4 described the outcomes of 48 patients with Durie-Salmon stage I disease by BMPC %. Only 17 patients had BMPC% > 50%, and the risk of death was 2.65 times higher compared to patients who had < 50% BMPC%. In another retrospective review that evaluated 267 patients between 1977 and 1983, BMPC% > 70% was a predictor for worse survival.5 The BMPC% was also assessed as part of a prognostic index and scoring system in MM patients.6 However, these are old reports with a small number of patients, and it is unknown whether BMPC% was an independent predictor of outcomes, as genetic information was not available at that time.
We therefore evaluated the prognostic impact of BMPC% at diagnosis in the current era.
Patients and Methods
We performed a retrospective review of 1426 patients with a new diagnosis of MM assessed at Mayo Clinic Rochester (Rochester, MN) between January 2004 and 2018. A prospectively maintained database was used, and review of medical records was performed as required. International Myeloma Working Group diagnostic criteria were used to diagnose patients with active MM.7,8 The study was approved by the Mayo Clinic institutional review board. All patients had an estimation of their BMPC% by morphologic evaluation of the bone marrow aspirate and biopsy samples, and the highest percentage was used. Flow cytometry data of the bone marrow results were only used if the BMPC% was higher using this method. The data were collected via a prospectively maintained database, and the documentation of BMPC% was done from the reports. Although an independent review would have been ideal, this was not logistically possible.
The receiver operating characteristic curve was used to determine the best cut point of BMPC% that predicted higher mortality, which was 60%. Hence, patients with BMPC% ≥ 60% were compared to patients with BMPC% < 60%. Only 4% of patients had 50% to 60% BMPC%, and only 11% had 60% to 70% BMPC%. The median BMPC% was 50% for all patients across the whole time interval studied. From 2004 to 2010, the median BMPC% was 50%, and from 2011 to 2018, it was also 50%, suggesting that the interobserver variability was not significantly different across time. Depending on the data, a t test or a Wilcoxon rank-sum test were used to evaluate continuous variables, and a chi-square test or a Fisher exact test were used to test categorical data. Patients were considered to have high-risk abnormalities if they had any of the following findings on bone marrow plasma-cell fluorescence in-situ hybridization (FISH): t(4;14), t(14;16), t(14;20), and del(17p).2,9 We only recently started testing for 1q in our patients, so we did not include it as conferring high risk, because we did not have this information available in enough patients. Progression-free survival (PFS) was defined from the time of diagnosis to disease progression or death from any cause. OS was defined from the time of diagnosis to death from any cause. PFS and OS analysis were performed by the Kaplan-Meier method. All tests were 2 sided; P < .05 was considered statistically significant.
Univariate analysis (for PFS and OS) using the Cox proportional hazards model was performed for age, R-ISS, creatinine levels, and BMPC%. The hazard ratio (HR) and 95% confidence intervals were reported. Only statistically significant factors associated with PFS and OS on univariate analysis were included in the multivariate models. Statistical analysis was performed with JMP Pro 14.0 (SAS Institute, Cary, NC).
Results
We identified 1426 patients who had a new diagnosis of MM. The median age of the cohort was 66 years, and 562 patients (39%) had BMPC% ≥ 60%. The baseline characteristics of patients with BMPC% < 60% versus ≥ 60% are listed in Table 1. Patients with BMPC% ≥ 60% were more likely to have advanced stage disease, as 27% of the BMPC% ≥ 60% group had R-ISS stage III disease compared to 8% in patients with BMPC% < 60% (P < .0001). As expected, the median serum monoclonal (M spike), serum free light chain, and β2 microglobulin values were higher in this group (respectively, 3.7 vs. 2.8 g/dL, P < .0001; 80 vs. 34 mg/dL, P < .0001; 5.77 vs. 3.5 μμ/mL, P < .0001). Interestingly, patients with BMPC% ≥ 60% were also more likely to have high-risk FISH (28% vs. 18%; P = .0005). They also had a higher median plasma-cell labeling index (1.1 vs. 0.6; P < .0001). Therapy, including chemotherapy and autologous stem-cell transplantation, was similar in both groups (Table 1; P = .2).
Table 1.
Baseline Patient Characteristics by BMPC% Group
| Characteristic | BMPC% <60% (N = 864) | BMPC% ≥60% (N = 562) | P |
|---|---|---|---|
| Age (years) | 66.4 (58.8-73.3) | 66.3 (58.7-73.5) | .7 |
| Male | 531 (61) | 313 (56) | .03* |
| ISS | <.0001* | ||
| 1 | 229 (30) | 73 (14) | |
| 2 | 381 (49) | 184 (35) | |
| 3 | 162 (21) | 272 (51) | |
| R-ISS | <.0001* | ||
| 1 | 116 (18) | 40 (9) | |
| 2 | 483 (74) | 283 (64) | |
| 3 | 51 (8) | 116 (27) | |
| FISH | .0005* | ||
| Standard risk | 419 (82) | 245 (72) | |
| High risk | 91 (18) | 95 (28) | |
| FISH results | |||
| Trisomies | 329 (56) | 226 (56) | .9 |
| t(11;14) | 111 (19) | 85 (21) | .4 |
| t(6;14) | 5 (1) | 6 (1) | .3 |
| t(4;14) | 44 (7) | 43 (11) | .08 |
| t(14;16) | 22 (4) | 24 (6) | .1 |
| t(14;20) | 5 (1) | 5 (1) | .5 |
| 1q+ | 32 (4) | 26 (5) | .4 |
| del(17p)/p53 mutated | 58 (10) | 59 (15) | .02* |
| PCLI | 0.6 (0.2-1.2) | 1.1 (0.6-2.2) | <.0001* |
| Hemoglobin (g/dL) | 11.6 (10.3-13) | 10 (9-11.3) | <.0001* |
| Platelets | 224 × 10^9/1 (176-272) | 201 × 10^9/1 (142-247) | <.0001* |
| WBC | 5.4 × 10^9/l (4.2-7.1) | 5.3 (4-7) | .2 |
| Creatinine (mg/dL) | 0.9 (0.9-1.3) | 1.1 (0.9-1.6) | <.0001* |
| LDH (U/L) | 162 (135-190) | 166 (137-208) | <.0001* |
| M spike (g/dL) | 2.8 (1.9-3.7) | 3.72 (2.5-5.) | <.0001* |
| Abnormal FLC (mg/dL) | 34 (7-124) | 80 (10-303) | <.0001* |
| β2 Microglobulin (μg/mL) | 3.5 (2.57-5.1) | 5.77 (3.8-9.75) | <.0001* |
| Lytic lesions | 478 (63) | 374 (71) | .004* |
| Therapy received | .2 | ||
| ASCT | 308 (36.6) | 184 (33) | |
| IMiD only | 259 (30.8) | 158 (28.4) | |
| PI only | 38 (4.5) | 28 (5) | |
| Alkylator only | 39 (4.6) | 21 (4) | |
| Steroids only | 29 (3.5) | 32 (5.7) | |
| IMiD + PI | 73 (8.7) | 51 (9) | |
| PI + alkylator | 88 (10.5) | 78 (14) | |
| No treatment | 7 (0.8) | 4 (0.9) |
Data are presented as n (%) or median (interquartile range) unless otherwise indicated.
Abbreviations: ASCT = autologous stem-cell transplantation; BMPC% = bone marrow plasma cell percentage; del = deletion; FISH = fluorescence in-situ hybridization; FLC = free light chain; IMiD = immunomodulatory drug; ISS = International Staging System; LDH = lactate dehydrogenase; M = monoclonal; PCLI = plasma-cell labeling index; PI = proteasome inhibitor; R-ISS = Revised International Staging System; WBC = white blood cell.
Statistically significant (P < .05).
The median PFS and OS for the whole cohort were 26.6 and 65.9 months, respectively. Between the two groups, the median PFS was shorter for those with BMPC% ≥ 60% compared to < 60% (respectively, 22.6 vs. 32.1 months; P < .0001) (Figure 1A). Also, for OS, the median was shorter for the high BMPC% group (53.4 vs. 75.4 months; P < .0001) (Figure 1B). We specifically looked at the interaction between FISH risk status and the BMPC%. For standard-risk FISH, patients with BMPC% ≥ 60% had a shorter median PFS compared to patients with BMPC% < 60% (26.1 vs. 32.7 months; P = .02) (Figure 1C). Also, the median OS was shorter in patients with BMPC% ≥ 60% (66 vs. 96.5 months; P = .04) (Figure 1D). For high-risk FISH, the median PFS and OS were shorter in patients with BMPC% ≥ 60% compared to < 60% (15.8 vs. 23.2 months, P = .001; and 32.9 vs. 64 months, P = .001, respectively; Figure 1E, F).
Figure 1.
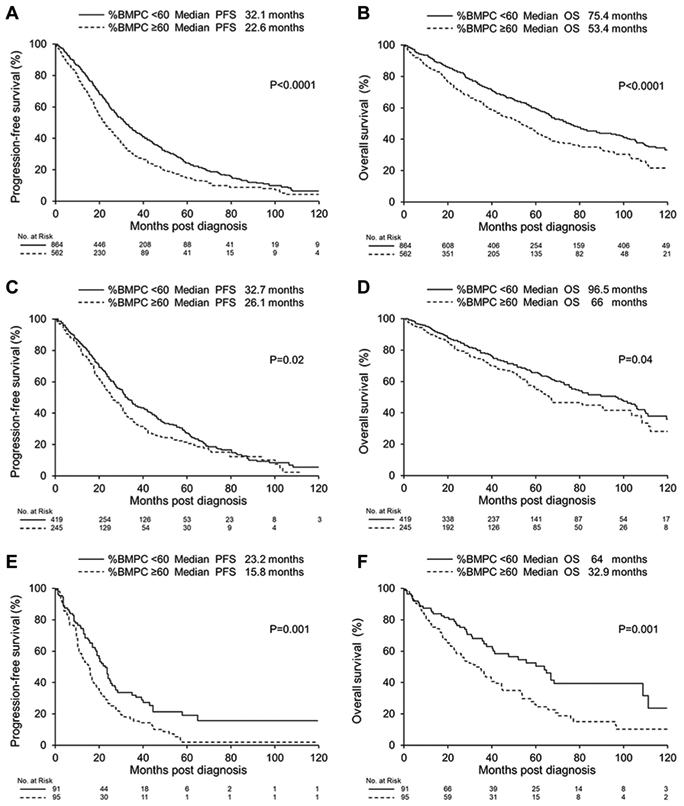
Kaplan-Meier PFS and OS Curves (A) PFS and (B) OS according to BMPC% at Diagnosis. (C) PFS and (D) OS in Patients With Standard-risk Disease Assessed by FISH. (E) PFS and (F) OS in Patients With High-risk Disease Assessed by FISH
Abbreviations: BMPC% = bone marrow plasma-cell percentage; FISH = fluorescence in-situ hybridization; PFS = progression-free survival; OS = overall survival.
We also evaluated the prognostic effect of BMPC% across two time intervals, 2004-2010 and 2011-2018. During the first time interval, the median PFS for patients with BMPC% ≥ 60% was 20.4 months compared to 28 months in patients with BMPC% < 60% (P = .002). For the second time interval, the median PFS was also shorter for the high BMPC% group (24.4 vs. 36.1 months; P < .0001). For OS, the median OS was shorter for BMPC% ≥ 60% versus < 60% for both the first interval (median OS: 50.9 vs. 68.6 months; P = .001) and the second interval (median OS: 55.6 vs. not reached; P = .0001).
On univariate analysis, predictive variables for PFS were age ≥ 65 years (HR, 1.33; P < .0001), R-ISS (1-2 vs. 3) (HR, 0.49; P < .0001), creatinine > 2 mg/dL (HR, 1.4; P = .0006), and BMPC% ≥ 60% (HR, 1.4; P < .0001). On the multivariate analysis for PFS, age ≥ 65 years (HR, 1.46; P < .0001), R-ISS (1-2 vs. 3) (HR, 0.49; P < .0001), and BMPC% ≥ 60% (HR, 1.23; P = .015) were predictive (Table 2). For the univariate analysis of OS, age ≥ 65 years (HR, 1.88; P < .0001), R-ISS (1-2 vs. 3) (HR, 0.38; P < .0001), creatinine > 2 mg/dL (HR, 1.66; P < .0001), and BMPC% ≥ 60% (HR, 1.44; P < .0001) were all predictive. On the multivariate analysis for OS, age ≥ 65 years (HR, 2.23; P < .001), R-ISS (1-2 vs. 3) (HR, 0.41; P < .0001), and BMPC% ≥ 60% (HR, 1.24; P = .02) were predictive (Table 2).
Table 2.
Univariate and Multivariate Analysis for PFS and OS
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | P | HR (95% CI) | P |
| PFS | ||||
| Age ≥65 years | 1.33 (1.16-1.53) | <.0001* | 1.46 (1.25-1.71) | <.0001* |
| R-ISS (1-2 vs. 3) | 0.49 (0.40-0.60) | <.0001* | 0.49 (0.39-0.62) | <.0001* |
| Creatinine >2 mg/dL | 1.4 (1.57-1.71) | .0006* | 0.93 (0.72-1.22) | .63 |
| BMPC% ≥60% | 1.4 (1.24-1.62) | <.0001* | 1.23 (1.03-1.52) | .015* |
| OS | ||||
| Age ≥65 years | 1.88 (1.60-2.21) | <.0001* | 2.23 (1.84-2.70) | <.0001* |
| R-ISS (1-2 vs. 3) | 0.38 (0.31-0.48) | <.0001* | 0.41 (0.32-0.52) | <.0001* |
| Creatinine >2 mg/dL | 1.66 (1.34-2.07) | <.0001* | 1.18 (0.88-1.57) | .25 |
| BMPC% ≥60% | 1.44 (1.23-1.69) | <.0001* | 1.24(1.03-1.49) | .02* |
Abbreviations: BMPC% = bone marrow plasma cell percentage; CI = confidence interval; HR = hazard ratio; OS = overall survival; PFS = progression-free survival; R-ISS = Revised International Staging System.
Statistically significant (P < .05).
Discussion
In our cohort, patients who had BMPC% ≥ 60% had a shorter median PFS and OS, which appeared to be independent of conventional risk factors such as age and R-ISS. Few old reports evaluated the prognostic impact of BMPC% at diagnosis,3-5 and having more than 50% to 70% BMPC% was associated with inferior outcomes. In one report that evaluated only 48 patients, the relative risk of death was 2.65 for patients with BMPC% > 50%, compared to 0.31 for those with BMPC% < 50%.4 Cherng et al5 evaluated 267 patients, and having BMPC% > 70% was associated with a HR that was 2.2 times higher than those with BMPC% < 30%. However, the number of patients was small and the diagnosis was mainly done using the criteria of the Chronic Leukemia–Myeloma Task Force.10 Also, the treatment of MM was limited, and FISH risk was not established.
In one retrospective report that evaluated 787 MM patients, having a BMPC% ≥ 30% was associated with shorter median OS compared to BMPC% < 30%.11 However, the multivariate analysis did not include ISS, and FISH was not available at that time. In another study that evaluated MM disease burden using magnetic resonance imaging of the bone marrow, patients who had more estimated bone marrow involvement had worse survival,12 even in a multivariate analysis that included ISS. Metabolic tumor volume assessed by positron emission tomography scan is currently being investigated as a marker for evaluating disease burden in MM; patients with higher metabolic tumor volume values had worse PFS and OS.13,14 Metabolic tumor volume values also correlated positively with the BMPC%.13
The relationship between FISH risk and the BMPC% is interesting. One could speculate that the higher proportion of high-risk FISH markers in the ≥ 60% group may be a reflection of a more aggressive disease evolution and/or disease diagnosed later. The median OS was nearly half of that in patients with high-risk FISH and BMPC% < 60%. This suggests that the increased BMPC% can aggravate the effect of FISH on outcomes, and patients with higher disease burden at diagnosis might have worse outcomes. The prognostic effect of BMPC% persisted and was even more profound during the 2011-2018 time interval compared to 2004-2010, suggesting that it still could be applied to patients diagnosed in the current era.
The bone marrow microenvironment is affected by plasma cells, and in one study, the BMPC% at diagnosis correlated positively with the number of mesenchymal stromal cells that expressed STRO-1 but negatively with the number of osteoblasts.15 These mesenchymal cells are a rich source of osteoclasts and plasma-cell activating factors, which could mediate plasma-cell growth and survival.15 Having a higher burden of disease at diagnosis could enhance the interaction of plasma cells with the microenvironment, making disease control more difficult.
Our study is limited by its retrospective nature. Staging data were missing in some patients. Also, the treatment for MM has changed over the years. Despite this, we found that the BMPC% at diagnosis was predictive for PFS and OS, even in a multivariate analysis that included known prognostic factors for MM, including R-ISS. This likely reflected higher disease burden at diagnosis (more high-risk FISH and higher plasma-cell labeling index), which affects outcomes. The prognostic effect of BMPC% was not affected by the long time interval of our study, and the impact persisted across different time intervals.
Clinical Practice Points.
Previous reports have suggested that higher bone marrow plasma cell percentage (BMPC%) is associated with worse outcomes. However, these reports had small numbers of patients, and it is unknown whether BMPC% was an independent predictor of outcomes, as genetic information was not yet available. In the current era, the impact of BMPC% at diagnosis of multiple myeloma is not well described.
BMPC% ≥ 60% was found in 39% of all patients, and median PFS was shorter for these patients compared to BMPC% < 60%. Median OS was also shorter for the higher BMPC% group.
For standard-risk FISH, patients with BMPC% ≥ 60% had a shorter median PFS compared to patients with BMPC% < 60%. Median OS was shorter in patients with BMPC% ≥ 60%.
For high-risk FISH, median PFS and OS were shorter in patients with BMPC% ≥ 60% compared to < 60%.
Multivariate analysis concluded that age ≥ 65 years, R-ISS (1-2 vs. 3), and BMPC% ≥ 60% were predictive for both PFS and OS.
BMPC% ≥ 60% at diagnosis is predictive for PFS and OS.
Footnotes
Disclosure
The authors have stated that they have no conflict of interest.
References
- 1.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23:3412–20. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: a report from International Myeloma Working Group. J Clin Oncol 2015; 33:2863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartl R, Frisch B, Burkhardt R, et al. Bone marrow histology in myeloma: its importance in diagnosis, prognosis, classification and staging. Br J Haematol 1982; 51:361–75. [DOI] [PubMed] [Google Scholar]
- 4.Cavo M, Baccarani M, Gobbi M, Lipizer A, Tura S. Prognostic value of bone marrow plasma cell infiltration in stage I multiple myeloma. Br J Haematol 1983; 55:683–90. [DOI] [PubMed] [Google Scholar]
- 5.Cherng NC, Asal NR, Kuebler JP, Lee ET, Solanki D. Prognostic factors in multiple myeloma. Cancer 1991; 67:3150–6. [DOI] [PubMed] [Google Scholar]
- 6.Corrado C, Santarelli MT, Pavlovsky S, Pizzolato M. Prognostic factors in multiple myeloma: definition of risk groups in 410 previously untreated patients: a Grupo Argentino de Tratamiento de la Leucemia Aguda study.J Clin Oncol 1989;7:1839–44. [DOI] [PubMed] [Google Scholar]
- 7.International Myeloma Working G Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121:749–57. [PubMed] [Google Scholar]
- 8.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15:e538–48. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar SV. Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol 2018; 93:981–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proposed guidelines for protocol studies. II. Plasma cell myeloma. Prepared by a Committee of the Chronic Leukemia—Myeloma Task Force, National Cancer Institute. Cancer Chemother Rep 1968; 3:17–39. [PubMed] [Google Scholar]
- 11.Qian J, Jin J, Luo H, et al. Analysis of clinical characteristics and prognostic factors of multiple myeloma: a retrospective single-center study of 787 cases. Hematology 2017; 22:472–6. [DOI] [PubMed] [Google Scholar]
- 12.Ailawadhi S, Abdelhalim AN, Derby L, et al. Extent of disease burden determined with magnetic resonance imaging of the bone marrow is predictive of survival outcome in patients with multiple myeloma. Cancer 2010; 116:84–92. [DOI] [PubMed] [Google Scholar]
- 13.Fonti R, Larobina M, Del Vecchio S, et al. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med 2012; 53:1829–35. [DOI] [PubMed] [Google Scholar]
- 14.McDonald JE, Kessler MM, Gardner MW, et al. Assessment of total lesion glycolysis by 18F FDG PET/CT significantly improves prognostic value of GEP and ISS in myeloma. Clin Cancer Res 2017; 23:1981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noll JE, Williams SA, Tong CM, et al. Myeloma plasma cells alter the bone marrow microenvironment by stimulating the proliferation of mesenchymal stromal cells. Haematologica 2014; 99:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


