Abstract
Nicotinic acetylcholine receptors can regulate inflammation primarily through the vagus nerve via the cholinergic anti-inflammatory pathway. α9α10 nicotinic receptors (nAChRs) are a new promising target for chronic pain and inflammation. Recently, α9α10 selective α-conotoxin antagonists were shown to have antinociception effect in neuropathic and tonic inflammatory pain animal models. However, limited data available on the role of α9α10 nAChRs in experimental colitis. In this study, we report for the first time, the role of α9α10 nAChRs in the dextran sodium sulfate (DSS) experimental animal colitis model. We determined the effect of the α9α10 nAChRs antagonist, α-conotoxin RgIA (α-RgIA) in DSS-induced colitis model in adult male and female C57BL/6J mice. DSS solution was freely given in the drinking water for seven consecutive days, and tap water was given on the 8th day. We then sacrificed mice on day 8 to examine the entire colon. Disease severity, colon tissue histology, and tumor necrosis factor-α (TNF-α) were evaluated. The lower doses (0.02 and 0.1 nmol/mouse, s.c.) of α-RgIA treatment in DSS-treated mice were inactive, whereas the higher dose (0.2 nmol/mouse, s.c.) reversed the disease activity index (DAI) score , loss of body weight, total histological damage score, as well as the colonic level of TNF-α compared to the DSS control group. Moreover, the highest dose of α-RgIA (0.2 nmol/mouse, s.c.) significantly rescued the colon length shortening in DSS-treated mice compared to the DSS-control mice. The availability of α9*-selective conotoxins has opened new avenues in pharmacology research and potential targets in inflammatory disorders.
Keywords: α9α10 nAChRs, α-RgIA, Colitis, Mice
1. Introduction
The α9α10 nicotinic acetylcholine receptor (nAChR) subtype is a promising new target for pain and inflammation (Hone and McIntosh, 2018; Romero et al., 2017). The α9α10 nAChRs have a distinct pharmacological and anatomical distribution profile among the nAChRs and α10 subunits form functional heteropentamer nicotinic receptors when co-expressed with α9 subunits in oocytes (Elgoyhen et al., 2001; Sgard et al., 2002), with α9(2)α10(3) being the major functional receptor (Plazas et al., 2005). α9 and α10 subunits have restricted anatomical tissue expression (Gotti et al., 2006; Nashmi and Lester, 2006). While their expression in the CNS is sparse and limited (Elgoyhen et al., 2001; Mihara et al., 2017; Sgard et al., 2002), gene transcripts for α9 or α10 have been reported within hair cells of the inner ear (Elgoyhen et al., 2001, 1994; Hiel et al., 1996; Vetter et al., 2007), dorsal root ganglion (DRG) neurons (Lips et al., 2002) and in rat intestinal mesothelial cells and ileal muscle layer (Mihara et al., 2017).
In addition, both α9 and α10 are present in many immune cells (Fujii et al., 2017; Galvis et al., 2006; Kawashima et al., 2012, 2007; Lustig et al., 2001; Peng et al., 2004; Wessler and Kirkpatrick, 2008) such as lymphocytes, and macrophages. Recently, (St-Pierre et al., 2016) reported that nicotine inhibited IL-1β, TNF-α, and IL-12 production in bone marrow-derived monocytes and stimulated IL-10, the anti-inflammatory cytokine, secretion via α7 and α9 nAChRs immune regulatory pathways. In addition functional α7 and α9 nicotinic receptors have been demonstrated in monocyte ATP-mediated release of IL-1β (Hecker et al., 2015; Richter et al., 2016; Zakrzewicz et al., 2017).
Administration of α9α10 selective antagonists showed an antinociception effect in neuropathic and tonic inflammatory pain animal models. For example, α-conotoxin RgIA, a potent and selective α9α10 nAChR antagonist, reduced mechanical hypersensitivity in animal models of nerve injury (Ellison et al., 2006; Livett et al., 2006; Vincler et al., 2006), and chemotherapy-induced neuropathy (Pacini et al., 2016; Wala et al., 2012). In addition, GeXIVA a potent α9α10 nAChR antagonist (Luo et al., 2015), produced long-lasting antinociceptive effects in the rat neuropathic pain model (Li et al., 2016).
However, to our knowledge, the α9α10 nAChRs subunit involvement has not been investigated in the murine experimental colitis model. In our previous study, we reported that oral nicotine administration dose-dependently reduced the clinical parameters of dextran sodium sulfate (DSS) colitis in mice (AlSharari et al., 2013). In addition, we and others investigated the role of α7 nAChRs in mouse colitis models (Abdrakhmanova et al., 2010; AlSharari et al., 2017; Costa et al., 2012; Salaga et al., 2016). The use of α-conotoxin RgIA, an α9α10 nAChRs selective antagonist that distinguishes between α7 and α9α10 nAChRs (Ellison et al., 2006; Terlau and Olivera, 2004), enabled us to identify a possible role for α9* nAChRs in colitis. In the present study, we used dextran sulfate sodium (DSS) which is widely used in animal model of colitis and shows resemblance to human ulcerative colitis (UC) in both clinical and histopathologic findings (Cooper et al., 1993; Gaudio et al., 1999; Okayasu et al., 1990). The aim of the present study was to determine whether α-conotoxin RgIA, an α9α10 nAChRs selective antagonist, would prevent and alleviate the various measures and signs of colitis in mice induced by DSS.
2. Materials and Methods
2.1. Animals
Male and female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). We used approximately 50% male and 50% female in our studies. The animals were 8–10 weeks of age at the start of the experiments, weighing 25–30 g and were group-housed (a maximum six per cage) in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility with ad libitum access to food and water. Mice were housed under standard conditions for a minimum of 1 week before experimentation. In the first set of experiments, were we measured the disease activity index (DAI) score, % body weight change, and colon length, eleven mice per group were used; in the second set of experiments, were we determined total histological score , ten mice per group were used. Further, seven mice per group were used to evaluate the level of colonic tumor necrosis factor-alpha (TNF-α) Levels. Experiments were performed during the light cycle (7:00 am to 7:00 pm) and were approved under application approval numbers AM10305 and AM10142 by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Drugs
α-Conotoxin, α-RgIA was synthesized, as described previously (Ellison et al., 2006; McIntosh et al., 2005). α-RgIA was dissolved in physiological saline (0.9% sodium chloride) and administered by subcutaneous (s.c.) injection at the doses of 0.02, 0.1, and 0.2 nmol per mouse at a volume of 0.3 ml/mouse. The doses of α-RgIA were selected in the current study based on pilot experiments. dextran sodium sulfate (DSS), (molecular weight, 36–50,000 kilodaltons) was purchased from ICN Biomedicals Inc (Aurora, OH).
2.3. DSS-Induced colitis Model
DSS in drinking water (2.5 %) (wt/vol) was given for seven days, and on day 8 replaced with normal drinking water. Controls consisted of age- and time-matched mice that received regular tap drinking. The average daily consumption of DSS by mice was 95–105 mg/mouse/day (data not shown). In the first study, which focused on determining the impact of α-RgIA on Disease Activity Index (DAI), body weigh change and colon length; mice were allocated to the following experimental treatments: (1) control (water only), (2) water + 0.2 nmol α-RgIA (3) DSS + saline (4) DSS + 0.02 nmol α-RgIA, (5) DSS + 0.1 nmol α-RgIA, (6) DDS + 0.2 nmol α-RgIA. The second study included examining the effect of the α-RgIA on the total histological score and estimation of colonic TNF-α level; mice were allocated to the following experimental treatments: (1) control (water only), (2) water + 0.2 nmol α-RgIA (3) DSS + saline (4) DSS + 0.02 nmol α-RgIA, (5) DDS + 0.2 nmol α-RgIA. α-RgIA was injected to mice three days before and for seven days after the induction of colitis.
2.3.1. Assessment of the Severity of Colitis: Disease Activity Index
Disease Activity Index (DAI) scores historically have correlated well with the pathologic findings in a DSS-induced model of IBD (Cooper et al., 1993). DAI is the combined score of four clinical parameters, including a) weight loss, b) stool consistency, c) rectal irritation, and d) blood in the stool. Scores were defined as follows: for weight: 0, no loss; 1, 5%–10%; 2, 10%–15%; 3, 15%–20%; and 4, 20% weight loss; for irritation around the anal area; 0, normal; 1, mild irritation; 2, moderate irritation; 3, severe irritation; for stool consistency: 0, normal; 1, mild loose stool; 2, moderate loose stool and 3, diarrhea; and for bleeding: 0, no blood; 1, presence of blood (Hemoccult II positive; Beckman Coulter, Fullerton, CA); and 2, gross blood. Total DAI score ranged from 0–12. Body weights were measured daily from days 0–8 at the same time of the day (9:00 am), and results were expressed % change from day 0 weight. On day 8, after replacing the DSS with water, DAI scores were measured, and mice were then sacrificed, and the abdominal cavity was opened, the colon was immediately removed and the colon length (cm) measured.
2.3.2. Colonic Histology Assessment
Seven days after the beginning of the DSS treatment, mice were sacrificed, and the colon was removed. Formalin-fixed colon segments were paraffin-embedded, and 3-μm sections were stained with hematoxylin-eosin (H&E). Colonic damage was scored based on a published scoring system that considers architectural derangements, epithelium changes, goblet cell depletion, ulceration, and degree of inflammatory cells infiltrate in a blinded fashion (Iba et al., 2003). The histological scoring system was used to evaluate the degree of colitis. The total histological score ranged from 0 to 12, which represented the sum of scores from 0 to 3 (0= none, 1= 0–5 %, 2= 5–10%, 3= > 10%) for loss of epithelium, (0= none, 1= 0–10 %, 2= 10–20%, 3= > 20%) for crypt damage, (0= none, 1= mild, 2= moderate, and 3= severe) for each of depletion of goblet cells and infiltration of inflammatory cells. Each section was scored for each feature separately by establishing the product of the grade for that feature and the percentage involvement in the loss of epithelium and crypt damage features (in a range from 0 to 3 for each feature). The number of inflammatory cells infiltration in 10 randomly selected power fields (40X) was counted, and the number per 10 fields was calculated. The scores were assigned by one experienced pathologist with no knowledge of the group being examined in each analysis. The histological colitis score of individual mice represents the sum of the different histological sub scores. Light microscope images were acquired with an Axioscope AX10 microscope and Axiovision 4.6 software (Carl Zeiss, Inc.). The effect of α-RgIA on the histological damage was assessed at the lowest and highest dose (0, 0.02, and 0.2 nmol).
2.3.3. Tumor Necrosis Factor-Alpha (TNF-α) Levels
In a separate group of mice, animal groups were allocated to the following treatments: (1) control (water only), (2) α-RgIA (0, 0.02, and 0.2 nmol) + 2.5% DSS. TNF-α colonic levels were measured as recently described by our group (AlSharari et al., 2013). The colonic samples were homogenized in 1 mL of Tris-HCl buffer containing protease inhibitors (Sigma-Aldrich Inc., St. Louis, MO, USA). Samples were centrifuged for 30 min, and the supernatant was frozen at − 80°C until assay. Cytokine level (TNF-α) was determined using an enzyme-linked immunosorbent assay commercial kit (Quantikine M murine; R&D Systems, Minneapolis, MN). The effect of α-RgIA on TNF-α colonic levels was assessed at the lowest and highest dose (0, 0.02, and 0.2 nmol).
2.4. Statistical Analysis
Data were analyzed using the GraphPad Prism software version 8.0.2 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M.; we used a non-parametric test (Kruskal-Wallis followed by Dunn’s multiple comparison tests to determine the significant differences in the DAI score and total histologic score. For parametric tests, we used unpaired two-tailed t-test to determine the pathological effects of DSS administration to the mice, the results of Vehicle-DSS treated group of mice were compared to Water-Saline treated group of mice. Additionally, ordinary one-way analysis of variance (ANOVA) was used to analyze the impact of α-RgIA treatment doses compared to vehicle-DSS treated mice. Significant overall ANOVA were followed by Tukey’s test post hoc test when appropriate. All differences were considered significant at *P < 0.05.
3. Results
3.1. Effect of repeated subcutaneous α-RgIA administration in DSS-treated C57BL/6J mice on DAI, body weight, and colon length
To determine if α-RgIA has a preventive effect on DSS induced colitis, mice were given repeated subcutaneous doses of α-RgIA (0, 0.02, 0.1, 0.2 nmol/mouse) once daily. The non-parametric test (Kruskal-Wallis with Dunn’s multiple comparison tests) showed that DSS at a concentration of 2.5% produced a significant increase in the disease activity index (DAI score) in the mice were signs seen as irritation around anal area, diarrhea, and the presence of blood in the stool at day eight compared to vehicle control, P< 0.0001 (Fig.1A). In addition, α-RgIA significantly reversed the disease activity index at a dose of 0.2 nmol/mouse, P<0.01 (Fig.1A). Likewise, DSS resulted in a significant decrease in body weight percentage compared to the control group of mice, t=11.84, df=18, P< <0.0001 (Fig.1B). Further, one-way analysis revealed that repeated administration of α-RgIA only at doses of 0.1 and 0.2 nmol/mouse significantly reversed the body weight percentage decrease in the DSS-treated mice, F (3, 36) = 12.55, P<0.0001 (Fig.1B). Similarly, the colon of mice treated with DSS only displayed a significant length shortening in comparison to the water treated mice, t=9.448, df=20, P<0.0001 (Fig.1C). Furthermore, only the highest dose of α-RgIA (0.2 nmol/mouse) significantly rescued the colon length shortening treated with DSS, F (3, 40) = 10.10, P<0.0001; whereas α-RgIA at doses of 0.02 and 0.1 nmol/mouse failed to rescue the colon shortening of mice treated with DSS P>0.05 (Fig.1C).
Fig. 1:
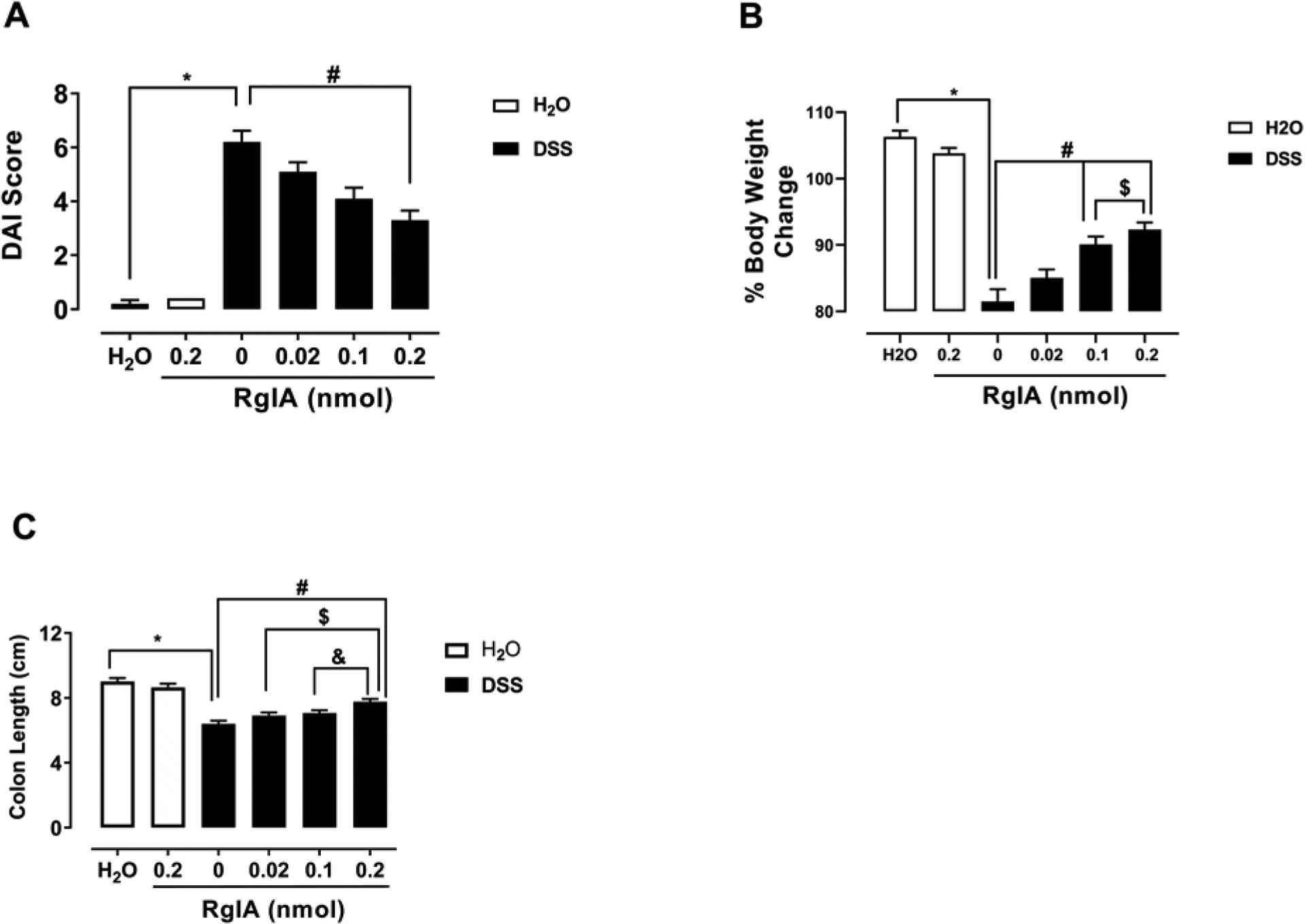
α-RgIA treatment reverses clinical signs of colitis in a mouse model. A) The repeated administration of α-RgIA only at dose of 0.2 nmol/mouse, s.c. reverses the increased DAI induced by DSS; * P<0.0001 DSS-Saline vs. Water-Saline; # P<0.01 DSS-Saline vs. α-RgIA at dose of 0.2 nmol/mouse. B) The repeated administration of α-RgIA at doses of 0.1 and 0.2 nmol/mouse, s.c. attenuate the decreased percentage of body weight change induced by DSS; * P<0.0001 DSS-Saline vs. Water-Saline; # P<0.0001 DSS-Saline vs. α-RgIA at dose of 0.1 and 0.2 nmol/mouse; $ P<0.01 α-RgIA 0.1 vs. 0.2 nmol/mouse. C) The repeated administration of α-RgIA only at dose of 0.2 nmol/mouse, s.c. rescued the shortening of the colon length; * P<0.0001 DSS-Saline vs. Water-Saline; # P<0.0001 DSS-Saline vs. α-RgIA at a dose of 0.2 nmol/mouse; $ P<0.01 α-RgIA 0.02 vs. 0.2 nmol/mouse; & P<0.05 α-RgIA 0.1 vs. 0.2 nmol/mouse; DAI, Disease Activity Index; DSS, dextran sodium sulfate n= 11 mice/group; data expressed as mean ± SEM.
3.2. Effect of repeated subcutaneous α-RgIA treatment in DSS-treated C57BL/6J mice on histological damage, and TNF-α colonic levels
We further examined the effect of DSS on the histological changes and TNF-α level in mouse colon and whether α-RgIA treatment would reverse the pathological changes induced by DSS. For that, analyzing the data for the mice treated with DSS only with the non-parametric test (Kruskal-Wallis with Dunn’s multiple comparison tests) showed a significant increase in the total histologic score compared to the water treated mice, P<0.001 (Fig. 2A). Additionally, the Dunn’s multiple comparison test revealed that mice repeatedly treated with α-RgIA only at a dose of (0.2 nmol/mouse) significantly reversed the increased total histologic score, P<0.05; whereas the 0.02 dose of α-RgIA failed to reverse the increased total histological score, P>0.05 (Fig. 2A). Finally, there was a significant elevation in the colonic level of the TNF-α in mice treated with DSS only, t=4.359, df=12, P<0.0001 (Fig.2B). Importantly, the highest α-RgIA dose (0.2 nmol/mouse) significantly reduced the elevated level of colonic level of the TNF-α, F (2, 18) = 4.529, P=0.0255; however, the α-RgIA dose of 0.02 nmol/mouse did not significantly reduced the elevated level of colonic level of the TNF-α in mice treated with DSS, P>0.05 (Fig.2B).
Fig. 2:
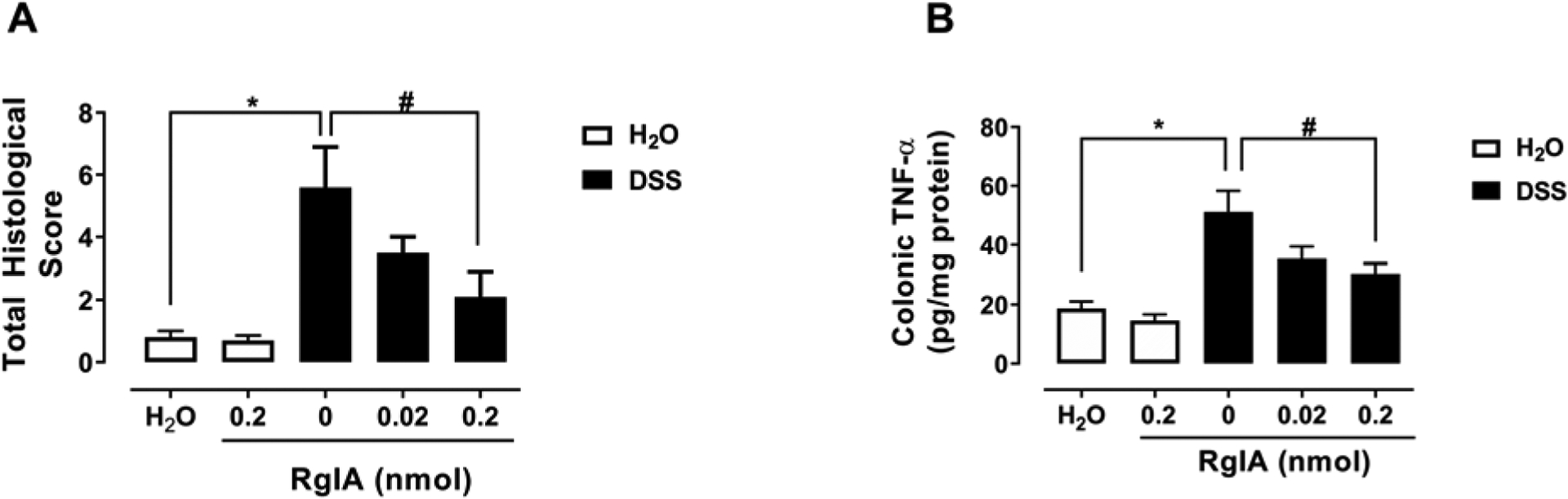
α-RgIA treatment attenuates the colon histopathological changes and inflammation marker. A) The repeated administration of the highest dose of α-RgIA (0.2 nmol/mouse) s.c. attenuates total histological score; * P<0.001 DSS-Saline vs. Water-Saline; # P<0.05 DSS-Saline vs. α-RgIA 0.2 nmol/mouse, n= 10 samples/group. B) and B) The repeated administration of the highest dose of α-RgIA (0.2 nmol/mouse) reduces the increased level of colonic TNF-α; * P<0.0001 DSS-Saline vs. Water-Saline; # P<0.05 DSS-Saline vs. α-RgIA 0.2 nmol/mouse, n= 7 samples/group; data expressed as mean ± SEM.
4. Discussion
In the present study, we explored the effects of in vivo systemic administration of α-RgIA, a selective α9α10 antagonist, in a mouse model of experimental colitis. We demonstrate for the first time that α-RgIA reduces the severity of DSS-induced colitis pathology in mice. These findings suggest that α9α10 nAChRs may play an important role in endogenous pro-inflammatory mechanisms required for colitis initiation and evolution.
The repeated daily s.c. administration of α-RgIA improved several clinical signs of colitis in a dose-related manner. Treatment decreased the DAI scores, attenuated the decrease in colon length and the loss of body weight with the highest dose of 0.2 nmol/animal significantly reducing all of these signs. In addition, α-RgIA treatment completely reversed DSS-induced histological damage and an increase of colonic TNF α levels. In non-DSS control mice, RgIA treatment did not significantly alter any of the parameters measured. It is possible that higher doses of α- RgIA would have fully reversed all the colitis signs. Indeed, the doses used in our mouse studies are much lower (5-fold lower on an mg/kg basis) than those reported to be fully active in rat models of nerve injury and chemotherapy-induced neuropathy (Di Cesare Mannelli et al., 2014; Pacini et al., 2016). α-Conotoxins are charged peptides, so they are unlikely to cross the blood-brain barrier (BBB). Thus, the action of RgIA most likely occurs at peripheral sites. α-RgIA may act in the DSS-induced colitis model through antagonism of α9α10 nAChRs on immune cells in the enteric nervous system, to reduce inflammation development and disease activity. The release of pro-inflammatory cytokines from immune cells such as macrophages play an important role in the pathogenesis of several inflammatory disease conditions, including inflammatory bowel diseases. We speculate that α-RgIA may decrease migration or infiltration of cytokine-producing cells into the site of inflammation. Recently, (Richter et al., 2016), reported the importance of α9 and α10 nAChR subunits for the anti-inflammatory signaling, IL-1β release, in pharmacological, gene-silencing, and gene-deficient mice studies. In line with these findings, (Zakrzewicz et al., 2017), demonstrated the need for α7, α9 and α10 nAChR subunits for IL-1β release mediated via the adenosine triphosphate (ATP) signaling mechanism in monocytes.
We have previously reported that female mice are less sensitive than male animals to the anti-inflammatory effects of α7 nAChR agonists and positive allosteric modulators in a the DSS colitis model (AlSharari et al., 2017),. In the present study, we investigated the role of α9α10 subtypes. These subtypes are different in terms of distribution, functionality, and pharmacological response to nicotine. In addition, it has been shown recently that RgIA4, a potent and selective α9α10 antagonist, reverses and prevent aspects of oxaliplatin-induced peripheral neuropathy in mice (Christensen et al., 2017) and in rats (Romero et al., 2017) without any reported sex differences.
The limited anatomical expression of α9α10 nAChRs might be an advantage in targeting these nAChRs, which also, could decrease the possibility of undesirable effects. Indeed, administration of α9α10 nicotinic antagonists did not produce toxicity in the rodent after systemic injection (McIntosh et al., 2009; Vincler et al., 2006; Vincler and McIntosh, 2007). In addition, since the α9α10 selective α-RgIA is an antagonist, tolerance is less likely to develop.
Conotoxin-based compounds have been tested in human clinical trials as analgesics for pain therapy. For example, ω-conotoxin MVIIA is an FDA approved drug known as Prialt® or ziconotide for the amelioration of severe and chronic pain. Ziconotide’s effect is mediated by blocking N-type Ca2+ channels (Deer et al., 2019; Safavi-Hemami et al., 2019). The development of α9α10 nAChR selective conotoxins would open new avenues in pharmacology research and potential targets in the pain states and inflammatory disorders due to the unique properties of this receptor.
α-conotoxin RgIA reduces the signs of experimental colitis in mice
α9α10 nAChRs are possible targets to treat inflammatory pain.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grant DA-019377 to M.I.D. and GM103801 to JMM. The authors would like to thank the deanship of scientific research, and research center, college of pharmacy, King Saud University, Riyadh, Saudi Arabia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The University of Utah has patented conotoxins, including α-RgIA on which JMM is listed as an inventor. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The first author and all co-authors have seen and approved the final version of the paper and have agreed to its submission for publication.
References
- Abdrakhmanova GR, AlSharari S, Kang M, Damaj MI, Akbarali HI, 2010. {alpha}7-nAChR-mediated suppression of hyperexcitability of colonic dorsal root ganglia neurons in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol 299, G761–8. 10.1152/ajpgi.00175.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSharari SD, Akbarali HI, Abdullah RA, Shahab O, Auttachoat W, Ferreira GA, White KL, Lichtman AH, Cabral GA, Damaj MI, 2013. Novel insights on the effect of nicotine in a murine colitis model. J. Pharmacol. Exp. Ther 344, 207–17. 10.1124/jpet.112.198796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSharari SD, Bagdas D, Akbarali HI, Lichtman PA, Raborn ES, Cabral GA, Carroll FI, McGee EA, Damaj MI, 2017. Sex Differences and Drug Dose Influence the Role of the α7 Nicotinic Acetylcholine Receptor in the Mouse Dextran Sodium Sulfate-Induced Colitis Model. Nicotine Tob. Res 19, 460–468. 10.1093/ntr/ntw245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SB, Hone AJ, Roux I, Kniazeff J, Pin J-P, Upert G, Servent D, Glowatzki E, McIntosh JM, 2017. RgIA4 Potently Blocks Mouse α9α10 nAChRs and Provides Long Lasting Protection against Oxaliplatin-Induced Cold Allodynia. Front. Cell. Neurosci 11, 219 10.3389/fncel.2017.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ, 1993. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest 69, 238–49. [PubMed] [Google Scholar]
- Costa R, Motta EM, Manjavachi MN, Cola M, Calixto JB, 2012. Activation of the alpha-7 nicotinic acetylcholine receptor (α7 nAchR) reverses referred mechanical hyperalgesia induced by colonic inflammation in mice. Neuropharmacology 63, 798–805. 10.1016/j.neuropharm.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Deer TR, Pope JE, Hanes MC, McDowell GC, 2019. Intrathecal Therapy for Chronic Pain: A Review of Morphine and Ziconotide as Firstline Options. Pain Med. 20, 784–798. 10.1093/pm/pny132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Cinci L, Micheli L, Zanardelli M, Pacini A, McIntosh MJ, Ghelardini C, McIntosh JM, Ghelardini C, 2014. α-Conotoxin RgIA protects against the development of nerve injury-induced chronic pain and prevents both neuronal and glial derangement. Pain 155, 1986–1995. 10.1016/j.pain.2014.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S, 1994. α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79, 705–15. 10.1016/0092-8674(94)90555-x [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J, 2001. α10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc. Natl. Acad. Sci. U. S. A 98, 3501–6. 10.1073/pnas.051622798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison M, Haberlandt C, Gomez-Casati ME, Watkins M, Elgoyhen AB, McIntosh JM, Olivera BM, 2006. α-RgIA: A Novel Conotoxin That Specifically and Potently Blocks the α9α10 nAChR † , ‡. Biochemistry 45, 1511–1517. 10.1021/bi0520129 [DOI] [PubMed] [Google Scholar]
- Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, Kawashima K, 2017. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci 134, 1–21. 10.1016/j.jphs.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Galvis G, Lips KS, Kummer W, 2006. Expression of nicotinic acetylcholine receptors on murine alveolar macrophages. J. Mol. Neurosci 30, 107–8. 10.1385/JMN:30:1:107 [DOI] [PubMed] [Google Scholar]
- Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, Caprilli R, 1999. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig. Dis. Sci 44, 1458–75. 10.1023/a:1026620322859 [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F, 2006. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci 27, 482–91. 10.1016/j.tips.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Hecker A, Küllmar M, Wilker S, Richter K, Zakrzewicz A, Atanasova S, Mathes V, Timm T, Lerner S, Klein J, Kaufmann A, Bauer S, Padberg W, Kummer W, Janciauskiene S, Fronius M, Schweda EKH, Lochnit G, Grau V, 2015. Phosphocholine-Modified Macromolecules and Canonical Nicotinic Agonists Inhibit ATP-Induced IL-1β Release. J. Immunol 195, 2325–34. 10.4049/jimmunol.1400974 [DOI] [PubMed] [Google Scholar]
- Hiel H, Elgoyhen AB, Drescher DG, Morley BJ, 1996. Expression of nicotinic acetylcholine receptor mRNA in the adult rat peripheral vestibular system. Brain Res. 738, 347–52. 10.1016/s0006-8993(96)01046-3 [DOI] [PubMed] [Google Scholar]
- Hone AJ, McIntosh JM, 2018. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 592, 1045–1062. 10.1002/1873-3468.12884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba Y, Sugimoto Y, Kamei C, Masukawa T, 2003. Possible role of mucosal mast cells in the recovery process of colitis induced by dextran sulfate sodium in rats. Int. Immunopharmacol 3, 485–491. 10.1016/S1567-5769(02)00299-0 [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T, Moriwaki Y, Misawa H, 2012. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. 91, 1027–32. 10.1016/j.lfs.2012.05.006 [DOI] [PubMed] [Google Scholar]
- Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H, 2007. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 80, 2314–2319. 10.1016/j.lfs.2007.02.036 [DOI] [PubMed] [Google Scholar]
- Li X, Hu Y, Wu Y, Huang Y, Yu S, Ding Q, Zhangsun D, Luo S, 2016. Anti-hypersensitive effect of intramuscular administration of αO-conotoxin GeXIVA[1,2] and GeXIVA[1,4] in rats of neuropathic pain. Prog. Neuropsychopharmacol. Biol. Psychiatry 66, 112–9. 10.1016/j.pnpbp.2015.12.005 [DOI] [PubMed] [Google Scholar]
- Lips KS, Pfeil U, Kummer W, 2002. Coexpression of alpha 9 and alpha 10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience 115, 1–5. 10.1016/s0306-4522(02)00274-9 [DOI] [PubMed] [Google Scholar]
- Livett BG, Sandall DW, Keays D, Down J, Gayler KR, Satkunanathan N, Khalil Z, 2006. Therapeutic applications of conotoxins that target the neuronal nicotinic acetylcholine receptor. Toxicon 48, 810–29. 10.1016/j.toxicon.2006.07.023 [DOI] [PubMed] [Google Scholar]
- Luo S, Zhangsun D, Harvey PJ, Kaas Q, Wu Y, Zhu X, Hu Y, Li X, Tsetlin VI, Christensen S, Romero HK, McIntyre M, Dowell C, Baxter JC, Elmslie KS, Craik DJ, McIntosh JM, 2015. Cloning, synthesis, and characterization of αO-conotoxin GeXIVA, a potent α9α10 nicotinic acetylcholine receptor antagonist. Proc. Natl. Acad. Sci. U. S. A 112, E4026–35. 10.1073/pnas.1503617112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs PA, 2001. Molecular cloning and mapping of the human nicotinic acetylcholine receptor alpha10 (CHRNA10). Genomics 73, 272–83. 10.1006/geno.2000.6503 [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Absalom N, Chebib M, Elgoyhen AB, Vincler M, 2009. Alpha9 nicotinic acetylcholine receptors and the treatment of pain. Biochem. Pharmacol 78, 693–702. 10.1016/j.bcp.2009.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JM, Plazas PV, Watkins M, Gomez-Casati ME, Olivera BM, Elgoyhen AB, 2005. A Novel α-Conotoxin, PeIA, Cloned from Conus pergrandis, Discriminates between Rat α9α10 and α7 Nicotinic Cholinergic Receptors. J. Biol. Chem 280, 30107–30112. 10.1074/jbc.M504102200 [DOI] [PubMed] [Google Scholar]
- Mihara T, Otsubo W, Horiguchi K, Mikawa S, Kaji N, Iino S, Ozaki H, Hori M, 2017. The anti-inflammatory pathway regulated via nicotinic acetylcholine receptors in rat intestinal mesothelial cells. J. Vet. Med. Sci 79, 1795–1802. 10.1292/jvms.17-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Lester HA, 2006. CNS localization of neuronal nicotinic receptors. J. Mol. Neurosci 30, 181–4. 10.1385/JMN:30:1:181 [DOI] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R, 1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98, 694–702. 10.1016/0016-5085(90)90290-h [DOI] [PubMed] [Google Scholar]
- Pacini A, Micheli L, Maresca M, Branca JJV, McIntosh JM, Ghelardini C, Di Cesare Mannelli L, 2016. The α9α10 nicotinic receptor antagonist α-conotoxin RgIA prevents neuropathic pain induced by oxaliplatin treatment. Exp. Neurol 282, 37–48. 10.1016/j.expneurol.2016.04.022 [DOI] [PubMed] [Google Scholar]
- Peng H, Ferris RL, Matthews T, Hiel H, Lopez-Albaitero A, Lustig LR, 2004. Characterization of the human nicotinic acetylcholine receptor subunit alpha (alpha) 9 (CHRNA9) and alpha (alpha) 10 (CHRNA10) in lymphocytes. Life Sci. 76, 263–80. 10.1016/j.lfs.2004.05.031 [DOI] [PubMed] [Google Scholar]
- Plazas PV, Katz E, Gomez-Casati ME, Bouzat C, Elgoyhen AB, 2005. Stoichiometry of the α9α10 nicotinic cholinergic receptor. J. Neurosci 25, 10905–12. 10.1523/JNEUROSCI.3805-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Mathes V, Fronius M, Althaus M, Hecker A, Krasteva-Christ G, Padberg W, Hone AJ, McIntosh JM, Zakrzewicz A, Grau V, 2016. Phosphocholine - an agonist of metabotropic but not of ionotropic functions of α9-containing nicotinic acetylcholine receptors. Sci. Rep 6, 28660 10.1038/srep28660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero HK, Christensen SB, Di Cesare Mannelli L, Gajewiak J, Ramachandra R, Elmslie KS, Vetter DE, Ghelardini C, Iadonato SP, Mercado JL, Olivera BM, McIntosh JM, 2017. Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc. Natl. Acad. Sci. U. S. A 114, E1825–E1832. 10.1073/pnas.1621433114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi-Hemami H, Brogan SE, Olivera BM, 2019. Pain therapeutics from cone snail venoms: From Ziconotide to novel non-opioid pathways. J. Proteomics 190, 12–20. 10.1016/j.jprot.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaga M, Blomster LV, Piechota-Polańczyk A, Zielińska M, Jacenik D, Cygankiewicz AI, Krajewska WM, Mikkelsen JD, Fichna J, 2016. Encenicline, an α7 Nicotinic Acetylcholine Receptor Partial Agonist, Reduces Immune Cell Infiltration in the Colon and Improves Experimental Colitis in Mice. J. Pharmacol. Exp. Ther 356, 157–69. 10.1124/jpet.115.228205 [DOI] [PubMed] [Google Scholar]
- Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F, 2002. A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit. Mol. Pharmacol 61, 150–9. 10.1124/mol.61.1.150 [DOI] [PubMed] [Google Scholar]
- St-Pierre S, Jiang W, Roy P, Champigny C, LeBlanc É, Morley BJ, Hao J, Simard AR, 2016. Nicotinic Acetylcholine Receptors Modulate Bone Marrow-Derived Pro-Inflammatory Monocyte Production and Survival. PLoS One 11, e0150230 10.1371/journal.pone.0150230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlau H, Olivera BM, 2004. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev 84, 41–68. 10.1152/physrev.00020.2003 [DOI] [PubMed] [Google Scholar]
- Vetter DE, Katz E, Maison SF, Taranda J, Turcan S, Ballestero J, Liberman MC, Elgoyhen AB, Boulter J, 2007. The alpha10 nicotinic acetylcholine receptor subunit is required for normal synaptic function and integrity of the olivocochlear system. Proc. Natl. Acad. Sci. U. S. A 104, 20594–9. 10.1073/pnas.0708545105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincler M, McIntosh JM, 2007. Targeting the alpha9alpha10 nicotinic acetylcholine receptor to treat severe pain. Expert Opin. Ther. Targets 11, 891–7. 10.1517/14728222.11.7.891 [DOI] [PubMed] [Google Scholar]
- Vincler M, Wittenauer S, Parker R, Ellison M, Olivera BM, McIntosh JM, 2006. Molecular mechanism for analgesia involving specific antagonism of alpha9alpha10 nicotinic acetylcholine receptors. Proc. Natl. Acad. Sci. U. S. A 103, 17880–4. 10.1073/pnas.0608715103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wala EP, Crooks PA, McIntosh JM, Holtman JR, 2012. Novel small molecule α9α10 nicotinic receptor antagonist prevents and reverses chemotherapy-evoked neuropathic pain in rats. Anesth. Analg 115, 713–720. 10.1213/ANE.0b013e31825a3c72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ, 2008. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol 154, 1558–71. 10.1038/bjp.2008.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewicz A, Richter K, Agné A, Wilker S, Siebers K, Fink B, Krasteva-Christ G, Althaus M, Padberg W, Hone AJ, McIntosh JM, Grau V, 2017. Canonical and Novel Non-Canonical Cholinergic Agonists Inhibit ATP-Induced Release of Monocytic Interleukin-1β via Different Combinations of Nicotinic Acetylcholine Receptor Subunits α7, α9 and α10. Front. Cell. Neurosci 11, 189 10.3389/fncel.2017.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]


