Abstract
Stress exposure can produce profound change in physiology and behavior that can impair health and well-being. Of note, stress exposure is linked to anxiety disorders and depression in humans. The widespread impact of these disorders warrants investigation into treatments to mitigate the harmful effects of stress. Pharmacological treatments fail to help many with these disorders, so recent work has focused on non-pharmacological alternatives. One of the most promising of these alternatives is environmental enrichment (EE). In rodents, EE includes social, physical, and cognitive stimulation for the animal, in the form of larger cages, running wheels, and toys. EE successfully reduces the maladaptive effects of various stressors, both as treatment and prophylaxis. While we know that EE can have beneficial effects under stress conditions, the morphological and molecular mechanisms underlying these behavioral effects are still not well understood. EE is known to alter neurogenesis, dendrite development, and expression of neurotrophic growth factors, effects that vary by type of enrichment, age, and sex. To add to this complexity, EE has differential effects in different brain regions. Understanding how EE exerts its protective effects on morphological and molecular levels could hold the key to developing more targeted pharmacological treatments. In this review, we summarize the literature on the morphological and molecular consequences of EE and stress in key emotional regulatory pathways in the brain, the hippocampus, prefrontal cortex, and amygdala. The similarities and differences amongst these regions provide some insight into stress-EE interaction that may be exploited in future efforts toward prevention of, and intervention in, stress-related diseases.
Keywords: Environmental Enrichment, Stress, Molecular Mechanisms, Amygdala, Prefrontal Cortex, Hippocampus
1. Introduction to the Interaction of Environmental Enrichment (EE) and Stress
1.1. EE and Stress: Overview
Environmental enrichment (EE) was first described by Hebb in 1947. Hebb demonstrated that allowing rats to move freely in his house, rather than standard cage housing, improved their performance on cognitive tasks1. Since then, numerous studies have investigated the effects of EE, both behaviorally and mechanistically. While EE protocols can vary greatly between labs, EE typically consists of improvements to the physical, cognitive, and/or social components of housing2–6. Enriched cage environments usually house multiple rodents together in the presence of toys and enclosures. For example, our lab uses 1 m3 wire mesh cages to house 10 Sprague-Dawley rats (Figure 1). Rats can interact with each other, climb on the wire mesh, and access a variety of toys and shelters that are rotated weekly7,8. Animals housed in enriched conditions such as these are often protected from the negative physiological or psychological impact of physical and emotional challenges/stressors, as compared with standard housed controls2–4. The beneficial effects of EE extend to humans, in that humans who engage in socially, cognitively, and physically stimulating activities often show improvements in mood and cognition9–11. These improvements are often similar to those achieved with medication but without side effects, so EE presents a unique non-pharmacological opportunity to treat a variety of disorders. Consequently, EE has considerable promise as a means of increasing stress resilience.
Figure 1: Example of EE housing.
Images of the EE apparatus used in our lab. EE cages are 1m3, have wire mesh walls for climbing, contain toys that are rotated weekly, and house 10 rats. This design provides physical, cognitive, and social stimulation, which are all traditional elements of EE. (A) Empty cage. (B) Cage with rats.
It is important to note that for the purposes of this review, we define EE as housing in a complex environment with opportunities for social interaction. This definition does not include paradigms focused purely on exercise, e.g. running wheels alone. EE is not a unilateral manipulation, but involves addition of multiple, diverse elements to stimulate the animal across different domains. Animals may engage in more physical activity as a result of EE housing via play or climbing, but exercise is not the sole or even primary manipulation. We are also not considering standard home cage enrichment (social housing, nylon bones, crinkle paper, huts, etc.) in our definition of EE. While of clear potential benefit to individuals, cage enrichment alone does not afford the spectrum of complexity associated with EE, including exercise opportunities, choice of companion animals, choices of enclosures, and voluntary investigation of familiar and novel stimuli (toys). It is however worth considering that introduction of mandatory enrichment in accordance with regulatory guidelines may well have beneficial effects in and of themselves, which may impact the magnitude of enhanced activity imparted by EE (as well as potentially occlude any number of endpoints previously observed in single-housed, unenriched rats)12–15. The experiments cited in this review largely abide by this definition of EE, and any exceptions will be noted.
It is similarly important to define what we refer to as ‘stress’, since this term can have different interpretations. Here, we consider stress to be a ‘real or perceived threat to homeostasis or well-being’16. Note we do not give the term a valence: e.g., vigorous exercise, enjoyed by many, causes engagement of physiological responses (HPA axis activation and autonomic drive) that are indistinguishable from responses to noxious stimuli. Here intense exercise is interpreted as a very real threat (requiring mobilization of physiological responses to mobilize energy resources), but one that can be managed through efficient counter-regulatory adaptations, likely by the brain.
1.2. Conceptual Understanding of EE and Stress Interactions
Multiple hypotheses have been proposed to explain how EE conveys beneficial effects on behavior, some based on the contention that EE acts as a mild stressor. While EE is typically considered a positive experience, over time it is sufficient to generate physiological changes consistent with mild stress, e.g., elevated corticosterone (CORT) secretion, body weight reduction, increased adrenal mass, etc. Such observations support the ‘cross-stressor adaptation hypothesis’, which proposes that exercise and stress act upon similar mechanisms in a way that enables exercise to better prepare an animal to cope with stressors and vice versa17,18. Exercise is a component of EE, and may provide activation of physiological responses, to which added social and experiential stimuli may synergize. Cross-stressor adaptation is consistent with the well-known phenomenon of stress habituation, whereby repeated exposure to the same stressor decreases subsequent responses19,20. Thus, it is possible that EE may work via a similar mechanism, serving to temper physiological processes associated with repeated mild stressor exposure.
This notion that EE acts as a mild stressor is further consistent with the ‘inoculation stress hypothesis’, which posits that the variety of morphological and molecular changes initiated by EE can improve stress resilience3,21–24. Indeed, engagement of stress pathways by EE is suggested by functional connectivity studies in the brains of EE rats, whereby chronic connectivity among stress-processing brain regions is increased by EE, while connectivity between all regions is decreased25,26. This shift in connectivity was associated with improved cognition, suggesting that EE rearranges circuitry in a way that improves information processing across the brain, strengthening important connections and weakening inefficient ones.
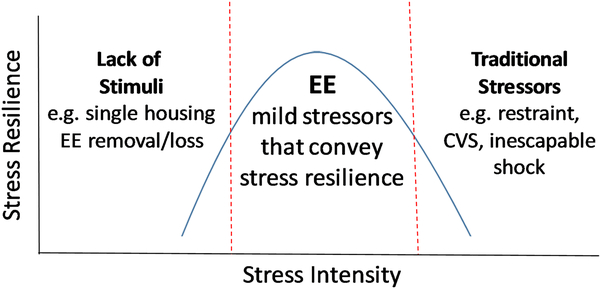
The hypothesis that EE mildly activates stress-related circuitry to improve overall brain function also aligns with the notion that stimulus intensity and behavioral effects typically obey an inverted U-shaped function, with low or high physiological responses driving inferior performance. For example, it is clear that increments in stress hormones (CORT, norepinephrine (NE), epinephrine) can improve performance of avoidance memory tasks, which are then impaired when hormone levels are driven higher. Thus, stress exposure can be adaptive and beneficial when appropriately controlled27–31. It is thought that EE may enable sufficient stimulation to improve function over that of animals lacking stimuli without pushing the animal into high, maladaptive levels of stress (Figure 2)20,32. In this way, EE is adaptive, building stress resilience and ameliorating negative behavioral consequences of stress3,20,27.
Figure 2: The position of EE on the inverted-U shaped function of stress intensity.
Stress intensity and behavioral effects exist on a spectrum where too much or too little can impair function. However, there is an optimum amount of stress that is protective and conveys stress resilience. The positive simulation of EE likely falls in this beneficial part of the spectrum, suggesting that EE provides just enough mild stress to guard against other stressors.
Despite that fact that EE causes mild physiological responses, it is also true that it is perceived as rewarding. Rats will lever-press for access to a running wheel or social counterparts, and they demonstrate conditioned place preference for compartments that contain novel, rather than familiar, objects33,34. EE also activates reward circuitry and dopamine (DA) production35. These findings suggest that EE is intrinsically rewarding and positive manipulation, making it a potentially important intervention to build stress resilience36. So while EE activates physiological domains of the stress response, it is generally perceived as rewarding, indicating that the context of the stress response matters.
Overall, EE appears to physically and psychologically improve animals’ capacities to cope with stress3,11,28–3. These benefits act both prophylactically in preparation for future stressors, and as a means of minimizing the impact of previously experienced stressors. This concept of improved function in EE conditions also appears to hold true in humans with depression and anxiety disorders, as exercise, cognitive training, and social contact all assist in amelioration of mood symptoms9–11,37. We will explore the mechanisms thought to play a role in this conceptual relationship between EE and stress in later sections.
1.3. Behavioral Effects of EE and Stress
On a behavioral level, EE confers anxiolytic, anti-depressive, and pro-cognitive effects2–5. Animals exposed to EE generally habituate to novelty faster, thereby reducing their anxiety-like behavior and improving their ability to cope with challenges10,38,39. This faster habituation is seen in open field (OF) tests, in which EE animals show increased center time and less locomotion. These behaviors suggest that the animals are less cautious about the novel environment and more exploratory24,40,41. Decreased anxiety-like behavior is observed in the elevated plus maze (EPM), where open arm times are increased in animals exposed to EE41–44. It seems likely that this increased habituation/decreased anxiety is a result of the more constant novelty animals experience in EE, as their environment (toys and shelters) changes frequently4,45. Similar beneficial effects are also observed in the domains of mood and cognitive processing. Beneficial effects of EE on mood are manifested as decreased anhedonia in the sucrose preference test (SPT)46–48, increased active coping in the forced swim test (FST)46–50, and increased resilience to social defeat51. EE also improves learning and memory in passive avoidance tests52, spatial memory in the radial arm maze53,54 and Morris water maze24,39,55–60, and reversal learning in operant touchscreen tasks61.
These effects are observed under both baseline and stress conditions. In non-stressed animals, simple addition of EE helps improve overall function. When various stressors are applied, these benefits are magnified, with EE ameliorating or even reversing detrimental effects of stress. For example, EE can rescue anxiety-like behaviors in the OF and EPM and depression-like behaviors in the SPT and FST induced by restraint, chronic variable stress, and social defeat51,62–64. EE rescues stress-induced increases in freezing during contextual and cued fear conditioning and ameliorates avoidance behaviors related to post-traumatic stress disorder (PTSD), suggesting beneficial effects on fear reactivity and emotional regulation65–69. In the context of stress, EE can work both prophylactically and therapeutically. EE exposure prior to stress can lessen its maladaptive effects, whereas EE exposure after stress can improve recovery2,3.
While EE conveys some level of behavioral benefit in most cases, these effects can vary by EE paradigm, as well as the age, strain, and sex of the animal. For example, longer EE exposures (several weeks) typically have a greater impact on more behaviors than acute exposure (hours to days)4. EE applied in adolescence also tends to have stronger effects than that initiated in adulthood65. Certain strains of mice may exhibit more aggression in group housing than rats or other mouse strains, resulting in attenuation of the benefits of EE70. Females and males may respond to certain components of EE differently, resulting in variable behaviors between sexes71. While these differences make it difficult to draw parallel conclusions across studies, they do not change the fact that EE is primarily beneficial and neuroprotective, regardless of specific technical details. These differential behavioral effects of EE across conditions have been reviewed elsewhere2,3.
1.4. Physiological Effects of EE and Stress
A variety of physiological responses are affected by EE, including hormones and cytokines. CORT release, which is an important factor in stress-related phenotypes, can be altered by EE3,19,72. Prior work suggests that EE increases basal CORT and, in some cases, increases adrenal mass, the latter finding reflecting long-term activation of the HPA axis73–76. In contrast, EE can blunt CORT responses to novel stressors, either by lowering peak responses or quickening return to baseline44,73,74,77. This suggests increased basal HPA axis drive in EE animals, along with smaller and more quickly resolved stress responses. Such improved stress processing would be in line with the resiliency phenotypes described above. It is important to note that chronic stress exposure typically causes sensitization rather than blunting of CORT responses19,20,78. These data indicate that while chronic HPA axis drive may contribute to beneficial actions of EE, it is not sufficient to mimic the impact of chronic stress. It is also important to note that while EE can activate the HPA axis, this activation is not perceived as stressful since EE is largely a rewarding, rather than aversive, stimulus.
A causal role of HPA axis activation in effects of EE is called into question by the lack of generalizability of CORT and ACTH elevations between laboratories. While most groups report increased basal and decreased CORT release during stress, contradictory results have also been found. Most studies suggest that basal CORT is increased by EE73–76, but others have found decreases77 or no change20,79. Similarly, most suggest that CORT responses to stress are decreased by EE44,73,74,77, but others report increases7 or no change62,75,80. Similar varied results are seen with adrenocorticotropic hormone (ACTH), both basally and in response to stress60,81,82. While it is important to note that these studies differ in terms of rat strain, EE protocol, and sampling times, CORT and ACTH differences across studies suggest that beneficial actions of EE cannot be directly attributed to HPA axis properties alone. It should be noted that discrete stress hormone sampling may fail to capture the dynamic nature of HPA axis drive. Chronic changes in HPA axis drive can be assessed via increases in adrenal weight; however, studies measured this endpoint again found contradictory results. Some reported increased adrenal weight in response to EE8,73,83, while others found no change7,74,84. Thus, while EE experience likely causes episodic or cumulative increases in CORT secretion, the data do not unequivocally support HPA axis drive as a primary cause of the beneficial actions of EE on physiology and behavior.
Stress-buffering by EE extends to sympathoadrenal responses. EE can blunt epinephrine release73 and stress-related increases in heart rate85–89, again pointing to an improved regulation of stress responses in general.
EE also modulates the immune system in a way that may promote resilience. EE is typically anti-inflammatory under basal conditions, although it can also increase the efficiency of the immune response4,41,80,86. Many of these immune studies focus specifically on exercise10,45,90, but cognitive and social EE have shown similar effects4,91,92. EE reduces production of circulating pro-inflammatory factors, such as interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNFα), and interferon gamma (IFNγ), and promotes production of circulating anti-inflammatory factors, such as interleukin 10 (IL-10) and interleukin 1 receptor antagonist (IL-1Ra)4,80,93. While these effects may be secondary to exercise-induced increases in interleukin 6 (IL-6) release from muscles94; toys and social EE can also lower plasma IL-1β and TNFα4,91, making the source of these changes unclear. This shift in cytokine production promotes an anti-inflammatory state at baseline, preventing potentially deleterious effects of excessive inflammatory responses when they are not required to combat a viral or bacterial challenge, while enhancing the body’s ability to deal with these challenges. Beyond cytokines, EE stimulates leukocytosis, increasing the proliferation of macrophages, neutrophils, monocytes, and natural killer cells41,45,95. Macrophage chemotaxis and phagocytosis are also increased by EE45. These changes in cellular immunity better prepare the immune system to respond to and resolve challenges. As elevations in many of these pro-inflammatory cytokines have been implicated in stress and psychiatric disease96,97, this anti-inflammatory profile with improved responsivity is expected to promote general health and stress resilience, thereby playing a role in the beneficial effects of EE.
2. EE Mechanisms in Stress-Processing Brain Regions
The central nervous system is the primary location for processing EE and stressors, as well as for generating the physiological effects and resiliency discussed above. EE promotes numerous actions in the brain that likely drive behavioral effects. Understanding these actions will be critical for understanding how EE is able to ameliorate maladaptive effects of stress. Therefore, this review focuses on the molecular and morphological effects of EE in various stress-processing brain regions in adult rodents, as well as how these changes may contribute to the interaction between EE and stress. Specifically, we will focus on the hippocampus, prefrontal cortex, and amygdala, three regions which are critical to processing stressful stimuli and are differentially impacted by EE19. The hippocampus and prefrontal cortex typically exert top-down control to terminate or alleviate responses to stressors, whereas the amygdala enhances stress responses. We will identify mechanisms consistently altered in these regions which present interesting targets for future resilience research, as well as unique mechanisms which could hold the key to understanding the complex effects of EE. Additionally, we will explore potentially negative effects of EE and important future directions for the field.
2.1. Morphological Effects and Synaptic Plasticity
The morphological effects of EE under baseline and stress conditions are summarized in Table 1 and Table 2, respectively.
Table 1:
Morphological Effects of EE under Baseline Conditions
| Category | Hippocampus | Prefrontal Cortex | Amygdala | References |
|---|---|---|---|---|
| Neurogenesis | EE ↑ neurogenesis | 5,38,101,102,105 | ||
| Cell Survival | EE ↑ cell proliferation | 43,114,115 | ||
| EE ↓ apoptosis, autophagy, oxidative stress | 67,68,113,116 | |||
| EE ↓ oxidative stress | 63,110,116 | |||
| Dendritic Complexity | EE ↑ dendritic branching and spine density | 111,119–121 | ||
| EE ↑ spine density in IL/PL | 126 | |||
| EE ↑ or ↓ complexity in Cg3 | 124,125 | |||
| EE ↑ dendritic branching in OFC | 124 | |||
| No effects | 20,62 | |||
| Electrophysiology | EE ↑ LTP | 130–133 | ||
| EE ↑ cell excitability | 100 | |||
| Plasticity Markers | EE ↑ synaptophysin, PSD95, synapsin I | 64,136,138–140 | ||
| EE ↑ synaptophysin and synapsin I | 136,139,145 | |||
| EE ↑ synaptophysin | 65 | |||
| Other Cell Types | EE ↑ PV interneurons | 153 | ||
| EE ↑ microglia and astrocyte # | 154–156 | |||
| EE ↑ IBA1 and GFAP | 99,156 | |||
| EE ↑ endothelial cell proliferation and angiogenesis | 10,119,162 | |||
| EE ↑ endothelial cell proliferation | 162 |
Table 2:
Morphological Effects of EE and Stress Interaction
| Target | Hippocampus | Prefrontal Cortex | Amygdala | References |
|---|---|---|---|---|
| Neurogenesis | EE block stress-induced decreases in neurogenesis | 65,97,98,107,206 | ||
| Cell Survival | EE block stress-induced increases in cell death | 56,102,112,113 | ||
| Dendritic Complexity | EE block stress-induced decreases in dendritic complexity | 64,123 | ||
| EE block chronic stress-induced dendritic branching | 84,129 | |||
| EE cannot block acute stress-induced dendritic branching | 62 | |||
| Electrophysiology | EE block stress-induced decreases in LTP and LTD | 134,135 | ||
| Plasticity and Activity Markers | EE block stress-induced decreases in synaptophysin, PSD95, and synapsin I | 64,138 | ||
| EE ↑ or ↓ Fos responses to stress | 51,60,141,142 | |||
| EE ↑ or ↓ Fos responses to stress | 51,60,142,146,147 | |||
| EE ↓ Fos responses to stress | 60,141,142,148,149 | |||
| Other Cell Types | EE block stress-induced loss of PV | 67 | ||
| EE block stress-induced loss of PV | 67 | |||
| EE block stress-induced loss of PV | 40 | |||
| EE block stress-induced decreases in GFAP | 63 | |||
| EE block stress-induced increases in GFAP | 63 |
2.1.1. Cellular Proliferation and Survival
One of the most widely studied effects of EE is adult neurogenesis in the hippocampus5,56,98–100. This effect was first recognized by Kempermann et al., 1997, using BrdU to identify newborn neurons in the dentate gyrus (DG) of mice exposed to EE101. Since then, many groups have also found this EE-induced increase in neurogenesis under baseline conditions both in mice and rats5,38,102–106. Moreover, EE can block stress-induced decreases in hippocampal neurogenesis, thought to contribute to the negative consequences of stress on behavior59,65,97,98,107. While most often associated with the exercise component of EE10,108,109, cognitive and social EE can also increase neurogenesis66,104,110,111. Indeed, the more complex forms of EE tend to induce more lasting effects than exercise alone66,104,110. EE also promotes health and survival of existing neurons, as it reduces spontaneous and stress-induced cell death in the hippocampus56,102,112,113. This protective effect of EE occurs in mice and rats under both baseline and stress conditions43,114,115, possibly as a result of decreased apoptosis113, autophagy68, and oxidative stress67,116. Given the role of the hippocampus in inhibiting stress responses, improved cell survival conveyed by EE likely contributes to its ability to improve stress resilience.
Neurogenesis is a phenomenon largely restricted to the DG of the hippocampus101, and is not thought to occur in other stress-regulatory limbic circuits (e.g. prefrontal cortex or amygdala). Direct measures of cell survival in EE have not been measured in the prefrontal cortex or amygdala under basal conditions or following stress. Similar protection from oxidative stress has been described in the prefrontal cortex63,110,116 and hippocampus, suggesting that benefits of EE on the hippocampus likely extend to cortical neurons as well. We will explore these effects further in later sections.
2.1.2. Dendritic Complexity
In addition to improving cellular health, EE increases the plasticity of hippocampal neurons, manifest as increased dendritic complexity (both branching and spine density)2,4,117,118. Under baseline conditions, EE increases arborization and spinogenesis in both apical and basal dendrites111,119–121. This effect is opposite to that seen with stress alone, which decreases the branching and length of apical and basal dendrites in the hippocampus106,122. When applied together, EE is able to block these stress-induced decreases and ultimately increase overall dendritic branching64,123. This increased connectivity between neurons likely allows for more rapid communication and ultimately more efficient information processing that likely help animals to cope with and recover from stressful situations26.
Three studies examined the effects of EE on dendrites in the prefrontal cortex under baseline conditions. One found that complex housing increased basal dendrite branching in the orbitofrontal cortex (OFC) and decreased basilar branch length in the anterior cingulate cortex (Cg3)124. Another found increased spine density without changes in branching in the Cg3125. The third found that complex housing increased overall dendritic complexity and spine density in the infralimbic (IL) and prelimbic (PL) cortices126. Overall, the data indicates that EE has different effects in individual prefrontal cortex subregions, biased toward increased complexity. Increased dendritic complexity in the prefrontal cortex is expected to oppose the decreased complexity caused by chronic stress127, although this effect has yet to be directly investigated.
Interestingly, EE and stress-induced changes in dendritic complexity in the amygdala, specifically the basolateral amygdala (BLA), are in the opposite direction to those seen in the hippocampus and prefrontal cortex. The BLA is the focus of many of these studies, as it is particularly important to processing psychological stressors19. Chronic stress alone increases dendritic complexity of projection neurons in the BLA84,106,122,128. This finding is in line with the pro-stress nature of the amygdala, namely that stress increases amygdalar activity and drives stress-related behavioral phenotypes19. EE alone does not appear to alter these endpoints129. However, when EE and chronic stress are run concurrently in adulthood, EE is able to block stress-induced increases in BLA dendritic branching and spine density129. This finding also occurs with adult EE after adolescent stress84. However, EE failed to reverse increases in BLA dendritic arborization caused by acute stress62, suggesting that the protective effects of EE in the amygdala only emerge after chronic stress. The differences in these results from those in the hippocampus or prefrontal cortex make the amygdala particularly interesting with respect to the interaction of EE and stress.
2.1.3. Synaptic Plasticity and Activity Markers
These changes in neuronal number and complexity translate to functional differences in EE and stress. Electrophysiological properties of the hippocampus change in response to EE alone, which strengthens long term potentiation (LTP) in pyramidal cells of the DG5,10,130 and CA1100,130–133. EE alters both the pre- and post-synaptic excitability in these regions to ultimately improve hippocampal neuroplasticity, likely contributing to the improved learning and memory of EE-treated animals100. EE also blocks stress-induced decreases in LTP and long term depression (LTD)134,135. These functional consequences of EE and stress have been further demonstrated using molecular markers of synaptic plasticity and neuronal activation. EE increases synaptophysin expression throughout the hippocampus64,136–139 and blocks stress-induced decreases in this plasticity markers64,138. Exercise alone stimulates similar changes in post synaptic density protein 95 (PSD95) and synapsin I137,140, suggesting that these changes may also exist in EE. Studies examining markers of neuronal activity in the hippocampus have yielded less consistent results. Some have found that EE increases the Fos response to stress51,60, while others have found decreases in Fos and delta-FosB141,142. These differences likely resulted from variations in stress and EE paradigms, or a species difference between mice and rats, although none of these factors appear to be the sole cause of differential responses. None of these studies conducted further colocalization analysis to identify the Fos-associated cell types, so it is difficult to draw conclusions about the functional implications of these results. However, studies utilizing cytochrome c oxidase (CCO) found that EE decreases the long-term metabolic capacity of the hippocampus, suggesting that EE improves the processing efficiency of hippocampal cells25,143,144. This heightened activity likely further enhances the ability of this region to adapt to novel stimuli, including stress. Altogether, these EE and stress-induced changes to membrane properties, synaptic plasticity markers, and activity support the above morphological observations. EE improves synaptic plasticity in the hippocampus, both under baseline and stress conditions, putatively guarding against the maladaptive effects of stress.
While fewer studies examined these markers in the prefrontal cortex, this region appears to show similar changes in synaptic plasticity as the hippocampus. EE increases synaptophysin and synapsin I in the prefrontal cortex136,139,145. Further exploration of electrophysiological properties, other plasticity markers, and stress interactions would be interesting for a more complete comparison. Again, the Fos results are conflicting in this region, with different studies finding that EE can increase51,146,147 or decrease60,142 Fos and delta-FosB responses to stress. CCO studies again demonstrate decreased metabolic capacity, and thus improved processing efficiency, in the prefrontal cortex25,143,144. These similarities further suggest that EE exerts similar effects on the hippocampus and prefrontal cortex.
One study found that exercise increases synaptophysin in the amygdala, suggesting increased synaptic plasticity65. However, more studies focusing on EE specifically are required to be confident that the amygdala response is the same as the hippocampus and prefrontal cortex in this case. In terms of neuronal activation, the amygdala Fos studies were relatively more consistent. EE often reduces stress-induced Fos responses in the amygdala60,141,142,148,149, suggesting that EE blocks the hyperactivation of the amygdala during stress. However, other studies found that EE increased Fos responses to stress147,150, so we cannot definitively make this conclusion. Overall, it appears EE dampens stress via opposite effects in the hippocampus (increased activity) and amygdala (decreased activity). Additionally, CCO studies suggest that EE exerts minimal effects on the metabolic activity of the amygdala under baseline conditions, providing another difference from the other regions25,143,144. Further studies are warranted to determine if this endpoint changes under stress conditions.
2.1.4. Other Cell Types
EE also influences interneurons, glia, and vasculature. Stress can often disrupt the function of parvalbumin (PV) interneurons, impairing their inhibitory control over excitatory neurons151,152. On the other hand, EE can increase PV immunoreactivity in the hippocampus under baseline conditions153 and reverse stress-induced loss of PV protein levels67. This effect is thought to be mediated by the ability of EE to ameliorate oxidative stress67,116, and it likely improves the inhibitory tone of the hippocampus. Similar EE protection from stress-induced PV loss has been suggested in the prefrontal cortex67 and amygdala40.
EE can increase the number of microglia and astrocytes in the hippocampus under baseline conditions154–156, but this has not been explored in the context of stress. This finding is further supported by increased levels of the microglia marker ionized calcium binding adaptor molecule 1 (IBA1) and the astrocyte marker glial fibrillary acidic protein (GFAP) in EE rats99,156. Additionally, EE rescues stress-induced decreases in hippocampal GFAP, suggesting that EE’s protective effects extend to astrocytes63. This improved support would further enhance the efficiency hippocampal signaling and is expected to contribute to EE-induced neurogenesis92,157–160. Although some studies have noted increased gliogenesis in the neocortex161, direct measures of microglia and astrocytes have not been made in the prefrontal cortex. In the amygdala, EE blocked stress-induced increases of GFAP, suggesting that EE reduces stress-induced increases in amygdalar astrocytes63. This amygdala effect is again opposite of that seen in the hippocampus.
EE can also increase the vasculature in the hippocampus, via enhanced endothelial cell proliferation and angiogenesis10,119,162. This effect would increase delivery of nutrients and clearance of waste, adding to the improved efficiency of the hippocampus during EE. Increased endothelial cell proliferation was also noted in the prefrontal cortex162. While vasculature has not been directly studied during stress or in the amygdala, EE-induced alterations in the neurotrophin vascular endothelial growth factor (VEGF) suggest that this proposed vascular effect is likely plays a role in both63,66,121. VEGF and other molecular mechanisms underlying all these morphological and plasticity effects will be explored in the next section.
2.2. Molecular Effects
The molecular effects of EE under baseline and stress conditions are summarized in Table 3 and Table 4, respectively.
Table 3:
Molecular Effects of EE under Baseline Conditions
| Category | Hippocampus | Prefrontal Cortex | Amygdala | References |
|---|---|---|---|---|
| Neurotrophins | EE ↑ BDNF and TrkB | 99,103,113,163,164,166,266 | ||
| EE ↑ BDNF | 163,164 | |||
| No effects | 65,66,129 | |||
| EE ↑ VEGF, NGF, NT-3, IGF-1, GDNF | 66,79,113,115,121,163,165,170,172 | |||
| EE ↑ VEGF, NGF | 66 | |||
| Hormones | EE ↑ GR expression and translocation | 63,170,173,185 | ||
| EE alters CRH receptor expression | 188,189 | |||
| Neurotransmitters | EE ↑ AMPA, NMDA, glutamate transporter | 5,83,135,196–198 | ||
| No effects | 203,206 | |||
| EE ↓ D1 and DAT | 204,206 | |||
| EE alters 5-HT system | 114,200,207 | |||
| EE ↓ NE | 69 | |||
| Immune Markers | EX ↑ CX3CR1 | 212 | ||
| EE ↓ TNFα | 180 | |||
| Intracellular Signaling | EE ↑ PKA and MAPK pathway activity (↑LTP) | 131,215 | ||
| EE ↑ CREB activity (↑ BDNF) | 58,113,165,174,176 | |||
| EX ↓ ROS, iNOS; ↑HSP70 (↑ antioxidants) | 216–218 | |||
| No effects | 65,129 |
Table 4:
Molecular Effects of EE and Stress Interaction
| Target | Hippocampus | Prefrontal Cortex | Amygdala | References |
|---|---|---|---|---|
| Neurotrophins | EE block stress-induced decreases in BDNF | 50,63,64,66,68,168,267 | ||
| EE block stress-induced decreases in BDNF | 63,180 | |||
| EE block stress-induced increases in BDNF | 63,129 | |||
| EE block stress-induced decreases in VEGF, NGF, NT-3, IGF-1, GDNF | 50,63,171,172,66,79,113,115,121,163,165,170 | |||
| EE block stress-induced decreases in VEGF and IGF-1 | 63,181 | |||
| EE cannot block stress-induced increases in VEGF and NGF | 63 | |||
| Hormones | EE block stress-induced decreases in GR expression and translocation | 63,186,187 | ||
| EE ↑ GR response to stress | 53 | |||
| EE ↓ CORT response to stress | 192 | |||
| EE ↑ IL control over HPA axis | 51,193 | |||
| EE block stress-induced increases in GR expression and translocation | 194 | |||
| EE ↑ NPY response to stress | 184,190,191 | |||
| EE ↑ NPY response to stress | 191 | |||
| EE block stress-induced increases in CRH receptors | 191,195 | |||
| Neurotransmitters | EE alters 5-HT and NE response to stress | 69,199–201,227 | ||
| EE ↑ 5-HT response to stress | 184 | |||
| EE blunt DA and ACh release during stress | 192,205,206 | |||
| Immune Markers | EE and EX blunt stress-induced increases IL-1β, TNFα, IL-6, IL-1Rα, CCL2, CCL3, CXCL12 | 4,93,99,208,209 | ||
| EE block stress-induced increases of IL-1β | 180 | |||
| Intracellular Signaling | EE block stress-induced decreases in CREB activity (↑ BDNF) | 58,113,165,174,176 | ||
| EE block stress-induced activation of ERK-MAPK-CREB pathway (↓ GR) | 194 | |||
| EE block stress-induced EGR-1 (↓ neuronal activation) | 194 | |||
| EE blunt stress-induced increases in PKM pathway activity (↑ GR signals) | 186 | |||
| EE block stress-induced decreases in SIRT1/miR-134 pathway (↑ VEGF) | 64 |
2.2.1. Neurotrophins
Increased neurotrophic activity in the hippocampus of EE animals is proposed as a primary molecular mechanisms for stress-protective effects of EE2–4,118. Hippocampal brain derived neurotrophic factor (BDNF), in particular, is increased by EE under basal conditions99,103,113,140,163–166. EE prevents stress-induced decreases in BDNF, which are believed to contribute to the maladaptive behavioral effects of stress50,63,64,66,68,167,168. The receptor for BDNF, tropomyosin receptor kinase B (TrkB), is also increased by EE and exercise50,169. EE stimulates other neurotrophins in the hippocampus as well, including VEGF, nerve growth factor (NGF), neurotrophin-3 (NT-3), insulin-like growth factor-1 (IGF-1) and glial cell-derived neurotrophic factor (GDNF), under basal and stress conditions50,63,171–173,66,79,113,115,121,163,165,170. Neurotrophins generally promote the survival and proliferation of neurons. BDNF and NGF specifically increase dendritic complexity163,174–176, while VEGF increases angiogenesis and spinogenesis66,121,177,178. Therefore, these molecules are expected to contribute to the positive morphological changes induced by EE in stress exposed animals noted above103,179.
EE-induced alterations of BDNF levels in the prefrontal cortex are similar to those seen in the hippocampus. EE increases BDNF in the prefrontal cortex under basal conditions163,164, and rescues stress-induced decreases in BDNF63,180. Similar protective effects have been found with VEGF63 and IGF-1181. However, another study found no change in VEGF69 and NGF, NT-3, and GDNF have not been examined in this context. While further studies are needed to fully compare the prefrontal cortex to the hippocampus, it appears that these regions share similar neurotrophin responses to EE and stress.
Neurotrophins in the amygdala are also impacted by EE and stress, but this region is again different from the hippocampus and prefrontal cortex. EE does not impact baseline levels of BDNF in the amygdala65,66,129, while stress alone increases BDNF here182,183. When animals experience both, EE blocks stress-induced increases in BDNF63,129. This directionality is opposite to that of the hippocampus where increased BDNF contributes to stress resilience, suggesting that increased amygdalar BDNF may instead promote, rather than ameliorate, stress. Despite this difference in direction, EE still counteracts this effect to improve resilience to chronic stress. However, one study specifically examining PTSD-related stress found that EE enhanced the stress-induced upregulation of BDNF184, suggesting that this effect may be sensitive to specific stressors. The other neurotrophins are not well-studied in this region. One study found no change in VEGF63, while another noted increases in VEGF and NGF in response to EE66. These conflicting results add to the complexity of the interaction of EE and stress in the amygdala, and suggest that the amygdala is more sensitive to different types of stress than the hippocampus and prefrontal cortex.
2.2.2. Hormones
The HPA axis is a critical element of the stress response, and glucocorticoid receptors (GR) in the brain are key to regulation of this system. As discussed above, CORT, the ligand for GR, is altered by EE and stress, although the directionality of this change is inconsistent between studies. In the hippocampus however, EE consistently increases GR expression under baseline conditions, while stress decreases GR expression63,170,173,185. Together, EE blocks stress-induced loss of GR63,186,187. This protective effect extends to GR translocation186, indicating that GR increases not only the number of GR receptors, but also their activation. This effect would increase the responsivity of the hippocampus to CORT stimulation, which would enhance feedback and ultimately dampen HPA responsiveness19,186. This finding supports the notion of a blunted stress response in EE animals, which agrees with the majority of CORT studies discussed above and further describes the role of the HPA axis in EE-induced stress resilience. Along the same lines, toys can decrease FKBP5 expression in the hippocampus66. FKBP5 negatively regulates GR, so a decrease in its expression would support this concept of increased GR activity in EE. Corticotropin releasing hormone (CRH), another element of the HPA axis, can also be altered by EE; although, different studies have found increases188 or decreases189 in this hormone and its receptors. The conflicting nature of these studies makes it difficult to draw conclusions about CRH in the hippocampus at this point in time. Another factor that may be important to EE is neuropeptide Y (NPY), a neuropeptide typically associated with PTSD. EE enhances NPY responses to stress and the expression of NPY-Y1 receptors. These receptors are thought to contribute to stress resilience184,190,191, so NPY may be a relatively unrecognized effector of EE.
While these mechanisms have been less studied in the prefrontal cortex, it appears that this region does share some characteristics with the hippocampus. EE induced a trend toward increased expression of GR in the prefrontal cortex in response to restraint stress, although this effect was not statistically significant53. Microdialysis examining free CORT in the prefrontal cortex showed that EE decreases the CORT response to restraint within this region192. Both of these results support the idea that EE decreases the responsivity of the HPA axis to stress: more GR suggests better feedback, which, in this region, would more quickly return the CORT response to baseline19,53,192. Lesion studies suggest that EE enhances the control of the IL over the HPA axis, further supporting this concept193. Beyond GR, EE increased CRH and CRHR2 in the prefrontal cortex; however, aggression between mice may have been confounding in this case189. No studies have examined NPY in this region.
The amygdala again responds to EE and stress differently than the hippocampus or prefrontal cortex. Stress often increases GR translocation in the amygdala19,183, which drives rather than dampens stress responsiveness. EE can block this stress-induced GR translocation194, suggesting that EE still opposes stress effects in this region, even though the directionality of the effects are switched. While further studies are needed to replicate this finding, the continued differential effects between regions is intriguing. EE blocks stress-induced increases in CRHR1 in the amygdala191,195, theoretically decreasing the excitatory output of the amygdala to the HPA axis and promoting resilience19,183. For NPY, EE increased NPY-Y1 expression in the amygdala, which is unexpectedly in agreement with the findings in the hippocampus. The exact mechanisms involved in NPY’s role have yet to be elucidated but may reveal a rare functional similarity between the hippocampus and amygdala.
2.2.3. Neurotransmitters
EE also alters neurotransmission across the brain, under both basal and stress conditions. In the hippocampus, EE increases the expression of glutamate receptors, specifically α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)5,83,135,196,197 and N-methyl-D-aspartate (NMDA) receptors197,198. EE decreases glutamate transporter expression, which would also increase glutamate neurotransmission via decreased astrocytic uptake of glutamate in the synaptic cleft198. These changes are consistent with the increases in LTP found in EE. Unfortunately, these glutamate EE studies did not examine stress, so, while these EE effects are expected to improve cognition and resilience, further studies are needed. Hippocampal serotonin (5-HT) and norepinephrine (NE) are also altered in the context of EE and stress. Some found that EE decreased the responsiveness of serotonin to stress69,199, while others found that this was increased200. Others noted increased expression of serotonin 1A (5-HT1A) receptors and enhanced serotonin turnover69,201. While these effects are somewhat contradictory, they seem to suggest that EE improves the buffering capacity of the serotonergic system in the hippocampus, which could aid in stress coping69. Norepinephrine studies conflict, showing increases200 and decreases69 in response to stress, so it is difficult to draw conclusions about this system.
Neurotransmitter systems in EE and stress have received more attention in the prefrontal cortex. It appears that the glutamatergic system is less involved in the prefrontal effects of EE, with more changes being noted in the dopaminergic, cholinergic, and serotonergic systems. Glutamate and gamma aminobutyric acid (GABA) levels and receptors are unchanged by EE under both baseline and stress conditions202,203. Several changes occur in the dopaminergic system when EE is applied in baseline conditions, including reduced function and expression of dopamine 1 (D1) receptors and decreased dopamine transporter (DAT) surface expression204,205. EE animals also release less dopamine when they are stressed192,206. A similar blunted stress response was observed with acetylcholine (ACh)192,205, suggesting that EE reduces the responsivity of these two systems in the prefrontal cortex. EE effects on serotonin are more uncertain, with most studies showing that EE increases its activity and others showing decreases114,200,207. Overall, the notion that EE modulates different neurotransmitter systems in the hippocampus and prefrontal cortex is interesting and demonstrates that unique effects exist in every region.
Less information is available regarding the amygdala. One study found that EE increases the serotonergic response to stress184, and another found that it lowered norepinephrine under basal conditions only69. Clearly, more studies will be needed to understand changes in neurotransmission in the amygdala in response to EE and stress.
2.2.4. Immune Markers
Another domain that EE is known to modulate is the immune system. As discussed above, EE has many effects on the peripheral immune system, which likely have reciprocal effects with the central nervous system4,208. Within the hippocampus, EE further modulates various cytokines and chemokines4,93,208. EE typically blunts stress-induced increases in immune factors such as IL-1β, TNFα, IL-6, IL-1Ra, C-C motif chemokine ligand 2 (CCL2), C-C motif chemokine ligand 3 (CCL3), and C-X-C motif chemokine 12 (CXCL12) here4,93,99,208,209. One study found increases in these factors, but aggression between mice could have been confounding189. It is important to note that these findings are associated with either exercise and stress93,208 or EE and immune challenge99,209. Together, EE would be expected to reduce stress-induced increases in inflammation-associated markers, which would likely be neuroprotective210,211. However, more targeted studies are needed to clarify the role of the central immune system in EE and stress interactions specifically. For example, the above morphological results pointed to increased numbers microglia and astrocytes in the hippocampus of EE animals154–156, yet here these glia appear to release fewer cytokines in response to stress. While a little counterintuitive, this mismatch does align with the decreased basal activity but increased responsiveness of the peripheral immune system noted above, suggesting that EE exerts similar immune effects throughout the body. Along the lines of improved efficiency, exercise can increase the expression of CX3C chemokine receptor 1 (CX3CR1), which would theoretically improve communication between neurons and microglia211,212. There is likely a complex interplay between a shift in activity and expression of various cytokines that influence the overall activity of the hippocampus in EE and stress that we cannot yet dissect.
Immune-related factors have not been well-studied in the prefrontal cortex or amygdala. In the prefrontal cortex, EE can block stress-induced increases of IL-1β and reduce basal TNFα180, suggesting that the prefrontal cortex is again behaving like the hippocampus. However, another study found no such changes in the prefrontal cortex189. These factors have yet to be examined in the amygdala. Given the unclear results in the hippocampus and the growing literature that the immune system plays a key role in stress responses211,213,214, further studies of EE, stress, and the central immune system would be valuable.
2.2.5. Intracellular Signaling
Going one level deeper, EE can modulate the activity of various intracellular signaling pathways and molecules in the hippocampus. EE increases the sensitivity of the protein kinase A (PKA)-cAMP pathway, increasing its ability to respond to and convey signals, ultimately enhancing hippocampal plasticity and LTP131. EE also stimulates extracellular signal-regulated kinase (ERK)/mitogen activated protein kinase (MAPK) signaling, specifically the Ras-GRF2-ERK-MAPK pathway in newborn neurons, which aids in their survival215. Stress-induced increases in protein kinase M (PKM) activity are rescued by EE, which aids in synaptic remodeling and is believed to result from the increased expression of GR activity discussed earlier186. EE-induced increases in AMPA receptors also stimulate the expression and activity of serum and glucocorticoid-regulated kinase 1 (SGK), a downstream kinase which mediates learning and memory197. Downstream of BDNF, the signaling molecule cAMP response element-binding protein (CREB) mediates its pro-survival effects. CREB expression and phosphorylation are increased by EE, suggesting that BDNF stimulated by EE affects downstream targets58,113,165,174,176. The expected pathways involved in VEGF have been even more thoroughly mapped. Here, EE is thought to downregulate miR-107, which upregulates hypoxia-inducible factor 1-alpha (HIF-1α), which then increases VEGF. VEGF then acts through its receptor Flk-1 to stimulate spinogenesis and convey resilience121. EE rescues stress-induced decreases in the histone deacetylase sirtuin 1 (SIRT1) and increases in miR-134, changes believed to improve synaptic plasticity and cognition64. While these mechanistic studies are currently limited in number, they hold the key to understanding the improved efficiency of the hippocampus in EE and revealing key molecular pathways that may be targeted pharmacologically.
Intracellular EE effects in the prefrontal cortex are less well studied. Lesion studies demonstrate that the IL is necessary for the positive behavioral effects of EE, which supports the importance of this region in EE and stress effects (but does not offer a specific mechanism)51. Exercise decreases reactive oxygen species (ROS) and inducible nitric oxide synthase (iNOS) in the prefrontal cortex216, an effect thought to be mediated by increased heat shock protein 70 (HSP70) expression (a chaperone protein known to improve cell survival)216–218. These antioxidative actions of EE are expected to improve function of prefrontal cortex neurons and combat enhanced reactive oxygen species production associated with chronic stress67,110,116,151.
EE reduces stress-induced activation of the ERK-MAPK-CREB pathway and increases in neuronal activity marker, early growth response protein 1 (EGR-1), expression in the BLA194. These effects support the idea that EE blunts the response of the amygdala to stress, which would act to dampen pro-stress signals from this region19,183,194. These stress-related effects do not appear to be present in EE animals under baseline conditions65,129, mirroring the early observations with dendritic complexity that stress is needed to reveal EE effects in the amygdala. Overall EE has few effects in the amygdala under standard conditions, but its effects emerge when stress is applied, improving the resilience of the animal.
2.3. Effects in Other Brain Regions
This review chose to focus on three major stress-related brain regions; however, EE effects extend beyond these regions and synergistic effects throughout the brain may contribute to the protective nature of EE. Many of the cardinal studies of EE examined the somatosensory cortices. EE consistently increases dendritic complexity219–222, synaptic plasticity223–225, and neurotrophins171 in these areas. Similar effects have been noted in the hypothalamus, where EE increases dendritic complexity226, and cerebellum, where EE increases BDNF164. In the nucleus accumbens, EE increases DA release, CREB activation, and delta-FosB expression in non-stressed animals48,227,228. It also attenuates immediate-early gene induction during stress and drug administration228. These nucleus accumbens alterations are expected to contribute to the anti-addictive effects of EE, which have been reviewed elsewhere229. In general, these other regions tend to resemble EE effects in the hippocampus and prefrontal cortex, rather than those in the amygdala.
3. Comparison of Brain Regions
All together, these results suggest that the neuroprotective effects of EE against stress arise from heterogeneous mechanisms throughout the brain in adult rodents. EE and stress both modulate a variety of morphological and molecular factors (Figure 3). However, the direction of these changes is dependent upon the region, the EE paradigm, and the presence of stress. This heterogeneity is both intriguing and challenging, for common mechanisms could present novel therapeutic targets and unique mechanisms present new opportunities to truly understand how EE conveys resilience.
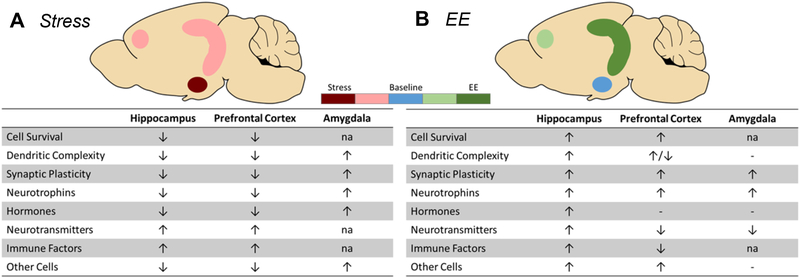
Figure 3: Independent effects of EE and stress alone in corticolimbic regions.
Summary of the effects of (A) stress and (B) EE alone on various morphological and molecular endpoints. Coloring reflects the intensity of EE and stress effects in each region. ↑ = increased activity. ↓ = decreased activity. − = no change. na = no data available.
3.1. Common Mechanisms
If we can identify a mechanism of EE that is consistent throughout the brain, it may be possible to target that molecule/pathway pharmacologically. EE in humans is even more heterogeneous than it is in rodents11. There are various ways for humans to attain greater stimulation from their surroundings, in a way that resembles EE in rodents11,230. For example, people can exercise or spend time with friends or perform cognitively stimulating activities, but these conditions are difficult to control. Human equivalents of EE have been shown to provide numerous benefits, in terms of mood and cognition. EE-like activities including exercise, cognitive training, social stimulation, and video games can help people to cope with stress and convey anti-depressive effects similar to those seen in rodents9,10,37,231. Some of the molecular mechanisms discussed here have also been recapitulated in humans. For example, cognitive training can prevent hippocampal atrophy, and exercise can increase BDNF in the blood9,11. Ideally, people would select to engage in a range of these EE-like activities, protecting them from future stressors and helping them recover from past struggles. However, this is often not practical for a variety of circumstances, both internal and external. These challenges introduce the need for development of “enviromimetic” drugs, which are drugs designed to mimic and enhance EE-induced therapeutic effects230,232. For example, exercise and antidepressants can have synergistic effects to ameliorate maladaptive behaviors138,233–235. In order to maximize beneficial effects and develop more targeted drugs, we need to understand how EE works and identify targets that change consistently throughout the brain. This effort would avoid unintended negative side effects in one region from opposing the expected benefits in other regions. While brain-wide data is not available, we can use the present information regarding highly stress-responsive regions to begin identifying molecular targets that could be manipulated to recapitulate the stress resilience of EE.
One mechanism that is mostly consistent across regions is increased synaptic plasticity. EE increased synaptophysin expression in the hippocampus, prefrontal cortex, and amygdala65,136,139. While this is just one measure, it suggests that EE increases the overall plasticity of brain, allowing it to adapt to novel (and possibly stressful) stimuli more rapidly. While directly targeting synaptophysin is unlikely in humans, stimulating neurotrophic factors that promote synaptic plasticity could be one potential approach to mimic EE effects174. Another consistent effect of EE was reduction of stress-induced oxidative stress at the cellular level. While presently these effects are linked to PV interneurons, the improved overall cell survival in EE animals suggests that other cells benefit from this action40,67,112,218. These results suggest that providing stressed individuals with supplemental antioxidants could improve their stress resilience in a similar manner as EE. Further studies are required to identify more consistent mechanisms in EE, as many of the other endpoints examined here showed regional differences.
3.2. Unique Mechanisms
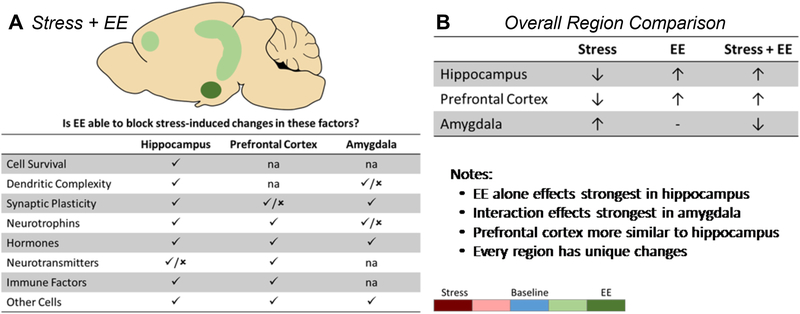
Unfortunately for enviromimetics, the hippocampus, prefrontal cortex, and amygdala showed more differences in their responses to EE and stress than similarities. However, these differences present interesting opportunities to truly dissect the mechanisms underlying EE effects and their role in promoting stress resilience (Figure 4).
Figure 4: Differential interaction of EE and stress in corticolimbic regions.
(A) Summary of the ability of EE to block stress effects on various endpoints. (B) Overall comparison of changes within each region and observations regarding similarities and differences. Coloring reflects the intensity of EE and stress effects in each region. ✓ = EE block stress effects. ✗ = EE does not block stress effects. ↑ = increased activity. ↓ = decreased activity. − = no change. na = no data available.
While the hippocampus is the most studied of these regions, the prefrontal cortex and amygdala are also critical to stress responsivity, with the hippocampus and prefrontal cortex inhibiting stress-related endpoints and the amygdala enhancing them19. In most cases, EE serves to increase the activity and functionality of the hippocampus and prefrontal cortex, while decreasing that of the amygdala. In the context of stress, these effects would all be predicted to ameliorate negative physiological or psychological consequences, even though they are in different directions. So even though these differential effects make recapitulating EE difficult, common functional effects may promote resiliency in EE animals.
While many endpoints demonstrated this pattern of resilience, the molecular mechanisms at play in each region were often different. Between the hippocampus and prefrontal cortex, the primary differences were the neurotransmitter systems that respond to EE and stress. Minor differences were found in dendritic complexity and intracellular signaling, but studies directly comparing the two regions using the same paradigm are lacking. Other than these endpoints, many of the EE and stress effects were shared between the hippocampus and prefrontal cortex. The amygdala was certainly the most unique of these regions, often showing opposite EE and stress effects. For example, EE blocked stress-induced increases in dendritic branching, neuronal activation, BDNF expression, and GR activity in the amygdala. In contrast, EE blocked stress-induced decreases in the same endpoints in the hippocampus. Again, these opposing functions are consistent with EE conveying resilience against stress in both regions. However, the fact that so many endpoints from so many domains exhibit the same differences is impressive and demonstrates the truly unique nature of the amygdala. It will be interesting to see if these stark differences hold as more region-specific studies are conducted.
It is notable that the amygdala appears to be especially susceptible to stress and important to the stress-responsive elements of EE. Many of the EE changes in the hippocampus were found under baseline conditions, suggesting that the hippocampus benefits the most from EE alone. On the other hand, the EE effects in the amygdala only became evident in the presence of stress, with the effects growing stronger with more chronic stressors. This points to the amygdala as a particularly important target when an animal is stressed. The prefrontal cortex tends to fall in the middle of this stress-responsive spectrum. These observations point to the amygdala as a key region governing the interaction between EE and stress that should be the focus of future mechanistic research.
4. Challenges of EE
4.1. Heterogeneous Nature of Current Research
While the ability of EE to oppose stress-related behavioral problems is exciting, we need to recognize that EE is a complex paradigm with multiple elements, each of which may have different effects on the present endpoints. Variations in exercise, social, and cognitive components can impact the effectiveness of EE236,237, making it difficult to derive the precise causal element of the molecular effects with our current definition of EE that encompasses these diverse components. The heterogenous nature of EE paradigms likely causes different studies to fall at different points along the inverted U-shaped curve of stress experience, eliciting differential levels of stress resilience. Paradigms that are too weak may fail to exert any effect, while paradigms that are too strong may cause excessive “stress” that is itself harmful. Theoretically, EE that achieves an optimum level of stimulation that trains the circuitry but is not deleterious in itself, should have maximum beneficial effects on an animal’s stress resilience. Finding this precise balance between the diverse components of EE is an important next step in this field, and studies directly comparing different elements of EE will help to delineate the source of different beneficial effects66,109,111.
While the present review focused on EE in adults, EE actions vary greatly by the age of the animal, with adolescent EE conveying stronger neuroplasticity effects and aged EE magnifying increases in neurotrophins and neurotransmitters192,238. EE and stress interactions can also vary by the age at which each stimulus is presented144. These age differences have been reviewed elsewhere202,239, but should be kept in mind when contemplating translational implications of EE. Differences in species and strain can also influence how EE and stress interact. For example, Wistar rats often exhibit stronger EE responses, both positive and negative, than other rat strains73. Additionally, certain strains of mice sometimes show aggression and establish stronger dominance hierarchies. This can make EE stressful for subordinate mice and may dampen beneficial effects189,240. However, this aggression is not seen in a majority of rat studies and is often limited to particular strains of mice180.
Sex differences have also been noted. Some groups have suggested that females are generally more sensitive to EE than males, exhibiting stronger changes in synaptic plasticity and protective effects against stress-induced anxiety-like behaviors, BDNF expression, and hormone responses39,81,111,116,151. Females also appear to be most responsive to the social components of EE, while males are more impacted by cognitive and physical EE71,241,242. However, a lack of studies directly comparing adult males and females makes it difficult to draw conclusions about on the extent of these differences. More studies comparing sex differences in early-life EE have suggested further differences in cognition, sociability, and HPA axis responses, but developmental confounds limit the current application of these results188,238,243,244. While further studies directly comparing males and females are certainly warranted, EE still appears to increase resilience in females71,116,136, suggesting that EE’s beneficial actions can present differently and arise from a variety of mechanisms.
4.2. Maladaptive Effects of EE Removal – Relevance to Loss
An additional challenge that is emerging in EE research is the potentially negative consequences of EE removal (ER). While some of the beneficial effects of EE appear to remain if animals are removed from EE and placed into standard housing117,245, there can be maladaptive consequences from the loss of EE or exercise itself7,8,246,247. These harmful effects are largely related to mood and appear to be particularly relevant to stress-related domains. For example, ER increases depression-like and anxiety-like behaviors when compared to control animals that never experienced EE7,8,247. These ER effects should be considered when designing EE experiments, since removing animals from EE prior to behavioral testing could be confounding.
Beyond complicating EE studies, ER presents an interesting stressor in and of itself. Typically, stressing an animal involves exposure to an externally imposed stimulus. The stressful nature of ER revolves around loss of positive reinforcers and is internally generated7. Loss is recognized by the Research Domain Criteria as a negative valence construct, and is broadly described as deprivation of something perceived as valuable248. In humans, loss can result from the death of a loved one, losing a job, a health crisis, or financial struggles248–251. While loss is recognized in humans and can even precipitate the development of depression252–254, it has received little attention in clinical or basic stress research. This largely results from difficulties in tracking loss in humans and modeling loss in animals. However, studies that have examined loss have yielded interesting similarities between human loss and ER. For example, both cause weight gain and generate hypoactive HPA axis responses to stress. These phenotypes differ from those observed with major depression and chronic stress, setting loss apart as a unique phenomenon7,255–257. As such, ER presents an interesting opportunity to study the mechanisms underlying loss and could be of great translational value for numerous people suffering from loss. This novel use of EE demonstrates the flexibility of the field and points to the opportunity for new discoveries in future research.
5. Future Directions
5.1. Links to the Inoculation Stress Hypothesis of EE and Importance of the Microenvironment
Despite the heterogeneous nature of EE and its mechanisms, one consistent pattern that emerged from all of this work is that EE improves the efficiency of stress circuitry and fine-tunes its ability to respond appropriately to various challenges. This observation is in line with the conceptual framework of the inoculation stress hypothesis, which proposes that repeated exposure to novel, diverse stimuli in EE prepares an individual to cope with future stress3. It also aligns well with several related theories including the cross-stressor adaptation hypothesis, stress habituation, and the inverted-U theory of stress resilience18,19,27. The variety of EE-related changes in the brain suggests that benefits are conferred by multiple mechanisms. EE induces changes in neurons, glia, endothelial cells, and numerous communication molecules that are shared between them. Consequently, EE is capable of altering all aspects of synaptic microenvironments across multiple brain areas, including neurons, glia, and blood vessels, as well as the extracellular matrix and extracellular space surrounding the so-called “tetrapartite” synapse258–261. By modulating the neurotrophins, hormones, neurotransmitters, immune factors, etc. in this microenvironment, EE may be able to promote holistic changes that impact all of the cells in the system. This broad control enables EE to increase the overall efficiency of signaling within each region; ultimately fine-tuning entire microenvironments of neurons to function more effectively. All together, these changes may provide for improved intercellular communications that can withstand energetic challenges promoted by stress exposure, a perspective supported by studies demonstrating that EE improves the efficiency of connectivity between brain regions by weakening unnecessary connections and strengthening important ones25,26. Overall, the stress-resiliency conveyed by EE appears to be the sum of many morphological and molecular changes working in tandem to improve the efficiency of stress circuitry.
5.2. Need for More Holistic Analyses
Future studies of EE and stress should consider this holistic nature of EE when trying to elucidate the interaction of the two. There is a particular need for more comprehensive omics studies of EE and stress117. Only a few groups have performed such studies, and their application is limited here by a lack of region-specificity or focus on addiction and adolescence262–265. These studies have noted EE effects similar to those reviewed here and then identified more specific molecules involved in each. Similar studies focused on EE and stress in adults, particularly examining regional differences, would rapidly advance the field. This type of information will be invaluable both in efforts to develop novel therapies for stress-related disorders and in deepening our understanding of the complex relationship between EE and stress.
HIGHLIGHTS.
Environmental enrichment (EE) can improve stress resilience in adulthood.
EE improves the overall efficiency of stress-processing corticolimbic regions.
EE differentially impacts the hippocampus, prefrontal cortex, and amygdala.
These effects may reveal novel therapeutic targets in stress-related disorders.
Acknowledgments
FUNDING: MH049698, MH119814
ABBREVIATIONS
- 5-HT
Serotonin
- 5-HT1A
Serotonin 1A Receptor
- ACh
Acetylcholine
- ACTH
Adrenocorticotrophic Hormone
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
Brain Derived Neurotrophic Factor
- BLA
Basolateral Amygdala
- CCL2
C-C Motif Chemokine Ligand 2
- CCL3
C-C Motif Chemokine Ligand 3
- CCO
Cytochrome C Oxidase
- Cg3
Anterior Cingulate Cortex
- CORT
Corticosterone
- CREB
cAMP Response Element-Binding protein
- CRH
Corticotropin Releasing Hormone
- CX3CR1
CX3C Chemokine Receptor 1
- CXCL12
C-X-C Motif Chemokine 12
- DA
Dopamine
- D1
Dopamine Receptor 1
- DAT
Dopamine Transporter
- DG
Dentate Gyrus
- EE
Environmental Enrichment
- EGR-1
Early Growth Response protein 1
- EPM
Elevated Plus Maze
- ER
Enrichment Removal
- ERK
Extracellular signal-Regulated Kinase
- FST
Forced Swim Test
- GABA
Gamma Aminobutyric Acid
- GDNF
Glial cell-Derived Neurotrophic Factor
- GFAP
Glial Fibrillary Acidic Protein
- GR
Glucocorticoid Receptor
- HIF-1α
Hypoxia-Inducible Factor 1-alpha
- HPA
Hypothalamic-Pituitary-Adrenal
- HSP70
Heat Shock Protein 70
- IBA1
Ionized calcium Binding Adaptor molecule 1
- IFNγ
Interferon gamma
- IGF-1
Insulin-like Growth Factor 1
- IL
Infralimbic
- IL-10
Interleukin 10
- IL-1Ra
Interleukin 1 Receptor antagonist
- IL-1β
Interleukin 1 Beta
- IL-6
Interleukin 6
- iNOS
inducible nitric oxide synthase
- LTD
Long-Term Depression
- LTP
Long-Term Potentiation
- MAPK
Mitogen Activated Protein Kinase
- NE
Norepinephrine
- NGF
Nerve Growth Factor
- NMDA
N-methyl-D-aspartate
- NPY
Neuropeptide Y
- NT-3
Neurotrophin-3
- OF
Open Field
- OFC
Orbitofrontal Cortex
- PKA
Protein Kinase A
- PKM
Protein Kinase M
- PL
Prelimbic
- PSD95
Post Synaptic Density protein 95
- PTSD
Post-Traumatic Stress Disorder
- PV
Parvalbumin
- ROS
Reactive Oxygen Species
- SGK
Serum and Glucocorticoid-Regulated Kinase 1
- SIRT 1
Sirtuin 1
- SPT
Sucrose Preference Test
- TNFα
Tumor Necrosis Factor alpha
- TrkB
Tropomyosin receptor kinase B
- VEGF
Vascular Endothelial Growth Factor
Footnotes
DECLARATION OF INTEREST: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Hebb D The effects of early experience on problem-solving at maturity. Am Psychol 2, 306–307 (1947). [Google Scholar]
- 2.Fox C, Merali Z & Harrison C Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav. Brain Res. 175, 1–8 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Crofton EJ, Zhang Y & Green TA Inoculation stress hypothesis of environmental enrichment. Neurosci. Biobehav. Rev. 49, 19–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhal G, Jaehne EJ, Corrigan F & Baune BT Cellular and molecular mechanisms of immunomodulation in the brain through environmental enrichment. Front. Cell. Neurosci. 8, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Praag H, Kempermann G & Gage FH Neural consequences of enviromental enrichment. Nat. Rev. Neurosci. 1, 191–198 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Simpson J & Kelly JP The impact of environmental enrichment in laboratory rats-Behavioural and neurochemical aspects. Behav. Brain Res. 222, 246–264 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Smith BL et al. Behavioral and physiological consequences of enrichment loss in rats. Psychoneuroendocrinology 77, 37–46 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morano R, Hoskins O, Smith BL & Herman JP Loss of Environmental Enrichment Elicits Behavioral and Physiological Dysregulation in Female Rats. Front. Behav. Neurosci. 12, 287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatchard T, Ting JJ & Messier C Translating the impact of exercise on cognition: Methodological issues in animal research. Behav. Brain Res. 273, 177–188 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Voss MW, Vivar C, Kramer AF & van Praag H Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences vol. 17 525–544 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers J, Renoir T & Hannan AJ Gene-environment interactions informing therapeutic approaches to cognitive and affective disorders. Neuropharmacology 145, 37–48 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Bailoo JD et al. Effects of cage enrichment on behavior, welfare and outcome variability in female mice. Front. Behav. Neurosci 12, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AL & Corrow DJ Modifications to Husbandry and Housing Conditions of Laboratory Rodents for Improved Well-being. https://academic.oup.com/ilarjournal/article-abstract/46/2/140/910141. [DOI] [PubMed]
- 14.Svenson KL & Paigen B Recommended housing densities for research mice: filling the gap in data-driven alternatives. J. • Rev • www.fasebj.org doi: 10.1096/fj.201801972R. [DOI] [PMC free article] [PubMed]
- 15.Couto M & Cates C Laboratory guidelines for animal care in Methods in Molecular Biology vol. 1920 407–430 (Humana Press Inc., 2019). [DOI] [PubMed] [Google Scholar]
- 16.Smith SM & Vale WW The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci vol. 8 www.dialoguescns.org (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arvidson E, Dahlman AS, Börjesson M, Gullstrand L & Jonsdottir IH Exercise training and physiological responses to acute stress: Study protocol and methodological considerations of a randomised controlled trial. BMJ Open Sport Exerc. Med. 4, 1–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dishman RK et al. Neurobiology of exercise. Obesity 14, 345–356 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Ulrich-Lai YM & Herman JP Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashokan A, Sivasubramanian M & Mitra R Seeding Stress Resilience through Inoculation. Neural Plast 2016, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons D Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front. Behav. Neurosci 3, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutter M Implications of resilience concepts for scientific understanding. Ann. N. Y. Acad. Sci. 1094, 1–12 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Selye H Stress without Distress BT - Psychopathology of Human Adaptation. 137–146 (1976) doi: 10.1007/978-1-4684-2238-2_9. [DOI] [Google Scholar]
- 24.Larsson F, Winblad B & Mohammed AH Psychological stress and environmental adaptation in enriched vs. Impoverished housed rats. Pharmacol. Biochem. Behav. 73, 193–207 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Sampedro-Piquero P et al. Environmental Enrichment Results in Both Brain Connectivity Efficiency and Selective Improvement in Different Behavioral Tasks. Neuroscience 388, 374–383 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Sampedro-Piquero P & Begega A Environmental Enrichment as a Positive Behavioral Intervention Across the Lifespan. Curr. Neuropharmacol. 15, 459–470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapolsky RM Stress and the brain: Individual variability and the inverted-U. Nat. Neurosci. 18, 1344–1346 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Salehi B, Cordero MI & Sandi C Learning under stress: The inverted-U-shape function revisited. Learn. Mem. 17, 522–530 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Anderson CR Coping behaviors as intervening mechanisms in the inverted-U stress-performance relationship. J. Appl. Psychol. 61, 30–34 (1976). [PubMed] [Google Scholar]
- 30.Wilke PK, Gmelch WH & Lovrich NP Stress and Productivity: Evidence of the Inverted U Function. Public Product. Rev. 9, 342 (1985). [Google Scholar]
- 31.Northoff G & Tumati S “Average is good, extremes are bad” – Non-linear inverted U-shaped relationship between neural mechanisms and functionality of mental features. Neuroscience and Biobehavioral Reviews vol. 104 11–25 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Zhang C, Ji Y & Yang L Biological and Psychological Perspectives of Resilience: Is It Possible to Improve Stress Resistance? Frontiers in Human Neuroscience vol. 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevins RA & Bardo MT Conditioned increase in place preference by access to novel objects: Antagonism by MK-801. Behav. Brain Res. 99, 53–60 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Venniro M et al. Volitional social interaction prevents drug addiction in rat models. Nat. Neurosci. 21, 1520–1529 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louilot A, Le Moal M & Simon H Differential reactivity of dopaminergic neurons in the nucleus accumbens in response to different behavioral situations. An in vivo voltammetric study in free moving rats. Brain Res. 397, 395–400 (1986). [DOI] [PubMed] [Google Scholar]
- 36.Ulrich-Lai YM et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc. Natl. Acad. Sci. U. S. A. 107, 20529–20534 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clemenson GD, Deng W & Gage FH Environmental enrichment and neurogenesis: From mice to humans. Current Opinion in Behavioral Sciences vol. 4 56–62 (2015). [Google Scholar]
- 38.Fuss J et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus 20, 364–376 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Hendershott TR, Cronin ME, Langella S, McGuinness PS & Basu AC Effects of environmental enrichment on anxiety-like behavior, sociability, sensory gating, and spatial learning in male and female C57BL/6J mice. Behav. Brain Res. 314, 215–225 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Urakawa S et al. Rearing in enriched environment increases parvalbumin-positive small neurons in the amygdala and decreases anxiety-like behavior of male rats. BMC Neurosci. 14, 13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benaroya-Milshtein N et al. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur. J. Neurosci. 20, 1341–1347 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Friske JE & Gammie SC Environmental enrichment alters plus maze, but not maternal defense performance in mice. Physiol. Behav. 85, 187–194 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Leal-Galicia P, Saldívar-González A, Morimoto S & Arias C Exposure to environmental enrichment elicits differential hippocampal cell proliferation: Role of individual responsiveness to anxiety. Dev. Neurobiol. 67, 395–405 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Roy V, Belzung C, Delarue C & Chapillon P Environmental enrichment in BALB/c mice: Effects in classical tests of anxiety and exposure to a predatory odor. Physiol. Behav. 74, 313–320 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Arranz L et al. Environmental Enrichment Improves Age-Related Immune System Impairment: Long-Term Exposure Since Adulthood Increases Life Span in Mice. Rejuvenation Res 13, 415–428 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Brenes Sáenz JC, Villagra OR & Fornaguera Trías J Factor analysis of Forced Swimming test, Sucrose Preference test and Open Field test on enriched, social and isolated reared rats. Behav. Brain Res. 169, 57–65 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Brenes JC, Rodríguez O & Fornaguera J Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol. Biochem. Behav. 89, 85–93 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Green TA et al. Environmental Enrichment Produces a Behavioral Phenotype Mediated by Low Cyclic Adenosine Monophosphate Response Element Binding (CREB) Activity in the Nucleus Accumbens. Biol. Psychiatry 67, 28–35 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llorens-Martín M, Tejeda GS & Trejo JL Antidepressant and Proneurogenic Influence of Environmental Enrichment in Mice: Protective Effects vs Recovery. Neuropsychopharmacology 36, 2460–2468 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seong HH, Park JM & Kim YJ Antidepressive Effects of Environmental Enrichment in Chronic Stress–Induced Depression in Rats. Biol. Res. Nurs. 20, 40–48 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Lehmann ML & Herkenham M Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J. Neurosci. 31, 6159–6173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leger M et al. Environmental enrichment improves recent but not remote memory in association with a modified brain metabolic activation profile in adult mice. Behav. Brain Res. 228, 22–29 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Garrido P et al. Differential effects of environmental enrichment and isolation housing on the hormonal and neurochemical responses to stress in the prefrontal cortex of the adult rat: Relationship to working and emotional memories. J. Neural Transm. 120, 829–843 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Veena J et al. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J. Neurosci. Res. 87, 831–843 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Wright RL & Conrad CD Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behav. Brain Res 187, 41–47 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nilsson M, Perfilieva E, Johansson U, Orwar O & Eriksson PS Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 39, 569–578 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Bouet V, Freret T, Dutar P, Billard JM & Boulouard M Continuous enriched environment improves learning and memory in adult NMRI mice through theta burst-related-LTP independent mechanisms but is not efficient in advanced aged animals. Mech. Ageing Dev. 132, 240–248 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Williams BM et al. Environmental enrichment: Effects on spatial memory and hippocampal CREB immunoreactivity. Physiol. Behav. 73, 649–658 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Segovia G, Yagüe AG, García-Verdugo JM & Mora F Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res. Bull. 70, 8–14 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Reichmann F, Painsipp E & Holzer P Environmental Enrichment and Gut Inflammation Modify Stress-Induced c-Fos Expression in the Mouse Corticolimbic System. PLoS One 8, 54811 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeleznikow-Johnston A, Burrows EL, Renoir T & Hannan AJ Environmental enrichment enhances cognitive flexibility in C57BL/6 mice on a touchscreen reversal learning task. Neuropharmacology 117, 219–226 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Novaes LS, dos Santos NB, Perfetto JG, Goosens KA & Munhoz CD Environmental enrichment prevents acute restraint stress-induced anxiety-related behavior but not changes in basolateral amygdala spine density. Psychoneuroendocrinology 98, 6–10 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Shilpa BM, Bhagya V, Harish G, Srinivas Bharath MM & Shankaranarayana Rao BS Environmental enrichment ameliorates chronic immobilisation stress-induced spatial learning deficits and restores the expression of BDNF, VEGF, GFAP and glucocorticoid receptors. Prog. Neuro-Psychopharmacology Biol. Psychiatry 76, 88–100 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Shen J et al. The enriched environment ameliorates chronic unpredictable mild stress-induced depressive-like behaviors and cognitive impairment by activating the SIRT1/miR-134 signaling pathway in hippocampus. J. Affect. Disord. 248, 81–90 (2019). [DOI] [PubMed] [Google Scholar]
- 65.O’Leary JD, Hoban AE, Cryan JF, O’Leary OF & Nolan YM Differential effects of adolescent and adult-initiated voluntary exercise on context and cued fear conditioning. Neuropharmacology 145, 49–58 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Tanichi M et al. Differential effects of voluntary wheel running and toy rotation on the mRNA expression of neurotrophic factors and FKBP5 in a post-traumatic stress disorder rat model with the shuttle-box task. Biochem. Biophys. Res. Commun. 501, 307–312 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Sun XR et al. Amelioration of oxidative stress-induced phenotype loss of parvalbumin interneurons might contribute to the beneficial effects of environmental enrichment in a rat model of post-traumatic stress disorder. Behav. Brain Res. 312, 84–92 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Takahashi T, Shimizu K, Shimazaki K, Toda H & Nibuya M Environmental enrichment enhances autophagy signaling in the rat hippocampus. Brain Res. 1592, 113–123 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Hendriksen H, Prins J, Olivier B & Oosting RS Environmental enrichment induces behavioral recovery and enhanced hippocampal cell proliferation in an antidepressant-resistant animal model for PTSD. PLoS One 5, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McQuaid RJ, Audet MC & Anisman H Environmental enrichment in male CD-1 mice promotes aggressive behaviors and elevated corticosterone and brain norepinephrine activity in response to a mild stressor. Stress 15, 354–360 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Girbovan C & Plamondon H Environmental enrichment in female rodents: Considerations in the effects on behavior and biochemical markers. Behav. Brain Res. 253, 178–190 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Venzala E, García-García AL, Elizalde N & Tordera RM Social vs. environmental stress models of depression from a behavioural and neurochemical approach. (2013) doi: 10.1016/j.euroneuro.2012.05.010. [DOI] [PubMed]
- 73.Moncek F, Duncko R, Johansson BB & Jezova D Effect of environmental enrichment on strees related systems in rats. J. Neuroendocrinol. 16, 423–431 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Konkle ATM, Kentner AC, Baker SL, Stewart A & Bielajew C Environmental-enrichment-related variations in behavioral, biochemical, and physiologic responses of Sprague-Dawley and Long Evans rats. J. Am. Assoc. Lab. Anim. Sci. 49, 427–436 (2010). [PMC free article] [PubMed] [Google Scholar]
- 75.Benaroya-Milshtein N et al. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur. J. Neurosci. 20, 1341–1347 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Fediuc S, Campbell JE & Riddell MC Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol 100, 1867–1875 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Belz EE, Kennell JS, Czambel RK, Rubin RT & Rhodes ME Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol. Biochem. Behav. 76, 481–486 (2003). [DOI] [PubMed] [Google Scholar]
- 78.Bhatnagar S & Dallman M Neuroanatomical basis for facilitation of hypothalamic-pituitary- adrenal responses to a novel stressor after chronic stress. Neuroscience 84, 1025–1039 (1998). [DOI] [PubMed] [Google Scholar]
- 79.Pham TM et al. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience 94, 279–286 (1999). [DOI] [PubMed] [Google Scholar]
- 80.Marashi V, Barnekow A & Sachser N Effects of environmental enrichment on males of a docile inbred strain of mice. Physiol. Behav. 82, 765–776 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Belz EE, Kennell JS, Czambel RK, Rubin RT & Rhodes ME Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol. Biochem. Behav. 76, 481–486 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Schrijver NCA, Bahr NI, Weiss IC & Würbel H Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol. Biochem. Behav. 73, 209–224 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Mlynarik M, Johansson BB & Jezova D Enriched environment influences adrenocortical response to immune challenge and glutamate receptor gene expression in rat hippocampus in Annals of the New York Academy of Sciences vol. 1018 273–280 (New York Academy of Sciences, 2004). [DOI] [PubMed] [Google Scholar]
- 84.Koe AS, Ashokan A & Mitra R Short environmental enrichment in adulthood reverses anxiety and basolateral amygdala hypertrophy induced by maternal separation. Transl. Psychiatry 6, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharp JL, Zammit TG, Azar TA & Lawson DM Stress-like Responses to Common Procedures in Male Rats Housed Alone or with Other Rats. Contemp. Top. Lab. Anim. Sci. 41, 8–14 (2002). [PubMed] [Google Scholar]
- 86.Marashi V, Barnekow A, Ossendorf E & Sachser N Effects of different forms of environmental enrichment on behavioral, endocrinological, and immunological parameters in male mice. Horm. Behav 43, 281–292 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Sharp J, Azar T & Lawson D Effects of a complex housing environment on heart rate and blood pressure of rats at rest and after stressful challenges. J. Am. Assoc. Lab. Anim. Sci. 53, 52–60 (2014). [PMC free article] [PubMed] [Google Scholar]
- 88.Ravenelle R, Santolucito HB, Byrnes EM, Byrnes JJ & Donaldson ST Housing environment modulates physiological and behavioral responses to anxiogenic stimuli in trait anxiety male rats. Neuroscience 270, 76–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Azar TA, Sharp JL & Lawson DM Effects of cage enrichment on heart rate, blood pressure, and activity of female Sprague-Dawley and spontaneously hypertensive rats at rest and after acute challenges. J. Am. Assoc. Lab. Anim. Sci 51, 339–344 (2012). [PMC free article] [PubMed] [Google Scholar]
- 90.Marie A, Petersen W & Pedersen BK The anti-inflammatory effect of exercise. J Appl Physiol 98, 1154–1162 (2005). [DOI] [PubMed] [Google Scholar]
- 91.Scarola SJ, Perdomo Trejo JR, Granger ME, Gerecke KM & Bardi M Immunomodulatory Effects of Stress and Environmental Enrichment in Long-Evans Rats (Rattus norvegicus). Comp. Med 69, 35–47 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ziv Y et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 9, 268–275 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Eyre H & Baune BT Neuroimmunological effects of physical exercise in depression. Brain, Behavior, and Immunity vol. 26 251–266 (2012). [DOI] [PubMed] [Google Scholar]
- 94.Petersen AMW & Pedersen BK The role of IL-6 in mediating the anti-inflammatory effects of exercise. in Journal of Physiology and Pharmacology vol. 57 43–51 (2006). [PubMed] [Google Scholar]
- 95.Kaufman JC, Harris TJ, Higgins J & Maisel AS Exercise-Induced Enhancement of Immune Function in the Rat. http://ahajournals.org (1994). [DOI] [PubMed]
- 96.Wohleb ES, Franklin T, Iwata M & Duman RS Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 17, 497–511 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Renoir T, Pang TY & Hannan AJ Effects of environmental manipulations in genetically targeted animal models of affective disorders. Neurobiology of Disease vol. 57 12–27 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Veena J, Srikumar BN, Raju TR & Shankaranarayana Rao BS Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci. Lett. 455, 178–182 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Williamson LL, Chao A & Bilbo SD Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain. Behav. Immun. 26, 500–510 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohline SM & Abraham WC Environmental enrichment effects on synaptic and cellular physiology of hippocampal neurons. Neuropharmacology vol. 145 3–12 (2019). [DOI] [PubMed] [Google Scholar]
- 101.Kempermann G, Kuhn H & Gage F More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (1997). [DOI] [PubMed] [Google Scholar]
- 102.Kempermann G & Gage FH Experience-dependent regulation of adult hippocampal neurogenesis: Effects of long-term stimulation and stimulus withdrawal. Hippocampus 9, 321–332 (1999). [DOI] [PubMed] [Google Scholar]
- 103.Rossi C et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 24, 1850–1856 (2006). [DOI] [PubMed] [Google Scholar]
- 104.Grégoire CA, Bonenfant D, Le Nguyen A, Aumont A & Fernandes KJL Untangling the influences of voluntary running, environmental complexity, social housing and stress on adult hippocampal neurogenesis. PLoS One 9, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fabel K Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front. Neurosci. (2009) doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vyas A, Mitra R, Shankaranarayana Rao BS & Chattarji S Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 22, 6810–6818 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schloesser RJ, Lehmann M, Martinowich K, Manji HK & Herkenham M Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry 15, 1152–1163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yau SY et al. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience 222, 289–301 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Vivar C, Potter MC & van Praag H All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Top. Behav. Neurosci. 15, 189–210 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mármol F, Sánchez J, Torres MN & Chamizo VD Environmental enrichment in the absence of wheel running produces beneficial behavioural and anti-oxidative effects in rats. Behav. Processes 144, 66–71 (2017). [DOI] [PubMed] [Google Scholar]
- 111.Moreno-Jiménez EP, Jurado-Arjona J, Avila J & Martín ML The social component of environmental enrichment is a pro-neurogenic stimulus in adult c57BL6 female mice. Front. Cell Dev. Biol. 7, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gould E, Tanapat P, Rydel T & Hastings N Regulation of hippocampal neurogenesis in adulthood. in Biological Psychiatry vol. 48 715–720 (2000). [DOI] [PubMed] [Google Scholar]
- 113.Young D, Lawlor PA, Leone P, Dragunow M & During MJ Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nature Medicine vol. 5 http://medicine.nature.com (1999). [DOI] [PubMed] [Google Scholar]
- 114.Leger M et al. Environmental enrichment duration differentially affects behavior and neuroplasticity in adult mice. Cereb. Cortex 25, 4048–4061 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Birch AM, McGarry NB & Kelly ÁM Short-term environmental enrichment, in the absence of exercise, improves memory, and increases NGF concentration, early neuronal survival, and synaptogenesis in the dentate gyrus in a time-dependent manner. Hippocampus 23, 437–450 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Mármol F, Rodríguez CA, Sánchez J & Chamizo VD Anti-oxidative effects produced by environmental enrichment in the hippocampus and cerebral cortex of male and female rats. Brain Res. 1613, 120–129 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Kempermann G Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 20, 235–245 (2019). [DOI] [PubMed] [Google Scholar]
- 118.Nithianantharajah J & Hannan AJ Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709 (2006). [DOI] [PubMed] [Google Scholar]
- 119.Beauquis J, Roig P, de Nicola AF & Saravia F Short-term environmental enrichment enhances adult neurogenesis, vascular network and dendritic complexity in the hippocampus of type 1 diabetic mice. PLoS One 5, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hutchinson KM et al. Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity. Neurobiol. Learn. Mem. 97, 250–260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang YF, Yang CH, Huang CC & Hsu K Sen. Vascular endothelial growth factor-dependent spinogenesis underlies antidepressant-like effects of enriched environment. J. Biol. Chem. 287, 40938–40955 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitra R, Jadhav S, McEwen BS, Vyas A & Chattarji S Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A. 102, 9371–9376 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ashokan A, Hegde A, Balasingham A & Mitra R Housing environment influences stress-related hippocampal substrates and depression-like behavior. Brain Res. 1683, 78–85 (2018). [DOI] [PubMed] [Google Scholar]
- 124.Comeau WL, McDonald RJ & Kolb BE Learning-induced alterations in prefrontal cortical dendritic morphology. Behav. Brain Res. 214, 91–101 (2010). [DOI] [PubMed] [Google Scholar]
- 125.Kolb B, Gorny G, Söderpalm AHV & Robinson TE Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse 48, 149–153 (2003). [DOI] [PubMed] [Google Scholar]
- 126.Ashokan A, Lim JWH, Hang N & Mitra R Complex housing causes a robust increase in dendritic complexity and spine density of medial prefrontal cortical neurons. Sci. Rep 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kolb B & Gibb R Plasticity in the prefrontal cortex of adult rats. Front. Cell. Neurosci. 9, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mitra R & Sapolsky RM Effects of enrichment predominate over those of chronic stress on fear-related behavior in male rats. Stress 12, 305–312 (2009). [DOI] [PubMed] [Google Scholar]
- 129.Ashokan A, Hegde A & Mitra R Short-term environmental enrichment is sufficient to counter stress-induced anxiety and associated structural and molecular plasticity in basolateral amygdala. Psychoneuroendocrinology 69, 189–196 (2016). [DOI] [PubMed] [Google Scholar]
- 130.Buschler A & Manahan-Vaughan D Brief environmental enrichment elicits metaplasticity of hippocampal synaptic potentiation in vivo. Front. Behav. Neurosci. (2012) doi: 10.3389/fnbeh.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duffy SN, Craddock KJ, Abel T & Nguyen PV Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn. Mem. 8, 26–34 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Artola A et al. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur. J. Neurosci. 23, 261–272 (2006). [DOI] [PubMed] [Google Scholar]
- 133.Irvine GI, Logan B, Eckert M & Abraham WC Enriched environment exposure regulates excitability, synaptic transmission, and LTP in the dentate gyrus of freely moving rats. Hippocampus 16, 149–160 (2006). [DOI] [PubMed] [Google Scholar]
- 134.Bhagya VR, Srikumar BN, Veena J & Shankaranarayana Rao BS Short-term exposure to enriched environment rescues chronic stress-induced impaired hippocampal synaptic plasticity, anxiety, and memory deficits. J. Neurosci. Res. 95, 1602–1610 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Gagné J et al. AMPA receptor properties in adult rat hippocampus following environmental enrichment. Brain Res. 799, 16–25 (1998). [DOI] [PubMed] [Google Scholar]
- 136.Lambert TJ, Fernandez SM & Frick KM Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiol. Learn. Mem. 83, 206–216 (2005). [DOI] [PubMed] [Google Scholar]
- 137.Lin TW et al. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol. Learn. Mem. 97, 140–147 (2012). [DOI] [PubMed] [Google Scholar]
- 138.Liu C et al. Enriched environment combined with fluoxetine ameliorates depression-like behaviors and hippocampal SYP expression in a rat CUS model. Brain Res. Bull. 135, 33–39 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Nithianantharajah J, Levis HJ & Murphy M Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiol. Learn. Mem. 81, 200–210 (2004). [DOI] [PubMed] [Google Scholar]
- 140.Vaynman SS, Ying Z, Yin D & Gomez-Pinilla F Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 1070, 124–130 (2006). [DOI] [PubMed] [Google Scholar]
- 141.Lopes DA et al. Environmental enrichment decreases avoidance responses in the elevated T-maze and delta FosB immunoreactivity in anxiety-related brain regions. Behav. Brain Res. 344, 65–72 (2018). [DOI] [PubMed] [Google Scholar]
- 142.Grimm JW et al. Effects of acute or chronic environmental enrichment on regional Fos protein expression following sucrose cue-reactivity testing in rats. Brain Struct. Funct. 221, 2817–2830 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sampedro-Piquero P, Zancada-Menendez C, Begega A, Rubio S & Arias JL Effects of environmental enrichment on anxiety responses, spatial memory and cytochrome c oxidase activity in adult rats. Brain Res. Bull. 98, 1–9 (2013). [DOI] [PubMed] [Google Scholar]
- 144.González-Pardo H, Arias JL, Vallejo G & Conejo NM Environmental enrichment effects after early stress on behavior and functional brain networks in adult rats. PLoS One 14, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sampedro-Piquero P, Arias JL & Begega A Behavioral testing-related changes in the expression of Synapsin I and glucocorticoid receptors in standard and enriched aged Wistar rats. Exp. Gerontol. 58, 292–302 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Venebra-Muñoz A et al. Enriched environment attenuates nicotine self-administration and induces changes in ΔfosB expression in the rat prefrontal cortex and nucleus accumbens. Neuroreport 25, 694–698 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Watanasriyakul WT et al. Protective neuroendocrine effects of environmental enrichment and voluntary exercise against social isolation: evidence for mediation by limbic structures. Stress 22, 603–618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nikolaev E, Kaczmarek L, Zhu SW, Winblad B & Mohammed AH Environmental manipulation differentially alters c-Fos expression in amygdaloid nuclei following aversive conditioning. Brain Res. 957, 91–98 (2002). [DOI] [PubMed] [Google Scholar]
- 149.Zarrindast MR et al. GABAA receptors in the basolateral amygdala are involved in mediating morphine reward. Brain Res. 1006, 49–58 (2004). [DOI] [PubMed] [Google Scholar]
- 150.Lambert K et al. Natural-enriched environments lead to enhanced environmental engagement and altered neurobiological resilience. Neuroscience 330, 386–394 (2016). [DOI] [PubMed] [Google Scholar]
- 151.Liu F. fang et al. NOX2 Mediated-Parvalbumin Interneuron Loss Might Contribute to Anxiety-Like and Enhanced Fear Learning Behavior in a Rat Model of Post-Traumatic Stress Disorder. Mol. Neurobiol. 53, 6680–6689 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Page CE, Shepard R, Heslin K & Coutellier L Prefrontal parvalbumin cells are sensitive to stress and mediate anxiety-related behaviors in female mice. Sci. Rep. 9, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suzuki H et al. Role of neuropsin in parvalbumin immunoreactivity changes in hippocampal basket terminals of mice reared in various environments. Front. Cell. Neurosci. 8, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ehninger D et al. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 345, 69–86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Walsh RN, Budtz-Olsen OE, Penny JE & Cummins RA The effects of environmental complexity on the histology of the rat hippocampus. J. Comp. Neurol. 137, 361–365 (1969). [DOI] [PubMed] [Google Scholar]
- 156.Soffié M, Hahn K, Terao E & Eclancher F Behavioural and glial changes in old rats following environmental enrichment. Behav. Brain Res. 101, 37–49 (1999). [DOI] [PubMed] [Google Scholar]
- 157.Glezer I, Simard AR & Rivest S Neuroprotective role of the innate immune system by microglia. Neuroscience vol. 147 867–883 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Ekdahl CT, Kokaia Z & Lindvall O Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience vol. 158 1021–1029 (2009). [DOI] [PubMed] [Google Scholar]
- 159.Sierra A et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Viola GG et al. Morphological changes in hippocampal astrocytes induced by environmental enrichment in mice. Brain Res. 1274, 47–54 (2009). [DOI] [PubMed] [Google Scholar]
- 161.Altman J & Das GD Autoradiographic examination of the effects of enriched environment on the rate of glial multiplication in the adult rat brain. Nature 204, 1161–1163 (1964). [DOI] [PubMed] [Google Scholar]
- 162.Ekstrand J, Hellsten J & Tingström A Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neurosci. Lett. 442, 203–207 (2008). [DOI] [PubMed] [Google Scholar]
- 163.Ickes BR et al. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp. Neurol. 164, 45–52 (2000). [DOI] [PubMed] [Google Scholar]
- 164.Angelucci F et al. Increased concentrations of nerve growth factor and brain-derived neurotrophic factor in the rat cerebellum after exposure to environmental enrichment. Cerebellum 8, 499–506 (2009). [DOI] [PubMed] [Google Scholar]
- 165.Mohammed AH et al. Environmental enrichment and the brain. Prog. Brain Res. 138, 109–133 (2002). [DOI] [PubMed] [Google Scholar]
- 166.Takahashi T, Morinobu S, Iwamoto Y & Yamawaki S Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology (Berl). 189, 165–173 (2006). [DOI] [PubMed] [Google Scholar]
- 167.Zheng H et al. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav. Brain Res. 168, 47–55 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ting C-Y et al. Concurrent blood–brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 33, 704–712 (2012). [DOI] [PubMed] [Google Scholar]
- 169.Greenwood BN, Strong PV, Foley TE & Fleshner M A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus 19, 988–1001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Olsson T, Mohammed AH, Donaldson LF, Henriksson BG & Seckl JR Glucocorticoid receptor and NGFI-A gene expression are induced in the hippocampus after environmental enrichment in adult rats. Mol. Brain Res. 23, 349–353 (1994). [DOI] [PubMed] [Google Scholar]
- 171.Torasdotter M, Metsis M, Henriksson BG, Bengt Winblad & Mohammed, A. H. Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav. Brain Res. 93, 83–90 (1998). [DOI] [PubMed] [Google Scholar]
- 172.Cassilhas RC et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 202, 309–317 (2012). [DOI] [PubMed] [Google Scholar]
- 173.Mohammed AH et al. Environmental influences on the central nervous system and their implications for the aging rat. Behav. Brain Res. 57, 183–191 (1993). [DOI] [PubMed] [Google Scholar]
- 174.Nowacka M & Obuchowicz E BDNF and VEGF in the pathogenesis of stress-induced affective diseases: An insight from experimental studies. Pharmacol. Reports 65, 535–564 (2013). [DOI] [PubMed] [Google Scholar]
- 175.McAllister AK, Lo DC & Katz LC Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15, 791–803 (1995). [DOI] [PubMed] [Google Scholar]
- 176.Jha S et al. Antidepressive and BDNF effects of enriched environment treatment across ages in mice lacking BDNF expression through promoter IV. Transl. Psychiatry 6, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Storkebaum E, Lambrechts D & Carmeliet P VEGF: Once regarded as a specific angiogenic factor, now implicated in neuroprotection. BioEssays vol. 26 943–954 (2004). [DOI] [PubMed] [Google Scholar]
- 178.Sun F-Y & Guo X Molecular and cellular mechanisms of neuroprotection by vascular endothelial growth factor. J. Neurosci. Res. 79, 180–184 (2005). [DOI] [PubMed] [Google Scholar]
- 179.Pham TM, Winblad B, Granholm AC & Mohammed AH Environmental influences on brain neurotrophins in rats. Pharmacol. Biochem. Behav. 73, 167–175 (2002). [DOI] [PubMed] [Google Scholar]
- 180.McQuaid RJ, Dunn R, Jacobson-Pick S, Anisman H & Audet MC Post-weaning environmental enrichment in male CD-1 mice: Impact on social behaviors, corticosterone levels and prefrontal cytokine expression in adulthood. Front. Behav. Neurosci. 12, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Duman CH et al. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav. Brain Res. 198, 366–371 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bennett MR & Lagopoulos J Stress and trauma: BDNF control of dendritic-spine formation and regression. Progress in Neurobiology vol. 112 80–99 (2014). [DOI] [PubMed] [Google Scholar]
- 183.Boyle LM A neuroplasticity hypothesis of chronic stress in the basolateral amygdala. Yale J. Biol. Med. 86, 117–125 (2013). [PMC free article] [PubMed] [Google Scholar]
- 184.Varman DR & Rajan KE Environmental enrichment reduces anxiety by differentially activating serotonergic and neuropeptide y (NPY)-ergic system in Indian field mouse (Mus booduga): An animal model of post-traumatic stress disorder. PLoS One 10, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sampedro-Piquero P, Begega A & Arias JL Increase of glucocorticoid receptor expression after environmental enrichment: Relations to spatial memory, exploration and anxiety-related behaviors. Physiol. Behav. 129, 118–129 (2014). [DOI] [PubMed] [Google Scholar]
- 186.Zanca RM et al. Environmental enrichment increases glucocorticoid receptors and decreases GluA2 and protein kinase M Zeta (PKMζ) trafficking during chronic stress: A protective mechanism? Front. Behav. Neurosci. 9, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wisłowska-Stanek A et al. Corticosterone modulates fear responses and the expression of glucocorticoid receptors in the brain of high-anxiety rats. Neurosci. Lett. 533, 17–22 (2013). [DOI] [PubMed] [Google Scholar]
- 188.Kentner AC, Lima E, Migliore MM, Shin J & Scalia S Complex Environmental Rearing Enhances Social Salience and Affects Hippocampal Corticotropin Releasing Hormone Receptor Expression in a Sex-Specific Manner. Neuroscience 369, 399–411 (2018). [DOI] [PubMed] [Google Scholar]
- 189.McQuaid RJ, Audet MC, Jacobson-Pick S & Anisman H The differential impact of social defeat on mice living in isolation or groups in an enriched environment: Plasma corticosterone and monoamine variations. Int. J. Neuropsychopharmacol. 16, 351–363 (2013). [DOI] [PubMed] [Google Scholar]
- 190.Lach G et al. Short-term enriched environment exposure facilitates fear extinction in adult rats: The NPY-Y1 receptor modulation. Neuropeptides 55, 73–78 (2016). [DOI] [PubMed] [Google Scholar]
- 191.Hendriksen H et al. Re-exposure and environmental enrichment reveal NPY-Y1 as a possible target for post-traumatic stress disorder. Neuropharmacology 63, 733–742 (2012). [DOI] [PubMed] [Google Scholar]
- 192.Segovia G, Arco A Del & Mora, F. Environmental enrichment, prefrontal cortex, stress, and aging of the brain. J. Neural Transm. 116, 1007–1016 (2009). [DOI] [PubMed] [Google Scholar]
- 193.Ronzoni G, Antón M, Mora F, Segovia G & Del Arco A Infralimbic cortex controls the activity of the hypothalamus-pituitary-adrenal axis and the formation of aversive memory: Effects of environmental enrichment. Behav. Brain Res. 297, 338–344 (2016). [DOI] [PubMed] [Google Scholar]
- 194.Novaes LS et al. Environmental enrichment protects against stress-induced anxiety: Role of glucocorticoid receptor, ERK, and CREB signaling in the basolateral amygdala. Neuropharmacology 113, 457–466 (2017). [DOI] [PubMed] [Google Scholar]
- 195.Sztainberg Y, Kuperman Y, Tsoory M, Lebow M & Chen A The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol. Psychiatry 15, 905–917 (2010). [DOI] [PubMed] [Google Scholar]
- 196.Naka F, Narita N, Okado N & Narita M Modification of AMPA receptor properties following environmental enrichment. Brain Dev. 27, 275–278 (2005). [DOI] [PubMed] [Google Scholar]
- 197.Lee EHY, Hsu WL, Ma YL, Lee PJ & Chao CC Enrichment enhances the expression of sgk, a glucocorticoid-induced gene, and facilitates spatial learning through glutamate AMPA receptor mediation. Eur. J. Neurosci. 18, 2842–2852 (2003). [DOI] [PubMed] [Google Scholar]
- 198.Andin J, Hallbeck M, Mohammed AH & Marcusson J Influence of environmental enrichment on steady-state mRNA levels for EAAC1, AMPA1 and NMDA2A receptor subunits in rat hippocampus. Brain Res. 1174, 18–27 (2007). [DOI] [PubMed] [Google Scholar]
- 199.Galani R et al. The behavioral effects of enriched housing are not altered by serotonin depletion but enrichment alters hippocampal neurochemistry. Neurobiol. Learn. Mem. 88, 1–10 (2007). [DOI] [PubMed] [Google Scholar]
- 200.Brenes JC, Padilla M & Fornaguera J A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav. Brain Res. 197, 125–137 (2009). [DOI] [PubMed] [Google Scholar]
- 201.Rasmuson S et al. Environmental enrichment selectively increases 5-HT1A receptor mRNA expression and binding in the rat hippocampus. Mol. Brain Res. 53, 285–290 (1998). [DOI] [PubMed] [Google Scholar]
- 202.Mora F, Segovia G & del Arco A Aging, plasticity and environmental enrichment: Structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res. Rev. 55, 78–88 (2007). [DOI] [PubMed] [Google Scholar]
- 203.Bakos J et al. Enriched environment influences hormonal status and hippocampal brain derived neurotrophic factor in a sex dependent manner. Neuroscience 164, 788–797 (2009). [DOI] [PubMed] [Google Scholar]
- 204.Zhu J, Apparsundaram S, Bardo MT & Dwoskin LP Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J. Neurochem. 93, 1434–1443 (2005). [DOI] [PubMed] [Google Scholar]
- 205.Del Arco A, Segovia G, Garrido P, de Blas M & Mora F Stress, prefrontal cortex and environmental enrichment: Studies on dopamine and acetylcholine release and working memory performance in rats. Behav. Brain Res. 176, 267–273 (2007). [DOI] [PubMed] [Google Scholar]
- 206.Segovia G, Del Arco A, de Blas M, Garrido P & Mora F Effects of an enriched environment on the release of dopamine in the prefrontal cortex produced by stress and on working memory during aging in the awake rat. Behav. Brain Res. 187, 304–311 (2008). [DOI] [PubMed] [Google Scholar]
- 207.Darna M, Beckmann JS, Gipson CD, Bardo MT & Dwoskin LP Effect of environmental enrichment on dopamine and serotonin transporters and glutamate neurotransmission in medial prefrontal and orbitofrontal cortex. Brain Res. 1599, 115–125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Eyre HA, Papps E, Baune BT, Grimm O & Horowitz M Treating depression and depression-like behavior with physical activity: an immune perspective. (2013) doi: 10.3389/fpsyt.2013.00003. [DOI] [PMC free article] [PubMed]
- 209.Jurgens HA & Johnson RW Environmental enrichment attenuates hippocampal neuroinflammation and improves cognitive function during influenza infection. Brain. Behav. Immun. 26, 1006–1016 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Colpo GD, Leboyer M, Dantzer R, Trivedi MH & Teixeira AL Immune-based strategies for mood disorders: facts and challenges. Expert Rev. Neurother. 18, 139–152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wohleb ES Neuron-microglia interactions in mental health disorders: ‘For better, and for worse’. Front. Immunol. 7, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Eisinger BE & Zhao X Identifying molecular mediators of environmentally enhanced neurogenesis. Cell Tissue Res. 371, 7–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Szepesi Z, Manouchehrian O, Bachiller S & Deierborg T Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 12, 323 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liu CH et al. Role of inflammation in depression relapse. J. Neuroinflammation 16, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Darcy MJ, Trouche S, Jin SX & Feig LA Ras-GRF2 mediates long-term potentiation, survival, and response to an enriched environment of newborn neurons in the hippocampus. Hippocampus 24, 1317–1329 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu X, Yang LJ, Fan SJ, Jiang H & Pan F Swimming exercise effects on the expression of HSP70 and iNOS in hippocampus and prefrontal cortex in combined stress. Neurosci. Lett. 476, 99–103 (2010). [DOI] [PubMed] [Google Scholar]
- 217.Streicher JM The role of heat shock proteins in regulating receptor signal transduction. Mol. Pharmacol. 468–474 (2019) doi: 10.1124/mol.118.114652. [DOI] [PubMed] [Google Scholar]
- 218.Kacimi R & Yenari MA Pharmacologic heat shock protein 70 induction confers cytoprotection against inflammation in gliovascular cells. Glia 63, 1200–1212 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Greenough WT & Volkmar FR Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp. Neurol. 40, 491–504 (1973). [DOI] [PubMed] [Google Scholar]
- 220.Jung CKE & Herms J Structural Dynamics of Dendritic Spines are Influenced by an Environmental Enrichment: An In Vivo Imaging Study. (2012) doi: 10.1093/cercor/bhs317. [DOI] [PubMed]
- 221.Diamond MC et al. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comp. Neurol. 128, 117–125 (1966). [DOI] [PubMed] [Google Scholar]
- 222.Leggio MG et al. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav. Brain Res. 163, 78–90 (2005). [DOI] [PubMed] [Google Scholar]
- 223.Briones TL, Klintsova AY & Greenough WT Stability of synaptic plasticity in the adult rat visual cortex induced by complex environment exposure. Brain Res. 1018, 130–135 (2004). [DOI] [PubMed] [Google Scholar]
- 224.Turner AM & Greenough WT Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 329, 195–203 (1985). [DOI] [PubMed] [Google Scholar]
- 225.Diamond MC, Krech D & Rosenzweig MR The effects of an enriched environment on the histology of the rat cerebral cortex. J. Comp. Neurol. 123, 111–119 (1964). [DOI] [PubMed] [Google Scholar]
- 226.Mitra R & Sapolsky RM Short-term enrichment makes male rats more attractive, more defensive and alters hypothalamic neurons. PLoS One 7, 36092 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Greenwood BN & Fleshner M Exercise, stress resistance, and central serotonergic systems. Exerc. Sport Sci. Rev. 39, 140–149 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang Y et al. Overexpression of DeltaFosB in nucleus accumbens mimics the protective addiction phenotype, but not the protective depression phenotype of environmental enrichment. Front. Behav. Neurosci 8, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Solinas M, Thiriet N, Chauvet C & Jaber M Prevention and treatment of drug addiction by environmental enrichment. Progress in Neurobiology vol. 92 572–592 (2010). [DOI] [PubMed] [Google Scholar]
- 230.Hannan AJ Review: Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol. Appl. Neurobiol. 40, 13–25 (2014). [DOI] [PubMed] [Google Scholar]
- 231.Clemenson GD & Stark CEL Behavioral/Cognitive Virtual Environmental Enrichment through Video Games Improves Hippocampal-Associated Memory. (2015) doi: 10.1523/JNEUROSCI.2580-15.2015. [DOI] [PMC free article] [PubMed]
- 232.McOmish CE, Burrows EL & Hannan AJ Identifying novel interventional strategies for psychiatric disorders: integrating genomics, ‘enviromics’ and gene-environment interactions in valid preclinical models. Br. J. Pharmacol. 171, 4719–4728 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Possamai F et al. Influence of enrichment on behavioral and neurogenic effects of antidepressants in Wistar rats submitted to repeated forced swim test. Prog. Neuro-Psychopharmacology Biol. Psychiatry 58, 15–21 (2015). [DOI] [PubMed] [Google Scholar]
- 234.Alboni S et al. Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain. Behav. Immun. 58, 261–271 (2016). [DOI] [PubMed] [Google Scholar]
- 235.Tanti A et al. Region-dependent and stage-specific effects of stress, environmental enrichment, and antidepressant treatment on hippocampal neurogenesis. Hippocampus 23, 797–811 (2013). [DOI] [PubMed] [Google Scholar]
- 236.Mustroph ML et al. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience 219, 62–71 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kobilo T et al. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn. Mem 18, 605–609 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Peña Y, Prunell M, Rotllant D, Armario A & Escorihuela RM Enduring effects of environmental enrichment from weaning to adulthood on pituitary-adrenal function, pre-pulse inhibition and learning in male and female rats. Psychoneuroendocrinology 34, 1390–1404 (2009). [DOI] [PubMed] [Google Scholar]
- 239.Mattson MP, Duan W, Lee J & Guo Z Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: Molecular mechanisms. Mechanisms of Ageing and Development vol. 122 757–778 (2001). [DOI] [PubMed] [Google Scholar]
- 240.McQuaid RJ, Audet MC, Jacobson-Pick S & Anisman H Environmental enrichment influences brain cytokine variations elicited by social defeat in mice. Psychoneuroendocrinology 38, 987–996 (2013). [DOI] [PubMed] [Google Scholar]
- 241.Elliott BM & Grunberg NE Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav. Brain Res. 165, 187–196 (2005). [DOI] [PubMed] [Google Scholar]
- 242.Brown KJ & Grunberg NE Effects of housing on male and female rats: Crowding stresses males but calms females. Physiol. Behav. 58, 1085–1089 (1995). [DOI] [PubMed] [Google Scholar]
- 243.Kokras N et al. Neuroplasticity-related correlates of environmental enrichment combined with physical activity differ between the sexes. Eur. Neuropsychopharmacol. 29, 1–15 (2019). [DOI] [PubMed] [Google Scholar]
- 244.Welberg L, Thrivikraman KV & Plotsky PM Combined pre- and postnatal environmental enrichment programs the HPA axis differentially in male and female rats. Psychoneuroendocrinology 31, 553–564 (2006). [DOI] [PubMed] [Google Scholar]
- 245.Vega-Rivera NM et al. The neurogenic effects of an enriched environment and its protection against the behavioral consequences of chronic mild stress persistent after enrichment cessation in six-month-old female Balb/C mice. Behav. Brain Res. 301, 72–83 (2016). [DOI] [PubMed] [Google Scholar]
- 246.Lle Nader J et al. Loss of Environmental Enrichment Increases Vulnerability to Cocaine Addiction. Neuropsychopharmacology 37, 1579–1587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nishijima T et al. Cessation of voluntary wheel running increases anxiety-like behavior and impairs adult hippocampal neurogenesis in mice. Behav. Brain Res. 245, 34–41 (2013). [DOI] [PubMed] [Google Scholar]
- 248.National Instititute of Mental Health. Domain: Negative Valence Systems. Research Domain Criteria (RDoC) (2018). [Google Scholar]
- 249.Heim C, Owens MJ, Plotsky PM & Nemeroff CB The role of early adverse life events in the etiology of depression and posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 821, 194–207 (1997). [DOI] [PubMed] [Google Scholar]
- 250.Tennant C Work-related stress and depressive disorders. Journal of Psychosomatic Research vol. 51 697–704 (2001). [DOI] [PubMed] [Google Scholar]
- 251.Wiseman TA, Curtis K, Lam M & Foster K Incidence of depression, anxiety and stress following traumatic injury: A longitudinal study. Scand. J. Trauma. Resusc. Emerg. Med. 23, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hardeveld F, Spijker J, De Graaf R, Nolen WA & Beekman ATF Recurrence of major depressive disorder and its predictors in the general population: Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Psychol. Med 43, 39–48 (2013). [DOI] [PubMed] [Google Scholar]
- 253.Kendler KS, Karkowski LM & Prescott CA Stressful life events and major depression: Risk period, long-term contextual threat, and diagnostic specificity. J. Nerv. Ment. Dis. 186, 661–669 (1998). [DOI] [PubMed] [Google Scholar]
- 254.Kendler KS, Karkowski LM & Prescott CA Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156, 837–841 (1999). [DOI] [PubMed] [Google Scholar]
- 255.Slavich GM & Shields GS Assessing Lifetime Stress Exposure Using the Stress and Adversity Inventory for Adults (Adult STRAIN): An Overview and Initial Validation. Psychosom. Med 80, 17–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lam JCW, Shields GS, Trainor BC, Slavich GM & Yonelinas AP Greater lifetime stress exposure predicts blunted cortisol but heightened DHEA responses to acute stress. Stress Heal 1–12 (2018) doi: 10.1002/smi.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shields GS et al. Recent life stress exposure is associated with poorer long-term memory, working memory, and self-reported memory. Stress 20, 598–607 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dityatev A & Rusakov DA Molecular signals of plasticity at the tetrapartite synapse. Current Opinion in Neurobiology vol. 21 353–359 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Smith ACW, Scofield MD & Kalivas PW The tetrapartite synapse: Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Research vol. 1628 29–39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chelini G, Pantazopoulos H, Durning P & Berretta S The tetrapartite synapse: a key concept in the pathophysiology of schizophrenia. European Psychiatry vol. 50 60–69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.De Sousa RT et al. Genetic Studies on the Tripartite Glutamate Synapse in the Pathophysiology and Therapeutics of Mood Disorders. Neuropsychopharmacology 42, 787–800 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhang Y et al. Convergent transcriptomics and proteomics of environmental enrichment and cocaine identifies novel therapeutic strategies for addiction. Neuroscience 339, 254–266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hüttenrauch M, Salinas G & Wirths O Effects of Long-Term Environmental Enrichment on Anxiety, Memory, Hippocampal Plasticity and Overall Brain Gene Expression in C57BL6 Mice. Front. Mol. Neurosci 9, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rampon C et al. Effects of environmental enrichment on gene expression in the brain. Proc. Natl. Acad. Sci. U. S. A. 97, 12880–12884 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lacar B et al. Nuclear RNA-seq of single neurons reveals molecular signatures of activation. Nat. Commun 7, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Griffin ÉW, Bechara RG, Birch AM & Kelly ÁM Exercise enhances hippocampal-dependent learning in the rat: Evidence for a BDNF-related mechanism. Hippocampus 19, 973–980 (2009). [DOI] [PubMed] [Google Scholar]
- 267.Adlard PA & Cotman CW Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience 124, 985–992 (2004). [DOI] [PubMed] [Google Scholar]