Introduction
National organizations and experts in the field have identified a pressing need to understand the healthcare delivery factors that contribute to the process of bereavement among caregivers.1-3 Advance care planning (ACP) is one such factor. ACP is defined as an ongoing process to prepare patients and their caregivers for future “in the moment” decisions, like those at the end-of-life.4 ACP consists of three components: 1) completing a living will, 2) designating a healthcare surrogate (the first two components are collectively known as an advance directive), and 3) participating in end-of-life discussions.5 Evidence suggests that the impact of ACP is optimized when people are fully engaged in all three components.6-9 Yet, cancer patients and their families generally do not fully engage,10-13 resulting in multiple levels of potential ACP engagement, and complicating overall determination of the effect of ACP on patient and caregiver outcomes.
To date, the impact of ACP is primarily examined and therefore understood from the perspective of its individual components in individual studies.6, 14-17 Less is understood about the impact of ACP when examined as a whole, that is, from a person-centered perspective (e.g., patient-level ACP engagement). Furthermore, proximal outcomes of ACP (e.g., documentation of an advance directive) are more commonly examined in ACP research than distal outcomes, like caregiver bereavement.18-19
Qualitative findings suggest that ACP has lasting impacts on caregivers during bereavement.20-24 Yet, quantitative findings of this relationship are limited,25 mixed,26-28 or not supported.29 Further, evidence suggests that caregivers’ perceptions of the cancer decedents’ end-of-life experiences are more influential on bereavement outcomes than the actual end-of-life care delivery itself. 30-32 Thus, caregivers’ perceptions of cancer decedents’ end-of-life experiences is an important outcome in understanding the process of caregiver bereavement risk for complicated grief, and little is known about how varying levels of ACP engagement may influence this outcome.
What is known is that various aspects of end-of-life care delivery influence caregivers’ perceptions of cancer decedents’ end-of-life experiences. Positive caregiver perceptions have typically resulted from end-of-life experiences where caregivers’ felt that the death was expected, care was aligned with the patient preferences, treatment approaches generally emphasized comfort, and patients died in comfort in their preferred place of death.33-40 When these perceptions are negative (i.e., resulting from perceived patient suffering, lack of pain relief, a focus on life prolonging treatments, hospital-based deaths), caregivers are susceptible to significant adverse health outcomes (e.g., depression, anxiety) during bereavement.16, 34, 41, 42 Thus, this study aimed to examine the relative impact of varying levels of ACP engagement among cancer decedents on caregivers’ perceptions of the end-of-life experience. It was hypothesized that increasing levels of ACP engagement (i.e., more full engagement) would be significantly associated with caregiver perceptions of a more positive end-of-life experience, compared to no ACP engagement.
Methods
Design and Participants
A secondary analysis of the 2002-2014 waves of the Health and Retirement Study (HRS) Exit Interview data was conducted.43 The HRS is sponsored by the National Institute on Aging (grant number NIA U01 AG009740) and is conducted by the University of Michigan. The HRS includes a nationally representative sample of over 20,000 Americans age 50 years and older. Data is collected from HRS participants longitudinally every two-years. When an HRS participant dies, a one-time HRS Exit Interview is conducted with a “proxy informant.” The proxy informant (hereafter, caregiver) is the person most knowledgeable about the patient’s health, family, and financial circumstances, with priority given to a surviving spouse or a close family member.44 Part of the HRS Exit Interview includes items that address the caregivers’ retrospective report of circumstances around the decedent’s end-of-life experience. This study analyzed these portions of the HRS Exit Interview data. The 2002-2014 waves of the HRS Exit Files included 9,243 death cases, of which, 2,172 were cancer related. The analyzed sample included the 983 cancer death cases in which end-of-life decisions occurred. A designation of non-human subjects research was obtained from a university-based IRB for this study.
Measures
Levels of ACP engagement.
Levels of ACP engagement were determined using three dichotomous (yes/no) HRS Exit survey items closest to the three components of ACP. First, the item, “Did [First Name] provide written instructions about the treatment or care [he/she] wanted to receive during the final days of [his/her] life?” was used to indicate living will completion. The item, “Did [First name] (also) make any legal arrangement for a specific person or persons to make decisions about [his/her] care or medical treatment if [h/she] could not make those decision [himself/herself]?” was used to indicate healthcare surrogate designation. Finally, the item, “Did [First Name] ever discuss with you or anyone else the treatment or care [he/she] wanted to receive in the final days of [his/her] life?” was used to indicate end-of-life discussion participation. Caregivers could endorse each item separately, so a total of eight levels of engagement were initially defined (Table 1). To address the relatively small numbers in some levels, the eight levels were reduced to five for the analysis (Table 1). These five levels encompassed a full ACP engagement level (endorsement of all three components), three partial ACP engagement levels (endorsement of one or two components – augmented end-of-life discussions, documents only, end-of-life discussions only), and a no ACP engagement level (lack of endorsement of all three components).
Table 1.
Levels of ACPa Engagement
[n = 960, missing = 23 (2%)]
| ACP Component Endorsement | Levels of Engagement | |||
|---|---|---|---|---|
| LWb | HCSc | EOLDd | Class of ACP completion |
n (%) |
| Yes | Yes | Yes | Full ACP | 354 (36) |
| Yes | No | Yes | Augmented EOLD |
174 (17.7) |
| No | Yes | Yes | ||
| Yes | Yes | No | Documents only |
151 (15.4) |
| Yes | No | No | ||
| No | Yes | No | ||
| No | No | Yes | EOLD only | 140 (14.2) |
| No | No | No | No ACP | 141 (14.3) |
advance care planning
living will
healthcare surrogate
end-of-life discussion
Caregivers’ Perceptions of the End-of-life Experience.
This latent variable was constructed using seven categorical HRS Exit items (place of death, death expectation, preferences honored, all care, limit care, withhold care, and comfort care) that were consistent with factors that have been documented to influence caregiver perceptions’ of the end-of-life experience (either positively or negatively) in prior research.16, 33-42 These items were also consistent with other measurement items that have been used to assess caregiver perceptions of the end-of-life experience.45-48
Analysis
Structural equation modeling (SEM) in Mplus749 was utilized to test the hypothesis using two steps: 1) measurement modeling and 2) path analysis. While traditional regression models rely solely on measured (observed) variables, SEM allows examination of relationships that contain latent or unobserved variables.50 Many phenomena in health science research are not directly measurable, therefore, multiple observed variables are used to approximate the underlying latent variable.51 Thus, this analytic approach allows for more complex modeling, accommodating a multitude of variables rather than single variables to represent the complexity of the phenomena under study, and helps to reduce measurement error.50
In the measurement modeling component of this analysis, seven observed variables in the HRS survey were theorized to be part of a single underlying latent variable, caregivers’ perceptions of the end-of-life experience. Given the theoretical and evidentiary support for the selection of these items,16, 33-42 a confirmatory factor analysis approach was utilized to test whether the latent variable explained the responses to the seven HRS Exit items. Each of these seven items were dichotomized to indicate a response that reflected circumstances that would be associated with either a positive or negative perception of the end-of-life experience (Table 2, Appendix). Then a series of measurement models were tested (Appendix). The weighted least square mean and variance (WLSMV) estimator was used due to the categorical nature of the items. Adequate model fit indices included: 1) a non-significant Chi-square statistic (χ2) indicated by a p value > .05, 2) a .95 or higher on the Comparative Fit Index (CFI), and 3) a .06 or lower for the root mean squared error of approximation (RMSEA).52
Table 2.
Caregivers’ Perceptions of the End-of-life Experience Latent Dependent Variable
(n = 983)
| Item | Perception Coding | 0 | 1 | |
|---|---|---|---|---|
| Negative (0) | Positive (1) | n (%) | n (%) | |
| All care | All care possible | Not all care possible | 240 (24.4) | 725 (73.8) |
| Limit care | Did not limit | Limited | 292 (29.7) | 659 (67) |
| Withhold care | Did not withhold | Withheld | 416 (42.3) | 543 (55.2) |
| Comfort care | Not comfort focused | Comfort focused | 48 (4.9) | 926 (94.2) |
After model re-specification, good model fit was achieved with four of the initial seven items (all care, limit care, withhold care, and comfort care), χ2 (df = 2) = 2.63, p = 0.268, CFI = .999, RMSEA = .018. Factor loadings for each item were all significant and ranged from .48 to .93 (Figure 1), providing evidence of convergent validity of the measurement model.50 These four items were dependent items in the HRS Exit survey, that is, the items were only answered if the caregivers reported end-of-life decisions were made in the final days of the patient’s life. Thus, the path analysis was limited to just those cancer death cases that involved end-of-life decisions.
Figure 1. Structural Equation Model.
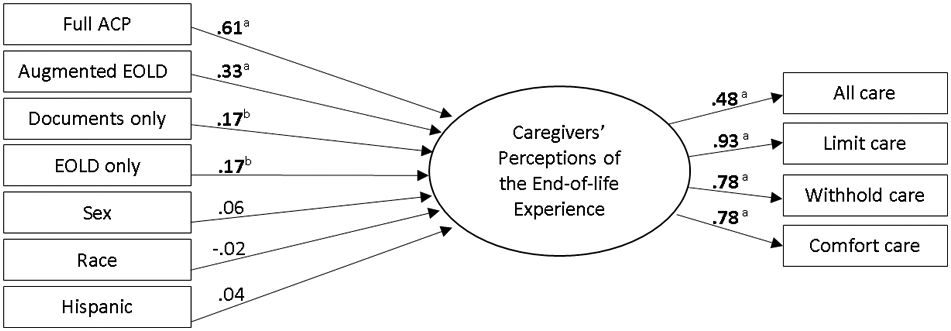
EOLD = end-of-life discussions; Coefficients are standardized betas; ap < .001, bp = .001
For the path analysis, the independent variable was dummy coded so that each level of ACP engagement was compared to the reference level of “no ACP engagement.” Sex, race, and Hispanic ethnicity were included as covariates in the analysis due to their documented relationship with the variables of interest.6, 15, 53-55 Significant path coefficients were defined as a p < .05. Standardized beta coefficients provided a basis of comparing the relative impact of each of the independent variables on the identified latent dependent variable. The magnitude of the R2 value was defined as follows: .02 a small, .13 a medium, and .26 a large effect size.56
Results
Sample characteristics
The cancer decedents’ characteristics in the analyzed sample were similar to those in the total sample of cancer decedents (Table 3). The majority (85%) of caregiver respondents were spousal or child caregivers. Cancer decedents were primarily 65 years or older (85%), White (80%), and had at least a high school education (69%). The majority of cancer decedents were engaged in the individual components of ACP (living will, 51%; health care surrogate, 63%; end-of-life discussion, 69%) (Table 3). However, only 36% of cancer decedents fully engaged in all three ACP components, 14% were not engaged at all, and the remainder were spread across the three levels of partial engagement (Table 1).
Table 3.
Cancer Decedent Characteristics
|
Cancer Decedents N = 2,172 |
Analyzed Sample of Cancer Decedents n = 983 |
|
|---|---|---|
| M (SD) | M (SD) | |
| Age | 76 (10.1) | 76 (10.2) |
| n (%) | n (%) | |
| Age range | ||
| 64 or less | 307 (14.1) | 144 (14.6) |
| 65 or more | 1865 (85.9) | 839 (85.4) |
| Sex | ||
| Male | 996 (45.9) | 509 (51.8) |
| Female | 1174 (54.1) | 474 (48.2) |
| Race | ||
| White/Caucasian | 1537 (70.8) | 789 (80.3) |
| Non-White/Caucasian | 581 (26.7) | 191 (19.4) |
| Hispanicity | ||
| Hispanic | 149 (6.9) | 57 (5.8) |
| Non-Hispanic | 1967 (90.6) | 925 (94.1) |
| Marital status | ||
| Married | 944 (43.5) | 406 (41.3) |
| Not married | 877 (40.3) | 422 (42.9) |
| Education (in years) | ||
| 0 | 13 (0.6) | 6 (0.6) |
| 1-11 | 546 (25.1) | 295 (30) |
| 12 (High school) | 758 (34.9) | 311 (31.6) |
| 13-15 | 407 (18.7) | 199 (20.3) |
| 16 (College graduate) | 193 (8.9) | 87 (8.9) |
| 17 or more | 200 (9.2) | 79 (8) |
| Religious service attendance | ||
| Some religious service attendance | 1246 (57.4) | 565 (57.5) |
| No religious service attendance | 875 (40.3) | 393 (40) |
| Caregiver relationship to decedent | ||
| Spouse/Partner | 947 (43.6) | 385 (39.2) |
| Child | 867 (39.9) | 453 (46.1) |
| Other | 358 (16.5) | 145 (14.8) |
| Duration of final illness | ||
| One or two hours (no warning) | 25 (1.2) | 9 (.9) |
| Less than a day | 53 (2.4) | 21 (2.1) |
| Less than a week | 171 (7.9) | 92 (9.4) |
| Less than a month | 327 (15.1) | 171 (17.4) |
| Less than a year | 898 (41.3) | 407 (41.4) |
| More than a year | 677 (31.2) | 275 (28) |
| Advance care planning by component | ||
| Completed a living will | 1022 (47.1) | 501 (51) |
| Designated a healthcare surrogate | 1236 (56.9) | 617 (62.8) |
| Participated in end-of-life discussion | 1338 (61.6) | 677 (68.9) |
Impact of the levels of ACP engagement
The path analysis revealed that all levels of ACP engagement were significantly associated with caregivers’ perceptions of the end-of-life experiences, after controlling for the effects of sex, race, and Hispanic ethnicity (full ACP, B = 1.41, SE = 0.14, p < .001; augmented end-of-life discussions, B = 0.97, SE = 0.16, p < .001; documents only, B = 0.51, SE = 0.15, p = .001; end-of-life discussions only, B = 0.52, SE = 0.16, p = .001). However, the relative impact of each of these levels was not equal. The standardized coefficients demonstrated that increasing levels of ACP engagement (i.e., more full engagement) had larger associations in magnitude with caregivers’ perceptions, positively, than when compared to no ACP engagement (full ACP, beta = 0.61, SEbeta = 0.05, p < .001; augmented end-of-life discussions, beta = 0.33, SEbeta = 0.05, p < .001; documents only, B = 0.17, SE = 0.05, p = .001; end-of-life discussions only, B = 0.17, SE = 0.05, p = .001). Thus, full ACP engagement was associated with the greatest relative impact on the bereaved caregivers’ perceptions, positively, with each of the partial levels of ACP engagement having a lesser impact, positively. The R2 value indicated a medium effect size (.209), and signified that 21% of the variation in caregivers’ perceptions of the end-of-life experience was explained by the model.
Discussion
This study adds to the mounting evidence that cancer patients are unevenly engaged in the components of ACP, with nearly half of the analyzed sample only partially engaged. This study also provides evidence of the human cost of this partial engagement as these varying levels of ACP engagement were found to significantly influence caregivers’ perceptions of the end-of-life experience. While any engagement in ACP was better than none, the relative impact of each level of engagement was not equal. Full engagement had a greater impact on the caregivers’ perceptions of the end-of-life experience than each of the partial levels of ACP engagement. Such findings are consistent with recommendations that emerged from the seminal SUPPORT trial, which suggested the diminished role of advance directives (i.e., partial engagement) when they are implemented in the absence of discussions.8-9 Thus, cancer patients and their caregivers may not be realizing the full benefits of ACP when they are only partially engaged.
This makes partial engagers in ACP, and not just non-engagers, important targets in ACP intervention research and in clinical practice. Yet, cancer patients with pre-existing evidence of some form of ACP (i.e., partial engagement) are sometimes excluded from ACP interventions.11, 57 This might be counterproductive as it may be easier to convert a partial engager to full engagement than to convince a non-engager to engage. Such exclusions deprive cancer patients and their caregivers the opportunity for repeated exposure to ACP over time and for more full ACP engagement leading up to the end-of-life.58 Designing research or clinical based interventions to also address both partial engagement and non-engagement in ACP may ultimately contribute to increased likelihood of full engagement with subsequent improved end-of-life experiences among cancer patients and better bereavement adjustment among caregivers.
Findings from the measurement modeling component of the analysis also provide support for the complex interrelationship of factors that contribute to caregivers’ perceptions of the end-of-life experience. Bereaved caregivers may reflect on a broad range of factors in processing the end-of-life experience.59-60 Thus, the four items comprising the latent dependent variable in this study offered advantages over single item measures of caregiver reflections.40 While this measurement model does not replace that of other well-established bereaved caregiver surveys,35, 46 this analysis demonstrates how multiple items from the HRS Exit Interview can function as a measurement model. Even though all seven items did not hold in the model, repeated measurement modeling with these items is warranted in future analyses of the HRS data, which has been increasingly utilized to understand the impact of ACP.13-14, 61-62
Limitations
This study has several limitations. The retrospective nature of the HRS Exit Interviews combined with the large window of time allowed for the interviews also introduces the potential for recall bias. This is an important consideration as caregivers may have a tendency to reframe certain situations or symptom experiences more positively with the passage of time.63 However, 72% (n = 710) of the interviews were conducted within a year of the cancer decedent’s death. Additionally, several potentially important characteristics of the caregiver respondents (e.g., extent of caregiving these “proxy informants” actually provided, educational level) were lacking in the dataset, and could not be accounted for in the analysis. The HRS Exit items pertaining to the three components of ACP were also limited to yes/no responses. ACP is a care planning process that evolves over the illness trajectory, so the global nature of these ACP indicators provided a limited view of the scope of the ACP that may have occurred among the cancer decedents. Finally, this analysis was reduced to cancer death cases where end-of-life decisions were made. Thus, the influence of the levels of ACP engagement may not be generalizable to contexts where end-of-life decisions are not required. However, because ACP is intended to function as an act of preparation for later “in-the-moment decision-making,”4 this analysis notably examined the impact of ACP in the context in which it is theorized to benefit patients and caregivers the most, an end-of-life decision-making context.
Implications
Findings from this study support the need to address varying levels of ACP engagement and aim for fuller ACP engagement in future ACP research and in clinical practice. This may require the development or modification of clinical practice guidelines and organizational policies that require systematic ACP facilitation across the illness trajectory, beyond the presence of logistical supports for the electronic storage of ACP documents.64 These systematic supports should encourage the implementation of ACP as a process, recognizing that readiness to engage in ACP and preferences for end-of-life care evolve over time.4, 58 Such approaches may entail the design of embedded clinical decision support tools in the electronic medical record to engender repeated exposure to ACP across the cancer illness trajectory. To do so, may also require training of the interprofessional team (e.g., communication trainings) to ensure these ACP-related workflow processes are high quality.64 Additionally, varying levels of ACP engagement are not unique to cancer patients;65 thus, patient-centered evaluations of ACP should be considered among patients and caregivers facing other chronic life limiting diseases. Further, findings from this study suggest that successful ACP should also be considered in terms of its impact on more distal outcomes (e.g., caregiver bereavement outcomes) in future ACP research. This contrasts with expert opinion in the field, where proximal outcomes (e.g., documentation of a living will) are more commonly prioritized when characterizing successful ACP.18, 66 Finally, as some cancer patients may never desire to engage in ACP,67 the role of other factors that prepare patients and their caregivers for the end-of-life needs to be considered. Broader processes of care that impact the quality of end-of-life care warrant further investigation, rather than focusing on single mechanisms, like ACP.68
Conclusion
The results of this study extend the current ACP literature by demonstrating the impact of ACP, not from the perspective of its individual components, but from the perspective of how cancer patients are actually engaging in the behavior. The study’s findings demonstrated that when cancer decedents were engaged in advance care planning as a whole, more positive impacts on caregiver perceptions’ of the end-of-life experience occurred, compared to cancer decedents who had engaged in only some of its parts. Thus, uneven engagement in ACP serves as an important clinically modifiable target that has the potential to improve the end-of-life care experience among cancer patients and the perceptions of those experiences among bereaved caregivers. These findings may have implications for addressing caregiver bereavement adjustment in future research.
Supplementary Material
Table 4.
Predictors of the Caregivers’ Perceptions of the End-of-life Experience
[n = 934, missing = 49 (5%)]
| B | SEB | beta | SEbeta | p-value | |
|---|---|---|---|---|---|
| Full ACPa | 1.41 | 0.14 | .61 | .05 | <.001 |
| Augmented EOLDb | 0.97 | 0.16 | .33 | .05 | <.001 |
| Documents only | 0.51 | 0.15 | .17 | .05 | 0.001 |
| EOLD only | 0.52 | 0.16 | .17 | .05 | 0.001 |
| Sex | 0.11 | 0.09 | .06 | .04 | 0.22 |
| Race | −0.04 | 0.10 | −.02 | .04 | 0.648 |
| Hispanic | 0.18 | 0.17 | .04 | .04 | 0.29 |
advance care planning
end-of-life discussion
Contributor Information
Kristin Levoy, NewCourtland Center for Transitions and Health, University of Pennsylvania School of Nursing; Philadelphia, PA; United States.
Harleah Buck, University of South Florida College of Nursing; Tampa, FL; United States.
Victoria Behar-Zusman, University of Miami School of Nursing and Health Studies; Coral Gables, FL; United States.
References
- 1.Hay A, Hall CW, Sealey M, Lobb EA, Breen LJ. Developing a practice-based research agenda for grief and bereavement care. Death Stud. 2019:1–11. doi: 10.1080/07481187.2019.1636897 [DOI] [PubMed] [Google Scholar]
- 2.Committee on Approaching Death. Dying in America: improving quality and honoring individual preferences near the end of life Institute of Medicine. Washington, D.C.: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 3.National Institute of Nursing Research. End-of-life and palliative care [Internet]. n.d. Available from: https://www.ninr.nih.gov/newsandinformation/iq/eolpc-workshop. Accessed January 8, 2020.
- 4.Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4):256–61. doi: 10.7326/0003-4819-153-4-201008170-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Department of Health and Human Services, Assistant Secretary for Planning and Evaluation, Office of Disability Aging and Long-Term Care Policy. Advance directives and advance care planning: report to congress. 2008. Available from: https://aspe.hhs.gov/sites/default/files/pdf/75811/ADCongRpt.pdf. Access January 8, 2020.
- 6.Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28(8):1000–25. doi: 10.1177/0269216314526272 [DOI] [PubMed] [Google Scholar]
- 7.Houben CHM, Spruit MA, Groenen MTJ, Wouters EFM, Janssen DJA. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc. 2014;15(7):477–89. doi: 10.1016/j.jamda.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 8.Teno JM, Licks S, Lynn J, Wenger N, Connors AF, Phillips RS, et al. Do advance directives provide instructions that direct care? J Am Geriatr Soc. 1997;45(4):508–12. doi: 10.1111/j.1532-5415.1997.tb05179.x [DOI] [PubMed] [Google Scholar]
- 9.Teno J, Lynn J, Wenger N, Phillips RS, Murphy DP, Connors AF, et al. Advance directives for seriously ill hospitalized patients: effectiveness with the patient self-determination act and the SUPPORT intervention. J Am Geriatr Soc. 1997;45(4):500–7. doi: 10.1111/j.1532-5415.1997.tb05178.x [DOI] [PubMed] [Google Scholar]
- 10.Dow LA, Matsuyama RK, Ramakrishnan V, Kuhn L, Lamont EB, Lyckholm L, et al. Paradoxes in advance care planning: the complex relationship of oncology patients, their physicians, and advance medical directives. J Clin Oncol. 2010;28(2):299–304. doi: 10.1200/JCO.2009.24.6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein AS, Volandes AE, Chen LY, Gary KA, Li Y, Agre P, et al. A randomized controlled trial of a cardiopulmonary resuscitation video in advance care planning for progressive pancreas and hepatobiliary cancer patients. J Palliat Med. 2013;16(6):623–31. doi: 10.1089/jpm.2012.0524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemminger L, Pittman C, Korones D, Serventi J, Ladwig S, Holloway R, et al. Palliative and end-of-life care in glioblastoma: defining and measuring opportunities to improve care. Neurooncol Pract. 2017;4(3):182–188. doi: 10.1093/nop/npw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narang A, Wright AA, Nicholas LH. Trends in advance care planning in patients with cancer results from a national longitudinal survey. JAMA Oncol. 2015;1(5):601–8. doi: 10.1001/jamaoncol.2015.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bischoff K, Sudore R, Miao Y, Boscardin W, Smith A. Advance care planning and the quality of end-of-life care in older adults. J Am Geriatr Soc. 2013;61(2):209–14. doi: 10.1111/jgs.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203. doi: 10.1200/JCO.2009.25.4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665. doi: 10.1001/jama.300.14.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higel T, Alaoui A, Bouton C, Fournier J. Effect of Living Wills on End-of-Life Care: A Systematic Review. J Am Geriatr Soc. 2019;67(1):164–71. doi: 10.111/jgs.15630 [DOI] [PubMed] [Google Scholar]
- 18.Sudore RL, Heyland DK, Lum HD, Rietjens JAC, Korfage IJ, Ritchie CS, et al. Outcomes that define successful advance care planning: A Delphi panel consensus. J Pain Symptom Manage. 2018;55(2):245,255.e8. doi: 10.1016/jpainsymman.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walczak A, Butow PN, Bu S, Clayton JM. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Educ Couns. 2016;99(1):3–16. doi: 10.1016/j.pec.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 20.Fried T, O’Leary J. Using the experiences of bereaved caregivers to inform patient- and caregiver-centered advance care planning. J Gen Intern Med. 2008;23(10):1602–7. doi: 10.1007/s11606-008-0748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebert RS, Schulz R, Copeland VC, Arnold RM. Preparing family caregivers for death and bereavement. Insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37(1):3–12. doi: 10.1016/j.jpainsymman.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 22.Patel MI, Moore D, Coker TR. End-of-life cancer care redesign: patient and caregiver experiences in a lay health worker-led intervention. Am J Hosp Palliat Care. 2019;36(12):1081–8. doi: 10.1177/1049909119847967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piil K, Jarden M. Bereaved caregivers to patients with high-grade glioma: a qualitative explorative study. Journal Neurosci Nurs. 2018;50(2):94–9. doi: 10.1097/JNN.0000000000000348 [DOI] [PubMed] [Google Scholar]
- 24.Norton S, Wittink M, Duberstein P, Prigerson H, Stanek S, Epstein R. Family caregiver descriptions of stopping chemotherapy and end-of-life transitions. Support Care Cancer. 2019;27(2):669–75. doi: 10.1007/s00520-018-4365-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song M, Ward SE, Fine JP, Hanson LC, Lin F, Hladik GA, et al. Advance care planning and end-of-life decision making in dialysis: a randomized controlled trial targeting patients and their surrogates. Am J Kidney Dis. 2015;66(5):813–22. doi: 10.1052/j.ajkd.2015.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duberstein PR, Maciejewski PK, Epstein RM, Fenton JJ, Chapman B, Norton SA, et al. Effects of values and options in cancer care communication intervention on personal caregiver experiences of cancer care and bereavement outcomes. J Palliat Med. 2019;22(11):1394–1400. doi: 10.1089/jpm.2019.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overbeek A, Korfage IJ, Hammes BJ, van dH, Rietjens JAC. Experiences with and outcomes of advance care planning in bereaved relatives of frail older patients: a mixed methods study. Age Ageing. 2019;48(2):299–306. doi: 10.1093/ageing/afy184 [DOI] [PubMed] [Google Scholar]
- 28.Ahluwalia SC, Chen C, Raaen L, Motala A, Walling AM, Chamberlin M, et al. A systematic review in support of the National Consensus Project Clinical Practice Guidelines for Quality Palliative Care, fourth edition. J Pain Symptom Manage. 2018;56(6):831–70. doi: 10.1016/j.jpainsymman.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 29.Johnson SB, Butow PN, Bell ML, Detering K, Clayton JM, Silvester W, et al. A randomised controlled trial of an advance care planning intervention for patients with incurable cancer. Br J Cancer. 2018;119(10):1182. doi: 10.1038/s41416-018-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrido MM, Prigerson HG. The end-of- life experience: modifiable predictors of caregivers' bereavement adjustment. Cancer. 2014;120(6):918–25. doi: 10.1002/cncr.28495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai W, Prigerson HG, Li C, Chou W, Kuo S, Tang ST. Longitudinal changes and predictors of prolonged grief for bereaved family caregivers over the first 2 years after the terminally ill cancer patient’s death. Palliat Med. 2016;30(5):495–503. doi: 10.1177/0269216315603261 [DOI] [PubMed] [Google Scholar]
- 32.Wilson DM, Cohen J, Eliason C, Deliens L, Macleod R, Hewitt JA, et al. Is the bereavement grief intensity of survivors linked with their perception of death quality? Int J Palliat Nurs. 2019;25(8):398–405. doi: 10.12968/ijpn.2019.25.8.398 [DOI] [PubMed] [Google Scholar]
- 33.Cagle JG, Kovacs PJ. Informal caregivers of cancer patients: perceptions about preparedness and support during hospice care. J Gerontol Soc Work. 2011;54(1):92–115. doi: 10.1080/01634372.2010.534547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cagle J, Pek J, Clifford M, Guralnik J, Zimmerman S. Correlates of a good death and the impact of hospice involvement: findings from the national survey of households affected by cancer. Support Care Cancer. 2015;23(3):809–18. doi: 10.1007/s00520-014-2404-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death: initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17–31. [DOI] [PubMed] [Google Scholar]
- 36.Ersek M, Miller SC, Wagner TH, Thorpe JM, Smith D, Levy CR, et al. Association between aggressive care and bereaved families’ evaluation of end-of-life care for veterans with non-small cell lung cancer who died in Veterans Affairs facilities. Cancer. 2017;123(16):3186–94. doi: 10.1002/cncr.30700 [DOI] [PubMed] [Google Scholar]
- 37.Hebert RS, Prigerson HG, Schulz R, Arnold RM. Preparing caregivers for the death of a loved one: a theoretical framework and suggestions for future research. J Palliat Med. 2006;9(5):1164–71. [DOI] [PubMed] [Google Scholar]
- 38.Higgins PC, Garrido MM, Prigerson HG. Factors predicting bereaved caregiver perception of quality of care in the final week of life: implications for health care providers. J Palliat Med. 2015;18(10):849–57. doi: 10.1089/jpm.2015.29001.hp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khandelwal N, Curtis JR, Freedman VA, Kasper JD, Gozalo P, Engelberg RA, et al. How often is end-of-life care in the United States inconsistent with patients' goals of care? J Palliat Med. 2017;20(12):1400. doi: 101.1089/jpm.2017.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen MK, Neergaard MA, Jensen AB, Bro F, Guldin M. Do we need to change our understanding of anticipatory grief in caregivers? A systematic review of caregiver studies during end-of-life caregiving and bereavement. Clin Psychol Rev. 2016;44:75–93. doi: 10.1016/j.cpr.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 41.Ylitalo N, Valdimarsdttir U, Onelv E, Dickman PW, Steineck G. Guilt after the loss of a husband to cancer: is there a relation with the health care provided? Acta Oncol. 2008;47(5):870–8. doi: 10.10180/02841860701766145 [DOI] [PubMed] [Google Scholar]
- 42.Allen JY, Haley WE, Small BJ. Bereavement outcomes among spousal hospice caregivers: Relief, rumination, and perceived patient suffering. Death Stud. 2019:1–9. doi: 10.1080/07481187.2019.1648331 [DOI] [PubMed] [Google Scholar]
- 43.Health and Retirement Study, (HRS 2002 Exit – HRS 2014 Exit) public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging; (grant number NIA U01AG009740). Ann Arbor, MI, 2020. [Google Scholar]
- 44.Servais MA. Overview of HRS public data files for cross-sectional and longitudinal analysis [Internet]. 2010. Available from https://hrs.isr.umich.edu/sites/default/files/biblio/OverviewofHRSPublicData.pdf. Accessed January 8, 2020.
- 45.Aoun S, Bird S, Kristjanson LJ, Currow D. Reliability testing of the FAMCARE-2 scale: measuring family carer satisfaction with palliative care. Palliat Med. 2010;24(7):674–81. doi: 10.1177/0269216310373166 [DOI] [PubMed] [Google Scholar]
- 46.Lendon JP, Ahluwalia SC, Walling AM, Lorenz KA, Oluwatola OA, Anhang Price R, et al. Measuring experience with end-of-life care: a systematic literature review. J Pain Symptom Manage. 2015;49(5):904,915.e3. doi: 10.1016/j.jpainsymman.2014.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patrick DL, Engelberg RA, Curtis JR. Evaluating the quality of dying and death. J Pain Symptom Manage. 2001;22(3):717–26. [DOI] [PubMed] [Google Scholar]
- 48.U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion [Internet]. 2018. Available from https://www.cherp.research.va.gov/CHERP/PROMISE/The_PROMISE_Survey.asp. Accessed January 8, 2020.
- 49.Muthén LK, Muthén BO. Mplus user’s guide. 8th ed. Los Angeles: Muthén & Muthén; 2017. [Google Scholar]
- 50.Garson DG. Structural equation modeling [Kindle-reader version]. Asheboro, NC: Statistical Publishing Associates; 2015. [Google Scholar]
- 51.Deng L, Yang M, Marcoulides KM. Structural equation modeling with many variables: a systematic review of issues and developments. Front Psychol. 2018;9:1–14. doi: 10.3389/fpsyg.2018.00580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- 53.Jonnalagadda S, Lin JJ, Nelson JE, Powell CA, Salazar-Schicchi J, Berman AR, et al. Racial and ethnic differences in beliefs about lung cancer care. Chest. 2012;142(5):1251–8. doi: 10.1378/chest.12-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loggers ET, Maciejewski PK, Jimenez R, Nilsson M, Paulk E, Stieglitz H, et al. Predictors of intensive end-of-life and hospice care in Latino and white advanced cancer patients. J Palliat Med. 2013;16(10):1249–54. doi: 10.1089/jpm.2013.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao JK, Anderson LA, Lin F, Laux JP. Completion of advance directives among U.S. consumers. Am J Prev Med. 2014;46(1):65–70. doi: 10.1016/j.amepre.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 57.Pautex S, Herrmann FR, Zulian GB. Role of advance directives in palliative care units: a prospective study. Palliat Med. 2008;22(7):835–41. doi: 10.1177/0269216308094336 [DOI] [PubMed] [Google Scholar]
- 58.Levoy K, Salani DA, Buck H. A systematic review and gap analysis of advance care planning intervention components and outcomes among cancer patients using the Transtheoretical Model of Health Behavior Change. J Pain Symptom Manage. 2019;57(1):118,139.e6. doi: 10.1016/j.jpainsymman.2018.10.502 [DOI] [PubMed] [Google Scholar]
- 59.Fritsch J, Petronio S, Helft PR, Torke AM. Making decisions for hospitalized older adults: ethical factors considered by family surrogates. J Clin Ethics. 2013;24(2):125–34. [PMC free article] [PubMed] [Google Scholar]
- 60.Torke A, Petronio S, Sachs G, Helft P, Purnell C. A conceptual model of the role of communication in surrogate decision making for hospitalized adults. Patient Educ Couns. 2012;87(1):54–61. doi: 10.1016/j.pec/2011.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicholas LH, Bynum JPW, Iwashyna TJ, Weir DR, Langa KM. Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia. Health Aff. 2014;33(4):667–74. doi: 10.1377/hlthaff.2013.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tschirhart EC, Du Q, Kelley AS. Factors influencing the use of intensive procedures at the end of life. J Am Geriatr Soc. 2014;62(11):2088–94. doi: 10.1111/jgs.13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McPherson CJ, Addington-Hall J. How do proxies' perceptions of patients' pain, anxiety, and depression change during the bereavement period? J Palliat Care. 2004;20(1):12–9. [PubMed] [Google Scholar]
- 64.Arnett K, Sudore RL, Nowels D, Feng CX, Levy CR, Lum HD. Advance care planning: understanding clinical routines and experiences of interprofessional team members in diverse health care settings. Am J Hosp Palliat Med. 2017;34(10):946–53. doi: 10.1177/1049909116666358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hash J, Bodnar-Deren S, Leventhal E, Leventhal H. Chronic illness with complexity: association with self-perceived burden and advance care planning. OMEGA. 2018;77(4):364–85. doi: 10.1177/0030222816675250 [DOI] [PubMed] [Google Scholar]
- 66.Rietjens JAC, Sudore RL, Connolly M, Van Delden JJ, Drickamer MA, Droger M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):543–51. doi: 10.1016/S1470-2045(17)30582-X [DOI] [PubMed] [Google Scholar]
- 67.Johnson S, Butow P, Kerridge I, Tattersall M. Advance care planning for cancer patients: a systematic review of perceptions and experiences of patients, families, and healthcare providers. Psychooncology. 2016;25(4):362–86. doi: 10.1002/pon.3926 [DOI] [PubMed] [Google Scholar]
- 68.Johnson S, Kerridge I, Butow PN, Tattersall MHN. Advance care planning: is quality end of life care really that simple? Intern Med J. 2017;47(4):390–4. doi: 10.1111/imj.13389 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


