Abstract
The CIDE (cell death-inducing DFF45-like effector) family composed of CIDEA, CIDEB, CIDEC/FSP27 (fat-specific protein 27), has a critical role in growth of lipid droplets. Of these, CIDEB and CIDEC2/FSP27B are abundant in the liver, and the steatotic livers, respectively. Hepatocyte nuclear factor 4α (HNF4α) has an important role in lipid homeostasis because liver-specific HNF4α-null mice (Hnf4aΔHep mice) exhibit hepatosteatosis. We investigated whether HNF4α directly regulates expression of CIDE family genes. Expression of Cideb and Fsp27b was largely decreased in Hnf4aΔHep mice, while expression of Cidea was increased. Similar results were observed only in CIDEC2, the human orthologue of the Fsp27b, in human hepatoma cell lines in which HNF4α expression was knocked down. Conversely, overexpression of HNF4α strongly induced CIDEC2 expression in hepatoma cell lines. Furthermore, HNF4α transactivated Fsp27b by direct binding to an HNF4α response element in the Fsp27b promoter. In addition, Fsp27b is known to be transactivated by CREBH that is regulated by HNF4α, and expression of CREBH was induced by HNF4α in human hepatoma cells. Co-transfection of HNF4α and CREBH resulted in synergistic transactivation and induction of Fsp27b compared to that of HNF4α or CREBH alone. These results suggest that HNF4α, in conjunction with CREBH, plays an important role in regulation of Fsp27b expression.
Keywords: HNF4α, CIDE family, Fsp27b, CREBH, Liver
1. Introduction
Hepatocyte nuclear factor 4α (HNF4α) is a member of the nuclear receptor superfamily and is a master regulator of the differentiation status of hepatocytes and binds to about 12% of the genes in human hepatocytes and positively regulates hepatocyte-specific gene expression [1-4]. Liver-specific Hnf4a-null mice (Hnf4aΔHep mice) were found to exhibit hepatosteatosis, partially due to a defect of VLDL secretion by downregulation of microsomal triglyceride transfer protein (Mttp) and many apolipoproteins including Apob [5], indicating that HNF4α has an important role in lipid homeostasis in liver.
Among many target genes of HNF4α, the CIDEB promoter was transactivated by HNF4α [6,7]. CIDEB is a member of the cell death-inducing DEF45-like effector (CIDE) protein family with CIDEA and CIDEC/FSP27 (fat-specific protein 27) that are involved in growth of lipid droplets. Of these, Cidea is induced in livers of HFD-fed and ob/ob mice [8,9], and is conversely reduced in mouse livers that are resistant to hepatosteatosis [10]. Thus, CIDEA plays an important role in the development of hepatosteatosis. Cideb is abundantly expressed in liver, and Cideb-deficient mice are resistant to HFD-induced hepatosteatosis [11]. Furthermore, CIDEB may be a critical factor controlling VLDL secretion by interaction with APOB [12], revealing that CIDEB is essential for the maintenance of hepatic lipid homeostasis. Fsp27 is induced hepatosteatotic ob/ob mouse livers by PPARγ [8]. Fsp27 was found to exist as two variants, the first identified Fsp27a, and a Fsp27b with 10 additional amino acids [13]. FSP27B protein is more stable than FSP27A protein, but their biochemical functions are similar. Fsp27b is enriched in ob/ob mouse livers, and overexpression of Fsp27b induced lipid accumulation in mouse liver [13]. Furthermore, cyclin AMP responsive element binding protein H (CREBH) transactivates the Fsp27b and induces expression of Fsp27b and CIDEC2, a human orthologue of Fsp27b. Hepatic Fsp27a/b was also reported to be transactivated by PPARα, PPARγ, and LXRα through the respective binding sites in the promoter region [8,14-16].
In this study, we investigated involvement of the CIDE family in hepatosteatotic development of Hnf4aΔHep mice. Expression of Cidea was increased, while expression of Cideb and Fsp27b was markedly decreased in Hnf4aΔHep mice. Similar results were also obtained in human hepatoma cell lines in which HNF4α was overexpressed and silenced. In addition, HNF4α transactivated Fsp27b through a binding site in the promoter region, and transactivation and expression of Fsp27b/CIDEC2 was strongly induced in the presence of CREBH. These findings reveal that Fsp27b/CIDEC2 is a novel target gene of HNF4α, and HNF4α and CREBH synergistically regulate Fsp27b expression.
2. Materials and methods
2.1. Animal
Liver-specific Hnf4a-null (Hnf4aΔHep) mice were described previously [5]. All experiments were performed with 45-day-old male Hnf4a-floxed (Hnf4af/f) and Hnf4aΔHep mice. Mice were housed in a pathogen-free animal facility under standard 12 h light/12 h dark cycle with ad libitum water and chow. All experiments with mice were carried out under Gunma University Animal Care and Experimentation Committee (Permission number 15–021).
2.2. Cell culture
HEK293T, HepG2, Huh7, and HLE/tet-HNF4A cells were cultured at 37 °C in Dulbecco’s modified Eagle’s medium (Wako, Osaka, Japan) containing 10% fetal bovine serum (HyClone, Logan, UT) and 100 units/ml penicillin and 100 μg/mL streptomycin (Wako). Tet-inducible HNF4α expressing HLE cells (HLE/tet-HNF4A) were generated using Tet-On 3G inducible expression system (Takara, Kusatsu, Japan). Expression of HNF4α in HLE/tet-HNF4A cells was induced by the addition of 500 ng/mL of doxycycline (Sigma-Aldrich, Tokyo, Japan) into the medium for 24 h.
2.3. RNA extraction, reverse-transcription, and quantitative PCR
Total RNA extracted from cell lines and mouse livers using Isogen II (Wako) was transcribed to cDNA using ReverTraAce qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan). cDNA was used for quantitative PCR using Luna qPCR Master Mix (New England Biolabs, Tokyo, Japan) and PrimeTime Gene Expression Master Mix (Integrated DNA Technologies, Tokyo, Japan) with the specific primers on a LightCycler 480 system II (Roche). Levels of mRNA expression were normalized relative to Gapdh and TBP mRNA as an internal control using ΔΔCt method. Nucleotide sequences of the primers are shown in Supplemental Table 1.
2.4. Transfection of siRNA
Ten nM of siRNA against human HNF4A mRNA and negative control (Sigma-Aldrich) were transfected into HepG2 and Huh7 cells with Lipofectamine RNAiMAX (Life Technologies). After 48 h of transfection, total RNA was harvested. Nucleotide sequences for the siRNA duplexes against human HNF4A are follows; rGrGrCrArGUrGrCrGUrGrGUrGrGrArCrArAdTdT and UUrGUrCrCrArCrCrArCrGrCrArCUrGrCrCdTdT.
2.5. Transient transfection and luciferase assays
HNF4α and CREBH(N) expression plasmids were transfected in Huh7 cells. At 48 h after transfection, the cells were lysed into Isogen II for RNA extraction. The wild-type Fsp27a/b promoters and the Fsp27b promoters with the mutated HNF4α and CREBH binding sites were cloned into pGL4.11 (Promega, Tokyo, Japan). These promoters were co-transfected into HEK293T cells with pGL4.74 (Promega) as an internal control and HNF4α, CREBH, PPARα, and RXRα expression plasmids using polyethyleneimine Max (Polyscience, Warrington, PA) as a transfection reagent. After 24 h, 10 μM of Wy, 14,643 (Sigma-Aldrich) was added to the medium of cells transfected PPARα and RXRα expression plasmids. At 48 h after transfection, promoter activities were measured using Dual-Glo Luciferase Assay System (Promega).
2.6. Electrophoretic mobility shift assay (EMSA)
EMSA was carried out using LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific) and nuclear extracts from HNF4α-overexpressed HEK293T cells. The following double-stranded probes were used; wild-type and the mutated HNF4α binding sites in the ornithine transcarbamylase (Otc) [2] and the Fsp27b promoters. Nucleotide sequences of the primers are shown in Supplemental Table 2. Nuclear extracts (3 μg) and the 5′-biotin labeled probes of the HNF4α binding sites for the Fsp27b promoter (wild-type) were added and the mixture was incubated on ice for 10 min. For competition experiments, a 50-fold excess of unlabeled probe was added to the reaction mixture and the mixture was incubated on ice for 10 min prior to the addition of the labeled probe. For supershift analysis, 1 μg of anti-HNF4α or anti-PPARβ antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the reaction mixture, and the mixture was incubated on ice for 10 min after the addition of the labeled probe. DNA-protein complexes were fractionated by 7% PAGE and blotted onto a Biodyne B Nylon membrane (Pall, Tokyo, Japan). DNA-protein complexes were visualized using detection module in the kit on an ImageQuant LAS4000.
2.7. Chromatin immunoprecipitation (ChIP)
ChIP using HepG2 cells and liver samples was performed according to a protocol using anti-HNF4α antibody and normal mouse IgG (Santa Cruz Biotechnology) [3]. Purified DNA was amplified by quantitative PCR using ΔΔCt method. Enrichment of the HNF4α binding site was normalized to the input samples compared with normal IgG antibody. The following primers were used for realtime PCR; Fsp27b and CIDEC2 promoters containing HNF4α binding site, and mouse Hmgcs2 and human MIR-194 genes without the HNF4α binding site as negative controls. Nucleotide sequences of the primers are shown in Supplemental Table 3.
2.8. Statistical analysis
All values are expressed as the mean ± standard derivation (S.D.). All data were analyzed by the Mann-Whitney U test for significant differences between the mean values of each group.
3. Results
3.1. Hepatic expression of CIDE and perilipin family in Hnf4aΔHep mice
Since Hnf4aΔHep mice exhibit fatty livers, hepatic expression of CIDE and perilipin family was investigated in Hnf4aΔHep mice. Expression of Cidea was increased in Hnf4aΔHep mice, while expression of Cideb and Fsp27, the mouse homologue of human CIDEC, was markedly decreased in Hnf4aΔHep mice (Fig. 1A). A new variant of Fsp27, Fsp27b was identified in liver, and Fsp27b was induced in hepatosteatotic ob/ob mice [13], revealing no significant difference in expression of Fsp27a, while Fsp27b was significantly reduced in Hnf4aΔHep mice. These results indicated that Fsp27b may be a novel HNF4α target gene. In addition, expression of Crebh encoding a transcription factor that is a positive regulator of Fsp27b, was decreased by nearly half in Hnf4aΔHep mice, in agreement with an earlier study revealing that HNF4α positively regulates Crebh expression [17]. Expression of Plin2-5 except for Plin1 was significantly increased in Hnf4aΔHep mice (Fig. 1B). Increased Plin2-5 in Hnf4aΔHep mice might be a secondary result of the hepatosteatosis in these mice and independent of direct HNF4α.
Fig. 1.
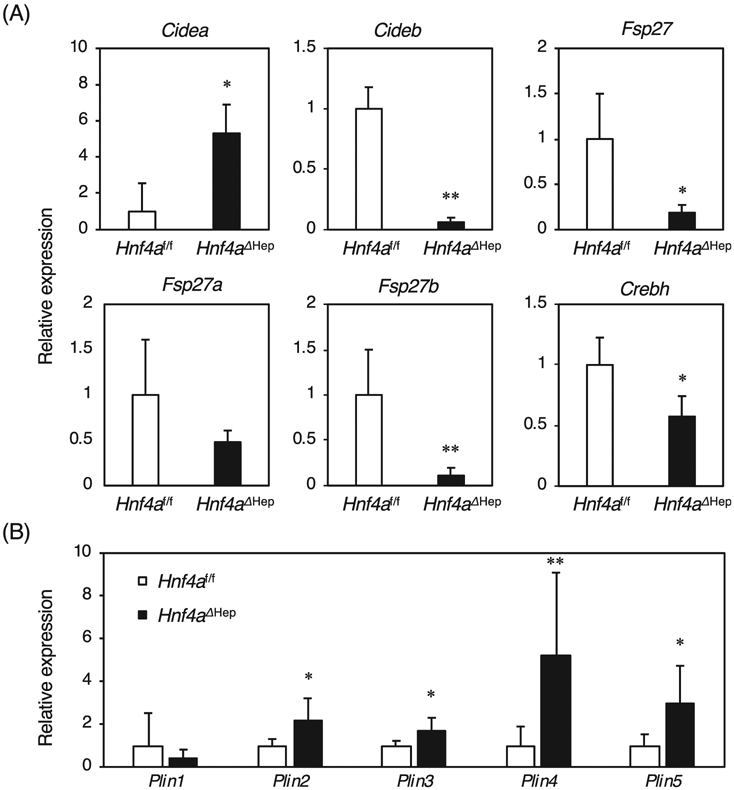
Hepatic expression of Cide and Plin family, and Crebh in Hnf4aΔHep mice. quantitative RT-PCR from total liver RNA of Hnf4af/f and Hnf4aΔHep mice (n = 6 for each genotype). The normalized expression in Hnf4aΔHep mice is presented relative to that in Hnf4af/f mice. Data are mean ± S.D. *, P < 0.05; **, P < 0.005 compared to Hnf4af/f mice.
3.2. Expression of CIDE family by HNF4α in human hepatoma cells
Expression of CIDE family mRNAs were analyzed by siRNA knockdown of HNF4α in human hepatoma cells (Fig. 2A). Knockdown of HNF4α in HepG2 and Huh7 cells showed that expression of CIDEA was not significantly different or not detected, and expression of CIDEB was slightly decreased even though the CIDEB is transactivated by HNF4α [6,7]. Expression of CIDEC that detect both CICEC1 encoding the human orthologue of the mouse Fsp27a, and CIDEC2 encoding the orthologue of the Fsp27b, was significantly decreased by HNF4α knockdown, and expression of CIDEC1 was significantly decreased by HNF4α knockdown only in HepG2 cells. Furthermore, expression of CIDEC2 was largely decreased by HNF4α knockdown in both cell lines, indicating that CIDEC2 may be positively regulated by HNF4α. Overexpression of HNF4α in Huh7 cells induced expression of CIDEA, CIDEB, and CIDEC2 (Fig. 2B). Overexpression of CREBH(N), an active form of CREBH [18], moderately induced expression of CIDEB. Expression of CIDEC1 was strongly induced even though CIDEC1 expression was not induced by CREBH(N) [13]. Induction of CIDEC2 by CREBH(N) was much stronger than that of CIDEC1. Furthermore, overexpression of both HNF4α and CREBH(N) synergistically induced CIDEB and CIDEC2 compared to HNF4α, or CREBH(N) alone. Thus, expression of CIDEB and CIDEC2 is induced in an HNF4α and CREBH(N)-dependent manner. In addition, a doxycycline-inducible HNF4α expression system was generated using human hepatoma-derived HLE cells (HLE/tet-HNF4A cells). Addition of doxycycline (DOX) did not induce expression of CIDEA and CIDEC1, but expression of CIDEB and CREBH was significantly induced by DOX. Notably, expression of CIDEC2 was largely induced by DOX, indicating that HNF4α is a strong inducer of CIDEC2.
Fig. 2.
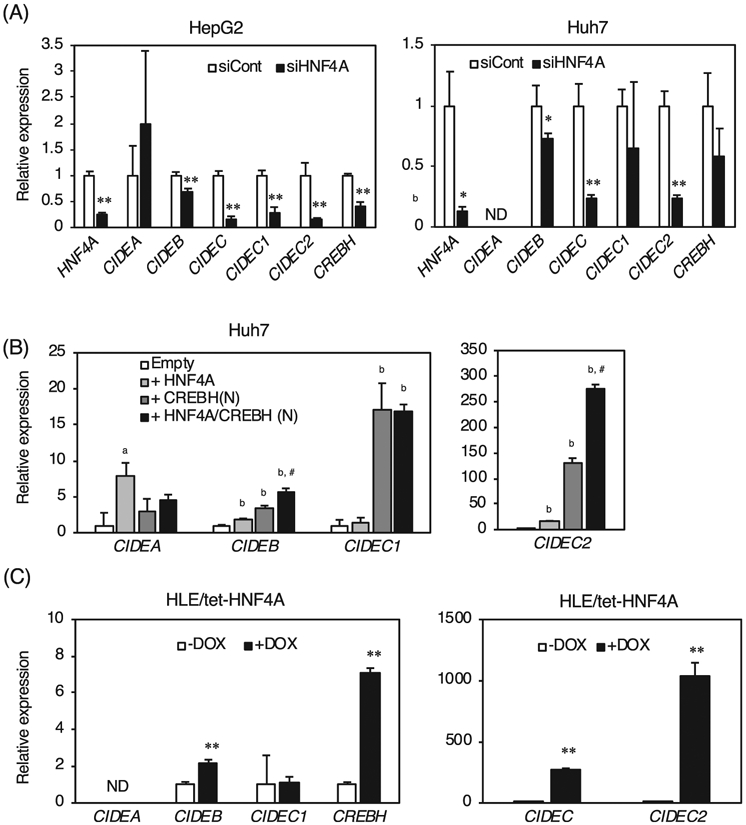
Hepatic expression of CIDE family and CREBH by HNF4α in human hepatoma cells. (A) quantitative RT-PCR from total RNA of HepG2 and Huh7 cells treated with negative control of siRNA (siCont) and siRNA for HNF4A (siHNF4A). (B) quantitative RT-PCR from total RNA of HNF4α-transfected Huh7 cells. (C) quantitative RT-PCR from total RNA of HLE/tet-HNF4A cells treated without or with doxycycline (−DOX, or + DOX) for 24 h. The normalized expression in Hnf4aΔHep mice, HNF4α-transfected cells, and the cells treated with doxycycline is presented relative to that in Hnf4af/f mice, empty vector-transfected cells, and the cells treated without doxycycline, respectively. Data are mean ± S.D. *, P < 0.05; **, P < 0.005 compared to the cells treated with negative control of siRNA, the cells transfected an empty, or the cells treated without doxycycline. a, P < 0.05; b, P < 0.01 compared to the cells transfected an empty. *, P < 0.05; #, P < 0.01 compared to the cells transfected CREBH(N) vector. ND; not detected.
3.3. Transactivation of the Fsp27b gene by HNF4α
To investigate whether the Fsp27b promoter is transactivated by HNF4α, a JASPAR database search was performed, and a high score HNF4α binding site was found at +922/+934 from the transcription start site of the Fsp27a (+1) and the Fsp27b (+946) genes with high conservation among the species (Fig. 3A and B). The Fsp27a promoter was positively regulated by PPARα through peroxisome proliferator response element (PPRE) at −218/−206 as described previously [15]. As expected, the promoter at −2467/+32 including the PPRE was transactivated by PPARα with RXRα and the ligand for PPARα, Wy-14,643, but this promoter was not transactivated by HNF4α (Fig. 3C). In contrast, the Fsp27b promoters at +33/+1026 and + 874/+1026 including the predicted HNF4α binding site were markedly transactivated by HNF4α, but the promoter at +950/+1026 without the HNF4α binding site was not transactivated by HNF4α. The +33/+1026 promoter with the mutated HNF4α binding site resulted in no transactivation by HNF4α, revealing that the Fsp27b promoter is transactivated in HNF4α-dependent manner. Furthermore, the +33/+1026 promoter and the promoter with the mutated HNF4α binding site was also transactivated by PPARα to the same degree, but the +874/+1026 promoter was not transactivated by PPARα, indicating that there may be a PPRE between +1026 and + 873. In addition, there was a binding site for CREBH responsive element (CRE) at +911/+919 just upstream of the HNF4α binding site, and CREBH is known to induce Fsp27b expression through this element [13]. When the wild-type Fsp27b promoter was used, HNF4α alone and CREBH alone equally transactivated the Fsp27b promoter by about 10-fold, and both HNF4α and CREBH strongly transactivated the Fsp27b promoter (Fig. 4A). When mutations were introduced into the HNF4α binding site, the promoter activities by HNF4α alone, and both HNF4α and CREBH were largely decreased, while the activity by CREBH alone was also decreased by half, indicating that the HNF4α binding site might be essential for full transactivation by CREBH. When mutations were introduced into the CRE, no difference in the promoter activity by HNF4α was observed compared to the wild-type promoter, but the activity by CREBH was largely decreased. Both HNF4α and CREBH reduced the promoter activity by half. Furthermore, mutations of both the HNF4α binding site and CRE dramatically decreased all promoter activities. These findings reveal that HNF4α and CREBH synergistically transactivate the Fsp27b promoter.
Fig. 3.
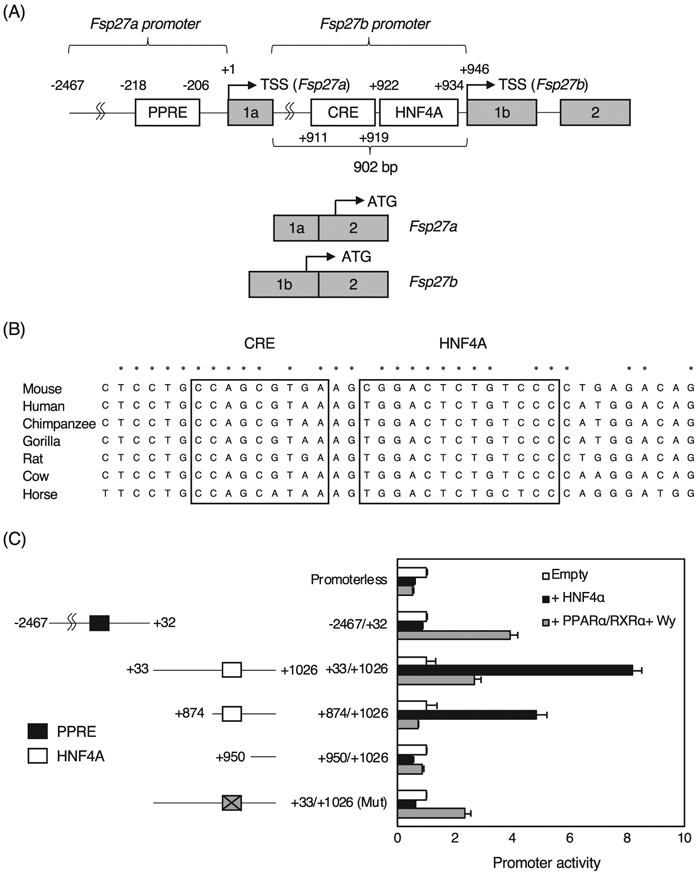
Analysis of the Fsp27a/b promoters. (A) Schematic structure of the Fsp27 promoter. Transcription start site of the Fsp27a and Fsp27b gene is shown as +1 and + 946, respectively. (B) Sequence alignment of the proximal promoter of the Fsp27b gene in mouse, human, chimpanzee, gorilla, rat, cow, and horse. CREBH-responsive element (CRE) and predicted HNF4α binding site are encircled as boxes. Completely conserved nucleotides among the species are shown as asterisk. (C) Promoter activity of the Fsp27 gene in HEK293T cells. The Fsp27 promoters were co-transfected with empty vector, HNF4α expression vector, and PPARα/RXRα expression vectors treated with 10 μM of Wy-14,643 into HEK293T cells. Mutations were introduced into the HNF4α in the +33/+1,026 promoter (gray square with a cross). HNF4α binding site and PPRE are shown as open and solid squares, respectively. The normalized activity ± S.D. was presented as relative activity based on empty vector-transfected cells. ND; not detected.
Fig. 4.
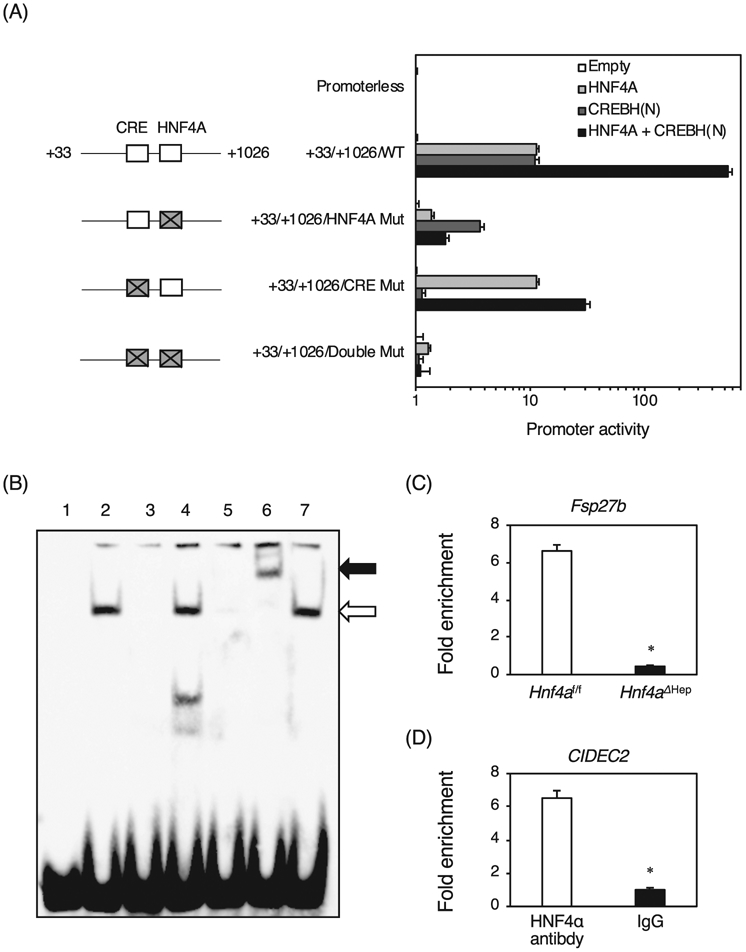
Promoter analysis of the Fsp27b promoter by HNF4α and CREBH, and identification of an HNF4α binding site in the Fsp27b promoter. (A) Promoter activity of the Fsp27b gene. The Fsp27b promoters were co-transfected with empty vector, HNF4α expression vector, and N-terminus-deleted activated CREBH expression vector, and both HNF4α and CREBH(N) expression vectors into HEK 293T cells. Mutations were introduced into the HNF4α and CREBH biding sites (CRE) (gray square with a cross). (B) EMSA. Nuclear extracts were incubated with biotin-labeled probe carrying the HNF4α binding sites in the Fsp27b promoter in the absence (lane 2) or presence of the unlabeled Fsp27b probe (lane 3), the mutated Fsp27b probe (lane 4), and the Otc probe (lane 5). For supershift analysis, anti-HNF4α and anti-PPARβ antibodies were added, respectively (lanes 6 and 7). Complex between HNF4α and the probe and supershifted complex are indicated by the lower and upper arrow, respectively. (C) Chromatin immunoprecipitation using the livers of Hnf4aΔHep and Hnf4af/f mice with anti-HNF4α antibody and normal goat IgG. The region containing the HNF4α binding site in the Fsp27b promoter and the region without an HNF4α binding site in the Hmgcs2 gene were amplified. The data from qPCR was normalized relative to the input and expressed as fold enrichment over data from IgG control. (D) Chromatin immunoprecipitation using HepG2 cells were performed with anti-HNF4α antibody and normal goat IgG. The region containing an HNF4α binding site in the human CIDEC2 gene and the region without HNF4α binding site in the human MIR194 gene were amplified. Data are mean ± S.D. *, P < 0.05 compared to Hnf4af/f mice and HNF4α antibody.
3.4. Binding of HNF4α in the Fsp27b promoter
To determine whether HNF4α directly binds to the predicted HNF4α binding site in the Fsp27b promoter, electrophoretic mobility shift analysis was performed (Fig. 4B). Nuclear extracts from HNF4α-overexpressed HEK293T cells bound to the identified HNF4α binding sites (lane 2, the lower arrow). This complex was diminished by the addition of unlabeled Fsp27b competitor and Otc competitor that contains a bonafide HNF4α binding site (lanes 3 and 5), but not the competitor that has mutations in the HNF4α binding site of the Fsp27b promoter (lane 4). Moreover, the complex was supershifted by anti-HNF4α antibody (lane 6, the upper arrow), but not the unrelated anti-PPARβ antibody (lane 7). Chromatin immunoprecipitation using the livers of Hnf4af/f and Hnf4aΔHep mice indicated that HNF4α in Hnf4af/f mice bound to the promoter region approximately 6.5-fold strongly compared to Hnf4aΔHep mouse livers (Fig. 4C). In addition, HNF4α bound to the predicted HNF4α binding site of the CIDEC2 promoter in HepG2 cells approximately 6.5-fold strongly compared to IgG control (Fig. 4D), suggesting that HNF4α directly and physiologically binds to the CIDEC2/Fsp27b promoters in human and mouse livers.
4. Discussion
We investigated whether CIDE family and perilipin family genes are involved in hepatosteatotic development in Hnf4aΔHep mice. CIDEB is transactivated by HNF4α [6,7], and expression of Cideb was strikingly decreased in Hnf4aΔHep mice as expected. However, inhibition of HNF4α slightly suppressed expression of CIDEB, and overexpression of HNF4α slightly induced expression of CIDEB in human hepatoma cells, indicating that HNF4α may weakly regulate CIDEDB expression in human liver. In Huh7 cells, CREBH induced CIDEB expression, and both HNF4α and CREBH synergistically induced CIBEB expression, indicating that both factors might be essential for CIDEB transactivation in human normal hepatocytes. Conversely, expression of Cidea was significantly increased in Hnf4aΔHep mice. Since Cidea expression is induced by PPARα and PPARγ [19], increased expression of Cidea in Hnf4aΔHep mice may be due to activation of PPARα and/or PPARγ by endogenous ligands. Curiously, expression of CIDEA was induced by HNF4α in Huh7 cells, but no induction was observed in HLE cells, suggesting that Cidea/CIDEA would not be a target of HNF4α. Furthermore, expression of Crebh was significantly decreased in Hnf4aΔHep mice as reported that HNF4α directly regulates Crebh [17], and expression of CREBH was also regulated by HNF4α in human hepatoma cells. Overexpression of CREBH did not induce expression of CIDEC1 in Huh7 cells [13], but our results showed that CREBH strongly induce expression of CIDEC1. Since we used probe-based qPCR for detection of CIDEC1, our results that CREBH upregulates CIDEC1 should be accurate. Interestingly, it was found that Fsp27b is a novel HNF4α target gene, and the HNF4α binding site in the Fsp27b promoter is located next to the CREBH binding site, indicating that HNF4α and CREBH might synergistically transactivate the Fsp27b through direct protein-protein interaction. Thus, HNF4α would strongly regulate Fsp27b/CIDEC2 through both direct regulation by HNF4α itself and indirect regulation by a HNF4α-CREBH axis.
Hnf4aΔHep mice exhibit hepatosteatosis, but expression of liver-enriched Cideb and steatotic liver-enriched Fsp27b was markedly decreased in Hnf4aΔHep mice. Conversely, expression of Cidea that was predominantly expressed in steatotic livers was increased in Hnf4aΔHep mice. Thus, HNF4α would play a central role to promote formation of lipid droplet by upregulation of Cideb as a main target and Fsp27b as a minor target in normal hepatocytes, that is, hepatic HNF4α is an essential factor required to maintain lipid homeostasis by a dual role to store excessive lipid as lipid droplets through CIDEB and FSP27B proteins, and to produce VLDL by induction of Apob and Mttp expression from lipid droplets as necessary.
However, it is still questionable whether altered expression of the CIDE family contributes to hepatosteatotic development in Hnf4aΔHep mice. In normal liver, expression of Cideb is most abundant, but expression of Cidea and Fsp27a/b is very low [8]. Expression of CIDEA is increased by about 5-fold, but expression of Cideb and Fsp27b is strongly decreased in Hnf4aΔHep mice. Thus, these results suggest that the hepatic total CIDE activity in growth of lipid droplets should be decreased in Hnf4aΔHep mice, indicating that hepatosteatosis would be improved in Hnf4aΔHep mice when only altered expression of CIDE family is considered. Conversely, expression of Plin2-5 was modestly increased in Hnf4aΔHep mice. Perilipins are the most abundant proteins that localize at the surface of lipid droplets, and protect lipid droplets from lipase action [20]. Thus, increased expression of Plin2-5 might be partly involved in hepatosteatotic development in Hnf4aΔHep mice by suppression of lipolysis. However, since many proteins are associated with lipid droplets [21], it is not easy to mechanistically explain hepatosteatotic development in Hnf4aΔHep mice by expression change of CIDE and perilipin family. Further analyses are required to elucidate the detailed mechanism of hepatosteatotic development in Hnf4aΔHep mice.
Supplementary Material
Acknowledgements
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant-in-Aid for Scientific Research, No. 16K08728 and 19K07474), Gunma University, Akita University, Nagoya University, Collaborative Investigation Project, the joint research program of the Institute for Molecular and Cellular Regulation, Gunma University, Bristol-Meyers Squibb, and Takeda Science Foundation.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrc.2020.05.070.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
References
- [1].Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA, Control of pancreas and liver gene expression by HNF transcription factors, Science 303 (2004) 1378–1381, 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Inoue Y, Hayhurst GP, Inoue J, Mori M, Gonzalez FJ, Defective ureagenesis in mice carrying a liver-specific disruption of hepatocyte nuclear factor 4alpha (HNF4alpha). HNF4alpha regulates ornithine transcarbamylase in vivo, J. Biol. Chem 277 (2002) 25257–25265, 10.1074/jbc.M203126200. [DOI] [PubMed] [Google Scholar]
- [3].Morimoto A, Kannari M, Tsuchida Y, Sasaki S, Saito C, Matsuta T, Maeda T, Akiyama M, Nakamura T, Sakaguchi M, Nameki N, Gonzalez FJ, Inoue Y, An HNF4alpha-microRNA-194/192 signaling axis maintains hepatic cell function, J. Biol. Chem 292 (2017) 10574–10585, 10.1074/jbc.M117.785592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Inoue Y, Peters LL, Yim SH, Inoue J, Gonzalez FJ, Role of hepatocyte nuclear factor 4alpha in control of blood coagulation factor gene expression, J. Mol. Med. (Berl.) 84 (2006) 334–344, 10.1007/s00109-005-0013-5. [DOI] [PubMed] [Google Scholar]
- [5].Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ, Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis, Mol. Cell Biol 21 (2001) 1393–1403, 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Da L, Li D, Yokoyama KK, Li T, Zhao M, Dual promoters control the cell-specific expression of the human cell death-inducing DFF45-like effector B gene, Biochem. J. 393 (2006) 779–788, 10.1042/BJ20051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Daigo K, Kawamura T, Ohta Y, Ohashi R, Katayose S, Tanaka T, Aburatani H, Naito M, Kodama T, Ihara S, Hamakubo T, Proteomic analysis of native hepatocyte nuclear factor-4alpha (HNF4alpha) isoforms, phosphorylation status, and interactive cofactors, J. Biol. Chem 286 (2011) 674–686, 10.1074/jbc.M110.154732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ, Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27, Cell Metabol. 7 (2008) 302–311, 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou L, Xu L, Ye J, Li D, Wang W, Li X, Wu L, Wang H, Guan F, Li P, Cidea promotes hepatic steatosis by sensing dietary fatty acids, Hepatology 56 (2012) 95–107, 10.1002/hep.25611. [DOI] [PubMed] [Google Scholar]
- [10].Kang HS, Okamoto K, Kim YS, Takeda Y, Bortner CD, Dang H, Wada T, Xie W, Yang XP, Liao G, Jetten AM, Nuclear orphan receptor TAK1/TR4-deficient mice are protected against obesity-linked inflammation, hepatic steatosis, and insulin resistance, Diabetes 60 (2011) 177–188, 10.2337/db10-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li JZ, Ye J, Xue B, Qi J, Zhang J, Zhou Z, Li Q, Wen Z, Li P, Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation, Diabetes 56 (2007) 2523–2532, 10.2337/db07-0040. [DOI] [PubMed] [Google Scholar]
- [12].Ye J, Li JZ, Liu Y, Li X, Yang T, Ma X, Li Q, Yao Z, Li P, Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B, Cell Metabol. 9 (2009) 177–190, 10.1016/j.cmet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- [13].Xu X, Park JG, So JS, Lee AH, Transcriptional activation of Fsp27 by the liver-enriched transcription factor CREBH promotes lipid droplet growth and hepatic steatosis, Hepatology 61 (2015) 857–869, 10.1002/hep.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aibara D, Matsusue K, Takiguchi S, Gonzalez FJ, Yamano S, Fat-specific protein 27 is a novel target gene of liver X receptor alpha, Mol. Cell. Endocrinol 474 (2018) 48–56, 10.1016/j.mce.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Langhi C, Baldan A, CIDEC/FSP27 is regulated by peroxisome proliferator-activated receptor alpha and plays a critical role in fasting- and diet-induced hepatosteatosis, Hepatology 61 (2015) 1227–1238, 10.1002/hep.27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aibara D, Matsuo K, Yamano S, Matsusue K, Fat-specific protein 27b is regulated by hepatic peroxisome proliferator-activated receptor gamma in hepatic steatosis, Endocr. J 67 (2020) 37–44, 10.1507/endo-crj.EJ19-0296. [DOI] [PubMed] [Google Scholar]
- [17].Luebke-Wheeler J, Zhang K, Battle M, Si-Tayeb K, Garrison W, Chhinder S, Li J, Kaufman RJ, Duncan SA, Hepatocyte nuclear factor 4alpha is implicated in endoplasmic reticulum stress-induced acute phase response by regulating expression of cyclic adenosine monophosphate responsive element binding protein H, Hepatology 48 (2008) 1242–1250, 10.1002/hep.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang M, Zhao S, Tan M, bZIP transmembrane transcription factor CREBH: potential role in non-alcoholic fatty liver disease (Review), Mol. Med. Rep 13 (2016) 1455–1462, 10.3892/mmr.2015.4749. [DOI] [PubMed] [Google Scholar]
- [19].Viswakarma N, Yu S, Naik S, Kashireddy P, Matsumoto K, Sarkar J, Surapureddi S, Jia Y, Rao MS, Reddy JK, Transcriptional regulation of Cidea, mitochondrial cell death-inducing DNA fragmentation factor alpha-like effector A, in mouse liver by peroxisome proliferator-activated receptor alpha and gamma, J. Biol. Chem 282 (2007) 18613–18624, 10.1074/jbc.M701983200. [DOI] [PubMed] [Google Scholar]
- [20].Sztalryd C, Brasaemle DL, The perilipin family of lipid droplet proteins: gatekeepers of intracellular lipolysis, Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862 (2017) 1221–1232, 10.1016/j.bbalip.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brasaemle DL, Dolios G, Shapiro L, Wang R, Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes, J. Biol. Chem 279 (2004) 46835–46842, 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


