Abstract
Despite the recent scientific advances made in cancer diagnositics and therapeutics, cancer still remains the second leading cause of death worldwide. Thus, there is a need to identify new potential biomarkers/molecular targets to improve the diagnosis and treatment of cancer patients. In this regard, long non-coding RNAs (lncRNAs), a type of non-coding RNA molecule, have been found to play important roles in diverse biological processes, including tumorigenesis, and may provide new biomarkers and/or molecular targets for the improved detection of treatment of cancer. For example, one lncRNA, tissue differentiation-inducing non-protein coding RNA (TINCR) has been found to be significantly dysregulated in many cancers, and has an impact on tumor development and progression through targeting pivotal molecules in cancer-associated signaling pathways. Hence, based on recent discoveries, herein, we discuss the regulatory functions and the underlying mechanisms of how TINCR regulates signaling pathways attributed to cancer hallmarks associated with the pathogenesis of various human cancers. We also highlight studies assessing its potential clinical utility as a biomarker/target for early detection, cancer risk stratification, and personalized cancer therapies.
Keywords: TINCR, Tissue differentiation-inducing non-protein coding RNA, Cancer, Biomarker, long non-coding RNA, LncRNA
Graphical Abstract

Introduction
Cancer initiation, promotion, progression, and metastasis involve a series of fundamental heterogeneous steps, resulting in challenges for its detection and treatment [1, 2]. Additionally, several factors are associated with cancer etiology, such as genetic mutations, environmental conditions, and alterations in the expression levels of the molecules involved in cellular signaling pathways and maintenance of genome stability [3]. Interestingly, more than 90% of the human genome is transcribed into non-coding RNAs (ncRNAs), while less than 2% of the genome is transcribed and translated into proteins [4, 5]. Long non-coding RNAs (lncRNAs), defined as RNA molecules of >200 nucleotides in length, do not code for annotated proteins, yet their abnormal expression has been shown to play essential roles in several cancer-associated signaling molecules/pathways such as Notch, mTOR, NF-kb, and Wnt/ β-catenin [6, 7]. Because of their roles in multiple signaling pathways, the dysregulation of lncRNA expression is thought to contribute to the development of various types of cancers as ascertained from their oncogenic or tumor-suppressive properties [8–10]. Furthermore, lncRNA expression has been correlated with many clinicopathological features of cancer patients, including age, gender, lymph node metastasis, Ki-67 levels, histological grade, tumor size, and tumor node metastasis stage [11, 12].
Tissue differentiation-induced non-coding RNA (TINCR), also known as placenta-specific protein 2 (PLAC2), is a lncRNA located on chromosome 19p13.3 in humans, and is abnormally expressed in many cancers (Figure 1) [13]. Recently, the role of TINCR has been investigated in association with cancers, including breast [14, 15], lung [16, 17], hepatocellular carcinoma [18], esophageal [19], colon [20–23], bladder [24], prostate [25], gastric [26–29], and oral squamous cell carcinoma [30] (Table 1). It was determined that overexpression of TINCR promoted cell proliferation and metastasis via activation of the Wnt/β-catenin signaling pathway in oral squamous cell carcinoma [30]. On the contrary, TINCR inhibited apoptosis by targeting the Krupple Like Factor 2 (KLF4) gene in gastric cancer [29]. Additionally, similar inferences were elucidated stating a significant association of TINCR levels, via sponging of miR-7–5p, and regulating the PI3K/Akt/mTOR signaling pathways to induce colorectal carcinogenesis [21].
Figure 1:
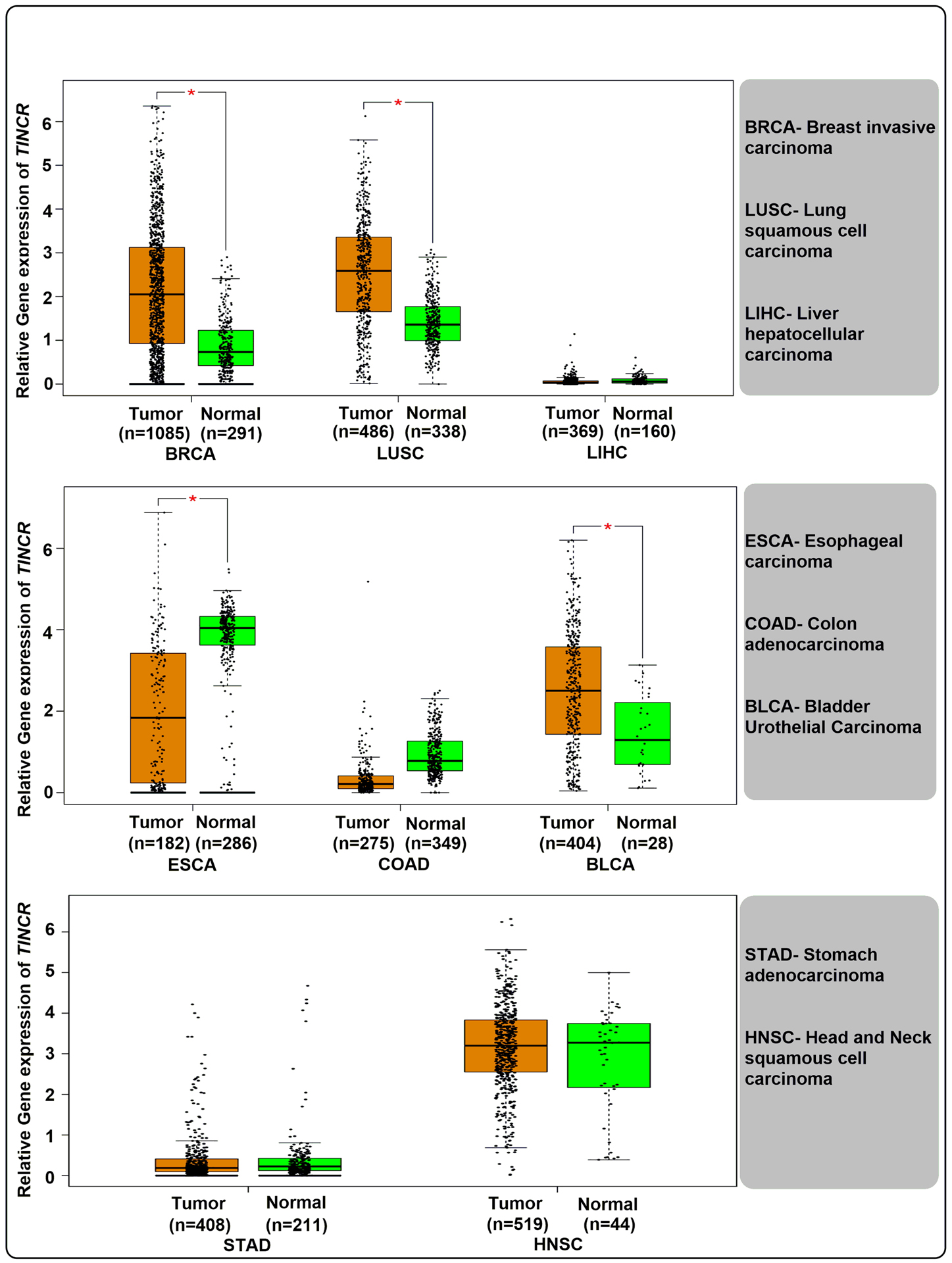
Relative gene expression levels of lncRNA TINCR in various cancers obtained from the TCGA database GEPIA (http://gepia.cancer-pku.cn). (Log2FC Cutoff = 1, p-value cut off = 0.01, jitter size =0.4). Analysis was performed using matched TCGA normal and GTEx data.
Table 1:
Characteristics of TINCR in different tissues/cell lines of various cancers.
| Cancer | Cancer type | Approx. Mean fold change in expression of TINCR compared to controls | Property | Validation Methods | Biological significance | Genes/Proteins/miRNAs/Pathways affected | Cell lines | References |
|---|---|---|---|---|---|---|---|---|
| Breast cancer | Breast carcinoma | ↑ 7.5 | Oncogenic | qRT-PCR, ChIP, Western blot analysis | Inhibits apoptosis, Promotes migration, invasion, cell proliferation, Metastasis, Induce EmT | ↑ SP1; ↓ miR-7; ↑ KLF4; ↓ Bax; ↓ Bcl-2; ↓ miR-125b; ↑ ERBB2; ↑ ERK ½; ↑ Akt signaling; ↓ E-cadherin; ↓ β-catenin; ↑ Vimentin; ↑ N-cadherin; ↑ snail-1 | MCF-7, MDA-MB-231, MDA-MB-435, MDA-MB-453, MDA-MB-468, SKBR-3, BT474, MCF-10A | [16, 44] |
| Lung cancer | Lung adenocarcinoma | ↓ 3.0 | Tumor suppressor | qRT-PCR, Western blot analysis | Inhibits cell proliferation and invasion | ↑ miR-544a; ↓ FBXW7; ↑ c-Myc; ↑ Cyclin E | HBE, A549, H322, H460, GLC-82, SPC-A1 | [18] |
| Non-Small Cell Lung Cancer | ↑ 1.8 | Oncogenic | qRT-PCR, Microarray analysis, Western blot analysis | Promotes migration, tumor growth, metastasis | ↑ BRAF; ↑MAPK signaling; ↑ CCND1; ↑MMP2; ↑ MMP9; ↑ MEK1/2 | MRC-5, PC9, H1299, H522, A549, 95D, 95C, H2172, 293T | [17] | |
| Esophageal cancer | Esophageal squamous cell carcinoma | ↑ 14.0 | Oncogenic | qRT-PCR | Promotes cell proliferation, migration, invasion and inhibits apoptosis | ↑ CLDN7; ↑ ANAX1; ↓ NF-kB signalling, ↓ ZNF50 | HEEC, TE-13, TE-1, KYSE-410, ECA-109 | [20] |
| Bladder cancer | Bladder urothelial carcinoma | ↑ 2.3 | Oncogenic | qRT-PCR | Promotes Cell proliferation and inhibits apoptosis | N.A | 5637, SW780, | [25] |
| Liver cancer | Hepatocellular carcinoma | ↑ 8.7 | Oncogenic | qRT-PCR, Western blot analysis | Promotes cell proliferation, migration, invasion | ↓ miR 137; ↓ miR 133a | HUH7 | [19] |
| Colorectal cancer | Colon carcinoma | ↓ 2.9 | Tumor suppressor | qRT-PCR, Western blot analysis, ChIP | Inhibits cell proliferation, metastasis, invasion and promotes apoptosis and cell cycle arrest | ↑ EpCAM; ↑ EpICD; ↑ Presenilin2; ↑ FHL2; ↑ Lef-1; ↑ β-catenin; ↑TCF4; ↑ c-Myc; ↑ WNT/β-catenin Signaling; ↓ CD36; ↓ PPAR-γ; ↓ PPAR signaling pathway; ↓ E-cadherin; ↓ EZR; ↑ miR-107 | RKO, LOVO, HCT116, SW480, SW620, HT29, LS174T, CCD 841 CON, | [21, 63] |
| Colon carcinoma | ↑ 5.0 | Oncogenic | Microarray analysis | Promotes Cell proliferation, migration and invasion | ↑ Sp1; ↑ EFGR; ↑ PIK3CD; ↑ mTOR; ↑ IRS-2; ↑PI3K/Akt/mTOR pathway; ↓ miR-7–5p | FHC, HCT116, HCT8, HT29, SW620 and SW480 | [22] | |
| Prostate cancer | Prostate cancer tissue | ↓ 4.0 | Tumor Suppressor | qRT-PCR, Western blot analysis | Inhibits Cell proliferation, migration and invasion | ↓ TRIP3 | P69, LNCaP, PC3, DU145 and 22Rv1 | [26] |
| Gastric cancer | Gastric carcinoma | ↑ 2.5 | Oncogenic | qRT-PCR, Western blot analysis | Inhibits apoptosis, Promotes cell proliferation, metastasis, Tumorigenecity, Cell growth | ↑ PDK1; ↓ miR-375; ↑ Akt; ↑ p53 gene; ↑ E2F1; ↑ SP1; ↑ BCL-2 gene; ↓ Caspase 3; ↓ CDKN1A/P21; ↓ CDKN2B/P15; ↓ KLF2 gene; ↑ E2F1 | KATO III, HGC-27, NCI-N87, SNU-1, SGC7901; BGC823; MGC803; MKN45; GES1 | [27, 88] |
| Oral cancer | Oral squamous cell carcinoma | ↑ 2.5 | Oncogenic | qRT-PCR; Western blot analysis; IHC | Promotes cell proliferation and metastasis | ↑ Wnt; ↑ β-catenin; ↑ WNT/β-catenin Signaling; ↑ MMP7; ↑ MMP9; ↑ Cyclin D1 | SCC-9 and CAL-27 | [31] |
As TINCR has been implicated in the pathogenesis of many cancers, in this review, we focus on the mechanistic roles of TINCR in contributing to oncogenesis by cancer type. We also discuss the clinical utility of the TINCR to as a novel approach for cancer diagnosis, prognosis, and as a potential therapeutic target.
BREAST CANCER
Breast cancer is the most prevalent type of malignancy among women worldwide, and the 5-year survival of tumor node metastasis (TNM) stage-IV has been estimated to be less than ~20% [9, 31]. Multiple factors correlate with high breast cancer incidence such as female gender, infertility issues, obesity, nulliparity, hormonal treatment for menopause, exposure to environmental ionizing radiation, excessive tobacco and alcohol use, diet, and genetic dysfunction (e.g., BRCA1 and BRCA2 mutations) [32–34]. Recently, many lncRNAs have been identified as critical regulators associated with breast malignancy, such as GAS5 [35], HOTAIR [36], CCAT [37], H19 [38], among others. Similarly, Liu et al. (2018) observed significantly higher, but varied changes (~3 to 16-fold), in the expression levels of TINCR in 24 breast cancer tissue samples compared to corresponding adjacent healthy tissues [15]. The authors also observed an ~8-fold higher expression of TINCR in MCF-7 breast cancer cell lines compared to immortalized human mammary epithelial cell lines MCF-10A [15]. Consistent with this, another group found an ~3-fold higher expression of TINCR in tissues of breast cancer patients compared to healthy controls [39]. At the molecular level, it was determined that the variation in the expression levels of TINCR in different patients group was largely due to the BRAC1/2 gene amplification status, and correlated with the HER2, estrogen, and progesterone levels of the breast cancer patients [39]. However, further studies are warranted with larger sample numbers to draw solid conclusions.
Kaplan-Meier survival curves have shown poor prognosis in breast cancer patients with higher TINCR expression, suggesting a tumorigenic role of TINCR in these patients. Consistent with this study, knockdown of TINCR was shown to suppress cellular migration and invasion, and stimulate apoptosis in MDA-MB-468 and MCF-7 human breast cancer cells [15]. Further, TINCR knockdown in MDA-MB-453 cells led to inhibition of cell proliferation and cell-cycle arrest at the G0/G1 stage [15]. In addition, knockdown of TINCR was found to upregulate Bax, an apoptotic regulator whose overexpression promotes cell death. The role of TINCR in breast cancer was also evaluated using a xenograft mouse model, and it was found that the weight of the tumors from the TINCR-depleted xenografts were on average ~0.8 g lighter compared to tumors derived from the mock-infected or wild-type MDA-MB-468 cells. At the molecular level, it was found that the Sp1 transcription factor activated the expression of TINCR by binding to its three putative GC-rich motifs, which in turn stimulated cell proliferation and suppressed apoptosis in breast cancer cells [29]. Mechanistically, TINCR was found to modulate the expression of Krupple Like Factor 4 (KLF4) by sponging the tumor suppressor miR-7 [40]. Similarly, in the case of gastric cancer, SP1 induced the upregulation of TINCR, and regulated apoptosis by affecting KLF2 mRNA stability [29]. The KLF transcription factor is known to stimulate a transition of cells from G0/G1 to S-phase of the cell cycle, and it also plays a role in cellular invasion and in the epithelial to mesenchymal transition (EMT) process.
Recently, Dong et al. (2019) assessed the association of TINCR with chemoresistance in breast cancer patient samples and cell lines [14]. By qRT-PCR analysis, the authors found that TINCR was up-regulated by ~2.5-fold (Table 1 and Figure 2) in trastuzumab-resistant (TR) breast cancer patients compared to trastuzumab-responsive breast cancer patients [14]. Similarly, they observed that trastuzumab-resistant human SKBR-3-TR and BT474-TR breast cancer cells showed an ~2.0-fold increase in TINCR levels compared to wild-type SKBR-3 breast cancer cells. The authors further noted that in the presence of trastuzumab, the SKBR-3-TR cells showed a higher rate of cell migration and invasion compared to the parental SKBR-3 cells, and lower apoptotic rates in the presence of trastuzumab [14]. Moreover, the down-regulation of TINCR significantly decreased the migration and invasion capacity of the SKBR-3-TR cells, which suggested that TINCR promoted trastuzumab resistance by facilitating the invasive potential and EMT characteristics of these breast cancer cells. The expression levels of some mesenchymal protein markers such as Vimentin and N-cadherin were increased in contrast to the level of epithelial proteins (e.g. β-catenin, E-cadherin) in trastuzumab-resistant breast cancer cells, which implicated the role of TINCR in breast cancer metastasis. Through the Targetscan database, miR-125b was found as a candidate miRNA regulated by TINCR. MiR-125b has been recognized as a tumor suppressor miRNA in breast cancer, capable of inhibiting glucose metabolism by downregulating the ERBB2 oncogene. Reduced levels of the ERBB2 protein can result in the deregulation of the ERK1/2 and AKT signaling pathways, which in turn can inhibit cell survival and the growth of breast cancer cells [41]. The overexpression of miR-125b was shown to downregulate the expression of Snail-1, a critical modulator of EMT in SKBR-3-TR and BT474-TR breast cancer cell lines [42]. It was also established that Snail-1 was a prime target of TINCR/miR-125b in the activation of trastuzumab-resistance induced EMT in HER2+ breast cancer patients [14]. Also, the upregulation of TINCR was found to be facilitated by H3K27 acetylation at its promoter region, which resulted in reduced activity toward trastuzumab treatment, providing a potential explanation for the correlation of TINCR with poor the prognosis of breast cancer patients who received trastuzumab therapy.
Figure 2:

Potential regulatory mechanisms of TINCR in the development and progression of a variety of cancer types.
Taken together, the above data suggested that the TINCR-miR-7-KLF4 and TINCR-miR-125b-ERBB2 axes play essential roles in contributing to tumorigenesis in breast cancer pathogenesis. Thus, modulation of the components of these signaling pathways may be manipulated to develop a targeted therapy for breast cancer patients. Moreover, TINCR may be considered as a potential biomarker for the diagnosis and prognosis of breast cancer patients.
LUNG CANCER
Lung cancer is the leading cause of cancer-related death worldwide, with 5-year median survival rates of less than 15% [43]. Earlier studies showed that a variety of lncRNAs are closely associated with the development and progression of lung cancer [44]. To better understand the correlation of TINCR with the occurrence and development of lung cancer, Liu et al. (2018) found significant downregulation (~2.9-fold) of TINCR expression in lung cancer tissue samples (n = 45), compared to healthy lung tissue samples [17]. Additionally, TINCR was significantly downregulated (~3-fold) in the A549 lung adenocarcinoma cell line when compared to normal human lung epithelial (HBE) cells [17]. Furthermore, TINCR depletion promoted cell growth, while its upregulation led to decreased levels of the Ki-67 protein (a cellular marker associated with cellular proliferation) in lung cancer cells. Similarly, the number of invasive cells significantly decreased after transfection with pcDNA-TINCR compared to an empty vector in A549 human lung cancer cells. To further investigate the molecular mechanisms associated with TINCR in correlation with lung cancer, RNA immunoprecipitation assays revealed an interaction of TINCR with miR-544a in lung cancer tissues [17]. Luciferase transcription-reporter assays revealed a mechanism where TINCR acted as a molecular sponge for miR-544a to regulate its expression. Additionally, miR-544a upregulation reversed the inhibition of TINCR on proliferation and invasion of lung cancer cells, while miR-544a inhibition decreased the promotion effects of TINCR. MiR-544 was previously reported to act as an oncogene and was found to increase cancer progression in several types of cancers [45]. In addition, the authors demonstrated that TINCR regulated the expression of the F-box and WD repeat domain-containing 7 (FBXW7) gene by competitively binding to miR-544a, and exerting its tumor-suppressive functions in lung cancer [17]. FBXW7 is a cell-cycle regulatory gene, which forms a ligase complex with Rictor (Rapamycin-insensitive companion of mTOR) that controls the ubiquitin-proteasome system (UPS) for the production and inhibition of cell cycle regulators, such as c-Myc and Cyclin E [46, 47] (see Figure 2). The downregulated expression of TINCR and FBXW7 was accompanied by enhanced cell proliferation and invasion in lung cancer, which led to cell-cycle activation and acceleration of the cells from the G1 to S phase in lung cancer cells. In contrast, the presence of TINCR enhanced the expression of FBXW7 by sponging miR-544a [17] (Figure 2). Overall, the above results suggested that decreased expression of TINCR enhanced the cellular proliferation and invasion through regulating the miR-544a/FBXW7 signaling axis, and may serve as a potential biomarker and therapeutic target for lung cancer.
In contrast to the above study, Zhu et al. (2018) reported that TINCR acted as an oncogene in non-small cell lung cancer (NSCLC) tissues, where the higher expression (~1.76-fold) was correlated with advanced tumor node metastasis stages, tumor size, metastasis, and poor survival in NSCLC patients [16]. Further, the authors demonstrated that higher levels of TINCR promoted migration and cell viability in low invasive 95C lung cancer cells. Mechanistically, TINCR was shown to physically interact with B-Raf Proto-oncogene (BRAF) at its N-terminal domain, referred to as the BRAF-specific region (BSR) [48]. The TINCR-BRAF interaction resulted in the activation of BRAF-kinase activity, which ultimately induced the MEK/ERK pathway and specially activated the MEK1/2 protein. MEK1/2 phosphorylation can trigger the oncogenic mitogen-activated protein kinase (MAPK) pathway, contributing to NSCLC oncogenesis. These results are contradictory to data presented by Liu et al. (2018), where they have demonstrated a tumor-suppressive role of TINCR in lung cancer [16, 17]. Thus, further studies are warranted prior to determining conclusions based on the clinical relevance of TINCR in lung cancer.
HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related deaths worldwide [49]. Recent studies have demonstrated the diagnostic and prognostic potential of miRNAs and lncRNAs as a novel biomarkers for the timely, noninvasive diagnosis of HCC [50]. Additionally, Tian et al., (2017) measured the expression levels of TINCR by qRT-PCR in 248 human HCC samples and their matched adjacent healthy tissues, and found significant upregulation (~8.7-fold) of TINCR in HCC tissues (Table 1) compared to healthy control samples [18]. Furthermore, the authors observed that upregulated expression of TINCR was positively correlated with enhanced tumor size, vascular invasion, and TNM stage. Additionally, HCC patients with higher TINCR expression demonstrated poorer overall survival compared to patients with lower TINCR expression, suggesting potential clinical significance [18]. The crosstalk between TINCR and miRNAs was analyzed by using the miRWalk and StarBase algorithm software programs. Additionally, employing dual-luciferase reporter assays, it was concluded that miR-137 and miR-133a significantly suppressed (~50%) the luciferase activity of pMirGLO-TINCR-WT when co-transfected with a -miR-137/miR-133a mimic in HUH7 HCC cell lines. In addition, the expression of TINCR was significantly inhibited in HUH7 cells when co-transfected with -miR-137/133a. These results suggested that TINCR expression was directly regulated by -miR-137/133a (Figure 2). The above results suggest that TINCR may provide a novel biomarker for HCC diagnosis and prognosis, which can be further explored as a potential therapeutic target; however, further investigation is needed for clinical application.
ESOPHAGEAL CANCER
Based on the histopathological data, esophageal cancer (EC) is often classified into two subtypes; esophageal adenocarcinoma and esophageal squamous cell carcinoma (ESCC). To elucidate the underlying mechanisms involved in the initiation and progression of EC, several lncRNAs were studied, such as POU3F3 [51], SPRY4-IT1 [52], FOXCUT [53] and HOTAIR [19, 54]. Similarly, TINCR, as a recently discovered regulatory molecule has been studied for the diagnosis and treatment of ESCC. Xu et al. (2016) analyzed the levels of TINCR in 56 ESCC patients using real-time PCR, where they found ~77% of specimens showed overexpression (~14-fold) of TINCR compared to healthy control samples [19] (Table 1). However, the authors did not report correlations between elevated TINCR expression levels and clinicopathological parameters such as age, family history, tumor size, tumor differentiation, cancer localization, invasion, or lymph node metastasis [19]. The authors also studied the loss and gain of function of TINCR in ESCC cell lines, where they found that an elevated level of TINCR promoted cell-cycle alterations, cell proliferation, migration, invasion, and inhibition of apoptosis in esophageal cancer cells [19]. Mechanistically, it was observed that TINCR regulated the expression of several cancer-associated molecules, e.g., claudin 7 (CLDN7) and Annexin-1 (ANAX1) mRNA levels were found to be positively corelated with an increased expression of TINCR in ESCC patients. The CLDN7 protein is involved in the production of tight junctions between epithelial cells [55], and the ANAX1 protein binds P65 and inhibits the NF-kB signaling pathway, resulting in the promotion of ESCC cell proliferation, and avoidance of apoptosis [56] (Figure 2). Since TINCR plays a direct role in the expression of these genes, perhaps these genes could be utilized in combination with TINCR as potential diagnostic and therapeutic biomarkers/targets for ESCC detection and treatment.
COLORECTAL CANCER
Despite recent advances in the available treatments, the 5-year survival rate for metastatic colorectal cancer (CRC) patients remains critically low (~10%) [57]. Studies have reported that several lncRNAs play critical roles in the regulation of pathways involved in in CRC [57]. In this regard, Zhang et al. (2016) observed a downregulated profile of TINCR (~1.5-fold) in CRC tissue when compared to adjacently paired healthy non-tumor tissues obtained from 44 CRC patients [58] (Table 1). Additionally, TINCR expression levels were significantly downregulated (~6-fold) in malignant LoVo, RKO, and SW620 human CRC cell lines when compared with non-malignant CRC cell lines such as HCT116, SW480, and LS174T. Of clinical relevance, TINCR expression was negatively correlated with serosal invasion, lymph metastasis, and tumor node metastasis, while dysregulated TINCR levels were positively correlated with differentiation, suggesting the significance of TINCR in CRC metastasis. The ectopic expression of TINCR in LoVo and RKO CRC cells demonstrated a significant decrease in the rate of proliferation and migration, while the knockdown of TINCR resulted in the opposite effects in HCT116 and SW480 cells [58]. Additionally, downregulated TINCR in HCT116 cells resulted in an increased proportion of cells in S-phase. Zhang et al. (2016) demonstrated that the upregulation of TINCR induced early apoptosis in RKO cells, and the upregulation of TINCR was associated with increased caspase-3 and caspase-9 proapoptotic protein levels. At a mechanistic level, TINCR specifically bound to the epithelial cell adhesion molecule (EpCAM), a 40 kD cell surface glycoprotein, and protected it from proteolytic cleavage. In contrast, downregulation of TINCR increased the availability of EpCAM, which ultimately led to the hydrolysis of EpCAM and resulted in the release of the EpCAM c-terminal, intracellular domain (EpICD) catalyzed by presenilin-2 [59] (Figure 2). The presence of excess EpICD was found to be associated with the activation of the WNT/β-catenin signaling pathway, where it interacted with FHL2 (four and a half LIM domains 2), Lef-1 (lymphoid enhancer-binding factor 1), and β-catenin, which forms a nuclear protein complex [60]. Zhang et al. (2016) also demonstrated the presence of a positive feedback loop between c-Myc and TINCR in CRC [58]. Additionally, Zhang et al. (2019) evaluated the expression of TINCR in 19 human CRC tissue samples and found it to be downregulated by ~1.3-fold compared to adjacent healthy tissues [20]. Several lncRNAs, including TINCR, are known to act as competitive endogenous RNAs (ceRNAs) by harboring multiple miRNA binding sites, thereby regulating their downstream targets by “sponging” those miRNAs. For example, TINCR has been shown to sponge miR-107 [20], which correlated with the increased progression of CRC. TINCR sponging has also been found to be associated with the activation of the CD36 gene, also known as a fatty acid translocase, which plays a critical role in various cancers, including mammary cancer and hepatocellular carcinoma [61]. Furthermore, CD36 activation can promote peroxisome proliferators-activated receptor (PPAR) signaling via PPAR-delta [20]. PPAR-delta, a component of the PPAR signaling cascade, can hinder the EMT process by increasing the protein levels of E-cadherin and EZR (Ezrin-Protein) [62]. EZR, a potential element of the Ezrin-Radixin Moesin (ERM) complex, is involved in connecting plasma membranes to the actin cytoskeleton [63]. Previous studies found that the increased expression of EZR by PPAR-delta inhibited EMT and metastasis in CRC [64, 65]. Thus, the TINCR/miR-107/CD36 interaction can dysregulate the PPAR signaling pathway, which may contribute to the development of CRC [20] (Figure 2).
In contrast to its tumor-suppressive association, TINCR has been reported to play an oncogenic role in CRC [21]. Yu et al. (2019) detected an upregulation (~5-fold) of TINCR in CRC cells and tissue samples relative to healthy colon cells and tissue samples. Based on chromatin immunoprecipitation (ChIP) and luciferase reporter assays, Yu et al. (2019) observed that the Sp1 transcription factor bound to the promoter region (−163 bp to −153 bp) of TINCR, and induced its transcription. They also identified interactions between TINCR and miR-7–5p through RNA pull-down by employing RNA immunoprecipitation (RIP) assays [21]. Furthermore, they found that the downregulation of miR-7–5p could partially abrogate the decrease in proliferation, migration, and invasion by TINCR downregulation. The Sp1-induced TINCR was able to target miR-7–5p, which acts as a tumor suppressor and promotes cell-cycle arrest and inhibits cell proliferation and metastasis in CRC. MiR-7–5p targets the 3’UTR of the epidermal growth factor receptor (EGFR) gene, which downregulates PIK3CD, a critical component of the PI3K pathway [66]. Similarly, miR-7–5p-mediated dysregulation of PIK3CD leads to reduced levels of mTOR and p-mTOR [66, 67]. Furthermore, miR-7–5p inhibits Akt and pAkt protein levels through targeting the 3’UTR of the IRS2 gene (Insulin Receptor Substrate 2), an upstream regulator of the Akt pathway (Fang et al., 2012). Thus, TINCR, via sponging of miR-7–5p, can promote cell proliferation, migration, invasion, and metastasis of CRC cells via the PI3K/Akt/mTOR signaling pathway [21] (Figure 2). These findings reveal a role of TINCR as a regulator of CRC progression and shed new light on our understanding of TINCR-mediated malignancy. In addition to aberrant expressional levels, TINCR gene polymorphisms can contribute to CRC susceptibility and progression. Zheng et al., 2017 identified two SNPs, rs2288947 and rs8105637, in the TINCR gene that were significantly associated with CRC susceptibility. In rs2288947, allele G correlated with a decreased risk (~23%) of CRC (OR=0.77; 95% CI=0.67–0.88; P-value=1.2×10−4), and lymph node metastasis (OR=0.77; 95% CI=0.63–0.94; P-value=0.011); whereas, with rs8105637, allele A correlated with an increased risk (~22%) of CRC (OR=1.22; 95% CI=1.09–1.37; P-value=6.2×10−4), and lymph node metastasis (OR=1.22; 95% CI=1.03–1.43; P-value=0.019) [22], demonstrating that TINCR polymorphisms could impact CRC etiology and metastasis.
Taken together, the results described above suggest that depending upon genetic variability of patients, TINCR can play either either a tumor suppressive or oncogenic role in CRC. Thus, further studies on the multiple roles of TINCR in CRC are warranted to determine its potential as a biomarker and/or therapeutic target for CRC.
BLADDER CANCER
Bladder cancer (BCa) accounts for ~95% of urothelial carcinomas, making BCa the most malignant cancer of the urinary tract [68]. Global statistics show that over a million new cases and ~15,000 mortalities have been reported annually [69, 70]. Despite recent advances in novel prognostic and diagnostic methods for BCa patients, frequent recurrence and metastasis persist [70]. Several studies have validated a significant correlation between BCa and dysregulation and various lncRNAs, such as H19 [71–73], PVT1 [74], SUMO1P3 [75].
Chen et al. (2016) have measured an overexpression (~2.3-fold) of TINCR in BCa tissues compared to paired healthy tissues. Additionally, overexpression of TINCR was associated with TNM staging, cell proliferation, and inhibition of apoptosis via obstruction of caspase 3 in SW780 and 5637 human BCa cell lines [24]. When a theophylline-RNAi “ON” and “OFF” switch was constructed to regulate TINCR expression levels in human SW780 and 5637 BCa cells, the authors observed an inhibition of cell proliferation and an increase in apoptosis, when TNCR expression was turned off [24]. However, the authors were unable to observe any correlations between TINCR upregulation and clinicopathological features of patients, such as gender, age, tumor size, histological grade, and lymph node metastasis [24].
Taken together, these findings suggest that TINCR plays an oncogenic role in BCa. Moreover, the theophylline-RNAi “ON”/”OFF” system may provide a novel approach toward effective therapy for BCa. However, further studies are warranted to understand the underlying molecular mechanisms of BCa etiology to develop improved treatments.
PROSTATE CANCER
Major advancements have been made in the development of novel prognostic biomarkers for prostate cancer (PCa), such as serum levels of prostate-specific antigen (PSA) and Gleason score (>7 for high-risk patients) [76, 77]. However, despite these recent improvements, the overall survival rate of PCa patients is ~31% in the United States [78]. Therefore, there is a compelling need to develop contemporary technology with enhanced diagnostic, prognostic, and therapeutic potential for PCa patients. In this regard, with the aid of the TCGA RNA-sequencing database, Dong et al. (2018) observed a significant downregulation (~2.5-fold) of TINCR expression in PCa tissue samples compared to healthy prostate tissue samples (N=52) [25]. This study was consistent with a previous result in 160 PCa patient specimens in which TINCR was found to be significantly downregulated (~4-fold) in prostate tumors compared to control samples [25]. Additionally, the authors observed an ~2.5-fold downregulation in human PCa cell lines (LNCaP, PC3, DU145, and 22Rv1) compared to standard human prostate epithelial cell lines (RWPE-1 and P69) [25]. To explore the relationship between TINCR expression and clinicopathological characteristics of PCa patients, it was found that decreased TINCR expression correlated with advanced TNM stages (T1-T2 vs. T3-T4: P=0.039), lymph node involvement (no versus yes: P=0.001), distant metastasis (no versus yes: P<0.001), and high Gleason score (<8 versus >8: P=0.003). However, no significant correlations were found between decreased TINCR expression levels and age, smoking status, or levels of serum prostate-specific antigen of PCa patients [25].
Mechanistically, TINCR was found to associate with thyroid hormone receptor-interacting protein 13 (TRIP13) mRNA. TRIP13 functions as an oncogene in multiple tumors [79, 80], and a negative association of TINCR expression with TRIP13 mRNA and protein expression in 374 PCa tissue samples was found using the TCGA database. Further, the author explored the role of TRIP13 in modulating cell proliferation, migration, and invasive potential in LNCaP and DU145 cells (Table 1 and Figure 2), and decreased cell proliferation, migration, and invasion occurred in DU145 cells transfected with pSilencer-TINCR and siRNA-TRIP13. In contrast, no significant alteration was observed in LNCaP cells [25].
The results discussed above may aide in the development of more effective strategies for the treatment of PCa by targeting both TINCR and TRIP13. However, further studies are required to better understand the underlying mechanisms involved.
GASTRIC CANCER
Gastric cancer (GC) has been identified as the fifth most commonly diagnosed cancer type, and the third leading cause of cancer death worldwide. [81]. A lack of early signs and symptoms leads to a poor prognosis, with a devastating 5-survival rate of only ~25% [82]. Unfortunately, genetic factors and distinct mechanisms contributing to the pathogenesis of GC remain largely unknown. However, recent studies have demonstrated a correlation between GC and TINCR. For instance, Chen et al. (2017) have studied the role(s) of TINCR in GC, where it functions as a competing endogenous RNA and regulates the expression of a 3-phosphoinositide-dependent protein kinase 1 (PDK1) by sponging miR-375 [26]. Chen et al. (2017) further analyzed the function of TINCR and PDK1 via qRT-PCR and western blotting, and found that TINCR levels were significantly upregulated (~2.5-fold) in GC tissue compared to non-tumorous tissue. Furthermore, altered expression of TINCR negatively regulated the expression of miR-375, where miR-375 elevated the expression of the PDK1 gene in HGC-27 and SNU-1 GC cells. It is thought that PDK1 can inhibit apoptosis by suppressing the expression of caspases 3 and 9 in GC cells [26, 83].
Xu et al. (2015) described a role for TINCR in tumor growth and GC development, where the Sp1 transcription factor was shown to induce the expression of TINCR by binding to its three GC-rich putative sites [(−163 to −153 bp (E1), −88 to −78 bp (E2) and −16 to −6 bp (E3)]. Additionally, luciferase-reporter transcription assays confirmed the abrogation of transcriptional activation of TINCR via the deletion of the Sp1-binding motif E1 [29]. Additional data have validated roles of TINCR in cell-cycle progression and deregulation of apoptosis by affecting the expression levels of KLF2, CDKN1A/P21 and CDKN2B/P15 [29]. Based on these findings, TINCR demonstrated the regulatory potential to modulate CDKN1A/P21 and CDKN2B/P15 gene expression after the degradation of KLF2 mRNA, which in turn resulted in GC cell growth by inhibiting the G1-phase arrest and promoting S-phase (Figure 2).
Similarly, Xu et al. (2017) assessed the correlation between TINCR and the E2F1 transcription factor, where E2F1 upregulated TINCR expression in GC. Functionally, ectopic expression of E2F1 promoted the cell cycle progression at the G1-S phase by binding to the promoter region of TINCR (+56 bp to +73 bp), and thus promoting transcriptional activation of TINCR [27].
Overall, the above findings suggest that TINCR is involved in GC etiology and might serve as a potential target in the treatment of GC. However, further studies are warranted to identify the molecular cross-talk between TINCR and distinct genes/proteins/transcription factors that contribute to mechanistic alterations involved in GC pathogenesis before TINCR may be considered as a potential biomarker for early diagnosis and/or treatment of GC.
ORAL SQUAMOUS CELL CARCINOMA
Despite recent advancements in novel diagnostic and prognostic techniques, early diagnosis of oral squamous cell carcinoma (OSCC) remains poor. Therefore, there is a need to discover new molecular markers for the early diagnosis of OSCC and/or serve as targets for therapy. LncRNAs can act as potential biomarkers and specific targets in the diagnosis and treatment of OSCC; for example, lncRNAs that have been identified as contributors of OSCC development include OIP5-AS1 [84], TUG1 [85], MEG3 [86], CASC2 [87] and HNF1A-AS1 [88].
Chen et al. (2019) demonstrated an association of TINCR as an oncogenic lncRNA promoting cell proliferation and invasion via regulation of the Wnt/β-catenin signaling pathway in OSCC [30]. The overexpression of TINCR (~2.5-fold) was regulated by lysine 27 acetylation on histone H3 (H3K27ac), where cAMP-response element-binding protein (CBP) facilitated enhanced acetylation of H3K27. Further, Creb Binding Protein (CBP) expression was upregulated in OSCC cells compared to HOK (Human Oral Keratinocytes) control cells. Consistent with this, treatment of human CAL-27 and SCC-9 OSCC cells with C646, a histone acetyltransferase (HAT) inhibitor, resulted in decreased levels of H3K27 acetylation, which corelated with decreased level of TINCR [30]. Atypical expression of TINCR in OSCC cells was found to promote cellular proliferation, which was measured by MTT assays in human SCC-9 and CAL-27 cells. Additionally, immunofluorescence results confirmed an increase in the Ki-67 protein, a well-known proliferation marker [89], and TINCR knockdown experiments significantly suppressed the invasive capacity of the CAL-27 and SCC-9 cells in a matrigel assay [89], highlighting the oncogenic role of TINCR in OSCC.
TINCR has also been shown to enhance tumor growth and metastasis in OSCC via upregulation of the Wnt/β-catenin signaling pathway. CBP, which increased H3K27 acetylation, also interacted with Wnt/β-catenin, where it functioned as a co-activator of Wnt/β-catenin-mediated transcription. Once Wnt was activated, it deactivated the β-catenin destruction complex and promoted β-catenin stability and nuclear translocation by forming a β-catenin-T-cell factor-4 (TCF-4) complex that transcriptionally activated the expression of Wnt/β-catenin signaling genes, such as Matrix Metallopeptidase-7 (MMP-7), Matrix Metallopeptidase-9 (MMP-9) and Cyclin D1 [30, 90, 91]. Therefore, TINCR-mediated activation of Wnt/β-catenin signaling promoted cell proliferation and metastasis, which is associated with poor prognosis of OSCC patients (Figure 2).
The involvement of TINCR as an oncogene in OSCC via manipulating different molecular mechanisms such as Wnt/β-catenin signaling can favor tumor growth and metastasis of OSCC cells. Thus, TINCR may provide a therapeutic target and/or may serve as a potential biomarker for the early prognosis of OSCC.
CONCLUSIONS AND PERSPECTIVES
Studies in the recent past have demonstrated the oncogenic and tumor suppressing potential of TINCR and its associated impact on various hallmarks of cancer such as cell survival and proliferation, invasion and metastasis, apoptosis, and drug responsiveness. Interestingly, TINCR has been reported to have tumor suppressor and/or oncogenic functions in cells. This may be accounted for a variety of factors, including tissue-specific functions, cell type, and/or genetic and epigenetic differences in patients. Based on the literature presented herein, we found that hepatocellular and esophageal carcinoma are best suited for novel diagnostic procedures using TINCR as a molecular marker. Additionally, Tian et al. (2017) and Xu et al. (2016) demonstrated its diagnostic potential in HCC and ESCC employing 248 and 56 tumor samples, respectively, where TINCR levels were highly upregulated in HCC (~8.7-fold) and ESCC (~14-fold). Conflicting results were observed in correlation studies of lung cancer with TINCR levels, where the study performed by Liv et al. (2018) demonstrated a downregulated profile (~2.9-fold), and that by Zhu et al. (2018), which demonstrated an upregulation of TINCR levels (~1.76-fold) in lung cancer patients [16]. These different profiles may be a result of varying population profiles and differences in patient genetics, epigenetics, and exposure to tobacco. Additionally, Tian et al. (2017) and Xu et al. (2016) were able to validate strong correlations between TINCR dysregulation and clinicopathological features, such age, gender, lymph node metastasis, Ki-67 level, histological grade, tumor size and tumor node metastasis stage in breast and bladder [11, 12]. Furthermore, TINCR has been found to regulate multiple genes and interlinking signaling patways associated with a variety of cancers (Figure 2). However, based on the pleiotropic nature of TINCR dysregulation, targeting TINCR may result in toxicity to both healthy normal cells and malignant cells. Therefore, additional studies are warranted to determine the potential of TINCR as a therapeutic target. Additionally, despite the recent advances in RNA biology, limitations such as the high cost of transcriptome analysis and sequencing of individual lncRNAs need to be addressed prior to its use for clinical applications. We are optimistic that this review will allow a better understanding of TINCR and its association with several cancers to act as a stepping stone for the future clinical application of this lncRNA as a diagnostic and/or therapeutic target for cancer patients.
Highlights:-.
Long non-coding RNA TINCR dysregulated in human cancers
TINCR modulates various hallmarks of cancer through targeting pivotal signaling pathways
TINCR possesses the therapeutic potential and may act as a promising biomarker for the diagnosis and prognosis of human cancers
Acknowledgements:
Funding-
AJ is thankful to the Department of Biotechnology, India for providing grant (6242-P30/RGCB/PMD/DBT/AKJN/2015), the Indian Council of Medical Research (5/13/81/2013-NCD-III), and an NIH/NCI grant to KMV (CA093729). Uttam Sharma is thankful to the Department of Science and Technology for DST-INSPIRE fellowship (IF180680)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest
Ethical approval: Since it’s a review article, therefore, this article does not contain any studies with human participants or animals performed by any of the authors.
References:
- [1].Castro-Giner F and Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med, 2020, 12(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gonzalez H, et al. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev, 2018, 32(19–20): 1267–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sever R and Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med, 2015, 5(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rinn JL and Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem, 2012, 81: 145–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Birney E, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature, 2007, 447(7146): 799–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li T, et al. Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget, 2016, 7(8): 8601–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rad SA, et al. Type 2 IDI performs better than type 1 for improving lycopene production in metabolically engineered E. coli strains. World J Microbiol Biotechnol, 2012, 28(1): 313–21 [DOI] [PubMed] [Google Scholar]
- [8].Tamang S, et al. SNHG12: An LncRNA as a Potential Therapeutic Target and Biomarker for Human Cancer. Front Oncol, 2019, 9: 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Malhotra A, et al. The regulatory roles of long non-coding RNAs in the development of chemoresistance in breast cancer. Oncotarget, 2017, 8(66): 110671–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Khandelwal A, et al. The emerging role of long non-coding RNA in gallbladder cancer pathogenesis. Biochimie, 2017, 132: 152–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tian T, et al. The Impact of lncRNA Dysregulation on Clinicopathology and Survival of Breast Cancer: A Systematic Review and Meta-analysis. Mol Ther Nucleic Acids, 2018, 12: 359–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xia Y, et al. The prognostic significance of long noncoding RNAs in bladder cancer: A meta-analysis. PLoS One, 2018, 13(6): e0198602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kretz M, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature, 2013, 493(7431): 231–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dong H, et al. Activation of LncRNA TINCR by H3K27 acetylation promotes Trastuzumab resistance and epithelial-mesenchymal transition by targeting MicroRNA-125b in breast Cancer. Mol Cancer, 2019, 18(1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [15].Liu Y, et al. Up-regulation of ceRNA TINCR by SP1 contributes to tumorigenesis in breast cancer. BMC Cancer, 2018, 18(1): 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhu ZJ and He JK. TINCR facilitates non-small cell lung cancer progression through BRAF-activated MAPK pathway. Biochem Biophys Res Commun, 2018, 497(4): 971–7 [DOI] [PubMed] [Google Scholar]
- [17].Liu X, et al. TINCR suppresses proliferation and invasion through regulating miR-544a/FBXW7 axis in lung cancer. Biomed Pharmacother, 2018, 99: 9–17 [DOI] [PubMed] [Google Scholar]
- [18].Tian F, et al. TINCR expression is associated with unfavorable prognosis in patients with hepatocellular carcinoma. Biosci Rep, 2017, 37(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu Y, et al. Long noncoding RNA, tissue differentiation-inducing nonprotein coding RNA is upregulated and promotes development of esophageal squamous cell carcinoma. Dis Esophagus, 2016, 29(8): 950–8 [DOI] [PubMed] [Google Scholar]
- [20].Zhang X, et al. LncRNA TINCR/microRNA-107/CD36 regulates cell proliferation and apoptosis in colorectal cancer via PPAR signaling pathway based on bioinformatics analysis. Biol Chem, 2019, 400(5): 663–75 [DOI] [PubMed] [Google Scholar]
- [21].Yu S, et al. SP1-induced lncRNA TINCR overexpression contributes to colorectal cancer progression by sponging miR-7–5p. Aging (Albany NY), 2019, 11(5): 1389–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zheng Y, et al. Genetic variation of long non-coding RNA TINCR contribute to the susceptibility and progression of colorectal cancer. Oncotarget, 2017, 8(20): 33536–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang ZY, et al. Loss of TINCR expression promotes proliferation, metastasis through activating EpCAM cleavage in colorectal cancer. Oncotarget, 2016, 7(16): 22639–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Z, et al. Theophylline controllable RNAi-based genetic switches regulate expression of lncRNA TINCR and malignant phenotypes in bladder cancer cells. Sci Rep, 2016, 6: 30798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dong L, et al. LncRNA TINCR is associated with clinical progression and serves as tumor suppressive role in prostate cancer. Cancer Manag Res, 2018, 10: 2799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen Z, et al. The long noncoding RNA, TINCR, functions as a competing endogenous RNA to regulate PDK1 expression by sponging miR-375 in gastric cancer. Onco Targets Ther, 2017, 10: 3353–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu TP, et al. E2F1 induces TINCR transcriptional activity and accelerates gastric cancer progression via activation of TINCR/STAU1/CDKN2B signaling axis. Cell Death Dis, 2017, 8(6): e2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma X, et al. Tag SNPs of long non-coding RNA TINCR affect the genetic susceptibility to gastric cancer in a Chinese population. Oncotarget, 2016, 7(52): 87114–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xu TP, et al. SP1-induced upregulation of the long noncoding RNA TINCR regulates cell proliferation and apoptosis by affecting KLF2 mRNA stability in gastric cancer. Oncogene, 2015, 34(45): 5648–61 [DOI] [PubMed] [Google Scholar]
- [30].Chen F, et al. lncRNA PLAC2 activated by H3K27 acetylation promotes cell proliferation and invasion via the activation of Wnt/betacatenin pathway in oral squamous cell carcinoma. Int J Oncol, 2019, 54(4): 1183–94 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [31].Chen L, et al. Trends in 5-year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res Treat, 2014, 147(3): 609–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peairs KS, et al. Screening for breast cancer. Semin Oncol, 2017, 44(1): 60–72 [DOI] [PubMed] [Google Scholar]
- [33].Cao Y and Giovannucci EL. Alcohol as a Risk Factor for Cancer. Semin Oncol Nurs, 2016, 32(3): 325–31 [DOI] [PubMed] [Google Scholar]
- [34].Nik-Zainal S, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature, 2016, 534(7605): 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li W, et al. Downregulation of LncRNA GAS5 causes trastuzumab resistance in breast cancer. Oncotarget, 2016, 7(19): 27778–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xue X, et al. LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene, 2016, 35(21): 2746–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cai Y, et al. [Suppression of long non-coding RNA CCAT2 improves tamoxifen-resistant breast cancer cells’ response to tamoxifen]. Mol Biol (Mosk), 2016, 50(5): 821–7 [DOI] [PubMed] [Google Scholar]
- [38].Si X, et al. LncRNA H19 confers chemoresistance in ERalpha-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget, 2016, 7(49): 81452–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu S, et al. Oncogenic long noncoding RNA landscape in breast cancer. Mol Cancer, 2017, 16(1): 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Reddy SD, et al. MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res, 2008, 68(20): 8195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Scott GK, et al. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J Biol Chem, 2007, 282(2): 1479–86 [DOI] [PubMed] [Google Scholar]
- [42].Kaufhold S and Bonavida B. Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention. J Exp Clin Cancer Res, 2014, 33: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Clarke M, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet, 2005, 366(9503): 2087–106 [DOI] [PubMed] [Google Scholar]
- [44].Khandelwal A, et al. Long non-coding RNA: A new paradigm for lung cancer. Mol Carcinog, 2015, 54(11): 1235–51 [DOI] [PubMed] [Google Scholar]
- [45].Pan C, et al. MiR-544 promotes immune escape through downregulation of NCR1/NKp46 via targeting RUNX3 in liver cancer. Cancer Cell Int, 2018, 18: 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Guo Z, et al. Rictor regulates FBXW7-dependent c-Myc and cyclin E degradation in colorectal cancer cells. Biochem Biophys Res Commun, 2012, 418(2): 426–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tu K, et al. Evaluation of Fbxw7 expression and its correlation with the expression of c-Myc, cyclin E and p53 in human hepatocellular carcinoma. Hepatol Res, 2012, 42(9): 904–10 [DOI] [PubMed] [Google Scholar]
- [48].Lavoie H and Therrien M. Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol, 2015, 16(5): 281–98 [DOI] [PubMed] [Google Scholar]
- [49].Yang JD, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].He Y, et al. Long noncoding RNAs: Novel insights into hepatocelluar carcinoma. Cancer Lett, 2014, 344(1): 20–7 [DOI] [PubMed] [Google Scholar]
- [51].Hu HB, et al. Three Circulating LncRNA Predict Early Progress of Esophageal Squamous Cell Carcinoma. Cell Physiol Biochem, 2016, 40(1–2): 117–25 [DOI] [PubMed] [Google Scholar]
- [52].Zhang HM, et al. High expression of long non-coding RNA SPRY4-IT1 predicts poor prognosis of clear cell renal cell carcinoma. Int J Clin Exp Pathol, 2014, 7(9): 5801–9 [PMC free article] [PubMed] [Google Scholar]
- [53].Pan F, et al. A novel long non-coding RNA FOXCUT and mRNA FOXC1 pair promote progression and predict poor prognosis in esophageal squamous cell carcinoma. Int J Clin Exp Pathol, 2014, 7(6): 2838–49 [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang S, et al. Long noncoding RNA HOTAIR as an independent prognostic marker in cancer: a meta-analysis. PLoS One, 2014, 9(8): e105538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gonzalez-Mariscal L, et al. Tight junction proteins. Prog Biophys Mol Biol, 2003, 81(1): 1–44 [DOI] [PubMed] [Google Scholar]
- [56].Zhang Z, et al. Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and inhibits its activation: anticancer effects in vitro and in vivo. Cancer Res, 2010, 70(6): 2379–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Roncucci L and Mariani F. Prevention of colorectal cancer: How many tools do we have in our basket? Eur J Intern Med, 2015, 26(10): 752–6 [DOI] [PubMed] [Google Scholar]
- [58].Zhang ZY, et al. Loss of TINCR expression promotes proliferation, metastasis through activating EpCAM cleavage in colorectal cancer. Oncotarget, 2016, 7(16): 22639–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schnell U, et al. EpCAM proteolysis: new fragments with distinct functions? Biosci Rep, 2013, 33(2): e00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Maetzel D, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol, 2009, 11(2): 162–71 [DOI] [PubMed] [Google Scholar]
- [61].Nath A, et al. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep, 2015, 5: 14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Milone MR, et al. Proteomic characterization of peroxisome proliferator-activated receptor-gamma (PPARgamma) overexpressing or silenced colorectal cancer cells unveils a novel protein network associated with an aggressive phenotype. Mol Oncol, 2016, 10(8): 1344–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gautreau A, et al. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol, 2000, 150(1): 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Haynes J, et al. Dynamic actin remodeling during epithelial-mesenchymal transition depends on increased moesin expression. Mol Biol Cell, 2011, 22(24): 4750–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang D and Dubois RN. Peroxisome proliferator-activated receptors and progression of colorectal cancer. PPAR Res, 2008, 2008: 931074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fang Y, et al. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology, 2012, 55(6): 1852–62 [DOI] [PubMed] [Google Scholar]
- [67].Trinh XB, et al. The VEGF pathway and the AKT/mTOR/p70S6K1 signalling pathway in human epithelial ovarian cancer. Br J Cancer, 2009, 100(6): 971–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Quan J, et al. LncRNA as a diagnostic and prognostic biomarker in bladder cancer: a systematic review and meta-analysis. Onco Targets Ther, 2018, 11: 6415–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rui X, et al. LncRNA GAS6-AS2 promotes bladder cancer proliferation and metastasis via GAS6-AS2/miR-298/CDK9 axis. J Cell Mol Med, 2019, 23(2): 865–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tuo Z, et al. LncRNA TP73-AS1 predicts the prognosis of bladder cancer patients and functions as a suppressor for bladder cancer by EMT pathway. Biochem Biophys Res Commun, 2018, 499(4): 875–81 [DOI] [PubMed] [Google Scholar]
- [71].Luo M, et al. Upregulated H19 contributes to bladder cancer cell proliferation by regulating ID2 expression. FEBS J, 2013, 280(7): 1709–16 [DOI] [PubMed] [Google Scholar]
- [72].Luo M, et al. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett, 2013, 333(2): 213–21 [DOI] [PubMed] [Google Scholar]
- [73].Ariel I, et al. The imprinted H19 gene as a tumor marker in bladder carcinoma. Urology, 1995, 45(2): 335–8 [DOI] [PubMed] [Google Scholar]
- [74].Zhuang C, et al. Tetracycline-inducible shRNA targeting long non-coding RNA PVT1 inhibits cell growth and induces apoptosis in bladder cancer cells. Oncotarget, 2015, 6(38): 41194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhan Y, et al. Increased expression of SUMO1P3 predicts poor prognosis and promotes tumor growth and metastasis in bladder cancer. Oncotarget, 2016, 7(13): 16038–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Misawa A, et al. Long non-coding RNAs and prostate cancer. Cancer Sci, 2017, 108(11): 2107–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bill-Axelson A, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med, 2014, 370(10): 932–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review (CSR) 1975–2017, 2020. https://seer.cancer.gov/csr/1975_2017/ [Google Scholar]
- [79].Sheng N, et al. TRIP13 promotes tumor growth and is associated with poor prognosis in colorectal cancer. Cell Death Dis, 2018, 9(3): 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Dazhi W, et al. Elevated expression of thyroid hormone receptor-interacting protein 13 drives tumorigenesis and affects clinical outcome. Biomark Med, 2017, 11(1): 19–31 [DOI] [PubMed] [Google Scholar]
- [81].Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018, 68(6): 394–424 [DOI] [PubMed] [Google Scholar]
- [82].Kunz PL, et al. Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol, 2012, 30(28): 3507–15 [DOI] [PubMed] [Google Scholar]
- [83].Zhou J, et al. MicroRNA-375 targets PDK1 in pancreatic carcinoma and suppresses cell growth through the Akt signaling pathway. Int J Mol Med, 2014, 33(4): 950–6 [DOI] [PubMed] [Google Scholar]
- [84].Li M, et al. Long noncoding RNA OIP5-AS1 promotes the progression of oral squamous cell carcinoma via regulating miR-338–3p/NRP1 axis. Biomed Pharmacother, 2019, 118: 109259. [DOI] [PubMed] [Google Scholar]
- [85].Liu S, et al. Long noncoding RNA TUG1 regulates the development of oral squamous cell carcinoma through sponging miR-524–5p to mediate DLX1 expression as a competitive endogenous RNA. J Cell Physiol, 2019, 234(11): 20206–16 [DOI] [PubMed] [Google Scholar]
- [86].Tan J, et al. LncRNA MEG3 suppresses migration and promotes apoptosis by sponging miR-548d-3p to modulate JAK-STAT pathway in oral squamous cell carcinoma. IUBMB Life, 2019, 71(7): 882–90 [DOI] [PubMed] [Google Scholar]
- [87].Xing HB, et al. Long noncoding RNA CASC2 alleviates the growth, migration and invasion of oral squamous cell carcinoma via downregulating CDK1. Eur Rev Med Pharmacol Sci, 2019, 23(11): 4777–83 [DOI] [PubMed] [Google Scholar]
- [88].Liu Z, et al. STAT3-induced upregulation of long noncoding RNA HNF1A-AS1 promotes the progression of oral squamous cell carcinoma via activating Notch signaling pathway. Cancer Biol Ther, 2019, 20(4): 444–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Miller I, et al. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep, 2018, 24(5): 1105–12 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Arensman MD, et al. The CREB-binding protein inhibitor ICG-001 suppresses pancreatic cancer growth. Mol Cancer Ther, 2014, 13(10): 2303–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Chen HJ, et al. The beta-catenin/TCF complex as a novel target of resveratrol in the Wnt/beta-catenin signaling pathway. Biochem Pharmacol, 2012, 84(9): 1143–53 [DOI] [PubMed] [Google Scholar]


