Abstract
Secondary S. pneumoniae infection is a significant cause of morbidity and mortality during influenza epidemics and pandemics. Multiple pathogenic mechanisms such as lung epithelial damage, and dysregulation of neutrophils and alveolar macrophages (AMs), have been suggested to contribute to the severity of disease. However, the fundamental reasons for influenza-induced susceptibility to secondary bacterial pneumonia remain unclear. Here we revisited these controversies over key pathogenic mechanisms in a lethal model of secondary bacterial pneumonia, with a S. pneumoniae strain that is innocuous to mice in the absence of influenza infection. Using a series of in vivo models, we demonstrate that rather than a systemic suppression of immune responses or neutrophil function, influenza infection activates IFN-γ receptor (IFN-γR) signaling and abrogates alveolar macrophage (AM)-dependent bacteria clearance, and thereby causes extreme susceptibility to pneumococcal infection. Importantly, using mice carrying conditional knockout of Ifngr1 gene in different myeloid cell subsets, we demonstrate that influenza-induced IFN-γR signaling in AMs impairs their antibacterial function, thereby enabling otherwise noninvasive S. pneumoniae to cause deadly pneumonia.
Introduction
Bacterial pneumonia after influenza is a leading cause of severe respiratory infections worldwide. In particular, secondary S. pneumoniae infection is a significant cause of morbidity and mortality during influenza epidemics and pandemics (1–4). However, the key mechanism underlying this devastating disease remains incompletely understood.
Multiple studies have suggested that lung epithelial damage, directly or indirectly caused by influenza infection, promotes bacterial invasion and systemic inflammation, thereby leading to excessive mortality after secondary bacterial infection (2, 5–8). To evaluate this possibility, we have recently developed a secondary bacterial infection model using S. pneumoniae TJO983, a serotype 14 strain that is innocuous to mice even after systemic challenge (9). Considering the high prevalence of “noninvasive” S. pneumoniae serotypes in human nasal carriage (10), we believe that this model is highly clinical-relevant. Importantly, this normally innocuous S. pneumoniae strain allows us to discern the local and systemic impact of influenza infection on innate antibacterial defense.
Defective bacterial control has recently been demonstrated as a primary cause of influenza-induced susceptibility to secondary bacterial infection. Many studies have suggested neutrophil dysregulation as a critical mechanism underlying defective antibacterial immunity (11–13). Conversely, we and others have shown that influenza inhibits alveolar macrophage (AM) responsiveness, by either functional impairment or direct depletion, thereby increasing host susceptibility to secondary bacterial pneumonia (14–19). Given these apparently disparate observations, it remains to be established the impact of influenza infection on neutrophil versus AM-mediated bacterial control, and their relative contribution to severe outcomes after secondary S. pneumoniae infection.
In this study, we revisited these controversies in our new mouse models of influenza/S. pneumoniae coinfection. We demonstrate that rather than a general suppression of innate immune response or neutrophil recruitment, preceding influenza infection disrupts AM-mediated bacterial clearance in the airway, and thereby enables otherwise innocuous S. pneumoniae to cause deadly pneumonia. Furthermore, using a series of conditional knockout mouse models, we show that this functional impairment is mediated, at least partially, through influenza-induced IFN-γ receptor signaling in AMs.
Materials and Methods
Murine model of viral and bacterial infection
Specific pathogen-free, C57BL/6 WT, Csf2rb−/− (20), Ifngr1−/−, Ifngr1fl/fl (21), LysMCre (22), Cd11cCre (23), Cx3cr1Cre (24), Mrp8Cre (25), Rag1−/−, Ifng−/− and mT/mG mice (26), as well as BALB/c WT, Rag2−/−Il2rg−/−, and Csf2/Il3tm1.1(CSF2 IL3)Flv (referred to as hIl3/Csf2 KI) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred at University of Nebraska Medical Center following IACUC guidelines. CD169-DTR mice were provided by the RIKEN BRC through the National Bio-Resource Project of the Mext, Japan, with approval from Drs. Kenji Kohno and Masato Tanaka. LysMCre, Cd11cCre, Cx3cr1Cre, and Mrp8Cre reporter mice were generated by crossing mT/mG mice with corresponding Cre+ mice. LysMCreIfngr1fl/fl, Cd11cCreIfngr1fl/fl, Cx3cr1CreIfngr1fl/fl, and Mrp8CreIfngr1fl/fl mice were generated by crossing Ifngr1fl/fl mice with corresponding Cre+ mice. All animal experiments were approved by University of Nebraska Medical Center, and all experiments were carried out in accordance with University of Nebraska Medical Center Assurance of Compliance with PHS Policy on humane Care and Use of Laboratory Animals, which is on file with the Office of Protection from Research Risks, NIH.
Viral challenge was performed with a sublethal dose of PR8 (~10 PFU/mouse) administered i.n. to anesthetized, sex and age-matched adult mice in 50 μl of sterile PBS. Titers of virus stocks and viral levels in the bronchoalveolar lavage fluids (BALF) and lungs of infected mice were determined by plaque assays on MDCK cell monolayers.
To induce S. pneumoniae infection, anesthetized mice were inoculated i.n. or i.p. with 50~100 μl of PBS containing 2×104 CFU of serotype 14 strain TJO983 unless otherwise specified. Bacterial burdens in the BALF and lungs were measured by sacrificing infected mice 24 h after infection, and plating serial 10-fold dilutions of each sample onto blood agar plates.
Bronchoalveolar lavage (BAL) cell analysis
BALF samples were collected by making an incision in the trachea and lavaging the lung twice with 0.8 ml PBS, pH 7.4. Total leukocyte counts were determined using a hemacytometer.
For flow cytometry analysis, BALF were incubated with 2.4G2 mAb against FcγRII/III, and stained with APC conjugated anti-CD11c (Biolegend), BUV395-conjugated anti-CD11b (BD Biosciences), FITC-conjugated or PE-Cy7-conjugated anti-Ly6G (clone 1A8, Biolegend), PerCP-Cy5.5-conjugated (eBiosciences) or PE-conjugated anti-Ly6C (BD Biosciences), and BV421-conjugated or PE-conjugated anti-Siglec-F (BD Biosciences) mAbs. The stained cells were analyzed on a BD LSRII-green using BD FACSDiva and FlowJo software analysis.
Determination of cytokine/chemokine production by ELISA
BALF were harvested and assayed for TNF-α and IFN-γ by ELISA using commercially available kits from BD Biosciences.
AM reconstitution in Csf2rb−/− mice
For isolation of AMs, BALF cells harvested from naïve WT mice were pelleted and resuspended in PBS for adoptive transfer. Csf2rb−/− recipient mice were anesthetized and 2~3×105 AMs were administered i.n. in 100 μl PBS. Mice were used for S. pneumoniae infection more than six weeks after receiving AM transfer. Reconstitution of AMs in the BAL was confirmed by flow cytometry.
Adoptive cell transfer to Rag1−/− mice
C57BL/6 Rag1−/− mice were injected i.p. with splenocytes (2×107 cells/mouse) isolated from naïve IFN-γ−/− or WT mice and infected with PR8 10 weeks after cell transfer. Influenza-induced airway recruitment of adoptively transferred T cells was confirmed by flow cytometric analysis.
Quantitative reverse transcription (RT)-PCR
AMs were purified using a Mouse CD11c Positive Selection Kit (STEMCELL Technologies). Total RNA derived from enriched AMs was characterized by using iScript Reverse Transcription and iTaqUniversal SYBR Green Supermix (Bio-Rad) on a Bio-Rad CFXConnet Real-Time system. Hprt specific primer sets were used to normalize the expression value for the genes of interest.
Diphtheria toxin (DT) treatment
Mice were i.p. injected with 50μg/kg body weight of DT (Sigma) four days before S. pneumoniae infection. The efficiency of AM depletion in CD169-DTR mice was confirmed by flow cytometry.
Neutrophil depletion
Neutrophils were depleted using anti-Gr-1 mAb RB6–8C5 (BioXCell). Specifically, starting at one day before bacterial infection, mice were injected i.p. with anti-Gr-1 mAb RB6–8C5 (0.1 mg/day) to deplete neutrophils or with rat IgG as a control. The efficiency of neutrophil depletion in bacterial-infected mice was confirmed by flow cytometry (27).
Statistics
Significant differences between experimental groups were determined using a two-tailed Student t-test (to compare two samples), an ANOVA analysis followed by Tukey’s multiple comparisons test (to compare multiple samples) or Mann Whitney test (nonparametric test) in GraphPad Prism 6 (La Jolla, CA). Survival analyses were performed using the log-rank test. For all analyses, a P value <0.05 was considered to be significant.
Results
AMs and neutrophils are critical for innate defense against airway and systemic S. pneumoniae infection, respectively
As airway-resident macrophages, AMs are crucial for immune defense against many respiratory infections (17, 27–29). However, neutrophils are commonly considered to play a more important role in killing of extracellular bacteria such as S. pneumoniae (30–32). Accordingly, an essential role of AMs in pneumococcal clearance remains uncertain. In this study, we evaluated the relative contribution of AMs and neutrophils to bacterial control during a low dose of S. pneumoniae serotype 14 strain TJO983 (Spn) infection alone.
It is known that AMs require GM-CSF (also known as CSF2) signaling for their development (29). Indeed, AMs (CD11c+Siglec-F+) were completely absent in CSF2 receptor β chain knockout (Csf2rb−/−) mice (Fig. 1A). To determine the role of AMs in bacterial clearance, we infected WT and Csf2rb−/− mice intranasally (i.n.) with 104 CFU/mouse of Spn; and 24 h later, we detected ~104-fold increased bacterial burdens in Csf2rb−/− lungs as compared with WT controls (Fig. 1B). Of particular interest, AM reconstitution in Csf2rb−/− mice, by intranasal transfer of AMs from naïve WT mice, was sufficient to restore bacterial clearance (Fig. 1A-B). In fact, there was no significant difference in lung bacterial burdens between WT and Csf2rb−/− mice after AM reconstitution (Fig. 1B). Together, these results demonstrate that AMs are not only critical but also sufficient for Spn clearance in the lower respiratory tract.
Figure 1. AMs are essential and sufficient for airway clearance of S. pneumoniae.
(A) The mean frequency of BALF AMs (CD11c+Siglec-F+), and (B) lung bacterial burdens (mean±SD) 24 h after Spn infection of B6 WT and Csf2rb−/− mice (n ≥ 5 mice/group). Csf2rb−/−+AM: Csf2rb−/− mice received adoptive transfer of WT AMs. **, P<0.01, Mann Whitney test. (C) The mean frequency of BALF CD11c+ and CD11b+ myeloid cells, and (D) lung bacterial burdens (mean±SD) 24 h after Spn infection of BALB/c WT, Rag2−/−Il2rg−/− and hIl3/Csf2 KI mice (n ≥ 5 mice/group). **, P<0.01, Mann Whitney test. (E) Animal survival after i.n. or i.p. challenge of B6 WT mice with 105 CFU Spn. Mice were treated anti-Gr1 antibodies (α-PMN) to deplete PMNs. **P< 0.01, log-rank test. Data shown are representative of at least two independent experiments.
Rag2−/−Il2rg−/− (also known as Rag2−/−γc−/−) mice are deficient in T, B and innate lymphoid cells (33). Due to knock-in (KI) replacement of mouse GM-CSF in Rag2−/−Il2rg−/− mice (34), human Il3/Csf2 KI mice are also devoid of AMs (Fig. 1C). Similar to Csf2rb−/− mice, hIl3/Csf2 KI mice exhibited 1000-fold increased bacterial burdens 24 h after Spn infection alone, as compared with both BALB/c WT and Rag2−/−Il2rg−/− controls (Fig. 1D). These results further confirm that AMs are essential and sufficient for innate clearance of airway Spn infection. On the other hand, we have shown that antibody-mediated depletion of neutrophils in WT mice has no effect on lung bacterial control (27). All WT mice survived i.n. Spn infection despite of neutrophil depletion (Fig. 1E). In contrast, neutrophil-depleted WT mice were highly susceptible to intraperitoneal (i.p.) Spn infection (Fig. 1E). Taken together, these results suggest that although dispensable for airway bacterial control, neutrophils are essential for innate defense against systemic Spn infection.
Influenza induces lethal susceptibility to airway but not systemic S. pneumoniae super-infection
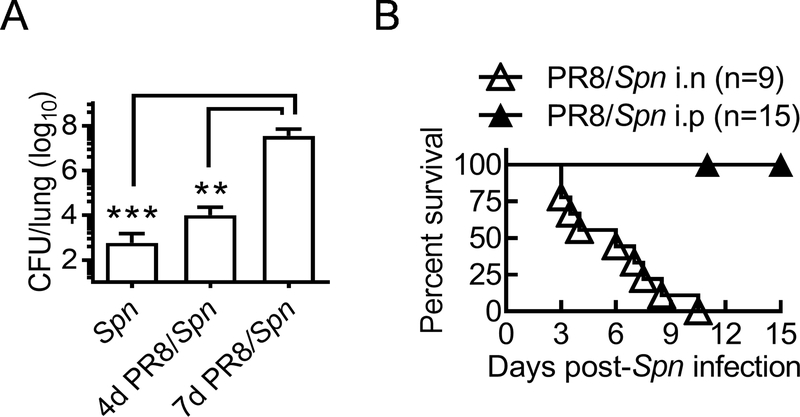
Based on the differential role of AMs and neutrophils in innate protection (Fig. 1), we next examined the impact of influenza infection on the local and systemic antibacterial defense. In agreement with our previous findings (15), on day seven after a low dose of PR8 virus challenge, WT mice become highly susceptible to Spn respiratory infection (Fig. 2A). Specifically, all day seven PR8-infected mice succumbed to i.n. Spn super-infection (Fig. 2B). In sharp contrast, all PR8-infected WT mice survived from i.p. Spn super-infection at the same time (Fig. 2B). This result suggests that neutrophil-dependent systemic antibacterial defense is relatively competent after influenza infection. Importantly, the distinct outcomes of local versus systemic Spn super-infection suggest that, rather than directly disrupting epithelial barrier to promote systemic dissemination, influenza infection impairs airway antibacterial immunity to enable otherwise noninvasive S. pneumoniae to cause lethal pneumonia.
Figure 2. Influenza induces lethal susceptibility to local but not systemic S. pneumoniae super-infection.
(A) Lung bacterial burdens (mean±SD) 24 h after i.n. challenge naïve (Spn), days 4 and 7 PR8-infected (PR8/Spn) B6 WT mice (n ≥ 4 mice/group) with Spn. (B) Survival of B6 WT mice after i.n. or i.p. super-infection with 2×104 Spn on day seven after PR8 infection. **P<0.01, ***P<0.001. Mann Whitney test. Data shown are representative of two independent experiments.
Preceding influenza infection abrogates AM-mediated bacterial clearance in the airway
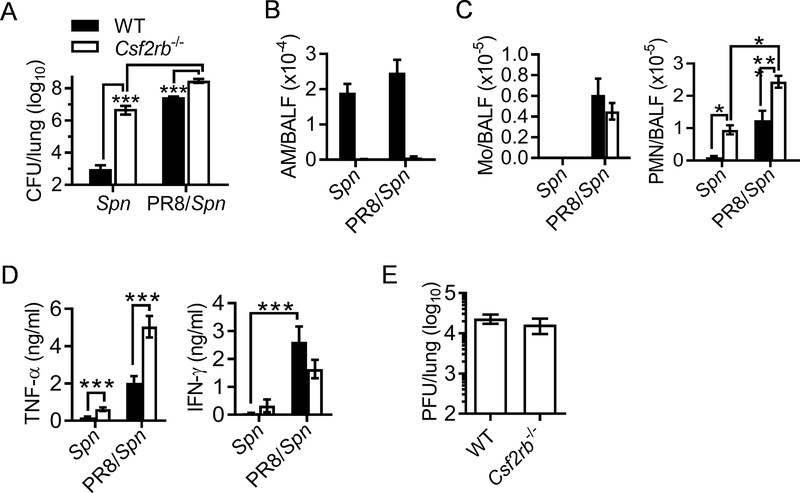
Considering that systemic antibacterial defense is relatively competent, we focused on airway antibacterial immune response to understand how influenza infection induces local susceptibility to pneumococcal infection. Using Csf2rb−/− mouse model, we first examined the impact of influenza infection on AM-associated immune responses. Due to the lack of AMs (Fig. 1A), Csf2rb−/− mice exhibited ~4-log-fold increases in lung CFU as compared with WT controls during Spn single infection (Fig. 3A). Conversely, compared with their corresponding Spn single-infected controls, PR8/Spn coinfection resulted in ~10-fold but >4-log-fold increased bacterial burdens in Csf2rb−/− and WT mice, respectively. Of particular interest, lung bacterial burdens were almost comparable between Spn single-infected Csf2rb−/− mice and PR8/Spn coinfected WT mice (Fig. 3A). These results indicate that similar to Csf2rb−/− mice devoid of AMs, AM-mediated bacterial clearance is completely absent in WT mice one week after influenza infection.
Figure 3. Influenza infection abolishes AM-mediated bacterial clearance during secondary pneumococcal infection.
(A) Lung bacterial burdens (mean±SD), (B) BALF CD11c+Siglec-F+ AM numbers (mean±SEM), (C) the numbers of CD11b+Ly6C+ monocytes (Mo) and CD11b+Ly6G+ neutrophils (PMN), (D) the levels of TNF-α and IFN-γ (mean±SEM), and (E) lung viral burdens (mean±SEM) 24 h after i.n. challenge of naïve (Spn) or day seven PR8-infected (PR8/Spn) B6 WT and Csf2rb−/− mice (n ≥ 5 mice/group) with Spn. ***P<0.001, Tukey’s multiple comparisons test. Data shown are representative of two independent experiments.
Notably, in contrast to AM-deficient Csf2rb−/− mice, the actual numbers of AMs in WT mice were not significantly affected at this early stage of coinfection (Fig. 3B), even though their percentages in the bronchoalveolar lavage fluid (BALF) decreased due to intensive inflammatory cell infiltration (Supplemental Fig. S1). Consistent with increased airway Ly6C+ monocytes and Ly6G+ neutrophils (Fig. 3C), we detected significantly increased inflammatory cytokines, particularly TNF-α and IFN-γ production after PR8/Spn coinfection (Fig. 3D). Furthermore, Csf2rb−/− mice exhibited increased TNF-α production and neutrophil recruitment after Spn single- or super-infection, as compared with corresponding WT controls (Fig. 3C-D). In line with other reported studies (29), we did not detect significant differences in viral burden between coinfected WT and Csf2rb−/− mice (Fig. 3E). Taken together, these data demonstrate that, rather than a general suppression of airway inflammatory response, influenza infection diminishes the antibacterial function of AMs, thereby leading to defective bacterial control during secondary pneumococcal infection.
A modest impairment of AM-independent bacterial clearance during influenza/Spn coinfection
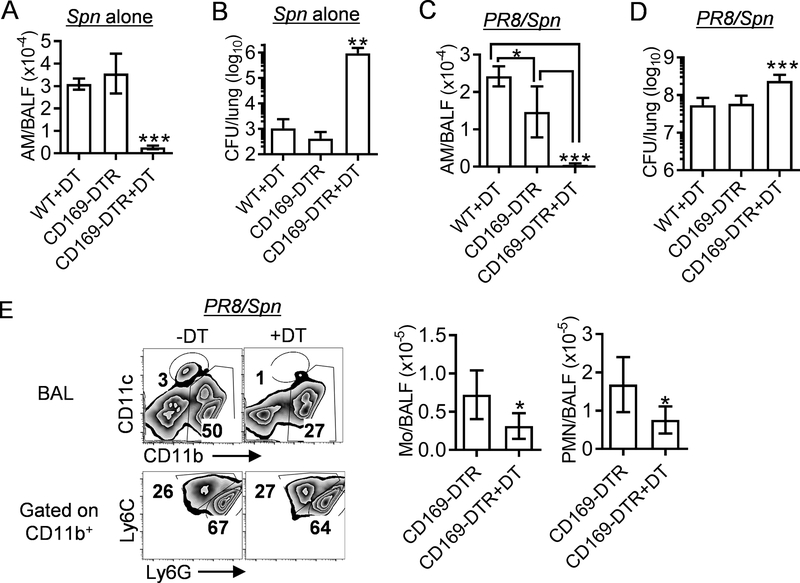
Notably, compared with Spn single-infection, Csf2rb−/− mice exhibited 10-fold further increased bacterial burdens after PR8/Spn coinfection (Fig. 3A). This increased susceptibility suggests that influenza also impairs AM-independent bacterial clearance during secondary pneumococcal infection. However, considering that a similar difference (10-fold) in lung CFU was detected between coinfected WT and Csf2rb−/− mice, it is also possible that this increased susceptibility is due to other immune defects in Csf2rb−/− mice, such as dendritic cell (DC) development. To exclude these inherent deficits, we temporarily depleted CD169+ macrophages in CD169-diphtheria toxin receptor (DTR) transgenic mice (35, 36). Similar to the key findings in Csf2rb−/− mice, diphtheria toxin (DT)-induced AM depletion in CD169-DTR mice resulted in ~1000-fold bacterial outgrowth after Spn single infection (Supplemental Fig. S2A & Fig. 4A-B).
Figure 4. Temporary AM depletion decreases neutrophil recruitment and promotes bacterial outgrowth in CD169-DTR mice during influenza/S. pneumoniae coinfection.
(A) The numbers of BALF AMs (CD11c+Siglec-F+), and (B) lung bacterial burdens 24 h after i.n. infection of B6 WT and CD169-DTR mice (mean±SD, n ≥ 4 mice/group) with Spn. (C) The numbers of BALF AMs, (D) lung bacterial burdens, and (E) the numbers of CD11b+Ly6C+ monocytes (Mo) and CD11b+Ly6G+ neutrophils (PMN) 24 h after i.n. challenge of day seven PR8-infected B6 WT and CD169-DTR mice (mean±SD, n ≥ 5 mice/group) with Spn. Naïve (A-B) or PR8-infected (C-E) mice were treated i.p. with DT (+DT) or PBS control four days before Spn infection. *P<0.05, **P<0.01***P<0.001, Tukey’s multiple comparisons test or t-test (E). Data shown are representative of two independent experiments.
To minimize the impact on antiviral immune response (37), we next administrated DT to induce AM depletion three days after PR8 infection (Supplemental Fig. S2B&Fig. 4C). As expected, PR8/Spn coinfection led to bacterial outgrowth in both WT and CD169-DTR mice (Fig. 4D). Furthermore, DT-induced AM depletion in CD169-DTR mice led to a modest but significant increase in their lung bacterial burden, as compared with both DT-treated WT and PBS-treated CD169-DTR controls (Fig. 4D). Interestingly, the numbers of inflammatory monocytes and neutrophils also significantly decreased in CD169-DTR mice after AM depletion (Fig. 4E), even though their TNF-α and IFN-γ levels remained unaffected (Supplemental Fig. S2C). We have shown that neutrophils are not required for airway bacterial clearance during Spn infection alone (27). However, as suggested by other reported studies (11, 12, 19), neutrophils play a compensatory protective role in bacterial clearance during influenza/S. pneumoniae coinfection. Thus, the modestly (~4-fold) increased bacterial burden in AM-depleted CD169-DTR mice is consistent with their decreased airway neutrophils during PR8/Spn coinfection, as compared with PBS-treated CD169-DTR controls (Fig. 4E). These results, together with findings in Csf2rb−/− mice (Fig. 3), suggest that in addition to the prime defect in AM-mediated direct bacterial clearance, influenza infection further suppresses compensatory protective mechanisms to exacerbate influenza/Spn coinfection.
Influenza-induced IFN-γ signaling enables noninvasive S. pneumoniae to cause lethal pneumonia
We next investigated the regulatory pathways underlying influenza-suppressed airway antibacterial immunity. Multiple animal studies have shown that influenza-induced cytokines suppress lung bacterial clearance (11–13, 38, 39). In particular, we have reported that influenza-induced IFN-γ increases susceptibility to secondary infection by highly virulent S. pneumoniae, i.e., serotype 2 strain D39 and serotype 3 strain A66.1 (15). In agreement, we found that influenza-induced IFN-γ signaling was responsible for suppressing innate clearance of S. pneumoniae TJO983 (Fig. 5A). Notably, in contrast to 100% mortality in WT mice, all IFN-γ receptor 1 knockout (Ifngr1−/−) survived PR8/Spn coinfection (Fig. 5B). These results indicate that activation of IFN-γ receptor signaling after influenza infection is sufficient to enable otherwise innocuous S. pneumoniae to cause lethal bacterial pneumonia.
Figure 5. Influenza-induced IFN-γ receptor signaling causes lethal susceptibility to secondary pneumococcal infection.
(A) Lung bacterial burdens (mean±SD, n ≥ 10 mice/group) at 24 h and (B) animal survival of B6 WT and Ifngr1−/− mice after i.n. challenge with Spn on day seven after PR8 infection. ***P<0.001, t-test. Data shown combined from two independent experiments.
Influenza-induced IFN-γ signaling in alveolar macrophages directly impairs their antibacterial capability
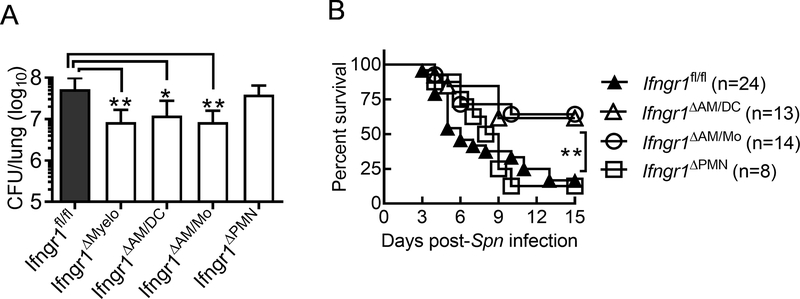
To further understand the cell-specific effect of IFN-γ receptor signaling on airway antibacterial immunity, we developed mouse models deleted of the Ifngr1 gene in myeloid cells (LysMCre), AMs/DCs (Cd11cCre), macrophages/monocytes (Cx3cr1Cre), or neutrophils (Mrp8Cre) (Supplemental Fig. S3). Compared with Ifngr1fl/fl controls, mice deficient in IFN-γ-responsive myeloid cells (LysMCreIfngr1fl/fl, referred to as Ifngr1ΔMyelo) exhibited significantly increased resistance to PR8/Spn coinfection (Fig. 6A). Similarly, mice carrying Ifngr1 deficiency in macrophages/monocytes (Cx3cr1CreIfngr1fl/fl, referred to as Ifngr1ΔAM/Mo) exhibited significantly improved lung bacterial clearance. In contrast, neutrophil-specific Ifngr1 deletion (Mrp8CreIfngr1fl/fl, referred to as Ifngr1ΔPMN) had no beneficial effect on lung bacterial clearance during coinfection, as indicated by comparable bacterial burdens between Ifngr1ΔPMN and Ifngr1fl/fl mice. These results establish that rather than regulation of neutrophil function, mononuclear phagocytes are responsive to the inhibitory effect of IFN-γ on airway antibacterial defense. Importantly, a selective deletion of Ifngr1 gene in CD11c+ cells (Cd11cCreIfngr1fl/fl, referred to as Ifngr1ΔAM/DC), mainly CD11c+Siglec-F+ AMs in the airway, resulted in significantly improved bacterial clearance after PR8/Spn coinfection (Fig. 6A). Taken together, these results indicate that IFN-γ signaling in AMs directly impairs their antibacterial function during influenza and S. pneumoniae coinfection.
Figure 6. Influenza-induced IFN-γ signaling in AMs directly impairs their antibacterial function during secondary pneumococcal infection.
(A) Lung bacterial burdens (mean±SD, n ≥ 8 mice/group) at 24 h and (B) animal survival of B6 Ifngr1fl/fl, LysMCreIfngr1fl/fl (Ifngr1ΔMyelo), Cd11cCreIfngr1fl/fl (Ifngr1ΔAM/DC), Cx3cr1CreIfngr1fl/fl (Ifngr1ΔAM/Mo), and Mrp8CreIfngr1fl/fl (Ifngr1ΔPMN) mice after i.n. challenge with Spn on day seven after PR8 infection. *P<0.05, **P<0.01, Tukey’s multiple comparisons test (A) or log-rank test (B). Data shown were combined from more than two independent experiments.
In accordance with their improved bacterial control, Ifngr1ΔAM/DC and Ifngr1ΔAM/Mo mice exhibited significantly increased survival from PR8/Spn coinfection, as compared with either Ifngr1ΔPMN animals or Ifngr1fl/fl controls (Fig. 6B). These in vivo findings further verify that IFN-γ signaling in AMs impairs their antibacterial function and thereby contributes to lethal pneumococcal pneumonia after influenza.
Discussion
In this study, we revisited the controversies over critical determinants of severe outcome in influenza and S. pneumoniae coinfection. We show that influenza infection enables otherwise noninvasive serotype 14 S. pneumoniae to cause lethal pneumonia in mice. Rather than disrupted mucosal integrity or general immune suppression, we found that this influenza-induced extreme susceptibility is attributable to IFN-γ signaling and abrogation of AM antibacterial function. Furthermore, by comparative analyses of mice with selective deletion of Ifngr1 gene in myeloid cells, AMs/DCs, macrophages/monocytes, or neutrophils, we demonstrate that influenza-induced IFN-γ signaling in AMs directly impairs their capability for bacterial control, thereby resulting in lethal bacterial outgrowth during secondary pneumococcal infection. Collectively, our current study contributes critical insights to the fundamental pathogenic mechanism of influenza and S. pneumoniae coinfection.
Multiple coinfection studies have found that, depending on the virulence of influenza virus and bacteria, the timing of bacterial super-infection, and the route of infection, a variety of outcomes are possible. Influenza virus replicates preferably in epithelial cells, and multiple studies have demonstrated that influenza-infected epithelial cells provide increased attachment sites for bacteria. These direct influenza-bacterial interactions can promote bacterial colonization in the upper respiratory tract (40), increase the risk of bacteria transmission into the normally sterile middle ear and lower respiratory tract, resulting in invasive diseases such as otitis media and pneumonia. On the other hand, many recent studies have demonstrated that dysregulation of host immune defenses critically contributes to the coinfection pathogenesis. For example, in a model of secondary systemic bacterial infection with L. monocytogenes, it has been shown that prior influenza infection leads to an increased inflammatory response in the lung but a suppression of systemic immune response (41, 42). Furthermore, it is proposed that the increased susceptibility to secondary bacterial infection is due to impaired ability to tolerate lung tissue damage (41). However, L. monocytogenes is not commonly associated with bacterial infections after influenza in humans.
The current understanding of influenza and S. pneumoniae coinfection in the lower respiratory tract is primarily built on animal models induced by highly virulent S. pneumoniae, such as serotype 2 and serotype 3 strains. These invasive bacterial strains alone can cause 100% mortality in mice after a high dose of infection, even in the absence of influenza infection. Accordingly, it has been suggested that influenza-induced epithelial damage promotes bacterial systemic dissemination, and thereby contributes to excessive mortality during secondary bacterial infection (8, 38, 43, 44). Using comparative mouse models, here we demonstrate that influenza-infected mice were more susceptible to i.n. than i.p. Spn super-infection (Fig. 2B). These surprising findings indicate that systemic antibacterial immunity is largely intact during influenza infection. Importantly, these results suggest that the acute disruption of lung integrity and therefore systemic bacterial spread is not the prime driver of lethal synergy between influenza and S. pneumoniae.
Using clodronate liposomes for AM depletion in naïve mice, we have previously demonstrated that AMs play a critical role in clearance of S. pneumoniae infection alone (27). However, clodronate liposomes also deplete other phagocytic cells, and therefore this approach is inapplicable for investigating AM-specific effect after influenza infection. In the current study, we employed both Csf2rb−/− and CD169-DTR mouse models to study the impact of influenza infection on AM-dependent and -independent bacterial control. We show in both models that in the absence of influenza infection, AMs are essential and sufficient for innate pneumococcal clearance; this antibacterial capability of AMs, however, diminishes after influenza infection, thereby resulting in extensive bacterial outgrowth during secondary pneumococcal infection.
It has been shown that influenza infection depletes AMs in BALB/c mice, especially after a high dose of viral infection (14). However, after a low dose of PR8 infection, the numbers of AMs were largely unaltered in B6 WT mice within 24 h after Spn super-infection (Fig. 3B). Thus, we conclude that influenza infection primarily impairs AM function to abrogate initial bacterial clearance. It should be noted that during PR8/Spn coinfection, the heightened bacterial burden is associated with an intensive inflammatory response in the lung. This progressive worsening of condition may eventually lead to AM depletion and lethal lung damage at the later stage of influenza and pneumococcal coinfection.
As airway sentinel cells, AMs produce pro-inflammatory cytokines that recruit and activate neutrophils to help control infection. This innate signaling function likely becomes critical when AM-mediated direct bacterial control diminishes after influenza infection. In line with that, it has been shown that influenza infection inhibits AM TLR signaling, and thereby reduces neutrophil recruitment and enhances susceptibility to secondary bacterial infection. Here we show that AM-depleted CD169-DTR mice have a reduced neutrophil response after PR8/Spn coinfection, in agreement with their increased bacterial burden. Therefore, we speculate that AMs are still capable of innate sensing during influenza/Spn coinfection, in an effort to facilitate neutrophil recruitment for compensatory bacterial clearance.
In the absence of influenza infection, airway clearance of a low dose of Spn does not require the presence of neutrophils (27), indicating that neutrophil dysregulation is not a prime deficit for bacterial control after influenza. On the other hand, neutrophils are critically involved in pneumococcal clearance after influenza infection (27), likely to compensate for AM dysfunction. Accordingly, some studies have suggested that suppression of lung inflammatory responses, i.e., inadequate acute cytokine/chemokine response and neutrophils, enhances influenza-induced susceptibility to secondary bacterial infection. In the current study, we show that compared with Spn single-infection, AM-deficient Csf2rb−/− mice exhibited 10-fold further increased bacterial burdens during PR8/Spn coinfection (Fig. 3A). This influenza-induced, AM-independent defect in bacterial control is probably due to neutrophil dysregulation. Nonetheless, this compensatory protective mechanism is too subtle to change the course and outcome of coinfection, as evidenced by the extensive neutrophil recruitment but heightened bacterial burden in both WT and Csf2rb−/− mice.
We have previously shown that T cell IFN-γ production suppresses acute (i.e., 4 h) bacterial clearance in the lung (15). In agreement, Ifng−/− mice showed ~10-fold reduced bacterial burdens as compared with WT controls at 24 h after TJO983 super-infection. Interestingly, Rag1−/− mice deficient in T and B cells exhibited 100-fold further reductions in bacterial CFU as compared with Ifng−/− mice (Supplemental Fig. S4A). In line with this, adoptive transfer of WT splenocytes into Rag1−/− mice led to significantly increased bacterial outgrowth during coinfection, as compared with Rag1−/− animals received Ifng−/− splenocytes (Supplemental Fig. S4B-C). These findings suggest that influenza infection induces both IFN-γ-dependent and -independent suppression of antibacterial immunity.
Recent evidence indicates that Type I IFN (IFN-I) also mediates host susceptibility to secondary bacterial infection after influenza. Furthermore, it has been shown that IFN-I inhibits bacterial phagocytosis by human macrophages (45, 46). It is noteworthy that influenza virus induces peak production of IFN-I and IFN-γ at the acute (~ 4 dpi) and recovery (~7 dpi) phase of viral infection, respectively (15, 47). Thus, the peak susceptibility to pneumococcal super-infection, i.e., 7 days after influenza infection (Fig. 2A), coincides with the production of IFN-γ but not IFN-I. In line with that, it has been shown that IFN-I suppresses neutrophil recruitment and thereby mediates susceptibility to secondary bacterial pneumonia, when mice were super-infected with S. pneumoniae on day 5 after influenza infection (11). Conversely, it has been shown that IFN-I protects AMs from viral replication in influenza-infected mice (48, 49). Taken together, these findings suggest that at least in our model, IFN-γ plays a dominant role in suppression of AM antibacterial function, as compared with influenza-induced IFN-I.
It is well established that scavenger receptors are critical for AM phagocytosis of unopsonized pneumococci (50, 51). We have measured mRNA levels of the macrophage mannose receptor (Mr, also called Cd206) and macrophage receptor with collagenous structure (Marco) (52), in AMs isolated from WT and Ifng−/− mice. Compared with AMs obtained from Ifng−/− mice, WT AMs exhibit significantly decreased Mr and Marco expression after influenza infection (Supplemental Fig. S5A). A similar effect of IFN-γ on AM scavenger receptor expression was observed in vitro (Supplemental Fig. S5B). Thus, it is likely that through down-regulation of phagocytic receptors, IFN-γ impairs the capability of AM for bacterial phagocytosis, thereby leading to increased susceptibility to S. pneumoniae infection.
Even though there are likely additional contributing factors in impairing AM antibacterial function after influenza, our current studies have demonstrated a fundamental role for IFN-γ signaling in promoting lethal susceptibility to secondary pneumococcal infection. Furthermore, using a series of conditional knockout mouse lines, we demonstrate that IFN-γ receptor signaling in macrophages/monocytes impairs their antibacterial function. Taken together, we conclude that influenza-induced IFN-γ signaling impairs direct bacterial clearance by AMs, thereby enabling otherwise noninvasive S. pneumoniae to cause deadly pneumonia.
Supplementary Material
Key points.
Influenza enables noninvasive Streptococcus pneumoniae to cause deadly pneumonia
Influenza mainly predisposes hosts to airway but not systemic bacterial infection
Influenza temporally abolishes innate bacterial clearance by alveolar macrophages
Acknowledgments
1. The authors thank University of Nebraska Medical Center (UNMC) Flow Cytometry Research Facility for assistance with FACS analysis. The UNMC Flow Cytometry Research Facility is supported by state funds from the Nebraska Research Initiative (NRI) and The Fred and Pamela Buffett Cancer Center’s National Cancer Institute Cancer Support Grant.
Footnote
This work was supported by National Institutes of Health R21 AI128527 and R01 HL118408 to K.S.
References
- 1.Metzger DW 2013. Immune dysfunction and bacterial coinfections following influenza. J Immunol 191: 2047–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morens DM, Taubenberger JK, and Fauci AS. 2008. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta RK, George R, and Nguyen-Van-Tam JS. 2008. Bacterial pneumonia and pandemic influenza planning. Emerg Infect Dis 14: 1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2009. Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1) virus infection - United States, April-August 2009. MMWR Morb Mortal Wkly Rep 58: 941–947. [PubMed] [Google Scholar]
- 5.Metzger DW, and Sun K. 2013. Immune dysfunction and bacterial coinfections following influenza. J Immunol 191: 2047–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCullers JA 2006. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 19: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, Ren A, Wang X, Fan X, Zhao Y, Gao GF, Cleary P, and Wang B. 2015. Influenza viral neuraminidase primes bacterial coinfection through TGF-beta-mediated expression of host cell receptors. Proc Natl Acad Sci U S A 112: 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussell T, and Cavanagh MM. 2009. The innate immune rheostat: influence on lung inflammatory disease and secondary bacterial pneumonia. Biochem Soc Trans 37: 811–813. [DOI] [PubMed] [Google Scholar]
- 9.Sun K, Johansen FE, Eckmann L, and Metzger DW. 2004. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J Immunol 173: 4576–4581. [DOI] [PubMed] [Google Scholar]
- 10.Weinberger DM, Harboe ZB, Viboud C, Krause TG, Miller M, Molbak K, and Konradsen HB. 2013. Serotype-specific effect of influenza on adult invasive pneumococcal pneumonia. J Infect Dis 208: 1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, and Deng JC. 2009. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest 119: 1910–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J, Wang D, Xu F, Gong Y, Wang H, Song Z, Li D, Zhang H, Li D, Zhang L, Xia Y, Xu H, Lai X, Lin S, Zhang X, Ren G, Dai Y, and Yin Y. 2014. Activation of IL-27 signalling promotes development of postinfluenza pneumococcal pneumonia. EMBO Mol Med 6: 120–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Moltedo B, and Moran TM. 2012. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J Virol 86: 12304–12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Califano D, Furuya Y, and Metzger DW. 2018. Effects of Influenza on Alveolar Macrophage Viability Are Dependent on Mouse Genetic Strain. J Immunol 201: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun K, and Metzger DW. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 14: 558–564. [DOI] [PubMed] [Google Scholar]
- 16.Smith AM, Adler FR, Ribeiro RM, Gutenkunst RN, McAuley JL, McCullers JA, and Perelson AS. 2013. Kinetics of coinfection with influenza A virus and Streptococcus pneumoniae. PLoS Pathog 9: e1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghoneim HE, Thomas PG, and McCullers JA. 2013. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol 191: 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura S, Davis KM, and Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 121: 3657–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Er JZ, Koean RAG, and Ding JL. 2019. Loss of T-bet confers survival advantage to influenza-bacterial superinfection. EMBO J 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robb L, Drinkwater CC, Metcalf D, Li R, Kontgen F, Nicola NA, and Begley CG. 1995. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc Natl Acad Sci U S A 92: 9565–9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Carrero JA, Uppaluri R, White JM, Archambault JM, Lai KS, Chan SR, Sheehan KC, Unanue ER, and Schreiber RD. 2013. Identifying the initiating events of anti-Listeria responses using mice with conditional loss of IFN-gamma receptor subunit 1 (IFNGR1). J Immunol 191: 4223–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clausen BE, Burkhardt C, Reith W, Renkawitz R, and Forster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8: 265–277. [DOI] [PubMed] [Google Scholar]
- 23.Caton ML, Smith-Raska MR, and Reizis B. 2007. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. The Journal of experimental medicine 204: 1653–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, and Jung S. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passegue E, Wagner EF, and Weissman IL. 2004. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 119: 431–443. [DOI] [PubMed] [Google Scholar]
- 26.Muzumdar MD, Tasic B, Miyamichi K, Li L, and Luo L. 2007. A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605. [DOI] [PubMed] [Google Scholar]
- 27.Bansal S, Yajjala VK, Bauer C, and Sun K. 2018. IL-1 Signaling Prevents Alveolar Macrophage Depletion during Influenza and Streptococcus pneumoniae Coinfection. J Immunol 200: 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arredouani MS, Palecanda A, Koziel H, Huang YC, Imrich A, Sulahian TH, Ning YY, Yang Z, Pikkarainen T, Sankala M, Vargas SO, Takeya M, Tryggvason K, and Kobzik L. 2005. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol 175: 6058–6064. [DOI] [PubMed] [Google Scholar]
- 29.Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, van Rooijen N, Vogel J, and Kopf M. 2014. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog 10: e1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis ML, and Surewaard BGJ. 2018. Neutrophil evasion strategies by Streptococcus pneumoniae and Staphylococcus aureus. Cell Tissue Res 371: 489–503. [DOI] [PubMed] [Google Scholar]
- 31.Steck P, Ritzmann F, Honecker A, Vella G, Herr C, Gaupp R, Bischoff M, Speer T, Tschernig T, Bals R, and Beisswenger C. 2019. Interleukin 17 Receptor E (IL-17RE) and IL-17C Mediate the Recruitment of Neutrophils during Acute Streptococcus pneumoniae Pneumonia. Infect Immun 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bou Ghanem EN, Clark S, Du X, Wu D, Camilli A, Leong JM, and Meydani SN. 2015. The alpha-tocopherol form of vitamin E reverses age-associated susceptibility to streptococcus pneumoniae lung infection by modulating pulmonary neutrophil recruitment. J Immunol 194: 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, Flavell RA, and Galan JE. 2010. A mouse model for the human pathogen Salmonella typhi. Cell host & microbe 8: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, Auerbach W, Eynon EE, Stevens S, Manz MG, and Flavell RA. 2011. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci U S A 108: 2390–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, and Kohno K. 2001. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol 19: 746–750. [DOI] [PubMed] [Google Scholar]
- 36.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, and Tanaka M. 2007. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest 117: 2268–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purnama C, Ng SL, Tetlak P, Setiagani YA, Kandasamy M, Baalasubramanian S, Karjalainen K, and Ruedl C. 2014. Transient ablation of alveolar macrophages leads to massive pathology of influenza infection without affecting cellular adaptive immunity. Eur J Immunol 44: 2003–2012. [DOI] [PubMed] [Google Scholar]
- 38.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, Blanc F, De Trez C, Van Maele L, Dumoutier L, Huerre MR, Eberl G, Si-Tahar M, Gosset P, Renauld JC, Sirard JC, Faveeuw C, and Trottein F. 2013. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol 87: 6911–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barthelemy A, Ivanov S, Fontaine J, Soulard D, Bouabe H, Paget C, Faveeuw C, and Trottein F. 2016. Influenza A virus-induced release of interleukin-10 inhibits the anti-microbial activities of invariant natural killer T cells during invasive pneumococcal superinfection. Mucosal Immunol. [DOI] [PubMed] [Google Scholar]
- 40.Rowe HM, Meliopoulos VA, Iverson A, Bomme P, Schultz-Cherry S, and Rosch JW. 2019. Direct interactions with influenza promote bacterial adherence during respiratory infections. Nat Microbiol 4: 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T, and Medzhitov R. 2013. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science 340: 1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamieson AM, Yu S, Annicelli CH, and Medzhitov R. 2010. Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection. Cell host & microbe 7: 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellis GT, Davidson S, Crotta S, Branzk N, Papayannopoulos V, and Wack A. 2015. TRAIL+ monocytes and monocyte-related cells cause lung damage and thereby increase susceptibility to influenza-Streptococcus pneumoniae coinfection. EMBO Rep 16: 1203–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goulding J, Godlee A, Vekaria S, Hilty M, Snelgrove R, and Hussell T. 2011. Lowering the threshold of lung innate immune cell activation alters susceptibility to secondary bacterial superinfection. J Infect Dis 204: 1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cardone M, Ikeda KN, Varano B, Belardelli F, Millefiorini E, Gessani S, and Conti L. 2014. Opposite regulatory effects of IFN-beta and IL-3 on C-type lectin receptors, antigen uptake, and phagocytosis in human macrophages. J Leukoc Biol 95: 161–168. [DOI] [PubMed] [Google Scholar]
- 46.Cooper GE, Pounce ZC, Wallington JC, Bastidas-Legarda LY, Nicholas B, Chidomere C, Robinson EC, Martin K, Tocheva AS, Christodoulides M, Djukanovic R, Wilkinson TM, and Staples KJ. 2016. Viral Inhibition of Bacterial Phagocytosis by Human Macrophages: Redundant Role of CD36. PLoS One 11: e0163889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun K, Torres L, and Metzger DW. 2010. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J Virol 84: 5007–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodgers BC, and Mims CA. 1982. Role of macrophage activation and interferon in the resistance of alveolar macrophages from infected mice to influenza virus. Infect Immun 36: 1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staples KJ, Nicholas B, McKendry RT, Spalluto CM, Wallington JC, Bragg CW, Robinson EC, Martin K, Djukanovic R, and Wilkinson TM. 2015. Viral infection of human lung macrophages increases PDL1 expression via IFNbeta. PLoS One 10: e0121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, and Kobzik L. 2004. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. The Journal of experimental medicine 200: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arredouani MS, Yang Z, Imrich A, Ning Y, Qin G, and Kobzik L. 2006. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. American journal of respiratory cell and molecular biology 35: 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu M, Gibbons JG, DeLoid GM, Bedugnis AS, Thimmulappa RK, Biswal S, and Kobzik L. 2017. Immunomodulators targeting MARCO expression improve resistance to postinfluenza bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol 313: L138–L153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.