Abstract
The COVID-19 pandemic has led to unprecedented stresses on modern medical systems, overwhelming the resource infrastructure in numerous countries while presenting a unique series of pathophysiologic clinical findings. Thrombotic coagulopathy is common in critically ill patients suffering from COVID-19, with associated high rates of respiratory failure requiring prolonged periods of mechanical ventilation. Here we report a case series of five patients suffering from profound, medically refractory COVID-19 associated respiratory failure who were treated with fibrinolytic therapy using tissue plasminogen activator (tPA, Alteplase). All five patients appeared to have an improved respiratory status following tPA administration: one patient had an initial marked improvement that partially regressed after several hours, one patient had transient improvements that were not sustained, and three patients had sustained clinical improvements following tPA administration.
Keywords: COVID-19, Acute Respiratory Distress Syndrome (ARDS), Tissue Plasminogen Activator (tPA), Pulmonary Failure, Fibrinolysis
INTRODUCTION
The COVID-19 pandemic and high rate of associated Acute Respiratory Distress Syndrome (ARDS) is placing an overwhelming burden on the healthcare system nationally. As mechanical ventilators and extracorporeal membrane oxygenation (ECMO) resources become exhausted, therapies to attenuate the severity of COVID-19-associated respiratory failure are urgently needed.
Approximately three-quarters of patients who die of COVID-19 meet the International Society for Thrombosis and Haemostasis (ISTH) criteria for disseminated intravascular coagulation (DIC), which is almost exclusively prothrombotic in COVID-19 patients with a thromboembolic complication rate in COVID-19 ICU patients of 31.4% (1–3). In contrast, only 0.6% of patients who survive meet DIC criteria (1). Laboratory hallmarks of COVID-19 critical illness include highly elevated fibrinogen levels together with elevated levels of D-dimer (4). COVID-19 pathology reports are demonstrating diffuse pulmonary and systemic microvascular thrombosis and occlusion (5). Although these findings seem much more marked in COVID-19 patients, this is in keeping with ARDS, regardless of cause (5,6). Numerous pre-clinical studies and a single clinical trial have shown improved survival in ARDS following the institution of fibrinolytic therapy (7,8). The pronounced clinical constellation of pulmonary and systemic thrombotic coagulopathy in COVID-19 critical illness with relatively normal lung compliance and high Alveolar-arterial oxygen gradients (9), along with the autopsy findings of diffuse pulmonary microvascular thrombosis, endorses the rationale that fibrinolytic therapy may have a biologically plausible role in treatment. The following is a case series using intravenous tissue plasminogen activator (tPA, alteplase) in five critically ill mechanically ventilated COVID-19 patients with thrombotic coagulopathy and ARDS.
CASE 1
A 39-year-old male with no past medical history (PMH) presented to a community hospital with a four-day history of shortness of breath, cough, and chest pain. Upon presentation he was hypoxic (room air oxygen saturation of 90%) and demonstrated bilateral patchy opacities on chest radiography and positive COVID-19 PCR testing. He rapidly declined within hours of admission to require endotracheal intubation, mechanical ventilation and Intensive Care Unit (ICU) admission. He was treated with ceftriaxone, azithromycin, hydroxychloroquine and supported with a lung protective ventilation strategy. On hospital day 5 (HD5) he was transferred to a tertiary care facility for further management given persistent severe, refractory hypoxemic respiratory failure; his organ failure was limited to single system (pulmonary). Admission laboratory work was notable for fibrinogen of 1,116 mg/dL (also his peak), D-dimer of 7,434 ng/mL, INR of 2.2, PTT of 34.1s, and platelet count of 344 k/uL
Despite sedation, neuromuscular blockade, lung protective ventilation with optimized PEEP of 16 cm H2O guided by esophageal manometry, his PaO2/FiO2(P/F) ratio was 81 on FiO2 of 80%. Prone positioning led to some improved oxygenation (P/F 110), but unfortunately over the subsequent 72 hours and trials of both inhaled epoprostenol and inhaled nitric oxide (iNO) his P/F ratios resided between 60-100, with the lowest ratios correlating with failed trials of supination from the prone position. Transthoracic echocardiography demonstrated normal biventricular systolic function. Throughout the clinical course his laboratory studies remained notable for extremely elevated fibrinogen and D-dimer levels consistent with a pro-thrombotic coagulopathy. Respiratory system and lung mechanics remained preserved throughout (static respiratory system compliance 35-45 mL/cmH2O and driving pressure <15cmH2O) despite severe hypoxemia and a large Alveolar-arterial oxygen gradient, consistent with pulmonary vascular occlusive phenomena as the primary pathophysiology.
Given lack of clinical improvement, a trial of tPA was initiated using a 25mg bolus over 2 hours, with an additional 25mg infused over the following 22 hours (8,10). Pre- and post-tPA bolus thromboelastography testing showed a low-normal value of fibrinolysis (LY30, %) of 0.2% (ref range 0-2.6%) (Table 1). D-dimer was already above reference range, so was unable to be used as a marker of fibrinolysis/clot degradation resulting from tPA therapy. A modest initial improvement in P/F ratio was observed within 4 hours (Figure 1A), however the response mostly subsided during the low-dose tPA maintenance infusion. Importantly, the patient was not anticoagulated during the maintenance tPA infusion. His fibrinogen went from 731mg/dL pre-tPA to 628mg/dL at completion of the 22-hour tPA infusion. Based on the lack of sustained clinical improvement, further therapies were considered, including the IL-6 receptor antagonist tocilizumab, which was administered.
Table 1.
Thromboelastography (TEG) parameters measured before, during, and after tPA administration in Case 1.
| Timepoint | R-time (min) | CRT-MA (mm) | CFF-MA (mm) | LY30 (%) | |
|---|---|---|---|---|---|
| Ref Range | (4.6-9.1) | (52-70) | (15-32) | (0-2.6) | |
| Pre-TPA Bolus #1 | 6.1 | 71.9 | 41.7 | 0.2 | |
| Post-TPA Bolus #1 | 7.7 | 70.7 | 36.0 | 0.2 | |
| Pre-TPA Bolus #2 | 7.6 | 72.1 | 39.1 | 0.2 | |
| Mid-TPA Bolus #2 | 7.5 | 67.3 | 24.3 | >22% | |
| Post-TPA Bolus #2 | 7.1 | 69.2 | 29.4 | 1.6 |
R-time describes the time to clot formation. MA (maximal amplitude) describes maximum clot strength. CRT-MA (Citrated Rapid TEG-MA) describes the maximum clot strength, but is obtained rapidly by accelerating coagulation. CFF (Citrated Functional Fibrinogen-MA) describes the fibrinogen contribution to clot strength (platelet contribution is excluded). LY30 is the % percent of clot lysis 30 minutes after maximal amplitude.
Figure 1.
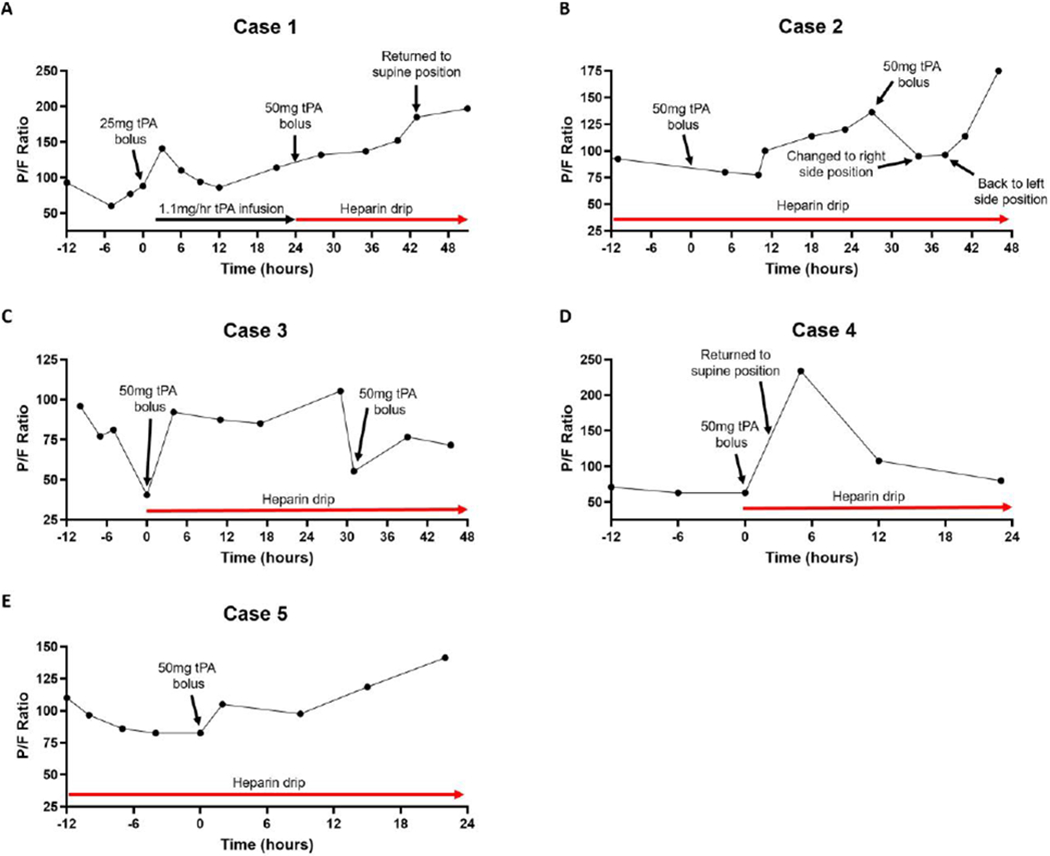
Graphs of P/F ratio as a function of time relative to when tPA was administered in (A) Case 1, (B) Case 2, (C) Case 3, (D) Case 4, and (E) Case 5.
His limited response to initial tPA therapy prompted concern for under-dosing of tPA based on the expected high levels of PAI-1 (11) and the modest dose he received relative to what is used in other thrombotic occlusive conditions (12), with further concern that lack of concomittant anticoagulation may have allowed for early re-thrombosis. Therefore a second bolus of 50mg of tPA over 2 hours was administered, this time with a simultaneous heparin infusion at 500u/hr. Thromboelastography showed low-normal LY30 values before and after tPA (0.2% and 1.6%, respectively), with an appropriate increase to LY30>22% during the tPA bolus (Table 1). After tPA bolus completion, heparin therapy was advanced to a target PTT of 60-80 seconds.
The patient’s oxygenation and P/F ratio progressively improved thereafter (Figure 1A), increasing to 197 after 24 hours and 227 at 36 hours despite returning the patient to the supine position, cessation of iNO and lifting of neuromuscular blockade. Seven days post-tPA the patient was successfully extubated and neurologically intact with no apparent complications of tPA therapy.
CASE 2
58-year-old male with PMH significant for hypertension and non-insulin dependent diabetes mellitus presented to the hospital with a 2-week history of shortness of breath and feeling “unwell” in the setting of a known outpatient diagnosis of COVID-19 confirmed by PCR analysis 4 days prior to admission. Upon presentation, he displayed hypoxia with an oxygen saturation of 78% on room air, which initially improved on 100% FiO2 via NRB, and chest radiography demonstrated bilateral infiltrates. Admission laboratory work was notable for a fibrinogen of 482 mg/dL (peak 1,021 mg/dL on HD5), D-dimer of 1,462 ng/mL, INR of 1.2, PTT of 30.2 seconds, and platelet count of 181 k/uL. Upon arrival to the ICU from the Emergency Department he was noted to have increased work of breathing, tachypnea to the 40’s, and recurrent desaturations despite 100% FiO2 on NRB, so was intubated on HD1.
He was treated with ceftriaxone, azithromycin, a therapeutic heparin drip and supported with a lung protective ventilation strategy, and by HD 3 was also chemically paralyzed. His respiratory status continued to deteriorate so a trial of prone positioning was attempted, however this movement led to significant respiratory decompensation so he was returned to his left side with some recovery. On HD5 his respiratory failure had continued to progress with P/F ratios persistently in the 90’s despite maximal ventilator strategies and FiO2 ranging from 80-100% tPA salvage therapy was initiated with a 50mg tPA (Alteplase) bolus over 2 hours with his heparin drip turned down to 500u/hr during the tPA bolus, with resumption of a therapeutic rate of his heparin drip after the tPA bolus was completed. His pre-tPA fibrinogen was 980 mg/dL and D-dimer was 2,124 ng/mL, post-tPA fibrinogen was 944 mg/dL and there was a spike in D-dimer to 7,094 ng/mL consistent with fibrinolysis of clot occurring after tPA administration. His P/F ratio immediately dipped in to 77-80 range, but then began to steadily climb up to 136 at 24-hours post-tPA, a 48% increase in P/F from pre-tPA (Figure 1B). The decision was made to repeat the 50mg tPA bolus to attempt further gains, which again led to an initial transient decrease in his P/F to the mid-90’s (that was also in the setting of a position change), but his P/F ratio then climbed up to 114 and then 175. His respiratory status remains improved as of the time of this submission with his P/F ratio up 90% from pre-tPA levels and measured in the same position (left side) he started tPA therapy in. No bleeding complications were noted during or after tPA therapy.
CASE 3
67-year-old male with PMH significant for hypertension, thyroid cancer status-post thyroidectomy and radioactive iodine presented to the hospital with a ten-day history of worsening shortness of breath, fatigue, fevers and dry cough. Upon presentation, he displayed hypoxia with an oxygen saturation of 80% on room air and chest radiography demonstrated bilateral patchy opacities. Admission laboratory work was notable for a fibrinogen 257 mg/dL (peak 709 mg/dL on HD 14), D-dimer 6,070 ng/mL, INR of 1.3, PTT of 32.1 seconds, and a platelet count of 212 k/uL. He was admitted to the ICU with acute hypoxemic respiratory failure with lung-protective ventilator settings and PEEP 16 on 100% FiO2, sedated and chemically paralyzed. A diagnosis of COVID-19 was confirmed by PCR analysis.
Following admission to the ICU his ventilator strategy was changed to APRV with a decrease in his FIO2 requirement to 50%. In consultation with infectious diseases, he was treated with ampicillin/sulbactam, hydroxychloroquine, and deemed not a candidate for other study trial medications. By HD 2 his renal function deteriorated, and he progressed to oligo-anuric acute kidney injury. Only short courses of continuous renal replacement therapy (approximately 8-12 hours per day) were able to be completed given excess demand for use of the limited dialysis machines amongst other critically ill patients throughout the hospital. He therefore remained with a severe mixed respiratory and metabolic acidosis as his ventilator requirements were necessarily increased (APRV Phigh 30-34, FIO2 50-75%). By HD 6, his D dimer was noted to be >35,000ng/mL and therapeutic anti coagulation was commenced using enoxaparin, titrated to anti-factor Xa activity levels (0.7-1.0). Unfortunately, his pulmonary function continued to deteriorate and he was no longer responding to 100% FiO2 despite multiple changes on the ventilator with P/F ratios now ranging from 70-105. He was deemed not to be a candidate for prone positioning given his tenuous hemodynamic status, large body habitus, severe acidosis (pH 7.1-7.2) and ongoing renal replacement requirements. On HD 16 the patient deteriorated (P/F ratio 77) and was commenced on a trial of inhaled nitric oxide with limited benefit – up to 30 ppm. Respiratory system and lung mechanics remained preserved throughout the entire course despite the severe hypoxemia with a large A-a gradient, similar to the previous 2 cases and consistent with what would be observed in pulmonary vascular occlusive phenomena.
The patient continued to decompensate with O2 saturation of 70% on 100% FiO2 (imputed P/F ratio 41, no arterial blood gas was available) and was unstable, so a trial of tPA (Alteplase) was initiated using a 50mg bolus over 2 hours and he was transitioned to a therapeutic heparin drip instead of enoxaparin. At 4 hours post-tPA initiation his P/F ratio was up to 92, a >2-fold increase and marked improvement (Figure 1C). Just over 24 hours after his initial bolus of tPA his P/F ratio was back down to 85, prompting a second 50mg tPA bolus with improvement of his P/F to 105 at 3-hours post-tPA initiation. The patient remained therapeutically heparinized during the second tPA challenge without any interruption in heparin administration. Unfortunately, his respiratory status declined again and a third 50mg tPA bolus was administered on HD18 given his prior improvements after tPA, but this time there was no response, his multiple organ failure progressed, and he expired a short time later.
CASE 4
27-year-old female with PMH significant for morbid obesity (BMI 57) and non-insulin dependent diabetes mellitus presented to the hospital with a seven-day history of cough, fever, and progressive dyspnea. She was profoundly hypoxic on hospital presentation with an oxygen saturation of 60% on room air, improved to 80% on FiO2 100% non-rebreather mask (NRB), and she was subsequently intubated. Chest radiography demonstrated bilateral patchy opacities with dense peripheral infiltrates. Admission laboratory work was notable for a fibrinogen 750 mg/dL (peak 856 mg/dL on HD 2 and 4), D-dimer of 2,240 ng/mL, INR of 1.0 and PTT of 34.1 seconds. She was admitted to the ICU with acute hypoxemic respiratory failure with lung-protective ventilator settings and PEEP 15 on 100% FiO2 with a P/F ratio of 61 despite sedation, chemical paralysis, and prone positioning. A diagnosis of COVID-19 was confirmed by PCR analysis.
The patient’s respiratory status remained tenuous with O2 saturations dipping to as low as 82% with a very modest improvement upon changing ventilator mode to APRV, and was too unstable for consideration of extracorporeal membrane oxygenation (ECMO) as it was felt she would not survive returning to the supine position to facilitate cannulation. Given her instability with P/F ratios in the 60’s despite prone positioning and maximal therapy the decision was made to administer a bolus of 50mg tPA over 2 hours while on a concomitant heparin drip at 500u/hr, followed by a tPA drip at 2mg/hr for 22 hours while on a therapeutic heparin drip with goal PTT 60-80s. Her pre-tPA fibrinogen was 756mg/dL and D-dimer was 4,040 ng/mL, post-tPA fibrinogen was 856 mg/dL and there was a spike in D-dimer to >20,000 ng/mL consistent with fibrinolysis/clot degradation occurring after tPA administration. The patient had a rapid improvement following administration of tPA allowing for return to the supine position within 3 hours, and at 5 hours post-tPA initiation her FiO2 was down to 50% and P/F ratio was 217 (Figure 1D). At the time of completion of her tPA infusion she had partial regression in that her P/F ratio had fallen to 71, but she remained in the supine position instead of prone and overall this was an improvement relative to her pre-tPA prone P/F ratio. No bleeding complications were noted during or after tPA therapy. While some sustained respiratory status improvements persist, she remains critically ill as of the time of this submission.
CASE 5
52-year-old male with PMH significant for aortic valve disease, Hodgkin’s lymphoma, and hyperlipidemia presented to a community hospital with a four-day history of fatigue, shortness of breath, body aches and fever. Upon presentation, he displayed hypoxia with an oxygen saturation of 82% on room air, which improved on 100% FiO2 via NRB, and chest radiography demonstrated bilateral infiltrates. Admission laboratory work was notable for a fibrinogen of 836 mg/dL (peak 1,070 mg/dL on HD4), D-dimer of 843 ng/mL, INR of 1.2, PTT of 27.8 seconds, and platelet count of 265 k/uL. He was immediately transferred to a tertiary care center for further management, where upon arrival to the ICU his O2 saturations were now 82% on 100% FiO2 NRB so he was intubated, sedated, and placed on mechanical ventilation. A diagnosis of COVID-19 was confirmed by PCR analysis.
He was treated with ceftriaxone, azithromycin, hydroxychloroquine, a therapeutic heparin drip and supported with a lung protective ventilation strategy, and by HD 3 was also chemically paralyzed. On HD6 his respiratory failure had continued to progress with P/F ratio of 97 and he was placed in the prone position with recovery of P/F ratio to >100. By HD12 his P/F ratio was consistently below 100 despite prone positioning and maximal ventilator strategies. tPA salvage therapy was initiated with a 50mg tPA (alteplase) bolus over 2 hours with his heparin drip turned down to 500u/hr during the tPA bolus, with resumption of a therapeutic rate of his heparin drip after the tPA bolus was completed. His pre-tPA fibrinogen was 365 mg/dL and D-dimer was 15,061 ng/mL, post-tPA fibrinogen was 373 mg/dL and there was a spike in D-dimer to 17,613 ng/mL consistent with fibrinolysis/clot degradation occurring after tPA administration. His P/F ratio immediately improved from 82 pre-tPA to 105 post-tPA (Figure 1E), which continued to improve throughout the day and his FiO2 was weaned from 80% to 70% that evening. At 24 hours post-tPA his P/F ratio had improved to 141 (>50% increase from pre-tPA) and he was returned to the supine position shortly after, which he tolerated. At 60 hours post-tPA he did develop some rectal bleeding felt to be related to the prolonged presence of a rectal tube in the setting of an ongoing therapeutic heparin drip, which required a 1 unit transfusion of packed red blood cells and temporary cessation of his heparin drip that was subsequently resumed without complication.
CONCLUSIONS
In summary, we report a case series of 5 patients who were treated with off-label intravenous administration of tPA (alteplase) for profound COVID-19 respiratory failure in the setting of an apparent thrombotic coagulopathy. All 5 patients appeared to have an improved respiratory status following tPA administration: 1 patient had an initial marked improvement that partially regressed after several hours, 1 patient had transient improvements that were not sustained, and 3 patients had sustained clinical improvements following tPA administration (1 of whom was successfully extubated 7 days after tPA administration). A prior case series of 3 patients treated with tPA (alteplase) for COVID-19 respiratory failure that used lower doses of tPA over longer periods of time and without concomitant heparin anticoagulation (13) demonstrated less dramatic effects that were less durable than what was observed with larger doses of tPA and concomitant heparin anticoagulation as described in the present case series. The universally observed spike in D-dimer following tPA administration also served to verify that clot was present in these patients and fibrinolysis/clot degradation occurred in response to tPA.
While the cases put forth in this manuscript all demonstrate what seems to be a temporal relationship between administration of tPA and improved respiratory status, there are no controls for comparison, so causation and efficacy cannot be ascribed to tPA with certainty. In a resource limited crisis such as the COVID-19 pandemic with possible shortages of ventilators and ECMO circuits, fibrinolytic therapy may be useful in select cases where laboratory markers and respiratory parameters point towards thrombotic coagulopathy with vascular occlusive pulmonary physiology. Notable in this regard is the near universal availability of tPA in most hospitals, and the potential for rapid escalation of industrial production and distribution to areas most affected by COVID-19.
Formal studies with larger patient groups, including a control group, will be required to demonstrate efficacy and safety, as well as identify the patient population that most benefits from tPA and the optimal dose and route for tPA administration. A Phase 2 multi-center randomized control trial of tPA in COVID-19 respiratory failure to answer these questions is now planned and about to open for enrollment (clinicaltrials.gov registration number NCT04357730). Until such studies are published, individual clinician considerations for off-label tPA therapy in COVID-19 patients with thrombotic coagulopathy and respiratory failure may be warranted when there is an imminent risk of death and no available options for escalation of care.
Acknowledgments
Conflicts of Interest: CDB, HBM, EEM, and MBY have patents pending related to both coagulation/fibrinolysis diagnostics and therapeutic fibrinolytics, and are passive co-founders and holds stock options in Thrombo Therapeutics, Inc. HBM and EEM have received grant support from Haemonetics and Instrumentation Laboratories. MBY has previously received a gift of Alteplase (tPA) from Genentech, and owns stock options as a co-founder of Merrimack Pharmaceuticals. All other authors have nothing to disclose.
REFERENCES
- 1.Tang N, Li D, Wang X, Sun Z, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost, ePub ahead of print (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thachil J. e. a., ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost, ePub ahead of print (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han H et al. , Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med, ePub ahead of print (2020). [DOI] [PubMed] [Google Scholar]
- 5.Fox SEAA, Harbert JL, Li G, Brown JQ, Vander Heide RS Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. MedRxiv (preprint) (2020). [Google Scholar]
- 6.Ware LB, Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 27, 337–349 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Liu C et al. , Meta-Analysis of Preclinical Studies of Fibrinolytic Therapy for Acute Lung Injury. Front Immunol 9, 1898 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardaway RM et al. , Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg 67, 377–382 (2001). [PubMed] [Google Scholar]
- 9.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. American journal of respiratory and critical care medicine. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore HB, Barrett CD, Moore EE, McIntyre RC, Moore PK, Talmor DS, Moore FA, Yaffe MB, Is There a Role for Tissue Plasminogen Activator (tPA) as a Novel Treatment for Refractory COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS)? J. Trauma Acute Care Surg, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware LB et al. , Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med 35, 1821–1828 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konstantinides S et al. , Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 347, 1143–1150 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue Plasminogen Activator (tPA) Treatment for COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS): A Case Series. J Thromb Haemost. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


