Abstract
Background and aims
Prevalent valvular calcification (VC) is associated with stroke but little is known about associations of VC progression with stroke.
Methods
Progression (interval increase >0 Agatston units/year) of aortic valvular calcification (AVC) and mitral annular calcification (MAC) was assessed by two cardiac CTs over a median of 2.4 years. We determined the risk of adjudicated total and ischemic stroke using Cox regression adjusted for cardiovascular disease (CVD) risk factors.
Results
We studied 5,539 MESA participants free of baseline CVD and atrial fibrillation. Baseline mean±SD age was 62±10 years; 53% were women; 83% had no progression of VC; 15%, progression at one site (AVC or MAC), and 3%, progression at both sites. Over a median of 12 years, 211 total and 167 ischemic strokes occurred. The number of sites with VC progression (range 0–2) was not associated with total and ischemic stroke (all p>0.05). We found MAC progression to be associated with increased risk of total stroke [adjusted hazard ratio (95% CI) 1.59 (1.11, 2.28)] and ischemic stroke [1.64 (1.10, 2.45)]. Results remained significant after further adjustment for baseline coronary artery calcification. After excluding participants with interim atrial fibrillation and coronary heart disease, findings were no longer statistically significant in fully-adjusted models. There was no interaction by age, sex, or race/ethnicity. There was no association with AVC progression and stroke.
Conclusions
Progression of MAC but not AVC over 2.4 years is associated with increased risk of total and ischemic stroke.
Keywords: Valvular calcification, stroke, epidemiology, aortic valvular calcification, mitral annular calcification
Introduction
Stroke is a leading cause of long-term disability and the 5th leading cause of death in the United States (US).1 Though the age-adjusted incidence rates of stroke have declined, the prevalence continues to increase with an estimated 2.5% of the adult US population having stroke between 2013 and 2016, with a projected increase of 20.5% by 2030 since 2012.1 Ischemic stroke can result from atherosclerotic cardiovascular disease (ASCVD) or cardio-embolism such as atrial fibrillation (AF).1, 2
The role of a baseline assessment of coronary artery calcium (CAC) and extra coronary calcification, markers of subclinical atherosclerosis, in predicting the risk of ASCVD, including stroke, has been well established in prior epidemiological studies.3–13 Calcifications of the left sided heart valves -mitral annular calcification (MAC) and aortic valve calcification (AVC) - can be easily detected by echocardiography or computed tomography (CT) imaging. Even when asymptomatic, valvular calcification (VC) has been linked to increased risk for stroke, myocardial infarction, and vascular death, independent of traditional ASCVD risk factors.6, 12, 13 Notably, MAC and its progression have also been associated with AF, and AF is a major risk factor for cardioembolic stroke.8
However, whether progression of VC, as a marker of worsening subclinical atherosclerosis or as a risk factor for AF,8 is associated with incident stroke is uncertain. The progression of VC may help identify individuals at higher risk of developing stroke independent of the impact of baseline calcification. Though the relationship between arterial calcification and probability of plaque rupture remains unclear,14 VC progression may help identify individuals who are more likely to suffer a cardioembolic phenomenon from increased burden of atherosclerosis.
The morbidity and mortality associated with stroke, and the increasing cost of health care, highlight the importance of continued research aimed at identifying individuals at risk for stroke, who may benefit from additional targeted therapeutic intervention strategies. Due to this knowledge deficit regarding the relationship of VC progression and stroke as stated earlier, we explored the impact of VC progression of MAC and AVC on incident total and ischemic stroke in a diverse multiethnic community-based cohort free from clinical ASCVD at baseline.
Materials and methods
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective epidemiological study involving a diverse ethnic population of men and women enrolled from six US communities.15 The study design and methods have been previously described15 and are available at http://www.mesa-nhlbi.org. In summary, a total of 6,814 White, African-American, Hispanic, or Chinese participants were enrolled from Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles County, CA between 2000 and 2002. At enrollment, participants were aged 45–84 years and free of clinical cardiovascular disease and AF. The institutional review board at each participating site approved the study, and each participant provided informed consent.
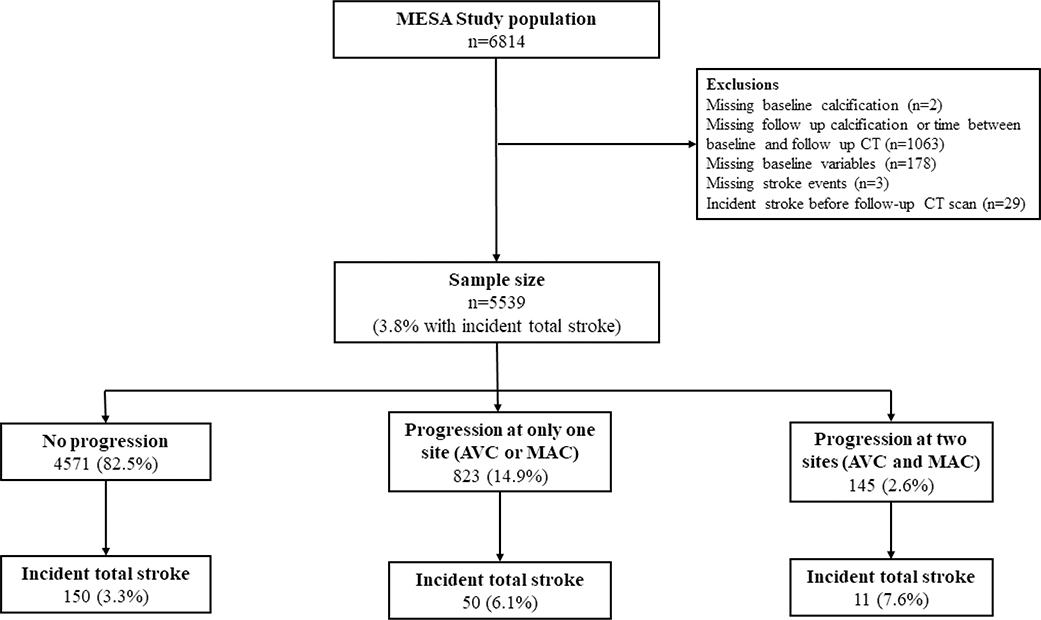
We included in our analysis 5,539 participants who had VC data assessed by cardiac CT at both Exam 1 (2000–2002) and Exam 2 (2002–2004) or Exam 3 (2004–2005) and were followed for the incidence of stroke (Figure 1). We excluded participants who had missing data on baseline VC (n=2), missing data on follow-up VC or the time between baseline and follow-up CT scans (n=1,063), missing covariates used in our main models (n=178), missing stroke events (n=3) and participants who developed stroke before the follow-up CT (n=29).
Figure 1.
Flow chart of study participants
Covariate ascertainment
Information on age, sex, race/ethnicity, education and smoking status was derived from enrollment interview and questionnaire. A physical activity survey was used to ascertain the total metabolic equivalent of task (MET)-minutes/week of vigorous and moderate physical activity. A medication inventory approach was used to determine use of anti-hypertensives and lipid lowering medications. Body mass index (BMI, kg/m2) was calculated as weight divided by height squared. The blood pressure was the average of the last two of three measurements using a Dinamap automated blood pressure device. Blood obtained after a 12-hour fast was used to measure total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglyceride at a central core lab (the Collaborative Studies Clinical Laboratory at Fairview–University Medical Center, Minneapolis, Minnesota). Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation. Diabetes was defined as a fasting glucose ≥126 mg/dL, use of diabetes medication or insulin, or self-report. The Chronic Kidney Disease Epidemiology Collaboration equation16 was used to ascertain participants’ estimated glomerular filtration rate (eGFR).
Valvular calcification measurement
We define VC to include AVC and MAC. At MESA baseline visit (Exam 1), and at either Exam 2 or 3 (randomly assigned), participants underwent ECG-gated cardiac CT scanning by electron-beam CT at three centers and a four-slice multi-detector row helical CT at the other three centers.17, 18 The Agatston scoring method was used to quantify VC.19 The scanning method, image reconstruction, and reading protocols have been previously reported.17 The equivalence across scanner types and inter-scanner reproducibility (kappa statistic of 0.94–0.96) has also been published.20, 21 Among the 5,539 participants included in our analysis, the mean, median, minimum and maximum time between baseline and follow-up cardiac CTs was 2.4, 2.4, 0.9 and 4.9 years.
Stroke ascertainment
This study includes stroke events that were ascertained from the follow-up CT scan to December 2016. Every 9–12 months, interim hospitalization was inquired from study participants or their next of kin. Medical records and death certifications were reviewed, and stroke events were adjudicated by a MESA committee, as detailed previously.22 This included fatal and nonfatal, ischemic, hemorrhagic, or unknown stroke type. Detailed description of MESA participant follow-up is available at http://www.mesa-nhlbi.org.
Coronary heart disease and atrial fibrillation events
In sensitivity analysis, we excluded individuals with incident coronary heart disease (CHD) events and incident AF events during follow-up. Incident CHD was defined as definite or probable myocardial infarction, resuscitated cardiac arrest, definite or probable angina (if followed by revascularization), and definite CHD death.23 Incident AF was ascertained by study ECGs at a follow-up visit consistent with AF, hospital discharge diagnoses, or Medicare inpatient and outpatient claims data for individuals enrolled in fee-for-service Medicare.24
Statistical analyses
Progression of VC was categorized as 0, 1, and 2 for no progression, progression at only one valve site (AVC or MAC) and progression at both sites (AVC and MAC) respectively. In another analysis, we created a dichotomous variable (AVC or MAC progression vs no AVC or MAC progression) by comparing participants with >0 Agatston units of change per year to those with ≤0 Agatston units of change per year between CT scans.8
We described baseline characteristics by progression of VC. We used multivariable-adjusted Cox proportional hazard regression to determine the hazard ratios (HRs) and 95% confidence intervals (CI) for incident total and ischemic stroke. Time to incident stroke was derived from the time of last follow-up CT scan to the stroke event. We ensured that the proportional hazards assumption was not violated using the Schoenfeld residuals in unadjusted models. Test for linear trend (p-for-trend) was derived by modeling the ordinal variable, VC progression, as a continuous variable. We assessed interactions by age, sex, and race/ethnicity.
We performed two supplemental analyses. First, we limited our analysis to participants with and without prevalent VC at baseline (i.e. Agatston score > 0) to explore if the association between VC progression and stroke depended on the presence or absence of baseline VC. In a second sensitivity analysis, we assessed if results differed after excluding participants with interim AF and CHD events.
We examined a hierarchy of models. Model 1 included age (continuous), sex (male, female), race/ethnicity (White, Black, Hispanic, Chinese), MESA site (six centers), CT scanner types (four types). Model 2 included Model 1+ educational status, BMI (continuous), smoking status (never, former, current), pack-years of smoking (continuous, natural log transformed), and physical activity (continuous, natural log transformed). Model 3 (our main model) included Model 2 + systolic blood pressure (continuous), diastolic blood pressure (continuous), use of anti-hypertensives (yes, no), use of lipid lowering medications (yes, no), total cholesterol (continuous), HDL-cholesterol (continuous), LDL-cholesterol (continuous), diabetes status (yes, no) and eGFR (continuous). Model 4 included Model 3 + baseline CAC scores (0, 1–99, 100–399, and ≥400), to determine if associations were independent of baseline CAC.
The models with the ordinal variable - no progression, progression at only one valve site and progression at both sites – were additionally adjusted for the time between baseline and follow-up CT scans. The other exposures included time by presenting progression as annual change (Agatston score/year) as so we did not additionally adjust for time between CT scans in these models.
Statistical analysis was performed using Stata (version 15.1, College Station, TX, USA) and a two-tailed p-value of <0.05 was considered to be statistically significant.
Results
Baseline characteristics
Of the 5,539 participants included, the mean ± SD age was 61.9 ± 10.2 years, 52.6% were women, 39.6% White, 27.2% Black, 21.2% Hispanic and 12.1% Chinese, 12.4% had prevalent AVC and 9.0% prevalent MAC. After a median of 2.4 years, 83% had no progression of VC, 14% had progression at only one site (AVC or MAC), and 3% had progression at both sites (AVC and MAC). Table 1 shows the baseline characteristics of MESA participants by the number of sites with VC progression. The mean age, BMI, systolic blood pressure, triglyceride, and median pack-years of cigarette use among ever-smokers was higher with a greater number of sites with VC progression. Similarly, the proportion of Whites, participants with diabetes, participants on anti-hypertensive and lipid-lowering medications, participants with CAC score of 100 to 399 and ≥400, and participants who developed interim AF and CHD were also higher with a great number of sites with VC progression. On the other hand, mean eGFR, HDL-cholesterol, median physical activity level, proportion of women, Blacks, Chinese, and participants with CAC score of zero were lower, with a greater number of sites with VC progression.
Table 1.
Baseline characteristics of study participants; MESA
| Characteristics | Total | Number of left valve sites with progression of calcification | Direction of association a | p-for-trend | ||
|---|---|---|---|---|---|---|
| No progression | One site only (AVC or MAC) | Both sites (AVC and MAC) | ||||
| N | 5539 | 4571 | 823 | 145 | ||
| Age, years | 61.9 ± 10.2 | 60.3 ± 9.8 | 68.7 ± 8.5 | 72 ± 7.1 | ▲ | <0.001 |
| Female | 2915 (52.6) | 2437 (53.3) | 413 (50.2) | 65 (44.8) | ▼ | 0.01 |
| Race/ ethnicity | ||||||
| White | 2192 (39.6) | 1737 (38.0) | 371 (45.1) | 84 (57.9) | ▲ | <0.001 |
| Black | 1504 (27.2) | 1287 (28.2) | 193 (23.5) | 24 (16.6) | ▼ | <0.001 |
| Hispanic | 1174 (21.2) | 956 (20.9) | 191 (23.2) | 27 (18.6) | ■ | 0.52 |
| Chinese | 669 (12.1) | 591 (12.9) | 68 (8.3) | 10 (6.9) | ▼ | <0.001 |
| Education | ||||||
| Less than high school | 909 (16.4) | 703 (15.4) | 183 (22.2) | 23 (15.9) | ▲ | <0.001 |
| High school or vocational school | 2266 (40.9) | 1867 (40.8) | 338 (41.1) | 61 (42.1) | ■ | 0.78 |
| College, graduate or professional school | 2364 (42.7) | 2001 (43.8) | 302 (36.7) | 61 (42.1) | ▼ | 0.003 |
| BMI, kg/m2 | 28.3 ± 5.4 | 28.1 ± 5.5 | 28.8 ± 5.4 | 28.8 ± 4.7 | ▲ | <0.001 |
| Current smoker | 668 (12.1) | 557 (12.2) | 105 (12.8) | 6 (4.1) | ■ | 0.12 |
| Pack-years of smoking in ever-smokers b | 16.0 (6.0 – 32.0) | 15.4 (5.8 – 30.0) | 19.4 (7.5 – 37.3) | 20.0 (6.0 – 43.5) | ▲ | <0.001 |
| Physical activity, MET-minutes/week b | 4125 (2055 – 7545) | 4230 (2100 – 7725) | 3780 (1713 – 6840) | 3300 (1680 – 6480) | ▼ | <0.001 |
| Systolic blood pressure, mmHg | 125.8 ± 21 | 124.4 ± 20.4 | 132.4 ± 22.7 | 133.4 ± 20.4 | ▲ | <0.001 |
| Diastolic blood pressure, mmHg | 71.8 ± 10.1 | 71.9 ± 10.1 | 71.5 ± 10.3 | 70.7 ± 9 | ■ | 0.06 |
| Antihypertensive use | 1783 (32.2) | 1348 (29.5) | 359 (43.6) | 76 (52.4) | ▲ | <0.001 |
| Lipid-lowering medication | 907 (16.4) | 671 (14.7) | 195 (23.7) | 41 (28.3) | ▲ | <0.001 |
| Total cholesterol, mg/dL | 193.5 ± 34.1 | 193.5 ± 34 | 194.7 ± 34.7 | 188.7 ± 31.4 | ■ | 0.75 |
| HDL-cholesterol, mg/dL | 51.2 ± 14.7 | 51.4 ± 14.7 | 50.7 ± 14.9 | 49.1 ± 14 | ▼ | 0.02 |
| LDL-cholesterol, mg/dL | 117.2 ± 30.9 | 117.3 ± 30.9 | 117.6 ± 31.3 | 112.4 ± 28.4 | ■ | 0.26 |
| Triglyceride, mg/dL | 125.5 ± 65.4 | 124 ± 65.3 | 132.4 ± 64.9 | 135.6 ± 68 | ▲ | <0.001 |
| Diabetes status | 642 (11.6) | 466 (10.2) | 145 (17.6) | 31 (21.4) | ▲ | <0.001 |
| eGFR, ml/min per 1.73 m2 | 77.8 ± 15.9 | 79 ± 15.6 | 72.6 ± 16.3 | 69.4 ± 16.3 | ▼ | <0.001 |
| Coronary artery calcium, Agatston units | ||||||
| 0 | 2857 (51.6) | 2603 (57.0) | 233 (28.3) | 21 (14.5) | ▼ | <0.001 |
| 1 – 99 | 1447 (26.1) | 1181 (25.8) | 227 (27.6) | 39 (26.9) | ■ | 0.35 |
| 100 – 399 | 723 (13.1) | 495 (10.8) | 190 (23.1) | 38 (26.2) | ▲ | <0.001 |
| ≥ 400 | 512 (9.2) | 292 (6.4) | 173 (21.0) | 47 (32.4) | ▲ | <0.001 |
| Interim AF and/ or CHD | 1077 (19.4) | 743 (16.3) | 277 (33.7) | 57 (39.3) | ▲ | <0.001 |
AVC = aortic valve calcium; AF = atrial fibrillation; BMI = body mass index; CHD = coronary heart disease; eGFR = estimated glomerular filtration rate; HDL = high density lipoprotein; LDL = low density lipoprotein; MAC= mitral annular calcium.
Data are presented as mean ± standard deviation for continuous variables and frequency (percentage) for categorical variables unless otherwise specified.
Direction of association from no progression to progression at both sites: ▲ = increasing; ■ = no association; ▼ = decreasing.
Data presented as median (interquartile interval).
Total stroke
We identified 211 cases of total stroke after a median of 12 years (62,463 person-years) with an unadjusted incidence rate (95% CI) of 3.38 (2.95 – 3.87) per 1,000 person-years. Progression of VC at 1 or 2 sites, compared to no progression, was associated with an increased risk of total stroke, but this did not reach statistical significance. In our demographic adjusted model 1, HRs (95% CI) of total stroke for progression of VC at one site and two sites were 1.41 (1.01 – 1.97) and 1.52 (0.81 – 2.86), respectively, when compared to participants with no VC progression (p-for-trend=0.03) (Table 2). Results were not significant in our fully adjusted model 3 (p-for-trend=0.08). However, progression of MAC specifically was associated with incident total stroke. Comparing progressors and non-progressors, the HR (95% CI) of total stroke for AVC was 1.11 (0.75 – 1.64) and for MAC was 1.64 (1.15 – 2.35) after adjusting for demographics (Table 3). Findings for MAC remained statistically significant in our fully adjusted model 3 [1.59 (1.11 – 2.28)] and after adjusting for baseline CAC in model 4 [1.48 (1.03 – 2.13)]. There was no statistically significant interaction found by age, sex, or race/ethnicity.
Table 2.
Hazard ratios (95% confidence interval) of incident total stroke associated with number of sites with valvular calcification progression, MESA, 2000–2016
| Number of left valve sites with progression of calcification | Total / p-for-trend | |||
|---|---|---|---|---|
| No progression | One site only (AVC or MAC) | Both sites (AVC and MAC) | ||
| N (row %) | 4571 (82.5%) | 823 (14.9%) | 145 (2.6%) | 5539 |
| Total stroke; n (%) | 150 (3.3%) | 50 (6.1%) | 11 (7.6%) | 211 (3.8%) |
| Person-years | 52774 | 8299 | 1390 | 624623 |
| Incidence rate a (95% CI) | 2.84 (2.42 – 3.34) | 6.02 (4.57 – 7.95) | 7.91 (4.38 – 14.29) | 3.38 (2.95 – 3.87) |
| Model 1 | 1 (reference) | 1.41 (1.01 – 1.97) | 1.52 (0.81 – 2.86) | 0.03 |
| Model 2 | 1 (reference) | 1.39 (0.99 – 1.94) | 1.58 (0.84 – 2.97) | 0.03 |
| Model 3 | 1 (reference) | 1.31 (0.94 – 1.84) | 1.45 (0.77 – 2.74) | 0.08 |
| Model 4 | 1 (reference) | 1.23 (0.88 – 1.73) | 1.33 (0.70 – 2.51) | 0.18 |
AVC= aortic valve calcium; MAC= mitral annular calcium.
Fonts in bold are statistically significant, p<0.05.
Incidence rate is unadjusted and per 1,000 person-years
Model 1: age, race/ethnicity, sex, MESA site, CT scanner type, and time between baseline and follow-up CT.
Model 2: Model 1+ educational status, BMI, smoking status, pack-years of smoking, and physical activity.
Model 3: Model 2 + systolic blood pressure, diastolic blood pressure, use of antihypertensives, use of lipid lowering medications, total cholesterol, HDL-cholesterol, LDL-cholesterol, diabetes status and eGFR.
Model 4: Model 3 + baseline coronary calcium.
Table 3.
Table3. Hazard ratios (95% confidence interval) of incident total and ischemic stroke associated with progression of valvular calcification, MESA, 2000–2016
| Progressors vs non-progressors | ||
|---|---|---|
| Aortic valve calcium | Mitral annular calcium | |
| Total stroke, n (%)Progressors vs non-progressors | 31 (5.6) vs 180 (3.6) | 41 (7.4) vs 170 (3.4) |
| N Progressors vs non-progressors | 556 vs 4983 | 557 vs 4982 |
| Model 1 | 1.11 (0.75 – 1.64) | 1.64 (1.15 – 2.35) |
| Model 2 | 1.10 (0.74 – 1.62) | 1.66 (1.16 – 2.38) |
| Model 3 | 1.02 (0.69 – 1.52) | 1.59 (1.11 – 2.28) |
| Model 4 | 0.96 (0.65 – 1.44) | 1.48 (1.03 – 2.13) |
| Ischemic stroke, n (%)Progressors vs non-progressors | 27 (4.9) vs 140 (2.8) | 34 (6.2) vs 133 (2.7) |
| N Progressors vs non-progressors | 552 vs 4943 | 550 vs 4945 |
| Model 1 | 1.20 (0.78 – 1.83) | 1.74 (1.17 – 2.58) |
| Model 2 | 1.17 (0.77 – 1.80) | 1.73 (1.16 – 2.57) |
| Model 3 | 1.07 (0.70 – 1.64) | 1.64 (1.10 – 2.45) |
| Model 4 | 1.01 (0.66 – 1.55) | 1.53 (1.02 – 2.29) |
Fonts in bold are statistically significant, p<0.05.
Model 1: age, race/ethnicity, sex, MESA site, CT scanner type.
Model 2: Model 1+ educational status, BMI, smoking status, pack-years of smoking, and physical activity.
Model 3: Model 2 + systolic blood pressure, diastolic blood pressure, use of antihypertensives, use of lipid lowering medications, total cholesterol, HDL-cholesterol, LDL-cholesterol, diabetes status and eGFR.
Model 4: Model 3 + baseline coronary calcium.
Progressors: participants with >0 Agatston units of change/year between CT scans
Non-progressors: participants with ≤0 Agatston units of change/year between CT scans
After excluding participants with interim AF and CHD (n=1,077), the HR (95% CI) for total stroke among participants with MAC progression compared to no MAC progression was 1.55 (0.96 – 2.48) in our fully adjusted model 3 (Supplemental Table 1). When stratified by participants with and without prevalent calcification at baseline (Agatston score>0), we did not find any statistically significant associations between AVC or MAC progression and total stroke risk (Supplemental Tables 2 and 3).
Ischemic stroke
We observed a total of 167 ischemic strokes during the study interval. Similar to total stroke, progression of VC at 1 or 2 sites, compared to no progression, was associated with an increased risk of ischemic stroke, but this did not reach statistical significance. After excluding participants with non-ischemic stroke events (n=44), the HRs (95% CI) of ischemic stroke for progression of VC at one site and two sites compared to no VC progression were 1.50 (1.03 – 2.17) and 1.72 (0.88 – 3.35), respectively (p-for-trend=0.02) (Table 4). Similar to our analysis for total stroke, associations were not significant in our main model 3 (p-for-trend=0.06, Table 4). There was no statistically significant association between AVC progression and ischemic stroke in any of the models (p>0.05, Table 3). However, progression of MAC was associated with ischemic stroke. In our main model, the HR (95% CI) of ischemic stroke among participants with MAC progression was 1.64 (1.10 – 2.45). This also remained statistically significant after adjusting for baseline CAC (Table 3). However, after excluding participants with interim AF and CHD, the HR (95% CI) for ischemic stroke was 1.57 (0.93 – 2.65) among participants with MAC progression (Supplemental Table 1). We did not find any statistically significant associations between AVC or MAC progression and ischemic stroke risk among participants with and without prevalent AVC or MAC at baseline (Supplemental Tables 2 and 3). Similarly, there was no significant interaction found by age, sex, or race/ethnicity for the associations between VC, AVC, and MAC progression and ischemic stroke.
Table 4.
Hazard ratios (95% confidence interval) of incident ischemic stroke associated with number of sites with valvular calcification progression, MESA, 2000–2016
| Number of left valve sites with progression of calcification | Total / p-for-trend | |||
|---|---|---|---|---|
| No progression | One site only (AVC or MAC) | Both sites (AVC and MAC) | ||
| N (row %) | 4537 (82.6%) | 814 (14.8%) | 144 (2.6%) | 5495 |
| Ischemic stroke; n (%) | 116 (2.6%) | 41 (5.0%) | 10 (6.9%) | 167 (3.0%) |
| Person-years | 52533 | 8235 | 1381 | 62149 |
| Incidence rate a (95% CI) | 2.21 (1.84 – 2.65) | 4.98 (3.67 – 6.76) | 7.24 (3.90 – 13.46) | 2.69 (2.31 – 3.13) |
| Model 1 | 1 (reference) | 1.50 (1.03 – 2.17) | 1.72 (0.88 – 3.35) | 0.02 |
| Model 2 | 1 (reference) | 1.45 (1.00 – 2.11) | 1.74 (0.89 – 3.41) | 0.02 |
| Model 3 | 1 (reference) | 1.34 (0.92 – 1.96) | 1.60 (0.82 – 3.13) | 0.06 |
| Model 4 | 1 (reference) | 1.26 (0.86 – 1.84) | 1.46 (0.74 – 2.87) | 0.14 |
AVC= aortic valve calcium; MAC= mitral annular calcium
Fonts in bold are statistically significant, p<0.05.
Incidence rate is unadjusted and per 1,000 person-years
Model 1: age, race/ethnicity, sex, MESA site, CT scanner type, and time between baseline and follow-up CT.
Model 2: Model 1+ educational status, BMI, smoking status, pack-years of smoking, and physical activity.
Model 3: Model 2 + systolic blood pressure, diastolic blood pressure, use of antihypertensives, use of lipid lowering medications, total cholesterol, HDL-cholesterol, LDL-cholesterol, diabetes status and eGFR.
Model 4: Model 3 + baseline coronary calcium.
Discussion
In this prospective community cohort study of individuals free from stroke at baseline, we found that the risk of total and ischemic stroke was higher, with a greater number of sites with VC progression, but this finding did not reach statistical significance in the overall cohort. Of note, over this relatively short period of 2.4 years, only 15% of participants experienced VC progression in 1 site, and only 3% in 2 sites. We however found that MAC progression was associated significantly with increased risk of total and ischemic stroke independent of ASCVD risk factors and baseline CAC when compared to participants without MAC progression. On the other hand, AVC progression was not associated with total or ischemic stroke.
Though we had initially hypothesized that VC progression in either valve bed would be a marker of worsening subclinical atherosclerosis and similarly associated with stroke, our findings suggest that MAC progression plays a greater role in stroke risk than AVC progression. A prior MESA study by O’Neal et al.8 found MAC progression to be associated with increased risk of incident AF while another MESA study showed that baseline MAC was a better predictor of total and ischemic stroke risk compared to AVC.11 Taken together, these studies, in addition to ours, highlight the role of MAC as a whole, baseline and progression, in predicting stroke risk. In addition, a median of 2.4 years may not have been a sufficient period to provide a clinically meaningful impact on stroke risk when assessing multiple valve sites (AVC and MAC) together. It is also possible that the mechanism of stroke risk associated with VC may vary amongst the different valve sites.
Several other mechanisms, such as cardio-embolism resulting from increased plaque burden, have been suggested to link MAC to stroke, aside from AF risk, and to share similar risk factors between MAC and stroke such as increasing age and hypertension.13, 25 Perhaps MAC is a marker of subclinical left atrial fibrosis or dysfunction that may increase the risk of left atrial thrombus formation.8 It is still unknown if the use of systemic anticoagulation and/or antiplatelets may reduce stroke risk in individuals with MAC. In the Framingham study, MAC presence as determined by echocardiography was shown to be associated with double the risk of stroke in the absence of AF.13 Perhaps interventions aimed at preventing MAC formation and progression may as well reduce stroke risk.
In our sensitivity analyses, excluding participants with interim AF and CHD, we found that the increased risk of total and ischemic stroke associated with MAC progression persisted in our demographic adjusted models and was lost after further adjustment for ASCVD risk factors. These observations may suggest that the associations were not only limited to MAC’s association with AF and CHD. We were also unable to show significant associations in our analyses involving participants with and without prevalent calcification at baseline (Agatston unit> 0). This may have been due to a lack of statistical power for subgroup analysis. We did note that among participants with prevalent calcification, there appeared to be an increased risk of both total and ischemic stroke among AVC and MAC progressors. This may suggest that the risk of stroke is increased once VC is established and is independent of the rate of progression. However, among individuals without baseline MAC, incident MAC was also suggestive for some increased stroke risk. Again, this may provide credence to the role of MAC as a whole in stroke risk prediction.
Limitations and strengths
Our study has some limitations worthy of mention. First, our observational study results may be prone to residual confounding. However, we did adjust for a number of covariates known to be associated with stroke risk. We also excluded interim AF and CHD in our sensitivity analysis. Second, our analysis assumed that the risk within the groups of progressors and non-progressors was homogenous. For example, among progressors, some individuals progressed from a zero score to a non-zero score, whereas others with prevalent VC with Agatston scores >0 at baseline just progressed further, and this risk might be different. However, we attempted to conduct a separate analysis in participants with and without prevalent calcification. Third, our findings may be limited by multiple testing given the multiple presentations of the composite exposure, different hierarchical models, and two stroke types evaluated.
On the other hand, our study has a number of important strengths. First, to our knowledge, this is the first study exploring the impact of VC progression on stroke risk in an ethnically diverse group of individuals. We utilized the well-characterized MESA cohort and could take into account a number of risk factors and potential confounders. Second, we provide some insight into the possible associations between VC progression and stroke beyond AF risk and ASCVD risk factors.
Conclusions
We found that the progression of MAC over 2.4 years was associated with increased risk of total and ischemic stroke over a median of 12 subsequent years. Further studies should confirm these associations in other populations and evaluate whether interventions aimed at reducing the risk of VC progression, most especially MAC progression, can impact the risk of stroke or whether the use of systemic anticoagulants or antiplatelets may reduce stroke risk in individuals with MAC progression.
Supplementary Material
Highlights.
We examined valve calcium progression with stroke risk in multiethnic cohort.
Progression of mitral annular calcium was associated with higher stroke risk.
This was independent of cardiovascular risk factors and coronary artery calcium.
However, progression of aortic valve calcium was not associated with stroke risk.
Acknowledgements
The authors thank the other investigators, the staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Financial support
This research was supported by R01 HL071739 and MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. Drs. Michos and Zhao are additionally funded by the Blumenthal Scholars Award in Preventive Cardiology at Johns Hopkins University.
Footnotes
Conflicts of interest
Dr. Budoff receives grant support from General Electric. The other authors do not have any disclosures.
Trial registration
The MESA cohort design is registered at clinicaltrials.gov as follows: https://clinicaltrials.gov/ct2/show/NCT00005487.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Benjamin EJ, Muntner P, Alonso A, et al. , Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association, Circulation, 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- [2].Ovbiagele B and Nguyen-Huynh MN, Stroke epidemiology: advancing our understanding of disease mechanism and therapy, Neurotherapeutics, 2011;8:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Budoff MJ, Young R, Burke G, et al. , Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA), European Heart Journal, 2018;39:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Budoff MJ, Nasir K, Katz R, et al. , Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA), Atherosclerosis, 2011;215:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hermann DM, Lehmann N, Gronewold J, et al. , Thoracic aortic calcification is associated with incident stroke in the general population in addition to established risk factors, European Heart Journal Cardiovascular Imaging, 2015;16:684–690. [DOI] [PubMed] [Google Scholar]
- [6].Fox CS, Vasan RS, Parise H, et al. , Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study, Circulation, 2003;107:1492–1496. [DOI] [PubMed] [Google Scholar]
- [7].Owens DS, Budoff MJ, Katz R, et al. , Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population, JACC Cardiovascular Imaging, 2012;5:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].O’Neal WT, Efird JT, Nazarian S, et al. , Mitral annular calcification progression and the risk of atrial fibrillation: results from MESA, European Heart Journal Cardiovascular Imaging, 2018;19:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim J, Budoff MJ, Nasir K, et al. , Thoracic aortic calcium, cardiovascular disease events, and all-cause mortality in asymptomatic individuals with zero coronary calcium: The Multi-Ethnic Study of Atherosclerosis (MESA), Atherosclerosis, 2017;257:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tison GH, Guo M, Blaha MJ, et al. , Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis, Journal of Cardiovascular Computed Tomography, 2015;9:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kianoush S, Al Rifai M, Cainzos-Achirica M, et al. , Thoracic extra-coronary calcification for the prediction of stroke: The Multi-Ethnic Study of Atherosclerosis, Atherosclerosis, 2017;267:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kizer JR, Wiebers DO, Whisnant JP, et al. , Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study, Stroke, 2005;36:2533–2537. [DOI] [PubMed] [Google Scholar]
- [13].Benjamin EJ, Plehn JF, D’Agostino RB, et al. , Mitral annular calcification and the risk of stroke in an elderly cohort, The New England Journal of Medicine, 1992;327:374–379. [DOI] [PubMed] [Google Scholar]
- [14].Wexler L, Brundage B, Crouse J, et al. , Coronary Artery Calcification: Pathophysiology, Epidemiology, Imaging Methods, and Clinical Implications, Circulation, 1996;94:1175–1192. [DOI] [PubMed] [Google Scholar]
- [15].Bild DE, Bluemke DA, Burke GL, et al. , Multi-Ethnic Study of Atherosclerosis: objectives and design, American Journal of Epidemiology, 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- [16].Levey AS and Stevens LA, Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions, American Journal of Kidney Disease, 2010;55:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carr JJ, Nelson JC, Wong ND, et al. , Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study, Radiology, 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- [18].Ezeigwe A, Fashanu OE, Zhao D, et al. , The novel inflammatory marker GlycA and the prevalence and progression of valvular and thoracic aortic calcification: The Multi-Ethnic Study of Atherosclerosis, Atherosclerosis, 2019;282:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Agatston AS, Janowitz WR, Hildner FJ, et al. , Quantification of coronary artery calcium using ultrafast computed tomography, Journal of the American College of Cardiology, 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- [20].Budoff MJ, Katz R, Wong ND, et al. , Effect of Scanner Type on The Reproducibility of Extracoronary Measures of Calcification: The Multi-Ethnic Study of Atherosclerosis, Academic Radiology, 2007;14:1043–1049. [DOI] [PubMed] [Google Scholar]
- [21].Budoff MJ, Takasu J, Katz R, et al. , Reproducibility of CT Measurements of Aortic Valve Calcification, Mitral Annulus Calcification, and Aortic Wall Calcification in the Multi-Ethnic Study of Atherosclerosis, Academic Radiology, 2006;13:166–172. [DOI] [PubMed] [Google Scholar]
- [22].Longstreth WT Jr., Gasca NC, Gottesman RF, et al. , Adjudication of Transient Ischemic Attack and Stroke in the Multi-Ethnic Study of Atherosclerosis, Neuroepidemiology, 2018;50:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Detrano R, Guerci AD, Carr JJ, et al. , Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups, New England Journal of Medicine, 2008; 358(13):1336–45. [DOI] [PubMed] [Google Scholar]
- [24].Heckbert SR, Wiggins KL, Blackshear C, et al. , Pericardial fat volume and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis and Jackson Heart Study, Obesity, 2017;25:1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kanjanauthai S, Nasir K, Katz R, et al. , Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA), Atherosclerosis, 2010;213:558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.